Abstract
The urinary bladder and lower urinary tract of domestic and wild carnivores can be parasitised by filamentous nematodes from the genus Pearsonema (syn. Capillaria). Infestations are often asymptomatic, but severe courses in dogs and cats have been described. Hosts are infested through the ingestion of earthworms (Lumbricidae) which act as intermediate hosts. Epidemiological studies of Pearsonema in raccoons (Procyon lotor) in North America are scarce and previous studies of urinary bladder parasites of European raccoons did not provide evidence of infestation. We examined urine sediment or rinse water from urinary bladders of 499 wild raccoons from Luxembourg, Poland and five study sites in Germany. Pearsonema eggs were found in the urine sediment of 31 (6.2%) raccoons. Infested animals were found in all study areas with prevalence values ranging from 3.7% to 8.7%. No significant difference in prevalence was found either between animals in urban and rural areas or between sexes and age classes. Based on their morphology, the eggs were likely to be P. plica. Considering their increasing density in Central Europe, raccoons may play a previously overlooked role in environmental contamination with Personema eggs.
Keywords: Pearsonema, Bladderworm, Procyon lotor, Raccoon, Wildlife, Reservoir host
Graphical abstract
Highlights
-
•
Invasive raccoons are reservoirs for Pearsonema spp. in Central Europe.
-
•
We tested urinary bladders from 499 raccoons from Luxembourg, Poland and Germany for the presence of Pearsonema eggs.
-
•
Thirty-one (6.2%) raccoons tested positive, with infections found in all study areas with a low prevalence.
-
•
This is the first report of Pearsonema spp. in raccoons in Europe and outside their original range in North America.
1. Introduction
In Europe, the urinary bladder and the lower urinary tract of domestic and wild carnivores can be parasitised by three species of filamentous nematodes from the genus Pearsonema (syn. Capillaria; family Capillaridae). The cosmopolitain bladderworm Pearsonema plica can been found in Canidae - especially red foxes (Vulpes vulpes) and dogs (Canis lupus familiaris) - and sometimes Felidae and Mustelidae (Moravec et al., 1987; Basso et al., 2013). P. mucronata infests several species of mustelids (Moravec et al., 1987; Varodi et al., 2017), while the presence of P. feliscati has been reported from domestic cats (Felis catus; Beugnet and Knaus, 2015) and sometimes from wild felids (Bagrade et al., 2003; Krone et al., 2008). Infestations with Pearsonema nematodes are usually asymptomatic, but severe cases of ‘urinary capillariosis’, characterised among others by dysuria, fever and urinary incontinence, have been described in both domestic dogs and cats (Beugnet and Knaus, 2015; Ilić et al., 2021).
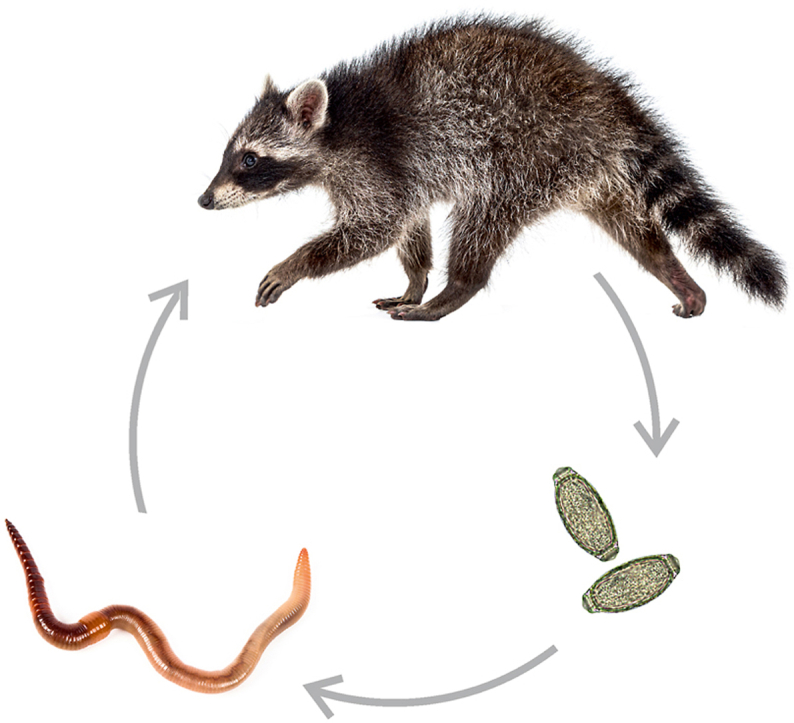
The life cycles of P. plica and P. mucronata are relatively similar. The carnivores act as final hosts and excrete the eggs of the nematodes via their urine. In the environment, Pearsonema eggs are ingested by earthworms (Lumbricidae), which act as obligate intermediate hosts and where the larvae develop to the L1 infective stage (Moravec et al., 1987). Final hosts become infested after ingestion of infested earthworms. In case of P. plica, the second stage larvae (L2) develop in the wall of the small intestine of the final host and remain there for 8–10 days. The third stage larvae (L3) migrate to the urinary bladder and develop into the mature nematode within 60 days of infestation (Low, 1999). The life cycle of P. feliscati has not been investigated, but is believed to be similar. Since cats eat earthworms only rarely, they are likely to become infested via paratenic hosts, i.e. their prey species that do feed on earthworms (Beugnet and Knaus, 2015).
Even though the Pearsonema nematodes and their pathogenic potential have been known for some time, prevalence studies in Central Europe have been conducted almost exclusively on the red fox, which is considered the main reservoir of P. plica in the region (Bork-Mimm and Rinder, 2011). The raccoon (Procyon lotor), a medium-sized carnivore that originated in North and Central America (Kaufmann, 1982), has been accidently or intentionally introduced into several Asian and European countries during the last century (Okuyama et al., 2020; Stubbe, 1999). Within Europe, the distributional focus of the raccoon is in Central Europe (Fischer et al., 2017). The species is particularly abundant and widespread in Germany and adjacent countries where the population can be traced back to several separate introduction events (Biedrzycka et al., 2014; Fischer et al., 2015; Maas et al., 2022). In recent decades, racoons have greatly increased their density and geographical distribution in Central Europe (Fischer et al., 2016) and are spreading rapidly into urban areas, where they live in close proximity to humans and domestic animals (Hohmann and Bartussek, 2011; Heddergott et al., 2020b).
Both in North America (Hamilton, 1936; Dexter, 1951) and Europe (Lutz, 1980; Engelmann et al., 2011; Michler, 2020), raccoons have been shown to consume earthworms. Consequently, while urinary bladder parasites are usually neglected in the numerous parasitological studies of raccoons in North America, Pearsonema spp. or P. plica infestations have been reported occasionally (Johnsen, 1970; Butterworth and Beverley-Burton, 1980; Cole and Shoop, 1987; Hamir and Rupprechtt, 1998; Hamir and Snyder, 1999). Outside their original range, only two German studies have investigated urinary bladder parasites and both failed to find evidence for the presence of Pearsonema spp. (Lux and Priemer, 1995; Gey, 1998). The aim of the present study was to determine the prevalence of Pearsonema spp. in wild raccoons in their core distribution areas in Central Europe in order to determine its role as a possible reservoir in view of the increasing contact with domestic dogs and cats.
2. Material and methods
2.1. Ethics statement
As an invasive species, raccoons are not legally protected in Luxembourg, Poland and Germany and can be harvested by licensed hunters outside the closed season without special permission. No animal was killed with the aim of providing samples for this study. All hunted individuals were legally shot and made available to the authors.
2.2. Sample collection
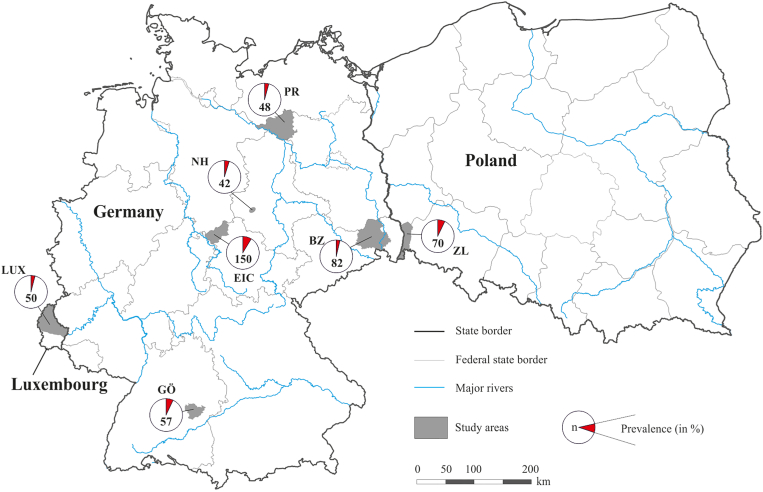
Between 2015 and 2019, urinary bladders were collected from 499 Central European raccoons that were legally hunted and part of a zoonotic pathogen control study (Heddergott et al., 2017; Heddergott et al., 2020a; Heddergott et al., 2020b; Solarczyk et al., 2021; Frantz et al., 2021). The selected study sites were characterised by a high raccoon densities (≥0.5 individuals per 100 ha; Fischer et al., 2016). We collected carcasses from northern Luxembourg (49° 45′N/06° 08′ E; study area 1500 km2), from Zgorzelec County in Poland (51° 09′N/15° 00′ E; 830 km2), as well as from five different German study areas: Prignitz (53° 7′ N/11° 44′ E; 600 km2); Nördliches Harzvorland (51° 52′ N/11° 19′ E; 50 km2; Hakel Wald); Eichsfeld (51° 20′ N/10° 10′ E; 800 km2); Bautzen (51° 11′ N/14° 26′ E; 800 km2); Göppingen (48° 42′ N/9° 39′ E; 300 km2) (Fig. 1). Raccoons were stored at −20 °C and the sex and and geographic origin of each animal was recorded. We determined the age-class of each raccoon based on the incremental growth lines in the cementum of a mandibular canine. Animals were classified either as juveniles (no growth line) or adults (one or more growth lines) (Heddergott et al., 2016). The dataset consisted of 237 males and 262 females, or of 275 adults and 224 juveniles. Altogether 429 raccoons were collected in rural areas and 70 in built-up areas with more than 10,000 people.
Fig. 1.
Sampling areas, sample size (n) and prevalence of Pearonema spp. in wild raccoons (Procyon lotor) from Luxembourg, Poland and Germany. Luxembourg: LUX Luxembourg; Poland: ZL administrative district of Zgorzelecki; Germany: NH Northern Harzvorland, Saxony-Anhalt and administrative district of BZ Bautzen, Saxony; EIC Eichsfeld, Thuringia; PR Prignitz, Brandenburg; GÖ Göppingen, Baden-Württemberg.
2.3. Urinary sediment examination
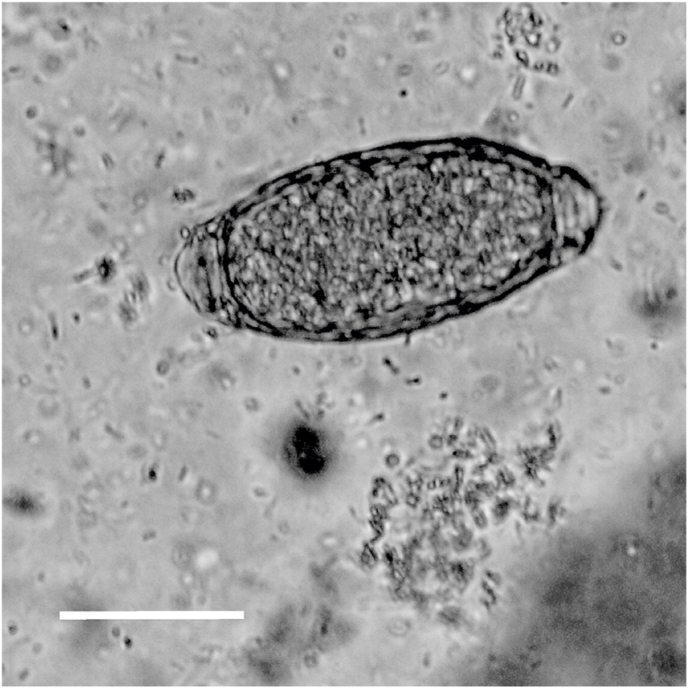
We identified infestation with Pearsonema sp. by detecting Pearsonema eggs through microscopic analysis of the urinary sediment. Pearsonema eggs are barrel-shaped and have a plug at both poles, with smooth or sculptured shells depending on the species (Fig. 2; Butterworth and Beverley-Burton, 1980). To minimize the risk posed by most infectious parasites, all raccoons were stored at −80 °C for ≥5 days prior to necropsy (Bork-Mimm and Rinder, 2011; Shafir et al., 2007). During necropsy, the urinary bladders were removed, ligated and stored in a tight plastic bags. We collected 10 ml of urine from the ligature, or washed empty bladders (n = 95) with milliQ water (Merck KGaA, Darmstadt, Germany) (Petersen et al., 2018). The urine or collected water was centrifuged at 400×g for 5 min using an EBA 200 (Hettich, Tuttlingen, Germany). Subsequently, 0.1 ml of the sediment/water was pipetted onto a slide. We examined the slides and counted the Pearsonema eggs using a Nikon E600 light microscope (NikonInc., Melville, New York, USA) (Studzińska et al., 2015).
Fig. 2.
Egg of Pearsonema spp. in urine sediment. The egg shows the characteristic features (barrel-shaped with bipolar plugs). Based on their morphology, the eggs were likely to be P. plica (see Discussion). Scale: 20 μm.
2.4. Morphometric analyses of eggs
Measurements of Pearsonema eggs were made using a digital image analysis system (Microimage 4, Microsoft Corp.) at 400× magnification following Borba et al. (2021). The measurements were performed on 25 undamaged eggs, selected regardless of their study area of origin. The following measurements were obtained: the length and width (diameter) of the eggs, the width and height of the plugs, and the thickness of the eggshell (see also Borba et al., 2021).
2.5. Statistical analyses
We used a χ2-test to test for differences in prevalence between study areas and habitat categories (urban vs. rural). We performed a logistic regression to test for an effect of sex and age on the presence of Pearsonema eggs. Given the relatively small amount of positive cases (see Results) we performed the regression without considering interactions. The statistical analyses were performed in R v.4.2.0 (R Core Team, 2022). Furthermore, we calculated the 95% confidence interval (CI) for the prevalence estimated for the entire data set and the different study areas using the “Wilson” Score interval method (Brown and Cat, 2011).
3. Results
Pearsonema eggs were found in 6.2% (31/499; 95% CI: 4.4–8.7) of the examined raccoons. Infested raccoons were found in all study areas in Germany, Poland and Luxembourg (Fig. 1; Table 1). While the prevalence of Pearsonema eggs varied from 3.7% (Bautzen) and 8.7% (in Eichsfeld), there was no significant difference in prevalence between the study areas (χ2 = 2.976, df = 6, p = 0.704). Three of the 70 raccoons (4.3%) originating from built-up areas were infested with a Pearsonema species, compared to 28 of 429 (6.5%) animals sampled in rural areas. This differences was not significant (χ2 = 2.976, df = 1, p = 0.610). Males (18/219; 8.2%) were more frequently infested than females 13/249; 5.2%) and adults (18/275; 6.5%) more frequently than juveniles (13/224; 5.8%). However, neither sex nor age class had a statistically significant influence on the probable presence of Pearsonema eggs (Table 2). Well over half of the Pearsonema-positive raccoons (n = 23; 74.2%) had a low egg load of ≤20 eggs per ml (Table 1).
Table 1.
Pearsonema egg load in sediment from washing of the bladder or urine sediment of raccoons (Procyon lotor) from Luxembourg, Poland and Germany.
| State | Region | No examined | No positive animals (in %) | 95% confidence interval | 1-10 eggs per ml (in %) | 11-20 eggs per ml (in %) | 21-30 eggs per ml (in %) | >30 eggs per ml (in %) |
|---|---|---|---|---|---|---|---|---|
| Luxembourg | 50 | 2 (4.0) | 1.1–13.4 | 1 (50.0) | – | 1 (50.0) | – | |
| Poland | Zgorzelecki | 70 | 5 (7.1) | 3.1–15.7 | 2 (40.0) | 2 (40.0) | 1 (20.0) | – |
| Germany | Northern Harzvorland | 42 | 2 (4.8) | 1.3–15.8 | – | 1 (50.0) | – | 1 (50.0) |
| Bautzen | 82 | 3 (3.7) | 1.3–10.3 | – | 2 (66.7) | 1 (33.3) | – | |
| Prignitz | 48 | 2 (4.2) | 1.2–14.0 | – | 2 (100.0) | – | – | |
| Göppingen | 57 | 4 (7.0) | 2.8–16.7 | 2 (50.0) | 1 (25.0) | 1 (25.0) | – | |
| Eichsfeld | 150 | 13 (8.7) | 5.1–14.3 | 6 (46.2) | 4 (30.8) | 2 (15.4) | 1 (7.6) | |
| Total | 499 | 31 (6.2) | 4.4–8.7 | 11 (35.5) | 12 (38.7) | 6 (19.4) | 2 (6.4) |
Table 2.
Results of logistic regression identifying predictors for the presence of Pearsonema spp. in raccoons (Procyon lotor).
| Predictor | Estimate | s.e. | z value | p value |
|---|---|---|---|---|
| (Intercept) | −2.89 | 0.32 | −9.11 | <0.001 |
| Sex-male | 0.47 | 0.38 | 1.25 | 0.213 |
| Age class-juvenile | −0.17 | 0.38 | −0.46 | 0.648 |
The estimate gives the change in the log odds of presence of the parasaite when considering males and juveniles relative to females and adults, respectively.
R2 Tjur = 0.004.
The 25 examined eggs had large pits that were consistent with the shell type I reticulate surface structure. The eggs had a length of (mean ± s.d.) 62.76 ± 1.23 μm and average width of 27.02 ± 0.57 μm. The plugs were 9.12 ± 0.66 μm wide and 4.46 ± 0.50 μm high. The thickness of the eggshell was on 2.09 ± 0.20 μm (Table 2).
4. Discussion
Even though the Pearsonema nematodes and the pathogenic risk they potentially pose to domestic cats and dogs have been known for some time, reliable reports on the prevalence of the parasites in Central European wild carnivores are relatively rare (Bork-Mimm and Rinder, 2011). A few studies on P. plica have focussed on prevalence in the red fox (Bork-Mimm and Rinder, 2011; but see Varodi et al., 2017; Petersen et al., 2018). There is very limited information on the prevalences of P. mucronata (but see Varodi et al., 2017) and P. feliscati in wildlife (but see Bagrade et al., 2003; Krone et al., 2008).
Certain identification of Pearsonema nematodes usually requires adult worms (Butterworth and Beverley-Burton, 1980; Moravec, 1982). Studies that diagnose infestation based on the presence of Pearsonema eggs in urinary sediment thus often report infestation without determination at the species level (e.g., Pelligra et al., 2020; Eleni et al., 2021). However, the differing morphological characteristics of their eggs ought to allow distinction between P. plica and P. feliscati (Enzie, 1951; Butterworth and Beverley-Burton, 1980). P. plica eggs have a reticulated shell characterised by large depressions and ridges, referred to as shell type I by Borba et al. (2021). Depending on the focus, light microscopy either shows eggshells of P. feliscati to be striated (Freitas et al., 1936; Enzie, 1951) or to have a pitted surface (Zajac and Conboy, 2012), with depressions that are not as large as those observed in P. plica (Borba et al., 2021).The sizes of the eggs of the different species partially overlap. P. plica eggs can be between 58 and 74 μm long and 23–31 μm wide (Enzie, 1951; Butterworth and Beverley-Burton, 1980; Borba et al., 2021). The eggs of P. feliscati appear to be somewhat shorter on average, ranging from 51 to 62 μm in length and 24–32 μm in width (Freitas et al., 1936). According to Pfeiffer et al. (1989), P. mucronata eggs are between 65 and 68 μm long and 28–31 μm wide.
Based on egg morphology, the Pearsonema eggs that we recovered from Central European raccoons in all likelihood were P. plica. All our morphological measurements of the eggs fall exactly within the range of values reported by Borba et al. (2021) for the corresponding features in P. plica (Table 3). In contrast, P. feliscati eggs can be shorter (Freitas et al., 1936) and P. mucronata eggs wider (Pfeiffer et al., 1989) relative to the sizes measured for Pearsonema eggs in the present study. In addition, our eggshells had the large pits that are characteristic of P. plica (but not P. feliscati). Finally, adult P. plica have been recovered from raccoons in North America (see below). While we cannot be categorical about the identity of the parasite, we thus believe that it is reasonable to presume that the nematode eggs we observed were indeed P. plica.
Table 3.
Selected morphometric measurements of Pearsonema plica eggs (Borba et al., 2021) and Pearsonema eggs measured in the present study.
| Measurement characteristics |
Borba et al. (2021),a |
Present examinationb |
|||
|---|---|---|---|---|---|
| Mean | Min-max | Mean | Min-max | ± SD | |
| Egg length (μm) | 62.62 | 60.30–65.32 | 62.76 | 60.18–65.02 | 1.23 |
| Egg width (μm) | 27.47 | 26.35–28.76 | 27.02 | 26.30–28.55 | 0.57 |
| Plug base width (μm) | 9.62 | 8.48–10.87 | 9.12 | 8.45–10.75 | 0.66 |
| Plug base height (μm) | 4.47 | 3.40–5.28 | 4.46 | 3.42–5.21 | 0.50 |
| Shell thickness (μm) | 2.24 | 1.17–2.55 | 2.09 | 1.74–2.49 | 0.20 |
±SD Standard deviation.
Examined eggs n = 30.
Examined eggs n = 25.
To our knowledge, the present Central European study is the first study outside North America to demonstrate infestation with Pearsonema spp. in raccoons. Two previous parasitological studies from Germany examined urinary bladders of raccoons but did not find evidence for the presence of Pearsonema spp. (Lux and Priemer, 1995; Gey, 1998). While we found a low prevalence of Pearsonema infestation (6.2%), similar values have been reported from the USA. Pearsonema sp. or P. plica worms were observed in 10.4% (20/193) of analysed animals from South Carolina, Virginia and Pennsylvania (Hamir and Rupprechtt, 1998), 10.6% from Alabama 5/47 (Johnsen, 1970) and 11.1% (7/63) from Oregon (Hamir and Snyder, 1999). In contrast, 57.9% (81/140) of raccoons from Ontario in Canada (Butterworth and Beverley-Burton, 1980) and 64.3% (45/70) from Kentucky in the USA (Cole and Shoop, 1987) were infested with P. plica.
The only documented route of Pearsonema infestation for carnivores is ingestion of Pearsonema-infested earthworms (Moravec et al., 1987). Raccoons have been shown to consume earthworms in both North America (Hamilton, 1936; Dexter, 1951) and Europe (Lutz, 1980; Engelmann et al., 2011; Michler, 2020). We did not find any differences in prevalence values between study areas, making it unlikely that the landscape characteristics of the study area influenced disease transmission. Nevertheless, there can be large differences in Pearsonema prevalence values between studies (see above). These differences possibly are due to regional differences in the importance of earthworms in the raccoons’ diet and/or to seasonal differences in the consumption of earthworms (Dexter, 1951; Rulison et al., 2012). For example, while earthworms overall represented 20% of the biomas found in raccoon scats in a study area in northeastern Germany (Michler, 2020), Bartoszewicz et al. (2008) did not detect earthworms in the scats of raccoons from neighbouring northwest Poland. In northeastern Germany, there were marked differences between the seasons, with earthworms almost completely absent from raccoon scats in winter (Michler, 2020). As has been speculated for cats (Beugnet and Knaus, 2015) and other wild carnivores (Petersen et al., 2018), it is also possible that raccoons become infested via paratenic hosts, i.e. by consuming prey species that feed on earthworms. The egg load (eggs per ml) of raccoons infested with Pearsonema was low (≤20 eggs per ml). Similarly low loads were reported for P. plica in red foxes from Bavaria in Germany (Bork-Mimm and Rinder, 2011), while higher values were report for red foxes (≥30 eggs per ml) from Denmark (Petersen et al., 2018).
This study is the first to report urinary capillariasis in free-ranging raccoons in European. Considering their increasing density, raccoons may thus play a previously overlooked role in environmental contamination with Personema eggs. Even more than red foxes, raccoons adapt to urban habitats (Hohmann and Bartussek, 2011), which probably leads to an increased risk of transmission of P. plica infestation to domestic animals. Further research may thus be necessary to assess whether raccoons play an important role in the epidemiology of Pearsonema spp.
Declaration of competing interest
There is not any conflict of Interest.
Acknowledgements
The following people contributed to the sampling effort: H Auer, S Becker, WD Becker, S Dietzel, M Dietrich, T Drössler, H Feller, K Gunkel, M Hartmann, JL Hammer, F Hummel, M Hoffmann, H Köhne, B König, G Lehmann, JK Lohmann, N Lenert, R Mock, D Müller, S Münder, KL Rumer, F Röse, L Scheer, L Schley, A Schneider, M Senge, O Sommerfeld, M Speck, AB Stytz, B Steidl, A Weißenstein, R Wildanger, BP Zimmer and P Zimmermann. Part of this study was supported by the Luxembourg National Research Fund (AFR 7484378). For the production and provision of the photo of the Pearsonema egg we thank Amer Alić.
References
- Bagrade G., Vismanis K., Kirjušina M., Ozoliņš J. Preliminary results on the helminthofauna of the Eurasian lynx (Lynx lynx) in Latvia. Acta Zool. Litu. 2003;13:3–7. doi: 10.1080/13921657.2003.10512536. [DOI] [Google Scholar]
- Bartoszewicz M., Okarma H., Zalewski A., Szczęsna J. Ecology of the raccoon (Procyon lotor) from western Poland. Ann. Zool. Fenn. 2008;45:291–298. doi: 10.5735/086.045.0409. [DOI] [Google Scholar]
- Basso W., Spänhauer Z., Arnold S., Deplazes P. Capillaria plica (syn. Pearsonema plica) infection in a dog with chronic pollakiuria: challenges in the diagnosis and treatment. Parasitol. Int. 2013;63:140–142. doi: 10.1016/j.parint.20013.09.002. [DOI] [PubMed] [Google Scholar]
- Beugnet F., Knaus M. In: Parasitoses and Vector Borne Diseases in Cats. Beugnet F., Halos L., editors. Merial; Lyon, France: 2015. Urinary capillariosis. [Google Scholar]
- Borba V.H., Martin C., Machado-Silva J.R., Xavier S.C.C., de Mello F.L., Iñignez A.M. Machine learning approach to support taxonomic species discrimination based on helminth collections data. Parasites Vectors. 2021;14:230. doi: 10.1186/s13071-021-04721-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bork-Mimm S., Rinder H. High prevalence of Capillaria plica infections in red foxes (Vulpes vulpes) in Southern Germany. Parasitol. Res. 2011;108:1063–1067. doi: 10.1007/s00436-010-2196-0. [DOI] [PubMed] [Google Scholar]
- Brown L.D., Cat T.T. DasGupta A. Interval estimation for a proportion. Stat. Sci. 2011;16:101–133. doi: 10.1214/ss/1009213286. [DOI] [Google Scholar]
- Biedrzycka A., Zalewski A., Bartoszewicz M., Okarma H., Jędrzejewska E. The genetic structure of raccoon introduced in Central Europe reflects multiple invasion pathways. Biol. Invasions. 2014;16:1611–1625. doi: 10.1007/s10530-013-0595-8. [DOI] [Google Scholar]
- Butterworth E.W., Beverley-Burton M. The taxonomy of capillaria spp. (nematoda: trichuroidea) in carnivorous mammals from Ontario, Canada. Syst. Parasitol. 1980;1:211–236. doi: 10.1007/BF00009847. [DOI] [Google Scholar]
- Cole R.A., Shoop W.L. Helminths of the raccoon (Procyon lotor) in western kenntucky. J. Parasitol. 1987;73:762–768. doi: 10.2307/3282410. [DOI] [PubMed] [Google Scholar]
- Dexter R.W. Earthworms in the winter diet of the opossum and raccoon. J. Mammal. 1951;32:464. doi: 10.1093/jmammal/32.4.464a. [DOI] [Google Scholar]
- Eleni C., Mariacher A., Grifoni G., Cardini E., Tonon S., Lombardo A., Barone A., Fichi G. Pathology of urinary bladder in Pearsonema spp. infected wildlife from Central Italy. Pathogens. 2021;10:474. doi: 10.3390/pathogens10040474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann A., Köhnemann B.A., Michler F.U. Nahrungsökologische Analyse von Exkrementen gefangener Waschbären (Procyon lotor L., 1758) aus dem Müritz-Nationalpark (Mecklenburg-Vorpommern) unter Berücksichtigung individueller Parameter. Beitr. J. Wildforsch. 2011;36:587–604. [Google Scholar]
- Enzie F.D. Do whipworms occur in domestic cats in North America? J. Am. Vet. Med. Assoc. 1951;119:210–213. [PubMed] [Google Scholar]
- Fischer M.L., Hochkirch A., Heddergott M., Schulze C., Anheyer-Behmenburg H.E., Lang J., Michler F.U., Hohmann U., Ansorge H., Hoffmann L., Klein R., Frantz A.C. Historical invasion recordscan be misleading: genetic evidence for multiple introductions ofinvasive raccoons (Procyon lotor) in Germany. PLoS One. 2015;10 doi: 10.1371/journal.pone.0125441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer M.L., Salgado I., Beninde J., Klein R., Frantz A.C., Heddergott M., Cullingham C.I., Kyle C.J., Hochkirch A. Multiple founder effects are followed by range expansion and admixture during the invasion process of the raccoon (Procyon lotor) in Europe. Divers. Distrib. 2017;23:409–420. doi: 10.1111/ddi.12538. [DOI] [Google Scholar]
- Fischer M.L., Sullivan M.J.P., Greiser G., Guerrero-Casado J., Heddergott M., Hohmann U., Keudling O., Lang J., Martin I., Michler F.U., Winter A., Klein R. Assessing and predicting the spread of non-native raccoons in Germany using hunting bag data and dispersal weighted models. Biol. Invasions. 2016;18:57–71. doi: 10.1007/s10530-015-0989-x. [DOI] [Google Scholar]
- Frantz A.C., Schleimer A., Wittische J., Heddergott M. Close spatial overlap between the genetic population boundaries of raccoons and the distribution of the raccoon roundworm in Germany. Hystrix. 2021;32:203–206. doi: 10.4404/hystrix-00444-2021. [DOI] [Google Scholar]
- Freitas J.F., Teixeira de, Lent H. vol. 31. Memórias do Instituto Oswaldo Cruz, Rio de Janeiro; 1936. p. 107. (Estudo sobre os Capillariinae parasitos de mammiferos: (Nematoda: Trichuroidea)). n. 1. [DOI] [Google Scholar]
- Gey A.B. 1998. pp. 1–203. (Synopsis der Parasitenfauna des Waschbären (Procyon lotor) unter Berücksichtigung von Befunden aus Hessen). – Diss., Justus-Liebig-Universität, Fachbereich Veterinärmedizin, Gießen. [Google Scholar]
- Hamilton W.J. The food and breeding habits of the raccoon. Ohio J. Sci. 1936;36:130–140. [Google Scholar]
- Hamir A.N., Rupprechtt C.E. A retrospective histopathological survey of capillariasis in raccoons from the eastern United States. J. Parasitol. 1998;84:180–182. doi: 10.2307/3284556. [DOI] [PubMed] [Google Scholar]
- Hamir A.N., Snyder D.E. A Retrospective histopathological survey of capillariasis in raccoons (Procyon lotor) from Oregon. J. Parasitol. 1999;6:1172–1174. doi: 10.2307/3285684. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Frantz A.C., Stubbe M., Stubbe A., Ansorge H., Osten-Sacken N. Seroprevalence and risk factors of Toxoplasma gondii infection in invasive raccoons (Procyon lotor) in Central Europa. Parasitol. Res. 2017;116:2335–2340. doi: 10.1007/s00436-017-5518-7. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Frantz A.C., Pohl D., Osten-Sacken N., Steinbach P. Detection of Cryptosporidium spp. infection in wild raccoons (Procyon lotor) from Luxembourg using an ELISA approach. Acta Parasitol. 2020;68:985–989. doi: 10.2478/s11686-020-00234-x. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Pohl D., Steinbach P., Cantú Salazar L., Müller F., Frantz A.C. Determinants and effects of sinus worm Skrjabingylus nasicola (Nematoda: metastrongyloidae) infestation in invasive American mink Neovison vison in Germany. Parasitol. Res. 2016;115:3449–3457. doi: 10.1007/s00436-016-5107-1. [DOI] [PubMed] [Google Scholar]
- Heddergott M., Steinbach P., Schwarz S., Anhemer-Behmenburg H.E., Sutor A., Schliephake A., Jeschke D., Striese M., Müller F., Meyer-Kayser E., Stubbe M., Osten-Sacken N., Krüger S., Gaede W., Runge M., Hoffmann L., Ansorge H., Conraths F.J., Frantz A.C. Geographic distribution of raccoon roundworm, Baylisascaris procyonis, Germany and Luxembourg. Emerg. Infect. Dis. 2020;26:821–823. doi: 10.3201/eid2604.191670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohmann U., Bartussek I. third ed. Verlag Oertel & Spörer; Reutlingen: 2011. Der Waschbär. [Google Scholar]
- Ilić T., Rogošić M., Gajić B., Aleksić J. Urinary capillariosis in dogs. Vet. Glas. 2021;75:20–32. doi: 10.2298/VETGL1910090031. [DOI] [Google Scholar]
- Johnsen S.A. 1970. (Biology of the Raccoon (Procyon lotor Varius Nilson and Goldman) in Alabama). Agricult Exp. Sat. Auburn Univ. [Google Scholar]
- Kaufmann J.H. In: Wild Mammals of North America. Chapman J.A., Feldhamer G.A., editors. John Hopkins University Press; Baltimore: 1982. Raccoon and allies; pp. 567–585. [Google Scholar]
- Krone O., Guminsky O., Meinig H., Herrmann M., Trinzen M., Wibbelt G. Endoparasite spectrum of wild cats (Felis silvestris Schreber, 1777) and domestic cats (Felis catus L.) from the Eifel, Pfalz region and Saarland, Germany. Eur. J. Wildl. Res. 2008;54:95–100. doi: 10.1007/s10344-007-0116-0. [DOI] [Google Scholar]
- Low D.L. Parassitosi delle vie urinarie superiori e inferiori del cane e del gatto. Nefrologia e urologia del cane e del gatto. 1999;35:949–953. [Google Scholar]
- Lutz W. Teilergebnisse der Nahrungsanalysen am waschbären (Procyon lotor [L.]) in nordhessen. Z. Jagdwiss. 1980;26:61–66. doi: 10.1007/BF02242007. [DOI] [Google Scholar]
- Lux E., Priemer J. Internationalen Symposiums über Erkrankungen der Zoo- und Wildtiere; Dresden: 1995. Zur Parasitierung wildlebender Waschbären unter dem Aspekt ihrer nordamerikanischen Herkunft. – Verhandlungsbericht des 37; pp. 429–434. [Google Scholar]
- Maas M., Tatem-Dokter R., Rijks J.M., Dam-Deisz C., Franssen F., van Bolhuis H., Heddergott M., Schleimer A., Schockert V., Lambinet C., Huber P., Redelijk T., Janssen R., Cruz A.P.L., Martinez I.C., Caron Y., Linden A., Lesenfants C., Paternostre J., van der Giessen J., Frantz A.C. Population genetics, invasion pathways and public health risks of the raccoon and its roundworm Baylisascaris procyonis in northwestern Europe. Transbound. Emer. Dis. 2022;69:2191–2200. doi: 10.1111/tbed.14218. [DOI] [PubMed] [Google Scholar]
- Michler B.A. Koproskopische Untersuchung zum Nahrungsspektrum des Waschbären Procyon lotor (Linné, 1758) im Müritz-Nationalpark (Mecklenburg-Vorpommern) unter Berücksichtigung des Artenschutzes und des Endoparasitenbefalls. – Wildtierforschung in Mecklenburg-Vorpommern. 2020;5:1–168. [Google Scholar]
- Moravec F. Proposal of a new systematic arrangement of nematodes of the family Capillariidae. Folia Parasitol. 1982;29:119–132. [PubMed] [Google Scholar]
- Moravec F., Prokopic J., Shlikas A.V. The biology of nematodes of the family Capillariidae Neveu-Lemaire, 1936. Folia Parasitol. 1987;34:39–56. [PubMed] [Google Scholar]
- Okuyama M.W., Shimozuru M., Nakai M., Yamaguchi E., Fujii K., Shimada K., Ikeda T., Tsubota T. Genetic population structure of invasive raccoons (Procyon lotor) in Hokkaido, Japan: unique phenomenon caused by pet escape or abandonment. Sci. Rep. 2020;10:8108. doi: 10.1038/s41598-020-64526-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelligra S., Guardone L., Riggio F., Parisi F., Maestrini M., Mariacher A., Perrucci S. Pearsonema spp. (family Capillariidae, order Enoplida) infection in domestic carnivores in central–Northern Italy and in a red fox population from central Italy. Animals. 2020;10:1607. doi: 10.3390/ani10091607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen H.H., Nielsen S.T., Larsen G., Holm E., Chriél M. Prevalence of Capillaria plica in Danish wild carnivores. Int. J. Parasitol. Parasites Wildl. 2018;7:360–363. doi: 10.1016/j.ijppaw.2018.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer A.S., Böckeler W., Lucius R. Parasiten der Haus-, Nutz- und Wildtiere Schleswig-Holsteins: parasiten der inneren Organe des Steinmarders (Martes foina) Z. Jagdwiss. 1989;35:100–112. doi: 10.1007/BF02242095. [DOI] [Google Scholar]
- R Core Team . R Foundation for Statistical Computing; Vienna: 2022. R: A Language and Environment for Statistical Computing. [Google Scholar]
- Rulison E.L., Luiselli L., Burke R.L. Relative impact of habitat and geography on raccoon diets. Am. Midl. Nat. 2012;168:231–246. doi: 10.1674/0003-0031-168.2.231. [DOI] [Google Scholar]
- Shafir S.C., Wang W., Sorvillo F.J., Wise M.E., Moore L., Sorvillo T., Eberhard M.L. Thermal death point of Baylisascaris procyonis eggs. Ermerg. Infect. Dis. 2007;13:172–173. doi: 10.3201/eid1301.060966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solarczyk P., Dabert M., Frantz A.C., Osten-Sacken M., Trzebny A., Wojtkowaik-Giera A., Heddergott M. Zoonotic Giardia duodenalis sub-assemblage BIV in wild raccoons (Procyon lotor) from Germany and Luxemburg. Zoonoses Public Health. 2021;68:538–543. doi: 10.1111/zph.12826. [DOI] [PubMed] [Google Scholar]
- Stubbe M. In: The Atlas of European Mammals. Jones A.J., Amori G., Bogdanowicz W., Krystufek B., Reijnders P., Spitzenberger F., Stubbe M., Thissen J.B.M., Vohralík V., Zima J., editors. Mitchell Academic Press; London: 1999. Procyon lotor (linnaeus, 1758) pp. 326–327. [Google Scholar]
- Studzińska M.B., Obara-Gałek J., Demkowska-Kutrzepa M., Tomczuk K. Diagnosis and therapy of Capillaria plica infection: report and literatura riview. Acta Parasitol. 2015;60:563–566. doi: 10.1515/ap-2015-0081. [DOI] [PubMed] [Google Scholar]
- Varodi E.I., Malega A.M., Kuzmin Y.I., Kornyushin V.V. Helminths of wild predatory mammals of Ukraine. Nematodes. Vestn. Zoo. 2017;51:187–202. doi: 10.1515/vzoo-2017-0026. [DOI] [Google Scholar]
- Zajac A., Conboy G.A. eighth ed. Wiley-Blackwell; Chichester, West Sussex, UK: 2012. Veterinary Clinical Parasitology. [Google Scholar]