Abstract
Background
Vulvodynia, vulvar pain of unknown origin lasting at least 3 months, affects 7% of American women. Dyspareunia, its frequent companion, renders sexual intercourse virtually impossible. Although few therapies are efficacious and rapid pain relief is rarely possible, there have been no sham/placebo-controlled studies of acupuncture for vulvodynia. Aims are to: 1) determine efficacy of acupuncture for vulvodynia, 2) explore duration of the acupuncture effect.
Methods
In a pretest/posttest randomized controlled, double-blind (practitioner-patient) efficacy trial of a standardized acupuncture protocol, we will randomize 80 participants 1:1 to either penetrating needle or skin-touch placebo needle groups. Both types of needles are designed to blind both the acupuncturist and participant. Participants with vulvodynia will insert and remove a tampon as a standardized stimulus and complete primary measures of vulvar pain (pain intensity) and secondary measures of dyspareunia (Female Sexual Function Index, FSFI dyspareunia subscale score) and sexual function (FSFI total score) pretreatment, after the 10th acupuncture session, and pain measures weekly until return to pretest levels. Upon study completion control group participants will be offered 10 free real acupuncture sessions.
Discussion
This is the first multi-needle multi-session RCT using double-blind acupuncture needles as a reliable sham. We hypothesize that controlling for baseline, at posttest there will be statistically significant less vulvar pain and dyspareunia and more sexual function over five weeks in the penetrating needle group compared to the skin touch placebo group.
Conclusion
This study is responsive to the need for efficacious pain management for women with vulvodynia.
ClinicalTrials.gov Identifier: NCT03364127.
Keywords: Acupuncture, Double-blind acupuncture needles, Placebo, Vulvodynia, Provoked vestibulodynia, Tampon test
1. Introduction
Our goal is to demonstrate the effects of acupuncture for the treatment of vulvodynia. Vulvodynia is chronic vulvar pain of unknown etiology that lasts at least 3 months and may be accompanied by other associated factors [1]. Up to 7% of American women have vulvodynia [2,3], a debilitating pain syndrome characterized by pain (burning, irritation, stinging or rawness) in the vulva, and dyspareunia that renders sexual intercourse virtually impossible [2,3] leaving these women desperate for relief. Not only are these women in pain, but they often lose their partners or have relationship difficulties due to their inability to have sexual intercourse [4,5]. Few therapies have been proven efficacious and rapid pain relief is unpredictable and rarely possible [[1], [2], [3]]. Americans spend $31 to $72 billion annually on treatments for this life altering pain syndrome [6]. After exhausting biomedical options, these women often turn to acupuncture [2,3].
In contrast to other pain conditions [7,8], there has been only 1 acupuncture sham-controlled study of vulvodynia which examined the feasibility and acceptability of using acupuncture augmentation of lidocaine for localized provoked vestibulodynia [9]. Only four studies, including one of ours [10], provide some evidence of the effect of acupuncture on vulvodynia [[11], [12], [13]]. In all of them, women had less pain, better quality of life, improved sexual health, and improved mental health. However, three were single-group uncontrolled acupuncture studies [[11], [12], [13]]. Our randomized wait-list controlled pilot study of 36 women with vulvodynia showed a statistically significant and clinically meaningful (>1.5 point) reduction [14] in vulvar pain (p < .01) and dyspareunia (p < .003) and an increase in overall sexual function (p < .04) after a 13-needle, 5-week, 10 session acupuncture protocol compared to a wait-list control group [10]. This standardized acupuncture treatment protocol [10] includes acupuncture points that relieve pain in the genitals. The results of our initial pilot study provide the first evidence from a two-group design that the acupuncture protocol could reduce pain intensity, pain during intercourse, and increase overall sexual function. Our findings, however, warrant stronger evidence to support the inference that the effect is indeed due to the acupuncture since neither ours nor any other study included a sham placebo control or provided follow-up data beyond immediate posttest, which means that the duration of the acupuncture effect is unknown. Also, our recent study testing the feasibility of using double-blind acupuncture needles in the same 13-needle, 10-session acupuncture protocol [15] paves the way to overcome this gap. Findings from these two studies support our capacity to conduct the first double-blind randomized controlled trial (RCT) of acupuncture for vulvodynia while exploring its duration of effect.
Our first aim is to compare the genuine penetrating needle group and the sham skin-touch placebo needle group for effects on the primary outcome, vulvar pain, and secondary outcomes, dyspareunia and sexual function. We hypothesize that, controlling for baseline, there will be a statistically significant difference between the genuine penetrating needle group and the sham skin-touch placebo group after treatment with less vulvar pain and dyspareunia and better sexual function in the penetrating needle group. Our second aim is to describe the duration of the acupuncture treatment and placebo effects weekly until pain returns to pretest levels or up to 12 weeks after posttest. For women with a clinically meaningful reduction in pain intensity (at least 1.5 points) [13] at posttest compared to pretest, we will describe the variability over time in vulvar pain intensity on a 0–10 pain intensity number scale (PINS) [16] after a tampon insertion-removal stimulus and thereby explore the duration of the effect by intervention groups, vulvodynia subgroups, and demographic subgroups (e.g., age, race, occupation). These findings will provide insights to guide future research on initial and maintenance acupuncture for vulvodynia.
2. Methods
2.1. Study design
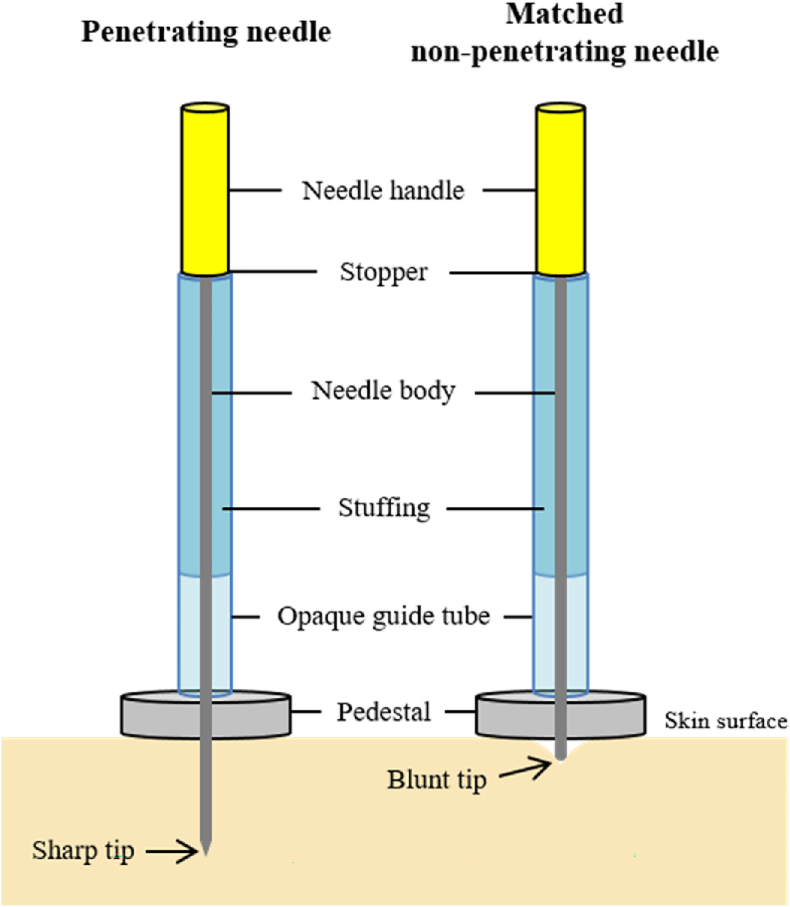
In this ongoing double-blind randomized controlled trial (RCT), we will compare the effects of double-blind acupuncture needles, one of which provides genuine skin penetration and the other sham placebo needle is skin-touch only (Fig. 1), on vulvar pain and dyspareunia in our 13-needle, 10-session, 5-week acupuncture treatment protocol [10]. We have previously described the double-blind needles, which look and feel the same to participant and acupuncturist, in detail in our feasibility study [15]. These double-blind needles provide a strong sham procedure (placebo) to mask the acupuncturist, participant, and research personnel to the type of needle used for the protocol. The study was approved by the UIC Institutional Review Board. The IRB at the University of Florida (UF) approved the study of de-identified data as exempt. Informed written consent will be obtained from all enrolled women.
Fig. 1.
A set of Double-Blind Needles.
2.2. Setting
The study data collection and acupuncture sessions are conducted at the UIC College of Nursing in a clinical research lab space with 5 private examination rooms. One room is set up with a gynecologic exam table for the gynecologic exams, and four rooms are set up with massage tables for the acupuncture treatments. Some of the acupuncture sessions are also conducted at two private acupuncture clinics in the greater Chicago metropolitan area to facilitate study participation.
2.3. Sample
A sample of 88 women with a diagnosis of vulvodynia, either provoked vestibulodynia or generalized vulvodynia, 18 years of age or older are being recruited from clinical and community settings. Eighty participants are expected to complete the study.
2.3.1. Recruitment
We are recruiting from the Chicago metropolitan area. An advertisement campaign is being conducted with study banners placed on Chicago Transit Authority buses and subway cars, Google ads, National Vulvodynia Association email blasts, through a study website (https://vulvodyniastudy.uic.edu/), and health care provider referrals, among other initiatives.
2.3.2. Retention strategies
We anticipate an attrition rate of no more than 10%, based on our two pilot studies [10,15] in which attrition was zero, even for the participants who did not like acupuncture. Women who have not had adequate pain relief from biomedical treatments are highly motivated to complete the acupuncture protocol. An important retention strategy is to engage the participants as active partners in the research by helping them understand the importance of their contributions. We believe our previous zero attrition rate is explained by our participant-centered study processes including: 1) scheduling data collection and acupuncture at times convenient to participants, including Saturdays, Sundays, and evenings; 2) some participants are very busy and often call to reschedule with only several hours’ notice; we always prioritized their schedules and accommodate them; 3) sending reminder emails or texts on the appointment day; and 4) collecting multiple methods of contacting participants (e-mail, cell phone, and home phone) to maximize follow-up. We are employing all 4 of these successful retention strategies for this study as well. Participants also receive $10 after each visit for transportation expenses. All participants assigned to the sham skin-touch placebo acupuncture group will be offered 10-free real acupuncture treatments upon study completion.
2.3.3. Eligibility criteria
Inclusion criteria are: 1) diagnosis of vulvodynia (provoked vestibulodynia or generalized vulvodynia), 2) ≥18 years old, 3) pain now score of 4 or higher on a 0–10 Pain Intensity Numbers Scale with tampon insertion and removal performed at the initial screening exam, 4) have never had acupuncture before, and 5) speaks and reads English. Women are included if they had never had previous acupuncture treatment(s) to minimize possible bias regarding guessing whether they had the penetrating or the placebo needles.
2.3.4. Exclusion criteria
To ensure that vulvar pain and/or dyspareunia is due to vulvodynia and not other co-morbidities, the exclusion criteria are very stringent. Potential participants are screened on the phone for the following conditions and excluded if any of the following are reported: 1) infectious conditions; 2) inflammatory conditions; 3) neoplastic disorders, cancer or pre-cancer of the vulva or Paget's disease; 4) neurologic disorders such as spinal nerve compression or pudendal nerve entrapment; 5) trauma to the genitals; 6) iatrogenic, history of chemotherapy, radiation or surgery of the genitals; 7) hormonal deficiencies; 8) co-morbid pelvic pain conditions (to avoid confounding pain outcomes); 9) pelvic inflammatory disease; 10) endometriosis; 11) pregnancy; 12) any of the following common comorbid conditions that have been active in the past 6 months: interstitial cystitis, painful bladder syndrome, irritable bowel syndrome, temporomandibular joint disorder, chronic fatigue syndrome, migraine headaches, fibromyalgia. Women receiving concurrent therapies that may decrease stress and/or muscle tension (i.e., concomitant physical therapy, biofeedback, massage, or other acupuncture sessions) are excluded. Women are also excluded if they have atrophic vaginitis as determined by a vaginal maturation index included as part of the gynecologic exam [17]. Potential participants deemed to be eligible are scheduled for a tampon test and a gynecologic exam at the UIC College of Nursing to confirm a vulvodynia diagnosis and type (provoked or generalized), and test for atrophic vaginitis.
2.3.5. Randomization and blinding
Women diagnosed with vulvodynia during the gynecologic exam are randomized 1:1 to either the genuine penetrating or the sham skin-touch placebo needle groups. Randomization is implemented and managed using REDCap software's randomization module adapted to achieve complete blinding of group allocation. We stratify women based on the type of vulvodynia determined during the exam (provoked vestibulodynia or generalized vulvodynia) and REDCap assigns the treatment arm as the next in sequence within permutated blocks for the separate strata. Study participants, acupuncturists, and research staff, and the principal investigator are blind to study assignment through the use of unique identifiers. The study statistician is aware of a group assignment code while an independent statistician is aware of the code meaning (genuine versus sham). We have previously described details of the randomization and blinding protocols for this double-blind RCT, including blinding assessment of acupuncturists and participants [18].
2.3.6. Sample power
Our approach to sample size estimation for Aim 1 comparing the two treatment groups is conservatively based on mean differences between groups at the end of treatment assuming 80% power, alpha = .05, and a two-sided test. Previously, we found a large to very large effect size of 1.05 for vulvar pain measured by a visual analogue scale comparing our acupuncture protocol to a waitlist control. For the proposed study, we recognize that a smaller effect size between the treatment arms should be expected since the sham placebo needle condition may result in more improvement in vulvar pain than a waitlist control condition. We estimated a minimum detectable effect size of 0.60–0.63 based on 80 of 88 participants completing the study. This minimum detectable effect size represents a 40–43% reduction of our 1.05 effect size. We anticipate that our actual power will be greater in models testing intervention efficacy when including covariates such as length of illness and diagnosis subtype and controlling for baseline pain intensity.
Aim 2 is a descriptive aim to describe the variability of treatment duration effects. Our wait-list controlled trial showed that 15/18 (83%) of intervention participants showed a clinically significant reduction in pain intensity of 1.5 points or more, but 3/18 (17%) of the wait-list control participants met this threshold for pain reduction. Based on these findings we conservatively estimate having 40 (50%) participants from both conditions who show a meaningful improvement following acupuncture or placebo treatment, a number more than sufficient to estimate preliminary estimates of treatment duration variability.
2.4. Procedures
A flow chart of the study protocol is included in Fig. 3. This study protocol consists of a 3-step eligibility process. For the first step, women complete an initial telephone screening with the research specialist. For the second step, if women fulfill the initial inclusion criteria and none of the exclusion criteria by telephone screening, they are scheduled for further screening for the presence of vulvar pain and dyspareunia at the UIC College of Nursing. Informed consent for study enrollment is then obtained and includes information stating that participants will be randomized to either the genuine penetrating needle or sham skin-touch placebo acupuncture groups. A urine pregnancy test is completed as acupuncture is contraindicated at some acupuncture points during pregnancy. Participants are then asked to fully insert and remove a regular size Tampax® tampon with cardboard applicator as a standard stimulus and note their pain intensity from 0 to 10. Women with pain of 4 or higher are eligible to continue and complete the baseline study measures using a tablet computer. For the third step, participants are escorted to a private exam room for a gynecologic exam to confirm the presence of vulvodynia and its type, provoked vestibulodynia (pain provoked by touch and localized to the vulvar vestibule) or generalized vulvodynia (spontaneous diffuse vulvar pain that may extend to the perineum and inner thighs) [19,20]. A vaginal smear is taken from all participants and is tested right after the exam for the presence of atrophic vaginitis, which is determined by if there are >15% parabasal cells per wet mount [17]. All gynecologic exams are performed by a urogynecologist or a certified nurse midwife. A trained, female research specialist is in attendance during all exams. Women confirmed to have a diagnosis of vulvodynia without atrophic vaginitis and with no other comorbidities are randomized via the REDCap randomization module to a needle packet number that corresponds to the genuine penetrating needle group or the sham skin-touch placebo groups. The participant is then escorted to a private exam room for acupuncture using the assigned numbered packet containing double blinded needles. The research specialist, participant, acupuncturist, principal investigator, and statistician are all blind to group assignment. Data collection and management are facilitated by the Research Electronic Data Capture (REDCap) [21,22], hosted at UIC. Upon completion of the 10th and final acupuncture session, participants complete all posttest study measures of vulvar pain, dyspareunia, and sexual function.
Fig. 3.
Flow chart of the study protocol.
2.5. Intervention
2.5.1. Standardized acupuncture point protocol
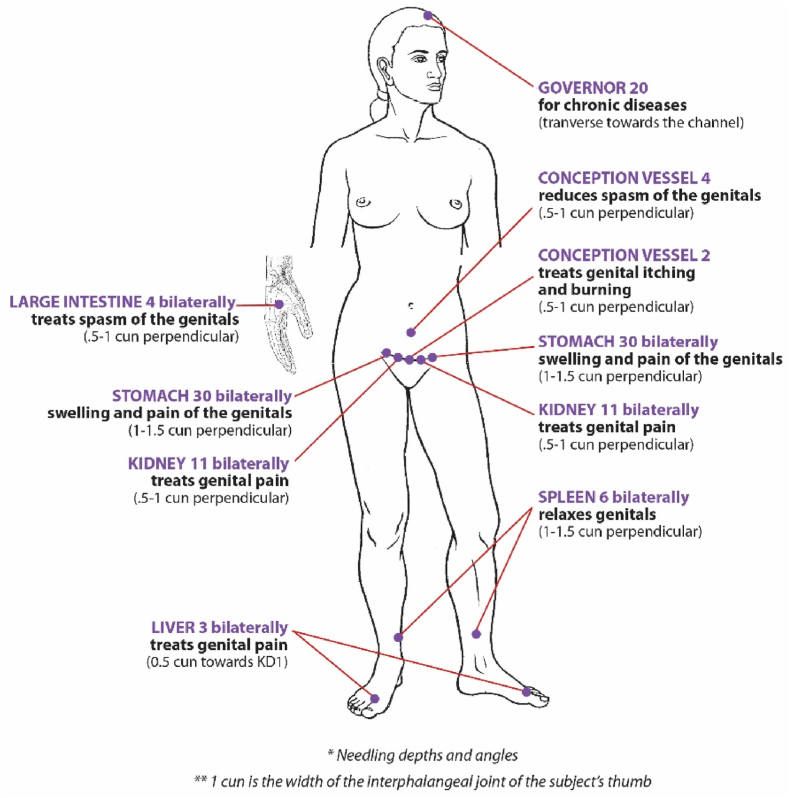
All participants receive a 13-needle standardized acupuncture protocol (Fig. 2) twice per week for 5 weeks, for a total of 10 sessions. Needles are retained for 45 min per session; Standards for Reporting Interventions in Clinical Trials of Acupuncture (STRICTA) [23] guidelines are adhered to. All efforts to control nonspecific therapeutic effects such as limiting conversation between acupuncturist and participant are maintained unless participant safety is compromised.
Fig. 2.
Standardized treatment protocol for vulvodynia.
Four acupuncturists, who are well-trained by the principal investigator in the study protocol and observed for fidelity, administer the 45-min acupuncture treatments twice a week for 5 weeks, for a total of 10 sessions. At every other visit the participant completes a pregnancy test. If pregnant, they will be terminated from the study. At pretest and at posttest participants insert and remove a tampon as a standardized stimulus and complete measures of vulvar pain, dyspareunia, and sexual function [24].
2.5.2. Entering the duration of the Acupuncture Effect Study Phase
If the pain from the posttest tampon insertion and removal after the 10th acupuncture treatment is equal to or higher than pretest levels, the participant does not achieve a reduction in vulvar pain after acupuncture and does not enter the next phase of the study. The Duration of the Acupuncture Effect Study Phase determines how long acupuncture treatments will reduce vulvar pain. If the participant's posttest tampon test score of vulvar pain intensity is at least 1.5 points lower than at pretest on a pain intensity numbers scale (PINS) of 0–10 (0 = no pain and 10 = pain as bad as it could be), the participant enters the Duration of the Acupuncture Effect Study Phase. Once per week, participants at home insert and remove a Tampax™ tampon with applicator until the provoked pain is equal or greater than the pretest tampon test score, for a maximum of 12 weeks. How long the acupuncture effect lasts will be determined by when provoked tampon pain returns to pre-acupuncture levels. Post tampon test pain scores are recorded by the participant once per week. Upon entering the Duration of the Acupuncture Effect Study Phase, REDCap automatically sends participants an email with a link to record their weekly pain scores in the vulvar pain diary.
2.5.3. Revealing participant allocation
Three days after completion of the Duration of the Acupuncture Effect Study Phase (up to 12 weeks post 10th acupuncture treatment) participants receive an email revealing their group assignment (genuine penetrating or sham skin-touch placebo acupuncture). The email is automatically generated by REDCap with data from the tool's randomization module, so that all key investigators and study personnel remain blind to group assignment. All participants who receive sham placebo acupuncture are offered 10 free regular acupuncture treatments at an acupuncturist's practice.
2.5.4. Fidelity of the study intervention
Acupuncturists are exactingly trained on the protocol and the use of the double-blind needles (Fig. 1). The research specialist prepares each needle for insertion and then hands it to the acupuncturist. Acupuncture needles are inserted according to the double-blind protocol checklist developed during our feasibility study. The research specialist observes the placement of every double-blind needle at every session to document the acupuncturists’ adherence to the protocol (Table 1).
Table 1.
Double-blind needle protocol checklist (acupuncture intervention procedural steps).
| Procedural Step Number | Acupuncturist (observed by research specialist): |
|---|---|
| 1 | Enters the exam room (participant placed previously in a supine position by the research specialist) |
| 2 | Does not speak to participant unless it is a matter of safety |
| 3 | Wipes the participant's skin with alcohol wipe at the 13 acupuncture points |
| 4 | Is handed the needle device from the research specialist and places the pedestal on the acupuncture point |
| 5 | Places the tip of thumb and index finger and holds the outer guide tube where it meets the pedestal while applying downward pressure |
| 6 | Taps the top of the needle handle with the pad of the index finger until it is even with the top of the outer guide tube |
| 7 | Removes the outer guide tube and hands it to the research specialist |
| 8 | Inserts the needle further while rotating alternately with the thumb of the insertion hand against the pad of the index finger, while applying downward pressure until the bottom of the needle handle touches the top of the inner guide tube Simultaneously the thumb and index finger of the other hand will stabilize the inner guide tube |
| 9 | Repeats steps 1–8 for all 13 needle insertions on all 13 acupuncture points |
| 10 | Retains all needles for 45 min |
| 11 | Leaves the exam room, but remains available for emergencies |
| 12 | After 45 min returns to the exam room |
| 13 | Does not speak to the participant unless it is a matter of safety |
| 14 | Removes all 13 needle devices one at a time Needles are withdrawn: 1) 15 mm or 2/3rd the length of the handle for the 5 mm and 10 mm insertion depths, or 2) 1 full length of the handle (20 mm) for the 18 mm insertion depths |
| 15 | Wipes each acupuncture point with an alcohol wipe after each needle device is removed |
| 16 | Places each of 13 needles as they are removed into an envelope with the participant's study number on it which will be sealed by research specialist |
| 17 | Leaves the exam room |
2.6. Measures
PAINReportIt is an expanded electronic version of the 1970 version of the McGill Pain Questionnaire [25] (Nursing Consultants LLC, Seattle, Washington) and is an interactive, touch screen method for pain assessment that can be self-administered by the patient and requires little or no computer experience and minimal or no provider time for administration [[26], [27], [28]]. We created a vulvodynia module housed within PAINReportIt that contains the self-report measures used for this study i.e., Pain Intensity Number Scale, Female Sexual Function Index, Protocol Acceptability Scale of Treating Vulvodynia with Acupuncture, and questions asking the participant what type of acupuncture they received (genuine penetrating or sham placebo) and questions asking the acupuncturist what type of acupuncture they administered. PAINReportIt has been found to be reliable and valid [28,29].
The Tampon Test enables investigators to obtain a vulvar pain score that considers the fact that women with vulvodynia who have provoked pain only could have no pain during all the study measurement sessions yet be unable to have intercourse because of pain. We will use Foster et al.‘s [30] tampon insertion and removal stimulus protocol before pretest and posttest measures and weekly for up to 12 weeks for the duration of effect phase. The tampon test provides a standard stimulus condition under which vulvar pain is measured and is appropriate for women with either provoked vestibulodynia or generalized vulvodynia. A random sample of 480 women were interviewed regarding their use of tampons [30]. Reported pain at the time of first use of tampons was the strongest risk factor for the development of vulvodynia (odds ratio, 2.4; 95% confidence interval, 1.1 to 4.9). Participants insert and remove (using the accompanying applicator) a regular size unlubricated Tampax™ tampon to provoke pain. In Foster et al.‘s 8-week clinical trial, change in the Tampon Test measure significantly correlated to: daily pain (r = 0.42), intercourse pain (r = 0.35), and cotton swab vestibular pain (r = 0.38). During the two-week baseline phase, vulvodynia-afflicted women reported stable mean Tampon Test scores 4.6 ± 2.6 (Week −2); 4.6 ± 2.7 (Week −1); and 4.7 ± 2.8 (Week 0) with moderate week-to-week reliability, (weighted Kappa = 0.52). Women with vulvodynia performed the Tampon Test 96.3% of the requested time, which was two-fold higher adherence than intercourse pain measurement (49.7%) [30,31].
The primary outcome, vulvar pain intensity (provoked by the tampon insertion and removal stimulus), is measured with the Pain Intensity Number Scale (PINS) [16], a tool commonly used as a measure of pain intensity. The PINS will also be used to measure duration of the treatment effect. The PINS provides ratio level data. The participant rates the pain intensity a number between 0 and 10, where 0 is no pain and 10 is pain as bad as it could be. The PINS and standardized instructions are embedded within. Investigators have reported concurrent (r = 0.80 to 0.89) [32,33] and construct [34,35] validity, and reliability and sensitivity [33,36] of the PINS. It is valid and reliable for patients with a variety of pain conditions [33,36]. A 1.5 point decrease in the PINS score is considered a clinically meaningful reduction in pain intensity [14].
For the secondary outcomes of dyspareunia and sexual function, participants complete the Female Sexual Function Index (FSFI). The FSFI is a 19-item self-report questionnaire designed to assess female sexual function by examining six domains: desire, arousal, lubrication, ability to orgasm, sexual satisfaction, and dyspareunia (pain with intercourse) [24,37]. Possible scores range from zero to five for each item and higher scores indicate improved function. For this secondary outcome, the FSFI defines sexual activity as caressing, foreplay, masturbation and vaginal intercourse; therefore, women who do not have sexual relations with a partner may still complete the tool. The FSFI has been found to be reliable and valid [37].
2.7. Post-intervention study measures
After the 10th and last acupuncture treatment, participants complete a 10-question acceptability questionnaire that assesses the acceptability of the study intervention, Protocol Acceptability Scale of Treating Vulvodynia with Acupuncture. It is reliable and valid and showed test-retest reliability over 4 weeks [27,28]. Participants also complete questions regarding what type of needle they thought was used in the study and their level of confidence in their guess. The acupuncturists are also asked questions about the needle type they thought they administered for each participant and their level of confidence in their guess.
2.8. Statistical analysis
A codebook was created with the variable names, descriptions, and value codes of each variable. Missing data will be minimized through our retention efforts but are inevitable, to some degree, in any longitudinal study. We will address missing data by intention to treat analyses (ITT), using the full information maximum likelihood (FIML) approach, which has been shown to produce unbiased parameter estimates and standard errors.
The focus of Aim 1 is to compare the penetrating needle group and the skin-touch placebo needle group for effects on the: (a) primary outcome, vulvar pain (PAINReportIt® average pain intensity), and (b) secondary outcomes, dyspareunia (FSFI dyspareunia) and sexual function (FSFI total). We will test for treatment group differences using separate general linear regression models for each primary and secondary outcome. In these models, the independent variable will be the treatment condition (penetrating needle versus non-penetrating placebo needle) and we will control for the baseline outcome measurement. We will explore the impact of hypothesized moderators and confounders such as types of vulvodynia, length of illness, and analgesic use.
The focus of Aim 2 is to describe the duration of the acupuncture treatment and placebo effects for up to 12 weeks after the 10th acupuncture treatment among participants who showed a clinically meaningful reduction in pain intensity. We will track participants’ vulvar pain levels weekly for up to 12 weeks following treatment completion using PINS scores after the tampon stimulus, noting the time-to-event, defined as return to their pretest value, at which point they will cease the follow-up measurements. We will explore treatment arm differences in the duration of treatment effect visually using the Kaplan-Meier plot. We will estimate the effects for group differences in survival curves with the log-rank test. While we are not powered for inferential tests, we will use Cox proportional hazard regression model to determine if the proportionality assumption is met and to explore treatment effect duration controlling for other correlates such as diagnosis type, illness duration, other clinical factors, or demographic characteristics. If not met, we will employ methods to learn about time-varying effects. These analyses will inform future studies.
3. Discussion
We anticipate that evidence of the efficacy of acupuncture for vulvodynia will provide insights to guide future research on initial and maintenance acupuncture for vulvodynia. Because there are so few treatments for vulvodynia that have been shown to be efficacious, new approaches for treating women with vulvodynia are sorely needed. Specifically, the purpose of this study is to respond to the need to develop efficacious pain management for women with vulvodynia by providing evidence for the efficacy of acupuncture.
4. Conclusion
Our goal is to demonstrate the effects of acupuncture for the pain of vulvodynia by performing the first double-blind randomized controlled trial of acupuncture as a treatment method. Evidence from this study will also aid in assessing the duration of the treatment effect of acupuncture thus providing insights about the need for maintenance treatment to control vulvar pain and dyspareunia. Findings will inform future studies for a pragmatic trial and translation to practice.
Funding
This publication was made possible in part by Grant Numbers R01 HD091210 and F31 NR019529 from the National Institute of Child Health and Human Development (NICHD), and the National Institute for Nursing Research (NINR). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NICHD or NINR. The final peer-reviewed manuscript is subject to the National Institutes of Health Public Access Policy. The Center for Clinical and Translational Science which hosted the data via REDCap was supported by the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant UL1TR002003. This publication is co-sponsored by the Rockefeller University Heilbrunn Family Center for Research Nursing through the generosity of the Heilbrunn Family and the National Center for Advancing Translational Sciences, National Institutes of Health, through Rockefeller University, Grant Number UL1 TR001866.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Dr. Nobuari Takakura and the Educational Foundation of Hanada Gakuen possess a US patent 6575992B1, a Canadian patent CA 2339223, a Korean patent 0478177, a Taiwan patent 150135, a Chinese patent ZL00800894.9 (Title: Safe needle, placebo needle and needle set for double blind), and two Japanese patents 4061397 (Title: Placebo needle, and needle set for double-blinding) and 4315353 (Title: Safe needle) on the needles described in this abstract. Dr. Takakura is a salaried employee of the Educational Foundation of Hanada Gakuen. Dr. Diana J. Wilkie is the founder and chairman of eNURSING LLC, a company without current ownership of the PAINReportIt software. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Acknowledgments
We thank the following acupuncturists for their dedication to this study: Jung Fu, Stephanie Marynus, Caroline Jung, Sunae Son.
Contributor Information
Judith M. Schlaeger, Email: jschlaeg@uic.edu.
Marie L. Suarez, Email: mlsuarez@uic.edu.
Jennifer E. Glayzer, Email: jshenk5@uic.edu.
William H. Kobak, Email: wkobak@uic.edu.
Monya Meinel, Email: meinemo@uic.edu.
Alana D. Steffen, Email: steffena@uic.edu.
Larisa A. Burke, Email: laburke@uic.edu.
Heather A. Pauls, Email: hpauls2@uic.edu.
Yingwei Yao, Email: y.yao@ufl.edu.
Miho Takayama, Email: takayama@tau.ac.jp.
Hiroyoshi Yajima, Email: yajima@tau.ac.jp.
Ted J. Kaptchuk, Email: tkaptchu@bidmc.harvard.edu.
Nobuari Takakura, Email: takakura@tau.ac.jp.
David Foster, Email: david_foster@urmc.rochester.edu.
Diana J. Wilkie, Email: diwilkie@ufl.edu.
References
- 1.Bornstein J., Goldstein A.T., Stockdale C.K., et al. ISSVD, ISSWSH, and IPPS consensus terminology and classification of persistent vulvar pain and vulvodynia. J. Sex. Med. 2015;13(4):607–612. doi: 10.1016/j.jsxm.2016.02.167. 2016. [DOI] [PubMed] [Google Scholar]
- 2.Haefner H.K., Collins M.E., Davis G.D., et al. The vulvodynia guideline. J. Low. Genit. Tract Dis. 2005;9(1):40–51. doi: 10.1097/00128360-200501000-00009. [DOI] [PubMed] [Google Scholar]
- 3.Stockdale C.K., Lawson H.W. Vulvodynia guideline update. J. Low. Genit. Tract Dis. 2013;18(2):93–100. doi: 10.1097/LGT.0000000000000021. 2014. [DOI] [PubMed] [Google Scholar]
- 4.Brotto L.A., Basson R., Gehring D. Psychological profiles among women with vulvar vestibulitis syndrome: a chart review. J. Psychosom. Obstet. Gynaecol. 2003;24(3):195–203. doi: 10.3109/01674820309039673. [DOI] [PubMed] [Google Scholar]
- 5.Basson R. The recurrent pain and sexual sequelae of provoked vestibulodynia: a perpetuating cycle. J. Sex. Med. 2012;9(8):2077–2092. doi: 10.1111/j.1743-6109.2012.02803.x. [DOI] [PubMed] [Google Scholar]
- 6.Xie Y., Shi L., Xiong X., Wu E., Veasley C., Dade C. Economic burden and quality of life of vulvodynia in the United States. Curr. Med. Res. Opin. 2012;28(4):601–608. doi: 10.1185/03007995.2012.666963. [DOI] [PubMed] [Google Scholar]
- 7.Vickers A.J., Cronin A.M., Maschino A.C., et al. Acupuncture for chronic pain: individual patient data meta-analysis. Arch. Intern. Med. 2012;172(19):1444–1453. doi: 10.1001/archinternmed.2012.3654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vickers A.J., Vertosick E.A., Lewith G., et al. Acupuncture for chronic pain: update of an individual patient data meta-analysis. J. Pain. 2018;19(5):455–474. doi: 10.1016/j.jpain.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rubin L.E.H., Mist S.D., Schnyer R.N., Chao M.T., Leclair C.M. Acupuncture augmentation of lidocaine for provoked, localized vulvodynia: a feasibility and acceptability study. J. Low. Genit. Tract Dis. 2019;23(4):279–286. doi: 10.1097/LGT.0000000000000489. [DOI] [PubMed] [Google Scholar]
- 10.Schlaeger J.M., Xu N., Mejta C.L., Park C.G., Wilkie D.J. Acupuncture for the treatment of vulvodynia: a randomized wait-list controlled pilot study. J. Sex. Med. 2015;12(4):1019–1027. doi: 10.1111/jsm.12830. [DOI] [PubMed] [Google Scholar]
- 11.Powell J., Wojnarowska F. Acupuncture for vulvodynia. J. R. Soc. Med. 1999;92(11):579–581. doi: 10.1177/014107689909201110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Danielsson I., Sjöberg I., Ostman C. Acupuncture for the treatment of vulvar vestibulitis: a pilot study. Acta Obstet. Gynecol. Scand. 2001;80(5):437–441. [PubMed] [Google Scholar]
- 13.Curran S., Brotto L.A., Fisher H., Knudson G., Cohen T. The ACTIV study: acupuncture treatment in provoked vestibulodynia. J. Sex. Med. 2010;7(2 Pt 2):981–995. doi: 10.1111/j.1743-6109.2009.01582.x. [DOI] [PubMed] [Google Scholar]
- 14.Younger J., McCue R., Mackey S. Pain outcomes: a brief review of instruments and techniques. Curr. Pain Headache Rep. 2009;13(1):39–43. doi: 10.1007/s11916-009-0009-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schlaeger J.M., Takakura N., Yajima H., et al. Double-blind acupuncture needles: a multi-needle, multi-session randomized feasibility study. Pilot. Feasibility Stud. 2018;4(1):72. doi: 10.1186/s40814-018-0265-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wilkie D.J., Savedra M.C., Holzemer W.L., Tesler M.D., Paul S.M. Use of the McGill Pain Questionnaire to measure pain: a meta-analysis. Nurs. Res. 1990;39(1):36–41. [PubMed] [Google Scholar]
- 17.Rakoff A. The endocrine factors in pelvic tumors, with a discussion of the Papanicolaou smear method for diagnosis. Radiology. 1948;50(2):190–201. doi: 10.1148/50.2.190. [DOI] [PubMed] [Google Scholar]
- 18.Steffen A.D., Burke L.A., Pauls H.A., et al. Double-blinding of an acupuncture randomized controlled trial optimized with clinical translational science award resources. Clin. Trials. 2020;17(5):545–551. doi: 10.1177/1740774520934910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schlaeger J.M., Patil C.L., Steffen A.D., et al. Sensory pain characteristics of vulvodynia and their association with nociceptive and neuropathic pain: an online survey pilot study. Pain Rep. 2019;4(2) doi: 10.1097/PR9.0000000000000713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Goldstein A.T., Pukall C.F., Brown C., Bergeron S., Stein A., Kellogg-Spadt S. Vulvodynia: assessment and treatment. J. Sex. Med. 2016;13(4):572–590. doi: 10.1016/j.jsxm.2016.01.020. [DOI] [PubMed] [Google Scholar]
- 21.Harris P.A., Taylor R., Minor B.L., et al. The REDCap consortium: building an international community of software platform partners. J. Biomed. Inf. 2019;95 doi: 10.1016/j.jbi.2019.103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Harris P.A., Taylor R., Thielke R., Payne J., Gonzalez N., Conde J.G. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J. Biomed. Inf. 2009;42(2):377–381. doi: 10.1016/j.jbi.2008.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.MacPherson H., Altman D.G., Hammerschlag R., et al. Revised standards for reporting interventions in clinical trials of acupuncture (STRICTA): extending the CONSORT statement. J. Alternative Compl. Med. 2010;16(10) doi: 10.1089/acm.2010.1610. ST-1-ST-14. [DOI] [PubMed] [Google Scholar]
- 24.Rosen R., Brown C., Heiman J., et al. The Female Sexual Function Index (FSFI): a multidimensional self-report instrument for the assessment of female sexual function. J. Sex Marital Ther. 2000;26(2):191–208. doi: 10.1080/009262300278597. [DOI] [PubMed] [Google Scholar]
- 25.Melzack R. The McGill Pain Questionnaire: major properties and scoring methods. Pain. 1975;1(3):277–299. doi: 10.1016/0304-3959(75)90044-5. [DOI] [PubMed] [Google Scholar]
- 26.Huang H.-Y., Wilkie D.J., Zong S.-P.S., et al. Developing a computerized data collection and decision support system for cancer pain management. Comput Inform Nurs. 2003;21(4):206–217. doi: 10.1097/00024665-200307000-00011. [DOI] [PubMed] [Google Scholar]
- 27.Wilkie D.J., Huang H.Y., Berry D.L., et al. Cancer symptom control: feasibility of a tailored, interactive computerized program for patients. Fam. Community Health. 2001;24(3):48–62. [PubMed] [Google Scholar]
- 28.Wilkie D.J., Judge M.K.M., Berry D.L., Dell J., Zong S., Gilespie R. Usability of a computerized PAINReportIt in the general public with pain and people with cancer pain. J. Pain Symptom Manag. 2003;25(3):213–224. doi: 10.1016/s0885-3924(02)00638-3. [DOI] [PubMed] [Google Scholar]
- 29.Schlaeger J.M., Patil C.L., Steffen A.D., Pauls H.A., Roach K.L., Thornton P.D., Hartmann D., Kobak W.H., Yao Y., Suarez M.L., Hughes T.L., Wilkie D.J. PAIN Reports; 2019. Sensory Pain Characteriatics and Their Association with Nociceptive and Neuropathic Pain: an Online Survey Pilot Study. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Foster D.C., Kotok M.B., Huang L.-S., et al. The tampon test for vulvodynia treatment outcomes research: reliability, construct validity, and responsiveness. Obstet. Gynecol. 2009;113(4):825. doi: 10.1097/AOG.0b013e31819bda7c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Foster D.C., Kotok M.B., Huang L.-S., et al. Oral desipramine and topical lidocaine for vulvodynia: a randomized controlled trial. Obstet. Gynecol. 2010;116(3):583–593. doi: 10.1097/AOG.0b013e3181e9e0ab. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Murphy D.F., McDonald A., Power C., Unwin A., MacSullivan R. Measurement of pain: a comparison of the visual analogue with a nonvisual analogue scale. Clin. J. Pain. 1987;3(4):197–200. [Google Scholar]
- 33.Wilkie D. Cancer pain quality: preliminary evidence to support sits-specific sensory scores when using the mcoill pain questionnaire (MPQ) Pain. 1990;41:S362. [Google Scholar]
- 34.Downie W., Leatham P., Rhind V., Wright V., Branco J., Anderson J. Studies with pain rating scales. Ann. Rheum. Dis. 1978;37(4):378. doi: 10.1136/ard.37.4.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jensen M.P., Karoly P. 1992. Self-report Scales and Procedures for Assessing Pain in Adults. [Google Scholar]
- 36.Coward D.D., Wilkie D.J. Metastatic bone pain: meanings associated with self-report and self-management decision making. Cancer Nurs. 2000;23(2):101–108. doi: 10.1097/00002820-200004000-00005. [DOI] [PubMed] [Google Scholar]
- 37.Masheb R.M., Lozano-Blanco C., Kohorn E.I., Minkin M.J., Kerns R.D. Assessing sexual function and dyspareunia with the Female Sexual Function Index (FSFI) in women with vulvodynia. J. Sex Marital Ther. 2004;30(5):315–324. doi: 10.1080/00926230490463264. [DOI] [PubMed] [Google Scholar]