Abstract
Background:
We characterized trends in statin eligibility and subsequent statin initiation among people with HIV (PWH) from 2001–2017 and identified predictors of statin initiation between 2014–2017.
Setting:
PWH participating in the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD) enrolled in twelve US cohorts collecting data on statin eligibility criteria/prescriptions from 2001–2017.
Methods:
We determined the annual proportion eligible for statins, initiating statins, and median waiting time (from statin eligibility to initiation). Eligibility was defined using ATP III guidelines (2001–2013) and ACC/AHA guidelines (2014–2017). We assessed initiation predictors in 2014–2017 among statin-eligible PWH using Poisson regression, estimating adjusted prevalence ratios (aPR) with 95% confidence intervals (95% CI).
Results:
Among 16,409 PWH, 7,386 (45%) met statin eligibility criteria per guidelines (2001–2017). From 2001–2013, statin eligibility ranged from 22–25%. Initiation increased from 13% to 45%. In 2014, 51% were statin-eligible, among whom 25% initiated statins, which increased to 32% by 2017. Median wait time to initiation among those we observed declined over time. Per 10-year increase in age, initiation increased 46% (aPR 1.46, 95% CI 1.29, 1.67). Per one-year increase in calendar year from 2014–2017, there was a 41% increase in the likelihood of statin initiation (aPR 1.41, 95% CI 1.25, 1.58).
Conclusions:
There is a substantial statins treatment gap, amplified by the 2013 ACC/AHA guidelines. Measures are warranted to clarify reasons we observe this gap, and if necessary, increase statin use consistent with guidelines including efforts to help providers identify appropriate candidates.
Keywords: HIV, statins, lipid-lowering medications, treatment gap
INTRODUCTION
As the population of people with HIV (PWH) ages, the prevalence of chronic non-HIV aging-related comorbidities including diabetes,1–3 liver disease,4,5 non-AIDS defining cancers,6,7 and cardiovascular disease (CVD)3,8–11 are rising. Studies have demonstrated increased risk for CVD comparing people with versus without HIV (accounting for known risk factors12), with estimates varying depending on the outcome measured.13,14 Possible mechanisms driving this increased risk include high prevalence of traditional risk factors (e.g. smoking), metabolic abnormalities resulting from ART regimens15,16 and HIV-associated elevated systemic inflammation and immune activation.17,18
Hydroxymethylglutaryl-CoA reductase inhibitors (statins) are commonly used medication that decrease CVD risk by reducing levels of low-density lipoprotein (LDL) cholesterol. It has been hypothesized that statins may act on HIV-specific mechanisms that reduce levels of inflammation and immune activation contributing to increased CVD risk.19–21 Usage of statins in the US is rising, with an estimated 27.8% of adults 40 and older receiving statins as of 2013, compared with 17.9% in 2003.22 The establishment of the 2013 American College of Cardiology/American Heart Association (ACC/AHA) Cholesterol Management Guidelines broadened clinical indications for statins compared to prior guidelines,23,24 resulting in increased eligibility.25 In the general population, there is a gap between eligible and “on treatment” patients, with a substantial proportion of persons having atherosclerotic cardiovascular disease, or other indications for statin use, not receiving statins.26,27
Despite the increased risk of CVD among PWH, this population is less likely to receive statins than those without HIV.28–35 Although there is evidence of this statin treatment gap among PWH, efforts to describe this have been limited by small size and were conducted among specific subgroups (e.g., among men or women only with HIV, within urban settings only), or prior to the ACC/AHA guideline implementation, precluding generalizability to the wider contemporary population of PWH in the US.29–40 Disparities in statin initiation have been reported by gender, hepatitis C coinfection, and race among PWH;28,33 however, it remains unclear what additional clinical and demographic factors are associated with statin initiation among PWH under the 2013 ACC/AHA guidelines. The establishment of these guidelines expanded statin eligibility at a time when substantial changes in care among PWH were also occurring. Treat-all policy was implemented in the year prior,41 and PWH were continually living longer into older ages. The timely examination of risk factors for statin initiation on a population-level should follow the ACC/AHA guidelines in large, observational settings.
Thus, we sought to characterize trends in statin eligibility and initiation in a nationally representative cohort of PWH in care in the US and to identify potential predictors of statin initiation among PWH eligible following the establishment of the ACC/AHA 2013 guidelines.
METHODS
Study Population
We utilized data from the North American AIDS Cohort Collaboration on Research Design (NA-ACCORD). The NA-ACCORD is a consortium of single-site/multisite cohorts among adults with HIV in the US and Canada, which has been previously described.42 Individuals are eligible for inclusion if they had two or more HIV visits in the twelve months prior to cohort inclusion screening, indicating linkage to HIV care. Each cohort has standardized methods of data collection, submitting data on enrolled participant characteristics, diagnoses, laboratory measures, prescribed medications, and vital status to the Data Management Core (University of Washington, Seattle, WA). Data are harmonized across cohorts and evaluated for quality control prior to being transmitted to the Epidemiology/Biostatistics Core (Johns Hopkins University, Baltimore, Maryland). Analyses for this study were conducted by the Epidemiology/Biostatistics Core. The NA-ACCORD is reviewed and approved by local institutional review boards (IRBs) and the Johns Hopkins School of Medicine IRB.
For this analysis, US cohorts were included that capture data for the components of the ATP III and ACC/AHA statin eligibility criteria, including smoking status (observed in at least 75% of the cohort), and statin prescriptions (Figure 1) (N=12). Inclusion was anchored on these parameters to ensure comprehensive capture of statin eligibility/initiation. Participants were included if they were under observation during 2001–2017, ≥18 years of age, and had covariates measured to determine statin eligibility. Missing smoking status was imputed using fully conditional specification multiple imputation with 20 iterations. One imputation out 20 was selected to measure smoking status.
Figure 1.
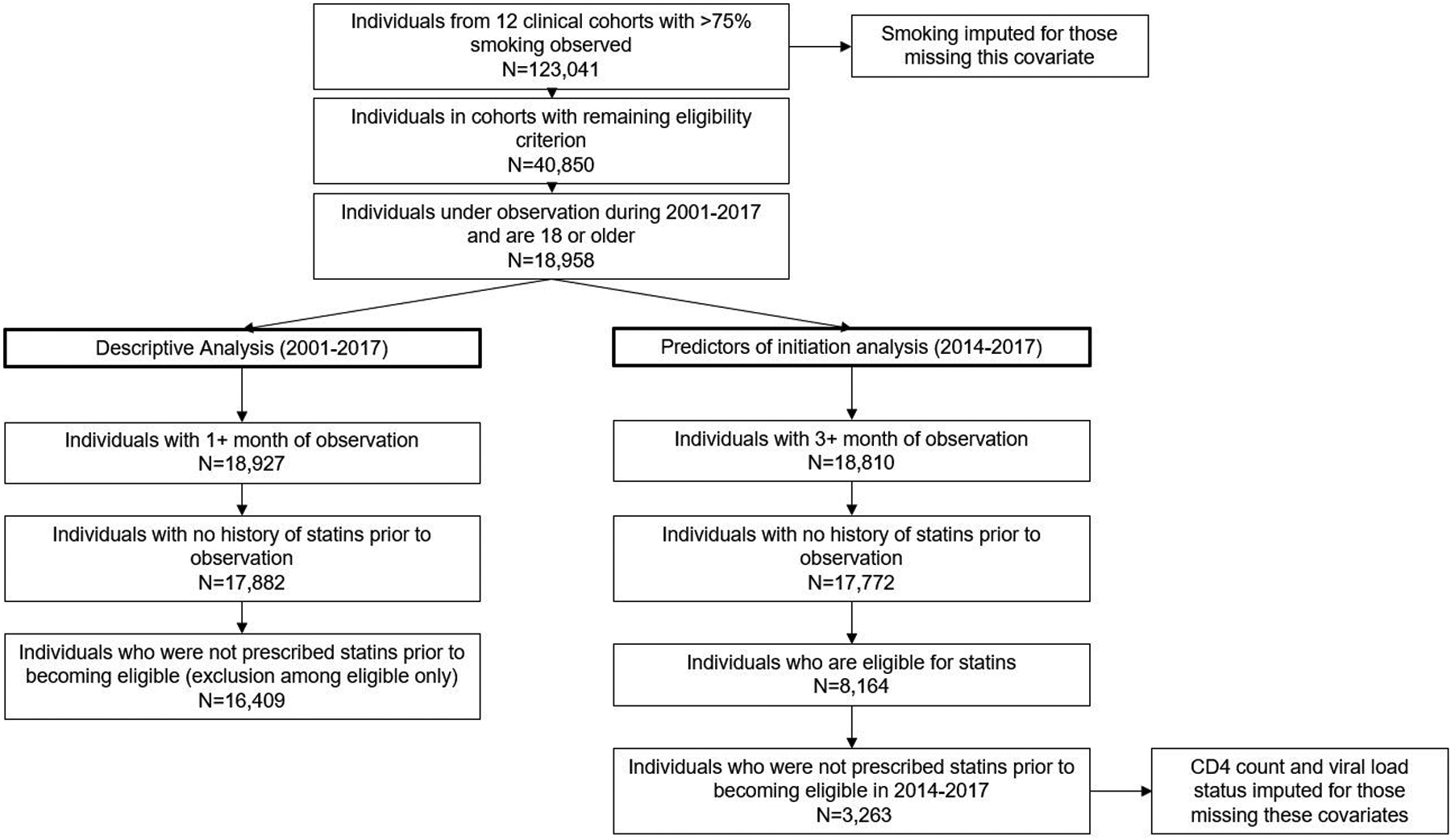
Flow diagram for inclusion into the analytic population for descriptive analysis and predictors of initiation analysis
Statin eligibility and statin initiation
Statin eligibility was assessed as a time-varying variable utilizing relevant clinical guidelines for the study period: the ATP III guidelines for 2001–2013, and the ACC/AHA guidelines for 2014–2017. Using ATP III guidelines, participants were eligible for statins if they had coronary heart disease and an LDL >100mg/dL; or ≤1 risk factors and LDL ≥190 mg/dL; or had ≥2 risk factors with 10-year predicted Framingham Risk Score (FRS) ≤20% and LDL ≥130 mg/dL; or were diabetic (HgA1c >6.5%, diabetes diagnosis, or diabetes medication) and had an FRS >20% and LDL ≥130 mg/dL. Risk factors included: smoking, hypertension (BP >140/90 mmHg or on antihypertensive medication), and age (45 or older in men, and 55 or older in women). Though family history of coronary heart disease is an additional criterion, these data were not available.
Participants were considered statin-eligible in 2014–2017 per ACC/AHA guidelines if they were diagnosed with CVD; or were diabetic and between the ages of 40–75; or had an LDL ≥190 mg/dL; or had a 10-year risk of CVD (defined as incidence non-fatal myocardial infarction, death due to coronary heart disease, or stroke) of 7.5% or more, and were between the ages of 40–75. Ten-year risk calculations were derived from pooled cohort equations,43 which have been externally validated, and incorporate age, race, sex, diabetes mellitus, current smoking status, total cholesterol, HDL cholesterol and systolic blood pressure.
Participants were defined as statin-eligible on the earliest date under observation they met any of the criteria for eligibility based on relevant guideline, and remained classified as statin eligible thereafter. Initiation of statins was defined as any first-time statin prescription of at least one month in duration within the study period.
For descriptive analyses spanning 2001–2017, participants were excluded if they had less than one month of observation or were prescribed statins prior to study entry/becoming statin-eligible (N=16,409) (Figure 1). For regression analyses, participants were additionally restricted to those who became eligible for statins using the 2013 ACC/AHA guidelines from 2014 to 2017 and were not on statins before becoming eligible (N = 3263).
Covariates
Additional demographic and clinical characteristics included: sex, race/ethnicity, mode of HIV transmission, hepatitis C virus (HCV) infection (defined as a positive hepatitis C antibody test, detectable HCV RNA or the presence of a genotype test), and calendar year of statin eligibility (the year a participant first identified as statin eligible). HIV-specific factors measured at study entry (for descriptive analyses) and at statin eligibility (for regression analyses) included: CD4 cell count (<200 cells/mm3, 200 – 350 cells/mm3, or ≤350 cells/mm3), undetectable HIV-1 RNA (viral load, ≤200 copies/mL), history of clinical AIDS, and ART regimen type (protease inhibitor (PI)-based, integrase inhibitor (INSTI)-based, Non-Nucleoside Reverse Transcriptase Inhibitor (NNRTI)-based, other/unknown ART, or ART naïve). For covariates measured at statin eligibility, the closest measurement to eligibility within 12 months prior to or 3 months following was utilized.
Statistical Analysis
Descriptive analyses included characterizing trends in statin eligibility and initiation from 2001–2017. Follow-up time began on 1/1/2001, enrollment into the NA-ACCORD, or the date when their respective cohorts began collecting data needed to classify statin eligibility status, whichever came last. The proportion of PWH eligible for statins, and the proportion who were prescribed (initiated) statins among those eligible were examined annually. Among statin initiators, the median waiting time from the date of statin eligibility to initiation was also calculated annually and graphically assessed, except for 2017 due to potentially having <12 months of follow-up observed. We hypothesized that there would be secular changes in clinician prescribing practices: as PWH were living longer on ART, and as this population continued aging, clinicians would more readily prescribe statins upon becoming eligible, especially under the ACC/AHA guidelines.
We identified predictors of statin initiation among PWH who were eligible for statins under the ACC/AHA guidelines (2014–2017). Participants in this portion of the analysis were followed from the date they were first identified as statin-eligible and were observed for statin initiation between 2014–2017. We estimated unadjusted and adjusted prevalence ratios (PRs) with 95% confidence intervals (95% CI) using Poisson regression with robust variance as an approximation to log binomial regression. Risk factors included: sex, race/ethnicity, age, mode of HIV transmission, smoking (ever vs. never), HCV infection status, year of statin eligibility, CD4 count, undetectable HIV viral load (≤200 copies/mL), history of clinical AIDS diagnosis, and ART regimen/treatment status. We additionally adjusted for: treated hypertension (yes vs. no, time-updated), systolic blood pressure (continuous, time-updated), low HDL (≤40 mg/dL vs. >40 mg/dL, time-updated), high LDL (≥130 mg/dL vs. <130 md/dL, time-updated) and high total cholesterol (≥240 mg/dL vs. <240 mg/dL, time-updated), diabetes (yes vs. no, time-updated), diagnosed with CVD (yes vs. no, time-updated), and high FRS (≥20% vs. <20%, time-updated). CD4 count and HIV viral load at statin eligibility were imputed using multiple imputation by chained equations as continuous variables and were then categorized accordingly as defined above. Seven percent had CD4 count and HIV viral load imputed (not overlapping). Subgroup analyses were conducted among those with CD4 count and HIV viral load observed (N=2,999).
RESULTS
The Statin Treatment Gap 2001–2017
Among NA-ACCORD participants meeting inclusion criteria (n=16,409), 7,386 (45%) were eligible for statins from 2001 through 2017 (Table 1). Compared with those who were not eligible, those observed to become statin-eligible were older (median age 53 vs. 40 years) and more commonly reported ever smoking (75% vs. 62%). Statin-eligible participants more often had high LDL (31% vs. 10%) and high total cholesterol (13% vs. 2%) at baseline relative to those who did not become statin-eligible, and had higher FRS scores (50% vs. 10% with intermediate to high-risk FRS). Thirty-one percent of statin-eligible participants were diagnosed with CVD, while 5% of non-statin-eligible participants were diagnosed with CVD. As CVD diagnosis is a criterion for statin eligibility under the ACC/AHA guidelines, we confirmed that the 5% of individuals diagnosed with CVD who were not eligible for statins were under observation when the ATP III guidelines were in effect. PWH who were eligible for statins were also more likely to have an undetectable HIV viral load at study entry (41% vs. 32%). Statin-eligible and non-eligible participants were similar with respect to sex, race/ethnicity, HIV risk group, HCV co-infection, ART regimen status, CD4 count at study entry, and history of a clinical AIDS diagnosis.
Table 1.
Characteristics among NA-ACCORD participants with no history of statins, 2001–2017
| Participants with no history of statin use, 2001–20171 | Eligible for statins in 2014–20172 | |||||||
|---|---|---|---|---|---|---|---|---|
| Never eligible | Eligible | Never Initiated | Initiated | |||||
| N=9,023 | N=7,386 | N=2,673 | N=590 | |||||
| Characteristic | n | % | n | % | n | % | n | % |
| Age | ||||||||
| 18-<40 | 5,720 | 63% | 2,044 | 28% | 491 | 18% | 52 | 9% |
| 40–49 | 2,414 | 27% | 2,894 | 39% | 763 | 29% | 157 | 27% |
| 50–59 | 775 | 8% | 1,938 | 26% | 1,076 | 40% | 247 | 42% |
| 60+ | 132 | 1% | 510 | 7% | 343 | 13% | 134 | 23% |
| Male | 7,684 | 85% | 6,336 | 86% | 2,265 | 85% | 496 | 84% |
| Race/ethnicity | ||||||||
| White | 3,867 | 43% | 3,613 | 49% | 1,116 | 42% | 275 | 47% |
| Black | 2,970 | 33% | 2,592 | 35% | 1,116 | 42% | 232 | 39% |
| Hispanic | 1,494 | 17% | 835 | 11% | 321 | 12% | 60 | 10% |
| Other/Unknown | 692 | 8% | 346 | 5% | 120 | 4% | 23 | 4% |
| HIV transmission risk | ||||||||
| MSM | 6,050 | 67% | 4,677 | 63% | 1,623 | 61% | 335 | 57% |
| IDU | 646 | 7% | 566 | 8% | 241 | 9% | 55 | 9% |
| Heterosexual | 1,873 | 21% | 1,791 | 24% | 685 | 26% | 162 | 27% |
| Other/Unknown | 454 | 5% | 352 | 5% | 124 | 5% | 38 | 6% |
| Ever HCV infected | 1,275 | 14% | 1,264 | 17% | 536 | 20% | 124 | 21% |
| Ever smoker(observed) | 5,604 | 62% | 5,559 | 75% | 1,975 | 74% | 409 | 69% |
| Ever smoker (imputed) | 471 | 5% | 53 | 1% | 19 | 1% | 4 | 1% |
| Diabetic | 149 | 2% | 529 | 7% | 303 | 11% | 151 | 26% |
| Low HDL (≤40 mg/dL) | 4,512 | 50% | 3,905 | 53% | 1,133 | 42% | 298 | 51% |
| High LDL (≥130 mg/dL) | 919 | 10% | 2,314 | 31% | 391 | 15% | 144 | 24% |
| High total cholesterol (≥ 240 mg/dL) | 219 | 2% | 951 | 13% | 162 | 6% | 80 | 14% |
| Diagnosed CVD | 439 | 5% | 2,254 | 31% | 1,517 | 57% | 326 | 55% |
| Framingham risk score | ||||||||
| low risk | 8,054 | 89% | 3,670 | 50% | 1,193 | 45% | 196 | 33% |
| intermediate | 939 | 10% | 3,481 | 47% | 1,431 | 54% | 364 | 62% |
| high risk | 30 | 0% | 235 | 3% | 49 | 2% | 30 | 5% |
| Treatment status | ||||||||
| Naïve | 3,754 | 42% | 2,599 | 35% | 257 | 14% | 79 | 14% |
| Initiated after entry | 3072 | 82% | 2396 | 92% | 194 | 75% | 70 | 89% |
| INSTI-based | 1,227 | 14% | 830 | 11% | 970 | 36% | 241 | 41% |
| PI-based | 1,795 | 20% | 2,015 | 27% | 633 | 24% | 111 | 19% |
| NNRTI-based | 1,693 | 19% | 1,545 | 21% | 623 | 23% | 126 | 21% |
| Other ART/unknown | 554 | 6% | 397 | 5% | 190 | 7% | 33 | 6% |
| CD4 count (cell/mm3) | ||||||||
| ≥350 (observed) | 5,128 | 57% | 4,105 | 56% | 1,773 | 66% | 409 | 69% |
| ≥350 (observed) | NA | - | NA | - | 145 | 5% | 16 | 3% |
| 200–349 (observed) | 1,834 | 20% | 1,408 | 19% | 355 | 13% | 86 | 15% |
| 200–349 (imputed) | NA | - | NA | - | 26 | 1% | 1 | 0% |
| <200 (observed) | 1,955 | 22% | 1,784 | 24% | 347 | 13% | 76 | 13% |
| <200 (imputed) | NA | - | NA | - | 27 | 1% | 2 | 0% |
| Unknown | 106 | 1% | 89 | 1% | NA | - | NA | - |
| Undetectable HIV RNA (observed) | 2,878 | 32% | 3,036 | 41% | 1,873 | 70% | 432 | 73% |
| Undetectable HIV RNA (imputed) | NA | - | NA | - | 124 | 5% | 12 | 2% |
| Missing HIV RNA | 235 | 3% | 218 | 3% | NA | - | NA | - |
| History of clinical AIDS diagnosis | 1,026 | 11% | 1,117 | 15% | 490 490 |
18% | 89 89 |
15% |
Abbreviations: MSM, men who have sex with men; IDU, HC injection drug use; HDL, high density lipoprotein; LDL, low density lipoprotein; CVD, cardiovascular disease; INTSI, integrase inhibitor; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Assessed at analysis entry
Assessed at statin eligibility
From 2001–2013, the proportion of individuals eligible for statins increased from 22% in 2001 to 26% in 2003 and remained at this proportion thereafter (Figure 2). Statin initiation among eligible PWH improved over time: in 2001 only 13% initiated statins, and by 2013, 45% among those eligible initiated statins. In 2014–2017, there was a notable increase in PWH eligible for statins in the newly developed ACC/AHA 2013 guidelines, ranging from 51%–54%. Statin initiation among eligible PWH was stable during this time, ranging from 25% to 32%.
Figure 2.
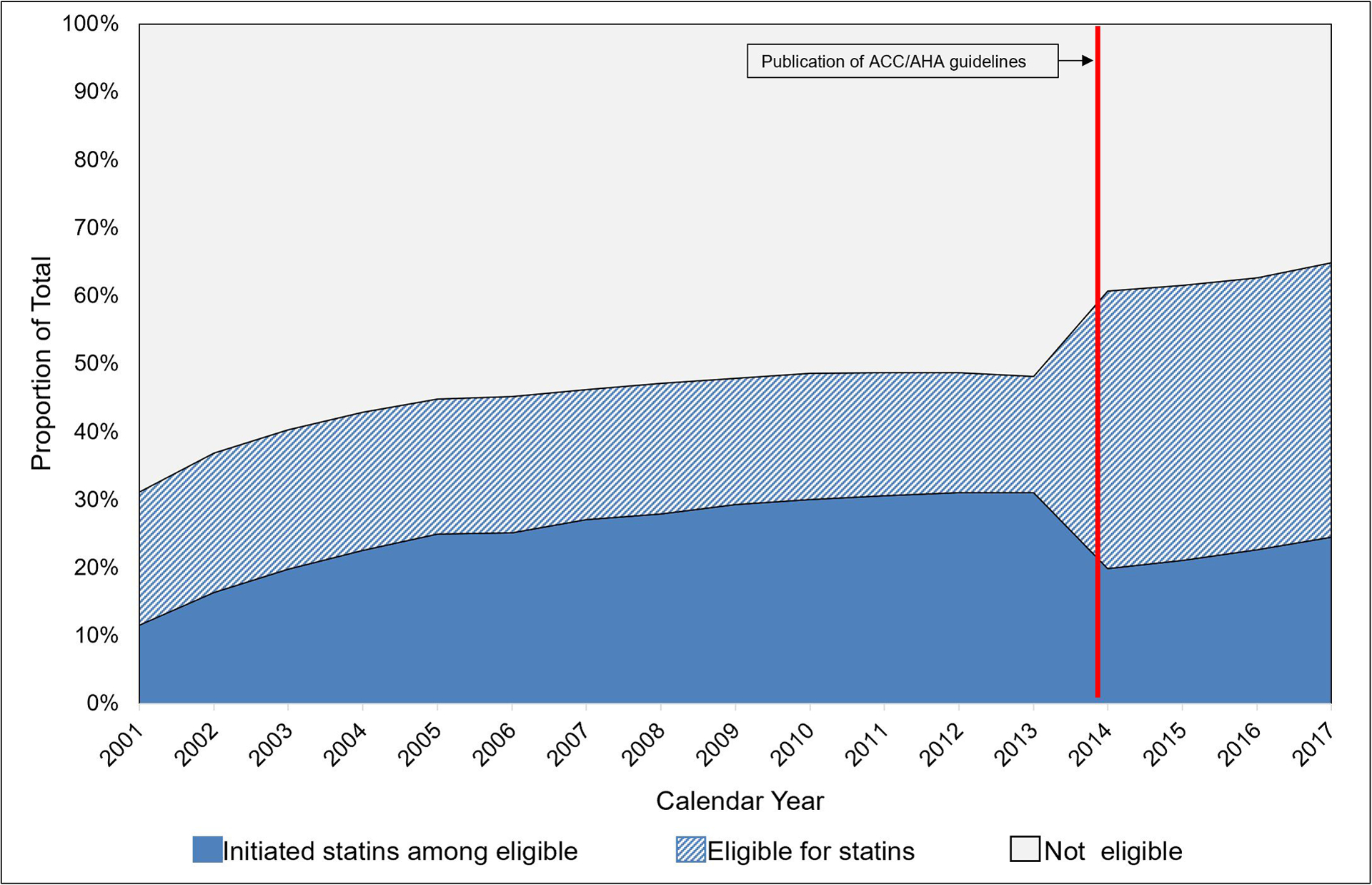
The statins treatment gap among NA-ACCORD participants with no history of statin use, 2001–2017 (N=16,409)
Note: ACC/AHA Guidelines were first published online November 13, 2013. Analyses implemented this new definition of statin eligibility starting in 2014.
The time between statin eligibility and initiation declined over time (Figure 3). In 2001, the median wait time among PWH who were statin eligible and observed to initiate statins was 30 months (IQR: 8, 70 months). By 2013, median wait time was 15 months (IQR: 2, 33 months) among those who were eligible that year. This fell to 2 months in median wait time by 2016 (IQR: 1, 6 months).
Figure 3.
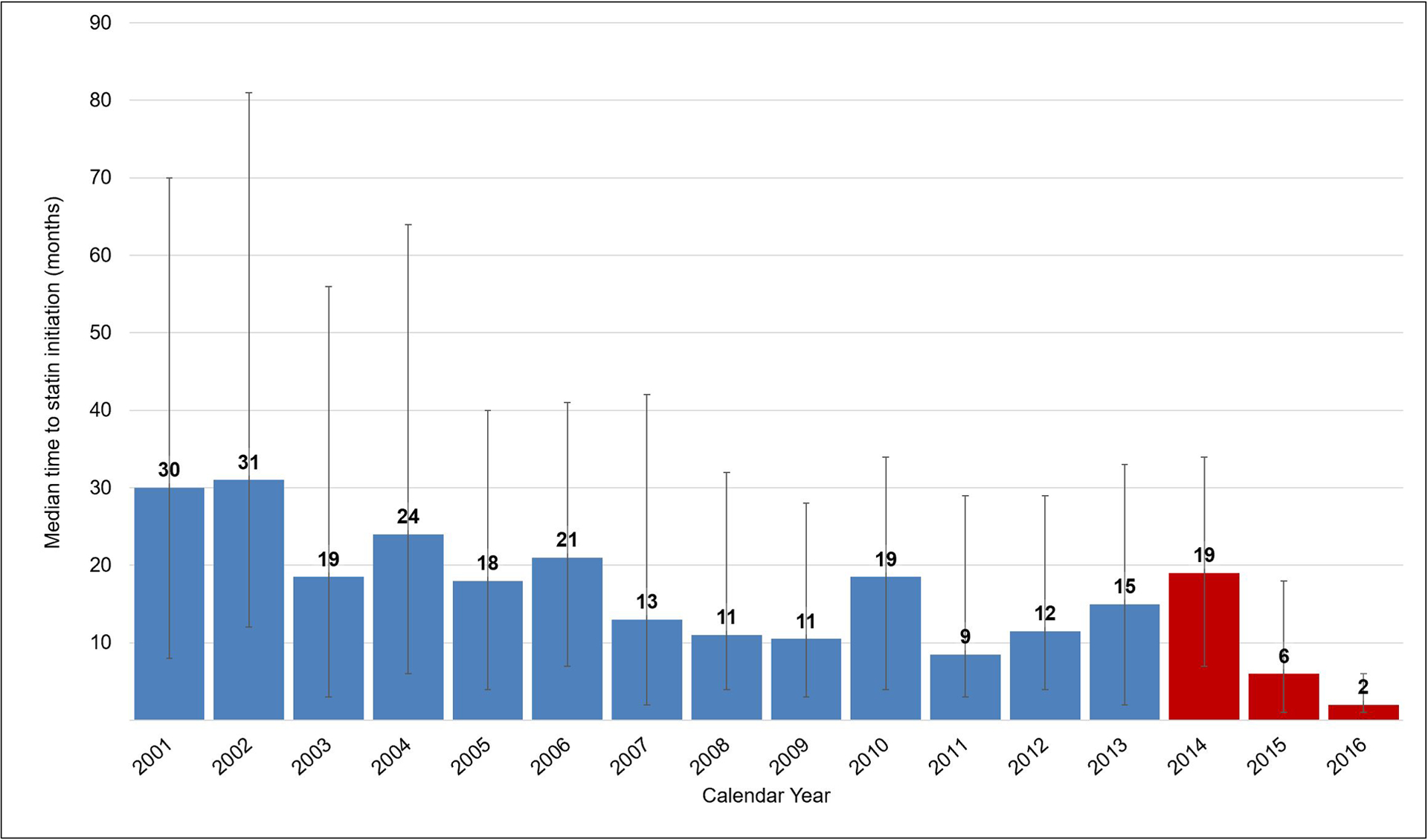
Median (IQR) months from statin eligibility to statin initiation among those becoming statin eligible 2001–2016 (N=2,188 initiators)
Statin eligibility defined as: date participant first met at least one of the criteria for statin eligibility for the time-appropriate guideline Time to statin initiation defined as: time between date of statin eligibility to the date of the first statin prescription of at least one month in duration Red shading indicates when ACC/AHA guidelines were implemented in the analysis (guidelines were first published online November 2013).
Predictors of Statin Initiation in 2014–2017
Among NA-ACCORD participants who were eligible for statins in 2014–2017, (n=3,263) 590 initiated statins (18%) (Table 1). More participants who initiated statins also had diabetes (26% vs. 11%) and high LDL cholesterol (24% vs. 15%). Initiators had higher FRS scores (high or intermediate: 67% vs. 56%). Other sociodemographic factors and HIV-related clinical factors were similarly distributed among statin-eligible participants by statin initiation status.
In unadjusted analyses, age (per 10-year increase) was associated with statin initiation (PR: 1.42, 95% CI 1.31, 1.55) (Table 2). Black and Hispanic PWH were 22% and 23% less likely to initiate statins compared with whites (PR: 0.78, 95% CI 0.66, 0.94; and PR 0.77, 95% CI 0.58,1.02, respectively). PWH who reported ever smoking were 25% less likely to initiate statins (PR: 0.75, 95% CI 0.62, 0.90) than people with no smoking history, while those with a history of a clinical AIDS diagnosis were 22% less likely to initiate statins (PR: 0.78, 95% CI 0.62, 0.98) than those without AIDS history. Per one-year increase calendar year of statin eligibility, there was a 46% increase in the likelihood of initiating statins (PR: 1.46, 95% CI 1.04, 2.06). PWH on a PI-based regimen we 44% less likely to initiate statins versus PWH on an INSTI-based regimen (PR: 0.56, 95% CI 0.44, 0.71), and those NNRTI-based regimens were 33% less likely to initiate statins (PR: 0.67, 95% CI 0.53, 0.84). Those with other/unknown ART regimen status were 49% more likely than those on an INSTI-based regiment to initiate statins (PR 1.49, 95% CI 1.13, 1.98). High total cholesterol was also associated with statin initiation (PR: 3.94, 95% CI 3.15, 4.95).
Table 2.
Predictors of statin initiation among those becoming eligible during 2014–2017 (N=3,263)
| Characteristic at study entry | PR1 | 95% CI | aPR1 | 95% CI |
|---|---|---|---|---|
| Age (every 10 years) | 1.42 | 1.31, 1.55 | 1.48 | 1.29, 1.70 |
| Sex | ||||
| Male | 1 | 1 | ||
| Female | 1.01 | 0.81, 1.27 | 1.11 | 0.78, 1.59 |
| Race and ethnicity | ||||
| White | 1 | 1 | ||
| Black | 0.78 | 0.66, 0.94 | 0.87 | 0.64, 1.17 |
| Hispanic | 0.77 | 0.58, 1.02 | 0.78 | 0.58, 1.04 |
| Other/unknown | 0.81 | 0.53, 1.25 | 0.95 | 0.55, 1.64 |
| HIV transmission risk | ||||
| MSM | 1 | 1 | ||
| IDU | 0.99 | 0.75, 1.32 | 0.97 | 0.69, 1.35 |
| Heterosexual | 1.08 | 0.89, 1.31 | 1.11 | 0.86, 1.42 |
| Other | 1.46 | 1.04, 2.06 | 1.21 | 0.71, 2.07 |
| Cigarette smoking | ||||
| Never | 1 | 1 | ||
| Ever | 0.75 | 0.62, 0.90 | 0.94 | 0.56, 1.58 |
| Year of statin eligibility (per 1-year increase) | 1.46 | 1.35, 1.58 | 1.41 | 1.25, 1.58 |
| Hepatitis C infection | ||||
| No | 1 | 1 | ||
| Yes | 0.96 | 0.79, 1.17 | 0.94 | 0.74, 1.20 |
| CD4 count (cells/mm3) | ||||
| ≥350 | 1 | 1 | ||
| 200–349 | 1.03 | 0.81, 1.30 | 1.05 | 0.81, 1.36 |
| <200 | 0.97 | 0.76, 1.25 | 0.90 | 0.64, 1.26 |
| HIV viral load (copies/mL) | ||||
| ≤200 | 1 | 1 | ||
| >200 | 1.08 | 0.89, 1.31 | 0.98 | 0.71, 1.33 |
| History of clinical AIDS diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 0.78 | 0.62, 0.98 | 0.84 | 0.63, 1.13 |
| HIV treatment regimen | ||||
| INSTI-based | 1 | 1 | ||
| Naïve | 1.20 | 0.92, 1.57 | 1.23 | 0.91, 1.66 |
| PI-based | 0.56 | 0.44, 0.71 | 0.68 | 0.42, 1.11 |
| NNRTI-based | 0.67 | 0.53, 0.84 | 0.81 | 0.51, 1.31 |
| Other/unknown | 1.49 | 1.13, 1.98 | 1.25 | 0.78, 2.02 |
| Diabetes | ||||
| No | 1 | 1 | ||
| Yes | 2.63 | 2.19, 3.16 | 3.16 | 2.06, 4.84 |
| Hypertension | ||||
| No | 1 | 1 | ||
| Yes | 1.27 | 1.07, 1.50 | 1.26 | 0.97, 1.62 |
| High total cholesterol | ||||
| No | 1 | 1 | ||
| Yes | 3.95 | 3.15, 4.95 | 2.72 | 1.19, 6.21 |
| CVD | ||||
| No | 1 | 1 | ||
| Yes | 1.07 | 0.90, 1.27 | 1.59 | 0.68, 3.74 |
| Low HDL | ||||
| No | 1 | 1 | ||
| Yes | 1.26 | 1.06, 1.48 | 1.33 | 1.06, 1.65 |
| High LDL | ||||
| No | 1 | 1 | ||
| Yes | 2.86 | 2.39, 3.43 | 2.11 | 1.08, 4.12 |
| Framingham risk score | ||||
| low | 1 | 1 | ||
| Intermediate | 1.57 | 1.31, 1.88 | 1.13 | 0.54, 2.34 |
| High | 3.27 | 2.28, 4.70 | 1.07 | 0.10, 11.28 |
| Systolic blood pressure | 0.97 | 0.80, 1.18 | 0.91 | 0.71, 1.16 |
Abbreviations: MSM, men who have sex with men; IDU, injection drug use; HDL, high density lipoprotein; LDL, low density lipoprotein; CVD, cardiovascular disease; INTSI, integrase inhibitor; PI, protease inhibitor; NNRTI, non-nucleoside reverse transcriptase inhibitor.
Bold signals p<0.05.
Approximated prevalence ratios using Poisson regression with robust variance
In adjusted analyses, 10-year age increase was associated with a 48% increased likelihood of initiating statins (aPR 1.48, 95% CI 1.29, 1.70). The association with increasing calendar year with increasing likelihood of initiation remained (aPR: 1.41, 95% CI 1.25, 1.58). Associations observed between race/ethnicity, history of smoking, history of an AIDS diagnosis, and treatment regimen became non-statistically significant, though qualitatively similar to unadjusted estimates. Adjustment factors that were criteria for statin eligibility were associated with increased likelihood of statin initiation, especially having high total cholesterol, diabetes, high LDL, and low HDL at statin eligibility. Findings from regression analyses were similar when restricted to individuals who had CD4 count and HIV viral load observed (results not shown).
DISCUSSION
Among PWH in care in the US from 2001–2017, the statin treatment gap—the gap between those eligible for statins and those initiating statins—persists but narrowed over time. While there have been improvements (increases) in statin initiation among PWH since 2001, less than half of those eligible by standard criteria had a statin prescription. This gap increased after the implementation of the ACC/AHA 2013 guidelines, which led to a higher proportion of PWH becoming eligible for statin therapy. Though regression analyses indicated increasing likelihood of statin prescription with each increase in calendar year from 2014–2017, the absolute increase in statin initiation following 2014 (25–32%) suggests limited early adoption of the ACC/AHA 2013 guidelines among clinicians. That said, the time from eligibility to initiation of statins appears to be declining over time, indicating improvements in timely delivery of statins prescriptions to PWH.
The increase in statin eligibility comparing the ATP III guidelines to the ACC/AHA guidelines among PWH has been previously observed,30,31,37 and the proportion eligible was within similar ranges.29–34,37 The estimated statins treatment gap in prior works varies by study and guidelines utilized, though our estimates of statin initiation were within what has been previously found among PWH in US populations.29–40,44 This variability may be explained by population differences, or use/availability of criteria utilized to define statin eligibility.
The treatment gap observed in PWH could be attributable to clinician prioritization of HIV-related clinical management, drug-drug interactions with ART, or specific contraindications for statins. Another important explanation for the treatment gap is polypharmacy and multimorbidity in PWH. Physicians may be reluctant to additionally prescribe statins due to high burden of comorbidities, reduced life expectancy for those with substantial physiologic frailty, and concerns about comorbidity medication interactions. Though medications for diabetes and hypertension were captured and included in our definitions for these conditions, which were adjustment factors, polypharmacy and medication complexity were not directly accounted for.
Our finding that advancing age was associated with statin initiation was expected given CVD risk increases with age, and older age is a criterion for statin eligibility. The crude association observed with smoking and statin initiation did not remain in adjusted analyses, and therefore is likely: a) confounded by other factors we assessed, or b) may be a proxy for lack of retention to HIV care45 leading to lower likelihood of attending visits to receive a statin prescription. The observation that being on either a PI-based or NNRTI-based regimen were negatively associated with statin initiation in unadjusted analyses may be attributed to drug-drug interactions, as PIs especially have established pharmacokinetic interactions with statins.46–48 Upon adjustment for calendar year of statin eligibility, these associations were no longer significant, suggesting that being on an INSTI-based regimen is a surrogate for calendar trends. These hypotheses would require further investigation.
The observation that Hispanic and Black individuals were less likely than white individuals to initiate statins in univariate regression analyses supports previous work.35 One other study further demonstrated that even when Black PWH are being prescribed CVD preventive medications to the same extent as white individuals, CVD risk factor control is poorer.49 Overall, the statin treatment gap disproportionally affects minority PWH in the US and addressing it may require provider- and clinic-level approaches.
We defined statin initiation using prescription data which rely on the assumption that medications are dispensed and taken. Prescription data are indicative of clinician-based as opposed to patient-based predictors of initiation. It is also possible that patients in the NA-ACCORD have a provider outside of their HIV care clinician that use a different electronic medical record system, which would lead to under-ascertainment of prescriptions. Given the proportion initiating statins is in line with other studies, we expect this under-ascertainment to be limited, and certainly would not eliminate the statins treatment gap we observed.
The short observation following implementation of the ACC/AHA guidelines limits our ability to describe the long-term implications of guidelines on statin eligibility and initiation, and to detect the effect of time-varying and/or long-lasting exposures on statin initiation. Our calculation of median wait time should be considered cautiously given truncation of data in later years, which could lead to an underestimation of time to initiation. Though individuals could have initiated statins after observation ended, among those we did observe, prescribing was being done more quickly. Statin-eligible individuals could have initiated statins after 2017, but we were unable to observe this, which may bias our estimate of time to initiation. Given the limited amount of follow-up (2014–2017) for our regression analysis, our estimates should be considered cross-sectional. The temporality of the associations cannot be confirmed and should be interpreted cautiously. Due to lack of information regarding family history of CVD, we may have underestimated statin eligibility using ATP III guidelines. One study also suggests that the Pooled Cohort Equations calculator utilized may underestimate ASCVD risk in PWH due to high prevalence of subclinical high-risk morphology coronary atherosclerotic plaque.50
Future work should assess predictors of statin initiation over longer duration of observation following the 2013 ACC/AHA guideline implementation and address polypharmacy among PWH. Additionally, measurement of longitudinal prescription data as a marker of continued use, or integration of claims or other dispensing/adherence data to correlate patient uptake of the prescription is encouraged. This would not only elucidate patient-level factors related to statin initiation, but also would lead to better estimation of the statin treatment gap in the US. As the results of the Randomized Trial to Prevent Vascular Events in HIV (REPREIVE) study51 –a large international trial assessing the effect of statins on cardiovascular health outcomes among PWH– are waited, evaluating facilitators and barriers to statin initiation remains an important area of research. Results from this trial will also be critical for understanding the safety and efficacy of statins among PWH, which must be addressed when considering statin initiation.52
Among PWH eligible for treatment in the US, statins remain underutilized, resulting from discordances in appropriate statin prescription that may constitute a substantial gap in clinical care. Whether this gap represents a true deficiency in care, is reflective of complex medical needs of PWH who are aging, or is a combination of both, requires further investigation. PWH, in addition to managing their HIV infection, face potentially accelerated aging, and have a high prevalence of age-related comorbidities, multimorbidity, and polypharmacy. Introduction of statins may not be a priority due to increased pill burden, or interaction with medications. Further, the ACC/AHA guidelines were developed for the general population, and there are no standardized guidelines for statins in the context of HIV. The AHA provided a framework for assessing cardiovascular disease risk for PWH, recommending statin initiation for “high-risk” patients.53 This framework was predicated on patients being treated and virally suppressed, limiting its applicability, as viral suppression and treatment status can be a fluid state that changes over time. Further, clinicians may be hesitant to prescribe statins in PWH: although there is long-term evidence of safety among healthy PWH, in practice these patients may be medically complex. Overall, our findings support clarifying the reasons why statins may not be prescribed to PWH, and if necessary, to increase statin utilization among PWH for CVD prevention in this high-risk population.
ACKNOWLEDGEMENTS
NA-ACCORD Collaborating Cohorts and Representatives:
AIDS Clinical Trials Group Longitudinal Linked Randomized Trials: Constance A. Benson and Ronald J. Bosch
AIDS Link to the IntraVenous Experience: Gregory D. Kirk
Emory-Grady HIV Clinical Cohort: Vincent Marconi and Jonathan Colasanti
Fenway Health HIV Cohort: Kenneth H. Mayer and Chris Grasso
HAART Observational Medical Evaluation and Research: Robert S. Hogg, Viviane Lima, P. Richard Harrigan, Julio SG Montaner, Benita Yip, Julia Zhu, and Kate Salters
HIV Outpatient Study: Kate Buchacz and Jun Li
HIV Research Network: Kelly A. Gebo and Richard D. Moore
Johns Hopkins HIV Clinical Cohort: Richard D. Moore
John T. Carey Special Immunology Unit Patient Care and Research Database, Case Western Reserve University: Jeffrey Jacobson
Kaiser Permanente Mid-Atlantic States: Michael A. Horberg
Kaiser Permanente Northern California: Michael J. Silverberg
Longitudinal Study of Ocular Complications of AIDS: Jennifer E. Thorne
MACS/WIHS Combined Cohort Study: Todd Brown, Phyllis Tien, and Gypsyamber D’Souza
Maple Leaf Medical Clinic: Graham Smith, Mona Loutfy, and Meenakshi Gupta
The McGill University Health Centre, Chronic Viral Illness Service Cohort: Marina B. Klein
Multicenter Hemophilia Cohort Study-II: Charles Rabkin
Ontario HIV Treatment Network Cohort Study: Abigail Kroch, Ann Burchell, Adrian Betts, and Joanne Lindsay
Parkland/UT Southwestern Cohort: Ank Nijhawan
Retrovirus Research Center, Universidad Central del Caribe, Bayamon Puerto Rico: Angel M. Mayor
Southern Alberta Clinic Cohort: M. John Gill
Study of the Consequences of the Protease Inhibitor Era: Jeffrey N. Martin and Steven G. Deeks
Study to Understand the Natural History of HIV/AIDS in the Era of Effective Therapy: Jun Li and John T. Brooks
University of Alabama at Birmingham 1917 Clinic Cohort: Michael S. Saag, Michael J. Mugavero, and Greer Burkholder
University of California at San Diego: Laura Bamford and Maile Karris
University of North Carolina at Chapel Hill HIV Clinic Cohort: Joseph J. Eron and Sonia Napravnik
University of Washington HIV Cohort: Mari M. Kitahata and Heidi M. Crane
Vanderbilt Comprehensive Care Clinic HIV Cohort: Timothy R. Sterling, David Haas, Peter Rebeiro, and Megan Turner
Veterans Aging Cohort Study: Kathleen McGinnis and Amy Justice
NA-ACCORD Study Administration:
Executive Committee: Richard D. Moore, Keri N. Althoff, Stephen J. Gange, Mari M. Kitahata, Jennifer S. Lee, Michael S. Saag, Michael A. Horberg, Marina B. Klein, Rosemary G. McKaig, and Aimee M. Freeman
Administrative Core: Richard D. Moore, Keri N. Althoff, and Aimee M. Freeman
Data Management Core: Mari M. Kitahata, Stephen E. Van Rompaey, Heidi M. Crane, Liz Morton, Justin McReynolds, and William B. Lober
Epidemiology and Biostatistics Core: Stephen J. Gange, Jennifer S. Lee, Brenna Hogan, Elizabeth Humes, Raynell Lang, Sally Coburn, Lucas Gerace, and Cameron Stewart
Sources of Funding:
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. This work was supported by National Institutes of Health grants F31CA247610, U01AI069918, F31AI124794, F31DA037788, G12MD007583, K01AI093197, K01AI131895, K23EY013707, K24AI065298, K24AI118591, K24DA000432, KL2TR000421, N01CP01004, N02CP055504, N02CP91027, P30AI027757, P30AI027763, P30AI027767, P30AI036219, P30AI050409, P30AI050410, P30AI094189, P30AI110527, P30MH62246, R01AA016893, R01DA011602, R01DA012568, R01 AG053100, R24AI067039, R34DA045592, U01AA013566, U01AA020790, U01AI038855, U01AI038858, U01AI068634, U01AI068636, U01AI069432, U01AI069434, U01DA03629, U01DA036935, U10EY008057, U10EY008052, U10EY008067, U01HL146192, U01HL146193, U01HL146194, U01HL146201, U01HL146202, U01HL146203, U01HL146204, U01HL146205, U01HL146208, U01HL146240, U01HL146241, U01HL146242, U01HL146245, U01HL146333, U24AA020794,U54GM133807, UL1RR024131, UL1TR000004, UL1TR000083, UL1TR002378, Z01CP010214 and Z01CP010176; contracts CDC-200-2006-18797 and CDC-200-2015-63931 from the Centers for Disease Control and Prevention, USA; contract 90047713 from the Agency for Healthcare Research and Quality, USA; contract 90051652 from the Health Resources and Services Administration, USA; the Grady Health System; grants CBR-86906, CBR-94036, HCP-97105 and TGF-96118 from the Canadian Institutes of Health Research, Canada; Ontario Ministry of Health and Long Term Care, and the Government of Alberta, Canada. Additional support was provided by the National Institute Of Allergy And Infectious Diseases (NIAID), National Cancer Institute (NCI), National Heart, Lung, and Blood Institute (NHLBI), Eunice Kennedy Shriver National Institute Of Child Health & Human Development (NICHD), National Human Genome Research Institute (NHGRI), National Institute for Mental Health (NIMH) and National Institute on Drug Abuse (NIDA), National Institute On Aging (NIA), National Institute Of Dental & Craniofacial Research (NIDCR), National Institute Of Neurological Disorders And Stroke (NINDS), National Institute Of Nursing Research (NINR), National Institute on Alcohol Abuse and Alcoholism (NIAAA), National Institute on Deafness and Other Communication Disorders (NIDCD), and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK).
Conflicts of Interest:
KN Althoff is a consultant to the All of Us Study (NIH) and serves on the scientific advisory board for TrioHealth.
FJ Palella is a consultant and/or on the Speakers’ Bureau for Gilead Sciences, Janssen, ViiV, and Merck.
MJS had a research grant to his institution from Gilead Sciences, Inc
JCM has a pending research grant paid to his institution from Gilead Sciences, Inc.
For the remaining authors, no conflicts were declared.
REFERENCES
- 1.Nansseu JR, Bigna JJ, Kaze AD, Noubiap JJ. Incidence and Risk Factors for Prediabetes and Diabetes Mellitus Among HIV-infected Adults on Antiretroviral Therapy. Epidemiology. 2018;29(3):431–441. doi: 10.1097/EDE.0000000000000815 [DOI] [PubMed] [Google Scholar]
- 2.Hernandez-Romieu AC, Garg S, Rosenberg ES, Thompson-Paul AM, Skarbinski J. Is diabetes prevalence higher among HIV-infected individuals compared with the general population? Evidence from MMP and NHANES 2009–2010. BMJ Open Diabetes Res Care. 2009;5:304. doi: 10.1136/bmjdrc-2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nix L, Tien PC. Metabolic Syndrome, Diabetes, and Cardiovascular Risk in HIV. doi: 10.1007/s11904-014-0219-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Price JC, Thio CL. Liver disease in the HIV-infected individual. Clin Gastroenterol Hepatol. 2010;8(12):1002–1012. doi: 10.1016/j.cgh.2010.08.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Joshi D, O’Grady J, Dieterich D, Gazzard B, Agarwal K. Increasing burden of liver disease in patients with HIV infection. Lancet. 2011;377(9772):1198–1209. doi: 10.1016/S0140-6736(10)62001-6 [DOI] [PubMed] [Google Scholar]
- 6.Shiels MS, Pfeiffer RM, Gail MH, et al. Cancer burden in the HIV-infected population in the United States. J Natl Cancer Inst. 2011. doi: 10.1093/jnci/djr076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Deeken JF, Tjen-A-Looi A, Rudek MA, et al. The Rising Challenge of Non-AIDS-Defining Cancers in HIV-Infected Patients. Clin Infect Dis. 2012;55(9):1228–1235. doi: 10.1093/cid/cis613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Triant VA. Cardiovascular disease and HIV infection. Curr HIV/AIDS Rep. 2013;10(3):199–206. doi: 10.1007/s11904-013-0168-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drozd DR, Kitahata MM, Althoff KN, et al. Increased Risk of Myocardial Infarction in HIV-Infected Individuals in North America Compared With the General Population. JAIDS J Acquir Immune Defic Syndr. 2017;75(5):568–576. doi: 10.1097/QAI.0000000000001450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Islam F, Wu J, Jansson J, Wilson D. Relative risk of cardiovascular disease among people living with HIV: a systematic review and meta-analysis. HIV Med. 2012;13(8):n/a–n/a. doi: 10.1111/j.1468-1293.2012.00996.x [DOI] [PubMed] [Google Scholar]
- 11.Boyd MA, Mocroft A, Ryom L, et al. Cardiovascular disease (CVD) and chronic kidney disease (CKD) event rates in HIV-positive persons at high predicted CVD and CKD risk: A prospective analysis of the D:A:D observational study. PLoS Med. 2017;14(11):e1002424. doi: 10.1371/journal.pmed.1002424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Freiberg MS, Chang C-CH, Kuller LH, et al. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173(8):614–622. doi: 10.1001/jamainternmed.2013.3728 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shah ASV, Stelzle D, Ken Lee K, et al. Global Burden of Atherosclerotic Cardiovascular Disease in People Living with the Human Immunodeficiency Virus: A Systematic Review and Meta-Analysis. Circulation. 2018;138(11):1100. doi: 10.1161/CIRCULATIONAHA.117.033369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.So-Armah K, Benjamin LA, Bloomfield GS, et al. HIV and cardiovascular disease. Lancet HIV. 2020;7(4):e279–e293. doi: 10.1016/S2352-3018(20)30036-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bourgi K, Jenkins CA, Rebeiro PF, et al. Weight gain among treatment-naïve persons with HIV starting integrase inhibitors compared to non-nucleoside reverse transcriptase inhibitors or protease inhibitors in a large observational cohort in the United States and Canada. J Int AIDS Soc. 2020;23(4). doi: 10.1002/JIA2.25484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.PF R, CA J, A B, et al. Risk of Incident Diabetes Mellitus, Weight Gain, and their Relationships with Integrase Inhibitor-based Initial Antiretroviral Therapy Among Persons with HIV in the US and Canada. Clin Infect Dis. September 2020. doi: 10.1093/CID/CIAA1403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bowman E, Funderburg NT. Lipidome Abnormalities and Cardiovascular Disease Risk in HIV Infection. Curr HIV/AIDS Rep. 2019. doi: 10.1007/s11904-019-00442-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsue PY, Waters DD. HIV infection and coronary heart disease: mechanisms and management. Nat Rev Cardiol. June 2019. doi: 10.1038/s41569-019-0219-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin treatment reduces markers of monocyte activation in HIV-infected subjects on antiretroviral therapy. Clin Infect Dis. 2014;58(4):588–595. doi: 10.1093/cid/cit748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Funderburg NT, Jiang Y, Debanne SM, et al. Rosuvastatin reduces vascular inflammation and T-cell and monocyte activation in HIV-infected subjects on antiretroviral therapy. J Acquir Immune Defic Syndr. 2015;68(4):396–404. doi: 10.1097/QAI.0000000000000478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eckard AR, McComsey GA. The role of statins in the setting of HIV infection. Curr HIV/AIDS Rep. 2015;12(3):305–312. doi: 10.1007/s11904-015-0273-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salami JA, Warraich H, Valero-Elizondo J, et al. National Trends in Statin Use and Expenditures in the US Adult Population From 2002 to 2013. JAMA Cardiol. 2017;2(1):56. doi: 10.1001/jamacardio.2016.4700 [DOI] [PubMed] [Google Scholar]
- 23.National Cholesterol Education Program (NCEP) Expert Panel on Detection E and T of HBC in A (Adult TPI (2002). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106(25):3143–3421. [PubMed] [Google Scholar]
- 24.Grundy SM, Cleeman JI, Bairey Merz CN, et al. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110(2):227–239. doi: 10.1161/01.CIR.0000133317.49796.0E [DOI] [PubMed] [Google Scholar]
- 25.Blum CB, Eckel RH, Goldberg AC, et al. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults. Am Hear Assoc Task Force Pract Guidel Circ. 2014;129(2):1–45. doi: 10.1161/01.cir.0000437738.63853.7a [DOI] [Google Scholar]
- 26.Lin I, Sung J, Sanchez RJ, et al. Patterns of Statin Use in a Real-World Population of Patients at High Cardiovascular Risk. www.jmcp.org JMCP J Manag Care Spec Pharm. 2016;22(685). http://www.jmcp.org/doi/pdf/10.18553/jmcp.2016.22.6.685. Accessed November 5, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang Q, Grabner M, Sanchez RJ, et al. Clinical Characteristics and Unmet Need Among Patients with Atherosclerotic Cardiovascular Disease Stratified by Statin Use. Am Heal drug benefits. 2016;9(8):434–444. http://www.ncbi.nlm.nih.gov/pubmed/28465771. Accessed June 18, 2019. [PMC free article] [PubMed] [Google Scholar]
- 28.Okeke NL, Chin T, Clement M, Chow S-C, Hicks CB. Coronary artery disease risk reduction in HIV-infected persons: a comparative analysis. AIDS Care. 2016;28(4):475–482. doi: 10.1080/09540121.2015.1099602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Kindi SG, Zidar DA, McComsey GA, Longenecker CT. Gender Differences in Statin Prescription Rate Among Patients Living With HIV and Hepatitis C Virus. Clin Infect Dis. 2016;63(7):993–994. doi: 10.1093/cid/ciw448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clement ME, Park LP, Navar AM, et al. Statin Utilization and Recommendations Among HIV- and HCV-infected Veterans: A Cohort Study. Clin Infect Dis. 2016;63(3):407–413. doi: 10.1093/cid/ciw289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Levy ME, Greenberg AE, Magnus M, Younes N, Castel A. Evaluation of Statin Eligibility, Prescribing Practices, and Therapeutic Responses Using ATP III, ACC/AHA, and NLA Dyslipidemia Treatment Guidelines in a Large Urban Cohort of HIV-Infected Outpatients. AIDS Patient Care STDS. 2018;32(2):58–69. doi: 10.1089/apc.2017.0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly SG, Krueger KM, Grant JL, et al. Statin Prescribing Practices in the Comprehensive Care for HIV-Infected Patients. JAIDS J Acquir Immune Defic Syndr. 2017;76(1):e26–e29. doi: 10.1097/QAI.0000000000001454 [DOI] [PubMed] [Google Scholar]
- 33.Riestenberg RA, Furman A, Cowen A, et al. Differences in statin utilization and lipid lowering by race, ethnicity, and HIV status in a real-world cohort of persons with human immunodeficiency virus and uninfected persons. Am Heart J. 2019;209:79–87. doi: 10.1016/J.AHJ.2018.11.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cortés YI, Reame N, Zeana C, et al. Cardiovascular Risk in HIV-Infected and Uninfected Postmenopausal Minority Women: Use of the Framingham Risk Score. J Women’s Heal. 2017;26(3):241. doi: 10.1089/JWH.2015.5736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blackman A, Pandit N, Pincus K. Comparing rates of statin therapy in eligible patients living with HIV versus uninfected patients. HIV Med. 2020;21(3):135–141. doi: 10.1111/HIV.12794 [DOI] [PubMed] [Google Scholar]
- 36.Lichtenstein KA, Armon C, Buchacz K, et al. Provider Compliance With Guidelines for Management of Cardiovascular Risk in HIV-Infected Patients. doi: 10.5888/pcd10.120083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mosepele M, Regan S, Massaro J, et al. Impact of the American College of Cardiology/American Heart Association Cholesterol Guidelines on Statin Eligibility Among Human Immunodeficiency Virus-Infected Individuals. Open Forum Infect Dis. 2018;5(12). doi: 10.1093/OFID/OFY326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brunet L, Mallon P, Fusco JS, et al. Switch from Tenofovir Disoproxil Fumarate to Tenofovir Alafenamide in People Living with HIV: Lipid Changes and Statin Underutilization. Clin Drug Investig. 2021;41(11):955–965. doi: 10.1007/s40261-021-01081-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagchi S, Rawan Faramand PP, Mian B Hossain SB, et al. Underutilization of Statins for Prevention of Cardiovascular Disease among Primarily African-American HIV-Infected Patients. J AIDS Clin Res. 2015;06(09):1–6. doi: 10.4172/2155-6113.1000499 [DOI] [Google Scholar]
- 40.Todd JV, Cole SR, Wohl DA, et al. Underutilization of Statins When Indicated in HIV-Seropositive and Seronegative Women. doi: 10.1089/apc.2017.0145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. https://files.aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Accessed May 15, 2020.
- 42.Gange SJ, Kitahata MM, Saag MS, et al. Cohort profile: the North American AIDS Cohort Collaboration on Research and Design (NA-ACCORD). Int J Epidemiol. 2007;36(2):294–301. doi: 10.1093/ije/dyl286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.David C Goff J, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the Assessment of Cardiovascular Risk. Circulation. 2014;129(25 SUPPL. 1):49–73. doi: 10.1161/01.CIR.0000437741.48606.98 [DOI] [PubMed] [Google Scholar]
- 44.Ladapo JA, Richards AK, DeWitt CM, et al. Disparities in the Quality of Cardiovascular Care Between HIV-Infected Versus HIV-Uninfected Adults in the United States: A Cross-Sectional Study. J Am Heart Assoc. 2017;6(11). doi: 10.1161/JAHA.117.007107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satre DD, Levine-Hall T, Sterling SA, et al. The relationship of smoking and unhealthy alcohol use to the HIV care continuum among people with HIV in an integrated health care system. Drug Alcohol Depend. 2021;219:108481. doi: 10.1016/j.drugalcdep.2020.108481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fichtenbaum CJ, Gerber JG, Rosenkranz SL, et al. Pharmacokinetic interactions between protease inhibitors and statins in HIV seronegative volunteers: ACTG study A5047. AIDS. 2002;16(4):569–577. doi: 10.1097/00002030-200203080-00008 [DOI] [PubMed] [Google Scholar]
- 47.Gebhardt A, Fichtenbaum CJ. Expert Opinion on Pharmacotherapy Current pharmacotherapy for the treatment of dyslipidemia associated with HIV infection Current pharmacotherapy for the treatment of dyslipidemia associated with HIV infection. 2019. doi: 10.1080/14656566.2019.1636033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gerber JG, Rosenkranz SL, Fichtenbaum CJ, et al. Effect of efavirenz on the pharmacokinetics of simvastatin, atorvastatin, and pravastatin: Results of AIDS Clinical Trials Group 5108 study. J Acquir Immune Defic Syndr. 2005;39(3):307–312. doi: 10.1097/01.qai.0000167156.44980.33 [DOI] [PubMed] [Google Scholar]
- 49.Galaviz KI, Varughese R, Agan BK, et al. The Intersection of HIV, Diabetes, and Race: Exploring Disparities in Diabetes Care among People Living with HIV. J Int Assoc Provid AIDS Care. 2020;19. doi: 10.1177/2325958220904241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zanni MV, Fitch KV, Feldpausch M, et al. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014;28(14):2061–2070. doi: 10.1097/QAD.0000000000000360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gilbert JM, Fitch KV, Grinspoon SK, MSN, Grinspoon SK, MD. HIV-Related Cardiovascular Disease, Statins, and the REPRIEVE Trial. Top Antivir Med. 2015;23(4):146–149. http://www.ncbi.nlm.nih.gov/pubmed/26713505. Accessed November 7, 2019. [PMC free article] [PubMed] [Google Scholar]
- 52.Justice A, Freiberg MS, Lo Re V. Should everyone ageing with HIV take a statin? 2015. doi: 10.1016/S2352-3018(14)00062-9 [DOI] [PubMed] [Google Scholar]
- 53.Feinstein MJ, Hsue PY, Benjamin LA, et al. Characteristics, Prevention, and Management of Cardiovascular Disease in People Living With HIV: A Scientific Statement From the American Heart Association. Circulation. 2019;140(2):e98. doi: 10.1161/CIR.0000000000000695 [DOI] [PMC free article] [PubMed] [Google Scholar]


