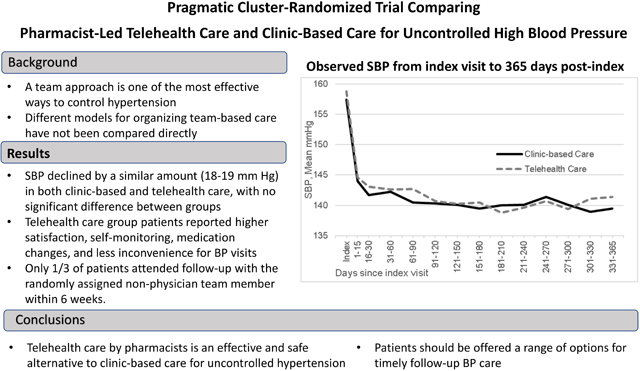
Abstract
Background:
A team approach is one of the most effective ways to lower blood pressure (BP) in uncontrolled hypertension, but different models for organizing team-based care have not been compared directly.
Methods:
A pragmatic, cluster-randomized trial compared two interventions in adult patients with moderately severe hypertension (BP ≥150/95 mmHg): 1) Clinic-Based Care using best practices and face-to face visits with physicians and medical assistants; and 2) Telehealth Care using best practices and adding home BP telemonitoring with home-based care coordinated by a clinical pharmacist or nurse practitioner. The primary outcome was change in systolic BP over 12 months. Secondary outcomes were change in patient-reported outcomes over 6 months.
Results:
Participants (N=3071 in 21 primary care clinics) were on average 60 years old, 47% male, and 19% Black. Protocol-specified follow-up within 6 weeks was 32% in Clinic-Based Care and 27% in Telehealth Care. BP decreased significantly during 12 months of follow-up in both groups, from 157/92 to 139/82 mmHg in Clinic-Based Care patients (adjusted mean difference −18/−10 mmHg) and 157/91 to 139/81 mmHg in Telehealth Care patients (adjusted mean difference −19/−10 mmHg), with no significant difference in systolic BP change between groups (−0.8 mmHg, 95% CI −2.84, 1.32). Telehealth Care patients were significantly more likely than Clinic-Based Care patients to report frequent home BP measurement, rate their BP care highly, and report that BP care visits were convenient.
Conclusions:
Telehealth care that includes extended team care is an effective and safe alternative to clinic-based care for improving patient-centered care for hypertension.
Keywords: Hypertension, pharmacist care, telehealth, telemonitoring, self-measured blood pressure, pragmatic trial, patient-reported outcomes
Graphical Abstract
Introduction
Elevated blood pressure (BP) is the largest modifiable risk factor contributing to all-cause and cardiovascular (CV) mortality in the U.S.1, 2 Decades of randomized trials have shown that treatment to lower levels of BP decreases the risk of future CV events,3 but better control of BP has been difficult to achieve at the population level. In the U.S., BP control to a goal of <140/90 mm Hg steadily improved from 32% in 1999 to 53% in 2010, held at just over 50% until 2014, but declined to 44% by 2018.4 In recognition of the negative effects of hypertension on population health, in 2020 the U.S. Surgeon General issued a Call to Action to Control Hypertension with three goals: to make hypertension control a national priority, to encourage community support, and to optimize patient care.5
Team-based care was among the top strategies to improve hypertension care recommended by the Surgeon General. Team-based care to improve BP control is an organizational intervention that uses new staff or changes the roles of existing staff who work with a primary care provider. In a recent review of 54 studies, the Community Preventive Services Task Force found strong evidence of effectiveness of team-based care for improving BP control and reducing systolic and diastolic blood pressure (SBP and DBP).6 Team-based care often incorporates patient self-monitoring of BP, a care improvement strategy also included in the Call to Action that has small effects on its own but may be synergistic with additional support interventions.7–10
In the previous Hyperlink 1 randomized trial, we combined home BP telemonitoring with team-based pharmacist-led telephone care in 450 consenting patients with uncontrolled hypertension at 16 primary care clinics.11 Patients who received this intervention achieved a 23/9 mm Hg BP reduction during 12 months, 10/5 mm Hg more than patients who received routine primary care, and experienced fewer cardiovascular events.11, 12 Research in other settings has shown similar BP improvement without the need for clinic visits.13–16 However, some group practices have achieved very high rates of BP control using quality-improvement methods without routine use of expanded care teams, home BP monitoring, or telehealth.17–19 Hyperlink 3 is a larger-scale, pragmatic trial in primary care clinics comparing the previously-tested pharmacist-led telehealth hypertension program with clinic-based care that is organized according to current best practices.
Methods
The data that support the findings of this study are available from the corresponding author upon reasonable request. Detailed methods for the study have been published.20 The study was designed as a cluster-randomized comparative effectiveness pragmatic trial in 21 HealthPartners primary care clinics. HealthPartners is a nonprofit integrated health system in Minnesota and western Wisconsin serving 1.2 million patients. Clinics were eligible to participate if they had a doctoral-level Medication Therapy Management (MTM) pharmacist21 onsite at least one half-day per week and used standardized methods to measure BP with validated oscillometric BP monitors in early 2017. All 21 eligible clinics agreed to participate. Two pairs of clinics were each randomized as a single unit due to co-location with shared MTM pharmacist and clinic management, resulting in a total of 19 randomized units (9 clinics [9 units] randomized to Clinic-Based Care and 12 clinics randomized to Telehealth Care, including all 4 of the co-located clinics [10 units]). The HealthPartners Institutional Review Board reviewed and approved the study protocol including a waiver of written informed consent for participation.
Patient eligibility was evaluated using automated real-time algorithms that ran upon BP entry into the electronic health record (EHR) during office encounters in randomized clinics. Patients were eligible if they 1) were age 18 to 85 years; 2) had ≥2 qualifying encounters with a hypertension diagnosis code within the last 24 months; 3) had an encounter with their designated primary care professional (PCP) in the last 12 months; 4) had the current encounter in the clinic where their assigned PCP practiced; 5) had SBP ≥ 150 mm Hg or DBP ≥ 95 mm Hg in the first BP and in a repeated BP at the current encounter; and 6) had SBP ≥ 150 mm Hg or DBP ≥ 95 mm Hg for the last measured BP at their most recent previous qualifying encounter. The BP entry criteria were chosen based on clinic capacity to conduct additional follow-up visits. Patients were excluded if they were 1) pregnant, 2) had stage 5 chronic kidney disease, 3) were in hospice care, or 4) permanently resided in a nursing home.
For eligible patient encounters, a best practice alert automatically displayed prompting the medical assistant (MA) to set up a referral order for hypertension follow-up in 1–2 weeks for the clinician to review and sign. The referral order defaulted to an intended provider/visit type depending on clinic randomization (MA for BP check for Clinic-Based Care, MTM pharmacist for Telehealth Care). Other follow-up options included PCP, cardiology, or nephrology. Clinicians were able to change the provider type or timing of follow-up from the defaulted choice on the referral order if they felt that a different choice was best for an individual patient, but Telehealth Care with home BP telemonitoring was only available for patients in telehealth clinics. The clinician signing the referral order served to enroll the eligible patient into the study and to denote the eligible encounter as the index visit from which follow-up time was calculated. Patients were enrolled over an 18-month period from November 15, 2017 to April 15, 2019 and followed for 24 months post-index.
The Hyperlink 3 interventions are summarized in Table 1. The Clinic-Based Care intervention was developed by the care system from then-current best practices recommendations that were affirmed in subsequent national guidelines.18, 19, 22–25 It relied on face-to-face visits with the PCP with the assistance of an MA and standardized workflows including: BP measurement exclusively using validated automated oscillometric BP monitors (Omron HEM 907XL);26 repeating BP if the initial BP was elevated; recognition of and action for uncontrolled BP via an evidence-based hypertension treatment protocol that included recommending lifestyle modification, monitoring adherence, and intensifying pharmacological treatment when BP was not at goal (preferably by adding a synergistic medication class and using low-cost generic medications); regular follow-up at 2–4-week intervals until BP was controlled; and a standing order for registered nurses to adjust anti-hypertensive medications. Performance of these components was supported by a hypertension registry and regular measurement and feedback of the care processes. Lastly, the pre-existing hypertension referral order process was used systematically to offer timely no-cost follow-up with an MA.
Table 1.
Components of the Clinic-Based and Telehealth Care Interventions
| Clinic-based Care Components | Telehealth Care Components |
|---|---|
| Index Visit | Index Visit |
| BP measurement with automated monitor | BP measurement with automated monitor |
| Repeat BP if 1st measurement ≥140/90 mm Hg | Repeat BP if 1st measurement ≥140/90 mm Hg |
| Recognition of uncontrolled BP by PCP | Recognition of uncontrolled BP by PCP |
| Action taken for uncontrolled BP by PCP | Action taken for uncontrolled BP by PCP |
| Refer to MA for hypertension follow-up visit | Refer to pharmacist for hypertension follow-up visit |
| Following Index Visit | Following Index Visit |
| Attend follow-up with MA to re-assess BP | Attend follow-up with pharmacist for intake visit |
| Ad hoc home BP monitoring Re-assess uncontrolled BP after 2–4 weeks | Pharmacist home-based care for uncontrolled home BP every 2-4 weeks Systematic home-based BP monitoring |
| Team-based care between pharmacist and PCP |
Abbreviations: BP – blood pressure; MA – medical assistant; PCP – primary care professional
The Telehealth Care intervention adapted and implemented a previous successful research-tested model and used a similar hypertension treatment protocol.11 It included all components of Clinic-Based Care and offered home BP telemonitoring and BP medication management by pharmacists. In one eligible large clinic with limited MTM pharmacist capacity, telehealth care management was done by nurse practitioners with assistance by registered nurses. However, for simplicity we refer to pharmacists carrying out the telehealth care management, since it was designed to be adaptable for shared coordination by other qualified members of primary care teams.
Enrolled Telehealth Care patients were offered a one-hour in-person intake visit with the pharmacist including a medication review, medication adherence assessment, lifestyle and nutrition counseling, titration of anti-hypertensive medication, and the opportunity to initiate home BP telemonitoring. For those patients who agreed to home monitoring, pharmacists also introduced the home monitor, trained on proper BP self-measurement, reviewed home BP goals (≥75% of home BPs <135/85 mmHg, 5 mmHg lower than clinic goal), and ordered the equipment from the vendor (AMC Health, New York, NY). Patients received equipment by mail with detailed instructions and technical assistance. Home BP devices automatically transmitted data to the EHR. Pharmacists conducted follow-up primarily by phone every 2–4 weeks, using the home BP goals to guide treatment intensification and counseling decisions. Patients continued Telehealth Care until ≥75% of home BPs were <135/85 mm Hg for three consecutive phone visits or until the pharmacist and patient agreed to discontinue. The Telehealth Care intervention was expected to last an average of 4 months, with flexibility as needed.27
Clinic-Based Care referral orders were added to a “referral work queue” used by clinic assistants for scheduling outreach. Clinic assistants placed up to two phone calls to patients who had not scheduled their appointments and sent a letter to those not reached by telephone. This process was similar in Telehealth Care clinics, except that pharmacist scheduling outreach was done by an MTM program coordinator. All clinics received feedback from the study on completion of follow-up visits. As a further backup in both groups, the hypertension registry was used to identify and contact hypertension patients with uncontrolled BP and no scheduled follow-up.
The primary outcome was SBP change from index to 12 months post-index. BP values that were routinely collected in clinical encounters were extracted from the EHR to estimate change in SBP (Figure S1). Other data extracted from the EHR included sex, age at index visit, race and ethnicity, DBP, antihypertensive medication orders, insurance payor, sodium, potassium, creatinine, and diagnostic codes. Secondary outcomes were change in patient-reported outcomes (PROs) between baseline and 6 months, collected by patient surveys. Baseline surveys were mailed within one week of the index visit, with telephone follow-up of initial non-responders by trained interviewers. Baseline respondents received follow-up surveys at 6, 12, and 24 months post-index. Survey questions included demographics, rating of general health, rating of BP care over the last 6 months,16 patient experience of hypertension care (modified from Patient Assessment of Chronic Illness Care survey or PACIC),28 frequency and sharing of BP measurements outside of clinic,16 confidence in managing blood pressure,16 side effects from medications (developed for this study), and overall burden of blood pressure care (modified from the Treatment Burden Questionnaire).29 The definition and sources for study outcomes are detailed in Table S1.
An a priori power analysis (power=0.80, two-sided α=0.05) estimated the minimum detectable standardized effect (MDSE) for a linear time by treatment parameter in a random coefficients model under assumptions of N=2000 (20 clinics, 100 patients per clinic), three SBP measures per patient, clinic intraclass correlation (ICC) in SBP values 0.01–0.03, and standard deviation (SD) for SBP 20.4 mm Hg. The estimated MDSE = 0.12–0.17 corresponded to a 2.53– 3.55 mm Hg differential change in SBP among patients in Telehealth relative to Clinic-Based Care clinics. We anticipated a 5 mm Hg greater reduction in SBP in patients in Telehealth compared with Clinic-Based Care.11, 30, 31 A 5 mm Hg reduction in SBP is a clinically important reduction that substantially lowers the risk of stroke and heart disease, and even smaller reductions of 2 or 3 mm Hg have clinically important effects.3, 32–36 The MDSE for between-groups differences in 6-month PROs was .24–.27 when ICC=0.02–0.03.
In the primary intention-to-treat (ITT) analysis, the between-group difference in SBP change was tested using a random coefficients model. All SBP values from index (day 0) through 365 days post-index were predicted from random clinic and patient intercepts and fixed effects for clinic-randomized treatment group, time in days elapsed from index to the SBP, the treatment by time interaction, index SBP and several key characteristics that were imbalanced: index age and DBP, sex, and Asian race. The study protocol analysis plan specified a linear relationship between time and SBP but anticipated a non-linear rate of change in SBP. We estimated a time relationship that incorporated spline knots at clinically meaningful lags following the index visit (days 1, 42, 90, 180). The model was adapted for the secondary PRO outcomes by replacing the normal distribution and identity link specifications with Poisson-log specifications, replacing the time parameters with an indicator to denote a 6-month survey PRO, and estimating a patient level scale parameter. We analyzed whether treatment effects differed among patient subgroups defined by sex, age (>=60 vs. <60 years), race (Black vs. White), socioeconomic status (Medicaid insurance vs. other payor), hypertension severity (number of current classes of antihypertensive medications at index) and comorbidity (diagnosed diabetes, cardiovascular disease at index).
To complement the ITT analysis we also conducted a per-protocol analysis to evaluate the comparative effect of the intervention on study outcomes, among patients who adhered to the study protocol as intended.37, 38 In Telehealth Care, patients were considered adherent to protocol if they: 1) attended an intake visit with an MTM pharmacist within 6 weeks of the index date, 2) submitted ≥1 home BP measurement, and 3) completed ≥1 follow-up visit with the pharmacist. Patients in Clinic-Based Care were considered adherent to protocol if they followed up with an MA within 6 weeks post-index. In sensitivity analyses, we also evaluated the effect of the intervention in those who were partially adherent to Telehealth Care (fulfilling one or two components). To account for potential bias due to post-randomization selection of patients who: 1) were enrolled, 2) were adherent to protocol, and 3) responded to surveys (for PROs), we calculated inverse probability weights (IPW) as a function of individuals’ propensity for enrollment, adherence, and survey response, respectively.39–42 Candidate variables for these propensity models were specified a priori and were selected for inclusion via a least absolute shrinkage and selection operator (LASSO) approach in which the Bayesian Information Criterion was optimized. Stabilized IPWs were truncated at the 1st and 99th percentiles and applied to outcome models among adherent patients that were otherwise analogous to the ITT models described above.42, 43 The propensity models are shown in Tables S2-4 and sample sizes are shown in Figure 1.
Figure 1.
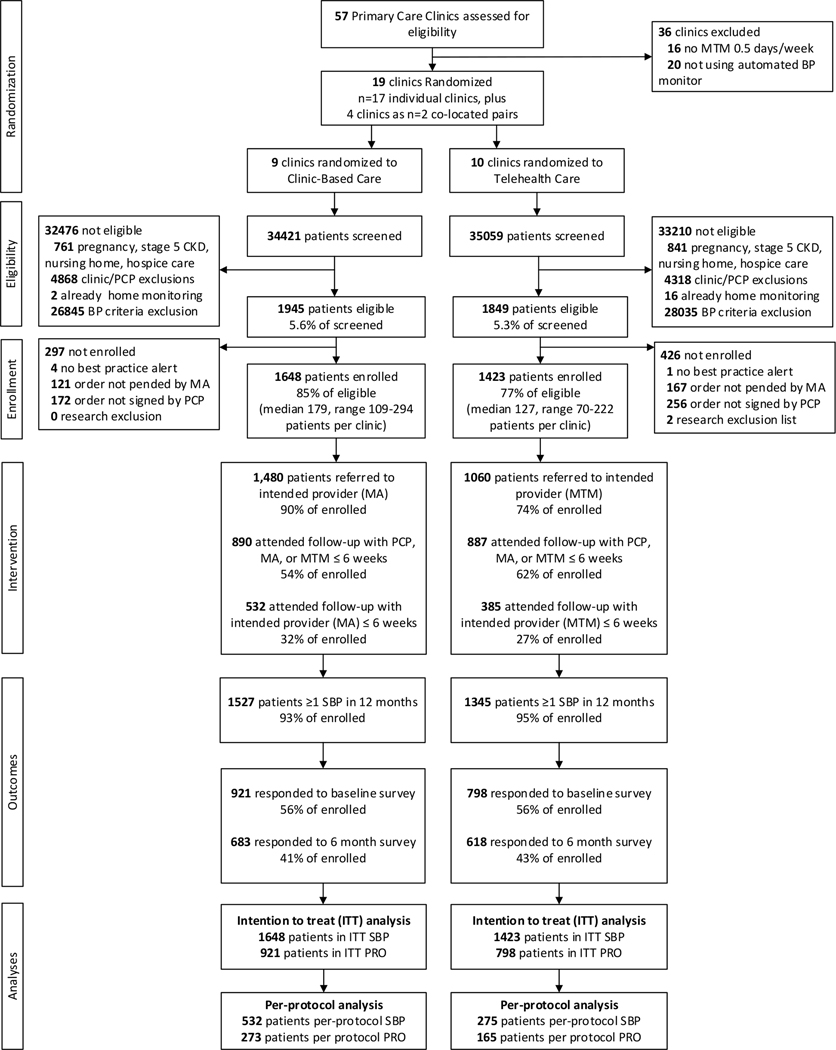
Hyperlink 3 participant flow diagram
Abbreviations: BP – blood pressure; CKD – chronic kidney disease; ITT – intention to treat; MA – medical assistant; MTM – Medication Therapy Management; PCP – primary care professional; PRO – patient-reported outcome; SBP – systolic blood pressure
Results
Participant Inclusion and Intervention Exposure
Figure 1 provides a flow diagram of participants in the study. Of 57 primary care clinics screened, 21 met study eligibility criteria. Patients aged 18–85 years who had an encounter during the 18-month enrollment period and two or more previous encounters with a hypertension diagnosis code within the last 24 months were potentially eligible: 34,421 in Clinic-Based Care and 35,509 in Telehealth Care clinics. Of these patients, 3794 (5.5%) met the remaining eligibility criteria. The eligible proportion and ineligibility reasons were similar in both groups. Hypertension follow-up orders for the 3794 eligible patients were signed for 85% of eligible patients in Clinic-Based Care and 77% in Telehealth Care.
PCPs were less likely to change the default follow-up from the intended provider in Clinic-Based Care (90% retained MA BP check) than in Telehealth Care (74% retained pharmacist). Most enrolled patients had follow-up within 6 weeks post-index with a PCP, MA, or pharmacist (54% in Clinic-Based Care and 62% in Telehealth Care). Of the enrolled patients, 532 (32%) in the Clinic-Based Care group and 385 (27%) in the Telehealth Care group attended a follow-up visit with the intended provider within 6 weeks post-index. Most Telehealth Care patients who attended the pharmacist visit within 6 weeks sent in ≥1 home BP measurement (80%) and had ≥1 phone visit with the pharmacist (71%).
Characteristics of Enrolled Study Patients
Enrolled patients had a mean age of 60 years and were about 47% male, 7% Asian, 19% Black, 69% White, and 2% Hispanic (Table 2). The mean BP at index was 158/92 mm Hg. About 15% of enrollees did not have any current classes of antihypertensive medication at index. The mean number of current antihypertensive classes was 1.7 (median=2). Diabetes was diagnosed in 25% and CV disease in 17% of enrolled patients. There were some differences in patient characteristics by treatment group (at least in part owing to a women’s health clinic, a geriatrics clinic and an international health clinic all being randomized to Telehealth Care): compared to Clinic-Based Care, Telehealth Care patients were about 4 years older (p < 0.02), more likely to be Asian (p=0.006), had lower DBP (p<0.02), less likely to be obese (p<0.03), and more likely to have CV disease (p<0.04).
Table 2.
Baseline characteristics of Hyperlink-enrolled patients
| Characteristic | All Clinics (N=3071) | Clinic-based Care Clinics (n=1648) | Telehealth Care Clinics (n=1423) | p-value |
|---|---|---|---|---|
| Age, Mean (SD) | 60.2 (14.4) | 58.3 (14.2) | 62.4 (14.2) | 0.02 |
| Male, n (%) | 1432 (46.6) | 814 (49.4) | 618 (43.4) | 0.15 |
| Race | ||||
| American Indian / Alaska Native, n (%) | 19 (0.6) | 15 (0.9) | 4 (0.3) | 0.06 |
| Asian, n (%) | 213 (6.9) | 92 (5.6) | 121 (8.5) | 0.006 |
| Black/African American, n (%) | 594 (19.3) | 329 (20.0) | 265 (18.6) | 0.69 |
| Hawaiian / Pacific Islander, n (%) | 3 (0.1) | 1 (0.1) | 2 (0.1) | 0.50 |
| Multiracial, n (%) | 15 (0.5) | 8 (0.5) | 7 (0.5) | 0.81 |
| Unknown, n (%) | 95 (3.1) | 59 (3.6) | 36 (2.5) | 0.43 |
| White, n (%) | 2132 (69.4) | 1144 (69.4) | 988 (69.4) | 0.99 |
| Hispanic ethnicity, n (%) | 60 (2.0) | 46 (2.8) | 14 (1.0) | 0.06 |
| Education | ||||
| n responders | 1688 | 908 | 780 | |
| ≤ High school or GED, n (%) | 563 (33.4) | 316 (34.8) | 247 (31.7) | 0.38 |
| Some college or technical school, n (%) | 599 (35.5) | 315 (34.7) | 284 (36.4) | 0.62 |
| 4-y college degree, n (%) | 308 (18.3) | 171 (18.8) | 137 (17.6) | 0.65 |
| >4-y college degree, n (%) | 218 (12.9) | 106 (11.7) | 112 (14.4) | 0.49 |
| Employment | ||||
| n responders | 1693 | 908 | 785 | |
| Full time, n (%) | 582 (34.4) | 351 (38.7) | 231 (29.4) | 0.05 |
| Part time, n (%) | 139 (8.2) | 71 (7.8) | 68 (8.7) | 0.54 |
| Retired, n (%) | 682 (40.3) | 332 (36.6) | 350 (44.6) | 0.23 |
| Otherwise not working for pay, n (%) | 290 (17.1) | 154 (17.0) | 136 (17.3) | 0.90 |
| Annual income | ||||
| n responders | 1481 | 798 | 683 | |
| ≤$20,000, n (%) | 307 (20.7) | 164 (20.6) | 143 (20.9) | 0.86 |
| $20,000-<$50,000, n (%) | 443 (29.9) | 258 (32.3) | 185 (27.1) | 0.13 |
| $50,000-<$100,000, n (%) | 469 (31.7) | 231 (29.0) | 238 (34.9) | 0.38 |
| >=$100,000, n (%) | 262 (17.7) | 145 (18.2) | 117 (17.1) | 0.74 |
| SBP, mm Hg, Mean (SD) | 158.0 (15.3) | 157.4 (15.4) | 158.8 (15.2) | 0.20 |
| DBP, mm Hg, Mean (SD) | 91.7 (13.9) | 93.1 (13.8) | 90.0 (13.8) | 0.02 |
| Number anti-hypertensive medication classes | ||||
| Mean (SD) | 1.7 (1.1) | 1.7 (1.1) | 1.7 (1.2) | 0.92 |
| 0, n (%) | 466 (15.2) | 245 (14.9) | 221 (15.5) | 0.60 |
| 1, n (%) | 1006 (32.8) | 538 (32.7) | 468 (32.9) | 0.89 |
| 2, n (%) | 915 (29.8) | 512 (31.1) | 403 (28.3) | 0.12 |
| 3+, n (%) | 684 (22.3) | 353 (21.4) | 331 (23.3) | 0.60 |
| BMI ≥30 kg/m2, N (%) | 1730 (57.1) | 987 (60.9) | 743 (52.7) | 0.03 |
| Diabetes, N (%) | 773 (25.2) | 407 (24.7) | 366 (25.7) | 0.81 |
| Cardiovascular disease, N (%) | 512 (16.7) | 247 (15.0) | 265 (18.6) | 0.04 |
Abbreviations: BMI – body mass index; DBP – diastolic blood pressure; GED – General Educational Development test; mm Hg – millimeters of mercury; kg/m2 – kilogram force per square meter; n or N – number; SBP – systolic blood pressure; SD – standard deviation; y – year.
Change in Systolic Blood Pressure from Baseline to 12 Months
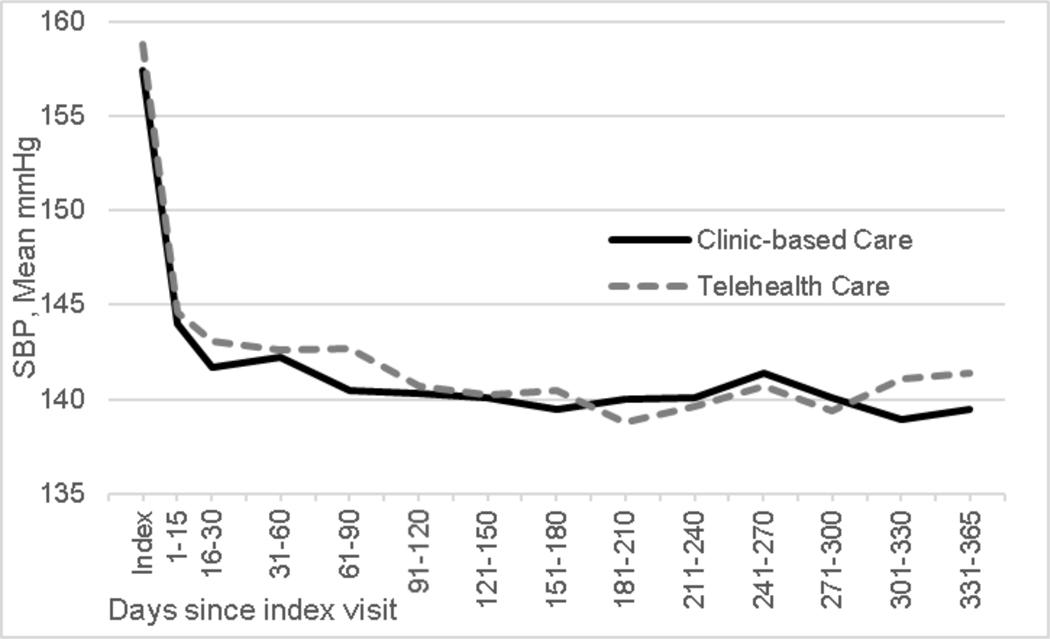
There were N=17545 SBP values (Clinic-Based Care n=8768; Telehealth Care n=8777) included in primary analysis. Model-estimated SBP was similar in the two groups at index (157.1 mm Hg Clinic-Based Care, 157.5 mm Hg in Telehealth Care). Estimated change in SBP over 365 days in Clinic-Based Care was −18.0 mm Hg (95% CI: −19.4, −16.5 mm Hg), to 139.2 mm Hg. The comparable change in Telehealth Care was −18.7 mm Hg (95% CI: −20.2, −17.2 mm Hg), to 138.8 mm Hg (Figure 2). The model-estimated between-groups difference in SBP change was −0.76 mm Hg (95% CI −2.84, 1.32 mm Hg; p=0.45).
Figure 2:
Observed systolic blood pressure (SBP) by treatment group from index visit to 365 days post-index
The per-protocol model-estimated difference in change in SBP between groups from day 0 to day 365 was −2.7 mm Hg (95% CI: −6.4, 1.0; p=0.159), slightly greater than in the ITT analysis, but less than the hypothesized 5 mm Hg effect size and not statistically significant in this smaller sample.
Change in Patient Reported Outcomes (PROs) from Baseline to 6 Months
The baseline survey was completed by 1719 of 3071 enrolled patients (56%) and the 6-month survey was completed by 1301 of enrolled patients that completed the baseline survey (76%), with similar response rates in Clinic-Based Care and Telehealth Care (Figure 1). The characteristics of baseline survey respondents and non-respondents are shown in Table S5 and of baseline survey respondents by randomized group in Table S6.
Several key PROs changed differentially over time in the hypothesized direction, while others did not change (Table 3). At baseline, less than 30% of patients rated their satisfaction with care as 9 or 10 vs. 0–8. Compared with the minimal change from baseline to 6 months in the Clinic-Based Care group, the Telehealth Care group had a higher proportion of patients who rated their care as 9 or 10 vs. 0–8 at 6 months (29.3% to 39.5%). The increase in satisfaction from baseline to 6 months was significantly greater in the Telehealth Care group than the Clinic-Based Care group (adjusted relative risk, RR 1.25 [ 1.02, 1.52]).
Table 3.
Patient reported outcomes at baseline and 6 months
| Clinic-Based Care | Telehealth Care | Intent to Treat | Per Protocol | |||||
|---|---|---|---|---|---|---|---|---|
| Survey Item | Baseline | 6 months | Baseline | 6 months | RR adj | 95% CI | RR adj | 95% CI |
| Survey respondents, n | 921 | 683 | 798 | 618 | ||||
| Overall rating of health, % (Excellent/very good vs good/fair/poor) | 24.6 | 27.8 | 29.3 | 32.2 | 0.96 | 0.80, 1.15 | 0.93 | 0.71, 1.21 |
| Rating of hypertension care, % (9–10 vs. 0–8) | 27.6 | 30.2 | 29.3 | 39.5 | 1.25 * | 1.02, 1.52 | 1.67 | 1.23, 2.27 |
| When receive BP care in past 6 months how often were you… (most times/always vs. sometimes/ generally not/never) | ||||||||
| Asked for ideas on treatment plan, % | 44.1 | 47.2 | 52.2 | 59.3 | 1.06 | 0.93, 1.20 | 1.15 | 0.94, 1.41 |
| Given treatment choices, % | 46.4 | 49.8 | 54.1 | 63.7 | 1.10 | 0.97, 1.25 | 1.21 | 0.99, 1.48 |
| Asked to talk about problems with medicines, % | 58.5 | 59.5 | 65.2 | 68.4 | 1.04 | 0.93, 1.15 | 1.27 | 1.08, 1.49 |
| Asked to talk about goals of BP care, % | 46.0 | 47.3 | 53.0 | 59.2 | 1.08 | 0.95, 1.23 | 1.33 | 1.11, 1.60 |
| Confident people involved in care on same page, % | 72.5 | 77.9 | 77.6 | 79.5 | 0.95 | 0.88, 1.02 | 0.99 | 0.87, 1.12 |
| Activities helpful for managing BP in last 6 months (Extremely/very vs. moderately/somewhat/not at all) | ||||||||
| Physical activity, % | 42.8 | 52.0 | 46.3 | 50.6 | 0.90 | 0.77, 1.06 | 0.99 | 0.74, 1.33 |
| Decreasing salt, % | 46.4 | 50.9 | 49.5 | 51.4 | 0.95 | 0.82, 1.11 | 1.14 | 0.87, 1.49 |
| Watching weight, % | 49.1 | 52.2 | 47.9 | 51.0 | 1.04 | 0.89, 1.22 | 1.20 | 0.93, 1.56 |
| Reducing stress, % | 44.7 | 49.1 | 49.7 | 50.4 | 0.92 | 0.78, 1.09 | 1.28 | 0.96, 1.72 |
| Limiting alcohol, % | 44.6 | 43.4 | 45.5 | 47.8 | 1.09 | 0.87, 1.37 | 0.99 | 0.62, 1.57 |
| Home BP monitoring | ||||||||
| Frequency, % (≥2x/week vs. less often) | 28.2 | 28.1 | 28.6 | 43.9 | 1.53 | 1.27, 1.85 | 1.99 | 1.46, 2.73 |
| Share home BP with care team, % (Y/N)† | 75.3 | 71.2 | 77.2 | 82.2 | 1.13 | 1.01, 1.26 | 1.44 | 1.19, 1.75 |
| How do you share home BP, % (electronically vs. other)‡ | 4.4 | 2.0 | 5.6 | 37.4 | 13.14 | 4.92, 35.12 | 13.58 | 3.65, 50.56 |
| BP treatment changed based on home BP, % (Y/N)‡ | 39.7 | 34.5 | 39.2 | 57.3 | 1.68 | 1.31, 2.17 | 2.24 | 1.45, 3.47 |
| For managing BP in last 6 months, confidence in ability to… (Extremely/very vs. moderately/somewhat/not at all) | ||||||||
| Contact care team, % | 69.4 | 71.7 | 73.8 | 78.1 | 1.02 | 0.94, 1.10 | 1.07 | 0.94, 1.22 |
| Measure BP at home, % | 54.8 | 57.7 | 62.8 | 69.0 | 1.04 | 0.94, 1.16 | 1.00 | 0.85, 1.18 |
| Know BP target numbers, % | 61.9 | 66.0 | 70.4 | 78.3 | 1.04 | 0.95, 1.13 | 1.10 | 0.96, 1.26 |
| Keep BP below target, % | 24.0 | 36.1 | 27.3 | 42.1 | 1.02 | 0.83, 1.25 | 1.21 | 0.88, 1.68 |
| Take BP medications, % | 82.0 | 84.6 | 85.7 | 89.4 | 1.01 | 0.95, 1.07 | 1.00 | 0.91, 1.10 |
| Problem with common symptoms that may be related to BP medications in last 6 months (Very big/big/moderate/some vs. none) | ||||||||
| Tiredness, % | 70.6 | 66.6 | 67.2 | 63.4 | 0.99 | 0.91, 1.08 | 1.02 | 0.88, 1.17 |
| Dizziness or lightheadedness, % | 43.6 | 37.7 | 40.8 | 39.1 | 1.11 | 0.95, 1.29 | 1.10 | 0.85, 1.43 |
| Swelling of feet or legs, % | 36.8 | 37.7 | 36.3 | 35.1 | 0.93 | 0.80, 1.08 | 0.95 | 0.74, 1.22 |
| Cough, % | 33.8 | 30.8 | 34.2 | 35.8 | 1.17 | 0.98, 1.40 | 1.22 | 0.92, 1.62 |
| Frequent urination, % | 55.4 | 50.5 | 55.4 | 49.4 | 0.98 | 0.87, 1.11 | 1.16 | 0.95, 1.42 |
| Sexual symptoms, % | 25.9 | 26.5 | 22.5 | 22.3 | 0.95 | 0.76, 1.19 | 0.90 | 0.64, 1.26 |
| Stopped medications due to symptoms (Y/N) | 23.2 | 15.3 | 20.5 | 16.7 | 1.22 | 0.89, 1.67 | 1.41 | 0.84, 2.36 |
| Satisfaction with medications, % (very/somewhat satisfied vs. neutral/somewhat/very dissatisfied) | 53.1 | 66.0 | 54.5 | 66.9 | 0.99 | 0.87, 1.12 | 1.06 | 0.84, 1.32 |
| Problem with frequency, time spent or inconvenience related to BP care last 6 months (Very big/big/moderate/some vs. none) | ||||||||
| Measuring BP, % | 32.3 | 26.9 | 29.5 | 29.4 | 1.21 | 0.97, 1.50 | 1.63 | 1.03, 2.58 |
| Clinic visits, % | 34.9 | 31.0 | 34.9 | 25.8 | 0.84 | 0.68, 1.03 | 0.74 | 0.51, 1.08 |
| Phone visits, % | 18.0 | 19.6 | 20.0 | 13.7 | 0.64 | 0.45, 0.92 | 0.47 | 0.21, 1.05 |
| Scheduling visits | 27.5 | 29.1 | 29.8 | 21.9 | 0.70 | 0.55, 0.89 | 0.54 | 0.36, 0.80 |
| Time away from work or responsibilities, % | 29.0 | 26.9 | 24.3 | 17.3 | 0.78 | 0.60, 1.01 | 0.46 | 0.28, 0.75 |
| Increasing physical activity, % | 43.5 | 38.4 | 43.6 | 43.8 | 1.14 | 0.98, 1.33 | 1.06 | 0.81, 1.37 |
| Lifestyle changes, % | 47.7 | 41.8 | 41.8 | 36.3 | 0.98 | 0.83, 1.16 | 1.22 | 0.93, 1.59 |
| Cost of care or medications, % | 34.2 | 29.0 | 31.2 | 22.8 | 0.90 | 0.73, 1.11 | 0.93 | 0.68, 1.29 |
Abbreviations: RR adj – adjusted relative risk; BP – blood pressure; CI – confidence interval; N – no; Y – yes;
Bold values indicate statistical significance (95% confidence interval that excludes 1.0)
Of people who reported measuring their BP outside of a clinic visit at least twice per month
Of people who said ‘yes’ to sharing their home BP with their care team
Compared with no change in frequency of home BP monitoring from baseline to 6 months in the Clinic-Based Care group, the Telehealth Care group had a significant increase in the proportion of patients who checked their BP ≥2 times per week (from 28.6% to 43.9%, RR 1.53 [1.27, 1.85]). The Telehealth Care group was also significantly more likely over time to 1) report that they shared their home BP data with their care team, 2) that they shared the data electronically, and 3) that someone on their health care team had ever changed their BP medication because of the home BP measurements.
Over 6 months, the Telehealth Care and Clinic-Based Care groups reported different reductions in burden related to BP care. The Telehealth Care reported significantly less burden from scheduling visits and attending phone visits. We did not observe a difference over time between groups in overall rating of health, rating of chronic illness care from items from the PACIC survey,61 helpfulness of lifestyle activities for managing BP, confidence in self-care, or BP medication side effects.
The per-protocol and ITT analyses of PROs generally had congruent results but point estimates of effect sizes for the statistically significant findings were generally of greater magnitude and confidence intervals were wider in the per-protocol analysis (Table 3). As in the ITT analysis, Telehealth Care had a significantly higher proportion of patients who rated their care as 9 or 10 vs. 0–8 at 6 months, but the adjusted RR was 1.68 (95% CI 1.23, 2.27) rather than 1.25 (95% CI 1.02, 1.52). The Telehealth Care group had a significantly greater change in the proportion of patients monitoring BP at home ≥2 times per week (RR 1.99 vs. 1.53 in the ITT analysis), sharing their home BP data with their care team (RR 1.44 vs. 1.13), sharing the data electronically (RR 13.6 vs. 13.1), and that someone on their health care team had ever changed their BP medication because of the BP measurements taken outside of clinic visits (RR 2.24 vs. 1.68). The Telehealth Care group also reported a greater increase in the burden of measuring BP (RR 1.63 vs. 1.21), and greater decrease in burden related to scheduling visits (RR 0.54 vs. 0.70) and time away from work or other responsibilities (RR 0.46 vs. 0.78). Unlike in the ITT analysis, the burden for phone visits in the per-protocol analysis was not significantly different in the two groups, although the point estimates of the RR were similar. Conversely, in the per-protocol analysis (but not in the ITT analysis) the Telehealth Care group reported less inconvenience related to time away from work or other responsibilities and were more likely to report being asked to talk about problems with medications and goals in caring for their BP than the Clinic-Based Care group.
Per-Protocol Sensitivity Analyses
The model-estimated difference in change in SBP between groups from day 0 to day 365 in the partial adherence models was not significantly different than zero, but the point estimates were intermediate between the point estimates in the pre-specified per-protocol analysis (−2.7 mm Hg) and the ITT analysis (0.8 mm Hg). A similar set of sensitivity analyses was done for the PRO outcomes. The estimates from these models were also generally closer to the null value and intermediate between the pre-specified per-protocol and the ITT results.
Other Outcomes
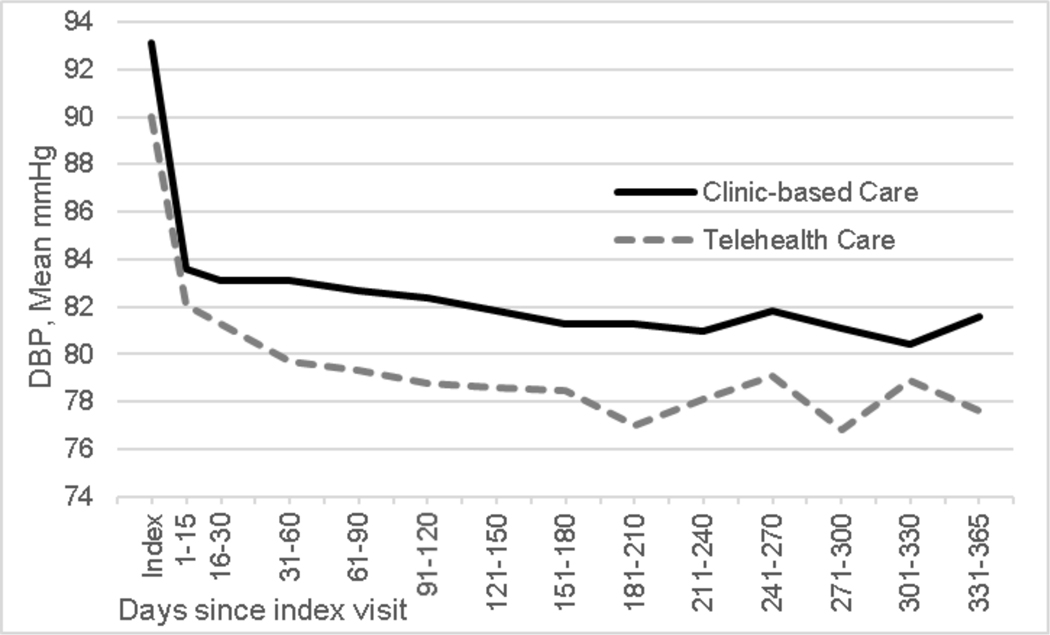
DBP differed by about 3 mm Hg in the two groups at baseline, likely due to the older age of the patients in the Telehealth Care group (93.1 mm Hg in Clinic-Based Care and 90.0 mm Hg in Telehealth Care). Adjusting for this difference and other covariates, model-estimated DBP changed non-linearly over 12 months by −10.0 mm Hg (95% CI −10.8, −9.1 mm Hg) in Clinic-Based Care and by −9.7 mm Hg (95% CI −10.6, −8.8 mm Hg) in Telehealth Care (Figure 3). The model-based difference in change in DBP between groups was 0.3 mm Hg (95% CI −1.0, 1.5 mm Hg).
Figure 3:
Observed diastolic blood pressure (DBP) by treatment group from index visit to 365 days post-index
Antihypertensive medication class additions were examined using EHR orders. On the index date about 1/3 of patients in both groups (31% in Telehealth Care and 32% in Clinic-Based Care) had an order for a new antihypertensive medication class added to their existing treatment regimen (adjusted OR 1.19, 95% CI 0.90, 1.56). Among patients without a medication class added on the index date, Telehealth Care patients (36%) were more likely than Clinic-Based Care patients (30%) to have a new medication class added to their treatment (adjusted OR 1.40, 95% CI 1.08, 1.83) over the next 12 months. Nevertheless, the number of current classes of antihypertensive medications was similar in both groups at baseline (mean=1.7) and at 12 months (mean=2.1).
Among patients who were eligible but not enrolled, 21% had an order for a new antihypertensive medication class added on the date of the visit when they became eligible, another 21% had a medication class added during the next 12 months, and 57% did not have any new antihypertensive medication classes added. The proportion of patients with newly added antihypertensive medication was significantly lower than in enrolled patients (index adjusted OR 0.45, 95% CI 0.36, 0.57; follow-up adjusted OR 0.41, 95% CI 0.33, 0.51).
There was no significant difference in change over time by treatment group for any safety indicators (diagnosis codes for dizziness, fainting or hypotension; hypokalemia, hyperkalemia, hyponatremia or reduced eGFR), whether analyzed as events per patient-year or as the proportion of patients with one or more events. There was also no differential change in laboratory values of potassium, sodium, or eGFR.
We observed no significant differences in SBP or DBP change over 12 months in Telehealth Care relative to Clinic-Based Care by patient subgroups defined by sex, age, race, diabetes, cardiovascular disease, and insurance type (Commercial/Medicare vs. Medicaid). There was, however, a significant difference in SBP change over 12 months in Telehealth Care relative to Clinic-Based Care by the number of medication classes at index (6.6 mm Hg, 95% CI 1.8, 11.3). Patients with 0–2 medication classes had more SBP reduction in Telehealth Care than Clinic-Based Care (−2.5 mm Hg [−4.9, −0.05]) while those with 3–6 medication classes had more SBP reduction in Clinic-Based Care than Telehealth Care (4.1 mm Hg [−0.04, 8.2]).
Discussion
The Hyperlink 3 pragmatic cluster-randomized trial compared two team-based care models for moderately severe uncontrolled hypertension, Clinic-Based Care and Telehealth Care, and found that they were both safe and effective for lowering BP. SBP declined significantly by a similar amount (18–19 mm Hg) in both groups from a baseline of 157 mm Hg to 139 mm Hg over 12 months of follow-up, with no significant difference between groups in SBP change over time (−0.76 mm Hg [95% CI −2.84, 1.32 mm Hg]). Similarly, DBP declined significantly by 10 mm Hg in both groups (from 93 mm Hg in Clinic-Based Care and 90 mm Hg in Telehealth Care) with no significant differences between groups in DBP change over time a (0.3 mm Hg [95% CI −1.0, 1.5 mm Hg]). There was no difference in key safety parameters from pre- to post-intervention between groups, including electrolyte disturbances, reduced kidney function, or diagnoses of dizziness, fainting, or hypotension.
Although most PROs did not change significantly between groups, several important ones did. There were clinically important and statistically significant changes over time in favor of improved satisfaction with hypertension care in Telehealth Care. Telehealth Care patients also reported a decrease in their sense of burden in caring for their hypertension (time and inconvenience related to scheduling visits and attending phone visits), and adherent patients reported more burden related to measuring BP, but less time away from work and other responsibilities. Improving satisfaction and convenience are goals of most health systems and may reduce barriers to care for marginalized populations. Although we did not observe a difference in between-group BP change, several patient-reported processes that could mediate improvements in BP were reported more frequently in the Telehealth Care group, including more monitoring of home BP, electronic sharing of BP data, and use of the home BP data by the care team to change treatment.
In pragmatic trials, low adherence to the randomly assigned interventions or substantial crossover from one intervention to the other is common.44–46 A per-protocol analysis can help determine whether the interventions have differential effectiveness when delivered as intended, and is an important analytic tool in pragmatic trials.38 Because adherence to both interventions was lower than expected, as measured by completion of key steps in Clinic-Based Care and Telehealth Care in under 1/3 of participants, we undertook a planned per-protocol analysis that accounted for measurable confounders and selection bias in enrollment, adherence, and survey completion. The SBP effect size was larger in the IPW per-protocol models (~3 mm Hg) compared with the ITT analysis (<1 mm Hg), and similarly, there were larger effect sizes in the per-protocol analysis for the PROs that were improved in the ITT analysis. The per protocol analyses had larger standard errors due to low adherence and use of IPW. Nevertheless, the general similarity of the results suggests that low adherence to the Telehealth Care intervention alone does not explain the lack of a between-group difference in SBP. In fact, in this population with moderately severe uncontrolled hypertension, it supports the conclusion that Clinic-Based Care and Telehealth Care were equally effective for lowering SBP, although there may be a differential effect related to the number of prescribed BP medications.
In retrospect, including a usual care group could have helped to distinguish whether the best practices incorporated in both the Clinic-Based Care and Telehealth Care clinics were responsible for the equivalent effectiveness for BP lowering compared with telehealth care. In particular, the best practice alert that we used to prompt enrollment via the hypertension referral order may have resulted in the unexpectedly high proportion of patients who had an antihypertensive medication class added at the index visit. Patients who were eligible for the trial but not enrolled were significantly less likely to have an antihypertensive medication added to treatment, even after adjusting for ways they may have differed from enrolled patients. Thus, we suspect that the similar BP reduction in both treatment groups could represent improvement in routine hypertension care that is sufficient to negate the advantage that previous trials have shown for extended team-based care and telemonitoring interventions.30, 47, 48 In the Hyperlink 1 trial mean baseline BP was 148/85 mm Hg.11 BP change from baseline to 12 months was −13/−4 mm Hg in the usual care group, which did not include many of the best practices in place for Hyperlink 3, and −23/−9 in the telemonitoring group, which had much higher adherence to protocol-specified pharmacist visits and home BP monitoring.27, 49 The BP reduction in Hyperlink 3 was substantially greater than that observed in the usual care group of Hyperlink 1, despite the potentially more difficult-to-treat patient population (higher baseline BP, treated with more medications, and not selected for interest in participating in research.)50
Pragmatic trial designs are meant to test interventions under realistic conditions, but require researchers to think about many potential trade-offs in implementation.51 The research team prioritized pragmatic design in the domains of recruitment, resources needed to deliver the telehealth intervention, flexibility of delivery of the telehealth intervention, flexibility of adherence to the intervention, and data collection.50 Although low-touch recruitment methods were successful in enrolling most of the eligible patients, there was much lower adherence to the follow-up visit than the 98% we observed in Hyperlink 1. Had we anticipated this important limitation, we could have planned for alternative methods to either make it easier to decline participation, enhance adherence to follow-up, allowed PCPs to manage the telehealth intervention, or offered patients more freedom of choice on intervention content and delivery.
Several additional factors are important to keep in mind when interpreting the study results. Hyperlink 3 enrolled patients with moderately severe uncontrolled hypertension who were prescribed a median of 2 antihypertensive medication classes at baseline. Some of the BP reduction we observed likely resulted from regression to the mean.52 Previous research suggests that self-monitoring and pharmacist management interventions are effective in patients with SBP ranging from 140–169 mm Hg,10, 16 but may be less effective as we observed in patients on ≥3 antihypertensive medications.10, 53 These patients made up about 20% of our study sample, and is likely a group for whom it is especially challenging to find additional effective and tolerable therapy. The study included both pharmacists and nurse practitioners to manage the telehealth intervention, but did not test a care model where telemonitoring was managed by a PCP and MA or RN, although that configuration would probably be quite appealing to some patients and PCPs. There were limitations on data collection owing to the pragmatic use of the EHR, so we lack information on some important care processes (eg, granular information on medication intensification). However, our previous research suggests that using BP measured in a research clinic, rather than EHR measurements, would not have changed the study results.54
A recent systematic review of 20 trials found that replacing or augmenting usual in-person care with video teleconferencing generally resulted in similar clinical effectiveness, health care use, patient satisfaction, and quality of life.55 However, none of these studies examined hypertension care or diagnosis. Future research and practice for improving hypertension care through remote monitoring and virtual care should focus on 1) offering a variety of evidence-based choices to patients and clinicians, 2) streamlining and supporting access to telehealth, and 3) adopting newly available technology to obtain accurate remote BP measures, and 4) developing sustainable reimbursement models for virtual care.
In summary, with low exposure to the pragmatic telehealth intervention that may be typical of what would be observed in clinical practice, both Telehealth and Clinic-Based Care were effective in lowering BP by 18–19/10 mm Hg with no statistically significant BP difference between groups. Despite low intervention exposure, there were more favorable PROs in the Telehealth Care group: patients were 26% more likely to highly rate their BP care experience, 55% more likely to report frequent self-monitoring of BP (and electronic sharing), and 68% more likely to report changes made to medications based on home BP. There was less inconvenience related to BP care visits, but more burden for measuring BP. We conclude that telehealth care by pharmacists is an effective and safe alternative to clinic-based care for improving patient-centered care for hypertension.
Perspectives
Comparing outcomes of different models of team-based care for uncontrolled hypertension may help primary care practices decide how to organize and allocate resources for this important aspect of CV risk reduction. Previous studies with highly-selected research volunteers had shown that home BP telemonitoring with pharmacist care management lowered BP more than usual in-person primary care. This study enrolled typical primary care patients with moderately severe uncontrolled hypertension. It compared 1) clinic-based care using best practices and face-to face visits with physicians and medical assistants; and 2) telehealth care including the same best practices, but with added home BP telemonitoring and home-based care coordinated by a clinical pharmacist or nurse practitioner. Only about 1/3 of patients in both groups attended the randomly assigned follow-up visit with the intended non-physician team member within 6 weeks. Clinic-based care and telehealth care were similarly effective in lowering BP by 18–19/10 mm Hg. Several patient-reported outcomes were more favorable in the telehealth care group: higher satisfaction with hypertension care, more frequent self-monitoring of home BP, perception that medications were changed based on home BP, and less inconvenience related to BP care visits. There was no difference in safety. In patients typical of those encountered in primary care practice, these results suggest that telehealth care by pharmacists is an effective and safe alternative to clinic-based care for uncontrolled hypertension. Because adherence to follow-up with a randomly-assigned non-physician team member was low, patients should be offered a range of options for timely follow-up care.
Supplementary Material
Pathophysiological Novelty and Relevance.
What is new?
Comparing outcomes of two models of team-based care for moderately severe uncontrolled hypertension in routine primary care: 1) clinic-based care using best practices and face-to face visits with physicians and medical assistants; and 2) telehealth care including the same best practices, but with added home BP telemonitoring and home-based care coordinated by a clinical pharmacist or nurse practitioner
What is relevant?
SBP declined significantly by a similar amount (18–19 mm Hg) in both groups from a baseline of 157 mm Hg to 139 mm Hg over 12 months of follow-up, with no significant difference between groups in SBP change over time (−0.76 mm Hg [95% CI −2.84, 1.32 mm Hg]).
Several patient-reported outcomes were more favorable in the telehealth care group: higher satisfaction with hypertension care, more frequent self-monitoring of home BP, perception that medications were changed based on home BP, and less inconvenience related to BP care visits.
Clinical/Pathophysiological Implications?
Telehealth care by pharmacists is an effective and safe alternative to clinic-based care for uncontrolled hypertension
Acknowledgments
We would like to thank our partners in the HealthPartners Care Group, including clinicians, staff, MTM pharmacists, the HealthPartners Health Plan, our external stakeholder advisory board and Data and Safety Monitoring Board members. We would also like to thank our patient advisory board members (Melinda Anderson; Daryl Coons, AS; Roger Karnow, BA, CPA; Robert Lee; Carlos Maeztu, MA; Lisa McCullen; Linda Mitchell; Barbara Taylor, MA) who contributed in countless ways to this research. Finally, we extend our gratitude to the patients who participated in the study.
Sources of Funding
Research reported in this article was funded through a Patient Centered Outcomes Research Institute© (PCORI©) Award (IHS-1507-31146). All statements in this report, including its findings and conclusions, are solely those of the authors and do not necessarily represent the official views of the Patient-Centered Outcomes Research Institute (PCORI), its Board of Governors or Methodology Committee.
Non-standard Abbreviations and Acronyms
- BMI
body mass index
- BP
blood pressure
- CI
confidence interval
- CKD
chronic kidney disease
- CV
cardiovascular
- DBP
diastolic blood pressure
- GED
General Educational Development test
- ICC
intraclass correlation
- ITT
intention to treat
- MA
medical assistant
- kg/m2
kilogram force per square meter
- MDSE
minimum detectable standardized effect
- mm Hg
millimeters of mercury
- MTM
Medication Therapy Management
- OR
odds ratio
- PACIC
Patient Assessment of Chronic Illness Care survey
- PCP
primary care professional
- PRO
patient-reported outcome
- RR
relative risk
- RR adj
adjusted relative risk
- SBP
systolic blood pressure
- SD
standard deviation
Footnotes
Clinical trial registration: www.clinicaltrials.gov NCT02996565
Disclosures
None of the authors reports a conflict of interest.
References
- 1.Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ and Ezzati M. The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS medicine. 2009;6:e1000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang Q, Cogswell ME, Flanders WD, Hong Y, Zhang Z, Loustalot F, Gillespie C, Merritt R and Hu FB. Trends in cardiovascular health metrics and associations with all-cause and CVD mortality among US adults. JAMA. 2012;307:1273–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blood Pressure Lowering Treatment Trialists Collaboration. Age-stratified and blood-pressure-stratified effects of blood-pressure-lowering pharmacotherapy for the prevention of cardiovascular disease and death: an individual participant-level data meta-analysis. Lancet (London, England). 2021;398:1053–1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Muntner P, Hardy ST, Fine LJ, Jaeger BC, Wozniak G, Levitan EB and Colantonio LD. Trends in Blood Pressure Control Among US Adults With Hypertension, 1999–2000 to 2017–2018. JAMA. 2020;324:1190–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.U.S. Department of Health and Human Services. The Surgeon General’s Call to Action to Control Hypertension. Washington, DC: U.S. Department of Health and Human Services, Office of the Surgeon General; 2020. [Google Scholar]
- 6.Guide to Community Preventive Services. TFFRS - Heart disease and stroke prevention: Team-based care to improve blood pressure control. https://www.thecommunityguide.org/content/tffrs-heart-disease-and-stroke-prevention-team-based-care-improve-blood-pressure-control. Accessed April 15, 2022.
- 7.Arrieta A, Woods JR, Qiao N and Jay SJ. Cost-benefit analysis of home blood pressure monitoring in hypertension diagnosis and treatment: an insurer perspective. Hypertension. 2014;64:891–6. [DOI] [PubMed] [Google Scholar]
- 8.Fletcher BR, Hartmann-Boyce J, Hinton L and McManus RJ. The Effect of Self-Monitoring of Blood Pressure on Medication Adherence and Lifestyle Factors: A Systematic Review and Meta-Analysis. Am J Hypertens. 2015;28:1209–1221. [DOI] [PubMed] [Google Scholar]
- 9.Uhlig K, Patel K, Ip S, Kitsios GD and Balk EM. Self-measured blood pressure monitoring in the management of hypertension: a systematic review and meta-analysis. Annals of internal medicine. 2013;159:185–94. [DOI] [PubMed] [Google Scholar]
- 10.Tucker KL, Sheppard JP, Stevens R, Bosworth HB, Bove A, Bray EP, Earle K, George J, Godwin M, Green BB, Hebert P, Hobbs FDR, Kantola I, Kerry SM, Leiva A, Magid DJ, Mant J, Margolis KL, McKinstry B, McLaughlin MA, Omboni S, Ogedegbe O, Parati G, Qamar N, Tabaei BP, Varis J, Verberk WJ, Wakefield BJ and McManus RJ. Self-monitoring of blood pressure in hypertension: A systematic review and individual patient data meta-analysis. PLoS medicine. 2017;14:e1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Margolis KL, Asche SE, Bergdall AR, Dehmer SP, Groen SE, Kadrmas HM, Kerby TJ, Klotzle KJ, Maciosek MV, Michels RD, O’Connor PJ, Pritchard RA, Sekenski JL, Sperl-Hillen JM and Trower NK. Effect of home blood pressure telemonitoring and pharmacist management on blood pressure control: a cluster randomized clinical trial. JAMA. 2013;310:46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Margolis KL, Dehmer SP, Sperl-Hillen J, O’Connor PJ, Asche SE, Bergdall AR, Green BB, Nyboer RA, Pawloski PA, Trower NK and Maciosek MV. Cardiovascular Events and Costs With Home Blood Pressure Telemonitoring and Pharmacist Management for Uncontrolled Hypertension. Hypertension. 2020;76:1097–1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Magid DJ, Olson KL, Billups SJ, Wagner NM, Lyons EE and Kroner BA. A pharmacist-led, American Heart Association Heart360 Web-enabled home blood pressure monitoring program. Cardiovasc Qual Outcomes. 2013;6:157–63. [DOI] [PubMed] [Google Scholar]
- 14.Bosworth HB, Powers BJ, Olsen MK, McCant F, Grubber J, Smith V, Gentry PW, Rose C, Van Houtven C, Wang V, Goldstein MK and Oddone EZ. Home blood pressure management and improved blood pressure control: results from a randomized controlled trial. Arch Intern Med. 2011;171:1173–80. [DOI] [PubMed] [Google Scholar]
- 15.McManus RJ, Mant J, Bray EP, Holder R, Jones MI, Greenfield S, Kaambwa B, Banting M, Bryan S, Little P, Williams B and Hobbs FD. Telemonitoring and self-management in the control of hypertension (TASMINH2): a randomised controlled trial. Lancet (London, England). 2010;376:163–72. [DOI] [PubMed] [Google Scholar]
- 16.Green BB, Cook AJ, Ralston JD, Fishman PA, Catz SL, Carlson J, Carrell D, Tyll L, Larson EB and Thompson RS. Effectiveness of home blood pressure monitoring, Web communication, and pharmacist care on hypertension control: a randomized controlled trial. JAMA. 2008;299:2857–2867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaffe MG, Lee GA, Young JD, Sidney S and Go AS. Improved blood pressure control associated with a large-scale hypertension program. JAMA. 2013;310:699–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shaw KM, Handler J, Wall HK and Kanter MH. Improving blood pressure control in a large multiethnic California population through changes in health care delivery, 2004–2012. Preventing chronic disease. 2014;11:E191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Feldman RD, Zou GY, Vandervoort MK, Wong CJ, Nelson SA and Feagan BG. A simplified approach to the treatment of uncomplicated hypertension: a cluster randomized, controlled trial. Hypertension. 2009;53:646–653. [DOI] [PubMed] [Google Scholar]
- 20.Margolis KL, Crain AL, Bergdall AR, Beran M, Anderson JP, Solberg LI, O’Connor PJ, Sperl-Hillen JM, Pawloski PA, Ziegenfuss JY, Rehrauer D, Norton C, Haugen P, Green BB, McKinney Z, Kodet A, Appana D, Sharma R, Trower NK, Williams R and Crabtree BF. Design of a pragmatic cluster-randomized trial comparing telehealth care and best practice clinic-based care for uncontrolled high blood pressure. Contemporary clinical trials. 2020;92:105939. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control and Prevention. Medication Therapy Management. Atlanta, GA. https://www.cdc.gov/dhdsp/pubs/guides/best-practices/pharmacist-mtm.htm. Accessed March 4, 2022. [Google Scholar]
- 22.Whelton PK, Carey RM, Aronow WS, Casey DE Jr., Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 23.American Medical Association in partnership with Johns Hopkins Medicine: STEPS in practice database. Measure, Act and Partner (M.A.P.) to help patients control blood pressure and ultimately prevent heart disease. https://www.stepsforward.org/modules/hypertension-blood-pressure-control. 2015.
- 24.American Medical Group Association Foundation. Measure Up Pressure Down. Alexandria, VA. http://www.measureuppressuredown.com/ Accessed July 18, 2019. [Google Scholar]
- 25.Centers for Disease Control and Prevention. Protocol for Controlling Hypertension in Adults. Atlanta, GA. https://millionhearts.hhs.gov/files/Hypertension-Protocol.pdf. 2013. Accessed April 15, 2022. [Google Scholar]
- 26.El Assaad MA, Topouchian JA, Darne BM and Asmar RG. Validation of the Omron HEM-907 device for blood pressure measurement. Blood Press Monit. 2002;7:237–241. [DOI] [PubMed] [Google Scholar]
- 27.Beran M, Asche SE, Bergdall AR, Crabtree B, Green BB, Groen SE, Klotzle KJ, Michels RD, Nyboer RA, O’Connor PJ, Pawloski PA, Rehrauer DJ, Sperl-Hillen JM, Trower NK and Margolis KL. Key components of success in a randomized trial of blood pressure telemonitoring with medication therapy management pharmacists. Journal of the American Pharmacists Association : JAPhA. 2018;58:614–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glasgow RE, Wagner EH, Schaefer J, Mahoney LD, Reid RJ and Greene SM. Development and validation of the Patient Assessment of Chronic Illness Care (PACIC). Med Care. 2005;43:436–444. [DOI] [PubMed] [Google Scholar]
- 29.Tran VT, Harrington M, Montori VM, Barnes C, Wicks P and Ravaud P. Adaptation and validation of the Treatment Burden Questionnaire (TBQ) in English using an internet platform. BMC medicine. 2014;12:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Proia KK, Thota AB, Njie GJ, Finnie RK, Hopkins DP, Mukhtar Q, Pronk NP, Zeigler D, Kottke TE, Rask KJ, Lackland DT, Brooks JF, Braun LT, Cooksey T and Community Preventive Services Task F. Team-based care and improved blood pressure control: a community guide systematic review. American journal of preventive medicine. 2014;47:86–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Santschi V, Chiolero A, Colosimo AL, Platt RW, Taffe P, Burnier M, Burnand B and Paradis G. Improving blood pressure control through pharmacist interventions: a meta-analysis of randomized controlled trials. Journal of the American Heart Association. 2014;3:e000718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Law M, Wald N and Morris J. Lowering blood pressure to prevent myocardial infarction and stroke: a new preventive strategy. Health Technol Assess. 2003;7:1–94. [DOI] [PubMed] [Google Scholar]
- 33.Lewington S, Clarke R, Qizilbash N, Peto R and Collins R. Age-specific relevance of usual blood pressure to vascular mortality: a meta-analysis of individual data for one million adults in 61 prospective studies. Lancet (London, England). 2002;360:1903–1913. [DOI] [PubMed] [Google Scholar]
- 34.Stamler R. Implications of the INTERSALT study. Hypertension. 1991;17:I16–20. [DOI] [PubMed] [Google Scholar]
- 35.Cook NR, Cohen J, Hebert PR, Taylor JO and Hennekens CH. Implications of small reductions in diastolic blood pressure for primary prevention. Arch Intern Med. 1995;155:701–709. [PubMed] [Google Scholar]
- 36.Sundstrom J, Arima H, Jackson R, Turnbull F, Rahimi K, Chalmers J, Woodward M and Neal B. Effects of blood pressure reduction in mild hypertension: a systematic review and meta-analysis. Annals of internal medicine. 2015;162:184–191. [DOI] [PubMed] [Google Scholar]
- 37.Hernan MA and Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9:48–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernan MA and Robins JM. Per-Protocol Analyses of Pragmatic Trials. The New England journal of medicine. 2017;377:1391–1398. [DOI] [PubMed] [Google Scholar]
- 39.Hernan MA, Hernandez-Diaz S and Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 40.Toh S and Hernan MA. Causal inference from longitudinal studies with baseline randomization. Int J Biostat. 2008;4:Article 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Robins JM, Hernan MA and Brumback B. Marginal structural models and causal inference in epidemiology. Epidemiology. 2000;11:550–560. [DOI] [PubMed] [Google Scholar]
- 42.Hernan MA, Brumback B and Robins JM. Marginal structural models to estimate the causal effect of zidovudine on the survival of HIV-positive men. Epidemiology. 2000;11:561–570. [DOI] [PubMed] [Google Scholar]
- 43.Cole SR and Hernan MA. Constructing inverse probability weights for marginal structural models. American journal of epidemiology. 2008;168:656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dember LM, Lacson E Jr., Brunelli SM, Hsu JY, Cheung AK, Daugirdas JT, Greene T, Kovesdy CP, Miskulin DC, Thadhani RI, Winkelmayer WC, Ellenberg SS, Cifelli D, Madigan R, Young A, Angeletti M, Wingard RL, Kahn C, Nissenson AR, Maddux FW, Abbott KC and Landis JR. The TiME Trial: A Fully Embedded, Cluster-Randomized, Pragmatic Trial of Hemodialysis Session Duration. Journal of the American Society of Nephrology : JASN. 2019;30:890–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Green BB, Vollmer WM, Keast E, Petrik AF and Coronado GD. Challenges in assessing population reach in a pragmatic trial. Prev Med Rep. 2019;15:100910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mitchell SL, Volandes AE, Gutman R, Gozalo PL, Ogarek JA, Loomer L, McCreedy EM, Zhai R and Mor V. Advance Care Planning Video Intervention Among Long-Stay Nursing Home Residents: A Pragmatic Cluster Randomized Clinical Trial. JAMA internal medicine. 2020;180:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McManus RJ, Mant J, Franssen M, Nickless A, Schwartz C, Hodgkinson J, Bradburn P, Farmer A, Grant S, Greenfield SM, Heneghan C, Jowett S, Martin U, Milner S, Monahan M, Mort S, Ogburn E, Perera-Salazar R, Shah SA, Yu LM, Tarassenko L, Hobbs FDR and investigators T. Efficacy of self-monitored blood pressure, with or without telemonitoring, for titration of antihypertensive medication (TASMINH4): an unmasked randomised controlled trial. Lancet (London, England). 2018;391:949–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Omboni S, McManus RJ, Bosworth HB, Chappell LC, Green BB, Kario K, Logan AG, Magid DJ, McKinstry B, Margolis KL, Parati G and Wakefield BJ. Evidence and Recommendations on the Use of Telemedicine for the Management of Arterial Hypertension: An International Expert Position Paper. Hypertension. 2020;76:1368–1383. [DOI] [PubMed] [Google Scholar]
- 49.Kerby TJ, Asche SE, Maciosek MV, O’Connor PJ, Sperl-Hillen JM and Margolis KL. Adherence to blood pressure telemonitoring in a cluster-randomized clinical trial. Journal of clinical hypertension (Greenwich, Conn). 2012;14:668–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Margolis KL, Crain AL, Green BB, O’Connor PJ, Solberg LI, Beran MS, Bergdall AR, Pawloski PA, Ziegenfuss JY, JaKa MM, Appana D, Sharma R, Kodet AJ, Trower NK, Rehrauer DJ, McKinney Z, Norton CK, Haugen P, Anderson JP, Crabtree BF, Norman SK and Sperl-Hillen JM. Comparison of explanatory and pragmatic design choices in a cluster-randomized hypertension trial: effects on enrollment, participant characteristics, and adherence. Trials 2022;23:673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Loudon K, Treweek S, Sullivan F, Donnan P, Thorpe KE and Zwarenstein M. The PRECIS-2 tool: designing trials that are fit for purpose. BMJ. 2015;350:h2147. [DOI] [PubMed] [Google Scholar]
- 52.Salam A, Atkins E, Sundstrom J, Hirakawa Y, Ettehad D, Emdin C, Neal B, Woodward M, Chalmers J, Berge E, Yusuf S, Rahimi K, Rodgers A and Blood Pressure Lowering Treatment Trialists C. Effects of blood pressure lowering on cardiovascular events, in the context of regression to the mean: a systematic review of randomized trials. Journal of hypertension. 2019;37:16–23. [DOI] [PubMed] [Google Scholar]
- 53.Asche SE, O’Connor PJ, Dehmer SP, Green BB, Bergdall AR, Maciosek MV, Nyboer RA, Pawloski PA, Sperl-Hillen JM, Trower NK and Margolis KL. Patient characteristics associated with greater blood pressure control in a randomized trial of home blood pressure telemonitoring and pharmacist management. J Am Soc Hypertens. 2016;10:873–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Margolis KL, Asche SE, Dehmer SP, Bergdall AR, Green BB, Sperl-Hillen JM, Nyboer RA, Pawloski PA, Maciosek MV, Trower NK and O’Connor PJ. Long-term outcomes of the effects of home blood pressure telemonitoring and pharmacist management on blood pressure among adults with uncontrolled hypertension: follow-up of a cluster randomized clinical trial. JAMA network open. 2018;1:e181617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Albritton J, Ortiz A, Wines R, Booth G, DiBello M, Brown S, Gartlehner G and Crotty K. Video Teleconferencing for Disease Prevention, Diagnosis, and Treatment: A Rapid Review. Ann Intern Med 2022;175(2):256–266. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.