Abstract
Background
US Centers for Disease Control and Prevention guidelines currently recommend triple-therapy antimicrobial treatment for anthrax meningitis. In the Kyrgyz Republic, a country with endemic anthrax, cutaneous anthrax patients are routinely hospitalized and treated successfully with only monotherapy or dual therapy. Clinical algorithms have been developed to identify patients with likely anthrax meningitis based on signs and symptoms alone. We sought to retrospectively identify likely meningitis patients in the Kyrgyz Republic using a clinical algorithm and evaluate risk factors and their outcomes by type of treatment.
Methods
We conducted a retrospective chart review of cutaneous anthrax patients in the Kyrgyz Republic from 2005 through 2012. Using previous methods, we developed a highly specific algorithm to categorize patients by meningitis status. We then evaluated patient risk factors, treatments, and outcomes by disease severity and meningitis status.
Results
We categorized 37 of 230 cutaneous anthrax patients as likely having meningitis. All 37 likely meningitis patients survived, receiving only mono- or dual-therapy antimicrobials. We identified underlying medical conditions, such as obesity, hypertension, and chronic obstructive pulmonary disease, and tobacco and alcohol use, as potential risk factors for severe anthrax and anthrax meningitis.
Conclusions
Based on our analyses, treatment of anthrax meningitis may not require 3 antimicrobials, which could impact future anthrax treatment recommendations. In addition, chronic comorbidities may increase risk for severe anthrax and anthrax meningitis. Future research should further investigate potential risk factors for severe anthrax and their impact on laboratory-confirmed meningitis and evaluate mono- and dual-therapy antimicrobial regimens for anthrax meningitis.
Keywords: anthrax, meningitis, treatment, Kyrgyz Republic
Based on our analyses, treatment of anthrax meningitis may not require 3 antimicrobials, which could impact future anthrax treatment recommendations. In addition, chronic comorbidities may increase risk for severe anthrax and anthrax meningitis.
Anthrax is caused by the zoonotic pathogen Bacillus anthracis and is often complicated by meningitis [1]. In 2014, the US Centers for Disease Control and Prevention (CDC) published guidelines for postexposure prophylaxis and treatment of anthrax that relied heavily on expert opinion in the absence of a formal evidence base [2–4]. In the 2014 document, 2 antimicrobials are recommended for treatment when meningitis can be ruled out [2]. If it cannot be ruled out, 3 antimicrobials are recommended for at least 2 weeks. Per recommendations, at least 1 of the 3 antimicrobials should be bactericidal, at least 1 should be a protein synthesis inhibitor, and all should have good central nervous system penetration.
In 2016, a clinical algorithm was developed using signs and symptoms to predict whether patients with systemic anthrax were likely to have confirmed meningitis [5]. This algorithm independently predicted meningitis using a 4-item assessment tool that included the following symptoms or signs: severe headache, altered mental status, meningeal signs, and other neurological signs. The presence of at least 1 of these 4 had an estimated sensitivity of 89% for identifying meningitis, while the presence of at least 2 made meningitis identification even more likely. More recently, univariate analysis of cutaneous anthrax patient data from 1950 through 2018 suggested confirmed meningitis could also be predicted in such patients by bacteremia anytime during hospitalization or by abdominal pain, lymphadenopathy, thoracic edema, or malignant pustule edema at presentation [6].
The Kyrgyz Republic is endemic for anthrax [7]. This Central Asian country has 6.6 million inhabitants; two-thirds live in rural settings. Agriculture and herding are the main economic activities and occur primarily on household farms and personal subsidiary plots. Most cases of anthrax are reported in the south, where herding and animal husbandry is common and the climate favors survival of B. anthracis spores in soil [7]. Animals contract anthrax through grazing, and humans are then infected through contact with dead or dying animals or contaminated by-products.
An analysis of cutaneous anthrax patients from the Kyrgyz Republic from 2005 through 2015 indicated that the vast majority were adult males exposed via slaughtering or butchering of sick farm animals. Most presented with mild disease, and none were reported to have meningitis [7]. Of the 74 categorized as having moderate or severe cutaneous anthrax, severe headache was noted for 65%; nausea and vomiting, 9%; confusion, 3%; and abdominal discomfort, 3%. Bacteremia was noted in one-third. Though none of these patients treated with 1 or 2 antimicrobials died, when they were discussed at a meeting, it was suggested some might have met the 2016 clinical criteria for anthrax meningitis. Data suggesting that patients with anthrax meningitis may be treated with fewer than 3 antimicrobials would be highly informative for physicians treating naturally occurring anthrax and for biopreparedness efforts.
To this end, we analyzed a case series of cutaneous anthrax patients in the Kyrgyz Republic. We developed a highly specific algorithm based on previous methods to categorize patients by meningitis status. We then applied it to our dataset to identify patients likely to have meningitis and evaluated risk factors, treatments, and outcomes.
METHODS
We abstracted medical chart data for all cutaneous anthrax patients hospitalized in 6 hospitals in Osh, Kyrgyz Republic, from 2005 through 2012. We abstracted information on demographics, medical and social history, symptoms, clinical course, outcome of current anthrax illness, laboratory testing, antimicrobial susceptibility results, and treatment. Data were abstracted in Russian into Epi Info version 7.1 (CDC, Atlanta, GA). These data were machine translated from Russian into English using Google Translate, and the translation was reviewed by a bilingual author (J. B.). There was some overlap in hospital sites and years between this study and the previous study [7], and approximately 40% of the anthrax patients were included in both analyses. However, patient information was wholly re-abstracted for this study using the current tool regardless of inclusion in the prior study.
Variable Definitions
The diagnosis of cutaneous anthrax was based on a history of exposure to sick or dead animals or animal products in a patient with clinical findings compatible with anthrax and supportive laboratory tests. Confirmatory laboratory tests in the Kyrgyz Republic include Gram stain, blood or lesion culture, and the anthraxin skin test [8]. Patients were categorized at presentation as having mild, moderate, or severe cutaneous anthrax per Kyrgyz convention [7]. Antimicrobial susceptibility testing was performed as a routine part of hospitalization; details can be found in the Supplementary Materials.
For this analysis, patients were considered to have fever or chills if any of the following terms were mentioned in their chief complaint: “fever,” “chills,” or “increased body temperature.” We defined measured fever as recorded temperature of >38°C, tachycardia as a recorded heart rate of ≥100 beats per minute, and tachypnea as a recorded respiratory rate of >20 breaths per minute, all at admission. We defined systolic and diastolic hypertension as a reported blood pressure at admission of systolic ≥130 mm Hg and diastolic ≥90 mm Hg, respectively. We defined systolic and diastolic hypotension as a reported blood pressure at admission of systolic <90 mm Hg and diastolic <60 mm Hg, respectively. We used pediatric cutoffs for temperature, tachycardia, and tachypnea based on Goldstein et al [9].
We categorized antimicrobials each patient received during hospitalization by the number of courses and concurrent antimicrobials received. A patient was categorized as receiving monotherapy alone if they only received 1 antimicrobial during hospitalization, sequential monotherapy if they received sequential courses of various monotherapies, dual therapy alone if they received a single course of 2 concurrent antimicrobials, dual therapy followed by monotherapy if they received a course of 2 concurrent antimicrobials followed by monotherapy with a different antimicrobial, and triple therapy if they received a course of 3 concurrent antimicrobials.
Algorithm to Identify Patients With Likely Anthrax Meningitis
We used data on adult systemic cutaneous anthrax cases abstracted from English-language literature published from 1880 through 2018 to develop a highly specific algorithm for categorizing the Kyrgyz patients into likely meningitis or likely nonmeningitis using previously developed methods [5, 10]. While the methods were the same, we selected different initial signs and symptoms compared with the previously published algorithms because meningeal signs and other neurological signs were not recorded in the abstracted Kyrgyz medical records. Additionally, new signs associated with meningitis in cutaneous anthrax patients have recently been proposed [6]. We selected the algorithm with one of the highest specificities, a reasonable sensitivity, and a modest proportion of unclassified cases and applied it to our dataset.
Our final algorithm included fever/chills (sign or symptom), nausea/vomiting, severe headache, altered mental status, and malignant pustule edema. Patients having 3 or more meningitis triage criteria were considered “likely meningitis,” while those with 0 or 1 were considered “likely nonmeningitis.” Patients with 2 triage criteria could not be classified. Based on the published literature dataset, the algorithm was highly specific (validation cohort: 93% specificity; 95% confidence interval [CI], 83%–100%), had moderate sensitivity (validation cohort: 69% sensitivity; 95% CI, 44%–94%), and left 20% of cases unclassified in the validation cohort. Results of all algorithm combinations can be found in Supplementary Table 1.
Since the proportion of patients with reported malignant pustule edema in our dataset was substantially higher than the proportion in the literature-derived dataset, we conducted a sensitivity analysis using an alternative definition of malignant pustule edema. We categorized anyone as having malignant pustule edema if any of the following terms were included in their chief complaint: “swell,” “edema,” or “inflamed.” The definitions of the other meningitis triage criteria were retained from the main analysis, and we categorized likely meningitis and likely nonmeningitis following the main analysis methods.
Analyses
We compared demographics, social and medical history, clinical presentation, laboratory results, and outcomes for patients based on their severity and meningitis classifications. Patients unclassified by the meningitis algorithm were excluded from meningitis analyses. Analyses were performed in Epi Info 7.1 and SAS version 9.4 (SAS Institute, Cary, NC). We used the χ2 test for differences for comparisons of categorical variables, the analysis of variance for comparisons of continuous variables, and univariate logistic regression to calculate odds ratios.
The Bioethical Committee at the International Higher School of Medicine in Bishkek, Kyrgyz Republic, approved the study.
RESULTS
Patient Cohort and Illness Severity
We identified and abstracted information on 230 cutaneous anthrax patients from 6 hospitals. The majority (60%) were admitted to 2 hospitals: Uzgen (37%) and Kara-Suu (23%). Most patients were aged 18–49 years (83%) and male (88%; Table 1). Patients with mild and moderate disease were younger (88% aged <50 years) than those with severe disease (32% aged <50 years).
Table 1.
Patient Demographics, Social History, and Chronic Conditions by Severity Among Cutaneous Anthrax Patients, Kyrgyz Republic, 2005–2012
| Characteristic | Overall (N = 230) |
Mild (N = 167) |
Moderate (N = 44) |
Severe (N = 19) |
P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Demographics | |||||||||
| Age, years | <.0001 | ||||||||
| <18 | 2 | (0.9) | 2 | (1.2) | 0 | (0) | 0 | (0) | |
| 18–49 | 190 | (82.6) | 147 | (88.0) | 37 | (84.1) | 6 | (31.6) | |
| ≥50 | 38 | (16.5) | 18 | (10.8) | 7 | (15.9) | 13 | (68.4) | |
| Male | 203 | (88.3) | 147 | (88.0) | 38 | (86.4) | 18 | (94.7) | .6 |
| Occupation | .02 | ||||||||
| Agriculture | 140 | (60.9) | 111 | (66.5) | 16 | (36.4) | 13 | (68.4) | |
| Industrya | 5 | (2.2) | 4 | (2.4) | 1 | (2.3) | 0 | (0) | |
| Construction | 22 | (9.6) | 14 | (8.4) | 8 | (18.2) | 0 | (0) | |
| Service sector | 21 | (9.1) | 15 | (9.0) | 5 | (11.4) | 1 | (5.3) | |
| Unemployedb | 42 | (18.3) | 23 | (13.8) | 14 | (31.8) | 5 | (26.3) | |
| Social history | |||||||||
| Smoker (male)c | <.0001 | ||||||||
| Current | 93 | (45.8) | 53 | (36.1) | 29 | (76.3) | 11 | (61.1) | |
| Former | 20 | (9.9) | 11 | (7.5) | 4 | (10.5) | 5 | (27.8) | |
| Never | 89 | (43.8) | 83 | (56.5) | 5 | (13.2) | 1 | (5.6) | |
| Alcohol use (male)c | <.0001 | ||||||||
| Current | 28 | (13.8) | 22 | (15.0) | 3 | (7.9) | 3 | (16.7) | |
| Former | 51 | (25.1) | 23 | (15.6) | 19 | (50.0) | 9 | (47.4) | |
| Never | 124 | (61.1) | 102 | (69.4) | 16 | (42.1) | 6 | (33.3) | |
| Chronic conditions | |||||||||
| Obesity | 53 | (23.0) | 22 | (13.2) | 16 | (36.4) | 15 | (78.9) | <.0001 |
| Diabetes | 4 | (1.7) | 0 | (0) | 2 | (4.5) | 2 | (10.5) | .001 |
| Hypertension | 40 | (17.4) | 11 | (6.6) | 14 | (31.8) | 15 | (78.9) | <.0001 |
| Chronic obstructive pulmonary disease | 53 | (23.0) | 28 | (16.8) | 13 | (29.5) | 12 | (63.2) | <.0001 |
| Otherd | 1 | (0.4) | 0 | (0) | 0 | (0) | 1 | (5.3) | .004 |
| Number of chronic conditions | <.0001 | ||||||||
| 0 | 137 | (59.6) | 121 | (72.5) | 16 | (36.4) | 0 | (0) | |
| 1 | 51 | (22.2) | 33 | (19.8) | 14 | (31.8) | 4 | (21.1) | |
| 2 | 26 | (11.3) | 11 | (6.6) | 11 | (25.0) | 4 | (21.1) | |
| 3 | 16 | (7.0) | 2 | (1.2) | 3 | (6.8) | 11 | (57.9) | |
Includes butcher, slaughterhouse worker, tanner.
Includes housewives, pensioners, students.
All female patients reported being never smokers and never drinking alcohol, except 1 who reported being a current smoker and 1 who reported being a former drinker.
One patient reported renal failure.
Overall, 60% of patients reported no underlying conditions (Table 1). However, this varied by severity status; all patients with severe anthrax reported underlying conditions, while only a quarter (27%) of mild anthrax patients did so. While 46% of male patients reported being current smokers, only 1 female patient reported the same. Among males, never smoking was more often seen with mild than severe anthrax (57% vs 6%). Alcohol use also varied by sex and severity; 14% of male patients, but no female patients, reported current consumption of alcohol. Among male patients, never drinking was more often seen with mild than severe anthrax (69% vs 33%).
Almost all (98%) patients were described as having malignant pustule edema (Table 2). Other common symptoms overall included fever or chills (52%), headache (27%), nausea with or without vomiting (17%), and severe headache (7%). Two patients with severe illness reported confusion. Hypertension, both systolic and diastolic, was most commonly observed in patients with severe illness. While not commonly observed in patients with mild or moderate illness, tachycardia and tachypnea were observed in most (58% and 53%, respectively) patients with severe illness.
Table 2.
Symptoms, Signs, Laboratory Tests, and Treatment by Severity Among Cutaneous Anthrax Patients, Kyrgyz Republic, 2005–2012
| Characteristic | Overall (N = 230) |
Mild (N = 167) |
Moderate (N = 44) |
Severe (N = 19) |
P Value | ||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | ||
| Symptoms at presentation | |||||||||
| Fever or chills | 120 | (52.2) | 70 | (41.9) | 32 | (72.7) | 18 | (94.7) | <.0001 |
| Headache | 63 | (27.4) | 1 | (0.6) | 43 | (97.7) | 19 | (100) | <.0001 |
| Severe headache | 17 | (7.4) | 0 | (0) | 5 | (11.4) | 12 | (63.2) | <.0001 |
| Nausea or vomiting | 39 | (17.0) | 29 | (17.4) | 9 | (20.5) | 1 | (5.3) | .5 |
| Abdominal pain | 2 | (0.9) | 1 | (0.6) | 0 | (0) | 1 | (5.3) | .3 |
| Confusion | 2 | (0.9) | 0 | (0) | 0 | (0) | 2 | (10.5) | <.0001 |
| Malignant pustule edemaa | 226 | (98.3) | 165 | (98.8) | 43 | (97.7) | 18 | (94.7) | .5 |
| Vital signs at presentation | |||||||||
| Measured feverb | 54 | (23.5) | 0 | (0) | 37 | (84.1) | 17 | (89.5) | <.0001 |
| Tachycardiac | 11 | (4.8) | 0 | (0) | 0 | (0) | 11 | (57.9) | <.0001 |
| Tachypnead | 13 | (5.7) | 2 | (1.2) | 1 | (2.3) | 10 | (52.6) | <.0001 |
| Systolic hypertensione | 68 | (29.6) | 36 | (21.6) | 18 | (40.9) | 14 | (73.7) | <.0001 |
| Diastolic hypertensione | 28 | (12.2) | 7 | (4.2) | 8 | (18.2) | 13 | (68.4) | <.0001 |
| Laboratory tests | |||||||||
| White blood cell count ×109/L, mean (SD) | 7.2 | (2.2) | 6.8 | (2.0) | 8.4 | (2.3) | 8.6 | (2.6) | <.0001 |
| Lesion culture positivea | 117 | (50.9) | 87 | (52.1) | 21 | (47.7) | 9 | (47.4) | .6 |
| Lesion Gram stain positive | 106 | (46.1) | 76 | (45.5) | 24 | (54.5) | 6 | (31.6) | .2 |
| Blood culture positivea | 20 | (8.7) | 3 | (1.8) | 9 | (20.5) | 8 | (42.1) | <.0001 |
| Positive culture or Gram staina | 202 | (87.8) | 147 | (88.0) | 39 | (88.6) | 16 | (84.2) | .9 |
| Positive culture, Gram stain, or anthraxin testa | 213 | (92.6) | 157 | (94.0) | 40 | (90.9) | 16 | (84.2) | .3 |
| Treatment | <.0001 | ||||||||
| Monotherapy alone | 195 | (84.8) | 153 | (91.6) | 42 | (95.5) | 0 | (0) | |
| Sequential monotherapy | 15 | (6.5) | 14 | (8.4) | 1 | (2.3) | 0 | (0) | |
| Dual therapy alone | 3 | (1.3) | 0 | (0) | 1 | (2.3) | 2 | (10.5) | |
| Dual therapy followed by monotherapy | 17 | (7.4) | 0 | (0) | 0 | (0) | 17 | (89.5) | |
| Triple therapy | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | |
| Outcome | |||||||||
| Fatal | 0 | (0) | 0 | (0) | 0 | (0) | 0 | (0) | 1.0 |
| Length of hospital stay, mean (SD), days | 18.0 | (4.3) | 17.3 | (4.2) | 18.4 | (3.1) | 23.5 | (3.5) | <.0001 |
Abbreviation: SD, standard deviation.
There was 1 patient with unspecified malignant pustule edema results, 6 patients with unspecified blood culture results, 7 patients with unspecified lesion culture results, and 1 patient with unspecified anthraxin results.
Measured fever: recorded temperature at admission >38°C for patients aged ≥18 years or >38.5°C for patients aged 13–17 years.
Tachycardia: recorded heart rate at admission of ≥100 beats per minute for patients aged ≥18 years or >110 beats per minute for patients aged 13–17 years.
Tachypnea: recorded respiratory rate at admission of >20 breaths per minute for patients aged ≥18 years or >14 breaths per minute for patients aged 13–17 years.
Systolic and diastolic hypertension: recorded blood pressure at admission of systolic ≥130 mm Hg and diastolic ≥90 mm Hg, respectively.
The proportion of patients with bacteriemia increased with disease severity; only 3 patients with mild disease (2%) had B. anthracis isolated from their blood compared with 42% of patients with severe disease (Table 2). Anthrax was confirmed in 213 patients (93%) by laboratory testing using positive culture, Gram stain, or anthraxin test. In the remaining 17 patients, the diagnosis was established based on clinical and epidemiological data alone.
All patients received antimicrobials during their hospitalization; regimens varied according to patient severity on presentation. Most (85%) patients received antimicrobial monotherapy with penicillin, ciprofloxacin, or doxycycline for 10 days. All patients with severe disease received 2 concurrent antimicrobials for 10 days; for most (90%), this was followed by a course of monotherapy. Among patients who began with dual therapy (n = 20), 14 (70%) received 2 bactericidal antimicrobials, 5 (25%) received 1 bactericidal and 1 protein synthesis inhibitor, and 1 received 2 protein synthesis inhibitors. No patients received 3 antimicrobials concurrently, required a ventilator, were admitted to an intensive care unit, or died.
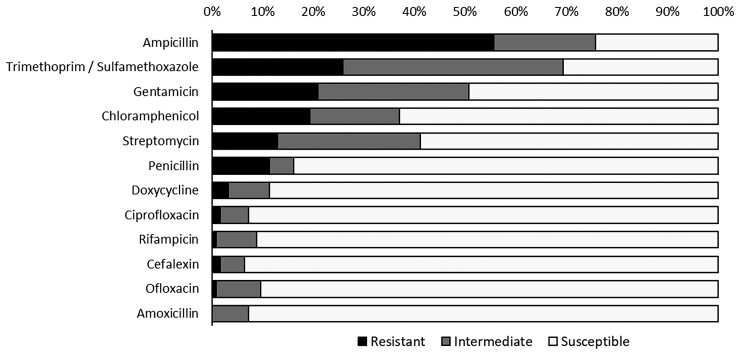
Records of susceptibility testing showed ampicillin resistance to be most common (56%), followed by resistance to trimethoprim/sulfamethoxazole (26%) and gentamicin (21%; Figure 1). Susceptibility patterns did not differ greatly by severity classification (Supplementary Table 2). All patients reported antimicrobial use for a prior illness in the year before their current anthrax illness (Supplementary Table 3).
Figure 1.
Antimicrobial susceptibility testing results based on the agar-based disk diffusion method for 124 cutaneous anthrax patients with positive blood or lesion culture results, Kyrgyz Republic, 2005–2012.
Anthrax Meningitis Algorithm
We categorized 89 patients (39%) as likely nonmeningitis as they met 0 (n = 1) or 1 (n = 88) of the meningitis triage criteria. We categorized 37 patients (16%) as likely meningitis as they met 3 (n = 34) or 4 (n = 3) of the meningitis triage criteria. The remaining 104 patients (45%) met 2 of the meningitis triage criteria, meaning there was insufficient information to determine if they had meningitis or not.
Risk Factors for Likely Meningitis
Compared with likely nonmeningitis patients, likely meningitis patients were more likely to be aged >50 years and have underlying chronic conditions (Table 3). More than half (54%) were obese, 46% reported hypertension, and 35% reported chronic obstructive pulmonary disease (COPD). By comparison, 76% of likely nonmeningitis patients reported no underlying medical conditions. Male patients with likely nonmeningitis were more likely to be never smokers (54%) or never drinkers (71%) compared with likely meningitis patients (16% and 56%, respectively). Chronic conditions, ever smoker, and any reported alcohol use increased the odds of likely meningitis even after controlling for age (Supplementary Table 4).
Table 3.
Patient Demographics, Social and Medical History, and Presenting Signs and Symptoms by Meningitis Status Among Cutaneous Anthrax Patients, Kyrgyz Republic, 2005–2012
| Characteristic | Likely Nonmeningitis (N = 89) | Likely Meningitis (N = 37) | Odds Ratio (95% Confidence Interval) |
P Value | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Demographics | ||||||
| Age, years | ||||||
| <18 | 1 | (1.1) | 0 | (0) | ... | ... |
| 18–49 | 76 | (85.4) | 28 | (75.7) | Reference | ... |
| ≥50 | 12 | (13.5) | 9 | (24.3) | 2.0 (.8–5.4) | .1 |
| Male | 78 | (87.6) | 32 | (86.5) | 0.9 (.3–2.8) | .9 |
| Chronic conditions | ||||||
| Obesity | 9 | (10.1) | 20 | (54.1) | 10.5 (4.1–26.9) | <.0001 |
| Diabetes | 0 | (0) | 4 | (10.8) | 13.7 (2.3–∞) | .007 |
| Hypertension | 4 | (4.5) | 17 | (45.9) | 18.1 (5.5–59.6) | <.0001 |
| Chronic obstructive pulmonary disease | 15 | (16.9) | 13 | (35.1) | 2.7 (1.1–6.4) | .03 |
| Number of chronic conditions | ||||||
| 0 | 68 | (76.4) | 9 | (24.3) | Reference | ... |
| 1 | 15 | (16.9) | 12 | (32.4) | 6.0 (2.2–16.9) | .0006 |
| 2 | 5 | (5.6) | 6 | (16.2) | 9.1 (2.3–35.9) | .0017 |
| 3 | 1 | (1.1) | 10 | (27.0) | 75.6 (8.6–661.7) | <.0001 |
| Social history | ||||||
| Smoker (male)a | ||||||
| Never | 42 | (53.8) | 5 | (15.6) | Reference | ... |
| Former | 2 | (2.6) | 7 | (21.9) | 29.4 (4.7–182.3) | .005 |
| Current | 34 | (43.6) | 19 | (59.4) | 4.7 (1.6–13.9) | .0003 |
| Alcohol use (male)a | ||||||
| Never | 55 | (70.5) | 18 | (56.3) | Reference | ... |
| Former | 9 | (11.5) | 11 | (34.4) | 3.7 (1.3–10.5) | .01 |
| Current | 14 | (17.9) | 3 | (9.4) | 0.7 (.2–2.5) | .5 |
| Illness presentation | ||||||
| Anthrax severity category | ||||||
| Mild | 81 | (91.0) | 12 | (32.4) | Reference | ... |
| Moderate | 7 | (7.9) | 12 | (32.4) | 11.6 (3.8–35.2) | <.0001 |
| Severe | 1 | (1.1) | 13 | (35.1) | 87.8 (10.5–732.8) | <.0001 |
| Fever or chills | 3 | (3.4) | 34 | (91.9) | 324.9 (62.5–∞) | <.0001 |
| Headache | 9 | (10.1) | 25 | (67.6) | 18.5 (7.0–49.0) | <.0001 |
| Severe headache | 0 | (0) | 17 | (45.9) | 100.0 (20.5–∞) | <.0001 |
| Nausea or vomiting | 0 | (0) | 21 | (56.8) | 151.0 (31.1–∞) | <.0001 |
| Confusion | 0 | (0) | 2 | (5.4) | 5.9 (0.7–∞) | .08 |
| Malignant pustule edemab | 85 | (95.5) | 37 | (100) | 1.6 (.2–∞) | .3 |
| Measured feverc | 2 | (2.2) | 25 | (67.6) | 90.6 (19.0–432.0) | <.0001 |
| Heart rate per minute, mean (SD) | 80.4 | (5.4) | 87.8 | (10.4) | 1.1 (1.1–1.2) | <.0001 |
| Tachycardiad | 0 | (0) | 10 | (27.0) | 43.7 (8.6–∞) | <.0001 |
| Respirations per minute, mean (SD) | 17.5 | (1.4) | 19.5 | (2.3) | 1.9 (1.4–2.5) | <.0001 |
| Tachypneae | 1 | (1.1) | 9 | (24.3) | 27.5 (3.5–∞) | .0001 |
| Systolic hypertensionf | 17 | (19.1) | 16 | (43.2) | 3.2 (1.4–7.5) | .006 |
| Diastolic hypertensionf | 2 | (2.2) | 14 | (37.8) | 26.5 (5.6–124.9) | <.0001 |
Abbreviation: SD, standard deviation.
All female patients reported being never smokers and never drinking alcohol except 1 who reported being a current smoker and 1 who reported being a former drinker.
There was 1 patient with unspecified malignant pustule edema results.
Measured fever: recorded temperature at admission >38°C for patients aged ≥18 years or >38.5°C for patients aged 13–17 years.
Tachycardia: recorded heart rate at admission of ≥100 beats per minute for patients aged ≥18 years or >110 beats per minute for patients aged 13–17 years.
Tachypnea: recorded respiratory rate at admission of >20 breaths per minute for patients aged ≥18 years or >14 breaths per minute for patients aged 13–17 years.
Systolic and diastolic hypertension: recorded blood pressure at admission of systolic ≥130 mm Hg and diastolic ≥90 mm Hg, respectively.
Almost all (91%) likely nonmeningitis patients were classified as having mild disease at presentation. Compared with likely nonmeningitis patients, likely meningitis patients were more likely to present with tachycardia (27% vs 0%), tachypnea (24% vs 1%), systolic hypertension (43% vs 19%), and diastolic hypertension (38% vs 2%). They also had higher rates of bacteremia (30% vs 2%) and slightly higher median white blood cell and platelet counts (Table 4). The 1 patient with laboratory-confirmed meningitis was classified as likely meningitis by the algorithm.
Table 4.
Patient Laboratory Results and Treatments by Meningitis Status Among Cutaneous Anthrax Patients, Kyrgyz Republic, 2005–2012
| Characteristic | Likely Nonmeningitis (N = 89) | Likely Meningitis (N = 37) | Odds Ratio (95% Confidence Interval) |
P Value | ||
|---|---|---|---|---|---|---|
| n | (%) | n | (%) | |||
| Laboratory results on admission | ||||||
| White blood count ×109/L, mean (SD) | 6.5 | (1.9) | 8.0 | (2.5) | 1.4 (1.1–1.7) | .001 |
| Hemoglobin g/L, mean (SD) | ||||||
| Male | 127 | (6.2) | 130 | (5.0) | 1.1 (1.0–1.2) | .03 |
| Female | 126 | (10) | 120 | (7.3) | 0.9 (.8–1.1) | .2 |
| Hematocrit, mean (SD) | ||||||
| Male | 0.45 | (0.02) | 0.44 | (0.03) | 0 (0–0) | .001 |
| Female | 0.43 | (0.04) | 0.43 | (0.03) | 0.07 (0–∞) | .9 |
| Platelets ×109/L, mean (SD) | 261 | (55) | 281 | (36) | 1.0 (1.0–1.0) | .05 |
| Lesion culture positivea | 37 | (41.6) | 19 | (51.4) | 1.4 (.6–3.0) | .4 |
| Lesion Gram stain positive | 51 | (57.3) | 20 | (54.1) | 1.9 (.7–5.0) | .2 |
| Blood culture positivea | 2 | (2.2) | 11 | (29.7) | 17.1 (3.6–82.4) | .0004 |
| Positive culture or Gram staina | 77 | (86.5) | 34 | (91.9) | 1.8 (.5–6.7) | .4 |
| Positive culture, Gram stain, or anthraxin testa | 84 | (94.4) | 35 | (94.6) | 1.0 (.2–5.6) | 1.0 |
| Treatment and outcome | ||||||
| Antimicrobial treatment combinations | ||||||
| Monotherapy alone | 83 | (93.3) | 21 | (56.8) | Reference | ... |
| Sequential monotherapy | 5 | (5.6) | 2 | (5.4) | 1.6 (.1–10.5) | .9 |
| Dual therapy alone | 0 | (0) | 3 | (8.1) | 14.4 (2.1–∞) | .01 |
| Dual therapy followed by monotherapy | 1 | (1.1) | 11 | (29.7) | 41.8 (5.5–∞) | <.0001 |
| Triple therapy | 0 | (0) | 0 | (0) | ... | ... |
| Fatal | 0 | (0) | 0 | (0) | ... | ... |
| Length of hospital stay, mean (SD), days | 16.9 | (3.8) | 19.8 | (4.8) | 1.2 (1.1–1.3) | .002 |
Abbreviation: SD, standard deviation.
There were 6 patients with unspecified blood culture results, 7 patients with unspecified lesion culture results, and 1 patient with unspecified anthraxin results.
A higher proportion of likely nonmeningitis patients (93%) received monotherapy alone compared with likely meningitis patients; however, more than half of likely meningitis patients also received monotherapy treatment alone (57%; Table 4). Only 1 likely nonmeningitis patient received dual therapy followed by monotherapy, compared with 30% of likely meningitis patients. Patients with likely meningitis had longer hospital stays than likely nonmeningitis patients, staying on average 19.8 days (±4.8) compared with 16.9 (±3.8) days.
Using the alternative definition in the sensitivity analysis, we classified 82 patients (36%) as having malignant pustule edema compared with 226 patients (98%) in the main analysis, leading to only 8 patients being classified as likely meningitis in the sensitivity analysis. Trends and differences between likely meningitis and likely nonmeningitis patients were similar for the main and sensitivity analyses (Supplementary Table 5).
DISCUSSION
In this study, we extracted information on 230 cutaneous anthrax patients in the Kyrgyz Republic and categorized 37 patients as likely having meningitis using a clinical algorithm. This is the first application of an anthrax meningitis screening tool to a dataset not involved in its creation. All 37 patients survived, despite receiving only mono- or dual-therapy antimicrobials, in contrast to the US CDC guidelines that recommend triple therapy for anthrax meningitis patients. We also identified chronic diseases (eg, obesity, hypertension, COPD) and tobacco and alcohol use as potential risk factors for severe anthrax disease and anthrax meningitis, which might help clinicians identify at-risk patients.
Previously, algorithms developed to identify anthrax patients with meningitis used 80% of the data to create the algorithm and 20% to validate it [5, 10]. In our study, we used published cases of systemic cutaneous anthrax and laboratory-confirmed anthrax meningitis to create our primary and validation samples and then applied the final algorithm to the Kyrgyz case series. Our algorithm identified fever and chills, nausea and vomiting, malignant pustule edema, severe headache, and altered mental status as being triage criteria for anthrax meningitis, all of which have been documented in the literature as being associated with meningitis [5, 6]. Application of our algorithm to the Kyrgyz dataset categorized 16.1% of the cases as likely meningitis. Although Lanska [1], in a review of anthrax meningitis, suggested that meningitis developed in 5% of cutaneous cases, the percentage of Kyrgyz patients categorized as likely meningitis in our study is consonant with Hendricks et al, in which 55 of 340 (16.2%) adults hospitalized for cutaneous anthrax were confirmed with meningitis [11]. Although our final algorithm left 45% of patients in the Kyrgyz case series unclassified, we were primarily focused on minimizing false positives for this analysis to evaluate treatment and outcomes for patients most likely to have meningitis. In other circumstances, such as the development of a potential triage tool for treatment, an algorithm that maximizes sensitivity or minimizes the proportion of unclassified cases may be more important.
Certain chronic comorbidities, such as diabetes mellitus, and tobacco and alcohol use have been linked to more severe disease for many infectious diseases, including other types of bacterial meningitis and coronavirus disease 2019 [12–15]. To our knowledge, we are the first to report these as risk factors for anthrax meningitis and severe anthrax disease. Metabolic syndrome, which encompasses conditions such as obesity, hypertension, and diabetes mellitus, has been linked to increased permeability in the blood–brain barrier [16], which could be a mechanism for the increased meningitis risk.
Prior to this study, only 12 survivors of anthrax meningitis had been identified in the English medical literature since 1880 [17–27]. We identified an additional 37 possible survivors, a 308% increase; none received 3 concurrent antimicrobials. Even when we restricted our definition of malignant pustule edema from our screening tool as part of a sensitivity analysis, we still identified 8 patients as likely having meningitis (a 67% increase); all survived with, at most, dual therapy. The large difference in survival rates from previously published literature could partially be due to a past bias toward reporting only the most severe patients and milder forms of anthrax meningitis potentially going undiagnosed and unpublished.
While the 2014 US CDC guidelines recommend 3 antimicrobials in patients with anthrax meningitis, our analysis suggests 2 might suffice in certain contexts. However, since meningitis was not clinically confirmed in our study, future research is needed among confirmed anthrax meningitis patients. We noted no added benefit regarding mortality or length of stay for likely meningitis patients who were treated with 2 compared with 1 antimicrobial or patients who received a protein synthesis inhibitor and a bactericidal antimicrobial compared with those who received other combinations. However, each category had only a handful of patients. Similarly, US CDC guidelines recommend dual therapy for systemic patients without meningitis. Our study suggests monotherapy may suffice for cutaneous patients without meningitis and may warrant further evaluation.
We saw high levels of resistance to commonly used antimicrobials in our patients and higher levels of antimicrobial resistance compared with other studies [28, 29]. Differences in laboratory methods, patient populations, and study years could have resulted in this discrepancy. As in many countries around the world, Kyrgyz farmers routinely feed their livestock antimicrobials to promote growth and prevent disease, which could lead to an increase in resistant bacteria in livestock that could be passed on to humans [30]. Additionally, in the Kyrgyz Republic, over-the-counter antimicrobials are widely used to self-medicate for a variety of illnesses [31]. Our data reflected this: all patients reported taking ampicillin and 1–3 other antimicrobials during the past year to treat other illnesses. Together, the widespread antimicrobial use in animals and for self-treatment in humans could be contributing to the high levels of antimicrobial resistance seen in our analysis [32, 33].
Our study has several key strengths. The 230 patients with cutaneous anthrax who we identified from 6 hospitals in Osh (2005 through 2012) represent a comparatively large patient sample for a rare global disease and is more than twice the number passively reported to the Kyrgyz Ministry of Health for this timeframe and region (n = 85). Second, because it is standard practice in the Kyrgyz Republic to hospitalize all diagnosed anthrax patients regardless of severity, we could access complete hospital records for all patients and collect information on clinical course, treatment, and laboratory testing.
We also acknowledge 5 important limitations to our study. First, we could not confirm meningitis in patients classified by our algorithm because confirmatory laboratory testing was not performed. Additionally, because all patients presented with cutaneous anthrax, meningitis was rarely suspected, and pertinent signs and symptoms were not recorded in the charts. However, our algorithm did successfully classify the 1 patient in our sample tested and confirmed for meningitis during hospitalization as likely meningitis. Second, the definition of malignant pustule edema, one of our meningitis triage criteria, is not standardized and may vary by hospital and setting. In our sample, 98% of patients reported malignant pustule edema compared with 17% in the dataset used to create the algorithm. To address this difference, we conducted a sensitivity analysis that identified malignant pustule edema in 36% of patients based on chief complaint. This analysis classified fewer patients as likely meningitis, but had similar trends, giving us confidence in our results. Third, the B. anthracis susceptibility testing on Kyrgyz patient cultures did not match Clinical and Laboratory Standards Institute standards [34], so results may differ. Disk diffusion methods can produce variable results for β-lactam testing on B. anthracis, and our findings here should be verified by broth microdilution [35]. Fourth, like all retrospective chart reviews, only information recorded at the time of hospitalization was available. This information may be incomplete or inaccurate at the individual patient level, and reporting biases may arise from potential cases that did not seek care. Self-reported variables that carry social stigmas, such as alcohol use, may not have been accurate or uniformly collected across hospitals and clinicians. Finally, these findings among Kyrgyz patients may not directly apply to other populations due to demographic differences.
Future research should delve into the issue of comorbidities as risk factors for severe anthrax and laboratory-confirmed meningitis. Human research could include biomarkers of inflammation, including iron metabolism, and chronic disease animal models might help elucidate pathophysiologic mechanisms. The utility of triage tools similar to ours should be evaluated in non-Kyrgyz populations, as well as their accuracy using confirmatory laboratory testing for meningitis. Prospective clinical studies should be carried out in endemic areas that currently use 1 or 2 antimicrobial regimens for treatment of hospitalized patients to assess their effectiveness against laboratory-confirmed anthrax meningitis.
CONCLUSIONS
Anthrax is a rare but highly pathogenic disease of global concern. Obesity, hypertension, and COPD were associated with severe anthrax and likely meningitis. Extra resources may need to be devoted to such patients. In low-resource endemic areas or following a B. anthracis mass event, triage tools could be useful for differentiating patients with likely meningitis. Furthermore, based on our analyses, treatment of anthrax meningitis may not require 3 antimicrobials, which could impact anthrax treatment in the future.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the authors' affiliated institutions.
Financial support. This project was supported by the Centers for Disease Control and Prevention and the Office of the Assistant Secretary for Preparedness and Response.
Supplement sponsorship. This article appears as part of the supplement “Anthrax Preparedness,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Supplementary Material
Contributor Information
Ainura Kutmanova, Department of Infectious Diseases, International Higher School of Medicine, Bishkek, Kyrgyz Republic.
Saparbai Zholdoshev, Department of Epidemiology, Microbiology with a course of Infectious Diseases, Osh State University, Osh, Kyrgyz Republic.
Katherine M Roguski, Division of High-Consequence Pathogens and Pathology, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Melis Sholpanbay uulu, Department of Infectious Diseases, Kyrgyz State Medical Academy, Bishkek, Kyrgyz Republic.
Marissa K Person, Division of High-Consequence Pathogens and Pathology, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Rachel Cook, Division of High-Consequence Pathogens and Pathology, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Julia Bugrysheva, Division of Preparedness and Emerging Infections, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Patrick Nadol, CDC Kyrgyzstan, US Centers for Disease Control and Prevention, Bishkek, Kyrgyz Republic.
Aisuluu Buranchieva, Department of Infectious Diseases, International Higher School of Medicine, Bishkek, Kyrgyz Republic.
Lira Imanbaeva, Department of Infectious Diseases, International Higher School of Medicine, Bishkek, Kyrgyz Republic.
Ainura Dzhangazieva, Department of Infectious Diseases, International Higher School of Medicine, Bishkek, Kyrgyz Republic.
William A Bower, Division of High-Consequence Pathogens and Pathology, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine Hendricks, Division of High-Consequence Pathogens and Pathology, US Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
References
- 1. Lanska DJ. Anthrax meningoencephalitis. Neurology 2002; 59:327–34. [DOI] [PubMed] [Google Scholar]
- 2. Hendricks KA, Wright ME, Shadomy SV, et al. Centers for Disease Control and Prevention expert panel meetings on prevention and treatment of anthrax in adults. Emerg Infect Dis 2014; 20:e130687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bradley JS, Peacock G, Krug SE, et al. Pediatric anthrax clinical management. Pediatrics 2014; 133:e1411-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Meaney-Delman D, Zotti ME, Creanga AA, et al. Special considerations for prophylaxis for and treatment of anthrax in pregnant and postpartum women. Emerg Infect Dis 2014; 20:e130611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Katharios-Lanwermeyer S, Holty JE, Person M, et al. Identifying meningitis during an anthrax mass casualty incident: systematic review of systemic anthrax since 1880. Clin Infect Dis 2016; 62:1537–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Thompson JM, Cook R, Person MK, et al. Risk factors for death or meningitis in adults hospitalized for cutaneous anthrax, 1950–2018: a systematic review. Clin Infect Dis 2022; 75:S459–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kutmanova A, Doganay M, Zholdoshev S. Human anthrax in Kyrgyz Republic: epidemiology and clinical features. J Infect Public Health 2020; 13:1161–5. [DOI] [PubMed] [Google Scholar]
- 8. Shlyakhov E, Rubinstein E. Evaluation of the anthraxin skin test for diagnosis of acute and past human anthrax. Eur J Clin Microbiol Infect Dis 1996; 15:242–5. [DOI] [PubMed] [Google Scholar]
- 9. Goldstein B, Giroir B, Randolph A. International Pediatric Sepsis Consensus Conference: definitions for sepsis and organ dysfunction in pediatrics. Pediatr Crit Care Med 2005; 6:2–8. [DOI] [PubMed] [Google Scholar]
- 10. Binney S, Person MK, Traxler RM, Cook R, Bower WA, Hendricks K. Algorithms for the identification of anthrax meningitis during a mass casualty event based on a systematic review of systemic anthrax from 1880 through 2018. Clin Infect Dis 2022; 75:S468–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hendricks K, Person MK, Bradley JS, et al. Clinical features of patients hospitalized for all routes of anthrax, 1880–2018: a systematic review. Clin Infect Dis 2022; 75:S341–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Brouwer MC, Tunkel AR, van de Beek D. Epidemiology, diagnosis, and antimicrobial treatment of acute bacterial meningitis. Clin Microbiol Rev 2010; 23:467–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. World Health Organization . Fact sheet on the SDGs: Alcohol consumption and sustainable development (2020). Available at: https://www.euro.who.int/en/health-topics/health-policy/sustainable-development-goals/publications/2017/fact-sheets-on-the-sustainable-development-goals-sdgs-health-targets/fact-sheet-on-the-sdgs-alcohol-consumption-and-sustainable-development-2020. Accessed 13 April 2022.
- 14. Lundbo LF, Benfield T. Risk factors for community-acquired bacterial meningitis. Infect Dis (Lond) 2017; 49:433–44. [DOI] [PubMed] [Google Scholar]
- 15. van de Beek D, Brouwer M, Hasbun R, Koedel U, Whitney CG, Wijdicks E. Community-acquired bacterial meningitis. Nat Rev Dis Primers 2016; 2:16074. [DOI] [PubMed] [Google Scholar]
- 16. Van Dyken P, Lacoste B. Impact of metabolic syndrome on neuroinflammation and the blood-brain barrier. Front Neurosci 2018; 12:930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Shanahan RH, Griffin JR, Von Auersperg AP. Anthrax meningitis; report of a case of internal anthrax with recovery. Am J Clin Pathol 1947; 17:719–22. [DOI] [PubMed] [Google Scholar]
- 18. Weinstein L, Oliver CS. The treatment of human anthrax with penicillin. Am Pract Dig Treat 1948; 2:533–8. [PubMed] [Google Scholar]
- 19. Tahernia AC, Hashemi G. Survival in anthrax meningitis. Pediatrics 1972; 50:329–33. [PubMed] [Google Scholar]
- 20. Tengio FU. Anthrax meningitis. Report of two cases. East Afr Med J 1973; 50:337–9. [PubMed] [Google Scholar]
- 21. Nalin DR, Sultana B, Sahunja R, et al. Survival of a patient with intestinal anthrax. Am J Med 1977; 62:130–2. [DOI] [PubMed] [Google Scholar]
- 22. Khanna N, Gokul BN, Ravikumar R, et al. Successfully treated primary anthrax meningitis. Indian J Pathol Microbiol 1989; 32:315–7. [PubMed] [Google Scholar]
- 23. Tabatabaie P, Syadati A. Bacillus anthracis as a cause of bacterial meningitis. Pediatr Infect Dis J 1993; 12:1035–7. [DOI] [PubMed] [Google Scholar]
- 24. Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 2001; 7:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faghihi G, Siadat AH. Cutaneous anthrax associated with facial palsy: case report and literature review. J Dermatolog Treat 2003; 14:51–3. [DOI] [PubMed] [Google Scholar]
- 26. Bindu M, Vengamma B, Kumar G. Anthrax meningoencephalitis successfully treated. Eur J Neurol 2007; 14:e18. [DOI] [PubMed] [Google Scholar]
- 27. Booth M, Donaldson L, Cui X, et al. Confirmed Bacillus anthracis infection among persons who inject drugs, Scotland, 2009–2010. Emerg Infect Dis 2014; 20:1452–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Ortatatli M, Karagoz A, Percin D, Kenar L, Kilic S, Durmaz R. Antimicrobial susceptibility and molecular subtyping of 55 Turkish Bacillus anthracis strains using 25-loci multiple-locus VNTR analysis. Comp Immunol Microbiol Infect Dis 2012; 35:355–61. [DOI] [PubMed] [Google Scholar]
- 29. Cavallo JD, Ramisse F, Girardet M, Vaissaire J, Mock M, Hernandez E. Antibiotic susceptibilities of 96 isolates of Bacillus anthracis isolated in France between 1994 and 2000. Antimicrob Agents Chemother 2002; 46:2307–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Silbergeld EK, Graham J, Price LB. Industrial food animal production, antimicrobial resistance, and human health. Annu Rev Public Health 2008; 29:151–69. [DOI] [PubMed] [Google Scholar]
- 31. Versporten A, Bolokhovets G, Ghazaryan L, et al. Antibiotic use in eastern Europe: a cross-national database study in coordination with the WHO Regional Office for Europe. Lancet Infect Dis 2014; 14:381–7. [DOI] [PubMed] [Google Scholar]
- 32. McEwen SA, Collignon PJ. Antimicrobial resistance: a one health perspective. Microbiol Spectr 2018; 6(2). doi:10.1128/microbiolspec.ARBA-0009-2017 [DOI] [PubMed] [Google Scholar]
- 33. Zarb P, Goossens H. Human use of antimicrobial agents. Rev Sci Tech 2012; 31:121–33. [DOI] [PubMed] [Google Scholar]
- 34. Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th Ed. CLSI standard M07. Wayne, PA: Clinical and Laboratory Standards Institute, 2018. [Google Scholar]
- 35. Mohammed MJ, Marston CK, Popovic T, Weyant RS, Tenover FC. Antimicrobial susceptibility testing of Bacillus anthracis: comparison of results obtained by using the National Committee for Clinical Laboratory Standards broth microdilution reference and Etest agar gradient diffusion methods. J Clin Microbiol 2002; 40:1902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.