Abstract
Background:
Germline mutation of CDH1 is rare and leads to hereditary diffuse gastric cancer (DGC).
Methods:
Patients (pts) with CDH1 mutation who underwent multidisciplinary counseling followed by open prophylactic total gastrectomy (PTG) by a single surgeon were reviewed.
Results:
Fifty-four pts with a median age of 41 years (16–70 years) underwent PTG between 2006–2021. Median operative time was 161 minutes, and median hospital stay was 7 days (range 6–12). There were 5 complications (9.2%) within 30 days, and two complications (pulmonary embolism and pancreatitis) required readmission. There were no anastomotic leaks. The pathologic analysis of the first 10 pts included the entire gastric mucosa, revealing a median of 15 foci of DGC (range 5–136). The subsequent 44 pts with more limited analysis had a median of 2 foci (range 0–5), and two pts (3.7%) had no foci identified. Median maximum weight loss was 19%. In long-term follow-up (median 4.6 years) of 20 pts, median global QOL was 2.0 (very good), the majority had persistent difficulty with certain foods or liquids, and all stated they would again elect PTG over surveillance endoscopy.
Conclusions:
PTG can be performed safely at high-volume referral centers with very good QOL but nutritional sequelae persist.
Keywords: Germline CDH1 mutation, Hereditary diffuse gastric cancer, Prophylactic gastrectomy
INTRODUCTION
Gastric cancer is the third highest cause of cancer death worldwide with over one million new cases diagnosed annually leading to more than 750,000 deaths 1. The incidence is twice as high in males than in females 2. The major risk factor of gastric cancer is infection with Helicobacter pylori (H. pylori), which can colonize the stomach early in life and cause chronic inflammation that over years or decades leads to gastric intestinal metaplasia, dysplasia, and eventually adenocarcinoma 3. H. pylori infection and gastric cancer incidence vary widely in different regions with the highest rates in East Asia, Eastern Europe, and South America 1.
In 1965, Lauren described two distinct histological subtypes of gastric adenocarcinomas, intestinal and diffuse 4. The intestinal type exhibits components of glandular, solid, or intestinal architecture as well as tubular structures. The diffuse type demonstrates single cells or poorly cohesive cells with intracytoplasmic mucin (a.k.a. signet ring cells) infiltrating the gastric wall, and progressive disease can ultimately lead to linitis plastica (a.k.a “leather bottle” stomach).
It is estimated that 90% of gastric cancer cases arise in the sporadic setting, whereas familial clustering is observed in the remaining 10% 5. Early onset familial gastric cancer was first described in three families of Maori descent from New Zealand in 1964 6. In 1998, Parry Guilford and colleagues published their study of early onset diffuse gastric cancer in a large Maori kindred in New Zealand. They carried out genetic linkage analysis and found significant linkage to markers flanking the CDH1 gene, which encodes for E-cadherin, and sequencing found a mutation in CDH1 leading to a truncated gene product 7. This group subsequently identified additional germline mutations in the CDH1 gene associated with familial gastric cancer. Hundreds of pathogenic CDH1 germline mutations have subsequently been identified in diffuse gastric cancer families in multiple different countries 8.
The original estimates of lifetime risk of developing HDGC in individuals with germline CDH1 mutation were up to 70% or greater 9,10. However, this estimate of lifetime risk was subject to ascertainment bias, as most identified cases were derived from kindreds with high burden of HDGC amongst multiple family members. As additional kindreds with weaker phenotypic expression (lower penetrance) have been reported among individuals undergoing germline CDH1 testing for conditions other than diffuse gastric cancer, the risk estimates for HDGC in the setting of germline CDH1 mutation have declined. Currently, the lifetime risk of HDGC in individuals with germline CDH1 mutation is estimated at 37–42% for men and 25–33% for women 11,12. Females with germline CDH1 mutation also carry a 39–55% risk of developing lobular breast cancer.
Pathological evaluation of prophylactic gastrectomy specimens in HDGC patients has been characterized by one or more microscopic infiltrates of signet-ring cell carcinoma that underlie normal appearing mucosa 13. This feature limits the use of conventional white-light endoscopy as a potentially screening modality in high risk patient populations that carry pathogenic variants in the CDH1 gene.
Individuals with germline pathogenic CDH1 mutations face difficult decisions with respect to cancer screening and risk reducing surgeries and benefit most from evaluation at referral centers with expertise in this rare condition. These referral centers should have a multidisciplinary team of genetic counselors, gastroenterologists, dieticians, surgical oncologists, breast surgeons, plastic surgeons, and pathologists. Prophylactic total gastrectomy can be offered to individuals with pathogenic germline CDH1 variants generally starting at 20 years old. The rates of morbidity and even mortality following total gastrectomy correlate with surgeon volume and institution volume 14. Thus, prophylactic total gastrectomies are best performed at high-volume gastric cancer referral centers. Here we report our experience of CDH1 gene mutation carriers undergoing prophylactic total gastrectomies by a single surgeon at two different gastric cancer referral centers over a 15-year period.
METHODS
Patients
All patients with germline CDH1 mutations who were referred to a single surgeon (S.S.Y) and underwent prophylactic total gastrectomy between April 2006 and June 2021 are included in this series. Most patients were evaluated before surgery by a multidisciplinary team that included a genetic counselor, gastroenterologist, dietician, and surgical oncologist. Those that did not see all team members declined certain consultations. A complete history and physical examination were performed, CDH1 mutation genetic testing records were obtained, and prior endoscopy reports and other relevant diagnostic studies were reviewed. Patient information was entered into a prospective database and included demographics, pre-operative height, weight, body mass index (BMI), detailed family history of gastric and breast cancer, pre-operative clinical investigations, operative details, pathological assessment, length of stay, and complications (≤30 days and >30 days).
The Institutional Review Boards approved this study, and informed consents were obtained preoperatively from all patients. The majority of patients agreed to be contacted for future inquiries, and these patients were last contacted between August and November of 2021 for follow-up information.
Prophylactic total gastrectomy
Prophylactic total gastrectomy was performed using an open technique via an upper midline incision. In order to ensure complete removal of all gastric mucosa at risk for malignant transformation, the proximal gastric division was performed at least 1 cm above the squamo-columnar junction and the distal division across the duodenum was performed at least 2 cm beyond the pylorus. The total gastrectomy generally incorporated all or portions of perigastric lymph node stations 1–6 15.
Reconstruction was performed with a Roux-en-Y esophagojejunostomy with a 50–60 cm Roux retrocolic Roux limb. The anastomosis technique between the end of the esophagus and jejunum changed over time. The first 10 anastomoses were hand-sewn in two layers as previously described 16. Subsequent anastomoses were performed using an EndoGIA Ultra linear stapler (45 mm purple load) between the posterior wall of the esophagus and the anti-mesenteric wall of the Roux limb, and the common enterotomy was closed with interrupted 3–0 silks sutures as previously described 17. In four cases, large body habitus or hiatal hernia did not allow for this linear anastomosis so the Orvil anvil combined with an EEA circular stapler were used 18.
For pathologic analysis of the first ten operations, the entire stomach was fixed in formalin, and the entire mucosa was mapped and examined microscopically. This required a median of 340 sections and up to 470 sections 19,20. Subsequently, more limited analysis of the gastric specimen was performed In general, an initial limited analysis of gastric mucosa was performed to identify one or more foci of diffuse gastric cancer; more extensive analysis was only performed if one or more foci of diffuse gastric cancer were not discovered on initial analysis.
Post-operative Follow-up
Recommended follow-up for patients was clinic visits every 3–6 months for 12 months and annually thereafter. All patients were started postoperatively on a daily multivitamin with iron and monthly injections of vitamin B12 or sublingual vitamin B12. At each clinic visit, patients had their weight recorded; blood tests included a CBC, chemistry panel (including electrolytes, BUN, creatinine, liver functions test, and calcium), vitamin B12 level, vitamin D, and iron studies. The majority of patients were from out-of-state and elected to do their follow-up appointments locally with their primary care physician or other physicians.
To evaluate long-term outcomes, patients were contacted in the fall of 2021 and asked to complete a questionnaire that included 30 questions that we developed (see Supplemental Materials).
RESULTS
Patients
Fifty-four patients from 25 different families with known CDH1 mutations were referred to a single surgeon (SSY) for prophylactic total gastrectomy from 2006 to 2021. There were 35 females and 19 males with median age of 41 years (range 16 to 70 years). The first patient to undergo prophylactic total gastrectomy for germline CDH1 mutation at our institution has been previously described 21. Figure 1 demonstrates the pedigree of the first patient who underwent prophylactic total gastrectomy in this cohort.
Figure 1.
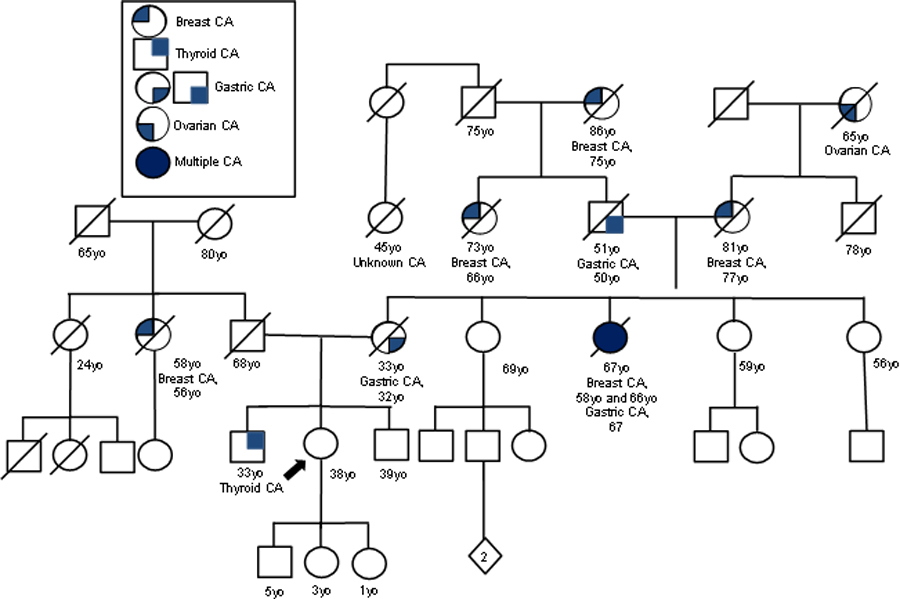
Pedigree of First Patient’s Family. Circles represent female family members, and squares male family members. The diamond represents the sex is unknown. Slashes indicate persons who have died, and the numbers beneath the circles or squares are age at death or age at the time the pedigree was created. The first patient is shown by the arrow. Modified from Chung DC et al. 21.
Mutations
Table 1 summarizes the kindred, age, gender, and mutation type of all 54 patients with pathogenic CDH1 variants. There were 25 families or kindreds. Four families (12%) were identified as harboring missense mutations (with one of these missense mutations at the start codon), 2 families (12%) had splice site mutations, and 4 families (16%) were identified with large deletions. Another nine families (36%) carried insertions and deletions that resulted in frameshift mutations, which in turn created premature stop codons in CDH1 and produced truncated E-cadherin protein. Finally, six families (24%) harbored single base pair substitutions that resulted in nonsense mutations. Thus, the majority of patients in this cohort had insertion/deletion (indel) mutations in CDH1.
Table 1:
CDH1 Mutations in 25 Families/Kindreds
| Kindred | Patient | Age/Sex | CDH1 Mutation | Protein change | Description |
|---|---|---|---|---|---|
| A | 1 | 38F | c.2195G>A | p.R732Q | Missense mutation |
| B | 2, 18, 19, 26, 27, 42, 53 | 44M, 21F, 16F, 20F, 20F, 22M, 30F | c.1682insA | Frameshift mutation leading to premature stop codon | |
| C | 3 | 43m | c.1901C>T | p.A634V | Missense mutation |
| D | 4, 5, 6, 21 | 42M, 38F, 41M, 21F | 48+1 G>A | G to A at the +1 position of intron 1 (splice site mutation) | |
| E | 7, 8, 9, 28 | 50F, 49M, 26M, 38M | c.1003C>T | p.R335* | Nonsense mutation |
| F | 10, 14 | F27, 50F | c.3G>A | p.M1I | Missense mutation at start codon |
| G | 11 | 40F | Deletion entire CDH1 gene | Deletion of entire CDH1 gene | |
| H | 12, 13, 15, 16, 17, 20 | 48F, 55F, 49F, 22F, 57M, 25F | c.308G>A | p.W103* | Nonsense mutation |
| I | 22, 25 | 52F, 27F | c.1147C>T | p.Q363* | Nonsense mutation |
| J | 23 | 58M | c.1893insA | Frameshift mutation leading to premature stop codon | |
| K | 24, 29 | 55F, 33F | c.1979insT | p.D662* | Frameshift mutation leading to premature stop codon |
| L | 30 | 41F | c.1063delT | p.Leu355* | Frameshift mutation leading to premature stop codon |
| M | 31 | 42F | c.2293C>T | p.Q765* | Nonsense mutation |
| N | 32 | 46M | c.1999delC | Frameshift mutation leading to premature stop codon | |
| O | 33, 34 | 29M, 25F | c.2287G>T | p.Glu763* | Nonsense mutation |
| P | 35, 36, 43 | 54F, 51F | c.1895_1896delAC | p.His632Argfs*30 | Frameshift mutation leading to premature stop codon |
| Q | 37 | 52F | c.1137G>A | p.Thr379= | G to non-G change at the last nucleotide of an exon (splice site mutation) |
| R | 38 | 48F | c.1008G>T | p.E336D | Missense mutation |
| S | 39 | 51F | 5’UTR_3’UTRdel | Deletion | Deletion spans the entire CDH1 gene from 5’UTR to 3’UTR |
| T | 40, 41, 44, 45, 48 | 55F, 35F, 54F, 32M, 30M | c.1792C>T | p.R598* | Nonsense mutation |
| U | 46, 47 | 43M, 38M | c.2064_2065delTG | p.Cys688* | Frameshift mutation leading to premature stop codon |
| V | 49 | 50M | Deletion exons 4–16 | Predicted to results in loss of 85% of CDH1 coding sequence | |
| W | 50, 51 | 70F, 43M | c.1046del | p.Leu355* | Frameshift mutation leading to premature stop codon |
| X | 52 | 32M | c.1145del | p.Gly382Valfs*11 | Frameshift mutation leading to premature stop codon |
| Y | 54 | 32F | Deletion entire CDH1 gene | Deletion of entire CDH1 gene |
Thirteen families/kindreds had only one patient undergo prophylactic gastrectomy. The remaining 14 families/kindreds had between 2 and 7 members undergo prophylactic gastrectomy.
Preoperative studies
All patients had an upper endoscopy with multiple, random biopsies obtained prior to prophylactic surgery and no gastric masses were identified. Eight patients (15%) were found to have one (n=6), two (n=1), or three (n=1) microscopic foci of diffuse gastric cancer on surveillance biopsies. Eleven patients had pre-operative CT scans, all of which were unremarkable.
Prophylactic gastrectomy
The interval between genetic testing and identification of CDH1 positive gene carrier status and gastrectomy was a mean of 15.8 months (median of 5.3 months; range of 1.2–82.5 months). All 54 patients underwent prophylactic total gastrectomy by a single surgeon (SSY). The median operative time for all patients was 161 minutes (range 116–308 minutes). The initial 10 patients underwent surgery with a senior surgical resident as the assistant and with a hand-sewn anastomosis, leading to a median operating time of 245 minutes (range 187–308). The subsequent patients underwent surgery with a surgical oncology fellow as the assistant and with a stapled anastomosis, leading to a median operating time of 157 minutes (range 116–188). Median blood loss was 100 ml (range 25–1000), and no patient received a blood transfusion.
Histologic Results
The pathological analysis of the surgical specimens changed during the course of this series. For the first 10 patients, the pathological analysis has been previously described 20. In summary, the entire gastric mucosa was embedded into 120–252 blocks and examined in up to 490 sections. Microscopic foci of adenocarcinoma were divided into signet ring cell carcinoma in situ (SCI, Tis), intramucosal adenocarcinoma (IMC, T1a). The median number of cancer foci was 15 (range 5–136). The subsequent 44 patients had a more limited pathological analysis of the gastrectomy specimen. The median number of foci of early (Tis or T1a) diffuse gastric cancer was 2 (range 0 – >15). Two patients (3.7%) had no foci of gastric cancer identified. Figure 2A shows the relationship between patient age and number of cancer foci. The Pearson correlation coefficient for age and number of foci for all patients is 0.024, for the initial 10 patient with more extensive pathologic analysis is 0.025, and for the subsequent 44 patients is 0.011. Thus, there is little or no correlation between age and number of foci.
Figure 2.
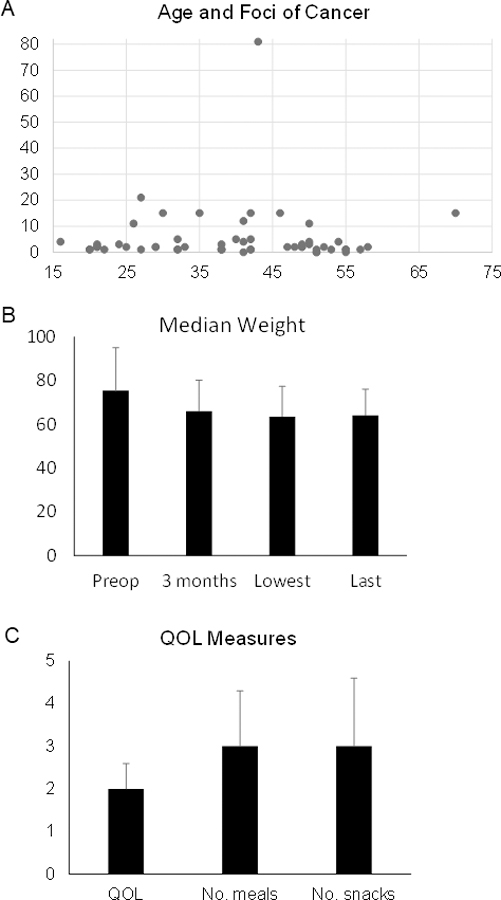
A. Graph showing patient age and number of foci of cancer. B. Graph showing median weight of patients at various time points. C. Graph showing QOL measure. Bars represent standard deviation.
Postoperative outcomes
Contrast study of the esophagojejunal anastomosis was generally performed 3–4 days following surgery in 49 of 54 patients, and all studies showed no anastomotic leak. None of the 54 patients, including the 5 patients that did not have a contrast study, developed an anastomotic leak. Patients were generally started on sips of clears on postoperative day (POD) 3–4, clears ad lib on POD 4–5, and a mechanical soft diet on POD 6. Pain control generally was maintained initially with an epidural catheter, and patients were transitioned to oral pain medication once the patient was tolerating liquids. Median hospital stay was 7 days and the range was 6–12 days.
Early complications identified within 30 days of surgery were observed in 5 subjects (9.2% of patients) where two required hospital re-admission. One patient was discharged on POD 7 and returned 2 days later with a pulmonary embolism. The patient was admitted for anticoagulation. One patient was discharged on POD 8 and returned on POD 16 with mild pancreatitis that resolved with medical management. The other three complications included one patient with a urinary tract infection requiring antibiotics and two patients with superficial incision cellulitis that resolved with antibiotics. There was one possible case of anastomotic stricture. This patient underwent one dilation of the esophagojejunostomy a few months after surgery at a local institution but his symptoms were subsequently determined likely to be secondary to either esophageal dysmotility or Roux limb stasis rather than stricture.
None of these patients had a jejunostomy feeding tube placed at the time of surgery. One patient required subsequent placement of a percutaneous jejunostomy tube (PEJ). This patient was 21 years old woman when she had her surgery, and initially had poor oral intake after surgery which was slow to improve. A PEJ was placed about 2 months after surgery and remained in place until 7 months after surgery, at which point the patient was able to maintain her weight without tube feeds. No patient required parenteral nutrition.
Four patients (7.4%) experienced late complications (>30 day) of surgery that required surgical intervention. Two years following prophylactic total gastrectomy, one of these patients (Patient #1) presented to the emergency department with significant abdominal pain and bilious emesis and imaging was suspicious for small bowel intussusception at the jejunojejunostomy. Exploratory laparotomy confirmed a jejuno-jejunal intussusception that required resection and reconstruction. Patient #19 presented 8 months after gastrectomy with a small bowel obstruction requiring laparotomy at an outside hospital. Patients #22 and #53 developed incisional hernias and had laparoscopic repairs with mesh placement 1.5 years and 8 months after gastrectomy, respectively.
Weight loss
Figure 2B shows the median weight of patients at various time points. Prior to surgery, the median weight was 75.4 kg (68 kg for women, 84.4 kg for men) and the median BMI was 25.6 (25.3 for women, 26.6 for men). Follow-up weight was available in 42 patients. The median percentage of maximum weight loss, compared to preoperative weight, was 19% (range 0–39%). The lowest weight was reached 1–10 months after surgery with a median of 3 months. Long term follow-up (2–15 years) weight was available in 21 patients, and long-term weight was a median of 15% less than the preoperative weight. Thus, the average patient gained back 4% of their original weight loss.
Long-term outcomes
To evaluate long-term outcomes, patients were contacted in the fall of 2021 and asked to complete a 30 question survey. Many patients could not be contacted due to missing current contact information or did not return the questionnaire. Of 54 patients, 20 patients (37%) responded. The median time from surgery to completion of the survey was 55.5 months (range 30–182 months). The median age of respondents was 50 years old (range 24–74 years old), and there were 10 women and 10 men. No patient suffered a recurrence of gastric cancer. Using the Centers for Disease Control and Prevention health-related quality of life instrument 22, the median response was 2.0 (very good) (Fig. 2C). Three patients answered 1 (excellent), eight patients answered 2 (very good) to 2.5, three patients answered 3 (good) to 3.5. No patient answered 4 (fair) or 5 (poor). Five of 20 patients (25%) reported symptoms that interfered with quality of life including frequent or urgent bowel movements (n=1) and fatigue (n=3).
Median size of meal compared to a preoperative normal meal was a median of 75% (range 25–100%). Median number of meals and snacks per day were 3.0 (range 2–8) and 3.0 (range 1.5–6), respectively (Fig. 2C). All but one patient had problems with certain foods or liquids. Common difficult foods or liquids included dairy, water, sugar or carbohydrate rich foods, fried foods, and certain vegetables. Seven patients (37%) reported episodes of heartburn while 3 patients (16%) had problems with bowel movements (e.g. diarrhea or constipation).
All patients were instructed after surgery to take a multivitamin with iron orally and vitamin B12 either parenterally or sublingually for life. All patients were still getting routine blood work every 3–12 months. Four patients had stopped the multivitamin and two patients had stopped vitamin B12 because their blood tests found no deficiencies off these supplements. Sixteen of 20 patients (80%) described taking additional vitamins or supplements including calcium, vitamin D, iron infusions, and vitamin C. The majority of these vitamins and supplements were recommended by physicians after blood testing.
Thirteen patients (65%) had persistent symptomatic issues with abnormal blood sugar levels years after surgery. Seven patients still had low blood sugar after eating, 3 had high, and 3 had both. Three patients reported other gastrointestinal issues including colitis (n=1), poor esophageal motility (n=1), and pancreatic insufficiency (n=1).
Non-gastrointestinal medical conditions developed after surgery in several patients. This included hypertension (n=1), anxiety/depression (n=1), and osteopenia (n=4). Five patients had subsequent non-orthopedic major surgeries including prophylactic mastectomies (n=3) and cholecystectomy (n=2).
In response to the question “If you had to do it all over again, would you choose prophylactic total gastrectomy or screening endoscopies?” all 20 respondents answered prophylactic total gastrectomy. Patients were also given the opportunity to include any additional comments. Two patients stated that increased nutrition support would have been helpful. One patient commented that increased mental health resources would have been helpful.
DISCUSSION
It has been more than 20 years since the discovery that germline pathogenic variants in the CDH1 gene cause hereditary diffuse gastric cancer 7. There are few published series on prophylactic total gastrectomy for germline CDH1 mutation given the rarity of this mutation and even fewer studies providing long-term follow-up of these patients following gastrectomy. Herein, we describe 54 patients with germline CDH1 pathogenic mutations who underwent prophylactic total gastrectomy. All but one patient had a strong family history of gastric cancer. Pre-operative endoscopy identified one or more foci of microscopic diffuse gastric cancer in only eight patients (15%). Following prophylactic total gastrectomy, patients were hospitalized for a median of 7 days. There were no anastomotic leaks in any subject, and the 30-day complication rate was 9.2%. Pathological analysis of gastrectomy specimens identified one or more foci diffuse gastric cancer in 52 patients (96.3%), and thus only 2 patients (3.7%) had no foci of diffuse gastric cancer identified. Median weight loss was 19%, with a weight nadir on average at three months. In long-term follow-up of 19 patients, quality of life was good to excellent, and most patients had persistent issues with meal size and certain foods or liquids.
The International Gastric Cancer Linkage Consortium (IGCLC) has published consensus management guidelines and indications for genetic testing in individuals suspected of having HDGC 23. Family criteria include: (1) two or more cases in family regardless of age, with at least one DGC, (2) one or more case of DGC at any age, and one or more case of lobular breast cancer at age <70 years old, in different family members, (3) two or more cases of lobular breast cancer in family members <50 years old. More recent modifications have further expanded these criteria 24. Individuals who meet criteria should be provided genetic counseling and tested for germline mutations in CDH1 as well as CTNNA1 and PALB2 which are genes also associated with diffuse type gastric cancer 25–27.
In this series, the majority of patients had insertions and deletions (indels) mutations in CDH1, which is consistent with larger population data. In published reports, pathogenic CDH1 mutations have been identified throughout the gene, including all 16 exons as well as introns 8,28. Small indels nonsense mutations, splice site mutations, large exon deletions, and missense mutations have all been reported, with the most common type of mutation being indels, as 38% of identified pathogenic CDH1 mutations are indels 28,29. Nonsense mutations account for 17% of known mutations, and splice site mutations, large deletions, and missense mutations make up 21%, 9%, and 16% of identified mutations, respectively 29. The effect of missense mutations is controversial, as often times E-cadherin full-length protein is produced and expressed at normal levels even in the presence of a germline CDH1 missense mutation 29. Furthermore, phenotypic expression has not been shown to correlate with the type of mutation, or its location in CDH1, highlighting a need for further research on the function of CDH1 mutations 8,28,29.
Individuals with a germline CDH1 mutation have a lifetime risk of developing HDGC of 37–42% for men and 25–33% for women, and the mean age of developing a gastric cancer is 47 years old 11,12. The risk of developing gastric cancer may vary in each family depending on how many gastric cancer cases have been found. Roberts et al. estimated the risk in families with 3 or more gastric cancer cases as 64% in men and 47% for women [11]. This risk decreased to 27% for men and 24% for women in families with 2 or less gastric cancer cases.
Prophylactic total gastrectomy is often recommended for healthy CDH1-positive individuals, however, the optimal age for prophylactic surgery is debated. The International Gastric Cancer Linkage Consortium (IGCLC) states “Where possible, surgery is recommended in early adulthood, generally between 20 and 30 years of age” 23. For those undergoing screening endoscopic evaluation, the Cambridge method involves a minimum of 30 random biopsies from 5 separate areas of the stomach which can detect occult signet ring cell carcinoma foci in up to 61% of patients 30,31. Since 90% or more of those with germline CDH1 mutation will have one or more microscopic foci of diffuse gastric cancer on prophylactic total gastrectomy 24, the clinical significance of such foci on random biopsy is unclear. Early foci of diffuse gastric cancer in HDGC patients is characterized by infiltrates of signet ring cells that underlie normal-appearing mucosa 13. In our series, preoperative endoscopy with random biopsies identified occult foci of diffuse gastric cancer in 15% of patients. The ability of serial screening endoscopies to identify clinically significant diffuse gastric cancer is unknown. In one study of CDH1-positive individuals who underwent endoscopic screening, there were 22 patients who underwent more than one endoscopy 32. Twelve patients ultimately had surgery, 11 had stage 0 or IA disease, and one patient had stage II (T3N0M0) disease.
The creation of the esophagojejunal anastomosis after total gastrectomy can be technically demanding, and reconstruction-related complications such as anastomotic leak and stricture account for a significant proportion of post-operative morbidity 33,34. In a retrospective review of adverse events within 90 days of total gastrectomy for 238 non-CDH1 patients with gastric cancer, esophagojejunal anastomotic leak requiring invasive intervention occurred in 11% of patients 33. Even among the usually younger and healthier CDH1-positive individuals that receive prophylactic total gastrectomy, esophageal anastomotic leak rates between 8–26% have been reported 35–39. Anastomotic stricture rates of up to 21% have been reported following esophagojejunostomy with the highest rates occurring with circular staplers 40. In this series of 54 patients, there were no anastomotic leaks and one possible anastomotic stricture.
The early and late postoperative morbidity in this series of patients compares favorably with other series. In one surgical series from Newfoundland, Canada, 23 patients underwent prophylactic total gastrectomy 36. In this series, 3 patients with venous thromboembolism, 2 had anastomotic leaks, and 1 had an intra-abdominal abscess. One patient was hospitalized for 107 days. In a series of 101 CDH1-postive patients, the 30-day major complication rate was 16%, the 30–90 day major complication rate was 18%, and one patient died from multi-system organ failure 41. A final study from the Netherlands observed complications in 8 of 26 CDH1-postive patients (31%), 5 of whom required surgical intervention within a year of surgery 42. Thus even at gastric cancer referral centers with significant experience, there can be significant complications from this type of prophylactic surgery.
Numerous studies have demonstrated a relationship between morbidity/mortality after gastrectomy and hospital volume as well as patient health. One retrospective review of 29,599 US National Cancer Database (NCDB) patients with gastric cancer found that, while only 7.8% of patients were treated at a high volume hospital (in this study defined as 17 or more cases per year), those that did experienced a significantly lower 30-day mortality than those that underwent gastrectomy at a low-volume hospital (2.1% vs 3.5%, p<0.01) 43. Another study of 2733 gastric cancer patients in Texas observed lower in-hospital mortality for patients treated at high volume hospitals (0.9% and 2.6% for patients with and without comorbidities, respectively) than those at low volume hospitals (2.4% and 5.6%). High volume hospital patients also had lower rates of adverse events (7.3% and 18%) than low volume hospital patients (17.9% and 31%). In multivariate analysis, treatment at a high volume hospital strongly predicted lower inpatient morbidity (OR 0.56, p = 0.013) and mortality (OR 0.39, p=0.019) 44. In terms of patient health, a study by Ogata et al. found that the most important predictors of early and late mortality following gastrectomy were age, Eastern Cooperative Oncology Group performance status (ECOG-PS), Charlson Comorbidity Index (CCI), and psoas muscle mass index (PMI, an indicator of sarcopenia) 45.
There are clear nutritional consequences following total gastrectomy, and thus routine laboratory testing and long-term follow-up are needed. Routine laboratory work should include a complete blood count (CBC), electrolytes, BUN, creatinine, and liver function tests. Total gastrectomy results in loss of intrinsic factor secretion, significantly impairing vitamin B12 absorption, and predisposes to iron malabsorption and deficiency Additionally, calcium and vitamin D absorption are also diminished. For total gastrectomy patients, we have always recommended that they take a multivitamin with iron orally and vitamin B12 either by intramuscular injection (1000 mcg monthly) or sublingually (methylcobalamin 1500 mcg daily) 46. Total calcium, albumin, ionized calcium, PTH, and 25-hydroxyvitamin D levels can be used to evaluate for calcium or vitamin D deficiency. In addition, bone mineral density can be tested. One can have normal calcium levels with low calcium absorption given the large endogenous supply of calcium in bones. For those at risk of calcium deficiency and osteoporosis, one can either increase consumption of calcium rich foods to 1,500 mg/day or take calcium citrate 1,200 to 1,500 mg per day in divided doses 47. Iron studies should be performed, and ferrous sulfate 200 mg (elemental iron 57 mg) three times per day can be added if there is iron deficiency. Fat malabsorption occurs in about 10% of patients after gastrectomy and is more common when the duodenum is bypassed. This is due to alterations in gastric lipase secretions, exocrine pancreatic insufficiency, altered cholecystokinin release, pancreaticobiliary asynchrony, or small intestinal bacterial overgrowth (SIBO). This can lead to deficiencies in vitamins A, D, E, and K.
Post-operative issues such as early dumping syndrome (secondary to hyperosmotic carbohydrate loads) and diarrhea (secondary to rapid transit or malnutrition) occur to some degree in the majority of patients, and can be severe immediately following surgery but tend to improve over time 48,49. Dumping and diarrhea can improve over time and can be alleviated with appropriate nutritional counseling and diet modification. Patients can also experience lactose intolerance, and bacterial overgrowth resulting in malabsorption and bloating can also occur. Patients are initially instructed to eat small amounts frequently over the course of the day, limit food and beverages high in sugar, avoid fried/ greasy foods, and focus on high protein foods. After several months, most patients are able to eat three small to moderate meals a day with snacks in-between.
Females with germline CDH1 mutation also carry a 39–55% risk of developing lobular breast cancer 11,12. Annual surveillance with breast MRI is thus recommended beginning at age 30 23. Lobular breast cancer often does not form a discrete mass or form microcalcifications so the utility of mammogram is lower than that for infiltrating ductal carcinoma 50. Bilateral risk-reducing mastectomies can also be considered. Three of the 35 women in this series underwent bilateral risk-reducing mastectomies after their prophylactic total gastrectomy.
Given we are still relatively early in the experience regarding prophylactic total gastrectomy for germline CDH1 mutation, there remain certain unresolved issues. First, the timing of prophylactic surgery is still unclear. The earliest reported case of a germline E-cadherin mutation carrier developing gastric cancer is 14 years old, and the median age in developing clinically apparent gastric cancer is around 47 years old 11,12. In this series, the median age for prophylactic surgery was 41 years old and the range was 16–70 years old. Second, given that total gastrectomy is a significant undertaking, a more accurate ability to estimate risk may improve patient selection for prophylactic surgery and reduce the morbidity associated with gastrectomy in the individuals who will not ultimately develop gastric cancer. Third, there is an increasing number of individuals and families who are found to have pathogenic germline CDH1 mutation who have no family history of gastric cancer 27. These individuals and families likely have a lower risk of developing gastric cancer compared to kindred with gastric cancer, but the relative risk reduction remains unknown.
In summary, individuals with germline CDH1 mutation face complex decisions, and thus benefit from counseling from an experienced, multidisciplinary team. Prophylactic total gastrectomy for CDH1-positive individuals at risk for HDGC can be accomplished with acceptable short-term morbidity when performed at high volume gastric cancer centers. While prophylactic total gastrectomy results in permanent nutritional sequelae, all respondents to a survey years after surgery reported good to excellent QOL and would again choose surgery over surveillance endoscopies if given the choice again. Future research into this rare genetic syndrome may allow for better risk stratification and surveillance strategies, thus focusing prophylactic total gastrectomy toward the subset of CDH1-positive individuals who are at highest risk of developing a clinically relevant cancer.
Supplementary Material
Synopsis:
Individuals with germline CDH1 mutation face complex decisions, and thus benefit from counseling from an experienced, multidisciplinary team. Prophylactic total gastrectomy for CDH1-positive individuals at risk for HDGC can be accomplished with acceptable short-term morbidity when performed at high volume gastric cancer centers. While prophylactic total gastrectomy results in permanent nutritional sequelae, all respondents to a survey years after surgery reported good to excellent QOL and would again choose surgery over surveillance endoscopies if given the choice again.
Funding:
The article was supported by Herbert Irving Columba Cancer Center Support Grant (CCSG) P30CA013696 (Sam Yoon, ssy2129@cumc.columbia.edu), the DeGregorio Family Foundation, and Stand Up To Cancer.
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Data availability statement:
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
REFERENCES
- 1.Sung H, Ferlay J, Siegel RL, et al. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin 2021; 71(3): 209–49. [DOI] [PubMed] [Google Scholar]
- 2.Armstrong DK, Bundy B, Wenzel L, et al. Intraperitoneal cisplatin and paclitaxel in ovarian cancer. N Engl J Med 2006; 354(1): 34–43. [DOI] [PubMed] [Google Scholar]
- 3.Hooi JKY, Lai WY, Ng WK, et al. Global Prevalence of Helicobacter pylori Infection: Systematic Review and Meta-Analysis. Gastroenterology 2017; 153(2): 420–9. [DOI] [PubMed] [Google Scholar]
- 4.Lauren P The Two Histological Main Types of Gastric Carcinoma: Diffuse and So-Called Intestinal-Type Carcinoma. An Attempt at a Histo-Clinical Classification. Acta Pathol Microbiol Scand 1965; 64: 31–49. [DOI] [PubMed] [Google Scholar]
- 5.Zanghieri G, Di Gregorio C, Sacchetti C, et al. Familial occurrence of gastric cancer in the 2-year experience of a population-based registry. Cancer 1990; 66(9): 2047–51. [DOI] [PubMed] [Google Scholar]
- 6.Jones EG. Familial Gastric Cancer. N Z Med J 1964; 63: 287–96. [PubMed] [Google Scholar]
- 7.Guilford P, Hopkins J, Harraway J, et al. E-cadherin germline mutations in familial gastric cancer. Nature 1998; 392(6674): 402–5. [DOI] [PubMed] [Google Scholar]
- 8.Hansford S, Kaurah P, Li-Chang H, et al. Hereditary Diffuse Gastric Cancer Syndrome: CDH1 Mutations and Beyond. JAMA Oncol 2015; 1(1): 23–32. [DOI] [PubMed] [Google Scholar]
- 9.Caldas C, Carneiro F, Lynch HT, et al. Familial gastric cancer: overview and guidelines for management. J Med Genet 1999; 36(12): 873–80. [PMC free article] [PubMed] [Google Scholar]
- 10.Kaurah P, MacMillan A, Boyd N, et al. Founder and recurrent CDH1 mutations in families with hereditary diffuse gastric cancer. JAMA 2007; 297(21): 2360–72. [DOI] [PubMed] [Google Scholar]
- 11.Roberts ME, Ranola JMO, Marshall ML, et al. Comparison of CDH1 Penetrance Estimates in Clinically Ascertained Families vs Families Ascertained for Multiple Gastric Cancers. JAMA Oncol 2019; 5(9): 1325–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xicola RM, Li S, Rodriguez N, et al. Clinical features and cancer risk in families with pathogenic CDH1 variants irrespective of clinical criteria. J Med Genet 2019; 56(12): 838–43. [DOI] [PubMed] [Google Scholar]
- 13.Fitzgerald RC, Caldas C. Clinical implications of E-cadherin associated hereditary diffuse gastric cancer. Gut 2004; 53(6): 775–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim CY, Nam BH, Cho GS, et al. Learning curve for gastric cancer surgery based on actual survival. Gastric Cancer 2016; 19(2): 631–8. [DOI] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer A. Japanese gastric cancer treatment guidelines 2018 (5th edition). Gastric Cancer 2021; 24(1): 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mathisen DJ, Grillo HC, Wilkins EW Jr, Moncure AC, Hilgenberg AD. Transthoracic esophagectomy: a safe approach to carcinoma of the esophagus. Ann Thorac Surg 1988; 45(2): 137–43. [DOI] [PubMed] [Google Scholar]
- 17.Chang KK, Patel MS, Yoon SS. Linear-Stapled Side-to-Side Esophagojejunostomy with Hand-Sewn Closure of the Common Enterotomy After Prophylactic and Therapeutic Total Gastrectomy. J Gastrointest Surg 2017; 21(4): 712–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rino Y, Yukawa N, Kano K, et al. Our connection procedure for an EEA XL stapler and anvil head using EEA OrVil for laparoscopic total or proximal gastrectomy. Asian J Endosc Surg 2018; 11(3): 280–3. [DOI] [PubMed] [Google Scholar]
- 19.Pandalai PK, Lauwers GY, Chung DC, Patel D, Yoon SS. Prophylactic total gastrectomy for individuals with germline CDH1 mutation. Surgery 2011; 149(3): 347–55. [DOI] [PubMed] [Google Scholar]
- 20.Fujita H, Lennerz JK, Chung DC, et al. Endoscopic surveillance of patients with hereditary diffuse gastric cancer: biopsy recommendations after topographic distribution of cancer foci in a series of 10 CDH1-mutated gastrectomies. Am J Surg Pathol 2012; 36(11): 1709–17. [DOI] [PubMed] [Google Scholar]
- 21.Chung DC, Yoon SS, Lauwers GY, Patel D. Case records of the Massachusetts General Hospital. Case 22–2007. A woman with a family history of gastric and breast cancer. N Engl J Med 2007; 357(3): 283–91. [DOI] [PubMed] [Google Scholar]
- 22.Yin S, Njai R, Barker L, Siegel PZ, Liao Y. Summarizing health-related quality of life (HRQOL): development and testing of a one-factor model. Popul Health Metr 2016; 14: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Blair VR, McLeod M, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical practice guidelines. Lancet Oncol 2020; 21(8): e386–e97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gamble LA, Heller T, Davis JL. Hereditary Diffuse Gastric Cancer Syndrome and the Role of CDH1: A Review. JAMA Surg 2021; 156(4): 387–92. [DOI] [PubMed] [Google Scholar]
- 25.Benusiglio PR, Colas C, Guillerm E, et al. Clinical implications of CTNNA1 germline mutations in asymptomatic carriers. Gastric Cancer 2019; 22(4): 899–903. [DOI] [PubMed] [Google Scholar]
- 26.Clark DF, Michalski ST, Tondon R, et al. Loss-of-function variants in CTNNA1 detected on multigene panel testing in individuals with gastric or breast cancer. Genet Med 2020; 22(5): 840–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Petridis C, Shinomiya I, Kohut K, et al. Germline CDH1 mutations in bilateral lobular carcinoma in situ. Br J Cancer 2014; 110(4): 1053–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Luo W, Fedda F, Lynch P, Tan D. CDH1 Gene and Hereditary Diffuse Gastric Cancer Syndrome: Molecular and Histological Alterations and Implications for Diagnosis And Treatment. Front Pharmacol 2018; 9: 1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Figueiredo J, Melo S, Carneiro P, et al. Clinical spectrum and pleiotropic nature of CDH1 germline mutations. J Med Genet 2019; 56(4): 199–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Post RS, Vogelaar IP, Carneiro F, et al. Hereditary diffuse gastric cancer: updated clinical guidelines with an emphasis on germline CDH1 mutation carriers. J Med Genet 2015; 52(6): 361–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mi EZ, Mi EZ, di Pietro M, et al. Comparative study of endoscopic surveillance in hereditary diffuse gastric cancer according to CDH1 mutation status. Gastrointest Endosc 2018; 87(2): 408–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Friedman M, Adar T, Patel D, et al. Surveillance Endoscopy in the Management of Hereditary Diffuse Gastric Cancer Syndrome. Clin Gastroenterol Hepatol 2021; 19(1): 189–91. [DOI] [PubMed] [Google Scholar]
- 33.Selby LV, Vertosick EA, Sjoberg DD, et al. Morbidity after Total Gastrectomy: Analysis of 238 Patients. J Am Coll Surg 2015; 220(5): 863–71 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Inokuchi M, Otsuki S, Fujimori Y, Sato Y, Nakagawa M, Kojima K. Systematic review of anastomotic complications of esophagojejunostomy after laparoscopic total gastrectomy. World J Gastroenterol 2015; 21(32): 9656–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y, Kingham K, Ford JM, et al. A prospective study of total gastrectomy for CDH1-positive hereditary diffuse gastric cancer. Ann Surg Oncol 2011; 18(9): 2594–8. [DOI] [PubMed] [Google Scholar]
- 36.Hebbard PC, Macmillan A, Huntsman D, et al. Prophylactic total gastrectomy (PTG) for hereditary diffuse gastric cancer (HDGC): the Newfoundland experience with 23 patients. Ann Surg Oncol 2009; 16(7): 1890–5. [DOI] [PubMed] [Google Scholar]
- 37.Lewis FR, Mellinger JD, Hayashi A, et al. Prophylactic total gastrectomy for familial gastric cancer. Surgery 2001; 130(4): 612–7; discussion 7–9. [DOI] [PubMed] [Google Scholar]
- 38.Haverkamp L, van der Sluis PC, Ausems MG, et al. Prophylactic Laparoscopic Total Gastrectomy with Jejunal Pouch Reconstruction in Patients Carrying a CDH1 Germline Mutation. J Gastrointest Surg 2015; 19(12): 2120–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seevaratnam R, Coburn N, Cardoso R, Dixon M, Bocicariu A, Helyer L. A systematic review of the indications for genetic testing and prophylactic gastrectomy among patients with hereditary diffuse gastric cancer. Gastric Cancer 2012; 15 Suppl 1: S153–63. [DOI] [PubMed] [Google Scholar]
- 40.Zuiki T, Hosoya Y, Kaneda Y, et al. Stenosis after use of the double-stapling technique for reconstruction after laparoscopy-assisted total gastrectomy. Surg Endosc 2013; 27(10): 3683–9. [DOI] [PubMed] [Google Scholar]
- 41.Vos EL, Salo-Mullen EE, Tang LH, et al. Indications for Total Gastrectomy in CDH1 Mutation Carriers and Outcomes of Risk-Reducing Minimally Invasive and Open Gastrectomies. JAMA Surg 2020; 155(11): 1050–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.van der Kaaij RT, van Kessel JP, van Dieren JM, et al. Outcomes after prophylactic gastrectomy for hereditary diffuse gastric cancer. Br J Surg 2018; 105(2): e176–e82. [DOI] [PubMed] [Google Scholar]
- 43.Ju MR, Blackwell JM, Zeh HJ, Yopp AC, Wang SC, Porembka MR. Redefining High-Volume Gastric Cancer Centers: The Impact of Operative Volume on Surgical Outcomes. Ann Surg Oncol 2021; 28(9): 4839–47. [DOI] [PubMed] [Google Scholar]
- 44.Ikoma N, Kim B, Elting LS, Shih YT, Badgwell BD, Mansfield P. Trends in Volume-Outcome Relationship in Gastrectomies in Texas. Ann Surg Oncol 2019; 26(9): 2694–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ogata Y, Hatta W, Ohara Y, et al. Predictors of early and late mortality after the treatment for early gastric cancers. Dig Endosc 2022; 34(4): 816–25. [DOI] [PubMed] [Google Scholar]
- 46.Kim HI, Hyung WJ, Song KJ, Choi SH, Kim CB, Noh SH. Oral vitamin B12 replacement: an effective treatment for vitamin B12 deficiency after total gastrectomy in gastric cancer patients. Ann Surg Oncol 2011; 18(13): 3711–7. [DOI] [PubMed] [Google Scholar]
- 47.Oh HJ, Yoon BH, Ha YC, et al. The change of bone mineral density and bone metabolism after gastrectomy for gastric cancer: a meta-analysis. Osteoporos Int 2020; 31(2): 267–75. [DOI] [PubMed] [Google Scholar]
- 48.Grant JP, Chapman G, Russell MK. Malabsorption associated with surgical procedures and its treatment. Nutr Clin Pract 1996; 11(2): 43–52. [DOI] [PubMed] [Google Scholar]
- 49.Tovey FI, Godfrey JE, Lewin MR. A gastrectomy population: 25–30 years on. Postgrad Med J 1990; 66(776): 450–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Michael M, Garzoli E, Reiner CS. Mammography, sonography and MRI for detection and characterization of invasive lobular carcinoma of the breast. Breast Dis 2008; 30: 21–30. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.


