Abstract
Monocytes respond to lipopolysaccharide (LPS) stimulation with a rapid expression of the tumor necrosis factor (TNF) gene. Upon repeated LPS stimulation there is, however, little production of TNF mRNA and protein; i.e., the cells are tolerant to LPS. Analysis of NF-κB proteins in gel shift assays demonstrated that the DNA binding activity that is induced by LPS stimulation in tolerant cells consists mainly of p50-p50 homodimers. Since p50 can bind to DNA but lacks a transactivation domain, this may explain the blockade of TNF gene expression. We now show that in the monocytic cell line Mono Mac 6, this inability to respond can be largely ascribed to NF-κB, since a reporter construct directed by a trimeric NF-κB motif is strongly transactivated by LPS stimulation of naive cells whereas LPS-tolerant cells exhibit only low activity. Also, Western blot analyses of proteins extracted from purified nuclei showed mobilization of threefold-higher levels of p50 protein in tolerant compared to naive cells, while mobilization of p65 was unaltered. Overexpression of p50 in HEK 293 cells resulted in a strong reduction of p65-driven TNF promoter activity at the levels of both luciferase mRNA and protein. These data support the concept that an upregulation of p50 is instrumental in LPS tolerance in human monocytes.
Lipopolysaccharide (LPS) produced by gram-negative bacteria will induce a pronounced activation of monocytes and macrophages, a process mediated by the CD14 cell surface receptor (35, 44). LPS-induced activation of monocytes leads to the expression of a whole body of inflammatory mediators, including tumor necrosis factor (TNF). TNF is a master cytokine that regulates a plethora of inflammatory processes; in gram-negative sepsis, TNF forms a central element in the pathophysiology of the disease.
Exposure to LPS for a short period of time (1 h) may lead to enhanced responses after secondary stimulation, a phenomenon frequently seen in neutrophils and termed priming. LPS-primed neutrophils show enhanced responses with respect to leukotrienes, reactive oxygen, and enzyme release typically after stimulation with formylmethionyl-leucyl-phenylalanine (29, 34). Priming may also be observed for macrophages with respect to cytokine gene expression (13). Here specific dose and time requirements have to be met. On the other hand, longer periods (days) of exposure to LPS will lead after a second LPS stimulation to reduced cytokine production by monocytes/macrophages, a phenomenon termed LPS tolerance.
Decreased responses of tolerant monocytes have been documented not only for TNF but also for interleukin-1 (IL-1), IL-6, and other cytokines, for arachidonic acid metabolites, for responses like fever, and for LPS-induced death rate (reviewed in reference 37).
In classical models of tolerance (for instance, to β-adrenergic drugs), the respective cell surface receptor is downregulated or uncoupled from downstream signalling (12). This does not appear to be the case in LPS tolerance since here the CD14 receptor is unchanged or even enhanced in cell surface expression (17, 22, 45). Furthermore, signal transduction can still occur to some extent since NF-κB was shown in some studies to be still mobilized upon secondary stimulation (11).
Characterization of the mobilized NF-κB proteins revealed a predominance of p50-p50 homodimers (8, 45). Since these p50 molecules lack a transactivation domain, we have hypothesized that upon binding to the promoter of, for instance, the TNF gene, the p50 homodimers block transactivation by other NF-κB/Rel family members.
This hypothesis was based on studies of DNA binding in gel shift assays that do not allow for direct quantitation of the proteins involved. We now show in Western blot analysis that upon LPS stimulation, the p65 protein is mobilized into the nucleus of tolerant cells as efficiently as in naive cells. This demonstrates that the signal transduction pathways leading to IκB degradation and subsequent nuclear location of p65/Rel-A are still intact in LPS tolerance. On the other hand, Western blotting for p50 protein after LPS stimulation showed threefold-higher levels in tolerant compared to naive cells. With this predominance of p50, the transactivation of an exclusively NF-κB-directed reporter gene is strongly reduced, and we show for the first time that overexpression of p50 can, in fact, block p65-driven transactivation of the human TNF promoter. These data provide direct evidence for the central role of p50 of NF-κB in preventing gene expression in LPS tolerance.
(This work is part of S. Kastenbauer’s thesis, completed at the Ludwig-Maximilian-Universititaet Muenchen.)
MATERIALS AND METHODS
Cell culture.
HEK 293 cells (kindly provided by D. Kuprash, Moscow, Russia) were seeded at 5 × 105 cells per well in six-well plates (product no. 3506; Costar, Bodenheim, Germany) in RPMI 1640 (Biochrom, Berlin, Germany) supplemented with l-glutamine (2 mm), penicillin (200 U/ml) plus streptomycin (200 μg/ml) (product no. 043-05140 H; Gibco), and 10% fetal calf serum. The cell line Mono Mac 6 was established from a patient with monoblastic leukemia (43). The cell line shows many characteristics of a human blood monocyte, including phagocytosis and expression of the CD14 LPS receptor. These cells were cultured in 24-well plates in RPMI 1640 with l-glutamine, penicillin, streptomycin, nonessential amino acids (product no. 043-01140 H; Gibco) oxalacetic acid-sodium pyruvate-insulin supplement (product no. O-5003; Sigma, Deisenhofen, Germany), and 10% fetal calf serum. The cells were controlled for absence of mycoplasma (38) on a weekly basis. Stimulation with LPS (Salmonella minnesota L-6261; Sigma) was done in complete culture medium with a standard dose of 1 μg/ml. For tolerance induction, Mono Mac 6 cells were cultured for 2 to 3 days with or without LPS at 20 ng/ml followed by stimulation for 1 to 6 h with LPS at 1 μg/ml.
TNF bioassay.
TNF bioactivity was measured in the WEHI 164/actinomycin D assay as described elsewhere (40). In brief, WEHI 164 cells were exposed to actinomycin D for 2 h, and the washed cells were added to threefold serial dilutions of supernatants. After overnight culture, viability was determined by using 2,3-bis(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide (XTT, product no. 4251; Sigma), and concentrations (units per milliliter) were calculated with reference to a standard supernatant.
TNF reverse transcriptase PCR (RT-PCR).
TNF mRNA expression was determined essentially as described elsewhere (6). In brief, cell lysates were taken after 1 h of stimulation, and total RNA was isolated and reverse transcribed with oligo(dT) primers. The cDNA was then amplified with specific primers, and after 32 cycles the product was separated on a 1.4% agarose gel in the presence of ethidium bromide. As an external control, the housekeeping enzyme α-enolase was amplified (24).
Gel shift analysis.
Nuclear extracts from cells stimulated as described above were prepared as described previously (3). The protein concentration was determined by the method of Bradford, using a commercial kit (product no. 500-0006; Bio-Rad, Munich, Germany), and 6 to 10 μg of protein was incubated with different double-stranded κB human TNF-605 oligonucleotides: coding strand 5′ AGCT TCCCCGGGGCTGTCCCAGG 3′
noncoding strand 3′ AGGGGCCCCGACAGGGTCCAGCT 5′ These oligonucleotides were labeled by fill-in reaction with [32P]dATP (Amersham, Braunscheig, Germany), and 50,000 cpm of the relevant double-stranded oligonucleotide was used per assay. The binding reaction was performed for 15 min at room temperature as previously described (7). The complexes were separated by gel electrophoresis on a 4% polyacrylamide gel and run with 0.25× Tris buffer containing borate and EDTA.
Western blotting.
Cells were disrupted by Dounce homogenization in the presence of a proteinase inhibitor cocktail, and nuclei were purified over a sucrose gradient. Nuclear proteins were extracted, and 20 to 30 μg of protein/lane was separated on 4 to 12% Tris-glycine gels (product no. EC60385; Novex [obtained via Anamed, Offenbach, Germany]) and transferred to Hybond ECL (enhanced chemiluminescence) nitrocellulose membranes (product no. RPN 2020D; Amersham) by electroblotting. Membranes were reacted with 1:5,000 dilutions of antibodies against p50 (1141) and p65 (1226) that were kindly provided by Nancy Rice (Frederick, Md.). After reaction with F(ab)2 goat anti-rabbit immunoglobulin-peroxidase conjugate (1:10,000; product no. A6667; Sigma), blots were incubated with ECL reagent (product no. RPN 2106; Amersham) and exposed to Hyperfilm ECL (product no. RPN 3103; Amersham).
Plasmids and transfection.
The pTNF-1064 luci-β reporter plasmid containing the human TNF 5′ region was obtained by exchanging the mouse β-globin promoter of the pβTATA.luci-β reporter plasmid for the HindIII/BglII fragment from the TNF 5′ region pxP2 luciferase construct (45). The pβTATA.luci reporter plasmid contains 3′ to the luciferase gene the rabbit β-globin intron (15). The 3×κB.luc construct contains three copies of the prototypic NF-κB sequence from the mouse kappa light-chain enhancer cloned upstream of the TATA box of the pβTATA.luci reporter plasmid (15).
For DEAE-dextran transfection, the Mono Mac 6 cells were harvested on day −1, split 1:2, and reseeded to ensure log-phase growth. On day 0, they were adjusted to 107/ml in RPMI 1640 without serum. One milliliter of cells was then admixed with DEAE-dextran (product no. D-1162; Sigma) at 62.5 μg/ml (final concentration) and a total of 5 μg of reporter plasmid. After incubation for 90 min at 37°C, dimethyl sulfoxide (product no. 1.02931; Merck, Darmstadt, Germany) was added at 10% (final concentration) for 3 min at room temperature. Cells were then washed three times and seeded at 5 × 105/ml in 2-ml volumes per well in 24-well plates. After overnight culture, 105 cells were then stimulated with different LPS doses for various length of times as given below. The standard dose of LPS was 1 μg/ml, and the standard time for stimulation was 4 h for luciferase mRNA analysis and 6 h for luciferase protein analysis.
For cotransfection analysis, HEK 293 cells were seeded at 5 × 105 cells per well in six-well plates (product no. 3506; Costar) and cultured overnight. Duplicates of two wells each were then transfected by the calcium phosphate method with luciferase reporter plasmid alone (0.5 μg), with luciferase reporter plasmid plus 0.25 μg of RcCMVp65 (25) (kindly provided by P. A. Baeuerle, Freiburg, Germany), or with luciferase reporter plasmid plus 0.25 μg of RcCMVp65 plus either 2.5 μg of RcCMVp50 expression plasmid or 2.5 μg of RcCMV empty expression plasmid. Cells were washed after 6 h, and reporter gene activity was measured after 24 h.
Analysis of luciferase DNA and mRNA.
The PCR procedure was performed essentially as described elsewhere (33). In brief, pellets of 105 cells each were lysed in 200 μl of RNAzol (WAK-Chemie, Bad Homburg, Germany). After addition of chloroform-isoamyl alcohol (24:1, vol/vol) and centrifugation at 10,000 × g, the aqueous phase was harvested and plasmid DNA and mRNA were isolated. This material was used directly for with 22 to 30 cycles of PCR with 5′ and 3′ primers that anneal to the plasmid DNA. For RT-PCR, the isolated mRNA was reverse transcribed with oligo(dT) primers and murine leukemia virus RT. The cDNA was then amplified for 34 to 40 cycles with the 3′ primer spanning the intron of the luciferase reporter gene (15). Products were separated on 1.4% agarose gels containing ethidium bromide. Laser densitometry was used to quantify Polaroid negative films, and the amount of luciferase mRNA was expressed as the percentage of plasmid DNA signal.
Analysis of luciferase protein.
Luciferase activity in cell lysates was determined by using a model LB9501 luminometer (Berthold, Wildbad, Germany) and the luciferase assay system (E1500) from Promega (Madison, Wis.).
Statistics.
For statistical analysis, Student’s t test (paired, log transformation) was used.
RESULTS
TNF gene expression in LPS tolerance.
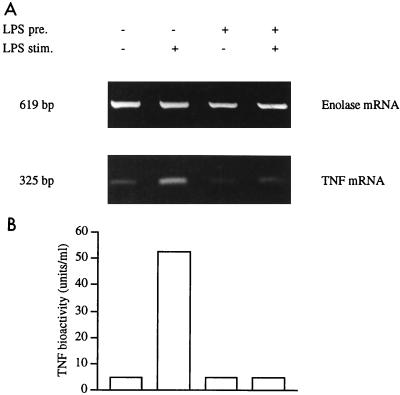
Human monocytic Mono Mac 6 cells in the absence of LPS produced little or no TNF mRNA and protein (Fig. 1A, lane 1). Stimulation with LPS at 1 μg/ml rapidly induced TNF mRNA within 1 h, and TNF protein in the supernatant showed a strong increase at 6 h (lane 2). When precultured with LPS at a low dose (20 ng/ml) for 2 days, the cells produced little TNF mRNA and protein without additional stimulation (lane 3); after stimulation with LPS at 1 μg/ml, there was no substantial expression of the TNF gene (lane 4). These data confirm earlier observations that the Mono Mac 6 cells which are stimulated during preculture with a low dose of LPS become tolerant to a second stimulation with a high dose of LPS (11, 45).
FIG. 1.
Induction of LPS tolerance in human Mono Mac 6 cells. Mono Mac 6 cells were precultured for 48 h with or without LPS (20 ng/ml), washed, and stimulated with or without LPS at 1 μg/ml for 1 h (for mRNA determination) or for 6 h (for protein analysis). Isolated RNA was reverse transcribed and amplified for α-enolase (32 cycles) and for TNF (34 cycles). Densitometry values for TNF, corrected for enolase prevalence, were 17.8, 48.7, 9.1, and 17.4% for lanes 1 to 4. In an average of four experiments, −/+ cells (A, lane 2) gave a value of 58.4 ± 16.9%, while for +/+ cells (lane 4) the value was 26.2 ± 15.0% (P < 0.01). Assay of supernatants from the same experiment for TNF protein in the WEHI 164/actinomycin D bioassay (B) gave values of <5 U/ml for all cultures except the LPS-stimulated naive cells in lane 2, which produced 52.2 U/ml. In an average of four experiments, −/+ cells (lane 2) gave 132.7 ± 58.3 U/ml and +/+ cells (lane 4) gave 9.8 ± 6.1 U/ml (P < 0.01).
Reporter gene mRNA analysis: LPS dose response.
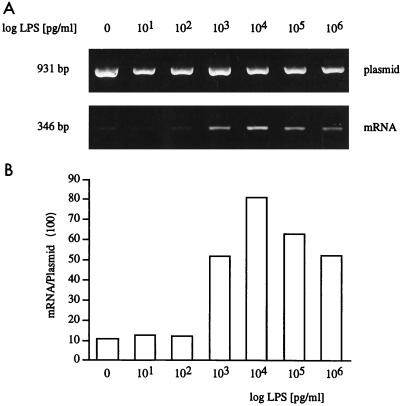
Previous studies have shown that LPS tolerance is regulated at the level of TNF transcription (45). In earlier studies we analyzed the human TNF promoter by using standard luciferase reporter constructs (45). In the present study, we used reporter gene constructs that contain a large 3′ intron which is spliced out with maturation of the mRNA. By using a transintron primer, the RNA for luciferase after reverse transcription can therefore be specifically amplified by PCR (15). Determination of plasmid DNA with a 3′ primer that anneals downstream of the intron can then be used as an internal control to correct for different levels of plasmid in different samples of an experiment. When Mono Mac 6 cells were transfected with such TNF promoter-directed luciferase reporter constructs, LPS stimulation rapidly induced luciferase mRNA over a wide range of LPS doses (Fig. 2). Doses as low as 1 ng/ml induced a significant response; in some experiments not shown, doses of 100 and even 10 pg of LPS/ml were sufficient to transactivate the construct.
FIG. 2.
LPS dose dependence of human TNF promoter-directed mRNA expression in Mono Mac 6 cells. Mono Mac 6 cells were transfected by DEAE-dextran and after overnight culture were stimulated with different doses of LPS for 4 h. RNA and plasmid DNA were isolated, and plasmid was amplified directly from cell lysates with 22 PCR cycles; mRNA was reverse transcribed, and cDNA was amplified by using one 5′ primer combined with one 3′ transintron primer (40 cycles). Products were separated on a 1.4% agarose gel (A). (B) Densitometry values for mRNA, expressed as percentage of the plasmid DNA. Data are representative of one experiment of five performed.
Analysis of the time course revealed an induction of luciferase mRNA at 1 h, and an optimum response was seen at 4 h (data not shown). The LPS dose of 1 μg/ml and the time point of 4 h were therefore used for all further stimulation experiments.
TNF promoter activity in LPS tolerance.
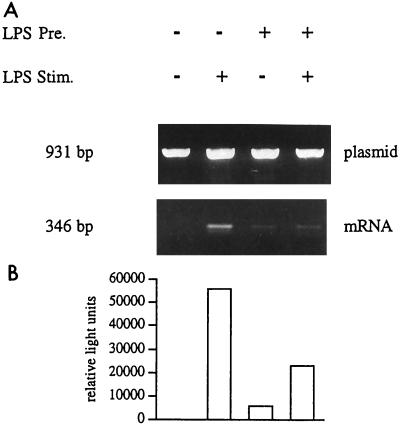
For analysis of TNF promoter activity, naive and tolerant Mono Mac 6 cells were transfected with the pTNF-1064 luci-β promoter reporter construct by using DEAE-dextran, precultured with or without LPS at 20 ng/ml for 2 days, washed, and stimulated with LPS at 1 μg/ml for 4 h. LPS stimulation of the naive cells gave the expected strong response for luciferase mRNA (26-fold activation compared to the unstimulated control [Fig. 3A, lane 2). Cells precultured with LPS gave, however, only a minimal (threefold) transactivation (lane 4). Determination of luciferase enzyme activity gave a similar pattern, with a high activity in LPS-stimulated naive cells (19-fold induction compared to the control [lane 2]), while tolerant cells showed only a minimal (twofold) response (lane 4). These data indicate that transactivation of the human TNF promoter reflects the pattern of response seen for expression of the endogenous TNF gene in LPS-tolerant human Mono Mac 6 monocytes.
FIG. 3.
TNF promoter activity in naive and LPS-tolerant Mono Mac 6 cells. Mono Mac 6 cells were transfected, cultured for 2 days with or without LPS (20 ng/ml), washed, and stimulated with LPS at 1 μg/ml. RNA and plasmid DNA were isolated; the plasmid DNA was amplified directly (30 cycles), while mRNA was reverse transcribed and amplified by using the 3′ transintron primer together with the 5′ primer (40 cycles). Products were separated on a 1.4% agarose gel (A). Densitometry values for mRNA are expressed as proportions of the plasmid DNA are 2.5, 65.9, 7.6, and 24.0% for lanes 1 to 4. Cell samples from the same experiment were taken for three freeze-thaw cycles, and luciferase enzyme activity was determined in the lysates (B). Values for luciferase were 5,218, 98,742, 9,623, and 21,931 relative light units for lanes 1 to 4. Data are representative of one experiment of three performed. The average fold induction for both mRNA and protein was significantly lower in LPS-stimulated tolerant cells than in LPS-stimulated naive cells. For mRNA, levels of induction were 39.8- and 4.4-fold in naive and tolerant cells, respectively; for luciferase protein, they were 13.9- and 2.1-fold for naive and tolerant cells, respectively (P < 0.05 for both mRNA and protein).
NF-κB activity in LPS tolerance.
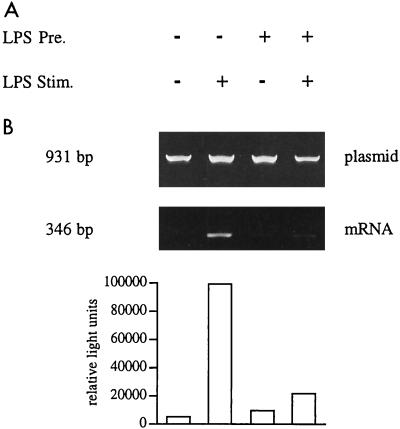
One crucial transcription factor controlling expression of the TNF gene is NF-κB (27, 30, 36, 42). Previous studies using a human immunodeficiency virus long terminal repeat reporter construct had indicated that function was reduced in LPS tolerance (45). We now have analyzed the activity of a luciferase reporter construct that contains a trimeric NF-κB binding motif (15). With this construct, we obtained essentially the same pattern of results as seen with the TNF promoter-directed reporter plasmid. Again naive Mono Mac 6 cells gave a strong induction of luciferase mRNA and protein after LPS stimulation (142- and 158-fold, respectively [Fig. 4, lane 2). Tolerant cells showed only a minimal induction of mRNA after LPS stimulation (1.3-fold). Luciferase enzyme activity was still high in unstimulated tolerant cells (lane 3), and fold induction compared to the unstimulated precultured cells (lane 4) was reduced to 3.9-fold. These data indicate that the reduced response of the human TNF promoter seen in LPS-tolerant monocytic Mono Mac 6 cells may be largely due to a reduced activity of the NF-κB transcription factor complex.
FIG. 4.
Promoter activity of the 3×κB construct in naive and LPS-tolerant Mono Mac 6 cells. Mono Mac 6 cells were transfected, cultured for 2 days with or without LPS (20 ng/ml), washed, and stimulated with LPS at 1 μg/ml. RNA and plasmid DNA were isolated; the plasmid DNA was amplified directly (27 cycles), while mRNA was reverse transcribed and amplified by using the 3′ transintron primer together with the 5′ primer (40 cycles). Products were separated on a 1.4% agarose gel (A). Densitometry values for mRNA are expressed as proportions of the plasmid DNA are 0.4, 56.7, 8.4, and 11.1% for lanes 1 to 4. Cell samples from the same experiment were taken for three freeze-thaw cycles, and luciferase enzyme activity was determined in the lysates (B). Values for luciferase were 354, 55,743, 6,022, and 23,208 relative light units for lanes 1 to 4. Data are representative of one experiment of three performed. The average fold induction for mRNA was significantly lower in LPS-stimulated tolerant cells (1.0-fold) compared to LPS-stimulated naive cells (81.8-fold; P < 0.01). The reduction of fold induction at the protein level in tolerant cells was not significant (P > 0.05).
Nuclear NF-κB proteins in LPS tolerance.
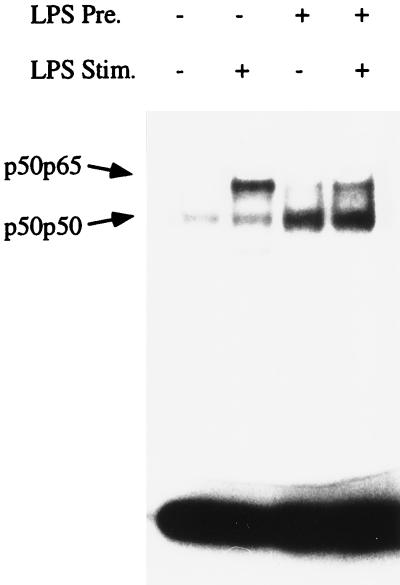
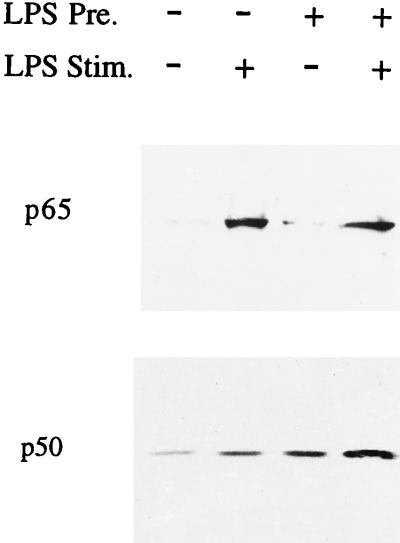
Next we analyzed the DNA binding activity for NF-κB in nuclear extracts of naive and tolerant Mono Mac 6 cells. Similar to what had been reported earlier (45), naive unstimulated cells showed a constitutive high-mobility band (Fig. 5, lane 1); with LPS stimulation, an additional low-mobility band was detected (lane 2). As shown previously, the low-mobility upper band consisted of p50-p65 heterodimers whereas the high-mobility lower band consisted of p50-p50 homodimers. In LPS-stimulated cells (lane 2), the p50-p65 band was clearly stronger (>2-fold) than the p50-p50 band. In LPS-tolerant cells, there was a higher intensity for the p50-p50 band even without secondary stimulation (lane 3). With secondary stimulation there again was mobilization of p50-p65, but p50-p50 predominated, the level being twofold higher than that of p50-p65 (lane 4). These data on DNA binding activity suggest that there is more p50 protein in the nuclei of LPS-tolerant cells. To address this point directly, we analyzed by Western blotting the abundance of p65 and p50 in highly purified nuclei. Figure 6 (upper panel) demonstrates an LPS-induced mobilization of p65 in tolerant cells similar to what is seen in naive cells, which indicates that tolerant cells still respond to LPS stimulation by mobilization of NF-κB. Analysis of p50 revealed some constitutive p50 in naive cells and a twofold increase after LPS stimulation (lower panel, lanes 1 and 2). Cells precultured with LPS contain higher amounts of p50 in the nuclei, and there was a further twofold increase after stimulation with LPS (lower panel, lanes 3 and 4). In an average of three experiments, the intensity of the p50 band in stimulated tolerant cells (lane 4) was threefold higher than in stimulated naive cells (lane 2).
FIG. 5.
Increase of p50 homodimers in tolerant Mono Mac 6 cells. Mono Mac 6 cells were cultured for 2 days with or without LPS (20 ng/ml), washed, and stimulated with LPS at 1 μg/ml. Nuclear extracts were admixed with a double-stranded radiolabeled probe representing the −605 motif of the human TNF promoter; after separation under nondenaturing conditions, the dried polyacrylamide gel was exposed to an X-ray film. The lower band represents p50-p50; the upper band represents p50-p65. Laser densitometry shows that the ratios of p50-p65 to p50-p50 are 2.44 in lane 2 and 0.44 in lane 4. Data are of one experiment of three performed.
FIG. 6.
Increase of p50 protein in nuclei of tolerant Mono Mac 6 cells. Nuclei were generated by Dounce homogenization in the presence of a protease inhibitor cocktail followed by purification over a sucrose gradient. Extracts were separated by gel electrophoresis and blotted on nitrocellulose. Blots were stained with an anti-p65 or anti-p50 antibody and developed by using ECL. In the upper panel densitometry for p65 gave values of 3.0, 41.8, 6.2, and 44.9 for lanes 1 to 4, respectively (one of three experiments). The difference between lanes 3 and 4 was significant (P < 0.05). In the lower panel, densitometry for p50 gave values of 2.6, 6.9, 10.6, and 24.3 arbitrary units for lanes 1 to 4, respectively (one of three experiments). The difference between lanes 2 and 4 was significant (P < 0.05).
Overexpression of p50 blocks the human TNF promoter.
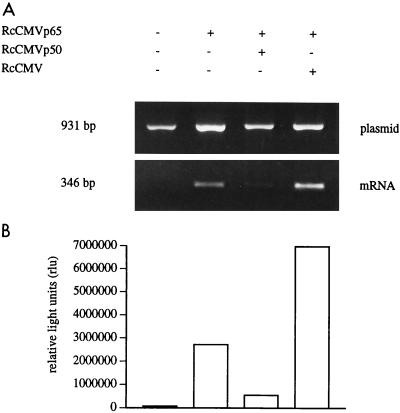
The upregulated p50 protein in nuclear extracts of LPS-tolerant Mono Mac 6 cells suggests that p50 homodimers may bind to DNA and block expression of the TNF gene. We therefore examined whether overexpression of p50 can in fact block transactivation of the TNF promoter-driven luciferase reporter gene. For this, HEK 293 cells were transfected by calcium phosphate coprecipitation with the TNF promoter reporter plasmid plus a cytomegalovirus promoter-driven p65 expression plasmid. Figure 7 demonstrates that p65 can efficiently induce transcription from this construct, leading to high levels of luciferase mRNA and protein (lane 2). Cotransfection of p65 with an empty cytomegalovirus expression plasmid led to some nonspecific enhancement of expression (lane 4); when p65 was cotransfected with a p50 expression plasmid, however, there was a strong suppression of transactivation (lane 3) which on average was 6-fold for mRNA and 11-fold for enzyme activity. These data demonstrate that high levels of p50 can block transactivation of the human TNF promoter.
FIG. 7.
Effect of p50 overexpression on transactivation of the human TNF promoter. HEK 293 cells were calcium phosphate transfected with 0.5 μg of pTNF-1064 luci-β reporter plasmid and, as indicated with 0.25 μg of RcCMVp65 without or together with 2.5 μg of RcCMVp50 or RcCMV empty vector. Luciferase mRNA and enzyme activity were measured after 24 h. Plasmid-corrected mRNA values (A) were 3.0, 46.2, 21.9, and 103.3% for lanes 1 to 4; luciferase enzyme activities (B) were 66,193, 2,708,986, 538,750, and 6,960,601 relative light units for lanes 1 to 4. Data are representative of three and four experiments for mRNA and protein, respectively. The average fold reduction by p50 compared to empty plasmid was 6.1-fold for mRNA and 10.9-fold for enzyme activity (P < 0.01).
DISCUSSION
When monocytes are stimulated with LPS repeatedly, the initially strong response is minimal such that only low amounts of the proinflammatory cytokine TNF are produced. This LPS tolerance can be considered a protective mechanism that prevents damage to the body by avoiding excessive inflammation, for instance, in sepsis patients (5, 23, 32).
LPS tolerance may be regulated at the translational (20) and posttranslational (46) levels, but most studies have demonstrated a decrease in transcripts for TNF in tolerant cells (11, 21, 31). In the present study, we have confirmed that LPS tolerance in Mono Mac 6 cells accompanies a decreased level of TNF protein and mRNA. We now have analyzed in this system the regulation of the human TNF promoter by using a luciferase reporter gene construct that allows for determination of luciferase mRNA (15).
The approach of looking at mRNA rather then at the enzyme activity of a reporter protein is advantagous since it directly analyzes the transcription product independent of regulatory effects that may influence luciferase protein activity during or after translation. In fact, in some instances (e.g., Fig. 4) the analysis of mRNA gave a clearer pattern of results.
In Mono Mac 6 cells transfected with such constructs, transactivation can occur at low levels of LPS. For the pTNF-1064 luci-β reporter plasmid, for instance, 1 ng of LPS/ml will give a good response, but in some experiments responses were noted at even the lower level of 10 pg/ml. Also, when the 3×κB.luc construct was used, LPS levels of 10 to 100 pg/ml induced significant reporter gene activity (data not shown).
These data on behavior of the TNF promoter in LPS tolerance are consistent with earlier work (45). Much of the activity of the TNF promoter is controlled by the transcription factor NF-κB (27, 30, 42). For the human promoter, sites at −605 and −97 have been implicated (30, 36, 42). To further analyze a possible contribution of NF-κB to LPS tolerance, we have analyzed the activity of an artificial construct that contains a trimeric NF-κB motif (15). In tolerant cells this construct showed strongly reduced LPS-induced transactivation (Fig. 4), further indicating that LPS tolerance goes along with a reduced transactivating activity of the NF-κB complex.
The human TNF promoter is controlled by additional transcription factors such as C/EBP (25, 32), but we found no change in DNA binding activity for C/EBP in LPS-tolerant Mono Mac 6 cells (unpublished data). In earlier work, we noted that LPS-tolerant Mono Mac 6 cells upregulate p50 of NF-κB such that there is a predominance of p50 homodimers (45). The same phenomenon has been demonstrated for primary human monocytes (39) and in the mouse macrophage cell line P388 (41). In gel shift analyses, these p50 homodimers are detectable only when a DNA binding motif with three cytosines at the 3′ end, leading to a DNA sequence that is partially palindromic, is used (8). In the present study, we extend the finding of upregulated p50 by demonstrating with Western blotting a threefold increase of p50 protein in nuclei of tolerant cells compared to naive cells.
The p50 NF-κB protein lacks a transactivation domain, and p50-p50 homodimers when bound to DNA cannot transactivate but instead block access of transactivating complexes like p50-p65. This will then result in a blockade of gene expression. Consistent with this scenario overexpression of p50 has been shown to downregulate the human immunodeficiency virus long terminal repeat and the IL-2 promoter (9, 14). As shown in the present report, overexpression of p50 in HEK 293 cells results in a strong reduction of transactivation of the pTNF-1064 luci-β reporter plasmid (Fig. 7). These data demonstrate for the first time that overexpression of p50 can, in fact, block activity of the human TNF promoter. The results support our concept that the upregulated p50 may be instrumental in LPS tolerance.
This conclusion is consistent with findings of Goldring et al., who analyzed the murine inducible nitric oxide synthase promoter by in vivo footprinting (10). In these analyses, the NF-κB sites were found occupied in LPS-tolerized murine macrophages. The LPS-tolerized cells also show a strong increase of a p50-p50 complex without secondary stimulation, suggesting that these homodimers occupy the NF-κB sites of the inducible nitric oxide synthase gene. The data suggest that upon secondary stimulation, the homodimers prevent access of p65-containing heterodimers and thereby block transactivation.
With respect to stimulation by superantigen and by TNF, an upregulation of p50 of NF-κB has been noted in other cellular systems of tolerance (18, 28), suggesting that the mechanism of p50-mediated blockade of gene expression may have a more general importance. On the other hand, LPS tolerance in other systems involving brief periods of primary LPS exposure may be due to a blockade that is located further upstream in the signalling cascade such that elements like IκB kinases are not activated at all (16). Also, it has been reported that in LPS-tolerant rat cells, all of the NF-κB mobilization is blocked because of a depletion of p50 and p65 in the cytosol (1). Still, data on LPS tolerance in rat macrophages may not necessarily translate directly into the human system. In the rat, G proteins are altered in LPS tolerance (2), while no such alterations are detected in human cells (4).
Consistent with our concept, LaRue and McCall (19) noted that in LPS-tolerant human monocytic cells IκB degradation still occurred, suggesting that NF-κB can still be mobilized. While there are several mechanisms that may control TNF gene expression in LPS tolerance, previous studies and the present report demonstrate a role for upregulated p50 in blocking TNF transcription.
ACKNOWLEDGMENTS
This work was supported by grants from Fritz Thyssen Stiftung, from Deutsche Forschungsgemeinschaft (SFB 217 and ZI 288/1-1), and from the VerUm Stiftung.
We acknowledge the expert technical assistance of G. Sulski and I. Petersmann and the generous provision of antibodies by Nancy Rice.
REFERENCES
- 1.Blackwell T S, Blackwell T R, Christman J W. Induction of endotoxin tolerance depletes nuclear factor-κB and suppresses its activation in rat alveolar macrophages. J Leukoc Biol. 1997;62:885–891. doi: 10.1002/jlb.62.6.885. [DOI] [PubMed] [Google Scholar]
- 2.Coffee K A, Halushka P V, Ashton S H, Tempel G E, Wise W C, Cook J A. Endotoxin tolerance is associated with altered GTP-binding protein function. J Appl Physiol. 1992;73:1008–1013. doi: 10.1152/jappl.1992.73.3.1008. [DOI] [PubMed] [Google Scholar]
- 3.Dignam J D, Lebovitz R M, Roeder R G. Accurate transcription initiation by RNA polymerase II in a soluble extract from isolated mammalian nuclei. Nucleic Acids Res. 1983;11:1475–1489. doi: 10.1093/nar/11.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Durando M, Ashton S H, Makhlouf M A, Simmons-Wagner R, Halushka P V, Cook J A. Endotoxin-induced desensitization of THP-1 cells is not associated with altered G protein binding or content. J Endotoxin Res. 1997;4:97–103. [Google Scholar]
- 5.Ertel W, Kremer J-P, Kenney J, Steckholzer U, Jarrar D, Trentz O, Schildberg F W. Downregulation of proinflammatory cytokine release in whole blood from septic patients. Blood. 1995;85:1341–1347. [PubMed] [Google Scholar]
- 6.Frankenberger M, Pechumer H, Ziegler-Heitbrock H W L. Interleukin-10 is upregulated in LPS tolerance. J Inflamm. 1995;45:56–63. [PubMed] [Google Scholar]
- 7.Frankenberger M, Pforte A, Sternsdorf T, Passlick B, Baeuerle P A, Ziegler-Heitbrock H W L. Constitutive nuclear NF-κB in cells of the monocyte lineage. Biochem J. 1994;304:87–94. doi: 10.1042/bj3040087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frankenberger M, Ziegler-Heitbrock H W L. LPS tolerance in monocyte/macrophages: three 3′ cytospins are required in the DNA binding motif for detection of upregulated NF-κB p50 homodimers. Immunobiology. 1997;198:81–90. doi: 10.1016/s0171-2985(97)80029-0. [DOI] [PubMed] [Google Scholar]
- 9.Franzoso G, Bours V, Park S, Tomita-Yamaguchi M, Kelly K, Siebenlist U. The candidate oncoprotein Bcl-3 is an antagonist of p50/NF-κB-mediated inhibition. Nature. 1992;359:339–342. doi: 10.1038/359339a0. [DOI] [PubMed] [Google Scholar]
- 10.Goldring C E, Reveneau S, Pinard D, Jeannin J F. Hyporesponsiveness to lipopolysaccharide alters the composition of NF-kappaB binding to the regulatory regions of inducible nitric oxide synthase gene. Eur J Immunol. 1998;28:2960–2970. doi: 10.1002/(SICI)1521-4141(199809)28:09<2960::AID-IMMU2960>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 11.Haas J G, Bäuerle P A, Riethmüller G, Ziegler-Heitbrock H W L. Molecular mechanisms in down-regulation of tumor necrosis factor expression. Proc Natl Acad Sci USA. 1990;87:9563–9567. doi: 10.1073/pnas.87.24.9563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hausdorff W P, Caron M G, Lefkowitz R J. Turning off the signal: desensitization of β-adrenergic receptor function. FASEB J. 1990;4:2881–2889. [PubMed] [Google Scholar]
- 13.Hirohashi N, Morrison D C. Low-dose lipopolysaccharide (LPS) pretreatment of mouse macrophages modulates LPS-dependent interleukin-6 production in vitro. Infect Immun. 1996;64:1011–1015. doi: 10.1128/iai.64.3.1011-1015.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kang S M, Tran A C, Grilli M, Lenardo M J. NF-κB subunit regulation in nontransformed CD4+ T lymphocytes. Science. 1992;256:1452–1456. doi: 10.1126/science.1604322. [DOI] [PubMed] [Google Scholar]
- 15.Kastenbauer S, Wedel A, Frankenberger M, Wirth T, Ziegler-Heitbrock H W L. Analysis of promoter activity by polymerase chain reaction amplification of reporter gene mRNA. Anal Biochem. 1996;233:137–139. doi: 10.1006/abio.1996.0018. [DOI] [PubMed] [Google Scholar]
- 16.Kohler N G, Joly A. The involvement of an LPS inducible IκB kinase in endotoxin tolerance. Biochem Biophy Res Commun. 1997;232:602–607. doi: 10.1006/bbrc.1997.6337. [DOI] [PubMed] [Google Scholar]
- 17.Labeta M O, Durieux J-J, Spagnoli G, Fernandez N, Wijdenes J, Herrmann R. CD14 and tolerance to lipopolysaccharide: biochemical and functional analysis. Immunology. 1993;80:415–423. [PMC free article] [PubMed] [Google Scholar]
- 18.Lægreid A, Thommesen L, Gullstein Jahr T, Sundan A, Espevik T. Tumor necrosis factor induces lipopolysaccharide tolerance in a human adenocarcinoma cell line mainly through the TNF p55 receptor. J Biol Chem. 1995;270:25418–25425. doi: 10.1074/jbc.270.43.25418. [DOI] [PubMed] [Google Scholar]
- 19.LaRue K E A, McCall C E. A labile transcriptional repressor modulates endotoxin tolerance. J Exp Med. 1994;180:2269–2275. doi: 10.1084/jem.180.6.2269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marchant A, Gueydan C, Houzet L, Amraoui Z, Sels A, Huez G, Goldman M, Kruys V. Defective translation of tumor necrosis factor mRNA in lipopolysaccharide-tolerant macrophages. J Inflamm. 1996;46:114–123. [PubMed] [Google Scholar]
- 21.Mathison J C, Virca G D, Wolfson E, Tobias P S, Glaser K, Ulevitch R J. Adaptation to bacterial lipopolysaccharide controls lipopolysaccharide-induced tumor necrosis factor production in rabbit macrophages. J Clin Investig. 1990;85:1108–1118. doi: 10.1172/JCI114542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mathison J, Wolfson E, Steinemann S, Tobias P, Ulevitch R. Lipopolysaccharide (LPS) recognition in macrophages. J Clin Investig. 1993;92:2053–2059. doi: 10.1172/JCI116801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Munoz C, Carlet J, Fitting C, Bisset B, Blériot J-P, Cavaillon J-M. Dysregulation of in vitro cytokine production by monocytes during sepsis. J Clin Investig. 1991;88:1747–1754. doi: 10.1172/JCI115493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pechumer H, Bender-Götze C, Ziegler-Heitbrock H W L. Detection of neuron specific gamma enolase (NSE) mRNA in normal human leukocytes by PCR with nested primers. Lab Investig. 1993;69:743–749. [PubMed] [Google Scholar]
- 25.Pope R M, Leutz A, Ness S A. C/EBP beta regulation of the tumor necrosis factor alpha gene. J Clin Investig. 1994;94:1449–1455. doi: 10.1172/JCI117482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schmitz M L, Bäuerle P A. The p65 subunit is responsible for the strong transcription activating potential of NF-κB. EMBO J. 1991;10:3805–3817. doi: 10.1002/j.1460-2075.1991.tb04950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shakov A N, Collart M A, Vassalli P, Nedospasov S A, Jongeneel C V. κB-type enhancers are involved in LPS-mediated transcriptional activation of the TNF-α gene in primary macrophages. J Exp Med. 1990;171:35–47. doi: 10.1084/jem.171.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sundstedt A, Sigvardsson M, Leanderson T, Hedlund G, Kalland T, Dohlsten M. In vivo anergized CD4+ T cells express perturbed AP-1 and NF-κB transcription factors. Proc Natl Acad Sci USA. 1996;93:979–984. doi: 10.1073/pnas.93.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Surette M E, Dallaire N, Jean N, Picard S, Borgeat P. Mechanisms of the priming effect of lipopolysaccharides on the biosynthesis of leukotriene B4 in chemotactic peptide-stimulated human neutrophils. FASEB J. 1998;12:1521–1531. doi: 10.1096/fasebj.12.14.1521. [DOI] [PubMed] [Google Scholar]
- 30.Trede N S, Tsytsykova A V, Chatila T, Goldfeld A E, Geha R S. Transcriptional activation of the human TNF-α promoter by superantigen in human monocytic cells: role of NF-κB. J Immunol. 1995;155:902–908. [PubMed] [Google Scholar]
- 31.Virca G D, Kim S Y, Glaser K B, Ulevitch R J. Lipopolysaccharide induces hyporesponsiveness to its own action in RAW 264.7 cells. J Biol Chem. 1989;264:21951–21956. [PubMed] [Google Scholar]
- 32.Wedel A, Sulski G, Ziegler-Heitbrock H W L. CCAAT/enhancer binding protein is involved in the expression of the tumor necrosis factor gene in human monocytes. Cytokine. 1996;8:335–341. doi: 10.1006/cyto.1996.0046. [DOI] [PubMed] [Google Scholar]
- 33.Wilson C S, Seatter S C, Rodriguez J L, Bellingham J, Clair L, West M A. In vivo endotoxin tolerance: impaired LPS-stimulated TNF release of monocytes from patients with sepsis, but not SIRS. J Surg Res. 1997;69:101–106. doi: 10.1006/jsre.1997.5040. [DOI] [PubMed] [Google Scholar]
- 34.Worthen G S, Seccombe J F, Clay K L, Guthrie L A, Johnston R. The priming of neutrophils by lipopolysaccharide for production of intracellular platelet-activating factor. Potential role in mediation of enhanced superoxide secretion. J Immunol. 1988;140:3553–3559. [PubMed] [Google Scholar]
- 35.Wright S D, Ramos R A, Robias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 36.Yao J, Mackman N, Edgington T S, Fan S-T. Lipopolysaccharide induction of the tumor necrosis factor-α promoter in human monocytic cells. J Biol Chem. 1997;272:17795–17801. doi: 10.1074/jbc.272.28.17795. [DOI] [PubMed] [Google Scholar]
- 37.Ziegler-Heitbrock H W L. Molecular mechanism in tolerance to lipopolysaccharide. J Inflamm. 1995;45:13–26. [PubMed] [Google Scholar]
- 38.Ziegler-Heitbrock H W L, Burger R. Rapid removal of mycoplasma from cell lines mediated by a direct effect of complement. Exp Cell Res. 1987;173:388–394. doi: 10.1016/0014-4827(87)90279-5. [DOI] [PubMed] [Google Scholar]
- 39.Ziegler-Heitbrock H W L, Frankenberger M, Wedel A. Tolerance to lipopolysaccharide in human blood monocytes. Immunobiology. 1995;193:217–223. doi: 10.1016/s0171-2985(11)80546-2. [DOI] [PubMed] [Google Scholar]
- 40.Ziegler-Heitbrock H W L, Käfferlein E, Haas J G, Meyer N, Ströbel M, Weber C, Flieger D. Gangliosides suppress tumor necrosis factor production in human monocytes. J Immunol. 1992;148:1753–1758. [PubMed] [Google Scholar]
- 41.Ziegler-Heitbrock H W L, Petersmann I, Frankenberger M. p50 (NF-κB1) is upregulated in LPS tolerant P388D1 murine macrophages. Immunobiology. 1997;198:73–80. doi: 10.1016/s0171-2985(97)80028-9. [DOI] [PubMed] [Google Scholar]
- 42.Ziegler-Heitbrock H W L, Sternsdorf T, Liese J, Belohradsky B, Weber C, Wedel A, Schreck R, Bäuerle P, Ströbel M. Pyrrolidine dithiocarbamate inhibits NF-κB mobilization and TNF production in human monocytes. J Immunol. 1993;151:6986–6993. [PubMed] [Google Scholar]
- 43.Ziegler-Heitbrock H W L, Thiel E, Fütterer A, Herzog V, Wirtz A, Riethmüller G. Establishment of a human cell line (Mono Mac 6) with characteristics of mature monocytes. Int J Cancer. 1988;41:456–461. doi: 10.1002/ijc.2910410324. [DOI] [PubMed] [Google Scholar]
- 44.Ziegler-Heitbrock H W L, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]
- 45.Ziegler-Heitbrock H W L, Wedel A, Schraut W, Ströbel M, Wendelgass P, Sternsdorf T, Bäuerle P, Haas J G, Riethmüller G. Tolerance to lipopolysaccharide involves mobilization of nuclear factor κB with predominance of p50 homodimers. J Biol Chem. 1994;269:17001–17004. [PubMed] [Google Scholar]
- 46.Zuckerman S H, Evans G F, Snyder Y M, Roeder W D. Endotoxin-macrophage interaction: post-translational regulation of tumor necrosis factor expression. J Immunol. 1989;143:1223–1227. [PubMed] [Google Scholar]