Abstract
Background
Anthrax is endemic to many countries, including the United States. The causative agent, Bacillus anthracis, poses a global bioterrorism threat. Without effective antimicrobial postexposure prophylaxis (PEPAbx) and treatment, the mortality of systemic anthrax is high. To inform clinical guidelines for PEPAbx and treatment of B. anthracis infections in humans, we systematically evaluated animal anthrax treatment model studies.
Methods
We searched for survival outcome data in 9 scientific search engines for articles describing antimicrobial PEPAbx or treatment of anthrax in animals in any language through February 2019. We performed meta-analyses of efficacy of antimicrobial PEPAbx and treatment for each drug or drug combination using random-effects models. Pharmacokinetic/pharmacodynamic relationships were developed for 5 antimicrobials with available pharmacokinetic data. Monte Carlo simulations were used to predict unbound drug exposures in humans.
Results
We synthesized data from 34 peer-reviewed studies with 3262 animals. For PEPAbx and treatment of infection by susceptible B. anthracis, effective monotherapy can be accomplished with fluoroquinolones, tetracyclines, β-lactams (including penicillin, amoxicillin-clavulanate, and imipenem-cilastatin), and lipopeptides or glycopeptides. For naturally occurring strains, unbound drug exposures in humans were predicted to adequately cover the minimal inhibitory concentrations (MICs; those required to inhibit the growth of 50% or 90% of organisms [MIC50 or MIC90]) for ciprofloxacin, levofloxacin, and doxycycline for both the PEPAbx and treatment targets. Dalbavancin covered its MIC50 for PEPAbx.
Conclusions
These animal studies show many reviewed antimicrobials are good choices for PEPAbx or treatment of susceptible B. anthracis strains, and some are also promising options for combating resistant strains. Monte Carlo simulations suggest that oral ciprofloxacin, levofloxacin, and doxycycline are particularly robust choices for PEPAbx or treatment.
Keywords: anthrax, treatment, postexposure prophylaxis, drug-resistant, antimicrobial
To inform clinical guidelines for antimicrobial postexposure prophylaxis and treatment of Bacillus anthracis infections, we systematically evaluated and summarized animal model studies. The meta-analyses provide promising, clinically relevant options for postexposure prophylaxis and treatment of susceptible and resistant B. anthracis.
Bacillus anthracis, the etiologic agent of anthrax, is distributed worldwide [1]. Naturally occurring anthrax cases usually follow direct contact with infected animals or their contaminated byproducts. Because the spores can be aerosolized and natural or laboratory engineered resistance is possible, B. anthracis is a potential bioweapon [2–4].
Anthrax mortality is high, particularly for ingestion and inhalation anthrax and anthrax meningitis, even with treatment [5, 6]. Given the high mortality rates of anthrax, timely and effective postexposure prophylaxis (PEP) and treatment are critical to minimizing morbidity and mortality rates. PEP should include anthrax vaccine PEP and antimicrobial PEP (PEPAbx).
To inform clinical guidelines, we conducted a systematic review (SR) and meta-analyses of animal models and leveraged translational pharmacokinetic (PK)/pharmacodynamic (PD) approaches to support robust options for PEPAbx and treatment of anthrax. This article is part of a Centers for Disease Control and Prevention (CDC) effort to determine the most efficacious options for PEPAbx and treatment of anthrax in humans for naturally occurring and multidrug-resistant strains.
METHODS
We searched 9 scientific databases from inception through February 2019 for articles containing terms that captured anthrax, randomized controlled trials in animals, antimicrobials generally, and the specific classes and antimicrobials detailed in Supplementary Figure 1. All foreign-language articles were translated. The SR followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines for conducting and reporting systematic literature reviews. The system we used to identify full-text articles for inclusion in this review is shown by Maxson et al [7, Supplementary Figure 1]. Data were abstracted from studies that used antimicrobials for PEPAbx or treatment following animal exposures to B. anthracis. A time frame exception was made for a single article involving meningitis [8].
Two of the authors (K. C. S. and K. H.) abstracted aggregate data for treatment and control arms on the following topics: B. anthracis challenge; antimicrobials used for PEPAbx or treatment (ie, class, dose, route, interval, duration, and trigger for start of treatment), minimal inhibitory concentrations (MICs); PK/PD parameters, and outcome (eg, death and pathology results). After data entry, discrepancies were discussed by the abstractors until they reached consensus.
Exclusions
Study arms were excluded from the main SR analysis if they included sheep, cows, or dogs; added a vaccine or antitoxin to the antimicrobial(s); lacked at least 1 treatment-control pair of arms or a clear antimicrobial dose description; used antimicrobials that were unapproved by the Food and Drug Administration or unavailable in the United States; assessed only preexposure prophylaxis, PK, or PD; or had extremely high B. anthracis exposures (eg, ≥750 times the lethal dose in 50% of animals). Some studies meeting exclusion criteria were nevertheless analyzed separately from the main analysis on request of a CDC workgroup that reviewed the data.
Definitions
For studies using susceptible strains of B. anthracis, study arms with ciprofloxacin or doxycycline were designated as positive (ie, efficacious) controls. Infection was determined based on the presence of fever, bacteremia, or toxemia. An antimicrobial arm was classified as PEPAbx if the animals lacked infection and if the antimicrobial was started ≤24 hours after exposure. Only aerosol exposures were included for PEPAbx arms. An antimicrobial arm was classified as treatment if the antimicrobial was started for evidence of infection or began >24 hours after exposure. All exposure routes (eg, intravenous, subcutaneous, intranasal, head-only aerosol) were included for treatment arms. Study arms in which vegetative B. anthracis was instilled intracranially were classified as treatment.
Analysis
Study quality was assessed on a 24-point scale comprising 4 main topics: (1) animal descriptions, (2) exposures, (3) antimicrobial descriptions, and (4) outcomes (Supplementary Figure 2). Quality scores were categorized as low (0–5 points), fair (6–13 points), good (14–17 points), and high (18–24 points) based on natural breaks in a histogram of the scores and discussions with an internal CDC steering committee.
Within studies, we calculated odds ratios (ORs) and 95% confidence intervals (CIs) for each study arm compared to its nontreated controls; ORs were then visually compared. ORs with overlapping CIs were considered to be not meaningfully different and the corresponding study arms were combined to improve statistical power.
Meta-analyses were performed to combine the results of individual studies on animal mortality data to estimate an overall OR and 95% CI for survival. The meta-analyses used conditional binomial-normal (or generalized linear mixed effects) models with a random-effects model option that allowed the treatment effect to differ from study to study. The conditional binomial-normal model was chosen because it was the most resilient against data challenges, such as 2-by-2 tables with 0 s (zero cells) and imbalanced comparison groups. However, ORs could not be calculated at all if the 2-by-2 table had 2 zero cells. Study arms that prevented OR calculation were removed from the meta-analysis and described separately.
For PK/PD analysis, we incorporated information on the doses, dosing intervals, and routes of administration for published studies in mice, rabbits, and nonhuman primates (NHPs) that evaluated PEPAbx or treatment of anthrax [9]. We assumed plasma protein binding was similar in mice and humans for levofloxacin and ciprofloxacin (approximately 30% bound), as well as for doxycycline (approximately 80%–90% bound) [10]. Known differences in protein binding were incorporated for dalbavancin (93% bound in humans vs 98.4% in mice) [11, 12] and oritavancin (85% bound in humans vs 93.6% in mouse serum) [13, 14].
Based on published human PK data, we performed Monte Carlo simulations with small (20% coefficient of variation) and moderately large (30% coefficient of variation) variability to predict the overall free (ie, non–protein-bound) drug exposures (area under the unbound plasma concentration-time curve [fAUC]) in healthy persons exposed to B. anthracis and in patients with early anthrax. These fAUC values were used to calculate the fAUC/MIC and then to calculate the probability of achieving the derived PK/PD target. The PK/PD breakpoint was defined as the highest MIC with a ≥98% probability of attaining the PK/PD target that is associated with near-maximal survival in animal studies. A complete description of the methods is provided in Supplementary Materials.
RESULTS
We identified 62 sources: 53 articles, 5 reports, 2 abstracts, 1 slide set, and 1 other source that described >800 study arms with >12 000 animals. Following application of exclusion criteria, 34 sources remained that described 27 antimicrobials and 294 study arms with 3262 animals, including 329 NHPs, 807 rabbits, 1328 mice, 138 guinea pigs, and 660 hamsters (Supplementary Table 1).
Meta-analyses results are displayed in 5 groups according to the susceptibility of the infecting B. anthracis strain and the type of therapy. Table 1 shows monotherapy for PEPAbx or treatment of infections with susceptible B. anthracis strains [12, 14–38, (unpublished data)]. Table 2 shows monotherapy for PEPAbx or treatment of infections with resistant strains [38, 39]. Table 3 shows combination therapies that included ciprofloxacin for resistant and sensitive strains [18, 19, 39]. Table 4 summarizes meta-analyses for anthrax meningitis treatment studies in rabbits [8, 40, 41]. Table 5 shows PEPAbx studies meeting exclusion criteria analyzed on anthrax workgroup request [17, 42–44]. Most studies of monotherapy against susceptible B. anthracis strains accrued a quality score of 10–20 points. Studies of monotherapy for resistant strains (Table 2), combination therapy (Table 3), anthrax meningitis (Table 4), and analyses run at the behest of the workgroup (Table 5) accrued 9–18 points. ORs for individual antimicrobials versus no therapy are summarized on logarithmic scales for PEPAbx and treatment in Figure 1 and for combination therapy and anthrax meningitis in Figure 2.
Table 1.
Odds of Survival for Monotherapy Studies for Which the Exposures Were Not Antibiotic-Resistant Strains of Bacillus anthracis
| [Study References] Animal Modela | Cidal or Static | Class | Antimicrobials | PEPAbx or Rx | No. of Studies | Quality Scoreb | OR (95% CI) vs No Treatment | I2 c | No. Studies | OR (95% CI) vs Ciprofloxacin | I2 | No. Studies | OR (95% CI) vs Doxycycline | I 2 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [12]M, [14]M, [15]M, [16]M, [28]NHP, [29]M, [30]M, [31]NHP, [32]M, [33]NHP | C | Fluoroquinolone | Ciprofloxacin | PEPAbx | 10 | 16,10,15,14,18, 12,14,19,13,18 |
11.5 (5.2–25.3)d | N | … | … | … | 3 | 1.1 (0.7–1.8) | N |
| [12]M, [34]R, [35]M, [unpublished]NHP |
C | Fluoroquinolone | Levofloxacin | PEPAbx | 4 | 16,14,8, 19 |
124.8 (0.4–42 865) | N | 1 | 2.2 (0.2–29.8) | … | … | … | … |
| [35]M,NHP, [36]NHP | C | Penicillin | Amoxicillin | PEPAbx | 1,1 | 8,14 | 357 (6.4–19 943)d 1345 (76.2–23 762)d,e |
… | … | … | … | … | … | … |
| [37]NHP, [unpublished]NHP | C | Penicillin | Penicillin | PEPAbx | 2 | 14,8 | 4.1 (0.9–19.9) | N | … | … | … | … | … | … |
| [25]NHP, [28]NHP | C | Penicillin | Procaine penicillin Gf | PEPAbx | 2 | 14,18 | 8.2 (0–5 846 972) | N | 1 | 0.3 (0–3.5) | 1 | 0.3 (0–3.1) | ||
| [35]M,NHP, [36]NHP, [36]NHP | C | Penicillin/β-lactamase inhibitor | Amoxicillin clavulanate | PEPAbx | 1,1,1 | 14,18,8 | 187 (3.2–10 884)d 45.0 (0.7–3042) 1617 (91.7–24 498)d,e |
… | … | … | … | … | … | … |
| [12]M | C | Glycopeptide | Dalbavancing | PEPAbx | 1 | 16 | 3381 (63.7–179 559)d | … | … | …h | … | … | … | … |
| [14]M | C | Glycopeptide | Oritavancing | PEPAbx | 1 | 10 | 46.3 (2.6–816)d | … | 1 | 0.1 (0–0.8)d | … | … | … | … |
| [30]M | C | Lipopeptide | Daptomycing | PEPAbx | 1 | 14 | 81.0 (4.4–1505)d | … | 1 | 1.9 (0.2–21.5) | … | … | … | … |
| [15]M, [16]M, [26]M, [28]NHP, [38]H | S | Tetracycline | Doxycycline | PEPAbx | 5 | 15,14,19,18, 12 |
5.4 (3.2–9.0)d | N | 3 | 0.9 (0.6–1.3) | N | … | … | … |
| [38]H | S | Tetracycline | Minocycline | PEPAbx | 1 | 12 | 1489 (84.4–26 267)d | … | … | … | … | 1 | 99.0 (5.9–1651)d | … |
| [16]M | S | Tetracycline | Omadacycline | PEPAbx | 1 | 14 | 50.4 (13.9–182)d | … | 1 | 0.2 (0–3.6) | 1 | 1.1 (0.4–3.1) | … | |
| [38]M | S | Tetracycline | Tetracycline | PEPAbx | 1 | 12 | 17.9 (7.8 - 40.9)d | … | … | … | … | 1 | 1.1 (0.6–2.0) | … |
| [35]M, [unpublished]NHP | S | Macrolide | Azithromycin | PEPAbx | 1,1 | 8,19 | 25.0 (1.1–562.8)d 7.5 (0.3–186)e |
… | … | … | … | … | … | … |
| [35]Mi, [unpublished]NHP | S | Macrolide | Clarithromycin | PEPAbx | 1 | 8,19 | 16.2 (0.6–441.7)h | … | … | … | … | … | … | … |
| [12]M, [14]M, [15]M, [16]M [17]GP,R, [18]R, [19]NHP |
C | Fluoroquinolone | Ciprofloxacin | Rx | 7 | 16,10,15,14, 11,12,18 | 12.5 (3.9–40.5)d | N | … | … | … | 2 | 0.8 (0.6–1.2) | N |
| [20]R, [21]R, [22]R, [23]R | C | Fluoroquinolone | Levofloxacin | Rx | 4 | 11,20,14,16 | 337 (0.5–210 268) | H | … | … | … | … | … | … |
| [18]R | C | Carbapenem | Imipenemj | Rx | 1 | 11 | 63.0 (2.5–1558)d | … | … | … | … | … | … | … |
| [24]Ri, [unpublished]NHP | C | Penicillin | Penicillin | Rx | 1 | 9,8 | 24.1 (1.0–567)d,h | … | … | … | … | … | … | … |
| [25]NHP | C | Penicillin | Procaine penicillinf | Rx | 1 | 14 | 24.1 (1.0–567)d | … | … | … | … | … | … | … |
| [18]R | C | Penicillin/beta-lactamase inhibitor | Amoxicillin clavulanatek | Rx | 1 | 12 | 25.0 (1.1–563)d | … | … | … | … | … | … | … |
| [18]R | C | Glycopeptide | Vancomycin | Rx | 1 | 12 | 52.7 (2.3–1211)d | … | … | … | … | … | … | … |
| [12]M | C | Glycopeptide | Dalbavancin | Rx | 1 | 16 | 138 (6.8–2780)d | … | 1 | 0.7 (0–15.9) | … | … | … | … |
| [14]M | C | Glycopeptide | Oritavancin | Rx | 1 | 10 | 39.0 (2.1–734)d | … | 1 | 0.3 (0.1–1.1) | … | … | … | … |
| [16]M, [17]GP,R, [26]M, [27]M | S | Tetracycline | Doxycycline | Rx | 4 | 14,11,19,11 | 4.9 (2.1–11.6)d | N | 1 | 1.6 (0.9–2.8) | … | … | … | … |
| [unpublished data]R | S | Tetracycline | Eravacycline | Rx | 1 | 19 | 369 (6.4–21191)d | … | … | … | … | … | … | … |
| [16]M | S | Tetracycline | Omadacycline | Rx | 1 | 14 | 9.8 (1.9–50.6)d | … | 1 | 0.4 (0.1–3.2) | 1 | 0.6 (0.1–4.1) | … | |
| [19]NHP | S | Lincosamide | Clindamycin | Rx | 1 | 18 | 24.4 (1.0–581)d | … | … | … | … | … | … | … |
| [18]R | S | Macrolide | Clarithromycink | Rx | 1 | 12 | 5.5 (0.3–111) | … | … | … | … | … | … | … |
| [18]R | S | Oxazolidinones | Linezolid | Rx | 1 | 12 | 17.2 (0.8–381) | … | … | … | … | … | … | … |
| [18]R | C | Rifamycin | Rifampin | Rx | 1 | 12 | 3.4 (0.2–74.4) | … | … | … | … | … | … | … |
Abbreviations: C, bactericidal; CI, confidence interval; OR, odds ratio; PEPAbx, antimicrobial postexposure prophylaxis; Rx, treatment; S, bacteriostatic.
Animal model used in the relevant arms of the above studies: M, mouse; R, rabbit; NHP, nonhuman primate; GP, guinea pig; H, hamster.
Quality score categorization: fair (6–13 points); good (14–17 points); high (18–24 points).
Heterogeneity (I2) Key: N = 0–24%; L = 25–49%; M = 50–74%; H = 75–100%.
Significant at P < .05.
Both ORs are shown as both studies had zero cells and could not be combined.
Only available in the United States as intramuscular formulation for humans, although animals may have received other formulations or routes of administration.
Only available in the United States as intravenous formulation for humans, although animals may have received other formulations or routes of administration.
At least one study removed to allow odds ratio calculation.
This study was removed to allow odds ratio calculation.
Only available in the United States as an intravenous formulation in combination with cilastatin for humans; animals received only imipenem, which may have used another route of administration.
Only available in the United States as oral formulation for humans, although animals may have received other formulations or routes of administration.
Table 2.
Odds of Survival for Monotherapy Studies With Antibiotic Resistant Strains of Bacillus anthracis
| [Study References] Animal Modela | Cidal or Static | Class | Antimicrobials | PEPAbx or Rx | Strain | No. of Studies | Quality Scoreb | OR (95% CI) vs No Treatment |
|---|---|---|---|---|---|---|---|---|
| [38]M,H | S | Tetracycline | Doxycycline | PEPAbx | H-7 (pBC16)c | 1 | 12 | 0.1 (0–0.4)d |
| [38]M,H | S | Tetracycline | Minocycline | PEPAbx | H-7 (pBC16) | 1 | 12 | 3.9 (2.0–7.8)d |
| [38]M,H | S | Tetracycline | Tetracycline | PEPAbx | H-7 (pBC16) | 1 | 12 | 0.6 (0.3–1.2) |
| [39]M | C | Fluoroquinolone | Ciprofloxacin | Rx | CIP-R Ames | 1 | 13 | 3.9 (0.3–45.6) |
Abbreviations: C, bactericidal; CI, confidence interval; CIP-R, ciprofloxacin-resistant; OR, odds ratio; PEPAbx, antimicrobial postexposure prophylaxis; Rx, treatment; S, bacteriostatic.
Animal model used in the relevant arms of the above studies: M, mouse; H, hamster.
Quality score categorization: fair (6–13 points); good (14–17 points); high (18–24 points).
Strain H-7 has plasmid pBC16 which confers tetracycline resistance.
Significant at P < .05.
Table 3.
Odds of Survival for Antimicrobial Combinations Including Ciprofloxacin for Treatment of Animals Infected With Sensitive or Resistant Bacillus anthracis Strains
| Antimicrobial 2 | Antimicrobial 3 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| [Study References] Animal Modela | Cidal or Static | Class | Antimicrobial | Cidal or Static | Class | Antimicrobial | Strain | Quality Scoreb | OR (95% CI) vs No Treatmente |
OR (95% CI) vs Ciprofloxacin Monotherapy |
| [39]M | C | Carbapenem | Meropenem | S | Oxazolidinones | Linezolid | CIP-R Ames | 13 | 81.0 (4.4–1505)c | 21.0 (1.8–248)c |
| [39]M | C | Carbapenem | Meropenem | S | Lincosamide | Clindamycin | CIP-R Ames | 13 | 81.0 (4.4–1505)c | 21.0 (1.8–248)c |
| [39]M | C | Carbapenem | Meropenem | C | Rifamycin | Rifampin | CIP-R Ames | 13 | 81.0 (4.4–1505)c | 21.0 (1.8–248)c |
| [39]M | C | Carbapenem | Meropenem | S | Tetracycline | Doxycycline | CIP-R Ames | 13 | 21.0 (1.8–248)c | 5.4 (0.8–36.9) |
| [39]M | C | Penicillin | Penicillin | S | Oxazolidinones | Linezolid | CIP-R Ames | 13 | 36.0 (2.7–476)c | 9.3 (1.2–7.3)c |
| [39]M | C | Penicillin | Penicillin | S | Tetracycline | Doxycycline | CIP-R Ames | 13 | 81.0 (4.4–1505)c | 21.0 (1.8–248)c |
| [39]M | C | Rifamycin | Rifampin | S | Oxazolidinones | Linezolid | CIP-R Ames | 13 | 133 (4.8–3674)c | 45.0 (2.0–1007)c |
| [39]M | C | Rifamycin | Rifampin | S | Lincosamide | Clindamycin | CIP-R Ames | 13 | 36.0 (2.7–476)c | 9.3 (1.2–7.3)c |
| [18]R, [19]NHP | S | Lincosamide | Clindamycin | Vollum,d,e Amesd |
12,18 | 32.1 (1.4–752)c 24.4 (1.03 - 581)c | 0.1 (0–2.6)f | |||
Abbreviations: C, bactericidal; CI, confidence interval; CIP-R, ciprofloxacin-resistant; OR, odds ratio; S, bacteriostatic.
Animal model used in the relevant arms of the above studies: M, mouse; R, rabbit; NHP, nonhuman primate.
Quality score categorization: fair (6–13 points); good (14–17 points); high (18–24 points).
Significant at P < .05.
Antibiotic sensitive.
American Type Culture Collection 14578.
Only the study performed in NHPs [19] was included in the odds ratio calculation.
Table 4.
Odds of Survival for Treatment Studies Using a Rabbit Model of Anthrax Meningitis With a Susceptible Vollum Strain of Bacillus anthracis
| [Study References] Animal Modela | Cidal or Static | Class | Antimicrobials | Dosing Route | Animals, No.b | Quality Scorec | OR (95% CI) vs No Treatmentd |
|---|---|---|---|---|---|---|---|
| [8]R | C | Penicillin/β-lactamase inhibitor | Amoxicillin-clavulanate | Intravenous/ subcutaneous |
12 | 13 | 153 (2.6–9077)e |
| [8]R | C | Penicillin | Ampicillin | Intravenous/ subcutaneous |
45 | 13 | 23.9 (1.2–479.2)e |
| [8]R | C | Penicillin/β-lactamase inhibitor | Ampicillin-sulbactam | Intravenous/ subcutaneous |
12 | 13 | 45.0 (1.5–1358)e |
| [40]R | C | Fluoroquinolone/carbapenem | Ciprofloxacin/meropenem | Intravenous/ ubcutaneous |
12 | 10 | 1.8 (0.1–54.3) |
| [40]R | S | Lincosamide | Clindamycin | Intravenous/ subcutaneous |
12 | 10 | 45.0 (1.5–1358)e |
| [8]R, [40]R | C | Carbapenem | Meropenem | Intravenous/ subcutaneous |
50 | 13,10 | 4.4 (0.2–87.6) |
| [40]R | C | Carbapenem | Meropenem | Intrathecal/ Intravenous |
12 | 10 | 153 (2.6–9077)e |
Abbreviations: C, bactericidal; CI, confidence interval; OR, odds ratio; S, bacteriostatic.
Animal model used in the relevant arms of the above studies: R, rabbit.
Number of treated and control animals.
Quality score categorization: fair (6–13 points); good (14–17 points); high (18–24 points).
Comparison with no-treatment control from Levy et al [41].
Significant at P < .05.
Table 5.
Odds of Survival for Monotherapy Studies That Met ≥1 Exclusion Criterion but Were Performed at Workgroup Request
| [Study References] Animal Modela | Cidal or Static | Class | Antimicrobials | PEPAbx or Rx | Reason for Exclusion | Quality Scoreb | OR (95% CI) vs No Treatment |
|---|---|---|---|---|---|---|---|
| [17]GP | C | Quinolone | Ofloxacin | PEPAbx | Exposure route (intranasal), vaccine | 11 | 171 (7.5–3910)c |
| [17]GP | C | Carbapenem | Imipenemd,e | PEPAbx | Exposure route (intranasal) | 11 | 29.9 (1.3–692)c |
| [42]GP | C | Cephalosporin | Cefazolinf | PEPAbx | Exposure route (intranasal) | 14 | 8.4 (0.4–177) |
| [43]M | S | Macrolide | Erythromycin ethylsuccinate | PEPAbx | Exposure route (intraperitoneal) | 9 | 21.0 (1.0–454) |
| [17]GP | C | Aminoglycocide | Gentamicinf | PEPAbx | Exposure route (intranasal) | 11 | 3.0 (0.1–83.4) |
| [44]M | S | Amphenicol | Chloramphenicold | PEPAbx | Exposure route (intraperitoneal) | 10 | 10.2 (0.6–184) |
| [42]GP | S | Sulfonamide | Trimethoprim-sulfamethoxazole | PEPAbx | Exposure route (intranasal) | 14 | 15.4 (0.8–305) |
Abbreviations: C, bactericidal; CI, confidence interval; OR, odds ratio; PEPAbx, antimicrobial postexposure prophylaxis; Rx, treatment; S, bacteriostatic.
Animal model used in the relevant arms of the above studies: M, mouse; GP, guinea pig.
Quality score categorization: fair (6–13 points); good (14–17 points); high (18–24 points).
Significant at P < .05.
Only available in the United States as intravenous formulation for humans.
Only available in the United States in combination with cilastatin for humans; animals received imipenem only, which may have used another route of administration.
Only available in the United States as intramuscular or intravenous formulation for humans, although animals may have received other formulations.
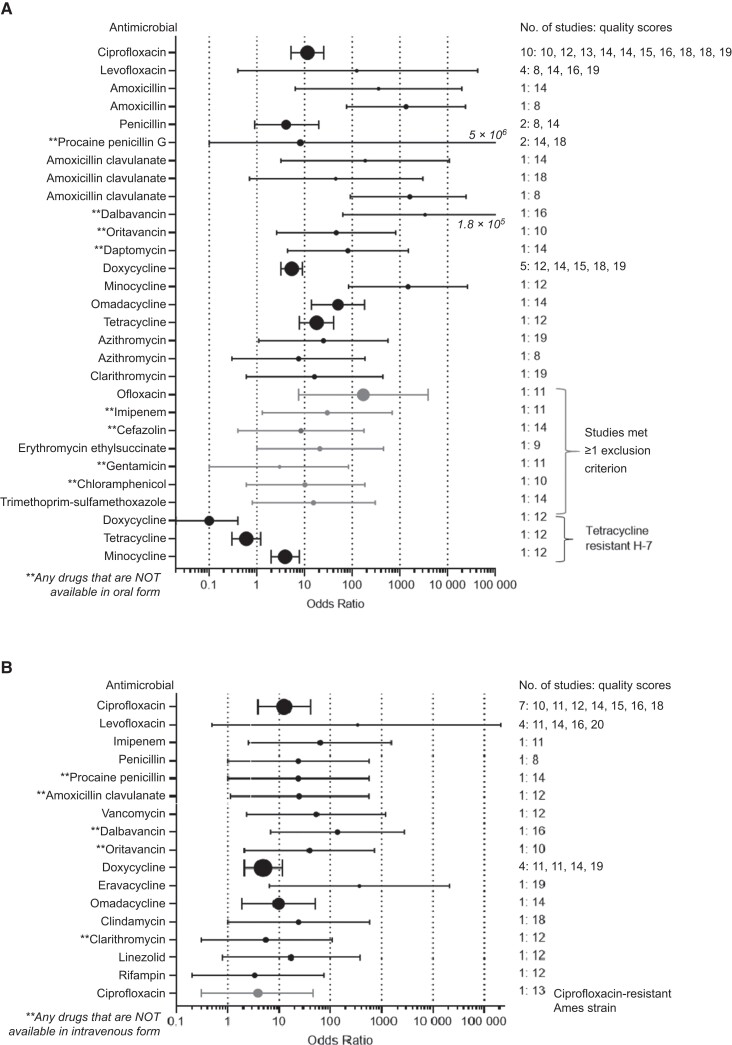
Figure 1.
Comparison of efficacies for specified antimicrobials used for postexposure prophylaxis (A) or treatment (B). A, Odds of survival (with 95% confidence interval [CI]) for postexposure prophylaxis monotherapy studies with specified antimicrobial compared with nontreated controls. B, Odds of survival (with 95% CI) for treatment monotherapy studies with specified antimicrobial compared with nontreated controls.
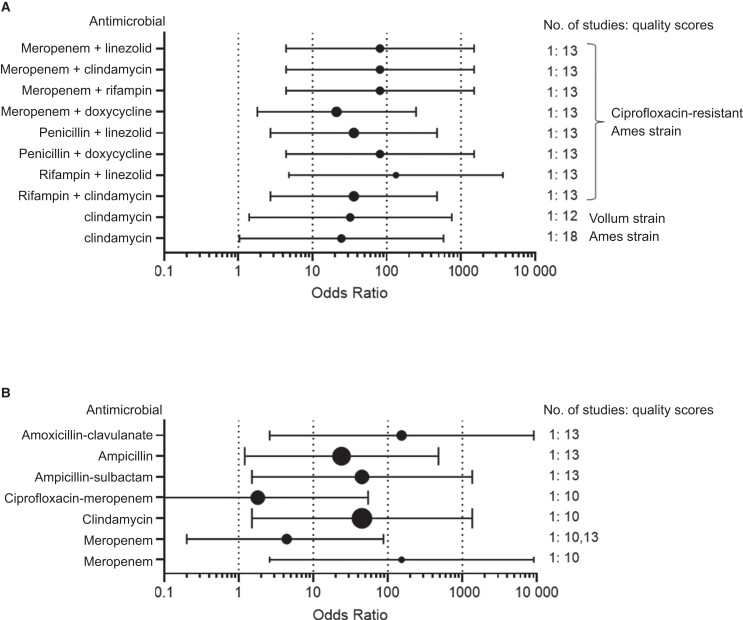
Figure 2.
Comparison of efficacies for specified antimicrobial combinations for treatment of susceptible or resistant Bacillus anthracis (A) and anthrax meningitis (B). A. Odds of survival (with 95% confidence interval [CI]) for antimicrobial combinations that included ciprofloxacin for treatment of animals infected with either sensitive or resistant strains. B, Odds of survival (with 95% CI) for treatment monotherapy studies using a rabbit model of anthrax meningitis with a susceptible strain with specified antimicrobials compared with nontreated controls. Due to limited data availability and high mortality rates, a control arm from one anthrax meningitis paper was used as a comparison for treatment arms in 2 other papers. The 3 papers were analyzed as if they were 1 paper.
Postexposure Prophylaxis Against Susceptible Strains of B. anthracis
For monotherapy PEPAbx following exposures to susceptible B. anthracis, ciprofloxacin showed efficacy superior to no therapy (Table 1). Amoxicillin, glycopeptides, and daptomycin all showed efficacy for PEPAbx. Three amoxicillin-clavulanate studies were included in the analysis but could not be combined owing to zero cells. When ORs were calculated, 2 of the 3 studies showed significant efficacy. Tetracyclines also showed efficacy for PEPAbx, with ORs ranging from 5.4 (95% CI, 3.2–9.0) for doxycycline to 1489 (84.4–26 267) for minocycline. Clarithromycin showed no efficacy for PEPAbx. As indicated in Table 1, the presence of zero cells precluded OR calculation for a second study using clarithromycin as PEPAbx. While the 2 azithromycin studies could not be combined (owing to zero cells), 1 showed efficacy while the other did not. Forest plots summarizing ciprofloxacin and doxycycline PEPAbx data appear in Supplementary Figure 3A and 3B.
For PEPAbx, levofloxacin, procaine penicillin G, daptomycin, doxycycline, and omadacycline had efficacies similar to ciprofloxacin, but oritavancin was less efficacious (OR, 0.1 [95% CI, 0–.8]; Table 1). Ciprofloxacin, procaine penicillin G, omadacycline, and tetracycline had efficacies similar to doxycycline, but minocycline was more efficacious (OR, 99.0 [95% CI, 5.9–1651]). Of PEPAbx study arms initially excluded for nonaerosol exposure route or addition of anthrax vaccine to antimicrobials, the ofloxacin and imipenem arms from 1 study were both efficacious (Table 5) [17].
Postexposure Prophylaxis Against Resistant Strains of B. anthracis
Neither doxycycline nor tetracycline were efficacious for PEPAbx of doxycycline-resistant B. anthracis (Table 2). In contrast, monotherapy PEPAbx with minocycline was efficacious (OR, 3.9 [95% CI, 2.0–7.8]) against this particular doxycycline-resistant strain [38].
Treatment of Infections from Susceptible Strains of B. anthracis
Eleven treatment studies assessed 2 widely used fluoroquinolones (ciprofloxacin and levofloxacin) currently recommended for first-line treatment of anthrax. Ciprofloxacin administration was associated with increased survival compared with the no-treatment control (OR, 12.5 [95% CI, 3.9–40.5]). The meta-analysis OR for levofloxacin did not differ significantly from the no-treatment control (OR, 337 [95% CI, .5–210 268]; Table 1). Forest plots summarizing treatment data for ciprofloxacin and levofloxacin appear in Supplementary Figure 3C and 3D.
Compared with no treatment, improved survival was found for the following cell-wall synthesis inhibitors: all studied β-lactams and glycopeptides (vancomycin, dalbavancin, and oritavancin; Table 1). One penicillin study in which all animals died was removed from analysis. For anthrax treatment by a protein synthesis inhibitor (PSI), doxycycline and the newer tetracyclines eravacycline (OR, 369 [95% CI, 6.4–21 191]) and omadacycline (9.8 9 [1.9–50.6]) improved survival, as did clindamycin (24.4 [1.03–581]) (Table 1). Some studies had positive controls: Efficacies for dalbavancin, oritavancin, doxycycline, and omadacycline were similar to that of ciprofloxacin (Table 1). Likewise, efficacies for ciprofloxacin and omadacycline were similar to that of doxycycline. The forest plot for doxycycline treatment data is available in Supplementary Figure 3E.
In treatment studies evaluating 2 antimicrobial combinations against sensitive strains of B. anthracis (Table 3), clindamycin plus ciprofloxacin demonstrated treatment efficacy compared with no treatment in rabbits [18] and NHPs [19]. However, clindamycin plus ciprofloxacin was not superior to ciprofloxacin monotherapy in NHPs.
Treatment of Infections from Resistant Strains of B. anthracis
Against a ciprofloxacin-resistant strain, ciprofloxacin monotherapy was not efficacious, as expected (Table 2). In contrast, combination therapy was more effective. Three antimicrobial combination regimens included ciprofloxacin, a β-lactam, and either a PSI or rifampin or ciprofloxacin, a PSI, and rifampin (Table 3). All combinations showed efficacy compared with no treatment. All combinations except ciprofloxacin, meropenem, and doxycycline showed efficacy compared with ciprofloxacin monotherapy [39].
Treatment of Anthrax Meningitis in a Rabbit Model
We included all available anthrax meningitis studies due to the extreme mortality rates associated with anthrax meningitis and the limited number of animal studies for this indication. In 2 anthrax meningitis studies using a rabbit model [8, 40], treatment efficacy was shown for penicillins (with or without clavulanate or sulbactam) and clindamycin (Table 4). Meropenem with or without ciprofloxacin was not efficacious in this animal model when dosed intravenously or subcutaneously.
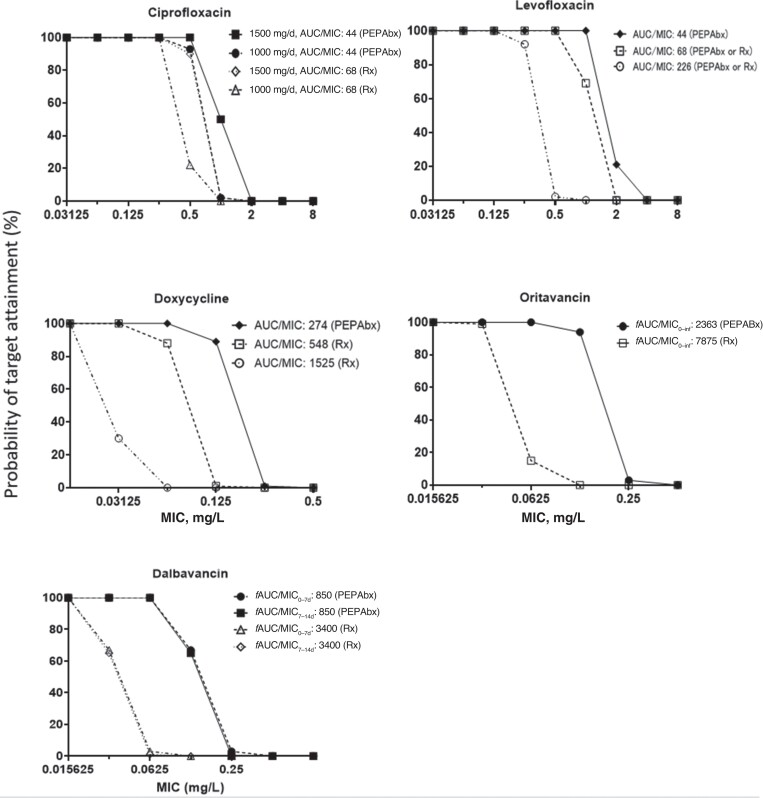
Monte Carlo Simulations to Predict Efficacy of Dosage Regimens
Monte Carlo simulations predicted the human unbound drug exposures for clinically relevant doses of ciprofloxacin, levofloxacin, doxycycline, oritavancin, and dalbavancin. Subsequently, efficacy was predicted based on the probability of PK/PD target attainment (Figure 3). These Monte Carlo–simulated efficacies were comparable for both small variability (representing PEPAbx) and moderately large variability (for treatment).
Figure 3.
Monte Carlo simulation results on the probability of pharmacokinetic/pharmacodynamic target attainment plotted against the minimum inhibitory concentration (MIC) for clinically relevant dosage regimens of 5 antimicrobials. The exposure targets for antimicrobial postexposure prophylaxis (PEPAbx) and treatment (Rx) are indicated, with more details in the Supplementary Materials. Abbreviations: d, day; AUC, area under the concentration-time curve; fAUC, area under the unbound plasma concentration-time curve; inf, infinity.
As described more comprehensively in the Supplementary Materials, for PEPAbx, a high degree of efficacy was predicted for 500 mg ciprofloxacin given every 12 hours (for MICs up to 0.25 mg/L), ciprofloxacin 500 mg every 8 hours (MICs up to 0.5 mg/L), levofloxacin 750 mg every 24 hours (MICs up to 0.5 to 1 mg/L), doxycycline 100 mg every 12 hours (MICs up to 0.0625 mg/L), oritavancin 1200 mg × 1 dose (MICs up to 0.0625 mg/L), and dalbavancin 1000 mg × 1 dose, then 500 mg a week later (MICs up to 0.0625 mg/L). When using the higher PK/PD targets for treatment, the highest MICs with robust probabilities of target attainment were predicted to be approximately 2-fold lower than those for PEPAbx (Figure 3). Of note, ciprofloxacin 500 mg every 12 hours was predicted to be highly efficacious up to an MIC of 0.25 mg/L for treatment.
DISCUSSION
In a wide-area release of B. anthracis, effective PEPAbx and treatment are needed to minimize morbidity and mortality rates. Models have estimated the impact of a large intentional aerosol release of B. anthracis spores. One kilogram of B. anthracis spores (approximately 1 × 1015) released upwind from 11.5 million city dwellers would infect 1.49 million people [45]. With timely treatment, but without PEP, approximately 670 000 people would be predicted to die (ie, 45% of those infected). With PEP, the casualty estimate could be reduced by as much as 80%, saving 537 000 to 547 000 lives [45]. The risk reduction afforded by PEPAbx begins immediately, while that afforded by vaccine PEP occurs only after immunity has developed. Thus, early PEPAbx is key to effective prophylaxis.
For this series of meta-analyses, we were preferentially interested in NHP, rabbit, mice, and guinea pig studies. The Food and Drug Administration identified NHPs and rabbits as acceptable models for product licensure studies under the Animal Rule [46, 47]. Moreover, mice and guinea pigs are considered acceptable for baseline screening of countermeasures [9], and many drug development programs extensively use dynamic in vitro and murine infection models to develop efficacious dosage regimens for patients [46–48].
Postexposure Prophylaxis
For PEPAbx of susceptible B. anthracis, monotherapies with ciprofloxacin, amoxicillin, amoxicillin-clavulanate, lipopeptides, glycopeptides, and tetracyclines were associated with improved survival compared with no-PEPAbx controls. Of the macrolides, only 1 of 2 studies with azithromycin was associated with survival, while clarithromycin was not associated with survival. Dalbavancin, oritavancin, daptomycin, and imipenem (combined with cilastatin) should also be effective but are only available intravenously and would therefore be less practical.
For PEPAbx of tetracycline-resistant B. anthracis, minocycline monotherapy improved survival, whereas doxycycline and tetracycline monotherapy did not. Because one of the anthrax toxins, lethal factor, is a metalloprotease, some of minocycline’s efficacy might be due to its metalloprotease inhibitory activity [49]. Thus, minocycline might be an option for some tetracycline-resistant B. anthracis strains once susceptibility has been established by laboratory testing.
Treatment
For treatment of susceptible B. anthracis infections, the odds of survival were increased compared with no-treatment controls for ciprofloxacin; β-lactams (with or without a β-lactamase inhibitor, including amoxicillin-clavulanate (available only orally in the United States), penicillin, procaine penicillin, and imipenem; glycopeptides; tetracyclines; and clindamycin. Although the meta-analysis OR for levofloxacin did not differ significantly from the no-treatment control, this was due to 1 study in which a relatively low dose of levofloxacin was delivered 48 to 96 hours after exposure and only 2 of 20 animals survived [22].
For treatment of ciprofloxacin-resistant B. anthracis, combinations of meropenem or penicillin with a PSI antimicrobial (linezolid, clindamycin, or doxycycline) or rifampin showed efficacy in mice. Rifampin combined with a PSI antimicrobial was also effective. Promising results for the combinations are plausible because the primary target site mutations that confer resistance to ciprofloxacin and other fluoroquinolones are not expected to affect β-lactams, rifampin, or PSIs [2]. However, fluoroquinolone use might increase the bacterial efflux of, and decrease susceptibility to, other antibiotics (including tetracyclines).
While doxycycline was the only tetracycline included in the combination studies (Table 3), the promising results for other tetracyclines in monotherapy against susceptible B. anthracis suggest that minocycline, omadacycline, and tetracycline might all be used in combination treatments against ciprofloxacin-resistant B. anthracis with primary target site mutations [2]. Newer tetracyclines that are less affected by efflux pump overexpression than tetracycline and doxycycline [50] also hold promise against fluoroquinolone-resistant strains, although future research is needed.
Susceptibility of suspected engineered strains should be evaluated swiftly following any intentional release to identify potentially engineered, resistant B. anthracis and enable rational treatment decisions [51]. While several combinations showed promising activity against ciprofloxacin-resistant B. anthracis, the possibility must be considered that a B. anthracis strain resistant to a specific antibiotic could be engineered. Point mutations at the active site of rifampin are common and clinically relevant and can confer high-level rifampin resistance [2], which prevents the use of rifampin as monotherapy. Likewise, older tetracyclines and fluoroquinolones may be affected by efflux-related resistance. Thus, these antimicrobials may prove nonefficacious against engineered, multidrug-resistant strains, and omadacycline, eravacycline, or minocycline may be better alternatives. Based on experience with other pathogens, β-lactams and glycopeptides may be promising choices, because emergence of resistance during therapy with these cell-wall synthesis inhibitors tends to be less common.
A penicillin combined with a β-lactamase inhibitor or a carbapenem (such as meropenem or imipenem) may overcome β-lactamase–related resistance. While susceptibility testing is essential, these cell-wall synthesis inhibitors may be used empirically if the status of penicillin-resistance is unknown. Of note, B. anthracis is naturally resistant to cephalosporins owing to a cephalosporinase that confers high-level resistance to cephalosporins. There were insufficient animal PK data for anthrax infections to establish PK/PD relationships for β-lactams. However, the half-life of β-lactams is much shorter in small animals (eg, mice) than in humans. Therefore, PEPAbx or treatment efficacy in mice should translate to humans, especially if β-lactams are dosed at short intervals or as prolonged infusions, because both lengthen the time the unbound drug concentrations remain above the MIC [52].
Our PK/PD analyses correlated doses and unbound drug exposures with outcomes in mice, rabbits, and NHPs. The application of PK/PD principles and available animal infection model data allowed us to predict probabilities of target attainment for PEPAbx and treatment in humans using clinically relevant dosage regimens and unbound drug exposures. Subsequent Monte Carlo simulations provided useful guidance and PK/PD breakpoints (ie, the highest MICs predicted to be treatable with good success) for clinically relevant antimicrobials.
Limitations
Our set of meta-analyses has several limitations, some inherent to our statistical approach. The model used in the meta-analyses could not combine study results and generate an OR for antimicrobials when the 2-by-2 table had 2 instances of zero cells. Some antimicrobials had large CIs because of small study sizes. Fewer than a third of the studies mentioned blinding of observers, conditions in which the animals were kept, inoculation dose by arm, humanization of the dose, randomization of exposures, PK data for the chosen antimicrobials in their animal model, use of NHPs, or immunological outcomes. No study accrued 24 quality points, and only 5 accrued >18 [21, 26, (unpublished data), 31]. Most low-quality studies were dropped from the analyses because they met exclusion criteria rather than for their low quality. However, it is possible that authors may have performed their experiments to a higher standard than was described in the respective methods sections. Some moderately high-quality studies were also dropped because of exclusion criteria—the most common reason being a nonaerosol mode of exposure in a PEPAbx study [53–56].
Because data on anthrax meningitis are limited, with only 2 articles describing meningitis in animal models falling within our time frame [40, 41], 1 article from 2020, identified by the workgroup, was included in the analysis [8]. These studies had the same methods and almost the same authors, but several methodologic flaws: 2 lacked control groups [8, 40], so controls from another study were used [41]; the model chosen, intracisternal injection of vegetative B. anthracis, may be confounded by trauma; antimicrobial PK data were usually lacking; antimicrobial concentrations were not assessed in cerebrospinal fluid; and the dosing regimen chosen for meropenem (40 mg/kg) was poor, as it provided coverage for only 4 of 24 hours in a day. Therefore, Table 4 should be interpreted with caution.
Some of the animal infection model data sets did not characterize the full exposure response relationships over a range of doses for PEPAbx or treatment. This led to uncertainty for the PK/PD target values, which we used during Monte Carlo simulations. Oritavancin displayed a clear exposure response relationship. However, neither ciprofloxacin nor dalbavancin displayed exposure response relationships, since all doses (including the lowest studied doses) provided near-maximal survival. Therefore, the PK/PD predictions for ciprofloxacin and dalbavancin are conservative. The exposure response data for levofloxacin primarily arose from rabbits, which are hypersensitive to anthrax [8]. Thus, the Monte Carlo simulations for levofloxacin borrowed the PK/PD targets from ciprofloxacin. When establishing PK/PD targets, we excluded studies with treatment onset later than 36 hours, because antimicrobial treatment becomes much less effective with late treatment onset [12, 14]. While acknowledging these sources of uncertainty, we simulated clinically relevant unbound drug exposures and used a range of targets values, including conservative (ie, high) PK/PD targets to support treatment choices. Monte Carlo simulations could be performed only for ciprofloxacin, levofloxacin, doxycycline, dalbavancin, and oritavancin because other antimicrobials lacked PK data in anthrax-infected animals.
Conclusions
In summary, these meta-analyses and PK/PD evaluations synthesized the available animal literature on PEPAbx and treatment of anthrax. A wide range of promising antimicrobials are available to combat susceptible strains for natural exposures, and several monotherapy and combination therapy options are available for resistant strains.
Future research is needed on PK/PD relationships for β-lactams, a wider range of tetracyclines, and antibiotic combinations and on anthrax meningitis. Moreover, mechanistic insights can be generated to define the postantibiotic effect for antimicrobials with long half-lives, which would help define dosing intervals and treatment durations. Overall, these data will further support the rational design of efficacious dosage regimens to combat anthrax.
Supplementary Data
Supplementary materials are available at Clinical Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Supplementary Material
Contributor Information
Jordan L Kennedy, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Jürgen B Bulitta, Department of Pharmacotherapy and Translational Research, University of Florida College of Pharmacy, Orlando, Florida, USA.
Kevin Chatham-Stephens, Division of Human Development and Disability, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Marissa K Person, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Rachel Cook, Oak Ridge Institute for Science and Education, CDC Fellowship Program, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Thitipong Mongkolrattanothai, Oak Ridge Institute for Science and Education, CDC Fellowship Program, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Eunjeong Shin, Department of Pharmacotherapy and Translational Research, University of Florida College of Pharmacy, Orlando, Florida, USA.
Patricia Yu, Division of Preparedness and Emerging Infections, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Maria E Negron, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
William A Bower, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Katherine Hendricks, Division of High-Consequence Pathogens and Pathology, Centers for Disease Control and Prevention, Atlanta, Georgia, USA.
Notes
Acknowledgments. The authors thank Joanna Taliano, MLS, for identifying the original “universe” of articles to sift through; Cassandra Tansey, DVM, for helping estimate animal weights based on age, breed, or both; Art Friedlander, MD, for helping identify cutoff points and exclusion criteria; George Drusano, MD, for his critique of the rabbit meningitis articles; Randy Elder, MD, Ray Slay, PhD, and Henry Heine, PhD, for helping think about and devise a quality score for the in vivo studies that were abstracted; and David Sue, PhD, for reviewing and commenting on the discussion.
Disclaimer. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC or the authors' affiliated institutions.
Financial support. This project was supported by the Centers for Disease Control and Prevention and the Office of the Assistant Secretary for Preparedness and Response.
Supplement sponsorship. This article appears as part of the supplement “Anthrax Preparedness,” sponsored by the Centers for Disease Control and Prevention.
Potential conflicts of interest. J. B. B. is a research consultant for MicuRx Therapeutics, and for Lupin. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Carlson CJ, Kracalik IT, Ross N, et al. The global distribution of Bacillus anthracis and associated anthrax risk to humans, livestock and wildlife. Nat Microbiol 2019; 4:1337–43. [DOI] [PubMed] [Google Scholar]
- 2. Athamna A, Athamna M, Abu-Rashed N, Medlej B, Bast DJ, Rubinstein E. Selection of Bacillus anthracis isolates resistant to antibiotics. J Antimicrob Chemother 2004; 54:424–8. [DOI] [PubMed] [Google Scholar]
- 3. Meselson M, Guillemin J, Hugh-Jones M, et al. The Sverdlovsk anthrax outbreak of 1979. Science 1994; 266:1202–8. [DOI] [PubMed] [Google Scholar]
- 4. Jernigan JA, Stephens DS, Ashford DA, et al. Bioterrorism-related inhalational anthrax: the first 10 cases reported in the United States. Emerg Infect Dis 2001; 7:933–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Holty JE, Bravata DM, Liu H, Olshen RA, McDonald KM, Owens DK. Systematic review: a century of inhalational anthrax cases from 1900 to 2005. Ann Intern Med 2006; 144:270–80. [DOI] [PubMed] [Google Scholar]
- 6. Abramova FA, Grinberg LM, Yampolskaya OV, Walker DH. Pathology of inhalational anthrax in 42 cases from the Sverdlovsk outbreak of 1979. Proc Natl Acad Sci U S A 1993; 90:2291–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Maxson T, Kongphet-Tran T, Mongkolrattanothai T. Systematic review of in vitro antimicrobial susceptibility testing for Bacillus anthracis, 1947–2019. Clin Infect Dis 2022; 75:S373–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sittner A, Ben-Shmuel A, Glinert I, et al. Using old antibiotics to treat ancient bacterium-β-lactams for Bacillus anthracis meningitis. PLoS One 2020; 15:e0228917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Welkos S, Bozue J, Twenhafel N, Cote C. Animal models for the pathogenesis, treatment, and prevention of infection by Bacillus anthracis. Microbiol Spectr 2015; 3:Tbs-0001-2012. [DOI] [PubMed] [Google Scholar]
- 10. Zhou J, Tran BT, Tam VH. The complexity of minocycline serum protein binding. J Antimicrob Chemother 2017; 72:1632–4. [DOI] [PubMed] [Google Scholar]
- 11. Andes D, Craig WA. In vivo pharmacodynamic activity of the glycopeptide dalbavancin. Antimicrob Agents Chemother 2007; 51:1633–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Heine HS, Purcell BK, Bassett J, Miller L, Goldstein BP. Activity of dalbavancin against Bacillus anthracis in vitro and in a mouse inhalation anthrax model. Antimicrob Agents Chemother 2010; 54:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Arhin FF, Belley A, McKay G, et al. Assessment of oritavancin serum protein binding across species. Antimicrob Agents Chemother 2010; 54:3481–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Heine HS, Bassett J, Miller L, et al. Efficacy of oritavancin in a murine model of Bacillus anthracis spore inhalation anthrax. Antimicrob Agents Chemother 2008; 52:3350–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Heine HS, Bassett J, Miller L, et al. Determination of antibiotic efficacy against Bacillus anthracis in a mouse aerosol challenge model. Antimicrob Agents Chemother 2007; 51:1373–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Steenbergen J, Tanaka SK, Miller LL, Halasohoris SA, Hershfield JR. In vitro and in vivo activity of omadacycline against two biothreat pathogens, Bacillus anthracis and Yersinia pestis. Antimicrob Agents Chemother 2017; 61:05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Weiss S, Kobiler D, Levy H, et al. Antibiotics cure anthrax in animal models. Antimicrob Agents Chemother 2011; 55:1533–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Weiss S, Altboum Z, Glinert I, et al. Efficacy of single and combined antibiotic treatments of anthrax in rabbits. Antimicrob Agents Chemother 2015; 59:7497–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Vietri NJ, Tobery SA, Chabot DJ, et al. Clindamycin protects nonhuman primates against inhalational anthrax but does not enhance reduction of circulating toxin levels when combined with ciprofloxacin. J Infect Dis 2021; 223:319–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kammanadiminti S, Patnaikuni RK, Comer J, Meister G, Sinclair C, Kodihalli S. Combination therapy with antibiotics and anthrax immune globulin intravenous (AIGIV) is potentially more effective than antibiotics alone in rabbit model of inhalational anthrax. PLoS One 2014; 9:e106393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Migone TS, Bolmer S, Zhong J, et al. Added benefit of raxibacumab to antibiotic treatment of inhalational anthrax. Antimicrob Agents Chemother 2015; 59:1145–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Peterson JW, Moen ST, Healy D, et al. Protection afforded by fluoroquinolones in animal models of respiratory infections with Bacillus anthracis, Yersinia pestis, and Francisella tularensis. Open Microbiol J 2010; 4:34–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yee SB, Hatkin JM, Dyer DN, Orr SA, Pitt ML. Aerosolized Bacillus anthracis infection in New Zealand white rabbits: natural history and intravenous levofloxacin treatment. Comp Med 2010; 60:461–8. [PMC free article] [PubMed] [Google Scholar]
- 24. Barnes JM. Penicillin and B. anthracis. J Pathol Bacteriol 1947; 59:113–25. [DOI] [PubMed] [Google Scholar]
- 25. Gochenour WS Jr., Gleiser CA, Tigertt WD. Observations on penicillin prophylaxis of experimental inhalation anthrax in the monkey. J Hyg (Lond) 1962; 60:29–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Grossman TH, Anderson MS, Drabek L, et al. The fluorocycline TP-271 Is efficacious in models of aerosolized Bacillus anthracis infection in BALB/c mice and cynomolgus macaques. Antimicrob Agents Chemother 2017; 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kalns J, Morris J, Eggers J, Kiel J. Delayed treatment with doxycycline has limited effect on anthrax infection in BLK57/B6 mice. Biochem Biophys Res Commun 2002; 297:506–9. [DOI] [PubMed] [Google Scholar]
- 28. Friedlander AM, Welkos SL, Pitt ML, et al. Postexposure prophylaxis against experimental inhalation anthrax. J Infect Dis 1993; 167:1239–43. [DOI] [PubMed] [Google Scholar]
- 29. Gill SC, Rubino CM, Bassett J, et al. Pharmacokinetic-pharmacodynamic assessment of faropenem in a lethal murine Bacillus anthracis inhalation postexposure prophylaxis model. Antimicrob Agents Chemother 2010; 54:1678–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Heine HS, Bassett J, Miller L, Purcell BK, Byrne WR. Efficacy of daptomycin against Bacillus anthracis in a murine model of anthrax spore inhalation. Antimicrob Agents Chemother 2010; 54:4471–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kao LM, Bush K, Barnewall R, et al. Pharmacokinetic considerations and efficacy of levofloxacin in an inhalational anthrax (postexposure) rhesus monkey model. Antimicrob Agents Chemother 2006; 50:3535–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Heine H. Susceptibility and efficacy of cethromycin in an animal model of anthrax. In: Interscience Conference on Antimicrobial Agents and Chemotherapy. San Francisco, CA: Amer Society for Microbiology. 2006: 41.
- 33. Nelson M, Stagg AJ, Stevens DJ, et al. Post-exposure therapy of inhalational anthrax in the common marmoset. Int J Antimicrob Agents 2011; 38:60–4. [DOI] [PubMed] [Google Scholar]
- 34. Leffel EK, Bourdage JS, Williamson ED, Duchars M, Fuerst TR, Fusco PC. Recombinant protective antigen anthrax vaccine improves survival when administered as a postexposure prophylaxis countermeasure with antibiotic in the New Zealand white rabbit model of inhalation anthrax. Clin Vaccine Immunol 2012; 19:1158–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Slay RM, Hewitt JA, Crumrine M. Determination of the postexposure prophylactic benefit of oral azithromycin and clarithromycin against inhalation anthrax in cynomolgus macaques. Clin Infect Dis 2022; 75:S411–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Slay RM, Hatch GJ, Hewitt JA. Evaluation of amoxicillin and amoxicillin-clavulanate (Augmentin) for antimicrobial postexposure prophylaxis following Bacillus anthracis inhalational exposure in cynomolgus macaques. Clin Infect Dis 2022; 75:S402–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Henderson DW, Peacock S, Belton FC. Observations on the prophylaxis of experimental pulmonary anthrax in the monkey. J Hyg (Lond) 1956; 54:28–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pomerantsev AP, Shishkova NA, Marinin LI. Comparison of therapeutic effects of antibiotics of the tetracycline group in the treatment of anthrax caused by a strain inheriting tet-gene of plasmid pBC16 [in Russian]. Antibiot Khimioter 1992; 37:31–4. [PubMed] [Google Scholar]
- 39. Heine HS, Shadomy SV, Boyer AE, et al. Evaluation of combination drug therapy for treatment of antibiotic-resistant inhalation anthrax in a murine model. Antimicrob Agents Chemother 2017; 61:e00788–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ben-Shmuel A, Glinert I, Sittner A, et al. Treating anthrax-induced meningitis in rabbits. Antimicrob Agents Chemother 2018; 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Levy H, Glinert I, Weiss S, et al. The central nervous system as target of Bacillus anthracis toxin independent virulence in rabbits and guinea pigs. PLoS One 2014; 9:e112319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Altboum Z, Gozes Y, Barnea A, Pass A, White M, Kobiler D. Postexposure prophylaxis against anthrax: evaluation of various treatment regimens in intranasally infected guinea pigs. Infect Immun 2002; 70:6231–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Madrid PB, Chopra S, Manger ID, et al. A systematic screen of FDA-approved drugs for inhibitors of biological threat agents. PLoS One 2013; 8:e60579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Jones WI Jr., Klein F, Lincoln RE, Walker JS, Mahlandt BG, Dobbs JP. Antibiotic treatment of anthrax infection in mice. J Bacteriol 1967; 94:609–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Wein LM, Craft DL. Evaluation of public health interventions for anthrax: a report to the Secretary's Council on Public Health Preparedness. Biosecur Bioterror 2005; 3:348–56. [DOI] [PubMed] [Google Scholar]
- 46. Roberts R, McCune SK. Animal studies in the development of medical countermeasures. Clin Pharmacol Ther 2008; 83:918–20. [DOI] [PubMed] [Google Scholar]
- 47. Hanna KE. Extraordinary measures for countermeasures to terrorism: FDA's ‘Animal Rule.’ Hastings Cent Rep 2002; 32:9. [PubMed] [Google Scholar]
- 48. Bulitta JB, Hope WW, Eakin AE, et al. Generating robust and informative nonclinical in vitro and in vivo bacterial infection model efficacy data to support translation to humans. Antimicrob Agents Chemother 2019; 63:e02307–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Modheji M, Olapour S, Khodayar MJ, Jalili A, Yaghooti H. Minocycline is more potent than tetracycline and doxycycline in inhibiting MMP-9 in vitro. Jundishapur J Nat Pharm Prod 2016; 11:e27377. [Google Scholar]
- 50. Zhanel GG, Esquivel J, Zelenitsky S, et al. Omadacycline: a novel oral and intravenous aminomethylcycline antibiotic agent. Drugs 2020; 80:285–313. [DOI] [PubMed] [Google Scholar]
- 51. Milatovic D, Braveny I. Development of resistance during antibiotic therapy. Eur J Clin Microbiol 1987; 6:234–44. [DOI] [PubMed] [Google Scholar]
- 52. Drusano GL. Antimicrobial pharmacodynamics: critical interactions of ‘bug and drug.’ Nat Rev Microbiol 2004; 2:289–300. [DOI] [PubMed] [Google Scholar]
- 53. Brook I, Giraldo DE, Germana A, et al. Comparison of clarithromycin and ciprofloxacin therapy for Bacillus anthracis Sterne infection in mice with or without 60Co gamma-photon irradiation. J Med Microbiol 2005; 54(pt 12):1157–62. [DOI] [PubMed] [Google Scholar]
- 54. Steward JA, Lever MS, Simpson AJH, Sefton AM, Brooks TJG. Post-exposure prophylaxis of systemic anthrax in mice and treatment with fluoroquinolones. J Antimicrob Chemother 2004; 54:95–9. [DOI] [PubMed] [Google Scholar]
- 55. Vietri NJ, Purcell BK, Lawler JV, et al. Short-course postexposure antibiotic prophylaxis combined with vaccination protects against experimental inhalation anthrax. Proc Natl Acad Sci USA 2006; 103:7813–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Vietri NJ, Purcell BK, Tobery SA, et al. A short course of antibiotic treatment is effective in preventing death from experimental inhalational anthrax after discontinuing antibiotics. J Infect Dis 2009; 198:336–41. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.