Abstract
The status of vitamin D in individuals infected with human immunodeficiency virus (HIV), particularly in naïve as well as treated patients, has never been reported in the Pakistani population. A cross-sectional study was performed to measure vitamin D in individuals infected with HIV living in various districts of the Punjab, Pakistan. 1000 persons attending various treatment centers of the Punjab were screened for HIV, hepatitis B virus (HBV), hepatitis C virus (HCV), and Syphilis. Total 398 patients met inclusion criteria and vitamin D level was measured in respective cases by using enzyme-linked immunosorbent assay (ELISA) technique. 232 samples from the healthy population were also included in present research. Demographic history and clinical parameters regarding HIV disease were evaluated. Comparison of variables was done to find out the link between vitamin D levels and characteristics of HIV infected persons and comparison to that of healthy individuals was performed. Among 398 HIV patients vitamin D deficiency and insufficiency was found among 15 % and 39 % while majority of the control participants had sufficient levels of vitamin D (78 %). Most of the HIV infected individuals were males (68.6 %) and had age between 24 and 47 years (67.8 %). A significant relationship was found for vitamin D level, lifestyle and CD4 count among HIV + ve non acquired immunodeficiency syndrome (AIDS) subjects (95 % CI; p < 0.001, p = 0.09). For HIV + ve AIDS patients vitamin D had a significant relationship with lifestyle along with HIV viral load and CD4 count. Hypovitaminosis D prevails among the HIV infected population of Punjab, Pakistan.
Keywords: Hypovitaminosis D, HIV/AIDS, HBV/HCV, ELISA, CD4 count
1. Introduction
Vitamin D, a steroid hormone, can be synthesized endogenously or exogenously via food or dietary supplements (Ali, 2020) and is a standard hormone characterized for its pivotal role in skeletal and mineral homeostasis. After exposure to UV rays from the sun, 7-dihydrocholesterol in the skin is converted to preVitD3. PreVitD3 is then transformed into cholecalciferol via spontaneous isomerization (VitD3) and is carried to the kidney and gets hydroxylated to form 1, 25 dihydroxycholecalciferol (1,25(OH)2D) or calcitriol and locally activated by CYP27B1 (Kim et al., 2020) in numerous tissues including immune cells, brain, breast, prostate and smooth muscles (Dusso et al., 2005, Jiménez-Sousa et al., 2018). The major hormonal activity of vitamin D is linked with intestinal calcium transport, renal calcium absorption, insulin secretion, osteogenesis, blood pressure regulation, and apoptosis (Skrobo et al., 2018). In order to initiate all biological processes and to be transported to all tissues 1,25(OH)2D form a complex with vitamin d-binding protein (DBP), an essential substrate to maintain the level of vitamin D in the body (Callejo et al., 2020). Vitamin D action process is interceded by interaction with high-affinity transcription factor VDR. The d-VDR complex modulates expression of gene at the transcriptional stage (Prietl et al., 2013). In addition to classical function, vitamin D also involves the following non-classical functions; immune responses, hormonal secretion, cell proliferation, and maturation (Xu et al., 2020, Dimitrov et al., 2021).
Vitamin D acts as a potent immunomodulator expressing intracellular VDR on macrophages, monocytes, and lymphocytes (T and B cells) (Holick, 2007, Bishop et al., 2020). The first evidence of vitamin D's effect on the immune cells in both innate and adaptive immune responses emerged approximately-three years ago (Penna et al., 2005, Bikle, 2009). It regulates the innate immune system by increasing the synthesis of β2 defensins and cationic antimicrobial peptide (CAMP), cathelicidin by macrophages and monocytes, hence enhancinptig their antibacterial activity (Dai et al., 2010). The immunomodulatory effect of vitamin D in adave immune response suggests that it inhibits T-helper1 cell (Th1) activation and synchronizes T-helper2 cell (Th2), T regulatory cell (Treg) and T-helper17 cell activity (Th17) (Boonstra et al., 2001). Vitamin D effect on cell differentiation is affirmed through antigen-presenting (APC) cells and dendritic cells, involved in T cells differentiation (Bishop et al., 2020). Dendritic cell differentiation within a vitamin-D microenvironment regulates a “tolerogenic state” characterized by decreased inflammatory cytokine levels (i.e., IL-12 AND TNF-) and enhanced anti-inflammatory cytokine levels (IL-10), which predominantly upregulate the development of regulatory T cells and promote cell death of autoreactive T-cells (Unger et al., 2009, Bishop et al., 2020).
Vitamin D importance in regulating immune responses revealed that its insufficiency is linked with an increased risk of a large number of comorbidities, including metabolic disorders, cardiovascular disorders, osteoporosis, diabetes, autoimmune diseases, cognitive disorders, and certain malignancies (Colotta et al., 2017, Charoenngam and Holick, 2020). Vitamin D deficiency is a worldwide condition with a high occurrence in the general population of both developing (Middle East and subcontinent) and developed nations (Raza et al., 2019).
Clinically, hypovitaminosis is described as a serum D (25[OH]D) concentration of 20 ng/ml (Holick, 2007) in both the general population and HIV-infected individuals (Lappe et al., 2007). Sunlight deprivation, black race, malnutrition, low nutritional intake, obesity, high altitude living, and usage of medicines that stimulate catabolism of vitamin D, like glucocorticoids and anticonvulsants, are all well known vitamin D deficiency risk factors (Bischoff-Ferrari et al., 2009). The deficiency of vitamin D is also linked with the prevalence of opportunistic infections and HIV exacerbation, leading to death in untreated patients (Bischoff-Ferrari et al., 2009).
Vitamin D helps to increase the lifespan of individuals infected with HIV through a therapy i.e., highly active antiretroviral therapy (HAART) but the risk of comorbidities remains elevated in comparison to the general population, most likely due to immune-suppression (Collins et al., 2020). Another study described that vitamin D and CD4 + T cells made an undeviating association and decreased absolute CD4 + T cells were found among HIV-positive people who were vitamin D deficient after starting HAART (Rosenvinge et al., 2010).
A metabolite of vitamin D, (25[OH]D), is served to determine the serum vitamin D concentration in humans (DeLuca, 2004). Although the 1,25-dihydroxy vitamin D (1,25[OH]2D) is the active vitamin D metabolite, routinely it can not be estimated due to its stringent regulation and short half life (Wasserman and Rubin, 2010). Quantifying 1,25[OH]2D alongside 25[OH]D may help to identify other diseases of 1- hydroxylation, 25[OH]D renal conversion to its active form, and irregular extra-renal synthesis of 1,25[OH]2D (systemic infections, sarcoidosis, and hematological disorders) (Wasserman and Rubin, 2010). There is a pressing need to investigate the vitamin D levels of the HIV-infected population. Immunocompromised population has an increased risk of suffering hypovitaminosis-related consequences. Consequently, the present study intends to investigate vitamin D levels in immunocompromised populations, i.e. HIV-infected individuals from different areas of Punjab, Pakistan.
2. Materials and methods
2.1. Study design and sample collection
In order to conduct this study, n = 1000 subjects were recruited between 2018 and 2019 from Voluntary Confidential Counseling and Testing (VCCT) Centers in Punjab, including Allied hospital Faisalabad, DHQ hospital Chiniot, Aziz Bhatti Shaheed Hospital Gujrat, Benazir Bhutto Shaheed hospital Rawalpindi, DHQ hospital Bahawalnagar, and DHQ hospital DG Khan (Fig. S1). Control blood samples (n = 232) were drawn from healthy populations that have not any known infectious or metabolic disorder. An informed consent about the study was obtained from patients infected with HIV and controls prior to sample collection. A questionnaire was created per the sample collecting inclusion and exclusion criteria. HIV positive status was a prerequisite for inclusion criteria. The co-infected patients with HBV, HCV and syphilis were excluded. The research questionnaire assessed demographic history (age, gender, district, and history of HIV treatment) and lifestyle (urban and rural).
The 5CC Ethylenediaminetetraacetic acid (EDTA) whole blood was taken using the World Health Organization (WHO)-recommended venipuncture method. The whole blood was used to obtain a CD4 count, after which plasma was isolated for HIV-PCR by centrifugation. These specimens were then processed in the Advanced diagnostic laboratory. Following the rapid screening test, samples infected with hepatitis B, C, and/or syphilis were excluded from this investigation. Also, subjects who were drug addicts or had infections following surgery were excluded. The present research work was recommended by the research ethics and biosafety committee of the Institute.
2.2. Rapid testing for HIV
Initially, samples were tested for HIV Ag/Ab, HBV, and HCV using “AlereTM HIV Ag/Ab,” “AlereTM HBs Ag,” and “SD Bioline anti-HCV Ab” quick diagnostic machines, respectively.
2.3. CD4 cell count
The CD4 count was performed on “PIMATM analyzer” using 25 µl whole blood in PIMATM CD4 cartridge. It is an image-based, automated immunological assay intended for rapid in vitro measurement of CD4 cells (T helper cells).
2.4. RNA extraction
Selected samples were treated for RNA extraction using a kit (QIAamp Viral RNA Mini Kit), and a buffer containing guanidine-thiocyanate was used to lyse and homogenize the samples in order to purify and collect the intact RNA. To ensure the appropriate binding conditions, ethanol was added and then any contaminants present were washed away using the RNeasy Mini spin column. Then, high-quality RNA was eluted in 70 ul of AVE buffer.
2.5. RNA amplification
HIV RNA was amplified using the Artus HI Virus-1 RG RT-PCR Kit (the limit of detection for HIV viral load was 71 IU/ml). For PCR amplification, a 50 µl reaction mix was prepared in the “Rotor Gene” cup by adding 30 µl MM (master mix) and 20 µl of extracted RNA. “Rotorgene” was used to amplify the reaction mixture. After performing RT-PCR for HIV viral load, 398 HIV-positive samples were chosen for additional vitamin D analysis.
2.6. 25(OH)D estimation
The vitamin D assay was performed by EUROIMMUN 25-OH vitamin D test kit (Noori et al., 2018). The vitamin D status was categorized as sufficient (≥30 ng/ml), insufficient (11–29 ng/ml), or deficient (≤10 ng/ml) (Ginde et al., 2009).
2.7. Statistical analysis
Using the laboratory management information system (LMIS) software of PACP-ADL, the patient's age, gender, and area of residence were recorded. SPSS 28.0.0.0 was used to tabulate and analyze the data for all conducted tests and demographic variables. Chi square test was done to see the association of serum 25(OH) D and p-value<0.05 was considered as significant.
3. Results
3.1. Regional distribution
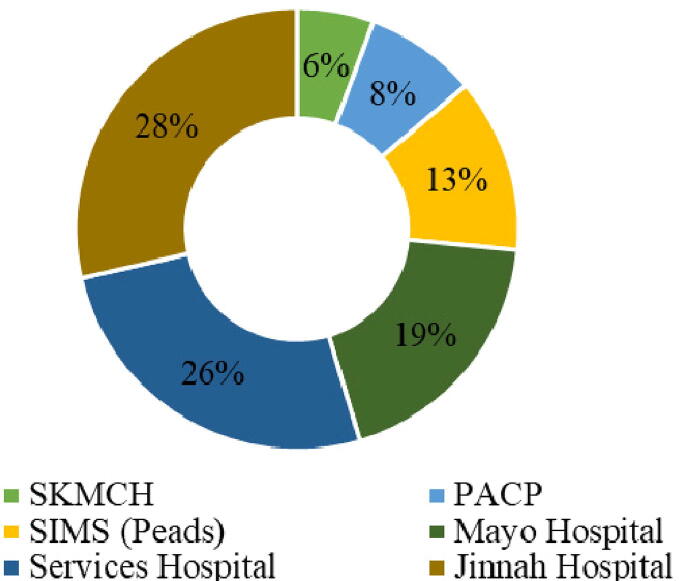
Blood samples received from various HIV treatment centers of the Punjab i.e., Aziz Bhatti Shaheed hospital (ABS) Gujrat, Allied hospital Faisalabad, Benazir Bhutto Shaheed hospital (BBSH) Rawalpindi, DHQ hospital Bahawalnagar, DHQ hospital Chiniot, DHQ hospital DG Khan and various places from Lahore (Fig. S1). Most of the HIV samples in Lahore (n = 196) region were collected at Jinnah Hospital Lahore 56 (28.6 %) followed by Services Hospital Lahore 51 (26 %) (Fig. 1).
Fig. 1.
Samples collected from various places of Lahore district (Punjab) from period of March 2018 to July 2019.
3.2. Clinical characteristics of patients and controls
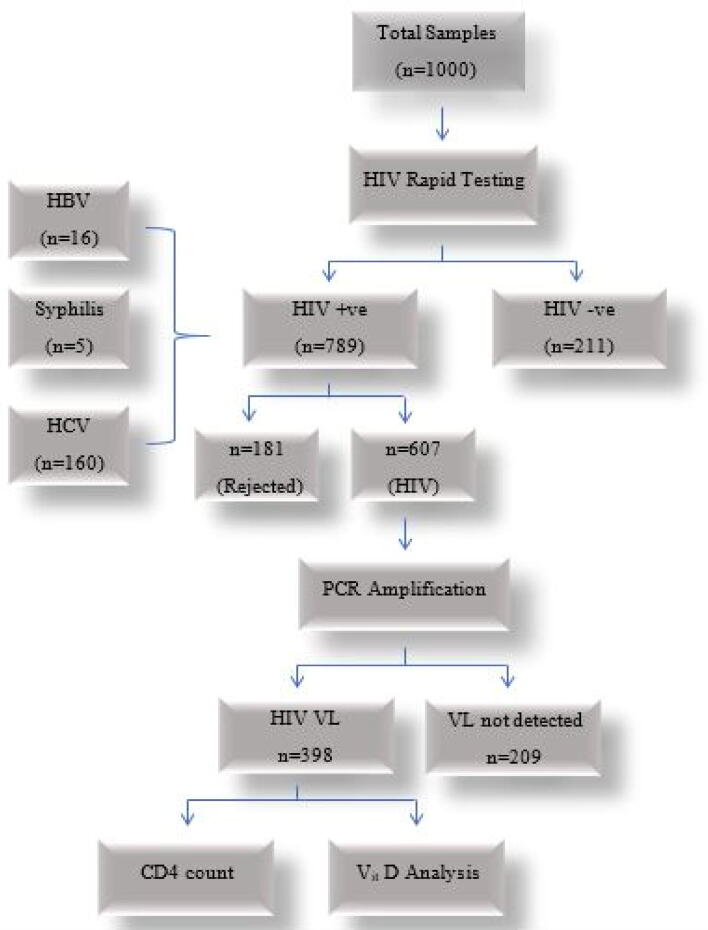
A total number of 789 HIV positive samples were obtained after HIV ICT screening out of total 1000 samples and 181 samples were rejected for HCV, HBV and Syphilis co-infection as an exclusion criteria. A subject was considered hepatitis C or B positive if one had anti-HCV antibody or HBsAg positive on ICT device. The remaining 607 samples were further processed for PCR amplification to rule out for the detection of viral load. After following all the screening steps, 398 samples with HIV viral load were preferred for CD4+ count assessment and Vitamin D analysis (Fig. 2). Demographic characteristics of the HIV positive patients such as age, gender, lifestyle and use of cART were investigated. Most of the studied patients were males 275 (69.09 %). TGs were also reported in present study but in low numbers i.e., 6 (1.5 %). Most of the studied population 270 (68 %) was aged between “24–47 years” followed by “1–23 years” 92 (23 %) and was mostly from urban areas i.e., 256 (64.30 %). HIV risk groups were also identified in the studied population. Among them 138 (34.6 %) were intravenous drug users (IDUs) and the general population also had high frequency 90 (22.6 %) after IDUs. In the present study, 279 (70.1 %) samples were of newly diagnosed cases. Controls were also categorized for age and gender (Table 1). Most of the controls were males 155 (67 %) and 139 (60 %) aged between “24–47 years”. In the present study, most of HIV cases have viral load ≤ 10,000 i.e., 233 (56.03 %). CD4 count was also categorized and most of the patients had “200–499 count/µl” i.e., 154 (38.7 %).
Fig. 2.
Flow scheme to evaluate patient samples for experimental setup.
Table 1.
Demographic and clinical characteristics of cases and control subjects.
| Characteristics | No. (%) of participants |
|---|---|
| Cases n = 398 | |
| Sex | |
| Male | 273 (68.6) |
| Female | 119 (29.9) |
| Transgender | 6 (1.5) |
| Age, years | |
| 1–23 | 92 (23.1) |
| 24–47 | 270 (67.8) |
| 48–71 | 36 (9.1) |
| Lifestyle | |
| Rural | 141 (35.4) |
| Urban | 257 (64.6) |
| Risk Groups | |
| Female sex workers | 50 (12.5) |
| General population | 90 (22.6) |
| Intravenous drug users | 138 (34.6) |
| Male sex workers | 70 (17.5) |
| Men who have sex with men | 50 (12.5) |
| Treatment Status | |
| On Therapy | 119 (30) |
| Newly Diagnosed | 279 (70) |
| CD4 count (cells/ul) | |
| <200 | 131 (33) |
| 200–500 | 154(39) |
| >500 | 113(28) |
| Viral Load (copies/ml) | |
| ≤10,000 | 179 (45) |
| >10,000 | 219 (55) |
| Vitamin D | |
| Deficient | 60 (15) |
| Insufficient | 155 (39) |
| Sufficient | 183 (46) |
| Controls n = 232 | |
| Sex | |
| Male | 136 (58.6) |
| Female | 96 (41.4) |
| Age | |
| 1–23 | 74 (31.8) |
| 24–47 | 139 (59.9) |
| 48–71 | 19 (8.1) |
| Vitamin D | |
| Deficient | 2 (0.86) |
| Insufficient | 48 (20.62) |
| Sufficient | 182 (78.42) |
3.3. Deficiency of serum 25(OH)D in population infected with HIV and healthy individuals
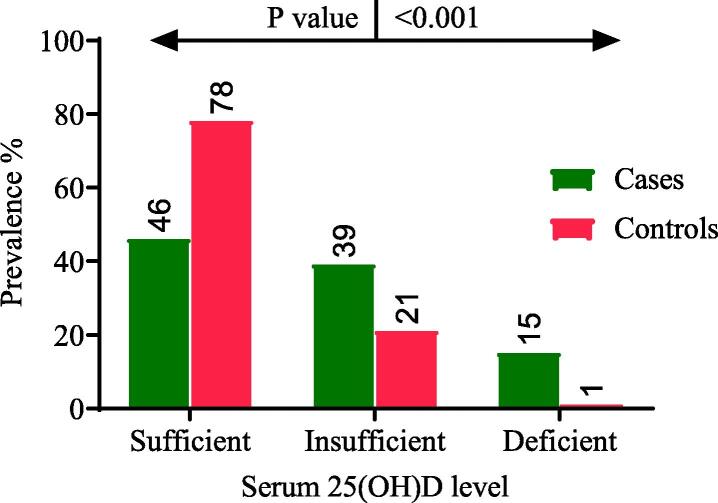
In the current investigation, deficiency and insufficiency of vitamin D was found in 62 (15.6 %) and 156 (39.2 %) patients, respectively. Most of the control participants had sufficient levels of vitamin D 182 (78 %). Results of statistical analysis indicate a noteworthy link of vitamin D deficiency with HIV cases (p < 0.001). The concentration of 25(OH) D in the population infected with HIV and non infected population (controls) has been shown in Fig. 3.
Fig. 3.
Comparison of 25(OH) D levels among cases and controls.
3.4. Serum 25(OH)D association with HIV + ve and AIDS -ve subjects
Among HIV positive, non AIDS cases, all vitamin D categories including vitamin D sufficiency, insufficiency, and deficiency were found in 130, 104, and 33 subjects, respectively. The male population had insufficient 25(OH)D levels (65.6 %). A comparable vitamin D association was found in relation to lifestyle and CD4 count of the participants (p < 0.001 and p = 0.009) as shown in Table 2.
Table2.
Characteristics of HIV positive, Non-AIDS subjects (CD4 > 200; n = 267).
|
Parameter |
Categories |
D: <10 (n = 33) |
IS: 11–29 (n = 104) |
S: >30 (n = 130) |
Chi2 | p Value |
|---|---|---|---|---|---|---|
| No. (%) | No. (%) | No. (%) | ||||
| Gender | Male | 16 (48.4) | 68 (65.6) | 90 (69.7) | * | * |
| Female | 17 (51.6) | 31 (30.3) | 39 (30.3) | |||
| Transgender | 0 | 5 (4.1) | 1 (1) | |||
| Age | 1–23 | 7 (21) | 21 (20) | 37 (28) | 4.93 | 0.29 |
| 24–47 | 25 (76) | 78 (75) | 82 (64) | |||
| 48–71 | 1 (3) | 5 (5) | 11 (8) | |||
| Lifestyle | Rural | 26 (79) | 28 (27) | 32 (25) | 76.2 | <0.001 |
| Urban | 7 (21) | 76 (73) | 98 (75) | |||
| cART status | On therapy | 11 (33) | 35 (34) | 42 (32) | 0.09 | 0.95 |
| Treatment naive | 22 (67) | 69 (66) | 88 (68) | |||
| CD4 count | 200–500 | 24 (73) | 62 (60) | 68 (52) | 9.5 | 0.09 |
| >500 | 9(27) | 42 (40) | 62 (48) | |||
| HIV Viral load | ≤10,000 | 15 (45) | 64 (62) | 72 (55) | 5.87 | 0.53 |
| >10,000 | 18 (55) | 40 (38) | 58 (45) |
*Data was not distributed among all categories so chi2 was not applicable.
3.5. Serum 25(OH)D association with HIV + ve & AIDS + ve subjects
Various parameters of AIDS patients (CD4 count 〈2 0 0) in different categories with respect to vitamin D were analyzed by using SPSS version 28.0. The categorization was done on the basis of amount of vitamin D such as ≤ 10 ng/ml was considered as deficient patient (n = 27), 11 to 29 ng/ml was insufficient patients (n = 51) and ≥ 30 ng/ml was considered as sufficient patients (n = 53). In the category of gender, male and female were included. Deficiency and insufficiency was prevalent among males (70.37 % & 76.4 %) as compared to female patients (29.6 % & 23.5 %). Lifestyles of AIDS patients were classified in terms of rural and urban. Vitamin D deficiency was more prevalent among rural area participants (70.4 %) while insufficiency was seen among urban cases in high frequency (70.5 %). Lifestyle of AIDS patients was significantly linked with concentration of vitamin D (p < 0.001). Among HIV viral load and CD4 count categories, a significant relationship was determined in relation to 25(OH)D with lower CD4 count and viral load ≥ 10,0000 copies/ml (p = 0.01) (Table 3).
Table 3.
HIV positive, AIDS patients (CD4 count < 200; n = 131).
|
Parameter |
Categories |
D: ≤10 (n = 27) |
IS: 11--29 (n = 51) |
S: ≥30 (n = 53) |
Chi2 | p VALUE |
|---|---|---|---|---|---|---|
| No.(%) | No.(%) | No.(%) | ||||
| Gender | Male | 19(70.4) | 39(76.5) | 41(77.4) | 1.5 | 0.471 |
| Female | 8(29.6) | 12(23.5) | 12(22.6) | |||
| Age | 1–23 | 6(22) | 10 (19.6) | 11(20.7) | 7.84 | 0.09 |
| 24–47 | 19(70) | 35(68.6) | 31(58.4) | |||
| 48–71 | 2(7.4) | 6(11.7) | 11(20.7) | |||
| Lifestyle | Rural | 19 (70.4) | 15 (29.5) | 21 (39.6) | 36.22 | <0.001 |
| Urban | 8 (29.6) | 36 (70.5) | 32 (60.4) | |||
| cART status | On therapy | 6(22.3) | 16(31.4) | 9(16.9) | 5.62 | 0.05 |
| Treatment Naïve | 21(77.7) | 35(68.6) | 44(83.1) | |||
| CD4 count | ≤100 | 7(26) | 24(47.1) | 23(43.4) | 10.48 | 0.01 |
| 100–199 | 20(74) | 27(52.9) | 30 (56.6) | |||
| Viral load | ≤10,000 | 3(11.1) | 15(29.4) | 10(18.8) | 10.29 | 0.01 |
| >10,000 | 24(88.9) | 36 (70.5) | 43 (81.2) |
3.6. Relationship among different variables
Pearson correlation depicted the weak negative correlation of vitamin D with viral load and CD4 count (-0.02, −0.025). However, the correlation was non-significant at CI = 95, p > 0.05 (Table S1).
4. Discussion
A condition in which serum vitamin D levels are low known as hypovitaminosis D which correlates a number of medical conditions significantly transitioning bone health. In clinical practice, hypovitaminosis D is generally assessed by insufficient 25-hydroxy vitamin D status in serum. The worldwide cumulative agreement suggests that below the optimal range (<30 ng/ml) is considered as vitamin D deficiency (Holick, 2007, Vieth et al., 2007). However, some studies propose the 25–80 ng/ml as optimal range and consider < 20 ng/ml as more apt to explicate deficiency (Ross et al., 2011). The current work reports that deficiency (15 %) and insufficiency (39 %) of vitamin D was higher among HIV infected individuals than local Pakistani population. Oyedele and Adeyemi reported the variance in prevalence rate of deficiency of vitamin D from 10 to 73 % in the population infected with HIV (Oyedele and Adeyemi, 2012).
Above and beyond the certain discrepancies in heterogeneous study population, the researchers also ruled out the geographic and demographic differences distressing the concentrations of vitamin D in the individuals under trials. They also appraise the schematic approaches and the controversial cut-off points for low vitamin D levels as significant limitations in cross-sectional research involving HIV patients. Other trials stated that HIV-positive carriers frequently encounter vitamin D deficiency (Rodríguez et al., 2009, Mueller et al., 2010) even on successful combined antiretroviral therapy (cART). Well-known risk factors impairing bone maintenance and attributing to low levels of 25(OH)D in HIV infection include minimized exposure to sunlight and nutritive intake, insufficient absorption, fatty liver, and renal damage encouraged anomalous vitamin D activation, altered bioavailability of non-hydroxylated vitamin D in adipose tissue, and the antiretroviral treatment intervening vitamin D metabolism (Cozzolino et al., 2003). Though progressive age is a typical indicator for hypovitaminosis D (Vescini et al., 2011), this relationship was not significant in the present work (p = 0.29; p = 0.09), and is consistent with prior findings in HIV carriers (Wasserman and Rubin, 2010, Dao et al., 2011, Allavena et al., 2012). Allavena et al. did not observe substantial correlation between age and VDD, one of the justifications may comprise the samples from younger HIV cohorts with few patients over 60 years of age, identical to the current research, which consisted majority of patients ranging within 23–47 years of age (Allavena et al., 2012). The current work revealed that insignificant results of gender and hypovitaminosis D (p = 0.471) consistent with other studies conducted on HIV infected individuals (Wasserman and Rubin, 2010, Allavena et al., 2012, Kwan et al., 2012). The majority of the population in study belonged to urban areas 257 (64.6 %). A notable link was observed between lifestyle and vitamin D status of HIV/AIDS & HIV/non AIDS patients (p < 0.001). Vitamin DD was mostly observed among rural populations. Although there are certain confounding factors for VDD in urban populations such as the sedentary lifestyle, growing urbanization and industrial development may also reduce contact with sunlight but the people usually take supplements in order to meet vitamin deficiencies.
The association among 25(OH)D concentrations along with viral load and CD4+ T-cell count is contradictory. Few clinical trials approved a positive correlation (Aziz et al., 2013) while some failed to reveal a significant association (Gedela et al., 2014)). Another study described that lower CD4+ T cell count is linked with deficiency of vitamin D in individuals infected with HIV (Zhang et al., 2017). Present study findings are in good agreement with the results of Zhang et al. (2017) i.e., a significant relationship is established in CD4 count and concentration of vitamin D among HIV/AIDS (p = 0.01) in the current study. Several other investigations have also associated lower 25(OH)D concentration to lower CD4+ T cell counts (Welz et al., 2010, Sudfeld et al., 2012). Because of complexity in their nature, several mechanisms involved in elucidating the link between chronic HIV disease and resultant 25(OH)D decrease are unclear. The individuals with immunosuppression are usually susceptible to a number of clinical complications; one may hypothesize a cause of HIV infection related to VDD. Secondly, as the viral infection progresses to chronic inflammation, elevated certain pro-inflammatory cytokines like TNF-α may interfere with the parathyroid hormone secretion (PTH). The PTH is the stimulatory hormone for the active form of vitamin D i.e. 1,25-dihydroxyvitamin D and its reduced release may result in renal 1α-hydroxylase impairment. Third, patients with low immunity and decreased CD4+ immune cells are more prone to infectious complications and they may have a reduced sun exposure as a contributing factor for hypovitaminosis D. Hospitalization may also be considered an option for food intake limitations, insufficient absorption and eventually malnutrition (Mansueto et al., 2015).
In the current work, viral load showed no significant relationship with vitamin D status in HIV/Non-AIDS (p = 0.53) however, HIV/AIDS subjects had a link among these parameters (p = 0.01). Bearden et al. (2013) reported a delicate association, statistically insignificant (p = 0.36), between HIV viral load and vitamin D levels. Among HIV/Non-AIDS subjects, most of the patients had viral load ≤ 10000 while the majority in HIV/AIDS group had > 10000. So increased prevalence VDD in the HIV/AIDS subjects could be explained by the likelihood of interactions between lipopolysaccharide (LPS), toll-like receptor (TLR) signaling pathways, HIV viremia, and proinflammatory cytokines with activation of 25-hydroxyvitamin D-1α-hydroxylase (CYP27B1) in macrophages. Uncontrolled HIV viral loads elvate LPS and proinflammatory cytokines through impaired gut-linked tissues (Anselmi et al., 2007, Brenchley and Douek, 2008). The up-regulation of 1,25(OH)2D receptor-specific Cyp24 mRNA and cathelicidin mRNA was not determined in the absence of vitamin D [25(OH)D] indicating the crucial role of vitamin D in the regulation of such molecules (Liu et al., 2006).
In the current investigation, a remarkable borderline relationship was determined in 25(OH)D levels between naïve and patients under antiretroviral therapy among HIV/AIDS subjects (p = 0.05). Aziz et al. (2013) findings showed recovered 25(OH)D3 levels in HAART-treated HIV carriers as compared to the newly infected patients. This recovery is ascribed to the sufficient vitamin D supplementation in patients infected with HIV. There are certain confounders such as the kind of ART given to the patient. Moreover, the present study didn’t include certain individual antiretroviral drugs. Efavirenz interacts with the enzymes responsible for vitamin D metabolism (cytochrome P450 monooxygenases) and potentially induces the enzymes involved in hydroxylation of vitamin D3 to 25(OH)D3 (CYP3A4) and catabolism of 1,25(OH)2D to inactive forms (CYP24) along with reduced CYP2R1 transcription in HIV populations (Kim et al., 2012).
Hypovitaminosis D is a risk factor for developing comorbidities including infectious diseases as well as immune disorders in patients infected with HIV. The status of vitamin D of a large cohort of HIV infected patients in comparison to that of the general population particularly in naïve as well as treated patients, is under researched in Pakistani population.
5. Conclusion
In conclusion, the present work documents the pervasiveness of vitamin D insufficiency or deficiency among a large (n = 398) group of HIV-infected subjects in comparison with that in the general population. HIV subjects have a greater pervasiveness of VDD deficiency in comparison to the local population. Vitamin D deficiency occurrence rate was greater in male population infected with HIV and those living in urban areas of Punjab, Pakistan. A strong relationship was determined between the lifestyle and vitamin D level. The significant associations of low concentrations of 25(OH)D, HIV viral load, and CD4 count among AIDS individuals culminate the requirement to assess insufficiency or deficiency of vitamin D as routine care of HIV infected population.
Funding
No funding was obtained.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.sjbs.2022.103484.
Contributor Information
Wajiha Kanwal, Email: wajiha.phd.mmg@pu.edu.pk.
Abdul Rehman, Email: rehman.mmg@pu.edu.pk.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- Ali N. Role of vitamin D in preventing of COVID-19 infection, progression and severity. J. Infect. Public Health. 2020;10:1373–1380. doi: 10.1016/j.jiph.2020.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allavena C., Delpierre C., Cuzin L., Rey D., Viget N., Bernard J., Guillot P., Duvivier C., Billaud E., Raffi F. High frequency of vitamin D deficiency in HIV-infected patients: effects of HIV-related factors and antiretroviral drugs. J. Antimicrob. Chemother. 2012;67(9):2222–2230. doi: 10.1093/jac/dks176. [DOI] [PubMed] [Google Scholar]
- Anselmi, A., Vendrame, D., Rampon, O., et al. 2007. Immune reconstitution in human immunodeficiency virus type 1-infected children with different virological responses to anti-retroviral therapy. Clin. Exp. Immunol. 150(3), 442–450. [DOI] [PMC free article] [PubMed]
- Aziz M., Livak B., Burke-Miller J., French A.L., Glesby M.J., Sharma A., Young M., Villacres M.C., Tien P.C., Golub E.T., Cohen M.H., Adeyemi O.M. Vitamin D insufficiency may impair CD4 recovery among women’s interagency HIV study participants with advanced disease on HAART. AIDS. 2013;27(4):573–578. doi: 10.1097/QAD.0b013e32835b9ba1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bearden A., Abad C., Gangnon R., Sosman J.M., Binkley N., Safdar N. Cross-sectional study of vitamin D levels, immunologic and virologic outcomes in HIV-infected adults. J. Clin. Endocrinol. Metabol. 2013;98:1726–1733. doi: 10.1210/jc.2012-4031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D. Nonclassic actions of vitamin D. J. Clin. Endocrinol. Metabol. 2009;94:26–34. doi: 10.1210/jc.2008-1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari H.A., Willett W.C., Wong J.B., Stuck A.E., Staehelin H.B., Orav E.J., Thoma A., Kiel D.P., Henschkowski J. Prevention of nonvertebral fractures with oral vitamin D and dose dependency: a meta-analysis of randomized controlled trials. Arch. Intern. Med. 2009;169:551–561. doi: 10.1001/archinternmed.2008.600. [DOI] [PubMed] [Google Scholar]
- Bishop E., Ismailova A., Dimeloe S.K., Hewison M., White J.H. Vitamin D and immune regulation: antibacterial, antiviral, anti-inflammatory. J. Biomed. Mater. Res. Plus. 2020;5:e10405. doi: 10.1002/jbm4.10405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonstra A., Barrat F.J., Crain C., Heath V.L., Savelkoul H.F.J., O’Garra A. 1α, 25-Dihydroxyvitamin D3 has a direct effect on naive CD4+ T cells to enhance the development of Th2 cells. J. Immunol. 2001;167(9):4974–4980. doi: 10.4049/jimmunol.167.9.4974. [DOI] [PubMed] [Google Scholar]
- Brenchley J.M., Douek D.C. The mucosal barrier and immune activation in HIV pathogenesis. Curr. Opin. HIV AIDS. 2008;3(3):356–361. doi: 10.1097/COH.0b013e3282f9ae9c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callejo M., Mondejar-Parreno G., Esquivel-Ruiz S., Olivencia M.A., Moreno L., Blanco I., Escribano-Subias P., Cogolludo A., Barbera J.A., Perez-Vizcaino F. Total, bioavailable, and free vitamin D levels and their prognostic value in pulmonary arterial hypertension. J. Clin. Med. 2020;9:448. doi: 10.3390/jcm9020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charoenngam N., Holick M.F. Immunologic effects of vitamin D on human health and disease. Nutrients. 2020;12:2097. doi: 10.3390/nu12072097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins L.F., Moran C.A., Oliver N.T., Moanna A., Lahiri C.D., Colasanti J.A., Kelley C.F., Nguyen M.L., Marconi V.C., Armstrong W.S., Ofotokun I., Sheth A.N. Clinical characteristics, comorbidities and outcomes among persons with HIV hospitalized with coronavirus disease 2019 in Atlanta, Georgia. AIDS. 2020;34:1789–1794. doi: 10.1097/QAD.0000000000002632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colotta F., Jansson B., Bonelli F. Modulation of inflammatory and immune responses by vitamin D. J. Autoimmun. 2017;85:78–97. doi: 10.1016/j.jaut.2017.07.007. [DOI] [PubMed] [Google Scholar]
- Cozzolino M., Vidal M., Arcidiacono M.V., Tebas P., Yarasheski K.E., Dusso A.S. HIV-protease inhibitors impair vitamin D bioactivation to 1, 25-dihydroxyvitamin D. AIDS. 2003;17(4):513–520. doi: 10.1097/00002030-200303070-00006. [DOI] [PubMed] [Google Scholar]
- Dai X., Sayama K., Tohyama M., Shirakata Y., Hanakawa Y., Tokumaru S., Yang L., Hirakawa S., Hashimoto K. PPARγ mediates innate immunity by regulating the 1α, 25-dihydroxyvitamin D3 induced hBD-3 and cathelicidin in human keratinocytes. J. Dermatol. Sci. 2010;60:179–186. doi: 10.1016/j.jdermsci.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Dao C.N., Patel P., Overton E.T., Rhame F., Pals S.L., Johnson C., Bush T., Brooks J.T. HIV StUtNHo, Investigators AitEoET: Low vitamin D among HIV-infected adults: Prevalence of and risk factors for low vitamin D Levels in a cohort of HIV-infected adults and comparison to prevalence among adults in the US general population. Clin. Infect. Dis. 2011;52:396–405. doi: 10.1093/cid/ciq158. [DOI] [PubMed] [Google Scholar]
- DeLuca H.F. Overview of general physiologic features and functions of vitamin D. Am. J. Clin. Nutr. 2004;80:1689S–1696S. doi: 10.1093/ajcn/80.6.1689S. [DOI] [PubMed] [Google Scholar]
- Dimitrov V., Barbier C., Ismailova A., Wang Y., Dmowski K., Salehi-Tabar R., Memari B., Groulx-Boivin E., White J.H. Vitamin D-regulated gene expression profiles: Species-specificity and cell-specific effects on metabolism and immunity. Endocrinology. 2021;162:bqaa218. doi: 10.1210/endocr/bqaa218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dusso A.S., Brown A.J., Slatopolsky E. Vitamin D. Am. J. Physiol. Renal Physiol. 2005;289(1):F8–F28. doi: 10.1152/ajprenal.00336.2004. [DOI] [PubMed] [Google Scholar]
- Gedela K., Edwards S.G., Benn P., Grant A.D. Prevalence of vitamin D deficiency in HIV-positive, antiretroviral treatment-naïve patients in a single centre study. Int. J. STD AIDS. 2014;25(7):488–492. doi: 10.1177/0956462413515194. [DOI] [PubMed] [Google Scholar]
- Ginde A.A., Liu M.C., Camargo C.A. Demographic differences and trends of vitamin D insufficiency in the US population, 1988–2004. Archives of internal medicine. 2009;169(6):626–632. doi: 10.1001/archinternmed.2008.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holick M.F. Vitamin D deficiency. N. Engl. J. Med. 2007;357:266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- Jiménez-Sousa M.A., Martínez I., Medrano L.M., Fernández-Rodríguez A., Resino S. Vitamin D in human immunodeficiency virus infection: influence on immunity and disease. Front. Immunol. 2018;9:458. doi: 10.3389/fimmu.2018.00458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Gandhi V., Psevdos G., Espinoza F., Park J., Sharp V. Evaluation of vitamin D levels among HIV-infected patients in New York City. AIDS Res. Hum. Retrovir. 2012;28(3):235–241. doi: 10.1089/AID.2011.0040. [DOI] [PubMed] [Google Scholar]
- Kim H.A., Perrelli A., Ragni A., Retta F., De Silva T.M., Sobey C.G., Retta S.F. Vitamin D deficiency and the risk of cerebrovascular disease. Antioxidants. 2020;9:327. doi: 10.3390/antiox9040327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwan C.K., Eckhardt B., Baghdadi J., Aberg J.A. Hyperparathyroidism and complications associated with vitamin D deficiency in HIV-infected adults in New York City. New York. AIDS Res. Hum. Retrovir. 2012;28(9):1025–1032. doi: 10.1089/aid.2011.0325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lappe J.M., Travers-Gustafson D., Davies K.M., Recker R.R., Heaney R.P. Vitamin D and calcium supplementation reduces cancer risk: results of a randomized trial. Am. J. Clin. Nutr. 2007;85:1586–1591. doi: 10.1093/ajcn/85.6.1586. [DOI] [PubMed] [Google Scholar]
- Liu P.T., Stenger S., Li H., et al. Toll-like receptor triggering of a vitamin D-mediated human antimicrobial response. Science. 2006;311(5768):1770–1773. doi: 10.1126/science.1123933. [DOI] [PubMed] [Google Scholar]
- Mansueto, P., Seidita, A., Vitale, G., Gangemi, S., Iaria, C., Cascio, A. 2015 Vitamin D deficiency in HIV infection: Not only a bone disorder. Biomed. Res. Int. 2015. 735615. [DOI] [PMC free article] [PubMed]
- Mueller N.J., Fux C.A., Ledergerber B., Elzi L., Schmid P., Dang T., Magenta L., Calmy A., Vergopoulos A., Bischoff-Ferrari H.A. High prevalence of severe vitamin D deficiency in combined antiretroviral therapy-naive and successfully treated Swiss HIV patients. AIDS. 2010;24:1127–1134. doi: 10.1097/QAD.0b013e328337b161. [DOI] [PubMed] [Google Scholar]
- Noori N.M., Nakhaee Moghadam M., Teimouri A., Pakravan A., Boryri T. 25-hydroxy vitamin D serum levels in congenital heart disease (CHD) children compared to controls. Int. J. Pediatr. 2018;6(8):8129–8138. [Google Scholar]
- Oyedele T., Adeyemi O.M. High prevalence of vitamin D deficiency in HIV-infected adults: what are the future research questions? Curr. HIV/AIDS Rep. 2012;9(1):1–4. doi: 10.1007/s11904-011-0101-9. [DOI] [PubMed] [Google Scholar]
- Penna G., Roncari A., Amuchastegui S., Daniel K.C., Berti E., Colonna M., Adorini L. Expression of the inhibitory receptor ILT3 on dendritic cells is dispensable for induction of CD4+ Foxp3+ regulatory T cells by 1, 25-dihydroxyvitamin D3. Blood. 2005;106:3490–3497. doi: 10.1182/blood-2005-05-2044. [DOI] [PubMed] [Google Scholar]
- Prietl B., Treiber G., Pieber T.R., Amrein K. Vitamin D and immune function. Nutrients. 2013;5:2502–2521. doi: 10.3390/nu5072502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raza, A., Syed, J.G., Ali, F.M., Khan, M.D., Khan, M.A., Haleem, F., Naeem, R. 2019. Incidence of vitamin D deficiency in different seasons in the adult Karachi population. [DOI] [PMC free article] [PubMed]
- Rodríguez M., Daniels B., Gunawardene S., Robbins G.K. High frequency of vitamin D deficiency in ambulatory HIV-positive patients. AIDS Res. Hum. Retrovir. 2009;25(1):9–14. doi: 10.1089/aid.2008.0183. [DOI] [PubMed] [Google Scholar]
- Rosenvinge M.M., Gedela K., Copas A.J., Wilkinson A., Sheehy C.A., Bano G., Hay P.E., Pakianathan M.R., Sadiq S.T. Tenofovir-linked hyperparathyroidism is independently associated with the presence of vitamin D deficiency. J. Acquir. Immun. Defic. Syndr. 2010;54:496–499. doi: 10.1097/qai.0b013e3181caebaa. [DOI] [PubMed] [Google Scholar]
- Ross A.C., Manson J.E., Abrams S.A., Aloia J.F., Brannon P.M., Clinton S.K., Durazo-Arvizu R.A., Gallagher J.C., Gallo R.L., Jones G. The 2011 report on dietary reference intakes for calcium and vitamin D from the Institute of Medicine: What clinicians need to know. J. Clin. Endocrinol. Metabol. 2011;96:53–58. doi: 10.1210/jc.2010-2704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrobot A., Demkow U., Wachowska M. Immunomodulatory role of vitamin D: A review. Adv. Exp. Med. Biol. 2018;1108:13–23. doi: 10.1007/5584_2018_246. [DOI] [PubMed] [Google Scholar]
- Sudfeld, C.R., Wang, M., Aboud, S., Giovannucci, E.L., Mugusi, F.M., Fawzi, W.W. 2012. Vitamin D and HIV progression among Tanzanian adults initiating antiretroviral therapy. PloS One 7, e40036. [DOI] [PMC free article] [PubMed]
- Unger, W.W., Laban, S., Kleijwegt, F.S., van der Slik, A.R., Roep, B.O. 2009. Induction of Treg by monocyte‐derived DC modulated by vitamin D3 or dexamethasone: differential role for PD‐L1. Eur. J. Immunol. 39, 3147-3159. [DOI] [PubMed]
- Vescini F., Cozzi-Lepri A., Borderi M., Re M.C., Maggiolo F., De Luca A., Cassola G., Vullo V., Carosi G., Antinori A., Tozzi V., Monforte A.d. Prevalence of hypovitaminosis D and factors associated with vitamin D deficiency and morbidity among HIV-infected patients enrolled in a large Italian cohort. J. Acquir. Immune Defic. Syndr. 2011;58(2):163–172. doi: 10.1097/QAI.0b013e31822e57e9. [DOI] [PubMed] [Google Scholar]
- Vieth R., Bischoff-Ferrari H., Boucher B.J., Dawson-Hughes B., Garland C.F., Heaney R.P., Holick M.F., Hollis B.W., Lamberg-Allardt C., McGrath J.J., Norman A.W., Scragg R., Whiting S.J., Willett W.C., Zittermann A. The urgent need to recommend an intake of vitamin D that is effective. Am. J. Clin. Nutr. 2007;5:649–650. doi: 10.1093/ajcn/85.3.649. [DOI] [PubMed] [Google Scholar]
- Wasserman P., Rubin D.S. (2010) Highly prevalent vitamin D deficiency and insufficiency in an urban cohort of HIV-infected men under care. AIDS patient care and STDs. 2010;24:223–227. doi: 10.1089/apc.2009.0241. [DOI] [PubMed] [Google Scholar]
- Welz T., Childs K., Ibrahim F., Poulton M., Taylor C.B., Moniz C.F., Post F.A. Efavirenz is associated with severe vitamin D deficiency and increased alkaline phosphatase. AIDS. 2010;24:1923–1928. doi: 10.1097/QAD.0b013e32833c3281. [DOI] [PubMed] [Google Scholar]
- Xu Y., Baylink D.J., Chen C.-S., Reeves M.E., Xiao J., Lacy C., Lau E., Cao H. The importance of vitamin d metabolism as a potential prophylactic, immunoregulatory and neuroprotective treatment for COVID-19. J. Transl. Med. 2020;18:1–12. doi: 10.1186/s12967-020-02488-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L., Tin A., Brown T.T., Margolick J.B., Witt M.D., Palella F.J., Kingsley L.A., Hoofnagle A.N., Jacobson L.P., Abraham A.G. Vitamin D deficiency and metabolism in HIV-infected and HIV-uninfected men in the Multicenter AIDS Cohort Study. AIDS Res. Hum. Retrovir. 2017;33(3):261–270. doi: 10.1089/aid.2016.0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.