Abstract
Purpose
We employ nnU-Net, a state-of-the-art self-configuring deep learning-based semantic segmentation method for quantitative visualization of hemothorax (HTX) in trauma patients, and assess performance using a combination of overlap and volume-based metrics. The accuracy of hemothorax volumes for predicting a composite of hemorrhage-related outcomes — massive transfusion (MT) and in-hospital mortality (IHM) not related to traumatic brain injury — is assessed and compared to subjective expert consensus grading by an experienced chest and emergency radiologist.
Materials and methods
The study included manually labeled admission chest CTs from 77 consecutive adult patients with non-negligible (≥50 mL) traumatic HTX between 2016 and 2018 from one trauma center. DL results of ensembled nnU-Net were determined from fvefold cross-validation and compared to individual 2D, 3D, and cascaded 3D nnU-Net results using the Dice similarity coefcient (DSC) and volume similarity index. Pearson’s r, intraclass correlation coefcient (ICC), and mean bias were also determined for the best performing model. Manual and automated hemothorax volumes and subjective hemothorax volume grades were analyzed as predictors of MT and IHM using AUC comparison. Volume cut-ofs yielding sensitivity or specifcity≥90% were determined from ROC analysis.
Results
Ensembled nnU-Net achieved a mean DSC of 0.75 (SD: ± 0.12), and mean volume similarity of 0.91 (SD: ± 0.10), Pearson r of 0.93, and ICC of 0.92. Mean overmeasurement bias was only 1.7 mL despite a range of manual HTX volumes from 35 to 1503 mL (median: 178 mL). AUC of automated volumes for the composite outcome was 0.74 (95%CI: 0.58–0.91), compared to 0.76 (95%CI: 0.58–0.93) for manual volumes, and 0.76 (95%CI: 0.62–0.90) for consensus expert grading (p = 0.93). Automated volume cut-offs of 77 mL and 334 mL predicted the outcome with 93% sensitivity and 90% specificity respectively.
Conclusion
Automated HTX volumetry had high method validity, yielded interpretable visual results, and had similar performance for the hemorrhage-related outcomes assessed compared to manual volumes and expert consensus grading. The results suggest promising avenues for automated HTX volumetry in research and clinical care.
Keywords: Deep learning, nnU-Net, Computed tomography, Trauma, Hemothorax, Segmentation, Quantitative visualization, Artificial intelligence machine learning
Introduction
Chest trauma collectively results in a quarter of all fatal traumatic injuries annually, and hemorrhage into the thoracic cavity is an important proximate cause of early morbidity and mortality [1–7]. Since admission computed tomography (CT) is rapid and allows concurrent monitoring by the trauma team, the modality is routinely employed in patients sufciently hemodynamically stable for transfer to a trauma bay-adjacent scanner. Hemothorax refects the total amount of bleeding into the chest cavity from the time of injury and manifests as dependently layering accumulations of blood within the pleural space [3]. Hemothorax is difcult to quantify with chest x-ray, and CT is generally superior for characterization of thoracic injury [2, 8, 9]. The unobstructed anatomic detail aforded by CT opens the possibility for voxelwise quantifcation of hemothorax, and could facilitate objective personalized prognostication and decision support with respect to hemorrhage control; however, manual or semi-automated voxelwise measurement is labor-intensive and not feasible at the point of care [10, 11].
There is currently no universal and clinically validated system for grading hemothorax size on imaging, with coarse subjective descriptors such as “small” or “moderate” routinely used in the clinical setting [5, 12, 13]. Because the thoracic cavity can accommodate accumulation of a large volume of blood, traumatic hemothorax may result in exsanguination and mortality. Massive transfusion is an important life-saving intervention and there is an unmet need for objective criteria to guide the transfusion strategy in patients with chest trauma and hemothorax [14, 15].
Automated methods for voxelwise segmentation and quantification have been described for features of hemorrhage in other body cavities, including extraperitoneal pelvic hematomas [16, 17] and hemoperitoneum [18]. Segmentation methods must be robust at multiple scales given the wide range of hemothorax volumes encountered.
The purpose of this pilot study is to assess method validity of deep learning-based automated hemothorax segmentation and quantification and explore the potential clinical role of automated hemothorax volumetry for predicting need for massive transfusion and hemorrhage-related mortality.
Materials and methods
Study dataset
De-identified admission contrast-enhanced chest CT studies that were performed on arrival at a level I trauma center were retrospectively compiled into the study dataset as part of an IRB-approved HIPAA compliant study. Consecutive adult (age ≥ 18 years) patients between August 2016 and December 2018 who experienced blunt or penetrating thoracic trauma were queried for the keyword “hemothorax” within radiologic reports, returning 97 patients. Four patients were excluded because of lack of archived CT images or clinical data. Ten cases with incomplete imaging data or corrupted meta-data could not be successfully loaded into the segmentation software and were also excluded.
Labeling in all patients was performed using 3D slicer [19] (details provided below), and trace hemothoraces (≤ 50 mL, reflecting a small fraction of one unit of transfused blood) expected to be too small to impact haemorrhage-related outcomes were excluded a priori (n = 6). The final dataset consisted of 77 labeled CT studies. These patients’ charts were reviewed by a research assistant for hemorrhag-erelated outcomes that were used to generate a composite endpoint including massive transfusion (MT-, defined as at least 4 units of packed red blood cells (PRBC) in a 4-h period or at least 10 units of PRBC over 24 h [20, 21]); or in-hospital mortality (IHM), excluding fatalities from traumatic brain injury (head abbreviated injury severity score of 6).
Image acquisition
Thoracic CT studies were completed using either a duals-ource 128-section (Siemens Force; Siemens, Erlangen, Germany) or 64-section (Brilliance; Philips Healthcare, Andover, MA) scanners located within the trauma center. The scanning protocol included injection of 100 mL of intravenous contrast (Omnipaque [iohexol; 350 mg of iodine per milliliter]; GE Healthcare, Chicago, IL) and image acquisition in the arterial phase at 120 kVp (Philips) or 120 kVp equivalent blend (Siemens) at 150–159 reference mAs. Studies were archived with 1.5–3-mm section thickness.
Qualitative grading
Blinded qualitative evaluation of hemothoraces was performed by consensus of two board-certifed radiologists — a chest radiologist with over 15 years of experience and an emergency radiologist with 10 years of experience. In keeping with clinical practice, hemothorax size was graded in a gestalt fashion as small, moderate, or large. The following guideline was used to inform qualitative grading [5]: small hemothoraces have an estimated volume less than 250 mL, with pleural thickness less than 1.5 cm; moderate hemothoraces have estimated volumes between 250 and 1000 mL, with pleural thickness between 1.5 and 4 cm; and large hemothoraces have a volume > 1000 mL with pleural thickness >4 cm.
Manual labeling
Each study was converted to Neuroimaging Informatics Technology Initiative (NifTI) file format and manually labeled by trained staf at the voxel level using the segment editor module in 3D slicer (version 4.10.2, www.slicer.org). The 3D ROI paint tool was employed with thresholding between −20 and 100 HU to account for image noise and beam hardening from adjacent ribs. Labels were created in the axial planes with further editing in the coronal and sagittal planes. A morphologic smoothing flter was uniformly applied to eliminate label artifact. All studies underwent further editing by a radiologist. Manual hemothorax volumes were calculated using voxel counting with the slicer quantifcation module.
High-level overview of the deep learning method
Convolutional neural networks with encoder and decoder architectures such as U-Net form the backbone of contemporary automated semantic segmentation methods [22]. At baseline, U-Net and similar fully convolutional neural networks (FCNs) involve gradual hierarchical increases in size of receptive fields in progressively deeper layers and are therefore suboptimal for segmentation of targets that vary greatly in scale [23]. Traumatic bleeding in the large body cavities, including pelvic hematoma [17], hemoperitoneum [18], and hemothorax, ranges widely in volumes and best results have been achieved using tailored multiscale solutions.
In recent years, numerous approaches have proliferated to address multiscale problems in cross-sectional imaging. Commonly, low-resolution networks that maximize global contextual awareness are combined with high resolution networks that produce fine detail segmentations, operating in a cascaded [17] or tandem [18, 24–26] fashion. nnU-Net, introduced in Nature in 2021 by Isensee et al. [27], is based on the principle that design choices in pre-processing and hyper-parameter tuning, including intensity thresholding, cropping, slice thickness resampling, data augmentation, batch size, and learning rate [28], have more impact on performance than do architectural variations. nnU-net is self-configuring, automatically determining the aforementioned design choices in a data-driven manner derived from best results on over 20 public cross-sectional imaging datasets. The selected design choices produce a unique dataset and pipeline fingerprint for the new data at hand. The rules-based method for determining the fingerprint is based on features which include median number of positive voxels, spacing and size anisotropy, number of training cases, and imaging modality. It is shorthand for “no new U-Net” as it employs generic 2D, 3D, and cascaded 3D U-Net architectures in fivefold cross-validation using a Dice similarity coefficient loss function. Ensembled results of the three methods are also determined, and the best-performing method (2D, 3D, cascaded 3D, or ensembled) is selected.
In spite of the simplicity of the backbone architectures, nnU-Net surpasses most existing highly-specialized solutions for 53 diferent segmentation tasks from international biomedical image segmentation challenges, and serves as a state-of-the-art out-of-the-box automated segmentation tool that is robust for multiscale problems [27].
Statistical analyses
nnU-Net training and validation were performed with an RTX 3090 NVIDIA GPU with 24 GB of RAM using Python version 3.7.11. The best performing model was selected based on highest mean Dice similarity coefcient (DSC) and volume similarity [29]. Correlation and agreement between automated and manual volumes were further interrogated using intraclass correlation coefcient (ICC), Pearson’s r, and mean bias derived from Bland–Altman analysis.
Median automated volumes were compared by composite outcome using the Mann–Whitney U test. Automated volumes were compared to manual volumes and qualitative consensus expert grading for the composite outcome of massive transfusion and in-hospital mortality using the area under the ROC curve (AUC). Statistical comparisons were performed using the DeLong method. Receiver operating characteristic curve (ROC) analysis was also used to determine volume thresholds resulting in ≥90% sensitivity and ≥90% specifcity.
Results
Ensembled nnU-Net was selected for further analysis as it demonstrated the best overall combined performance using overlap and volume-based segmentation metrics, achieving high mean volume similarity of 0.91 — equivalent to high-resolution 3D and cascaded 3D methods — and highest mean DSC of 0.75 (Table 1). The distribution of manual and automated (ensembled nnU-Net) hemothorax volumes ranged between 35 and 1503 mL (manual) and 34 and 1451 mL (auto), with median volumes of 178 mL (manual) and 185 mL (auto). Standard quantitative imaging biomarker evaluation metrics [30] comparing manual to automated volumes included an ICC of 0.92 (95%CI: 0.88 to 0.95), and Pearson’s r of 0.93 (95%CI: 0.90 to 0.96). Pearson’s r values ≥ 0.80 are indicative of strong correlation and ICC values ≥ 0.75 are considered to indicate excellent agreement [31]. The automated method had a mean overmeasurement bias of 1.7 mL compared to manual volumes.
Table 1.
Performance metrics for nnU-Net
| nnU-Net component network | Mean DSC (± SD) | Volume similarity score (± SD) |
|---|---|---|
|
| ||
| 2D U-Net | 0.65 (± 0.14) | 0.87 (± 0.13) |
| 3D U-Net (high resolution) | 0.74 (± 0.12) | 0.91 (± 0.10) |
| 3D U-Net (cascaded) | 0.74 (± 0.12) | 0.91 (± 0.10) |
| Ensembled nnU-Net | 0.75 (± 0.12) | 0.91 (± 0.10) |
Patient baseline characteristics are provided in Table 2. Among the 77 patients included in the study, the median age was 50 years (interquartile range [IQR] 36–62), and 74% were male. Mean Abbreviated Injury Score (AIS) score was 3.2 (SD: 0.6), and mean injury severity score (ISS) was 24.2 (SD: 10.3). Four patients (5%) suffered penetrating injuries, and the other 73 patients (95%) experienced blunt injuries. Twelve patients (16%) underwent massive transfusion, and 8 patients (10%) died during their hospital admission. A total of 15 patients (18%) had either or both outcomes.
Table 2.
Demographic and clinical characteristics
| Total cohort n = 77 | Composite outcome (MT or IHM) |
p value | ||
|---|---|---|---|---|
| Yes n = 15 | No n = 62 | |||
|
| ||||
| autoHTX vol (median [IQR]) | 185.5 [118.0, 277.8] | 341.1 [211.2, 485.6] | 158.4 [114.4, 263.1] | 0.004 |
| Age (median [IQR]) | 50 [36–62] | 62 [36–76] | 49 [36, 60] | 0.35 |
| Gender (n (%)) | ||||
| Male | 57 (74) | 11 (73) | 46 (74) | 1 |
| Female | 20 (26) | 4 (27) | 16 (26) | |
| HR (mean [SD]) | 94 [19] | 100 [19] | 94 [19] | 0.12 |
| SBP (mean [SD]) | 136 [33] | 103 [23] | 144 [30] | < 0.001 |
| Shock (SBP ≤ 90) (n (%)) | 6 (8) | 4 (27) | 2 (3) | 0.02 |
| Lactate (mean [SD]) | 3.8 [3.0] | 7.6 [4.5] | 2.9 [1.4] | < 0.001 |
| Mechanism (n (%)) | 0.58 | |||
| Blunt | 73 (95) | 15 (100) | 58 (93) | |
| Penetrating | 4 (5) | 0 (0) | 4 (7) | |
| Chest AIS (mean [SD]) | 3.2 [0.6] | 3.8 [0.7] | 3.1 [0.6] | < 0.001 |
| ISS (mean [SD]) | 24.2 [10.3] | 31.4 [10.7] | 22.5 [9.5] | 0.01 |
| Qualitative grade (n (%)) | < 0.001 | |||
| Mild | 28 (36) | 2 (13) | 26 (42) | |
| Moderate | 39 (51) | 6 (40) | 33 (53) | |
| Severe | 10 (13) | 7 (47) | 3 (5) | |
Two-tailed p-values less than 0.05 indicate statistical significance
Abbreviations: autoHTX vol, automated hemothorax volumes; IQR, interquartile range; HR, heart rate; SBP, admission systolic blood pressure; AIS, abbreviated injury scale; ISS, Injury Severity Score
Automated volumes were significantly higher in patients with the composite outcome (massive transfusion or in-hospital mortality) than those without (341.1 mL versus 158.4 mL, p = 0.004). Examples of automated segmentation results for a range of HTX volumes are illustrated in Fig. 1.
Fig. 1.
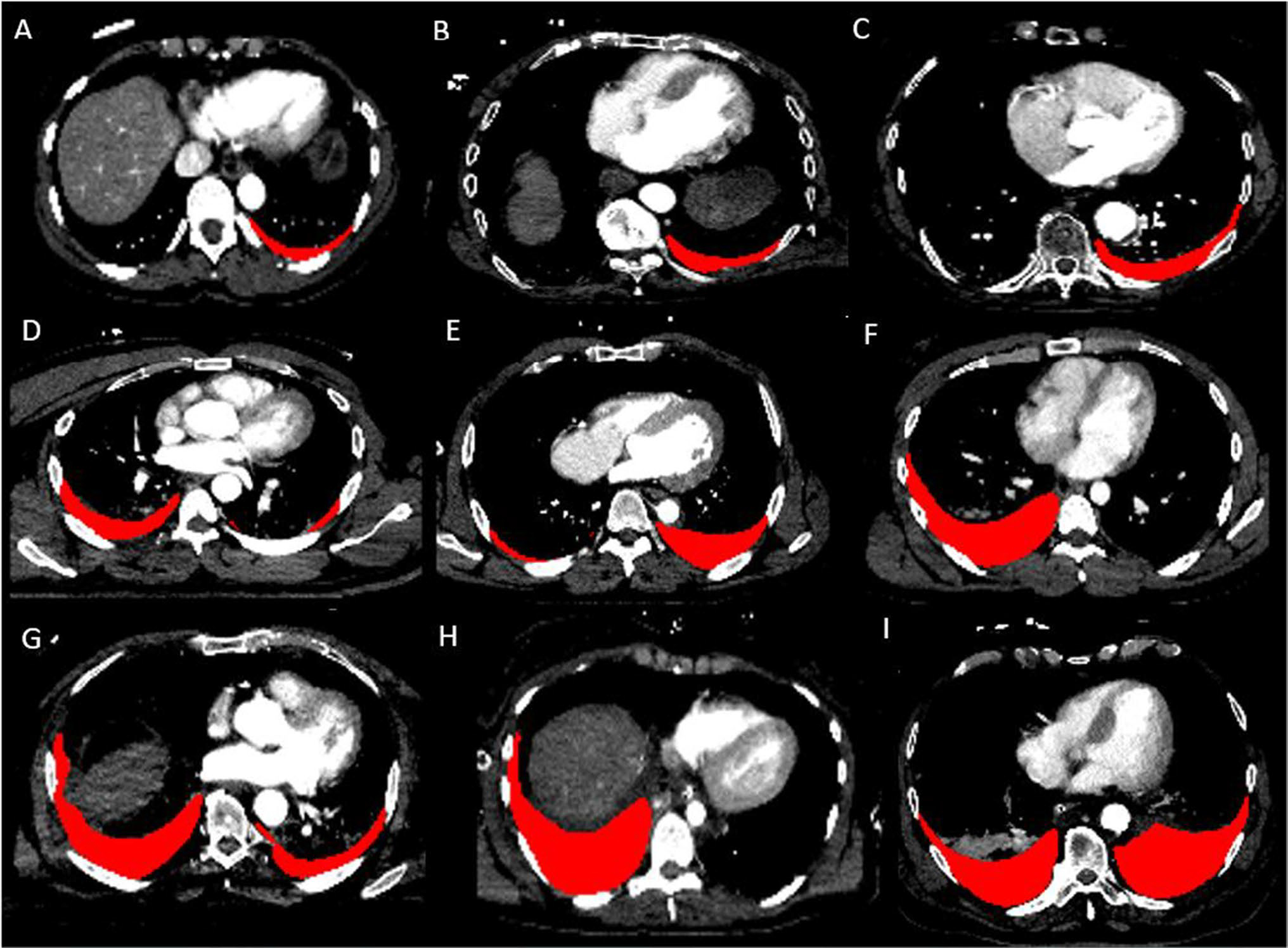
Automated results using nnU-Net are shown for a wide range of hemothorax volumes (A 96.6 mL, B 147.8 mL, C 185.4 mL, D 287.3 mL, E 419.6 mL, F 597.4 mL, G 714.0 mL, H 898.6 mL, I 1450.6 mL)
The AUCs of traumatic automated HTX volumes, manual volumes, and qualitative expert grading for the composite outcome were 0.74 (95%CI: 0.58–0.91), 0.76 (95%CI: 0.58–0.93), and 0.76 (95%CI: 0.62–0.90) (p = 0.93 for testing the null hypothesis that the three AUCs are equal). A traumatic automated HTX volume cut-off of 81 mL predicted the outcome with 93% sensitivity and 11% specificity and a volume cut-off 341 mL with 90% specificity and 53% sensitivity.
Discussion
Persistent hemorrhage into the pleural space may be fatal, and clinical decisions in managing traumatic hemothorax early in the course of treatment relate to addressing the origin of hemorrhage and transfusing to compensate for blood loss until the source can be controlled [1, 3, 13–15]. CT is integral to the evaluation of patients after severe thoracic trauma and is an important component of trauma care algorithms where intracavitary hemorrhage is suspected [4, 6]. Despite advancements in image technology that enables rapid acquisition of anatomic data, point of care hemothorax volume estimation is typically achieved with coarse subjective assessment. In comparison to broad, qualitative categorizations that are traditionally given in radiology reports, automated quantitative visualization of hemothorax could potentially provide objective information for prognosticating hemorrhage-related outcomes. Our automated hemothorax results demonstrate high method validity and outcome prediction on par with expert level qualitative grading undertaken in a controlled research setting. Automated hemothorax volumes that can be visually verified by any radiologist or trauma surgeon end-user are advantageous because they are rapid, consistent, and objective, not depending on experience level, subspecialization, reader fatigue, reading room distractions, or the lengthy study turnaround times of trauma CT exams, which can exceed 20–30 min [32–34]. By comparison, nnU-Net has inference times of under 2 min.
To engender trust among radiologists or trauma surgeons, hemothorax segmentation masks must conform to the boundaries of hemothorax while avoiding neighboring structures of similar attenuation values such as compressed lung. We achieved high-saliency interpretable visual results with corresponding high mean DSC values, volume similarity scores, correlation, and agreement using nnU-Net.
In this dataset, injury severity scores, and lactate levels were significantly higher in patients requiring massive transfusion or that expired from hemorrhage-related complications, and admission systolic blood pressure was significantly lower. This is likely accounted for by multicavitary hemorrhage, which is not addressed in this pilot study. Bleeding features on routine admission trauma CT are often leading indicators of hemodynamic collapse [4, 6, 35–37] and can soon be accounted for more holistically using similar deep learning-based quantitative visualization approaches. Proof-of-concept automated methods for quantitative visualization of pelvic hematoma volumes and hemoperitoneum are described [16–18], and comprehensive assessment of hemorrhage burden using automated hemothorax, hemoperitoneum, and pelvic hematoma segmentation is planned in future work scaled to larger datasets. Once containerized as software and deployed, high-throughput automated hemorrhage burden quantification tools will likely accelerate cross-institutional research and may play an important role in objective decision-making in the early management of patients with torso hemorrhage.
Additional outcomes specific to hemothorax may also be of interest, including the risk of retained hemothorax and empyema [13]. Other manifestations of injury including lung contusion can impact the risk of developing traumatic acute respiratory distress syndrome (ARDS) [38], and in the future, automated multi-class voxelwise labeling and quantification of traumatic chest injuries on CT could facilitate prediction of a variety of outcomes based on multiple quantitative imaging parameters.
The computer vision aspect of our work is currently limited to the canonical task of segmentation. The method is currently not intended as a screening or workfow prioritization tool. A pipeline that screens for, segments, and measures hemothorax and hemorrhage in other body cavities autonomously in the clinical background and automatically notifes practitioners of critical quantitative results, as RAPID software has achieved for stroke imaging [39], is likely feasible but will require initial deep learning object detection steps with algorithms such as mask R-CNN preceding segmentation tasks [40, 41].
Finally, our pilot study used a CT dataset from a single institution. A large multi-institutional dataset will eventually be needed to ensure robust performance in the face of domain shift from scanner and scanning protocol heterogeneity and variable patient populations [42, 43] to achieve a technology readiness level suitable for deployment across institutions.
In conclusion, in this pilot study, we found that a state-of-the-art deep learning method resulted in objective and interpretable automated quantitative visualization of traumatic hemothorax, with hemorrhage-related outcome prediction comparable to expert qualitative grading. Future work will entail combining the method with similar pelvic hematoma and hemoperitoneum pipelines for comprehensive torso hemorrhage burden quantification, using initial detection algorithms to facilitate full-throughput autonomous function in the clinical background, and scaling to large multicenter datasets to ensure robustness.
Funding
NIH K08 EB027141-01A1 (PI: David Dreizin, MD).
Accelerated Translational Incubator Pilot (ATIP) award, University of Maryland (PI: David Dreizin, MD).
Footnotes
Conflict of interest David Dreizin, MD, is founder of TraumaVisual, LLC.
Code availability Code can be made available on github.
Declarations
Ethics approval IRB approval was obtained for this study at the University of Maryland School of Medicine.
Consent for study The IRB waived informed consent due to retrospective observational nature and deidentifed data.
Consent for publication IRB approved waiver of informed consent for publication of observational study of deidentified data as above.
Data availability
Labeled dataset has not been made available at this time.
References
- 1.Mowery NT, Gunter OL, Collier BR, Jose’J D Jr, Haut E, Hildreth A, Holevar M, Mayberry J, Streib E (2011) Practice management guidelines for management of hemothorax and occult pneumothorax. J Trauma Acute Care Surg 70(2):510–518 [DOI] [PubMed] [Google Scholar]
- 2.Sangster GP, González-Beicos A, Carbo AI, Heldmann MG, Ibrahim H, Carrascosa P, Nazar M, D’Agostino HB (2007) Blunt traumatic injuries of the lung parenchyma, pleura, thoracic wall, and intrathoracic airways: multidetector computer tomography imaging findings. Emerg Radiol 14(5):297–310 [DOI] [PubMed] [Google Scholar]
- 3.Mahmood I, Abdelrahman H, Al-Hassani A, Nabir S, Sebastian M, Maull K (2011) Clinical management of occult hemothorax: a prospective study of 81 patients. Am J Surg 201(6):766–769 [DOI] [PubMed] [Google Scholar]
- 4.Dreizin D, Munera F (2012) Blunt polytrauma: evaluation with 64-section whole-body CT angiography. Radiographics 32(3):609–631 [DOI] [PubMed] [Google Scholar]
- 5.Bilello JF, Davis JW, Lemaster DM (2005) Occult traumatic hemothorax: when can sleeping dogs lie? Am J Surg 190(6):844–848 [DOI] [PubMed] [Google Scholar]
- 6.Dreizin D, Munera F (2015) Multidetector CT for penetrating torso trauma: state of the art. Radiology 277(2):338–355 [DOI] [PubMed] [Google Scholar]
- 7.Demetri L, Aguilar MMM, Bohnen JD, Whitesell R, Yeh DD, King D, de Moya M (2018) Is observation for traumatic hemothorax safe? J Trauma Acute Care Surg 84(3):454–458 [DOI] [PubMed] [Google Scholar]
- 8.Stafford RE, Linn J, Washington L (2006) Incidence and management of occult hemothoraces. Am J Surg 192(6):722–726 [DOI] [PubMed] [Google Scholar]
- 9.Marts B, Durham R, Shapiro M, Mazuski JE, Zuckerman D, Sundaram M, Luchtefeld WB (1994) Computed tomography in the diagnosis of blunt thoracic injury. Am J Surg 168(6):688–692 [DOI] [PubMed] [Google Scholar]
- 10.Dreizin D, Bodanapally UK, Neerchal N, Tirada N, Patlas M, Herskovits E (2016) Volumetric analysis of pelvic hematomas after blunt trauma using semi-automated seeded region growing segmentation: a method validation study. Abdom Radiol 41(11):2203–2208 [DOI] [PubMed] [Google Scholar]
- 11.Battey TW, Dreizin D, Bodanapally UK, Wnorowski A, Issa G, Iacco A, Chiu W (2019) A comparison of segmented abdomin-opelvic fluid volumes with conventional CT signs of abdominal compartment syndrome in a trauma population. Abdom Radiol 44(7):2648–2655 [DOI] [PubMed] [Google Scholar]
- 12.Moy MP, Levsky JM, Berko NS, Godelman A, Jain VR, Haramati LB (2013) A new, simple method for estimating pleural effusion size on CT scans. Chest 143(4):1054–1059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Meyer DM (2007) Hemothorax related to trauma. Thorac Cardiovasc Surg 17(1):47–55 [DOI] [PubMed] [Google Scholar]
- 14.Huang J-F, Hsu C-P, Fu C-Y, Yang C-HO, Cheng C-T, Liao C-H, Kuo I-M, Hsieh C-H (2021) Is massive hemothorax still an absolute indication for operation in blunt trauma? Injury 52(2):225–30 [DOI] [PubMed] [Google Scholar]
- 15.Ma H-S, Ma J-H, Xue F-L, Fu X-N, Zhang N (2016) Clinical analysis of thoracoscopic surgery combined with intraoperative autologous blood transfusion in the treatment of traumatic hemothorax. Chin J Traumatol 19(6):371–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dreizin D, Zhou Y, Chen T, Li G, Yuille AL, McLenithan A, Morrison JJ (2020) Deep learning-based quantitative visualization and measurement of extraperitoneal hematoma volumes in patients with pelvic fractures: potential role in personalized forecasting and decision support. J Trauma Acute Care Surg 88(3):425–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dreizin D, Zhou Y, Zhang Y, Tirada N, Yuille AL (2020) Performance of a deep learning algorithm for automated segmentation and quantification of traumatic pelvic hematomas on CT. J Digit Imaging 33(1):243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dreizin D, Zhou Y, Fu S, Wang Y, Li G, Champ K, Siegel E, Wang Z, Chen T, Yuille AL (2020) A multiscale deep learning method for quantitative visualization of traumatic hemoperitoneum at CT: assessment of feasibility and comparison with subjective categorical estimation. Radiol: Artif Intell 2(6):e190220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fedorov A, Beichel R, Kalpathy-Cramer J, Finet J, Fillion-Robin J-C, Pujol S, Bauer C, Jennings D, Fennessy F, Sonka M (2012) 3D Slicer as an image computing platform for the Quantitative Imaging Network. Magn Reson Imaging 30(9):1323–1341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackenzie CF, Wang Y, Hu PF, Chen S-Y, Chen HH, Hagegeorge G, Stansbury LG, Shackelford S, Group OS (2014) Automated prediction of early blood transfusion and mortality in trauma patients. J Trauma Acute Care Surg 76(6):1379–85 [DOI] [PubMed] [Google Scholar]
- 21.Parimi N, Hu PF, Mackenzie CF, Yang S, Bartlett ST, Scalea TM, Stein DM (2016) Automated continuous vital signs predict use of uncrossed matched blood and massive transfusion following trauma. J Trauma Acute Care Surg 80(6):897–906. 10.1097/TA.0000000000001047 [DOI] [PubMed] [Google Scholar]
- 22.Litjens G, Kooi T, Bejnordi BE, Setio AAA, Ciompi F, Ghafoorian M, Van Der Laak JA, Van Ginneken B, Sánchez CI (2017) A survey on deep learning in medical image analysis. Med Image Anal 42:60–88 [DOI] [PubMed] [Google Scholar]
- 23.Fang X, Yan P (2020) Multi-organ segmentation over partially labeled datasets with multi-scale feature abstraction. IEEE Trans Med Imaging 39(11):3619–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dreizin D, Chen T, Liang Y, Zhou Y, Paes F, Wang Y, Yuille AL, Roth P, Champ K, Li G, McLenithan A, Morrison JJ (2021) Added value of deep learning-based liver parenchymal CT volumetry for predicting major arterial injury after blunt hepatic trauma: a decision tree analysis. Abdom Radiol (NY) 46(6):2556–2566. 10.1007/s00261-020-02892-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zhou Y, Dreizin D, Li Y, Zhang Z, Wang Y, Yuille A (2019) Multi-scale attentional network for multi-focal segmentation of active bleed after pelvic fractures. In International Workshop on Machine Learning in Medical Imaging. Springer, Cham, pp 461–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou Y, Dreizin D, Wang Y, Liu F, Shen W, Yuille AL (2022) External attention assisted multi-phase splenic vascular injury segmentation with limited data. IEEE Trans Med Imaging 41(6):1346–1357. 10.1109/TMI.2021.3139637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Isensee F, Jaeger PF, Kohl SA, Petersen J, Maier-Hein KH (2021) nnU-Net: a self-configuring method for deep learning-based biomedical image segmentation. Nat Methods 18(2):203–211 [DOI] [PubMed] [Google Scholar]
- 28.Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A (2017) Deep learning: a primer for radiologists. Radiographics 37(7):2113–2131 [DOI] [PubMed] [Google Scholar]
- 29.Maier-Hein L, Reinke A, Christodoulou E, Glocker B, Godau P, Isensee F, Kleesiek J, Kozubek M, Reyes M, Riegler MA (2022) Metrics reloaded: pitfalls and recommendations for image analysis validation. arXiv preprint arXiv:2206.01653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kessler LG, Barnhart HX, Buckler AJ, Choudhury KR, Kondratovich MV, Toledano A, Guimaraes AR, Filice R, Zhang Z, Sullivan DC (2015) The emerging science of quantitative imaging biomarkers terminology and definitions for scientific studies and regulatory submissions. Stat Methods Med Res 24(1):9–26 [DOI] [PubMed] [Google Scholar]
- 31.Zou KH, Tuncali K, Silverman SG (2003) Correlation and simple linear regression. Radiology 227(3):617–628 [DOI] [PubMed] [Google Scholar]
- 32.Hunter TB, Taljanovic MS, Krupinski E, Ovitt T, Stubbs AY (2007) Academic radiologists’ on-call and late-evening duties. J Am Coll Radiol 4(10):716–719 [DOI] [PubMed] [Google Scholar]
- 33.Brant-Zawadzki MN (2007) Special focus—outsourcing after hours radiology: one point of view—outsourcing night call. J Am Coll Radiol 4(10):672–674 [DOI] [PubMed] [Google Scholar]
- 34.Banaste N, Caurier B, Bratan F, Bergerot J-F, Thomson V, Millet I (2018) Whole-body CT in patients with multipletraumas: factors leading to missed injury. Radiology 289(2):374–383 [DOI] [PubMed] [Google Scholar]
- 35.Dreizin D, Bodanapally U, Boscak A, Tirada N, Issa G, Nascone JW, Bivona L, Mascarenhas D, O’Toole RV, Nixon E (2018) CT prediction model for major arterial injury after blunt pelvic ring disruption. Radiology 287(3):1061–1069 [DOI] [PubMed] [Google Scholar]
- 36.Borror W, Gaski GE, Steenburg S (2019) Abdominopelvic bleed rate on admission CT correlates with mortality and transfusion needs in the setting of blunt pelvic fractures: a single institution pilot study. Emerg Radiol 26(1):37–44 [DOI] [PubMed] [Google Scholar]
- 37.Dreizin D, Champ K, Dattwyler M, Bodanapally U, Smith EB, Li G, Singh R, Wang Z, Liang Y (2022) Blunt splenic injury in adults: association between volumetric quantitative CT parameters and intervention. J Trauma Acute Care Surg. 10.1097/TA.0000000000003684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller PR, Croce MA, Bee TK, Qaisi WG, Smith CP, Collins GL, Fabian TC (2001) ARDS after pulmonary contusion: accurate measurement of contusion volume identifies high-risk patients. J Trauma Acute Care Surg 51(2):223–230 [DOI] [PubMed] [Google Scholar]
- 39.Laughlin B, Chan A, Tai WA, Moftakhar P (2019) RAPID automated CT perfusion in clinical practice. Pract Neurol 2019:41–55 [Google Scholar]
- 40.Zhang T, Song Z, Yang J, Zhang X, Wei J (2021) Cerebral hemorrhage recognition based on Mask R-CNN network. Sens Imaging 22(1):1–16 [Google Scholar]
- 41.Zapaishchykova A, Dreizin D, Li Z, Wu JY, Faghihroohi S, Unberath M (2021) An interpretable approach to automated severity scoring in pelvic trauma. In International Conference on Medical Image Computing and Computer-Assisted Interventio. Springer, Cham, pp 424–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Gomez C, Huang C-M, Unberath M (2021) INTRPRT: a systematic review of and guidelines for designing and validating transparent AI in medical image analysis. arXiv preprint arXiv:2112.12596 [Google Scholar]
- 43.Vlontzos A, Rueckert D, Kainz B (2022) A review of causality for learning algorithms in medical image analysis. arXiv preprint arXiv:2206.05498 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Labeled dataset has not been made available at this time.


