Abstract
The gut microbiota has recently gained attention due to its association with cardiovascular health, cancers, gastrointestinal disorders, and non-communicable diseases. One critical question is how the composition of the microbiota contributes to cardiovascular diseases (CVDs). Insightful reviews on the gut microbiota, its metabolites and the mechanisms that underlie its contribution to CVD are limited. Hence, the aim of this review was to describe linkages between the composition of the microbiota and CVD, CVD risk factors such as hypertension, diet, ageing, and sex differences. We have also highlighted potential therapies for improving the composition of the gut microbiota, which may result in better cardiovascular health.
Subject terms: Physiology, Cardiovascular diseases
Introduction
Humans are surrounded, both externally and internally, by a diverse range of microbes which profoundly affect wellbeing by interacting with skin, respiratory, and digestive systems. They self-organize, quickly mold to their changing environment and develop a complex ecosystem within an otherwise uninhabitable niche. The human halobiont is a very diverse assembly of microbial species which makes a singular functional unit [1]. The gastrointestinal tract harbors a complex community of over 100 trillion microbial cells that influence human physiology, metabolism, nutrition, and immune function. Therefore, the gut microbiota is considered a singular functional unit sometimes termed ‘metabolic organ’ [2]. Some research estimates suggest that human gut possesses ~1000 bacterial species with 100-fold more genes than those found in the human genome [3, 4].
The gut microbiota can exert healthy benefits as well as pathological effects on human health [5, 6]. Physiological functions of the microbiota include metabolism of food, fermentation of indigestible food, synthesis of vitamins, and forming an epithelial barrier and barricade against pathogenic bacteria [7]. Dysbiosis, a term referring to changes in the composition of the microbiota and its metabolites has been suggested to play a pivotal role in propagating inflammatory and metabolic diseases including gastrointestinal disorders, cancers, cardiovascular disease (CVD) [6], atherosclerosis, hypertension, kidney disease, heart disease, obesity, type 2 diabetes mellitus, and inflammatory bowel disease (Fig. 1) [5, 8]. Several metabolic pathways may mediate the pathogenic effects of an altered microbiota, including the trimethylamine (TMA)/trimethylamine N-oxide (TMAO) and the bile acids pathways [8]. TMAOs have been associated with increased risk for CVD [9]. In the last decade, the relationship between the microbiota and cardiovascular disease has become a major topic of interest. This review presents a detailed and comprehensive overview of the published literature in the last decade regarding some of the mechanisms, recent advances, diagnostic approaches, and clinical implications of the gut microbiota in contributing towards CVD.
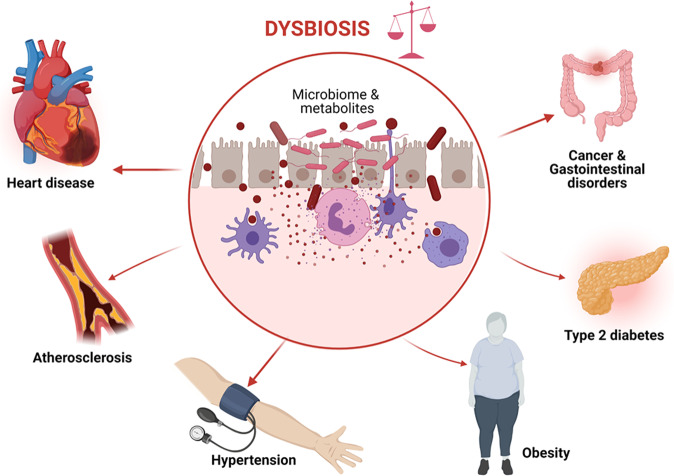
Fig. 1. Diseases associated with dysbiosis.
Abnormal changes in the composition of the microbiota (dysbiosis) is positively associated with pathogenesis and propagation of heart disease, atherosclerosis, hypertension, obesity, type 2 diabetes mellitus, cancer, and gastrointestinal disorders.
Gut microbiota, atherosclerosis and CVDs
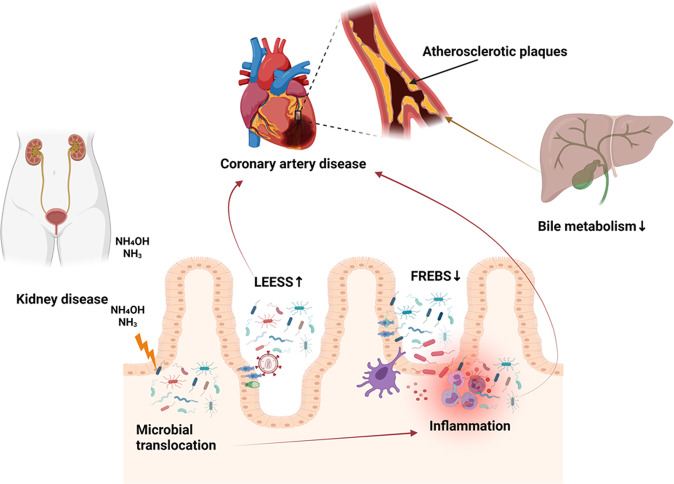
The source of many of the microorganisms that have been associated with atherosclerotic plaques, endothelial dysfunction, and resulting CVDs is their translocation from the gut into the systemic circulation. Metabolites produced by the microbiota may also promote kidney injury as they are concentrated and excreted in the kidney [10]. Conditions that increase microbial translocation from the gut, such as HIV infection, overproduction of TMAOs and urea have been linked to systemic inflammation, heart failure, and hypertension [11]. Microbial urease leads to overproduction of waste products, such as ammonia and ammonium hydroxide, which are especially important in patients with chronic kidney disease (CKD), whose urea excretion is already compromised [12]. Overproduction of ammonia and ammonium hydroxide disrupt the tight junctions between intestinal epithelial cells resulting in further enhancement of microbial translocation and systemic inflammation [8, 9]. While the precise mechanism by which the microbiota contributes to atherosclerosis remains unknown, dysbiosis has been consistently associated with a leaky gut, with abnormalities of lipid and glucose metabolism that are associated with inflammation, and with the size of atherosclerotic plaques, which ultimately contribute to the development and progression of CVD and to its prognosis [13]. It has been shown that atheromatous plaques of patients with coronary artery disease (CAD) contain pathogenic Staphylococcus species, Proteus vulgaris, Klebsiella pneumoniae, and Streptococcus species [7]. Their guts exhibit an increase in Lactobacillus, Streptococcus, Esherichia, Shigella and Enterococcus species, concomitant with a reduction in Faecalibacterium, Subdoligranulum, Roseburia, Eubacterium rectale and Bacteroides fragilis species, the latter group known to regulate T-cell functions in the gut mucosa with consequent anti-inflammatory effects and protection of the gut barrier [7, 14]. In patients at high risk for stroke, there is a reduction in butyrate-producing bacteria such as those of the Lachnospiraceae and Ruminococcaceae family, resulting in reduced fecal butyrate levels and concomitant increases in intestinal pathogens such as those of the Enterobacteriaceae and Veillonellaceae family [7]. Whether microbiota has a direct role in the pathogenesis of other CVDs such as abdominal aortic aneurysm (AAA) or peripheral artery disease (PAD) is yet unknown but likely, since they contribute to inflammatory processes and colonization of atheromatic plaques in blood vessels, thereby enhancing the progression of various atherosclerotic processes (Fig. 2). More studies are required to understand the mechanisms and so devise future therapeutic interventions. For example, reduced bile acid synthesis by a dysbiotic microbiota has been shown to decrease the amount of cholesterol eliminated via feces, with increases in absorption and plasma levels of low-density lipoproteins. This may be an additional mechanism that contributes to increased risk for atherosclerosis and CVD in subjects with a dysbiosis [7, 9, 15].
Fig. 2. Microbiota’s contribution to atherosclerosis and CVD.
Ammonia (NH3) and ammonium hydroxide (NH4OH) resulting from kidney disease or the action of microbial urease and HIV infection in the gut contributes to microbial translocation and systemic inflammation. Microbes colonize atherosclerotic plaques enhancing progression of various atherosclerotic processes. Dysbiosis contributes to decreased bile formation that results in decreased cholesterol elimination and increased plasma levels of low-density lipoproteins. LEESE Lactobacillus, Esherichia, Enterococcus, Shigella, and Streptococcus, FREBS Faecalibacterium, Roseburia, Eubacterium rectale, Bacteroides fragilis, and Subdoligranulum.
Gut microbiota and hypertension
The pathophysiology of hypertension involves various contributing factors including genetic, lifestyle, environmental, hormonal, inflammatory, and hemodynamic changes. Mounting evidence from human and animal studies suggests that gut microbiota play an indispensable function in the regulation of blood pressure [16–29]. The evidence for an association between gut microbiota and hypertension emanates from studies in murine models showing that rats lacking normal gut flora experience elevated blood pressure [29, 30]. Moreover, alterations in the composition of fecal microbiota have been linked to modulation of blood pressure and poor response to antihypertensive drugs [8]. Alpha diversity is the parameter that reflects microbial diversity within a particular ecosystem, as captured in a biological sample. Reduced alpha diversity of the microbiota has been identified in hypertensive patients. [17–25, 28]. Similar trends were observed in obesity, hyperinsulinemia, and dyslipidemia. Moreover, studies in humans demonstrated an association between a higher abundance of Gram-negative microbiota including Klebsiella, Parabacteroides, Desulfovibrio, and Prevotella and higher blood pressure levels, but not all studies confirmed this pattern [16, 18, 21, 26]. The cross-sectional HELIUS cohort study (HEalthy Life In an Urban Setting study) demonstrated positive correlations between Klebsiella spp. and Streptococcaceae spp. and blood pressure [24], and confirmed the results from previous studies [25, 26]. A causal relationship is suggested by experiments with fecal microbiota transplantation (FMT). It was clearly shown that germ-free (GF) mice, which received FMT from a hypertensive patient not only developed a similar gut microbiota as that of the donor, but also elevated systolic and diastolic blood pressures after 8 weeks when compared with GF mice that received FMT from normotensive donors [22]. Also, stroke-prone SHRs (spontaneously hypertensive rats) harbor a dysbiotic gut microbiota that differs significantly from that of normotensive WKY (Wistar-Kyoto) control rats. FMT from SHRs into WKY controls increased the systolic blood pressure of these otherwise normotensive rats [29]. Additional studies in Dahl salt-sensitive rats [31], angiotensin II infused mice [32], high salt treated mice [17], and deoxycorticosterone acetate-salt hypertensive mice [33] demonstrated that all these hypertensive animal models exhibit dysbiosis. Santisteban et al. recently showed that SHRs exhibit the pathophysiological changes and disrupted integrity of the gut epithelium, characteristic of other forms of dysbiosis [34]. Finally, it has been shown that abnormal intestinal permeability and dysbiosis can be reversed by treatment with the antihypertensive agent losartan [35], suggesting that the relationship between dysbiosis and blood pressure may be bidirectional.
Several studies have implicated high salt in contributing to the dysbiosis of both human and experimental animals. A seminal study from Muller and colleagues demonstrated that high salt treatment depleted Lactobacillus murinus from the gut microbiota, resulting in an increase in TH 17 cells and salt-sensitive hypertension, findings that were replicated in a pilot study in humans [17]. Since high salt depleted Lactobacillus spp. and raised blood pressure both in human and animals, this study indicates that the link between gut microbiota and hypertension is not species-specific. Interestingly, other studies demonstrated that either reduced salt or increasing Lactobacillus spp with probiotic treatment improved blood pressure regulation, arterial compliance, vascular function, and insulin sensitivity [17, 36, 37]. An elegant systematic review and meta-analysis of randomized, controlled trials showed that probiotics containing Lactobacillus spp are effective in blood pressure regulation if used in sufficient amount for at least 8 weeks [38].
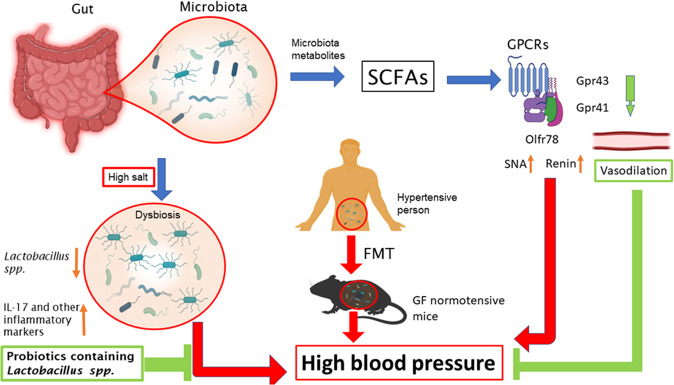
Short-chain fatty acids (SCFAs) resulting from microbiota metabolism have been linked to blood pressure mediated by G-protein coupled receptor (GPCR) pathways in renin secretion and blood pressure regulation [39]. Olfactory receptor (Olfr) 78 and GPR41 free fatty acid receptor stimulation by SCFA results in elevated and decreased BP, respectively [8]. SCFAs such as acetate and propionate produced by gut microbiota have antihypertensive effects by decreasing systemic inflammation and atherosclerotic lesions which are independent predictors of hypertension [39]. A composition of the microbiota characterized by abundant Lactobacilli is known to have BP lowering effects. Other SCFA produced by the gut microbiota such as lactate and butyrate also have a significant impact on BP through vasodilation and vasoconstriction mediated by GPR43, GPR41, and Olfr 78 [39]. A summary of the relationship between the gut microbiota and blood pressure is illustrated in Fig. 3.
Fig. 3. Gut microbiota and high blood pressure.
Microbiota metabolites SCFAs modulate distinct GPCRs and thereby affect blood pressure. For example, activation of Gpr43 and 41 results in vasodilation and blood pressure attenuation. In contrast, activation of olfr78 increases SNA and renin secretion resulting in blood pressure elevation. Moreover, high salt depletes lactobacillus spp. causing dysbiosis and activation of inflammatory immune response by releasing IL-17 and other inflammatory signaling molecules consequently causing blood pressure elevation. FMT is strong evidence to show that gut microbiota plays an indispensable role in the contribution of high blood pressure. SCFAs short-chain fatty acids, GPCRs G protein-coupled receptors, SNA sympathetic nerve activity, FMT fecal microbiota transplantation, GF germ free.
Although research data indicate a great potential to target the gut microbiota in contributing to treatment of hypertension by using probiotics, changing lifestyle, and diet, further research is warranted to better understand the role of various gut microbial species and their metabolites in the regulation of blood pressure and associated diseases. Further, it would be interesting to understand the interaction among environmental factors, gut microbial species, and blood pressure regulation.
Dietary lifestyle, microbiota, and CVD
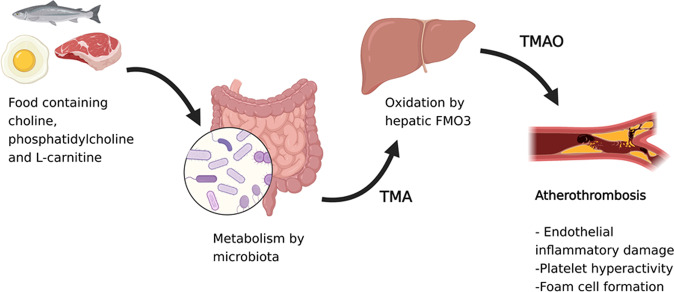
Evidence suggest that diet potentially modulates the gut microbiota by regulating the balance between pathogenic and beneficial microbes or microbial products [40]. Vegetarian diets foster a beneficial microbiota composition by increasing Prevotella enterotype whereas diets high in animal protein foster Bacteroides enterotype and other species associated with proatherogenic metabolites and CVD [40, 41]. The production of the proatherogenic metabolite TMAO, resulting from TMA oxidation by the liver enzyme flavin monooxygenase 3 and its release into the systemic circulation have been linked to coronary plaques, peripheral artery disease, the severity of CVD, and its complications including stroke, myocardial infarction, and death [42, 43]. Common dietary nutrients possessing a TMA moiety, such as the choline, phosphatidylcholine, and l-carnitine present in red meat, fish, and eggs after microbial metabolism, are the main contributors of TMAO-mediated effects that promote artherosclerosis [9]. The underlying mechanisms by which TMAO contributes to CVD remain unknown. However, preliminary evidence suggests that TMAO stimulates inflammatory pathways with activation of cells of the innate immunity response that propagate atherosclerosis. Also TMAO interferes with platelet function through stimulus-dependent calcium signaling, promoting atherothrombotic events (Fig. 4) [44].
Fig. 4. TMAO biosynthesis and metabolism.
Choline, phosphatidylcholine, and l-carnitine found in fish, red meat and eggs are metabolized into TMA by colonic microbiota. The TMA that enters the systemic circulation is oxidized into TMAO by FMO3 in the liver, which is released back into the circulation, leading to platelet and inflammatory pathway activation. Inflammatory injury in the endothelium, along with increased foam cell formation and platelet activation, contributes to the progression of atherosclerosis and development of atherothrombotic events.
Diets rich in fiber such as whole grains increase the acetate-producing Bifidobacteriaceae, which are protective against pathogenic bacteria, lower blood pressure, improve insulin sensitivity, and decrease cardiac hypertrophy and fibrosis [8, 45]. Polyphenols, a large class of aromatic compounds found in plant-based beverages have been shown to improve cardiovascular health through their antiplatelet and anti-inflammatory actions, and by inducing nitric oxide formation in blood vessels, promoting vasodilation and improving gut microbiota with increased Firmicutes and decreased Bacteroides. Quercetin, a member of the subclass of flavonoid polyphenols increases the abundance of Bacteroides vulgatus and Akkermansia muciniphila and concomitantly reduces Eubacterium cylindroides and Bilophilia wadsworthia to reduce the risk for diet-induced obesity which is a risk factor for CVD and hypertension. Quercetin also improves cellular energy homeostasis, fatty acid oxidation, and availability of nitric oxide by upregulating adenosine monophosphate-activated protein kinase (AMPK) expression.
Diet induced alterations in the gut microbial composition may also trigger disease states via immune activation. Regulatory T cells (Tregs) are essential immune cells to maintain immunologic self-tolerance that are categorized into two; thymus-derived and peripherally derived Tregs [46]. Importantly, SCFAs, especially butyrate, are known to induce the differentiation of peripherally derived Tregs in the colon, through G-protein coupled receptors [47]. This process is crucial to limit inflammatory activation. Furthermore, SCFAs are essential nutrients for Tregs as well as colonic epithelial cells [48]. Therefore, reduced consumption of fermentable dietary fibers may decrease colonic Treg population and predispose to chronic inflammatory states by reducing the abundance of SCFA-forming bacteria [49].
There is increasing evidence showing the link between high dietary salt and hypertension by modulation of the composition and function of the gut microbiota [50, 51]. Additional blood pressure phenotypes termed as salt sensitive and salt resistant blood pressure have an impact on the magnitude of the effect of salt on the gut microbiota. Excess dietary salt alters the gut microbiota and activates dendritic cells that in turn activates T cells and stimulate production of interleukin 17 (IL-17), tumor necrosis factor alpha (TNF-α) and interferon gamma (IFN-γ) leading to hypertension [51]. A high-salt diet may likely also disrupt the gut barrier, which results in systemic inflammation, insulin resistance, and increased blood pressure [52]. These studies indicate a link between the diet and the gut microbiota. However, more studies are needed to understand the underlying mechanisms.
Sex differences in cardiovascular events and microbiota
Sex differences have been proposed to account for differential effects of gut microbiota and resulting metabolite effects on cardiovascular health owing to hormonal regulation and differences in dietary intake between males and female adults [39]. Sex differences in the composition of gut microbiota have been shown in both animal and human studies, although inconsistently. Using two different mice strains, Elderman et al. [53] showed that male mice had lower microbial diversity than female counterparts, with almost 12% of the variance in diversity explained by sex. Similarly, analysis of 89 different mice strains revealed significant differences in microbial composition between the sexes, although the direction of the change in each strain varied, suggesting that the influence of sex on microbiota may depend on the animal overall genotype [54].
Human studies showed similar inconsistencies. In the Human Microbiome Project Consortium males had decreased Bacteriodes and increased Prevotella populations [55], whereas in other studies, these findings were not replicated [56]. The Belgian Flemish Gut Flora Project and the Dutch LifeLines-DEEP study revealed that sex had the 10th effect size among the 69 factors shown to associate significantly with overall microbiota variation [56]. Investigation of cohorts from different ethnicities did not reveal a consistent sex-dependent microbial composition [56]. Therefore, whether sex effects on the microbiota are a determinant of the different cardiovascular risk profiles in men and women is a question awaiting answers in future studies.
The intersection between aging and the gut microbiota in cardiovascular disease
Aging is associated with adverse cardiovascular health regardless of one’s biological sex and race [57]. The primary acquisition of microbiota occurs by exposure to the maternal one and this is heavily influenced by birth mode. The microbiota later matures, starting to resemble an adult microbiota as early as 2 years of age. During aging, there is additional exposure to external microbes through diet and contact with other environment factors such as farm animals and pets [58]. This maturation of the gut microbiota influences the development of the immune system and in some individuals it may provide an imprint for increased risk of inflammatory diseases such as inflammatory bowel syndrome, obesity, and hypertension [59]. Aging is also associated with reduction in bacterial diversity, unusual phylum proportions and decline in health promoting bacteria species [60, 61]. Specifically, the aging microbiota has been characterized by a reduction in the Firmicutus:Bacteroidetes ratio [62, 63] and by overpopulation of facultative anaerobes [64]. In conventionally housed mice, microbial dysbiosis, intestinal permeability, and circulating bacterial products increase with age, whereas these changes are not observed in germ-free mice, which live longer [65]. FMT from old donors is sufficient to induce phenotypes associated with aging in young recipients. For example, FMT from aged mice increases fat body mass, and food consumption, thus inducing an obesogenic phenotype in previously healthy adult mice [66]. Interestingly, metformin attenuates obesity in old mice by increasing mucin production and goblet cell mass in the gut. Metformin-induced improvement in gut health leads to decreased low-grade inflammation, a very important phenomenon seen in the elderly population that has been named inflammaging [67, 68]. Indeed, whereas healthy microbiota is associated with attenuation of markers of inflammation [64], old microbiota induces differential regulation of pathways including T cell differentiation, B-cell development, and recognition of microbes by pattern recognition receptors in young mice, further supporting a role of the gut microbiota in inflammation [69]. TMAO supplementation induces an aging-like endothelial dysfunction via reduced nitric oxide bioavailability and increased superoxide-driven oxidative stress in young mice [70]. Since TMAO and p-cresylsulfate are eliminated through the kidney [71], the age-related decline in kidney function, which is seen in both men and women, may exacerbate the systemic accumulation of these metabolites further enhancing pathways that lead to cardiovascular disease [72]. Age-associated inflammation is a risk factor for adverse cardiovascular events, thus, therapeutic approaches that target the gut microbiota may be a potential approach to promote healthy aging.
Diagnostic and research approaches on gut microbiota state
Although the gut microbiota is too numerous to characterize, a few analytical tools are available to aid in the study of specific organisms of interest to disease. Metagenomic analysis is one of the powerful tools currently used to reconstruct microbial species and their function by examining genetic sequences. Quantification of TMAO in systemic blood is helpful in assessing CVD severity and complications. For example, elevated TMAO is present in patients with stable PAD and is a significant predictor of acute coronary syndrome, stroke, and death [7], in some cases independent of traditional risk factors [7]. In addition to TMAO, plasma levels of choline and betaine are elevated in patients with chronic heart failure. The pathogenic mechanisms of TMAO in heart failure have been previously described [11].
Drugs and the microbiota
Certain microbial species in the gut can inactivate or lessen the potency of drugs prescribed to aid the management of CVDs. The therapeutic effects of statins are attenuated by abundant presence of Lactobacillus, Eubacterium, Faecalibacterium, and Bifidobacterium and decreased proportion of genus Clostridium [5], which renders these drugs relatively ineffective in decreasing LDL levels. Similarly, treatment of atrial fibrillation, atrial flutter, and heart failure using digoxin may not be efficacious when Eggerthella lenta strains are abundant, since they inactivate this drug [5]. Conversely, therapeutic drugs may alter the microbiota. For example, metformin, the glucose lowering drug used in diabetes mellitus treatment, cancers, CVD and other conditions increases the amount of pathogenic Escherichia-Shigella species [5].
Microbiota potential therapy targets to improve cardiovascular health
We have reviewed the evidence that diet has a profound impact on microbiota composition and consequently, disease. Specific vegetarian diets that foster a good microbiota environment that is protective against CVD are recommended as the mainstay to prevent or attenuate adverse cardiovascular effects via modulation of the gut microbiota [73]. Dietary supplementation with polyphenols is quite beneficial for cardiovascular health [8].
Caloric restriction and caloric restriction mimetics are emerging as additional tools to modulate the gut microbiota and thereby promote health. Intermittent fasting and molecules such as polyphenols and beta-hydroxybutyrate decrease blood pressure by modulating the gut microbiota and attenuating inflammatory pathways [74–77]. Intermittent fasting is associated with changes in the gut microbiota including enrichment of species of the genus Lactobacillus, Oscillospira, and Ruminococcus and reduction of species of the genus Akkermansia, Bacteroides, and Bifidobacterium [78]. Most notably, the resulting gut microbial changes are associated with changes in bile acid metabolism. Microbes are responsible for modifying primary bile acids, synthesized in the liver and released into the small intestine, to form secondary bile acids [79]. Bile acids activate receptors such as the farnesoid x receptor (FXR) and TGR5 to modulate inflammation, blood pressure and vascular function [80–83]. Intermittent fasting improves availability of bile acids, which are depleted in disease states, and attenuates hypertension [84–86]. Thus, available evidence supports modulating the gut microbiota via calorie restriction modalities that improve health through mechanisms such as bile acid signaling.
Administration of probiotics (live bacteria) may offer a protection against CVDs [73]. In murine models, administration of Lactobacillus plantarum, and Lactobacillus rhamnosus GR-1 mitigated the effects of left ventricular hypertrophy, heart failure and myocardial infarction [8]. Prebiotics, nondigestible food ingredients, are known to promote bifidobacterial species and acetate-producing bacteria, thus improve gut microbiota composition and cardiovascular health [39, 44, 50]. Acetate regulates many pathways related to cardiovascular health including the upregulation of early growth response protein 1 (Egr1) transcription factor that decreases inflammation, cardiac fibrosis, and hypertrophy [7].
FMT, commonly used in treatment of Clostridium difficile infection and in inflammatory bowel diseases like ulcerative colitis is another emerging technique that has also been targeted to mitigate CVD [7, 8]. Evidence that FMT improves the components of the metabolic syndrome has been already provided [7]. However, the risk for introducing pathogenic microbes and toxins and increasing the risk for other pathological processes is high. An example reviewed above is that FMT from a hypertensive to a germ-free mice induces hypertension in the latter [7]. Hence, implementation of microbiota transplantation is still a challenge that needs further investigations.
Targeted therapy against gut microbiota metabolites such as TMAO may prove to be helpful as the administration of 3,3-dimethyl-1-butanol which blocks the TMA/TMAO ameliorated the harmful effects of a high-sugar and high-fat Western die on cardiac health [7].
Clinical implications of gut microbiota and future aspects for research
It is clear that gut microbiota science has far-reaching clinical implications for management of CVDs. Diagnosis, prognosis, and monitoring of CVDs can be supplemented with characterization, quantification, and to a lesser extent, transplantation of specific gut microbiota and its metabolites. Dietary supplementation remains the safest probable method to improve the gut microbiota and its associated detrimental cardiovascular effects.
Conclusion
Dysbiosis increases the risk for various CVDs through several mechanisms. It is associated with microbial translocation from the gut into the interstitium and perivascular tissues resulting in systemic inflammation, abnormalities of lipid and glucose metabolism, atherosclerosis, and hypertension. Western diet and timing of feeding contribute to the risk for CVD by modulating the gut microbiota. High dietary salt intake contributes to dysbiosis and development of hypertension and increases the risk for various CVDs. Sex-dependent microbial composition is emerging as one of the risk factors for CVD. However, there is still scarcity of data on this subject and it warrants further investigation. Aging is associated with a decline in health-promoting bacteria species and with enhancement of metabolic pathways that lead to cardiovascular disease. Thus, the gut microbiota is intricately involved in various CVDs. Understanding this relationship is critical for future targeted therapy to prevent and improve CVDs and to ameliorate cardiovascular adverse events. Quantification of TMAOs is an important marker for prognosis of certain cardiovascular events such as stroke, heart attack and death. Use of pre- and probiotics and TMAO inhibitors, has great potential for future therapy in managing CVDs.
Summary
What is known about the topic
The gut microbiota affects cardiovascular health. Although the mechanisms are unknown, the composition of the gut microbiota modulates risk for cardiovascular disease.
Leaky gut is associated with inflammation. Microbial translocation elicits an inflammatory cascade that may exacerbate existing disease or induce cardiovascular diseases.
What this study adds
Dysbiosis is associated with increased risk for specific diseases. Abnormal composition of the gut microbiota is linked to the pathogenesis and propagation of heart disease, atherosclerosis, hypertension, obesity, type 2 diabetes mellitus, cancer, and gastrointestinal disorders.
High salt diet depletes lactobacillus spp. causing dysbiosis and activation of inflammatory immune response by releasing IL-17 and other inflammatory signaling molecules consequently causing blood pressure elevation.
Gut metabolites contribute to vascular injury and thrombotic events. Choline, phosphatidylcholine, and l-carnitine found in fish, red meat, and eggs are metabolized into compounds that activate platelets and activate inflammatory pathways resulting in endothelial injury, and contributing to the progression of atherosclerosis and development of atherothrombotic events.
Author contributions
SKM, BH, JH, VH, LAE, JI, SR, and MS wrote different sections of the manuscript. CLL and FE edited and reviewed the manuscript. AK conceptualized the study and framework and finalized the manuscript as well as obtained funding for the manuscript. All authors contributed to article reviews, edited and approved the final version of this manuscript.
Funding
This work was supported by the Fogarty International Center of the National Institutes of Health grants D43 TW009744 and D43 TW009337 (SKM), K01HL130497, R03HL155041, and R01HL144941 (AK). The content is solely the responsibility of the authors and does not represent the official views of the National Institutes of Health.
Data availability
Not applicable.
COMPETING INTERESTS
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Avery EG, Bartolomaeus H, Maifeld A, Marko L, Wiig H, Wilck N, et al. The gut microbiome in hypertension. Circulation Res. 2021;128:934–50. doi: 10.1161/CIRCRESAHA.121.318065. [DOI] [PubMed] [Google Scholar]
- 2.Guinane CM, Cotter PD. Role of the gut microbiota in health and chronic gastrointestinal disease: understanding a hidden metabolic organ. Ther Adv Gastroenterol. 2013;6:295–308. doi: 10.1177/1756283X13482996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ley RE, Peterson DA, Gordon JI. Ecological and evolutionary forces shaping microbial diversity in the human intestine. Cell. 2006;124:837–48. doi: 10.1016/j.cell.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 4.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao Y, Wang Z. Gut microbiome and cardiovascular disease. Curr Opin Cardiol. 2020;35:207–18. doi: 10.1097/HCO.0000000000000720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou W, Cheng Y, Zhu P, Nasser MI, Zhang X, Zhao M. Implication of gut microbiota in cardiovascular diseases. Oxid Med Cell Longev. 2020;2020:5394096. doi: 10.1155/2020/5394096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ahmad AF, Dwivedi G, O’Gara F, Caparros-Martin J, Ward NC. The gut microbiome and cardiovascular disease: current knowledge and clinical potential. Am J Physiol-Heart Circ Physiol. 2019;317:H923–H938.. doi: 10.1152/ajpheart.00376.2019. [DOI] [PubMed] [Google Scholar]
- 8.Tang WHW, Kitai T, Hazen SL. Gut microbiota in cardiovascular health and disease. Circ Res. 2017;120:1183–96. doi: 10.1161/CIRCRESAHA.117.309715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Witkowski M, Weeks TL, Hazen SL. Gut microbiota and cardiovascular disease. Circ Res. 2020;127:553–70. doi: 10.1161/CIRCRESAHA.120.316242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain A, Li XH, Chen WN. An untargeted fecal and urine metabolomics analysis of the interplay between the gut microbiome, diet and human metabolism in Indian and Chinese adults. Sci Rep. 2019;9:9191. doi: 10.1038/s41598-019-45640-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Y, Wang Y, Ke B, Du J. TMAO: how gut microbiota contributes to heart failure. Transl Res. 2021;228:109–25. doi: 10.1016/j.trsl.2020.08.007. [DOI] [PubMed] [Google Scholar]
- 12.Wong J, Piceno YM, DeSantis TZ, Pahl M, Andersen GL, Vaziri ND. Expansion of urease- and uricase-containing, indole- and p-cresol-forming and contraction of short-chain fatty acid-producing intestinal microbiota in ESRD. Am J Nephrol. 2014;39:230–7. doi: 10.1159/000360010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toubal A, Kiaf B, Beaudoin L, Cagninacci L, Rhimi M, Fruchet B, et al. Mucosal-associated invariant T cells promote inflammation and intestinal dysbiosis leading to metabolic dysfunction during obesity. Nat Commun. 2020;11:3755. doi: 10.1038/s41467-020-17307-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tuomisto S, Huhtala H, Martiskainen M, Goebeler S, Lehtimäki T, Karhunen PJ. Age-dependent association of gut bacteria with coronary atherosclerosis: Tampere Sudden Death Study. PLoS ONE. 2019;14:e0221345. doi: 10.1371/journal.pone.0221345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsui M, Fukunishi S, Nakano T, Ueno T, Higuchi K, Asai A. Ileal bile acid transporter inhibitor improves hepatic steatosis by ameliorating gut microbiota dysbiosis in NAFLD model mice. mBio. 2021;12:e0115521. doi: 10.1128/mBio.01155-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brantsæter AL, Myhre R, Haugen M, Myking S, Sengpiel V, Magnus P, et al. Intake of probiotic food and risk of preeclampsia in primiparous women: The Norwegian Mother and Child Cohort Study. Am J Epidemiol. 2011;174:807–15. doi: 10.1093/aje/kwr168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, et al. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–9. doi: 10.1038/nature24628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang T, Santisteban MM, Rodriguez V, Li E, Ahmari N, Carvajal JM, et al. Gut dysbiosis is linked to hypertension. Hypertension. 2015;65:1331–40. doi: 10.1161/HYPERTENSIONAHA.115.05315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Filippis F, Pellegrini N, Vannini L, Jeffery IB, La Storia A, Laghi L, et al. High-level adherence to a Mediterranean diet beneficially impacts the gut microbiota and associated metabolome. Gut. 2016;65:1812–21. doi: 10.1136/gutjnl-2015-309957. [DOI] [PubMed] [Google Scholar]
- 20.Dan X, Mushi Z, Baili W, Han L, Enqi W, Huanhu Z, et al. Differential analysis of hypertension-associated intestinal microbiota. Int J Med Sci. 2019;16:872–81. doi: 10.7150/ijms.29322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De la Cuesta-Zuluaga J, Mueller NT, Álvarez-Quintero R, Velásquez-Mejía EP, Sierra JA, Corrales-Agudelo V, et al. Higher fecal short-chain fatty acid levels are associated with gut microbiome dysbiosis, obesity, hypertension and cardiometabolic disease risk factors. Nutrients. 2019;11:51. doi: 10.3390/nu11010051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. doi: 10.1186/s40168-016-0222-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sun S, Lulla A, Sioda M, Winglee K, Wu MC, Jacobs DR, et al. Gut microbiota composition and blood pressure. Hypertension. 2019;73:998–1006. doi: 10.1161/HYPERTENSIONAHA.118.12109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Verhaar BJH, Collard D, Prodan A, Levels JHM, Zwinderman AH, Bäckhed F, et al. Associations between gut microbiota, faecal short-chain fatty acids, and blood pressure across ethnic groups: the HELIUS study. Eur Heart J. 2020;41:4259–67. doi: 10.1093/eurheartj/ehaa704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yan Q, Gu Y, Li X, Yang W, Jia L, Chen C et al. Alterations of the gut microbiome in hypertension. Front Cell Infect Microbiol. 2017. 10.3389/fcimb.2017.00381. [DOI] [PMC free article] [PubMed]
- 26.Kim S, Goel R, Kumar A, Qi Y, Lobaton G, Hosaka K, et al. Imbalance of gut microbiome and intestinal epithelial barrier dysfunction in patients with high blood pressure. Clin Sci. 2018;132:701–18. doi: 10.1042/CS20180087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huart J, Leenders J, Taminiau B, Descy J, Saint-Remy A, Daube G, et al. Gut microbiota and fecal levels of short-chain fatty acids differ upon 24-hour blood pressure levels in men. Hypertension. 2019;74:1005–13. doi: 10.1161/HYPERTENSIONAHA.118.12588. [DOI] [PubMed] [Google Scholar]
- 28.Jackson MA, Verdi S, Maxan M-E, Shin CM, Zierer J, Bowyer RCE, et al. Gut microbiota associations with common diseases and prescription medications in a population-based cohort. Nat Commun. 2018;9:2655. doi: 10.1038/s41467-018-05184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Adnan S, Nelson JW, Ajami NJ, Venna VR, Petrosino JF, Bryan RM, et al. Alterations in the gut microbiota can elicit hypertension in rats. Physiol Genomics. 2017;49:96–104. doi: 10.1152/physiolgenomics.00081.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yan X, Jin J, Su X, Yin X, Gao J, Wang X, et al. Intestinal flora modulates blood pressure by regulating the synthesis of intestinal-derived corticosterone in high salt-induced hypertension. Circ Res. 2020;126:839–53. doi: 10.1161/CIRCRESAHA.119.316394. [DOI] [PubMed] [Google Scholar]
- 31.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, et al. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–97. doi: 10.1152/physiolgenomics.00136.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaye DM, Shihata WA, Jama HA, Tsyganov K, Ziemann M, Kiriazis H, et al. Deficiency of prebiotic fiber and insufficient signaling through gut metabolite-sensing receptors leads to cardiovascular disease. Circulation. 2020;141:1393–403. doi: 10.1161/CIRCULATIONAHA.119.043081. [DOI] [PubMed] [Google Scholar]
- 33.Marques FZ, Nelson E, Chu P-Y, Horlock D, Fiedler A, Ziemann M, et al. High-fiber diet and acetate supplementation change the gut microbiota and prevent the development of hypertension and heart failure in hypertensive mice. Circulation. 2017;135:964–77. doi: 10.1161/CIRCULATIONAHA.116.024545. [DOI] [PubMed] [Google Scholar]
- 34.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, et al. Hypertension-linked pathophysiological alterations in the gut. Circulation Res. 2017;120:312–23. doi: 10.1161/CIRCRESAHA.116.309006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robles-Vera I, Toral M, Visitación N, de la, Sánchez M, Gómez-Guzmán M, Muñoz R, et al. Changes to the gut microbiota induced by losartan contributes to its antihypertensive effects. Br J Pharmacol. 2020;177:2006–23. doi: 10.1111/bph.14965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toral M, Gómez-Guzmán M, Jiménez R, Romero M, Sánchez M, Utrilla MP, et al. The probiotic Lactobacillus coryniformis CECT5711 reduces the vascular pro-oxidant and pro-inflammatory status in obese mice. Clin Sci. 2014;127:33–45. doi: 10.1042/CS20130339. [DOI] [PubMed] [Google Scholar]
- 37.Chen L, He FJ, Dong Y, Huang Y, Wang C, Harshfield GA, et al. Modest sodium reduction increases circulating short-chain fatty acids in untreated hypertensives. Hypertension. 2020;76:73–79. doi: 10.1161/HYPERTENSIONAHA.120.14800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Khalesi S, Sun J, Buys N, Jayasinghe R. Effect of probiotics on blood pressure. Hypertension. 2014;64:897–903. doi: 10.1161/HYPERTENSIONAHA.114.03469. [DOI] [PubMed] [Google Scholar]
- 39.Razavi AC, Potts KS, Kelly TN, Bazzano LA. Sex, gut microbiome, and cardiovascular disease risk. Biol Sex Differ. 2019;10:29. doi: 10.1186/s13293-019-0240-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, et al. Diet rapidly and reproducibly alters the human gut microbiome. Nature. 2014;505:559–63. doi: 10.1038/nature12820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kahleova H, Rembert E, Alwarith J, Yonas WN, Tura A, Holubkov R, et al. Effects of a low-fat vegan diet on gut microbiota in overweight individuals and relationships with body weight, body composition, and insulin sensitivity. A Randomized Clinical Trial. Nutrients. 2020;12:E2917. doi: 10.3390/nu12102917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bin Waleed K, Lu Y, Liu Q, Zeng F, Tu H, Wei Y, et al. Association of trimethylamine N-oxide with coronary atherosclerotic burden in patients with non-ST-segment elevation myocardial infarction. Medicine. 2020;99:e20794. doi: 10.1097/MD.0000000000020794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sheng Z, Tan Y, Liu C, Zhou P, Li J, Zhou J, et al. Relation of circulating trimethylamine N-oxide with coronary atherosclerotic burden in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2019;123:894–8. doi: 10.1016/j.amjcard.2018.12.018. [DOI] [PubMed] [Google Scholar]
- 44.Zhu Y, Li Q, Jiang H. Gut microbiota in atherosclerosis: focus on trimethylamine N‐oxide. APMIS. 2020;128:353–66. doi: 10.1111/apm.13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chleilat F, Schick A, Reimer RA. Microbiota changes in fathers consuming a high prebiotic fiber diet have minimal effects on male and female offspring in rats. Nutrients. 2021;13:820. doi: 10.3390/nu13030820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas S, Izard J, Walsh E, Batich K, Chongsathidkiet P, Clarke G, et al. The host microbiome regulates and maintains human health: a primer and perspective for non-microbiologists. Cancer Res. 2017;77:1783–812. doi: 10.1158/0008-5472.CAN-16-2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim CH, Park J, Kim M. Gut microbiota-derived short-chain fatty acids, T cells, and inflammation. Immune Netw. 2014;14:277–88. doi: 10.4110/in.2014.14.6.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Smith PM, Howitt MR, Panikov N, Michaud M, Gallini CA, Bohlooly-Y M, et al. The microbial metabolites, short-chain fatty acids, regulate colonic Treg cell homeostasis. Science. 2013;341:569–73. doi: 10.1126/science.1241165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vaziri ND, Liu S-M, Lau WL, Khazaeli M, Nazertehrani S, Farzaneh SH, et al. High amylose resistant starch diet ameliorates oxidative stress, inflammation, and progression of chronic kidney disease. PLoS ONE. 2014;9:e114881. doi: 10.1371/journal.pone.0114881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Naqvi S, Asar TO, Kumar V, Al-Abbasi FA, Alhayyani S, Kamal MA, et al. A cross-talk between gut microbiome, salt and hypertension. Biomed Pharmacother. 2021;134:111156. doi: 10.1016/j.biopha.2020.111156. [DOI] [PubMed] [Google Scholar]
- 51.Elijovich F, Laffer CL, Sahinoz M, Pitzer A, Ferguson JF, Kirabo A. The gut microbiome, inflammation, and salt-sensitive hypertension. Curr Hypertens Rep. 2020. 10.1007/s11906-020-01091-9. [DOI] [PMC free article] [PubMed]
- 52.Tanaka M, Itoh H. Hypertension as a metabolic disorder and the novel role of the gut. Curr Hypertens Rep. 2019;21:63. doi: 10.1007/s11906-019-0964-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Elderman M, Hugenholtz F, Belzer C, Boekschoten M, van Beek A, de Haan B, et al. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol Sex Differ. 2018;9:26. doi: 10.1186/s13293-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Org E, Mehrabian M, Parks BW, Shipkova P, Liu X, Drake TA, et al. Sex differences and hormonal effects on gut microbiota composition in mice. Gut Microbes. 2016;7:313–22. doi: 10.1080/19490976.2016.1203502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ding T, Schloss PD. Dynamics and associations of microbial community types across the human body. Nature. 2014;509:357–60. doi: 10.1038/nature13178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kim YS, Unno T, Kim B-Y, Park M-S. Sex differences in gut microbiota. World J Men’s Health. 2020;38:48–60. doi: 10.5534/wjmh.190009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.North BJ, Sinclair DA. The intersection between aging and cardiovascular disease. Circulation Res. 2012;110:1097–108. doi: 10.1161/CIRCRESAHA.111.246876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yatsunenko T, Rey FE, Manary MJ, Trehan I, Dominguez-Bello MG, Contreras M, et al. Human gut microbiome viewed across age and geography. Nature. 2012;486:222–7. doi: 10.1038/nature11053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishimwe JA. Maternal microbiome in preeclampsia pathophysiology and implications on offspring health. Physiol Rep. 2021;9:e14875. doi: 10.14814/phy2.14875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Claesson MJ, Cusack S, O’Sullivan O, Greene-Diniz R, Weerd H, de, Flannery E, et al. Composition, variability, and temporal stability of the intestinal microbiota of the elderly. Proc Natl Acad Sci USA. 2011;108:4586–91. doi: 10.1073/pnas.1000097107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woodmansey EJ, McMurdo MET, Macfarlane GT, Macfarlane S. Comparison of compositions and metabolic activities of fecal microbiotas in young adults and in antibiotic-treated and non-antibiotic-treated elderly subjects. Appl Environ Microbiol. 2004;70:6113–22. doi: 10.1128/AEM.70.10.6113-6122.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Biagi E, Franceschi C, Rampelli S, Severgnini M, Ostan R, Turroni S, et al. Gut microbiota and extreme longevity. Curr Biol. 2016;26:1480–5. doi: 10.1016/j.cub.2016.04.016. [DOI] [PubMed] [Google Scholar]
- 63.Mariat D, Firmesse O, Levenez F, Guimarăes V, Sokol H, Doré J, et al. The Firmicutes/Bacteroidetes ratio of the human microbiota changes with age. BMC Microbiol. 2009;9:123. doi: 10.1186/1471-2180-9-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Biagi E, Nylund L, Candela M, Ostan R, Bucci L, Pini E, et al. Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS ONE. 2010;5:e10667. doi: 10.1371/journal.pone.0010667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Thevaranjan N, Puchta A, Schulz C, Naidoo A, Szamosi JC, Verschoor CP, et al. Age-associated microbial dysbiosis promotes intestinal permeability, systemic inflammation, and macrophage dysfunction. Cell Host Microbe. 2017;21:455–.e4. doi: 10.1016/j.chom.2017.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Binyamin D, Werbner N, Nuriel-Ohayon M, Uzan A, Mor H, Abbas A, et al. The aging mouse microbiome has obesogenic characteristics. Genome Med. 2020;12:87. doi: 10.1186/s13073-020-00784-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ahmadi S, Razazan A, Nagpal R, Jain S, Wang B, Mishra SP, et al. Metformin reduces aging-related leaky gut and improves cognitive function by beneficially modulating gut microbiome/goblet cell/mucin axis. J Gerontol A Biol Sci Med Sci. 2020;75:e9–e21.. doi: 10.1093/gerona/glaa056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Calder PC, Bosco N, Bourdet-Sicard R, Capuron L, Delzenne N, Doré J, et al. Health relevance of the modification of low grade inflammation in ageing (inflammageing) and the role of nutrition. Ageing Res Rev. 2017;40:95–119. doi: 10.1016/j.arr.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 69.Fransen F, van Beek AA, Borghuis T, Aidy SE, Hugenholtz F, van der Gaast-de Jongh C, et al. Aged gut microbiota contributes to systemical inflammaging after transfer to germ-free mice. Front Immunol. 2017;8:1385. doi: 10.3389/fimmu.2017.01385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Brunt VE, Gioscia-Ryan RA, Casso AG, VanDongen NS, Ziemba BP, Sapinsley ZJ, et al. Trimethylamine-N-Oxide promotes age-related vascular oxidative stress and endothelial dysfunction in mice and healthy humans. Hypertension. 2020;76:101–12. doi: 10.1161/HYPERTENSIONAHA.120.14759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li DY, Tang WHW. Contributory role of gut microbiota and their metabolites toward cardiovascular complications in chronic kidney disease. Semin Nephrol. 2018;38:193–205. doi: 10.1016/j.semnephrol.2018.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wang Z, Bergeron N, Levison BS, Li XS, Chiu S, Jia X, et al. Impact of chronic dietary red meat, white meat, or non-meat protein on trimethylamine N-oxide metabolism and renal excretion in healthy men and women. Eur Heart J. 2019;40:583–94. doi: 10.1093/eurheartj/ehy799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Moszak M, Szulińska M, Bogdański P. You are what you eat—the relationship between diet, microbiota, and metabolic disorders—a review. Nutrients. 2020;12:1096. doi: 10.3390/nu12041096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hügel HM, Jackson N, May B, Zhang AL, Xue CC. Polyphenol protection and treatment of hypertension. Phytomedicine. 2016;23:220–31. doi: 10.1016/j.phymed.2015.12.012. [DOI] [PubMed] [Google Scholar]
- 75.Potì F, Santi D, Spaggiari G, Zimetti F, Zanotti I. Polyphenol health effects on cardiovascular and neurodegenerative disorders: a review and meta-analysis. Int J Mol Sci. 2019;20:E351. doi: 10.3390/ijms20020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chakraborty S, Galla S, Cheng X, Yeo J-Y, Mell B, Singh V, et al. Salt-responsive metabolite, β-hydroxybutyrate, attenuates hypertension. Cell Rep. 2018;25:677–.e4. doi: 10.1016/j.celrep.2018.09.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ishimwe JA, Garrett MR, Sasser JM. 1,3-Butanediol attenuates hypertension and suppresses kidney injury in female rats. Am J Physiol Ren Physiol. 2020;319:F106–F114.. doi: 10.1152/ajprenal.00141.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Beli E, Yan Y, Moldovan L, Vieira CP, Gao R, Duan Y, et al. Restructuring of the gut microbiome by intermittent fasting prevents retinopathy and prolongs survival in db/db mice. Diabetes. 2018;67:1867–79. doi: 10.2337/db18-0158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winston JA, Theriot CM. Diversification of host bile acids by members of the gut microbiota. Gut Microbes. 2020;11:158–71. doi: 10.1080/19490976.2019.1674124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Cao Y, Xiao Y, Zhou K, Yan J, Wang P, Yan W, et al. FXR agonist GW4064 improves liver and intestinal pathology and alters bile acid metabolism in rats undergoing small intestinal resection. Am J Physiol Gastrointest Liver Physiol. 2019;317:G108–G115.. doi: 10.1152/ajpgi.00356.2017. [DOI] [PubMed] [Google Scholar]
- 81.Fu ZD, Klaassen CD. Increased bile acids in enterohepatic circulation by short-term calorie restriction in male mice. Toxicol Appl Pharmacol. 2013;273:680–90. doi: 10.1016/j.taap.2013.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo C, Xie S, Chi Z, Zhang J, Liu Y, Zhang L, et al. Bile acids control inflammation and metabolic disorder through inhibition of NLRP3 inflammasome. Immunity. 2016;45:802–16. doi: 10.1016/j.immuni.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 83.Han M, Li S, Xie H, Liu Q, Wang A, Hu S, et al. Activation of TGR5 restores AQP2 expression via the HIF pathway in renal ischemia-reperfusion injury. Am J Physiol-Ren Physiol. 2021;320:F308–F321.. doi: 10.1152/ajprenal.00577.2020. [DOI] [PubMed] [Google Scholar]
- 84.Shi H, Zhang B, Abo-Hamzy T, Nelson JW, Ambati CSR, Petrosino JF, et al. Restructuring the gut microbiota by intermittent fasting lowers blood pressure. Circ Res. 2021;128:1240–54. doi: 10.1161/CIRCRESAHA.120.318155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Thaiss CA, Zeevi D, Levy M, Zilberman-Schapira G, Suez J, Tengeler AC, et al. Transkingdom control of microbiota diurnal oscillations promotes metabolic homeostasis. Cell. 2014;159:514–29. doi: 10.1016/j.cell.2014.09.048. [DOI] [PubMed] [Google Scholar]
- 86.Voigt RM, Forsyth CB, Green SJ, Engen PA, Keshavarzian A. Chapter Nine - Circadian Rhythm and the Gut Microbiome. In: Cryan JF, Clarke G (eds). International Review of Neurobiology. Academic Press; 2016. p. 193–205. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.