Abstract
Osteoporosis is a systemic metabolic bone disease characterized by the descending bone mass and destruction of bone microstructure, which tends to result in the increased bone fragility and associated fractures, as well as high disability rate and mortality. The relation between gut microbiota and bone metabolism has gradually become a research hotspot, and it has been verified that gut microbiota is closely associated with reduction of bone mass and incidence of osteoporosis recently. As a novel “organ transplantation” technique, fecal microbiota transplantation (FMT) mainly refers to the transplantation of gut microbiota from healthy donors to recipients with gut microbiota imbalance, so that the gut microbiota in recipients can be reshaped and play a normal function, and further prevent or treat the diseases related to gut microbiota disorder. Herein, based on the gut–bone axis and proven regulatory effects of gut microbiota on osteoporosis, this review expounds relevant basic researches and clinical practice of FMT on osteoporosis, thus demonstrating the potentials of FMT as a therapeutic option for osteoporosis and further providing certain reference for the future researches.
Keywords: Fecal microbiota transplantation, Gut microbiota, Osteoporosis, The elderly
Introduction
Osteoporosis is a systemic metabolic bone disease characterized by the descending bone mass and destruction of bone microstructure, which tends to result in the increased bone fragility and fractures [1, 2]. Therein, the brittle fractures caused by osteoporosis mainly occur in stress concentration parts, including the vertebral bodies, hip and distal radius, which significantly enhances the disability rate and the mortality of the middle-aged and elderly individuals [3–5]. Moreover, in accordance with previous studies, more than 150 million individuals worldwide suffer from osteoporosis every year, and one-third of women and one-fifth of men around the world might suffer from osteoporotic fractures at least once during their whole life [6, 7], which is a major challenge for the patients themselves, their families and social medical security system [8, 9].
Currently, the prevention and treatment of the osteoporosis mainly depend on the interventions of various kinds of drugs and the adjustment of lifestyles of middle-aged and elderly people [10]. The drugs for osteoporosis mainly include bisphosphonates, selective estrogen receptor modulators, hormone replacement agents, calcitonin, and so on, which have already obtained the effective clinical efficacy and safety [11]. However, the potential complications and corresponding side effects of partial drugs are need to be concerned. For example, the bisphosphonates may result in the severe bone, joint or muscle discomfort in partial patients [12]. Long-term use of alendronate is associated with the occurrence of femoral intertrochanteric fracture and femoral shaft fracture [13]. As a kind of selective estrogen receptor modulator, raloxifene may enhance risk of venous thromboembolism and stroke [14]. In hormone replacement therapy, estrogen might increase the risk of endometrial hyperplasia and cancers in the elderly women, and enhances the incidence rates of gallstone disease and venous thromboembolism by two to three times [15]. Hence, the existing side effects of drugs limit its long-term use in the osteoporosis, and it is urgent to explore alternative approaches with fewer side effects to help get out of this dilemma.
Regarding this, numerous studies in recent years have revealed that there is a close relationship between the gut microbiota and bone metabolism [16, 17]. As the largest microbial repository in human body, the dynamic balance of intestinal micro-ecosystem is closely associated with the health of body. Meanwhile, the changes in internal and external environment of body can also disrupt the balance between the gut microbiota and body, triggering kinds of inflammatory and metabolic diseases, such as obesity, inflammatory bowel disease, diabetes, rheumatoid arthritis, osteoporosis, and so on [18–22]. Moreover, in previous critical reviews, based on concept of “brain-gut-bone” axis, we have also summarized the modulatory effect and implication of the gut microbiota to osteoporosis [23], and regulative effect and repercussion of probiotics and prebiotics on osteoporosis [24]. Hence, in view of the close association between the gut microbiota and bone metabolism, it is suggested that gut microbiota may be regarded as a potential target for preventing and treating the osteoporosis to a certain extent.
In addition, the current interventional strategies for gut microbiota in clinical and scientific research stages mainly include the antibiotic application, dietary adjustment, supplementation of probiotics and prebiotics, fecal microbiota transplantation (FMT), and so on [25–28]. Therein, the FMT, as a novel “organ transplantation” technique, has gradually attracted the close attention of researchers. Specifically, the core meaning of FMT is to extract the beneficial microbiota from the feces of healthy people or animals after the screening, centrifugation, filtration and other steps, and transplant it into the gastrointestinal tract of corresponding recipients, so that the microbiota in the recipients can be reshaped and play a normal function, and further prevent or treat the diseases related to gut microbiota disorder [29]. Meanwhile, FMT can also reshape the intestinal micro-ecology of recipients, improve the inflammatory, immune and metabolic status of the recipient's intestine, and provide a new treatment concept and approach for various intestinal and non-intestinal diseases [30]. In a single FMT, the time for the receptors’ microbiota to maintain the normalization is generally 3–6 months [31]. Since each human body has its own internal environment, the healthy microbiota system can be successfully colonized soon after FMT. However, due to the effects of its own internal environment and external interventions, partial bacterial components might slowly disappear after 3 to 6 months, and need to be transplanted again [32, 33].
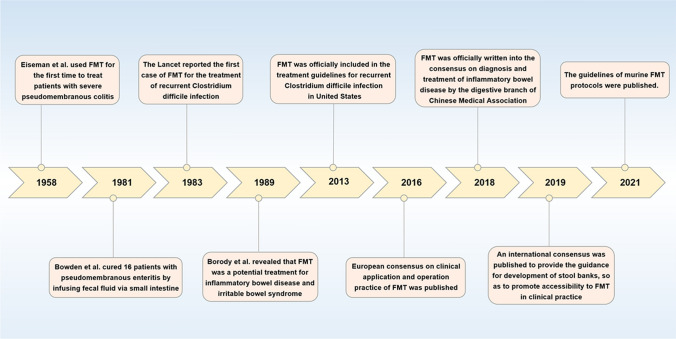
To date, FMT has been gradually verified to be effective for a variety of intestinal diseases, such as irritable bowel syndrome (IBD), Clostridium difficile infection, colon cancer, and so on [23, 34, 35]. FMT has exhibited a cure rate of more than 90% in clinical trials for the treatment of recurrent Clostridium difficile infection and accompanied with less side effects. More importantly, FMT is regarded as potential life-saving “last chance” for the treatment of recurrent Clostridium difficile infection, which has been written into the diagnosis and treatment guidelines [23, 34]. The development history and significant time nodes of FMT since the modern times are exhibited in Fig. 1 [36–44]. Moreover, FMT has been widely explored to many metabolic and immune diseases, such as the obesity, diabetes, fatty liver, arthritis, psoriasis and osteoporosis [45–49]. Herein, combined with relevant studies and based on the relevant basic studies and clinical practice, this current review is aimed to discuss the potential mechanisms, application, therapeutic feasibility, and existing challenges of FMT on osteoporosis and propose the corresponding prospect, thus demonstrating the potentials of FMT as a therapeutic option for the osteoporosis and further provide certain reference for future researches.
Fig. 1.
The development history and significant time nodes of FMT since modern times
The relationship between gut microbiota and osteoporosis
Gut microbiota is a collection of the microorganisms colonized in the host intestine, including about 10 trillion bacteria, and its total gene number is 150 times that of host cells. The gut microbiota is mainly composed of dominant bacteria (obligate anaerobic bacteria) and the secondary bacteria (aerobic bacteria or facultative anaerobic bacteria), including beneficial bacteria, harmful bacteria and neutral bacteria [50–52]. Currently, the relationship between gut microbiota and bone metabolism has gradually become a research hotspot, especially in recent years, it has been verified that gut microbiota is closely associated with the reduction of bone mass and incidence of osteoporosis [53–55]. Gut microbiota might alter the relative activity of the osteoclasts and osteoblasts by affecting its intestinal metabolites, host metabolism, inflammatory and immune system, thus influencing the bone metabolism. Moreover, in a previous review, based on concept of “brain–gut–bone” axis, we have summarized the effects of gut microbiota on osteoporosis through the brain–gut bidirectional regulation, immune regulation and endocrine regulation [23].
Regarding this, in terms of animal studies, Tu et al. [56] revealed that by mimicking postmenopausal osteoporosis, estrogen deficiency may cause the various host changes, such as the impaired intestinal mucosal barrier function, imbalance of gut microbiota composition and abundance, and enhanced immune response. During this process, the metabolites of intestinal pathogenic bacteria are able to enter into the body circulation through the damaged intestinal mucosal barrier, further induce the immune response mediated by CD4 + T cells, generate various kinds of pro-osteoclastogenic cytokines (such as interleukin-1β (IL-1β), IL-6, tumor necrosis factor-α (TNF-α)), and ultimately mediate the activation of osteoclasts and enhancement of bone resorption [57, 58]. Yan et al. [59] verified that the beneficial bacteria can ferment the dietary fiber in food to produce short-chain fatty acids (SCFAs), and SCFAs can decrease the local pH value of intestine and reduce the formation of complexes between calcium ions and phosphorus in intestine, thus promoting the calcium absorption and regulating the bone metabolism. Sjögren et al. [60] indicated that the expression of TNF-α and IL-6 in the germ-free (GF) mice was reduced, the number of CD4 + T cells was less than that of normal mice, and the number of osteoclasts in the bones was reduced and the bone quality was better than that of the normal mice, suggesting that gut microbiota may be involved in the osteoclastogenesis by regulating the immune system and local inflammatory responses. Huo et al. [61] also suggested that the GF mice were able to exhibit the strong activity of hypothalamic pituitary adrenal (HPA) axis under mild stress, and the function of HPA axis could return to normal after supplementing an appropriate number of probiotics and prebiotics. Meanwhile, gut microbiota could also directly or indirectly regulate a variety of hormones or chemicals (such as 5-hydroxytryptamine (5-HT), epinephrine (E), vasoactive intestinal polypeptide (VIP), γ-aminobutyrate (GABA), norepinephrine (NE), and so on), and these substances will influence distal organs and body functions after being released into blood, thus speculating that gut microbiota may regulate bone metabolism through neuroendocrine metabolic pathways [62, 63].
In terms of the clinical researches, Fuhrman et al. [64] conducted a study on 60 randomly selected healthy postmenopausal women, and results indicated that the fecal microbial diversity and relative abundance of Clostridium and Bacillus were positively correlated with urinary estrogen metabolites, and the diversity of gut microbiota increased with proportion of hydroxylated estrogen metabolites in the urine. Several studies have indicated that prebiotics, such as inulin, Fructooligosaccharides and Galactooligosaccharides, can contribute to the increase of the number of probiotics (Bifidobacteria, Lactobacillus and Clostridium butyricum), promote the secretion of SCFAs, reduce the intestinal PH value, improve the solubility of calcium in intestinal cavity, increase the calcium absorption by body, and ultimately enhance the contents and density of bone minerals in individuals [65, 66]. Moreover, Abrams et al. [67] revealed in a population-based study that supplementing Fructooligosaccharides for a duration of 9 days to 1 year could enhance the intestinal calcium absorption, and the continued supplementation of it for more than 1 year might enable to prevent bone loss. Similarly, van den Heuvel et al. [68] revealed in a previous study that supplementation of Transgalactooligosaccharides could increase the intestinal calcium absorption in postmenopausal women, so as to further enhance the bone mass. In a double-blind, placebo-controlled, randomized, and multi-center study, Jones et al. [69] revealed that supplementing Lactobacillus reuteri was associated with enhancement of the contents of serum 25 hydroxyvitamin D in the body, thereby increasing the synthesis of vitamin D and further preventing osteoporosis.
Collectively, more and more studies have gradually verified that gut microbiota can regulate the metabolism, immunity and inflammation of the body. However, with the enhancement of age, alteration of lifestyle, and alteration of physiological and immune characteristics, the intestinal mucosal barrier damage induced by aging and estrogen deficiency may lead to a surge of inflammatory reaction in the intestine and circulation, which could further contribute to the ecological imbalance of gut microbiota. On the contrary, the imbalance of gut microbiota may also increase the intestinal permeability, thereby enhancing the leakage of gut microbiota and its metabolites, and such positive feedback process might enhance with aging. Meanwhile, it can also act as a significant regulator of bone metabolism, affect the generation of osteoclasts, and play a critical role in regulation of bone. The emerging studies on the regulation of bone metabolism by probiotics and prebiotics have also suggested that the increase of beneficial bacteria can promote the enhancement of bone mass and reverse the bone loss, which can be regarded as a target for the prevention and treatment of osteoporosis. However, the researches on the relationship between gut microbiota and osteoporosis are still in the initial stage, and more studies are needed to further clarify its deep-level mechanisms in future.
The potential regulation of FMT on bone metabolism
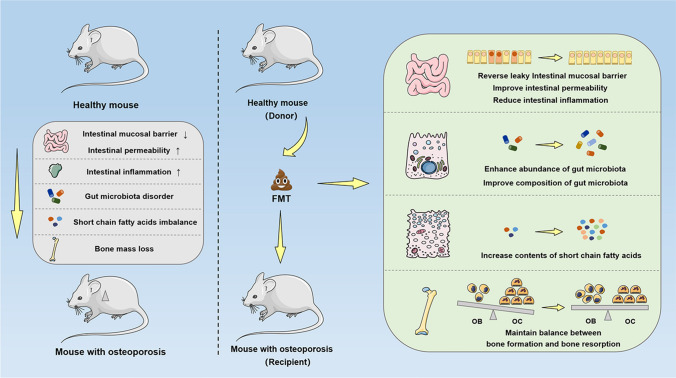
On the one hand, bone remodeling is a dynamic and balanced process, which is mainly composed of the osteoblast-mediated bone formation and the osteoclast-mediated bone resorption [70]. On the other hand, the underlying mechanisms of FMT mainly lie in reconstruction of normal intestinal micro-ecology, which participates in the regulation of bone metabolism by correcting gut microbiota disorder, reversing intestinal metabolites (SCFAs, indole derivatives, vitamins, cholic acids, polyamines) imbalance, repairing the intestinal mucosal barrier, regulating the immune response, and improving the intestinal permeability. Meanwhile, different from the application of probiotics, prebiotics and other single microbial targets, FMT maintains the integrity of whole gut microbiota and metabolites during the overall process, which could retain the original function of gut microbiota to the greatest extent, significantly improve gut microbiota-related disorder, and restore the homeostasis of intestinal microenvironment more quickly and efficiently. Moreover, given that the diseases-induced physiological disorders may alter the composition and abundance of gut microbiota, and conversely, gut microbiota disorder can also trigger or exacerbate diseases. Hence, the experiment of transferring host phenotype by the means of FMT is essential in researches of disease pathogenesis. Herein, the potential regulatory mechanisms involving the FMT on bone metabolism are represented as follows, and the Fig. 2 exhibits the potential mechanism diagrams.
Fig. 2.
The potential mechanism diagrams of regulatory mechanisms involving FMT on bone metabolism. Based on the gut–bone axis, the underlying mechanisms of FMT mainly lie in reconstruction of normal intestinal micro-ecology, which could participate in the regulation of bone metabolism by repairing intestinal mucosal barrier, regulating immune response, improving intestinal permeability, correcting gut microbiota disorder, and reversing intestinal metabolites imbalance. FMT fecal microbiota transplantation, OB osteoblast, OC osteoclast
The involvement of intestinal metabolites
Accumulating research evidence indicates that a variety of intestinal metabolites, including SCFAs, vitamins, 5-HT, cholic acids, polyamines and indole derivatives, are involved in the regulation of bone metabolism. Therein, SCFAs are mainly produced by the bacterial fermentation of amino acids or indigestible carbohydrates in the colon, and its specific components include acetic acid, propionic acid, butyric acid, and so on, which is one of the most vital metabolites of intestinal beneficial bacteria in the process of FMT [71–73]. Furthermore, after performing FMT, the SCFAs may influence the process of bone metabolism via various mechanisms, including inhibiting inflammatory response, improving intestinal calcium absorption, promoting osteoblast differentiation via regulatory T cells (Tregs), directly inhibiting osteoclast differentiation, and so on [20, 74–76]. Yan et al. [59] also highlighted in a previous study that SCFAs indirectly participate in the protection of bone mass by affecting the level of serum insulin-like growth factor-1 (IGF-1). Hence, it is recognized that the implementation of FMT may enhance the contents of SCFAs, so as to further participate in the regulation of bone metabolism through various different mechanisms, maintain the balance between bone formation and bone resorption, and effectively obtain the purposes of protecting bone mass and preventing osteoporosis.
Next, in addition to the SCFAs, other intestinal metabolites produced after FMT may also be involved in the regulation of bone metabolism. For example, 5-HT is able to be produced by the culture of Corynebacterium, Streptococcus, and Escherichia coli [77–79]. As a neurotransmitter produced by intestinal chromaffin cells, more than 90% of 5-HT is synthesized by the intestine, and it can be targeted by osteoblasts and participates in regulation of osteoblast proliferation via the signal pathways, such as Htr1b, Htr2a, Htr2b, and so on [80]. The vitamins (including vitamin C, vitamin D, vitamin K) are also significant for maintaining the balance between bone formation and bone resorption [81, 82]. As a common intestinal mucosal growth factor, polyamine is also accompanied with the process of FMT and verified to be conducive to protecting bone mass [83]. Thus, it is recognized that the FMT might regard the intestinal metabolites as one of the bridges to play a role in balancing bone formation and bone resorption, so as to further protect the bone mass and prevent osteoporosis.
The involvement of intestinal mucosal barrier
Intestinal mucosal barrier is a physical barrier and the first line of defense against intestinal pathogens. It can not only selectively absorb the water and nutrients, but also restrict the invasion of exogenous harmful antigens into the host [84]. Meanwhile, the intestinal mucosal barrier is also an interface between the host and gut microbiota, which is a significant link in the occurrence and development of osteoporosis mediated by gut microbiota [85, 86]. With regard to this, Schepper et al. [87] suggested that the intervention of antibiotics on normal mice may cause gut microbiota disorder, damaged intestinal mucosal barrier and enhancement of intestinal permeability, which further caused the bone loss and induced osteoporosis. Chen et al. [88] also indicated that the supplementation of lactulose could reverse ovariectomy (OVX)-induced bone loss in mice by improving intestinal mucosal barrier function, reducing inflammatory response, and optimizing composition and abundance of gut microbiota and SCFAs. Li et al. [89] also revealed that puerarin modulated the gut microbiota disorder to elicit the anti-osteoporosis effects in OVX-induced rats through improving bone microenvironment through repairing the intestinal mucosal integrity and regulating the SCFAs levels. Li et al. [90] demonstrated that tuna bone powder could ameliorate the glucocorticoid-induced osteoporosis in mice by blocking pro-inflammatory cytokines, coregulating signaling pathways, repairing intestinal mucosal barrier and modulating gut microbiota. Wang et al. [91] indicated that the supplementation of Prevotella histicola could prevent OVX-induced bone loss in mice through improving intestinal mucosal barrier function, reducing intestinal inflammatory response, and optimizing composition and abundance of gut microbiota. More importantly, Ma et al. [46] also reported that using young healthy rats as donors and providing the feces as materials to conduct the FMT might alleviate the senile osteoporosis in the aged rats through enhancing the composition and abundance of gut microbiota and improving intestinal mucosal barrier function. Wang et al. [92] suggested that the dysbiosis of gut microbiota by transferring the feces from senile osteoporotic rats to young rats can induce osteoporosis, and the changed gut microbiota and impaired intestinal mucosal barrier contributed to the pathogenesis of osteoporosis. Zhang et al. [93] also showed that FMT plays an active role in remodeling the gut microbiota and improving the bone loss in OVX-induced mice with osteoporosis. Specifically, by correcting imbalance of gut microbiota, improving the level of SCFAs, optimizing intestinal permeability and inhibiting release of pro-inflammatory cytokines, FMT inhibited the excessive generation of osteoclasts and obtained the balance between bone formation and bone absorption, thus ameliorating the bone loss in OVX-induced mice with osteoporosis. Notably, these studies provide the direct and strong evidence that FMT is able to improve the bone mass and prevent the osteoporosis.
The involvement of immune regulation
A variety of immune factors and immune cells in the intestine can participate in the regulation of bone metabolism by maintaining the balance between the osteoblasts and osteoclasts [94]. Meanwhile, the gut microbiota also affects the production of cytokines and differentiation of lymphocytes in the lamina propria of intestinal mucosa, especially the differentiation of CD4 + T cells to T helper cells 17 (Th17 cells) and Treg cells [95]. In a previous study, Atarashi et al. [96] showed that the transplantation of Clostridium IV and XIVa isolated from the normal mice into GF mice could enhance the number of systemic and lamina propria Treg cells in vivo. Moreover, SCFAs were also involved in inducing the differentiation of Treg cells, and Treg cells can further influence osteoclastogenesis by secreting the IL-4, IL-10 and transforming growth factor-β (TGF-β). Moreover, the estrogen could also activate the Treg cells and enhance the production of TGF-β, or indirectly inhibit the formation of osteoclasts and prevent postmenopausal osteoporosis by inhibiting the differentiation of Th17 cells and decreasing production of TNF-α and receptor activator of nuclear factor-κ B ligand (RANKL) through the estrogen receptor [97]. In addition, Goto et al. [98] revealed that the transplantation of segmental filamentous bacteria (SFB) into GF mice increased the number of Th17 cells, resulting in enhanced levels of IL-1β, IL-17 and TNF-α, inducing the expression of RANKL, and thereby promoting the formation of osteoclasts and inducing the bone resorption. As a kind of intestinal colonization bacterium, SFB could also induce the serum amyloid A protein (SAA) in the terminal ileum, which can further promote the differentiation of Th17 cells [99].
The involvement of endocrine regulation
As a virtual endocrine organ, the gut microbiota can directly produce or regulate a variety of hormonal chemicals, which are released into blood to regulate the functions of distant organs and systems [100]. A variety of hormones produced by gut microbiota are also the neurotransmitters of the central nervous system (CNS). For example, the GABA produced by Lactobacillus is one of the most significant inhibitory transmitters in the brain, while the monoamines (such as NE and dopamine) produced by the Bacillus phlei are excitatory transmitters, which play an indirect role in complex endocrine network, especially on HPA axis, resulting in close relationship between the secretion of estrogen, glucocorticoid and gut microbiota [101, 102]. In a FMT experiment, Schepper et al. [103] transplanted the gut microbiota of the glucocorticoid-induced osteoporosis mice to the mice treated with antibiotics for one week, and then observed that the bone mass of recipient mice decreased, indicating that effects of glucocorticoid on bone mass can be transferred via FMT. Thus, the potential therapeutic feasibility and effects of FMT on osteoporosis and its corresponding mechanisms have broad research prospects and application space. In future, more and more in-depth explorations are still needed in terms of the basic researches and clinical trials.
The current operative approaches and security of FMT
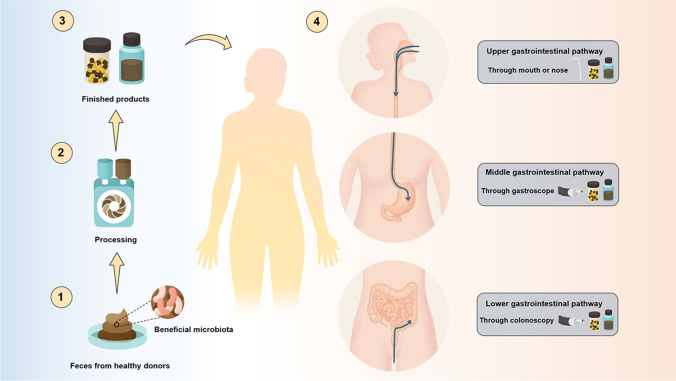
The normal gut microbiota tends to be in a harmonious and stable state. However, when the number of certain bacteria increases, decreases or lacks, the body will exhibit corresponding pathological state [104]. FMT is usually applied to relieve or treat the gut microbiota imbalance-related diseases, mainly by centrifuging and purifying feces collected from the healthy patients to obtain the functional bacteria, and then transferred to recipient's intestinal tract via upper gastrointestinal pathway, middle gastrointestinal pathway and lower gastrointestinal pathway, thus reconstructing the stable intestinal micro-ecological environment, relieving the relevant symptoms and treating the diseases [105–107]. With regard to this, whether FMT can obtain the expected efficacy depends on several aspects, mainly including the extraction method and obtained components of fecal bacteria, the filtration steps and preservation methods of bacterial liquids, the dose, frequency and location of fecal bacteria infusion, the operative approaches, and so on [108, 109]. Therein, the aspect of operative approaches of FMT plays a significant role in the efficacy of FMT [110]. Herein, the current operative approaches and the security of FMT are shown as follow, and Fig. 3 exhibits the corresponding illustration. Moreover, the main mode of operation, advantages and drawbacks of each operative approach are also summarized in Table 1.
Fig. 3.
The preparation process and current main operative approaches of FMT
Table 1.
The main mode of operation, advantages and drawbacks of operative approaches for FMT
| Approaches | Main mode of operation | Advantages | Drawbacks |
|---|---|---|---|
| Upper gastrointestinal pathway |
1. Oral administration 2. Nasogastric tubes |
1. Easy to perform and have relatively low overall risk 2. Protect the FMT materials from the gastrointestinal environment 3. No need for expensive instruments 4. Less damage to patients after repeated use 5. Well tolerated by patients, especially for the elderly patients 6. Reduce the potential probability of diseases transmission |
1. Asphyxia may be caused by the bacterial fluid reflux or suction 2. If the human body itself has an obstruction, the transplanted bacterial fluid may not reach targeted intestinal tract accurately |
| Middle gastrointestinal pathway |
1. Naso-intestinal tubes 2. Mid-gastrointestinal pathway-mediated TET |
1. Reduce the bacterial translocation 2. Improve the efficacy of FMT 3. The mid-gastrointestinal pathway-mediated TET is convenient and easy to maintain 4. The mid-gastrointestinal pathway-mediated TET does not require further confirmation of the intestinal position by other medical instruments |
1. The incidence of adverse events was relatively high, including diarrhea, cramp and constipation, and so on 2. It is expensive for the patients who require repeated FMT 3. It is accompanied with high risk of operation |
| Lower gastrointestinal pathway |
1. Enema 2. Colonoscopy 3. Colonic pathway-mediated TET |
1. Enema is a less invasive and relatively simple operation 2. It can completely display the situation of colon and biopsy suspicious tissues, which is conducive for the diseases staging 3. It allows operators to directly assess the intestinal inflammation and infuse a sufficient amount of donor fecal bacteria at the appropriate site 4. The gut microbiota can be accurately transplanted into the influenced intestinal segment, and the bacteria can further be retained in the targeted intestinal segment 5. Sufficient amount of donor fecal bacteria can be accurately infused, which can improve the treatment efficiency |
1. Enema is accompanied with the risk of the bacteria of FMT is not able to reach the colon and splenic flexure 2. The incidence of adverse events was relatively high, including aggravating intestinal reactions and causing intestinal perforation 3. The patients with severe conditions may not able to tolerate colonoscopy transplantation or relevant anesthesia procedures 4. It is expensive for the patients who require repeated FMT |
FMT fecal microbiota transplantation, TET trans-endoscopic enteral tubing
Upper gastrointestinal pathway
Among the routes of the upper gastrointestinal pathway, the oral administration and nasogastric tubes are widely used, which are easy to be performed and have relatively low overall risk. However, during this process, the asphyxia may be caused by bacterial fluid reflux or suction, and if the body itself has obstruction, the transplanted bacterial fluid may not reach the intestinal tract accurately. Therein, the oral approaches mainly refer to encapsulated fecal bacteria materials, which is prepared by mixing the extracted fecal bacteria with a cryoprotectant (mainly glycerol), followed by multiple packaging to protect the FMT materials from gastrointestinal environment [111, 112]. Meanwhile, the operators could further select the material of capsule shell according to the expected release position. Currently, the commercially available capsule shells are often targeted at the release of stomach or colon, and are formulated to ensure the survival rate and the colonization of bacteria along with the gastrointestinal tract [113]. Collectively, the FMT through the upper gastrointestinal pathway has the advantages of no need for expensive instruments, less damage to patients after repeated use, and is well tolerated by patients. This pathway is suitable for the patients who are not able to tolerate the use of naso-intestinal tube and gastroscopic transplantation, and the patients who require the oral customized bacteria. Moreover, during the process of making capsules, fungi, parasites, viruses and partial inflammatory mediators that may exist in asymptomatic donors can also be removed to reduce potential probability of diseases transmission [114, 115].
Middle gastrointestinal pathway
Middle gastrointestinal pathway mainly refers to the operations of naso-intestinal transplantation and trans-endoscopic enteral tubing (TET), and TET is able to realize the whole intestinal administration [116]. Therein, the naso-intestinal tubes are stretched and straightened through the guide wire, which can pass through the pylorus automatically under the condition of normal gastrointestinal motility and further reduce the bacterial translocation when used for FMT. With regard to this, in a randomized controlled trial, van Nood et al. [117] reported that the FMT through the naso-intestinal tubes was superior to the use of vancomycin alone in the treatment of Clostridium difficile infection, while the patients in FMT group also experienced the adverse events, such as diarrhea, cramp and constipation. Moreover, TET specifically refers to the endoscopic-assisted implantation of a fixed tube and the fixation in the deep part of intestinal tract, while the external end communicates with the outside along the intestinal tract. TET mainly includes the types of colonic pathway-mediated TET and mid-gastrointestinal pathway-mediated TET. Colonic pathway-mediated TET requires the application of colonoscopy, and it is expensive to use this route for repeated FMT. In contrast, mid-gastrointestinal pathway-mediated TET is more convenient and easier to maintain [118]. Since mid-gastrointestinal pathway-mediated TET does not require further confirmation of intestinal position by X-rays or other medical instruments after endoscopic surgery, for the patients who are not able to undergo bowel preparation for colonoscopy, or who require both the repeated FMT and enteral nutrition, the mid-gastrointestinal pathway-mediated TET is also the primary option [119].
Lower gastrointestinal pathway
Lower gastrointestinal pathway mainly refers to operations of enema, colonoscopy, and colonic pathway-mediated TET. Therein, the enema is a less invasive and relatively simple operation. Enema is well tolerated by patients and does not require the expensive instruments, which effectively decreases the risk of operation. However, this approach requires the retention of infused fecal suspension for a relatively long time, the patient remains supine to reduce the fecal excretion, while the repeated enemas are easy to be accepted by patients [120]. In addition, the proposition that whether the bacteria of FMT can be retained in intestinal segment by enema is still unclear, and this approach is also accompanied with the risk of not reaching the colon and splenic flexure, which requires multiple perfusions to obtain the curative effect and longer operation time to make up for the defect of the low gut microbiota retention rate. Meanwhile, due to the retention of bacterial fluid, this approach may not be suitable for the patients with anal sphincter relaxation or urinary incontinence [121]. Moreover, in terms of the colonoscopy and colonic pathway-mediated TET, it is equipped with a variety of advantages, mainly including: (1) It is able to completely display the situation of colon and biopsy suspicious tissues, which is conducive for the diseases identification and staging; (2) It allows operators to directly assess the intestinal inflammation and infuse a sufficient amount of donor fecal bacteria at appropriate sites; (3) Gut microbiota can be accurately transplanted into the influenced intestinal segment, and bacteria can further be retained in targeted intestinal segment; (4) Sufficient amount of donor fecal bacteria could be accurately infused, which can improve the treatment efficiency to a certain extent. However, these operations are also accompanied by partial adverse events, including aggravating intestinal reactions, causing intestinal perforation, and patients with severe conditions may not able to tolerate colonoscopy transplantation or relevant anesthesia procedures [122, 123].
The clinical application prospect and existing challenges of FMT on osteoporosis
To date, more and more patients around the world have benefited from FMT, and the medical community of numerous countries is also actively working to promote the wider clinical application of FMT [124]. Based on the proven regulatory effects of gut microbiota on the osteoporosis, the gut microbiota disorder might enhance the risk of occurrence and progression of osteoporosis, and further normalizing the composition and abundance of gut microbiota could reconstruct the gut–bone axis and maintain the balance between bone formation and bone resorption, so as to prevent and treat the osteoporosis. Hence, FMT is regarded as a novel approach of reconstructing the gut microbiota and a novel target for the prevention and treatment of osteoporosis at the present and future stages.
In addition, although FMT has become increasingly mature as a treatment method for the common gastrointestinal and metabolic diseases, there are still certain problems to be solved in the clinical prevention and treatment of osteoporosis. For one thing, the discomfort associated with current FMT approaches through traditional methods, such as nasogastric tube and naso-intestinal tube, may reduce the compliance of patients, as well as concomitant adverse events. In addition to this, the administration frequency of FMT is also inconclusive, mainly including single administration, and intermittent or continuous administration. The repeated FMT can improve the success rate of treatment to a certain extent, and the success of FMT may also depend on amount of suspension of transplanted [125, 126]. For another thing, the choice of FMT approaches might be influenced by the clinical experience of specific operators. Currently, the most suitable FMT approach has not been verified in clinical practice [127]. With regard to this, Paramsothy et al. [128] collected and sorted out the knowledge and experience of 52 gastroenterologists on the FMT in past. Therein, 37% of them regarded that the most appropriate FMT approach was colonoscopy-assisted FMT, 17% thought it was naso-intestinal route, 13% thought it was enema, and 8% thought it was oral capsules. Hence, it is concluded that each FMT approach has its advantages and drawbacks, and the specific FMT approach should be comprehensively selected in accordance with the type of diseases, position to obtain the effects, professional judgment of operators, and psychological acceptance of the patients. Additionally, the utilization of human-derived gut microbiota and complications associated with severe infection are indeed a safety issue worthy of attention. Especially in the era of multi-infectious disease pandemics represented by the coronavirus disease 2019 (COVID-19), this issue is particularly noteworthy [129–131]. Several studies have showed that during the process of COVID-19 infection, the expression of intestinal angiotensin-converting enzyme 2 (ACE2) is down-regulated, resulting in the decreased secretion of antimicrobial peptides (AMPs) and improved survival of pathogens [132, 133]. With the global spread of COVID-19 pandemic, it is urgent to take preventive measures to screen FMT donors for COVID-19 to prevent the potential risk of COVID-19 transmission. Indeed, COVID-19 might be transmitted from asymptomatic donors to recipients via FMT, especially those who are negative for COVID-19 through respiratory samples but positive for stool samples [134, 135]. To address this vital issue, FMT donor screening should be rigorous and follow the guidelines from different jurisdictions around the world.
Nevertheless, although the current application of FMT in prevention and treatment of osteoporosis is still immature, to further realize the wide application of FMT, the specific solutions include: (1) Deepen the basic researches of FMT in the fields of osteoporosis and bone metabolism, thus providing a solid theoretical basis for its further clinical trials. Meanwhile, during the process of further clinical trials, gradually expand the research samples of FMT in prevention and treatment of osteoporosis, and deeply understand the relevant mechanisms on the gut–bone axis; (2) Further researches require appropriate phenotypic analysis of patients with osteoporosis and rational application of techniques, including functional imaging and deep microbial pyrosequencing. Using high-throughput sequencing techniques, comprehensively analyze the composition and abundance of gut microbiota in the patients with osteoporosis, and deeply analyze the microbiota structure and functional metabolism characteristics in specific environment, so as to provide a theoretical basis for FMT to improve osteoporosis and then conduct individualized targeted FMT therapy [136]. Hence, it is recognized that the application of precision medicine to conduct the personalized FMT for different individuals is a novel research direction on this field in future; (3) On the basis of current FMT-related researches, further explore the technique of synthetic microbiota transplantation (SMT) [137, 138]. The SMT mainly refers to the extraction of bacteria from the feces of healthy individuals, cultures it in vitro, and then uses conventional FMT approaches, such as oral administration and naso-intestinal tube, to treat patients with osteoporosis; (4) On the basis of FMT, Zhang et al. [139] proposed the concept of washing microbiota transplantation (WMT) for the first time through intelligent separation system and strict quality control system. WMT belongs to the category of FMT, while the fecal bacteria have been washed more strictly, which could also further promote the development of FMT to a certain extent. Moreover, various previous clinical evidence and animal studies have verified that WMT has significant clinical therapeutic value for the patients with inflammatory bowel diseases, and also significantly reduces the incidence rate of adverse events without reducing the efficacy of FMT [140]. Currently, there is still no related research on application of WMT in the prevention and treatment of osteoporosis, while its future prospect is still worthy of our expectation and belief; (5) As another indelible aspect related to the efficacy of FMT, it is also essential to further optimize the extraction method of fecal bacteria, develop the filtration steps and preservation methods of bacterial liquids, and explore more proper guidelines of doses, frequency and location of infusion; (6) Based on existing studies, it is necessary to further explore how the gut microbiota plays a critical role in human health and the related mechanisms by which FMT works. More high-quality researches still need to be promoted to screen out which patients are suitable for the FMT and whether there are definite age, gender, race, physical quality, genetic correlation, and other requirements for potential fecal bacteria donors [38]. Meanwhile, it is essential to identify the safe, effective and stable methods to implement FMT for different individuals, and design scientific randomized controlled trials and long-term follow-up planning to obtain a reliable analysis of the efficacy and safety of FMT on osteoporosis in future.
Ultimately, during the process of FMT, the therapeutic stools are collected from the healthy donors, and the success or failure of the treatment is likely to be determined by the composition of the donors’ fecal microbiota. Thus, the screening and evaluation of donors are crucial. Regarding this, in combination with previous studies and relevant guidelines, the selection criteria for recruiting donors could be summarized as follows: (1) Individuals aged 12 to 30, and living and working in the same area as the recipients; (2) Preference is given to the healthy adolescents aged 18 to 25 with normal body mass index (BMI) and normal bowel habits; (3) Preference is given to those with more than two long-lived people (over 80 years old) in their family; (4) The donors should have a basic regular life schedule, proper exercise, a balanced and diversified diet, good mental health, and a regular bowel movement [141]. Moreover, the exclusion criteria mainly include: (1) Individuals who have used antibiotics in recent 3 months; (2) Individuals with a history of traumatic infections, allergies and immune disorders in the past years; (3) Individuals with the abnormal defecation habits; (4) Individuals with a suspected or definite pathogen infection; (5) Individuals with a history of cancer or living in special environments [142]. In addition, especially for the prevention and treatment of patients with osteoporosis via FMT, the selection of donors pays more attention to the screening of bone, family history of osteoporosis and bone-related genetic history. Specifically, the donor's bone mineral density (BMD) should be within the normal range (T value >—1), have adequate dietary intake of calcium, vitamins and other bone-related nutrients, have adequate and regular sleep, regularly participate in various forms of exercise, have no history of hormone use, and so on.
Conclusion and perspectives
With the increasing understanding of microbe–host interactions in recent years, the involvement of gut microbiota has become a novel, ingenious and non-negligible way to regulate the host health. On the basis of gut-bone axis and proven regulatory effects of gut microbiota on osteoporosis, more and more attention has been paid to the role of FMT in regulating bone metabolism and maintaining balance between bone formation and bone absorption. Indeed, the application of FMT in the prevention and treatment of osteoporosis is a novel proposition full of unknowns and challenges. It still needs to be recognized that although the osteoporosis can be divided into multiple subtypes, which subtypes of patients are eligible for the FMT treatment, and whether there are specific subtypes of patients who can obtain greater benefits in the process of FMT treatment, these questions are still worthy of further exploration and consideration in future.
Currently, although FMT has been widely applied in treatment of gastrointestinal diseases, the FMT as a potential treatment method for the osteoporosis has rarely been reported, and further large-scale animal experiments and potential human prospective studies are still needed, as well as the randomized controlled trials for exploring efficacy and incidence of adverse events of FMT, and the changes in the structure and function of gut microbiota after the FMT treatment. Notably, FMT is expected to become an effective supplement or even an alternative to the traditional drugs for the prevention and treatment of osteoporosis, and has broad research and application prospects. In the further researches, with in-depth exploration of donors screening, patients’ preparation, transplantation routes, fresh storage of fecal bacterial fluid, times of transplantation, combined medication and other issues, we can fully expect that the standard, efficient and safe FMT will bring novel hope for the prevention and treatment of patients with osteoporosis in future.
Acknowledgements
Not applicable.
Author contributions
YWZ and YFR: wrote main manuscript text. MMC, YJL, RLZ, MTW and QY: prepared figures. All authors reviewed the manuscript.
Fundings
This current work was supported by grants from Winfast Charity Foundation Project (YL20220525); and Jiangsu Elderly Health Research Project, Key Project of Elderly Health Research Project (LKZ2022010).
Declarations
Conflict of interest
All the authors declare that they have no potential conflict of interests, nor biomedical financial interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Reid IR. A broader strategy for osteoporosis interventions. Nat Rev Endocrinol. 2020;16:333–339. doi: 10.1038/s41574-020-0339-7. [DOI] [PubMed] [Google Scholar]
- 2.Kendler DL, Marin F, Zerbini CAF, Russo LA, Greenspan SL, Zikan V, Bagur A, Malouf-Sierra J, Lakatos P, Fahrleitner-Pammer A, Lespessailles E, Minisola S, Body JJ, Geusens P, Möricke R, López-Romero P. Effects of teriparatide and risedronate on new fractures in post-menopausal women with severe osteoporosis (VERO): a multicentre, double-blind, double-dummy, randomised controlled trial. Lancet. 2018;391:230–240. doi: 10.1016/s0140-6736(17)32137-2. [DOI] [PubMed] [Google Scholar]
- 3.Zhuang HF, Wang PW, Li YZ, Lin JK, Yao XD, Xu H. Analysis of related factors of brittle hip fracture in postmenopausal women with osteoporosis. Orthop Surg. 2020;12:194–198. doi: 10.1111/os.12605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chawla A, Kwek EBK. Fractures in patients with osteopetrosis, insights from a single institution. Int Orthop. 2019;43:1297–1302. doi: 10.1007/s00264-018-4167-5. [DOI] [PubMed] [Google Scholar]
- 5.Zhang YW, Lu PP, Li YJ, Dai GC, Chen MH, Zhao YK, Cao MM, Rui YF. Prevalence, characteristics, and associated risk factors of the elderly with hip fractures: a cross-sectional analysis of NHANES 2005–2010. Clin Interv Aging. 2021;16:177–185. doi: 10.2147/cia.S291071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang YW, Lu PP, Li YJ, Dai GC, Cao MM, Xie T, Zhang C, Shi L, Rui YF. Low dietary choline intake is associated with the risk of osteoporosis in elderly individuals: a population-based study. Food Funct. 2021;12:6442–6451. doi: 10.1039/d1fo00825k. [DOI] [PubMed] [Google Scholar]
- 7.Zhang YW, Lu PP, Li YJ, Wang H, Zhao YK, Chen H, Rui YF. Short report: relationship between self-reported sleep characteristics and falls-associated fractures in elderly individuals: a population-based study. Psychol Health Med. 2022 doi: 10.1080/13548506.2022.2119482. [DOI] [PubMed] [Google Scholar]
- 8.Kanis JA, Harvey NC, McCloskey E, Bruyère O, Veronese N, et al. Algorithm for the management of patients at low, high and very high risk of osteoporotic fractures. Osteoporos Int. 2020;31:1–12. doi: 10.1007/s00198-019-05176-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Shi L, Zhang C, Rui Y-F. Dietary protein intake in relation to the risk of osteoporosis in middle-aged and older individuals: a cross-sectional study. J Nutr Health Aging. 2022;26:252–258. doi: 10.1007/s12603-022-1748-1. [DOI] [PubMed] [Google Scholar]
- 10.Söreskog E, Lindberg I, Kanis JA, Åkesson KE, Willems D, Lorentzon M, Ström O, Berling P, Borgström F. Cost-effectiveness of romosozumab for the treatment of postmenopausal women with severe osteoporosis at high risk of fracture in Sweden. Osteoporos Int. 2021;32:585–594. doi: 10.1007/s00198-020-05780-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Estell EG, Rosen CJ. Emerging insights into the comparative effectiveness of anabolic therapies for osteoporosis. Nat Rev Endocrinol. 2021;17:31–46. doi: 10.1038/s41574-020-00426-5. [DOI] [PubMed] [Google Scholar]
- 12.Wysowski DK, Chang JT. Alendronate and risedronate: reports of severe bone, joint, and muscle pain. Arch Intern Med. 2005;165:346–347. doi: 10.1001/archinte.165.3.346-b. [DOI] [PubMed] [Google Scholar]
- 13.Lenart BA, Neviaser AS, Lyman S, Chang CC, Edobor-Osula F, Steele B, van der Meulen MC, Lorich DG, Lane JM. Association of low-energy femoral fractures with prolonged bisphosphonate use: a case control study. Osteoporos Int. 2009;20:1353–1362. doi: 10.1007/s00198-008-0805-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barrett-Connor E, Mosca L, Collins P, Geiger MJ, Grady D, Kornitzer M, McNabb MA, Wenger NK. Effects of raloxifene on cardiovascular events and breast cancer in postmenopausal women. N Engl J Med. 2006;355:125–137. doi: 10.1056/NEJMoa062462. [DOI] [PubMed] [Google Scholar]
- 15.Watts NB, Bilezikian JP, Camacho PM, Greenspan SL, Harris ST, Hodgson SF, Kleerekoper M, Luckey MM, McClung MR, Pollack RP, Petak SM. American association of clinical endocrinologists medical guidelines for clinical practice for the diagnosis and treatment of postmenopausal osteoporosis. Endocr Pract. 2010;16:1–37. doi: 10.4158/ep.16.s3.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu JH, Chen CY, Liu ZZ, Luo ZW, Rao SS, et al. Extracellular vesicles from child gut microbiota enter into bone to preserve bone mass and strength. Adv Sci. 2021;8:2004831. doi: 10.1002/advs.202004831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Biver E, Berenbaum F, Valdes AM, Araujo de Carvalho I, Bindels LB, et al. Gut microbiota and osteoarthritis management: An expert consensus of the European society for clinical and economic aspects of osteoporosis, osteoarthritis and musculoskeletal diseases (ESCEO) Ageing Res Rev. 2019;55:100946. doi: 10.1016/j.arr.2019.100946. [DOI] [PubMed] [Google Scholar]
- 18.Franzosa EA, Sirota-Madi A, Avila-Pacheco J, Fornelos N, Haiser HJ, et al. Gut microbiome structure and metabolic activity in inflammatory bowel disease. Nat Microbiol. 2019;4:293–305. doi: 10.1038/s41564-018-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu Z, Dai X, Zhang H, Shi R, Hui Y, et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat Commun. 2020;11:855. doi: 10.1038/s41467-020-14676-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, d'Aigle J, Atadja L, Quaicoe V, Honarpisheh P, Ganesh BP, Hassan A, Graf J, Petrosino J, Putluri N, Zhu L, Durgan DJ, Bryan RM, Jr, McCullough LD, Venna VR. Gut microbiota-derived short-chain fatty acids promote poststroke recovery in aged mice. Circ Res. 2020;127:453–465. doi: 10.1161/circresaha.119.316448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Xu Y, Wang N, Tan HY, Li S, Zhang C, Zhang Z, Feng Y. Panax notoginseng saponins modulate the gut microbiota to promote thermogenesis and beige adipocyte reconstruction via leptin-mediated AMPKα/STAT3 signaling in diet-induced obesity. Theranostics. 2020;10:11302–11323. doi: 10.7150/thno.47746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chevalier C, Kieser S, Çolakoğlu M, Hadadi N, Brun J, Rigo D, Suárez-Zamorano N, Spiljar M, Fabbiano S, Busse B, Ivanišević J, Macpherson A, Bonnet N, Trajkovski M. Warmth prevents bone loss through the gut microbiota. Cell Metab. 2020;32:575–590.e577. doi: 10.1016/j.cmet.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang YW, Li YJ, Lu PP, Dai GC, Chen XX, Rui YF. The modulatory effect and implication of gut microbiota on osteoporosis: from the perspective of “brain-gut-bone” axis. Food Funct. 2021;12:5703–5718. doi: 10.1039/d0fo03468a. [DOI] [PubMed] [Google Scholar]
- 24.Zhang YW, Cao MM, Li YJ, Dai GC, Lu PP, Zhang M, Bai LY, Chen XX, Zhang C, Shi L, Rui YF. The regulative effect and repercussion of probiotics and prebiotics on osteoporosis: involvement of brain-gut-bone axis. Crit Rev Food Sci Nutr. 2022 doi: 10.1080/10408398.2022.2047005. [DOI] [PubMed] [Google Scholar]
- 25.Becattini S, Taur Y, Pamer EG. Antibiotic-induced changes in the intestinal microbiota and disease. Trends Mol Med. 2016;22:458–478. doi: 10.1016/j.molmed.2016.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weingarden AR, Vaughn BP. Intestinal microbiota, fecal microbiota transplantation, and inflammatory bowel disease. Gut microbes. 2017;8:238–252. doi: 10.1080/19490976.2017.1290757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gomez-Arango LF, Barrett HL, Wilkinson SA, Callaway LK, McIntyre HD, Morrison M, Dekker Nitert M. Low dietary fiber intake increases Collinsella abundance in the gut microbiota of overweight and obese pregnant women. Gut microbes. 2018;9:189–201. doi: 10.1080/19490976.2017.1406584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders ME, Merenstein DJ, Reid G, Gibson GR, Rastall RA. Probiotics and prebiotics in intestinal health and disease: from biology to the clinic. Nat Rev Gastroenterol Hepatol. 2019;16:605–616. doi: 10.1038/s41575-019-0173-3. [DOI] [PubMed] [Google Scholar]
- 29.Korpela K, Helve O, Kolho KL, Saisto T, Skogberg K, Dikareva E, Stefanovic V, Salonen A, Andersson S, de Vos WM. Maternal fecal microbiota transplantation in cesarean-born infants rapidly restores normal gut microbial development: a proof-of-concept study. Cell. 2020;183:324–334.e325. doi: 10.1016/j.cell.2020.08.047. [DOI] [PubMed] [Google Scholar]
- 30.Taur Y, Coyte K, Schluter J, Robilotti E, Figueroa C, et al. Reconstitution of the gut microbiota of antibiotic-treated patients by autologous fecal microbiota transplant. Sci Transl Med. 2018 doi: 10.1126/scitranslmed.aap9489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guirro M, Costa A, Gual-Grau A, Herrero P, Torrell H, Canela N, Arola L. Effects from diet-induced gut microbiota dysbiosis and obesity can be ameliorated by fecal microbiota transplantation: a multiomics approach. PLoS ONE. 2019;14:e0218143. doi: 10.1371/journal.pone.0218143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Staley C, Khoruts A, Sadowsky MJ. Contemporary applications of fecal microbiota transplantation to treat intestinal diseases in humans. Arch Med Res. 2017;48:766–773. doi: 10.1016/j.arcmed.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Tkach S, Dorofeyev A, Kuzenko I, Boyko N, Falalyeyeva T, Boccuto L, Scarpellini E, Kobyliak N, Abenavoli L. Current status and future therapeutic options for fecal microbiota transplantation. Medicina. 2022 doi: 10.3390/medicina58010084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Juul FE, Garborg K, Bretthauer M, Skudal H, Øines MN, Wiig H, Rose Ø, Seip B, Lamont JT, Midtvedt T, Valeur J, Kalager M, Holme Ø, Helsingen L, Løberg M, Adami HO. Fecal microbiota transplantation for primary clostridium difficile infection. N Engl J Med. 2018;378:2535–2536. doi: 10.1056/NEJMc1803103. [DOI] [PubMed] [Google Scholar]
- 35.De Palma G, Lynch MD, Lu J, Dang VT, Deng Y, et al. Transplantation of fecal microbiota from patients with irritable bowel syndrome alters gut function and behavior in recipient mice. Sci Transl Med. 2017 doi: 10.1126/scitranslmed.aaf6397. [DOI] [PubMed] [Google Scholar]
- 36.Borody TJ, George L, Andrews P, Brandl S, Noonan S, Cole P, Hyland L, Morgan A, Maysey J, Moore-Jones D. Bowel-flora alteration: a potential cure for inflammatory bowel disease and irritable bowel syndrome? Med J Aust. 1989;150:604. doi: 10.5694/j.1326-5377.1989.tb136704.x. [DOI] [PubMed] [Google Scholar]
- 37.Inflammatory Bowel Disease Group, Chinese Society of Gastroenterology, Chinese Medical Association Chinese consensus on diagnosis and treatment in inflammatory bowel disease (2018, Beijing) J Dig Dis. 2021;22:298–317. doi: 10.1111/1751-2980.12994. [DOI] [PubMed] [Google Scholar]
- 38.Cammarota G, Ianiro G, Tilg H, Rajilić-Stojanović M, Kump P, Satokari R, Sokol H, Arkkila P, Pintus C, Hart A, Segal J, Aloi M, Masucci L, Molinaro A, Scaldaferri F, Gasbarrini G, Lopez-Sanroman A, Link A, de Groot P, de Vos WM, Högenauer C, Malfertheiner P, Mattila E, Milosavljević T, Nieuwdorp M, Sanguinetti M, Simren M, Gasbarrini A. European consensus conference on faecal microbiota transplantation in clinical practice. Gut. 2017;66:569–580. doi: 10.1136/gutjnl-2016-313017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Luz M, Waizbort RF. Fecal microbiota transplants in the treatment of pseudomembranous colitis (1958–2013): priority of discovery and thought styles in the academic literature. Historia, Ciencias, Saude Manguinhos. 2020;27:859–878. doi: 10.1590/s0104-59702020000400009. [DOI] [PubMed] [Google Scholar]
- 40.Secombe KR, Al-Qadami GH, Subramaniam CB, Bowen JM, Scott J, Van Sebille YZA, Snelson M, Cowan C, Clarke G, Gheorghe CE, Cryan JF, Wardill HR. Guidelines for reporting on animal fecal transplantation (GRAFT) studies: recommendations from a systematic review of murine transplantation protocols. Gut microbes. 2021;13:1979878. doi: 10.1080/19490976.2021.1979878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cammarota G, Ianiro G, Kelly CR, Mullish BH, Allegretti JR, Kassam Z, Putignani L, Fischer M, Keller JJ, Costello SP, Sokol H, Kump P, Satokari R, Kahn SA, Kao D, Arkkila P, Kuijper EJ, Vehreschild MJG, Pintus C, Lopetuso L, Masucci L, Scaldaferri F, Terveer EM, Nieuwdorp M, López-Sanromán A, Kupcinskas J, Hart A, Tilg H, Gasbarrini A. International consensus conference on stool banking for faecal microbiota transplantation in clinical practice. Gut. 2019;68:2111–2121. doi: 10.1136/gutjnl-2019-319548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bowden TA, Jr, Mansberger AR, Jr, Lykins LE. Pseudomembraneous enterocolitis: mechanism for restoring floral homeostasis. Am Surg. 1981;47:178–183. [PubMed] [Google Scholar]
- 43.Schwan A, Sjölin S, Trottestam U, Aronsson B. Relapsing clostridium difficile enterocolitis cured by rectal infusion of homologous faeces. Lancet. 1983;2:845. doi: 10.1016/s0140-6736(83)90753-5. [DOI] [PubMed] [Google Scholar]
- 44.Bakken JS, Polgreen PM, Beekmann SE, Riedo FX, Streit JA. Treatment approaches including fecal microbiota transplantation for recurrent Clostridium difficile infection (RCDI) among infectious disease physicians. Anaerobe. 2013;24:20–24. doi: 10.1016/j.anaerobe.2013.08.007. [DOI] [PubMed] [Google Scholar]
- 45.Rinott E, Youngster I, Yaskolka Meir A, Tsaban G, Zelicha H, et al. Effects of diet-modulated autologous fecal microbiota transplantation on weight regain. Gastroenterology. 2021;160:158–173.e110. doi: 10.1053/j.gastro.2020.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ma S, Wang N, Zhang P, Wu W, Fu L. Fecal microbiota transplantation mitigates bone loss by improving gut microbiome composition and gut barrier function in aged rats. PeerJ. 2021;9:e12293. doi: 10.7717/peerj.12293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Aron-Wisnewsky J, Clément K, Nieuwdorp M. Fecal microbiota transplantation: a future therapeutic option for obesity/diabetes? Curr DiabRep. 2019;19:51. doi: 10.1007/s11892-019-1180-z. [DOI] [PubMed] [Google Scholar]
- 48.Lu C, Chen J, Yi C, Han J, Shi Q, Li J, Liu B, Zhou J, Su X. Gut microbiota mediated the protective effects of tuna oil on collagen-induced arthritis in mice. Food Funct. 2021;12:5387–5398. doi: 10.1039/d1fo00709b. [DOI] [PubMed] [Google Scholar]
- 49.Kragsnaes MS, Kjeldsen J, Horn HC, Munk HL, Pedersen JK, Just SA, Ahlquist P, Pedersen FM, de Wit M, Möller S, Andersen V, Kristiansen K, Kinggaard Holm D, Holt HM, Christensen R, Ellingsen T. Safety and efficacy of faecal microbiota transplantation for active peripheral psoriatic arthritis: an exploratory randomised placebo-controlled trial. Ann Rheum Dis. 2021;80:1158–1167. doi: 10.1136/annrheumdis-2020-219511. [DOI] [PubMed] [Google Scholar]
- 50.Pennycook JH, Scanlan PD. Ecological and evolutionary responses to antibiotic treatment in the human gut microbiota. FEMS Microbiol Rev. 2021 doi: 10.1093/femsre/fuab018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milani C, Duranti S, Bottacini F, Casey E, Turroni F, Mahony J, Belzer C, Delgado Palacio S, Arboleya Montes S, Mancabelli L, Lugli GA, Rodriguez JM, Bode L, de Vos W, Gueimonde M, Margolles A, van Sinderen D, Ventura M. The first microbial colonizers of the human gut: composition, activities, and health implications of the infant gut microbiota. Microbiol Mol Biol Rev MMBR. 2017 doi: 10.1128/mmbr.00036-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Loo YT, Howell K, Chan M, Zhang P, Ng K. Modulation of the human gut microbiota by phenolics and phenolic fiber-rich foods. Compr Rev Food Sci Food Safety. 2020;19:1268–1298. doi: 10.1111/1541-4337.12563. [DOI] [PubMed] [Google Scholar]
- 53.Ling CW, Miao Z, Xiao ML, Zhou H, Jiang Z, et al. The association of gut microbiota with osteoporosis is mediated by amino acid metabolism: multiomics in a large cohort. J Clin Endocrinol Metab. 2021;106:e3852–e3864. doi: 10.1210/clinem/dgab492. [DOI] [PubMed] [Google Scholar]
- 54.Jia X, Jia L, Mo L, Yuan S, Zheng X, He J, Chen V, Guo Q, Zheng L, Yuan Q, Xu X, Zhou X. Berberine ameliorates periodontal bone loss by regulating gut microbiota. J Dent Res. 2019;98:107–116. doi: 10.1177/0022034518797275. [DOI] [PubMed] [Google Scholar]
- 55.Lu L, Tang M, Li J, Xie Y, Li Y, Xie J, Zhou L, Liu Y, Yu X. Gut microbiota and serum metabolic signatures of high-fat-induced bone loss in mice. Front Cell Infect Microbiol. 2021;11:788576. doi: 10.3389/fcimb.2021.788576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Tu MY, Han KY, Chang GR, Lai GD, Chang KY, Chen CF, Lai JC, Lai CY, Chen HL, Chen CM. Kefir peptides prevent estrogen deficiency-induced bone loss and modulate the structure of the gut microbiota in ovariectomized mice. Nutrients. 2020 doi: 10.3390/nu12113432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu H, Gu R, Zhu Y, Lian X, Wang S, Liu X, Ping Z, Liu Y, Zhou Y. D-mannose attenuates bone loss in mice via Treg cell proliferation and gut microbiota-dependent anti-inflammatory effects. Ther Adv Chronic Dis. 2020;11:2040622320912661. doi: 10.1177/2040622320912661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jia L, Tu Y, Jia X, Du Q, Zheng X, Yuan Q, Zheng L, Zhou X, Xu X. Probiotics ameliorate alveolar bone loss by regulating gut microbiota. Cell Prolif. 2021;54:e13075. doi: 10.1111/cpr.13075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yan J, Herzog JW, Tsang K, Brennan CA, Bower MA, Garrett WS, Sartor BR, Aliprantis AO, Charles JF. Gut microbiota induce IGF-1 and promote bone formation and growth. Proc Natl Acad Sci USA. 2016;113:E7554–e7563. doi: 10.1073/pnas.1607235113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sjögren K, Engdahl C, Henning P, Lerner UH, Tremaroli V, Lagerquist MK, Bäckhed F, Ohlsson C. The gut microbiota regulates bone mass in mice. J Bone Miner Res. 2012;27:1357–1367. doi: 10.1002/jbmr.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Huo R, Zeng B, Zeng L, Cheng K, Li B, Luo Y, Wang H, Zhou C, Fang L, Li W, Niu R, Wei H, Xie P. Microbiota modulate anxiety-like behavior and endocrine abnormalities in hypothalamic-pituitary-adrenal axis. Front Cell Infect Microbiol. 2017;7:489. doi: 10.3389/fcimb.2017.00489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Han H, Yi B, Zhong R, Wang M, Zhang S, Ma J, Yin Y, Yin J, Chen L, Zhang H. From gut microbiota to host appetite: gut microbiota-derived metabolites as key regulators. Microbiome. 2021;9:162. doi: 10.1186/s40168-021-01093-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Qi X, Yun C, Pang Y, Qiao J. The impact of the gut microbiota on the reproductive and metabolic endocrine system. Gut microbes. 2021;13:1–21. doi: 10.1080/19490976.2021.1894070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Fuhrman BJ, Feigelson HS, Flores R, Gail MH, Xu X, Ravel J, Goedert JJ. Associations of the fecal microbiome with urinary estrogens and estrogen metabolites in postmenopausal women. J Clin Endocrinol Metab. 2014;99:4632–4640. doi: 10.1210/jc.2014-2222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tousen Y, Uehara M, Abe F, Kimira Y, Ishimi Y. Effects of short-term fructooligosaccharide intake on equol production in Japanese postmenopausal women consuming soy isoflavone supplements: a pilot study. Nutr J. 2013;12:127. doi: 10.1186/1475-2891-12-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cronin BE, Allsopp PJ, Slevin MM, Magee PJ, Livingstone MB, Strain JJ, McSorley EM. Effects of supplementation with a calcium-rich marine-derived multi-mineral supplement and short-chain fructo-oligosaccharides on serum lipids in postmenopausal women. Br J Nutr. 2016;115:658–665. doi: 10.1017/s0007114515004948. [DOI] [PubMed] [Google Scholar]
- 67.Abrams SA, Griffin IJ, Hawthorne KM, Liang L, Gunn SK, Darlington G, Ellis KJ. A combination of prebiotic short- and long-chain inulin-type fructans enhances calcium absorption and bone mineralization in young adolescents. Am J Clin Nutr. 2005;82:471–476. doi: 10.1093/ajcn.82.2.471. [DOI] [PubMed] [Google Scholar]
- 68.van den Heuvel EG, Schoterman MH, Muijs T. Transgalactooligosaccharides stimulate calcium absorption in postmenopausal women. J Nutr. 2000;130:2938–2942. doi: 10.1093/jn/130.12.2938. [DOI] [PubMed] [Google Scholar]
- 69.Jones ML, Martoni CJ, Prakash S. Oral supplementation with probiotic L. reuteri NCIMB 30242 increases mean circulating 25-hydroxyvitamin D: a post hoc analysis of a randomized controlled trial. J Clin Endocrinol Metab. 2013;98:2944–2951. doi: 10.1210/jc.2012-4262. [DOI] [PubMed] [Google Scholar]
- 70.Yu W, Zhong L, Yao L, Wei Y, Gui T, Li Z, Kim H, Holdreith N, Jiang X, Tong W, Dyment N, Liu XS, Yang S, Choi Y, Ahn J, Qin L. Bone marrow adipogenic lineage precursors promote osteoclastogenesis in bone remodeling and pathologic bone loss. J Clin Investig. 2021 doi: 10.1172/jci140214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sun J, Xu J, Ling Y, Wang F, Gong T, Yang C, Ye S, Ye K, Wei D, Song Z, Chen D, Liu J. Fecal microbiota transplantation alleviated Alzheimer's disease-like pathogenesis in APP/PS1 transgenic mice. Transl Psychiatry. 2019;9:189. doi: 10.1038/s41398-019-0525-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kim JH, Kim K, Kim W. Gut microbiota restoration through fecal microbiota transplantation: a new atopic dermatitis therapy. Exp Mol Med. 2021;53:907–916. doi: 10.1038/s12276-021-00627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Bajaj JS, Gavis EA, Fagan A, Wade JB, Thacker LR, Fuchs M, Patel S, Davis B, Meador J, Puri P, Sikaroodi M, Gillevet PM. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2021;73:1688–1700. doi: 10.1002/hep.31496. [DOI] [PubMed] [Google Scholar]
- 74.Zhao S, Jang C, Liu J, Uehara K, Gilbert M, Izzo L, Zeng X, Trefely S, Fernandez S, Carrer A, Miller KD, Schug ZT, Snyder NW, Gade TP, Titchenell PM, Rabinowitz JD, Wellen KE. Dietary fructose feeds hepatic lipogenesis via microbiota-derived acetate. Nature. 2020;579:586–591. doi: 10.1038/s41586-020-2101-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chen B, Wei J, Zhu R, Zhang H, Xia B, Liu Y, Dai X, Ye Z, Tian Y, Li R, Zhao D, Mo F, Orekhov AN, Gao S, Brὃmme D, Wang L, Zhang D. Fructus Ligustri Lucidi aqueous extract promotes calcium balance and short-chain fatty acids production in ovariectomized rats. J Ethnopharmacol. 2021;279:114348. doi: 10.1016/j.jep.2021.114348. [DOI] [PubMed] [Google Scholar]
- 76.Park J, Kim M, Kang SG, Jannasch AH, Cooper B, Patterson J, Kim CH. Short-chain fatty acids induce both effector and regulatory T cells by suppression of histone deacetylases and regulation of the mTOR-S6K pathway. Mucosal Immunol. 2015;8:80–93. doi: 10.1038/mi.2014.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Beilharz JE, Kaakoush NO, Maniam J, Morris MJ. Cafeteria diet and probiotic therapy: cross talk among memory, neuroplasticity, serotonin receptors and gut microbiota in the rat. Mol Psychiatry. 2018;23:351–361. doi: 10.1038/mp.2017.38. [DOI] [PubMed] [Google Scholar]
- 78.Esmaili A, Nazir SF, Borthakur A, Yu D, Turner JR, Saksena S, Singla A, Hecht GA, Alrefai WA, Gill RK. Enteropathogenic Escherichia coli infection inhibits intestinal serotonin transporter function and expression. Gastroenterology. 2009;137:2074–2083. doi: 10.1053/j.gastro.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wang H, Liu W, Shi F, Huang L, Lian J, Qu L, Cai J, Xu Z. Metabolic pathway engineering for high-level production of 5-hydroxytryptophan in Escherichia coli. Metab Eng. 2018;48:279–287. doi: 10.1016/j.ymben.2018.06.007. [DOI] [PubMed] [Google Scholar]
- 80.Hodge JM, Wang Y, Berk M, Collier FM, Fernandes TJ, Constable MJ, Pasco JA, Dodd S, Nicholson GC, Kennedy RL, Williams LJ. Selective serotonin reuptake inhibitors inhibit human osteoclast and osteoblast formation and function. Biol Psychiat. 2013;74:32–39. doi: 10.1016/j.biopsych.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 81.Rizzoli R, Biver E. Are probiotics the new calcium and vitamin D for bone health? Curr Osteoporos Rep. 2020;18:273–284. doi: 10.1007/s11914-020-00591-6. [DOI] [PubMed] [Google Scholar]
- 82.Thomas RL, Jiang L, Adams JS, Xu ZZ, Shen J, Janssen S, Ackermann G, Vanderschueren D, Pauwels S, Knight R, Orwoll ES, Kado DM. Vitamin D metabolites and the gut microbiome in older men. Nat Commun. 2020;11:5997. doi: 10.1038/s41467-020-19793-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Feng YL, Cao G, Chen DQ, Vaziri ND, Chen L, Zhang J, Wang M, Guo Y, Zhao YY. Microbiome-metabolomics reveals gut microbiota associated with glycine-conjugated metabolites and polyamine metabolism in chronic kidney disease. Cell Mol Life Sci. 2019;76:4961–4978. doi: 10.1007/s00018-019-03155-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Raya-Sandino A, Luissint AC, Kusters DHM, Narayanan V, Flemming S, Garcia-Hernandez V, Godsel LM, Green KJ, Hagen SJ, Conway DE, Parkos CA, Nusrat A. Regulation of intestinal epithelial intercellular adhesion and barrier function by desmosomal cadherin desmocollin-2. Mol Biol Cell. 2021;32:753–768. doi: 10.1091/mbc.E20-12-0775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pontarollo G, Mann A, Brandão I, Malinarich F, Schöpf M, Reinhardt C. Protease-activated receptor signaling in intestinal permeability regulation. FEBS J. 2020;287:645–658. doi: 10.1111/febs.15055. [DOI] [PubMed] [Google Scholar]
- 86.Van Spaendonk H, Ceuleers H, Witters L, Patteet E, Joossens J, Augustyns K, Lambeir AM, De Meester I, De Man JG, De Winter BY. Regulation of intestinal permeability: the role of proteases. World J Gastroenterol. 2017;23:2106–2123. doi: 10.3748/wjg.v23.i12.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Schepper JD, Collins FL, Rios-Arce ND, Raehtz S, Schaefer L, Gardinier JD, Britton RA, Parameswaran N, McCabe LR. Probiotic lactobacillus reuteri prevents postantibiotic bone loss by reducing intestinal dysbiosis and preventing barrier disruption. J Bone Miner Res. 2019;34:681–698. doi: 10.1002/jbmr.3635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chen X, Zhang Z, Hu Y, Cui J, Zhi X, Li X, Jiang H, Wang Y, Gu Z, Qiu Z, Dong X, Li Y, Su J. Lactulose suppresses osteoclastogenesis and ameliorates estrogen deficiency-induced bone loss in mice. Aging Dis. 2020;11:629–641. doi: 10.14336/ad.2019.0613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Li B, Liu M, Wang Y, Gong S, Yao W, Li W, Gao H, Wei M. Puerarin improves the bone micro-environment to inhibit OVX-induced osteoporosis via modulating SCFAs released by the gut microbiota and repairing intestinal mucosal integrity. Biomed Pharmacother. 2020;132:110923. doi: 10.1016/j.biopha.2020.110923. [DOI] [PubMed] [Google Scholar]
- 90.Li J, Yang M, Lu C, Han J, Tang S, Zhou J, Li Y, Ming T, Wang ZJ, Su X. Tuna bone powder alleviates glucocorticoid-induced osteoporosis via coregulation of the NF-κB and Wnt/β-catenin signaling pathways and modulation of gut microbiota composition and metabolism. Mol Nutr Food Res. 2020;64:e1900861. doi: 10.1002/mnfr.201900861. [DOI] [PubMed] [Google Scholar]
- 91.Wang Z, Chen K, Wu C, Chen J, Pan H, Liu Y, Wu P, Yuan J, Huang F, Lang J, Du J, Xu J, Jin K, Chen L. An emerging role of Prevotella histicola on estrogen deficiency-induced bone loss through the gut microbiota-bone axis in postmenopausal women and in ovariectomized mice. Am J Clin Nutr. 2021;114:1304–1313. doi: 10.1093/ajcn/nqab194. [DOI] [PubMed] [Google Scholar]
- 92.Wang N, Ma S, Fu L. Gut microbiota dysbiosis as one cause of osteoporosis by impairing intestinal barrier function. Calcif Tissue Int. 2022;110:225–235. doi: 10.1007/s00223-021-00911-7. [DOI] [PubMed] [Google Scholar]
- 93.Zhang Y-W, Cao M-M, Li Y-J, Lu P-P, Dai G-C, Zhang M, Wang H, Rui Y-F. Fecal microbiota transplantation ameliorates bone loss in mice with ovariectomy-induced osteoporosis via modulating gut microbiota and metabolic function. J Orthop Transl. 2022;37:46–60. doi: 10.1016/j.jot.2022.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tsukasaki M, Takayanagi H. Osteoimmunology: evolving concepts in bone-immune interactions in health and disease. Nat Rev Immunol. 2019;19:626–642. doi: 10.1038/s41577-019-0178-8. [DOI] [PubMed] [Google Scholar]
- 95.Liu YJ, Tang B, Wang FC, Tang L, Lei YY, Luo Y, Huang SJ, Yang M, Wu LY, Wang W, Liu S, Yang SM, Zhao XY. Parthenolide ameliorates colon inflammation through regulating Treg/Th17 balance in a gut microbiota-dependent manner. Theranostics. 2020;10:5225–5241. doi: 10.7150/thno.43716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Atarashi K, Tanoue T, Shima T, Imaoka A, Kuwahara T, Momose Y, Cheng G, Yamasaki S, Saito T, Ohba Y, Taniguchi T, Takeda K, Hori S, Ivanov II, Umesaki Y, Itoh K, Honda K. Induction of colonic regulatory T cells by indigenous Clostridium species. Science. 2011;331:337–341. doi: 10.1126/science.1198469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yu M, Pal S, Paterson CW, Li JY, Tyagi AM, Adams J, Coopersmith CM, Weitzmann MN, Pacifici R. Ovariectomy induces bone loss via microbial-dependent trafficking of intestinal TNF+ T cells and Th17 cells. J Clin Investig. 2021 doi: 10.1172/jci143137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Goto Y, Panea C, Nakato G, Cebula A, Lee C, Diez MG, Laufer TM, Ignatowicz L, Ivanov II. Segmented filamentous bacteria antigens presented by intestinal dendritic cells drive mucosal Th17 cell differentiation. Immunity. 2014;40:594–607. doi: 10.1016/j.immuni.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ivanov II, Atarashi K, Manel N, Brodie EL, Shima T, Karaoz U, Wei D, Goldfarb KC, Santee CA, Lynch SV, Tanoue T, Imaoka A, Itoh K, Takeda K, Umesaki Y, Honda K, Littman DR. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139:485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Han Q, Wang J, Li W, Chen ZJ, Du Y. Androgen-induced gut dysbiosis disrupts glucolipid metabolism and endocrinal functions in polycystic ovary syndrome. Microbiome. 2021;9:101. doi: 10.1186/s40168-021-01046-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bravo JA, Forsythe P, Chew MV, Escaravage E, Savignac HM, Dinan TG, Bienenstock J, Cryan JF. Ingestion of Lactobacillus strain regulates emotional behavior and central GABA receptor expression in a mouse via the vagus nerve. Proc Natl Acad Sci USA. 2011;108:16050–16055. doi: 10.1073/pnas.1102999108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clarke G, Stilling RM, Kennedy PJ, Stanton C, Cryan JF, Dinan TG. Minireview: gut microbiota: the neglected endocrine organ. Mol Endocrinol. 2014;28:1221–1238. doi: 10.1210/me.2014-1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Schepper JD, Collins F, Rios-Arce ND, Kang HJ, Schaefer L, Gardinier JD, Raghuvanshi R, Quinn RA, Britton R, Parameswaran N, McCabe LR. Involvement of the gut microbiota and barrier function in glucocorticoid-induced osteoporosis. J Bone Miner Res. 2020;35:801–820. doi: 10.1002/jbmr.3947. [DOI] [PubMed] [Google Scholar]
- 104.Honda K, Littman DR. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 105.Zhong M, Buch H, Wen Q, Long C, Cui B, Zhang F. Colonic transendoscopic enteral tubing: route for a novel, safe, and convenient delivery of washed microbiota transplantation in children. Gastroenterol Res Pract. 2021;2021:6676962. doi: 10.1155/2021/6676962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Zhang T, Long C, Cui B, Buch H, Wen Q, Li Q, Ding X, Ji G, Zhang F. Colonic transendoscopic tube-delivered enteral therapy (with video): a prospective study. BMC Gastroenterol. 2020;20:135. doi: 10.1186/s12876-020-01285-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Long C, Yu Y, Cui B, Jagessar SAR, Zhang J, Ji G, Huang G, Zhang F. A novel quick transendoscopic enteral tubing in mid-gut: technique and training with video. BMC Gastroenterol. 2018;18:37. doi: 10.1186/s12876-018-0766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Woodworth MH, Neish EM, Miller NS, Dhere T, Burd EM, Carpentieri C, Sitchenko KL, Kraft CS. Laboratory testing of donors and stool samples for fecal microbiota transplantation for recurrent clostridium difficile infection. J Clin Microbiol. 2017;55:1002–1010. doi: 10.1128/jcm.02327-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Papanicolas LE, Wang Y, Choo JM, Gordon DL, Wesselingh SL, Rogers GB. Optimisation of a propidium monoazide based method to determine the viability of microbes in faecal slurries for transplantation. J Microbiol Methods. 2019;156:40–45. doi: 10.1016/j.mimet.2018.12.001. [DOI] [PubMed] [Google Scholar]
- 110.Ramai D, Zakhia K, Ofosu A, Ofori E, Reddy M. Fecal microbiota transplantation: donor relation, fresh or frozen, delivery methods, cost-effectiveness. Ann Gastroenterol. 2019;32:30–38. doi: 10.20524/aog.2018.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fuentes S, de Vos WM. How to manipulate the microbiota: fecal microbiota transplantation. Adv Exp Med Biol. 2016;902:143–153. doi: 10.1007/978-3-319-31248-4_10. [DOI] [PubMed] [Google Scholar]
- 112.Ng SC, Kamm MA, Yeoh YK, Chan PKS, Zuo T, Tang W, Sood A, Andoh A, Ohmiya N, Zhou Y, Ooi CJ, Mahachai V, Wu CY, Zhang F, Sugano K, Chan FKL. Scientific frontiers in faecal microbiota transplantation: joint document of Asia-Pacific Association of Gastroenterology (APAGE) and Asia-Pacific Society for Digestive Endoscopy (APSDE) Gut. 2020;69:83–91. doi: 10.1136/gutjnl-2019-319407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Costello SP, Hughes PA, Waters O, Bryant RV, Vincent AD, Blatchford P, Katsikeros R, Makanyanga J, Campaniello MA, Mavrangelos C, Rosewarne CP, Bickley C, Peters C, Schoeman MN, Conlon MA, Roberts-Thomson IC, Andrews JM. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA. 2019;321:156–164. doi: 10.1001/jama.2018.20046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Khanna S, Pardi DS, Kelly CR, Kraft CS, Dhere T, Henn MR, Lombardo MJ, Vulic M, Ohsumi T, Winkler J, Pindar C, McGovern BH, Pomerantz RJ, Aunins JG, Cook DN, Hohmann EL. A novel microbiome therapeutic increases gut microbial diversity and prevents recurrent clostridium difficile infection. J Infect Dis. 2016;214:173–181. doi: 10.1093/infdis/jiv766. [DOI] [PubMed] [Google Scholar]
- 115.Youngster I, Russell GH, Pindar C, Ziv-Baran T, Sauk J, Hohmann EL. Oral, capsulized, frozen fecal microbiota transplantation for relapsing Clostridium difficile infection. JAMA. 2014;312:1772–1778. doi: 10.1001/jama.2014.13875. [DOI] [PubMed] [Google Scholar]
- 116.Wen Q, Liu KJ, Cui BT, Li P, Wu X, Zhong M, Wei L, Tu H, Yuan Y, Lin D, Hsu WH, Wu DC, Yin H, Zhang FM. Impact of cap-assisted colonoscopy during transendoscopic enteral tubing: a randomized controlled trial. World J Gastroenterol. 2020;26:6098–6110. doi: 10.3748/wjg.v26.i39.6098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.van Nood E, Vrieze A, Nieuwdorp M, Fuentes S, Zoetendal EG, de Vos WM, Visser CE, Kuijper EJ, Bartelsman JF, Tijssen JG, Speelman P, Dijkgraaf MG, Keller JJ. Duodenal infusion of donor feces for recurrent Clostridium difficile. N Engl J Med. 2013;368:407–415. doi: 10.1056/NEJMoa1205037. [DOI] [PubMed] [Google Scholar]
- 118.Zhong M, Sun Y, Wang HG, Marcella C, Cui BT, Miao YL, Zhang FM. Awareness and attitude of fecal microbiota transplantation through transendoscopic enteral tubing among inflammatory bowel disease patients. World J Clin Cases. 2020;8:3786–3796. doi: 10.12998/wjcc.v8.i17.3786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ding X, Li Q, Li P, Zhang T, Cui B, Ji G, Lu X, Zhang F. Long-term safety and efficacy of fecal microbiota transplant in active ulcerative colitis. Drug Saf. 2019;42:869–880. doi: 10.1007/s40264-019-00809-2. [DOI] [PubMed] [Google Scholar]
- 120.Liu J, Miyake H, Zhu H, Li B, Alganabi M, Lee C, Pierro A. Fecal microbiota transplantation by enema reduces intestinal injury in experimental necrotizing enterocolitis. J Pediatr Surg. 2020;55:1094–1098. doi: 10.1016/j.jpedsurg.2020.02.035. [DOI] [PubMed] [Google Scholar]
- 121.Hsu WH, Wang JY, Kuo CH. Current applications of fecal microbiota transplantation in intestinal disorders. Kaohsiung J Med Sci. 2019;35:327–331. doi: 10.1002/kjm2.12069. [DOI] [PubMed] [Google Scholar]
- 122.Kao D, Roach B, Silva M, Beck P, Rioux K, Kaplan GG, Chang HJ, Coward S, Goodman KJ, Xu H, Madsen K, Mason A, Wong GK, Jovel J, Patterson J, Louie T. Effect of oral capsule- vs colonoscopy-delivered fecal microbiota transplantation on recurrent clostridium difficile infection: a randomized clinical trial. JAMA. 2017;318:1985–1993. doi: 10.1001/jama.2017.17077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lahtinen P, Jalanka J, Hartikainen A, Mattila E, Hillilä M, Punkkinen J, Koskenpato J, Anttila VJ, Tillonen J, Satokari R, Arkkila P. Randomised clinical trial: faecal microbiota transplantation versus autologous placebo administered via colonoscopy in irritable bowel syndrome. Aliment Pharmacol Ther. 2020;51:1321–1331. doi: 10.1111/apt.15740. [DOI] [PubMed] [Google Scholar]
- 124.Hanssen NMJ, de Vos WM, Nieuwdorp M. Fecal microbiota transplantation in human metabolic diseases: from a murky past to a bright future? Cell Metab. 2021;33:1098–1110. doi: 10.1016/j.cmet.2021.05.005. [DOI] [PubMed] [Google Scholar]
- 125.Holvoet T, Joossens M, Vázquez-Castellanos JF, Christiaens E, Heyerick L, Boelens J, Verhasselt B, van Vlierberghe H, De Vos M, Raes J, De Looze D. Fecal microbiota transplantation reduces symptoms in some patients with irritable bowel syndrome with predominant abdominal bloating: short- and long-term results from a placebo-controlled randomized trial. Gastroenterology. 2021;160:145–157.e148. doi: 10.1053/j.gastro.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 126.Wang H, Cui B, Li Q, Ding X, Li P, Zhang T, Yang X, Ji G, Zhang F. The safety of fecal microbiota transplantation for crohn's disease: findings from a long-term study. Adv Ther. 2018;35:1935–1944. doi: 10.1007/s12325-018-0800-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Khoruts A, Sadowsky MJ, Hamilton MJ. Development of fecal microbiota transplantation suitable for mainstream medicine. Clin Gastroenterol Hepatol. 2015;13:246–250. doi: 10.1016/j.cgh.2014.11.014. [DOI] [PubMed] [Google Scholar]
- 128.Paramsothy S, Walsh AJ, Borody T, Samuel D, van den Bogaerde J, Leong RW, Connor S, Ng W, Mitchell HM, Kaakoush NO, Kamm MA. Gastroenterologist perceptions of faecal microbiota transplantation. World J Gastroenterol. 2015;21:10907–10914. doi: 10.3748/wjg.v21.i38.10907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Nicholson MR, Hourigan SK, Conrad M, Goyal A, Jensen K, Kelsen J, Kennedy M, Weatherly M, Kahn SA. Current challenges in fecal microbiota transplantation for clostridioides difficile infection in children. Am J Gastroenterol. 2021;116:1954–1956. doi: 10.14309/ajg.0000000000001350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xiao F, Tang M, Zheng X, Liu Y, Li X, Shan H. Evidence for gastrointestinal infection of SARS-CoV-2. Gastroenterology. 2020;158:1831–1833.e1833. doi: 10.1053/j.gastro.2020.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.DeFilipp Z, Bloom PP, Torres Soto M, Mansour MK, Sater MRA, Huntley MH, Turbett S, Chung RT, Chen YB, Hohmann EL. Drug-resistant E. coli bacteremia transmitted by fecal microbiota transplant. New Engl J Med. 2019;381:2043–2050. doi: 10.1056/NEJMoa1910437. [DOI] [PubMed] [Google Scholar]
- 132.Zhang L, Ghosh SK, Basavarajappa SC, Chen Y, Shrestha P, Penfield J, Brewer A, Ramakrishnan P, Buck M, Weinberg A. HBD-2 binds SARS-CoV-2 RBD and blocks viral entry: strategy to combat COVID-19. iScience. 2022;25:103856. doi: 10.1016/j.isci.2022.103856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Sekar PC, Srinivasan E, Chandrasekhar G, Paul DM, Sanjay G, Surya S, Kumar N, Rajasekaran R. Probing the competitive inhibitor efficacy of frog-skin alpha helical AMPs identified against ACE2 binding to SARS-CoV-2 S1 spike protein as therapeutic scaffold to prevent COVID-19. J Mol Model. 2022;28:128. doi: 10.1007/s00894-022-05117-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ianiro G, Mullish BH, Kelly CR, Kassam Z, Kuijper EJ, et al. Reorganisation of faecal microbiota transplant services during the COVID-19 pandemic. Gut. 2020;69:1555–1563. doi: 10.1136/gutjnl-2020-321829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Khanna S, Tande A, Rubin DT, Khoruts A, Kahn SA, Pardi DS. Fecal microbiota transplantation for recurrent c difficile infection during the COVID-19 pandemic: experience and recommendations. Mayo Clin Proc. 2021;96:1418–1425. doi: 10.1016/j.mayocp.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shahinas D, Silverman M, Sittler T, Chiu C, Kim P, Allen-Vercoe E, Weese S, Wong A, Low DE, Pillai DR. Toward an understanding of changes in diversity associated with fecal microbiome transplantation based on 16S rRNA gene deep sequencing. iBio. 2012 doi: 10.1128/mBio.00338-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lagier JC. Faecal microbiota transplantation: from practice to legislation before considering industrialization. Clin Microbiol Infect. 2014;20:1112–1118. doi: 10.1111/1469-0691.12795. [DOI] [PubMed] [Google Scholar]
- 138.Khan R, Roy N, Ali H, Naeem M. Fecal microbiota transplants for inflammatory bowel disease treatment: synthetic- and engineered communities-based microbiota transplants are the future. Gastroenterol Res Pract. 2022;2022:9999925. doi: 10.1155/2022/9999925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang T, Lu G, Zhao Z, Liu Y, Shen Q, Li P, Chen Y, Yin H, Wang H, Marcella C, Cui B, Cheng L, Ji G, Zhang F. Washed microbiota transplantation vs. manual fecal microbiota transplantation: clinical findings, animal studies and in vitro screening. Protein Cell. 2020;11:251–266. doi: 10.1007/s13238-019-00684-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Zhang T, Li P, Wu X, Lu G, Marcella C, Ji X, Ji G, Zhang F. Alterations of Akkermansia muciniphila in the inflammatory bowel disease patients with washed microbiota transplantation. Appl Microbiol Biotechnol. 2020;104:10203–10215. doi: 10.1007/s00253-020-10948-7. [DOI] [PubMed] [Google Scholar]
- 141.Colman RJ, Rubin DT. Fecal microbiota transplantation as therapy for inflammatory bowel disease: a systematic review and meta-analysis. J Crohns Colitis. 2014;8:1569–1581. doi: 10.1016/j.crohns.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Haifer C, Kelly CR, Paramsothy S, Andresen D, Papanicolas LE, et al. Australian consensus statements for the regulation, production and use of faecal microbiota transplantation in clinical practice. Gut. 2020;69:801–810. doi: 10.1136/gutjnl-2019-320260. [DOI] [PubMed] [Google Scholar]