Abstract
Background
A substantial proportion of patients with heart failure (HF) with preserved ejection fraction (HFpEF) present with normal natriuretic peptide (NP) levels. The pathophysiology and natural history for this phenotype remain unclear.
Methods and results
Consecutive subjects undergoing invasive cardiopulmonary exercise testing for unexplained dyspnoea at Mayo Clinic in 2006–18 were studied. Heart failure with preserved ejection fraction was defined as a pulmonary arterial wedge pressure (PAWP) ≥15 mmHg (rest) or ≥25 mmHg (exercise). Patients with HFpEF and normal NP [N-terminal of the pro-hormone B-type natriuretic peptide (NT-proBNP) < 125 ng/L] were compared with HFpEF with high NP (NT-proBNP ≥ 125 ng/L) and controls with normal haemodynamics. Patients with HFpEF and normal (n = 157) vs. high NP (n = 263) were younger, yet older than controls (n = 161), with an intermediate comorbidity profile. Normal NP HFpEF was associated with more left ventricular hypertrophy and worse diastolic function compared with controls, but better diastolic function, lower left atrial volumes, superior right ventricular function, and less mitral/tricuspid regurgitation compared with high NP HFpEF. Cardiac output (CO) reserve with exercise was preserved in normal NP HFpEF [101% predicted, interquartile range (IQR): 75–124%], but this was achieved only at the cost of higher left ventricular transmural pressure (LVTMP) (14 ± 6 mmHg vs. 7 ± 4 mmHg in controls, P < 0.001). In contrast, CO reserve was decreased in high NP HFpEF (85% predicted, IQR: 59–109%), with lower LVTMP (10 ± 8 mmHg) compared with normal NP HFpEF (P < 0.001), despite similar PAWP. Patients with high NP HFpEF displayed the highest event rates, but normal NP HFpEF still had 2.7-fold higher risk for mortality or HF readmissions compared with controls (hazard ratio: 2.74, 95% confidence interval: 1.02–7.32) after adjusting for age, sex, and body mass index.
Conclusion
Patients with HFpEF and normal NP display mild diastolic dysfunction and preserved CO reserve during exercise, despite marked elevation in filling pressures. While clinical outcomes are not as poor compared with patients with high NP, patients with normal NP HFpEF exhibit increased risk of death or HF readmissions compared with patients without HF, emphasizing the importance of this phenotype.
Keywords: Diastolic heart failure, Exercise tolerance, Mortality, Natriuretic peptides, Obesity
Structured Graphical Abstract
See the editorial comment for this article ‘BNP: Biomarker Not Perfect in heart failure with preserved ejection fraction’, by Sanjiv J. Shah, https://doi.org/10.1093/eurheartj/ehac121.
See the editorial comment for this article ‘BNP: Biomarker Not Perfect in heart failure with preserved ejection fraction’, by Sanjiv J. Shah, https://doi.org/10.1093/eurheartj/ehac121.
Introduction
Heart failure (HF) with preserved ejection fraction (HFpEF) afflicts over half of all patients with HF, and there are few effective treatments. Patients with HFpEF share common haemodynamic abnormalities, defined by an elevated pulmonary arterial wedge pressure (PAWP).1–4 While many patients with HFpEF display an elevation in PAWP that is present at rest, a substantial proportion of patients has evidence of circulatory congestion exclusively during exercise. Such patients frequently exhibit normal or near-normal plasma natriuretic peptide (NP) levels.
A recent consensus document has proposed a new universal definition of HF, which requires elevation in NP levels or objective evidence of pulmonary or systemic congestion to meet diagnostic criteria for HF.5 This definition conflicts with prior studies showing that a substantial proportion of patients with HFpEF display unequivocal haemodynamic evidence of HF when evaluated invasively, despite the presence of normal NP levels.1,2,6 Multiple studies have shown that patients with HFpEF and elevated NP levels have an increased risk of adverse events, indicating its role in risk stratification.6–8 Although the presence of normal NP levels in some patients with HFpEF is recognized in the new European HF guidelines, no study has yet compared event rates in patients with HFpEF and normal NP levels to patients without HFpEF.9
We hypothesized that patients with HFpEF and normal NP levels would display impairments in cardiac and vascular function, more adverse exercise haemodynamics, and poorer outcomes when compared with controls without HF, but that these abnormalities would be less pronounced when compared with patients with elevated NP levels. To test this hypothesis, we performed a detailed comparison of these groups using clinical, echocardiographic, and invasive haemodynamic exercise data in tandem with long-term clinical follow-up.
Methods
Study population and design
This prospective cohort study includes a contemporary population of consecutive patients with New York Heart Association (NYHA) functional Class II–III dyspnoea undergoing invasive haemodynamic assessment at rest and during exercise in the Mayo Clinic Rochester catheterization laboratory between February 2006 and March 2018. Heart failure with preserved ejection fraction was defined by signs and symptoms of HF (i.e. dyspnoea or fatigue) with a left ventricular ejection fraction ≥50% and elevated PAWP ≥ 15 mmHg at rest and/or ≥25 mmHg during exercise, according to current guidelines.1,4 Only subjects with measurement of N-terminal of the pro-hormone of B-type NP (NT-proBNP) levels at the time of evaluation were considered for this analysis. High NP HFpEF was defined as those with NT-proBNP levels ≥125 or ≥375 ng/L in atrial fibrillation (high NP group), and normal NP HFpEF was defined as those with NT-proBNP <125 or <375 ng/L in atrial fibrillation. A sensitivity analysis with slightly higher cut-offs that have been used in the literature (>220 and >660 ng/L) was also performed.4 Controls were defined as those with symptoms of NYHA Class II–III exertional dyspnoea but normal haemodynamics at rest and during exercise, including normal PAWP, a mean pulmonary arterial pressure (mPAP) ≤20 mmHg or pulmonary vascular resistance (PVR) <3 WU at rest, and an mPAP ≤ 30 mmHg or total pulmonary resistance <3 WU during exercise.10,11 The cause of dyspnoea in these patients was deemed related to deconditioning and/or psychogenic mechanisms.4,12
Patients with a history of reduced ejection fraction (<50%), unstable coronary disease, primary valvular disease, cardiac amyloidosis, hypertrophic cardiomyopathy, or constrictive pericarditis were excluded. The study complies with the Declaration of Helsinki and the Mayo Clinic Institutional Review Board has approved the study protocol. All study participants provided written informed consent. All authors had full access to the data, took responsibility for its integrity, contributed to the writing of the manuscript, and agreed to this report as written.
Transthoracic echocardiography measurements
Comprehensive transthoracic echocardiography was performed by experienced sonographers according to contemporary guidelines.13,14 Global right ventricular size and function were scaled semi-quantitatively as normal, borderline, mild, mild–moderate, moderate, moderate–severe, or severe enlargement and dysfunction, respectively. Mitral and tricuspid valve regurgitation severity was reported on a semi-quantitative scale as normal, trivial, mild, mild–moderate, moderate, moderate–severe or severe, after integration of a visual estimate by colour Doppler imaging with available quantitative measurements according to contemporary guidelines.15
Invasive cardiopulmonary exercise testing
Right heart and right radial artery catheterization were performed as previously described.2,16 Following baseline haemodynamic assessment, subjects performed a supine cycle exercise test with first stage at 20 W for 5 min, followed by 20 W increments in workload until subject-reported exhaustion (2 min stages). Cardiopulmonary pressures were measured at end-expiration by a single observer, taken as the average over three cardiac cycles or five cycles in atrial fibrillation. Arterial and mixed venous oxygen samples were obtained at baseline, 20 W, and peak exercise for calculation of cardiac output (CO) using the direct Fick method. Oxygen uptake (VO2) was measured from expired gas analysis (MedGraphics, St. Paul, MN, USA). Pulmonary arterial compliance was calculated as the ratio of stroke volume over pulmonary arterial pulse pressure. Left ventricular transmural pressure (LVTMP), which reflects left ventricular pre-load independent of right heart filling and pericardial restraint, was estimated as PAWP minus right atrial pressure.17 Effective arterial elastance (Ea) was calculated as 0.9 times arterial systolic blood pressure over stroke volume and total arterial compliance as stroke volume divided by arterial pulse pressure. The expected CO increase with exercise was based on its normal linear correlation with VO2 that requires a ∼6 mL/min rise in CO for every 1 mL/min increase in VO2 under normal circumstances.18 Cardiac output reserve was then taken as the observed increase in CO with exercise divided by expected.
Clinical outcome
Vital status was determined from the Mayo Clinic registration database and the Rochester Epidemiology Project death database, which ascertains mortality data from medical records, death certificates, obituaries, and notices of death in the local newspapers. Data on all Minnesota deaths are obtained from the State of Minnesota annually. Heart failure hospitalizations were determined from the Mayo Clinic electronic medical record and adjudicated by a single cardiologist (K.O.). Patient follow-up was initiated on the day of cardiac catheterization. Patients were censored at last follow-up contact or 10 December 2020, whichever came first.
Statistical analysis
Continuous variables are expressed as mean ± standard deviation if normally distributed, or otherwise as median (interquartile range, IQR). The one-way analysis of variance and Kruskal–Wallis H test were used as indicated for comparisons among groups, with individual groups compared with the Tukey HSD or Steel–Dwass test as indicated in case of a significant result. Categorical data are expressed as percentages and compared with Fisher’s exact test. Correlations between haemodynamics and NT-proBNP levels were assessed with Pearson’s correlation coefficient r, after log-transformation of NT-proBNP that was not normally distributed. The Kaplan–Meier method was used to construct survival curves, with the log-rank test used for comparison between groups and individual curves compared post hoc. A Cox-proportional hazards model was used to calculate the hazard ratio (HR) with corresponding 95% confidence interval (95% CI), with adjustments for age, sex, and body mass index. To account for potential differences in NT-proBNP levels due to comorbid conditions such as hypertension, diabetes, and atrial fibrillation, a sensitivity analysis matching subjects for these baseline variables in addition to their age was performed. Statistical significance was always set at a two-tailed probability level of <0.05. As the present study was focussed on mechanistic and outcome data rather than clinical trial results that may guide treatment decisions, no correction for multiple hypothesis testing was performed. All statistics were performed using JMP 14.1.0. (SAS Institute, Cary, NC, USA).
Results
Study population
During the study period, 880 subjects underwent invasive cardiopulmonary exercise testing at the Mayo Clinic catheterization lab. From this group, 581 fulfilled study criteria and comprised the study population (Supplementary material online, Figure S1). There were 161 control subjects and 420 patients with HFpEF, with NT-proBNP levels of 73 ng/L (30–144 ng/L) and 331 ng/L (96–977 ng/L), respectively (P < 0.0001). In the HFpEF group, 157 were classified with normal NP (60%), whereas 263 had high NP (40%). According to the continuous H2FPEF score, the pre-test probability for HFpEF before invasive cardiopulmonary exercise testing was 34% (16 – 48%) in controls, 80% (55–94%) in the normal NP group, and 93% (77–98%) in the high NP group.12 Alternatively, the HFA-PEFF score was 2 (1–3), 3 (2–3), and 5 (3–6) in the same groups, respectively (P < 0.001). Baseline characteristics of the study population are presented in Table 1. Compared with patients with HFpEF and high NP, those with normal NP were younger, more obese, with less atrial fibrillation, better renal function, higher haemoglobin levels, and less frequent use of diuretics and beta blockers. Compared with controls, they were older, with more comorbidities, and more frequent use of diuretics and beta blockers. A minority of control subjects (28/161 or 17%) had high NP levels. Their baseline characteristics have been provided in Supplementary material online, Table 1.
Table 1.
Baseline characteristics of the study population
| Control subjects without HFpEF (n = 161) | HFpEF with normal NP (n = 157) | HFpEF with high NP (n = 263) | P-value for HFpEF with normal NP vs. controls | P-value for HFpEF with normal vs. high NP | |
|---|---|---|---|---|---|
| Age (years) | 54 ± 13 | 63 ± 11 | 71 ± 10 | <0.001 | <0.001 |
| Men/women* (%) | 45/55 | 43/57 | 40/60 | N/A | N/A |
| Body mass index (kg/m2) | 27.7 ± 5.2 | 35.3 ± 7.2 | 32.1 ± 7.6 | <0.001 | <0.001 |
| Left ventricular ejection fraction (%) | 65 ± 5 | 65 ± 5 | 64 ± 6 | N/A | N/A |
| Comorbidities | |||||
| Hypertension | 63% | 92% | 95% | <0.001 | 0.232 |
| Diabetes | 13% | 27% | 28% | 0.002 | 0.823 |
| Obesity | 32% | 79% | 57% | <0.001 | <0.001 |
| Coronary artery disease | 18% | 30% | 35% | 0.013 | 0.288 |
| Paroxysmal AF | 5% | 20% | 16% | <0.001 | 0.727 |
| Persistent/permanent AF | 1% | 7% | 34% | <0.001 | <0.001 |
| COPD | 6% | 16% | 13% | 0.003 | 0.459 |
| Laboratory results | |||||
| NT-proBNP (ng/L)a | 73 (30–144) | 65 (37–109) | 790 (350–1506) | 0.863 | <0.001 |
| Haemoglobin (g/dL) | 13.6 ± 1.5 | 13.3 ± 1.4 | 12.8 ± 1.7 | 0.202 | 0.001 |
| eGFR (mL/min/1.73 m2) | 79 ± 20 | 72 ± 17 | 57 ± 19 | 0.002 | <0.001 |
| Medication use | |||||
| Renin–angiotensin system blocker | 26% | 45% | 48% | <0.001 | 0.462 |
| Beta blocker | 24% | 45% | 63% | <0.001 | <0.001 |
| Diuretic | 22% | 54% | 70% | <0.001 | <0.001 |
| Continuous H2FPEF score probability (%)a,b | 34 (16–48) | 80 (55–94) | 93 (77–98) | <0.001 | <0.001 |
| HFA-PEFF scorea,b | 2 (1–3) | 3 (2–3) | 5 (3–6) | <0.001 | <0.001 |
All three-group comparisons were first tested using ANOVA (or Kruskal–Wallis H test for non-parametric distributions); if the results of this test were not significant (indicated by an asterisk), no further between group testing was performed and individual group comparison P-values are indicated as N/A.
AF, atrial fibrillation; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate according to the Chronic Kidney Disease Epidemiology Collaboration formula; HFpEF, heart failure with preserved ejection fraction; NP, natriuretic peptide; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide.
Reported as median (interquartile range).
Cardiac morphology and function in heart failure with preserved ejection fraction with normal natriuretic peptide levels
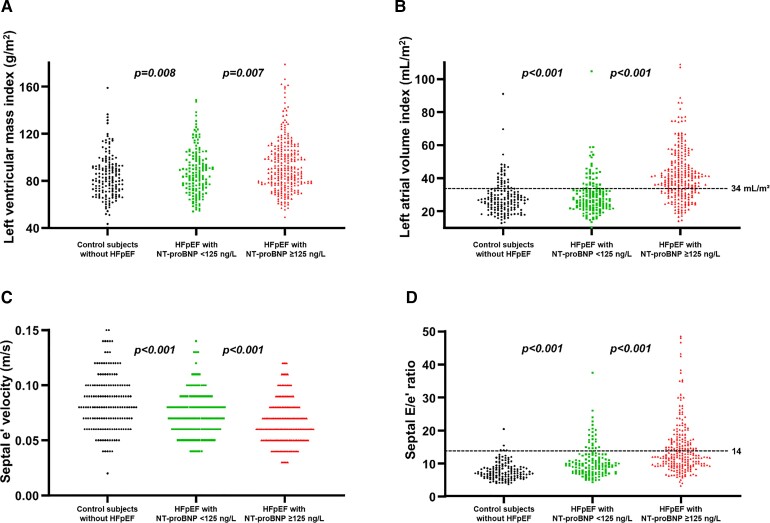
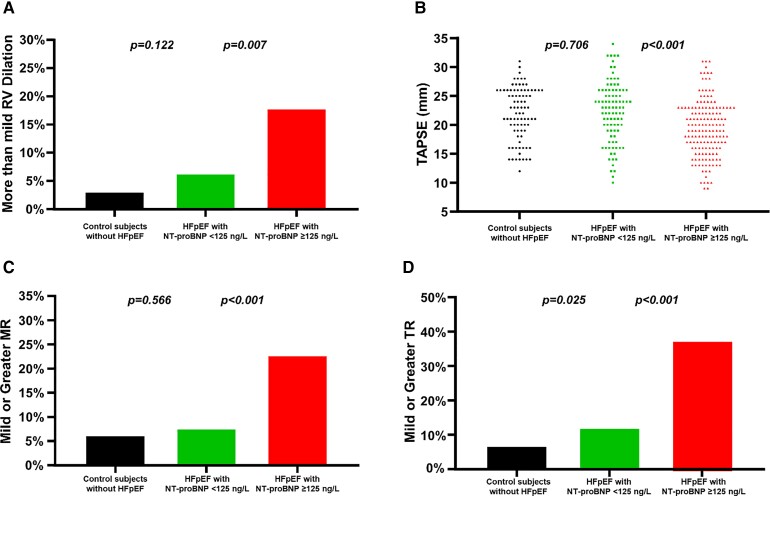
Patients with HFpEF and normal NP had significantly more pronounced left ventricular hypertrophy and worse diastolic function with higher left ventricular filling pressures when compared with controls (Table 2 and Figure 1). However, abnormalities were generally mild and often overlapping with what would be considered the normal range. Right heart function was preserved with similar low incidence of mitral and tricuspid valve regurgitation. In contrast, patients with HFpEF and high NP had a higher left ventricular mass index, more left atrial dilation, and elevated left ventricular filling pressures (Figure 1). In addition, they had more right ventricular remodelling and dysfunction and more significant secondary mitral and tricuspid valve regurgitation (Figure 2).
Table 2.
Cardiac structure and function
| Control subjects without HFpEF (n = 161) | HFpEF with normal NP (n = 157) | HFpEF with high NP (n = 263) | P-value for HFpEF with normal NP vs. controls | P-value for HFpEF with normal vs. high NP | |
|---|---|---|---|---|---|
| Dimensions | |||||
| Interventricular septal thickness (mm) | 9.7 ± 1.5 | 10.8 ± 1.8 | 10.9 ± 2.0 | <0.001 | 0.885 |
| Posterior wall thickness (mm) | 9.5 ± 1.4 | 10.3 ± 1.5 | 10.2 ± 1.6 | <0.001 | 0.988 |
| Left ventricular end-diastolic diameter (mm)* | 48 ± 5 | 49 ± 5 | 49 ± 5 | N/A | N/A |
| Left ventricular end-systolic diameter (mm)* | 31 ± 4 | 32 ± 4 | 32 ± 4 | N/A | N/A |
| Left atrial volume (mL) | 52 (42–67) | 59 (49–71) | 80 (64–100) | 0.003 | <0.001 |
| Left atrial volume index (mL/m2) | 27 (22–32) | 27 (22–33) | 40 (31–49) | 0.772 | <0.001 |
| Left ventricular hypertrophy indices | |||||
| Relative wall thickness | 0.40 ± 0.06 | 0.42 ± 0.07 | 0.42 ± 0.08 | 0.015 | 0.789 |
| Concentric remodellinga | 48 (30%) | 68 (43%) | 122 (46%) | 0.012 | 0.540 |
| Left ventricular mass (g) | 166 ± 47 | 195 ± 58 | 191 ± 56 | <0.001 | 0.774 |
| Left ventricular mass index (g/m2) | 84 ± 18 | 89 ± 22 | 94 ± 23 | 0.124 | 0.068 |
| Left ventricular hypertrophyb | 61 (38%) | 91 (58%) | 158 (61%) | <0.001 | 0.670 |
| Diastolic function | |||||
| E-wave velocity (m/s) (n = 579) | 0.7 (0.6–0.8) | 0.8 (0.6–0.9) | 0.9 (0.7–1.2) | 0.029 | <0.001 |
| A-wave velocity (m/s) (n = 579) | 0.6 (0.5–0.8) | 0.7 (0.6–0.9) | 0.6 (0–0.9) | 0.039 | <0.001 |
| E/A ratio (n = 486) | 1.17 (0.83–1.43) | 1.00 (0.75–1.24) | 1.11 (0.78–1.75) | 0.024 | 0.049 |
| Septal e′ velocity (cm/s) (n = 571) | 8.5 ± 2.5 | 7.4 ± 2.0 | 6.6 ± 2.0 | <0.001 | <0.001 |
| Septal E/e′ (n = 571) | 8.3 (6.7–10.0) | 10.0 (8.0–13.5) | 13.9 (10.0–19.5) | <0.001 | <0.001 |
| Lateral e′ velocity (cm/s) (n = 515) | 11.2 ± 3.4 | 9.0 ± 2.7 | 8.5 ± 2.7 | <0.001 | 0.347 |
| Lateral E/e′ (n = 515) | 6.0 (5.0–7.8) | 8.8 (6.9–11.3) | 10.9 (8.5–15.0) | <0.001 | <0.001 |
| Right ventricle | |||||
| Dilatation > mild | 5 (3.1%) | 10 (6.4%) | 47 (17.9%) | 0.170 | <0.001 |
| Dilatation > moderate | 1 (0.6%) | 1 (0.6%) | 13 (4.9%) | 0.986 | 0.017 |
| TAPSE (mm) (n = 334) | 22 ± 5 | 22 ± 5 | 19 ± 5 | 0.951 | <0.001 |
| Tricuspid annular s′ (cm/s) (n = 481) | 13.1 ± 2.6 | 13.2 ± 2.6 | 11.8 ± 3.0 | 0.984 | <0.001 |
| Mitral valve regurgitation | |||||
| Moderate or greater | 10 (6.2%) | 12 (7.6%) | 60 (22.8%) | 0.615 | <0.001 |
| More than moderate* | 1 (0.6%) | 0 (0%) | 6 (2.3%) | N/A | N/A |
| Tricuspid valve regurgitation | |||||
| Moderate or greater | 11 (6.8%) | 19 (12.1%) | 100 (38.0%) | 0.106 | <0.001 |
| More than moderate | 3 (1.9%) | 2 (1.3%) | 40 (15.2%) | 0.673 | <0.001 |
All three-group comparisons were first tested using ANOVA (or Kruskal–Wallis H test for non-parametric distributions); if the results of this test were not significant (indicated by an asterisk), no further between group testing was performed and individual group comparison P-values are indicated as N/A.
HFpEF, heart failure with preserved ejection fraction; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide; TAPSE, tricuspid annular plane systolic excursion.
Concentric remodelling defined as relative wall thickness >0.42.
Left ventricular hypertrophy defined as left ventricular mass index ≥115 g/m2 in men or ≥95 g/m2 in women.
Figure 1.
(A) Left ventricular mass index, (B) left atrial volume index, (C) septal e′ velocity, and (D) septal E/e′ ratio in control subjects, patients with heart failure with preserved ejection fraction and N-terminal of the pro-hormone B-type natriuretic peptide <125 ng/L vs. ≥125 ng/L in sinus rhythm or <375 ng/L vs. ≥375 ng/L in atrial fibrillation. E, transmitral early velocity on pulsed-wave Doppler; e′, septal mitral annular early velocity on pulsed-wave tissue Doppler; HFpEF, heart failure with preserved ejection fraction; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide.
Figure 2.
(A) Right ventricular dilation, (B) tricuspid annular plane systolic excursion, (C) mitral regurgitation, and (D) tricuspid regurgitation in control subjects, patients with heart failure with preserved ejection fraction and N-terminal of the pro-hormone B-type natriuretic peptide <125 ng/L vs. ≥125 ng/L in sinus rhythm or <375 ng/L vs. ≥375 ng/L in atrial fibrillation. HFpEF, heart failure with preserved ejection fraction; MR, mitral regurgitation; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide; RV, right ventricular; TAPSE, tricuspid annular plane systolic excursion; TR, tricuspid regurgitation.
Rest and exercise haemodynamics
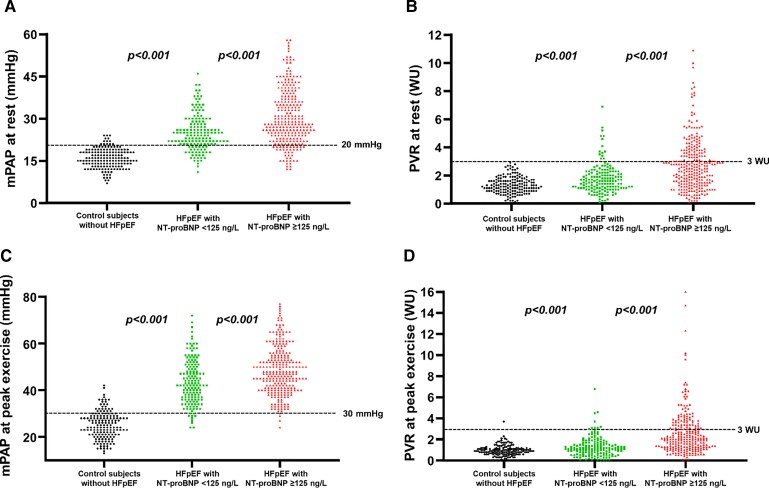
Patients with HFpEF and normal NP displayed significant haemodynamic abnormalities that were apparent at rest and became more pronounced during exercise (Table 3). In 98 of the normal NP HFpEF patients (62%), PAWP was elevated ≥15 mmHg at rest. Low CO <4 L/min with an indexed value <2.5 L/min/m2 was observed in 26 patients (17%). Compared with control subjects, patients with HFpEF and normal NP had a lower heart rate and higher blood pressure (Table 3). Despite higher cardiac filling pressures and a higher left ventricular pre-load reflected by the LVTMP, stroke volume index was reduced in the normal NP HFpEF group, whereas PVR was increased (Table 3). Findings of elevated cardiac filling pressures and lower stroke volume were more pronounced in patients with HFpEF and high NP as compared with normal NP HFpEF. Pulmonary vascular resistance was normal in the vast majority of normal NP HFpEF (91% with PVR < 3 WU) but was normal in only 65% of patients with HFpEF and elevated NP (Figure 3). Natriuretic peptide levels correlated with total pulmonary resistance (r = 0.56; P < 0.001), PVR (r = 0.46; P < 0.001), and mPAP (r = 0.52; P < 0.001), with less robust correlations with PAWP (r = 0.44; P < 0.001) and CO (r = −0.36; P < 0.001).
Table 3.
Invasive haemodynamic assessment at rest and during exercise
| Control subjects without HFpEF (n = 161) | HFpEF with normal NP (n = 157) | HFpEF with high NP (n = 263) | P-value for HFpEF with normal NP vs. controls | P-value for HFpEF with normal vs. high NP | |
|---|---|---|---|---|---|
| Rest | |||||
| Heart rate (b.p.m.) | 74 ± 13 | 71 ± 12 | 70 ± 12 | 0.083 | 0.782 |
| Systolic blood pressure (mmHg) | 135 ± 23 | 148 ± 23 | 146 ± 25 | <0.001 | 0.740 |
| Right atrial pressure (mmHg) | 5 ± 2 | 10 ± 4 | 11 ± 5 | <0.001 | <0.001 |
| Systolic pulmonary arterial pressure (mmHg) | 26 ± 6 | 37 ± 9 | 46 ± 16 | <0.001 | <0.001 |
| Mean pulmonary arterial pressure (mmHg) | 16 ± 3 | 25 ± 6 | 30 ± 10 | <0.001 | <0.001 |
| Pulmonary arterial wedge pressure (mmHg) | 9 ± 3 | 16 ± 5 | 18 ± 6 | <0.001 | <0.001 |
| Left ventricular transmural pressure (mmHg) | 4 ± 2 | 6 ± 4 | 7 ± 5 | <0.001 | 0.059 |
| Cardiac output (L/min) | 5.70 ± 1.65 | 5.60 ± 1.63 | 4.77 ± 1.52 | 0.851 | <0.001 |
| Cardiac index (L/min/m2) | 2.93 ± 0.80 | 2.57 ± 0.66 | 2.34 ± 0.65 | <0.001 | 0.003 |
| Stroke volume index (mL/m2) | 40.4 ± 10.4 | 36.8 ± 8.9 | 34.2 ± 10.5 | 0.004 | 0.036 |
| Systemic vascular resistance (dynes.s.cm–5) | 1318 ± 379 | 1412 ± 430 | 1590 ± 583 | 0.210 | 0.001 |
| Total arterial compliance (mL/mmHg) | 1.26 (0.97–1.60) | 1.16 (0.84–1.40) | 0.89 (0.67–1.22) | 0.022 | <0.001 |
| Effective arterial elastance (mmHg/mL) | 1.66 ± 0.56 | 1.77 ± 0.55 | 2.12 ± 0.87 | 0.333 | <0.001 |
| Pulmonary vascular resistance (WU) | 1.3 (0.9–1.7) | 1.6 (1.1–2.2) | 2.5 (1.5–3.7) | <0.001 | <0.001 |
| Pulmonary arterial compliance (mL/mmHg) | 5.18 ± 2.04 | 4.27 ± 1.83 | 3.02 ± 1.59 | <0.001 | <0.001 |
| Peak exercise | |||||
| Heart rate (b.p.m.) | 119 ± 23 | 106 ± 18 | 98 ± 22 | <0.001 | <0.001 |
| Systolic blood pressure (mmHg) | 170 ± 33 | 186 ± 28 | 173 ± 35 | <0.001 | 0.001 |
| Right atrial pressure (mmHg) | 7 ± 4 | 18 ± 7 | 22 ± 8 | <0.001 | <0.001 |
| Systolic pulmonary arterial pressure (mmHg) | 39 ± 9 | 62 ± 14 | 70 ± 17 | <0.001 | <0.001 |
| Mean pulmonary arterial pressure (mmHg) | 25 ± 6 | 44 ± 10 | 49 ± 11 | <0.001 | <0.001 |
| Pulmonary arterial wedge pressure (mmHg) | 15 ± 5 | 31 ± 6 | 32 ± 6 | <0.001 | 0.545 |
| Left ventricular transmural pressure (mmHg) | 7 ± 4 | 14 ± 6 | 10 ± 8 | <0.001 | <0.001 |
| Arterial oxygen saturation (%) | 97 (95–98) | 96 (94–97) | 95 (92–97) | <0.001 | 0.008 |
| Cardiac output (L/min) | 11.5 ± 3.29 | 10.5 ± 3.28 | 7.53 ± 2.57 | 0.011 | <0.001 |
| Cardiac index (L/min/m2) | 5.87 ± 1.59 | 4.81 ± 1.39 | 3.71 ± 1.19 | <0.001 | <0.001 |
| Stroke volume index (mL/m2) | 49.6 ± 12.7 | 45.4 ± 12.6 | 38.8 ± 13.0 | 0.014 | <0.001 |
| Systemic vascular resistance (dynes.s.cm–5) | 759 ± 263 | 882 ± 342 | 1024 ± 397 | 0.029 | <0.001 |
| Pulmonary vascular resistance (WU) | 0.9 (0.7–1.2) | 1.1 (0.6–1.7) | 2.1 (1.3–3.4) | 0.010 | <0.001 |
All three-group comparisons were first tested using ANOVA (or Kruskal–Wallis H test for non-parametric distributions); if the results of this test were not significant (indicated by an asterisk), no further between group testing was performed and individual group comparison P-values are indicated as N/A.
HFpEF, heart failure with preserved ejection fraction; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide.
Figure 3.
(A) Mean pulmonary arterial pressure at rest; (B) pulmonary vascular resistance at rest; (C) mean pulmonary arterial pressure at peak exercise; and (D) pulmonary vascular resistance at peak exercise in control subjects, patients with heart failure with preserved ejection fraction and N-terminal of the pro-hormone B-type natriuretic peptide <125 ng/L vs. ≥125 ng/L in sinus rhythm or <375 ng/L vs. ≥375 ng/L in atrial fibrillation. HFpEF, heart failure with preserved ejection fraction; mPAP, mean pulmonary arterial pressure; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide; PVR, pulmonary vascular resistance.
Cardiac output reserve was preserved in the normal NP HFpEF group based on published normative values (101% predicted, IQR: 75–124%), but this was significantly lower when compared with controls (112% predicted, IQR: 91–135; P = 0.02 vs. normal NP HFpEF). Notably, controls achieved this higher peak CO with a significantly lower pre-load, defined by LVTMP, as compared with patients with HFpEF and normal NP (Supplementary material online, Figure S2). In contrast, patients with HFpEF and high NP had more severely reduced CO reserve with exercise (85% predicted, IQR: 59–109%; P < 0.001 vs. normal NP group, P < 0.001 vs. controls). When adjusted for differences in beta blocker use, HFpEF with high vs. low NP still had a lower CO reserve (2.5 ± 2.0 L/min vs. 4.6 ± 3.0 L/min; P < 0.001). While PAWP increased to a similar extent as in the normal NP and high HFpEF groups, the increase in LVTMP was lower in high NP HFpEF. At peak effort compared with rest, LVTMP increased with 100% (25–200%) in controls, with 104% (43–263%) in patients with HFpEF and normal NP, and with 40% (−18%; 143%) in HFpEF with high NP (P = 0.188 for low NT-proBNP group vs. controls; P < 0.001 for low vs. high NT-proBNP group). N-terminal of the pro-hormone B-type natriuretic peptide levels correlated with lower exercise CO (r = −0.57; P < 0.001), higher PVR (r = 0.46; P < 0.001), and mPAP (r = 0.42; P < 0.001), and to a lesser extent, with higher PAWP (r = 0.33; P < 0.001).
Clinical outcome in heart failure with preserved ejection fraction with normal natriuretic peptide levels
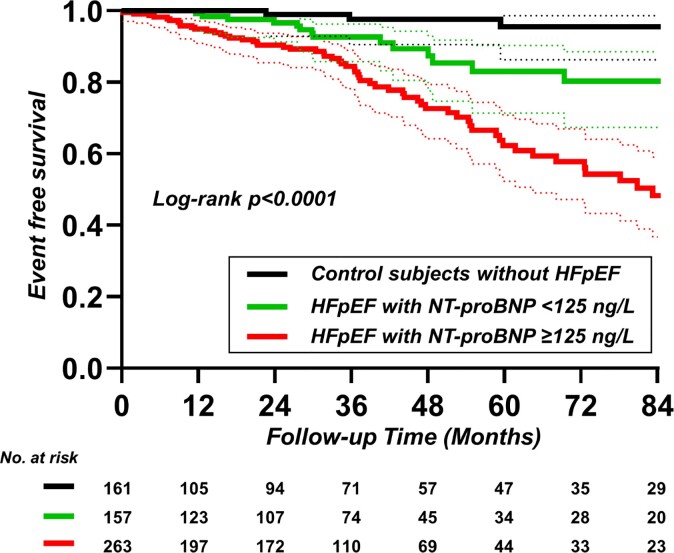
Over a median follow-up duration of 32 months (IQR: 8–54 months), there were 105 end point events, including 58 deaths (10%) and 47 HF hospitalizations (8%). As compared with patients with HFpEF and high NP, patients with HFpEF and normal NP displayed lower risk of death or HF hospitalization (HR: 0.38, 95% CI: 0.22–0.65; Figure 4 and Table 4). However, compared with controls, the risk for all-cause mortality or HF hospitalization was over three-fold higher in patients with HFpEF and normal NP (HR: 4.28, 95% CI: 1.29–8.33; Figure 4 and Table 4). In a Cox model adjusting for age, sex, and body mass index, patients with normal NP HFpEF displayed a lower risk for all-cause mortality or HF hospitalization compared with patients with high NP (HR: 0.41, 95% CI: 0.24–0.72; Table 4) and a higher risk compared with controls (HR: 2.74, 95% CI: 1.02–7.32; Table 4 and Supplementary material online, Tables S2 and S3).
Figure 4.
Freedom from all-cause mortality or heart failure readmission in control subjects; patients with heart failure with preserved ejection fraction and N-terminal of the pro-hormone B-type natriuretic peptide <125 ng/L vs. ≥125 ng/L. HFpEF, heart failure with preserved ejection fraction; NT-proBNP, N-terminal of the pro-hormone B-type natriuretic peptide.
Table 4.
Univariate and multivariate models for all-cause mortality or heart failure readmission
| Univariate model | Multivariate model | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Normal vs. high NP HFpEF | 0.38 (0.22–0.65) | <0.001 | 0.41 (0.24–0.72) | 0.002 |
| Normal NP HFpEF vs. control subjects | 4.28 (1.29–8.33) | 0.013 | 2.74 (1.02–7.32) | 0.045 |
| Age, per 1 year | 1.05 (1.03–1.07) | <0.001 | 1.02 (0.998–1.05) | 0.089 |
| Female gender | 0.66 (0.43–1.01) | 0.059 | 0.58 (0.37–0.89) | 0.014 |
| Body mass index, per 1 kg/m2 | 1.02 (0.99–1.04) | 0.225 | 1.01 (0.98–1.04) | 0.552 |
CI, confidence interval; HFpEF, heart failure with preserved ejection fraction; HR, hazard ratio; NP, natriuretic peptides.
Sensitivity analyses
In a sensitivity analysis, after matching the study groups for age, hypertension, diabetes, and atrial fibrillation, results were very much aligned with the overall population (Supplementary material online, Tables S4–S6 and Figure S3). In a sensitivity analysis using higher cut-offs of >220 and >660 ng/L for high NP in sinus rhythm and atrial fibrillation, respectively, results were virtually the same (Supplementary material online, Tables S7–S9 and Figures S4–S7).
Discussion
The present study provides detailed cardiac and haemodynamic phenotyping along with natural history data for the poorly described population of patients with HFpEF and normal NP levels. Patients with normal NP HFpEF were compared with controls with dyspnoea due to non-cardiac causes and HFpEF with elevated NP. The major findings are as follows: (i) a substantial proportion of patients with normal NT-proBNP referred for invasive haemodynamic exercise testing were found to display HFpEF according to the gold standard invasive criteria; (ii) compared with control patients with non-cardiac dyspnoea, patients with HFpEF and normal NP had a higher left ventricular mass and worse diastolic function, but right ventricular function was preserved; (iii) invasive haemodynamic assessment at rest showed that cardiac filling and pulmonary pressures in patients with HFpEF and normal NP were elevated to values intermediate between controls with non-cardiac dyspnoea and high NP HFpEF, whereas the high NP HFpEF cohort displayed the most profound cardiac functional and haemodynamic abnormalities, with notably greater prevalence of right ventricular remodelling and dysfunction, and more significant secondary mitral and tricuspid insufficiency; (iv) during exercise, patients with HFpEF and normal NP had less CO reserve compared with controls, at the cost of a greater increase in both LVTMP (i.e. left ventricular pre-load) and PAWP; (v) although the outcome of patients with HFpEF and normal NP was better than the outcome in high NP HFpEF, the risk for mortality or HF hospitalization was nearly 3 times greater in patients with normal NP HFpEF compared with individuals with non-cardiac dyspnoea. These findings provide new insight into the pathophysiology and clinical importance of the large population of patients with HFpEF and normal NP levels (Structured Graphical Abstract).
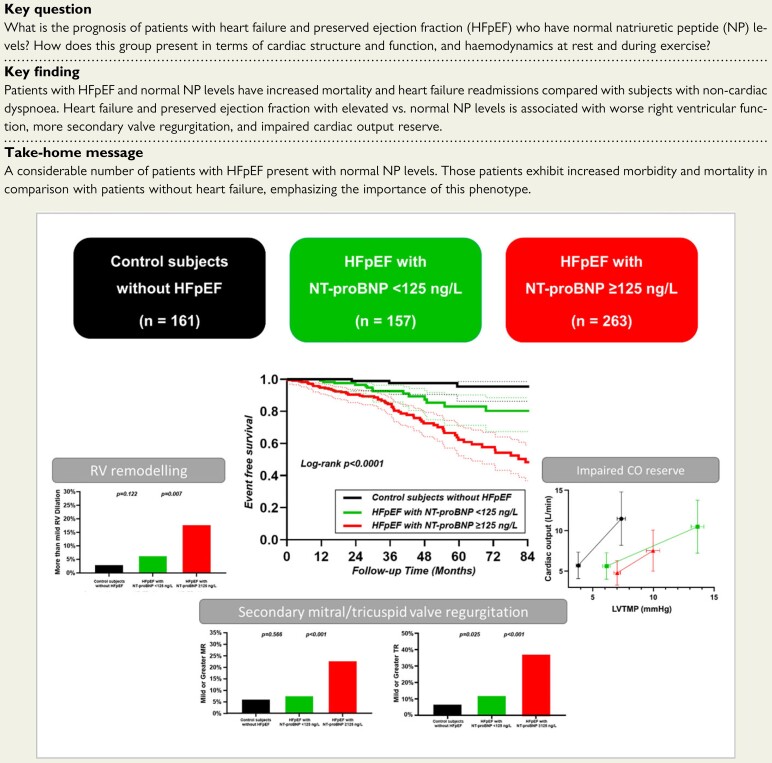
Structured Graphical Abstract.
As compared to control subjects without heart failure (black), patients with HFpEF and low NTproBNP levels (<125 ng/L, green) displayed increased risk for the combined endpoint of heart failure hospitalization or death, with greater reliance on an increase in left ventricular transmural pressure (LVTMP) to increase cardiac output during exercise. As compared to patients with HFpEF and lower NTproBNP, those with elevated NTproBNP (red) displayed the greatest risk for heart failure hospitalization or death, with more severely impaired cardiac output reserve, greater right ventricular (RV) remodeling, and higher prevalence of secondary (functional) mitral and tricuspid insufficiency.
Natriuretic peptide plays a central role in the guideline-recommended diagnostic work up in patients with suspected HFpEF.4,9,19 It has recently been argued that NP testing should be used as part of the universal definition of HF.20 Indeed, most randomized clinical trials in HFpEF use NP as part of the entry criteria with the goal to enrich for patients at higher cardiovascular risk and to ensure enrolment of patients with true HFpEF.21–24 The present study shows that patients with HFpEF and normal NP levels constitute a group with clear, unequivocal cardiac and vascular abnormalities that fulfil a priori definitions of cardiac failure, as shown in prior studies. To our knowledge, the present study shows for the first time that patients with HFpEF and normal NP display an increased risk of adverse outcome compared with controls with non-cardiac dyspnoea. This is important because these two patient groups are difficult to distinguish by NP levels or echocardiography, but the present data show the importance of correctly identifying patients with this specific phenotype who also require treatment, even as they are often excluded from clinical trials.2 Indeed, the present data raise serious questions with the practice of using NP levels as a necessary component to establish the diagnosis of HF, as sensitivity would be insufficient.
The present study confirms and extends upon a seminal study from Anjan et al. 6 that included resting cardiac and outcome assessments. In agreement with the earlier study, we found that patients with HFpEF and normal vs. high NP were younger, more obese, and less likely to have right ventricular dysfunction, atrial fibrillation, and kidney disease. Both the present and former studies have demonstrated increased risk for adverse events in patients with high NP as compared with normal NP HFpEF. However, the Anjan study did not include a control group without HFpEF, and the present study shows that in addition to previously demonstrated cardiac abnormalities present in these patients, there is also greater risk of death and HF hospitalization over nearly 3 years of follow-up.
While the severity of most abnormalities in cardiovascular structure and function existed on a continuum moving from control to normal to high NP HFpEF, the most striking differences between normal and high NP HFpEF were in the underlying severity of pulmonary vascular disease, right HF, and functional atrioventricular valve regurgitation. The cross-sectional nature of this study does not permit conclusions to be drawn regarding disease progression, but prior studies have shown that the latter findings are reflective of more advanced HFpEF.25 Patients with HFpEF and normal NP levels displayed reduced CO reserve with exercise as compared with controls, although deficits were not dramatic. Despite a relatively preserved CO reserve, these patients required marked elevations in PAWP during exercise, which was similar to that observed among patients with high NP HFpEF.
Together with prior studies, the present study suggests that in HFpEF, NP elevation is generally more reflective of abnormalities in biventricular (and atrial) function rather than pointing towards isolated left ventricular pathology. Notably, right heart function is one of the strongest prognosticators in HFpEF.16,26 Patients with HFpEF commonly develop right ventricular dilation and remodelling over time, resulting in progressive tricuspid valve regurgitation, development of systemic venous congestion, and subsequent organ failure.27,28 This progression is hastened by the development of atrial fibrillation, an indicator of left atrial myopathy and another comorbidity that was much more common in high NP HFpEF, even after employing NP-specific criteria to define the normal range in the present study. Combined left and right HF in HFpEF with high NP leads to greater accumulation of extravascular lung water during exertion.29 The increase in PAWP increases fluid transit into the pulmonary interstitium, while elevated central venous pressure from right HF impedes lymphatic clearance promoting lung oedema.28,29
Two post-hoc analyses from clinical trials have shown that patients with HFpEF and lower NP levels respond more favourably to mineralocorticoid receptor antagonists and angiotensin receptor blockers as compared with patients with higher NP patients.30,31 The results of the present study support the hypothesis that this lack of responsiveness in high NP HFpEF may be related to more advanced stages of HFpEF, wherein abnormalities in the right heart, left atrium, and pulmonary vasculature may require alternative treatment approaches and targets.
Study limitations
This study was performed at a tertiary centre in patients referred for invasive testing, introducing bias. Furthermore, individuals in the control group are more ill than asymptomatic healthy adults in that they presented with symptoms of dyspnoea related to deconditioning and/or psychogenic causes and were referred for invasive testing with clear risk factors for HFpEF such as hypertension and obesity. If anything, this would only be expected to bias our results toward the null, as a truly normal comparator group would be expected to have even better cardiac function, exercise reserve, and clinical outcomes. The fact that the control group also presented with similar symptoms of dyspnoea as normal NP HFpEF but hardly experienced any mortality or HF events also reinforces the clinical relevance of normal NP HFpEF. Importantly, in the absence of invasive testing, one cannot readily discern normal NP HFpEF from controls, so inclusion of this control group is scientifically necessary to achieve the study objectives. There are multiple cut-offs to define normal vs. high NP but we used values most commonly applied in the literature, including those incorporated into the current diagnostic guidelines for HFpEF, with results consistent when a more liberal cut-off was used (Supplementary material online, Tables S7–S9 and Figures S4–S7).4
Conclusions
Patients with HFpEF and normal NP levels display clear abnormalities in cardiac structure, function, and haemodynamic characteristic of HF, and importantly experience increased risk of death or HF hospitalization when compared with patients with non-cardiac dyspnoea. Further study is warranted to better understand the longitudinal course of cardiac changes in patients with HFpEF and future trials should consider use of normal and high NP to help guide tailoring of treatment to underlying pathophysiology.
Supplementary Material
Acknowledgements
The authors thank the staff of the Earl Wood Catheterization Laboratory and the patients who agreed to participate in research, allowing for this study to be completed.
Contributor Information
Frederik H Verbrugge, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA; Centre for Cardiovascular Diseases, University Hospital Brussels, Brussels, Belgium; Faculty of Medicine and Life Sciences, Biomedical Research Institute, Hasselt University, Hasselt, Belgium.
Kazunori Omote, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Yogesh N V Reddy, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Hidemi Sorimachi, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Masaru Obokata, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Barry A Borlaug, Department of Cardiovascular Diseases, Mayo Clinic, Rochester, MN, USA.
Funding
F.H.V. is supported by a Fellowship of the Belgian American Educational Foundation (B.A.E.F.) and by the Special Research Fund (BOF) of Hasselt University (BOF19PD04). B.A.B. is supported by R01 HL128526 from the National Institutes of Health.
Supplementary material
Supplementary material is available at European Heart Journal online.
Conflict of interest
none declared.
References
- 1. Borlaug BA, Nishimura RA, Sorajja P, Lam CSP, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circ Heart Fail 2010;3:588–595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obokata M, Kane GC, Reddy YNV, Olson TP, Melenovsky V, Borlaug BA. Role of diastolic stress testing in the evaluation for heart failure with preserved ejection fraction: a simultaneous invasive-echocardiographic study. Circulation 2017;135:825–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Reddy YNV, Olson TP, Obokata M, Melenovsky V, Borlaug BA. Hemodynamic correlates and diagnostic role of cardiopulmonary exercise testing in heart failure with preserved ejection fraction. JACC Heart Fail 2018;6:665–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Pieske B, Tschope C, de Boer RA, Fraser AG, Anker SD, Donal E, et al. How to diagnose heart failure with preserved ejection fraction: the HFA–PEFF diagnostic algorithm: a consensus recommendation from the Heart Failure Association (HFA) of the European Society of Cardiology (ESC). Eur Heart J 2019;40:3297–3317. [DOI] [PubMed] [Google Scholar]
- 5. Bozkurt B, Coats AJS, Tsutsui H, Abdelhamid CM, Adamopoulos S, Albert N, et al. Universal definition and classification of heart failure: a report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and writing committee of the universal definition of heart failure: endorsed by the Canadian Heart Failure Society, Heart Failure Association of India, Cardiac Society of Australia and New Zealand, and Chinese Heart Failure Association. Eur J Heart Fail 2021;23:352–380. [DOI] [PubMed] [Google Scholar]
- 6. Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol 2012;110:870–876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Bishu K, Deswal A, Chen HH, LeWinter MM, Lewis GD, Semigran MJ, et al. Biomarkers in acutely decompensated heart failure with preserved or reduced ejection fraction. Am Heart J 2012;164:763–770.e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. van Veldhuisen DJ, Linssen GCM, Jaarsma T, van Gilst WH, Hoes AW, Tijssen JGP, et al. B-type natriuretic peptide and prognosis in heart failure patients with preserved and reduced ejection fraction. J Am Coll Cardiol 2013;61:1498–1506. [DOI] [PubMed] [Google Scholar]
- 9. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J 2021;42:3599–3726. [DOI] [PubMed] [Google Scholar]
- 10. Kovacs G, Herve P, Barbera JA, Chaouat A, Chemla D, Condliffe R, et al. An official European Respiratory Society statement: pulmonary haemodynamics during exercise. Eur Respir J 2017;50:1700578. [DOI] [PubMed] [Google Scholar]
- 11. Simonneau G, Montani D, Celermajer DS, Denton CP, Gatzoulis MA, Krowka M, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J 2019;53:1801913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Reddy YNV, Carter RE, Obokata M, Redfield MM, Borlaug BA. A simple, evidence-based approach to help guide diagnosis of heart failure with preserved ejection fraction. Circulation 2018;138:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2015;28:1–39.e14. [DOI] [PubMed] [Google Scholar]
- 14. Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr 2016;29:277–314. [DOI] [PubMed] [Google Scholar]
- 15. Zoghbi WA, Adams D, Bonow RO, Enriquez-Sarano M, Foster E, Grayburn PA, et al. Recommendations for noninvasive evaluation of native valvular regurgitation: a report from the American Society of Echocardiography developed in collaboration with the Society for Cardiovascular Magnetic Resonance. J Am Soc Echocardiogr 2017;30:303–371. [DOI] [PubMed] [Google Scholar]
- 16. Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J 2016;37:3293–3302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Borlaug BA, Reddy YNV. The role of the pericardium in heart failure: implications for pathophysiology and treatment. JACC Heart Fail 2019;7:574–585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Abudiab MM, Redfield MM, Melenovsky V, Olson TP, Kass DA, Johnson BD, et al. Cardiac output response to exercise in relation to metabolic demand in heart failure with preserved ejection fraction. Eur J Heart Fail 2013;15:776–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA focused update of the 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines and the Heart Failure Society of America. Circulation 2017;136:e137–e161. [DOI] [PubMed] [Google Scholar]
- 20. Cleland JGF, Pfeffer MA, Clark AL, Januzzi JL, McMurray JJV, Mueller C, et al. The struggle towards a universal definition of heart failure—how to proceed? Eur Heart J 2021;42:2331–2343. [DOI] [PubMed] [Google Scholar]
- 21. Pitt B, Anker SD, Böhm M, Gheorghiade M, Køber L, Krum H, et al. Rationale and design of MinerAlocorticoid Receptor antagonist Tolerability Study-Heart Failure (ARTS-HF): a randomized study of finerenone vs. eplerenone in patients who have worsening chronic heart failure with diabetes and/or chronic kidney disease. Eur J Heart Fail 2015;17:224–232. [DOI] [PubMed] [Google Scholar]
- 22. Solomon SD, McMurray JJV, Anand IS, Ge J, Lam CSP, Maggioni AP, et al. Angiotensin–neprilysin inhibition in heart failure with preserved ejection fraction. N Engl J Med 2019;381:1609–1620. [DOI] [PubMed] [Google Scholar]
- 23. Armstrong PW, Lam CSP, Anstrom KJ, Ezekowitz J, Hernandez AF, O’Connor CM, et al. Effect of vericiguat vs placebo on quality of life in patients with heart failure and preserved ejection fraction: the VITALITY-HFpEF randomized clinical trial. JAMA 2020;324:1512–1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ibrahim NE, Burnett JC Jr, Butler J, Camacho A, Felker GM, Fiuzat M, et al. Natriuretic peptides as inclusion criteria in clinical trials: a JACC: heart failure position paper. JACC Heart Fail 2020;8:347–358. [DOI] [PubMed] [Google Scholar]
- 25. Obokata M, Reddy YNV, Melenovsky V, Pislaru S, Borlaug BA. Deterioration in right ventricular structure and function over time in patients with heart failure and preserved ejection fraction. Eur Heart J 2019;40:689–697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Melenovsky V, Hwang S-J, Lin G, Redfield MM, Borlaug BA. Right heart dysfunction in heart failure with preserved ejection fraction. Eur Heart J 2014;35:3452–3462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Verbrugge FH, Dupont M, Steels P, Grieten L, Malbrain M, Tang WHW, et al. Abdominal contributions to cardiorenal dysfunction in congestive heart failure. J Am Coll Cardiol 2013;62:485–495. [DOI] [PubMed] [Google Scholar]
- 28. Verbrugge FH, Guazzi M, Testani JM, Borlaug BA. Altered hemodynamics and end-organ damage in heart failure: impact on the lung and kidney. Circulation 2020;142:998–1012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reddy YNV, Obokata M, Wiley B, Koepp KE, Jorgenson CC, Egbe A, et al. The haemodynamic basis of lung congestion during exercise in heart failure with preserved ejection fraction. Eur Heart J 2019;40:3721–3730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Anand IS, Rector TS, Cleland JG, Kuskowski M, McKelvie RS, Persson H, et al. Prognostic value of baseline plasma amino-terminal pro-brain natriuretic peptide and its interactions with irbesartan treatment effects in patients with heart failure and preserved ejection fraction: findings from the I-PRESERVE trial. Circ Heart Fail 2011;4:569–577. [DOI] [PubMed] [Google Scholar]
- 31. Anand IS, Claggett B, Liu J, Shah AM, Rector TS, Shah SJ, et al. Interaction between spironolactone and natriuretic peptides in patients with heart failure and preserved ejection fraction: from the TOPCAT trial. JACC Heart Fail 2017;5:241–252. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.