Abstract
Background and Purpose:
Early diagnosis of cognitive impairment is important because symptoms can be delayed through therapies. Synaptic disconnections are the key characteristic of dementia, and through non-linear complexity analysis of brain function, it is possible to identify long-range synaptic disconnections in the brain.
Methods:
We investigated the capability of a novel upper-extremity function (UEF) dual-task paradigm in the functional MRI (fMRI) setting, where the participant flexes and extends their arm while counting, to differentiate between cognitively normal (CN) and those with mild cognitive impairment (MCI). We used multiscale entropy (MSE) complexity analysis of the blood oxygen-level dependent time-series across neural networks and brain regions. Outside of the fMRI, we used the UEF dual-task test while the elbow kinematics were measured using motion sensors, to record the motor function score.
Results:
Results showed 34% lower MSE values in MCI compared to CN (p<0.04 for all regions and networks except cerebellum when counting down by one; effect size=1.35±0.15) and a negative correlation between MSE values and age (average r2 of 0.30 for counting down by one and 0.36 for counting backward by three). Results also showed an improvement in the logistic regression model sensitivity by 14–24% in predicting the presence of MCI when brain function measure was added to the motor function score (kinematics data).
Conclusions:
Current findings suggest that combining measures of neural network and motor function, in addition to neuropsychological testing, may provide an accurate tool for assessing early-stage cognitive impairment and age-related decline in cognition.
Keywords: fMRI, Dual-task Function, Entropy, Repeatability, Alzheimer’s disease, Aging
Introduction
The average life-expectancy worldwide is increasing, and subsequently, the proportion of older adults is growing faster than ever, leading to a surge in age-related illnesses such as dementia.1,2 An estimated 6% of adults live with some form of cognitive impairment and some may remain undiagnosed during their lifetime.1,3 In the clinical setting, some individuals with cognitive impairment are too frail, anxious, or unwilling to undergo testing via existing assessment tools, which unfortunately leads to late-in-life diagnosis of Alzheimer’s disease (AD).2,3 Additionally, AD is an incurable disease in its late stages, but major complications can be delayed through motor or cognitive-based therapies especially in early stages of the disease (see2 for a review). Therefore, it is crucial to detect AD earlier in life, possibly when it is still considered mild cognitive impairment (MCI).1
Prior work demonstrated associations between dual-task performance and increased risk for AD or MCI,4–6 which is mostly due to the well-known fact that simultaneous decline in motor and cognitive performance occurs with neurodegenerative diseases.7–9 It is hypothesized that compensatory processes in cortical and subcortical brain regions are required to allow simultaneous motor and cognitive performance among cognitively impaired older adults.10 Additionally, assessment of dual-task performance is important because it represents real-life conditions, such as performing daily activities that require divided attention.5,11–13 We previously developed an upper-extremity function (UEF) test, which involves flexion and extension of the elbow, to detect AD and even MCI-related dual-task deficits.14 This test is highly applicable to clinical settings, especially where patients are too frail to perform dual-task assessments that involve walking. We have previously validated the UEF test to identify MCI and AD based on kinematics and kinetics parameters of upper-extremity performance.15–17 The UEF motor score is established based on motor function variability within dual-task performance, which involved UEF motor task and a cognitive task of counting backwards. In addition to being easy-to-perform, one advantage of the UEF test is its capability for assessment inside the functional MRI (fMRI) setting. In the current study, we implemented the UEF test in the fMRI setting to study brain function during dual-task performance.
To study brain function, non-linear complexity analysis of single-channel Electroencephalography (EEG), fMRI, or functional near infrared spectroscopy (fNIRS) time-series are utilized to determine the interconnectivity of neural networks, especially the connection between sub-cortical and cortical regions where disconnections can lead to altered pathways and activity in AD.10,18–22 Further, hypoactivation due to AD can be attributed to neuronal death and subsequent disruptions to synaptic connections.23,24 All of these factors can lead to a low value of nonlinear complexity of the brain function in AD, which can be quantified using entropy analysis. Previous work suggests that the time series with repeating elements arise from a more ordered (deterministic) system (less complexity), which is attributed to characteristics of declining cognitive health due to neurodegenerative diseases.25–27 On the other hand, an increased value of entropy suggests that the interconnectivity between neuronal groups can lead to a more chaotic and unpredictable time series, which can be attributed to a complex and dynamic system characteristic of a healthy brain.18,21 Nevertheless, resting state fMRI has been often studied previously to show how the complexity of neural networks is associated with cognitive impairment.19,28–30
To this end, the goal of the current study was to investigate differences in the brain function between cognitively normal (CN) and MCI older adults, while they were challenged by the UEF dual-task performance. Based on these previous observations, our hypothesis is that complexity of brain function is a physiological correlate of cognitive impairment, and we expect to see a less complex brain function behavior among MCI compared to CN, which can be used as a measure for cognitive impairment assessment. We also expect to observe a significant association between age and brain function complexity, with less complexity with increased age. Finally, we assessed the association between brain function complexity and dual-task motor performance outside of the MRI setting. The hypothesis was that a less complex brain function behavior would be associated with a worse dual-task motor performance.
Methods
Participants
CN and MCI participants (≥65 years) were recruited from assisted living facilities, community aging centers, and internal medicine and geriatric clinics at the Banner University Medical Center, in Tucson, AZ, from September 2017 to June 2018. A brief screening questionnaire related to fMRI safety was administered to determine the eligibility of participants. Inclusion criteria were being 65 years or older, being able to understand study instructions, and proficiency in English language. Participants were excluded if they had MRI contraindications, which include implanted metal or electrical devices such as heart pacemakers, neurostimulators, metal hip replacements, and similar implants. However, those with orthopedic implants eight weeks post-surgery were allowed. Furthermore, participants were excluded if they had known disorders associated with severe motor function deficits (e.g., stroke and Parkinson’s disease) and upper-extremity disorders (e.g., severe shoulder or elbow osteoarthritis). Additionally, participants with active psychiatric disorders were excluded. Any other factors that in the investigator’s judgement may affect patient safety or compliance were considered as exclusion criteria. Before participating in the study, a written informed consent according to the principles expressed in the Declaration of Helsinki was obtained from each participant.31 Participants were informed about the benefits, possible negative consequences, and their right to withdraw from the study at any time without any repercussion. This pilot study was approved by the University of Arizona Institutional Review Board.
Cognitive assessments
Cognitive status of each participant was determined based on Montreal Cognitive Assessment (MoCA score ≥ 26 representing CN). Other neuropsychological tests based on the National Alzheimer’s Coordinating Center Neuropsychological Uniform Data Set of the Alzheimer’s Disease Centers, included: 1) Rey Auditory Test; 2) Wechsler Adult Intelligence Scale (WAIS) Digit Span subtest; 3) WAIS-R Digit Symbol Test; 4) Trails Making Test; 5) Stroop Color and Word Test; 6) The Controlled Oral Word Association Test; and 7) Wisconsin Card Sorting Test. These standardized tests were included to assess several cognitive domains including memory, attention, working memory, cognitive inhibition, cognitive flexibility, reasoning, and complex problem-solving. Scores from neuropsychological tests were adjusted with normative age and education level based on previous work.32
UEF measures outside of the MRI
To assess the motor function outside of the MRI room, participants were asked to perform two UEF function tests, which consisted of a 60-second self-selected normal pace elbow flexion and a cognitive task of either counting numbers backwards by ones (Cog 1) or threes (Cog 2). Assessments were administered by trained researchers and all participants were given the same instructions for consistency. Two wearable motion sensors (LEGSys tri-axial gyroscope sensors, sample frequency = 100 Hz, BioSensics LLC, Cambridge, MA) were applied to the wrist and upper-arm of the dominant side using elastic bands. The sensors measured upper-extremity kinematics during elbow flexion, which were then used to obtain outcome parameters related to motor function variability and speed. Subsequently, these parameters were used to calculate two motor UEF scores. The UEF scores (range: cognitive normal = 0 - cognitive impairment = 100) were determined by summing points given based on UEF dual-task performance and computing the percent difference from maximum possible points. Points were assigned based on variable comparisons to previously determined ranges for the UEF parameters, including: 1) flexion number; 2) range of motion variability (coefficient of variation of the flexion angle range), and 3) flexion variability (coefficient of variation of time distances between consecutive angular velocity peaks). Readers are referred to previous work for details regarding UEF motor scores.16
Brain scanning
A 3-Tesla Siemens Skyra MRI machine (Siemens Healthineers, Erlangan, Germany) was utilized for performing fMRI, which used blood oxygenation level-dependent (BOLD) contrast echo-planar imaging with the following sequence parameters: echo time of 30 msec, repetition time of 2 sec, 1.9 mm in-plane spatial resolution, and a 2 mm slice thickness with 62 slices for covering the whole brain. Each participant performed four trials; trial 1 and 2 were identical with similar cognitive task of counting backwards by one and trial 3 and 4 were also identical with similar cognitive tasks of counting backwards by three (Figure 1). The duration of each trial was six minutes and included six thirty-second task blocks and six thirty-second rest periods in between, starting with a rest period. The following task trials were repeated twice in each trial: 1) counting backwards (cognitive task); 2) UEF (motor task); and 3) combined counting and UEF (dual task) (Figure 1). For the UEF motor task, while laying down on the fMRI setup, participants flexed and extended the elbow of the dominant arm as consistently as possible with a self-selected pace for 30 seconds. Participants had their heads fixed with a head mount within the MRI bore and were instructed to maintain their upper arm motionless. All participants included in this study exhibited less than 1.5 mm of displacement magnitude as well as less than 1.5° angular motion along each axis, which were within the acceptable ranges of head motion for fMRI analysis.33 During each trial, participants were instructed to perform actions displayed on a screen in the MRI bore. The resting periods were added to allow for BOLD response to return to a baseline condition after each task. Similar to UEF outside of the MRI, we had two cognition tasks: 1) counting numbers backward by ones (Cog 1: Trial 1&2); and 2) counting numbers backward by threes (Cog 2: Trial 3&4). The first rest block in each trial lasted 50 seconds instead of 30 seconds to account for the necessary initial transition into steady-state magnetization, where the first 20 s (N = 10) were discarded.34
Figure 1:
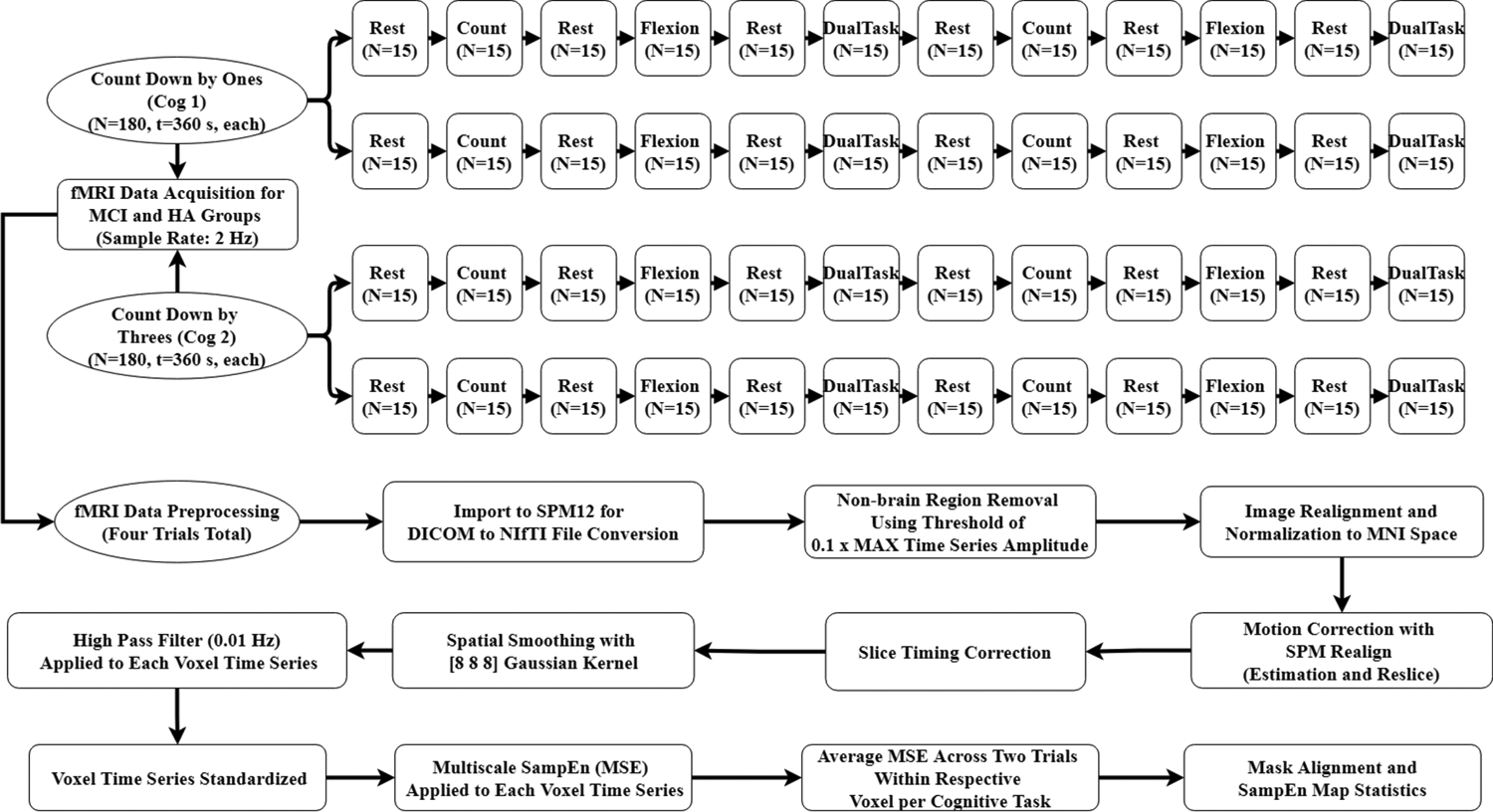
Flow chart demonstrating pre-processing steps in SPM12 and the customized MATLAB program for processing fMRI data. Our dual-task paradigm consisted of 30 s (N=15 data points) blocks for rest, counting (cognitive task), flexion (motor task), and counting + flexion (dual-task) conditions. Two trials resulted in two whole time series (N = number of data points, 180 data points each, for a time (t) of 260 second (s)), which underwent complexity analysis. Cog 1: counting down by ones; and Cog 2: counting down by threes; CN: cognitively normal; MCI: mild cognitive impairment; SMP: statistical parametric mapping; DICOM: digital imaging and communications in medicine; NIfTI: neuroimaging informatics technology initiative; MNI: Montreal Neurological Institute; SampEn: sample entropy; MSE: multiscale entropy; Hz: Hertz
fMRI data preprocessing
The fMRI images were preprocessed using Statistical Parametric Mapping (SPM)12 (Wellcome Department of Imaging, Neuroscience, London, UK) and custom MATLAB software. The preprocessing included (Figure 1): 1) DICOM to NIfTI conversion using the SPM12 import tool; 2) non-brain region removal using a threshold of 0.1 multiplied by the maximum voxel time series amplitude;29 3) image realignment and normalization to Montreal Neurological Institute (MNI)23 space (resampled into 3mm × 3mm × 3 mm voxels) utilizing custom MATLAB program; 4) head motion correction using SPM12 Realign (Estimation and Reslice) from the spatial pre-processing section;29 5) slice-timing correction using Hanning-windowed Sinc temporal interpolation35,36 included in SPM12; 6) spatial smoothing using a Gaussian Kernel of 8mm full width at half maximum;23,28,37,38 7) high pass filter of 0.01 Hz (128s) for each voxel time series;29,39 and 8) standardization of each voxel to a mean of zero and standard deviation of unity. The preprocessed data were afterwards used for complexity analysis across the whole brain using a custom MATLAB program (Mathworks, Sherborn, MA).
Sample entropy (SampEn) and multiscale entropy (MSE) analysis
Due to the low sampling frequency of the fMRI data, we used SampEn for brain function complexity analysis, since it is more reliable for smaller data sets.40 SampEn quantifies the signal complexity as the logarithmic likelihood of pattern reproducibility within a time series.41 MSE utilizes the SampEn formula but across multiple time scales in two steps.42 For our complexity analysis, first, a course-graining procedure was performed using the following equation:
| (Eq. 1) |
Where N is the original time series length, l is the time scale, and y1 is the resulting time series. A time scale of one, typically, results in the original time series, while others represent the system dynamics across different scale factors. The advantage of MSE over SampEn (scale 1) is that higher scale factors can improve the signal-to-noise ratio of a short time series.43 For the current study scale 6 was selected as suggested by previous work.44 The SampEn algorithm was then performed on the resulting course-grained time series as:
| (Eq. 2) |
Where N is the length of the time series, m is the pattern length for comparison, r is the tolerance for radius of similarity, and P is the probability of radius falling within the tolerance level r.40 SampEn was calculated here using a pattern length of m = 2 and a tolerance of r = 0.2 × SD, where SD is the standard deviation of the original time series.19
A custom MATLAB program was utilized to calculate MSE at each voxel for each time series. A threshold of 0.1 times the maximum signal was considered for determining voxels within the brain29 and subsequently, MSE maps were generated for these brain voxels.23,43 Standard masks for general regions were generated using the Wake Forest University PickAtlas toolbox (Wake Forest University, School of Medicine, Winston Salem, NC), which generates masks based on the Talairach Daemon database.45,46 Using the masking process, we included the gray matter of five general regions of frontal, parietal, temporal, and occipital lobes, and cerebellum. Further, a custom MATLAB program was utilized to modify the 90 functional region of interest atlas from the functional imaging in neuropsychiatric disorders lab47 for generating eight functional neural network masks associated with MCI and AD. The selected neural networks for complexity analysis included basal ganglia (motor symptoms and amyloid-β deposition,48 default mode (dorsal and ventral default mode associated with amyloid-β and tau pathology and episodic memory),49–53 central executive (right and left executive control associated with executive and compensatory cognitive control),49,54,55 language (amyloid-β and tau pathology),56 precuneus (disruption of functional connectivity due to AD,56,57 salience (anterior and posterior salience with altered brain function due to AD),58,59 sensorimotor (connectively alterations due to AD),49,60,61 and visuospatial (amyloid-β and tau pathology)56 networks for complexity analysis. The average MSE was calculated for two similar trials (average of Trail 1 and 2 for Cog 1 and Trail 3 and 4 for Cog 2 – See Figure 1) for all MSE maps created.
Statistical analysis
Univariate analysis of variance (ANOVA) was utilized to evaluate the differences in demographic characteristics and neuropsychological tests between the two cognitive groups of CN and MCI. Similarly, differences in SampEn and MSE values for each brain region were assessed via univariate ANOVA. The analyses were repeated after replacing cognitive groups with age. Further, multivariable ANOVA was used, with age, cognitive group, sex, and body mass index (BMI) (with significant association with SampEn or MSE) as independent variables and SampEn and MSE as the dependent variables.
We evaluated the associations between UEF scores (outside MRI), SampEn, and MSE values with cognition status (CN or MCI) as the dependent variable utilizing multiple logistic regression models; UEF scores, and SampEn (or MSE) values were considered as hypothesis covariates, and age, sex, and BMI (with significant association with SampEn or MSE) as adjusting covariates. To determine predictive covariates, first we tested for collinearity between independent variables (i.e., UEF scores, SampEn, MSE, age, and BMI) using variance inflation factor (VIF) values. A VIF cutoff value larger than 10 was considered an indication of presence of collinearity.62 We then employed a stepwise procedure based on Akaike information criterion values; subsequently, we computed the area under the curve (AUC) with 95% confidence interval utilizing receiver operating characteristics curves for each predictive model.
Finally, Pearson or Spearman’s rank correlation (based on the distribution of the data) was applied for assessing relationships between UEF scores (outside MRI) and SampEn (or MSE) values, as well as the relationship between SampEn (or MSE) values with age and MoCA scores. All data was analyzed using the JMP statistical program (version 14.2.0, copyright 2018 SAS institute Inc), and statistical significance was indicated when p<0.05.
Results
Participants
Among 24 participants that were recruited for this study, eight were classified as CN (age = 73.63 ± 3.70 years; BMI = 25.67 ± 4.74 kg/m2; and 50% female) and nine were MCI (age = 78.78 ± 8.39 years; BMI = 27.53 ± 5.29 kg/m2; and 77.8% female), while the rest were excluded based on several factors, including severe motion artifacts within fMRI data (n=2), left handedness (n=3), hyperintensities within white matter due to brain cancer (n=1), and lost to follow-up for the neuropsychological testing (n=1). None of the demographics were significantly different between the cognitive groups (p>0.13). The MoCA and WAIS-R Digit Symbol neuropsychological tests were significantly different between the groups (p≤0.05, Table 1). Of note, although expected trends were observable across other neuropsychological tests and UEF motor scores, these parameters were not significantly different between the cognitive groups due to our small sample of participants.
Table 1.
Average values of participant demographics and neuropsychological test results.
| Demographic information | CN (n=8) | MCI (n=9) | p-value (effect size) |
|---|---|---|---|
| Female, n (% of group) | 4.00 (50%) | 7.00 (77.8%) | 0.23 |
| Age, years (SD) | 73.63 (3.70) | 78.78 (8.39) | 0.13 (0.79) |
| Stature, cm (SD) | 169.70 (11.64) | 161.57 (9.96) | 0.14 (0.75) |
| Body Mass, kg (SD) | 74.20 (17.12) | 71.81 (14.54) | 0.76 (0.15) |
| BMI, kg/m2 (SD) | 25.67 (4.74) | 27.53 (5.29) | 0.46 (0.37) |
| Neuropsychological tests | |||
| UEF Cognitive Score 1 (SD) | 0.43 (0.27) | 0.57 (0.21) | 0.25 (0.58) |
| UEF Cognitive Score 2 (SD) | 0.45 (0.33) | 0.72 (0.33) | 0.12 (0.82) |
| Stroop Word (SD) | 87.00 (12.83) | 75.33 (18.71) | 0.16 (0.73) |
| Stroop Color (SD) | 63.13 (14.21) | 49.33 (13.44) | 0.06 (1.00) |
| Stroop Inhibition (SD) | 35.25 (7.76) | 24.56 (14.14) | 0.08 (0.94) |
| MoCA (SD) | 27.63 (2.26) | 19.11 (3.33) | < 0.01 (2.99) * |
| Rey Auditory (SD) | 53.38 (18.06) | 38.25 (18.21) | 0.12 (0.83) |
| COWAT (SD) | 37.75 (7.83) | 33.22 (9.12) | 0.29 (0.53) |
| WAIS-R Digit Symbol (SD) | 51.25 (5.95) | 36.67 (17.23) | 0.04 (1.13) * |
| Card Sort (SD) | 68.00 (10.84) | 72.50 (20.04) | 0.62 (0.23) |
n: number of participants in each group; CN: cognitively normal; MCI: mild cognitive impairment; SD = standard deviation; BMI = body mass index; UEF = upper extremity function; MoCA = Montreal cognitive assessment; COWAT = controlled oral word association test; and WAIS-R = Wechsler adult intelligence scale.
A significant difference between parameters (p<0.05) is indicated with the asterisk.
SampEn and MSE
Univariate ANOVA tests showed significant effect of age on MSE values among all five brain regions across both Cog 1 and Cog 2 conditions (r2≥0.31 and p≤0.02, Table 2 and Figure 2). On the other hand, univariate results showed significant associations between cognitive groups and MSE values within all brain regions except cerebellum for Cog 1, and only for frontal region for Cog 2 (p≤0.03, effect size=1.35±0.16 for significant associations, Table 2). Across all regions except cerebellum, MSE on average was 30% and 28% lower in the MCI group compared to CN (Table 2 and Figure 3) for Cog 1 and Cog 2 conditions, respectively. Additionally, multivariable analyses showed that when age and cognitive group were both included as independent variables, there were significant differences between cognitive groups in MSE for frontal and parietal brain regions for Cog 1 and frontal region for Cog 2 condition (p≤0.05, Table 2). Of note, gender and BMI were not included in the multivariable analyses because they were not associated with MSE results and were not significantly different between the cognitive groups.
Table 2.
Results of univariate analysis of variance for each of age (in years) or cognitive groups as independent variable, and multivariable analysis with age and cognitive group as independent variables.
| Brain Regions | MSE – Mean (SD) | Univariate p-value (effect size or r2) |
Multivariable p-value | |||
|---|---|---|---|---|---|---|
| CN | MCI | Cognitive group | Age | Cognitive group | Age | |
| Cerebellum - Cog 1 | 1.26 (0.30) | 1.00 (0.34) | 0.11 (0.82) | 0.02 (0.31)* | 0.40 | 0.03* |
| Cerebellum - Cog 2 | 1.26 (0.20) | 1.05 (0.48) | 0.24 (0.57) | <0.01 (0.42)** | 0.84 | 0.01* |
| Frontal - Cog 1 | 1.49 (0.10) | 1.14 (0.29) | 0.01 (1.57)* | 0.27 (0.08) | 0.03* | 0.15 |
| Frontal - Cog 2 | 1.51 (0.08) | 1.13 (0.45) | 0.01 (1.20)* | 0.28 (0.08) | 0.03* | 0.25 |
| Occipital - Cog 1 | 1.39 (0.22) | 1.00 (0.40) | 0.03 (1.23)* | <0.01 (0.46)** | 0.11 | 0.01* |
| Occipital - Cog 2 | 1.34 (0.10) | 1.04 (0.49) | 0.16 (0.85) | <0.01 (0.48)** | 0.60 | 0.01* |
| Parietal - Cog 1 | 1.50 (0.07) | 1.13 (0.35) | 0.01 (1.46)* | <0.01 (0.37)** | 0.04* | < 0.01* |
| Parietal - Cog 2 | 1.54 (0.24) | 1.17 (0.53) | 0.07 (0.91) | <0.01 (0.38)** | 0.29 | 0.01* |
| Temporal - Cog 1 | 1.48 (0.09) | 1.22 (0.27) | 0.02 (1.27)* | <0.01 (0.39)** | 0.10 | < 0.01* |
| Temporal - Cog 2 | 1.53 (0.15) | 1.23 (0.51) | 0.06 (0.80) | <0.01 (0.50)** | 0.27 | 0.01* |
| Neural Networks | MSE – Mean (SD) | Univariate p-value (effect size or r2) |
Multivariable p-value | |||
| CN | MCI | Cognitive group | Age | Cognitive group | Age | |
| Basal Ganglia - Cog 1 | 1.37 (0.19) | 0.97 (0.38) | 0.02 (1.33)* | 0.06 (0.22) | 0.07 | 0.05* |
| Basal Ganglia - Cog 2 | 1.38 (0.10) | 0.98 (0.51) | 0.05 (1.10)* | 0.03 (0.27)* | 0.16 | 0.11 |
| Default Mode - Cog 1 | 1.42 (0.15) | 1.03 (0.34) | 0.01 (1.44)* | <0.01 (0.40)** | 0.04* | 0.00* |
| Default Mode - Cog 2 | 1.42 (0.27) | 1.07 (0.42) | 0.06 (0.99) | 0.01 (0.36)* | 0.24 | 0.01* |
| Central Executive - Cog 1 | 1.47 (0.08) | 1.10 (0.33) | 0.01 (1.54)* | 0.08 (0.19) | 0.03* | 0.04* |
| Central Executive - Cog 2 | 1.50 (0.15) | 1.12 (0.45) | 0.02 (1.14)* | 0.05 (0.23) | 0.10 | 0.09 |
| Language - Cog 1 | 1.48 (0.12) | 1.17 (0.33) | 0.02 (1.25)* | <0.01 (0.38)** | 0.11 | 0.01* |
| Language - Cog 2 | 1.57 (0.08) | 1.19 (0.36) | 0.05 (1.47)* | <0.01 (0.43)** | 0.21 | 0.01* |
| Precuneus - Cog 1 | 1.40 (0.17) | 0.96 (0.41) | 0.01 (1.41)* | <0.01 (0.48)** | 0.05* | < 0.01 |
| Precuneus - Cog 2 | 1.34 (0.10) | 1.02 (0.52) | 0.17 (0.85) | <0.01 (0.54)** | 0.66 | < 0.01* |
| Salience - Cog 1 | 1.47 (0.15) | 1.05 (0.35) | 0.01 (1.56)* | 0.13 (0.14) | 0.03* | 0.09 |
| Salience - Cog 2 | 1.46 (0.23) | 1.04 (0.58) | 0.02 (0.96)* | 0.22 (0.10) | 0.08 | 0.17 |
| Sensorimotor - Cog 1 | 1.42 (0.14) | 1.09 (0.37) | 0.03 (1.16)* | 0.02 (0.33)* | 0.14 | 0.02* |
| Sensorimotor - Cog 2 | 1.42 (0.15) | 1.14 (0.44) | 0.17 (0.83) | <0.01 (0.49)** | 0.63 | 0.01* |
| Visual - Cog 1 | 1.37 (0.24) | 0.98 (0.39) | 0.03 (1.22)* | <0.01 (0.48)** | 0.12 | 0.01* |
| Visual - Cog 2 | 1.32 (0.10) | 1.02 (0.41) | 0.17 (1.00) | <0.01 (0.37)** | 0.62 | 0.01* |
| Visuospatial - Cog 1 | 1.53 (0.08) | 1.17 (0.36) | 0.01 (1.40)* | 0.11 (0.16) | 0.06 | 0.03* |
| Visuospatial - Cog 2 | 1.57 (0.24) | 1.18 (0.54) | 0.04 (0.93)* | 0.01 (0.34)* | 0.17 | 0.02* |
MSE: multiscale entropy, SD: standard deviation; CN: cognitively normal; MCI: mild cognitive impairment; Cog 1: counting down by ones; and Cog 2: counting down by threes.
A significant association is indicated with the asterisk (* for p<0.05 and ** for p<0.01).
Figure 2:
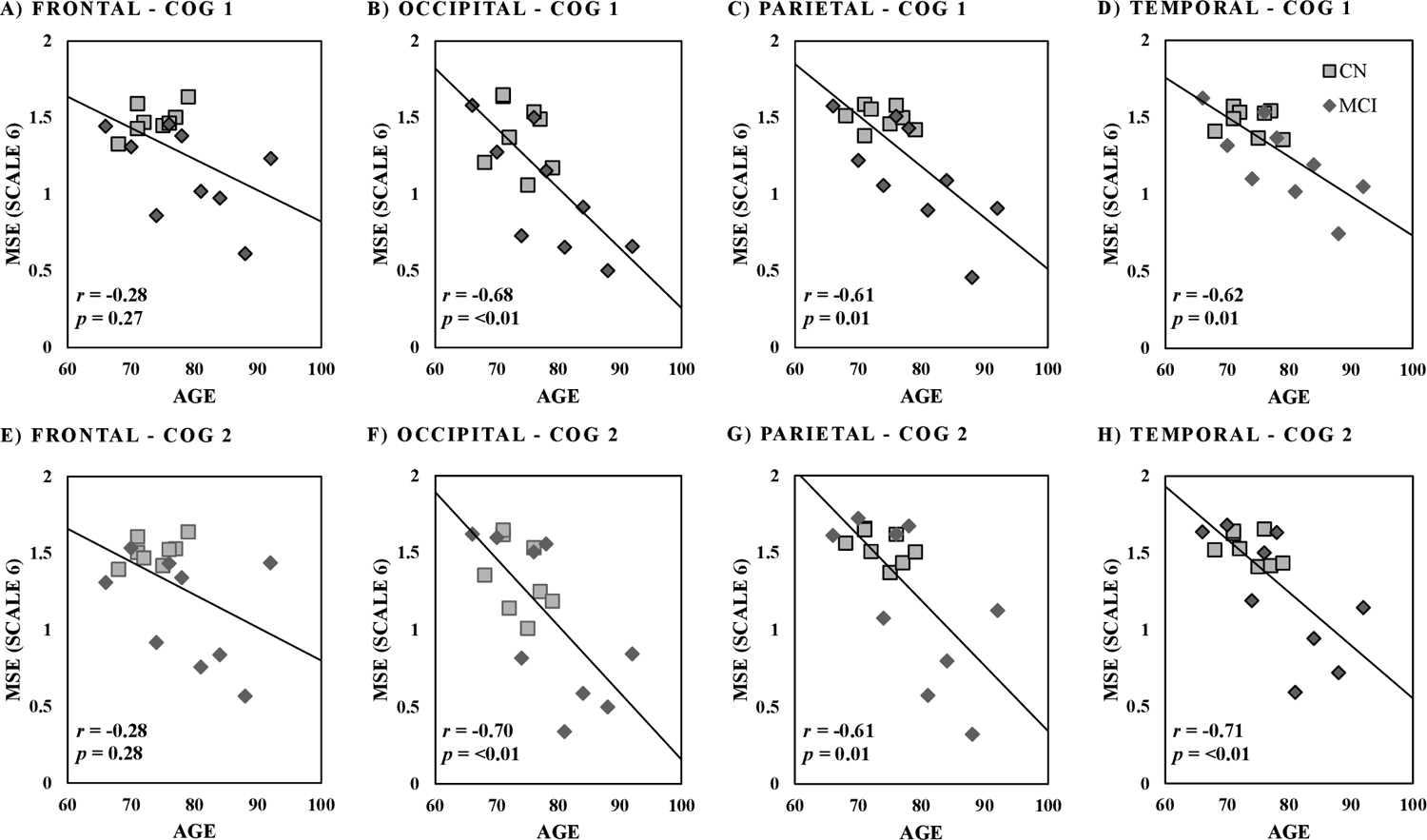
Spearman correlation between age (in years) and multiscale entropy (MSE scale 6) values across brain regions for Cog 1 of counting by ones (A-D) and Cog 2 of counting backward by threes (E-H). p-value cutoff is 0.05.
Figure 3:
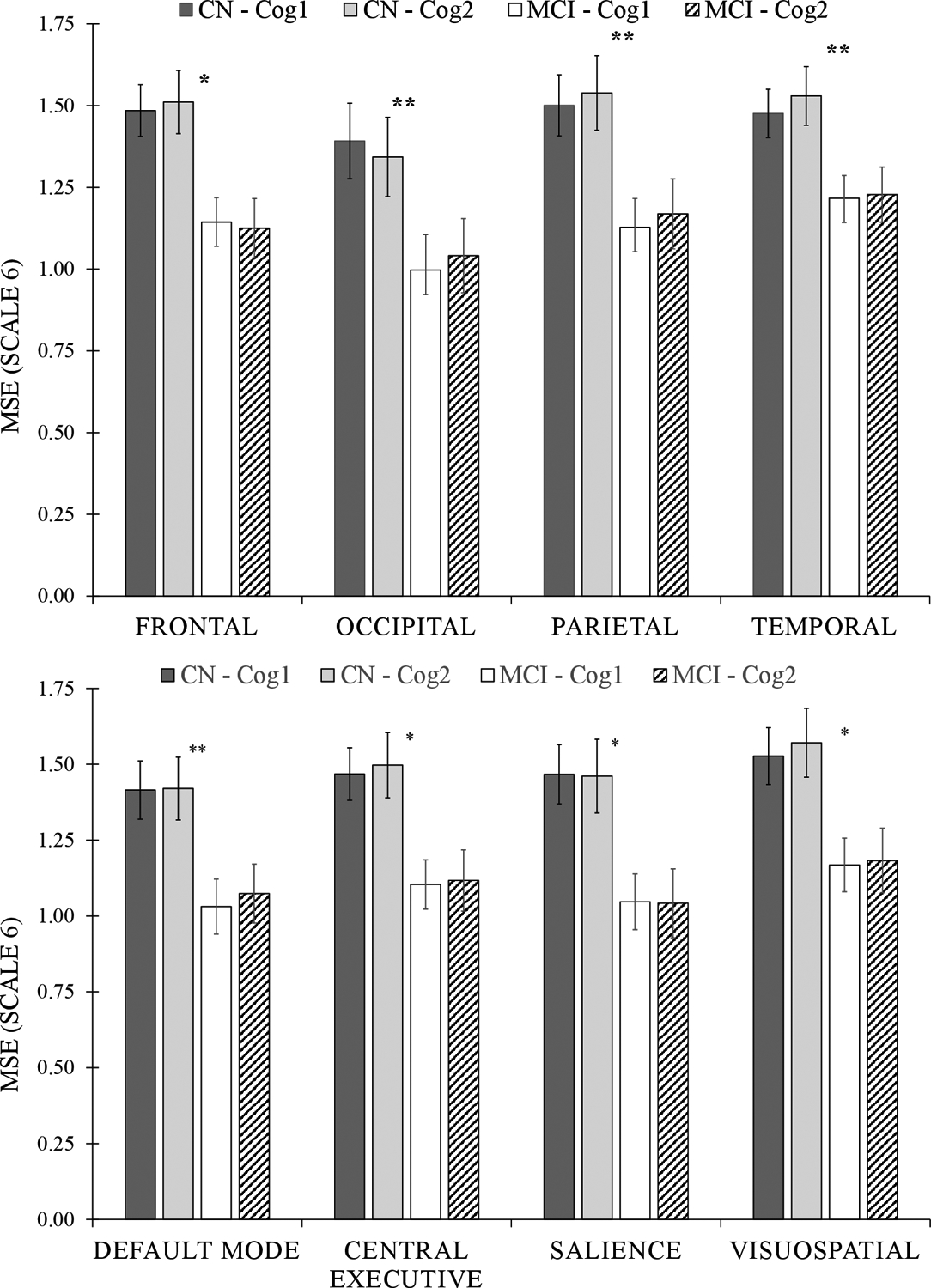
Results from univariate analysis of variance models for differences in multiscale entropy (MSE Scale 6) between cognitive groups across brain regions (top) and neural networks (bottom). A significant between-group (CN versus MCI) difference is indicated with the asterisk (* for p<0.05 and ** for p<0.01). Cog 1: counting down by ones; and Cog 2: counting down by threes; CN: cognitively normal; and MCI: mild cognitive impairment.
Results for neural networks showed significant univariate association between age and MSE for all neural networks including basal ganglia, default mode, central executive, language, precuneus, salience, sensorimotor, visual, and visuospatial across both Cog 1 and Cog 2 conditions (r2≥0.23 and p≤0.05, Table 2 and Figure 4). Univariate results showed significant differences in MSE between MCI and CN for all neural networks under the Cog 1 condition (p≤0.04, effect size=1.34±0.16, Table 2), and for basal ganglia, central executive, language, salience, and visuospatial networks under the Cog 2 condition (p≤0.05, effect size=1.12±0.22 for significant associations, Table 2). Multivariable analysis showed significant group differences across the default mode, central executive, precuneus, and salience neural networks, when adjusted with age, under the Cog 1 condition (Table 2). Overall, within both univariate and multivariate analyses, the strongest association between MSE and cognitive status was observed for executive control and salience networks, with respective 34% and 40% lower MSE for MCI compared to CN (Table 2 and Figure 3).
Figure 4:
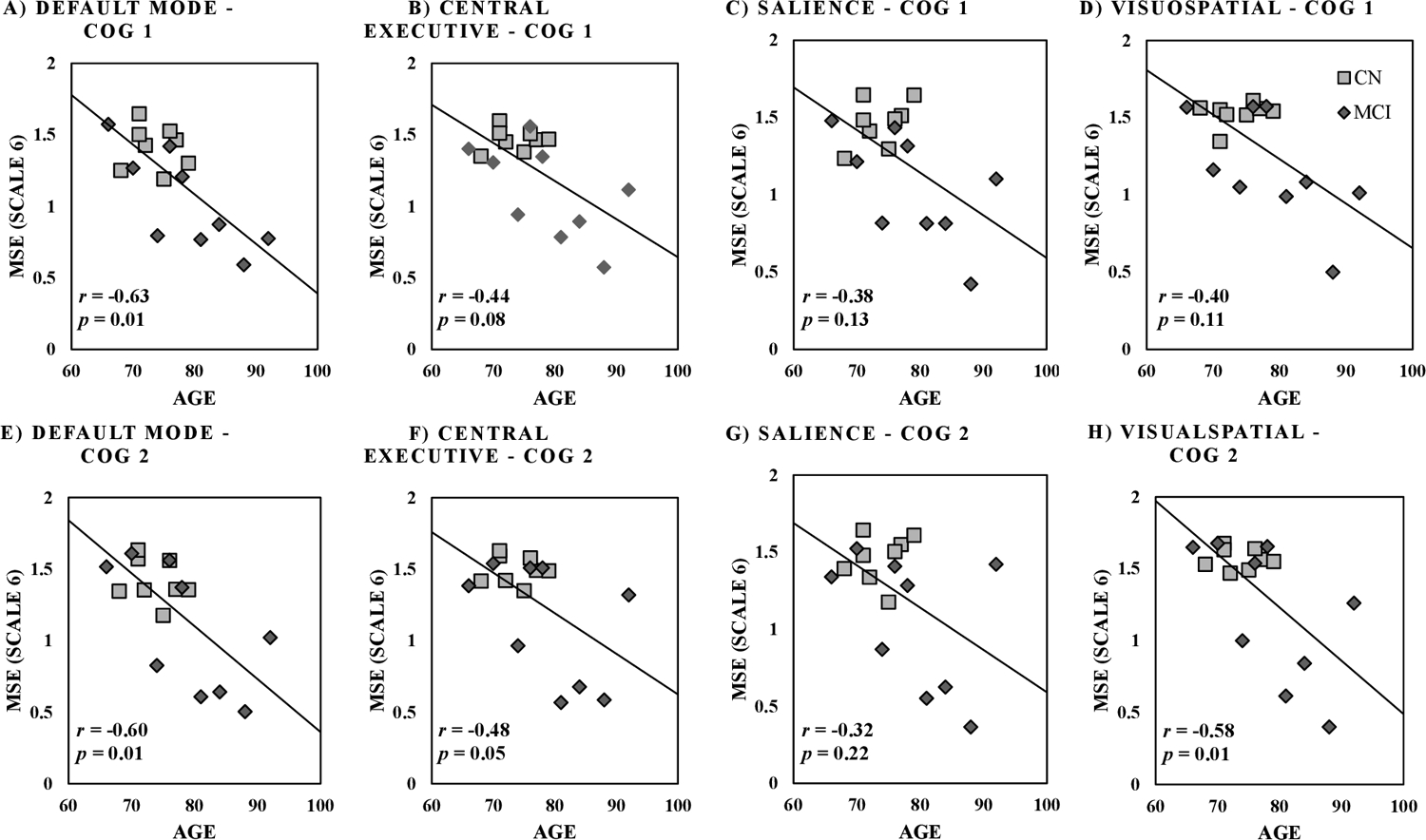
Spearman correlation between age (in years) and multiscale entropy (MSE scale 6) values across neural networks for Cog 1 of counting by ones (A-D) and Cog 2 of counting backward by threes (E-H). p-value cutoff is 0.05.
Logistic results showed that the frontal lobe and salience network were best predictors of cognitive status (AUC of 0.92 and 0.89 for frontal lobe and salience network for Cog 1 and 0.86 and 0.81 for Cog 2). Prediction of cognitive status improved when MSE of the frontal lobe or salience network were included to the UEF scores, where the AUC went from 0.72 to 0.93 and 0.88 for Cog 1 and from 0.74 to 0.88 and 0.83 for Cog 2, respectively (Table 3).
Table 3.
Multivariable ordinal logistic prediction models for cognition status.
| UEF Score Only - Counting Down by Ones (Sensitivity = 0.67; Specificity = 0.75; AUC = 0.72) | ||||||
| Estimate | Standard Error | χ2 | P value | Lower 95% | Upper 95% | |
| Intercept | −1.29 | 1.28 | 1.02 | 0.31 | −4.19 | 1.07 |
| UEF Cognitive Score | 2.85 | 2.45 | 1.36 | 0.24 | −1.47 | 8.65 |
| MSE Only - Counting Down by Ones (Sensitivity = 0.89; Specificity = 0.75; AUC = 0.92) | ||||||
| Intercept | 22.67 | 13.60 | 2.78 | 0.10 | 5.12 | 60.61 |
| Frontal Lobe | −16.23 | 9.55 | 2.89 | 0.09 | −42.72 | −3.80 |
| MSE Only - Counting Down by Ones (Sensitivity = 0.78; Specificity = 0.75; AUC = 0.89) | ||||||
| Intercept | 10.87 | 5.92 | 3.38 | 0.07 | 2.68 | 26.56 |
| Salience Network | −8.15 | 4.29 | 3.60 | 0.06 | −19.51 | −2.11 |
| Combined - Counting Down by Ones (Sensitivity = 0.89; Specificity = 0.75; AUC = 0.93) | ||||||
| Intercept | 25.58 | 15.70 | 2.65 | 0.10 | 4.36 | 67.80 |
| UEF Cognitive Score | 4.60 | 4.61 | 0.99 | 0.32 | −4.15 | 15.77 |
| Frontal Lobe | −19.50 | 11.63 | 2.81 | 0.09 | −50.73 | −4.13 |
| Combined - Counting Down by Ones (Sensitivity = 0.78; Specificity = 0.75; AUC = 0.88) | ||||||
| Intercept | 10.46 | 6.51 | 2.58 | 0.11 | 0.99 | 27.55 |
| UEF Cognitive Score | 2.72 | 3.45 | 0.62 | 0.43 | −4.39 | 10.81 |
| Salience Network | −8.69 | 4.95 | 3.08 | 0.08 | −22.41 | −1.95 |
| UEF Score Only - Counting Down by Threes (Sensitivity = 0.67; Specificity = 0.75; AUC = 0.72) | ||||||
| Intercept | −1.39 | 1.14 | 1.49 | 0.22 | −4.12 | 0.64 |
| UEF Cognitive Score | 2.55 | 1.67 | 2.32 | 0.13 | −0.44 | 6.44 |
| MSE Only - Counting Down by Threes (Sensitivity = 0.89; Specificity = 0.75; AUC = 0.86) | ||||||
| Intercept | 22.01 | 13.48 | 2.67 | 0.10 | 4.08 | 57.20 |
| Frontal Lobe | −15.40 | 9.28 | 2.75 | 0.10 | −39.71 | −3.01 |
| MSE Only - Counting Down by Threes (Sensitivity = 0.67; Specificity = 0.75; AUC = 0.81) | ||||||
| Intercept | 7.67 | 5.06 | 2.30 | 0.13 | 1.24 | 21.80 |
| Salience Network | −5.69 | 3.59 | 2.51 | 0.11 | −15.67 | −0.99 |
| Combined - Counting Down by Threes (Sensitivity = 0.89; Specificity = 0.75; AUC =0.88) | ||||||
| Intercept | 23.71 | 17.59 | 1.82 | 0.18 | 1.98 | 71.27 |
| UEF Cognitive Score | −0.43 | 2.63 | 0.03 | 0.87 | −6.34 | 4.83 |
| Frontal Lobe | −16.42 | 11.57 | 2.02 | 0.16 | −47.96 | −2.31 |
| Combined - Counting Down by Threes (Sensitivity = 0.89; Specificity = 0.75; AUC = 0.83) | ||||||
| Intercept | 6.49 | 5.38 | 1.45 | 0.23 | −1.49 | 21.17 |
| UEF Cognitive Score | 1.03 | 1.98 | 0.27 | 0.60 | −2.80 | 5.47 |
| Salience Network | −5.23 | 3.60 | 2.11 | 0.15 | −15.23 | −0.22 |
AUC: area under curve of receiver operating characteristic curve; and UEF: upper-extremity function; and MSE: multiscale entropy.
A significant association of p<0.05 is indicated with the asterisk.
Finally, UEF motor score measured outside of the fMRI was significantly associated with MSE values of five brain regions and six neural networks within Cog 2 condition (p≤0.05 and ρ≥0.49 for Spearman, Table 4), including cerebellum, frontal, occipital, parietal, and temporal lobes and default mode, central executive, language, salience, sensorimotor, visual, and visuospatial networks. On the other hand, for the Cog 1 condition, only the occipital lobe and visual and visuospatial networks showed MSE values that were significantly associated with UEF motor scores (p≤0.05 and ρ≥0.49 for Spearman, Table 4). Results showed significant associations between MSE values within frontal and parietal brain regions and the salience network with MoCA scores for Cog 1 (p≤0.05 and ρ≥0.48 for Spearman, Table 4) and frontal region MSE values with MoCA score for Cog 2 (p=0.03 and ρ = 0.52 for Spearman, Table 4).
Table 4:
Correlations between brain function multiscale entropy with upper-extremity function motor score and Montreal cognitive assessment (MoCA).
| Brain Regions | Motor Score | MoCA | ||
|---|---|---|---|---|
| Spearman ρ | p-value | Spearman ρ | p-value | |
| Cerebellum Cog1 | −0.44 | 0.07 | 0.23 | 0.37 |
| Cerebellum Cog2 | −0.54 | 0.03 | 0.16 | 0.55 |
| Frontal Cog1 | −0.25 | 0.33 | 0.60 | 0.01 |
| Frontal Cog2 | −0.61 | 0.01 | 0.52 | 0.03 |
| Occipital Cog1 | −0.49 | 0.05 | 0.31 | 0.23 |
| Occipital Cog2 | −0.49 | 0.05 | 0.13 | 0.62 |
| Parietal Cog1 | −0.39 | 0.12 | 0.48 | 0.05 |
| Parietal Cog2 | −0.57 | 0.02 | 0.16 | 0.55 |
| Temporal Cog1 | −0.45 | 0.07 | 0.35 | 0.16 |
| Temporal Cog2 | −0.68 | 0.00 | 0.26 | 0.31 |
| Neural Network | Motor Score | MoCA | ||
| Spearman ρ | p-value | Spearman ρ | p-value | |
| Basal Ganglia Cog1 | −0.23 | 0.37 | 0.38 | 0.13 |
| Basal Ganglia Cog2 | −0.45 | 0.07 | 0.18 | 0.48 |
| Default Mode Cog1 | −0.36 | 0.15 | 0.40 | 0.12 |
| Default Mode Cog2 | −0.56 | 0.02 | 0.17 | 0.51 |
| Executive Control Cog1 | −0.44 | 0.08 | 0.40 | 0.12 |
| Executive Control Cog2 | −0.63 | 0.01 | 0.36 | 0.16 |
| Language Cog1 | −0.46 | 0.06 | 0.38 | 0.13 |
| Language Cog2 | −0.67 | 0.00 | 0.26 | 0.32 |
| Precuneus Cog1 | −0.47 | 0.06 | 0.40 | 0.11 |
| Precuneus Cog2 | −0.45 | 0.07 | 0.05 | 0.85 |
| Salience Cog1 | −0.32 | 0.22 | 0.51 | 0.04 |
| Salience Cog2 | −0.53 | 0.03 | 0.40 | 0.12 |
| Sensorimotor Cog1 | −0.47 | 0.05 | 0.32 | 0.22 |
| Sensorimotor Cog2 | −0.56 | 0.02 | 0.12 | 0.65 |
| Visual Cog1 | −0.51 | 0.04 | 0.30 | 0.25 |
| Visual Cog2 | −0.38 | 0.14 | 0.08 | 0.76 |
| Visuospatial Cog1 | −0.51 | 0.04 | 0.30 | 0.24 |
| Visuospatial Cog2 | −0.50 | 0.04 | 0.23 | 0.37 |
MSE: multiscale entropy; Cog 1: counting down by ones; and Cog 2: counting down by threes.
A significant association of p<0.05 is indicated with the asterisk.
Discussion
Complexity and Brain Function
As hypothesized, complexity analysis revealed significant differences in brain activity between CN and MCI older adults. Overall, a lower time-domain complexity of brain activity was observed among older adults with signs of early-stage cognitive impairment, compared to CN. A lower entropy value is associated with neuronal death and synaptic disconnections linked with memory loss;18,21,25 the less connections between neurons, the less complex (more predictable) the signal becomes, as there is less neuronal activity in a given brain area.25 Based on the current findings, the observed differences in brain function complexity were more pronounced across frontal, parietal, and temporal brain regions, and default mode, central executive, precuneus, and salience brain networks. Previous research suggests that synaptic losses occur unevenly throughout the cortical regions in the AD brain, where severity is highest in the frontal lobe and lowest within the occipital lobe;63 additionally, synaptic losses begin within the temporal lobe or more specifically within the limbic entorhinal cortex, and radiate outward from the hippocampus as a linear function of hippocampal volume loss.63,64 It has been previously found that AD affects the following brain networks: the default mode, central executive, and salience. Dysfunction in one of these networks can affect the performance of the other twoS65 Specifically, the salience network can affect the switching between the default mode and central executive when performing self-referential, conscious motor control, or memory-based tasks.64–66 Results from the current study suggest that fMRI complexity analysis is sensitive to detect these gradual alterations in brain function within specific brain regions/networks.
Further, a significant association was observed between age and brain function complexity; older participants showed less brain function complexity compared to younger individuals (Table 2 and Figure 2 and 4). Previous research done by Smith et al. suggested similar trends of complexity reduction with healthy aging, reporting significant differences in MSE between young (age of 23±2 years) and older (age of 66 ± 3 years) adults.67 In another study, a negative significant correlation was reported between approximate entropy and age, specially across frontal, parietal, limbic, temporal, and cerebellum regions.68 All these results, in agreement to the current findings, confirm the theory of higher complexity for healthier and more robust physiological systems.27
Current findings suggest that complexity analysis of the brain function from cortical regions may provide a stronger indicator of early-stage AD, compared to subcortical deep brain regions. The effect of cognitive impairment on complexity of brain function has been investigated in previous research using fMRI, EEG, and more recently fNIRS. In agreement with current findings, resting state fMRI studies showed smaller complexity due to cognitive impairment in cortical brain regions, including temporal, parietal, occipital, and frontal lobes.20,69 The observed smaller complexity of fNIRS and EEG signal in AD also support the fact that measuring the complexity of cortical brain regions is a reliable methodology for assessing cognitive impairment, rather than deep brain regions.70,71 This is primarily due to the well-known phenomena of increased recruitment of cortical neurons when brain damage occurs due to the plasticity of cortical brain regions,63,64 the capacity of which reduces in AD.63 Furthermore, cortical brain regions connect directly to sub-cortical regions like the hippocampus,63,64,66 where early AD has been identified with volume loss analysis. It is supported by evidence that complexity analysis of lower temporal frequencies similar to the current setting can reflect long range interconnectivity between neural populations (e.g., cortical and sub-cortical regions) and their respective synaptic disconnections and loss of signal complexity.43 Subsequently, it is possible to detect brain damage early via observation of complexity in cortical time-series.
Unlike most research focusing on resting-state brain function, the current study employed an upper-extremity motor task to assess brain function complexity between healthy and cognitively impaired older adults. Previous motor task-based fMRI studies utilized finger-tapping and imagery gait to determine brain function alterations due to AD. These studies found that atrophy in the hippocampus was directly correlated with hyper- and hypo-activations throughout the brain, evidence of compensatory processes for motor-based control, and deficits in the deactivation of specific regions in the default mode.64,66 Current findings suggest that consistent normal-speed elbow flexion motor task may provide advantages over previously implemented approaches. First, UEF is measurable with sensors; although motor performance was not measured in the current study due to equipment limitations, UEF motor task can be measured using fMRI-friendly accelerometers or cameras. Further, UEF may provide a better direct assessment of motor performance compared to imagery tasks (e.g., imagery gait), since previous work suggested difference in imagery and actual task in terms of both brain function and the task performance.66,72 Further, although gait dual-tasking has been commonly validated for cognitive impairment assessment,73–75 it relies heavily on procedural memory systems, while novel motor functions that requires some degree of skill learning, such as UEF, may increase the demand on attentional resources and working memory to better reveal cognitive impairment.76,77
In addition to UEF as a novel method for performing motor task in the fMRI setting, we also employed the task of counting numbers in combination with UEF (dual-task UEF). In our previous work we showed association between UEF and gait dual-task performance, within which, a better prediction of cognitive impairment (15% higher accuracy) was achieved using UEF motor variability parameters compared to gait measures.14 Further, in association with cognitive components, we previously observed that visuospatial/executive, attention, delayed recall, and orientation MoCA subcategories were better correlated with the UEF dual-task performance.17 These observations agree with the current fMRI findings, which show association between cognitive impairment and brain function complexity. For instance, previous work suggested that default mode is linked to episodic memory,78 frontoparietal to executive function,54 and central executive to attention during dual-tasking.79
Clinical Implications
Recent studies suggest that motor deficits may be considered as early markers of Alzheimer’s disease and cognitive impairment in general.64,66,72 Current findings provided evidence that by assessing brain function in combination with motor performance we would be able to improve cognitive impairment detection. Further, complexity of brain function in cortical regions were mostly affected by cognitive impairments. These findings showed potential for developing a quick and objective measure of cognitive decline using UEF and brain function measurement tools such as EEG and fNIRS. Once targeted neural networks are identified, it is possible to limit the confounding effects due to motion artifacts and reduce the duration of assessments by implementing EEG and fNIRS, because of higher temporal frequency of these devices. These technologies can help with early and objective screening of cognitive decline to establish a baseline, facilitate tracking cognition over time, and ensure appropriate care for cognitive health.
Limitations and Future Direction
The following limitations exist, which warrant future research. First, the sample size of the two CN and MCI groups was small. The effect size hints at the potential for a larger sample size to yield more significant results. Second, the present study only included MCI older adults and no AD patients, so we were unable to determine whether the observed trend of smaller complexity for MCI would be expandable to AD patients as well. We advise that this study be upscaled in future research and include older adults with AD. Our study also consisted of a very low sampling rate within the fMRI (<0.5 Hz); therefore, assessing complexity changes across paradigm blocks, such as transitioning from resting-state to dual-task condition, was not possible. In future work, changes in complexity measures, especially dual-task costs, should be investigated within fNIRS and/or EEG setup with higher sampling frequencies.
Conclusion
In the current work, a significant difference in brain function complexity, measured by entropy analysis, was found between CN and MCI participants while performing a series of tasks including elbow flexion, counting numbers, and both elbow flexion and counting simultaneously. MCI participants showed on average 28% smaller brain function complexity across frontal, occipital, parietal, and temporal regions, and 35% smaller brain function complexity across default mode, central executive, salience, and visuospatial neural networks. Further, there were significant negative correlations between brain function complexity and age. Using brain function measures, MCI identification was improved by 14–24% compared to models that included only dual-task motor performance. Accordingly, current findings suggest that combining brain and motor function measures may provide an accurate tool for assessing early-stage cognitive impairment among older adults.
Acknowledgments and Disclosure:
Thanks to coordinators for their significant contribution in coordinating study staff, and to Scott Squire for assisting with fMRI data collection. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding:
This project was supported by an award from Bio5 Rapid Grants (Improving Health Technology and Research Initiative Fund - TRIF). Dr. Toosizadeh was supported by National Institute of Health (NIH/NIA, R21AG059202). Dr. Chou was supported by National Institute of Health (NIH/NIA, R01AG062543).
References
- [1].Athilingam P, Visovsky C, Elliott AF, Rogal PJ. Cognitive screening in persons with chronic diseases in primary care: Challenges and recommendations for practice. Am J Alzheimers Dis Other Demen 2015;30:547–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Takizawa C, Thompson PL, van Walsem A, Faure C, Maier WC. Epidemiological and economic burden of Alzheimer’s disease: A systematic literature review of data across europe and the United States of America. J Alzheimers Dis 2014;43:1271–84. [DOI] [PubMed] [Google Scholar]
- [3].Bradford A, Kunik ME, Schulz P, Williams SP, Singh H. Missed and delayed diagnosis of dementia in primary care: Prevalence and contributing factors. Alzheimer Dis Assoc Disord 2009;23:306–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Camicioli R, Howieson D, Lehman S, Kaye J. Talking while walking: The effect of a dual task in aging and Alzheimer’s disease. Neurology 1997;48:955–8. [DOI] [PubMed] [Google Scholar]
- [5].Montero-Odasso M, Muir SW, Speechley M. Dual-task complexity affects gait in people with mild cognitive impairment: The interplay between gait variability, dual tasking, and risk of falls. Arch Phys Med Rehabil 2012;93:293–9. [DOI] [PubMed] [Google Scholar]
- [6].Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: Divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc 2003;51:1633–7. [DOI] [PubMed] [Google Scholar]
- [7].Cognitive aging: progress in understanding and opportunities for action. Washington, D.C.: National Academies Press; 2015. [PubMed] [Google Scholar]
- [8].Verhaeghen P, Steitz DW, Sliwinski MJ, Cerella J. Aging and dual-task performance: A meta-analysis. Psychol Aging 2003;18:443–60. [DOI] [PubMed] [Google Scholar]
- [9].Volkow ND, Gur RC, Wang GJ, et al. Association between decline in brain dopamine activity with age and cognitive and motor impairment in healthy individuals. Am J Psychiatry 1998;155:344–9. [DOI] [PubMed] [Google Scholar]
- [10].Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol 2001;58:498–504 [DOI] [PubMed] [Google Scholar]
- [11].Hausdorff JM, Yogev G, Springer S, Simon ES, Giladi N. Walking is more like catching than tapping: gait in the elderly as a complex cognitive task. Exp Brain Res 2005;164:541–8. [DOI] [PubMed] [Google Scholar]
- [12].Kearney FC, Harwood RH, Gladman JRF, Lincoln N, Masud T. The relationship between executive function and falls and gait abnormalities in older adults: A systematic review. Dement Geriatr Cogn Disord 2013;36:20–35. [DOI] [PubMed] [Google Scholar]
- [13].Ruiz M, Peña M, Cohen A, et al. Physical and cognitive function assessment to predict postoperative outcomes of abdominal surgery. J Surg Res 2021;267:495–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Ehsani H, Mohler MJ, O’Connor K, Zamrini E, Tirambulo C, Toosizadeh N. The association between cognition and dual-tasking among older adults: the effect of motor function type and cognition task difficulty. Clin Interv Aging 2019;14:659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Asghari M, Ehsani H, Cohen A, Tax T, Mohler J, Toosizadeh N. Nonlinear analysis of the movement variability structure can detect aging-related differences among cognitively healthy individuals. Hum Mov Sci 2021;78:102807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Toosizadeh N, Ehsani H, Wendel C, Zamrini E, Connor KO, Mohler J. Screening older adults for amnestic mild cognitive impairment and early-stage Alzheimer’s disease using upper-extremity dual-tasking. Sci Rep 2019;9:10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Toosizadeh N, Najafi B, Reiman EM, et al. Upper-Extremity dual-task function: an innovative method to assess cognitive impairment in older adults. Front Aging Neurosci 2016;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Jelles B, Van Birgelen J, Slaets J, Hekster R, Jonkman E, Stam C. Decrease of non-linear structure in the EEG of Alzheimer patients compared to healthy controls. Clin Neurophysiol 1999;110:1159–67. [DOI] [PubMed] [Google Scholar]
- [19].Sokunbi MO, Staff RT, Waiter GD, et al. Inter-individual differences in fmri entropy measurements in old age. IEEE Trans Biomed Eng 2011;58:3206–14. [DOI] [PubMed] [Google Scholar]
- [20].Sun J, Wang B, Niu Y, et al. Complexity analysis of EEG, MEG, and fMRI in mild cognitive impairment and Alzheimer’s disease: A review. Entropy 2020;22:239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Tononi G. Complexity and coherency: Integrating information in the brain. Trends Cogn Sci 1998;2:474–84. [DOI] [PubMed] [Google Scholar]
- [22].Yang AC, Huang C-C, Yeh HL, et al. Complexity of spontaneous BOLD activity in default mode network is correlated with cognitive function in normal male elderly: a multiscale entropy analysis. Neurobiol Aging 2013;34:428–38. [DOI] [PubMed] [Google Scholar]
- [23].Niu Y, Wang B, Zhou M, et al. Dynamic complexity of spontaneous BOLD activity in Alzheimer’s disease and mild cognitive impairment using multiscale entropy analysis. Front Neurosci 2018;12:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Yang AC, Wang S-J, Lai K-L, et al. Cognitive and neuropsychiatric correlates of EEG dynamic complexity in patients with Alzheimer’s disease. Prog Neuropsychopharmacol Biol Psychiatry 2013;47:52–61. [DOI] [PubMed] [Google Scholar]
- [25].Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol 2004;115:1490–505. [DOI] [PubMed] [Google Scholar]
- [26].Lipsitz LA. Dynamics of stability: The physiologic basis of functional health and frailty. J Gerontol A Biol Sci Med Sci 2002;57:B115–B25. [DOI] [PubMed] [Google Scholar]
- [27].Lipsitz LA. Physiological complexity, aging, and the path to frailty. Sci Aging Knowledge Environ 2004;2004:pe16. [DOI] [PubMed] [Google Scholar]
- [28].Sokunbi MO. Sample entropy reveals high discriminative power between young and elderly adults in short fMRI data sets. Front Neuroinform 2014;8:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Sokunbi MO, Fung W, Sawlani V, Choppin S, Linden DEJ, Thome J. Resting state fMRI entropy probes complexity of brain activity in adults with ADHD. Psychiatry Res Neuroimaging 2013;214:341–8. [DOI] [PubMed] [Google Scholar]
- [30].Sokunbi MO, Gradin VB, Waiter GD, et al. Nonlinear complexity analysis of brain fmri signals in schizophrenia. PLoS One 2014;9:e95146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].World Medical Association. World Medical Association declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013;310:2191. [DOI] [PubMed] [Google Scholar]
- [32].Malek-Ahmadi M, Powell JJ, Belden CM, et al. Age- and education-adjusted normative data for the Montreal Cognitive Assessment (MoCA) in older adults age 70–99. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn 2015;22:755–61. [DOI] [PubMed] [Google Scholar]
- [33].Yang AC, Huang C-C, Liu M-E, et al. The APOE E4 Allele affects complexity and functional connectivity of resting brain activity in healthy adults. Hum Brain Mapp 2014; 35:3238–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Muresan L, Renken R, Roerdink JB, Duifhuis H. Position-history and spin-history artifacts in fMRI time series. In: Clough AV, Chen C-T, eds. Proc Spie 4683: Medical Imaging; 2002. [Google Scholar]
- [35].Grootoonk S, Hutton C, Ashburner J, et al. Characterization and correction of interpolation effects in the realignment of fMRI time series. Neuroimage 2000;11:49–57. [DOI] [PubMed] [Google Scholar]
- [36].Sladky R, Friston KJ, Tröstl J, Cunnington R, Moser E, Windischberger C. Slice-timing effects and their correction in functional MRI. Neuroimage 2011;58:588–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Sarraf S, Tofighi G. DeepAD: Alzheimer′ s disease classification via deep convolutional neural networks using MRI and fMRI. bioRxiv 2017:070441 (January, 14, 2017) 10.1101/070441 [DOI] [Google Scholar]
- [38].Sarraf S, Tofighi G. Classification of Alzheimer’s disease using fmri data and deep learning convolutional neural networks. arXiv 2016;160308631 [cs]. (March, 29, 2016) 10.48550/arXiv.1603.08631 [DOI] [Google Scholar]
- [39].Ashby FG. Statistical analysis of fMRI data. Cambridge, Mass: MIT Press; 2011:332. [Google Scholar]
- [40].Richman JS, Moorman JR. Physiological time-series analysis using approximate entropy and sample entropy. Am J Physiol Heart Circ Physiol 2000;278:H2039–H49. [DOI] [PubMed] [Google Scholar]
- [41].Kaffashi F, Foglyano R, Wilson CG, Loparo KA. The effect of time delay on approximate & sample entropy calculations. Physica D 2008;237:3069–74. [Google Scholar]
- [42].Costa M, Goldberger AL, Peng CK. Multiscale entropy analysis of complex physiologic time series. Phys Rev Lett 2002;89:068102. [DOI] [PubMed] [Google Scholar]
- [43].Wang DJJ, Jann K, Fan C, et al. Neurophysiological basis of multi-scale entropy of brain complexity and its relationship with functional connectivity. Front Neurosci 2018;12:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Niu Y, Wang B, Zhou M, et al. Dynamic complexity of spontaneous bold activity in Alzheimer’s disease and mild cognitive impairment using multiscale entropy analysis. Front Neurosci 2018;12:677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Lancaster JL, Summerlin JL, Rainey L, Freitas CS, Fox PT. The Talairach Daemon a database server for talairach atlas labels. NeuroImage (Orlando, Fla) 1997;5:S633. [Google Scholar]
- [46].Lancaster JL, Woldorff MG, Parsons LM, et al. Automated Talairach Atlas labels for functional brain mapping. Hum Brain Mapp 2000;10:120–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Shirer WR, Ryali S, Rykhlevskaia E, Menon V, Greicius MD. Decoding subject-driven cognitive states with whole-brain connectivity patterns. Cereb Cortex 2012;22:158–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Vöglein J, Paumier K, Jucker M, et al. Clinical, pathophysiological and genetic features of motor symptoms in autosomal dominant Alzheimer’s disease. Brain 2019;142:1429–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li HJ, Hou XH, Liu HH, Yue CL, He Y, Zuo XN. Toward systems neuroscience in mild cognitive impairment and Alzheimer’s disease: A meta-analysis of 75 fMRI studies. Hum Brain Mapp 2015;36:1217–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Kapogiannis D, Mattson MP. Disrupted energy metabolism and neuronal circuit dysfunction in cognitive impairment and Alzheimer’s disease. Lancet Neurol 2011;10:187–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Greicius MD, Srivastava G, Reiss AL, Menon V. Default-mode network activity distinguishes Alzheimer’s disease from healthy aging: evidence from functional MRI. Proc Natl Acad Sci 2004;101:4637–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Greicius MD, Krasnow B, Reiss AL, Menon V. Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci 2003;100:253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Zanto TP, Gazzaley A. Fronto-parietal network: flexible hub of cognitive control. Trends Cogn Sci 2013;17:602–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Cole MW, Reynolds JR, Power JD, Repovs G, Anticevic A, Braver TS. Multi-task connectivity reveals flexible hubs for adaptive task control. Nat Neurosci 2013;16:1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Watanabe H, Bagarinao E, Yokoi T, et al. Tau accumulation and network breakdown in Alzheimer’s disease. Adv Exp Med Biol 2019;1184:231–40. [DOI] [PubMed] [Google Scholar]
- [57].Yokoi T, Watanabe H, Yamaguchi H, et al. Involvement of the precuneus/posterior cingulate cortex is significant for the development of Alzheimer’s disease: a PET (THK5351, PiB) and resting fMRI study. Front Aging Neurosci 2018;10:304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Zhang L, Zuo X-N, Ng KK, et al. Distinct BOLD variability changes in the default mode and salience networks in Alzheimer’s disease spectrum and associations with cognitive decline. Sci Rep 2020;10:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Zhang M, Guan Z, Zhang Y, et al. Disrupted coupling between salience network segregation and glucose metabolism is associated with cognitive decline in Alzheimer’s disease–A simultaneous resting-state FDG-PET/fMRI study. Neuroimage Clin 2022;34:102977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Zhang Z, Zheng H, Liang K, et al. Functional degeneration in dorsal and ventral attention systems in amnestic mild cognitive impairment and Alzheimer’s disease: an fMRI study. Neurosci Lett 2015;585:160–5. [DOI] [PubMed] [Google Scholar]
- [61].Li R, Wu X, Fleisher AS, Reiman EM, Chen K, Yao L. Attention-related networks in Alzheimer’s disease: A resting functional MRI study. Hum Brain Mapp 2012;33:1076–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].O’Brien RM. A caution regarding rules of thumb for variance inflation factors. Qual Quan Int J Methodol 2007;41:673–90. [Google Scholar]
- [63].Coleman P, Federoff H, Kurlan R. A focus on the synapse for neuroprotection in Alzheimer disease and other dementias. Neurology 2004;63:1155–62. [DOI] [PubMed] [Google Scholar]
- [64].Agosta F, Rocca MA, Pagani E, et al. Sensorimotor network rewiring in mild cognitive impairment and Alzheimer’s disease. Hum Brain Mapp 2010;31:515–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Menon V. Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011;15:483–506. [DOI] [PubMed] [Google Scholar]
- [66].Allali G, van der Meulen M, Beauchet O, Rieger SW, Vuilleumier P, Assal F. The neural basis of age-related changes in motor imagery of gait: An fMRI study. J Gerontol A Biol Sci Med Sci 2014;69:1389–98. [DOI] [PubMed] [Google Scholar]
- [67].Smith RX, Yan L, Wang DJJ. Multiple time scale complexity analysis of resting state FMRI. Brain Imaging Behav 2014;8:284–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Sokunbi MO, Cameron GG, Ahearn TS, Murray AD, Staff RT. Fuzzy approximate entropy analysis of resting state fMRI signal complexity across the adult life span. Med Eng Phys 2015;37:1082–90. [DOI] [PubMed] [Google Scholar]
- [69].Wang B, Niu Y, Miao L, et al. Decreased complexity in Alzheimer’s disease: Resting-state fMRI evidence of brain entropy mapping. Front Aging Neurosci 2017;9:378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Labate D, Foresta FL, Morabito G, Palamara I, Morabito FC. Entropic measures of EEG complexity in Alzheimer’s disease through a multivariate multiscale approach. IEEE Sensors J 2013;13:3284–92. [Google Scholar]
- [71].Maturana-Candelas A, Gómez C, Poza J, Pinto N, Hornero R. EEG characterization of the Alzheimer’s disease continuum by means of multiscale entropies. Entropy 2019;21:544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [72].Beauchet O, Launay CP, Sejdić E, Allali G, Annweiler C. Motor imagery of gait: a new way to detect mild cognitive impairment? J NeuroEngineering Rehabil 2014;11:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Lamoth CJ, van Deudekom FJ, van Campen JP, Appels BA, de Vries OJ, Pijnappels M. Gait stability and variability measures show effects of impaired cognition and dual tasking in frail people. J Neuroeng Rehabil 2011;8:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Montero-Odasso M, Bergman H, Phillips NA, Wong CH, Sourial N, Chertkow H. Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr 2009;9:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sheridan PL, Solomont J, Kowall N, Hausdorff JM. Influence of executive function on locomotor function: divided attention increases gait variability in Alzheimer’s disease. J Am Geriatr Soc 2003;51:1633–7. [DOI] [PubMed] [Google Scholar]
- [76].Ehsani H, Mohler MJ, O’connor K, Zamrini E, Tirambulo C, Toosizadeh N. The association between cognition and dual-tasking among older adults: the effect of motor function type and cognition task difficulty. Clin Interv Aging 2019;14:659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Toosizadeh N, Najafi B, Reiman EM, et al. Upper-extremity dual-task function: an innovative method to assess cognitive impairment in older adults. Front Aging Neurosci 2016;8:167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Buckner RL. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci 2005;25:7709–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].D’Esposito M, Detre JA, Alsop DC, Shin RK, Atlas S, Grossman M. The neural basis of the central executive system of working memory. Nature 1995;378:279–81. [DOI] [PubMed] [Google Scholar]


