Abstract
Background
The deliberate use of Bacillus anthracis spores is believed by the US government to be a high bioweapons threat. The first line of defense following potential exposure to B. anthracis spores would be postexposure prophylaxis with antimicrobials that have activity against B. anthracis. Additional therapies to address the effects of toxins may be needed in systemically ill individuals. Over the last 2 decades, the United States government (USG) collaborated with the private sector to develop, test, and stockpile 3 antitoxins: anthrax immunoglobulin intravenous (AIGIV), raxibacumab, and obiltoxaximab. All 3 products target protective antigen, a protein factor common to the 2 exotoxins released by B. anthracis, and hamper or block the toxins’ effects and prevent or reduce pathogenesis. These antitoxins were approved for licensure by the United States Food and Drug Administration based on animal efficacy studies compared to placebo.
Methods
We describe USG-sponsored pre- and postlicensure studies that compared efficacy of 3 antitoxins in a New Zealand White rabbit model of inhalation anthrax; survival following a lethal aerosolized dose of B. anthracis spores was the key measure of effectiveness. To model therapeutic intervention, intravenous treatments were started following onset of antigenemia.
Results
In pre- and postlicensure studies, all 3 antitoxins were superior to placebo; in the postlicensure study, raxibacumab and obiltoxaximab were superior to AIGIV, but neither was superior to the other.
Conclusions
These data illustrate the relative therapeutic benefit of the 3 antitoxins and provide a rationale to prioritize their deployment.
Keywords: Inhalation anthrax, antitoxin, Bacillus anthracis, protective antigen, New Zealand white rabbit
Pre- and postlicensure inhalation anthrax studies compared efficacy of a polyclonal and 2 monoclonal antibodies against protective antigen in New Zealand White rabbits. Each antitoxin administered at antigenemia onset improved outcomes over placebo. Monoclonal antibodies were superior to polyclonal.
Bacillus anthracis, the etiologic agent of anthrax, is a gram-positive, rod-shaped, aerobic and/or facultative anaerobic, spore-forming bacterium. The cutaneous form of anthrax has a low mortality rate with treatment [1]. The gastrointestinal and inhalation forms have much higher mortality, even with treatment [2, 3].
Germinated B. anthracis spores express virulence factors, including a phagocytosis-resistant capsule, and 3 toxin proteins: protective antigen (PA), lethal factor (LF), and edema factor (EF). PA and LF combine to form lethal toxin (LT), and PA and EF to form edema toxin (ET) [4]. LT severely reduces antigen-specific T- and B-cell immunity [5], effectively disarming the immune system [6]. ET promotes edema of the liver, intestine, and skin [4]. Together, they can lead to shock and death.
Each B. anthracis bacterium can form an environmentally stable endospore [7] that can be mass-produced, aerosolized, and used to deliberately infect large populations that inhale them [8]. Following the 2001 civilian attacks in the United States (US), where mortality approached 50% for the inhalation anthrax cases, the United States government (USG) re-emphasized countermeasure development for inhalation anthrax, including development of antibody-based antitoxins. Because inhalation anthrax cases are rare and randomized controlled human clinical trials would be unethical to conduct, development of countermeasures such as anthrax antitoxins relies on the US Food and Drug Administration (FDA) Animal Rule [9]. New Zealand white (NZW) rabbits [10–12] are 1 of 2 animal models developed to evaluate candidate countermeasures for efficacy in B. anthracis aerosol challenge studies.
This article describes National Institutes of Health (NIH)– and Biomedical Advanced Research and Development Authority (BARDA)–sponsored studies that were conducted to evaluate the efficacy of antitoxin products in NZW rabbits. The NIH-sponsored studies were performed at Battelle Biomedical Research Center (BBRC) and the University of Texas Medical Branch (UTMB) from 2013 through 2016, and the BARDA-sponsored studies were performed at BBRC from 2015 through 2016.
The NIH studies evaluated 5 investigational antitoxins in their early development. Mechanisms of action for the 5 products are outlined in Table 1 [13–19]. Two of the antitoxins evaluated by NIH were later FDA-approved: the monoclonal antitoxins raxibacumab [20] and obiltoxaximab [21]. Animal efficacy data for the other 3 investigational products—a polyclonal anthrax immune globulin intravenous (AIGIV-Anthrivig), MDX-1303, and AVP-21D9—in the NIH studies are described in the text of the Supplementary Materials.
Table 1.
Antitoxins Evaluated in the United States Government–Funded Pre- and Postlicensure Studies
| Generic Name | Study Sponsora | Product Name/Brand Name | Also Known As | FDA Approval | Manufacturer | Description | Mechanism of Action |
|---|---|---|---|---|---|---|---|
| Raxibacumab | NIH/BARDA | … | … | 2012 | Emergent Biosolutions | … | Prevents binding of PA to anthrax toxin receptor, disrupting formation of the PA heptamer plasma membrane pore, impeding internalization of LT and ET [13] |
| Obiltoxaximab | NIH/BARDA | Anthim | … | 2016 | Elusys Therapeutics | … | Prevents binding of PA to anthrax toxin cellular receptors, preventing formation of the PA plasma membrane pore and blocking internalization of lethal factor and edema factor [14] |
| AIGIV | BARDA | Anthrasil | … | 2015 | Emergent Biosolutions | Purified human immune globulin from BioThrax-vaccinated donors | Toxin-neutralizing antibodies directed at PA; act by preventing or impeding internalization of LT and ET [15, 16] |
| AIGIV | NIH | Anthrivig | … | … | Emergent Biosolutions | Purified human immune globulin from BioThrax-vaccinated donors | Toxin-neutralizing antibodies directed at PA; act by preventing or impeding internalization of LT and ET [15, 16] |
| ... | NIH | Valortim | MDX-1303 | … | Pharmathene, Inc. | … | Tethers PA, free or bound, to a cell surface receptor, impeding attachment to the anthrax toxin receptor and internalization [17] |
| ... | NIH | … | AVP-21D9 | … | Emergent Biosolutions | … | Binds to PA63 and disrupts formation of the heptamer pore [18, 19] |
Abbreviations: AIGIV, anthrax immunoglobulin intravenous; BARDA, Biomedical Advanced Research and Development Authority; ET, edema toxin; FDA, United States Food and Drug Administration; LT, lethal toxin; NIH, National Institutes of Health; PA, protective antigen.
The NIH sponsored the prelicensure studies and BARDA sponsored the postlicensure studies.
BARDA funded a head-to-head efficacy study of 3 approved antitoxins, which was performed in 2 phases: Phase 1 compared AIGIV-Anthrasil [22] and raxibacumab; phase 2 compared obiltoxaximab and raxibacumab. The results of each phase were combined and statistically compared. This article presents survival and morbidity data from the pre- and postlicensure studies that illustrate their relative therapeutic benefit. Collectively these data depict the products’ relative effectiveness in the NZW rabbit model and can be used to inform antitoxin prioritization for treatment.
METHODS
NIH Prelicensure Studies
Study Locations
The study was performed in 2 phases at separate locations. Phase 1 evaluated raxibacumab and was performed at BBRC, located in West Jefferson, Ohio. Phase 2 evaluated obiltoxaximab and was performed at UTMB in Galveston, Texas.
Animals and Animal Husbandry and Monitoring
For phase 1, 88 specific-pathogen-free NZW rabbits (Oryctolagus cuniculus; 50% male, 50% female) weighing 2.5–4.5 kg were procured by BBRC without regard to age from Covance (Denver, Pennsylvania) in 3 shipments. All had preplaced, surgically implanted vascular access ports (VAPs) and temperature and activity transmitters. The animals were split into 4 test groups and an untreated control group with 16 animals each; there were 8 extra animals. Only the 16 raxibacumab and 16 untreated animals for phase 1 are discussed here; the other 48 animals are described in the Supplementary Materials (Text, Tables 1-3, and Figure 1). For phase 2, 26 NZW rabbits (50% male, 50% female) weighing 3.0–3.45 kg were purchased without regard to age from Covance. All had preimplanted VAPs. Ten animals were used to test obiltoxaximab and 6 were untreated controls. The remaining 10 rabbits are described in the Supplementary Materials (Text, Table 1, and Figure 2). In both phases, identities were confirmed by ear tag number before and after each procedure. VAP patency was maintained to ensure sterility for blood draws and antitoxin and placebo administration. Blood was collected from VAPs 4 times a day for 3 days, then weekly from day 7 to 28.
In phase 1, each animal was observed for clinical signs of infection between 24 hours postchallenge to B. anthracis and up to 96 hours (4 days) postchallenge. Animals were observed every 6 ± 2 hours beginning about 12 Pm on day 1 postchallenge and ending approximately 12 Pm on day 4 postchallenge. Beginning at 96 hours postchallenge, animals were observed twice a day until day 28 postchallenge. In phase 2, each animal was observed at least 4 times daily for the first 14 days, then twice daily for the remainder of the 28-day period. In compliance with US Department of Agriculture guidelines, all phase 1 and 2 rabbits were housed individually in stainless steel cages with automatic watering systems.
Serum samples were promptly evaluated by PA-electrochemiluminescent (PA-ECL) assay for antigenemia, the trigger for treatment [23].
Anthrax Challenge
Prior to the start of the BBRC study, the original 88 rabbits were randomized into 5 groups of 16 animals each and 1 extra group of 8 (50% male, 50% female). For phase 1, animals were exposed, muzzle only, to a lethal challenge of 200 median lethal dose (LD50) (2 × 107) B. anthracis Ames spores. The total accumulated tidal volume of each animal and the spore concentrations measured during aerosolization were used to estimate the aerosol volume needed to achieve the target dose. For phase 2, the challenge dose and exposure methodology resembled those used in phase 1 (Table 2).
Table 2.
Prelicensure Study Metrics for the Evaluation of Raxibacumab and Obiltoxaximab in Rabbits Exposed to a Lethal Challenge
| Phase | Arm | No. | % Male | Mean Weight, kga (min–max) | Mean Spore Challenge, LD50 | Start Trigger, Hours Postchallenge | Antitoxin | Nonsurvivors, Mean Time to Death, h (min–max) | Survivors, n | |
|---|---|---|---|---|---|---|---|---|---|---|
| Dose | Route | |||||||||
| 1 | Raxibacumab | 16 | 50 | 3.3 (2.8–3.6) | 180 | 28.8b | 20 mg/kg (2.5 mL/kg) | Single IV bolus injection/VAP | 84 (54–115) | 10 |
| 1 | Placebo | 16 | 50 | 3.3 (3.0–3.8) | 193 | 29.2b | 3.6 mL/kg | Slow IV infusion | 80 (56–121) | 0 |
| 2 | Obiltoxaximab | 10 | 50 | 3.2 (3.0–3.5) | 275 | NDc | 20 mg/kg in 2 mL | Single IV bolus injection/VAP | 42 (36–48) | 8 |
| 2 | Placebo | 6 | 50 | 3.3 (2.9–3.5) | 291 | NDc | 2 mL | Single IV bolus injection/VAP | 70 (36–96) | 0 |
Abbreviations: IV, intravenous; LD50, median lethal dose; ND, not done; VAP, vascular access port.
Animals weighed on study day –1 (Battelle) or day of challenge (University of Texas Medical Branch).
Median time from challenge to abnormal.
Obiltoxaximab group: protective antigen (PA) detected approximately 19.8 hours postchallenge; placebo group: PA detected approximately 21 hours postchallenge; treatment initiated 3–4 hours postantigenemia.
Randomization and Treatment
Animals at BBRC were randomized to treatment groups based on their shipment. Animals in each phase were randomly assigned to test or control groups. For phase 1, raxibacumab was provided by the Centers for Disease Control and Prevention (CDC) and administered in a blinded fashion at a dose of 20 mg/kg as a single bolus. Toxin-neutralizing assays (TNA), rather than individual antitoxin assays, were used to assess the dose accuracy of antitoxin solutions for each animal [24].
For phase 2, obiltoxaximab (as a stock solution) was diluted in 0.9% sodium chloride, and administered at a dose of 20 mg/kg as a single bolus. For this study, dosages were not blinded, and dose concentrations were not confirmed. In both phases, placebo (0.9% sodium chloride for injection) was administered at a dose of either 2.5 mL/kg or 3.6 mL/kg.
Necropsy and Histopathology
For phase 1, complete gross necropsies were performed on animals that succumbed to challenge or that were euthanized after either being found moribund or surviving to day 28. Necropsies were performed by a board-certified pathologist blind to animal assignment. Formalinized sections of at least brain, lungs, liver, spleen, and mediastinal lymph nodes were examined. The findings were graded on a semi-quantitative scale of 1–4 for severity (with 1 being minimal changes and 4 being maximal), and scores were averaged for each lesion by test group. Animals in phase 2 were not necropsied.
Statistical Testing
For both phases 1 and 2, statistical significance of time-to-death Kaplan–Meier plots between the test groups and their respective control groups was determined using a pairwise, log-rank test adjusted for multiple comparisons with a Bonferroni correction. No statistical testing was performed across the 2 phases.
BARDA Postlicensure Studies
Study Location
The study, including the histopathology, was performed in 2 phases at BBRC. Phase 1 compared AIGIV-Anthrasil and raxibacumab, and phase 2 evaluated obiltoxaximab efficacy.
Animals and Animal Husbandry and Monitoring
For phase 1, 160 specific-pathogen-free NZW rabbits (Oryctolagus cuniculus; 50% male, 50% female) weighing 2.5–5.0 kg were procured without regard to age from Covance. All had preplaced VAPs and temperature transponders. Of the 160, 148 were used in the experiment, and the remainder were kept as potential replacements. For phase 2, 102 rabbits with similar characteristics were procured from Covance. For both phases, identities were confirmed by ear tag number before and after each procedure. Rabbits were housed in individual steel cages with ad libitum access to water, placed on a 12-hour light/dark cycle, and fed Purina Mills rabbit chow. VAP patency was maintained to ensure sterility for blood draws and antitoxin and placebo administration.
Animals were visually observed every 6 hours for the first 4 days postchallenge, after which they were observed twice a day. Temperatures were measured twice a day. To test for bacteremia and PA, blood was collected from VAPs on days 7, 14, and 28 postchallenge; 4 times a day prior to treatment; shortly after the 12-, 24-, 48-, and 72-hour timepoints posttreatment; and in surviving animals at terminal euthanasia. PA was assessed by PA-ECL in fresh serum samples soon after collection and by PA enzyme-linked immunosorbent assay (PA-ELISA) in batched frozen (≤−70°C) samples [23].
Anthrax Challenge
Animals in both phases were randomized into groups of 24–25 (∼50% male) for challenge, then randomly assigned a challenge day and challenge order. Aqueous B. anthracis was aerosolized [23]. Rabbits were exposed, muzzle only, to a target aerosol dose of 200 LD50 of B. anthracis Ames spores [10]. The aerosol challenge duration was based on an estimated aerosol challenge concentration and a cumulative minute volume that was gathered in “real time” throughout the exposure.
Randomization and Treatment
Using Stata statistical software, blocks of 11–15 animals in each phase were individually randomized to antitoxin or placebo by a statistician who was not otherwise involved in the study.
For phase 1, AIGIV-Anthrasil was provided by the sponsor and administered in a blinded fashion at a dose of 15 TNA units per animal. Raxibacumab was provided by the sponsor, diluted using 0.9% sodium chloride, and administered in a blinded fashion at the FDA-approved dose of 40 mg/kg. For phase 2, obiltoxaximab was provided by the sponsor, diluted with 0.9% sodium chloride, and administered in a blinded fashion at the FDA-approved dose of 16 mg/kg. In both phases, placebo (0.9% sodium chloride for injection) was administered in a blinded fashion at a dose of 4.7 mL/kg.
Necropsy and Histopathology
Complete gross necropsies were performed on animals that succumbed to challenge or that were euthanized after either being found moribund or surviving to day 28. Necropsies were performed by a board-certified pathologist blind to animal assignment and included examination of the external surface of the body; all orifices; and the cranial, thoracic, and peritoneal cavities and their contents. Formalinized sections were examined for at least brain, lungs, liver, spleen, and mediastinal lymph nodes.
Statistical Testing
Statistical analyses were conducted with SAS (version 9.3) except for LASSO logistic regression modeling, which was performed using R (version 3.1.3). To assess whether animals in each group were similar, descriptive statistics were calculated for sex, weight, quantitative bacteremia, PA-ELISA, and PA-ECL. Geometric means and 95% confidence intervals (CIs) were calculated for challenge dose. Values less than the limit of detection (LOD) or the lower limit of quantification (LLOQ) were replaced with one-half of the LOD or LLOQ, respectively.
Survival proportions and 95% Clopper–Pearson CIs were calculated for each group, and Fisher exact tests were used to assess differences in the proportions of animals surviving antitoxins compared to placebo. For each group, Kaplan–Meier median time to death (hours) and 95% CIs were calculated. Log-rank tests were used to determine differences among the groups in terms of time to death. This survival analysis was repeated after excluding nonbacteremic animals.
Time from end of challenge to positive bacteremia, PA-ELISA, and PA-ECL; time to treatment; and time to death were calculated for each animal, and differences were assessed using log-rank tests. Geometric means, 95% CIs, and Clopper–Pearson 95% CIs were calculated for bacteremia and PA-ELISA. Clopper-Pearson CIs were also calculated for PA-ECL. Analysis of variance models were fitted to the log-transformed quantitative bacteremia or PA-ELISA data with an effect for group. The models were used to test for significant group effects and Tukey multiple comparison procedure was used to determine which pairs differed significantly. A Fisher's exact test was used to identify differences in the proportions of animals that were bacteremic or positive for PA-ELISA or PA-ECL.
RESULTS
NIH Prelicensure Studies
Descriptive Epidemiology for Rabbit Populations
Baseline characteristics such as sex and weight for the 88 rabbits in phase 1 and the 26 rabbits in phase 2 are summarized in Table 2. No differences were observed between treatment groups.
Aerosol Challenge
Mean spore challenge values were calculated by treatment group for each phase (Table 2). For phase 1, the mean spore challenge for all animals in all groups was 187 ± 9 LD50. For phase 2, the mean spore challenge for all animals in all groups was 283 ± 11 LD50.
Start of Treatment
Treatment was initiated within 3–4 hours of antigenemia onset via intravenous bolus or slow infusion based on animal weights the day prior to exposure.
Primary Outcomes
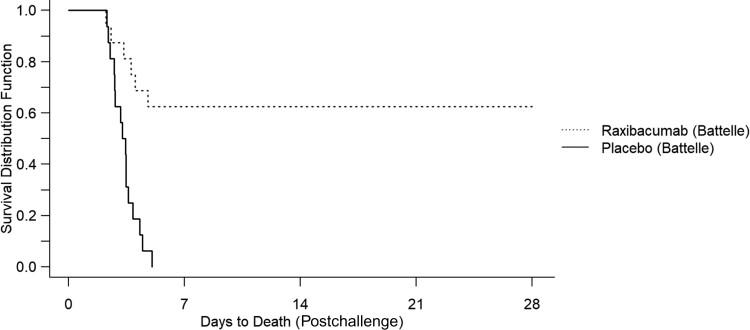
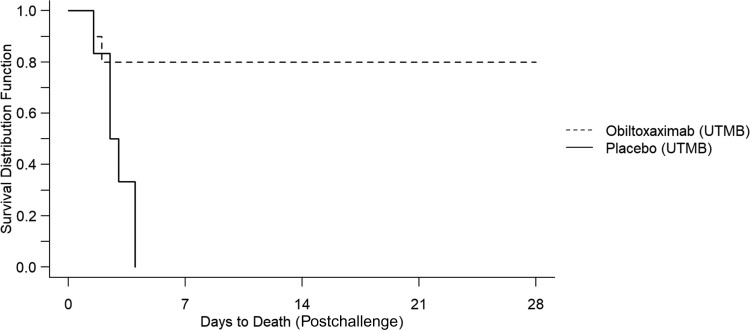
Table 2 displays the proportions of animals surviving anthrax challenge with each treatment option. In both phases, all placebo-treated animals died. In phase 1, 10 of 16 animals treated with raxibacumab survived (Figure 1). In phase 2, 8 of 10 animals treated with obiltoxaximab survived (Figure 2).
Figure 1.
Kaplan–Meier curve representing time-to-death and survival data for raxibacumab against inhalation anthrax in rabbits.
Figure 2.
Kaplan–Meier curve representing time-to-death and survival data for obiltoxaximab against inhalation anthrax in rabbits. Abbreviation: UTMB, University of Texas Medical Branch.
Statistical Comparisons for Primary Outcomes
In phase 1, survival in the raxibacumab arm exceeded that of the placebo arm (P = .0002). In phase 2, survival in the obiltoxaximab arm exceeded that of the placebo arm (P = .024) (Table 3).
Table 3.
Statistical Comparisons of Survival by Antitoxin in Prelicensure Studies
| Phase | Arm | No. | Nonsurvivors, Mean ± SD Time to Death, h (min–max) | Survivors, n (%) |
P Values for Survival Comparisons vs Placebo (P < .05) |
|---|---|---|---|---|---|
| 1 | Raxibacumab | 16 | 83 ± 23 (54–115) | 10 (63) | .00032a |
| 1 | Placebo | 16 | 80 ± 19 (56–121) | 0 (0) | |
| 2 | Obiltoxaximab | 10 | 42 ± 8 (36–48) | 8 (80) | .024b |
| 2 | Placebo | 6 | 70 ± 23 (36–96) | 0 (0) |
Abbreviation: SD, standard deviation.
Time-to-death pairwise log-rank test analysis with Bonferroni correction; without Bonferroni correction, P = .00008.
Time-to-death pairwise log-rank test with Bonferroni correction, P = .05 is statistically significant; without Bonferroni correction, P = .006.
Secondary Outcomes (Histopathology) for Phase 1
Lesions, including microscopic findings, observed in rabbits found dead or euthanized due to morbidity were consistent with anthrax as the cause of death (Table 4 [male] and Table 5 [female]). Most findings were graded as minimal to mild. Moderate-to-marked lesions of mediastinal lymph nodes were noted in both males and females for raxibacumab- and placebo-treated groups.
Table 4.
Histopathologic Examination and Scoring of Microscopic Observations in Challenged Male Rabbits That Died or Were Euthanized in Phase 1 of the Prelicensure Study, by Antitoxin Type
| Tissue | Observation | Animals Scored per Group/Average Severitya | ||
|---|---|---|---|---|
| AIGIV (7 Examined) | Raxibacumab (4 Examined) | Placebo (8 Examined) | ||
| Brain | Bacteria | 7/1.4 | 3/1.7 | 6/1.5 |
| Fibrin | 2/1.5 | 2/1.0 | 1/1.0 | |
| Hemorrhage | 2/2.5 | 3/1.3 | 2/1.0 | |
| Inflammation | 2/1.5 | 1/2.0 | 1/1.0 | |
| Necrosis | 2/2.0 | 1/1.0 | 0/0.0 | |
| Vasculitis | 2/1.5 | 1/1.0 | 0/0.0 | |
| Liver | Bacteria | 4/1.0 | 1/1.0 | 5/1.4 |
| Degeneration/necrosis | 3/1.3 | 4/1.3 | 4/1.5 | |
| Infiltrate, cellular | 1/1.0 | 1/1.0 | 1/1.0 | |
| Inflammation | 2/1.0 | 0/0.0 | 3/1.0 | |
| Leukocytosis | 5/1.0 | 3/1.0 | 7/1.0 | |
| Lungs | Bacteria | 5/1.4 | 1/1.0 | 6/1.8 |
| Edema | 2/1.5 | 3/1.0 | 5/1.4 | |
| Fibrin | 3/1.7 | 3/1.0 | 5/1.0 | |
| Hemorrhage | 2/1.5 | 1/1.0 | 3/1.0 | |
| Inflammation | 5/1.6 | 4/1.3 | 7/1.4 | |
| Mineralization | 1/1.0 | 0/0.0 | 0/0.0 | |
| Thrombosis | 1/2.0 | 0/0.0 | 0/0.0 | |
| Lymph nodes/mediastinal | Bacteria | 5/1.6 | 3/1.7 | 6/3.3 |
| Fibrin | 7/1.7 | 4/2.8 | 8/2.6 | |
| Hemorrhage | 5/2.0 | 4/1.8 | 7/1.7 | |
| Infiltrate, cellular | 0/0.0 | 0/0.0 | 1/1.0 | |
| Inflammation | 4/1.0 | 3/1.0 | 7/1.1 | |
| Necrosis | 6/1.7 | 4/3.3 | 8/3.0 | |
| Spleen | Bacteria | 5/1.6 | 4/1.3 | 6/2.0 |
| Fibrin | 4/2.3 | 4/2.3 | 7/1.6 | |
| Hemorrhage | 5/1.4 | 3/1.7 | 5/1.6 | |
| Necrosis | 7/2.1 | 4/2.5 | 7/2.0 | |
| Pigment | 3/1.3 | 2/1.0 | 3/1.0 | |
Abbreviation: AIGIV, anthrax immunoglobulin intravenous.
Severity score: 1, minimal (least detectable lesion); 2, mild (easily discernable lesion); 3, moderate (change affecting a large area); 4, marked (lesion that approached maximal).
Table 5.
Histopathologic Examination and Scoring of Microscopic Observations in Challenged Female Rabbits That Died or Were Euthanized in Phase 1 of the Prelicensure Study, by Antitoxin Type
| Tissue | Observation | Animals Scored per Group/Average Severitya | ||
|---|---|---|---|---|
| AIGIV (6 Examined) | Raxibacumab (2 Examined) | Placebo (8 Examined) | ||
| Brain | Bacteria | 6/1.7 | 2/1.0 | 8/1.6 |
| Fibrin | 2/2.0 | 2/1.5 | 0/0.0 | |
| Hemorrhage | 3/2.0 | 2/1.5 | 3/1.7 | |
| Inflammation | 2/2.5 | 2/3.5 | 1/1.0 | |
| Necrosis | 2/2.5 | 2/1.5 | 0/0.0 | |
| Vasculitis | 2/1.5 | 2/1.0 | 0/0.0 | |
| Liver | Bacteria | 3/1.0 | 1/1.0 | 6/1.3 |
| Degeneration/necrosis | 6/1.5 | 0/0.0 | 5/1.4 | |
| Hemorrhage | 0/0.0 | 0/0.0 | 1/1.0 | |
| Inflammation | 3/1.0 | 0/0.0 | 2/1.0 | |
| Leukocytosis | 6/1.0 | 2/1.0 | 7/1.0 | |
| Lungs | Bacteria | 4/1.8 | 0/0.0 | 8/2.0 |
| Edema | 4/1.0 | 0/0.0 | 3/1.7 | |
| Fibrin | 4/1.0 | 2/2.0 | 5/1.6 | |
| Hemorrhage | 1/1.0 | 1/3.0 | 1/2.0 | |
| Inflammation | 6/1.5 | 2/2.5 | 7/1.3 | |
| Vasculitis | 0/0.0 | 0/0.0 | 0/0.0 | |
| Thrombosis | 2/1.0 | 2/1.0 | 2/1.0 | |
| Lymph nodes/mediastinal | Bacteria | 5/2.2 | 0/0.0 | 8/3.9 |
| Fibrin | 5/1.8 | 2/3.0 | 8/2.5 | |
| Hemorrhage | 3/2.0 | 2/1.5 | 8/2.1 | |
| Inflammation | 2/1.5 | 1/1.0 | 7/1.3 | |
| Necrosis | 6/2.0 | 2/3.0 | 8/3.3 | |
| Spleen | Bacteria | 6/1.3 | 1/1.0 | 8/2.8 |
| Fibrin | 5/1.6 | 2/1.0 | 6/2.0 | |
| Hemorrhage | 4/1.5 | 1/1.0 | 6/1.3 | |
| Necrosis | 6/1.7 | 2/2.0 | 8/2.4 | |
| Pigment | 4/1.5 | 4/1.5 | 5/1.2 | |
Abbreviation: AIGIV, anthrax immunoglobulin intravenous.
Severity score: 1, minimal (least detectable lesion); 2, mild (easily discernable lesion); 3, moderate (change affecting a large area); 4, marked (lesion that approached maximal).
BARDA Postlicensure Studies
Descriptive Epidemiology for Rabbit Populations
Baseline characteristics such as sex and weight for the 148 rabbits in phase 1 and the 102 rabbits in phase 2 are summarized in Table 6. No significant differences were observed between the 3 groups given antitoxins.
Table 6.
Postlicensure Study Metrics for the Evaluation of Anthrax Immunoglobulin Intravenous, Raxibacumab, and Obiltoxaximab in Rabbits Exposed to a Lethal Challenge
| Phase | Arm | No. | % Male | Mean ± SD Weight, kg2 | Mean ± SD Spore Challenge, LD50 | Antitoxin | Nonsurvivors, Median Time to Death, h (min–max) | Survivors, n (Proportion) | |
|---|---|---|---|---|---|---|---|---|---|
| Dose | Route | ||||||||
| 1 | AIGIV- Anthrasil | 68 | 51 | 3.1 ± 0.24 | 230 ± 43 | 15 U/animal (4.7 mL/animal) | Slow IV infusion | 110 (94–143) | 15 (22) |
| 1 | Raxibacumab | 68 | 51a | 3.2 ± 0.28b | 230 ± 46 | 40 mg/kg (4.7 mL/kg) | Slow IV infusion | (106, NC) | 35 (51) |
| 1 | Placebo | 12 | 30c | 3.2 ± 0.33d | 232 ± 48 | 4.7 mL/kg | Slow IV infusion | 86 (47–107) | 0 (0) |
| 2 | Raxibacumab | 26 | 51a | 3.2 ± 0.28b | 195 ± 51 | 40 mg/kg (4.7 mL/kg) | Slow IV infusion | (77, NC) | 15 (58) |
| 2 | Obiltoxaximab | 68 | 53 | 3.3 ± 0.24 | 184 ± 56 | 16 mg/kg (4.7 mL/kg) | Slow IV infusion | (117, NC) | 39 (57) |
| 2 | Placebo | 8 | 30c | 3.2 ± 0.33d | 182 ± 65 | 4.7 mL/kg | Slow IV infusion | 75 (56–106) | 0 (0) |
Abbreviations: AIGIV, anthrax immunoglobulin intravenous; IV, intravenous; LD50, median lethal dose; SD, standard deviation; NC, not calculable.
Percentage male administered raxibacumab was only provided for phase 1 and 2 combined.
Mean weight for rabbits administered raxibacumab was only provided for phase 1 and 2 combined.
Percentage male administered placebo was only provided for phase 1 and 2 combined.
Mean weight for rabbits administered placebo was only provided for phase 1 and 2 combined.
Aerosol Challenge
Mean spore challenge values were calculated by treatment group for each phase (Table 6). For phase 1, the mean spore challenge for all animals in all groups was 230 ± 44 LD50. For phase 2, the mean spore challenge for all animals in all groups was 187 ± 55 LD50.
Start of Treatment
Antitoxin treatment was initiated within 3 hours of confirming antigenemia and the dose used was based on animal weights the day prior to exposure. In both phases, most (58%–88%) animals had detectable PA and almost all were bacteremic by 30 hours postchallenge.
Primary Outcomes
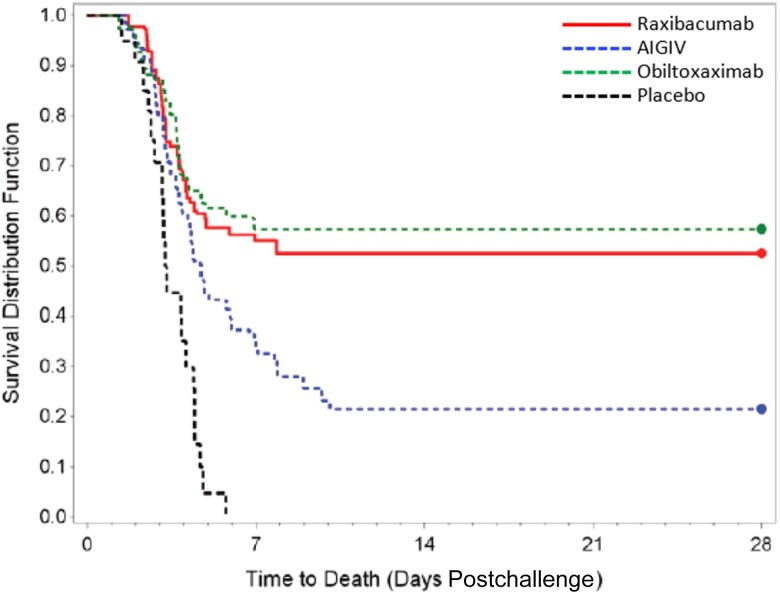
Table 6 displays the percentage of animals surviving anthrax challenge by treatment option. In both phases, all placebo-treated animals died. Just over a fifth of the animals (22%) treated with AIGIV-Anthrasil survived. Similar proportions of animals treated with raxibacumab (51% for phase 1 and 58% for phase 2) and obiltoxaximab (57%) survived (Figure 3).
Figure 3.
Kaplan–Meier curve representing time-to-death and survival data by group for all animals. Abbreviation: AIGIV, anthrax immunoglobulin intravenous.
Statistical Comparisons for Primary Outcomes
Because there were no significant differences in the percentage of animals that survived nor their times to death between the 2 phases, the raxibacumab and placebo arms for phase 1 and 2 were combined for statistical purposes (Table 7). Survival in the AIGIV-Anthrasil arm was higher than the combined placebo arms (P = .02). Survival in the combined raxibacumab arms was higher than the combined placebo arms (P < .0001) and the AIGIV-Anthrasil arm (P = .0007). Survival in the obiltoxaximab arm was higher than the combined placebo arms (P = <.0001) and the AIGIV-Anthrasil arm (P = .0004). Finally, obiltoxaximab and raxibacumab had similar survival (P = .64). Excluding nonbacteremic animals (n = 3) did not change these results. There was a statistical difference in time to positive PA test between the phase 1 and phase 2 raxibacumab, but not placebo, arms. This may be due to small sample size. There was no difference in challenge dose between phase 1 and phase 2 (P = .9021).
Table 7.
Statistical Comparisons of Survival by Antitoxin in Postlicensure Studies
| Phase | Arm | No. | Nonsurvivors, Median Time to Death, h (min–max) | Survivors, No. (Proportion [95% CI]) | P Values for Survival Comparisons | |||
|---|---|---|---|---|---|---|---|---|
| Versus Placeboa | Versus Placebob | Versus AIGIVb | Versus Raxibacumabb | |||||
| 1 | AIGIV-Anthrasil | 68 | 110 (94–143) | 15 (0.22 [.13–.34]) | .0185 | .0001 | ||
| 1, 2 | Raxibacumabc | 94 | (117, NC) | 50 (0.53 [.43–.64]) | <.0001 | <.0001 | .0007 | |
| 2 | Obiltoxaximab | 68 | (117, NC) | 39 (0.57 [.45–.69]) | <.0001 | <.0001 | .0004 | .6405 |
| 1, 2 | Placeboc | 20 | 78.3 (63.4–106.1) | 0 (0.00 [.00–.17]) | ||||
Abbreviations: AIGIV, anthrax immunoglobulin intravenous; CI, confidence interval; NC, not calculable.
Fisher's exact tests.
Log-rank tests.
Values shown are phase 1 and 2 combined.
Secondary Outcomes (Histopathology)
Regardless of treatment, all lesions observed in nonsurviving animals were consistent with anthrax as a cause of death. Microscopic lesions in numerous tissues from both phase 1 and 2 studies included heterophilic inflammation, fibrin exudation, hemorrhage, edema, necrosis, and/or lymphoid depletion. Most animals had large bacilli in at least 1 organ. Some antitoxin-treated animals that succumbed had no visible bacteria in their tissues.
Death was generally attributed to sepsis (indicated by inflammatory lesions in multiple organs) or brain inflammation/necrosis. Brain lesions (inflammation, necrosis, and hemorrhage) affected 64% of the animals in the raxibacumab arm, 62% in the obiltoxaximab arm, 32% in the AIGIV-Anthrasil arm, and 40% in the placebo arm. No rabbits surviving to scheduled termination had lesions suggestive of anthrax.
DISCUSSION
The high fatality in the 2001 anthrax letter attacks spurred the development of new treatments to improve survival. Studied treatments included therapies that targeted LT and ET. Four monoclonal antibodies (mAbs) and 2 polyclonal antibodies, each directed at PA, the protein factor common to both toxins, were developed and evaluated for efficacy in experimentally challenged NZW rabbits. This article describes efficacy data for 2 of the 4 above-mentioned mAbs (raxibacumab and obiltoxaximab) and 1 polyclonal antibody (AIGIV-Anthrasil), all of which are FDA-approved therapies. The Supplementary Materials describe efficacy data for the other 2 mAbs (MDX-1303 and AVP-21D9) and the other polyclonal antibody (AIGIV-Anthrivig), all of which are unapproved therapies.
Rabbits in NIH-sponsored prelicensure studies were challenged with lethal doses of B. anthracis Ames spores (2 × 107 colony-forming units). Raxibacumab (20 mg/kg) and obiltoxaximab (20 mg/kg) administered at the onset of antigenemia protected 63% and 80% of NZW rabbits, respectively; none of the placebo-treated animals survived. The FDA-approved adult doses of raxibacumab and obiltoxaximab are 40 mg/kg and 16 mg/kg, respectively. The raxibacumab dose in the prelicensure study was 50% lower than the approved dose, while the obiltoxaximab dose was 25% higher than the approved dose. These 2 preliminary studies demonstrated these antitoxins provide significant survival benefits in animals.
To inform policy decisions for the deployment of antitoxins in an anthrax emergency, BARDA compared relative antitoxin efficacies through postlicensure studies designed to mimic the operational use of the products. The products were diluted to ensure that equal volumes (4.7 ml/kg) that aligned with approved human dosing were administered to each animal. All 3 products were administered by slow infusion so that the time to maximum concentration for each would be similar and mimic human dosing. Treatment was synchronized across the arms so that each modality was given to similarly ill animals; treatment was started once PA was detected in the bloodstream. The animals and treatment order were randomized, and staff were blinded to the treatment. The groups were demographically similar and received similar challenge doses, suggesting that the results could be pooled for analysis. Raxibacumab and obiltoxaximab were twice as effective as AIGIV-Anthrasil, and the mAb product efficacies were comparable to one another. Almost two-thirds (>60%) of animal fatalities treated with mAbs had brain lesions; brain lesions were less common in animals treated with AIGIV-Anthrasil or placebo (<40%). This supports the notion that antitoxins would likely be ineffective once B. anthracis has crossed the blood-brain barrier.
The greater efficacy observed for mAbs vs polyclonal therapies agrees with previous findings for Ebola and coronavirus disease 2019 (COVID-19). Clinical trials using polyclonal serum for patients with either Ebola or COVID-19 showed survival advantage if used early in infection but no advantage when used later in patients with severe cases [25]. In contrast, monoclonal cocktails (Regeneron EB-3 or mAb114 for Ebola and various products from Regeneron, Eli Lilly, etc, for COVID-19) provided a survival advantage when used after the onset of severe symptoms [26, 27].
Because a limited amount of human protein can be administered to rabbits, a palliative effect of a higher AIGIV-Anthrasil dose may have been missed. The polyclonal product AIGIV comprises an antibody pool from individuals vaccinated with anthrax vaccine adsorbed and has a panoply of antibodies against a variety of antigens. However, antibodies against PA only constitute a small percentage of the total human antibody pool. In contrast, mAbs are composed solely of antibodies against PA. Given the difference in drug composition, product-specific release criteria (a set of criteria with predefined specifications that a drug substance or drug product must meet by testing to be considered acceptable for its intended use), and dosing, it is difficult to directly compare the potency among these products. However, a TNA assay [24] performed by the CDC has estimated the neutralizing activity per FDA-approved adult dose to be 1.4- and 7-fold higher with raxibacumab and obiltoxaximab, respectively, than with AIGIV-Anthrasil. Although not evaluated in the comparative animal efficacy studies we describe here, the approved AIGIV-Anthrasil dosing allows discretionary doubling of the dose for patients with severe disease [20]. Doubling the AIGIV-Anthrasil dose would theoretically reduce the difference in estimated neutralizing activity between the mAbs and AIGIV-Anthrasil by half.
CONCLUSIONS
The 3 FDA-approved antitoxins were evaluated in rabbit models of anthrax under similar conditions. Each antitoxin, administered at the onset of antigenemia, resulted in improved survival over placebo. In the prelicensure study, both mAbs were superior to placebo, but neither was superior to the other. In the postlicensure study, all 3 antitoxins were superior to placebo and the mAbs were superior to AIGIV-Anthrasil, but neither mAb was superior to the other.
In summary, polyclonal antibodies are more efficacious than the placebo, and mAbs are more efficacious than polyclonal antibodies. Separate studies have demonstrated that antitoxins administered with antimicrobials provide additional benefit over antimicrobials alone and that antitoxins do not interfere with antimicrobials [28, 29]. Overall, the data suggest that mAbs should be deployed first in the event of an exposure, in conjunction with antibiotics.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. R. S. thanks Mr Eric Chu of Leidos for providing statistical analysis and generation of some the Kaplan-Meier curves used in this manuscript.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or other USG agencies, including the US Army.
Financial support. This project was supported by the Centers for Disease Control and Prevention and the Office of the Assistant Secretary for Preparedness and Response.
Supplement sponsorship. This article appears as part of the supplement “Anthrax Preparedness,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest . The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Supplementary Material
Contributor Information
Raymond M Slay, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Bethesda, Maryland, USA.
Rachel Cook, Oak Ridge Institute for Science and Education, CDC Fellowship Program, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine Hendricks, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
David Boucher, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Washington, District of Columbia, USA.
Michael Merchlinsky, Office of the Assistant Secretary for Preparedness and Response, Biomedical Advanced Research and Development Authority, Washington, District of Columbia, USA.
References
- 1. Davies JC. A major epidemic of anthrax in Zimbabwe. The experience at the Beatrice Road Infectious Diseases Hospital, Harare. Cent Afr J Med 1985; 31:176–180. [PubMed] [Google Scholar]
- 2. Beatty ME, Ashford DA, Griffin PM, Tauxe RV, Sobel J. Gastrointestinal anthrax: review of the literature. Arch Intern Med 2003; 163:2527–31. [DOI] [PubMed] [Google Scholar]
- 3. Holty J-EC, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 2006; 144:270–80. [DOI] [PubMed] [Google Scholar]
- 4. Moayeri M, Leppla SH, Vrentas C, Pomerantsev A, Liu S. Anthrax pathogenesis. Ann Rev Microbiol 2015; 69:185–208. [DOI] [PubMed] [Google Scholar]
- 5. Leysath CE, Monzingo AF, Maynard JA, et al. Crystal structure of the engineered neutralizing antibody M18 complexed to domain 4 of the anthrax protective antigen. J Mol Biol 2009; 387:680–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu S, Moayeri M, Leppla S. Anthrax lethal and edema toxins in anthrax pathogenesis. Trends Microbiol 2014; 22:317–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ngetich W. Review of anthrax: a disease of animals and humans. Intl J Agric Environ Bioresearch 2019; 4:123–34. [Google Scholar]
- 8. Goel AK. Anthrax: a disease of biowarfare and public health importance. World J Clin Cases 2015; 3:20–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hanna KE. Extraordinary measures for countermeasures to terrorism: FDA's “Animal Rule”. Hastings Cent Rep 2002; 32(4):9. [PubMed] [Google Scholar]
- 10. Zaucha GM, Pitt ML, Estep J, Ivins B, Friedlander AM. The pathology of experimental anthrax in rabbits exposed by inhalation and subcutaneous inoculation. Arch Pathol Lab Med 1998; 122:982–92. [PubMed] [Google Scholar]
- 11. Twenhafel NA. Pathology of inhalational anthrax animal models. Vet Pathol 2010; 47:819–30. [DOI] [PubMed] [Google Scholar]
- 12. Gutting B. Deterministic models of inhalational anthrax in New Zealand white rabbits. Biosecur Bioterror 2014; 12:29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kummerfeldt CE. Raxibacumab: potential role in the treatment of inhalational anthrax. Infect Drug Resist 2014; 7:101–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Leysath CE, Monzingo AF, Maynard JA, et al. Crystal structure of the engineered neutralizing antibody M18 complexed to domain 4 of the anthrax protective antigen. J Mol Biol 2009; 387:680–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Malkevich NV, Basu S, Rudge TL Jr, et al. Effect of anthrax immune globulin on response to BioThrax (anthrax vaccine adsorbed) in New Zealand white rabbits. Antimicrob Agents Chemother 2013; 57:5693–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Mytle N, Hopkins RJ, Malkevich NV, et al. Evaluation of intravenous anthrax immune globulin for treatment of inhalation anthrax. Antimicrob Agents Chemother 2013; 57:5684–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Wang F, Ruther P, Jiang I, et al. Human monoclonal antibodies that neutralize anthrax toxin by inhibiting heptamer assembly. Hum Antibodies 2004; 13:105–10. [PubMed] [Google Scholar]
- 18. Riddle V, Leese P, Blanset D, Adamcio M, Meldorf M, Lowy I. Phase I study evaluating the safety and pharmacokinetics of MDX-1303, a fully human monoclonal antibody against Bacillus anthracis protective antigen, in healthy volunteers. Clin Vaccine Immunol 2011; 18:2136–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vitale L, Blanset D, Lowy I, et al. Prophylaxis and therapy of inhalational anthrax by a novel monoclonal antibody to protective antigen that mimics vaccine-induced immunity. Infect Immun 2006; 74:5840–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. US Food and Drug Administration . Full prescribing information: raxibacumab. Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2021/125349s026lbl.pdf. Accessed 1 March 2022.
- 21. US Food and Drug Administration . Full prescribing information: Anthim (obiltoxaximab). Available at: www.accessdata.fda.gov/drugsatfda_docs/label/2019/125509s015lbl.pdf.
- 22. US Food and Drug Administration . Full prescribing information: Anthrasil (anthrax immune globulin intravenous [human]). Available at: www.fda.gov/media/91577. Accessed 1 March 2022.
- 23. Henning LN, Comer JE, Stark GV, et al. Development of an inhalational Bacillus anthracis exposure therapeutic model in cynomolgus macaques. Clin Vaccine Immunol 2012; 19:1765–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li H, Soroka SD, Taylor TH, et al. Standardized, mathematical model-based and validated in vitro analysis of anthrax lethal toxin neutralization. J Immunol Methods 2008; 333:89–106. [DOI] [PubMed] [Google Scholar]
- 25. Thijssen M, Devos T, Ejtahed H-S, Amini-Bavil-Olyaee S, Pourfathollah AA, Pourkarim MR. Convalescent plasma against COVID-19: a broad-spectrum therapeutic approach for emerging infectious diseases. Microorganisms 2020; 8:1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mahase E. Covid-19: Regeneron's antibody combination cuts deaths in seronegative patients, trial finds. BMJ 2021; 373:n1570. [DOI] [PubMed] [Google Scholar]
- 27. Joyner MJ, Rickey EC, Senefeld JW, Klassen SA. Convalescent plasma antibody levels and the risk of death from Covid-19. N Engl J Med 2021; 384:1015–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Migone T-S, Bolmer S, Zhong J, et al. Added benefit of raxibacumab to antibiotic treatment of inhalational anthrax. Antimicrob Agents Chemother 2015; 59:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kammanadiminti S, Patnakikuni RK, Comer J, Meister G, Sinclair C, Kodihalli S. Combination therapy with antibiotics and anthrax immune globulin intravenous (AIGIV) is potentially more effective than antibiotics alone in rabbit model of inhalational anthrax. PLoS One 2014; 9:e106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.