Abstract
Multiple lines of evidence have increasingly suggested a pathogenic connection between rheumatoid arthritis (RA) and the mechanisms of type 2 diabetes (T2D) in a vicious circle perpetuated by glucose derangement and inflammatory mediators. These findings have been further reinforced by clinical studies showing that the inhibition of interleukin (IL)-1 and IL-6 may allow the treatment of RA and concomitant T2D at the same time. Interestingly, IL-1 inhibition induced a more evident reduction of glycated haemoglobin (HbA1c) in patients with concomitant RA and T2D than in previous studies on IL-1 inhibition in patients with this metabolic disease alone. Thus, the inflammatory pathogenic mechanisms of T2D could be exaggerated in the context of a rheumatic disease, possibly explaining these findings. In fact, IL-1 inhibition could not only palliate glycaemia, but also decrease the progressive decline in insulin secretion associated with T2D, interfering with apoptosis of β-cells, improving their function, and ameliorating the peripheral insulin resistance. Moreover, the maintenance of clinical remission of rheumatic disease could further improve the glucose derangement and reduce the occurrence of T2D in RA. On these bases, the presence of T2D may allow the physicians to perform a better profile of patients with RA according to the principles of precision medicine, tailoring the medical treatment to the individual characteristics. In this context, the benefits of targeting the inflammatory process, mainly by IL-1 inhibition, may be suggested in patients with RA and concomitant T2D.
Key Points
| Multiple lines of evidence have increasingly suggested a pathogenic connection between the RA process and the mechanisms of T2D in a vicious circle perpetuated by glucose derangement and inflammatory mediators. |
| Some clinical studies showed that the inhibition of inflammatory cytokines, mostly IL-1, may allow the treatment of RA and concomitant T2D at the same time. |
| The presence of T2D may allow the physicians to perform a better profile of patients with RA according to the principles of precision medicine, tailoring the medical treatment to the individual characteristics. |
Introduction
Rheumatoid arthritis (RA) is a chronic inflammatory disease characterised by synovitis, cartilage damage, and bone erosions, which is associated with an increased morbidity and mortality compared with the general population [1]. Patients with RA are burdened by an increased risk of accelerated atherosclerosis and cardiovascular disease (CVD) [2–5]. This finding is partially explained by the enhanced prevalence of “traditional” cardiovascular (CV) risk factors, including smoking habit, overweight, lipid metabolism alterations, high blood pressure, and glucose derangement. The underlying systemic rheumatoid inflammatory process, along with genetic factors, and therapies, may play an important role in synergising with “traditional” CV risk to enhance the risk of CVD. In this context, glucose metabolism abnormalities, insulin resistance (IR), and type 2 diabetes (T2D), are frequently observed in patients with RA [6–9]. Insulin resistance is defined as an altered biologic response to insulin in target tissues associated with the necessity for an enhanced release of this hormone to obtain a quantitatively normal response to glucose [10]. Thus, IR results in a compensatory increase of insulin production, leading to hyperinsulinaemia. The latter predicts the development of T2D [10], which occurs when pancreatic ß-cell function fails to compensate for IR, with a consequent hyperglycaemia, due to either impaired synthesis or impaired insulin function [11]. Patients with RA have an increased prevalence of IR, about 40 %, in respect to the general population [12–15]. Furthermore, an increased prevalence, of almost 15 %, of T2D is reported in those patients [16]. A recent meta-analysis also showed that patients with RA have an approximately 1.5-fold higher risk of developing T2D than the general population [16]. Interestingly, a poorly controlled RA disease activity has been reported to predict the occurrence of T2D in these patients [6, 17]. Conversely, the achievement and maintenance of the clinical remission, underlying the abrogation of the inflammatory process, is associated with a low probability to develop this comorbidity [2]. These findings may lead to hypothesise that the inflammatory process of RA may be implicated in the development of concomitant metabolic diseases [15, 18]. On these bases, multiple lines of evidence have proposed the role of the immune system in contributing to the pathogenesis of IR and T2D; thus, suggesting the possible efficacy of biologic disease-modifying anti-rheumatic drugs (bDMARDs) in treating the glucose derangement [19, 20]. The bDMARDs are immunosuppressive drugs successfully used in patients with RA leading to a significant clinical improvement and reduction of the joint damage in the long term. Interestingly, a systematic review and meta-analysis found that the administration of bDMARDs may be associated with a reduced incidence of T2D in patients with RA than those treated with other drugs [21]. Therefore, common therapeutic targets may exist in patients with RA and concomitant T2D proposing a possible “bidirectional” treatment [22, 23]. In this context, some studies have recently reported that some bDMARDs may have a beneficial role in improving the glucose derangement of patients with RA [24–26].
In this work we aimed to discuss the available evidence, from pathogenic mechanisms to treatment, about the influence of therapeutic options in RA on glucose derangement in patients with concomitant T2D.
Pathogenic Links Between RA and T2D
The Pathogenic Role of Insulin in the Context of Rheumatic Diseases
Insulin is the main hormone regulating glucose homeostasis and it acts through the transmembrane insulin receptors. The latter are expressed on multiple target cells including hepatocytes, adipocytes, synoviocytes and muscle cells [27]. Intriguingly, these receptors may also be found on the surface membrane of immune cells [27, 28]. In fact, these cells need glucose to produce energy, and through its receptors, insulin exerts its hypoglycaemic function and behaves as a growth-like factor as well as a cytokine regulator [29, 30]. Therefore, this hormone may also exert immunomodulatory effects on the immune system in addition to well-known metabolic effects [31, 32]. In this context, it has been shown that hyperglycaemia has negative effects on the immune cells leading to the production of advanced glycation end products and reactive oxygen species, which in turn may stimulate the generation of various pro-inflammatory mediators [33]. Thus, insulin may have a role in reducing the “glucose toxicity” and cell stress, exerting an anti-inflammatory effect [28]. Other evidence performed in healthy nondiabetic subjects explored the effects of insulin on polymorphonuclear leukocytes functions [34]. On those cells, this hormone has shown to have the ability to induce the production of pro-inflammatory mediators. Taking together these findings, a possible role of insulin in contributing to the induction of an aberrant immune response may be speculated during rheumatic diseases [28].
Inflammatory Pathogenic Mechanisms of T2D may be Exacerbated by RA
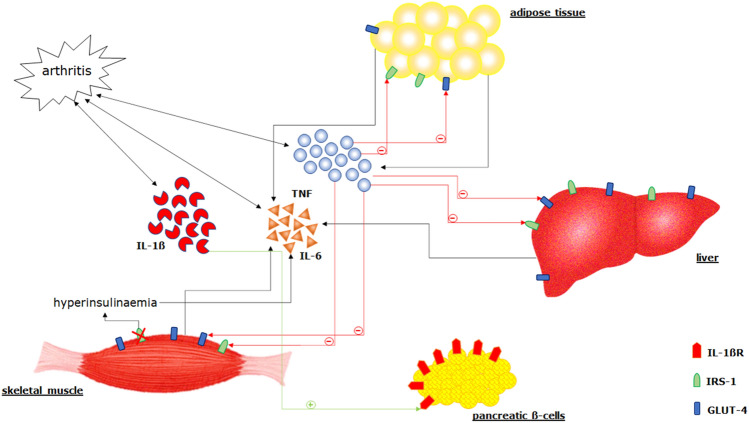
Multiple features may be associated with occurrence of T2D in the context of RA [5, 15, 16]. Inactivity, disability, sedentary behaviour, obesity, and glucocorticoid (GC) therapy are known to negatively affect the glucose metabolism and to favour the occurrence of T2D [35]. A recent study demonstrated a strong association between some RA pathogenic mediators and the risk of incident T2D [6]. Interleukin (IL)-1α, IL-1β, and IL-6 predicted the occurrence of this comorbidity during the follow-up in patients with RA [6], suggesting that inflammatory mediators may contribute to the development of the metabolic disease [18]. In fact, as reported in Fig. 1, these molecules may contribute to altering blood lipid levels, inducing endothelial dysfunction, and enhancing oxidative stress; thus, increasing the risk of CVD and metabolic diseases [36, 37]. In this context, it has been shown that the pancreatic β-cell has a high density of IL-1β receptors [38]. On the basis of an overexpression of IL-1β, as observed in RA, β-cells may become more susceptible to the negative effects of this cytokine but also of other inflammatory mediators, such as IL-6 and tumour necrosis factor (TNF) [39]. In turn, these molecules may also attract macrophages and additional immune cells, which infiltrate the pancreatic islets [39]. These alterations may first lead to β-cell dysfunction and consequently to their apoptosis, resulting in a progressive glucose derangement and the consequent occurrence of T2D [40]. Furthermore, β-cells and other insulin-sensitive tissues, stressed by the high glucose levels, may produce elevated levels of IL-1β via the hyper-expression of nucleotide-binding domain and leucine-rich repeat containing family pyrin 3 (NLRP3) inflammasome in perpetuating this pathogenic vicious circle [39, 41]. Moreover, high levels of glucose may strongly influence the pro-inflammatory status of monocytes derived from patients with RA and concomitant T2D, leading to the production of IL-1β [39]. A work by Almeida-Santiago et al, has shown a direct relationship between IL-1RA, the receptor antagonist of IL-1β, and β-cell function, thus further reinforcing the role of this pathway in glucose derangement during RA [42].
Fig. 1.
The inflammatory pathogenic mechanisms associated with glucose derangement may be exacerbated by rheumatoid arthritis (RA). Interleukin (IL)-1β, IL-6, and TNF may contribute to the development of glucose derangement and type 2 diabetes (T2D). β-cell has a high density of IL-1β receptors; based on the overexpression of IL-1β, as observed in RA, β-cells may become more susceptible to the negative effects of this cytokine but also of other inflammatory mediators, such as IL-6 and TNF. In turn, these other molecules may also attract macrophages and other immune cells, which infiltrate the pancreatic islets. The consequent inflammatory infiltration is characterised largely by macrophages. These alterations may first lead to β-cell dysfunction and consequently to their apoptosis, with the result of a progressive glucose derangement and the consequent occurrence of T2D. Furthermore, β cells and other insulin-sensitive tissues, stressed by the high glucose levels, may produce elevated amounts of IL-1β via the hyper-expression of nucleotide-binding domain and leucine-rich repeat containing family pyrin 3 (NLRP3) inflammasome in perpetuating this pathogenic vicious circle. Tumour necrosis factor (TNF) may be of some relevance in the development of both insulin resistance (IR) and of T2D since it is produced in the adipose tissue. This cytokine may reduce the expression of glucose transporter type 4 (GLUT4), an insulin-regulated glucose transporter mainly located in adipocytes, skeletal and cardiac muscles, thereby reducing glucose uptake. Moreover, serine phosphorylation of insulin receptor substrate-1 (IRS-1) induced by TNF may inhibit the insulin receptor and may antagonise its signal. Interleukin-6 is produced by both adipocytes and macrophages within adipose tissues, skeletal muscle, and liver, and it is hyper-activated in RA. Furthermore, hyperinsulinemia may lead to an increase in IL-6 blood levels, thus leading an additional vicious cycle involving a pro-inflammatory molecule and glucose abnormalities
In addition, a pathogenic role of TNF and IL-6 has been also proposed in this setting. Tumour necrosis factor is of crucial importance in the pathogenesis of RA [43]. In fact, it is implicated in the development of arthritis by activation of other cytokines, chemokine and endothelial-cell adhesion molecule expression, promotion of angiogenesis, suppression of regulatory T cells, and amplification of osteoclast differentiation and activation [37, 44]. Tumour necrosis factor may be of some relevance in the development of both IR and T2D since it is produced in the adipose tissue [45]. This cytokine may reduce the expression of glucose transporter type 4 (GLUT4), an insulin-regulated glucose transporter mainly located in adipocytes, skeletal and cardiac muscles, thereby reducing glucose uptake [46]. Moreover, serine phosphorylation of insulin receptor substrate-1 (IRS-1) induced by TNF may inhibit insulin receptor and may antagonise its signal [47]. This mediator is also able to decrease the fatty acid oxidation leading to increase of plasma free fatty acid levels [48]. Furthermore, TNF together with IL-6 may have a negative effect on insulin signalling, thus promoting IR [49–53]. Interleukin-6 is produced by adipocytes and macrophages within adipose tissues, skeletal muscle, and liver, and it is hyper-activated in RA [54]. In addition, hyperinsulinaemia may lead to an increase in IL-6 blood levels [55, 56]; thus, leading to an additional vicious cycle involving a pro-inflammatory molecule and glucose abnormalities [56–58]. In fact, IL-6 values are correlated with obesity grade, and negatively with IR [59]. In contrast, some studies have shown some possible favourable effects of this cytokine on IR, stimulating the production of glucagon-like peptide-1 from the gut and pancreas [60, 61]. Furthermore, IL-6-deficient mice may develop mature-onset obesity [61]. This controversial role of IL-6 on metabolic processes may be explained by its complex signalling pathways and different experimental conditions. Additionally, activated T cells, which play a central role in the pathogenesis of RA, may also be involved in the development of IR and T2D [62–65]. Interestingly, during T2D, a higher percentage of memory, whereas lower naive CD4+ T cells may be observed [66]. Furthermore, another study identified higher blood percentage of CD4+ memory T cells in T2D patients with CVD, compared with those without [67]. In addition, the rheumatoid pro-inflammatory environment is amplified by the JAnus Kinase/Signal Transducer and Activator of Transcription (JAK/STAT) signalling pathway [68]. These enzymes have a crucial role in the intracellular signalling transduction of several cytokines, contributing to joint inflammation and damage in RA [69]. Interestingly, hyperglycaemia seems to activate this pathway and dysregulation of the leptin-induced JAK/STAT signalling pathways could play a key role in IR in T2D [70–72]. Moreover, adipocytes produce some molecules acting on JAK/STAT signalling pathway in target tissues further contributing to glucose derangement [73].
Taking together the findings about the inflammatory contribution to the pathogenesis of T2D, it is possible to hypothesise that these mechanisms may be exacerbated in the context of RA, thus proposing possible common therapeutic targets for patients affected by arthritis and T2D.
The Effects of RA Therapeutic Agents on Glucose Abnormalities
Glucocorticoids
During RA, GCs are widely used since they rapidly provide symptomatic relief, suppress disease activity, and significantly slow the progression of progressive radiological damage in these patients [74, 75]. However, the cumulative exposure to GCs is associated with an increased occurrence of CVD [14, 76–78]. These drugs may alter lipid and glucose metabolism, increase blood pressure, and lead to endothelial dysfunction, especially considering the cumulative dosages in the long term [79]. As far as T2D is concerned, many studies found a high cumulative or daily GC dosage could reduce the glucose tolerance due to IR and pancreatic cells dysfunction in patients with RA [8, 13, 14, 80–82]. Although the exact underlying mechanisms are still unknown, genetic disposition, age, and obesity, as well as chronic inflammation, are recognised as risk factors for the development of IR/T2D, and GCs represent an additional factor in patients with RA [83–87]. However, it must be pointed out that the negative effects of GCs may be balanced by the positive effects on inflammation [80, 88]. In fact, GCs could positively affect the rheumatoid inflammatory process associated with the occurrence of CVD and metabolic disease [88]. In this regard, a study evaluated the dose-related effects of GCs on glucose metabolism parameters and RA disease activity [80]. Short-term treatment with high-dose prednisone did not deteriorate glycaemic parameters, but was associated with an improvement [80]. Furthermore, a prospective cohort study evaluated the risk of developing CV disease in patients with RA exposed to GCs [88]. The adjustments for disease activity and disability revealed no relationship between GC and CVD [88]. Based on these observations, GCs could be associated with positive effects on glucose abnormalities due to rheumatoid inflammatory process, but predictable side effects should be carefully considered and monitored when administering these drugs in patients with RA in the long term.
Methotrexate
Methotrexate (MTX) is one of the most widely used drugs in patients with RA [89, 90]; this is an anti-folate cellular immunosuppressant mainly inhibiting dihydrofolate reductase [91]. Methotrexate mechanisms of action may result in a variety of anti-inflammatory effects, inhibiting the proliferation and inducing apoptosis of activated immune cells, decreasing the release of pro-inflammatory cytokines, like IL-1β, IL-6, and TNF, and simultaneously increasing those anti-inflammatories, mainly IL-4 and IL-10 [92]. Recently, direct effects of MTX on glucose metabolism have been proposed [93]. A prolonged treatment with MTX increased skeletal muscle GLUT-4 expression in T2D murine models, and was also related to a significant reduction of glucose and insulin serum concentrations [93]. In addition, treatment with MTX, compared with other drugs, may be able to reduce the levels of glycated haemoglobin (HbA1c) [94]. In particular, an association between higher levels of erythrocyte MTX polyglutamate, the intracellular form of MTX, and the reduced levels of HbA1c [95] has been observed, thus, suggesting a possible beneficial effect of this drug on glucose derangement in patients with RA.
IL-1 Inhibitors
In recent years, based on its pathogenic contribution, multiple lines of evidence have suggested the role of IL-1 inhibition in improving glucose metabolism abnormalities (Table 1) [22, 96]. Anakinra is a recombinant form of human IL-1 receptor antagonist, and it works as a competitive inhibitor of IL-1β. Many studies analysed its clinical role in improving glycaemic control in T2D reporting the possible usefulness in these patients [97–100]. In a placebo-controlled study, anakinra induced a significant improvement of glucose metabolism, as demonstrated by the significant reduction of HbA1c [97]. The same group also demonstrated that this improvement could be maintained after anakinra withdrawal in a 39-week follow-up [100]. More recently, the clinical efficacy of canakinumab, a monoclonal antibody against IL-1β, has been proposed on CVD, reducing inflammatory markers and CV events in patients characterised by a high risk [101, 102]. In this context, the possibility that canakinumab could decrease the occurrence of T2D has been also tested [103]. Although the incidence of T2D was not prevented, canakinumab reduced values of HbA1c during the first 6–9 months of treatment, despite no consistent long-term benefits on this parameter. Taking together these findings and the well-known pathogenic role of IL-1β in rheumatic diseases, the potential clinical usability of IL-1 inhibition has been tested in RA patients with T2D as a possible “bidirectional” therapy [104–106]. In a clinical trial, patients with RA and concomitant T2D were randomised to receive anakinra or TNF inhibitors (TNFis) to evaluate a differential efficacy on HbA1c [104]. Patients in the anakinra group had a significant and marked reduction of HbA1c%, which has not been observed in the TNFi group after 6 months of follow-up (crude difference 0.93 HbA1c% between groups) [104]. Concerning RA, a progressive reduction of disease activity was observed in both groups without significant differences. Therefore, a benefit of IL-1 inhibition in patients with RA and T2D has been reported, reaching the therapeutic targets of both diseases [104]. In addition, data derived from the long-term extension of this study, including patients with RA and T2D, suggested that the benefits of IL-1 inhibition on metabolic and inflammatory parameters could last longer than the first 6 months of follow-up [105]. Interestingly, a reduction of anti-diabetic drugs was also observed in anakinra-treated patients whereas an increase of anti-diabetic therapies was needed in TNFi-treated patients to reach a reduction of HbA1c [105]. In this trial [104, 105], a persistent good clinical response has been observed in patients randomised to anakinra in regard to RA disease activity. These findings may appear to be in conflict with previous results about the efficacy of this drug in RA [106, 107]. However, it must be pointed out that patients enrolled in this trial showed a short disease duration and were codified according to 2010 ACR/EULAR criteria [104]. Different from those fulfilling 1987 criteria, these patients may have a less severe disease course, developing a less severe radiological joint damage, and more often achieving clinical remission [108]. However, it must be pointed out that future studies are needed to fully clarify the efficacy of anakinra in RA.
Table 1.
Main studies investigating the efficacy of bDMARDs in T2D with or without RA
| First author, year | Type of study | Number of participants | Disease | Type of drug, dose, frequency | Follow-up | Outcome of interest | Value of assessment measure at baseline | Value of assessment measure at final observation | Difference between baseline and final observation |
|---|---|---|---|---|---|---|---|---|---|
| Larsen et al, 2007 [97] | Clinical trial | 67 | T2D | Anakinra, 100 mg, once daily | 13 weeks | HbA1c reduction | 8.7% | 8.4% | − 0.3% |
| Ridker et al, 2012 [101] | Clinical trial | 551 | T2D | Canakinumab, 150 mg, every 4 weeks | 4 months | HbA1c reduction | 7.4% | 7.1% | − 0.3% |
| Everett et al. 2018 [103] | Clinical trial | 10061 | Prior MI, with or without prediabetes or T2D | Canakinumab, 50 mg, every 12 weeks | 48 months | HbA1c reduction | 7.0% | 7.1% | +0.1% |
| Canakinumab, 150 mg, every 12 weeks | 48 months | HbA1c reduction | 7.1% | 7.1% | 0.0 | ||||
| Canakinumab, 300 mg, every 12 weeks | 48 months | HbA1c reduction | 7.2% | 7.1% | 0.0 | ||||
| Ruscitti et al, 2019 [104] | Clinical trial | 39 | RA and T2D | Anakinra, 100 mg, once daily | 6 months | HbA1c reduction | 7.7% | 6.7% | − 1.0% |
| Tam et al, 2007 [113] | Clinical trial | 19 | RA and T2D | Infliximab, 3 mg/kg, at Week 0, Weeks 2, 6 and 14 | 14 weeks | HOMA-IR reduction | 1.3 | 0.6 | − 0.7 |
| Stavropoulos-Kalinoglou et al, 2012 [114] | Observational study | 32 | RA and T2D |
Infliximab, 3 mg/kg, every 8 weeks Etanercept, 50 mg, every week Adalimumab, 40 mg, every 2 weeks |
6 months | HOMA-IR reduction | 2.6 | 2.4 | − 0.2 |
| Stagakis et al, 2012 [117] | Prospective study | 61 | RA and T2D |
Infliximab, 3 mg/kg, every 8 weeks Etanercept, 50 mg, every week Adalimumab, every 2 weeks |
12 weeks | HOMA-IR reduction | 7.0 | − 5.7 | − 12.7 |
| Otsuka et al, 2018 [122] | Observational study | 221 | RA and T2D | Tocilizumab, 8 mg/kg, every 4 weeks | 3 months | HbA1c reduction | 6.2% | 5.8% | − 0.4% |
| Genovese et al, 2020 [129] | Post hoc analyses of clinical trials | 184 | RA and T2D | Sarilumab 150 mg + csDMARDs, every 2 weeks | 24 weeks | HbA1c reduction | 6.9% | 6.4% | − 0.5% |
| Sarilumab 200 mg + csDMARDs, every 2 weeks | 24 weeks | HbA1c reduction | 7.0% | 6.3% | − 0.7% | ||||
| Sarilumab 200 mg, every 2 weeks | 24 weeks | HbA1c reduction | 6.6% | 6.2% | − 0.4% |
bDMARDS biologic disease-modifying anti-rheumatic drugs. csDMARDs conventional synthetic disease-modifying anti-rheumatic drugs, HbA1c glycated haemoglobin, HOMA-IR Homeostasis Model Assessment of Insulin Resistance, MI myocardial infarction, RA rheumatoid arthritis, T2D type 2 diabetes
Finally, in patients with RA and T2D, anakinra has been shown to improve both IR and IS, suggesting some possible mechanisms of action underlying the improvement of glucose abnormalities. Furthermore, IL-1 inhibition was positively associated with a reduction of some adipokines in patients with RA and T2D [109].
TNF Inhibitors
Tumour necrosis factor has been implicated in the development of IR and T2D, despite conflicting results of antagonising this cytokine in patients with this metabolic disease [110, 111]. In the context of RA, several studies analysed the effects of TNFis (i.e., adalimumab, etanercept, and infliximab) on glucose metabolism, mostly assessing IR and IS by Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) and Quantitative Insulin sensitivity ChecK Index (QUICKI), respectively [112–117]. In these studies, TNFis were associated with a positive effect on both HOMA-IR and QUICKI, mainly in those patients who were more insulin resistant than others [24, 112–118]. Furthermore, these positive effects were observed in the short term—within a few weeks following the administration of such drugs [24, 112–118].
In addition, it has been shown that a rapid reduction of IR together with improvement of IS may be observed in non-diabetic patients with RA who underwent a single infusion of infliximab [119]. Therefore, the short-term effect of the anti-TNF therapy on IR may be suggested in RA [119]. Although these studies have been limited by a relatively small sample size, all showed the potential beneficial role of inhibiting TNF in IR and IS, implicating this molecule in the development of glucose metabolism derangement in RA. Therefore, TNFi could have a role in the initial phases of IR before the development of T2D, as highlighted by the reduction of the incidence of T2D in patients with RA treated with such drugs [21, 120]. In fact, Lin et al [21], observed that new-onset T2D could be less frequent in patients treated by TNFi, with no differences among specific drugs, synthesising data from 22 randomised controlled trials and 3 cohort studies [21].
IL-6 Inhibitors
In the past decade, extensive clinical experience has established the efficacy of IL-6 inhibition in patients with RA, by tocilizumab or sarilumab, which are antagonists of IL-6 receptor. In this context, some studies investigated the efficacy of IL-6 inhibition on IR and T2D in patients with RA [121–126]. Tocilizumab showed a positive effect on IR as per reduction of HOMA-IR observed in these studies [124]. Furthermore, the improvement of leptin/adiponectin ratio, a measure of IR, has been reported following the administration of this drug [125]. Additionally, the effect of the administration of TNFi or tocilizumab and HbA1c has been investigated in patients with RA [122]. A significant reduction in HbA1c after starting these drugs has been observed, even if a more pronounced decrease was observed in those treated with tocilizumab rather than TNFi [122]. Another study analysed the correlation between the administration of tocilizumab and the improvement of IR in patients with RA, by using HOMA-IR and QUICKI [121]. A decrease of IR together with an improvement of IS in patients with RA has been observed, especially in those who were more insulin resistant [121]. However, other experiences failed to report this positive effect of tocilizumab on glucose derangement, advising the need of further studies to fully clarify the efficacy of this drug in this context [127, 128]. The efficacy of sarilumab on improving glucose metabolism was assessed in a post hoc analysis [129] of three randomised clinical trials in patients with RA with or without concomitant T2D [130–132]. This study aimed to assess the effect of sarilumab as monotherapy or in combination with conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs) on HbA1c, after 24 months, compared with either placebo or adalimumab monotherapy. The authors found that sarilumab as monotherapy or in combination may significantly reduce HbA1c than adalimumab monotherapy or placebo + MTX/csDMARDs, particularly in patients with T2D. Moreover, a more relevant reduction of HbA1c was reported in patients treated with sarilumab whose baseline HbA1c was ≥ 7 % [129].
Other bDMARDs and tsDMARDs
In the large background of RA therapies, other drugs with different mechanisms of action are presently available to treat these patients [89, 133].
Abatacept is a recombinant fusion protein formed by the extracellular domain of human cytotoxic T-lymphocyte-associated protein 4 and the modified Fc region of a human IgG1. This drug has been studied in patients with type 1 diabetes and T2D [134–137]. A recent study reported an increased activation of T cells in the bone marrow of T2D patients [135]. These authors showed that abatacept reduced this overactivation of immune cells in a murine model of diabetes [135]. In patients with RA, an improvement of IS has been observed following the administration of abatacept by an evaluation of an insulin sensitivity index in a 6-month follow-up study [25]. The authors also reported a concomitant reduction of HcA1c [25]. Conversely, in another study, no effect of abatacept on IR, assessed using HOMA-IR, has been observed [117]. In a large observational cohort study [136], the use of this drug was associated with a lower risk of developing T2D in patients with RA, than the administration of TNFi in a mean follow‐up of 368 days [136]. This result mirrored a previous study in which the incidence of T2D in RA patients was lower in patients treated with abatacept than MTX [137].
Rituximab is a chimeric monoclonal antibody to the cell surface antigen cluster differentiation 20 (CD20), which is found on mature B cells. The binding of rituximab with CD20 leads to circulating and tissue B cell depletion with a concurrent decrease in immunoglobulin (Ig)G and IgM levels. Chen et al evaluated the risk of T2D treatment intensification in patients with RA and concomitant T2D initiating a bDMARD [138]. In the univariate analysis, the rate of T2D treatment intensification was higher in patients treated with rituximab. However, in a multivariable-adjusted analysis, there was no difference between rituximab and other drugs. In this study, the administration of tofacitinib, a JAK-inhibitor, recently approved for patients RA, was also assessed. The rate of treatment intensification was lower in patients treated with tofacitinib than other drugs and was associated with a reduced administration of insulin to manage these patients [138]. Another JAK-inhibitor, which has been introduced in the treatment of RA, is baricitinib. The latter has been recently tested in decreasing diabetic kidney disease progression in patients with T2D in a Phase 2 clinical trial [139]. Baricitinib has been shown to reduce the albuminuria in these patients but also to decrease HbA1c after 24 weeks [139]. Its potential use in metabolic alterations has been also analysed in a murine high-fat/high-sugar diet model [140]. Interestingly, the use of the baricitinib showed a multi-organ protection against the deleterious effects of the high-fat/high-sugar diet exposure [140].
The Presence of T2D May Contribute to a Personalised Therapeutic Approach in Patients with RA
As shown in Fig. 1, multiple lines of evidence have increasingly suggested a pathogenic connection between the rheumatoid process and the mechanisms of T2D in a vicious circle perpetuated by glucose derangement and inflammatory mediators [22, 23]. These findings have been further reinforced by some clinical studies showing that the inhibition of IL-1 and IL-6 may simultaneously enable the therapeutic targeting of patients with RA and concomitant T2D [104, 129]. Interestingly, comparing these findings to the previous studies on T2D following administration of bDMARDs [97, 98, 100, 101], a more evident reduction of HbA1c has been observed in patients with RA and concomitant T2D (Table 1), mainly considering results about IL-1 inhibition. Thus, the inflammatory pathogenic mechanisms of the metabolic disease could be exaggerated in the context of a rheumatic disease possibly explaining these findings. In addition, IL-1 inhibition has been proposed to have disease modifying effects on T2D in this setting considering the reduction and the stoppage of the antidiabetic therapies, which have been observed in patients with RA [105]. In fact, despite the latest improvement of the antidiabetic therapeutic strategies, a percentage of patients with T2D, around 30 %, is still treated with insulin after failing a dual oral therapy [141, 142]. Contrarily, IL-1 inhibition could not only palliate glycaemia, but also decrease the progressive decline in insulin secretion associated with T2D, interfering with apoptosis of β-cells, improving their function, and improving the peripheral IR [96]. Moreover, the maintenance of clinical remission of the rheumatic disease could further improve the glucose derangement and reduce the occurrence of T2D in RA [2]. On these bases, the presence of T2D may allow physicians to perform a better profile of patients with RA according to the principles of precision medicine, tailoring the medical treatment to the individual characteristics [143–145]. In addition, in patients with RA and concomitant T2D, IL-1 inhibition could be considered a targeted therapeutic strategy, leading to a simultaneous improvement of both metabolic parameters and inflammatory signs. Furthermore, considering the effects of IL-1 inhibition in the prevention of CVD [102], it is possible to suggest that this therapeutic strategy may decrease the burden of CV risk in RA targeting the synergy between the inflammation and the glucose derangement. These findings may open the way for subsequent confirmatory studies to entirely elucidate the clinical role of IL-1 inhibition in patients with RA and concomitant T2D. However, it must be pointed out that further studies are needed to fully clarify the possible usefulness of additional therapeutic strategies, such as IL-6 and JAK inhibitors, which have also shown some efficacy in this context [146, 147].
Conclusions
In conclusion, the benefits of targeting the inflammatory process, mainly by IL-1 inhibition, may be suggested in patients with RA and concomitant T2D. Considering the pronounced decrease of HbA1c, it could be possible to hypothesise that the pro-inflammatory mechanisms of T2D could be exaggerated by RA in a pathogenic vicious circle perpetuated by glucose derangement and inflammation. Thus, the presence of T2D could identify a subset of RA possibly benefitting by IL-1 inhibition, although further studies are needed to elucidate this topic stratifying the patients according to their clinical picture and associated comorbidities.
Declarations
Conflict of interest
None declared.
Funding
Open access funding provided by Università degli Studi dell’Aquila within the CRUI-CARE Agreement.
Ethics approval
None required, this work represents a review of available evidence.
Consent to participate
None required, this work represents a review of available evidence.
Consent for publication
Not applicable.
Availability of data and materials
Not applicable, this work represents a review of available evidence.
Code availability
Not applicable.
Authors’ contributions
All authors meet all criteria for authorship in the ICMJE Recommendations, since all authors made substantial contributions to the conception of the article, the acquisition and interpretation of data. All authors contributed to the critical review and revision of the manuscript and approved the final version. All the authors agreed to be accountable for all aspects of the article.
References
- 1.Smolen JS, Aletaha D, McInnes IB. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. doi: 10.1016/S0140-6736(16)30173-8. [DOI] [PubMed] [Google Scholar]
- 2.Ruscitti P, Cipriani P, Liakouli V, Iacono D, Pantano I, Margiotta DPE, et al. Occurrence and predictive factors of high blood pressure, type 2 diabetes, and metabolic syndrome in rheumatoid arthritis: findings from a 3-year, multicentre, prospective, observational study. Clin Exp Rheumatol. 2021;39(5):995–1002. doi: 10.55563/clinexprheumatol/5r53em. [DOI] [PubMed] [Google Scholar]
- 3.Romano S, Salustri E, Ruscitti P, Carubbi F, Penco M, Giacomelli R. Cardiovascular and metabolic comorbidities in rheumatoid arthritis. Curr Rheumatol Rep. 2018;20(12):81. doi: 10.1007/s11926-018-0790-9. [DOI] [PubMed] [Google Scholar]
- 4.England BR, Thiele GM, Anderson DR, Mikuls TR. Increased cardiovascular risk in rheumatoid arthritis: mechanisms and implications. BMJ. 2018;361:k1036. doi: 10.1136/bmj.k1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Semb AG, Rollefstad S, Ikdahl E, Wibetoe G, Sexton J, Crowson C, et al. Diabetes mellitus and cardiovascular risk management in patients with rheumatoid arthritis: an international audit. RMD Open. 2021;7(2):e001724. doi: 10.1136/rmdopen-2021-001724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker JF, England BR, George M, Cannon G, Sauer B, Ogdie A, et al. Disease activity, cytokines, chemokines and the risk of incident diabetes in rheumatoid arthritis. Ann Rheum Dis. 2021;80(5):566–572. doi: 10.1136/annrheumdis-2020-219140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ruscitti P, Ursini F, Cipriani P, Ciccia F, Liakouli V, Carubbi F, et al. Prevalence of type 2 diabetes and impaired fasting glucose in patients affected by rheumatoid arthritis: results from a cross-sectional study. Medicine (Baltimore) 2017;96(34):e7896. doi: 10.1097/MD.0000000000007896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dessein PH, Joffe BI. Insulin resistance and impaired beta cell function in rheumatoid arthritis. Arthritis Rheum. 2006;54(9):2765–2775. doi: 10.1002/art.22053. [DOI] [PubMed] [Google Scholar]
- 9.Rosenvinge A, Krogh-Madsen R, Baslund B, Pedersen BK. Insulin resistance in patients with rheumatoid arthritis: effect of anti-TNFalpha therapy. Scand J Rheumatol. 2007;36(2):91–96. doi: 10.1080/03009740601179605. [DOI] [PubMed] [Google Scholar]
- 10.Lebovitz HE. Insulin resistance: definition and consequences. Exp Clin Endocrinol Diabetes. 2001;109(Suppl 2):S135–S148. doi: 10.1055/s-2001-18576. [DOI] [PubMed] [Google Scholar]
- 11.Brunton S. Pathophysiology of type 2 diabetes: the evolution of our understanding. J Fam Pract. 2016;65(4 Suppl):0416. [PubMed] [Google Scholar]
- 12.Chung CP, Oeser A, Solus JF, Avalos I, Gebretsadik T, Shintani A, et al. Prevalence of the metabolic syndrome is increased in rheumatoid arthritis and is associated with coronary atherosclerosis. Atherosclerosis. 2008;196(2):756–763. doi: 10.1016/j.atherosclerosis.2007.01.004. [DOI] [PubMed] [Google Scholar]
- 13.Giles JT, Danielides S, Szklo M, Post WS, Blumenthal RS, Petri M, et al. Insulin resistance in rheumatoid arthritis: disease-related indicators and associations with the presence and progression of subclinical atherosclerosis. Arthritis Rheumatol. 2015;67(3):626–636. doi: 10.1002/art.38986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoes JN, van der Goes MC, van Raalte DH, van der Zijl NJ, den Uyl D, Lems WF, et al. Glucose tolerance, insulin sensitivity and β-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis. 2011;70(11):1887–1894. doi: 10.1136/ard.2011.151464. [DOI] [PubMed] [Google Scholar]
- 15.Nicolau J, Lequerré T, Bacquet H, Vittecoq O. Rheumatoid arthritis, insulin resistance, and diabetes. Jt Bone Spine. 2017;84(4):411–416. doi: 10.1016/j.jbspin.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 16.Jiang P, Li H, Li X. Diabetes mellitus risk factors in rheumatoid arthritis: a systematic review and meta-analysis. Clin Exp Rheumatol. 2015;33(1):115–121. [PubMed] [Google Scholar]
- 17.Ruscitti P, Ursini F, Cipriani P, Liakouli V, Carubbi F, Berardicurti O, et al. Poor clinical response in rheumatoid arthritis is the main risk factor for diabetes development in the short-term: a 1-year, single-centre, longitudinal study. PLoS One. 2017;12(7):e0181203. doi: 10.1371/journal.pone.0181203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tian Z, Mclaughlin J, Verma A, Chinoy H, Heald AH. The relationship between rheumatoid arthritis and diabetes mellitus: a systematic review and meta-analysis. Cardiovasc Endocrinol Metab. 2021;10(2):125–131. doi: 10.1097/XCE.0000000000000244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li J, Chen Y, Liu Q, Tian Z, Zhang Y. Mechanistic and therapeutic links between rheumatoid arthritis and diabetes mellitus. Clin Exp Med. 2022 doi: 10.1007/s10238-022-00816-1. [DOI] [PubMed] [Google Scholar]
- 20.Matulewicz N, Karczewska-Kupczewska M. Insulin resistance and chronic inflammation. Postepy Hig Med Dosw (Online) 2016;70:1245–1258. [PubMed] [Google Scholar]
- 21.Lin C, Ji H, Cai X, Yang W, Lv F, Ji L. The association between the biological disease-modifying anti-rheumatic drugs and the incidence of diabetes: a systematic review and meta-analysis. Pharmacol Res. 2020;161:105216. doi: 10.1016/j.phrs.2020.105216. [DOI] [PubMed] [Google Scholar]
- 22.Ruscitti P, Cipriani P, Liakouli V, Carubbi F, Berardicurti O, Di Benedetto P, et al. The emerging role of IL-1 inhibition in patients affected by rheumatoid arthritis and diabetes. Rev Recent Clin Trials. 2018;13(3):210–214. doi: 10.2174/1574887113666180314102651. [DOI] [PubMed] [Google Scholar]
- 23.Giacomelli R, Ruscitti P, Alvaro S, Ciccia F, Liakouli V, Di Benedetto P, et al. IL-1β at the crossroad between rheumatoid arthritis and type 2 diabetes: may we kill two birds with one stone? Expert Rev Clin Immunol. 2016;12(8):849–855. doi: 10.1586/1744666X.2016.1168293. [DOI] [PubMed] [Google Scholar]
- 24.Burska AN, Sakthiswary R, Sattar N. Effects of tumour necrosis factor antagonists on insulin sensitivity/resistance in rheumatoid arthritis: a systematic review and meta-analysis. PLoS One. 2015;10(6):e0128889. doi: 10.1371/journal.pone.0128889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ursini F, Russo E, Letizia Hribal M, Mauro D, Savarino F, Bruno C, et al. Abatacept improves whole-body insulin sensitivity in rheumatoid arthritis: an observational study. Medicine (Baltimore) 2015;94(21):e888. doi: 10.1097/MD.0000000000000888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ruscitti P, Cipriani P, Cantarini L, Liakouli V, Vitale A, Carubbi F, et al. Efficacy of inhibition of IL-1 in patients with rheumatoid arthritis and type 2 diabetes mellitus: two case reports and review of the literature. J Med Case Rep. 2015;9:123. doi: 10.1186/s13256-015-0603-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Haeusler RA, McGraw TE, Accili D. Biochemical and cellular properties of insulin receptor signalling. Nat Rev Mol Cell Biol. 2018;19(1):31–44. doi: 10.1038/nrm.2017.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tripolino C, Ciaffi J, Pucino V, Ruscitti P, van Leeuwen N, Borghi C, et al. Insulin signaling in arthritis. Front Immunol. 2021;12:672519. doi: 10.3389/fimmu.2021.672519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen X, Liu Y, Zhang X. Topical insulin application improves healing by regulating the wound inflammatory response. Wound Repair Regen. 2012;20(3):425–434. doi: 10.1111/j.1524-475X.2012.00792.x. [DOI] [PubMed] [Google Scholar]
- 30.Yu T, Gao M, Yang P, Pei Q, Liu D, Wang D, et al. Topical insulin accelerates cutaneous wound healing in insulin-resistant diabetic rats. Am J Transl Res. 2017;9(10):4682–4693. [PMC free article] [PubMed] [Google Scholar]
- 31.van Niekerk G, Christowitz C, Conradie D, Engelbrecht AM. Insulin as an immunomodulatory hormone. Cytokine Growth Factor Rev. 2020;52:34–44. doi: 10.1016/j.cytogfr.2019.11.006. [DOI] [PubMed] [Google Scholar]
- 32.Sun Q, Li J, Gao F. New insights into insulin: the anti-inflammatory effect and its clinical relevance. World J Diabetes. 2014;5(2):89–96. doi: 10.4239/wjd.v5.i2.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Maciver NJ, Jacobs SR, Wieman HL, Wofford JA, Coloff JL, Rathmell JC. Glucose metabolism in lymphocytes is a regulated process with significant effects on immune cell function and survival. J Leukoc Biol. 2008;84(4):949–957. doi: 10.1189/jlb.0108024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Walrand S, Guillet C, Boirie Y, Vasson MP. In vivo evidences that insulin regulates human polymorphonuclear neutrophil functions. J Leukoc Biol. 2004;76(6):1104–1110. doi: 10.1189/jlb.0104050. [DOI] [PubMed] [Google Scholar]
- 35.Biswas A, Oh PI, Faulkner GE, Bajaj RR, Silver MA, Mitchell MS, et al. Sedentary time and its association with risk for disease incidence, mortality, and hospitalization in adults: a systematic review and meta-analysis. Ann Intern Med. 2015;162(2):123–132. doi: 10.7326/M14-1651. [DOI] [PubMed] [Google Scholar]
- 36.Løgstrup BB, Ellingsen T, Pedersen AB, Darvalics B, Olesen KKW, Bøtker HE, et al. Cardiovascular risk and mortality in rheumatoid arthritis compared with diabetes mellitus and the general population. Rheumatology (Oxford) 2021;60(3):1400–1409. doi: 10.1093/rheumatology/keaa374. [DOI] [PubMed] [Google Scholar]
- 37.McInnes IB, Schett G. The pathogenesis of rheumatoid arthritis. N Engl J Med. 2011;365(23):2205–2219. doi: 10.1056/NEJMra1004965. [DOI] [PubMed] [Google Scholar]
- 38.Mandrup-Poulsen T, Pickersgill L, Donath MY. Blockade of interleukin 1 in type 1 diabetes mellitus. Nat Rev Endocrinol. 2010;6(3):158–166. doi: 10.1038/nrendo.2009.271. [DOI] [PubMed] [Google Scholar]
- 39.Ruscitti P, Cipriani P, Di Benedetto P, Liakouli V, Berardicurti O, Carubbi F, et al. Monocytes from patients with rheumatoid arthritis and type 2 diabetes mellitus display an increased production of interleukin (IL)-1β via the nucleotide-binding domain and leucine-rich repeat containing family pyrin 3(NLRP3)-inflammasome activation: a possible implication for therapeutic decision in these patients. Clin Exp Immunol. 2015;182(1):35–44. doi: 10.1111/cei.12667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Scott DL, Wolfe F, Huizinga TW. Rheumatoid arthritis. Lancet. 2010;376(9746):1094–1108. doi: 10.1016/S0140-6736(10)60826-4. [DOI] [PubMed] [Google Scholar]
- 41.Berchtold LA, Prause M, Størling J, Mandrup-Poulsen T. Cytokines and pancreatic β-cell apoptosis. Adv Clin Chem. 2016;75:99–158. doi: 10.1016/bs.acc.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 42.Almeida-Santiago C, Quevedo-Abeledo JC, Hernández-Hernández V, de Vera-González A, Gonzalez-Delgado A, González-Gay MÁ, et al. Interleukin 1 receptor antagonist relation to cardiovascular disease risk in patients with rheumatoid arthritis. Sci Rep. 2022;12(1):13698. doi: 10.1038/s41598-022-18128-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Noack M, Miossec P. Selected cytokine pathways in rheumatoid arthritis. Semin Immunopathol. 2017;39(4):365–383. doi: 10.1007/s00281-017-0619-z. [DOI] [PubMed] [Google Scholar]
- 44.Schett G, Teitelbaum SL. Osteoclasts and arthritis. J Bone Miner Res. 2009;24(7):1142–1146. doi: 10.1359/jbmr.090533. [DOI] [PubMed] [Google Scholar]
- 45.Akash MSH, Rehman K, Liaqat A. Tumor necrosis factor-alpha: role in development of insulin resistance and pathogenesis of type 2 diabetes mellitus. J Cell Biochem. 2018;119(1):105–110. doi: 10.1002/jcb.26174. [DOI] [PubMed] [Google Scholar]
- 46.Guilherme A, Virbasius JV, Puri V, Czech MP. Adipocyte dysfunctions linking obesity to insulin resistance and type 2 diabetes. Nat Rev Mol Cell Biol. 2008;9(5):367–377. doi: 10.1038/nrm2391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Fasshauer M, Paschke R. Regulation of adipocytokines and insulin resistance. Diabetologia. 2003;46(12):1594–1603. doi: 10.1007/s00125-003-1228-z. [DOI] [PubMed] [Google Scholar]
- 48.Ruan H, Zarnowski MJ, Cushman SW, Lodish HF. Standard isolation of primary adipose cells from mouse epididymal fat pads induces inflammatory mediators and down-regulates adipocyte genes. J Biol Chem. 2003;278(48):47585–47593. doi: 10.1074/jbc.M305257200. [DOI] [PubMed] [Google Scholar]
- 49.Moller DE. Potential role of TNF-alpha in the pathogenesis of insulin resistance and type 2 diabetes. Trends Endocrinol Metab. 2000;11(6):212–217. doi: 10.1016/s1043-2760(00)00272-1. [DOI] [PubMed] [Google Scholar]
- 50.Kern PA, Saghizadeh M, Ong JM, Bosch RJ, Deem R, Simsolo RB. The expression of tumor necrosis factor in human adipose tissue. Regulation by obesity, weight loss, and relationship to lipoprotein lipase. J Clin Investig. 1995;95(5):2111–2119. doi: 10.1172/JCI117899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saghizadeh M, Ong JM, Garvey WT, Henry RR, Kern PA. The expression of TNF alpha by human muscle. Relationship to insulin resistance. J Clin Investig. 1996;97(4):1111–1116. doi: 10.1172/JCI118504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Mishima Y, Kuyama A, Tada A, Takahashi K, Ishioka T, Kibata M. Relationship between serum tumor necrosis factor-alpha and insulin resistance in obese men with Type 2 diabetes mellitus. Diabetes Res Clin Pract. 2001;52(2):119–123. doi: 10.1016/s0168-8227(00)00247-3. [DOI] [PubMed] [Google Scholar]
- 53.Hotamisligil GS, Peraldi P, Budavari A, Ellis R, White MF, Spiegelman BM. IRS-1-mediated inhibition of insulin receptor tyrosine kinase activity in TNF-alpha- and obesity-induced insulin resistance. Science. 1996;271(5249):665–668. doi: 10.1126/science.271.5249.665. [DOI] [PubMed] [Google Scholar]
- 54.Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, et al. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrinol Metab. 1997;82(12):4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- 55.Fasshauer M, Klein J, Lossner U, Paschke R. Interleukin (IL)-6 mRNA expression is stimulated by insulin, isoproterenol, tumour necrosis factor alpha, growth hormone, and IL-6 in 3T3-L1 adipocytes. Horm Metab Res. 2003;35(3):147–152. doi: 10.1055/s-2003-39075. [DOI] [PubMed] [Google Scholar]
- 56.Krogh-Madsen R, Plomgaard P, Keller P, Keller C, Pedersen BK. Insulin stimulates interleukin-6 and tumor necrosis factor-alpha gene expression in human subcutaneous adipose tissue. Am J Physiol Endocrinol Metab. 2004;86(2):E234–E238. doi: 10.1152/ajpendo.00274.2003. [DOI] [PubMed] [Google Scholar]
- 57.Park HS, Park JY, Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract. 2005;69(1):29–35. doi: 10.1016/j.diabres.2004.11.007. [DOI] [PubMed] [Google Scholar]
- 58.Kraakman MJ, Allen TL, Whitham M, Iliades P, Kammoun HL, Estevez E, et al. Targeting gp130 to prevent inflammation and promote insulin action. Diabetes Obes Metab. 2013;15(Suppl 3):170–175. doi: 10.1111/dom.12170. [DOI] [PubMed] [Google Scholar]
- 59.Klover PJ, Zimmers TA, Koniaris LG, Mooney RA. Chronic exposure to interleukin-6 causes hepatic insulin resistance in mice. Diabetes. 2003;52(11):2784–2789. doi: 10.2337/diabetes.52.11.2784. [DOI] [PubMed] [Google Scholar]
- 60.Ellingsgaard H, Ehses JA, Hammar EB, Van Lommel L, Quintens R, Martens G, et al. Interleukin-6 regulates pancreatic alpha-cell mass expansion. Proc Natl Acad Sci USA. 2008;105(35):13163–13168. doi: 10.1073/pnas.0801059105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wallenius V, Wallenius K, Ahrén B, Rudling M, Carlsten H, Dickson SL, et al. Interleukin-6-deficient mice develop mature-onset obesity. Nat Med. 2002;8(1):75–79. doi: 10.1038/nm0102-75. [DOI] [PubMed] [Google Scholar]
- 62.Korhonen R, Moilanen E. Abatacept, a novel CD80/86-CD28 T cell co-stimulation modulator, in the treatment of rheumatoid arthritis. Basic Clin Pharmacol Toxicol. 2009;104(4):276–284. doi: 10.1111/j.1742-7843.2009.00375.x. [DOI] [PubMed] [Google Scholar]
- 63.Hotamisligil GS. Inflammation, metaflammation and immunometabolic disorders. Nature. 2017;542(7640):177–185. doi: 10.1038/nature21363. [DOI] [PubMed] [Google Scholar]
- 64.Dasu MR, Devaraj S, Zhao L, Hwang DH, Jialal I. High glucose induces toll-like receptor expression in human monocytes: mechanism of activation. Diabetes. 2008;57(11):3090–3098. doi: 10.2337/db08-0564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Winer DA, Winer S, Shen L, Wadia PP, Yantha J, Paltser G, et al. B cells promote insulin resistance through modulation of T cells and production of pathogenic IgG antibodies. Nat Med. 2011;17(5):610–617. doi: 10.1038/nm.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Olson NC, Doyle MF, de Boer IH, Huber SA, Jenny NS, Kronmal RA, et al. Associations of circulating lymphocyte subpopulations with type 2 diabetes: cross-sectional results from the multi-ethnic study of atherosclerosis (MESA) PLoS One. 2015;10(10):e0139962. doi: 10.1371/journal.pone.0139962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rattik S, Engelbertsen D, Wigren M, Ljungcrantz I, Östling G, Persson M, et al. Elevated circulating effector memory T cells but similar levels of regulatory T cells in patients with type 2 diabetes mellitus and cardiovascular disease. Diab Vasc Dis Res. 2019;16(3):270–280. doi: 10.1177/1479164118817942. [DOI] [PubMed] [Google Scholar]
- 68.Ibrahim SSA, Salama MA, Selima E, Shehata RR. Sitagliptin and tofacitinib ameliorate adjuvant induced arthritis via modulating the cross talk between JAK/STAT and TLR-4/NF-κB signaling pathways. Life Sci. 2020;260:118261. doi: 10.1016/j.lfs.2020.118261. [DOI] [PubMed] [Google Scholar]
- 69.Favoino E, Prete M, Catacchio G, Ruscitti P, Navarini L, Giacomelli R, et al. Working and safety profiles of JAK/STAT signaling inhibitors. Are these small molecules also smart? Autoimmun Rev. 2021;20(3):102750. doi: 10.1016/j.autrev.2021.102750. [DOI] [PubMed] [Google Scholar]
- 70.Gurzov EN, Stanley WJ, Pappas EG, Thomas HE, Gough DJ. The JAK/STAT pathway in obesity and diabetes. FEBS J. 2016;283(16):3002–3015. doi: 10.1111/febs.13709. [DOI] [PubMed] [Google Scholar]
- 71.Peyron JG, Stanescu R, Stanescu V, Maroteaux P. Particular electrophoretic distribution of proteoglycans in the zones of regeneration of the arthrotic cartilage and study of their collagen. Rev Rhum Mal Osteoartic. 1978;45(10):569–576. [PubMed] [Google Scholar]
- 72.Al-Rasheed NM, Al-Rasheed NM, Hasan IH, Al-Amin MA, Al-Ajmi HN, Mahmoud AM. Sitagliptin attenuates cardiomyopathy by modulating the JAK/STAT signaling pathway in experimental diabetic rats. Drug Des Devel Ther. 2016;10:2095–2107. doi: 10.2147/DDDT.S109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Richard AJ, Stephens JM. Emerging roles of JAK-STAT signaling pathways in adipocytes. Trends Endocrinol Metab. 2011;22(8):325–332. doi: 10.1016/j.tem.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boers M, Verhoeven AC, Markusse HM, van de Laar MA, Westhovens R, van Denderen JC, et al. Randomised comparison of combined step-down prednisolone, methotrexate and sulphasalazine with sulphasalazine alone in early rheumatoid arthritis. Lancet. 1997;350(9074):309–318. doi: 10.1016/S0140-6736(97)01300-7. [DOI] [PubMed] [Google Scholar]
- 75.Gorter SL, Bijlsma JW, Cutolo M, Gomez-Reino J, Kouloumas M, Smolen JS, et al. Current evidence for the management of rheumatoid arthritis with glucocorticoids: a systematic literature review informing the EULAR recommendations for the management of rheumatoid arthritis. Ann Rheum Dis. 2010;69(6):1010–1014. doi: 10.1136/ard.2009.127332. [DOI] [PubMed] [Google Scholar]
- 76.Souverein PC, Berard A, Van Staa TP, Cooper C, Egberts AC, Leufkens HG, et al. Use of oral glucocorticoids and risk of cardiovascular and cerebrovascular disease in a population based case–control study. Heart. 2004;90(8):859–865. doi: 10.1136/hrt.2003.020180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis JM, 3rd, Maradit Kremers H, Crowson CS, Nicola PJ, Ballman KV, Therneau TM, et al. Glucocorticoids and cardiovascular events in rheumatoid arthritis: a population-based cohort study. Arthritis Rheum. 2007;56(3):820–830. doi: 10.1002/art.22418. [DOI] [PubMed] [Google Scholar]
- 78.Mazzantini M, Talarico R, Doveri M, Consensi A, Cazzato M, Bazzichi L, et al. Incident comorbidity among patients with rheumatoid arthritis treated or not with low-dose glucocorticoids: a retrospective study. J Rheumatol. 2010;37(11):2232–2236. doi: 10.3899/jrheum.100461. [DOI] [PubMed] [Google Scholar]
- 79.Moreland LW, O'Dell JR. Glucocorticoids and rheumatoid arthritis: back to the future? Arthritis Rheum. 2002;46(10):2553–2563. doi: 10.1002/art.10567. [DOI] [PubMed] [Google Scholar]
- 80.den Uyl D, van Raalte DH, Nurmohamed MT, Lems WF, Bijlsma JW, Hoes JN, et al. Metabolic effects of high-dose prednisolone treatment in early rheumatoid arthritis: balance between diabetogenic effects and inflammation reduction. Arthritis Rheum. 2012;64(3):639–646. doi: 10.1002/art.33378. [DOI] [PubMed] [Google Scholar]
- 81.Buttgereit F. Do the treatment with glucocorticoids and/or the disease itself drive the impairment in glucose metabolism in patients with rheumatoid arthritis? Ann Rheum Dis. 2011;70:1881–1883. doi: 10.1136/annrheumdis-2011-200388. [DOI] [PubMed] [Google Scholar]
- 82.Dessein PH, Joffe BI, Stanwix AE, Christian BF, Veller M. Glucocorticoids and insulin sensitivity in rheumatoid arthritis. J Rheumatol. 2004;31(5):867–874. [PubMed] [Google Scholar]
- 83.Giorgino F, Almahfouz A, Goodyear LJ, Smith RJ. Glucocorticoid regulation of insulin receptor and substrate IRS-1 tyrosine phosphorylation in rat skeletal muscle in vivo. J Clin Investig. 1993;91(5):2020–2030. doi: 10.1172/JCI116424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lambillotte C, Gilon P, Henquin JC. Direct glucocorticoid inhibition of insulin secretion. An in vitro study of dexamethasone effects in mouse islets. J Clin Investig. 1997;99(3):414–423. doi: 10.1172/JCI119175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Svenson KL, Lundqvist G, Wide L, Hällgren R. Impaired glucose handling in active rheumatoid arthritis: effects of corticosteroids and antirheumatic treatment. Metabolism. 1987;36(10):944–948. doi: 10.1016/0026-0495(87)90129-6. [DOI] [PubMed] [Google Scholar]
- 86.Han C, Robinson DW, Jr, Hackett MV, Paramore LC, Fraeman KH, Bala MV. Cardiovascular disease and risk factors in patients with rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. J Rheumatol. 2006;33(11):2167–2172. [PubMed] [Google Scholar]
- 87.Strehl C, Bijlsma JW, de Wit M, Boers M, Caeyers N, Cutolo M, et al. Defining conditions where long-term glucocorticoid treatment has an acceptably low level of harm to facilitate implementation of existing recommendations: viewpoints from an EULAR task force. Ann Rheum Dis. 2016;75(6):952–957. doi: 10.1136/annrheumdis-2015-208916. [DOI] [PubMed] [Google Scholar]
- 88.van Sijl AM, Boers M, Voskuyl AE, Nurmohamed MT. Confounding by indication probably distorts the relationship between steroid use and cardiovascular disease in rheumatoid arthritis: results from a prospective cohort study. PLoS One. 2014;9(1):e87965. doi: 10.1371/journal.pone.0087965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Burmester GR, Pope JE. Novel treatment strategies in rheumatoid arthritis. Lancet. 2017;389(10086):2338–2348. doi: 10.1016/S0140-6736(17)31491-5. [DOI] [PubMed] [Google Scholar]
- 90.Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate: an old new drug in autoimmune disease. Expert Rev Clin Immunol. 2014;10(11):1519–1530. doi: 10.1586/1744666X.2014.962996. [DOI] [PubMed] [Google Scholar]
- 91.Cipriani P, Ruscitti P, Carubbi F, Liakouli V, Giacomelli R. Methotrexate in rheumatoid arthritis: optimizing therapy among different formulations. Current and emerging paradigms. Clin Ther. 2014;36(3):427–435. doi: 10.1016/j.clinthera.2014.01.014. [DOI] [PubMed] [Google Scholar]
- 92.Cronstein BN, Aune TM. Methotrexate and its mechanisms of action in inflammatory arthritis. Nat Rev Rheumatol. 2020;16(3):145–154. doi: 10.1038/s41584-020-0373-9. [DOI] [PubMed] [Google Scholar]
- 93.Russo GT, Minutoli L, Bitto A, Altavilla D, Alessi E, Giandalia A, et al. Methotrexate increases skeletal muscle GLUT4 expression and improves metabolic control in experimental diabetes. J Nutr Metab. 2012;2012:132056. doi: 10.1155/2012/132056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rekedal LR, Massarotti E, Garg R, Bhatia R, Gleeson T, Lu B, et al. Changes in glycosylated hemoglobin after initiation of hydroxychloroquine or methotrexate treatment in diabetes patients with rheumatic diseases. Arthritis Rheum. 2010;62(12):3569–3573. doi: 10.1002/art.27703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.de Rotte MC, de Jong PH, den Boer E, Pluijm SM, Özcan B, Weel AE, et al. Effect of methotrexate use and erythrocyte methotrexate polyglutamate on glycosylated hemoglobin in rheumatoid arthritis. Arthritis Rheumatol. 2014;66(8):2026–2036. doi: 10.1002/art.38652. [DOI] [PubMed] [Google Scholar]
- 96.Donath MY, Dinarello CA, Mandrup-Poulsen T. Targeting innate immune mediators in type 1 and type 2 diabetes. Nat Rev Immunol. 2019;19(12):734–746. doi: 10.1038/s41577-019-0213-9. [DOI] [PubMed] [Google Scholar]
- 97.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, Seifert B, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356(15):1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 98.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses J, Seifert B, et al. Interleukin-1 receptor antagonist-treatment of patients with type 2 diabetes. Ugeskr Laeger. 2007;169(45):3868–3871. [PubMed] [Google Scholar]
- 99.Malozowski S, Sahlroot JT. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;357(3):302–303. doi: 10.1056/NEJMc071324. [DOI] [PubMed] [Google Scholar]
- 100.Larsen CM, Faulenbach M, Vaag A, Ehses JA, Donath MY, Mandrup-Poulsen T. Sustained effects of interleukin-1 receptor antagonist treatment in type 2 diabetes. Diabetes Care. 2009;32(9):1663–1668. doi: 10.2337/dc09-0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ridker PM, Howard CP, Walter V, Everett B, Libby P, Hensen J, et al. Effects of interleukin-1β inhibition with canakinumab on hemoglobin A1c, lipids, C-reactive protein, interleukin-6, and fibrinogen: a phase IIb randomized, placebo-controlled trial. Circulation. 2012;126(23):2739–2748. doi: 10.1161/CIRCULATIONAHA.112.122556. [DOI] [PubMed] [Google Scholar]
- 102.Ridker PM, Everett BM, Thuren T, MacFadyen JG, Chang WH, Ballantyne C, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377(12):1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 103.Everett BM, Donath MY, Pradhan AD, Thuren T, Pais P, Nicolau JC, et al. Anti-inflammatory therapy with canakinumab for the prevention and management of diabetes. J Am Coll Cardiol. 2018;71(21):2392–2401. doi: 10.1016/j.jacc.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 104.Ruscitti P, Masedu F, Alvaro S, Airò P, Battafarano N, Cantarini L, et al. Anti-interleukin-1 treatment in patients with rheumatoid arthritis and type 2 diabetes (TRACK): a multicentre, open-label, randomised controlled trial. PLoS Med. 2019;16(9):e1002901. doi: 10.1371/journal.pmed.1002901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ruscitti P, Berardicurti O, Cipriani P, Giacomelli R, TRACK study group Benefits of anakinra versus TNF inhibitors in rheumatoid arthritis and type 2 diabetes: long-term findings from participants furtherly followed-up in the TRACK study, a multicentre, open-label, randomised, controlled trial. Clin Exp Rheumatol. 2021;39(2):403–406. [PubMed] [Google Scholar]
- 106.Nuki G, Bresnihan B, Bear MB, McCabe D, European Group Of Clinical Investigators Long-term safety and maintenance of clinical improvement following treatment with anakinra (recombinant human interleukin-1 receptor antagonist) in patients with rheumatoid arthritis: extension phase of a randomized, double-blind, placebo-controlled trial. Arthritis Rheum. 2002;46(11):2838–2846. doi: 10.1002/art.10578. [DOI] [PubMed] [Google Scholar]
- 107.Cohen SB, Moreland LW, Cush JJ, Greenwald MW, Block S, Shergy WJ, Hanrahan PS, Kraishi MM, Patel A, Sun G, Bear MB, 990145 Study Group A multicentre, double blind, randomised, placebo controlled trial of anakinra (Kineret), a recombinant interleukin 1 receptor antagonist, in patients with rheumatoid arthritis treated with background methotrexate. Ann Rheum Dis. 2004;63(9):1062–1068. doi: 10.1136/ard.2003.016014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bykerk VP, Jamal S, Boire G, Hitchon CA, Haraoui B, Pope JE, et al. The Canadian Early Arthritis Cohort (CATCH): patients with new-onset synovitis meeting the 2010 ACR/EULAR classification criteria but not the 1987 ACR classification criteria present with less severe disease activity. J Rheumatol. 2012;39(11):2071–2080. doi: 10.3899/jrheum.120029. [DOI] [PubMed] [Google Scholar]
- 109.Ruscitti P, Ursini F, Cipriani P, Greco M, Alvaro S, Vasiliki L, et al. IL-1 inhibition improves insulin resistance and adipokines in rheumatoid arthritis patients with comorbid type 2 diabetes: An observational study. Medicine (Baltimore) 2019;98(7):e14587. doi: 10.1097/MD.0000000000014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Wood PR, Manning E, Baker JF, England B, Davis L, Cannon GW, et al. Blood glucose changes surrounding initiation of tumor-necrosis factor inhibitors and conventional disease-modifying anti-rheumatic drugs in veterans with rheumatoid arthritis. World J Diabetes. 2018;9(2):53–58. doi: 10.4239/wjd.v9.i2.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ferraz-Amaro I, Arce-Franco M, Muñiz J, López-Fernández J, Hernández-Hernández V, Franco A, et al. Systemic blockade of TNF-α does not improve insulin resistance in humans. Horm Metab Res. 2011;43(11):801–808. doi: 10.1055/s-0031-1287783. [DOI] [PubMed] [Google Scholar]
- 112.Seriolo B, Ferrone C, Cutolo M. Longterm anti-tumor necrosis factor-alpha treatment in patients with refractory rheumatoid arthritis: relationship between insulin resistance and disease activity. J Rheumatol. 2008;35(2):355–357. [PubMed] [Google Scholar]
- 113.Tam LS, Tomlinson B, Chu TT, Li TK, Li EK. Impact of TNF inhibition on insulin resistance and lipids levels in patients with rheumatoid arthritis. Clin Rheumatol. 2007;26(9):1495–1498. doi: 10.1007/s10067-007-0539-8. [DOI] [PubMed] [Google Scholar]
- 114.Stavropoulos-Kalinoglou A, Metsios GS, Panoulas VF, Nightingale P, Koutedakis Y, Kitas GD. Anti-tumour necrosis factor alpha therapy improves insulin sensitivity in normal-weight but not in obese patients with rheumatoid arthritis. Arthritis Res Ther. 2012;14(4):R160. doi: 10.1186/ar3900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kiortsis DN, Mavridis AK, Vasakos S, Nikas SN, Drosos AA. Effects of infliximab treatment on insulin resistance in patients with rheumatoid arthritis and ankylosing spondylitis. Ann Rheum Dis. 2005;64(5):765–766. doi: 10.1136/ard.2004.026534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Seriolo B, Paolino S, Ferrone C, Cutolo M. Impact of long-term anti-TNF-alpha treatment on insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2008;26:159–160. [PubMed] [Google Scholar]
- 117.Stagakis I, Bertsias G, Karvounaris S, Kavousanaki M, Virla D, Raptopoulou A, et al. Anti-tumor necrosis factor therapy improves insulin resistance, beta cell function and insulin signaling in active rheumatoid arthritis patients with high insulin resistance. Arthritis Res Ther. 2012;14(3):R141. doi: 10.1186/ar3874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Leporini C, Russo E, Angelo SD, Arturi F, Tripepi G, Peluso R, et al. Insulin-sensiting effects of tumor necrosis factor alpha inhibitors in rheumatoid arthritis: a systematic review and meta-analysis. Rev Recent Clin Trials. 2018;13(3):184–191. doi: 10.2174/1574887113666180314100340. [DOI] [PubMed] [Google Scholar]
- 119.Gonzalez-Gay MA, De Matias JM, Gonzalez-Juanatey C, Garcia-Porrua C, Sanchez-Andrade A, Martin J, et al. Anti-tumor necrosis factor-alpha blockade improves insulin resistance in patients with rheumatoid arthritis. Clin Exp Rheumatol. 2006;24(1):83–86. [PubMed] [Google Scholar]
- 120.Solomon DH, Massarotti E, Garg R, Liu J, Canning C, Schneeweiss S. Association between disease-modifying antirheumatic drugs and diabetes risk in patients with rheumatoid arthritis and psoriasis. JAMA. 2011;305(24):2525–2531. doi: 10.1001/jama.2011.878. [DOI] [PubMed] [Google Scholar]
- 121.Castañeda S, Remuzgo-Martínez S, López-Mejías R, Genre F, Calvo-Alén J, Llorente I, et al. Rapid beneficial effect of the IL-6 receptor blockade on insulin resistance and insulin sensitivity in non-diabetic patients with rheumatoid arthritis. Clin Exp Rheumatol. 2019;37:465–473. [PubMed] [Google Scholar]
- 122.Otsuka Y, Kiyohara C, Kashiwado Y, Sawabe T, Nagano S, Kimoto Y, et al. Effects of tumor necrosis factor inhibitors and tocilizumab on the glycosylated hemoglobin levels in patients with rheumatoid arthritis; an observational study. PLoS One. 2018;13(4):e0196368. doi: 10.1371/journal.pone.0196368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Fève B, Bastard JP. The role of interleukins in insulin resistance and type 2 diabetes mellitus. Nat Rev Endocrinol. 2009;5(6):305–311. doi: 10.1038/nrendo.2009.62. [DOI] [PubMed] [Google Scholar]
- 124.Schultz O, Oberhauser F, Saech J, Rubbert-Roth A, Hahn M, Krone W, et al. Effects of inhibition of interleukin-6 signalling on insulin sensitivity and lipoprotein (a) levels in human subjects with rheumatoid diseases. PLoS One. 2010;5(12):e14328. doi: 10.1371/journal.pone.0014328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zaletel J, Barlovic DP, Prezelj J. Adiponectin-leptin ratio: a useful estimate of insulin resistance in patients with type 2 diabetes. J Endocrinol Investig. 2010;33(8):514–518. doi: 10.1007/BF03346639. [DOI] [PubMed] [Google Scholar]
- 126.Ogata A, Morishima A, Hirano T, Hishitani Y, Hagihara K, Shima Y, et al. Improvement of HbA1c during treatment with humanised anti-interleukin 6 receptor antibody, tocilizumab. Ann Rheum Dis. 2011;70(6):1164–1165. doi: 10.1136/ard.2010.132845. [DOI] [PubMed] [Google Scholar]
- 127.Makrilakis K, Fragiadaki K, Smith J, Sfikakis PP, Kitas GD. Interrelated reduction of chemerin and plasminogen activator inhibitor-1 serum levels in rheumatoid arthritis after interleukin-6 receptor blockade. Clin Rheumatol. 2015;34(3):419–427. doi: 10.1007/s10067-014-2704-1. [DOI] [PubMed] [Google Scholar]
- 128.Tournadre A, Pereira B, Dutheil F, Giraud C, Courteix D, Sapin V, et al. Changes in body composition and metabolic profile during interleukin 6 inhibition in rheumatoid arthritis. J Cachexia Sarcopenia Muscle. 2017;8(4):639–646. doi: 10.1002/jcsm.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Genovese MC, Burmester GR, Hagino O, Thangavelu K, Iglesias-Rodriguez M, John GS, et al. Interleukin-6 receptor blockade or TNFα inhibition for reducing glycaemia in patients with RA and diabetes: post hoc analyses of three randomised, controlled trials. Arthritis Res Ther. 2020;22(1):206. doi: 10.1186/s13075-020-02229-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Burmester GR, Lin Y, Patel R, van Adelsberg J, Mangan EK, Graham NM, et al. Efficacy and safety of sarilumab monotherapy versus adalimumab monotherapy for the treatment of patients with active rheumatoid arthritis (MONARCH): a randomised, double-blind, parallel-group phase III trial. Ann Rheum Dis. 2017;76(5):840–847. doi: 10.1136/annrheumdis-2016-210310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fleischmann R, van Adelsberg J, Lin Y, Castelar-Pinheiro GD, Brzezicki J, Hrycaj P, et al. Sarilumab and nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis and inadequate response or intolerance to tumor necrosis factor inhibitors. Arthritis Rheumatol. 2017;69(2):277–290. doi: 10.1002/art.39944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Genovese MC, Fleischmann R, Kivitz AJ, Rell-Bakalarska M, Martincova R, Fiore S, et al. Sarilumab plus methotrexate in patients with active rheumatoid arthritis and inadequate response to methotrexate: results of a phase III study. Arthritis Rheumatol. 2015;67(6):1424–1437. doi: 10.1002/art.39093. [DOI] [PubMed] [Google Scholar]
- 133.Ferro F, Elefante E, Luciano N, Talarico R, Todoerti M. One year in review 2017: novelties in the treatment of rheumatoid arthritis. Clin Exp Rheumatol. 2017;35:721–734. [PubMed] [Google Scholar]
- 134.Rachid O, Osman A, Abdi R, Haik Y. CTLA4-Ig (abatacept): a promising investigational drug for use in type 1 diabetes. Expert Opin Investig Drugs. 2020;29(3):221–236. doi: 10.1080/13543784.2020.1727885. [DOI] [PubMed] [Google Scholar]
- 135.Santopaolo M, Sullivan N, Thomas AC, Alvino VV, Nicholson LB, Gu Y, et al. Activation of bone marrow adaptive immunity in type 2 diabetes: rescue by co-stimulation modulator abatacept. Front Immunol. 2021;12:609406. doi: 10.3389/fimmu.2021.609406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Desai RJ, Dejene S, Jin Y, Liu J, Kim SC. Comparative risk of diabetes mellitus in patients with rheumatoid arthritis treated with biologic or targeted synthetic disease-modifying drugs: a cohort study. ACR Open Rheumatol. 2020;2(4):222–231. doi: 10.1002/acr2.11124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ozen G, Pedro S, Holmqvist ME, Avery M, Wolfe F, Michaud K. Risk of diabetes mellitus associated with disease-modifying antirheumatic drugs and statins in rheumatoid arthritis. Ann Rheum Dis. 2017;76(5):848–854. doi: 10.1136/annrheumdis-2016-209954. [DOI] [PubMed] [Google Scholar]
- 138.Chen SK, Lee H, Jin Y, Liu J, Kim SC. Use of biologic or targeted-synthetic disease-modifying anti-rheumatic drugs and risk of diabetes treatment intensification in patients with rheumatoid arthritis and diabetes mellitus. Rheumatol Adv Pract. 2020;4(2):rkaa027. doi: 10.1093/rap/rkaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Collotta D, Hull W, Mastrocola R, Chiazza F, Cento AS, Murphy C, et al. Baricitinib counteracts metaflammation, thus protecting against diet-induced metabolic abnormalities in mice. Mol Metab. 2020;39:101009. doi: 10.1016/j.molmet.2020.101009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tuttle KR, Brosius FC, 3rd, Adler SG, Kretzler M, Mehta RL, Tumlin JA, et al. JAK1/JAK2 inhibition by baricitinib in diabetic kidney disease: results from a Phase 2 randomized controlled clinical trial. Nephrol Dial Transplant. 2018;33(11):1950–1959. doi: 10.1093/ndt/gfx377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Donath MY. Multiple benefits of targeting inflammation in the treatment of type 2 diabetes. Diabetologia. 2016;59(4):679–682. doi: 10.1007/s00125-016-3873-z. [DOI] [PubMed] [Google Scholar]
- 142.Selvin E, Parrinello CM, Daya N, Bergenstal RM. Trends in insulin use and diabetes control in the US: 1988–1994 and 1999–2012. Diabetes Care. 2016;39(3):e33–e35. doi: 10.2337/dc15-2229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Giacomelli R, Afeltra A, Bartoloni E, Berardicurti O, Bombardieri M, Bortoluzzi A, et al. The growing role of precision medicine for the treatment of autoimmune diseases; results of a systematic review of literature and Experts' Consensus. Autoimmun Rev. 2021;20(2):102738. doi: 10.1016/j.autrev.2020.102738. [DOI] [PubMed] [Google Scholar]
- 144.Giacomelli R, Afeltra A, Alunno A, Baldini C, Bartoloni-Bocci E, Berardicurti O, et al. International consensus: what else can we do to improve diagnosis and therapeutic strategies in patients affected by autoimmune rheumatic diseases (rheumatoid arthritis, spondyloarthritides, systemic sclerosis, systemic lupus erythematosus, antiphospholipid syndrome and Sjogren's syndrome)? The unmet needs and the clinical grey zone in autoimmune disease management. Autoimmun Rev. 2017;16(9):911–924. doi: 10.1016/j.autrev.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 145.Giacomelli R, Afeltra A, Alunno A, Bartoloni-Bocci E, Berardicurti O, Bombardieri M, et al. Guidelines for biomarkers in autoimmune rheumatic diseases—evidence based analysis. Autoimmun Rev. 2019;18(1):93–106. doi: 10.1016/j.autrev.2018.08.003. [DOI] [PubMed] [Google Scholar]
- 146.Ursini F, Russo E, Ruscitti P, Giacomelli R, De Sarro G. The effect of non-TNF-targeted biologics and small molecules on insulin resistance in inflammatory arthritis. Autoimmun Rev. 2018;17(4):399–404. doi: 10.1016/j.autrev.2017.11.030. [DOI] [PubMed] [Google Scholar]
- 147.Ursini F, Ruscitti P, Caio GPI, Manfredini R, Giacomelli R, De Giorgio R. The effect of non-TNF-targeted biologics on vascular dysfunction in rheumatoid arthritis: a systematic literature review. Autoimmun Rev. 2019;18(5):501–509. doi: 10.1016/j.autrev.2019.03.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable, this work represents a review of available evidence.