Summary
Background
Hearing impairment was recently identified as the most prominent risk factor for dementia. However, the mechanisms underlying the link between hearing impairment and dementia are still unclear.
Methods
We investigated the association of hearing performance with cognitive function, brain structure and cerebrospinal fluid (CSF) proteins in cross-sectional, longitudinal, mediation and genetic association analyses across the UK Biobank (N = 165,550), the Chinese Alzheimer's Biomarker and Lifestyle (CABLE, N = 863) study, and the Alzheimer's Disease Neuroimaging Initiative (ADNI, N = 1770) database.
Findings
Poor hearing performance was associated with worse cognitive function in the UK Biobank and in the CABLE study. Hearing impairment was significantly related to lower volume of temporal cortex, hippocampus, inferior parietal lobe, precuneus, etc., and to lower integrity of white matter (WM) tracts. Furthermore, a higher polygenic risk score (PRS) for hearing impairment was strongly associated with lower cognitive function, lower volume of gray matter, and lower integrity of WM tracts. Moreover, hearing impairment was correlated with a high level of CSF tau protein in the CABLE study and in the ADNI database. Finally, mediation analyses showed that brain atrophy and tau pathology partly mediated the association between hearing impairment and cognitive decline.
Interpretation
Hearing impairment is associated with cognitive decline, brain atrophy and tau pathology, and hearing impairment may reflect the risk for cognitive decline and dementia as it is related to bran atrophy and tau accumulation in brain. However, it is necessary to assess the mechanism in future animal studies.
Funding
A full list of funding bodies that supported this study can be found in the Acknowledgements section.
Keywords: Hearing impairment, Cognitive decline, Brain atrophy, CSF tau protein
Abbreviations: CSF, cerebrospinal fluid; PRS, polygenic risk score; ADNI, Alzheimer's Disease Neuroimaging Initiative; CABLE, Chinese Alzheimer's Biomarker and Lifestyle
Research in context.
Evidence before this study
Hearing impairment was recently identified as the most prominent risk factor for dementia, which emphasizes the need to investigate the mechanism of how hearing impairment is related to dementia. The pathological changes, such as neurofibrillary tangle (tau) and brain atrophy, occur a decade before dementia. Thus, it is possible that hearing impairment is associated with the pathological changes resulting in dementia. We searched PubMed for studies that examined the association of hearing impairment with the pathological changes before dementia, and limited evidence was available from these studies due to the sample sizes. Therefore, it remains necessary to investigate the relationship between hearing impairment and pathological biomarkers in large cohorts to explore the mechanism underlying the link between hearing impairment, pathological changes, and cognitive decline.
Added value of this study
Our study utilized 168,183 participants from three independent cohorts and identified that hearing impairment was associated with cognitive decline, brain atrophy and tau pathology, and these associations were validated in genetic association studies. In addition, mediation analyses showed that brain atrophy and tau pathology partly mediated the association between hearing impairment and cognitive decline. It suggested that hearing impairment is related to brain atrophy and tau accumulation in the brain and the risk for cognitive decline and dementia.
Implications of all the available evidence
Hearing impairment is regarded as the greatest risk for dementia among the 12 modifiable risk factors in 2020. Our investigation elucidated that hearing impairment is related to the risk for cognitive decline, brain atrophy and tau accumulation. Future studies are needed to test whether interventions of hearing impairment reduce the risk of dementia.
Introduction
Hearing impairment is a common problem for older adults, and its prevalence increases with age, affecting over 40% of the population aged ≥50 years, rising to 71% of the population aged ≥70 years.1 Recently, hearing impairment was identified as the most prominent risk factor for dementia among the 12 potentially modifiable factors, including less education, smoking and depression, and was associated with 8% of dementia cases.2 These developments emphasize the need to investigate the pathophysiological mechanism underlying the association between hearing impairment and dementia;1,3 and understanding the neural mechanisms may help to provide potential avenues for early intervention for dementia.4
It has been established that pathological changes, such as amyloid-β (Aβ) deposition, neurofibrillary tangle (tau) and brain atrophy, occur a decade before dementia.5 Therefore, it is possible that hearing impairment is related to pathological changes resulting in the occurrence of dementia. Since the auditory cortex is located in the temporal lobe, it is possible that the temporal cortex shows the most severe atrophy among brain regions in hearing impairment. The sensory deprivation hypothesis suggests that long-term auditory deprivation reallocates cognitive resources towards auditory cognition. Consequently, alongside the temporal cortex, hearing impairment may relate to the atrophy of the cortex associated with general cognitive process. Neuroimaging biomarkers have been documented to reflect the pathophysiological processes in the brain during the entire course of dementia.6,7 To date, a few studies have investigated the associations of hearing impairment with gray matter (GM) macrostructural size and white matter (WM) microstructural integrity in the brain, but few brain regions and WM tracts were consistently reported to be associated with hearing impairment in these studies.8, 9, 10, 11, 12, 13, 14 Moreover, since Aβ and tau protein in the cerebrospinal fluid (CSF) are strongly associated with Aβ and tau pathology in the brain, several investigations explored the association of hearing impairment with CSF proteins to reveal the effects of hearing impairment in pathology. Similarly, inconsistent results were obtained owing to the limitation of sample size.15,16 Therefore, it remains necessary to investigate the relationship between hearing impairment and neuroimaging and CSF biomarkers in large cohorts to explore the mechanisms underlying the link between hearing status, pathological changes, and cognitive function.
Additionally, hearing impairment is attributable to the cumulative effects of environmental and genetic factors and the contribution of genetic risk was estimated to be ∼50%.17 Thus far, over 150 loci have been identified as risk factors for hearing impairment which allowed the calculation of polygenic risk score (PRS) for hearing impairment.18 However, little is known about the correlation between genetic risk for hearing impairment and pathological and clinical changes before dementia. It is necessary to investigate whether PRS for hearing performance is associated with cognitive decline and pathological changes to further understand the mechanism of how hearing impairment is related to dementia.
Accordingly, here we investigated the association between hearing impairment and cognitive decline, brain structure and CSF proteins in the UK Biobank (N = 165,550),19 the CABLE (Chinese Alzheimer's Biomarker and Lifestyle, N = 863) study,20 and the ADNI (Alzheimer's Disease Neuroimaging Initiative, N = 1770) database21 to uncover the mechanisms of the association between hearing performance and cognitive function. Meanwhile, we calculated the PRS for hearing performance based on the GWAS (genome-wide association study) results of hearing tests in the UK Biobank to explore the correlation of PRS for hearing performance with cognitive decline and pathological changes to further understand the potential mechanism underlying the substantial association between hearing impairment and dementia.
Methods
Participants
The UK biobank
The UK Biobank is a prospective epidemiological study that involves over 500,000 individuals recruited in 22 centers across the UK between 2006 and 2010.22,23 The study has collected extensive questionnaire data, physical measurements, and biological samples. A subset of the cohort has been invited back to collect multimodal imaging data and repeat behavioral assessments since 2014. All participants are followed up for health conditions through linkage to national electronic health-related datasets. The health follow-up data used in the present study started at enrollment (2006–2010) and ended in January 2021. The variables used in this study are listed in Supplementary Table S1. All participants provided informed consent and the ethical approval was from the UK North West Multi-Centre Research Ethics Committee.
CABLE study
The Chinese Alzheimer's Biomarker and Lifestyle (CABLE) study is an ongoing large and independent cohort focused on determining the genetic and environmental modifiers of AD biomarkers and their utility in the early diagnosis in the northern Chinese Han population.20,24 All enrolled participants were between 40 and 90 years old, and each participant underwent comprehensive clinical, neuropsychological, and psychiatric evaluations as well as bio-sample (blood and CSF sample) collection to determine their diagnoses in compliance with the National Institute on Aging–Alzheimer's Association (NIA-AA) workgroup diagnostic criteria.25, 26, 27 The data used here are shown in Supplementary Table S1. Participants without dementia were included in our study. The CABLE cohort was conducted in accordance with the Helsinki Declaration, and the research program was approved by the Institutional Ethics Committee of Qingdao Municipal Hospital. All subjects or their proxies provided written consent.
ADNI database
The Alzheimer's Disease Neuroimaging Initiative (ADNI) is a large, multicenter, longitudinal neuroimaging study, launched in 2003.21,28 To date, ADNI have recruited over 3000 adults (ages 55 to 90), and our study included cognitively normal older individuals (CN) and patients with mild cognitive impairment (MCI). Participants provided detailed medical history and physical examination and offered biological samples, such as a cerebrospinal fluid (CSF) sample. The variables used in this study are listed in Supplementary Table S1. The study was approved by the institutional review boards of all participating centers and written informed consent was obtained from all participants or authorized representatives after extensive description of the ADNI based on the 1975 Declaration of Helsinki.
Hearing assessment
The UK Biobank used a speech-in-noise (SiN) test to assess the hearing performance of participants. The SiN test was quantified by the speech-reception-threshold (SRT) from the Digit Triplets Test. Detailed information about the SiN test has been described in previous studies.29,30 The hearing performance was evaluated using the SRT of the better hearing ear. The log-transformed SRT (calculated as log (SRT+13) was used due to the skewed distribution when hearing performance was used as a continuous measure. The cutoff point for SRT was −5.5 dB, suggesting that an SRT of > -5.5 dB was considered as a hearing impairment.29 Since the CABLE study and the ADNI database did not provide continuous evaluation on hearing function, we divided the participants into two groups (hearing impairment and hearing normal groups). The CABLE study used a questionnaire about hearing (“Do you have any difficulty with your hearing?”) to detect whether the participant was in a hearing impaired or hearing normal group. The subjects with the description of “hearing loss”, “decreased hearing”, “impaired hearing”, “hard of hearing”, “hearing problems”, “deaf”, “presbycusis”, or “wearing hearing aids” in the MH (Medical History) and PE (Physical Exam) datasets were defined as the hearing impairment group in the ADNI database, and the subjects without these descriptions in the MH and PE datasets were defined as in the hearing normal group.16
Polygenic risk score for hearing performance
We used the imputed genotype data from the UK Biobank resource, and we only included the loci with call rates >95%, minor allele frequency >1% and the Hardy–Weinberg equilibrium with P > 1 × 10−6 and the subjects who self-reported white British ancestry and undertook the hearing test in the subsequent analysis. Finally, a total of 5,938,284 loci from 123,425 participants entered the genome-wide association study (GWAS) to assess the association between genetic variation and hearing performance. We then calculated the PRS based on the selected single-nucleotide polymorphisms (SNPs) from the above GWAS using the PRSice platform (http://www.prsice.info). The PRS analysis required that the subjects of base data and target data share the same ancestry and there are no overlapping samples between the base and target data.31 The GWAS results (base data) were from the subjects who self-reported white British ancestry and undertook the hearing test. Thus, PRS of hearing performance at the threshold of P = 0.05 were computed for the subjects who self-reported white British ancestry and did not receive the hearing test (target data) in the UK Biobank. The PRS was also calculated at different thresholds of P value (1 × 10−4, 5 × 10−4, 1 × 10−3, 5 × 10−3, 1 × 10−2, 1 × 10−1, 5 × 10−1) for the sensitivity analysis.
Cognitive function
Cognitive tests were first administered via a touchscreen interface in the UK Biobank assessment center at the baseline visit and repeated at the following 3 visits. The primary cognitive outcome was fluid intelligence from the UK Biobank in our study,29 and numeric memory, prospective memory, reaction time, and the pairs matching tests from the UK Biobank were also utilized in the current study.19 These five tests captured general cognition, attention, memory, processing speed, and visual spatial functions of the participants. A description of the five cognitive tests is listed in the Supplementary Methods. The MMSE (Mini-mental state examination) and MoCA (Montreal Cognitive Assessment) were used to assess the cognitive function in the CABLE study and in the ADNI database.
Magnetic resonance imaging data
Quality-controlled neuroimaging data released by the UK Biobank were used in this study. Details of the dataset can be found online (https://biobank.ctsu.ox.ac.uk/crystal/docs/brain_mri.pdf). Structural MRI was processed with FreeSurfer. The Desikan-Killiany atlas was used to segment the cortex into 68 regions,32 and the ASEG atlas was used to segment the subcortex into 45 regions.33 FA (fractional anisotropy) and MD (mean diffusivity) were extracted from the preprocessed diffusion MRI. The JHU ICBM-DTI-81 atlas was used to derive 48 white matter tracts.34 The AutoPtx package from FSL was used to map 27 major tracts over the whole brain.35
CSF proteins
Fasting CSF samples of the CABLE cohort were drawn by the standard procedure of lumbar puncture and processed within 2 h after collection. Each specimen was centrifuged at 2000 g for 10 min, and stored in an enzyme-free EP (Eppendorf) tube at −80 °C until subsequent assays. Amyloid-beta (Aβ42), total tau (t-tau) and phosphorylated-tau (p-tau181) were detected with the ELISA kit.36 The detailed measurement methods of CSF proteins in ADNI were described in a previous paper.21 We excluded values over 3 standard deviations (SD) from the mean to avoid the effects of extreme values.
Statistical analysis
Cross-sectional analysis
Baseline differences between the two groups (the hearing impairment and hearing normal groups) were analysed using Student's t test for continuous variables and the Chi-square test for categorical variables. A multiple linear/logistic regression model was used to test the associations between hearing performance (or the PRS for hearing performance) and cognitive function, brain structure, and CSF proteins (Supplementary Table S1). The covariates including age, sex, body mass index (BMI), Townsend deprivation index, education qualification, smoking and drinking status were regressed out in the analysis within the UK Biobank, and the covariates used in the other two cohorts are listed in Supplementary Table S2. Since multiple tests were conducted, the P values after Bonferroni correction are reported and P < 0.05 was considered as statistically significant.
Longitudinal analysis
A within-subjects linear mixed-effects model was used to assess the relationship between cognitive function and hearing performance at four time points (with missing data removed) in the longitudinal analysis. In addition, the longitudinal associations between hearing impairment and primary cognitive outcome were also examined using a classic two-wave cross-lagged panel model (CLPM) implemented by the “Lavaan” package in R based on a structural equation model. Model parameters were estimated by maximum likelihood estimation. The standardized regression coefficients and P values are estimated throughout. Likewise, the P values after Bonferroni correction were calculated due to multiple tests used in the longitudinal study and P < 0.05 was regarded as statistical significance.
Mediation analysis
Mediation analyses which test whether the covariance between two variables can be explained by a third variable (the mediator) were conducted at the third time point (cross-section) in the UK Biobank and at baseline in the CABLE study. As the directional association of hearing impairment (or the PRS for hearing impairment) with cognitive decline was determined by the CLPM, we separately assessed the mediation effect of brain structure and CSF tau protein on the association between hearing impairment and cognitive decline. The same covariates as mentioned above were controlled for in the model. Mediation analysis was run in R software, version R 4.2.0 using the “Lavaan” package, with nonparametric bootstrapping with 10,000 iterations to estimate direct and indirect effects and P values between variables.
Ethics
The UK Biobank received ethical approval from the North West Multi-Centre Research Ethics Committee in the UK (REC reference 11/NW/0382). The present analyses were conducted under UK Biobank application number 19542. Written informed consent was obtained from each subject according to the Declaration of Helsinki. The CABLE study was conducted in accordance with the Helsinki Declaration, and the research program was approved by the Institutional Ethics Committee of Qingdao Municipal Hospital. All subjects or their proxies provided written consent. For the ADNI data, all participants provided written informed consent approved by the institutional review board of each ADNI participating institution.
Role of funding sources
The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Results
The characteristics of included subjects
In the 502,493 participants from the UK Biobank, 165,550 participants accepted the hearing (speech-in-noise, SiN) test at baseline. Of 165,550 participants with the hearing test, 14,113 (8.5%) participants were identified as in the hearing impairment group. Hearing impairment was associated with older age, male sex, lower socioeconomic status, smoking, alcohol intake, lower education, hypertension, diabetes, and higher BMI in the UK Biobank. The characteristics of the UK Biobank participants are shown in Table 1. In addition, Table 1 also shows the characteristics of participants in the CABLE study and in the ADNI database.
Table 1.
Characteristics of participants.
| Characteristics | UK Biobank (Hearing test - SiN)a |
CABLE study (Questionnaire)b |
ADNI (MH and PE)c |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Hearing normal |
Hearing impairment |
P | Hearing normal |
Hearing impairment |
P | Hearing normal |
Hearing impairment |
P | |
| (N = 151,437) | (N = 14,113) | (N = 715) | (N = 148) | (N = 1357) | (N = 413) | ||||
| Age (years) | 56.38 (8.14) | 60.39 (7.34) | <0.001 | 60.39 (11.70) | 66.29 (9.83) | <0.001 | 72.15 (6.97) | 75.53 (6.51) | <0.001 |
| Sex | |||||||||
| Female | 82,763 (54.65%) | 7401 (52.44%) | <0.001 | 408 (57.06%) | 103 (69.59%) | 0.006 | 705 (51.95%) | 129 (31.23%) | <0.001 |
| Male | 68,674 (45.35%) | 6712 (47.56%) | 307 (42.94%) | 45 (30.41%) | 652 (48.05%) | 284 (68.77%) | |||
| Educationd | 0.001 | 0.142 | 0.003 | ||||||
| Years | – | – | 10.07 (4.47) | 9.45 (4.67) | 16.13 (2.73) | 16.57 (2.59) | |||
| Higher | 60,368 (46.65%) | 4475 (44.96%) | – | – | – | – | |||
| Lower | 69,049 (53.35%) | 5478 (55.04%) | – | – | – | – | |||
| TDI | −1.20 (2.90) | −0.48 (3.22) | <0.001 | – | – | – | – | ||
| BMI (kg/m2) | 27.38 (4.81) | 27.91 (4.98) | <0.001 | 25.56 (4.40) | 25.47 (5.92) | 0.864 | – | – | |
| ApoE ε4 | 0.274 | 0.388 | 0.805 | ||||||
| 0 | 89,921 (71.63%) | 6966 (71.23%) | 605 (84.97%) | 122 (82.43%) | 795 (58.59%) | 242 (58.60%) | |||
| 1 | 32,756 (26.09%) | 2567 (26.25%) | 101 (14.19%) | 23 (15.54%) | 461 (33.97%) | 144 (34.87%) | |||
| 2 | 2867 (2.28%) | 247 (2.53%) | 6 (0.84%) | 3 (2.03%) | 101 (7.44%) | 27 (6.54%) | |||
| Smoking | 0.014 | 0.010 | 0.092 | ||||||
| No | 83,437 (55.27%) | 7592 (54.19%) | 517 (72.51%) | 91 (61.49%) | 746 (71.18%) | 227 (66.18%) | |||
| Yes | 67,513 (44.73%) | 6417 (45.81%) | 196 (27.49%) | 57 (38.51%) | 302 (28.82%) | 116 (33.82%) | |||
| Drinking | <0.001 | 0.205 | 0.590 | ||||||
| No | 6457 (4.27%) | 1405 (9.98%) | 497 (69.90%) | 95 (64.19%) | 1019 (97.23%) | 336 (97.96%) | |||
| Yes | 144,821 (95.73%) | 12,677 (90.02%) | 214 (30.10%) | 53 (35.81%) | 29 (2.77%) | 7 (2.04%) | |||
| Hypertension | <0.001 | 0.981 | 0.922 | ||||||
| No | 111,418 (73.70%) | 9114 (64.82%) | 445 (62.32%) | 93 (62.84%) | 601 (57.35%) | 195 (56.85%) | |||
| Yes | 39,766 (26.30%) | 4946 (35.18%) | 269 (37.68%) | 55 (37.16%) | 447 (42.65%) | 148 (43.15%) | |||
| Diabetes | <0.001 | 0.790 | 0.989 | ||||||
| No | 143,202 (94.79%) | 12,632 (90.02%) | 612 (85.71%) | 125 (84.46%) | 951 (90.74%) | 312 (90.96%) | |||
| Yes | 7873 (5.21%) | 1401 (9.98%) | 102 (14.29%) | 23 (15.54%) | 97 (9.26%) | 31 (9.04%) | |||
Abbreviations: SiN, Speech in Noise; CABLE, Chinese Alzheimer's Biomarker and Lifestyle; ADNI, Alzheimer's Disease Neuroimaging Initiative; MH, Medical History; PE, Physical Exam; TDI, Townsend deprivation index (socioeconomic status); BMI, Body Mass Index.
Data was shown as mean (SD) for continuous variables, and was show as number (percentage) for categorical variables.
P values were analysed using Student's t test for continuous variables and the Chi-square test for categorical variables.
Participants in the UK Biobank were categorized as hearing impairment (Speech reception threshold [SRT]>-5.5 dB) and hearing normal (SRT ≤ −5.5 dB) based on hearing test (SiN).
Participants in the CABLE study were categorized as hearing impairment and hearing normal based on a questionnaire about hearing problem.
Participants in the ADNI database were categorized as hearing impairment and hearing normal based on the results in Medical History (MH) and Physical Exam (PE) datasets.
Education in UK biobank categorized as higher (college/university degree or other professional qualification) or lower (others).
Hearing impairment is associated with cognitive decline
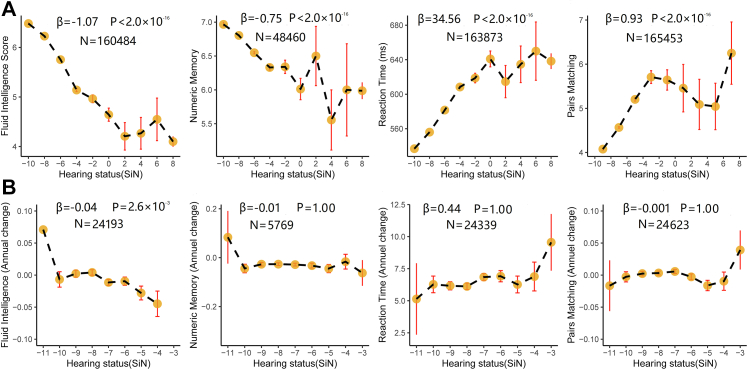
The performance in the hearing test was related to cognitive function in fluid intelligence (β = −1.07, Bonferroni corrected P < 2.0 × 10−16), numeric memory (β = −0.75, Bonferroni corrected P < 2.0 × 10−16), prospective memory (odds ratio [OR] = −1.02, Bonferroni corrected P < 2.0 × 10−16), reaction time (β = 34.56, Bonferroni corrected P < 2.0 × 10−12), and the pairs matching tests (β = 0.93, Bonferroni corrected P < 2.0 × 10−16) in the cross-sectional analysis from the UK Biobank (Fig. 1a, Supplementary Fig. S1). Likewise, hearing impairment was correlated with lower cognitive function in the MoCA (β = −1.60, P = 6.0 × 10−4) and MMSE (β = −0.52, P = 0.048) tests in the CABLE study (Supplementary Fig. S2), but the correlation between hearing function and MMSE disappeared after Bonferroni correction (MoCA, Bonferroni corrected P = 0.001; MMSE, Bonferroni corrected P = 0.097). There was no significant association between hearing status and cognitive function assessed by MMSE and MoCA in the ADNI database (Supplementary Table S3). In the longitudinal analysis, lower performance in the hearing test was associated with a higher rate of decline of cognitive function in fluid intelligence (β = −0.04, Bonferroni corrected P = 2.6 × 10−3), but was not related to the change of numeric memory (β = −0.01, Bonferroni corrected P = 1.00), reaction time (β = 0.44, Bonferroni corrected P = 1.00), and pairs matchings (β = −0.001, Bonferroni corrected P = 1.00) in the UK Biobank (Fig. 1b). Further, we used a structural equation model to assess the changes between the baseline and the 4-year follow-up with potential confounders regressed out (see the “Methods” section). Poor hearing performance at baseline was significantly associated with worse cognitive function (fluid intelligence) in the follow-up (β = −0.038, Bonferroni corrected P = 1.5 × 10−7). Meanwhile, worse cognitive function at baseline was also significantly associated with poor hearing performance in the follow-up (β = −0.057, Bonferroni corrected P = 2.2 × 10−11) (Supplementary Fig. S3).
Fig. 1.
The association of hearing performance with cognitive function. a. The association of hearing performance with fluid intelligence, numeric memory, reaction time and pairs matching in the cross-sectional study; b. The association of hearing performance with fluid intelligence, numeric memory, reaction time and pairs matching in the longitudinal study. The association of hearing performance with cognitive function were investigated using the multiple linear regression models with the covariates regressed out including age, sex, body mass index, Townsend deprivation index, education qualification, smoking status, and drinking status in the UK Biobank. All P values were calculated after Bonferroni correction. Abbreviations: SiN, Speech in Noise.
The polygenic risk score for hearing performance is associated with cognitive decline
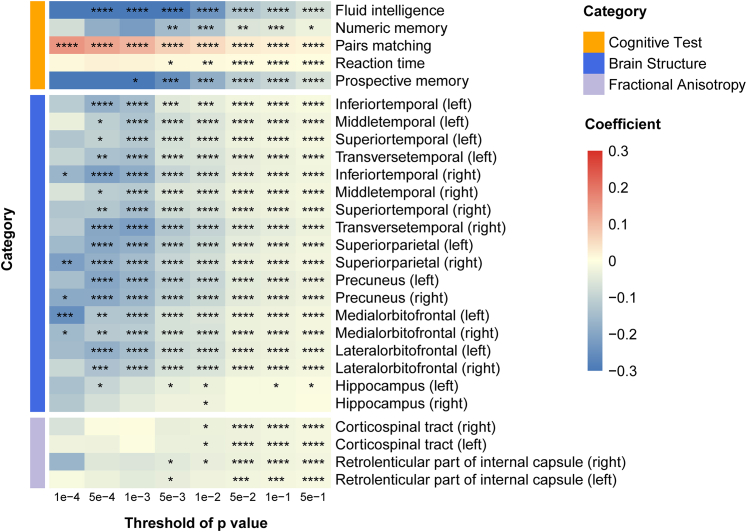
We then investigated the association between PRS for hearing performance and cognitive function in the UK Biobank. The PRS for hearing performance calculated at the threshold of P = 0.05 was associated with cognitive function in the fluid intelligence, numeric memory, prospective memory, reaction time, and pairs matching tests in the cross-sectional analysis within the multiple linear models with the covariates provided in the "Methods" section (Fig. 2). Further, the significant association survived Bonferroni correction (P < 0.05). In addition, these significant associations still existed in the sensitivity analyses where the PRS were calculated at different thresholds of P value (Supplementary Table S4).
Fig. 2.
The association of polygenic risk score for hearing performance with cognitive function and brain structure. The polygenic risk score (PRS) for hearing performance was associated with cognitive function, grey matter volume, and white matter microstructure integrity in the multiple linear regression models with the covariates including age, sex, body mass index, Townsend deprivation index, education qualification, smoking status, drinking status, and imaging scanning sites (only for brain imaging) in the UK Biobank. Abbreviations: PRS, polygenic risk scores; “∗” P < 0.05; “∗∗” P < 0.01; “∗∗∗” P < 0.005; “∗∗∗∗” P < 0.001.
Hearing impairment is associated with brain structure
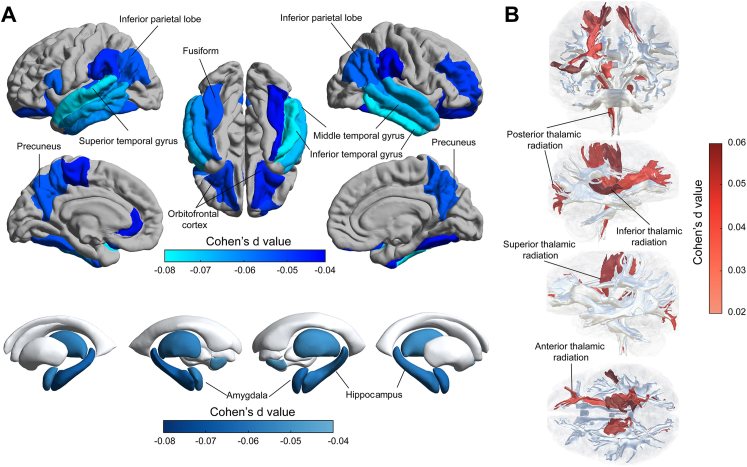
There were 38,438 participants who received the hearing test and MRI examination in the UK Biobank. Poor hearing performance was significantly associated with reduced volumes bilaterally of the superior, middle, and inferior temporal gyrus, hippocampus, precuneus, inferior parietal lobe and supramarginal gyrus, fusiform, and orbitofrontal cortex; and of the left transverse temporal gyrus, left rostral anterior cingulate and right rostral middle frontal gyrus (Fig. 3a, Supplementary Table S5). Likewise, poor hearing performance was significantly associated with lower volumes bilaterally of the amygdala, thalamus, and nucleus accumbens (Fig. 3b, Supplementary Table S5). Poor hearing performance was associated with a lower FA bilaterally of the fornix crus and stria terminalis, and of the right posterior thalamic radiation; and with a higher MD bilaterally of the superior and posterior thalamic radiation, and of the right inferior thalamus radiation and superior fronto-occipital fasciculus (Fig. 3b, Supplementary Table S6). All these significant associations were obtained after controlling for the covariates provided in the “Methods” section and after Bonferroni correction.
Fig. 3.
The association of hearing performance with brain structure. a. Lower volume of cortical and subcortical regions was associated with poor hearing performance; b. Higher mean diffusivity of white matter tracts was associated with poor hearing performance. The hearing performance was measured using the speech-in-noise test, in which the higher speech-reception-threshold indicated the poorer hearing performance. The association between hearing performance and brain structure were investigated in the multiple linear regression models with the covariates regressed out including age, sex, body mass index, Townsend deprivation index, education qualification, smoking status, drinking status, and imaging scanning sites in the UK Biobank.
The polygenic risk score for hearing performance is associated with brain structure
The association between PRS for hearing performance and brain structure was also tested in 20,899 subjects from the UK Biobank. Higher PRS for hearing impairment (at the threshold of P = 0.05) was associated with lower volume bilaterally of the transverse, superior, and middle temporal gyrus, precuneus, supramarginal gyrus, insula, superior parietal gyrus, cuneus, lateral and medial orbitofrontal cortex, etc. in the multiple linear models with the covariates regressed out (Fig. 2, Supplementary Table S7). The PRS of hearing impairment at the threshold (P = 0.01) was significantly associated with the volume of the hippocampus, but the association disappeared after Bonferroni correction (Supplementary Table S7). Besides, the PRS at the threshold of P = 0.05 was correlated with the FA of bilateral corticospinal tracts, and right retrolenticular part of the internal capsule in the multiple linear models (Fig. 2, Supplementary Table S8). Further, these associations of PRS with the volume of grey matter and the FA of white matter tracts were still significant after Bonferroni correction and at different thresholds of P values in the sensitivity analysis (Fig. 2).
Hearing impairment is associated with CSF proteins
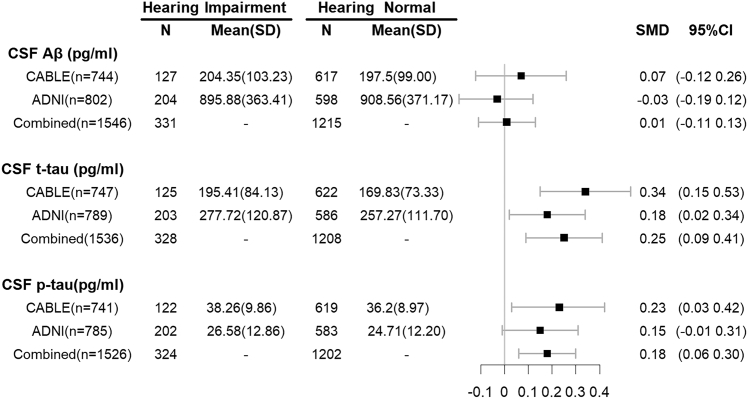
We investigated the association of hearing impairment with the CSF proteins in 747 participants from the CABLE study. The levels of CSF t-tau and p-tau181 were significantly higher in the hearing impairment group than in the hearing normal group. Moreover, hearing impairment was significantly associated with a higher level of CSF t-tau (β = 23.75, P = 0.001) and p-tau181 (β = 1.95, P = 0.032) after adjustment for age, gender, and APOE ε4 status, but only the association between hearing impairment and CSF t-tau survived Bonferroni correction (t-tau, Bonferroni corrected P = 0.004; p-tau181, Bonferroni corrected P = 0.096). In addition, hearing impairment was not related to the level of CSF Aβ42 (β = 7.94, P = 0.42) in the CABLE study. Furthermore, we explored the association in another independent 802 participants from the ADNI database, and detected that hearing impairment was associated with a higher level of CSF t-tau (β = 19.89, P = 0.027), although the relationship disappeared after multiple correction (Bonferroni corrected P = 0.080). Likewise, hearing impairment did not affect the level of CSF p-tau181 (β = 1.68, P = 0.087) and Aβ42 (β = 9.63, P = 0.73) in the ADNI database. Finally, pooled analysis of the above two cohorts showed that hearing impairment was associated with higher levels of CSF t-tau (standardized mean difference [SMD] = 0.25, Bonferroni corrected P = 5.19 × 10−3) and p-tau181(SMD = 0.18, Bonferroni corrected P = 0.012), but was not associated with CSF Aβ42 (SMD = 0.07, Bonferroni corrected P > 0.05) (Fig. 4).
Fig. 4.
The association of hearing performance with CSF proteins. Higher level of CSF t-tau and p-tau were associated with poor hearing performance. The SMD (Standard Mean Difference) and 95% CI were from the meta-analysis with random-effects. Abbreviations: CSF, cerebrospinal fluid; CABLE, Chinese Alzheimer's Biomarker and Lifestyle; ADNI, Alzheimer's Disease Neuroimaging Initiative; SD, Standard Deviation; SMD, Standard Mean Difference; CI, Confidence Interval.
Brain structure and CSF tau mediated the association of hearing impairment with cognitive decline
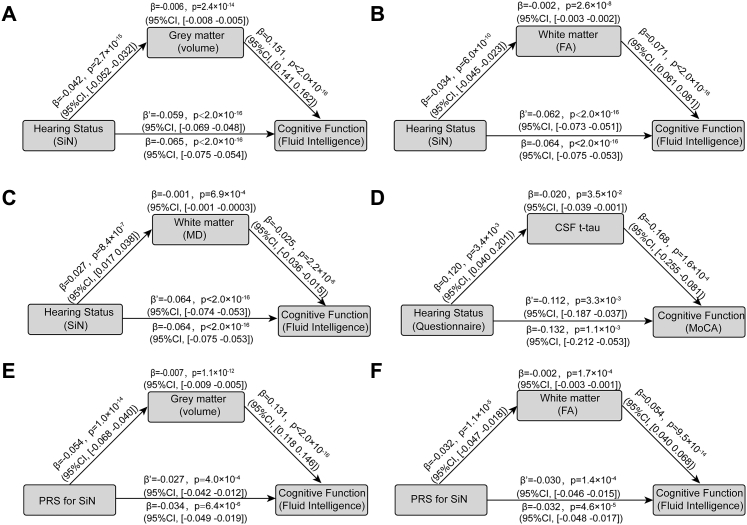
We conducted a mediation analysis to identify whether the brain regions associated with hearing performance mediated the relationship between hearing performance and cognitive function. The mean volume of these brain regions (Fig. 3) mediated the association of hearing impairment with lower cognitive function in the fluid intelligence test (β = −0.006, P = 2.4 × 10−14, 95%CI = −0.008 to −0.005) (Fig. 5a). Moreover, the mean FA (β = −0.002, P = 2.6 × 10−8, 95%CI = −0.003 to −0.002) (Fig. 5b) and MD (β = −0.001, P = 6.9 × 10−4,95%CI = −0.001 to −0.0003) (Fig. 5c) of the white matter tracts also mediated the association between hearing performance and cognitive function. Finally, CSF tau protein partly mediated the association between hearing performance and cognitive function on MoCA (β = −0.020, P = 3.5 × 10−2, 95%CI = −0.039 to −0.001) (Fig. 5d).
Fig. 5.
Mediation analysis of the association between hearing performance and cognitive function. a. Mediation analysis: the mediation implemented by brain regions from hearing performance on cognitive function was significant (β = −0.006, P = 2.4 × 10−14, 95%CI: −0.008 to −0.005); b. Mediation analysis: the mediation implemented by fractional anisotropy of white matter tracts from hearing performance on cognitive function was significant (β = −0.002, P = 2.6 × 10−8, 95%CI: −0.003 to −0.002); c. Mediation analysis: the mediation implemented by mean diffusivity of white matter tracts from hearing performance on cognitive function was significant (β = −0.001, P = 6.9 × 10−4,95%CI: −0.001 to −0.0003); d. Mediation analysis: the mediation implemented by CSF tau protein from hearing performance on cognitive function (β = −0.020, P = 3.5 × 10−2, 95%CI: −0.039 to −0.001). e. Mediation analysis: the mediation implemented by the volume of brain regions from the PRS for hearing performance on cognitive function (β = −0.007, P = 1.1 × 10−12, 95%CI: −0.009 to −0.005). f. Mediation analysis: the mediation implemented by the FA of white matter tracts from the PRS for hearing performance on cognitive function (β = −0.002, P = 1.7 × 10−4, 95%CI: −0.003 to −0.001). The indirect and direct effects and P values were estimated using nonparametric bootstrapping with 10,000 iterations with the “Lavaan” package in R software, version 4.2.0. Abbreviations: SiN, Speech in Noise; CSF, Cerebrospinal Fluid; MoCA, Montreal Cognitive Assessment; CI, Confidence Interval; FA, Fractional anisotropy; MD, Mean diffusivity; PRS, polygenic risk score.
In addition, mediation analyses were also performed to test whether the brain structure (GW volume and WM integrity) mediated the association between PRS for hearing performance and cognitive function. It was found that the mean volume of grey matter (β = −0.007, P = 1.1 × 10−12, 95%CI: −0.009 to −0.005) and the mean FA of white matter tracts (β = −0.007, P = 1.1 × 10−12, 95%CI: −0.009 to −0.005) mediated the association between PRS at the P value of 0.05 and fluid intelligence (Fig. 5e and f).
Discussion
This investigation showed that hearing impairment was significantly associated with poorer cognitive function in the cross-sectional and longitudinal investigations in the UK Biobank, and these associations were verified in the CABLE study and in the genetic association study. This research also showed that hearing impairment was significantly associated with lower volume of the temporal cortex, hippocampus, amygdala, precuneus, inferior parietal lobe, fusiform gyrus, orbitofrontal cortex, etc. The regions with the lowest volume in the hearing impaired group were in the superior temporal gyrus, which is auditory association cortex,37 and which also includes language-related regions.38 These regions have connectivity with the parietal cortex39 and orbitofrontal cortex.40 Hearing impairment was also markedly related to lower microstructural integrity of WM tracts. Likewise, the genetic association study validated the correlation of hearing impairment with the reduced WM volume and WM integrity. Moreover, hearing impairment was detected to be significantly associated with CSF t-tau and p-tau181 protein in the pooled analysis of the CABLE study and the ADNI database. Mediation analyses showed that GM volume, WM integrity, and CSF tau protein partly mediated the association between hearing performance and cognitive function.
Growing evidence is showing that hearing impairment is the largest risk for dementia,2 and we investigated the potential mechanism for how hearing impairment contributes to dementia in this research. Our study showed that hearing impairment was a risk factor for cognitive decline. Moreover, the neuroimaging analysis demonstrated that poor hearing performance was significantly associated with lower volume of the temporal cortex including the superior temporal auditory association cortical areas, hippocampus and precuneus, which are the most vulnerable brain regions related to dementia.41 This study also showed that poor hearing performance was associated with a higher level of CSF tau protein. The mediation analysis showed that brain structure and CSF tau protein partly mediated the association between hearing impairment and cognitive decline. Since speech-in-noise perception used in the UK Biobank is a proxy for central auditory function rather than peripheral hearing function, these findings taken together suggest that central hearing dysfunction is a marker of neurodegenerative brain pathology and incipient dementia.42, 43, 44 These results indicate that hearing impairment is related to the atrophy of brain structure, the accumulation of tau pathology in the brain, and the increased risk for cognitive decline and dementia.
Our study found that poor hearing performance was significantly associated with lower cognitive function in the cross-sectional and longitudinal analyses in the UK Biobank. However, the relationship between cognitive function and hearing performance appeared non-monotonic in the cross-sectional analyses (Fig. 1a). This may result from the large variation in sample size across the range of hearing status (from 39 to 45,019) and the small sample size increasing the risk of bias. In the longitudinal analyses, poor hearing performance was still correlated with fluid intelligence. Besides, structural equation model identified the relationship between hearing performance and cognitive function. In addition, hearing impairment was related to lower MoCA score in the CABLE study, but this relationship was not replicated in the ADNI database. The majority of the participants in the UK Biobank and in the CABLE study were cognitively normal subjects, but the ADNI database includes many MCI subjects. Differences in the subjects included in these cohorts may explain the difference in the cognitive associations with hearing performance.
These results showed that hearing impairment was associated with lower volume of the superior, middle, inferior, and transverse temporal gyrus, hippocampus, amygdala, precuneus, inferior parietal lobe, orbitofrontal cortex, rostral middle frontal gyrus (part of the prefrontal cortex), etc. The genetic association analyses also showed that hearing impairment was related to lower volume of the temporal cortex, precuneus, etc. These results are consistent with previous findings that hearing impairment is associated with lower volumes of the temporal cortex, hippocampus, inferior parietal lobe, precuneus, and amygdala.8,9,45,46 We also found that hearing impairment was strongly associated with lower microstructural integrity of the thalamic radiations, crus fornix and stria terminalis, consistent with previous findings.10,13 The superior and middle temporal gyri contain auditory association cortex. Although these auditory cortical regions showed the lowest volumes related to hearing impairment, many other brain regions also had lower volumes, so that quite widespread changes in the brain are associated with the memory and cognitive problems associated with hearing impairment, including the inferior temporal gyrus (ITG), prefrontal cortex (PFC), inferior parietal lobe (IPL), orbitofrontal cortex (OFC) and medial temporal lobe (amygdala and hippocampus).3 These findings are not inconsistent with the sensory deprivation hypothesis that auditory deprivation due to hearing impairment results in compensatory cortical reorganisation to support auditory perception which precludes other cognitive processes. In addition, the brain regions correlated with hearing impairment (precuneus, prefrontal cortex, inferior parietal lobe, etc.) closely overlapped the default mode network targeted by Alzheimer's disease, and the hearing impairment was associated with CSF tau but not with CSF Aβ42. The evidence presented here indicates that hearing dysfunction acts as a proximity marker for the emergence of clinical dementia.47
In addition, we assessed the relationship between hearing impairment and the levels of CSF Aβ42, t-tau and p-tau181. Hearing impairment was associated with a high level of tau protein (t-tau) but not the level of Aβ42, which was verified in another independent cohort (ADNI database). This is consistent with previous findings that poor hearing performance is associated with elevated tau level rather than Aβ deposition.11,15,16 Furthermore, mediation analysis identified that CSF tau protein mediated the link between hearing impairment and cognitive decline. Given that tau pathology was more strongly associated with the cognitive phenotype compared to Aβ pathology,47,48 these results further support the relationship between hearing impairment, tau pathology and cognitive decline. Previous studies observed that auditory processes in hearing impairment resulted in higher neural activity in response to the reduced input, and higher neural activity was associated with tau deposition in human studies.49,50
The current investigation has several strengths. The large study population, the long duration of follow-up, and various endophenotypes improved the precision of the findings and facilitated exploration of the mechanism how hearing impairment affect cognitive decline. Our study included three cohorts, which allowed us to replicate the results from one cohort in another. The differences described in the methods used to assess hearing performance and cognitive function in these study cohorts may result in the differences in the results, and the differences in the methodology between these cohorts did not allow the combination of samples. However, the speech reception threshold and hearing symptom questionnaires did reflect the hearing level, and fluid intelligence, MMSE, and MoCA represented cognitive function. Furthermore, the results from these cohorts with different hearing and cognitive measures were consistent (e.g., hearing impairment was associated with lower cognitive function in the UK Biobank and in the CABLE study). This further verifies the robustness of our results. Recent studies identified that higher genetic risk for AD also is related to hearing status,29,51 and it is possible that there is a long pre-clinical phase of dementia associated with neural changes that impairs auditory processing.52 Here, the longitudinal analysis showed that poor hearing performance predicted cognitive decline, and impaired cognitive function also predicted poorer hearing performance in the follow-up. This evidence supports the common cause hypothesis that hearing impairment and cognitive function may share a common neurodegenerative etiology.1 Part of the interest and utility of the findings described here is that hearing impairment can be detected years before dementia is diagnosed, and may be a useful indicator or biomarker.
Overall, our study identified that hearing impairment is related to cognitive decline, brain atrophy, and tau accumulation.3 The genetic association analyses furthermore confirmed the correlation of hearing performance with cognitive function and brain structure. The CSF profile identified that hearing impairment is associated with CSF tau protein. Mediation analyses showed that brain atrophy and tau pathology mediated the association between hearing impairment and cognitive decline. The investigation investigated potential mechanisms whereby hearing impairment is associated with increased the risk for cognitive decline and dementia. However, our study did not resolve whether hearing impairment is causally related to dementia, or whether hearing impairment and dementia share the same common neurodegenerative aetiology.3,52, 53, 54 Further research is needed to confirm the mechanisms underlying the association between hearing impairment and dementia in future interventional and animal studies.
Contributors
All authors had full access to all the data in the study and accept responsibility to submit for publication. W Cheng and JT Yu conceptualized and designed the study. HF Wang, W Zhang, Y Li, L Wang and J Kang analysed and interpreted the data. YH Ma performed the measurement of CSF proteins. HF Wang, W Zhang and ET Rolls wrote and revised the manuscript. W Cheng, JT Yu and J Feng advised, and checked the manuscript. All authors contributed to and approved the final manuscript.
Data sharing statement
The data analysed are available from the UK Biobank (https://biobank.ctsu.ox.ac.uk). The ADNI data used here were from the ADNI database (https://adni.loni.usc.edu/). The data from the CABLE study and the code used in this study are available from the corresponding author on reasonable request.
Declaration of interests
The authors declare no conflict of interest related to this work.
Acknowledgments
This study utilized the UK Biobank Resource under application number 19542. The authors gratefully thank all the participants and professionals contributing to the UK Biobank. This study was supported by grants from the Science and Technology Innovation 2030 Major Projects (2022ZD0211600), National Natural Science Foundation of China (No. 82071997, No. 82071201), Shanghai Rising-Star Program (No. 21QA1408700), Medical Engineering Fund of Fudan University (yg2021-013), National Key R&D Program of China (2018YFC1312904, 2019YFA0709502), Shanghai Municipal Science and Technology Major Project (No.2018SHZDZX01) and ZHANGJIANG LAB, Tianqiao and Chrissy Chen Institute, the State Key Laboratory of Neurobiology and Frontiers Center for Brain Science of the Ministry of Education, Fudan University, China, the 111 Project (No. B18015), and Taishan Scholars Program of Shandong Province (tsqn201812157). The authors thanks all the researchers and participants in the ADNI initiative. As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. A complete listing of ADNI investigators can be found at: http://adni.loni.usc.edu/wp-content/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.ebiom.2022.104336.
Contributor Information
Jin-Tai Yu, Email: jintai_yu@fudan.edu.cn.
Wei Cheng, Email: wcheng@fudan.edu.cn.
Appendix A. Supplementary data
References
- 1.Slade K., Plack C.J., Nuttall H.E. The effects of age-related hearing loss on the brain and cognitive function. Trends Neurosci. 2020;43(10):810–821. doi: 10.1016/j.tins.2020.07.005. [DOI] [PubMed] [Google Scholar]
- 2.Livingston G., Huntley J., Sommerlad A., et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet. 2020;396(10248):413–446. doi: 10.1016/S0140-6736(20)30367-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Johnson J.C.S., Marshall C.R., Weil R.S., Bamiou D.E., Hardy C.J.D., Warren J.D. Hearing and dementia: from ears to brain. Brain. 2021;144(2):391–401. doi: 10.1093/brain/awaa429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafari Z., Kolb B.E., Mohajerani M.H. Age-related hearing loss and tinnitus, dementia risk, and auditory amplification outcomes. Ageing Res Rev. 2019;56 doi: 10.1016/j.arr.2019.100963. [DOI] [PubMed] [Google Scholar]
- 5.Jack C.R., Jr., Knopman D.S., Jagust W.J., et al. Hypothetical model of dynamic biomarkers of the Alzheimer's pathological cascade. Lancet Neurol. 2010;9(1):119–128. doi: 10.1016/S1474-4422(09)70299-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bucci M., Chiotis K., Nordberg A. Alzheimer's disease profiled by fluid and imaging markers: tau PET best predicts cognitive decline. Mol Psychiatr. 2021;26(10):5888–5898. doi: 10.1038/s41380-021-01263-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jack C.R., Jr., Bennett D.A., Blennow K., et al. NIA-AA Research Framework: toward a biological definition of Alzheimer's disease. Alzheimers Dement. 2018;14(4):535–562. doi: 10.1016/j.jalz.2018.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ren F., Ma W., Li M., et al. Gray matter atrophy is associated with cognitive impairment in patients with presbycusis: a comprehensive morphometric study. Front Neurosci. 2018;12:744. doi: 10.3389/fnins.2018.00744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Uchida Y., Nishita Y., Kato T., et al. Smaller hippocampal volume and degraded peripheral hearing among Japanese community dwellers. Front Aging Neurosci. 2018;10:319. doi: 10.3389/fnagi.2018.00319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Croll P.H., Vernooij M.W., Reid R.I., et al. Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement. 2020;16(11):1515–1523. doi: 10.1002/alz.12151. [DOI] [PubMed] [Google Scholar]
- 11.Parker T., Cash D.M., Lane C., et al. Pure tone audiometry and cerebral pathology in healthy older adults. J Neurol Neurosurg Psychiatry. 2020;91(2):172–176. doi: 10.1136/jnnp-2019-321897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ma W., Li M., Gao F., et al. DTI analysis of presbycusis using voxel-based analysis. Am J Neuroradiol. 2016;37(11):2110–2114. doi: 10.3174/ajnr.A4870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rigters S.C., Cremers L.G.M., Ikram M.A., et al. White-matter microstructure and hearing acuity in older adults: a population-based cross-sectional DTI study. Neurobiol Aging. 2018;61:124–131. doi: 10.1016/j.neurobiolaging.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 14.Belkhiria C., Vergara R.C., Martín S.S., et al. Cingulate cortex atrophy is associated with hearing loss in presbycusis with cochlear amplifier dysfunction. Front Aging Neurosci. 2019;11(APR):97. doi: 10.3389/fnagi.2019.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tuwaig M., Savard M., Jutras B., et al. Deficit in central auditory processing as a biomarker of pre-clinical alzheimer's disease. J Alzheimers Dis. 2017;60:1589–1600. doi: 10.3233/JAD-170545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu W., Zhang C., Li J.Q., et al. Age-related hearing loss accelerates cerebrospinal fluid tau levels and brain atrophy: a longitudinal study. Aging (Albany NY) 2019;11(10):3156–3169. doi: 10.18632/aging.101971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cherny S.S., Livshits G., Wells H.R.R., et al. Self-reported hearing loss questions provide a good measure for genetic studies: a polygenic risk score analysis from UK Biobank. Eur J Hum Genet. 2020;28(8):1056–1065. doi: 10.1038/s41431-020-0603-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lewis M.A., Schulte B.A., Dubno J.R., Steel K.P. Investigating the characteristics of genes and variants associated with self-reported hearing difficulty in older adults in the UK Biobank. BMC Biol. 2022;20(1):150. doi: 10.1186/s12915-022-01349-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y., Sahakian B.J., Kang J., et al. The brain structure and genetic mechanisms underlying the nonlinear association between sleep duration, cognition and mental health. Nature Aging. 2022;2:425–437. doi: 10.1038/s43587-022-00210-2. [DOI] [PubMed] [Google Scholar]
- 20.Xu W., Tan L., Su B.J., et al. Sleep characteristics and cerebrospinal fluid biomarkers of Alzheimer's disease pathology in cognitively intact older adults: the CABLE study. Alzheimers Dement. 2020;16(8):1146–1152. doi: 10.1002/alz.12117. [DOI] [PubMed] [Google Scholar]
- 21.Wang H.F., Shen X.N., Li J.Q., et al. Clinical and biomarker trajectories in sporadic Alzheimer's disease: a longitudinal study. Alzheimers Dement (Amst) 2020;12(1) doi: 10.1002/dad2.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lourida I., Hannon E., Littlejohns T.J., et al. Association of Lifestyle and genetic risk with incidence of dementia. JAMA. 2019;322(5):430–437. doi: 10.1001/jama.2019.9879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkinson T., Schnier C., Bush K., et al. Identifying dementia outcomes in UK Biobank: a validation study of primary care, hospital admissions and mortality data. Eur J Epidemiol. 2019;34(6):557–565. doi: 10.1007/s10654-019-00499-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu H., Meng L., Bi Y.L., et al. Tau pathologies mediate the association of blood pressure with cognitive impairment in adults without dementia: the CABLE study. Alzheimers Dement. 2021;18:53–64. doi: 10.1002/alz.12377. [DOI] [PubMed] [Google Scholar]
- 25.Albert M.S., DeKosky S.T., Dickson D., et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):270–279. doi: 10.1016/j.jalz.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.McKhann G.M., Knopman D.S., Chertkow H., et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sperling R.A., Aisen P.S., Beckett L.A., et al. Toward defining the preclinical stages of Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7(3):280–292. doi: 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Veitch D.P., Weiner M.W., Aisen P.S., et al. Using the Alzheimer's Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer's disease. Alzheimers Dement. 2021;18:824–857. doi: 10.1002/alz.12422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brenowitz W.D., Filshtein T.J., Yaffe K., et al. Association of genetic risk for Alzheimer disease and hearing impairment. Neurology. 2020;95(16):e2225–e2234. doi: 10.1212/WNL.0000000000010709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stevenson J.S., Clifton L., Kuźma E., Littlejohns T.J. Speech-in-noise hearing impairment is associated with an increased risk of incident dementia in 82,039 UK Biobank participants. Alzheimers Dement. 2021;18:445–456. doi: 10.1002/alz.12416. [DOI] [PubMed] [Google Scholar]
- 31.Choi S.W., Mak T.S., O'Reilly P.F. Tutorial: a guide to performing polygenic risk score analyses. Nat Protoc. 2020;15(9):2759–2772. doi: 10.1038/s41596-020-0353-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Desikan R.S., Ségonne F., Fischl B., et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage. 2006;31(3):968–980. doi: 10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- 33.Fischl B., Salat D.H., Busa E., et al. Whole brain segmentation: automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33(3):341–355. doi: 10.1016/s0896-6273(02)00569-x. [DOI] [PubMed] [Google Scholar]
- 34.Wakana S., Jiang H., Nagae-Poetscher L.M., van Zijl P.C., Mori S. Fiber tract-based atlas of human white matter anatomy. Radiology. 2004;230(1):77–87. doi: 10.1148/radiol.2301021640. [DOI] [PubMed] [Google Scholar]
- 35.de Groot M., Vernooij M.W., Klein S., et al. Improving alignment in Tract-based spatial statistics: evaluation and optimization of image registration. Neuroimage. 2013;76:400–411. doi: 10.1016/j.neuroimage.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ma Y.H., Wang Y.Y., Tan L., et al. Social networks and cerebrospinal fluid biomarkers of Alzheimer's disease pathology in cognitively intact older adults: the CABLE study. J Alzheimers Dis. 2021;81(1):263–272. doi: 10.3233/JAD-201426. [DOI] [PubMed] [Google Scholar]
- 37.Rolls E.T. Oxford University; Oxford: 2021. Brain Computations: What and How. [Google Scholar]
- 38.Rolls E.T., Deco G., Huang C.C., Feng J. The human language effective connectome. Neuroimage. 2022;258 doi: 10.1016/j.neuroimage.2022.119352. [DOI] [PubMed] [Google Scholar]
- 39.Rolls E.T., Deco G., Huang C.C., Feng J. The human posterior parietal cortex: effective connectome, and its relation to function. Cerebr Cortex. 2022 doi: 10.1093/cercor/bhac266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rolls E.T., Deco G., Huang C.C., Feng J. The human orbitofrontal cortex, vmPFC, and anterior cingulate cortex effective connectome: emotion, memory, and action. Cerebr Cortex. 2022 doi: 10.1093/cercor/bhac070. [DOI] [PubMed] [Google Scholar]
- 41.Doré V., Villemagne V.L., Bourgeat P., et al. Cross-sectional and longitudinal analysis of the relationship between Aβ deposition, cortical thickness, and memory in cognitively unimpaired individuals and in Alzheimer disease. JAMA Neurol. 2013;70(7):903–911. doi: 10.1001/jamaneurol.2013.1062. [DOI] [PubMed] [Google Scholar]
- 42.Tarawneh H.Y., Menegola H.K., Peou A., Tarawneh H., Jayakody D.M.P. Central auditory functions of Alzheimer's disease and its preclinical stages: a systematic review and meta-analysis. Cells. 2022;11(6) doi: 10.3390/cells11061007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gates G.A., Beiser A., Rees T.S., D'Agostino R.B., Wolf P.A. Central auditory dysfunction may precede the onset of clinical dementia in people with probable Alzheimer's disease. J Am Geriatr Soc. 2002;50(3):482–488. doi: 10.1046/j.1532-5415.2002.50114.x. [DOI] [PubMed] [Google Scholar]
- 44.Gates G.A., Anderson M.L., McCurry S.M., Feeney M.P., Larson E.B. Central auditory dysfunction as a harbinger of Alzheimer dementia. Arch Otolaryngol Head Neck Surg. 2011;137(4):390–395. doi: 10.1001/archoto.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin F.R., Ferrucci L., An Y., et al. Association of hearing impairment with brain volume changes in older adults. Neuroimage. 2014;90:84–92. doi: 10.1016/j.neuroimage.2013.12.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Belkhiria C., Vergara R.C., San Martin S., et al. Insula and amygdala atrophy are associated with functional impairment in subjects with presbycusis. Front Aging Neurosci. 2020;12:102. doi: 10.3389/fnagi.2020.00102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jack C.R., Jr., Wiste H.J., Botha H., et al. The bivariate distribution of amyloid-β and tau: relationship with established neurocognitive clinical syndromes. Brain. 2019;142(10):3230–3242. doi: 10.1093/brain/awz268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Griffiths T.D., Lad M., Kumar S., et al. How can hearing loss cause dementia? Neuron. 2020;108(3):401–412. doi: 10.1016/j.neuron.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kocagoncu E., Quinn A., Firouzian A., et al. Tau pathology in early Alzheimer's disease is linked to selective disruptions in neurophysiological network dynamics. Neurobiol Aging. 2020;92:141–152. doi: 10.1016/j.neurobiolaging.2020.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Haan W., Mott K., van Straaten E.C.W., Scheltens P., Stam C.J. Activity dependent degeneration explains hub vulnerability in Alzheimer's disease. PLoS Comput Biol. 2012;8(8) doi: 10.1371/journal.pcbi.1002582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brenowitz W.D., Filshtein T.J., Yaffe K., et al. Functional hearing loss as a marker for early Alzheimer's disease and dementia: exploring the potential for reverse causation. Alzheimers Dement. 2020;16(S10) [Google Scholar]
- 52.Goll J.C., Kim L.G., Ridgway G.R., et al. Impairments of auditory scene analysis in Alzheimer's disease. Brain. 2012;135(Pt 1):190–200. doi: 10.1093/brain/awr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hardy C.J.D., Yong K.X.X., Goll J.C., Crutch S.J., Warren J.D. Impairments of auditory scene analysis in posterior cortical atrophy. Brain. 2020;143(9):2689–2695. doi: 10.1093/brain/awaa221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Golden H.L., Nicholas J.M., Yong K.X., et al. Auditory spatial processing in Alzheimer's disease. Brain. 2015;138(Pt 1):189–202. doi: 10.1093/brain/awu337. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.