Abstract
The neurological sequelae of Bacillus anthracis infection include a rapidly progressive fulminant meningoencephalitis frequently associated with intracranial hemorrhage, including subarachnoid and intracerebral hemorrhage. Higher mortality than other forms of bacterial meningitis suggests that antimicrobials and cardiopulmonary support alone may be insufficient and that strategies targeting the hemorrhage might improve outcomes. In this review, we describe the toxic role of intracranial hemorrhage in anthrax meningoencephalitis. We first examine the high incidence of intracranial hemorrhage in patients with anthrax meningoencephalitis. We then review common diseases that present with intracranial hemorrhage, including aneurysmal subarachnoid hemorrhage and spontaneous intracerebral hemorrhage, postulating applicability of established and potential neurointensive treatments to the multimodal management of hemorrhagic anthrax meningoencephalitis. Finally, we examine the therapeutic potential of minocycline, an antimicrobial that is effective against B. anthracis and that has been shown in preclinical studies to have neuroprotective properties, which thus might be repurposed for this historically fatal disease.
Keywords: anthrax, subarachnoid hemorrhage, intracerebral hemorrhage, neuroprotection
Intracranial hemorrhage plays a central role in the pathogenesis of anthrax meningoencephalitis. We examine interventions targeting the neurotoxicity of extravasated blood in more common forms of hemorrhagic stroke and their potential application to anthrax meningoencephalitis.
Bacillus anthracis is an infectious pathogen and a potential bioterrorism threat. Systemic dissemination carries a high mortality and is associated with multiorgan hemorrhage [1]. Leptomeningeal seeding, cerebrospinal fluid (CSF) dissemination, and parenchymal invasion result in fulminant anthrax meningoencephalitis, which is frequently associated with hemorrhage into the subarachnoid space, brain parenchyma, or ventricles (Figure 1). The prognosis for anthrax meningoencephalitis is universally poor: a recent case report documents only the 13th survivor [2].
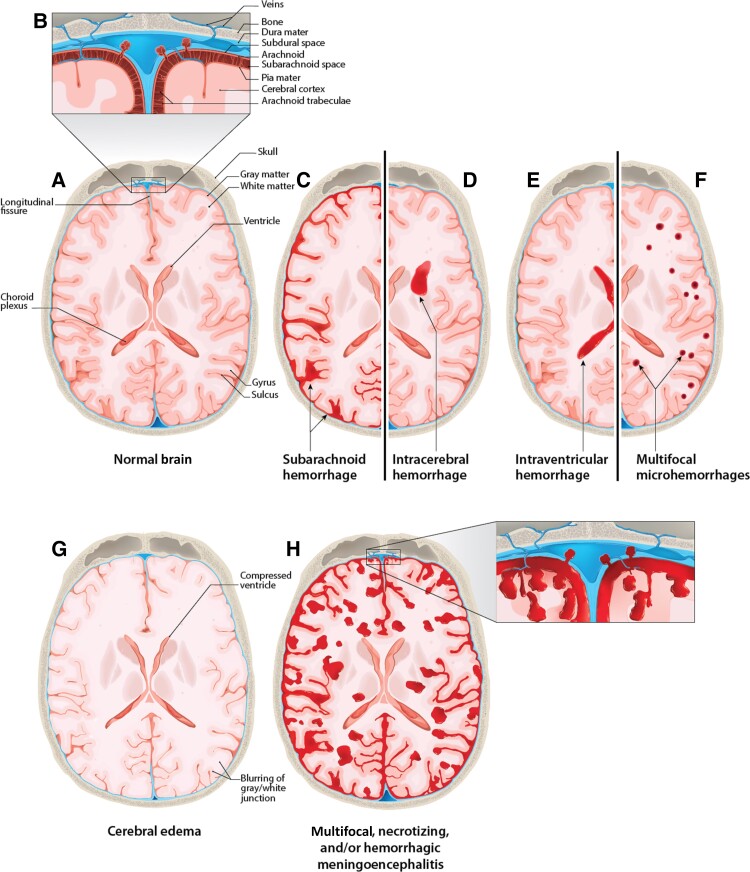
Figure 1.
Sections of normal brain and meninges illustrating the types of hemorrhage found in anthrax meningitis. A and B, Normal brain with meninges and other key anatomical features labeled. C, Subarachnoid hemorrhage. D, Intracerebral hemorrhage. E, Intraventricular hemorrhage. F, Diffuse microhemorrhages. G, Edema showing compressed ventricles, blurring of the gray/white junction, and loss of subarachnoid space. H, Multifocal, necrotizing, and/or hemorrhagic meningoencephalitis.
Current guidelines stress multimodal antibiotic regimens, antitoxins, and cardiopulmonary support but offer few recommendations regarding cerebral hemorrhage or the application of neurointensive care principles. Multidisciplinary study of intracranial hemorrhage and the toxicity of extravasated blood may improve our understanding and treatment of this highly lethal disease.
PATHOPHYSIOLOGY
In this section, we review key anatomical characteristics important for bacterial seeding and the effects of toxic anthrax moieties on the blood-brain barrier (BBB), with the goal of providing a mechanistic understanding linking anthrax meningoencephalitis and intracranial hemorrhage.
Seeding the Meninges and the Brain
Two types of anthrax meningitis are recognized: secondary, which is the most common, and primary. In secondary meningitis, B. anthracis inoculation occurs initially through the skin or by inhalation, ingestion, or injection, resulting in a systemic infection followed by secondary involvement of the meninges. The bacilli are first released into the lymphatic system by the death of phagocytic cells, then enter the bloodstream where they multiply and disseminate. As 20% of cardiac output is distributed to the brain, the brain and meninges are at high risk for bacterial seeding.
Anthrax meningitis is designated as “primary meningitis” when it occurs without a recognized, developed site of infection, such as skin, lung, or gastrointestinal tract, from which bacteremia could originate. It is possible that bacteremia occurs through routes of infection that include inhalation inoculation and growth of vegetative bacilli in the respiratory tract (upper and lower), with bacteremia occurring early in the infection prior to the development of recognized, focal infection. In nonanthrax meningitis, common bacterial pathogens also are presumed to first colonize mucosal surfaces, with subsequent bloodstream invasion and entry into CSF via the subarachnoid space, choroid plexus, or ventricles [3]. Alternatively, some have hypothesized that airborne spores that germinate in the nasopharynx may inoculate the brain without systemic involvement, because nasal lymphatics and the subarachnoid space are connected via the cribriform plate. These anatomical features may account for the high incidence of meningoencephalitis in those with respiratory tract inoculation and the occurrence in inhalation anthrax of meningoencephalitis with minimal lung abnormalities [4, 5].
Pathological studies of anthrax meningitis reveal a thickened, inflamed dura mater with infiltration by bacilli and neutrophils [6]. The dura is supplied by anterior, middle, posterior, and accessory meningeal arteries adjacent to the periosteum. Penetrating anastomotic arteries connect these major dural vessels to a fragile inner capillary network adjacent to the arachnoid. This delicate capillary network is prone to bleeding, and proximity to the leptomeninges presents an anatomical route for bacilli to breach the arachnoid and access the subarachnoid space.
The subarachnoid space contains an extensive vascular network that may be a site of seeding. Subarachnoid vessels are suspended in CSF and tethered by thin trabeculae, forming the delicate subarachnoid-pial complex. The inner capillary network of the dura and subarachnoid space may be an ideal environment for the growth of bacilli due to tissue-specific oxygen and carbon dioxide tensions, pH, and the rich blood supply. CSF pathways also provide an avenue for dissemination throughout the central nervous system (CNS), as confirmed in autopsy studies demonstrating bacilli in the CSF, brain parenchyma, or meninges [7].
Toxin Moieties
The pathogenesis of B. anthracis infection is mediated in part via 2 exotoxins, lethal toxin (zinc metalloprotease) and edema toxin (calmodulin-dependent adenylate cyclase) [8]. These exotoxins exert direct cytotoxic effects on neuronal dendritic cells; inhibit lymphocyte function; and promote barrier dysfunction, tissue edema, and hemorrhage via endothelial cell apoptosis and inhibition of platelet aggregation. Specific targeting of the BBB may explain the high incidence of intracranial hemorrhage with CNS infection. Bacillus anthracis metalloprotease InhA targets BBB tight junction proteins and contributes to BBB homeostatic imbalance and cerebral hemorrhage [9]. Metalloproteases are also implicated in the pathogenesis of hemorrhagic transformation in ischemic stroke, cerebral amyloid angiopathy, and Alzheimer’s disease. Their activation may be a common mechanism contributing to hemorrhagic stroke across multiple CNS diseases. To inhibit toxin production, the most recent anthrax treatment guidelines suggest that bactericidal antimicrobials should be supplemented with protein synthesis inhibitor antimicrobials [1].
Pathology
Meningeal seeding and BBB destruction result in edema; necrosis; suppurative meningitis; and—frequently, but not invariably—subarachnoid hemorrhage, multifocal intraparenchymal hemorrhage, or intraventricular hemorrhage (Figure 1). The exact mechanism of hemorrhage remains unclear. Although some studies suggest cytokine-mediated diffuse intravascular coagulopathy, small vessel necrosis from diffuse cerebral arteritis is frequently seen in pathologic specimens [10]. Intracranial hemorrhage may develop later in the disease process and is characteristic of more advanced, prolonged infection. In nonhuman primates (African green monkey; Chlorocebus aethiops), necropsy 4 days after aerosolized exposure showed severe suppurative meningitis with preservation of the pia and underlying parenchyma, whereas necropsy 7 days after aerosolized exposure revealed gross infection along with hemorrhagic breach of the pia with multifocal subarachnoid hemorrhage and intraparenchymal microhemorrhages [6].
INTRACRANIAL HEMORRHAGE
Both experimental and clinical studies suggest that intracranial hemorrhage is common when B. anthracis infection involves the brain. The diffusely red-appearing leptomeninges are classically described as “Cardinal’s cap” (Figure 2). A study in nonhuman primates reported meningeal hemorrhages in 54% and hemorrhage within the brain parenchyma in 31% [10]. Multiple case reports document hemorrhagic meningitis after exposure to cutaneous, ingestion, and inhalation anthrax. Magnetic resonance imaging (MRI) and computed tomography (CT) studies frequently show hemorrhage within the subarachnoid space, deep gray matter, or ventricles, with diffuse meningeal enhancement (Figure 3) [11]. Haight reviewed 95 cases of anthrax meningitis that occurred from 1874 through 1952 and described hemorrhagic meningitis in 100% and cortical hemorrhages in 50% of patients [12]. Complete autopsies of 38 individuals who died from inhalation anthrax in a 1979 outbreak showed hemorrhagic meningoencephalitis in 21 cases (55%) [7]. In a series of 132 cases of anthrax meningitis, the CSF was hemorrhagic in 57 (43%) [13]. Katharios-Lanwermeyer et al [13] and Lanska [14] reported an increased likelihood of death with hemorrhagic CSF. However, these figures may be misleading, as case series and autopsy series are subject to sampling bias.
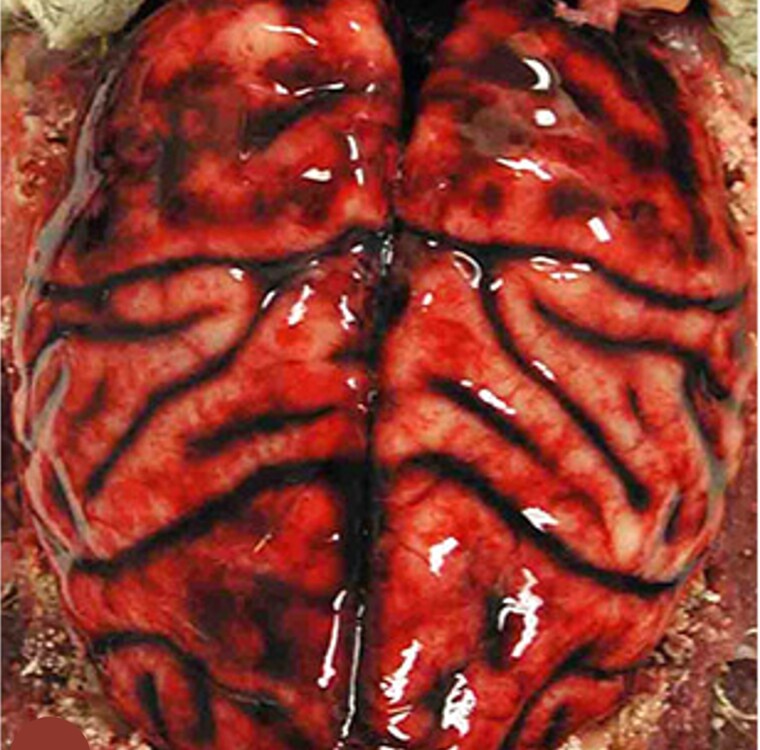
Figure 2.
Anthrax meningoencephalitis with subarachnoid hemorrhage. Brain of an African green monkey (Chlorocebus aethiops) that was infected by an aerosolized lethal dose of anthrax spores and died 9 days after exposure. The dura mater is reflected. There is diffuse subarachnoid hemorrhage over the cortical surfaces, classically referred to as “Cardinal’s cap.” From N. A. Twenhafel, unpublished.
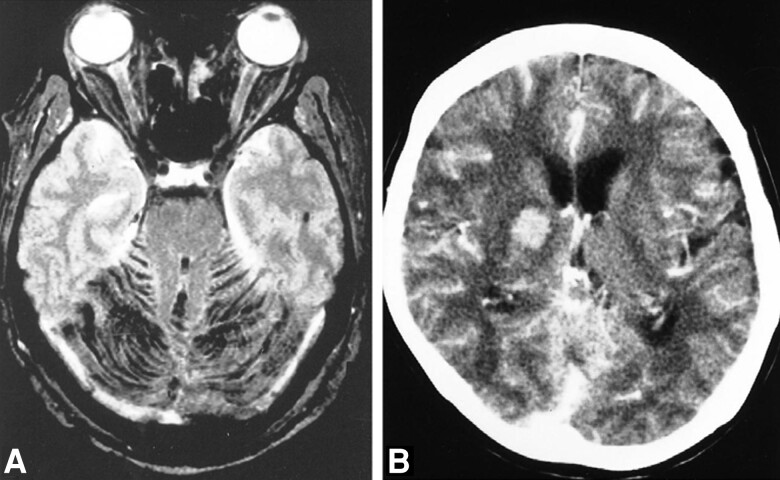
Figure 3.
Neuroradiological imaging of anthrax meningoencephalitis. A, Magnetic resonance imaging of a 53-year-old with anthrax meningoencephalitis. Axial T2-weighted gradient-echo images show diffuse dark signal intensities indicating hemorrhage within the infratentorial subarachnoid space. B, Computed tomography scan with contrast in a 72-year-old with anthrax meningoencephalitis. There is diffuse leptomeningeal enhancement and intraparenchymal hemorrhage within the right deep gray matter. Adapted from Kim et al [11], with permission.
The CDC recently conducted a systematic review of the 492 patients hospitalized for systemic anthrax through 2018 for whom adequate clinical data were available (unpublished data, Marissa K. Person). Of these, 157 (32%) patients developed meningitis and 96 of the 157 (61%) suffered intracranial hemorrhage diagnosed by lumbar puncture, CT, or MRI. Similar to previous findings, fatalities were more common in cases with intracranial hemorrhage than those without (64% vs 18%) (unpublished data, Marissa K. Person).
The timing of intracranial hemorrhage during infection remains unclear. Lanska reviewed 70 cases of anthrax meningitis and identified CSF studies in 43 patients [14]. Only 8 underwent serial lumbar puncture, however, limiting insight into hemorrhage evolution. Case reports suggest that hemorrhage may be related to more severe infection and prolonged inflammation. Rangel and Gonzalez described a 14-year-old boy in Mexico with anthrax meningitis who died within 12 hours of admission [15]. The initial CSF profile was negative for hemorrhage, but repeat lumbar puncture 5 hours later showed markedly bloody fluid.
BLOOD IS TOXIC TO THE BRAIN
The role of extravasated blood in the pathophysiology and clinical outcome of anthrax meningoencephalitis has not been specifically studied. We discuss the neurotoxic components of blood and review standard treatments in common types of hemorrhagic stroke, namely aneurysmal subarachnoid hemorrhage and spontaneous intracerebral hemorrhage. We postulate that principles of neurointensive care and treatment regimens used in these diseases may be applicable to the multimodal treatment of hemorrhagic anthrax meningoencephalitis (Table 1).
Table 1.
Clinical Practice Guidelines for Anthrax Meningitis Accompanied by Intracranial Bleeding
| Pathology | Clinical Problem | Potential Treatment | Comments |
|---|---|---|---|
| Anthrax infection | Presumed or confirmed anthrax meningitis | Consult a neurocritical care specialist. | Follow all normal standards of care. |
| Hypotension | Impaired cerebral autoregulation predisposing to cerebral hypoperfusion | Maintain euvolemia through strict monitoring of fluid intake and output, serial bedside ultrasound volume assessments, and target mean arterial pressures of 70–100 mm Hg [21, 23]. | These are widely practiced neurointensive care measures that minimize cerebral hypoperfusion and early brain injury. While clinical trials are lacking, these measures may apply to hemorrhagic anthrax meningoencephalitis in which cerebral autoregulatory mechanisms may be impaired. |
| Subarachnoid hemorrhage | Cerebral vasospasm and neuronal death | Administer nimodipine (60 mg every 4 h or, with hypotension, 30 mg every 2 h), as this is an FDA-approved drug for subarachnoid hemorrhage [24, 47]. | With clear benefit in aneurysmal subarachnoid hemorrhage, and promising preclinical results in infectious and neuroinflammatory disorders, the neuroprotective effects of nimodipine may merit consideration in the multimodal treatment of anthrax meningoencephalitis. |
| Intracranial hemorrhage | Expansion of intracerebral hemorrhage | Maintain systolic blood pressure ≤150 mm Hg or mean arterial pressure ≤110 mm Hg to minimize intracerebral hemorrhage expansion [48]. | With Bacillus anthracis likely injuring both the structural integrity of the cerebral vasculature and autoregulatory mechanisms, treatment of hypertension may be a reasonable, safe principle to minimize intracerebral hemorrhage expansion. |
| Intracranial swelling | Elevated intracranial pressure | Administer hypertonic saline (3% or 23.4%) or mannitol (0.5–1 g/kg) in patients with symptoms of brain swelling, as this may reduce the likelihood of cerebral herniation and death [36, 38, 49]. | In anthrax meningoencephalitis, combined hemorrhage and infection may predispose to malignant, rapidly fatal brain swelling. |
| Inflammation secondary to intracranial hemorrhage | Bacillus anthracis infection and neuronal death | Consider including minocycline (up to 10 mg/kg/d) in treatment regimens as it crosses the BBB well, and there is preclinical evidence that it is neuroprotective. | The combined antimicrobial and neuroprotective effects of minocycline might be uniquely applicable to the treatment of fulminant anthrax meningitis, offering therapies against both bacilli and intracranial hemorrhage. |
Abbreviations: BBB, blood-brain barrier; FDA, United States Food and Drug Administration.
Toxic Components of Blood
The neurotoxicity of extravasated blood in the brain is reviewed in detail by Stokum et al [16] and is briefly summarized here. Neuronal apoptosis is directly or indirectly activated by blood components such as thrombin. Xue et al found that human neurons die when exposed to thrombin in vitro, and intracerebral injection of autologous blood in vivo results in microglial activation, neutrophil accumulation, and cerebral edema [17]. Free iron produces reactive oxygen species and free radicals that promote neuroinflammation, cerebral edema, and neuronal and astrocyte cell death [18]. Hemin degrades hydrogen peroxide, producing free radicals leading to oxidative neural damage and widespread neuronal cell death [19]. Fibrinogen, complement, leukocytes, platelets, oxyhemoglobin, and methemoglobin also show strong preclinical evidence of neuronal and glial toxicity and are considered major contributors to cerebral edema, vasospasm, and hydrocephalus following extravasation into the subarachnoid space, brain parenchyma, or ventricles.
SUBARACHNOID HEMORRHAGE
In a review of 132 cases of anthrax meningitis, subarachnoid hemorrhage detected by lumbar puncture was found in 57 (43%) [13]. Notably, this figure may underestimate the incidence of intracranial subarachnoid hemorrhage because, in experimental anthrax meningoencephalitis, hemorrhage and inflammation frequently do not extend far from the cranial vault and so would not be detected by lumbar puncture (N. A. T., unpublished data). Extrapolating from our understanding of aneurysmal subarachnoid hemorrhage, blood within the subarachnoid space promotes neuroinflammation, excitotoxicity, apoptosis, and cerebral circulatory dysfunction, which may superimpose on the neurological injury associated with infectious meningitis. In the following section, we review management principles for aneurysmal subarachnoid hemorrhage that may be applicable to the treatment of subarachnoid hemorrhage in anthrax meningoencephalitis.
Euvolemia and Normotension
Macro- and microcirculatory dysfunction are major sources of morbidity and mortality after aneurysmal subarachnoid hemorrhage. Cerebral autoregulation is frequently disrupted, and cerebral perfusion is highly dependent on volume status and blood pressure [20]. Current recommendations stress strict avoidance of hypovolemia and maintenance of euvolemia, as well as normotension to optimize cerebral perfusion following aneurysmal subarachnoid hemorrhage [21]. Euvolemia avoids substantial changes in blood viscosity and associated perturbations in global cerebral blood flow and oxygen-carrying capacity, and normal mean arterial pressure prevents reductions in cerebral blood flow with hypotension. While this principle optimizes cerebral perfusion to meet metabolic demand without stressing cardiopulmonary reserve, up to 30% of patients with subarachnoid hemorrhage will develop symptomatic circulatory dysfunction and 15% will suffer cerebral infarction despite aggressive measures.
Cerebrovascular autoregulation is disrupted in animal models of bacterial meningitis. Thus, cerebral perfusion, intracranial pressure, cerebral metabolism, and brain edema may be highly dependent on volume status and blood pressure with advanced CNS infection [22]. Prospective physiologic trials in humans also report impaired autoregulation in early phases of bacterial meningitis, highlighting the importance of blood volume and blood pressure control [23]. While clinical trials are lacking, strict monitoring of fluid intake and output, serial bedside ultrasound volume assessment, and target mean arterial pressures of 70–100 mm Hg are widely practiced neurointensive measures that minimize cerebral hypoperfusion and early brain injury. These measures may apply to hemorrhagic anthrax meningoencephalitis in which cerebral autoregulatory mechanisms may be impaired.
Nimodipine is the only drug approved by the United States Food and Drug Administration (FDA) for aneurysmal subarachnoid hemorrhage, as it reduces the incidence of poor neurological outcome by 40% [24]. Nimodipine has several neuroprotective effects, including vasodilatation of smooth muscle, protection from calcium-mediated neuronal excitotoxicity, and reduction in microthrombosis with improved microcirculatory function [21]. Nimodipine treatment is initiated immediately after subarachnoid hemorrhage, continued for 21 days, and dosed at 60 mg every 4 hours or 30 mg every 2 hours if hypotension is encountered.
Preclinical evidence suggests that nimodipine may also be beneficial in CNS infection. Paul et al reported significantly reduced intracranial pressures in experimental models of pneumococcal meningitis treated with nimodipine [25]. Hosoglu et al found improved vascular and neuronal function when nimodipine was added to standard antibiotic regimens for the treatment of pneumococcal meningitis [26]. Additional studies support nimodipine for the treatment of autoimmune encephalitis [27]. With clear benefit of nimodipine in aneurysmal subarachnoid hemorrhage and promising preclinical results in infectious and neuroinflammatory disorders, the neuroprotective effects of nimodipine may merit consideration in the multimodal treatment of anthrax meningoencephalitis.
Statins
The beneficial effects of statins are seen across multiple CNS pathologies, including stroke, Alzheimer’s disease, and select brain tumors [28]. Statins may be neuroprotective following aneurysmal subarachnoid hemorrhage, wherein they improve cerebral vasomotor reactivity, decrease glutamate-mediated neuronal excitotoxicity, and exert antioxidant and anti-inflammatory effects [21]. Some clinical trials suggest reduced vasospasm with statin therapy [29]. However, a meta-analysis of clinical trials failed to identify statistically significant improvements in neurological outcomes [30]. Nevertheless, pravastatin 40 mg daily or simvastatin 80 mg daily is frequently administered after subarachnoid hemorrhage.
Preclinical studies suggest statins also may be beneficial in meningitis and sepsis. In animal models of pneumococcal meningitis, Winkler et al found that simvastatin attenuates CNS leukocyte recruitment and systemic complications [31]. Importantly, decreased CSF leukocytes are associated with higher likelihood of survival in anthrax meningitis [13]. Braga Filho et al also report improved survival with simvastatin treatment in animal models of lethal sepsis [32]. With a favorable safety profile and preclinical evidence of neuroprotective properties, statins might be considered as adjunctive therapy in the treatment of anthrax meningoencephalitis with subarachnoid hemorrhage, with the understanding that definitive improvements in neurological outcomes are unproven.
INTRACEREBRAL HEMORRHAGE
Intracerebral hemorrhage (ICH) occurs commonly in anthrax meningoencephalitis, although its precise incidence is unknown. In experimental studies, hemorrhages within the brain parenchyma are identified in 31% of cases [10]. ICH leads to direct mechanical damage via dissection and compression of neural networks and secondary injury via neuroinflammation and perihematomal edema. In spontaneous ICH associated with hypertension, surgical evacuation is seldom pursued as it lacks demonstrated efficacy. Treatment is primarily supportive, focused on minimizing hemorrhage progression and counteracting the deleterious effects of progressive brain swelling. In the following section, we review principles in the management of spontaneous ICH that may be applicable to the management of ICH in anthrax meningoencephalitis.
Blood Pressure Control
Blood pressure elevation occurs in most patients with spontaneous ICH. Early control is hypothesized to reduce active extravasation of blood into the parenchyma and to reduce hyperemia that contributes to elevated intracranial pressure and cerebral edema. While maintaining systolic blood pressure ≤150 mm Hg or mean arterial pressure ≤110 mm Hg is safe and reduces absolute hemorrhage growth at 24 hours, a meta-analysis of multiple clinical trials failed to demonstrate this measure significantly improved neurological function or mortality [33].
Normotension remains a core neurointensive principle in the management of spontaneous intracerebral hemorrhage. In fulminant anthrax meningoencephalitis, uncontrolled hypertension may risk hemorrhage expansion in vascular beds compromised by bacilli toxins and metalloproteases. Rabbit models of meningitis also report alterations of cerebral autoregulation that may further exacerbate hemorrhage with hypertension [22]. With B. anthracis likely injuring both the structural integrity of the cerebral vasculature and autoregulatory mechanisms, treatment of hypertension may be a reasonable, safe principle to minimize intracerebral hemorrhage expansion.
Hyperosmolar Therapy
Perihematomal edema develops in the first few days after spontaneous ICH and may cause elevated intracranial pressure, mass effect, and midline shift [34]. Rapid progression is associated with worse functional outcomes [35]. Patients with asymptomatic perihematomal edema require no specific treatment except maintaining normal serum sodium levels, and there is no indication for the routine use of hyperosmolar agents in the absence of significant cerebral edema, mass effect, or neurological decline [36]. In patients with symptomatic perihematomal edema or elevated intracranial pressure, hypertonic saline (3% or 23.4%) or mannitol (0.5–1 g/kg) is a first-line treatment, and a target hypernatremia of 140–150 mEq/L is recommended [36]. Hyperosmolar therapy reduces elevated intracranial pressure but may not improve neurological outcome [37].
In bacterial meningitis, preclinical animal studies suggest a beneficial effect of hypertonic saline for the treatment of symptomatic cerebral edema. Liu et al reports that 3% hypertonic saline reduces intracranial pressure, improves cerebral perfusion pressure, inhibits aquaporin-4 expression, reduces cerebral edema, and attenuates neuronal injury, with superior effects compared to mannitol [38]. In anthrax meningoencephalitis, combined hemorrhage and infection may predispose to malignant, rapidly fatal brain swelling. In this context, hyperosmolar therapy might offer some chance of stabilization to reduce cerebral herniation and death.
MINOCYCLINE
Antimicrobial regimens that combine bactericidal activity and inhibition of protein synthesis, usually a combination of fluoroquinolones, carbapenems, and linezolid, are the primary treatment for anthrax meningitis [1]. Alternative antimicrobials with additional neuroprotective effects may be uniquely positioned to combat the hemorrhagic and infectious CNS insults in anthrax meningoencephalitis.
Minocycline is a second-generation tetracycline that is FDA-approved for the treatment of infections due to several microorganisms. Although not specifically approved for B. anthracis infection, minocycline is effective against this organism. In time-kill assays, minocycline combined with a broad-spectrum antimicrobial peptide enhances killing of B. anthracis [39]. Minocycline may effectively kill tetracycline-resistant strains of B. anthracis when resistance is mediated by a tetracycline efflux pump (tetL) but not other resistance mechanisms (David Sue, personal communication). Pomerantsev et al evaluated the in vivo and in vitro efficacy of minocycline against tetL-mediated tetracycline-resistant strains of B. anthracis [40]. While no therapeutic effect was observed with doxycycline, high therapeutic efficacy was found with minocycline.
Minocycline is highly lipophilic and readily crosses the BBB [41]. Recently, there has been interest in the neuroprotective effects of minocycline for the treatment of neurological diseases. In hemorrhagic and infectious CNS injuries, minocycline reduces secondary injury via anti-inflammatory, anti-apoptotic, and antioxidant effects. In animal models, minocycline suppresses microglial activation, cytokine release, and matrix metalloprotease activity [42]. Minocycline stabilizes the mitochondrial membrane and reduces caspase-dependent cell death pathways, a prominent mechanism of endothelial, astrocyte, and neuronal cell death in subarachnoid hemorrhage [43]. Intracranial hemorrhage also generates free radicals and reactive oxygen species, leading to secondary neural injury, and minocycline reduces oxidant-mediated cell damage via poly(ADP-ribose) polymerase 1 (PARP-1) inhibition [44].
Clinical trials evaluating minocycline in ischemic stroke and intracerebral hemorrhage suggest promising but inconclusive results. Meta-analysis of 7 randomized controlled trials evaluating the neuroprotective role of minocycline report favorable outcomes regarding 3-month neurological examination (National Institutes of Health Stroke Scale) and functional independence (Barthel index) after acute ischemic stroke but not intracerebral hemorrhage [45]. In intracerebral hemorrhage, the studies reported overall safety of minocycline when dosed up to 10 mg/kg/day, but additional, larger trials are needed to evaluate effects on hematoma expansion, perihematomal edema, and functional outcome. While clinical efficacy for anthrax treatment remains unproven, minocycline was found to be effective as chemoprophylaxis for meningococcal meningitis [46]. The combined antimicrobial and neuroprotective effects of minocycline may be uniquely applicable to the treatment of fulminant anthrax meningitis, offering therapies against both bacilli and intracranial hemorrhage.
CONCLUSIONS
Anthrax meningoencephalitis is both an infectious and a hemorrhagic disease that affects the brain. The compounding neurotoxicity of the infection and the hemorrhage contributes to a dismal prognosis despite modern antimicrobial and medical advancements. Historically, research efforts and clinical management have focused on antimicrobials, but an appreciation of the pathophysiology of intracranial hemorrhage suggests antimicrobials alone may be insufficient to improve outcome. A consideration of pharmaceuticals established in the treatment of aneurysmal subarachnoid hemorrhage and spontaneous ICH along with neurointensive principles may comprehensively address the multifaceted CNS insult of anthrax meningoencephalitis. The promise of combined therapy is nicely exemplified by minocycline, first commercially available in 1971, which could potentially be repurposed for its antimicrobial and neuroprotective properties. Future efforts to combat the hemorrhagic and infectious toxicity of anthrax meningoencephalitis will likely focus on the prevention of BBB destabilization, as it is better to prevent than treat brain bleeding.
Notes
Acknowledgments. We would like to thank visual information specialist Dan Higgins, MAMS, for illustrating Figure 1.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the authors' affiliated institutions.
Financial support. This project was supported by the Centers for Disease Control and Prevention and the Office of the Assistant Secretary for Preparedness and Response.
Supplement sponsorship. This article appears as part of the supplement “Anthrax Preparedness,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. The authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
Contributor Information
Nicholas Caffes, Department of Neurosurgery, University of Maryland School of Medicine, Baltimore, Maryland, USA.
Katherine Hendricks, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
John S Bradley, Department of Pediatrics, San Diego School of Medicine and Rady Children’s Hospital, University of California, San Diego, California, USA.
Nancy A Twenhafel, Division of Pathology, United States Army Medical Research Institute of Infectious Diseases, Frederick, Maryland, USA.
J Marc Simard, Departments of Neurosurgery, Pathology and Physiology, University of Maryland School of Medicine, Baltimore, Maryland, USA.
References
- 1. Hendricks KA, Wright ME, Shadomy SV, et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 2014; 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lombarte Espinosa E, Villuendas Uson MC, Arribas Garcia J, et al. Survival of patient with hemorrhagic meningitis associated with inhalation anthrax. Clin Infect Dis 2022; 75:S364–72. [DOI] [PubMed] [Google Scholar]
- 3. Schwerk C, Tenenbaum T, Kim KS, Schroten H. The choroid plexus—a multi-role player during infectious diseases of the CNS. Front Cell Neurosci 2015; 9:80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Vasconcelos D, Barnewall R, Babin M, et al. Pathology of inhalation anthrax in cynomolgus monkeys (Macaca fascicularis). Lab Invest 2003; 83:1201–9. [DOI] [PubMed] [Google Scholar]
- 5. Holty JE, Kim RY, Bravata DM. Anthrax: a systematic review of atypical presentations. Ann Emerg Med 2006; 48:200–11. [DOI] [PubMed] [Google Scholar]
- 6. Twenhafel NA. Pathology of inhalational anthrax animal models. Vet Pathol 2010; 47:819–30. [DOI] [PubMed] [Google Scholar]
- 7. Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci U S A 1993; 90:2291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Moayeri M, Leppla SH. The roles of anthrax toxin in pathogenesis. Curr Opin Microbiol 2004; 7:19–24. [DOI] [PubMed] [Google Scholar]
- 9. Mukherjee DV, Tonry JH, Kim KS, et al. Bacillus anthracis protease InhA increases blood-brain barrier permeability and contributes to cerebral hemorrhages. PLoS One 2011; 6:e17921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fritz DL, Jaax NK, Lawrence WB, et al. Pathology of experimental inhalation anthrax in the rhesus monkey. Lab Invest 1995; 73:691–702. [PubMed] [Google Scholar]
- 11. Kim HJ, Jun WB, Lee SH, Rho MH. CT and MR findings of anthrax meningoencephalitis: report of two cases and review of the literature. AJNR Am J Neuroradiol 2001; 22:1303–5. [PMC free article] [PubMed] [Google Scholar]
- 12. Haight TH. Anthrax meningitis: review of literature and report of two cases with autopsies. Am J Med Sci 1952; 224:57–69. [PubMed] [Google Scholar]
- 13. Katharios-Lanwermeyer S, Holty JE, Person M, et al. Identifying meningitis during an anthrax mass casualty incident: systematic review of systemic anthrax since 1880. Clin Infect Dis 2016; 62:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lanska DJ. Anthrax meningoencephalitis. Neurology 2002; 59:327–34. [DOI] [PubMed] [Google Scholar]
- 15. Rangel RA, Gonzalez DA. Bacillus anthracis meningitis. Neurology 1975; 25:525–30. [DOI] [PubMed] [Google Scholar]
- 16. Stokum JA, Cannarsa GJ, Wessell AP, Shea P, Wenger N, Simard JM. When the blood hits your brain: the neurotoxicity of extravasated blood. Int J Mol Sci 2021; 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xue M, Hollenberg MD, Yong VW. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci 2006; 26:10281–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Willmore LJ, Rubin JJ. Formation of malonaldehyde and focal brain edema induced by subpial injection of FeCl2 into rat isocortex. Brain Res 1982; 246:113–9. [DOI] [PubMed] [Google Scholar]
- 19. Robinson SR, Dang TN, Dringen R, Bishop GM. Hemin toxicity: a preventable source of brain damage following hemorrhagic stroke. Redox Rep 2009; 14:228–35. [DOI] [PubMed] [Google Scholar]
- 20. Harrigan MR. Hypertension may be the most important component of hyperdynamic therapy in cerebral vasospasm. Crit Care 2010; 14:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Daou BJ, Koduri S, Thompson BG, Chaudhary N, Pandey AS. Clinical and experimental aspects of aneurysmal subarachnoid hemorrhage. CNS Neurosci Ther 2019; 25:1096–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Tureen JH, Dworkin RJ, Kennedy SL, Sachdeva M, Sande MA. Loss of cerebrovascular autoregulation in experimental meningitis in rabbits. J Clin Invest 1990; 85:577–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Moller K, Larsen FS, Qvist J, et al. Dependency of cerebral blood flow on mean arterial pressure in patients with acute bacterial meningitis. Crit Care Med 2000; 28:1027–32. [DOI] [PubMed] [Google Scholar]
- 24. Pickard JD, Murray GD, Illingworth R, et al. Effect of oral nimodipine on cerebral infarction and outcome after subarachnoid haemorrhage: British aneurysm nimodipine trial. BMJ 1989; 298:636–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Paul R, Koedel U, Pfister HW. Reduction of intracranial pressure by nimodipine in experimental pneumococcal meningitis. Crit Care Med 2000; 28:2552–6. [DOI] [PubMed] [Google Scholar]
- 26. Hosoglu S, Ceviz A, Kemaloglu MS, et al. Effects of nimodipine on the cerebrovascular and neuronal changes during pneumococcal meningitis in the rat. Acta Microbiol Immunol Hung 1997; 44:271–9. [PubMed] [Google Scholar]
- 27. Ingwersen J, De Santi L, Wingerath B, et al. Nimodipine confers clinical improvement in two models of experimental autoimmune encephalomyelitis [manuscript published online ahead of print 23 February 2018]. J Neurochem 2018. [DOI] [PubMed] [Google Scholar]
- 28. Cucchiara B, Kasner SE. Use of statins in CNS disorders. J Neurol Sci 2001; 187:81–9. [DOI] [PubMed] [Google Scholar]
- 29. Sillberg VA, Wells GA, Perry JJ. Do statins improve outcomes and reduce the incidence of vasospasm after aneurysmal subarachnoid hemorrhage: a meta-analysis. Stroke 2008; 39:2622–6. [DOI] [PubMed] [Google Scholar]
- 30. Vergouwen MD, de Haan RJ, Vermeulen M, Roos YB. Effect of statin treatment on vasospasm, delayed cerebral ischemia, and functional outcome in patients with aneurysmal subarachnoid hemorrhage: a systematic review and meta-analysis update. Stroke 2010; 41:e47–52. [DOI] [PubMed] [Google Scholar]
- 31. Winkler F, Angele B, Pfister HW, Koedel U. Simvastatin attenuates leukocyte recruitment in experimental bacterial meningitis. Int Immunopharmacol 2009; 9:371–4. [DOI] [PubMed] [Google Scholar]
- 32. Braga Filho JAF, Abreu AG, Rios CEP, et al. Prophylactic treatment with simvastatin modulates the immune response and increases animal survival following lethal sepsis infection. Front Immunol 2018; 9:2137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Tsivgoulis G, Katsanos AH, Butcher KS, et al. Intensive blood pressure reduction in acute intracerebral hemorrhage: a meta-analysis. Neurology 2014; 83:1523–9. [DOI] [PubMed] [Google Scholar]
- 34. Urday S, Kimberly WT, Beslow LA, et al. Targeting secondary injury in intracerebral haemorrhage—perihaematomal oedema. Nat Rev Neurol 2015; 11:111–22. [DOI] [PubMed] [Google Scholar]
- 35. Murthy SB, Moradiya Y, Dawson J, et al. Perihematomal edema and functional outcomes in intracerebral hemorrhage: influence of hematoma volume and location. Stroke 2015; 46:3088–92. [DOI] [PubMed] [Google Scholar]
- 36. Dastur CK, Yu W. Current management of spontaneous intracerebral haemorrhage. BMJ 2017; 2:21–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wang X, Arima H, Yang J, et al. Mannitol and outcome in intracerebral hemorrhage: propensity score and multivariable intensive blood pressure reduction in acute cerebral hemorrhage trial 2 results. Stroke 2015; 46:2762–7. [DOI] [PubMed] [Google Scholar]
- 38. Liu S, Li L, Luo Z, et al. Superior effect of hypertonic saline over mannitol to attenuate cerebral edema in a rabbit bacterial meningitis model. Crit Care Med 2011; 39:1467–73. [DOI] [PubMed] [Google Scholar]
- 39. Cote CK, Blanco II, Hunter M, et al. Combinations of early generation antibiotics and antimicrobial peptides are effective against a broad spectrum of bacterial biothreat agents. Microb Pathog 2020; 142:104050. [DOI] [PubMed] [Google Scholar]
- 40. Pomerantsev AP, Shishkova NA, Marinin LI. Comparison of therapeutic effects of antibiotics of the tetracycline group in the treatment of anthrax caused by a strain inheriting tet-gene of plasmid pBC16 [in Russian]. Antibiot Khimioter 1992; 37:31–4. [PubMed] [Google Scholar]
- 41. Barza M, Brown RB, Shanks C, Gamble C, Weinstein L. Relation between lipophilicity and pharmacological behavior of minocycline, doxycycline, tetracycline, and oxytetracycline in dogs. Antimicrob Agents Chemother 1975; 8:713–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Stirling DP, Koochesfahani KM, Steeves JD, Tetzlaff W. Minocycline as a neuroprotective agent. Neuroscientist 2005; 11:308–22. [DOI] [PubMed] [Google Scholar]
- 43. Garcia-Martinez EM, Sanz-Blasco S, Karachitos A, et al. Mitochondria and calcium flux as targets of neuroprotection caused by minocycline in cerebellar granule cells. Biochem Pharmacol 2010; 79:239–50. [DOI] [PubMed] [Google Scholar]
- 44. Alano CC, Kauppinen TM, Valls AV, Swanson RA. Minocycline inhibits poly(ADP-ribose) polymerase-1 at nanomolar concentrations. Proc Natl Acad Sci U S A 2006; 103:9685–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Malhotra K, Chang JJ, Khunger A, et al. Minocycline for acute stroke treatment: a systematic review and meta-analysis of randomized clinical trials. J Neurol 2018; 265:1871–9. [DOI] [PubMed] [Google Scholar]
- 46. Guttler RB, Beaty HN. Minocycline in the chemoprophylaxis of meningococcal disease. Antimicrob Agents Chemother 1972; 1:397–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Liu GJ, Luo J, Zhang LP, et al. Meta-analysis of the effectiveness and safety of prophylactic use of nimodipine in patients with an aneurysmal subarachnoid haemorrhage. CNS Neurol Disord Drug Targets 2011; 10:834–44. [DOI] [PubMed] [Google Scholar]
- 48. Shi L, Xu S, Zheng J, Xu J, Zhang J. Blood pressure management for acute intracerebral hemorrhage: a meta-analysis. Sci Rep 2017; 7:14345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kamel H, Navi BB, Nakagawa K, Hemphill JC 3rd, Ko NU. Hypertonic saline versus mannitol for the treatment of elevated intracranial pressure: a meta-analysis of randomized clinical trials. Crit Care Med 2011; 39:554–9. [DOI] [PubMed] [Google Scholar]