Abstract
Osteoarthritis (OA) is the most prevalent joint disease characterized by degradation of articular cartilage, inflammation, and changes in periarticular and subchondral bone of joints. Osteoporosis (OP) is another systemic skeletal disease characterized by low bone mass and bone mineral density (BMD) accompanied by microarchitectural deterioration in bone tissue and increased bone fragility and fracture risk. Both OA and OP are mainly affected on the elderly people. Recent studies have shown that osteopontin (OPN) plays a vital role in bone metabolism and homeostasis. OPN involves these biological activities through participating in the proliferation, migration, differentiation, and adhesion of several bone-related cells, including chondrocytes, synoviocytes, osteoclasts, osteoblasts, and marrow mesenchymal stem cells (MSCs). OPN has been demonstrated to be closely related to the occurrence and development of many bone-related diseases, such as OA and OP. This review summarizes the role of OPN in regulating inflammation activity and bone metabolism in OA and OP. Furthermore, some drugs that targeted OPN to treat OA and OP are also summarized in the review. However, the complex mechanism of OPN in regulating OA and OP is not fully elucidated, which drives us to explore the depth effect of OPN on these two bone diseases.
Keywords: bone metabolism, inflammation, osteoarthritis, osteoporosis, osteopontin
Introduction
Osteoarthritis (OA), the most common aging-related joint pathology is a degenerative disease affecting all the structures of the joints. OA is mainly characterized by articular cartilage destruction along with changes occurring in other joint components including bone, menisci, synovium, ligaments, capsule, and muscles (1). Worldwide estimates that 9.6% of men and 18.0% of women aged over 60 years have symptomatic OA (2). Radiographic evidence of OA occurs in the majority of people by 65 years of age and in about 80% of those aged over 75 years (3).
Osteoporosis (OP) is defined as a systemic skeletal disease characterized by low bone mass and micro-architectural deterioration of bone tissue with a consequent increase in bone fragility and susceptibility to fracture (4). OP is a major risk factor for fractures of the hip, vertebrae, and distal forearm. It is thought of as a disease of old age which is present in 15% of those 50–59 years of age, but these figures increase quickly to 70% of those over 80 years of age (2). The relationship between OA and OP is complex and controversial. Kim et al. (5) conducted a meta-analysis and finally found the frequency of OP overall in men and women with OA was no different. However, according to the site of bone mineral density measurement, there was a higher prevalence of OP in the lumbar spine in both men and women compared to the matched controls. A cross-sectional study which was aimed to reveal the relationship between radiographic features of OA and bone mineral density (BMD) found that hand osteophytes and sclerosis exhibited a positive relationship with the BMDs of the lumbar spine and femoral neck while the knee and hand joint space narrowing presented a negative tendency to the BMD of the lumbar spine and femoral neck (6). Kasher et al. (7) confirmed that a higher hand OA score was significantly negatively correlated with arm and hand BMD measurements in males and females accompanied by a higher prevalence of wrist fracture, but the knee OA affection was positively associated with the elevated hip, spine, and total body BMD levels. A previous study also found the relationship between OA and OP was sexually different for the reason that femur neck and lumbar BMD and OA showed a positive tendency in women while BMD of the lumbar and pelvis in men was negatively correlated with OA (8). Hence, illuminating the relationship between OA and OP may uncover the relationship between OA and OP as well as the molecular or pathological factors that influence them that could help treat these diseases.
Some factors have been found to affect the progress of both OA and OP, including sex hormones, ethnicity, age, nutritional factors, genetic factors and physical activity. Aging is a predictor of radiographic OA, bone loss, development of OP, and fracture (2). Recently, a few studies found that the expression of OPN mRNA isolated from human OA cartilage was enhanced as compared with normal cartilage. OPN was shown to be upregulated in human OA chondrocytes (9). OPN in plasma, synovial fluid and articular cartilage is associated with progressive joint damage and is likely to be a useful biomarker for determining disease severity and progression in knee OA (10, 11). Additionally, high serum OPN level was also a significant risk factor causing menopausal OP and serum OPN levels could be used as a biomarker for the early diagnosis of OP in postmenopausal women (12). Thus, OPN may be involved in the molecular pathogenesis of OA and OP, it may play a role as a bridge between OA and OP. Herein, we summarize current understandings of the molecular mechanism of OPN in OA and OP, focusing on recent results that have examined the role of OPN between OA and OP.
The structure and function of OPN
OPN is a 44-75 KD multifunctional phosphoprotein secreted by many cell types such as osteoclasts, chondrocytes, synoviocytes, macrophages, lymphocytes, epithelial cells and vascular smooth muscle cells (SMC) and is present in the extracellular matrix of mineralized tissues and extra-cellular fluids, at sites of inflammation (13–15). It is encoded by the SPP1 gene and maps as a tandem array to the long arm of chromosome-4 (16), producing splicing variants of mRNA, full-length OPN and spliceosomal OPN (missing exons 4 or 5) (17, 18). The full-length OPN is cleaved by thrombin to form the thrombin-cleaved OPN (19). Both the native OPN and cleaved OPN could interact with integrin and play an important role in regulating inflammation, biomineralization, bone remodeling, immune functions, chemotaxis, and cell apoptosis (19, 20). Gene structure and chromosomal location identify OPN as a member of the small integrin-binding ligand N-linked glycoprotein (SIBLING) family. This protein also known as early T cell activation gene-1 (Eta-1) is abundant in bone, where it mediates important cell-matrix and cell-cell interactions (21). OPN expression is one of the important events involved in cartilage-to-bone transitions in fracture repair during the period of chondrocyte maturation (22, 23). OPN facilitates the attachment of osteoclasts to the bone matrix via interaction with cell surface αvβ3 integrin and CD44 (24, 25). OPN interacts with receptors such as integrin and CD44 to regulate physiological and pathological processes of proliferation, differentiation, inflammation, metabolism and tumor metastasis in chondrocytes, osteoblasts and osteoclasts (26–28). Previous studies have shown that OPN is involved in the occurrence, repair and maintenance of cartilage and subchondral bone metabolic homeostasis, suggesting that OPN is an important regulator of OA and OP and plays an important role in chondrocyte and osteocyte metabolism by regulating the extracellular matrix components of articular cartilage subchondral bone components (10, 29, 30).
OPN is widely distributed in many cells and tissues such as chondrocytes, plasma, synovial, osteoblasts and osteoclasts and plays regulatory roles in many diseases. Clinical studies have shown that OPN is involved in bone strength and remodeling, suggesting that serum OPN is positively correlated with the severity of OP and could be targeted as a biomarker for early diagnosis of postmenopausal osteoporosis (31, 32). Further, OPN is related to bone turnover and bone mineral density (BMD) and influences morphological formation and reconstruction (33–35). The high serum OPN level could result in a low BMD and OP in postmenopausal women (34, 35), moreover, the high level of OPN is associated with osteoporotic fractures in postmenopausal women, particularly at the lumbar spine (32). In addition, researchers have examined the relation between obesity and OP, and suggested obesity could lead to a large number of adipocytes and adipose tissue which may be greatly related to OP (36–38). Dai et al. (39) found that OPN secreted by the macrophages in epididymal white adipose tissue regulated the bone metabolism in high-fat diet (HFD)-induced obesity. The OPN selectively circulated to the bone marrow and promoted the degradation of the bone matrix by activating osteoclasts, both surgical removal of epididymal white adipose tissue and local injection of OPN-neutralizing antibodies or drugs aimed to deplete macrophages could ameliorate HFD-induced bone loss in mice. There is also evidence indicating that OPN is one of the most overexpression genes in the adipose tissue-derived from obese patients (40).
OPN is a critical intrinsic regulator that plays an important role in the pathogenesis and progress of OA. The plasma and synovial fluid level of OPN in OA patients are higher than in healthy adults, suggesting that OPN may be correlated with the severity of joint lesions in OA (11). Min et al. (41) investigated the serum of 249 people and found serum OPN was significantly increased in OA patients compared with control group, furthermore, they found there existed a gender difference in the concentration of OPN between the OA group and the control group. The gender difference in OPN expression was mainly presented that serum OPN level in the control group was lower in OA patients with the exact level of 2539.9 (pg/ml) and 5538.1 (pg/ml) in males, while the results of serum OPN level in the control group compared to OA patients were 1632.0 (pg/ml) and 4545.8 (pg/ml) in female. In addition, other researchers also found higher OPN mRNA and protein expression in synovial fluid of OA patients is closely related to the occurrence and development of OA (42), while OPN gene polymorphism could decrease the risk of OA (43). Recently, studies also show the expression of OPN is greatly increased in both the superficial zone and deep zone of articular cartilage of OA patients (9, 44). Animal experiments show that increased expression of OPN accelerates the turnover and remodeling of OA subchondral bone, promotes vessels formation in subchondral bone, mediates articular cartilage degeneration induced by subchondral bone metabolism, and accelerates the progression of OA (45). Some contradictory findings show OPN has a therapeutic value and mechanism in OA treatment. The mRNA and protein expression of OPN, CD44 is upregulated in OA chondrocytes, moreover, the upregulated OPN could delay chondrocyte degeneration and reduce cartilage matrix component loss by binding to CD44 and integrin via OPN/CD44/PI3K signaling pathway (46). CD44 is a cell surface protein that interacts with a variety of extracellular matrix (ECM) components whose principal ligands mainly include hyaluronan (HA) and OPN (47). The CD44 variants containing the exons v6 and v7 bind to the N- and C- terminal portions of OPN in an arginine-glycine-aspartic independent manner and further regulate the effect of OPN on bone metabolism (48). Zhang et al. (15) indicated that HA could upregulate OPN mRNA expression in OA fibroblast-like synoviocytes, and the high expression of OPN mRNA in OA may be a result of increased HA level of OA synovitis, finally, alleviating the severity and improving the symptoms of OA. OPN deficiency may enhance the senescence and apoptosis of OA chondrocytes, up-regulated the expression of pro-inflammation, and decrease the expression of COL2A1, finally accelerating the progress of chondrocytes and OA severity (27).
OPN involves inflammation in OA and OP
OPN is highly expressed in the process of inflammation. Many studies have characterized the function of OPN in inflammation activity, and it plays an important role in the pathogenesis of various inflammatory diseases such as OA. The previous study has shown that OPN is a proinflammatory cytokine that plays an important role in the pathogenesis of arthritis as summarized in Table 1 . There presents an elevated level of OPN in synovial fluid samples from RA patients, and the increased expression of OPN is correlated with the high level of multiple inflammatory cytokines including tumor necrosis factor-alpha (TNF-α) and interleukin-6(IL-6) (62). Another study also confirms the high protein expression of OPN in synovial fluid derived from advanced OA patients, significant differences and correlations are found among the thrombin-cleaved OPN, synovitis, and cartilage damage indicated by higher Kellgren-Lawrence scores (63). A long-term cohort study has shown the inflammatory mediators mainly including CRP, IL-6 accompanied by OPN significantly increased in arthroplasty patients the day after surgery and returned to and baseline six weeks later. It also found other inflammatory factors IL-1β and IL-8 showed the same up-regulated trend and reached the peak at 5 years post-surgery and returned to normal level at 10 years while the TNF-α level did not show any change preoperative or postoperative (53). The serum sample collected from total joint arthroplasty patients showed OPN and matrix metalloproteinase-9 (MMP-9) was greatly up-regulated, while MMP-9 was known to cleave OPN aimed to degrade Cola2a1 and upregulated by inflammatory markers such as IL-1, IL-6 and CRP (49). These biomarkers of inflammation were correlated with the progress of OA and total joint arthroplasty. The OPN level also significantly increased in synovial fluid samples from symptomatic primary knee osteoarthritis with ultrasound-confirmed joint effusion, moreover, the up-regulated OPN level presented associations between IL-8 and TNF which is responsible for pain, cartilage damage, clinical severity, and progression of OA (54). Wang, et al. (50, 51) also revealed that OPN could regulate the expression of various inflammatory factors including MMP-13, IL-6 and IL-8 which were significantly upregulated in OA tissues and associated with the pathogenesis of OA. Furthermore, many drugs have been devoted to investigating the relationship between OPN and inflammation factors in OA. Isorhamnetin could reduce knee swelling and alleviate cartilage damage in MIA-induced rats. The therapeutic effect was achieved mainly through inhibiting the expression of OPN and C-terminal telopeptide of type II collagen (CTX-II) accompanied by decreased levels of NO, PGE2, iNOS and COX- 2 (52). However, some controversial results found that OPN plays a protective role in OA. Tian et al. (27) compared the mRNA expression level of OPN from human OA chondrocytes and normal chondrocytes, and found the mRNA level of OPN was greatly suppressed in normal chondrocytes. OPN deficiency increased the expressions of Col10a1, IL-1β, TNF-ɑ, MMP-13, and ADAMTS-5 but decreased the expression of Col2a1, finally leading to higher rates of senescence and apoptosis of chondrocytes. Other researchers also found that OPN deficiency led to the induction of MMP-13 in instability-induced and aging-associated OA, which degrades a major component of the cartilage matrix protein type II collagen, indicating OPN plays a pivotal role in the progression of OA (55). Therefore, OPN might be a critical biomarker in the inflammatory activity of OA for either up-regulated OPN level or OPN deficiency could stimulate the secretion of inflammatory factors, damage articular cartilage, and aggravate the severity of OA.
Table 1 OPN involves inflammation activity in OA and OP.
| Reference | disease | Subjects | cytokines | Main results |
|---|---|---|---|---|
| Hannah Slovacek, et al. (49) | OA | Blood samples | MMP-9, ADAMTS-4 | OPN and ADAMTS-4 inversely fluctuated post-operatively of total joint arthroplasty upregulation of MMP-9 and OPN results in the downregulation of ADAMTS-4. |
| Jian Tian, et al. (27) | OA | Chondrocytes | IL-1β, TNF-ɑ, MMP-13, ADAMTS-5 | OPN deficiency increased the expressions of COL10A1, IL-1β, TNF-ɑ, MMP-13, and ADAMTS-5, but decreased the expression of COL2A1 in chondrocytes, finally accelerated OA progress. |
| Qiyuan Wang, et al. (50) | OA | Synoviocytes | MMP-13, IL-6, IL-8 | OPN upregulates expression of inflammatory factors MMP-13, IL-6 and IL-8 in OA tissues. Inhibiting OPN expression and synoviocyte proliferation is an efficient strategy for OA treatment. |
| Shenglong Li, et al. (51) | OA | Rat adipose mesenchymal stem cell | IL-1β | IL-1β treatment significantly inhibites the viability and migration of chondrocytes, enhances cell apoptosis andinduced cartilage damage by upregulating IL-1β, OPN, p53 and decreasing the expression of COL1A1, COL2A1, OCN, RUNX2. |
| Tsai Sen-Wei, et al. (52) | OA | Sprague-Dawley rats | NO, PGE2, iNOS, COX-2 | OPN level was inhibited by isorhamnetin accompanied with the down-regulated level of NO, PGE2, iNOS and COX-2 in MIA-induced OA rats. |
| Jean Cassuto, et al. (53) | OA | Blood samples | CRP, IL-6 | Inflammatory mediators CRP, IL-6 increased significantly on day one after surgery vs preoperative value and returned to baseline at 6 week in arthroplasty patients. |
| María García-Manrique, et al. (54) | OA | Blood samples and synovial fluid | IL-8, TNF | IL-8 in synovial fluid is related to clinical severity and progression in knee osteoarthritis. |
| Yuichiro Matsui, et al. (55) | OA | C57BL/6 mice | MMP-13 | OPN deficiency leads to the induction of MMP-1, which degrades a major component of the cartilage matrix protein type II collagen in mice. |
| Bao-Ming Tang, et al. (56) | OP | Sprague-Dawley rats | IL-1β, TNF-α, IL-6 | Inflammation mediated osteoporosis presented apparently down-regulated in OPN and OCN levels, higher serum IL-1β, TNF-α, IL-6 levels in ovariectomized rats. |
| Krzysztof Marycz, et al. (57) | OP | MC3T3-E1 cell lines | iNOS, TNF-α, Il-1β | NHAp/IO@miR-21/124 could enhance osteogenesis through increasing osteogenic markers Runx-2, OPN, Coll-1 level, inhibiting the expression of inflammatory markers TNF-α, iNOs or IL-1β in LPS-stimulated cells. |
| Xiang Gao, et al. (58) | OP | Lewis rats | TNF-α, IL-6 | Salvianolate treatment could increase Osterix, OPN, Runx2 level and decrease TNF-α, IL-6 level in serum and IL-1β protein in TNF-α-induced MC3T3-E1 osteoblasts, finally ameliorate osteopenia and improved bone quality. |
| Wei-Ming Li, et al. (59) | OP | C57BJ/6 L mice | TNF-α, IL-1β, IL-6, CCL-2 | Silenceing angiopoietin-like protein2 (ANGPTL2) reduces nuclear factor of activated T cell c1/4 (NFATC1/4) expressions in macrophage colony-stimulating factor -treated cells, decreases Runx2, OPN and Colla1 level and accompanied with down-regulating level pro-inflammatory cytokines such as TNF-α, IL-1β, IL-6 and CCL-2. |
| Chen Lei, et al. (60) | OP | C57B/L6 mice and osteoclasts | TNF-α, IL-1β, IL-6, and MMP-1 | MLN64-knockdown alleviates the severity of osteoporosis, down-regulates specific genes related to osteoclastogenesis including Runx2 and inflammatory factors such as TNF-α, IL-1β, IL-6, and MMP-1. |
| Yu-Qiong He, et al. (61) | OP | C57/BL6 mice and MC3T3-E1 osteoblast-like cells | IL-6, IL-1β and NO | Monotropein increases the proliferation and activity of ALP, bone matrix mineralization and OPN in osteoblastic MC3T3-E1 cells, decreases the production of IL-6, IL-1β and NO. |
Osteoporosis is a common disease in the aging population and multiple studies have shown that inflammation cytokines greatly influence the pathogenesis of OP (64, 65). Researchers have shown that many pro-inflammatory mediators including cytokines such as TNF-α, IL-1, IL-6, and IL-10 up-regulated in OP and participate in the process of bone resorption or bone mineral density (65–67). As a secreted protein, OPN is greatly related to the inflammatory response in OP, which further influences the process of bone remodeling as the Table 1 shown. The Animal experiment has shown that OPN and osteocalcin (OCN) level declined in inflammation-mediated osteoporosis (IMO) of ovariectomized rats. The IMO rats represented higher serum tartrate-resistant acid phosphatase (TRAP), CTX-I, and pro-inflammatory factors TNF-α, IL-1β, and IL-6 levels, accompanied by decreased femur BMD, bone mineral content (BMC) and distal femur cancellous bone in IMO rats (56). Gao, et al. (58) demonstrated rheumatoid arthritis induced bone loss and bone quality deterioration, with high bone turnover in collagen-induced arthritis (CIA) rats. The CIA rats showed higher levels of IL-6, and TNF-α in serum accompanied by decreased mRNA and protein levels of Osx (Osterix), OPN which is induced by a TNF-α-induced inflammatory medium in vitro. Osteoclasts are multinucleated cells essential for bone resorption and play a central role in the development of OP. The osteoclasts intervened by knock-down angiopoietin-like protein2 could promote expressions of pro-inflammatory cytokines including TNF-α, IL-1β, IL-6, and CCL-2, reduce Runx2, OPN, and Colla1 levels, finally improve bone loss and BMD in osteoporosis mice induced by ovariectomy (59). Some studies found many drugs could regulate the OPN level and inflammation response to alleviate the severity of OP. Monotropein significantly inhibited bone mass reduction and improved bone micro-architectures by enhancing bone formation in osteoporotic mice by suppressing the secretion of IL-6 and IL-1β induced by LPS. The experiment in vitro also increased the expression and activity of alkaline phosphatase (ALP) and OPN in osteoblasts (61). Other researchers also confirmed that increasing the expression level of OPN and Runx2 could promote bone remodeling and reduce bone loss in OP accompanied by inhibiting the expression of inflammatory markers TNF-α, iNOS, or IL-1β in LPS-stimulated osteoblastic cells (57, 60).
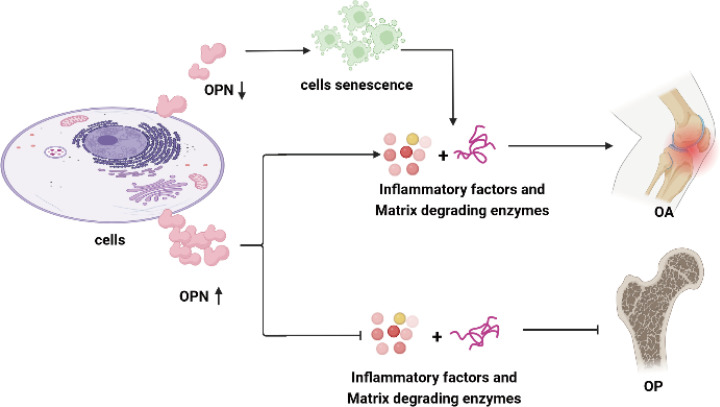
Taking together, the above results and studies mainly investigated the role of OPN and its regulating effect on inflammation activity in OA and OP, and further indicated overexpression of OPN or declined OPN levels in chondrocytes, synoviocytes, and osteoblasts could lead to metabolic disturbance in bone diseases as shown in Figure 1 .
Figure 1.
OPN regulates inflammatory activity in OA and OP. OPN plays a controversial role in the inflammation response in OA. Overexpression of OPN in chondrocytes, synoviocytes and subchondral bone stimulates the secretion of proinflammation cytokines and enhances the inflammation activity in OA. OPN-deficiency in chondrocytes leads to chondrocyte senescence and the secretion of cytokines and proteins degraded the articular cartilage. A high level of OPN suppresses the secretion of proinflammation cytokines and matrix-degrading enzymes, alleviates bone mass reduction, and promotes bone formation as well as matrix calcification in OP. The proinflammation cytokines include IL-1β, TNF-ɑ, IL-6, IL-8, NO, iNOS, COX-2, and CRP, while the articular cartilage matrix-degrading enzymes mainly refer to MMP-9, MMP-13, ADAMTS-4, ADAMTS-5 in this review.
OPN involves in cartilage and bone metabolism in OA and OP
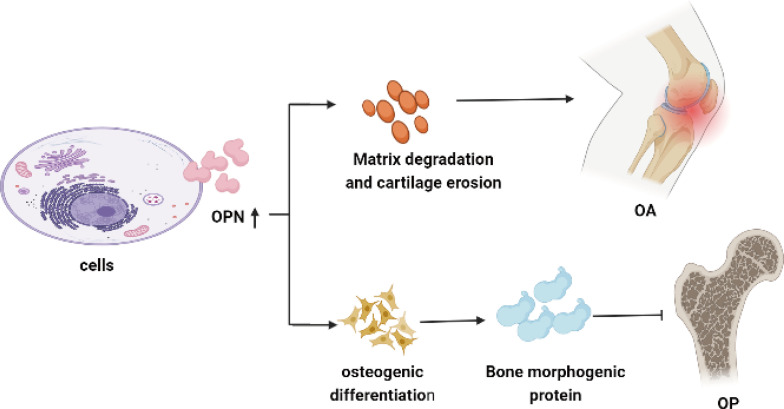
OPN is secreted by many types of cells, such as macrophages, lymphocytes, epithelial cells, vascular smooth muscle cells, chondrocytes, and synovial cells, which exist in a large number of cells and tissues (68–70). It is well known that abnormal expression of OPN in mRNA or protein level is correlated to the onset of OA. Up-regulated OPN and the phosphorylation of OPN in chondrocytes and synovial fluid greatly contribute to and aggravate the severity of OA (10, 19, 68). A previous study shows overexpression of OPN could promote the proliferation of chondrocytes, but suppressed their apoptosis through the NF-κB signaling pathway to regulate the pathological process of OA (70). The elevated OPN serum level is coincident with the tartrate-resistant acid phosphatase (TRAP)-positive osteoclasts and the extent of bone erosion in CIA mice, silencing of OPN using lentiviral-OPN short hairpin RNA presents an opposite result (71). Elmazoglu, et al. (72) found OPN and bone morphogenetic protein-7(BMP-7) were inhibited by S-allylcysteine accompanied by the suppressed inflammation response and decreased IL-1β, IL-6 in chondrocytes which finally alleviate the severity of OA. OPN also mediates subchondral bone remodeling and cartilage degeneration in OA. Animal study shows that higher level of OPN secreted by pre-osteoblasts and osteoblasts in subchondral bone could promote osteoclastogenesis and blood vessels formation in subchondral bone, accelerate the turnover and remodeling of subchondral bone and mediate articular cartilage degeneration induced by subchondral bone metabolism in anterior cruciate ligament transection and destabilization of the medial meniscus (ACLT + DMM) OA model (45). Furthermore, OPN is also on behalf of the osteogenic and chondrogenic differentiation progress, abnormal expression of OPN along with Runx2, Sox9, and OCN may induce metabolic disturbance and cartilage damage in OA (51, 73). Therefore, OPN plays a critical role in regulating the metabolism in chondrocytes, synovial cells, and subchondral bone which greatly influence the physiological process of joint, it should be precise modulated at a proper level for the prevention of onset and aggravation of OA as summarized in Table 2 and shown Figure 2 .
Table 2.
OPN involves bone metabolism in OA and OP.
| Reference | disease | Subjects | Test items | Main results |
|---|---|---|---|---|
| Elmazoglu, et al. (72) | OA | Human OA chondrocyte | BMP | S-allylcysteine exhibits anti-osteoarthritic properties through suppressing the expression of IL-1β, IL-6, OPN and BMP in chondrocytes. |
| Qian, et al. (71) | OA | Serum and synovial tissue | BMD | Increased serum OPN level in OA and RA is related to the activity of osteoclasts and bone erosion in CIA mice. |
| Lin, et al. (45) | OA | Subchondral bone and osteoclasts | OCN, Runx2, ALP | OPN promotes osteoclastogenesis in the subchondral bone, regulates OA subchondral bone metabolism and accelerates subchondral bone remodeling. |
| Cassuto, et al. (53) | OA | OA patients blood samples | BALP, OPG, sclerostin | Total hip arthroplasties patients present the second peak of inflammation five years post-surgery which may stimulate bone remodeling and new synthetic coupling, characterized by increasing bone anabolic transition markers BALP and RANKL/OPG. |
| Kulsirirat, et al. (73) | OA | Mesenchymal stem cells | Runx2, Sox9, OPN | Andrographolide enhance osteogenesis and chondrogenesis by increasing the expression of osteogenic and chondrogenic differentiation, including Runx2, OPN, Sox9. |
| Li, et al. (51) | OA | Adipose mesenchymal stem cell | OCN, Runx2 | IL-1β treatment inhibits the viability and migration of chondrocytes, enhances cell apoptosis, increase OPN level, decrease the mRNA and protein level of Col1a1, Col2a1, OCN, Runx2. Extracellular vesicles-chitosan oligosaccharide conjugates could reverse the effect of IL-1β on cartilage damage. |
| Tang, et al. (74) | OP | C57/B6L mice and chondrocytes | Sox9, CyclinD1, PTH1R, Osx, Runx2, ATF4, OCN, OPN | Runx1f/fCol2α1-cre mice exhibits impaired cartilage formation, decreases bone density, accompanied with decreased expression of osteoblast differentiation genes including Osx, Runx2, ATF4 and osteoblast marker genes including OCN and OPN in chondrocytes. |
| Xiao, et al. (75) | OP | C57BL/6 J mice | Vascular endothelial growth factor (VEGF) | Ovariectomy mice presents osteoporosis of the vertebra and osteochondral remodeling of the endplate with increased endplate porosity and decreased disc volume. |
| Li, et al. (76) | OP | Human osteoblast-like cells and mice | Collagen I, OCN, OPN | Aucubin increase cortical bone thickness, bone density and tighter trabecular bone in Dex-induced osteoporotic mouse model, up-regulates the expression of collagen I, OCN, OPN in cells. |
| Gu, et al. (77) | OP | Wistar rats | BMD, BMP2, Runx2, Collagen I, ALP | Anti-Osteoporosis Decoction (AOD) and Yougui Pill treatment effectively inhibits osteoporosis and reduces the broken trabecular bones, increases the level of BMD, ALP levels, accompanied by the increased expressions of BMP2, Runx2, Collagen I and OPN. |
| Zeng, et al. (78) | OP | Human bone marrow mesenchymal stem cells | Runx2, OCN, and OPN | Artesunate promotes osteoblasts differentiation related proteins Runx2, OCN and OPN expression through activating the Wnt signaling pathway in hBMSCs. |
| Dong, et al. (79) | OP | Mouse preosteoblast cell lines (MC3T3-E1) | ALP, OCN, OPN, and BMP2 | Anagliptin significantly increased matrix deposition and mineralization by osteoblasts, as evidenced by elevated levels of ALP, OCN, OPN, and BMP2. |
| Lei, et al. (60) | OP | C57B/L6 mice and osteoclasts | Runx2, OPN, TRAP | MLN64 was over-expressed during the process of osteoclast differentiation, up-regulation Runx2 and OPN. The in vivo study suggested that MLN64 deletion reversed streptozotocin-induced trabecular deleterious effects and stimulated bone remodeling. |
| Yin, et al. (80) | OP | Zebrafish | ALP, hydroxyproline, and TRAP | Evodiamine (EV) can alleviate dexamethasone-induced osteoporosis through increasing the area of bone formation, the content of hydroxyproline and the expression of ALP and TRAP in zebrafish. |
| Fan, et al. (81) | OP | Sprague-Dawley rats | BMD, ALP, OCN, BMP2, Runx2, TRAP | Myricetin could increase body weight gain and inhibit Dex-induced reduction in BMD, enhanced ALP activity, upregulated OCN, BMP2 and Runx2 levels accompanied with reduced TRAP activity and CTX level. |
| Wu, et al. (82) | OP | Human plasma samples | B lymphocyte chemoattractant (BLC) and BMP | Inflammatory cytokines were closely related with OP, BLC, OPN and IGFBP4 are greatly related to the pathogenesis of OP. |
| Lee, et al. (83) | OP | Female ICR mice | collagen type I, BMP2 and OPN | Osteo-F ameliorated bone loss by increasing bone forming molecules including BMP-2 and OPN in osteoporosis. |
| Marycz, et al. (84) | OP | MC3T3-E1cell lines | OPN, OCN and ALP | PRHD@MnFe2O4 protect osteoporosis through enhancing the expression of bone remodeling markers including OPN, osteocalcin (OCL) and ALP in pre-osteoblasts. |
| Shao, et al. (85) | OP | MC3T3-E1 cells | ALP, osteocalcin, OPN and BMP-2 | Trelagliptin increased the activity of ALP and promoted osteoblastic calcium deposition. Additionally, Trelagliptin upregulated ALP, osteocalcin, OPN, and BMP2 and Runx2 in MC3T3-E1 cells. |
Figure 2.
OPN involves in cartilage and bone metabolism in OA and OP. Overexpression of OPN enhances the proliferation of TRAP-positive osteoclasts and secretion of matrix metalloproteinase, leading to bone erosion and cartilage degeneration in OA. High levels of OPN could increase matrix deposition and enhance bone remodeling by promoting osteoblasts differentiation along with the elevated level of bone morphogenetic proteins, resulting in reducing the pathological changes of osteoporosis.
Osteoporosis is a systemic skeletal disorder characterized by systemic damage to bone mass and microstructure, which is caused by bone metabolism disorders and is the main reason for fragility fractures in aged people (30, 86, 87). Notably, OPN plays an important role in bone strength and bone remodeling. The role of OPN involved in cartilage and bone metabolism of OP has been greatly investigated in many previous studies and the effect of OPN levels on osteoporosis is gaining more and more attention, especially those OPN exits in serum, chondrocytes, osteoclasts, and osteoblasts.
A large number of previous studies through animal experiments have shown that OPN has a protective effect on osteoporosis and OPN-deficient mice by oophorectomy are resistant to osteoporosis (88–90). Chondrocyte-specific genes knockout mice also showed impaired cartilage formation, decreased bone density and an osteoporotic phenotype with the decreased osteoblast marker genes including OPN and OCN in osteoblasts, as well as the expression of osteoblast differentiation regulation genes such as Osx, Runx2 and ATF4 (74). Ovariectomy mice presented an osteoporosis phenotype mainly on osteochondral remodeling accompanied by increasing the endplate porosity and decreasing the bone volume, the changes of OPN, OCN, Osx in osteoblast and serum which influence the osteoblast differentiation is responsible for this pathological process (75, 76). Clinical studies have found that serum level of OPN, as biomarkers for early diagnosis of osteoporosis in postmenopausal women, is positively related to the severity of osteoporosis. The postmenopausal women have a higher OPN serum level compared to those premenopausal women, meanwhile the higher OPN level shows a negatively correlated relationship with the weight, height, and BMD (31, 32, 35). Previous scholars found the serum OPN level in the menopausal females was 15.4 (ng/ml) compared to non-menopausal females with a lower OPN level of 7.8 (ng/ml), moreover, the higher OPN level also indicated an approximately 2.97-fold risk of osteoporosis compared with the persons with low serum OPN levels (12). Vancea et al. (33–35) also confirmed the negatively relationships between OPN and BMD in the postmenopausal women, they considered postmenopausal women exhibited higher serum OPN levels may have a lower BMD and higher risk of OP than the premenopausal women. Wu et al. (82) adopted an extreme sampling design to systemically screen the OP-related cytokines in serum of postmenopausal women, further verified the OPN and BLC modulate the bone metabolism by inhibiting bone formation and promoting bone resorption. Since OPN has a major effect on osteoclasts through regulating the bone metabolism and bone remodeling, plenty of scholars have devoted great energy to investigating the role of OPN in OP and found new drugs targeting OPN to treat osteoporosis. Dong et al. (79) investigated the effects of anagliptin on the differentiation and mineralization of osteoblasts and discovered that anagliptin significantly increased matrix deposition and mineralization by increasing the activity of osteoblasts as evidenced by elevated levels of ALP, OCN, OPN, and BMP-2. Other studies also confirmed increased expression of OPN in mRNA or protein level of osteoclasts protects the osteoporosis to promote osteogenic differentiation and inhibits osteoclastogenesis. The potential therapeutic effects of elevated OPN activity on osteoporosis mainly reflected on increasing body weight gain and BMD, with the assistance effect of up-regulated level of ALP, OCN, BMP-2 and reduced tartrate-resistant acid phosphatase (TRAP) activity as well as C-terminal telopeptide of type I collagen (CTX-I) level (81, 85, 91). In addition, some scholars also found traditional Chinese medicine tonifying prescriptions have potential therapeutic effects on osteoporosis in ovariectomy-induced rat model. The traditional Chinese medicine mainly targeted to increase the expression of OPN, BMP-2 and Runx2 which finally resulted in reducing the broken trabecular bones in femur bones and increasing the activity of ALP combined with enhanced the content of total bone mineral density in osteoporosis rats (77). Due to the important biological effect of OPN on osteoporosis, its expression and physiological effect in mesenchymal stem cells (MSC) has been greatly studied. Recently, researchers confirmed that the therapeutical effect of MSCs on osteoporosis relied on the elevated extracellular Ca2+ promoted cell proliferation and matrix mineralization of MSCs. Moreover, the enhancement of MSCs under up-regulated extracellular Ca2+ condition is induced by the elevated OPN level (92). It is further found OPN not only significantly promoted the proliferation of MSCs, but also activated the migration and regulate the cell stiffness through integrin and the Wnt signaling pathway which accelerates the osteoblasts differentiation of MSCs indicated by overexpression of osteoblasts differentiation related proteins (78, 93, 94).
Pharmaceutical interventions of OPN in OA and OP
The complex effect of OPN means that regulating its expression is a complex and challenging process. Recently, some new and classic drugs have investigated the effect of OPN on OA and OP. Pharmaceutical interventions of OPN in OA have hitherto mainly focused on the OPN level in chondrocytes, synovial tissue and synoviocytes. Elmazoglu et al. (72) found that S-allylcysteine could inhibit the IL-1β, IL-6 and OPN in chondrocytes by suppressing the activity of the p-JNK/pan-JNK signaling pathway. The inhibition of OPN and these cytokines finally lead to the up-regulated level of peroxidase and type-II-collagen to delay the progress of OA. Li et al. (51) investigated the effect of chitosan oligosaccharides which are packed into the extracellular vesicles in OA. They verified that chitosan oligosaccharides could reverse IL-1β induced chondrocytes apoptosis and the inhibition of viability and migration of chondrocytes, furthermore, they also discovered the therapeutical effect of chitosan oligosaccharides on cartilage damage was by down-regulating the level IL-1β, OPN, and p53 accompanied by the increased level of Col2a1, OCN, and Runx2 via PI3K-Akt pathway. Isorhamnetin also reduced MIA-induced knee swelling by significant reduction of articular cartilage damage through regulating the OPN level in rats. The protective effect of isorhamnetin on OA was through the inhibition of OPN, NO, PGE2, iNOS and COX-2 (52). Slovacek et al. (49) showed that OPN and MMP-9 levels were significantly elevated in OA, the increased level of OPN and MMP-9 is stimulated by inflammatory markers, such as IL-1, IL-6, and CRP. Previous researchers found adiponectin aggravates bone erosion by promoting OPN production in synovial tissue while silencing of OPN with lentiviral-OPN short hairpin RNA could reduce the number of TRAP-positive osteoclasts and the extent of bone erosion in CIA mice (71). In addition, the microRNA(miR) could also influence the expression of OPN in OA. MiR-181c could greatly inhibit the effect of OPN on stimulating the secretion of MMP-13, IL-6, and IL-8 along with repressing synoviocyte proliferation which finally alleviated local inflammation activity and cartilage damage in joints, severed as a therapeutic strategy for OA (50).
The multiple effects of OPN on different types of cells indicates that modulation of OPN in OP represents a definite challenge. Our current understanding of pharmaceutical interventions of OPN in OP is discussed in the following sections and summarized in Table 3 . Gao et al. (58) found salvianolic treatment ameliorated osteopenia and improved bone quality in rats, the potential mechanism may rely on increasing Osx, OPN, and Col1a1 levels in TNF-α-induced MC3T3-E1 osteoblasts through regulating the RANKL/RANK/OPG signaling pathway. Monotropein was also found to play an important role in regulating the OPN metabolism in MC3T3-E1 cells. Research indicated that monocropping was able to increase the proliferation and activity of ALP, bone matrix mineralization and the expression of bone matrix protein OPN accompanied by decreasing the production of IL-6 and IL-1β via suppressing the activity of the NF-κB signaling pathway (61). Choi et al. (96) found that Palmul-tang could also increase bone mineral content and improve bone mineral density to treat OP. Furthermore, they indicated that the therapeutic effect of Palmul-tang mainly depended on up-regulating the expressions of BMP-2, Runx2, and Osx with its downstream factors ALP and OPN through the BMP-2 signaling pathway. Increased level of OPN also influenced the osteoblasts differentiation, matrix deposition and mineralization along with the high level of ALP, OCN, OPN, and BMP-2 which was related to the Wnt/β-catenin signaling pathway with the interventions of anagliptin and artesunate (78, 79). Apart from the above biomarkers such as BMP-2, Runx2, Osx and ALP, the serum OPN level was also negatively correlated with bone turnover markers mainly including parathyroid hormone, lumbar spine BMD, femoral neck BMD and positively associated with type I procollagen amino-terminal propeptide (PINP), carboxy-terminal cross-linking telopeptide of type I collagen (CTX) in the OP patients (35). Furthermore, they indicated CTX was independent predictor of serum OPN while vitamin D was not correlated to OPN in adults (32, 97). The animal model showed that up-regulated OPN could enhance the activity of ALP, OCN, and BMP-2 in dexamethasone-induced OP, the anti-osteoporosis function of myricetin in vivo may be due to the promoting osteogenic differentiation and matrix mineralization effect caused by OPN (83).
Table 3.
Drug therapies for treatment of OA and OP.
| Reference | Disease | Drugs | Subjects | Signaling pathways | Main results |
|---|---|---|---|---|---|
| Qiyuan Wang, et al. (50) | OA | miR-181c | Synoviocytes | / | MiR-181c significantly repressed synoviocyte proliferation, as well as the levels of OPN, MMP13, IL-6, and IL-8. |
| Shenglong Li, et al. (51) | OA | Chitosan oligosaccharides | Chondrocytes | / | Chitosan oligosaccharides could reverse the effect of IL-1β on inhibiting chondrocytes viability and migration, moreover, chitosan oligosaccharides could reverse the upregulated expression of IL-1β, OPN, and p53 induced by cartilage injury. |
| Tsai Sen-Wei, et al. (52) | OA | Isorhamnetin | Sprague-Dawley rats | / | Isorhamnetin may reduce MIA-induced knee swelling by significantly reduction of articular cartilage damage in rats. The protective effect of isorhamnetin on OA was through the inhibition of OPN, NO, PGE2, iNOS and COX-2. |
| Zubeyir Elmazoglu, et al. (72) | OA | S-allylcysteine | Human OA chondrocyte | p-JNK/pan-JNK signaling pathway | S-allylcysteine (SAC) inhibited reactive oxygen species (ROS), lipid hydroperoxides (LPO), IL-1β, IL-6, OPN and increased glutathione peroxidase (GPx) and type-II-collagen to control the progression of OA. |
| Qian, et al. (71) | OA | Adiponectin | Serum and synovial tissue | / | Adiponectin aggravates bone erosion by promoting OPN production in synovial tissue of rheumatoid arthritis. |
| Kulsirirat, et al. (73) | OA | Andrographolide | Mesenchymal stem cells | / | Andrographolide could upregulate the expression of genes related to osteogenic and chondrogenic differentiation, including Runx2, OPN, Sox9, and Aggrecan in mesenchymal stem cells. |
| Marycz, et al. (57) | OP | NHAp/IO@miR-21/124 | MC3T3-E1 cells | / | NHAp/IO@miR-21/124 could enhance osteogenesis through increasing osteogenic markers Runx-2, OPN, Coll-1 level. |
| Xiang Gao, et al. (58) | OP | Salvianolate | Lewis rats | RANKL/RANK/OPG signaling pathway | Salvianolate treatment ameliorate osteopenia and improved bone quality through increasing the Osterix, OPN, Runx2 level and decrease TNF-α, IL-6 level in serum. |
| Yu-Qiong He, et al. (61) | OP | Monotropein | C57/BL6 mice and MC3T3-E1 cells | NF-κB signaling pathway | Monotropein inhibited bone mass reduction and improved bone micro-architectures by enhancing bone formation and blocking increased secretion of inflammatory cytokines in osteoporotic mice. |
| Miranda, et al. 959) | OP | strontium ranelate | Wistar rats | NF-κB signaling pathway | Strontium ranelate-treated groups presented higher staining of OPN and BSP which was beneficial to bone healing and the expression of bone markers. |
| La Yoon Choi, et al. (96) | OP | Palmul-tang | Female ICR mice | BMP-2 signaling pathway | Palmul-tang replenished bone marrow cavity and increased collagen deposition in bone marrow cells of femur, furthermore, restored the bone minerals and improvement of bone integrity through increasing the expressions of BMP-2, Runx2 and OSX with its downstream factors, ALP, OPN and BSP-1. |
| Li, et al. (76) | OP | Aucubin | MG63 cells | / | Aucubin increase cortical bone thickness, bone density and tighter trabecular bone in Dex-induced osteoporotic mouse model through increasing the level of collagen I, OCN, OPN in cells. |
| Zeng, et al. (78) | OP | Artesunate | hBMSCs | Wnt signaling pathway | Artesunate increased the osteoblasts differentiation related protein expression through activating the Wnt signaling pathway. |
| Dong, et al. (79) | OP | Anagliptin | MC3T3-E1 cells | Wnt/β-catenin signaling pathway | Anagliptin increased matrix deposition and mineralization by increased the expression of ALP, OCN, OPN, and BMP-2. |
| Yin, et al. (80) | OP | Evodiamine | Zebrafish | MMP3-OPN-MAPK pathway | Evodiamine increased the area of bone formation through MMP3-OPN-MAPK pathway in zebrafish. |
| Fan, et al. (81) | OP | Myricetin | Sprague-Dawley rats | / | Myricetin promoted osteoblast differentiation and mineralization, accompanied by increases in BMP2, Runx2, ALP, OCN, Col1a and OPN levels. |
| Lee, et al. (83) | OP | Osteo-F | Female ICR mice | / | Osteo-F-treatment increased the Bone mineral content and BMD through up-regulating the mRNA expressions of collagen type I, BMP-2 and OPN in ovariectomized mice. |
| Huang, et al. (91) | OP | Psoralen | hBMSCs | TGF-β/Smad3 signaling pathway | Psoralen accelerates osteogenic differentiation by increasing the expression of BMP4, OPN, Osterix, Runx2, TGF-β1, TGF-β RI and p-Smad3 through activating the TGF-β/Smad3 pathway. |
| Marycz, et al. (84) | OP | PRHD@MnFe2O4 | MC3T3-E1 cells | / | PRHD@MnFe2O4 could be a potential agent in osteoporosis treatment through enhancing expression of bone remodeling genes mainly including OPN, OCL and ALP. |
Taking together, the pharmaceutical interventions of OPN in bone metabolism present different biological effects due to the multiple types of cells. Generally speaking, the current drug treatment mainly depended on reducing the OPN level in chondrocytes and synovial cells to suppress inflammatory activity alleviate cartilage erosion, and delay the course of OA. While it referred to OP, the pharmaceutical effect of drug intervention on OPN is to increase the expression level of OPN in osteoclasts and stem cells. It is contrary to the study of drug intervention in OA, the increased OPN level, as well as its related cytokines BMP-2, OCN, and Runx2, could promote bone formation and enhance bone mineral density to achieve the purpose of treating OP.
Conclusion
In recent years, there has been growing interest in trying to identify the physiological effect of OPN on skeleton diseases. OPN acts as a secreted protein that participates in the progress of bone remodeling and bone metabolism which are relevant to many bone metabolism disturbance diseases. OPN regulates the inflammation activity both in OA and OP, and plays controversial roles in the inflammation response for either overexpression of OPN level in chondrocytes and synoviocytes or OPN-deficiency in cells residents in joints may stimulate and enhance the inflammation activity in OA as the Figure 1 shown. While the higher level of OPN could suppress the secretion of proinflammation cytokines and inflammation activity, alleviate bone mass reduction, and promote bone formation as well as matrix calcification in OP.
As referred to as the role of cartilage and bone metabolism, OPN is a biological marker of OA severity. Overexpression of OPN enhances the proliferation of chondrocytes and TRAP-positive osteoclasts along with the osteogenic and chondrogenic differentiation progress, which finally leads to bone erosion and cartilage degeneration in OA. High levels of OPN could increase matrix deposition and enhance bone remodeling by promoting osteoblasts differentiation along with the elevated level of ALP, OCN, and BMP-2 which finally resulted in reducing the pain and pathological changes of osteoporosis. Low activity of OPN could increase fracture sensitivity in patients with osteoporosis, especially for those postmenopausal women as Figure 2 shown. Due to the above physiological role of OPN in OA and OP, we do deem that OA and OP influenced each other for the reason that OA is associated with bone formation as seen in subchondral sclerosis and osteophyte formation, and the tendency to accumulate bone in the subchondral area could increase the onset of OA. In contrast, once OA is established, the pain and reduced mobility reduce bone mass, particularly in the affected limb. The pain and loss of joint function in OA patients could result in muscle loss and postural instability, which subsequently increased fracture risk. OPN may serve as a bridge role in regulating bone metabolism and signal transduction between the disease of osteoarthritis and osteoporosis, and it should be precisely modulated at a proper level for either higher OPN level or lower OPN activity may induce to disrupt the balance of bone tissue. However, there are still many details and effects of OPN on bone metabolism and bone-related disease not been elucidated. Further in-depth studies on the physiological function of OPN provide new ideas and directions for exploring and clarifying the pathogenesis of bone metabolic diseases, targeting OPN may provide new clinical therapeutic orientations and values for OA and OP.
Author contributions
All the authors researched the data for the article, provided substantial contributions to discussions of its content, wrote the article and reviewed and/or edited the manuscript before submission. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key R&D Program of China (No.2019YFA0111900), the National Natural Science Foundation of China (No.81501923 and No. 82072506), Provincial Natural Science Foundation of Hunan (No.2020JJ3060), Administration of Traditional Chinese Medicine of Hunan Province (No.2021075), InnovationDriven Project of Central South University (No.2020CX045), Wu Jieping Medical Foundation (No.320.6750.2020-03-14), Rui E (Ruiyi) Emergency Medical Research Special Funding Project (No.R2019007), and Independent Exploration and Innovation Project for Postgraduate Students of Central South University (No.2021zzts1037).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Glossary
| OA | osteoarthritis |
| OP | osteoporosis |
| OPN | osteopontin |
| SMC | smooth muscle cells |
| SIBLING | small integrin-binding ligand N-linked glycoprotein |
| Eta-1 | early T cell activa-tion gene-1 |
| BMD | bone mineral density |
| CRP | C-reaction protein |
| ACLT | anterior cruciate ligament transection |
| DMM | destabilization of the medial meniscus |
| MMP-9 | matrix metalloproteinase 9 |
| OCN | osteocalcin |
| IMO | inflammation mediated osteoporosis |
| COX-2 | cyclooxygenase-2 |
| PGE2 | prostaglandin E2 |
| TRAP | tartrate-resistant acid phosphatase |
| BMC | bone mineral content |
| CIA | collagen-induced arthritis |
| ALP | alkaline phosphatase |
| CTX-I | C-terminal telopeptide of type I collagen |
| BMP-7 | bone morphogenetic protein-7 |
| IL | interleukin |
| TNF-α | tumor necrosis factor alpha |
| MSC | mesenchymal stem cells |
| NF-kB | nuclear factor kappa-B |
| iNOS | inducible nitric-oxide synthase |
| LPS | Lipopolysaccharide |
| ADAMTS | a disintegrin and metalloproteinase with thrombospondin motifs |
| Col2a1 | type II collagen alpha 1 chain |
| Sox9 | SRY-box transcription factor 9 |
| Acan | Aggrecan |
| Osx | Osterix |
| HFD | high-fat diet |
| ECM | extracellular matrix |
| HA | hyaluronan |
| miR | microRNA |
References
- 1. Hunter DJ, Felson DT. Osteoarthritis. BMJ (2006) 332(7542):639–42. doi: 10.1136/bmj.332.7542.639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ (2003) 81(9):646–56. [PMC free article] [PubMed] [Google Scholar]
- 3. Arden N, Nevitt MC. Osteoarthritis: Epidemiology. Best Pract Res Clin Rheumatol (2006) 20(1):3–25. doi: 10.1016/j.berh.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 4. Consensus Development Conference . Diagnosis, prophylaxis, and treatment of osteoporosis. Am J Med (1993) 94(6):646–50. doi: 10.1016/0002-9343(93)90218-e [DOI] [PubMed] [Google Scholar]
- 5. Kim D, Pirshahid AA, Li Y, Varghese T, Pope JE. Prevalence of osteoporosis in osteoarthritis: A systematic review and meta-analysis. Osteoporos Int (2022) 33(8):1687–93. doi: 10.1007/s00198-022-06376-0 [DOI] [PubMed] [Google Scholar]
- 6. Wen L, Shin MH, Kang JH, Yim YR, Kim JE, Lee JW, et al. The relationships between bone mineral density and radiographic features of hand or knee osteoarthritis in older adults: Data from the dong-gu study. Rheumatol (Oxford) (2016) 55(3):495–503. doi: 10.1093/rheumatology/kev377 [DOI] [PubMed] [Google Scholar]
- 7. Kasher M, Williams FMK, Freidin MB, Cherny S, Livshits G. An in-depth study of the associations between osteoarthritis- and osteoporosis-related phenotypes at different skeletal locations. Osteoporos Int (2020) 31(11):2197–208. doi: 10.1007/s00198-020-05504-y [DOI] [PubMed] [Google Scholar]
- 8. Jeon SH, Lee KG, Kim MS. Association of bone mineral density with the development of knee osteoarthritis in men and women: A cross-sectional study using the fourth and fifth Korea national health and nutrition examination surveys. Arch Osteoporos (2020) 15(1):117. doi: 10.1007/s11657-020-00793-6 [DOI] [PubMed] [Google Scholar]
- 9. Pullig O, Weseloh G, Gauer S, Swoboda B. Osteopontin is expressed by adult human osteoarthritic chondrocytes: Protein and mrna analysis of normal and osteoarthritic cartilage. Matrix Biol (2000) 19(3):245–55. doi: 10.1016/S0945-053x(00)00068-8 [DOI] [PubMed] [Google Scholar]
- 10. Gao SG, Li KH, Zeng KB, Tu M, Xu M, Lei GH. Elevated osteopontin level of synovial fluid and articular cartilage is associated with disease severity in knee osteoarthritis patients. Osteoarthritis Cartilage (2010) 18(1):82–7. doi: 10.1016/j.joca.2009.07.009 [DOI] [PubMed] [Google Scholar]
- 11. Honsawek S, Tanavalee A, Sakdinakiattikoon M, Chayanupatkul M, Yuktanandana P. Correlation of plasma and synovial fluid osteopontin with disease severity in knee osteoarthritis. Clin Biochem (2009) 42(9):808–12. doi: 10.1016/j.clinbiochem.2009.02.002 [DOI] [PubMed] [Google Scholar]
- 12. Chang IC, Chiang TI, Yeh KT, Lee H, Cheng YW. Increased serum osteopontin is a risk factor for osteoporosis in menopausal women. Osteoporos Int (2010) 21(8):1401–9. doi: 10.1007/s00198-009-1107-7 [DOI] [PubMed] [Google Scholar]
- 13. Denhardt DT NM. Osteopontin expression and function: Role in bone remodeling. J Cell Biochem Suppl (1998) 30-31):92–102. [PubMed] [Google Scholar]
- 14. Gravallese EM. Osteopontin: A bridge between bone and the immune system. J Clin Invest (2003) 112(2):147–9. doi: 10.1172/JCI19190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Zhang FJ, Gao SG, Cheng L, Tian J, Xu WS, Luo W, et al. The effect of hyaluronic acid on osteopontin and Cd44 mrna of fibroblast-like synoviocytes in patients with osteoarthritis of the knee. Rheumatol Int (2013) 33(1):79–83. doi: 10.1007/s00296-011-2339-3 [DOI] [PubMed] [Google Scholar]
- 16. Kothari AN, Arffa ML, Chang V, Blackwell RH, Syn WK, Zhang J, et al. Osteopontin-a master regulator of epithelial-mesenchymal transition. J Clin Med (2016) 5(4):39. doi: 10.3390/jcm5040039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. De Fusco C, Messina A, Monda V, Viggiano E, Moscatelli F, Valenzano A, et al. Osteopontin: Relation between adipose tissue and bone homeostasis. Stem Cells Int (2017) 2017:4045238. doi: 10.1155/2017/4045238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wen Y, Jeong S, Xia Q, Kong X. Role of osteopontin in liver diseases. Int J Biol Sci (2016) 12(9):1121–8. doi: 10.7150/ijbs.16445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yamaga M, Tsuji K, Miyatake K, Yamada J, Abula K, Ju YJ, et al. Osteopontin level in synovial fluid is associated with the severity of joint pain and cartilage degradation after anterior cruciate ligament rupture. PLoS One (2012) 7(11):e49014. doi: 10.1371/journal.pone.0049014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hattori T, Iwasaki-Hozumi H, Bai G, Chagan-Yasutan H, Shete A, Telan EF, et al. Both full-length and protease-cleaved products of osteopontin are elevated in infectious diseases. Biomedicines (2021) 9(8):1006. doi: 10.3390/biomedicines9081006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. O'Regan AW, Nau GJ, Chupp GL, Berman JS. Osteopontin (Eta-1) in cell-mediated immunity: Teaching an old dog new tricks. Immunol Today (2000) 21(10):475–8. doi: 10.1016/s0167-5699(00)01715-1 [DOI] [PubMed] [Google Scholar]
- 22. McKee MD NA. Osteopontin at mineralized tissue interfaces in bone, teeth, and osseointegrated implants: Ultrastructural distribution and implications for mineralized tissue formation, turnover, and repair. Microsc Res Tech (1996) 33(2):141–64. doi: [DOI] [PubMed] [Google Scholar]
- 23. Standal T BM, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol (2004) 3):179–84. [PubMed] [Google Scholar]
- 24. Chellaiah MA, Hruska KA. The integrin Alpha(V)Beta(3) and Cd44 regulate the actions of osteopontin on osteoclast motility. Calcif Tissue Int (2003) 72(3):197–205. doi: 10.1007/s00223-002-1025-6 [DOI] [PubMed] [Google Scholar]
- 25. Suzuki K, Zhu B, Rittling SR, Denhardt DT, Goldberg HA, McCulloch CA, et al. Colocalization of intracellular osteopontin with Cd44 is associated with migration, cell fusion, and resorption in osteoclasts. J Bone Miner Res (2002) 17(8):1486–97. doi: 10.1359/jbmr.2002.17.8.1486 [DOI] [PubMed] [Google Scholar]
- 26. Chen J SK, Mukherjee BB, Sodek J. Developmental expression of osteopontin (Opn) mrna in rat tissues: Evidence for a role for opn in bone formation and resorption. Matrix (1993) 13(2):113–23. doi: 10.1016/s0934-8832(11)80070-3 [DOI] [PubMed] [Google Scholar]
- 27. Tian J, Cheng C, Kuang SD, Su C, Zhao X, Xiong YL, et al. Opn deficiency increases the severity of osteoarthritis associated with aberrant chondrocyte senescence and apoptosis and upregulates the expression of osteoarthritis-associated genes. Pain Res Manag (2020) 2020:3428587. doi: 10.1155/2020/3428587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Yuan Y, Liu Q, Wu Z, Luo W. Mechanistic insight on the interaction between opn and integrin Alphanubeta3 in osteoarthritis. BioMed Res Int (2020) 2020:2905634. doi: 10.1155/2020/2905634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li L, Lv G, Wang B, Kuang L. Xist/Mir-376c-5p/Opn axis modulates the influence of proinflammatory M1 macrophages on osteoarthritis chondrocyte apoptosis. J Cell Physiol (2020) 235(1):281–93. doi: 10.1002/jcp.28968 [DOI] [PubMed] [Google Scholar]
- 30. Si J, Wang C, Zhang D, Wang B, Zhou Y. Osteopontin in bone metabolism and bone diseases. Med Sci Monit (2020) 26:e919159. doi: 10.12659/MSM.919159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Chiang TI, Chang IC, Lee HS, Lee H, Huang CH, Cheng YW. Osteopontin regulates anabolic effect in human menopausal osteoporosis with intermittent parathyroid hormone treatment. Osteoporos Int (2011) 22(2):577–85. doi: 10.1007/s00198-010-1327-x [DOI] [PubMed] [Google Scholar]
- 32. Fodor D, Bondor C, Albu A, Simon SP, Craciun A, Muntean L. The value of osteopontin in the assessment of bone mineral density status in postmenopausal women. J Investig Med (2013) 61(1):15–21. doi: 10.2310/JIM.0b013e3182761264 [DOI] [PubMed] [Google Scholar]
- 33. Cho EH, Cho KH, Lee HA, Kim SW. High serum osteopontin levels are associated with low bone mineral density in postmenopausal women. J Korean Med Sci (2013) 28(10):1496–9. doi: 10.3346/jkms.2013.28.10.1496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vancea A, Serban O, Fodor D. Relationship between osteopontin and bone mineral density. Acta Endocrinol (Buchar) (2021) 17(4):509–16. doi: 10.4183/aeb.2021.509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wei QS, Huang L, Tan X, Chen ZQ, Chen SM, Deng WM. Serum osteopontin levels in relation to bone mineral density and bone turnover markers in postmenopausal women. Scand J Clin Lab Invest (2016) 76(1):33–9. doi: 10.3109/00365513.2015.1087045 [DOI] [PubMed] [Google Scholar]
- 36. Gkastaris K, Goulis DG, Potoupnis M, Anastasilakis AD, Kapetanos G. Obesity, osteoporosis and bone metabolism. J Musculoskelet Neuronal Interact (2020) 20(3):372–81. [PMC free article] [PubMed] [Google Scholar]
- 37. Hu L, Yin C, Zhao F, Ali A, Ma J, Qian A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int J Mol Sci (2018) 19(2):360. doi: 10.3390/ijms19020360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Wang ZX, Luo ZW, Li FX, Cao J, Rao SS, Liu YW, et al. Aged bone matrix-derived extracellular vesicles as a messenger for calcification paradox. Nat Commun (2022) 13(1):1453. doi: 10.1038/s41467-022-29191-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Dai B, Xu J, Li X, Huang L, Hopkins C, Wang H, et al. Macrophages in epididymal adipose tissue secrete osteopontin to regulate bone homeostasis. Nat Commun (2022) 13(1):427. doi: 10.1038/s41467-021-27683-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Kiefer FW, Zeyda M, Todoric J, Huber J, Geyeregger R, Weichhart T, et al. Osteopontin expression in human and murine obesity: Extensive local up-regulation in adipose tissue but minimal systemic alterations. Endocrinology (2008) 149(3):1350–7. doi: 10.1210/en.2007-1312 [DOI] [PubMed] [Google Scholar]
- 41. Min S, Shi T, Han X, Chen D, Xu Z, Shi D, et al. Serum levels of leptin, osteopontin, and sclerostin in patients with and without knee osteoarthritis. Clin Rheumatol (2021) 40(1):287–94. doi: 10.1007/s10067-020-05150-z [DOI] [PubMed] [Google Scholar]
- 42. Qin LF WW, Fang H, Mao XZ, Huang GL, Chen Y, Zhou HD, et al. Expression of nf-Kb and osteopontin of synovial fluid of patients with knee osteoarthritis. Asian Pac J Trop Med (2013) 6(5):379–82. doi: 10.1016/S1995-7645(13)60042-5 [DOI] [PubMed] [Google Scholar]
- 43. Shang H, Hao Y, Hu W, Hu X, Jin Q. Opn gene locus is associated with the risk of knee osteoarthritis: A case-control study. Biosci Rep (2019) 39(2):BSR20182023. doi: 10.1042/BSR20182023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Martinez-Calleja A, Velasquillo C, Vega-Lopez M, Arellano-Jimenez MJ, Tsutsumi-Fujiyoshi VK, Mondragon-Flores R, et al. Osteopontin expression and localization of ca++ deposits in early stages of osteoarthritis in a rat model. Histol Histopathol (2014) 29(7):925–33. doi: 10.14670/HH-29.925 [DOI] [PubMed] [Google Scholar]
- 45. Lin C, Chen Z, Guo D, Zhou L, Lin S, Li C, et al. Increased expression of osteopontin in subchondral bone promotes bone turnover and remodeling, and accelerates the progression of oa in a mouse model. Aging (Albany NY) (2022) 14(1):253–71. doi: 10.18632/aging.203707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Liu Q, Zeng H, Yuan Y, Wang Z, Wu Z, Luo W. Osteopontin inhibits osteoarthritis progression Via the Opn/Cd44/Pi3k signal axis. Genes Dis (2022) 9(1):128–39. doi: 10.1016/j.gendis.2020.06.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Morath I, Hartmann TN, Orian-Rousseau V. Cd44: More than a mere stem cell marker. Int J Biochem Cell Biol (2016) 81(Pt A):166–73. doi: 10.1016/j.biocel.2016.09.009 [DOI] [PubMed] [Google Scholar]
- 48. Katagiri YU, Sleeman J, Fujii H, Herrlich P, Hotta H, Tanaka K, et al. Cd44 variants but not Cd44s cooperate with beta 1-containing integrins to permit cells to bind to osteopontin independently of arginine-Glycine-Aspartic acid, thereby stimulating cell motility and chemotaxis. Cancer Res (1999) 59(1):219–26. [PubMed] [Google Scholar]
- 49. Slovacek H, Khanna R, Poredos P, Jezovnik M, Hoppensteadt D, Fareed J, et al. Interrelationship of osteopontin, mmp-9 and Adamts4 in patients with osteoarthritis undergoing total joint arthroplasty. Clin Appl Thromb Hemost (2020) 26:1076029620964864. doi: 10.1177/1076029620964864 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wang Q, Wang W, Zhang F, Deng Y, Long Z. Neat1/Mir-181c regulates osteopontin (Opn)-mediated synoviocyte proliferation in osteoarthritis. J Cell Biochem (2017) 118(11):3775–84. doi: 10.1002/jcb.26025 [DOI] [PubMed] [Google Scholar]
- 51. Li S, Liu J, Liu S, Jiao W, Wang X. Chitosan oligosaccharides packaged into rat adipose mesenchymal stem cells-derived extracellular vesicles facilitating cartilage injury repair and alleviating osteoarthritis. J Nanobiotechno (2021) 19(1):343. doi: 10.1186/s12951-021-01086-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Tsai SW, Lin CC, Lin SC, Wang SP, Yang DH. Isorhamnetin ameliorates inflammatory responses and articular cartilage damage in the rats of monosodium iodoacetate-induced osteoarthritis. Immunopharmacol Immunotoxicol (2019) 41(4):504–12. doi: 10.1080/08923973.2019.1641723 [DOI] [PubMed] [Google Scholar]
- 53. Cassuto J, Folestad A, Gothlin J, Malchau H, Karrholm J. The key role of proinflammatory cytokines, matrix proteins, Rankl/Opg and Wnt/Beta-catenin in bone healing of hip arthroplasty patients. Bone (2018) 107:66–77. doi: 10.1016/j.bone.2017.11.004 [DOI] [PubMed] [Google Scholar]
- 54. Garcia-Manrique M, Calvet J, Orellana C, Berenguer-Llergo A, Garcia-Cirera S, Llop M, et al. Synovial fluid but not plasma interleukin-8 is associated with clinical severity and inflammatory markers in knee osteoarthritis women with joint effusion. Sci Rep (2021) 11(1):5258. doi: 10.1038/s41598-021-84582-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Matsui Y, Iwasaki N, Kon S, Takahashi D, Morimoto J, Matsui Y, et al. Accelerated development of aging-associated and instability-induced osteoarthritis in osteopontin-deficient mice. Arthritis Rheum (2009) 60(8):2362–71. doi: 10.1002/art.24705 [DOI] [PubMed] [Google Scholar]
- 56. Tang BM, Li ZW, Wang ZY. Perk activator Cct020312 prevents inflammation-mediated osteoporosis in the ovariectomized rats. Gynecol Endocrinol (2021) 37(4):342–8. doi: 10.1080/09513590.2021.1874904 [DOI] [PubMed] [Google Scholar]
- 57. Marycz K, Smieszek A, Marcinkowska K, Sikora M, Turlej E, Sobierajska P, et al. Nanohydroxyapatite (Nhap) doped with iron oxide nanoparticles (Io), mir-21 and mir-124 under magnetic field conditions modulates osteoblast viability, reduces inflammation and inhibits the growth of osteoclast - a novel concept for osteoporosis treatment: Part 1. Int J Nanomedicine (2021) 16:3429–56. doi: 10.2147/IJN.S303412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Gao X, Wu Q, Zhang X, Tian J, Liang D, Min Y, et al. Salvianolate ameliorates osteopenia and improves bone quality in prednisone-treated rheumatoid arthritis rats by regulating Rankl/Rank/Opg signaling. Front Pharmacol (2021) 12:710169. doi: 10.3389/fphar.2021.710169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li WM, Han CL, Liu C, Xing HY, Ding DC. Angptl2 deletion inhibits osteoclast generation by modulating nf-Kappab/Mapks/Cyclin pathways. Biochem Biophys Res Commun (2018) 503(3):1471–7. doi: 10.1016/j.bbrc.2018.07.065 [DOI] [PubMed] [Google Scholar]
- 60. Lei C, Xueming H, Ruihang D. Mln64 deletion suppresses rankl-induced osteoclastic differentiation and attenuates diabetic osteoporosis in streptozotocin (Stz)-induced mice. Biochem Biophys Res Commun (2018) 505(4):1228–35. doi: 10.1016/j.bbrc.2018.10.007 [DOI] [PubMed] [Google Scholar]
- 61. He YQ, Yang H, Shen Y, Zhang JH, Zhang ZG, Liu LL, et al. Monotropein attenuates ovariectomy and lps-induced bone loss in mice and decreases inflammatory impairment on osteoblast through blocking activation of nf-kappab pathway. Chem Biol Interact (2018) 291:128–36. doi: 10.1016/j.cbi.2018.06.015 [DOI] [PubMed] [Google Scholar]
- 62. Sharif SA, Du X, Myles T, Song JJ, Price E, Lee DM, et al. Thrombin-activatable carboxypeptidase b cleavage of osteopontin regulates neutrophil survival and synoviocyte binding in rheumatoid arthritis. Arthritis Rheum (2009) 60(10):2902–12. doi: 10.1002/art.24814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hasegawa M, Segawa T, Maeda M, Yoshida T, Sudo A. Thrombin-cleaved osteopontin levels in synovial fluid correlate with disease severity of knee osteoarthritis. J Rheumatol (2011) 38(1):129–34. doi: 10.3899/jrheum.100637 [DOI] [PubMed] [Google Scholar]
- 64. Yan L, Hu R, Tu S, Cheng WJ, Zheng Q, Wang JW, et al. Meta-analysis of association between il-6 -634c/G polymorphism and osteoporosis. Genet Mol Res (2015) 14(4):19225–32. doi: 10.4238/2015.December.29.32 [DOI] [PubMed] [Google Scholar]
- 65. Kany S, Vollrath JT, Relja B. Cytokines in inflammatory disease. Int J Mol Sci (2019) 20(23):6008. doi: 10.3390/ijms20236008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kawai T, Akira S. Tlr signaling. Cell Death Differ (2006) 13(5):816–25. doi: 10.1038/sj.cdd.4401850 [DOI] [PubMed] [Google Scholar]
- 67. Wang Z, Yang Y, He M, Wang R, Ma J, Zhang Y, et al. Association between interleukin-6 gene polymorphisms and bone mineral density: A meta-analysis. Genet Test Mol Biomarkers (2013) 17(12):898–909. doi: 10.1089/gtmb.2013.0223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Xu M, Zhang L, Zhao L, Gao S, Han R, Su D, et al. Phosphorylation of osteopontin in osteoarthritis degenerative cartilage and its effect on matrix metalloprotease 13. Rheumatol Int (2013) 33(5):1313–9. doi: 10.1007/s00296-012-2548-4 [DOI] [PubMed] [Google Scholar]
- 69. Icer MA, Gezmen-Karadag M. The multiple functions and mechanisms of osteopontin. Clin Biochem (2018) 59:17–24. doi: 10.1016/j.clinbiochem.2018.07.003 [DOI] [PubMed] [Google Scholar]
- 70. Sun PF, Kong WK, Liu L, Liu Y, Liu FM, Liu WJ, et al. Osteopontin accelerates chondrocyte proliferation in osteoarthritis rats through the nf-kappab signaling pathway. Eur Rev Med Pharmacol Sci (2020) 24(6):2836–42. doi: 10.26355/eurrev_202003_20647 [DOI] [PubMed] [Google Scholar]
- 71. Qian J, Xu L, Sun X, Wang Y, Xuan W, Zhang Q, et al. Adiponectin aggravates bone erosion by promoting osteopontin production in synovial tissue of rheumatoid arthritis. Arthritis Res Ther (2018) 20(1):26. doi: 10.1186/s13075-018-1526-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Elmazoglu Z, Aydin Bek Z, Saribas SG, Ozogul C, Goker B, Bitik B, et al. S-allylcysteine inhibits chondrocyte inflammation to reduce human osteoarthritis Via targeting rage, Tlr4, jnk, and Nrf2 signaling: Comparison with colchicine. Biochem Cell Biol (2021) 99(5):645–54. doi: 10.1139/bcb-2021-0004 [DOI] [PubMed] [Google Scholar]
- 73. Kulsirirat T, Honsawek S, Takeda-Morishita M, Sinchaipanid N, Udomsinprasert W, Leanpolchareanchai J, et al. The effects of andrographolide on the enhancement of chondrogenesis and osteogenesis in human suprapatellar fat pad derived mesenchymal stem cells. Molecules (2021) 26(7):1831. doi: 10.3390/molecules26071831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Tang CY, Chen W, Luo Y, Wu J, Zhang Y, McVicar A, et al. Runx1 up-regulates chondrocyte to osteoblast lineage commitment and promotes bone formation by enhancing both chondrogenesis and osteogenesis. Biochem J (2020) 477(13):2421–38. doi: 10.1042/BCJ20200036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Xiao ZF, He JB, Su GY, Chen MH, Hou Y, Chen SD, et al. Osteoporosis of the vertebra and osteochondral remodeling of the endplate causes intervertebral disc degeneration in ovariectomized mice. Arthritis Res Ther (2018) 20(1):207. doi: 10.1186/s13075-018-1701-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Li Y, Zhang Y, Zhang X, Lu W, Liu X, Hu M, et al. Aucubin exerts anti-osteoporotic effects by promoting osteoblast differentiation. Aging (Albany NY) (2020) 12(3):2226–45. doi: 10.18632/aging.102742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gu F JJ, Wang S, Feng T, Zhou Y, Ma Y, Shen S. An experimental research into the potential therapeutic effects of anti-osteoporosis decoction and yougui pill on ovariectomy-induced osteoporosis. Am J Transl Res (2019) 11(9):6032–9. [PMC free article] [PubMed] [Google Scholar]
- 78. Zeng HB, Dong LQ, Xu C, Zhao XH, Wu LG. Artesunate promotes osteoblast differentiation through mir-34a/Dkk1 axis. Acta Histochem (2020) 122(7):151601. doi: 10.1016/j.acthis.2020.151601 [DOI] [PubMed] [Google Scholar]
- 79. Dong C, Yang H, Wang Y, Yan X, Li D, Cao Z, et al. Anagliptin stimulates osteoblastic cell differentiation and mineralization. BioMed Pharmacother (2020) 129:109796. doi: 10.1016/j.biopha.2019.109796 [DOI] [PubMed] [Google Scholar]
- 80. Yin H, Wang J, Wu M, Ma Y, Wang S, Su Q. Preventive effects of evodiamine on dexamethasone-induced osteoporosis in zebrafish. Biomed Res Int (2019) 2019:5859641. doi: 10.1155/2019/5859641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Fan S, Gao X, Chen P, Li X. Myricetin ameliorates glucocorticoid-induced osteoporosis through the erk signaling pathway. Life Sci (2018) 207:205–11. doi: 10.1016/j.lfs.2018.06.006 [DOI] [PubMed] [Google Scholar]
- 82. Wu LF, Wang WY, Zhu DC, He P, Zhu K, Gui GP, et al. Protein array test detected three osteoporosis related plasma inflammatory cytokines in Chinese postmenopausal women. Cytokine (2020) 133:155166. doi: 10.1016/j.cyto.2020.155166 [DOI] [PubMed] [Google Scholar]
- 83. Lee H, Kim MH, Choi Y, Yang WM. Ameliorative effects of osteo-f, a newly developed herbal formula, on osteoporosis Via activation of bone formation. J Ethnopharmacol (2021) 268:113590. doi: 10.1016/j.jep.2020.113590 [DOI] [PubMed] [Google Scholar]
- 84. Marycz K, Kowalczuk A, Turlej E, Zachanowicz E, Tomaszewska A, Kulpa-Greszta M, et al. Impact of polyrhodanine manganese ferrite binary nanohybrids (PRHD@MnFe2O4) on osteoblasts and osteoclasts activities-A key factor in osteoporosis treatment. Materials (Basel) (2022) 15(11):3990. doi: 10.3390/ma15113990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Shao H, Wu R, Cao L, Gu H, Chai F. Trelagliptin stimulates osteoblastic differentiation by increasing runt-related transcription factor 2 (Runx2): A therapeutic implication in osteoporosis. Bioengineered (2021) 12(1):960–8. doi: 10.1080/21655979.2021.1900633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet (2019) 393(10169):364–76. doi: 10.1016/S0140-6736(18)32112-3 [DOI] [PubMed] [Google Scholar]
- 87. Johnston CB, Dagar M. Osteoporosis in older adults. Med Clin North Am (2020) 104(5):873–84. doi: 10.1016/j.mcna.2020.06.004 [DOI] [PubMed] [Google Scholar]
- 88. Yoshitake H, Rittling SR, Denhardt DT, Noda M. Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci U.S.A. (1999) 96(14):8156–60. doi: 10.1073/pnas.96.14.8156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Asou Y, Rittling SR, Yoshitake H, Tsuji K, Shinomiya K, Nifuji A, et al. Osteopontin facilitates angiogenesis, accumulation of osteoclasts, and resorption in ectopic bone. Endocrinology (2001) 142(3):1325–32. doi: 10.1210/endo.142.3.8006 [DOI] [PubMed] [Google Scholar]
- 90. Cao H, Cao B, Heazlewood CK, Domingues M, Sun X, Debele E, et al. Osteopontin is an important regulative component of the fetal bone marrow hematopoietic stem cell niche. Cells (2019) 8(9):985. doi: 10.3390/cells8090985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Huang Y, Liao L, Su H, Chen X, Jiang T, Liu J, et al. Psoralen accelerates osteogenic differentiation of human bone marrow mesenchymal stem cells by activating the tgf-Beta/Smad3 pathway. Exp Ther Med (2021) 22(3):940. doi: 10.3892/etm.2021.10372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lee MN, Hwang HS, Oh SH, Roshanzadeh A, Kim JW, Song JH, et al. Elevated extracellular calcium ions promote proliferation and migration of mesenchymal stem cells Via increasing osteopontin expression. Exp Mol Med (2018) 50(11):1–16. doi: 10.1038/s12276-018-0170-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Zou C, Luo Q, Qin J, Shi Y, Yang L, Ju B, et al. Osteopontin promotes mesenchymal stem cell migration and lessens cell stiffness Via integrin Beta1, fak, and erk pathways. Cell Biochem Biophys (2013) 65(3):455–62. doi: 10.1007/s12013-012-9449-8 [DOI] [PubMed] [Google Scholar]
- 94. Liu L, Luo Q, Sun J, Wang A, Shi Y, Ju Y, et al. Decreased nuclear stiffness Via fak-Erk1/2 signaling is necessary for osteopontin-promoted migration of bone marrow-derived mesenchymal stem cells. Exp Cell Res (2017) 355(2):172–81. doi: 10.1016/j.yexcr.2017.04.004 [DOI] [PubMed] [Google Scholar]
- 95. Miranda TS, Napimoga MH, De Franco L, Marins LM, Malta FS, Pontes LA, et al. Strontium ranelate improves alveolar bone healing in estrogen-deficient rats. J Periodontol. (2020) 91(11):1465–74. doi: 10.1002/JPER.19-0561 [DOI] [PubMed] [Google Scholar]
- 96. Choi Y, Kim MH, Nam YK, Kim JH, Cho HY, Yang WM. Palmul-tang, a Korean medicine, promotes bone formation Via bmp-2 pathway in osteoporosis. Front Pharmacol (2021) 12:643482. doi: 10.3389/fphar.2021.643482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. El Khassawna T, Bocker W, Govindarajan P, Schliefke N, Hurter B, Kampschulte M, et al. Effects of multi-Deficiencies-Diet on bone parameters of peripheral bone in ovariectomized mature rat. PLoS One (2013) 8(8):e71665. doi: 10.1371/journal.pone.0071665 [DOI] [PMC free article] [PubMed] [Google Scholar]