Abstract
The ubiquitous protozoan parasite Toxoplasma gondii is a major cause of morbidity and mortality in neonates and immunocompromised hosts. Both acute invasion and reactivation of latent infection result in an inflammatory reaction with lymphocytes, macrophages, and neutrophils. The mechanisms responsible for triggering the local host response to toxoplasmosis are not fully understood. Infection of monolayers of human HeLa epithelial cells and fibroblasts with T. gondii resulted in a marked increase in the expression of interleukin-8 (IL-8)-specific mRNA and secretion of the proinflammatory and chemoattractant cytokines interleukin-8 (IL-8), GROα, and MCP-1. Host cell invasion and lysis were required for this response, as tachyzoite lysates alone had no effect on IL-8 secretion. IL-8 release was dependent on the release of soluble host cell factors: IL-1α in HeLa cells and an additional mediator in fibroblasts. HT-29 epithelial cells, which lack IL-1α or another IL-8-inducing activity, did not release IL-8 after infection, although they were efficiently infected with T. gondii and increased IL-8 secretion in response to added IL-1α. These data suggest that proinflammatory chemokine secretion is an important host cell response to toxoplasmosis and that the release of IL-1α and other mediators from lysed host cells is critical for this chemokine response.
Toxoplasma gondii is one of the most common parasitic infections worldwide, causing life-threatening encephalitis in the immunocompromised host and congenital infections in newborns. After patients ingest infective tissue cysts or oocysts, released tachyzoites first invade and multiply in intestinal epithelial cells (5), and then spread to regional lymph nodes, leading to hematogenous and lymphatic dissemination (2). Tachyzoites can invade any nucleated mammalian cell in an active process requiring release of the contents of specific parasite organelles followed by formation of a parasitophorous vacuole and inhibition of phagosome-lysosome fusion (23). Tachyzoites divide rapidly within the specialized vacuole, resulting in lysis of the host cell, subsequent invasion of adjacent cells, and dissemination. Early lesions are characterized by necrotic areas containing tachyzoites surrounded by acute inflammation. In latent infection, tachyzoites convert to slowly growing bradyzoites, which can persist in tissues for life without eliciting an inflammatory response. Upon profound deterioration in immunity, however, reactivation of chronic (latent) infection can occur, eliciting an inflammatory reaction consisting of lymphocytes, macrophages, and neutrophils (2, 3, 24).
T lymphocytes, natural killer (NK) cells, and activated macrophages have been shown to play important roles in resistance to T. gondii infection (13, 14, 26). In murine models, depletion of both CD4+ and CD8+ T lymphocytes causes reactivation of chronic infection (12). NK cells are also critical, as SCID mice, without functional T cells, survive acute T. gondii infection for at least 2 weeks (15). NK cells are capable of lysing parasite-infected cells and releasing cytokines, a key effector mechanism in controlling acute toxoplasmosis (14, 15, 29). Gamma interferon (IFN-γ) activation of macrophages is particularly important, as infection is uniformly fatal in IFN-γ knockout mice (27, 30). Both interleukin-12 (IL-12) and tumor necrosis factor alpha (TNF-α) act synergistically to induce IFN-γ release, which limits parasite replication (11, 17, 27).
In addition to the resistance conferred by macrophages and T cells, neutrophils also play a role in host resistance to T. gondii. Neutrophils can phagocytose and kill opsonized Toxoplasma (32). In vivo support comes from the finding that mice deficient in inducible nitric oxide synthase (iNOS) succumbed to acute infection following depletion of neutrophils (28). Thus, it appears that neutrophils contribute to acute resistance against this parasite and might account for the ability of iNOS knockout mice to control infection in the apparent absence of macrophage killing function. Neutrophils might scavenge infected cells, secrete toxic products, or produce chemokines required for the recruitment of other effector cell populations (18).
Chemokines are a group of chemotactic polypeptides that are key mediators of leukocyte activation and chemotaxis (1, 20). They are divided into groups of related families based on the arrangement of cysteine residues in their amino-terminal domain (4, 22). The C-X-C or β-chemokines, of which IL-8 is a prototype, are primarily involved in the recruitment and activation of neutrophils, although they may attract other leukocyte populations (20). The C-C or β-chemokines (e.g., MCP-1) attract different leukocyte subsets, including monocytes, macrophages, and T cells (20). Because chemokines are important chemoattractants for neutrophils, macrophages, and T cells, which accumulate at the site of T. gondii infection, the present study investigated the potential role of chemokines in mediating the initial inflammatory response after acute T. gondii infection.
MATERIALS AND METHODS
Human cell lines.
The human colon adenocarcinoma cell line HT-29 (ATCC HTB 38) and HeLa cervix epithelioid carcinoma cells were obtained from the American Type Culture Collection (Rockville, Md.) and were grown in Dulbecco’s modified Eagle’s medium (DME, Irvine Scientific, Santa Ana, Calif.) supplemented with 10% fetal calf serum (FCS; Sigma Chemical, St. Louis, Mo.) and 1% penicillin-streptomycin-amphotericin B. Primary human foreskin fibroblasts (from Mark Sawyer [University of California, San Diego] or the American Type Culture Collection) were subcultured initially in DME supplemented with 10% FCS and 1% penicillin-streptomycin-amphotericin B at 37°C in 5% CO2–95% air until confluent and maintained subsequently with 2% FCS.
T. gondii tachyzoites.
T. gondii RH tachyzoites were cultured in primary human foreskin fibroblast cultures as described above in 2% FCS. Following cell lysis, T. gondii tachyzoites were harvested, centrifuged, washed twice with phosphate-buffered saline (PBS), and resuspended in DME prior to inoculation into cell cultures. Direct cell counts were determined in a hemocytometer.
Infection protocol.
Epithelial cells or fibroblasts were seeded into 24-well plates (Corning Costar Corp., Cambridge, Mass.) and grown to confluence. Fibroblasts required 3 to 7 days to reach 100% confluence, whereas HeLa and HT-29 cells became confluent within 1 to 3 days. When monolayers were confluent, the medium was removed and 102 to 106 washed tachyzoites in DME supplemented with 2% FCS and antibiotics were added. Controls included wells in which DME alone was added to the cell cultures following removal of the original medium. Cultures were incubated at 37°C for 6 to 72 h. Supernatants were removed, filtered through a 0.22-μm-pore-size filter to remove tachyzoites and debris, and stored at −80°C prior to performance of the assays.
The number of viable cells in the monolayer was determined by washing the cultures three times with PBS and detaching the monolayer with trypsin-EDTA. Cells were centrifuged at 1,000 rpm for 10 min and resuspended in 0.25% trypan blue in PBS, and the viable cells were counted. To confirm infection of HT-29 cells, infected monolayers were washed three times with PBS and detached with trypsin-EDTA. Host cells were disrupted by passage through a 27-gauge needle, and the number of released tachyzoites was determined by phase-contrast microscopy.
Cytokine assays.
Cytokine concentrations in culture supernatants and cell lysates were determined by enzyme-linked immunosorbent assay (ELISA) using the following pairs of capturing and detecting antibodies: goat anti-human IL-8 (R&D Systems Inc., Minneapolis, Minn.) and rabbit anti-human IL-8 (Endogen, Inc., Cambridge, Mass.); goat anti-human GROα (R&D Systems) and monoclonal mouse anti-human GROα (R&D Systems); goat anti-human MCP-1 (R&D Systems) and rabbit anti-human MCP-1 (Genzyme Corp., Boston, Mass.); goat anti-human ENA-78 (R&D Systems) and monoclonal mouse anti-human ENA-78 (R&D Systems); and goat anti-human IL-1α (R&D Systems) and monoclonal mouse anti-human IL-1α (Genzyme Corp.). Peroxidase-labeled goat anti-rabbit immunoglobulin G (IgG) and light chains (Biosource International, Camarillo, Calif.) or peroxidase-labeled goat anti-mouse IgG and light chains (Biosource International) were used as secondary antibodies. Sensitivities of the ELISAs were 50 pg/ml for IL-8, GROα and MCP-1, 100 pg/ml for ENA-78, and 10 pg/ml for IL-1α.
RNA extraction and reverse transcription-PCR (RT-PCR) analysis of IL-8 mRNA levels.
Tissue culture flasks (25 cm2; Corning Costar Corp.) were seeded with HeLa or HT-29 cells and grown to confluence. Washed tachyzoites (5 × 105) were then inoculated into each flask, and cultures were incubated for 8, 24, or 48 h. Uninfected monolayers were used as controls. Total cellular RNA was extracted by an acid guanidium thiocyanate-phenol-chloroform method (6). RNA was quantified by determining the optical density at 260 nm, and the integrity was confirmed by gel electrophoresis. Reverse transcription, PCR amplification, and quantitation of IL-8 mRNA were performed as described previously (16), using internal RNA standards. For each experiment, 1 μg of total cellular RNA was used. PCR products were electrophoresed and visualized by ethidium bromide staining, and photographs of the gels were taken with Polaroid 665 film. Band intensities were quantified by densitometry (GS-670 imaging densitometer; Bio-Rad Instruments, Hercules, Calif.), and the number of IL-8 transcripts was derived by determining the point at which the number of standard RNA transcripts was equivalent to the number of cellular target RNA transcripts (16).
Preparation of cell lysates and supernatants.
Tachyzoites were released from host cell monolayers by using a 27-gauge needle, separated from cell debris by filtration through 3.0-μm-pore-size Nuclepore filters, and resuspended in PBS. Tachyzoite lysates were prepared by three freeze-thaw cycles followed by sonication for 15 to 20 s in a Fisher Sonic Dismembrater (model 300).
Supernatants of uninfected monolayers were collected 48 h after fresh medium was added to the confluent monolayers. Supernatants of infected monolayers were collected after the monolayers were infected with 5 × 105 tachyzoites/25-cm2 flask, incubated for 48 h, and filtered to remove live tachyzoites prior to inoculation on fresh monolayers.
Lysates of uninfected monolayers were prepared by growing fibroblasts or HeLa cells to confluence in 25-cm2 tissue culture flasks. Cell monolayers were washed with PBS, removed by scraping, suspended in fresh medium, and subsequently sonicated on ice for 5 s. The lysates were added to uninfected monolayers in an amount equivalent to approximately twice the number of cells as the monolayer to which they were added. Lysates of infected monolayers were prepared in a similar manner after the monolayers were infected with 5 × 105 tachyzoites/flask and incubated for 48 h.
To test for an IL-8-inducing activity released by extracellular tachyzoites, purified tachyzoites were incubated at a concentration of 106/ml of DME for 4 h at 37°C, and the tachyzoites were removed by filtration. The supernatants were then added to fresh fibroblast monolayers in 24-well plates and incubated for 24 h, and secreted IL-8 was detected by ELISA. Controls included fibroblasts incubated with DME alone or stimulated with IL-1α (20 ng/ml).
RESULTS
T. gondii infection induces the release of chemokines by human fibroblasts and HeLa epithelial cells.
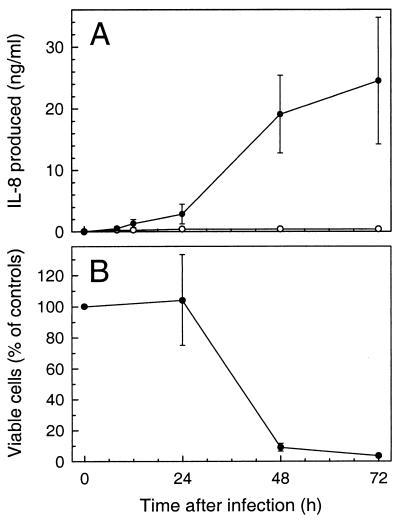
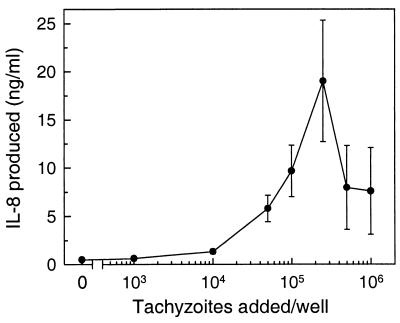
To characterize the factors which may elicit the acute inflammatory infiltrate detected in acute and reactivation toxoplasmosis (3, 24), we evaluated the release of chemokines, which are potent chemoattractants and activators of neutrophils and macrophages. We first assessed the chemokine response in a primary human fibroblast cell line, which has not been transformed for long-term culture propagation. Monolayers of primary human fibroblasts were infected with T. gondii, and secretion of the prototypic neutrophil chemoattractant IL-8 was determined by ELISA. As shown in Fig. 1A, T. gondii-infected fibroblasts secreted >900-fold more IL-8 than uninfected controls. A small increase in IL-8 secretion was first observed at 8 h postinfection, and maximal IL-8 production was seen in the period between 24 and 48 h after infection. Although IL-8 levels continued to increase over the 72-h period of the experiment, absolute levels of IL-8 production decreased in the 48- to 72-h period compared to the 24- to 48-h period after infection. This decrease probably was not due to decreased IL-8 production per cell but rather was related to a decrease in the numbers of viable fibroblasts remaining in the culture at 48 and 72 h after infection due to T. gondii-induced cell lysis (Fig. 1B). Both the magnitude of the IL-8 response (Fig. 2) and the extent of cell lysis (data not shown) were related to the size of the initial inoculum.
FIG. 1.
Increased IL-8 secretion by human fibroblasts in response to infection with T. gondii. (A) IL-8 secretion. Fibroblast monolayers in 24-well plates were infected with 2.5 × 105 tachyzoites/well (●) or left uninfected for controls (○). Supernatants were collected at the indicated times, and IL-8 concentrations were determined by ELISA. The increase in IL-8 secretion following T. gondii infection was significant at 48 and 72 h (P < 0.05, Student’s paired t test). (B) Viable cell counts. The number of viable cells in the fibroblast monolayers was determined by trypan blue dye exclusion following detachment of the cells with trypsin-EDTA. Values are reported as the percentage of uninfected monolayers at each time point (mean ± standard error of the mean; n = 4 to 6).
FIG. 2.
Relationship of T. gondii inoculum size and cumulative IL-8 secretion. Fibroblast monolayers in 24-well plates were infected with 103 to 106 tachyzoite/well and incubated for 48 h. IL-8 concentrations are shown as means ± standard errors of the means (n = 4).
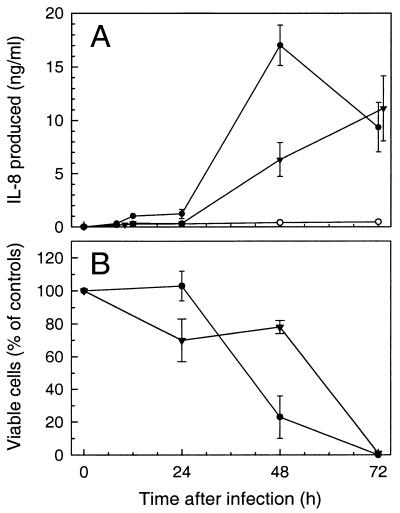
Since T. gondii also invades epithelial cells in vivo and in vitro (5, 10, 19), we next determined the cytokine response of infected HeLa epithelial cells. These cells are readily infected by T. gondii, and they have been previously shown to have well-defined cytokine responses to infection with other invasive pathogens (7, 8, 25). Infection of HeLa cells with T. gondii also markedly increased IL-8 production >60-fold at 48 h postinfection (Fig. 3). Similar to fibroblasts, maximal IL-8 secretion by HeLa cells in response to T. gondii infection was observed at higher inocula (106 tachyzoites/well), which also resulted in significant cell lysis (Fig. 3). Moreover, at a lower inoculum (2.5 × 105), maximal IL-8 secretion was not observed until 72 h postinfection, indicating that the time of maximal IL-8 secretion is dependent on the initial T. gondii inoculum.
FIG. 3.
Time dependence of maximal IL-8 secretion on the T. gondii inoculum. HeLa cell monolayers in 24-well plates were infected with 2.5 × 105 (▾) or 1 × 106 (●) tachyzoites/well, or left uninfected as controls (○), and incubated for 8 to 72 h. (A) IL-8 secretion. IL-8 concentrations were determined by ELISA. Data points represent means ± standard errors of the means (n = 4). IL-8 secretion at 48 and 72 h was significantly increased above controls (P < 0.02, Student’s paired t test). (B) Viable cell counts. The number of viable cells in the monolayers was determined by trypan blue dye exclusion following detachment of the cells with trypsin-EDTA. Values represent the means ± standard errors of the means of three or more determinations.
To investigate whether infection with T. gondii elicits secretion of cytokines other than IL-8, we determined the levels of three additional chemokines, GROα, MCP-1, and ENA-78, in supernatants from infected and control cultures. As shown in Table 1, the GROα response was very similar to that observed with IL-8; at 48 h after infection, infected HeLa cells secreted >100-fold-higher levels and fibroblasts secreted >30-fold-higher levels of GROα compared to uninfected controls. Infected fibroblasts also released significantly higher levels of MCP-1 compared with uninfected controls. Uninfected HeLa cells had significant baseline production of MCP-1, which doubled with infection by T. gondii. Neither cell line produced detectable levels of ENA-78 before or after infection with T. gondii.
TABLE 1.
Release of chemokines in response to infection with T. gondiia
| Cell type | Chemokine | Chemokines secreted (pg/ml)
|
Ratio, infected/control | |
|---|---|---|---|---|
| Control | +T. gondii | |||
| Fibroblasts | GROα | 51 ± 14 | 1,753 ± 512 | 34 |
| IL-8 | 262 ± 106 | 26,946 ± 6,106 | 103 | |
| MCP-1 | 833 ± 75 | 21,763 ± 6,176 | 26 | |
| HeLa cells | GROα | 165 ± 41 | 23,400 ± 2,990 | 142 |
| IL-8 | 286 ± 39 | 6,327 ± 1,600 | 22 | |
| MCP-1 | 37,656 ± 2,034 | 89,644 ± 14,969 | 2.4 | |
Confluent monolayers of fibroblasts and HeLa cells in 24-well plates were infected with 2.5 × 105 T. gondii tachyzoites/well and incubated for 48 h. Culture supernatants were collected, and levels of GROα, IL-8, and MCP-1 were determined by ELISA. Values represent the means ± standard errors of the means (n = 4 to 6). Secretion of all chemokines in infected cultures was significantly increased over that in uninfected controls (P < 0.02 by Student’s paired t test).
T. gondii infection of HeLa epithelial cells increases IL-8 mRNA expression.
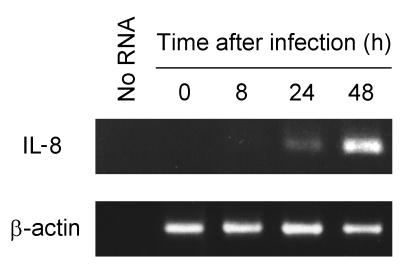
We next investigated whether increased IL-8 secretion after T. gondii infection was paralleled by increased IL-8 mRNA expression. Infection of HeLa cells with T. gondii increased IL-8 mRNA levels at 24 and 48 h after infection, as determined by RT-PCR analysis (Fig. 4). This finding was confirmed by quantitative RT-PCR using internal RNA standards, which showed a 50-fold increase in IL-8 mRNA levels at 24 h after infection (8 × 104 transcripts/μg of total RNA after infection versus 1.6 × 103 transcripts/μg of RNA in controls). In contrast to IL-8, levels of the mRNA for β-actin were not affected by T. gondii infection of HeLa cells (Fig. 4).
FIG. 4.
Increased IL-8 mRNA expression after T. gondii infection of HeLa cells. Monolayers of HeLa cells in 25-cm2 tissue culture flasks were infected with 5 × 105 T. gondii tachyzoites and incubated for the indicated periods. Total RNA was extracted, and levels of IL-8 and β-actin transcripts were determined by RT-PCR. Twenty microliters of each PCR mixture was electrophoresed and stained with ethidium bromide. As a negative control, RNA was omitted from reverse transcription and PCR amplification (No RNA).
IL-8 response to T. gondii infection requires live tachyzoites.
Increased cytokine secretion has also been reported after in vitro infection of epithelial cells by fungi and protozoa other than T. gondii, including Entamoeba histolytica and Aspergillus fumigatus (31, 33). For these pathogens, soluble products were found to be important for stimulating IL-8 release. Lysates of E. histolytica stimulated IL-8 secretion from human colonic epithelial cells in the absence of cell-cell contact (33), while released serine proteases of A. fumigatus induced the production of IL-8, IL-6, and MCP-1 in epithelial cells (31). Therefore, we investigated the ability of soluble cellular components of Toxoplasma to stimulate IL-8 secretion. Monolayers of fibroblasts were either infected with 5 × 105 viable tachyzoites/well or exposed to an equivalent number of sonicated tachyzoites. After 24 to 48 h of incubation, infection with live tachyzoites resulted in a 25- to 100-fold increase in IL-8 secretion (Fig. 1). In contrast, when the same number of sonicated tachyzoites were added to the fibroblast cultures, IL-8 secretion did not increase (data not shown). To rule out the possibility of active secretion of an IL-8-inducing activity by extracellular tachyzoites themselves, tachyzoites were incubated for 4 h at a concentration of 106/ml of DME, which is at least 1,000-fold higher than would be seen extracellularly in 24-h cultures. When the supernatants were added to fresh fibroblast monolayers for 24 h, a small increase in IL-8 was detected (0.56 ng/ml versus <0.05 ng/ml in unstimulated controls). This value was less than 10% of the stimulation induced by infection with the same number of live tachyzoites at 24 h (mean of 6.0 ng/ml). Therefore, it appears that infection by intact, viable tachyzoites is the primary factor in eliciting an increase in IL-8 production.
IL-1α is the principal mediator of T. gondii-induced IL-8 secretion in HeLa cells but not in fibroblasts.
Since maximal IL-8 secretion in response to T. gondii infection was observed at inocula which caused substantial lysis of fibroblasts and HeLa cells, we hypothesized that components of these host cells released during lysis may mediate the IL-8 response. Such a mechanism was previously described for infection with two other cytolytic pathogens, E. histolytica and Chlamydia trachomatis, where IL-1α was identified as the crucial mediator of increased cytokine production after infection (8, 25). To test a role of IL-1α in mediating the T. gondii-induced IL-8 response, monolayers of fibroblasts and HeLa cells were infected with T. gondii in the presence or absence of antibodies against IL-1α to block any potential IL-1α activity that may be released during the infection. As shown in Table 2, anti-IL-1α antibodies completely (>98%) blocked the IL-8 response of HeLa cells to T. gondii infection. In contrast, increased IL-8 production by T. gondii-infected fibroblasts was decreased by only 40% by addition of antibodies against IL-1α, although the same antibodies fully blocked IL-1α-induced IL-8 secretion by these cells. Furthermore, no additional inhibition was achieved with higher concentrations of anti-IL-1α antibodies (up to 100 μg/ml).
TABLE 2.
Effect of blocking IL-1α activity on IL-8 secretion by T. gondii-infected HeLa cells and fibroblastsa
| Stimulus added | Inhibitor added | IL-8 secreted (pg/ml)
|
|
|---|---|---|---|
| HeLa cells | Fibroblasts | ||
| None | None | 291 ± 15 | 26 ± 4 |
| T. gondii | None | 16,336 ± 3,024 | 14,436 ± 2,833 |
| Anti-IL-1α | 244 ± 147 | 8,641 ± 1,724 | |
| Goat IgG | 12,930 ± 915 | 15,794 ± 3,668 | |
| IL-1α | None | 23,979 ± 7,051 | 48,117 ± 4,335 |
| Anti-IL-1α | 363 ± 32 | 212 ± 64 | |
| Goat IgG | 26,079 ± 7,816 | 52,106 ± 11,210 | |
Confluent monolayers of HeLa cells and fibroblasts in 24-well plates were infected with 5 × 105 T. gondii tachyzoites/well. Goat antibodies against human IL-1α (20 μg/ml; R&D Systems) or normal goat IgG (20 μg/ml) was added at the start of the cultures and was present throughout the 72-h period. Parallel uninfected cultures were stimulated with IL-1α (20 ng/ml) for 24 h. Culture supernatants were collected, and IL-8 concentrations were determined by ELISA. Goat anti IL-1α antibodies did not interfere with the IL-8 ELISA (8). Values represent means ± standard errors of the means (n = 4).
To confirm a role of IL-1α in mediating the IL-8 response to T. gondii infection, we directly determined IL-1α levels in the supernatants of infected HeLa cells and fibroblasts. T. gondii infection of HeLa cells increased IL-1α levels in the supernatants at 48 h after infection in a dose-dependent manner. Thus, after infection with 2.5 × 105, 5 × 105, and 106 tachyzoites/well, IL-1α levels were 39 ± 12, 95 ± 35, and 197 ± 24 pg/ml, respectively, whereas uninfected control cells had <10 pg/ml in the supernatants. These data support a key role for IL-1α in mediating T. gondii-induced IL-8 secretion in HeLa cells. In contrast to HeLa cells, IL-1α appeared to play a minor role in the induction of IL-8 by T. gondii in fibroblasts (Table 2). Consistent with this finding, only low levels of IL-1α (<10 pg/ml) were detected in supernatants of T. gondii-infected fibroblasts.
Release of an IL-8-inducing activity from T. gondii-infected fibroblasts.
Because IL-1α release played a minor role in mediating the IL-8 response to T. gondii infection in fibroblasts, we next determined whether infected fibroblasts released an IL-8-inducing activity other than IL-1α or if the IL-8 response of these cells was a direct response to infection. For these experiments, 48-h supernatants from infected and control cells were tested for the ability to induce IL-8 secretion in cultures of uninfected fibroblasts (test cultures). Since the 48-h supernatants already contained substantial levels of IL-8 (Fig. 1), which could confound the assessment of additional IL-8 production, we stimulated the test cultures with 48-h supernatants for only a short period (2 h), sufficient to activate increased IL-8 expression (25). The supernatants were then removed by repeated washing, fresh medium was added, and IL-8 secretion by the test cultures was determined after an additional 6 h of incubation. This approach allowed us to assess IL-8 production by fibroblasts in the absence of any IL-8 from the original 48-h supernatants (the latter was confirmed by the observation that <100 pg of IL-8/ml was detected in the final washes used to remove the original supernatants). Using this approach, we found that 48-h supernatants from uninfected control fibroblasts had no significant effect on IL-8 secretion, whereas supernatants from T. gondii-infected fibroblasts increased IL-8 secretion by the test cultures >10-fold (Table 3). The IL-8-inducing activity present in the supernatants from infected fibroblasts could not be blocked by addition of antibodies against IL-1β or TNF-α and was blocked by only approximately 30% after addition of antibodies to IL-1α (Table 3).
TABLE 3.
Release of an IL-8-inducing activity by T. gondii-infected fibroblastsa
| Stimulus added | Source of stimulus | Inhibitor added | IL-8 secreted (pg/ml) |
|---|---|---|---|
| None | Control cells | None | 35 ± 7 |
| Supernatant | Control cells | None | 71 ± 23 |
| T. gondii-infected cells | None | 458 ± 57b | |
| Anti-IL-1α | 257 ± 43c | ||
| Anti-IL-1β | 396 ± 21 | ||
| Anti-TNF-α | 430 ± 29 | ||
| Lysate | Control cells | None | 292 ± 101 |
| T. gondii-infected cells | None | 2,436 ± 768b | |
| Anti-IL-1α | 1,799 ± 851 | ||
| Anti-IL-1β | 2,794 ± 1,318 | ||
| Anti-TNF-α | 2,419 ± 1,159 |
Fibroblast monolayers in T-25 flasks were infected with 5 × 105 T. gondii for 48 h, resulting in <50% lysis of the monolayer. Uninfected monolayers were used as controls. Supernatants and lysates of both infected and uninfected fibroblast monolayers were collected, filtered to remove free tachyzoites, and added to fresh, confluent fibroblast monolayers in 24-well plates, with or without goat antibodies against IL-1α, IL-1β, or TNF-α (20 μg/ml). Addition of normal goat IgG as an isotype control did not alter IL-8 levels. Following a 2-h incubation period, monolayers were washed and further incubated with fresh medium for 6 h. IL-8 levels were then determined by ELISA and are expressed as means ± standard errors of the means (n = 4 to 6).
Significantly increased over uninfected control cells (P < 0.02 by Student’s paired t test).
Significantly different from T. gondii-infected cells without inhibitors (P < 0.02).
To determine whether a preexisting activity was present in fibroblasts or if the IL-8-inducing activity was induced during the course of infection, lysates of uninfected and infected fibroblasts were added to fresh fibroblast monolayers. Greater than 5-fold stimulation of IL-8 secretion could be induced by uninfected fibroblast lysates, while >10-fold induction was detected after addition of lysates from infected cells. As with supernatants, the activity was not blocked by antibodies to IL-1β or TNF-α but was partially inhibited by antibodies to IL-1α (although not to a statistically significant extent) (Table 3). No additional effect was seen when all three antibodies were used in combination. These results suggest that a bioactive factor other than IL-1α, IL-1β, or TNF-α was upregulated and released from fibroblasts during infection with T. gondii and was important for stimulating IL-8 production.
T. gondii infection of HT-29 epithelial cells that do not contain an IL-8-inducing activity has no effect on IL-8 secretion.
The studies in HeLa cells and fibroblasts suggested that IL-1α or another IL-8-inducing activity is an important mediator of the IL-8 response to T. gondii infection. Based on these findings, we hypothesized that cells that do not express IL-1α or another IL-8-inducing activity might not respond to T. gondii infection with increased IL-8 secretion. To test this, we used HT-29 human intestinal epithelial cells which were shown previously to neither express IL-1α mRNA nor contain preformed IL-1α or another IL-8-inducing activity (8, 16). Monolayers of HT-29 cells were infected efficiently by T. gondii, as they released as many tachyzoites as, or even more than, HeLa cells when incubated with 2.5 × 105 tachyzoites/well for 48 h (data not shown). Furthermore, 80 to 100% of the HT-29 monolayer was lysed within 72 h of infection (at inocula of 5 × 104 to 1 × 106 tachyzoites/well). Nevertheless, T. gondii infection had no effect on IL-8 secretion by HT-29 cells at 24 to 72 h after infection (354 ± 38 pg of IL-8/ml from T. gondii-infected HT-29 cells versus 393 ± 21 pg/ml from uninfected controls; n = 4). Furthermore, IL-8 mRNA levels in HT-29 cells were not increased by infection with T. gondii (data not shown). The lack of an IL-8 response was not due to an inability of HT-29 cells to upregulate IL-8 secretion since stimulation of control HT-29 cells with IL-1α or TNF-α resulted in an approximately 200-fold increase in IL-8 production. Furthermore, T. gondii infection was not inhibitory for IL-8 secretion of HT-29 cells since infected and control cells increased IL-8 production to the same extent after IL-1α and TNF-α stimulation. HT-29 cells also did not secrete increased levels of MCP-1, GROα, or ENA-78 in response to T. gondii infection. Thus, despite efficient infection of HT-29 cells with resultant cell lysis, T. gondii infection failed to elicit a chemokine response from these cells that lack IL-1α or another IL-8-inducing activity.
DISCUSSION
Acute toxoplasmosis causes host cell lysis and an inflammatory infiltrate consisting of lymphocytes, macrophages, and neutrophils. A similar host response occurs when latent infection is reactivated in immunocompromised patients with impaired cellular immunity, particularly in patients with AIDS. We have shown that one signal for the observed cellular infiltrate after T. gondii infection may be the release of proinflammatory chemokines from infected cells. Infection of primary fibroblasts, as well as transformed epithelial cell lines, with T. gondii stimulates secretion of the proinflammatory chemokines IL-8, GROα, and MCP-1. Secretion of IL-8 by host cells is associated with increased IL-8 mRNA expression. The chemokine response is dependent on invasion by live tachyzoites and subsequent host cell lysis. Furthermore, supernatants or lysates from T. gondii-infected fibroblasts could elicit significant IL-8 secretion when added to uninfected fibroblasts, and IL-1α release played a crucial role in mediating the IL-8 response to infection in HeLa cells.
The release of chemokines may contribute to the inflammatory infiltrate that accompanies acute or reactivation toxoplasmosis. IL-8 and GROα are both chemoattractants for neutrophils (20), which accumulate at the site of acute T. gondii infection (24). In vitro studies have shown that human neutrophils inhibit T. gondii replication (32). The early pathology of T. gondii infection in mice mimics human disease with recruitment of neutrophils (5, 19, 27). Neutrophil depletion by the administration of antigranulocyte antibody markedly diminished early resistance of mice to Toxoplasma infection (28). In addition, mice with defective macrophage function from knockouts of iNOS were able to survive acute, but not persistent, infection as long as their neutrophils were intact (28), suggesting that neutrophils contribute to acute resistance to T. gondii infection.
Increased cytokine secretion in response to infection has been reported for invasive pathogens other than T. gondii, including enteroinvasive bacteria (7, 16), E. histolytica (8), Cryptosporidium parvum (21), and Chlamydia sp. (25). Two distinct time courses of cytokine responses are seen following infection with these pathogens. Infection with invasive enteric bacteria (e.g., Salmonella spp. or enteroinvasive E. coli) evoked a rapid (within 2 to 3 h) and transient (lasting 4 to 10 h) expression and secretion of C-X-C chemokines such as IL-8 (7, 16). Although E. histolytica does not actually invade cells, it does result in rapid host cell lysis with IL-8 secretion detectable within 2 to 4 h and maximum release by 4 to 8 h (8). In contrast, the first detectable IL-8 was not noted until 8 h following infection with T. gondii and failed to reach maximum release until 48 to 72 h. Similarly, upregulated expression and secretion of IL-8 were first detected 16 to 24 h after C. parvum infection of epithelial cells (21), while Chlamydia infection of epithelial cells required at least 48 h to cause a detectable IL-8 response (25). Slow inducers of a host proinflammatory cytokine response, such as T. gondii, C. parvum, and Chlamydia, induce little cytoskeletal changes in the host cells during invasion and require a prolonged period of intracellular development before lysing the host cell. In contrast, the rapid cytokine inducers, enteroinvasive E. coli and Salmonella, elicit extensive membrane ruffling and actin redistribution in host cells (9), while E. histolytica rapidly lyses and disrupts host cells (8).
Cytokine release by host cells following T. gondii infection appeared to require at least three steps: invasion by live tachyzoites, tachyzoite multiplication resulting in cell lysis, and a paracrine-acting factor in host cells that can be released upon lysis. In HeLa epithelial cells, upregulation of IL-8 production in uninfected neighboring cells is dependent on IL-1α release from lysed cells. Similarly, lysis of cells by E. histolytica also evoked an IL-1α-dependent release of IL-8 (8), although non-IL-1α-dependent mechanisms of IL-8 induction also exist (33). Thus, amplification of IL-8 release by IL-1α may be a conserved mechanism of host cell response to infection by lytic pathogens. An additional host cell factor appears to upregulate IL-8 production in fibroblasts. Only a relatively small fraction of the IL-8 response of fibroblasts could be inhibited by antibodies to IL-1α, and no inhibition occurred with antibodies to IL-1β or TNF-α, other cytokines which can elicit IL-8 secretion (1).
HT-29 cells, a colonic cell line which lacks preformed IL-1α or other IL-8-inducing activities (8, 16), failed to induce an IL-8 response after infection with T. gondii. Nevertheless, HT-29 cells secrete IL-8 upon stimulation with exogenous IL-1α and TNF-α, demonstrating that this cell line is not deficient in inducible IL-8 production (6). In addition, HT-29 cells were not resistant to infection by T. gondii, based on cell lysis studies and enumeration of intracellular tachyzoites.
These studies may shed light on the host inflammatory response and latency in T. gondii infection. Cell invasion or the intracellular presence of T. gondii alone is not sufficient to elicit an inflammatory cytokine response. Maximal IL-8 production in HeLa cells and fibroblasts was delayed for >24 h until significant cell lysis occurred. Slowly dividing bradyzoites would not cause cell lysis and thus the subsequent release of factors such as IL-1α to stimulate secretion of IL-8 by uninfected neighboring cells. In addition, certain host cells, such as HT-29, can be effectively infected by T. gondii, but infection elicits no inflammatory cytokine response because they lack appropriate paracrine factors. Induction of the host inflammatory response and the development of latent infection are likely to be multifactorial, but these studies suggest that release of IL-1α and other mediators from lysed host cells is critical to elicit proinflammatory chemokine secretion and provides an important signaling system to initiate the host inflammatory response to active T. gondii infection.
ACKNOWLEDGMENTS
This work was supported in part by grants from the University of California Universitywide AIDS Research Program (S.L.R.), the UCSD Academic Senate, the UCSD Center for AIDS Research (NIAID 5 P30 AI36214), the National Institutes of Health (AI41903), and the Crohns and Colitis Foundation of America (L.E.).
We thank Jennifer Smith and Scott Herdman for their expert assistance.
REFERENCES
- 1.Baggiolini M, Dewald B, Moser B. Interleukin-8 and related chemotactic cytokines—CXC and CC chemokines. Adv Immunol. 1994;55:97–179. [PubMed] [Google Scholar]
- 2.Bertoli F, Espino M, Arosemena J R, Fishback J L, Frenkel J K. A spectrum in the pathology of toxoplasmosis in patients with AIDS. Arch Pathol Lab Med. 1995;119:214–224. [PubMed] [Google Scholar]
- 3.Carrazana E J, Rossitch E, Samuels M A. Cerebral toxoplasmosis in the acquired immune deficiency syndrome. Clin Neurol Neurosurg. 1989;91:291–299. doi: 10.1016/0303-8467(89)90004-8. [DOI] [PubMed] [Google Scholar]
- 4.Curfs J H A J, Meis J F G M, Hoogkamp-Korstanje J A A. A primer on cytokines: sources, receptors, effects, and inducers. Clin Microbiol Rev. 1997;10:742–780. doi: 10.1128/cmr.10.4.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dupey J P, Speer C A, Shen S K, Kwok O C H, Blixt J A. Oocyst-induced murine toxoplasmosis: life cycle, pathogenicity, and stage conversion in mice fed Toxoplasma gondii oocysts. J Parasitol. 1997;83:870–882. [PubMed] [Google Scholar]
- 6.Eckmann L, Jung H C, Schurer-Maly C, Panja A, Morzycka-Wroblewska E, Kagnoff M F. Differential cytokine expression by human intestinal epithelial cells: regulated expression of interleukin-8. Gastroenterology. 1993;105:1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- 7.Eckmann L, Kagnoff M F, Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993;61:4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eckmann L, Reed S L, Smith J R, Kagnoff M F. Entamoeba histolytica trophozoites induce an inflammatory cytokine response by cultured human cells through the paracrine action of cytolytically released interleukin-1α. J Clin Investig. 1995;96:1269–1279. doi: 10.1172/JCI118161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella choleraesuis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 10.Garcia L W, Hemphill R B, Marasco W A, Ciano P S. Acquired immunodeficiency syndrome with disseminated toxoplasmosis presenting as an acute pulmonary and gastrointestinal illness. Arch Pathol Lab Med. 1991;115:459–463. [PubMed] [Google Scholar]
- 11.Gazzinelli R T, Hieny S, Wynn T A, Wolf S, Sher A. Interleukin 12 is required for the T-lymphocyte-independent induction of interferon γ by an intracellular parasite and induces resistance in T-cell-deficient hosts. Proc Natl Acad Sci USA. 1993;90:6115–6119. doi: 10.1073/pnas.90.13.6115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gazzinelli R, Xu Y, Heny S, Cheever A, Sher A. Simultaneous depletion of CD4+ and CD8+ T lymphocytes is required to reactivate chronic infection with Toxoplasma gondii. J Immunol. 1992;149:175–180. [PubMed] [Google Scholar]
- 13.Hakim F T, Gazzinelli R T, Denkers E, Hieny S, Shearer G M, Sher A. CD8+ T cells from mice vaccinated against Toxoplasma gondii are cytotoxic for parasite-infected or antigen-pulsed host cells. J Immunol. 1991;147:2310–2316. [PubMed] [Google Scholar]
- 14.Hauser W E, Tsai V. Acute Toxoplasma infection of mice induces spleen NK cells that are cytotoxic for T. gondii in vitro. J Immunol. 1986;136:313–319. [PubMed] [Google Scholar]
- 15.Hunter C A, Subauste C S, Van Cleave V H, Remington J S. Production of gamma interferon by natural killer cells from Toxoplasma gondii-infected SCID mice: regulation by interleukin-10, interleukin-12, and tumor necrosis factor alpha. Infect Immun. 1994;62:2818–2824. doi: 10.1128/iai.62.7.2818-2824.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung H C, Eckmann L, Yang S, Panja A, Fierer J, Morzycka-Wroblewska E, Kagnoff M F. A distinct array of proinflammatory cytokines is expressed in human colon epithelial cells in response to bacterial invasion. J Clin Investig. 1995;95:55–65. doi: 10.1172/JCI117676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kahn I A, Matsuura T, Kasper L H. Interleukin-12 enhances survival against acute toxoplasmosis. Infect Immun. 1994;62:1639–1642. doi: 10.1128/iai.62.5.1639-1642.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kasama T, Strieter R M, Lukacs N W, Lincoln P M, Burdick M D, Kunkel S L. Interferon gamma modulates the expression of neutrophil-derived chemokines. J Investig Med. 1995;43:58–67. [PubMed] [Google Scholar]
- 19.Khavkin T. Histological and ultrastructural studies of the interaction of Toxoplasma gondii tachyzoites with mouse omentum in experimental infection. J Protozool. 1981;28:317–325. doi: 10.1111/j.1550-7408.1981.tb02858.x. [DOI] [PubMed] [Google Scholar]
- 20.Kunkel S L, Lukacs N W, Chensue S W, Strieter R M. Chemokines and the inflammatory response. In: Remick D G, Friedland J S, editors. Cytokines in health and disease. New York, N.Y: Marcel Dekker; 1997. pp. 121–131. [Google Scholar]
- 21.Laurent F, Eckmann L, Savidge T C, Morgan G, Theodos C, Naciri M, Kagnoff M F. Cryptosporidium parvum infection of human intestinal epithelial cells induces the polarized secretion of C-X-C chemokines. Infect Immun. 1997;65:5067–5073. doi: 10.1128/iai.65.12.5067-5073.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luster A D. Chemokines-chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- 23.Mordue D G, Sibley L D. Intracellular fate of vacuoles containing Toxoplasma gondii is determined at the time of formation and depends on the mechanism of entry. J Immunol. 1997;159:4452–4459. [PubMed] [Google Scholar]
- 24.Navia B A, Petitio C K, Gold J W M, Cho E S, Jordan B D, Price R W. Cerebral toxoplasmosis complicating the acquired immune deficiency syndrome: clinical and neuropathological findings in 27 patients. Ann Neurol. 1986;19:224–238. doi: 10.1002/ana.410190303. [DOI] [PubMed] [Google Scholar]
- 25.Rasmussen S J, Eckmann L, Quayle A J, Shen L, Zhang Y, Anderson D J, Fierer J, Stephens R S, Kagnoff M F. Secretion of proinflammatory cytokines by epithelial cells in response to Chlamydia infection suggests a central role for epithelial cells in chlamydial pathogenesis. J Clin Investig. 1997;99:77–87. doi: 10.1172/JCI119136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Remington J S, Krahenbuhl J L, Mendenhall J W. A role for activated macrophages in resistance to infection with Toxoplasma. Infect Immun. 1972;6:829–834. doi: 10.1128/iai.6.5.829-834.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scharton-Kersten T M, Wynn T A, Denkers E Y, Bala S, Grunvald E, Hieny S, Gazzinelli R T, Sher A. In the absence of endogenous IFN-γ, mice develop unimpaired IL-12 responses to Toxoplasma gondii while failing to control acute infection. J Immunol. 1996;157:4045–4054. [PubMed] [Google Scholar]
- 28.Scharton-Kersten T M, Yap G, Magram J, Sher A. Inducible nitric oxide is essential for host control of persistent but not acute infection with the intracellular pathogen Toxoplasma gondii. J Exp Med. 1997;185:1261–1273. doi: 10.1084/jem.185.7.1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Subauste C S, Dawson L, Remington J S. Human lymphokine-activated killer cells are cytotoxic against cells infected with Toxoplasma gondii. J Exp Med. 1992;176:1511–1519. doi: 10.1084/jem.176.6.1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Suzuki Y, Orellana M A, Schreiber R D, Remington J S. Interferon-γ: the major mediator of resistance against Toxoplasma gondii. Science. 1988;240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 31.Tomee J F C, Wierenga A T J, Hiemstra P S, Kauffman H F. Proteases from Aspergillus fumigatus induce release of proinflammatory cytokines and cell detachment in airway epithelial cell lines. J Infect Dis. 1997;176:300–303. doi: 10.1086/517272. [DOI] [PubMed] [Google Scholar]
- 32.Wilson C B, Remington J S. Activity of human blood leukocytes against Toxoplasma gondii. J Infect Dis. 1979;140:890–895. doi: 10.1093/infdis/140.6.890. [DOI] [PubMed] [Google Scholar]
- 33.Yu Y, Chadee K. Entamoeba histolytica stimulates interleukin-8 from human colonic epithelial cells without parasite-enterocyte contact. Gastroenterology. 1997;112:1536–1547. doi: 10.1016/s0016-5085(97)70035-0. [DOI] [PubMed] [Google Scholar]