Abstract
Fever improves survival in acute infections, but the effects of increased core temperature on host defenses are poorly understood. Tumor necrosis factor alpha (TNF-α) is an early activator of host defenses and a major endogenous pyrogen. TNF-α expression is essential for survival in bacterial infections but, if disregulated, can cause tissue injury. In this study, we show that passively increasing core temperature in mice from the basal (36.5 to 37.5°C) to the febrile (39.5 to 40°C) range modifies systemic TNF-α expression in response to bacterial endotoxin (lipopolysaccharide). The early TNF-α secretion rate is enhanced, but the duration of maximal TNF-α production is shortened. We identified Kupffer cells as the predominant source of the excess TNF-α production in the warmer animals. The enhanced early TNF-α production observed at the higher temperature in vivo could not be demonstrated in isolated Kupffer cells or in precision-cut liver slices in vitro, indicating the participation of indirect pathways. Therefore, expression of the endogenous pyrogen TNF-α is regulated by increments in core temperature during fever, generating an enhanced early, self-limited TNF-α pulse.
The beneficial effects of fever in bacterial, fungal, and viral infections have been widely reported (reviewed in reference 22). Fever accelerates the resolution of human viral infections (12, 39) and shigellosis (28) and is positively correlated with survival in patients with gram-negative bacteremia (7, 39). Studies of induced hyperthermia in infected animals have provided evidence that an increase in body temperature may enhance host defenses. For example, housing herpesvirus-infected mice in a 38°C ambient environment increased their core temperature by approximately 2°C and increased survival to 100% compared with 0% survival in mice maintained at a normal laboratory temperature (1). Bell and Moore (2) reported similar protection by passive warming of rabies virus-infected mice. However, the mechanisms through which an increase in core temperature can improve survival in the infected host are incompletely understood.
Tumor necrosis factor alpha (TNF-α) is an early and essential activator of host defenses (9, 10, 30); however, inappropriate TNF-α expression can cause multiorgan failure, shock, and death (41). This apparent paradox has led to the evolution of redundant regulation of TNF-α expression. We previously reported that raising incubation temperature from basal (37°C) to febrile (38.5 to 40°C) levels reduced the duration of lipopolysaccharide (LPS)-induced TNF-α secretion in macrophages in vitro (13, 14). However, the ability of fever to regulate TNF-α expression in vivo has not been clearly determined. To address this question, we developed a mouse model in which endogenous thermoregulation was suspended with anesthesia and core temperature was rigorously controlled by immersion in a constant-temperature water bath. Based on our in vitro observations, we predicted that raising core temperature to febrile levels would attenuate systemic TNF-α production in vivo. We found that increasing core temperature from basal (36.5 to 37.5°C) to febrile (39.5 to 40°C) ranges immediately before or coincident with LPS challenge reduced the duration of TNF-α production but surprisingly enhanced the rate of early TNF-α production by Kupffer cells, leading to a self-limited TNF-α pulse in the warmer animals.
MATERIALS AND METHODS
Temperature control.
LPS purified from Escherichia coli 0111:B4 prepared according to the Boivin method was obtained from Difco (Detroit, Mich.). Preparation of LPS by this method preserves more of the associated proteins than does preparation by methods that make use of phenol (11, 35). Six- to eight-week-old male CD-1 mice weighing 25 to 30 g were purchased from Harlan-Sprague Co. (Indianapolis, Ind.), housed in the University of Maryland, Baltimore, animal facility under the supervision of a full-time veterinarian, and used within 4 weeks. All animal protocols were approved by the Institutional Animal Care and Use Committee of the University of Maryland, Baltimore. Mice were anesthetized subcutaneously with tribromoethanol (Sigma). The anesthetized animals were suspended in water baths (VWR; temperature variation, <0.2°C) to the level of the axillae. Body temperature was continuously monitored with rectal thermistors. When body temperature reached bath temperature, 0.25 ml of either LPS or vehicle (pyrogen-free saline) was administered as an intraperitoneal (i.p.) injection. To control for the effects of anesthesia and water immersion, we also studied a group of conscious, unrestrained mice at normal laboratory temperatures (22 to 24°C). To model endotoxemia or bacteremia in the setting of established infection in febrile hosts, we increased core temperature to febrile levels before administering LPS. The basal core temperature range in conscious mice was 36.5 to 37.5°C but fell to ambient levels within 30 min of induction of anesthesia. In the temperature-controlled mice, core temperature reached water bath temperature in less than 10 min and varied by <0.2°C during the experiments. To avoid the influence of diurnal cycling, all experiments were started at approximately the same time each day (between 8:00 a.m. and 10:00 a.m.).
Plasma TNF-α and LPS levels.
Mouse TNF-α was measured by a standard two-antibody enzyme-linked immunosorbent assay (ELISA) with a commercial antibody pair and a recombinant standard (Endogen, Boston, Mass.), a biotin-streptavidin-peroxidase detection system (Research Diagnostics Inc., Flanders, N.J.), and a commercial substrate preparation (Dako, Carpinteria, Calif.). The mouse TNF-α ELISA had a lower detection limit of 3 pg/ml and measured predominantly free, biologically active TNF-α. The results of the TNF-α ELISA correlated with the measurement of TNF-α activity by an L929 cell bioassay. In preliminary experiments, ELISA results were confirmed by the L929 cell bioassay. LPS was analyzed by a commercial colorimetric Limulus amebocyte lysate assay (Associates of Cape Cod, Falmouth, Mass.) with a minimal detection limit of 10 pg/ml. Clearance of circulating TNF-α was determined by measuring the clearance of exogenous human TNF-α levels in temperature-controlled, LPS-challenged mice. Mice received 1 ng of recombinant human TNF-α (Endogen) via the tail vein, heparinized blood was sequentially collected from the retro-orbital plexus, and the concentration of human TNF-α in plasma was measured by an ELISA with a monoclonal antibody pair specific for human TNF-α (Endogen). This ELISA had a lower detection limit of 1 pg/ml for human TNF-α and showed no cross-reactivity with 10 ng of mouse TNF-α per ml. The half-life of circulating human TNF-α was calculated with an iterative exponential curve-fitting algorithm (Deltagraph; Deltapoint).
Organ-specific TNF-α expression.
Organs were collected and snap frozen after the circulatory system was flushed with 10 ml of 4°C phosphate-buffered saline containing protease inhibitors (4 mM EDTA, 1 mM phenylmethylsulfonyl fluoride, and leupeptin at 1 μg/ml), injected through the left ventricle and drained from the left atrium. Organs were powdered under liquid nitrogen, homogenized in 1 ml of lysing buffer (150 mM NaCl, 25 mM Tris [pH 7.4], 1% Nonidet P-40, 4 mM EDTA, protease inhibitors), and cleared by centrifugation. Total protein concentrations in organ homogenates were measured with a commercial reagent (Bio-Rad, Mountain View, Calif.) and with bovine serum albumin (Sigma) as a standard. To minimize the background signal from endogenous biotin-containing proteins, 50 μl of a 3-mg/ml avidin solution (Sigma) was added to 470 μl of homogenate, and an ELISA was performed as described above except for a 20-min incubation with 20 μg of free biotin (Sigma) per ml added between the sample incubation and the addition of the detecting antibody. This method reduced background noise by >99% without affecting the specific cytokine signal. Cytokine concentrations in organ homogenates were standardized to total protein concentrations.
Immunostaining for TNF-α.
Formalin-fixed, paraffin-embedded, 8-μm-thick liver sections were incubated overnight with a 1:400 dilution of rabbit anti–mouse TNF-α antiserum (Genzyme, Cambridge, Mass.). TNF-α was detected with biotinylated goat anti-rabbit immunoglobulin G (Dako) and an avidin-biotin complex detection system (Vector, Burlingame, Calif.) in accordance with the manufacturer’s protocol. Groups of three animals were studied.
In vivo Kupffer cell depletion.
Liposome-encapsulated clodronate (0.1 ml) prepared as previously described (42) was injected via the tail vein 2 days prior to temperature controlling and LPS challenge. Control mice received 0.1 ml of pyrogen-free, sterile saline via tail vein injection. This method has been shown by rigorous histochemical analysis to deplete predominantly Kupffer cells and less predominantly splenic macrophages and to spare circulating monocytes and pulmonary and peritoneal macrophages (42).
Macrophage isolation and preparation of precision-cut liver slices.
Raw 264.7 macrophages were obtained from the American Type Culture Collection (Rockville, Md.) and maintained in RPMI 1640 supplemented with 10 mM HEPES (pH 7.3), 1 mM sodium pyruvate, 2 mM l-glutamine, 50 μg of streptomycin per ml, 50 U of penicillin per ml, and 10% heat-inactivated fetal calf serum (FCS; Hyclone, Logan, Utah) (CRPMI). Peritoneal macrophages were elicited by injecting mice with 1 ml of thioglycolate broth (Sigma) i.p. 4 days prior to sacrifice. Following sacrifice by anesthesia and cervical dislocation, cells were harvested by peritoneal lavage with 5 ml of 4°C FCS-free CRPMI and collected by centrifugation at 600 × g for 10 min. The macrophages were purified by adherence to plastic. The macrophages were preincubated with CRPMI–10% FCS at 37 or 40°C for 30 min and stimulated with 100 ng of LPS per ml, and culture supernatants were sequentially analyzed for TNF-α concentrations by an ELISA. Kupffer cells were isolated from livers by collagenase digestion and Nycodenz gradient centrifugation as previously described by ten Hagen et al. (40), except that Kupffer cells were purified by differential adherence in FCS-free CRPMI for 30 min (36) rather than by elutriation. Based on the morphologic appearance of Diff-Quik (Baxter Scientific Products, Miami, Fla.)-stained cells, the monolayers comprised >90% Kupffer cells. The monolayers were preincubated with CRPMI–10% FCS at 37 or 40°C for 30 min and stimulated with 5 μg of LPS per ml, and TNF-α concentrations in culture supernatants were sequentially analyzed by an ELISA. The viability of all macrophage cultures was determined to be >95% by trypan blue dye exclusion.
Precision-cut liver slices were prepared by cutting 175-μm-thick slices from 8-mm cores of liver tissue with a Krumdieck tissue slicer (Alabama Research and Development, Munford, Ala.) as previously described (24). The slices were cultured in CRPMI–10% FCS with continuous shaking (27, 37), preincubated at 37 or 40°C for 30 min, and stimulated with 50 μg of LPS per ml, and supernatants were sequentially analyzed for TNF-α concentrations. All solutions used for the isolation and culturing of Kupffer cells and liver slices contained 2 μg of polymyxin B (Sigma) per ml to prevent inadvertent activation by contaminating LPS. Evaporation was minimized by filling the outer wells of each culture plate with sterile water and sealing them with Parafilm. Volume loss was <5% over 24 h and was not different in the 37 and 40°C culture wells.
Data analysis.
All data are presented as means ± standard errors (SE). Differences among groups were tested by a Fisher protected least-squares difference test applied to a one-way analysis of variance. Survival was analyzed with a Gehan-Wilcoxon test of a Kaplan-Meier plot.
RESULTS
Influence of core temperature on circulating levels of TNF-α.
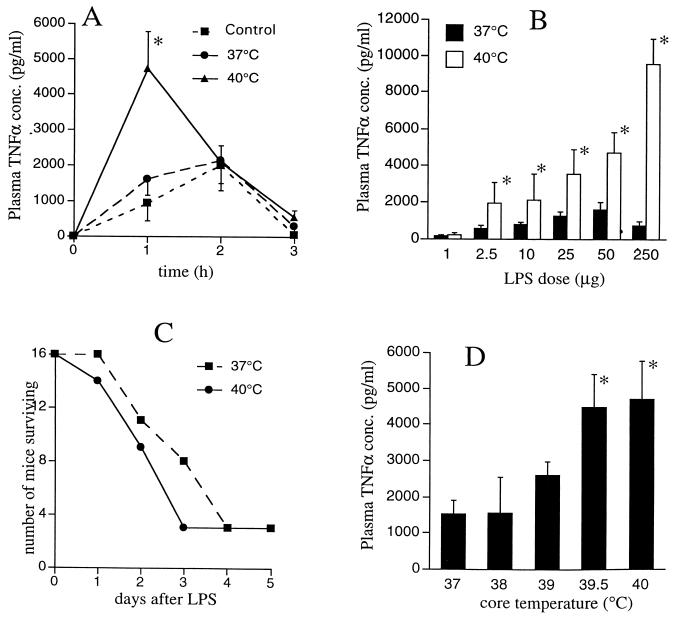
We initially compared LPS-induced TNF-α expression in mice temperature controlled at 37 and 40°C. Treatment i.p. with 50 μg of LPS induced the appearance of circulating TNF-α in both groups of mice. As we anticipated, based on our in vitro studies (13, 34a), plasma TNF-α levels peaked and began to decline earlier in the 40°C mice (Fig. 1A), indicating a shorter duration of TNF-α expression in the warmer mice. However, plasma TNF-α peaked at 2.2-fold-higher levels in the 40°C mice. The clearance of circulating TNF-α, as estimated by the half-life of an intravenous bolus of exogenous human TNF-α, was comparable in the 37 and 40°C mice (data not shown). Taken together, these data suggest that the early TNF-α production rate was greater but that the duration of maximal TNF-α expression was shorter in the warmer mice. The net result of these two temperature-dependent effects is an accelerated and enhanced but self-limited pulse of systemic TNF-α expression in the 40°C mice.
FIG. 1.
Effects of temperature controlling on plasma TNF-α expression. (A) Kinetics of TNF-α expression. Male CD-1 mice were temperature controlled by anesthesia with subcutaneous tribromoethanol and immersion in 37 or 40°C constant-temperature water baths. When the core temperature measured by a rectal thermistor probe reached the bath temperature (5 to 10 min), mice were injected i.p. with 50 μg of LPS. Control mice (not temperature controlled) were injected with LPS but remained conscious at a normal ambient temperature (22 to 24°C). Groups of six mice were sacrificed just before and 1, 2, or 3 h after LPS injection, and plasma TNF-α concentrations (conc.) were measured by an ELISA. Data are means ± SE. The asterisk indicates that the P value for 40°C mice versus 37°C mice or control mice was <0.02. (B) LPS dose response. Groups of five mice were temperature controlled at 37 or 40°C, injected i.p. with the indicated doses of LPS, and sacrificed 1 h later for quantitation of plasma TNF-α concentrations. An asterisk indicates that the P value for 40°C mice versus 37°C mice was <0.01. (C) Effects of core temperature on LPS-induced mortality. Groups of 16 mice were temperature controlled at 37 or 40°C and injected i.p. with 250 μg of LPS, and temperature controlling was continued for 3 h. The mice were allowed to recover, and survival was assessed over the next 5 days. Survival was analyzed with a Gehan-Wilcoxon test of a Kaplan-Meier plot (P, 0.198). (D) Effects of intermediate temperatures on TNF-α expression. Groups of five mice were temperature controlled at the indicated temperatures, injected i.p. with 50 μg of LPS, and sacrificed 1 h later for quantitation of plasma TNF-α concentrations. An asterisk indicates that the P value for mice at the indicated temperature versus 37°C mice at the same time point was <0.01.
The temperature-dependent enhancement of TNF-α expression was greater as the LPS dose was increased (Fig. 1B). While the LPS dose-response curve for plasma TNF-α expression was flat between 50- and 250-μg doses in the 37°C mice, as previously described (27), the LPS dose-response curve remained steeply positive over this range in the 40°C mice. While TNF-α is protective in the infected host (10, 30), it is also a central mediator of LPS-induced shock and death (3). In mice treated with 250 μg of LPS, survival time tended to be shorter in the 40°C than in the 37°C animals, but the difference did not reach statistical significance (P, 0.198) (Fig. 1C).
To define the threshold temperature required to amplify early TNF-α expression, we measured plasma TNF-α levels 1 h after LPS treatment in 37 and 40°C mice (Fig. 1D). The threshold for increasing plasma TNF-α levels consistently occurred between 39 and 39.5°C, a temperature that is within the normal murine febrile range (19). The effect of temperature controlling on TNF-α expression was critically dependent on the timing of the core temperature increase relative to the LPS challenge. While increasing the core temperature to 40°C coincident with LPS treatment modified TNF-α expression, delaying the temperature increase until 30 min after LPS challenge completely abrogated this effect (data not shown). To determine if anesthesia influenced the temperature-dependent, LPS-induced TNF-α expression, plasma TNF-α levels were measured 1 h after treatment with 250 μg of LPS in conscious mice maintained in 38 or 24°C cages. Core temperatures were 39.7 and 37.1°C in the two groups of animals, and plasma TNF-α levels were 5.6-fold higher in the warmer mice than in the mice housed in 24°C cages.
Major tissue site of temperature-dependent TNF-α expression.
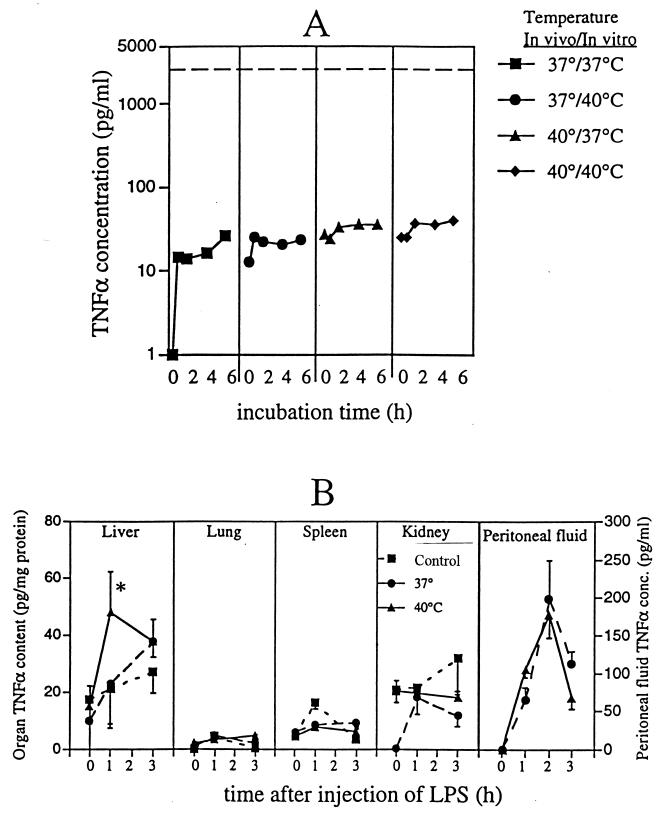
To determine if the excess plasma TNF-α in the 40°C mice was generated by circulating leukocytes, we measured TNF-α expression in blood in vitro. Heparinized blood was collected from 37 and 40°C mice 30 min after LPS challenge and incubated at 37 or 40°C (Fig. 2A). The peak TNF-α concentrations generated in blood samples in vitro were <1% of the peak plasma TNF-α levels attained in the mice in vivo (Fig. 2A) and were not affected by changes in either in vivo core temperature or in vitro incubation temperature, excluding circulating blood leukocytes as the predominant source of circulating TNF-α in either group of mice.
FIG. 2.
Source of excess TNF-α production in mice at 40°C. (A) Effect of temperature on TNF-α generation in blood ex vivo. Groups of five mice were temperature controlled at either 37 or 40°C and given a single 50-μg dose of LPS i.p., and temperature controlling was continued for 30 min. Heparinized (10 U/ml) blood was collected and incubated at the indicated in vitro temperature. At the indicated times, samples were diluted with 3 volumes of 4°C CRPMI, cells were removed by centrifugation, and TNF-α concentrations in the diluted plasma were measured by an ELISA. The broken line indicates the peak plasma TNF-α levels reached 1 h after LPS treatment in vivo in 40°C mice. (B) Effects of core temperature on organ-associated and peritoneal fluid TNF-α concentrations (conc.). Male CD-1 mice were anesthetized, temperature controlled at 37 or 40°C, and injected i.p. with 50 μg of LPS, and groups of five animals were sacrificed just before (0 h) or 1 or 3 h after LPS injection. Control (not temperature controlled) animals received LPS but remained conscious at a normal ambient temperature (22 to 24°C). After sacrifice, the circulation was flushed with 0.9% NaCl containing protease inhibitors, and the lungs, kidneys, liver, and spleen were snap frozen in liquid nitrogen. The frozen organs were powdered under liquid nitrogen, homogenized, and cleared by centrifugation, and TNF-α concentrations in the supernatants were measured by an ELISA. Levels were standardized to total protein concentrations. For quantitation of TNF-α in peritoneal fluid, groups of five mice were temperature controlled at either 37 or 40°C and injected i.p. with 250 μg of LPS, and temperature controlling was continued until sacrifice. Peritoneal lavage was done with 5 ml of FCS-free CRPMI, the cells were removed by centrifugation, and TNF-α concentrations were measured by an ELISA. Data are means ± SE. The asterisk indicates that the P value for 40°C mice versus 37°C and control mice was <0.05.
TNF-α is not uniformly expressed during endotoxemia (18). To determine if fever alters the tissue distribution of TNF-α and to identify the potential tissue sources of the excess circulating TNF-α in the 40°C mice, we measured TNF-α in peritoneal lavage fluid and homogenates of liver, spleen, lung, and kidney tissues obtained from LPS-challenged, temperature-controlled animals (Fig. 2B). These organs were selected for study because they either are important sources of systemic TNF-α expression (15) or are frequently injured in sepsis (6). As previously reported (15), the liver appeared to be the predominant source of TNF-α after LPS challenge. The peak total TNF-α content of the liver (93 ± 21 ng) was 2 to 3 orders of magnitude higher than the total TNF-α content of the spleen (0.10 ± 0.02 ng), lungs (0.06 ± 0.04 ng), kidneys (1.1 ± 0.1 ng), or peritoneal lavage fluid (0.98 ± 0.28 ng). Furthermore, of the organs analyzed, the liver was the only organ in which TNF-α accumulated more rapidly in the 40°C than in the 37°C mice, parallelling the changes in plasma TNF-α levels and suggesting that the liver was the predominant source of the excess circulating TNF-α in the 40°C mice.
Kupffer cells are the predominant source of liver-associated and circulating TNF-α.
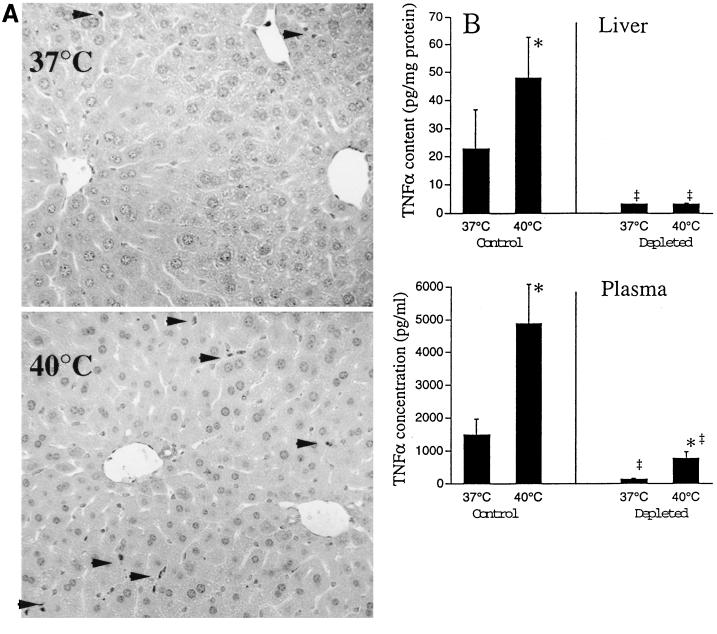
Among hepatic cells, Kupffer cells have the greatest capacity to secrete TNF-α, but marginated intravascular leukocytes and hepatocytes are also capable of TNF-α expression (27). To identify the cellular source of the excess hepatic TNF-α expression in the 40°C mice, we performed an immunohistochemical analysis for TNF-α in livers from 37 and 40°C mice 1 h after LPS challenge. Not only was TNF-α staining limited almost exclusively to Kupffer cells, but also the proportion of TNF-α-staining Kupffer cells was threefold higher in the 40°C than in the 37°C mice (Fig. 3A).
FIG. 3.
Source of excess hepatic TNF-α production in mice at 40°C. (A) TNF-α immunostaining. Mice were temperature controlled at 37 or 40°C, treated i.p. with 50 μg of LPS, and sacrificed 1 h later. Livers were analyzed for TNF-α by immunostaining with a rabbit anti–mouse TNF-α antibody and a streptavidin-biotin detection system. Representative micrographs from a total of three experiments are shown. Kupffer cells staining positively for TNF-α are indicated by arrowheads. (B) Kupffer cell depletion in vivo. Kupffer cells were depleted in groups of four mice by administration of a single 0.1-ml dose of liposome-encapsulated clodronate intravenously 2 days prior to LPS challenge. Control mice received 0.1 ml of saline. Mice were temperature controlled at 37 or 40°C, injected i.p. with 50 μg of LPS, and sacrificed 1 h later. TNF-α concentrations in plasma and liver homogenates were quantified by an ELISA. TNF-α concentrations in liver homogenates were standardized to total protein concentrations. Data are means ± SE. An asterisk indicates that the P value for 40°C versus 37°C mice was <0.05; a double dagger indicates that the P value for mice with depletion of Kupffer cells versus control mice was <0.01.
To confirm that Kupffer cells were the predominant source of the excess hepatic and circulating TNF-α present in 40°C animals, we analyzed LPS-induced TNF-α expression in temperature-controlled animals after depleting their Kupffer cells with intravenous liposome-encapsulated clodronate (42). This treatment reduced LPS-induced hepatic and plasma TNF-α levels by 87 and 91%, respectively, in the 37°C mice compared with the sham-depleted 37°C control mice and reduced excess hepatic and plasma TNF-α levels by 99 and 81%, respectively, in the 40°C mice compared with the sham-depleted mice (Fig. 3B). These data indicate that Kupffer cells are virtually the sole source of the excess hepatic TNF-α and the major source of the excess circulating TNF-α in the 40°C mice.
Mechanisms of temperature-dependent regulation of TNF-α expression.
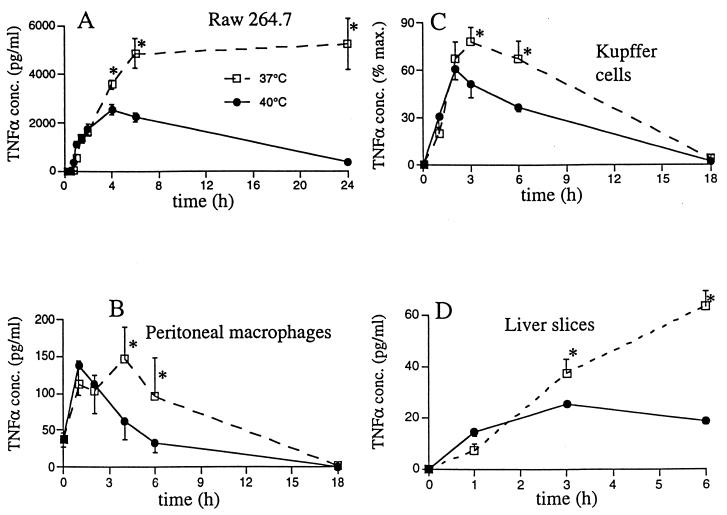
While the earlier peak and decline in Kupffer cell TNF-α production in the warmer mice was consistent with our previous in vitro studies (13), enhancement of the early TNF-α secretion rate was novel for in vivo studies. To determine if the enhanced TNF-α expression in the 40°C mice in vivo was caused by a unique response of Kupffer cells to higher temperatures, we analyzed the effects of 37 versus 40°C incubation temperatures on TNF-α secretion in freshly isolated Kupffer cell cultures (Fig. 4A), in thioglycolate-elicited peritoneal macrophages (Fig. 4B), and in the Raw 264.7 macrophage cell line (Fig. 4C). The TNF-α secretion patterns of all three macrophage types were similar. TNF-α expression peaked and declined earlier in the 40°C than in the 37°C cells, but there were no detectable differences in the initial TNF-α secretion rate between the 37 and the 40°C cells. These data suggest that raising core temperature directly limits the duration of Kupffer cell TNF-α production but that the increase in early Kupffer cell TNF-α production in vivo involves indirect mechanisms and is probably dependent on the in vivo environment.
FIG. 4.
Direct effect of increased incubation temperature on TNF-α expression in vitro. (A to C) Effects of febrile-level incubation temperature on TNF-α expression by macrophages in vitro. Kupffer cells (106 per well containing 1 ml of CRPMI–10% FCS) were isolated from the liver by collagenase digestion and plastic adherence and then preincubated at 37 or 40°C for 30 min, and 5 μg of LPS per ml was added. Peritoneal macrophages were obtained 4 days after i.p. injection of 1 ml of thioglycolate broth and purified by plastic adherence. Raw 264.7 macrophages and peritoneal macrophages (0.5 × 106 per well containing 1 ml of medium) were preincubated at 37 or 40°C for 30 min, and 100 ng of LPS per ml was added. Cell supernatants were sequentially collected, and TNF-α concentrations (conc.) were measured by an ELISA. Data are means ± SE. An asterisk indicates that the P value for 40°C versus 37°C cells was <0.05. (D) Effects of febrile-level incubation temperature on TNF-α expression in precision-cut liver slices in vitro. Liver slices (175 μm thick) were cut from 8-mm tissue cores with a Krumdieck tissue slicer. The slices were preincubated at 37 or 40°C for 30 min with continuous shaking. LPS (50 μg/ml) was added, and supernatants were sequentially collected for analysis of TNF-α concentrations by an ELISA. The asterisk is as defined for panel A.
We evaluated three possible mechanisms for the Kupffer cell-specific increase in TNF-α expression in the 40°C mice, including: (i) enhanced absorption of LPS from the peritoneum, (ii) modification of interactions among Kupffer cells and other cell types in the hepatic microenvironment, and (iii) release of a circulating factor that enhances Kupffer cell TNF-α expression. The plasma LPS concentrations 1 h after i.p. injection of 250 μg of LPS were virtually identical in the 37 and 40°C mice (2.34 ± 0.43 versus 2.29 ± 0.45 μg/ml; P, 0.89), suggesting that systemic LPS absorption was not increased in the 40°C mice. However, the possibility that both absorption and blood clearance of LPS were enhanced proportionately at 40°C remains to be excluded.
To determine if the enhanced TNF-α expression was mediated through complex interactions among Kupffer cells and neighboring cells, we analyzed TNF-α expression in freshly isolated precision-cut liver slices, in which the local microenvironment of the Kupffer cells is preserved (Fig. 4D). The temperature dependence of LPS-induced TNF-α expression in the liver slices and the isolated Kupffer cells (Fig. 4A) was similar. In both systems, Kupffer cells failed to demonstrate enhanced TNF-α expression during a 40°C incubation, suggesting that a factor(s) beyond the local hepatic microenvironment is required for this effect.
To determine if increasing the core temperature induces the release of a circulating factor that enhances Kupffer cell TNF-α expression, we stimulated liver slices with 50 μg of LPS per ml in the presence of 20% (vol/vol) plasma obtained from 37 or 40°C temperature-controlled mice 30 min after they were challenged with LPS (Table 1). This time point was chosen because amplification of TNF-α expression in vivo required that the core temperature be increased within 30 min of LPS challenge. The addition of plasma from the 40°C mice did not enhance TNF-α secretion in the liver slices, suggesting that the enhanced TNF-α production which occurred in the 40°C mice could not be ascribed to a stable circulating factor.
TABLE 1.
Increased core temperature does not induce a circulating enhancer of TNF-α expression
| Culture temp (°C) | TNF-α concn (pg/ml) in culture supernatants at the following time after LPS treatment and with the indicated plasmaa:
|
|||
|---|---|---|---|---|
| 1 h
|
2 h
|
|||
| 37°C | 40°C | 37°C | 40°C | |
| 37 | 5.2 ± 2.8 | 4.4 ± 3.1 | 15.7 ± 4.4 | 13.0 ± 2.3 |
| 40 | 7.7 ± 4.8 | 4.7 ± 2.3 | 22.5 ± 10.6 | 15.2 ± 2.4 |
Mice were temperature controlled at 37 or 40°C, challenged i.p. with 250 μg of LPS, and sacrificed 30 min later, and heparinized plasma was collected. Precision-cut liver slices were incubated in CRPMI–10% FCS, 50 μg of LPS per ml, and 20% (vol/vol) plasma from either 37 or 40°C mice. Culturing was performed at either 37 or 40°C, culture supernatants were collected after 1 and 2 h, and TNF-α was quantified by an ELISA. Results are means ± SE of three experiments.
DISCUSSION
We (13, 14) and others (16, 38) have reported that TNF-α expression by mononuclear phagocytes is inhibited upon exposure to febrile-range temperatures in vitro. The temperature-dependent inhibition of TNF-α expression, which is caused by the reduced stability of TNF-α mRNA (13) and the premature deactivation of TNF-α transcription (34a), occurs in the absence of heat shock protein 70 expression (13). In this study, we have extended these observations by analyzing the effects of febrile-range temperature on TNF-α expression in vivo. We found that increasing the core temperature from basal to febrile (40°C) levels preceding LPS challenge caused TNF-α production to be accelerated and enhanced but also to peak and decline earlier. This schedule models recurrent endotoxemia and bacteremia during fever, which often occur during serious bacterial infections (34). Three previous studies reported that increasing the core temperature in rodents either reduced (8, 21) or had no effect (4) on circulating TNF-α expression. However, in these studies the animals were warmed to temperatures above the normal febrile range and within the heat shock range (≥41°C). Furthermore, the core temperature increase in these studies either preceded LPS challenge by 6 to 7 h (4) or 24 h (21) or followed LPS challenge (8). In our model, delaying the increase in the core temperature for 30 min after the injection of LPS abrogated the enhancement of TNF-α expression. These data suggest that the effect of increasing the core temperature on TNF-α expression may be determined by the magnitude of the temperature increase and the timing of the LPS challenge relative to the core temperature change.
Our temperature-controlled mouse model was designed to isolate the effects of febrile temperature itself from the processes that generate fever. The use of anesthetized animals avoided the effects of physical stress (23) and provided more rapid and precise control of the core temperature. While anesthetic agents may potentially influence the acute-phase response, two lines of evidence suggested that the anesthetic agents used in this study did not interfere with the evaluation of temperature-dependent TNF-α expression in the temperature-controlled mice. First, circulating and organ-associated TNF-α levels were comparable in the 37°C temperature-controlled mice and in the conscious control animals with similar core temperatures. Second, the effects of passive warming on TNF-α expression were similar in anesthetized and conscious mice. Furthermore, all temperature-controlled mice were treated with the same anesthesia protocol.
The enhanced early TNF-α pulse reflects an increased initial rate of TNF-α generation but a decreased duration of maximal TNF-α expression in the warmer animals. The decreased duration of TNF-α expression appears to be a direct response of Kupffer cells to the higher temperature and is similar to temperature-dependent responses in other mononuclear phagocytes (13, 14, 16, 38). In contrast, the enhanced early rate of TNF-α generation by Kupffer cells at febrile core temperatures could not be reconstituted in vitro with freshly isolated Kupffer cells or with liver slices, in which local cell-cell interactions are preserved. We failed to detect a circulating enhancer of hepatic TNF-α expression in the warmer animals but have not excluded a labile circulating or intrahepatic enhancer of TNF-α expression. While circulating levels of LPS in mice at the two temperatures were comparable, modification of LPS bioactivity or distribution within the liver may account for the temperature-dependent modification of TNF-α expression. Within the liver, endothelial cells, hepatocytes, and Kupffer cells can each bind LPS via scavenger receptors (31). While TNF-α expression is predominantly limited to Kupffer cells, endothelial cells and hepatocytes contribute to LPS clearance. The intrahepatic distribution of LPS is influenced by its modification, primarily by binding to soluble proteins. For example, when complexed with ApoE, LPS is redirected from Kupffer cells to hepatocytes in vivo (31). On the other hand, when complexed with LPS-binding protein, LPS efficiently binds to CD14 (43), the membrane-bound LPS receptor on Kupffer cells. The influence of changes in temperature on the modification and intrahepatic distribution of LPS has not been reported.
While our results obtained with LPS-challenged mice provide proof of the principle that increases in core temperature to the usual febrile range can modify an early inflammatory response, caution must be exercised in applying these observations to actual bacterial infections. While LPS is clearly the predominant gram-negative bacterial activation signal for macrophages (11, 26), there are important differences in the biological behaviors of the purified LPS used in the present study and bacterium-associated LPS, including tissue distribution (17), specific activity (26), and sensitivity to inhibition by polymyxin B (26). The method used to purify LPS also affects its biological activity (11, 35). In the rat, purified LPS and LPS associated with viable E. coli localizes exclusively to hepatic Kupffer cells 1 h after intravenous injection, but the purified LPS is partially redistributed to hepatocytes by 8 h after administration. Since TNF-α generation peaked within 1 to 2 h after LPS challenge in the present study, the difference in the late intrahepatic distribution of different forms of LPS is not likely to be relevant to our results. Antibiotic treatment causes the release from gram-negative bacteria of soluble LPS (26), which may be an important inducer of sepsis during gram-negative bacterial infections. While LPS is the predominant macrophage activator in the supernatants of antibiotic-treated gram-negative bacteria (26), this form of LPS may be functionally distinct from chemically purified LPS. Experiments comparing the temperature dependence of cytokine expression induced by purified, native, and bacterium-associated forms of LPS are in progress.
While fever is generally considered to be beneficial in the infected host, we found that survival after LPS challenge was not improved and tended to be shorter in the 40°C mice than in the 37°C mice. Plasma TNF-α levels were 13-fold higher in the warmer animals. Increases in core temperature are associated with increases in metabolic rate and cardiac index (29, 33). While TNF-α is essential for survival in the infected host (10, 30), the mice were challenged with LPS, a nonreplicating agonist, rather than with viable pathogens. Thus, enhanced antimicrobial defenses would not be beneficial, and the increased risk of tissue injury might reduce survival in the 40°C mice after LPS challenge. Furthermore, while pretreating animals with heat shock has been reported to improve survival after LPS challenge (8, 20), the effects of heat shock and febrile temperatures are distinct (13, 14).
The enhanced TNF-α expression in the warmer mice was novel and raised the question of what survival advantage could possibly be provided by enhancing TNF-α generation during elevations of core temperature. We believe that this temperature-dependent response may have evolved as part of a host strategy to refine the regulation of systemic innate defenses. While the entry of LPS into the circulation from sites of gram-negative infection signals the host to enhance the innate defenses which protect against microbial dissemination (25, 32), LPS also continuously enters the circulation from the gut lumen under normal physiological conditions (5). Furthermore, LPS entry from the gut can increase during benign conditions (5). Thus, the context in which LPS is presented to the host may be as important as the LPS signal itself in directing an appropriate host response. We showed that core temperature may serve this function by modifying the stimulus-response relationship for LPS and TNF-α in hepatic Kupffer cells. Variations in core temperature within a narrow range may coregulate TNF-α expression, increasing the amplitude of the TNF-α response to bacterial pathogens when the risk of bacterial dissemination is high (e.g., during an acute infection) and decreasing the magnitude of the TNF-α response when the risk of bacterial dissemination is low. The early deactivation of TNF-α expression in febrile hosts may be an important counterregulatory mechanism that prevents prolonged exposure to high TNF-α levels. We conclude that the protective effects of fever in the infected host (1, 2, 28, 39) may be mediated in part by appropriate modification of the TNF-α response to circulating bacterial products.
ACKNOWLEDGMENTS
We thank Matthew Kluger, Simeon Goldblum, Barry Handwerger, Sheldon E. Greisman, Phillip Mackowiak, and Rose Viscardi for valuable time and helpful comments and Timothy Chen for assistance with the statistical analysis.
This work was supported by VA Merit Review grant 128444284-0005.
REFERENCES
- 1.Armstrong C. Some recent research in the field of neurotropic viruses with especial reference to lymphocytic choriomeningitis and herpes simplex. Mil Surg. 1942;91:129–145. [Google Scholar]
- 2.Bell J F, Moore G F. Effects of high ambient temperature on various stages of rabies virus infection in mice. Infect Immun. 1974;10:510–515. doi: 10.1128/iai.10.3.510-515.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beutler B, Milsark I, Cerami A. Passive immunization against cachectin/tumor necrosis factor protects mice from the lethal effect of endotoxin. Science. 1985;229:869–871. doi: 10.1126/science.3895437. [DOI] [PubMed] [Google Scholar]
- 4.Blake D, Bessey P, Karl I, Nunnally I, Hotchkiss R. Hyperthermia induces IL-1α or TNF-α after endotoxin. Lymphokine Cytokine Res. 1994;13:271–275. [PubMed] [Google Scholar]
- 5.Bocci V. The neglected organ: bacterial flora has a crucial immunostimulatory role. Perspect Biol Med. 1992;35:251–260. doi: 10.1353/pbm.1992.0004. [DOI] [PubMed] [Google Scholar]
- 6.Bone R C, Fisher C J J, Clemmer T P, Slotman G J, Metz C A, Balk R A. Sepsis syndrome: a valid clinical entity. Crit Care Med. 1989;17:389–393. [PubMed] [Google Scholar]
- 7.Bryant R E, Hood A F, Hood C E, Koenig M G. Factors affecting mortality of gram-negative rod bacteremia. Arch Intern Med. 1971;127:120–128. [PubMed] [Google Scholar]
- 8.Chu E K, Ribeiro S P, Slutsky A S. Heat stress increases survival rates in lipopolysaccharide-stimulated rats. Crit Care Med. 1997;25:1727–1732. doi: 10.1097/00003246-199710000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Cross A, Asher L, Seguin M, Yuan L, Kelly N, Hammack C, Sadoff J. The importance of a lipopolysaccharide-initiated, cytokine-mediated host defense mechanism in mice against extraintestinally invasive Escherichia coli. J Clin Investig. 1995;96:676–686. doi: 10.1172/JCI118110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cross A S, Sadoff J C, Kelly N, Bernton E, Gemski P. Pretreatment with recombinant murine tumor necrosis factor α/cachectin and murine interleukin 1 alpha protects mice from lethal bacterial infection. J Exp Med. 1989;169:2021–2027. doi: 10.1084/jem.169.6.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doe W F, Yang S T, Morrison D C, Betz S J, Henson P M. Macrophage stimulation by bacterial lipopolysaccharides. II. Evidence for differentiation signals delivered by lipid A and by protein rich fractions of lipopolysaccharides. J Exp Med. 1978;148:557–568. doi: 10.1084/jem.148.2.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dorn T F, DeAngelis C, Baumgardner R A, Mellits E D. Acetaminophen: more harm than good for chicken pox? J Pediatr. 1989;114:1045–1048. doi: 10.1016/s0022-3476(89)80461-5. [DOI] [PubMed] [Google Scholar]
- 13.Ensor J E, Crawford E K, Hasday J D. Warming macrophages to febrile range destabilizes tumor necrosis factor-α mRNA without inducing heat shock. Am J Physiol. 1995;269:C1140–C1146. doi: 10.1152/ajpcell.1995.269.5.C1140. [DOI] [PubMed] [Google Scholar]
- 14.Ensor J E, Wiener S M, McCrea K A, Viscardi R M, Crawford E K, Hasday J D. Differential effects of hyperthermia on macrophage interleukin-6 and tumor necrosis factor-α expression. Am J Physiol Cell Physiol. 1994;266:C967–C974. doi: 10.1152/ajpcell.1994.266.4.C967. [DOI] [PubMed] [Google Scholar]
- 15.Fong Y, Marano M A, Moldawer L L, Wei H, Calvano S E, Kenney J S, Allison A C, Cerami A, Shires G T, Lowry S F. The acute splanchnic and peripheral tissue metabolic response to endotoxin in humans. J Clin Investig. 1990;85:1896–1904. doi: 10.1172/JCI114651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fouqueray B, Philippe C, Amrani A, Perez J, Baud L. Heat shock prevents lipopolysaccharide-induced tumor necrosis factor-α synthesis by rat mononuclear phagocytes. Eur J Immunol. 1992;22:2983–2987. doi: 10.1002/eji.1830221133. [DOI] [PubMed] [Google Scholar]
- 17.Ge Y, Ezzell R M, Tompkins R G, Warren H S. Cellular distribution of endotoxin after injection of chemically purified lipopolysaccharide differs from that after injection of live bacteria. J Infect Dis. 1994;169:95–104. doi: 10.1093/infdis/169.1.95. [DOI] [PubMed] [Google Scholar]
- 18.Giroir B P, Johnson J H, Brown T, Allen G L, Beutler B. The tissue distribution of tumor necrosis factor biosynthesis during endotoxemia. J Clin Investig. 1992;90:693–698. doi: 10.1172/JCI115939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Habicht G S. Body temperature in normal and endotoxin-treated mice of different ages. Mech Aging Dev. 1981;16:97–104. doi: 10.1016/0047-6374(81)90037-3. [DOI] [PubMed] [Google Scholar]
- 20.Hotchkiss R, Nunnally I, Lindquist S, Taulien J, Perdrizet G, Karl I. Hyperthermia protects mice against the lethal effects of endotoxin. Am J Physiol. 1993;265:R1447–R1457. doi: 10.1152/ajpregu.1993.265.6.R1447. [DOI] [PubMed] [Google Scholar]
- 21.Kluger M J, Rudolph K, Soszynski D, Conn C A, Leon L R, Kozak W, Wallen E S, Moseley P L. Effect of heat stress on LPS-induced fever and tumor necrosis factor. Am J Physiol. 1997;273:R858–R863. doi: 10.1152/ajpregu.1997.273.3.R858. [DOI] [PubMed] [Google Scholar]
- 22.Kluger M J, Wieslaw K, Conn C A, Leon L, Soszynski S. The adaptive value of fever. In: Mackowiak P A, editor. Fever: basic mechanisms and management. 2nd ed. New York, N.Y: Raven Press; 1996. pp. 255–266. [Google Scholar]
- 23.Kozak W, Conn C A, Kluger M J. Lipopolysaccharide induces fever and depresses locomotor activity in unrestrained mice. Am J Physiol. 1994;35:R125–R135. doi: 10.1152/ajpregu.1994.266.1.R125. [DOI] [PubMed] [Google Scholar]
- 24.Krumdieck C L, Dos Santos J E, Ho K J. A new instrument for the rapid preparation of tissue slices. Anal Biochem. 1980;104:118–123. doi: 10.1016/0003-2697(80)90284-5. [DOI] [PubMed] [Google Scholar]
- 25.Landy M, Pillemer L. Increased resistance to infection and accompanying alteration in properdin levels following administration of bacterial lipopolysaccharides. J Exp Med. 1956;104:383–409. doi: 10.1084/jem.104.3.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leeson M C, Fujihara Y, Morrison D C. Evidence for lipopolysaccharide as the predominant proinflammatory mediator in supernatants of antibiotic-treated bacteria. Infect Immun. 1994;62:4975–4980. doi: 10.1128/iai.62.11.4975-4980.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luster M I, Germolec D R, Yoshida T, Kayama F, Thompson M. Endotoxin-induced cytokine gene expression and excretion in the liver. Hepatology. 1994;19:480–488. [PubMed] [Google Scholar]
- 28.Mackowiak P A, Wasserman S S, Levine M M. An analysis of the quantitative relationship between oral temperature and severity of illness in experimental shigellosis. J Infect Dis. 1992;166:1181–1184. doi: 10.1093/infdis/166.5.1181. [DOI] [PubMed] [Google Scholar]
- 29.Manthous C, Hall J, Olson D, Singh M, Chatila W, Pohlman A, Kushner R, Schmidt G, Wood L. Effect of cooling on oxygen consumption in febrile critically ill patients. Am J Respir Crit Care Med. 1995;151:10–14. doi: 10.1164/ajrccm.151.1.7812538. [DOI] [PubMed] [Google Scholar]
- 30.Marino M W, Dunn A, Grail D, Inglese M, Noguchi Y, Richards E, Jungbluth A, Wada H, Moore M, Williamson B, Basu S, Old L. Characterization of tumor necrosis factor-deficient mice. Proc Natl Acad Sci USA. 1997;94:8093–8098. doi: 10.1073/pnas.94.15.8093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rensen P C, van Ooosten M, van de Bilt E, van Eck M, Kulper J, van Berkel T J C. Human recombinant apolipoprotein E redirects lipopolysaccharide from Kupffer cells to liver parenchymal cells in rats in vivo. J Clin Investig. 1997;99:2438–2445. doi: 10.1172/JCI119427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rowley D. Rapidly induced changes in the level of non-specific immunity in laboratory animals. Br J Exp Pathol. 1956;37:223–234. [PMC free article] [PubMed] [Google Scholar]
- 33.Schumacker P, Rowland J, Saltz S, Nelson D, Wood L. Effects of hyperthermia and hypothermia on oxygen extraction by tissues during hypovolemia. J Appl Physiol. 1987;63:1246–1252. doi: 10.1152/jappl.1987.63.3.1246. [DOI] [PubMed] [Google Scholar]
- 34.Shenep J L, Flynn P M, Barrett F F, Stidham G L, Westenkirchner D F. Serial quantitation of endotoxemia and bacteremia during therapy for Gram-negative bacterial sepsis. J Infect Dis. 1988;157:565–568. doi: 10.1093/infdis/157.3.565. [DOI] [PubMed] [Google Scholar]
- 34a.Singh, I. S., et al. Submitted for publication.
- 35.Skidmore B J, Morrison D C, Chiller J M, Weigle W O. Immunologic properties of bacterial lipopolysaccharide (LPS). II. The unresponsiveness of C3H/HeJ mouse spleen cells to LPS-induced mitogenesis is dependent on the method used to extract LPS. J Exp Med. 1975;142:1488–1508. doi: 10.1084/jem.142.6.1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Smedsrod B, Pertoft H. Preparation of pure hepatocytes and reticuloendothelial cells in high yield from a single rat liver by means of Percoll centrifugation and selective adherence. J Leukocyte Biol. 1985;38:213–230. doi: 10.1002/jlb.38.2.213. [DOI] [PubMed] [Google Scholar]
- 37.Smith P F, Gandolfi A J, Krumdieck C L, Putnam C W, Zukoski C F, Davis W M, Brendel K. Dynamic organ culture of precision liver slices for in vitro toxicology. Life Sci. 1985;36:1367–1375. doi: 10.1016/0024-3205(85)90042-6. [DOI] [PubMed] [Google Scholar]
- 38.Snyder Y M, Guthrie L, Evans G F, Zuckerman S H. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukocyte Biol. 1992;51:181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- 39.Stanley E D, Jackson G G, Panusarn C, Rubenis M, Dirda V. Increased viral shedding with aspirin treatment of rhinovirus infection. JAMA. 1975;231:1248–1251. [PubMed] [Google Scholar]
- 40.ten Hagen T L M, Van Vianen W, Bakker-Woudenberg I A J M. Isolation and characterization of murine Kupffer cells and splenic macrophages. J Immunol Methods. 1996;193:81–91. doi: 10.1016/0022-1759(96)00045-2. [DOI] [PubMed] [Google Scholar]
- 41.Tracey K, Cerami A. Tumor necrosis factor: a pleiotropic cytokine and therapeutic target. Annu Rev Med. 1994;45:491–503. doi: 10.1146/annurev.med.45.1.491. [DOI] [PubMed] [Google Scholar]
- 42.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 43.Wright S, Tobias P, Ulevitch R, Ramos R. Lipopolysaccharide (LPS) binding protein opsonizes LPS-bearing particles for recognition by a novel receptor on macrophages. J Exp Med. 1989;170:1231–1241. doi: 10.1084/jem.170.4.1231. [DOI] [PMC free article] [PubMed] [Google Scholar]