Abstract
Background
COVID-19 vaccine distribution is at risk of further propagating the inequities of COVID-19, which in the United States (US) has disproportionately impacted the elderly, people of color, and the medically vulnerable. We sought to measure if the disparities seen in the geographic distribution of other COVID-19 healthcare resources were also present during the initial rollout of the COVID-19 vaccine.
Methods
Using a comprehensive COVID-19 vaccine database (VaccineFinder), we built an empirically parameterized spatial model of access to essential resources that incorporated vaccine supply, time-willing-to-travel for vaccination, and previous vaccination across the US. We then identified vaccine deserts—US Census tracts with localized, geographic barriers to vaccine-associated herd immunity. We link our model results with Census data and two high-resolution surveys to understand the distribution and determinates of spatially accessibility to the COVID-19 vaccine.
Results
We find that in early 2021, vaccine deserts were home to over 30 million people, >10% of the US population. Vaccine deserts were concentrated in rural locations and communities with a higher percentage of medically vulnerable populations. We also find that in locations of similar urbanicity, early vaccination distribution disadvantaged neighborhoods with more people of color and older aged residents.
Conclusion
Given sufficient vaccine supply, data-driven vaccine distribution to vaccine deserts may improve immunization rates and help control COVID-19.
Subject terms: Health services, Public health, Epidemiology, Disease prevention, Infectious diseases
Plain language summary
COVID-19 has affected the elderly, people of color, and individuals with chronic illnesses more than the general population. Large barriers to accessing the COVID-19 vaccine could make this problem worse. We used a website called VaccineFinder, which has information on the location of most COVID-19 vaccine doses in the US, to measure vaccine accessibility in early 2021. We then identified vaccine deserts, defined as small US regions with poor access to the COVID-19 vaccine. We found that over 10% of the US lived in a vaccine desert. Overall, we found that vaccines were less available to people in rural areas, people of color, and individuals with chronic illnesses. It will be important to reverse this pattern and ensure enough vaccines are sent to these communities to help reduce the spread of COVID-19.
Rader et al. model spatial accessibility to COVID-19 vaccines in the US, identifying vaccine deserts where access was insufficient during the first phase of the rollout. Rural areas and medically vulnerable populations are found to have poorer access to vaccines, as are areas with higher proportions of Black, Hispanic and older residents.
Introduction
Geographic access to medical resources is an essential component to maximizing the benefits of healthcare1. Individuals who must travel longer to access services are less likely to receive routine, acute, and pandemic-related care2–4. To achieve an equitable distribution of resources, spatial barriers should be minimized for all communities and access to care should be ensured for those who need it the most1. This is especially true for the distribution of the COVID-19 vaccine in the United States (US) given the notable disparities in the virus’s impact5. A disproportionate burden of COVID-19 morbidity and mortality has been experienced among individuals with a lower median income and Black and Hispanic persons6–8. These differences are associated with significant inequities in the ability to physically distance9,10, burden of co-morbidities7,11, percent of people in essential worker roles7,9,12, and systemic disparities in the likelihood to die without receiving care13. Further, reduced geographic access to SARS-CoV-2 testing in rural areas, areas with a higher percentage of non-White persons, and areas with a large percentage of uninsured individuals suggests that the disproportionate impact of the virus is likely understated14.
The introduction of multiple vaccines creates the potential to reduce the burden of COVID-19 in the US15. Mitigating disparities and ensuring a fair and equitable distribution of the COVID-19 vaccine are guiding principles of the Centers for Disease Control and Prevention (CDC) distribution recommendations16. However, an ideal distribution of the vaccine may be difficult to achieve because it relies on current medical supply chains and distribution locations, which are efficient but demographically biased14. The need to vaccinate large numbers of individuals at a highly elevated risk for COVID-19 (e.g., healthcare workers and long-term care residents and staff)17 led to initial allocation in most states being focused on hospitals and academic medical centers with supply later being expanded to other clinics and pharmacies. However, these facilities have been shown to be unequally distributed throughout the US and less geographically accessible to rural populations18–21. Reliance on these nodes for distribution of the COVID-19 vaccine can lead to vaccine deserts, where local supply is not sufficiently accessible or available.
Studies in Chicago, Baltimore and Los Angeles have shown a higher prevalence of low-access pharmacy deserts in minority communities and neighborhoods with higher rates of infectious disease19,22,23 while a state-wide study in Pennsylvania showed that pharmacy deserts tend to be in rural communities20. Scenario modeling suggests these pharmacy disparities may result in unequal distribution of the COVID-19 vaccine24, but to our knowledge no studies have measured geographic accessibility to confirmed vaccine locations or attempted to account for differences in vaccine supply. In addition, traditional definitions of resource deserts (e.g., food within 10 miles25), are insufficient to characterize the unique goals of rapid, population-wide immunization. To quantify accessibility of the COVID-19 vaccine, we utilize a unique comprehensive dataset of COVID-19 vaccine providers in the US and employ the enhanced two-step floating catchment area (E2SFCA) method18, a technique that has been used to study accessibility to various health resources including primary care physicians18, pharmacies26, and COVID-19 healthcare27. This flexible spatial model simultaneously incorporates supply, demand, travel time, and between-census-tract travel.
In this analysis, we seek to understand if COVID-19 vaccine doses were optimally allocated to unvaccinated individuals in communities that are most vulnerable to the virus during the early vaccine rollout. To accomplish this, vaccine distribution sites and vaccine supply quantities in the contiguous US states were identified in early 2021 from a comprehensive national database (VaccineFinder). Utilizing the E2SFCA method, we calculated a spatial accessibility score for each US Census tract, which was the outcome of interest. To connect the relationship of spatial accessibility to disease transmission, we define vaccine deserts with a novel modification of resource desert that comports with COVID-19 specific public health goals. Utilizing US Census demographic data and a high-resolution survey of 16.5 million Facebook users28–30, we find that early vaccine distribution resulted in vaccine deserts in rural locations and communities with medically vulnerable populations. To identify if disparities in spatial accessibility to the COVID-19 vaccine exist within communities of similar urbanicity, we apply spatial regression modeling. Our regression findings suggest early vaccination disadvantaged neighborhoods with more Black, Hispanic, and older aged residents. To understand this further, we explore a case-series of two highly racially segregated US cities: Detroit, Michigan and Chicago, Illinois. Lastly, we model potential vaccine deserts under conditions where supply is no longer constrained to understand relative contributions of supply shortages to vaccine deserts.
Methods
Demographic and population data
We gathered demographic and cartographic data from the US Census 2015-2019 American Community Survey31 for each census tract in the contiguous US with a non-zero population count (n = 72,042, Alaska and Hawaii excluded due to the unique geographic challenges). We calculated population density (people per square kilometer) and the proportional breakdowns by race, sex, median resident age, median income, health insurance type, and internet access. We gathered information about urbanicity (e.g., metropolitan, rural) from the US Department of Agriculture Rural-Urban Commuting Area (RUCA) codes32 and population counts from the Gridded Population of the World (v4) dataset33. Vaccination rates on February 16, 2021 for each US county were obtained from the National Center for Immunization and Respiratory Diseases34.
COVID-19 and medical burden data
We estimated the COVID-19 and medical burden for each census tract using data from the Delphi Group at Carnegie Mellon University U.S. COVID-19 Trends and Impact Survey (N = 16,533,319) conducted via the Facebook platform (April 6, 2020–February 11, 2021)28,29. This survey was chosen because its large sample size allowed for exploring fine spatial resolutions. Participants in this survey were recruited from clicking a banner on their Facebook newsfeed. The survey was hosted on Qualtrics and adults 18 years and older were surveyed after providing explicit informed consent30.
Medical burden was defined as the self-reported proportion of respondents with at least one pre-existing condition that could exacerbate COVID-19 severity (Supplementary Table). COVID-19 burden was defined as the proportion of surveys responding “yes” to the question “in the past 24 h, have you had direct contact with anyone who recently tested positive for COVID-19 (coronavirus)” for each week, which closely mirror government case counts in the US35,36, although the latter are only available at county-level resolutions (Supplementary Fig. 1).
COVID-19 vaccine locations and supply data
The location and supply of publicly accessible COVID-19 vaccine administration sites in the contiguous US were gathered from VaccineFinder, a comprehensive, national vaccine system maintained by Boston Children’s Hospital in collaboration with the CDC and Castlight Health. VaccineFinder receives registration information and daily reports of on-hand vaccine supply for each COVID-19 vaccine administration site from the CDC’s Vaccine Tracking System (VTrckS), the ordering platform for entities receiving COVID-19 vaccines37. VTrckS is the most comprehensive vaccine supply source because all 64 public health jurisdictions and pharmacies participating in the FRPP and receiving direct allocation from the federal government are enrolled38. All vaccine distribution sites are collected in the database, however each location can choose to display their location, vaccine availability, and/or appointment resources to the public on the web-based search tool (www.VaccineFinder.org).
We used all vaccine distribution sites (N = 37,287) that administered one or more COVID-19 vaccine dose on at least three separate days between February 17, 2021–March 16, 2021 (doses administered was imputed from supply levels, Supplementary Methods under dose administration days). The overall dose count for each site was determined by calculating the mean daily doses administered following each site’s receipt of its first reported dose. U.S. Department of Veteran Affairs (VA) locations (extracted from their publicly available database39) dispensing the vaccine were also included (N = 139). The supply levels for VA vaccine distribution sites were determined by the daily reported difference (February 19, 2021–March 17, 2021) in cumulative doses administered as reported on February 20, 2021.
Spatial accessibility outcome
We calculated spatial accessibility of vaccine supply using the E2SFCA18. Scores were undefined for a small number (n = 23) of census tracts, resulting in estimates for the majority (n = 72,019) of the populated tracts in the contiguous US. The first step of E2SFCA calculates the vaccine supply-to-population ratio (Rj) for each vaccine distribution site (j) based on the mean daily supply (Sj) of a site and the unvaccinated population (P) of its catchment area (k ∈ {dkj ≤ T}) (Supplementary Fig. 2, Step 1). A catchment area is defined as the set of all grid squares (k) where the distance (d) between that grid square and the vaccine distribution site is less than or equal to a threshold, T. Daily supply levels were multiplied by 248 to represent the total doses over the aspirational roll out period (January 1–September 6), assuming the current modeled supply was constant over time. September 6 (Labor Day) was chosen to coincide with the expiration of the majority of COVID-19 related unemployment benefits provided by the American Rescue Plan Act of 202140. These assumptions were chosen to reflect circumstances at the time of analysis. The model used here has the flexibility to incorporate alternative assumptions in the presence of expected change (increases in vaccine supply, etc.).
The catchment populations were calculated using unvaccinated population counts and weighted by the travel-time decay function (Wd) based on willingness to travel to receive the vaccine (see below for more details). Unvaccinated population counts and locations were calculated from the 1 km2 resolution gridded data33,34. The population count for each grid square was adjusted to only include the percentage unvaccinated by multiplying the grid total by the percentage unvaccinated (from the county the grid square is in) plus ½ the percentage partially vaccinated. For computational efficiency, we set T equal to 90 min.
| 1 |
The second step of the E2SFCA (Supplementary Fig. 2, Step 2) calculated the spatial accessibility value (A) for each census tract (i) by summing all the travel-time-weighted supply-to-population ratios (Rj Wd) for each vaccine distribution site within its own catchment area. Each census tract is represented by its population-weighted centroid41. As in the first step, the catchment area is delineated by a travel threshold around each census tract and the supply-to-population ratio is weighted by the travel-time decay function.
| 2 |
Travel time
Travel time (d) was determined by utilizing an impedance map of the quickest transversal time (e.g., car, public transportation, boat, etc.) across the gridded population surface of the US42–44. For each vaccine distribution location, travel time from all grid squares within 90 min were measured by calculating the lowest accumulated travel time across a friction surface between the vaccine location and the grid square. This calculation was made using Dijkstra’s algorithm45 which calculates the fastest total travel time between a source and destination point by tracing the fastest travel time between each pair of neighboring intervening points and optimizing the fastest cumulative path across these pairs. The same procedure was used to determine the catchment area for each census tract by measuring the travel time between each grid square and population-weighted centroid of the census track.
Time willing to travel to vaccine and travel-time decay function
To empirically define the time unvaccinated individuals would be willing to travel for the COVID-19 vaccine, we included travel time questions within a large-scale, nationally-representative, previously-validated46 web survey from OutbreaksNearMe (ONM) via the SurveyMonkey platform (survey fielded February 9–February 17, 2021, see reference47 for full survey language and Supplementary Table 2 for survey demographics). The proportion of respondents previously vaccinated for COVID-19 was calculated using survey weights. We then asked all unvaccinated survey participants: “What’s the most time you would be willing to travel to receive a COVID-19 vaccine? (please enter a time in minutes).” To ensure comparability to other health resources, we also asked “What’s the most time you would be willing to travel for a routine medical appointment? (please enter a time in minutes).” Responses were limited to 1–90 min (T in the spatial accessibility model). We received 23,288 responses among those (N = 26,466, 88.0%) presented the question. Travel times from survey respondents who indicated they were considering COVID-19 vaccination (e.g., responded “yes” or “not sure” when asked if they would get the vaccine) were used to define our travel-time decay function48,49 (W, Fig. 1, left, plotted as gray line) via a monotonic cubic spline fit to 1 minus the empirical cumulative distribution of these responses. The resulting spline50 approximates a continuous travel-time delay function (such as the commonly used Gaussian function51) but captures the different behavioral response to time benchmarks (e.g., perceived difference between 59 min and 61 min).
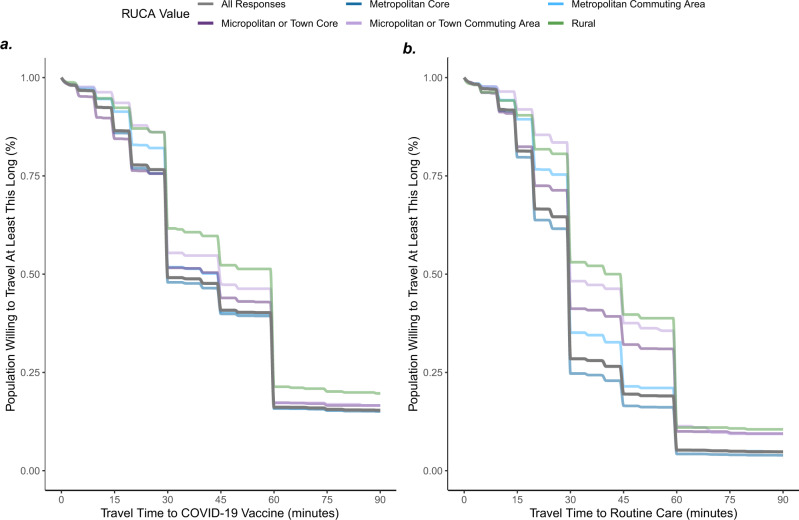
Fig. 1. Time willing to travel to COVID-19 vaccine compared to routine healthcare.
Responses (N = 23,288) from OutbreaksNearMe US national web survey on how long people were willing to travel to COVID-19 vaccine (a) compared to routine care (b). Lines are monotonic cubic splines fit to 1 minus the empirical cumulative distribution of these response times. Responses were limited to individuals considering a vaccine, defined as those who reported either “yes” or “not sure” when asked if they would get the vaccine. Results are broken down by U.S. Department of Agriculture Rural-Urban Commuting Area (RUCA) codes of the respondent’s self-reported zip code. Overall distribution of travel time to COVID-19 vaccine (a gray) was used as the travel-time decay function in E2SFCA model.
Definition of vaccine desert
Access deserts for essential resources (e.g., food, pharmacy) are typically defined in terms of a resource lacking within a specific travel-time or distance19,23,25. However, these definitions are restrictive given the lack of consideration of potential supply and our empirical analysis of the long times individuals will travel for a vaccine. Therefore, we used the E2SFCA accessibility score to incorporate supply and travel time into the vaccine distribution scheme model: assuming the vaccine sites available to each census tract have a constant supply for the 8-month period (January–September 6, 2021), each tract would have a travel-time weighted sum of Ai doses per person in their respective weighted catchment areas. Access strata therefore broadly represent cutoffs suggesting difficulty in reaching herd immunity (modeled at 85% based on variants circulating at time of analysis, assumptions detailed in Supplementary Methods under herd immunity estimates) in 8-months with one dose (vaccine desert, Ai < 0.85), immunizing all with one dose (low, 0.85 ≤ Ai < 1.0), difficulty immunizing all with two doses (medium, 1.0 ≤ Ai < 2.0), and sufficient accessibility (high, Ai ≥ 2.0). Note these thresholds are based on assumptions of the reproductive number, vaccine effectiveness, desired time to achieve herd immunity, a constant supply, and vaccines available and variants circulating at the time of analysis. Though a simplification, the model allows flexibility to relax these assumptions as additional data become available or if other public health priorities are selected. We have conducted a sensitivity analysis varying the accessibility score cutoff to 0.75 (based on lower herd immunity) and 0.95 (based on higher herd immunity) to assess this assumption.
Spatial regression model
Based on the known distribution of health resources52, we hypothesized that vaccine distribution would be a mixture of consolidated vaccine sites (e.g., medical centers) and intentionally spaced locations to capture markets (e.g., pharmacies, rural health clinics). This may create spatial autocorrelation and spillover such that the location of one vaccine distribution site (and resulting accessibility scores) may be dependent on the placement of sites around them. The E2SFCA also allows individuals to access sites across census boundaries, potentially amplifying correlation in accessibility scores when census tracts rely on overlapping distribution points. To quantify the determinants of accessibility accounting for autocorrelation of accessibility scores in neighboring census tracts and urban/rural confounding, we fit a spatial lag model:53,54
| 3 |
where the term g QAi represents the coefficient (g) and spatially lagged standardized accessibility score (QAi) from a weighted matrix (queen contiguity) of neighboring census tracts. Sensitivity analyses of k-nearest (k = 4) neighbor inverse distance weighted matrix was performed. We modeled the log of population density and multiple other predictor variables (X) for each census tract (i). The models include six basic multivariate models (urbanicity measured by RUCA code and population density in addition to a single demographic or disease burden metric) and one full multivariate model (all modeled variables detailed in Table 2). We then used Monte Carlo simulation to quantify the resulting direct impact55, to produce a coefficient similar to Ordinary Least Squares regression and irrespective of spillover effects to neighboring census tracts. Census tracts with incomplete demographic data (N = 390, 0.5%) and outlier (see Supplementary Methods under census tract outliers) accessibility scores (N = 1699, 2.3%) were excluded from regression analysis.
Flooded supply simulation
To understand vaccine deserts that may persist when vaccine distribution is abundant, we re-run our E2SFCA catchment area model setting the supply-to-population ratio (Rj) to 2. This implies all sites have enough vaccine to theoretically serve every person in their catchment area.
Calculations were performed in R version 3.6.2. The ONM survey was approved by the Boston Children’s Hospital Institutional Review Board (IRB-P00023700) and received a waiver of informed consent. The Delphi Group survey protocol required informed consent, collected via a Qualtrics survey platform. It was approved by the Carnegie Mellon University IRB (STUDY2020_00000162). This activity was reviewed by CDC and was conducted consistent with applicable federal law and CDC policy.§
§See e.g., 45 C.F.R. part 46, 21 C.F.R. part 56; 42 U.S.C.
§241(d); 5 U.S.C.
§552a; 44 U.S.C.
§3501 et seq.
Reporting summary
Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Results
Time willing to travel among those considering vaccination
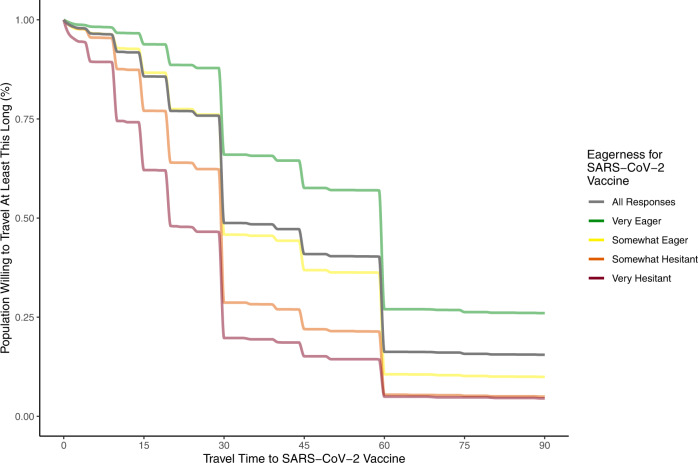
In the ONM survey47, 19% of survey respondents reported having received at least one dose of COVID-19 vaccine. The majority of ONM survey respondents (81%) were unvaccinated and, of these, most (N = 23,288) indicated “yes” (61.2%) or “not sure” (21.2%) when asked if they wanted the COVID-19 vaccine. These individuals considering the vaccine indicated they were willing to travel a median of 44.0 [IQR: 30.0–60.0] minutes to receive a COVID-19 vaccine (Fig. 1a) versus 33.9 [IQR: 20–45] to receive routine medical care (Fig. 1b). Respondents were on average willing to travel 10.6 min (paired two-sided t-test 95% CI: 10.2–10.9) longer to get a vaccine compared to routine care. Notably, while there was a substantial gap (12.1 min, two-sided t-test 95% CI: 9.9–14.3) between time willing to travel to routine care along an urban-rural divide (31.9 min for individuals in urban zip codes versus 44.0 min mean time in rural zip codes) this difference was reduced (6.8 min, two sided t-test 95% CI: 4.3–9.3) for travel to the COVID-19 vaccine. Willingness to travel was more strongly related to vaccine hesitancy (Fig. 2) than the urban-rural divide, with individuals who were “very eager” to get the vaccine willing to travel a mean of 27.2 min longer (95% CI: 25.9–28.5, p < 0.001) than those who were “very hesitant.”
Fig. 2. Population willingness to travel to COVID-19 vaccine by vaccine hesitancy.
Responses (N = 23,288) to OutbreaksNearMe U.S. national web survey on how long people were willing to travel for the COVID-19 vaccine stratified by self-reported vaccine hesitancy. Lines are monotonic cubic splines fit to 1 minus the empirical cumulative distribution of these response times. Responses were limited to individuals considering a vaccine, defined as those who reported either “yes” or “not sure” when asked if they would get the vaccine.
Geographic distribution of vaccine deserts
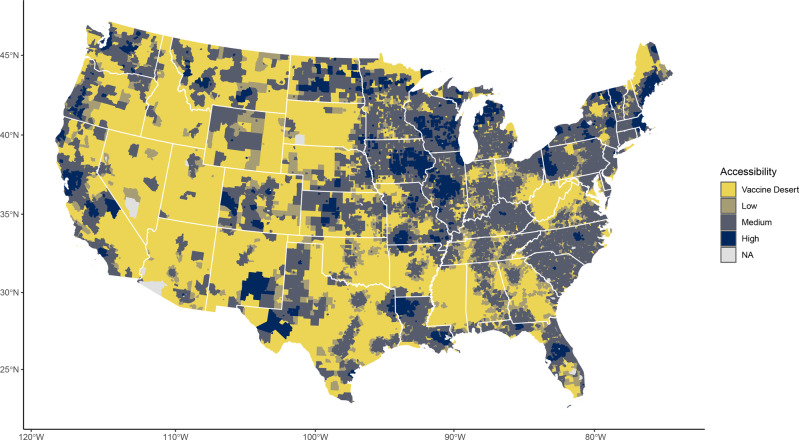
Using the self-reported time-willing-to-travel distribution to parametrize an E2SFCA model of accessibility to the COVID-19 vaccine sites, we found considerable geographic disparities of vaccine access across the US (Fig. 3). There was good accessibility to the vaccine in the Pacific region where over 93% of census tracts were categorized as medium or high accessibility (Table 1). Access was also found to be strong in New England and the Middle Atlantic where respectively only 3.2% and 4.1% of census tracts were categorized as vaccine deserts (Table 1). Conversely, 27.8% of tracts in the Mountain region were categorized as vaccine deserts as well as 18.9% in West- and 22.4% in East–South Central regions.
Fig. 3. COVID-19 vaccine deserts and accessibility scores across the US.
Map of United States Census tracts colored by their spatial accessibility to COVID-19 vaccine doses. Accessibility scores (Ai) were estimated by the enhanced two-step floating catchment area method. Access strata broadly represent cutoffs suggesting difficulty in reaching herd immunity in 8-months (Jan 1—American Labor Day) with one dose (vaccine desert, Ai < 0.85), immunizing all with one dose (low, 0.85 ≤ Ai < 1.0), difficulty immunizing all with two doses (medium, 1.0 ≤ Ai < 2.0), and sufficient accessibility (high, Ai ≥ 2.0). Under the modeled distribution scheme, if the vaccine sites available to each census tract have a steady supply for the rollout period, they would have a sum (accounting for travel-time decay) of Ai doses per person in their respective weighted catchment areas. No estimates (NA) were provided for non-populated census tracts and those (n = 23) where Ai was undefined.
Table 1.
Census tract demographics by accessibility score for the Contiguous United States
| Vaccine desert | Low accessibility | Medium accessibility | High accessibility | All | |
|---|---|---|---|---|---|
| N Tracts: 7999 | N Tracts: 4582 | N Tracts: 45,283 | N Tracts: 714,155 | N Tracts: 72,019 | |
| Column percent | Column percent | Column percent | Column percent | Row percent | |
| Total population (N, %) | 32,847,516 (10.2%) | 20,899,652 (6.5%) | 205,655,647 (63.8%) | 63,009,659 (19.5%) | 322,412,474 (100.0%) |
| Census division (N, %) | |||||
| New England | 109 (3.2%) | 215 (6.4%) | 1552 (46.2%) | 1486 (44.2%) | 3362 (4.7%) |
| Middle Atlantic | 408 (4.1%) | 323 (3.2%) | 7757 (77.0%) | 1582 (15.7%) | 10,070 (14.0%) |
| East North Central | 711 (6.1%) | 662 (5.7%) | 7361 (62.8%) | 2979 (25.4%) | 11,713 (16.3%) |
| West North Central | 523 (9.9%) | 272 (5.2%) | 3244 (61.6%) | 1231 (23.4%) | 5270 (7.3%) |
| South Atlantic | 1743 (12.9%) | 1148 (8.5%) | 9668 (71.3%) | 998 (7.4%) | 13,557 (18.8%) |
| East South Central | 994 (22.4%) | 614 (13.8%) | 2782 (62.7%) | 46 (1.0%) | 4436 (6.2%) |
| West South Central | 1533 (18.9%) | 682 (8.4%) | 5040 (62.2%) | 844 (10.4%) | 8099 (11.2%) |
| Mountain | 1448 (27.8%) | 499 (9.6%) | 2751 (52.7%) | 520 (10.0%) | 5218 (7.2%) |
| Pacific* | 530 (5.1%) | 167 (1.6%) | 5128 (49.8%) | 4469 (43.4%) | 10,294 (14.3%) |
| Rural-Urban designation (N, %) | |||||
| Metropolitan Core | 1856 (3.6%) | 2022 (3.9%) | 35,186 (68.2%) | 12,559 (24.3%) | 51,623 (71.7%) |
| Micropolitan/Town Core | 1468 (23.3%) | 772 (12.3%) | 3359 (53.3%) | 700 (11.1%) | 6299 (8.7%) |
| Metropolitan Commuting Area | 1832 (24.7%) | 941 (12.7%) | 4103 (55.2%) | 556 (7.5%) | 7432 (10.3%) |
| Micropolitan/Town Com. Area | 1472 (41.8%) | 485 (13.8%) | 1388 (39.4%) | 177 (5.0%) | 3522 (4.9%) |
| Rural | 1371 (43.6%) | 362 (11.5%) | 1247 (39.7%) | 163 (5.2%) | 3143 (4.4%) |
| Female (mean %, SD) | 49.8 (±5.1) | 50.4 (±4.7) | 50.9 (±4.8) | 50.9 (±4.3) | 50.8 (±4.8) |
| Missing (N, %) | 16 (0.2%) | 8 (0.2%) | 84 (0.2%) | 22 (0.2%) | 130 (0.2%) |
| Median age (mean years, SD) | 42.7 (±8.2) | 40.9 (±8.3) | 39.4 (±7.8) | 38.1 (±7.0) | 39.6 (±7.8) |
| Missing (N, %) | 16 (0.2%) | 9 (0.2%) | 102 (0.2%) | 24 (0.2%) | 151 (0.2%) |
| Median income (mean $1,000, SD) | 28.5 (±9.2) | 30.8 (±10.7) | 33.5 (±13.8) | 35.7 (±16.3) | 33.2 (±13.9) |
| Missing (N, %) | 27 (0.3%) | 12 (0.3%) | 142 (0.3%) | 32 (0.2%) | 213 (0.3%) |
| Race/ethnicity (mean %, SD) | |||||
| White | 80.4 (±20.8) | 79.8 (±20.9) | 72.0 (±25.2) | 66.4 (±26.2) | 72.3 (±25.1) |
| Black | 9.7 (±16.9) | 11.5 (±18.9) | 15.2 (±22.6) | 13.2 (±22.2) | 13.9 (±21.8) |
| Hispanic | 11.8 (±17.3) | 11.2 (±15.9) | 17.1 (±21.7) | 20.1 (±23.7) | 16.7 (±21.5) |
| American Indian or Alaskan Native* | 2.6 (±10.7) | 1.2 (±4.8) | 0.7 (±2.9) | 0.6 (±2.3) | 0.9 (±4.6) |
| Asian | 1.6 (±3.6) | 2.0 (±3.5) | 4.4 (±7.7) | 9.0 (±13.4) | 4.9 (±8.9) |
| Hawaiian or Pacific Islander* | 0.1 (±0.5) | 0.1 (±0.4) | 0.1 (±0.5) | 0.2 (±0.8) | 0.1 (±0.6) |
| Two or more races | 2.7 (±2.8) | 2.9 (±2.7) | 3.1 (±2.6) | 3.7 (±3.2) | 3.1 (±2.8) |
| Other race | 2.9 (±6.5) | 2.4 (±4.5) | 4.6 (±8.4) | 6.9 (±11.1) | 4.7 (±8.7) |
| Missing (N, %) | 16 (0.2%) | 8 (0.2%) | 84 (0.2%) | 22 (0.2%) | 130 (0.2%) |
| No internet access (mean %, SD) | 20.7 (±11.5) | 16.6 (±10.3) | 14.6 (±10.3) | 13.6 (±9.7) | 15.2 (±10.6) |
| Missing (N, %) | 30 (0.4%) | 23 (0.5%) | 206 (0.5%) | 45 (0.3%) | 304 (0.4%) |
| Insurance coverage (mean %, SD) | |||||
| Employer | 39.9 (±12.6) | 43.6 (±13.7) | 45.4 (±15.1) | 47.6 (±15.4) | 45.1 (±14.9) |
| Direct | 6.4 (±4.1) | 6.3 (±3.9) | 6.6 (±4.2) | 6.6 (±4.3) | 6.5 (±4.2) |
| Medicare | 6.8 (±3.4) | 6.0 (±3.4) | 5.4 (±3.2) | 4.8 (±2.6) | 5.5 (±3.2) |
| Medicaid | 15.2 (±9.5) | 14.2 (±9.5) | 15.7 (±12.2) | 17.3 (±13.4) | 15.8 (±12.1) |
| Veterans Administration | 0.4 (±0.5) | 0.3 (±0.5) | 0.3 (±0.5) | 0.2 (±0.5) | 0.3 (±0.5) |
| Tricare | 1.1 (±3.8) | 1.5 (±5.1) | 1.0 (±4.0) | 0.5 (±2.4) | 0.9 (±3.8) |
| Two or More | 6.4 (±3.3) | 6.4 (±3.2) | 6.0 (±3.3) | 5.7 (±3.1) | 6.0 (±3.2) |
| Uninsured | 10.4 (±6.6) | 9.5 (±6.8) | 9.0 (±7.3) | 7.3 (±6.0) | 8.8 (±7.0) |
| Missing (N, %) | 25 (0.3%) | 20 (0.4%) | 154 (0.3%) | 31 (0.2%) | 230 (0.3%) |
| Medical burden† (mean %, SD) | 53.5 (±5.9) | 52.5 (±5.4) | 49.0 (±6.2) | 46.5 (±6.7) | 49.2 (±6.5) |
| Missing (N, %) | 63 (0.8%) | 11 (0.2%) | 79 (0.2%) | 14 (0.1%) | 167 (0.2%) |
| COVID-19 burden† (mean %, SD) | 3.1 (±1.4) | 3.0 (±1.1) | 2.6 (±1.1) | 2.4 (±1.1) | 2.7 (±1.1) |
| Missing (N, %) | 63 (0.8%) | 11 (0.2%) | 79 (0.2%) | 14 (0.1%) | 167 (0.2%) |
*Alaska and Hawaii excluded from analysis.
†Percentage of survey respondents in each census tract who reported COVID-19 related pre-existing conditions (medical burden) or previous contact with COVID-19 positive individual (COVID-19 burden).
Vaccine deserts in relation to demography
Vaccine deserts are home to 10.2% (N = 32,847,516) of the US census estimated population. A sensitivity analysis varying the accessibility score cutoff for vaccine desert to 0.75 and 0.95, found that 21,289,659 (6.6%) and 45,385,859 (14.1%) people would live in a desert, respectively. When stratifying census tracts by RUCA code, spatial accessibility is higher in metropolitan cores (92.5% medium or high accessibility). This falls dramatically with RUCA classifications farther from metropolitan cores, dropping from 64.4% (micropolitan/town core) to 44.9% (rural area). Rural and micropolitan/town commuting areas had the worst accessibility; vaccine deserts comprised 43.6% and 41.8% of census tracts in these areas, respectively.
In contrast to other forms of vaccine access, disparities in spatial access largely reflect the demographics of the urban-rural divide. Vaccine deserts as compared to high accessibility locations are concentrated among older (4.5 year more), white (14.0% more), lower income ($7190 less), and uninsured (3.1% higher) census tract populations. Vaccine deserts also have a greater burden of those with COVID-19-associated medical conditions (6.9% higher), COVID-19 exposure (0.7% higher), and less access to the internet (7.1% less). These relationships scale monotonically, and low and medium accessibility areas have differences that generally fall linearly between vaccine deserts and high accessibility locations.
Determinants of spatial accessibility
To understand the determinants of accessibility scores accounting for urban-rural confounding, we performed a multivariate analysis and separate spatial regression models for each variable of interest while controlling for population density and urbanicity. While a simplification, a census tract’s accessibility score can be generally interpreted as the number of COVID-19 doses accessible to each unvaccinated individual living in it over the 248-day study period. According to a direct impact assessment from the multivariate model (best fitting according to Akaike information criterion), census tracts with the highest quartile of Black and Hispanic residents were associated with 0.024 and 0.016 lower accessibility scores respectively, when compared to the lowest quartile for each variable (Supplementary Data 1). Per 100,000 individuals in census tracts with the highest quartile of Black and Hispanic residents, this broadly translates to 2400 and 1600 fewerless accessible COVID-19 vaccine doses over the study period. Census tracts with older residents were associated with 200 fewer accessible doses per 100,000 people for each additional year of age, while census tracts with a higher prior COVID-19 burden were associated with 49,700 fewer accessible doses per 100,000 people for each additional percent of previous infection. We find the opposite effect for medical burden whereby, when controlling for population density, an increased percentage of individuals with self-reported pre-existing conditions was associated with 8300 more accessible doses per 100,000 people over the study period. Direction of results were robust to a sensitivity analysis with an alternative weighting matrix (Supplementary Data 2).
Case study of racial disparities in highly segregated urban centers
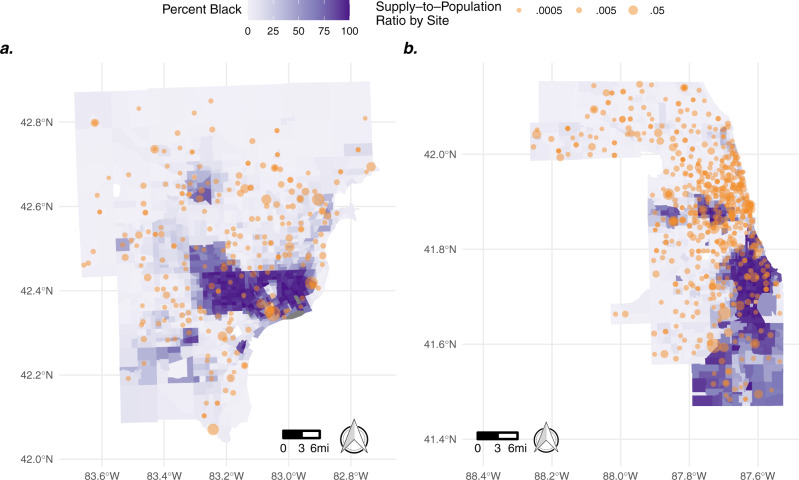
To explore differential access by race within areas of the similar urban composition, we focused on two highly racially segregated cities, Detroit (defined here: Wayne, Oakland, and Macomb Counties, MI) and Chicago (Cook County, IL)56. Consistent with our findings of high vaccine accessibility scores in metropolitan cores, neither of these cities have census tracts that meet the vaccine desert criteria, and most tracts have numerically high accessibility to the COVID-19 vaccine (Mean Ai = 1.8 in Detroit and 2.2 in Chicago). Despite this, we find notable racial disparities in the organization of vaccine distribution in both locations. We find census tracts with a higher proportion of Black residents were served by noticeably fewer vaccine distribution sites compared to nearby tracts with fewer black residents in both Detroit (38% less, Fig. 4a) and Chicago (28% less, Fig. 4b). Of note, vaccine distribution sites located in Black neighborhoods in Detroit were more centralized consisting of fewer locations with larger supply, while tracts with a larger percentage of non-Black residents were served by a distributed network of smaller sites. Numerically, there were 406 more daily doses available per 100,000 residents in majority (>50%) Black census tracks, despite these tracts being served by less vaccine distribution locations. Unlike in Detroit, in Chicago we found that the largest distribution sites were located in predominately non-Black tracts of the city. Quantitatively, this translated to majority Black census tracts in Chicago having 120 less daily doses available per 100,000 residents during the study period.
Fig. 4. Disparities in COVID-19 vaccine access in Detroit and Chicago, USA.
Map of census tracts around Detroit, Michigan (a) and Chicago, Illinois (b) by the percentage of residents who are Black (purple). Overlayed in orange are COVID-19 vaccine distribution locations sized by their supply-to-population ratio. Vaccine distribution sites (from Vaccines.gov) in both cities are sparser in census tracts with a higher percentage of Black residents.
Flooded supply simulation
When repeating our model under the assumption each vaccine distribution site has enough vaccine doses for every individual in its catchment area, we find that only 106 US census tracts persist as vaccine deserts (Supplementary Fig. 3) which is 1.3% of vaccine deserts under the present constrained supply conditions. These locations are primarily located in the rural Mountain and Pacific divisions of the US and the majority of them are outside or on the outskirts of the modeled 90-min distribution site catchment areas as evidenced by accessibility scores at or near zero (Supplementary Fig. 4).
Discussion
The distribution of the COVID-19 vaccine supply during the timeframe of February 19, 2021–March 17, 2021 has created vaccine deserts that have the potential to interfere with population-wide disease control. Based on an empirically parameterized spatial accessibility model, paired with an extensive national dataset of confirmed locations with vaccines and their doses administered, we find that the initial distribution scheme favored spatial accessibility within US metropolitan areas. In contrast, vaccine deserts, more often found in rural regions, had a preponderance of vulnerable populations. Vaccine deserts had more residents with self-reported COVID-19 exposures and pre-existing conditions as well as more individuals who lacked health insurance. When measuring determinants of accessibility while controlling for population density, we see that tracts with a higher proportion of Black residents, Hispanic residents and older residents had reduced spatial access to the vaccine. Together, these findings highlight that at the time of our analysis (February 19–March 17 2021) vaccine dissemination points within the US were not yet effectively placed to target those most vulnerable to COVID-19.
US populations living in rural census tracts had the lowest accessibility to the COVID-19 vaccine. While prior studies hypothesize that rural populations may be willing to travel further distances for health resources than those in urban areas57,58 we find these differences were diminished for the COVID-19 vaccine. Despite a large portion of urban populations’ willingness to travel longer for the vaccine than they would for routine care, this effect was reduced for those in rural census tracts. This finding may be related to rural populations reporting higher levels of vaccine hesitancy59, which our findings show had a stronger association with willingness to travel than geography. These regions may benefit the most from the COVID-19 vaccine due to their relatively higher medical burden60, older population61 and lower likelihood to consider adoption of mitigation measures such as face mask wearing46. We also find that convenience could play a large role in whether hesitant individuals would be willing to get vaccinated, suggesting increased spatial access may be a meaningful public health intervention. Only ~50% of the overall population reported willingness to travel >30 min for the vaccine and <20% would travel >1 h. These numbers were even smaller amongst hesitant individuals. Despite rural areas being less populated than urban ones, after incorporating these findings, our model suggests ~14% of the US lived in a vaccine desert at the time of analysis. Inability to reach spatially coupled rural regions with vaccinations presents the risk of intense outbreaks62 and may sustain persistent chains of transmission which are barriers to infection elimination63, although the latter has yet to be studied for SARS-CoV-2.
COVID-19 morbidity and mortality is not distributed equally across demographic groups and prior work has shown that within both urban9,13 and rural64 regions, communities with a higher proportion of minorities had worse outcomes. Ensuring equal access to the COVID-19 vaccine is an important part of mitigating these structural racism outcomes and preventing further propagation of these imbalances. Previous state-wide analyses have shown that pharmacy deserts are more likely to be in rural regions with a high white population20, while citywide analyses have shown they tend to be located in neighborhoods with more minorities19,22,23. Utilizing a 90-min catchment area to understand vaccine deserts (the definition of which is specific to COVID-19 immunization goals, but more flexible than the strict radius limits routinely used to study access to less scarce resources19,23), our results are congruent with both these findings. On the national level, we observe vaccine deserts in rural census tracts that were predominately white. Yet, when we remove urban-rural confounding and look within regions of similar spatial structure, we find the same racial disparities highlighted in the pharmacy desert literature, suggesting vaccine distribution strategies during this timeframe did not go far enough to address these disparities.
When adjusting for urban-rural confounding we find higher spatial accessibility for those with self-reported pre-existing conditions, which may highlight deliberate vaccine site placement or the likelihood of individuals who live near medical resources to receive diagnoses65. While self-reports are biased estimates of medical diagnoses, this also suggests that our general finding of higher burden in rural areas may be an underestimate given a rural region’s reduced access to diagnoses. We also find that, despite the initial targeting of older individuals for vaccination, census tracks with a higher median age had less spatial accessibility during the study period. This highlights how a concerted effort needs to be made to overcome demographic biases in infrastructure and optimally allocate medical resources to their intended target.
Importantly, the geospatial barriers discussed here only represent one type of vaccine access challenge. Social disparities (e.g., ability to take time off work, obtain child care, procure efficient transportation) suggest equal spatial barriers can manifest very differently66,67 and that vulnerable populations may be disproportionately affected by poor spatial access. For example, this is supported by evidence suggesting that individuals with lower income experienced a disproportionate reduction in access to health services in response to the pandemic68. The two-dose regimens for the selected COVID-19 vaccine formulations available at the time of analysis also created a need to access a vaccine distribution site twice within a short period (21 or 28 days), suggesting that spatial barriers may compound and/or affect dose completion rates. Additionally, the disparities in internet access in vaccine deserts suggest these regions may have had a harder time both identifying and accessing vaccine distribution locations due to the reliance on web-based tools (e.g., Vaccines.gov) to find vaccine availability and schedule appointments. These same disparities in internet access may affect the representativeness of the two surveys used here and cause undersampling in vaccine deserts (which census data shows have poorer internet access). Therefore, our results may underestimate the medical and COVID-19 burden in census tracts with poor spatial accessibility to the COVID-19 vaccine. Panel surveys are also only sampling individuals willing to complete them. For questions regarding vaccine intent, this group may not be representative of the broader US or hold true over time, as perceptions relating to vaccination can change quickly69.
The VaccineFinder dataset used in this analysis is likely the most comprehensive source of vaccine site and dose supply information given the mandate for COVID-19 vaccine providers enrolled in the Federal Retail Pharmacy Partnership (FRPP) to register in VTrckS. However, not all locations modeled may have been open or advertising to the public and we only included locations with evidence of dose administration on multiple occasions. These locations will likely change substantially as vaccine supply expands to more providers. Additionally, while we integrate locations from the Veterans Administration, our dataset is missing sites in states that only partially joined FRPP (e.g., West Virginia) and other important federal vaccine distribution channels such as Military Treatment Facilities, the Indian Health Service and Federally Qualified Health Centers. The missing sites are therefore likely not distributed at random which may bias the association between demographics and vaccine accessibility in either direction.
The current analysis also only measured spatial access from the perspective of the unvaccinated dose recipient (and assumed everyone currently unvaccinated in the population was eligible to receive a vaccine) based on static observations of vaccination locations in the early phases of the rollout process and the time-willing-to-travel of those who had yet to vaccinate (which ignores the long distances people may have been willing to travel in the first phase of the rollout). Distribution during this period was characterized by manufacturing constraints17 and supply-chain barriers (e.g., need for ultralow temperature freezers)70, as well as prioritized vaccination of healthcare workers, those over 65 years of age, and certain high-risk individuals17. The selected site locations during the time of this analysis favor urban areas to optimally reach these populations for vaccination. Populations with a high number of medical workers may be vaccinated relatively quickly, allowing some distribution nodes to broaden their population served. Conversely, populations with a high number of children under 18 have a large proportion of residents who may not yet be eligible for the vaccine. Additionally, this analysis did not account for natural immunity levels already in the population which is rapidly changing and may not be evenly geographically distributed. Here we have shown the importance of changes in the vaccine desert cutoff based on herd immunity, highlighting the sensitivity of this assumption.
Improving the supply of vaccines will cause the numerator of the vaccine supply-to-population ratio to rise and minimize the number of vaccine deserts as defined here. When scenario modeling a situation in which supply is abundant, we find that very few vaccine deserts remain suggesting that increases in supply will help alleviate the presently modeled deserts. However, as we remove supply barriers, travel time to vaccine sites will become the primary component of accessibility. These travel time metrics have been shown to have similar demographic disparities as the present results when measuring accessibility of other COVID-19 resources14,71,72. Further, even in locations such as Detroit and Chicago, where vaccine supply and spatial accessibility is numerically adequate (i.e., not a desert), locations of vaccine distribution sites may favor certain groups and exacerbate disparities. Coordinated efforts are required to ensure distribution is both data-driven and optimized for local circumstances.
As vaccine priorities evolve, states increase/shift distribution locations and supply, and one-dose vaccines are integrated into the supply-chain, the barriers identified here can be overcome and the results from this study period will change. This manuscript presents a novel utilization of a highly flexible model that can incorporate these future changes while also helping identify the need to direct vaccine supply to underserved communities. Controlling COVID-19 in the US requires extensive, well-orchestrated coordination of vaccine delivery at the national and local scales73. Here we show significant disparities in geographic access to the COVID-19 vaccine during the early stages of the vaccination program, a hurdle to this achievement. Eliminating COVID-19 vaccine deserts and spatial barriers to transmission control will require an ongoing commitment to optimizing distribution to align with continued expansion of populations prioritized to receive vaccines and simultaneous investment in resources to mitigate transportation access disparities. This will necessitate locally targeted interventions including active outreach campaigns, mobile vaccination clinics and other innovative health delivery mechanisms such as vaxathons74 and ride-share partnerships75.
Supplementary information
Description of Additional Supplementary Files
Acknowledgements
This work was supported by Flu Lab and Ending Pandemics. B.R., C.M.A. and J.S.B. acknowledge funding from Facebook Sponsored Research Agreement [INB1116217]. B.R. and J.S.B. acknowledge funding from Google.org via the Tides Foundation [TF2003–089662]. The authors thank Kate Miller, Ethan Roubenoff, and Caroline Westnedge for their insights and assistance. The authors thank the HealthMap VaccineFinder and research teams including Christopher Remmel, Gaurav Tuli and Autumn Gertz. The authors thank the SurveyMonkey research team including Jon Cohen and Jack Chen. The authors also thank the Castlight Health team including Vivek Salgar, Ankur Jain, Rajiv Kutty, Mukul Mittal, Gavin Thompson, Sophia Golden, Danielle Hershon, Mustafa Husaini, Ariel Klein, Venkatesh Hemanth Gorur, Kranthi Ravi, Akshay Mungrey, Terry Bennett, Phani Kumar Mallampati, Vaneet Loomba, Abhishek Jain, Vamshi Krishna Naidi, Umamaheshwara Gupta Karnati, Stephen Brokaw, Brian Quock, Deepak Mali, Milind Pandrangi, Ryan Scribner and Shahram Moatazedi. Boston Children’s Hospital’s High-Performance Computing Cluster Enkefalos 2 (E2) was used for research reported in this publication. Software used in the project was installed and configured by BioGrids76. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Author contributions
B.R., C.M.A., D.M.B., A.P., and J.S.B. conceived of the study. B.R., C.M.A., K.S., K.C., and J.S.B. wrote the first draft of the manuscript. K.S., L.W., K.H., D.M.B., L.L., A.P., and J.S.B. oversaw and implemented data collection. B.R., C.M.A., P.L.D., and K.H. contributed to analysis. B.R., C.M.A., K.S., P.L.D., K.C., L.W., J.M.R., K.H., M.F.P., J.C., C.M.W., D.M.B., L.L., A.P., J.S.B. contributed to the interpretation of the data and critical revision of the final manuscript. B.R. and J.S.B. have verified the underlying data. All authors have seen and approve the final text.
Peer review
Peer review information
Communications Medicine thanks the anonymous reviewers for their contribution to the peer review of this work.
Data availability
Real-time vaccine site location is available at VaccineFinder.org for locations that choose to publicly display. Vaccine dose supply is available: [https://data.cdc.gov/Vaccinations/Vaccines-gov-COVID-19-vaccinating-provider-locatio/5jp2-pgaw]. Source data for manuscript main figures available: [10.6084/m9.figshare.20513949]. Delphi Group COVID-19 Trends and Impact Survey data may requested via [https://cmu-delphi.github.io/delphi-epidata/].
Code availability
Code and travel-time decay function is available: github.com/computationalepi/E2SFCA and archived on Zenodo50.
Competing interests
The authors declare the following competing interests: JSB reports that VaccineFinder.org has received programmatic funding from the U.S. Centers for Disease Control and Prevention. The remaining authors have no competing interests to declare.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Anita Patel, John S. Brownstein.
Contributor Information
Benjamin Rader, Email: benjamin.rader@childrens.harvard.edu.
John S. Brownstein, Email: john.brownstein@childrens.harvard.edu
Supplementary information
The online version contains supplementary material available at 10.1038/s43856-022-00183-8.
References
- 1.Andrulis DP. Access to care is the centerpiece in the elimination of socioeconomic disparities in health. Ann. Int. Med. 1998;129:412–416. doi: 10.7326/0003-4819-129-5-199809010-00012. [DOI] [PubMed] [Google Scholar]
- 2.Charland KM, de Montigny L, Brownstein JS, Buckeridge DL. Clinic accessibility and clinic-level predictors of the geographic variation in 2009 pandemic influenza vaccine coverage in Montreal, Canada. Influenza Other Respir. Viruses. 2014;8:317–328. doi: 10.1111/irv.12227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Qato DM, et al. Pharmacy accessibility and cost-related underuse of prescription medications in low-income Black and Hispanic urban communities. J. Am. Pharm. Assoc. 2017;57:162–169.e1. doi: 10.1016/j.japh.2016.12.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Athas WF, Adams-Cameron M, Hunt WC, Amir-Fazli A, Key CR. Travel distance to radiation therapy and receipt of radiotherapy following breast-conserving surgery. J. Natl Cancer Inst. 2000;92:269–271. doi: 10.1093/jnci/92.3.269. [DOI] [PubMed] [Google Scholar]
- 5.Jean-Jacques, M. & Bauchner, H. Vaccine distribution—equity left behind? JAMA10.1001/jama.2021.1205 (2021). [DOI] [PubMed]
- 6.Chen JT, Krieger N. Revealing the unequal burden of COVID-19 by income, race/ethnicity, and household crowding: US county versus zip code analyses. J. Pub. Health Manag. Pract. 2021;27:S46–S56. doi: 10.1097/PHH.0000000000001263. [DOI] [PubMed] [Google Scholar]
- 7.Webb Hooper M, Nápoles AM, Pérez-Stable EJ. COVID-19 and racial/ethnic disparities. JAMA. 2020;323:2466–2467. doi: 10.1001/jama.2020.8598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The COVID Racial Data Tracker. The COVID Tracking Projecthttps://covidtracking.com/race (2020).
- 9.Sy, K. T. L., Martinez, M. E., Rader, B. & White, L. F. Socioeconomic disparities in subway use and COVID-19 outcomes in New York city. Am. J. Epidemiol.10.1093/aje/kwaa277 (2020). [DOI] [PMC free article] [PubMed]
- 10.Jay J, et al. Neighbourhood income and physical distancing during the COVID-19 pandemic in the United States. Nat. Hum. Behav. 2020;4:1294–1302. doi: 10.1038/s41562-020-00998-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kabarriti R, et al. Association of race and ethnicity with comorbidities and survival among patients with COVID-19 at an urban medical center in New York. JAMA Netw. Open. 2020;3:e2019795. doi: 10.1001/jamanetworkopen.2020.19795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Moore JT, et al. Disparities in incidence of COVID-19 among underrepresented racial/ethnic groups in counties identified as hotspots during June 5–18, 2020—22 states, February–June 2020. MMWR. Morb. Mortal. Wkly Report. 2020;69:1122–1126. doi: 10.15585/mmwr.mm6933e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogedegbe G, et al. Assessment of racial/ethnic disparities in hospitalization and mortality in patients with COVID-19 in New York city. JAMA Netw. Open. 2020;3:e2026881. doi: 10.1001/jamanetworkopen.2020.26881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rader B, et al. Geographic access to United States SARS-CoV-2 testing sites highlights healthcare disparities and may bias transmission estimates. J. Travel Med. 2020;2020:1–4. doi: 10.1093/jtm/taaa076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peiris M, Leung GM. What can we expect from first-generation COVID-19 vaccines? Lancet. 2020;396:1467–1469. doi: 10.1016/S0140-6736(20)31976-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.CDC. How CDC Is Making COVID-19 Vaccine Recommendations. https://www.cdc.gov/coronavirus/2019-ncov/vaccines/recommendations-process.html (2020).
- 17.Dooling K, et al. The advisory committee on immunization practices’ interim recommendation for allocating initial supplies of COVID-19 vaccine—United States, 2020. MMWR. Morb. Mortality Wkly Rep. 2020;69:1857–1859. doi: 10.15585/mmwr.mm6949e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Luo W, Qi Y. An enhanced two-step floating catchment area (E2SFCA) method for measuring spatial accessibility to primary care physicians. Health Place. 2009;15:1100–1107. doi: 10.1016/j.healthplace.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 19.Qato DM, et al. ‘Pharmacy deserts’ are prevalent in chicago’s predominantly minority communities, raising medication access concerns. Health Affairs. 2014;33:1958–1965. doi: 10.1377/hlthaff.2013.1397. [DOI] [PubMed] [Google Scholar]
- 20.Pednekar P, Peterson A. Mapping pharmacy deserts and determining accessibility to community pharmacy services for elderly enrolled in a State Pharmaceutical Assistance program. PLoS One. 2018;13:e0198173. doi: 10.1371/journal.pone.0198173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lam, O., Broderick, B. & Toor, S. How far do urban, suburban and rural Americans live from a hospital? Pew Research Centerhttps://www.pewresearch.org/fact-tank/2018/12/12/how-far-americans-live-from-the-closest-hospital-differs-by-community-type/ (2018).
- 22.Qin JZ, Diniz CP, Coleman JS. Pharmacy-level barriers to implementing expedited partner therapy in Baltimore, Maryland. Am. J. Obstet. Gynecol. 2018;218:e1–504.e6. doi: 10.1016/j.ajog.2018.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wisseh, C. et al. Social determinants of pharmacy deserts in Los Angeles county. J. Racial Ethn. Health Disparities10.1007/s40615-020-00904-6 (2020). [DOI] [PMC free article] [PubMed]
- 24.Ullrich, F. & Mueller, K. Pharmacy Vaccination Service Availability in Nonmetropolitan Counties. (Rural Health Research Gateway, 2020).
- 25.Ploeg, M. Ver, Nulph, D. & Williams, R. Mapping Food Deserts in the United States (U.S. Department of Agriculture, 2011).
- 26.Wang L, Ramroop S. Geographic disparities in accessing community pharmacies among vulnerable populations in the Greater Toronto Area. Can. J. Public Health. 2018;109:821–832. doi: 10.17269/s41997-018-0110-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kang JY, et al. Rapidly measuring spatial accessibility of COVID-19 healthcare resources: a case study of Illinois, USA. Int. J. Health Geogr. 2020;19:1–17. doi: 10.1186/s12942-020-00229-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Delphi Group. COVID Symptom Survey (Campus Ready, 2020).
- 29.Barkay, N. et al. Weights and methodology brief for the COVID-19 symptom survey by University of Maryland and Carnegie Mellon University, in partnership with Facebook. arXiv10.48550/arXiv.2009.14675 (2020).
- 30.Salomon JA, et al. The US COVID-19 trends and impact survey: continuous real-time measurement of COVID-19 symptoms, risks, protective behaviors, testing, and vaccination. Proc. Natl. Acad. Sci. USA. 2021;118:e2111454118. doi: 10.1073/pnas.2111454118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.U.S. Census Bureau. 2015–2019 American Community Survey (Esri Data Development, 2020).
- 32.United States Department of Agriculture Economic Research Service. Rural-Urban Commuting Area Codes. https://www.ers.usda.gov/data-products/rural-urban-commuting-area-codes.aspx (2014).
- 33.Doxsey-Whitfield E, et al. Taking advantage of the improved availability of census data: a first look at the gridded population of the world, Version 4. Pap. Appl. Geogr. 2015;1:226–234. doi: 10.1080/23754931.2015.1014272. [DOI] [Google Scholar]
- 34.U.S. Centers for Disease Control and Prevention, N. COVID-19 vaccinations in the United States, county (COVID-19 Vaccine Surveillance Public Data Access, 2020).
- 35.Reinhart, A. & Tibshirani, R. COVID-19 Symptom Surveys Through Facebook. https://delphi.cmu.edu/blog/2020/08/26/covid-19-symptom-surveys-through-facebook/ (2020).
- 36.Sudre, C. H. et al. Anosmia, ageusia, and other COVID-19-like symptoms in association with a positive SARS-CoV-2 test, across six national digital surveillance platforms: an observational study. Lancet Digital Health3, e577–e586 (2021). [DOI] [PMC free article] [PubMed]
- 37.Centers for Disease Control and Prevention. COVID-19 Vaccination Reporting Systems. https://www.cdc.gov/vaccines/covid-19/reporting/vtrcks/index.html (2022).
- 38.Centers for Disease Control and Prevention. Pharmacies Participating in the Federal Retail Pharmacy Program for COVID-19 Vaccination. https://www.cdc.gov/vaccines/covid-19/retail-pharmacy-program/participating-pharmacies.html (2020).
- 39.VA Access to Care. U.S. Department of Veterans Affairs. COVID-19 National Summary—VA Access to Care. https://www.accesstocare.va.gov/Healthcare/COVID19NationalSummary (2022).
- 40.Yarmuth, J. A. American Rescue Plan Act of 2021 (117th USA Congress, 2021).
- 41.Apparicio P, Abdelmajid M, Riva M, Shearmur R. Comparing alternative approaches to measuring the geographical accessibility of urban health services: distance types and aggregation-error issues. Int. J. Health Geogr. 2008;7:7. doi: 10.1186/1476-072X-7-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hulland EN, et al. Travel time to health facilities in areas of outbreak potential: maps for guiding local preparedness and response. BMC Med. 2019;17:1–16. doi: 10.1186/s12916-019-1459-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Weiss DJ, et al. A global map of travel time to cities to assess inequalities in accessibility in 2015. Nature. 2018;553:333–336. doi: 10.1038/nature25181. [DOI] [PubMed] [Google Scholar]
- 44.Delamater PL, Messina JP, Shortridge AM, Grady SC. Measuring geographic access to health care: raster and network-based methods. Int. J. Health Geogr. 2012;11:15. doi: 10.1186/1476-072X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dijkstra EW. A note on two problems in connexion with graphs. Numer. Math. 1959;1:269–271. doi: 10.1007/BF01386390. [DOI] [Google Scholar]
- 46.Rader, B. et al. Mask-wearing and control of SARS-CoV-2 transmission in the USA: a cross-sectional study. Lancet Digital Health3, e148–e157 (2021). [DOI] [PMC free article] [PubMed]
- 47.Wronski, L. et al. OutbreaksNearMe and SurveyMonkey COVID-19 syndromic surveillance survey instrument. figshare10.6084/m9.figshare.13568648.v3 (2022).
- 48.Chen X, Jia P. A comparative analysis of accessibility measures by the two-step floating catchment area (2SFCA) method. Int. J. Geogr. Inf. Sci. 2019;33:1739–1758. doi: 10.1080/13658816.2019.1591415. [DOI] [Google Scholar]
- 49.McGrail MR, Humphreys JS, Ward B. Accessing doctors at times of need-measuring the distance tolerance of rural residents for health-related travel. BMC Health Serv. Res. 2015;15:212. doi: 10.1186/s12913-015-0880-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.ComputationalEpi/E2SFCA. Willingness to Travel for the SARS-CoV-2 Vaccine.10.5281/ZENODO.7004875 (2022).
- 51.Delamater PL. Spatial accessibility in suboptimally configured health care systems: a modified two-step floating catchment area (M2SFCA) metric. Health Place. 2013;24:30–43. doi: 10.1016/j.healthplace.2013.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Weiss DJ, et al. Global maps of travel time to healthcare facilities. Nat. Med. 2020;26:1835–1838. doi: 10.1038/s41591-020-1059-1. [DOI] [PubMed] [Google Scholar]
- 53.Anselin, L. Spatial Econometrics: Methods and Models (Kluwer Academic, 2010).
- 54.Anselin L. Under the hood issues in the specification and interpretation of spatial regression models. Agri. Econ. 2002;27:247–267. doi: 10.1111/j.1574-0862.2002.tb00120.x. [DOI] [Google Scholar]
- 55.LeSage, J. & Pace, R. Introduction to Spatial Econometrics (CRC Press, 2009).
- 56.Salviati, C. The Persistent Effects of Residential Segregation (2018).
- 57.McGrail MR, Humphreys JS. Measuring spatial accessibility to primary care in rural areas: Improving the effectiveness of the two-step floating catchment area method. Appl. Geogr. 2009;29:533–541. doi: 10.1016/j.apgeog.2008.12.003. [DOI] [Google Scholar]
- 58.Luo W, Whippo T. Variable catchment sizes for the two-step floating catchment area (2SFCA) method. Health Place. 2012;18:789–795. doi: 10.1016/j.healthplace.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 59.Khubchandani, J. et al. COVID-19 vaccination hesitancy in the United States: a rapid national assessment. J. Community Health10.1007/s10900-020-00958-x (2021). [DOI] [PMC free article] [PubMed]
- 60.Mueller, J. T. et al. Impacts of the COVID-19 pandemic on rural America. Proc. Natl Acad. Sci. USA118, 2019378118 (2020). [DOI] [PMC free article] [PubMed]
- 61.Symens Smith, A. & Trevelyan, E. Older Population in Rural America. Census Bureauhttps://www.census.gov/library/publications/2019/acs/acs-41.html (2020).
- 62.Rader, B. et al. Crowding and the shape of COVID-19 epidemics. Nat. Med.26, 1–6 10.1038/s41591-020-1104-0 (2020). [DOI] [PubMed]
- 63.Metcalf CJE, Hampson K, Tatem AJ, Grenfell BT, Bjørnstad ON. Persistence in epidemic metapopulations: quantifying the rescue effects for measles, mumps, rubella and whooping Cough. PLoS One. 2013;8:e74696. doi: 10.1371/journal.pone.0074696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cheng KJG, Sun Y, Monnat SM. COVID‐19 death rates are higher in rural counties with larger shares of blacks and hispanics. J. Rural Health. 2020;36:602–608. doi: 10.1111/jrh.12511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kelly, C., Hulme, C., Farragher, T. & Clarke, G. Are differences in travel time or distance to healthcare for adults in global north countries associated with an impact on health outcomes? A systematic review. BMJ Open10.1136/bmjopen-2016-013059 (2016). [DOI] [PMC free article] [PubMed]
- 66.Khan AA. An integrated approach to measuring potential spatial access to health care services. Socio-Econ. Plan. Sci. 1992;26:275–287. doi: 10.1016/0038-0121(92)90004-O. [DOI] [PubMed] [Google Scholar]
- 67.Bissonnette L, Wilson K, Bell S, Shah TI. Neighbourhoods and potential access to health care: The role of spatial and aspatial factors. Health Place. 2012;18:841–853. doi: 10.1016/j.healthplace.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 68.Whaley CM, et al. Changes in health services use among commercially insured US populations during the COVID-19 pandemic. JAMA Netw. Open. 2020;3:e2024984. doi: 10.1001/jamanetworkopen.2020.24984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nguyen KH, et al. COVID-19 vaccination intent, perceptions, and reasons for not vaccinating among groups prioritized for early vaccination—United States, September and December 2020. Am J. Transplant. 2021;70:217–222. doi: 10.15585/mmwr.mm7006e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ford, T. & Schweik, C. M. The cold supply chain can’t reach everywhere—that’s a big problem for equitable COVID-19 vaccination. The Conversationhttps://theconversation.com/the-cold-supply-chain-cant-reach-everywhere-thats-a-big-problem-for-equitable-covid-19-vaccination-152057 (2021).
- 71.Rader, B., Whaley, C. M., Rogers, W. S., Brownstein, P. J. S. & Cantor, J. Assessment of geographic access to monoclonal antibodies in the United States. J. Travel Med.10.1093/jtm/taac018 (2022). [DOI] [PMC free article] [PubMed]
- 72.Khazanchi R, Powers SD, Rogawski McQuade ET, McManus KA. Inequities in the geographic accessibility of COVID-19 biomedical therapeutic trials in the United States. J. Gen. Intern. Med. 2021;36:3650–3653. doi: 10.1007/s11606-021-07081-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Omer SB, Yildirim I, Forman HP. Herd immunity and implications for SARS-CoV-2 control. JAMA. 2020;324:2095–2096. doi: 10.1001/jama.2020.20892. [DOI] [PubMed] [Google Scholar]
- 74.Stamm, D., Brandt, J. & Greeman, D. 2021. Walk-up 24-h ‘vaxathon’ looks to get coronavirus vaccines to those from philadelphia’s most at-risk neighborhoods. NBC10 Philadelphia, February 19.
- 75.Brownstein JS, Huston JE, Steingold L, Joyce MV. On-demand delivery of influenza vaccination. Ann. Intern. Med. 2015;163:806–807. doi: 10.7326/L15-5169. [DOI] [PubMed] [Google Scholar]
- 76.Morin, A. et al. Collaboration gets the most out of software. eLife2013, e01456 (2013). [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Description of Additional Supplementary Files
Data Availability Statement
Real-time vaccine site location is available at VaccineFinder.org for locations that choose to publicly display. Vaccine dose supply is available: [https://data.cdc.gov/Vaccinations/Vaccines-gov-COVID-19-vaccinating-provider-locatio/5jp2-pgaw]. Source data for manuscript main figures available: [10.6084/m9.figshare.20513949]. Delphi Group COVID-19 Trends and Impact Survey data may requested via [https://cmu-delphi.github.io/delphi-epidata/].
Code and travel-time decay function is available: github.com/computationalepi/E2SFCA and archived on Zenodo50.