Abstract
Background
Coffee (Coffea arabica) and tea (Camellia sinensis) are beverages consumed widely across the globe. Flavor enhancement of beverages is the prime interest for consumers and industry, but it is still a major challenge for researchers.
Objectives
In this work, we aimed to enhance the sensory characteristics and lower the caffeine content of tea and coffee by applying 2–6 μm mid-infrared wavelengths emitted through our recently invented Mid-Infrared Generating Atomizer (MIRGA) without creating any adverse effects.
Methodology
Two methods were followed: Direct MIRGA spraying over the packaged coffee or tea powder packets, and direct MIRGA spraying over the liquid coffee or tea. Controls were maintained in both methods. The treated samples were subjected to organoleptic tests by an expert panel and consumers.
Results
This study is supported by comprehensive field trials, including sensory attributes evaluation and laboratory analyses. In coffee, spraying resulted in 8% decaffeination and increase in theobromine and theophylline by 40% and 10–20%, respectively. In tea, caffeine and theobromine increased by 20–25% and 30%, respectively in addition to a 0.6–1.2% increase in thearubigins. A 20–30% lower amount of sprayed coffee or tea powder was required to prepare beverages with regular sensory characteristics. We have proven that the MIRGA technology applied to the products reduced the caffeine content in coffee, rendered them safe to consume, improved the taste and flavor, and induced health benefits. In addition, as the MIRGA platform contributed toward improving the product characteristics, it can also positively impact their price and affordability.
Conclusion
Applications of MIRGA technique and its benefits can be potentially scaled up and utilized for a variety of products used in daily life.
Keywords: MIRGA, 2–6 μm Mid-IR, Tea, Coffee, Taste, Flavor, Enhancement, Resource, Health, Savings, Economy
MIRGA; 2–6 μm Mid-R; Tea; Coffee; Taste; Flavor; Enhancement; Resource; Health; Savings; Economy.
1. Introduction
Coffee and tea are popular and desirable beverages around the world. It is estimated that globally around 2 billion cups of coffee and 3 billion cups of tea are consumed on a daily basis (Voora et al., 2019; website). These beverages contain the major alkaloid caffeine, which is a natural stimulant of the central nervous system. Nevertheless, excess caffeine intake causes abuse, dependence, and intoxication. Furthermore, a segment of the population is sensitive to caffeine and suffers from symptoms like palpitations or insomnia when drinking caffeinated beverages (Cappelletti et al., 2015). Hence removal or reduction of caffeine in coffee is needed (Colombo and Papetti, 2020; Park et al., 2012). Decaffeination is a challenge that needs to be tackled with technologies that do not pose health risks or pollute the environment (Marco et al., 2017). Current decaffeination technologies are laborious, cost-heavy, insufficient and yet to be explored for other beverages like tea.
Recently, electromagnetic radiation such as infrared, ultraviolet, and gamma rays are gaining momentum in food preservation and packaging. Thus, we chose the safer infrared region for this study. In the electromagnetic wave (EMW) spectrum (Supplementary Figure (i)), the mid-IR region is interesting for many applications since that region coincides with the internal vibration of most molecules (CORDIS, European Commission). At the molecular level, the interaction of mid-IR wavelength energy elicits rotational and vibrational modes through a change in dipole movement, leading to chemical bond alteration (Girard, 2014). These alterations ultimately lead to changes in the physicochemical characteristics of objects.
We fabricated a mid-infrared generating atomizer (MIRGA) as a way to alter chemical bond parameters, thereby potentiating any natural characters of the product. MIRGA is a harmless, economical atomizer containing an imbalanced ratio of ions suspended in water, which influence the natural potency of target substances by generating mid-IR while spraying. We designed MIRGA to accommodate the ions suspended in water in their fundamental state, which can move as free particles. The solution has very little background frequency, below that of cosmic events and of a human body emitting around 10 microns (Ashcroft, 2000; Sanders, 2014). MIRGA generates energy based on various processes like ionization produced by spraying, excitation/charging of water-based ionic solutions, leading to oscillation among the imbalanced ions (Verheest, 2000) in their excited state, which in turn results in the emission of photons (Keping and Yu, 2004; Fauchais et al., 2014). Even though a low electromagnetic field exists between the charged particles of the MIRGA’s ionic solution, during spraying the induced oscillation between these charged particles produces energy (Pople, 1999; Wendish and Brenguier; Singh, 2009; Prasad, 2017). Moreover, in MIRGA, we also simulated the energy gained (nearly 6-μm mid-IR) by the process of rainfall (i.e., small droplets with high internal energy, acquired kinetic energy, and emitted energy by breaking the surface tension). We then calibrated the ejection pressure of the atomizer to obtain a fine mist, minimizing the evaporation rate by altering the pH and density of the solution.
Depending on the pressure given to the plunger, MIRGA emits 2–6 μm mid-IR. In general, terahertz and mid-infrared wavelength rays are known to transmit through nearly any material without causing biological harm. Pereira and Shulika (2011) summarized an overall picture of how these radiations can be safely generated, detected, and applied. Specifically, MIR is of high interest since many biomolecular compounds have strong resonances in the MIR region. The precision, sterility, and versatility of MIR irradiation is opening up opportunities as explained recently by Toor et al. (2018).
In this study, we applied 2–6 μm mid-infrared (mid-IR) rays externally over commercially bought coffee and tea packages to decaffeinate the samples while enhancing their taste and aroma. We then evaluated the alkaloid content in both samples and also conducted sensory and toxicological tests. The objective is to offer coffee and tea with enhanced sensory qualities while being low in caffeine, which may be appealing to consumers who are sensitive to caffeine or fear the addictive potential of these products.
2. Materials and methods
2.1. Materials and sprayer
MIRGA (patent pending 201941048628 – details in the Supplementary Figure (i)) is a 20-mL capacity polypropylene plastic atomizer containing an inorganic (molar mass 118.44 g/mol) water-based solution. The sprayer unit has dimensions 86 × 55 × 11 mm, an orifice diameter of 0.375 mm, ejection volume 0.062 ± 0.005 mL, and ejection time 0.2 s. The average pressure is 3900 Pa, and the cone liquid back pressure is 2000 N/m2 (Supplementary Figure (ii)). During spraying, approximately 1-μg weight of water is lost as mist and the non-volatile material in the sprayed liquid has a concentration of 153 mg/mL. Depending on the pressure applied to the plunger, every spraying is designed to generate 2–6 μm as estimated by an FTIR (retro-reflector) interferometer instrument (Detector type D∗ [cm HZ1/2-1] MCT [2-TE cooled]) at Lightwind, Petaluma, CA, USA (raw data in the Supplementary data (i)).
Commercially available coffee powder of different weights, brands, quality, price, and packaging were tested in this study. We added a constant weight of milk, sugar, and water proportional to the weight of the coffee powder because coffee is generally mixed with milk and sugar in India. Sensory evaluations were solicited from a coffee expert panel (n:6) as well as from coffee makers and consumers. The same testing procedure was followed for tea.
Ethical statement and Informed consent: Since spraying was only external, the institutional review board (Ashram Siddha Research Institute) deemed sensory ethical approval as unnecessary. Informed consent was obtained from all the participants.
2.2. MIRGA method 1
Spraying was done at a distance of 0.25–0.50 m from packaged (polyethylene, paper) coffee and tea on one or both sides (Supplementary video (i)). This distance allows the MIRGA solution to form ion clouds, with oscillation and 2–6 μm mid-IR generation. The rays can penetrate the packaging and act on the stored coffee or tea powder inside the package. A closer spraying compared to the mentioned distance does not generate enough measurable energy.
Nine packets of the same brand and batch of coffee powder (100 g each) were purchased for testing. These packets were made of polyethylene (>51-micron thickness). They were marked as C (control) and with numbers from 1 to 8. Each numbered packet was MIRGA sprayed; the number corresponded to the number of sprayings (i.e., Packet 1 received one spraying, Packet 2 received two sprayings, and so on). The same procedure was done for tea. The 8-sprayed sample was expected to result in a denaturing of coffee and tea characteristics with the input of more energy.
2.3. MIRGA method 2
Following the aforementioned Method 1, it was found that for 100 g coffee packets, 3 sprayings were required to achieve the desirable effects. Thus, in this method, 3 and 8 MIRGA sprayings were performed on coffee packets available in local coffee shops, mass kitchens, restaurants, motels, hotels, hostels, and houses. For 100 g tea packets, 4 and 8 sprayings were performed.
2.4. Sample preparation for analysis
Coffee: A sample of 0.5 ± 0.001 g was weighed into a 50-mL volumetric flask. Approximately 25 mL of hot water (maximum temperature of 60 °C) was added and the sample was mixed until dissolved. After cooling to room temperature, 5.0 mL of acetonitrile was added and the sample was diluted to the mark with water.
Tea: A sample of 0.2 ± 0.001 g was weighed into an extraction tube. A methanol/water extraction mixture with 70% methanol (v/v) was prepared, and 5.0 mL of the hot methanol/water mixture (70 °C) was poured into the extraction tube, followed by vortexing. The extraction tube was heated in a water bath set at 70 °C for 10 min, followed by vortexing after 5 and 10 min. The supernatant was carefully decanted into a 10-mL volumetric flask. The extraction steps were repeated, and the two extracts were combined by adjusting to 10 mL with cool methanol/water extraction mixture, followed by thorough mixing. Then, 1.0 mL of the sample extract was diluted with stabilizing solution (10 % v/v acetonitrile with 500 μg/mL of EDTA and ascorbic acid) to adjust to 5 mL, mixed, and filtered through a 0.45-μm filter.
Samples were evaluated in duplicate, except for those used in HPLC analysis which was measured once.
2.5. Instrumental analysis
2.5.1. Alkaloid analysis (HPLC)
Caffeine, theobromine, and theophylline were analyzed. The methodology used was based on ISO 14502-2:2005 as reported by Bispo et al. (2002). An ultra-high performance liquid chromatography (UHPLC) “DIONEX Ultimate 3000” equipment was used. The system was fitted with a Phenomenex C18 column (5 μm; 250 × 4.6 mm, phenyl hexyl ligand). The solvent system was: Solvent A – 9% (v/v) acetonitrile, 2% (v/v) acetic acid with 20 μg/mL EDTA. Solvent B – 80% (v/v) acetonitrile, 2% (v/v) acetic acid with 20 μg/mL EDTA. A flow rate of 1 mL/min was used. The injection volume was 10 μL, using an autosampler. The column oven was set at 25 °C. The chromatographic conditions were: 100% solvent A for the initial 10 min, gradient elution over 15 min to 38% solvent B, followed by 10 min under these conditions (i.e., 62% solvent A + 38% solvent B). The UV/vis detector was set at 278 nm. All the solvents used were of HPLC grade and were filtered through a 0.45-micron membrane filter. Standards were procured from Sigma Aldrich.
2.5.2. Thearubigins
Tea samples were analyzed for thearubigins using a method following Takeo and Oosawa (1976), with modifications based on UPASI RC/CNR-03 SOP No. 23 dt.13/10/2014. The solvent extraction of tea extract was carried out in separating funnels with adequate shaking at every stage. Contents of thearubigins were calculated from absorbance values as shown in the Supplementary Figure (iii). The multiplication factors were derived from molar extinction coefficients of pure compounds (Roberts and Smith, 1963) and dilution factors.
2.5.3. Fourier transform infrared spectra (FT-IR)
A NICOLET 5700 FT-IR spectrometer was used for coffee analysis, and an IR AFFINITY I – FT-IR spectrophotometer (FTIR 7600, Shimadzu, Japan) was used for tea analysis. FTIR spectra were recorded in the 4000–400 cm−1 wave length range using a potassium bromide pellet, following Sun et al. (2004).
2.5.4. Powder X-ray diffraction (P-XRD)
A D8 Advance (Bruker) was used with Cu Kα radiation. The equipment was fitted with a high-speed wide-angle Lynx eye detector. Small angle scan for mesoporous materials was performed from 2θ = 0.5°.The sample was spread on a glass sample holder and the XRD pattern was recorded in the desired angle range. Crystallinity (%) was calculated as per Segal et al. (1959).
2.5.5. 1H-nuclear magnetic resonance (NMR)
A Bruker Avance III 400 MHz NMR equipment was used. A 10-mg sample was added to a clean NMR tube, and dissolved in approximately 0.5 mL of deuterated solvent (D2O/DMSO/CDCl3). The tube was then shaken gently to ensure that all the materials had dissolved (ultrasonication and heating were done whenever required). The spinner was placed in a sample depth gauge to ensure that the bottom of the NMR tube was not inserted too far into the NMR probe. Then, the sample was inserted into the NMR magnet position, and the spectrum was obtained following Verma and Kumar (2010). The spectrum was processed and the peaks observed were assigned.
2.5.6. Transmission electron microscopy (TEM)
A Jeol JM 2100TEM was used. Following Wen et al. (2015), an extremely small amount of material was suspended in water/ethanol (just enough to obtain a slightly turbid solution). The solution was homogenized using an ultrasonicator to disperse the particles. A drop of the solution was then drop casted on carbon-coated grid of 200 mesh. The grid was dried and fixed in the specimen holder.
2.6. Sensory analysis
Different brands of coffee and tea powder sachets available in local markets were used for the sensory analysis. The control, sprayed coffee and tea powder, and prepared coffee and tea drinks were subjected to sensory tests by the expert panel and also tasted by the consumer panel. The sensory test questionnaire is available in the Supplementary Figures (iv) and (v).
Coffee was prepared by brewing 15 g of coffee powder in 150 mL of a mixture of hot water, milk, and sugar. Tea was prepared by infusing 5 g of tea powder in 150 mL of a mixture of boiling water, milk and sugar.
The acceptability index used was a hedonic scale with a 9-point nominal ranking structure: 1 - Dislike extremely, 2 - Dislike very much, 3 - Dislike moderately, 4 - Dislike slightly, 5 - Neither like nor dislike, 6 - Like slightly, 7 - Like moderately, 8 - Like very much, 9 - Like extremely (Everitt, 2009; Wichchukit and O’ Mahony, 2014). All the brands and batches were tested individually. Some brands needed one or two less or more MIRGA sprayings to enhance or decrease the taste and flavor.
The sensory evaluation of coffee was conducted by a trained 6-member panel according to published procedures (Ngugi et al., 2015). The attributes such as appearance, aroma, flavor, bitterness, aftertaste, acidity, bitterness, sweetness, mouthfeel, and overall acceptance were scored using a 9-point hedonic scale. Sensory grading of tea was conducted in a similar manner, according to a previous publication (Adnan et al., 2013). The attributes scored were flavor, taste, color, and overall acceptability using the 9-point hedonic scale.
2.7. Toxicological analysis
We evaluated the MIRGA’s toxicity effect by cytotoxicity assays in-vitro using Vero, A549, and human dermal fibroblast cells.
3. Results and discussion
3.1. Sensory evaluation
The effect of MIRGA on the sensory qualities of samples was evaluated by the experts and consumer panel after 1–2 min of spraying. The sensory profiles of the samples are listed in Tables 1 and 2.
Table 1.
Sensory profiling of coffee.
| No of sprayings | Sensory attributes of coffee |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| Appearance | Aroma | Flavor | Aftertaste | Acidity | Bitterness | Sweetness | Mouthfeel | Overall acceptance | |
| Control | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a |
| 1 sprayed | 6.3 ± 0.5ab | 6.3 ± 0.8b | 5.8 ± 0.8ab | 5.3 ± 0.5b | 5.2 ± 0.4ab | 5.3 ± 0.5ab | 6.7 ± 0.5b | 6.0 ± 0.6b | 5.8 ± 0.4ab |
| 2 sprayed | 6.8 ± 0.8bc | 6.8 ± 0.8b | 6.3 ± 0.5ac | 7.7 ± 0.8c | 6.8 ± 0.8bc | 6.7 ± 0.5c | 7.7 ± 0.5bc | 7.8 ± 0.8c | 7.7 ± 0.5b |
| 3 sprayed | 8.2 ± 0.8d | 7.2 ± 0.4b | 7.3 ± 1.0c | 8.7 ± 0.5c | 7.0 ± 0.6cd | 6.3 ± 1.0bc | 8.5 ± 0.5c | 8.7 ± 0.5cd | 8.7 ± 0.5b |
| 4 sprayed | 7.8 ± 0.8cd | 6.3 ± 0.5b | 6.5 ± 0.8bc | 7.7 ± 0.5c | 6.3 ± 0.5bd | 6.0 ± 0.6ac | 8.0 ± 0.6c | 7.0 ± 0.9bc | 7.5 ± 1.0b |
| 5 sprayed | 6.5 ± 0.8bc | 5.5 ± 0.8ab | 6.2 ± 0.8ac | 6.3 ± 0.8ab | 5.7 ± 1.0acd | 5.0 ± 0.9a | 7.7 ± 0.5bc | 6.7 ± 0.5bc | 6.3 ± 1.2ab |
| 6 sprayed | 5.5 ± 0.5ab | 4.3 ± 1.0a | 4.5 ± 0.8a | 3.3 ± 1.2d | 2.3 ± 1.0e | 1.5 ± 0.5d | 5.5 ± 0.5ab | 4.2 ± 0.8a | 4.2 ± 0.8a |
| 7 sprayed | 3.5 ± 0.8e | 2.5 ± 0.5e | 2.5 ± 0.8d | 2.0 ± 0.6de | 1.8 ± 0.8ef | 1.2 ± 0.4d | 4.0 ± 0.9a | 1.8 ± 0.8e | 2.3 ± 1.0c |
| 8 sprayed | 1.2 ± 0.4f | 1.3 ± 0.5e | 1.8 ± 0.8d | 1.5 ± 0.8e | 1.3 ± 0.5f | 1.0 ± 0.0d | 2.2 ± 1.2d | 1.3 ± 0.5e | 1.3 ± 0.5c |
Data are means ± standard deviation. Different letters in the same column indicate significant differences by one-way ANOVA (p < 0.05).
Table 2.
Sensory profiling of Tea (mean ± std. dev.).
| Sensory attributes of Tea |
||||
|---|---|---|---|---|
| Flavor | Taste | Color | Overall acceptance | |
| Control | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a | 5.0 ± 0.0a |
| 1 sprayed | 6.3 ± 0.5b | 7.0 ± 0.6b | 5.2 ± 0.4a | 6.0 ± 0.6ab |
| 2 sprayed | 6.5 ± 0.5b | 7.2 ± 0.8bc | 6.5 ± 0.5b | 6.5 ± 0.8b |
| 3 sprayed | 7.5 ± 0.5bc | 7.5 ± 0.5bc | 7.3 ± 0.5b | 7.5 ± 0.5bc |
| 4 sprayed | 8.8 ± 0.4c | 8.3 ± 0.5c | 8.0 ± 0.6c | 8.5 ± 0.5c |
| 5 sprayed | 6.5 ± 0.8b | 5.1 ± 1.0a | 7.3 ± 0.5b | 6.5 ± 0.5b |
| 6 sprayed | 4.8 ± 0.8a | 4.0 ± 0.6a | 3.5 ± 0.8d | 4.8 ± 0.8a |
| 7 sprayed | 2.3 ± 0.5d | 2.0 ± 0.6d | 1.8 ± 0.8e | 3.0 ± 0.6d |
| 8 sprayed | 1.3 ± 0.5d | 1.2 ± 0.4d | 1.2 ± 0.4e | 1.3 ± 0.5e |
Data are means ± standard deviation. Different letters in the same column indicate significant differences by one-way ANOVA (p < 0.05).
The control sample had regular taste and flavor. Three sprayings received by the coffee and tea powders resulted in enhanced aroma and taste, solubility in saliva, fineness to the touch, and persistence of taste for longer time. Drinks prepared from the sprayed powders also had enhanced taste and flavor. Most of the consumers even drank a second or third cup. According to conversations with participants, no consumers who regularly drank the MIRGA sprayed samples felt any dependence or withdrawal symptoms. Also, sprayed coffee drinks did not produce any insomnia or other side effects in consumers who drank them before going to bed. One or two direct sprayings 0.25–0.50 m above the cupped coffee and tea also showed the same results. The refreshing effect of MIRGA sprayed coffee and tea was also enhanced compared to the control sample. Enhanced flavor was found to be retained for 8–16 months (brand-dependent) in the powdered form. In general, the consumers were more satisfied with the sprayed coffee and tea samples.
The data depicted in Tables 1 and 2 indicate substantial variations in the sensory attributes of coffee and tea after every spraying. The highest overall acceptability for coffee was achieved with 3 sprayings, and for tea with 4 sprayings. The improved sensory qualities of these samples are due to chemical bond modification and other changes at the molecular level. Nevertheless, excessive sprayings (i.e., 8 sprayings) downgraded the sensory qualities of both coffee and tea, resulting in the lowest sensory scores. These results suggest that mid-IR emitted by spraying influenced the sensory qualities of coffee and tea, and agree with Bagheri (2020), who reported that infrared roasting improved the taste, color, texture, and appearance of nuts. Moreover, Qu et al. (2018) reported the enhancement of fresh taste and aroma with far-infrared drying.
In addition, an amount of sprayed coffee powder 20–30% lower than that of the control was used to prepare beverages with regular taste and aroma. This represents cost savings and a healthier beverage. The taste and flavor-enhanced sprayed coffee and tea may be able to command a higher price in the market. Furthermore, coffee with improved sensory qualities can be produced easily and economically compared to alternatives like fermentation and genetic modification of coffee beans (Dolgin, 2019).
3.2. Results of instrumental analyses of coffee and tea
Changes in the alkaloid concentration, spectrometric peaks, crystal and particle structures, and fatty acid concentrations are attributable to the chemical bond alterations caused by MIRGA spraying. Recent studies have described a positive relationship between the infrared energy and physicochemical and sensory characteristics of foods. For instance, Aboud et al. (2019) described the advantages of infrared spectra on food quality improvement and food safety.
3.2.1. Alkaloids in coffee and tea
The raw data and detailed interpretations are provided in the Supplementary data (ii).
The effects of the mid-IR rays spraying on the chemistry of coffee and tea are summarized in Table 3.
Table 3.
Alkaloids in coffee and tea powder determined by HPLC.
| Alkaloids |
Control |
3 sprayings |
8 sprayings |
Remarks |
|---|---|---|---|---|
| Coffee (% dry matter basis) | ||||
| Caffeine | 2.88 | 2.66 | 2.64 | 8% decrease |
| Theobromine | 1.08 | 1.36 | 1.52 | 40% increase |
| Theophylline | 0.10 | 0.12 | 0.11 | 10–20% increase |
| Tea (% dry matter basis) | ||||
| Caffeine | 2.68 | 3.23 | 3.32 | 20–25% increase |
| Theobromine | 0.10 | 0.13 | 0.13 | 30% increase |
| Thearubigins | 9.47 | 9.53 | 9.59 | 0.6–1.2% increase |
Table 3 also shows that spraying resulted in 8% decrease in the caffeine content in coffee. However, in tea we observed a 20–25% caffeine increase.
Naturally, caffeine concentration is lower in tea powder than in coffee powder. It is possible that MIRGA enhanced the caffeine quantity in tea due to its coarse leaf powdery nature. And the caffeine content was substantially reduced in coffee powder due to its soft and fine nature (Bunker and McWilliams, 1979; Su, 2007). We have observed the enhanced/decreased effect of MIRGA according to the coarse or fine nature of other food products during our previous research.
Currently, decaffeination is achieved by solvent-based processes using methylene chloride or ethyl acetate. Another option is the Swiss water process, in which coffee beans are immersed in hot water to wash off the caffeine and flavor components, followed by use of a carbon filter to remove caffeine, channeling back the cleaned water into the coffee beans to restore their flavor. Supercritical carbon dioxide is a more recent method, and involves exposing the coffee beans to supercritical CO2 under high temperature and pressure to remove the caffeine. However, these methods have major drawbacks including a reduced sensory quality and difficult processing of the decaffeinated coffee beans and tea leaves. Supercritical CO2 is also costly (Petruzzello; Farah, 2009; Dasguptaa et al., 2014). Therefore, at least for coffee, MIRGA spraying seems to be a promising alternative as a decaffeination process since caffeine was decreased while the sensory qualities were improved.
In our study, theobromine and theophylline were found to be quantitatively increased (Weinberg and Bealer, 2002a). Theobromine– “The God of Foods” (Theo– God; Bromo– food) (Weinberg and Bealer, 2002b) is non-toxic and therapeutically used against many diseases (Garner, 1944; Smit, 2011; Martinez-Pinilla et al., 2015; Joseph, 2018). Theophylline is also used as a therapeutic drug against respiratory tract diseases (Jilani et al., 2019). Thearubigins, which are antioxidants, also increased by 0.6% and 1.2% in 4-sprayed and 8-sprayed tea samples, respectively.
3.2.2. FTIR spectra
-
(a)
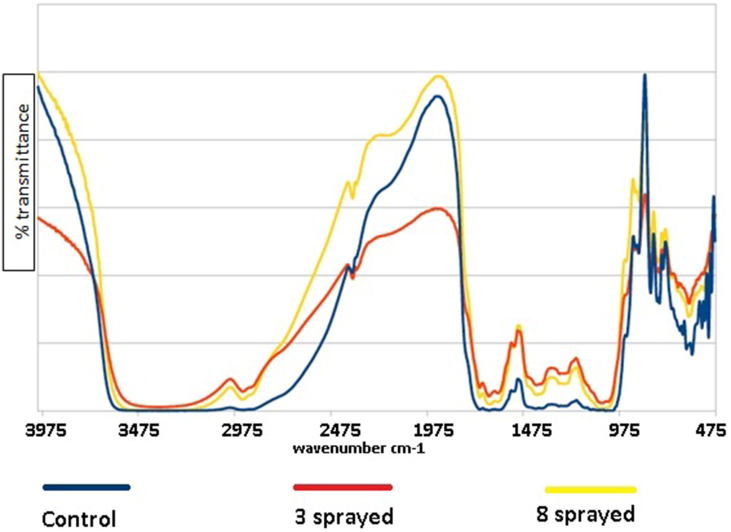
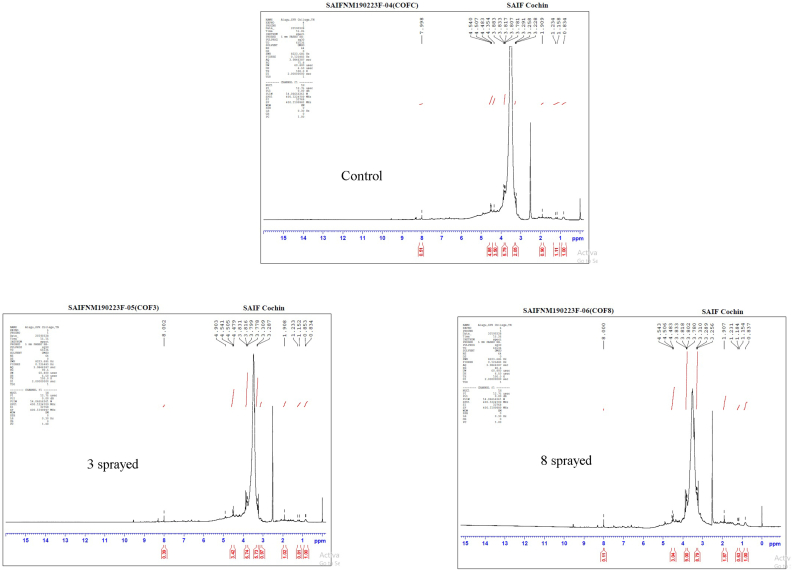
Coffee (Figure 1)
Figure 1.
FTIR spectra of coffee samples.
The control coffee sample showed apparent key peaks at the following (cm−1): 3374 (N–H), broad peak centered at 3000 (O–H, N–H), 2363 (unk), ∼1651 (C=N), 873 (C=C), 812 (C=C), 765 (C=C), 746 (C–H), 544 (C–Br), 530 (C–Br, C–I), and 494 (C–I). The proposed assignments are provided in parentheses.
The 3-sprayed sample showed apparent peaks at (cm−1): 2993 (C–H), 1065 (C–O), but is missing peaks at 544 (C–Br), 530 (C–Br, C–I), and 494 (C–I). These changes are associated with the aroma and taste.
The 8-sprayed sample showed peaks at 2993 cm−1, 1405 cm−1, 1241 cm−1, but is missing peaks in the 494 to 544 cm−1 range. These changes in the FTIR spectra are related to reduced aroma and taste.
-
(b)
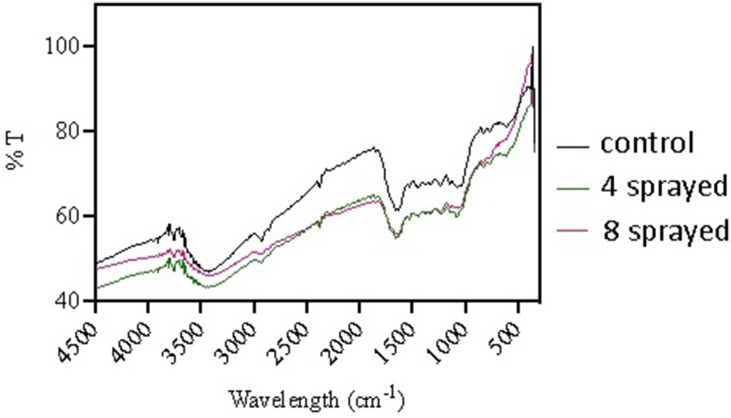
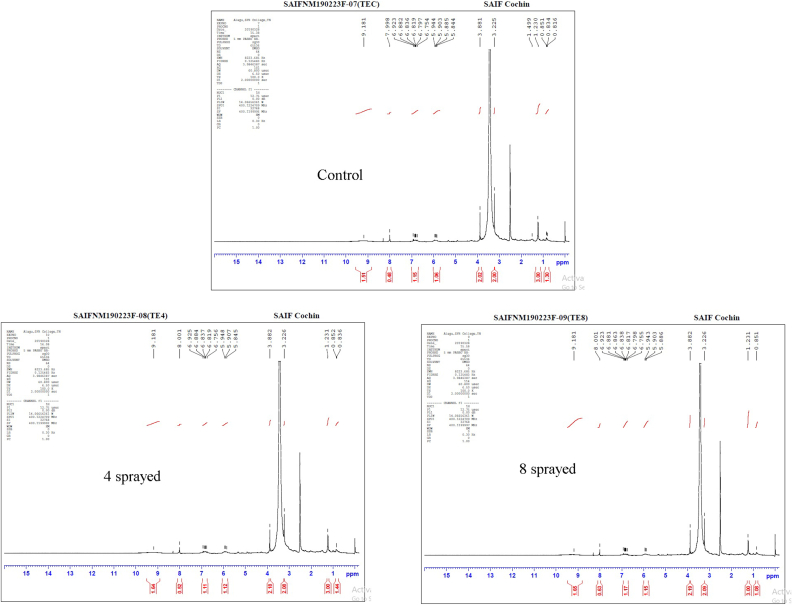
Tea (Figure 2)
Figure 2.
FTIR spectra of tea samples.
The control tea samples howed characteristic peaks at 1041 cm−1, 1442 cm−1, and 2924 cm−1, attributed to C–O, C–H, and C–H, respectively. The peak at 1627 cm−1 is normally due to the water content. The absorption bands at 1658 cm−1 and 1072 cm−1 were attributed to C=O and C–O, respectively. Peaks at 1527 and 1381 cm−1 are due to the CH groups. Additionally, the FTIR spectrum also displays a narrow absorption peak at 3448 cm−1, suggesting an abundant presence of –OH on the surface of the sample. Peaks at 600–800 cm−1 are due to C-X.
The 4-sprayed sample showed characteristic peaks at 1033 cm−1, 1442 cm−1, and 2924 cm−1, attributed to C–O, C–H, and C–H, respectively. The peak at 1627 cm−1 is normally due to the water content (intensity decreased). The absorption bands at 1658 cm−1 and 1072 cm−1 were attributed to C=O and C–O, respectively. Peaks at 1527 and 1373 cm−1 are due to the CH groups. Additionally, the FTIR spectrum also displays a narrow absorption peak at 3448 cm−1, suggesting an abundant presence of –OH on the sample surface. Peaks at 600–800 cm−1 are similar to those in the control sample and are due to C-X.
The 8-sprayed sample showed the characteristic peaks at 1033 cm−1, 1442 cm−1, and 2924 cm−1, attributed to C–O, C–H, and C–H, respectively. The peak at 1627 cm−1 is due to water. Both peaks at 1658 cm−1 and 1072 cm−1(C=O and C–O, respectively) are very weak or almost disappeared. The absorption band at 1658 cm−1 belongs to the C=O stretching. Peaks at 1527 and 1381 cm−1 are due to CH groups. Additionally, the FTIR spectrum also displays a narrow absorption peak at 3448 cm−1, suggesting an abundant presence of –OH on the sample surface. Peaks at 600–800 cm−1 are a bit weaker compared to other samples and are due to C-X.
Overall, the main difference among these spectra is the disappearance or reduced intensity of the C–O and C=O peaks in the 8-sprayed sample. This suggests a change in the tea structure upon 8 sprayings, which resulted in decreased aroma and increased bitter taste. The peak related to water decreased in the 4-sprayed sample, resulting in increased aroma and taste.
3.2.3. P-XRD
-
(a)
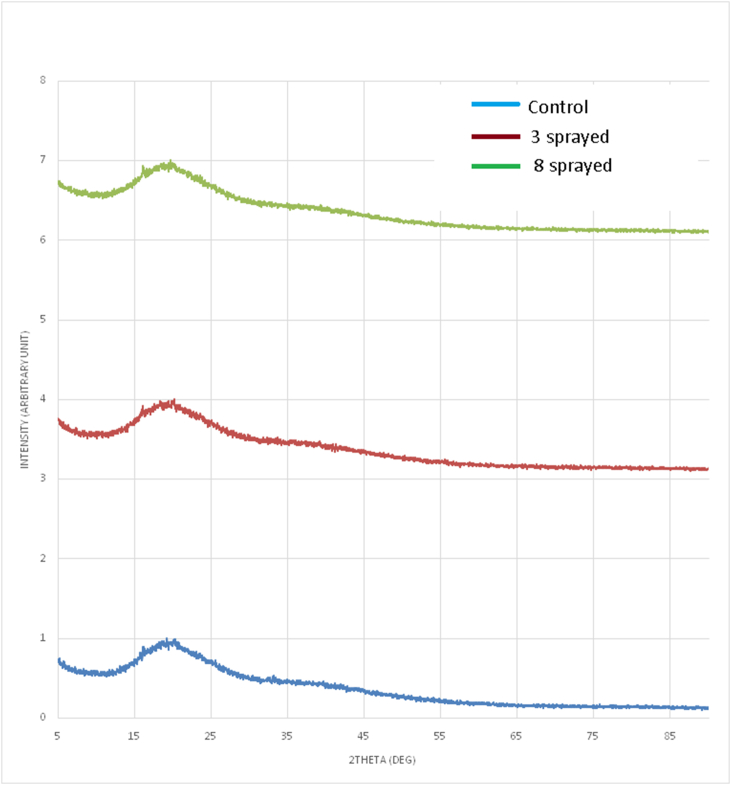
Coffee (Figure 3) (Supplementary table (i))
Figure 3.
PXRD spectra of coffee samples.
The control sample shows one broad peak in the 2-θrange 11.0° to 30.0°, located at 20.1°. Minor peaks overriding the broad peak are observed at 16.7°, 19.7°, and 20.8°.A broad diffraction peak implies that X-rays were scattered in many directions.
The 3-sprayed sample showed one broad peak in the 2-θ range 11.0°–30.0°, located at 20.0°. Broad minor peaks appeared at 16.5°, 18.9°, and 20.7°. The 3-sprayed sample showed a higher intensity of minor peaks overriding the broad peak.
The 8-sprayed sample showed one broad peak at 20.1°. Occurrence of broad minor peaks at16.8°and 20.1° was observed.
The XRD patterns agree with the signature peaks of green and roasted coffee (Perdana et al., 2018), and indicate a mixture of amorphous and crystalline phases in the sample. Broad peaks in all the samples corresponded to crystalline sucrose, in which the crystal structure is a monoclinic crystal system. All samples showed one broad peak around 20.0°, indicating that the majority of the phases in the sample are amorphous and X-rays scattered in many directions. Three very minor peaks were observed on the broad peaks of all samples.
-
(b)
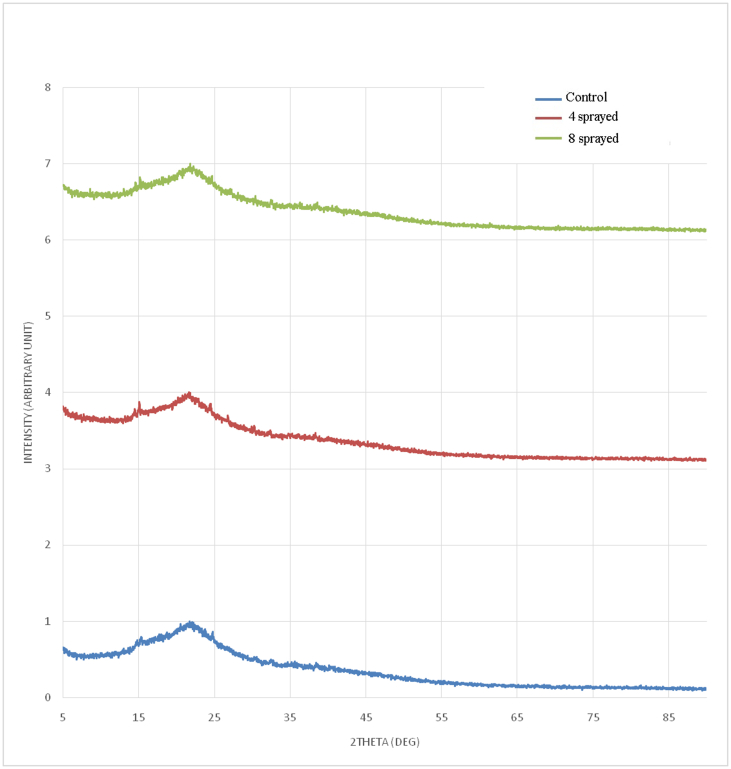
Tea (Figure 4) (Supplementary table (ii))
Figure 4.
PXRD spectra of tea samples.
The control sample shows one broad peak in the 2-θ range 8.0°–33.0°, located at 22.2°. This broad peak is compounded by minor peaks at 15.5°, 16.4°, and 24.6°. Other minor peaks outside the broad peak are at 33.3° and 39.3°.
The 4-sprayed sample shows one broad peak in the 2-θ range 12.0°–33.0°, located at 22.1°. This broad peak is compounded by minor peaks at 14.8°, 15.1°, 16.4°, 24.4°, 27.0°, 30.8°, and 32.8°. A minor peak outside the broad peak appeared at 39.1°.
The 8-sprayed sample shows one broad peak in the 2-θ range 12.0°–33.0°, located at 22.2°. This broad peak is compounded by minor peaks at 15.0°, 15.2°, 21.8°, 24.5°, 30.7°, and 32.9°. A minor peak outside the broad peak is seen at 39.4°.
All samples showed similar crystal structures. The XRD patterns observed agree with the signature broad peak of brewed tea leaves (Nath et al., 2014; Mahmood et al., 2017). The XRD patterns indicate a mixture of amorphous and crystalline phases in the sample. The 4-sprayed sample had the greatest number of minor peaks in its XRD; hence, its more favourable sensory properties. The broad peak at 22.0° indicates that the majority of phases in the sample are amorphous, and X-rays scattered in many directions. The control sample had the least number of minor peaks but had the broadest peak centred at 22.2°.
3.2.4. Proton NMR
-
(a)
Coffee (Figure 5)
Figure 5.
Proton NMR spectra of coffee samples.
Since one of the main compounds in coffee is quinic acid and there was no substantial change in the dimethyl sulfoxide derivative at 3.833 ppm in the evaluated samples, we used this peak as a reference to normalize the integral values in all three datasets. In order to analyze the data, peak integral and normalized peak integral values were sketched in two separate bar charts. As can be seen in the normalized peak integral chart (available in the Supplementary Fig (vi) & (Supplementary table (iii)), with respect to the control sample, fatty acid CH3 at 0.834 ppm, lactic acid –COOH at 1.158 ppm, mono-substituted ethylene –OCH = CH2 at 4.507 ppm, and non-condensed heteroaromatic ring C7H7NO2 at 7.998 ppm all consistently dropped in value as the spraying increased. However, the carbonic acid derivative at 3.291 ppm exhibited the opposite trend. The peak integral chart shows a consistent increase in the dimethyl sulfoxide derivative compound reference at 3.833 ppm as the spraying increased. These results suggest that the 3-sprayed sample was more positively impacted than the control sample, whereas the 8-sprayed sample was the least favorable (Badertscher et al., 2009; Toci et al., 2017).
-
(b)
Tea (Figure 6)
Figure 6.
Proton NMR spectra of tea samples.
Since one of the main compounds in tea is caffeine and there was no substantial change in the carbonic acid derivative HO(C=O)OH at 3.225 ppm in the evaluated samples, we used this peak as a reference to normalize the integral values in all three datasets. The peak integral and normalized peak integral values were sketched in two separate bar charts (available in the Supplementary Fig (vii) & (Supplementary table (iv)). As can be seen in the normalized peak integral chart, with respect to the control sample, fatty acid CH3 increased in the 4-sprayed sample, but decreased in the 8-sprayed sample. Aromatic hydrocarbon C6H5O at 6.923 ppm showed the opposite behavior. The peak integral chart showed an increase in the carbonic acid derivative reference HO(C=O)OH at 3.225 ppm in both 4-sprayed and 8-sprayed samples. These results suggest that the 4-sprayed sample showed more favorable characteristics than the control sample, while the 8-sprayed sample showed the least favorable result (Lillo et al., 2006; Badertscher et al., 2009; Ohno et al., 2011).
3.2.5. TEM
-
(a)
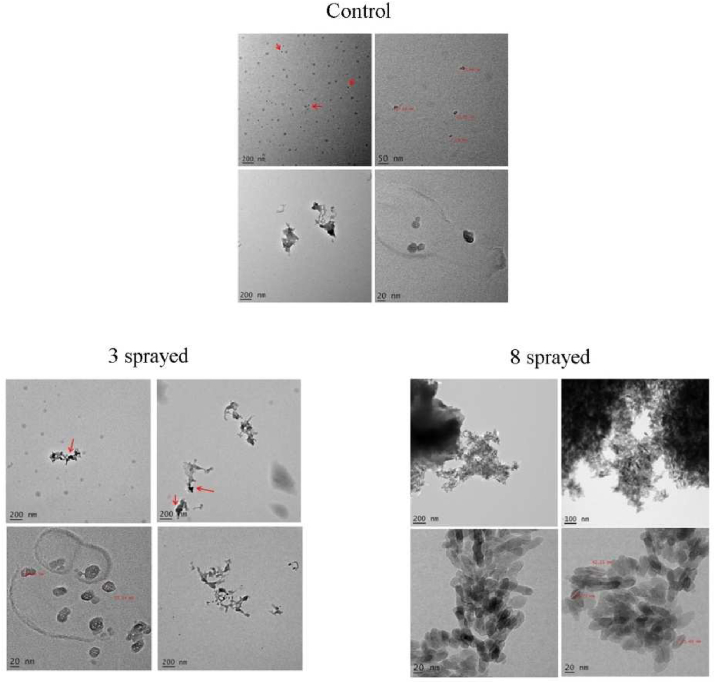
Coffee (Figure 7) (detailed interpretation in Supplementary Text (i))
Figure 7.
TEM images of coffee samples.
The control sample showed an average size (Feret’s diameter) of 400–800 nm, 30–40 nm (gray particles in the top left image), and 10–20 nm (dark particles in the top left image) for large and small particles, respectively. Large particles exhibited an amorphous shape, whereas small ones had ellipsoidal shape.
The 3-sprayed sample showed an average size (Feret’s diameter) of large and small particles of 200–400 nm, 40–60 nm (gray colored particles in the top left image), and 15–30 nm (dark particles in the bottom left image), respectively. Large particles showed the same shape as those in the control, but a smaller size of about half compared to the control. Small particles (either gray or dark types) showed a 10–20% increase in size and a combined crystalline and polycrystalline structure.
The 8-sprayed sample had a different structure with respect to the control and 3-sprayed samples. No amorphous large particles were observed. Small particles showed a pronounced prolate ellipsoid shape that was not observed in the control and 3-sprayed samples. Also, the main size (around the main axis) of the small particles was in a narrower range (20–30 nm). Small sized particles with crystalline structure were seen. Thus, 8 sprayings seem to be responsible for the changes in the interplanar lattice distance of the crystal phases (from 0.42 nm (3-sprayed) to 0.24 nm (8-sprayed), and for enhanced preferential orientation of the crystallites.
-
(b)
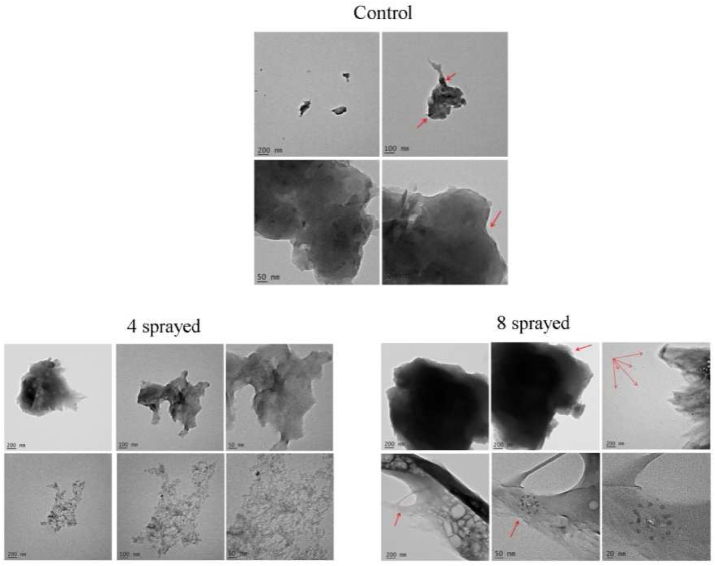
Tea (Figure 8) (Supplementary Text (ii))
Figure 8.
TEM images of tea samples.
The control sample showed large particles of 0.2 μm and small particles of 20–50 nm. Large particles were amorphous and smaller ones were ellipsoidal.
The 4-sprayed sample showed large particles of 0.5–1 μm and small particles of 4 nm. The size difference between large and small particles was far more noticeable than in the control. Structural changes also occurred and the particles were amorphous in shape.
The 8-sprayed sample showed large particles of around 2 μm and small particles of 15 nm. Size differences between large and small particles were far more noticeable than in the control. Structural changes occurred, and particles were polycrystalline.
The results of instrumental analyses demonstrated that MIRGA, depending on number of sprayings, altered the chemical bonds, structure, and chemical composition of the particles.
3.3. Toxicological analysis
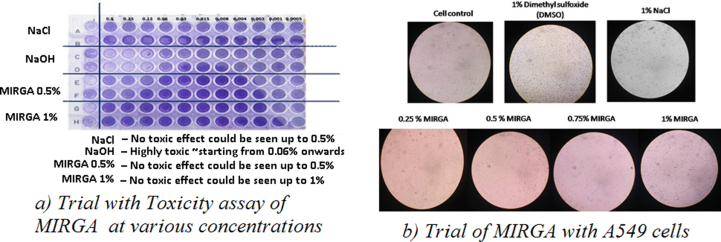
Even though, MIRGA generates safe 2–6 μm mid-IR energy, and spraying was done externally on packaged samples at a 0.25–0.50-m distance, we also evaluated MIRGA’s toxic effect. In vitro cytotoxicity assays proved that MIRGA-sprayed mist was non-toxic in any way (Figure 9).
Figure 9.
MIRGA’s toxicological studies.
3.4. Mode of action of MIRGA (detailed discussion in Supplementary Text (iii))
While spraying MIRGA, most of the generated mid-IR energy scatters through air and gets absorbed by receptor molecules, here, coffee and tea. Virtually these organic compounds absorb mid-IR radiation which causes a change in their molecule’s vibrational state from the lower ground state to an excited higher energy state (Girard, 2014). Nanostructured water layers of the coffee/tea are triggered upon the application of mid-IR radiation as the water molecules absorb energy in the infrared region (Sommer et al., 2008, 2011). This leads to changes in their chemical bonds (Mohan, 2004; Shankar, 2017), resulting in changes in the target’s physical and chemical characters and configuration (Atkins and Paula, 2011; Yi, 2012; Datta et al., 2014; Esmaeili, 2015). (Figure 10).
Figure 10.
Flowchart on action of MIRGA on coffee/tea.
Yousif and Haddad (2013) explained this process as the photodissociation of molecules caused by the absorption of photons from sunlight, including infrared radiation, visible light, and ultraviolet light, leading to changes in the molecular structure as revealed by our instrumental analyses. These changes in turn are reflected in a modification of the sensory properties of coffee and tea. Thus, depending on the number of MIRGA sprayings (MIR energy given), the chemical bond configurations and subsequent inherent physical and chemical characters of a receptor can be altered as desired.
In our nearly two-decades of research, we have observed that MIRGA-induced bond altered target substances do not show any adverse reaction upon consumption/use. Moreover, bond alteration commonly occurs when cooking or during digestion (Kowtaluk, 2006; Scanlon and Sanders, 2019). Williamson and Masters (2011) observed the influence of stereochemical configuration on food flavor. In the food industry, sensory attributes and shelf-life are enhanced by altering the food’s chemical bonds using various irradiation processes like radappertization, radicidation, and radurization (Sivasankar, 2014).
Ogundele and Kayitesi (2019) used an infrared emitter, which generated 1.3 μm–3.0 μm wavelength. This energy was absorbed by moisture-conditioned foods and the subsequent heat transfer mechanism resulted in changes in the biomolecules and their microstructures, which eventually modified the food. Abdelbasset et al. (2022) observed that infrared heating of foods preserved the aroma, taste, and acceptable quality characteristics of the treated foods. Tyagi et al. (2020) discussed the multi-dimensional application of infrared radiation in food industry. Results observed in the present study agree with these authors regarding the changes in the molecular and physicochemical nature of coffee and tea following mid-IR application. However, in the present study, a more convenient laser emitter (MIRGA) was developed and used. This is the first-time mid-IR spraying has been conducted to decaffeinate and improve the sensory qualities of coffee and tea.
Even though a variety of mid-IR emitters are now available (e.g., silicon photonic devices (CMOS Emerging Technologies, 2012), cascade lasers (quantum and inter-band) (Jung et al., 2017), non-cascade-based lasers, chalcogenide fiber-based photonic devices (Sincore et al., 2018), suspended-core tellurium-based chalcogenide fiber photonic devices (Wu et al., 2018), and infrared thermal emitters (Hsiao et al., 2021), these emitters are not as cost-effective as MIRGA, and also have not been tested in a domestic setting or with foods.
4. Conclusions
We present a facile strategy for the development of enhanced sensory attributes of beverages like coffee and tea. The MIRGA technology proposed here has several advantages over other decaffeination and sensory quality-enhancing methods, including avoidance of chemical solvents, improvement of sensory qualities of coffee and tea, ease of use in a domestic setting, and low cost. We demonstrated that the mid-IR induces changes in the chemistry of coffee and tea, thereby enhancing their taste and flavor, and modifying the content of alkaloids. Spraying resulted in coffee with lowered caffeine, but tea with a higher caffeine content. The amount of MIRGA sprayed coffee and tea powder used to prepare beverages with regular sensory characteristics was 20–30% lower than that of the control. The results of our work not only demonstrate an alternative strategy to promote wellness through improvement of everyday beverages, but also provide insights into the future prospects and potential of the MIRGA technique in the ever-expanding food industry.
Declarations
Author contribution statement
Umakanthan: Conceived and designed the experiments; Performed the experiments; Contributed reagents, materials, analysis tools or data; Wrote the paper.
Madhu Mathi: Performed the experiments; Analyzed and interpreted the data; Wrote the paper.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interest’s statement
The authors declare the following conflict of interests: In accordance with the journal’s policy and my ethical obligation as a researcher, we, the authors, are reporting that we together are the inventors and have applied for Indian patent for MIRGA (Application number: 201941048628) which is a major material employed in this study.
Additional information
No additional information is available for this paper.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Abdelbasset W.K., Alrawaili S.M., Elkholi S.M., Mahmoud E.I.D.M., Abd-Elghany A.A., Mahmoud M.Z. The role of infrared waves in increasing the quality of food products. Food Sci. Technol. 2022:42. [Google Scholar]
- Aboud S.A., Altemimi A.B., Al-HiIphy A.R.S., Yi-Chen L., Cacciola F. A comprehensive review on infrared heating applications in food processing. Molecules. 2019;24(22):4125. doi: 10.3390/molecules24224125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adnan M., Ahmad A., Ahmed A., Khalid N., Hayat I., Ahmed I. Chemical composition and sensory evaluation of tea (Camellia sinensis) commercialized in Pakistan. Pakistan J. Bot. 2013;45:901–907. [Google Scholar]
- Ashcroft F. University of California Press; California: 2000. Life at the Extremes: the Science of Survival; p. 122. [Google Scholar]
- Atkins P., Paula J. Oxford University Press; Oxford: 2011. Physical Chemistry for the Life Sciences; p. 365. [Google Scholar]
- Badertscher M., Bühlmann P., Pretsch E. Springer-Verlag Berlin Heidelberg; 2009. Structure Determination of Organic Compounds. [Google Scholar]
- Bagheri H. Application of infrared heating for roasting nuts. J. Food Qual. 2020:1–10. https://www.hindawi.com/journals/jfq/2020/8813047/ [Google Scholar]
- Bispo M.S., Veloso M.C.C., Pinheiro H.L.C., De Oliveira R.F.S., Reis J.O.N., De Andrade J.B. Simultaneous determination of caffeine, theobromine, and theophylline by high-performance liquid chromatography. J. Chromatogr. Sci. 2002;40(1):45–48. doi: 10.1093/chromsci/40.1.45. [DOI] [PubMed] [Google Scholar]
- Bunker M.L., McWilliams M. Caffeine content of common beverages. J. Am. Diet Assoc. 1979;74(1):28–32. [PubMed] [Google Scholar]
- Cappelletti S., Piacentino D., Sani G., Aromatario M. Caffeine: cognitive and physical performance enhancer or psychoactive drug? Curr. Neuropharmacol. 2015;13(1):71–88. doi: 10.2174/1570159X13666141210215655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CMOS Emerging Technologies CMOSET 2012: Abstracts, p49. 2012. https://books.google.co.in/books?id=3XVYC-yBgksC&pg=PA49&dq=mid+infra#v=onepage&q&f=false Available at:
- Coffee Association British. Coffee Facts. https://britishcoffeeassociation.org/coffee-consumption/
- Colombo R., Papetti A. Decaffeinated coffee and its benefits on health: focus on systemic disorders. Crit. Rev. Food Sci. Nutr. 2020;61(15):2506–2522. doi: 10.1080/10408398.2020.1779175. [DOI] [PubMed] [Google Scholar]
- CORDIS, European Commission New Advances in Mid-infrared Laser Technology, Compact, High-Energy, and Wavelength-Diverse Coherent Mid-infrared Source. https://cordis.europa.eu/project/rcn/99977/brief/en URL: (last accessed on 27.01.2019)
- Dasguptaa S., Mittala V., Choudharya P., Saxenab P., Joshipurac M.H. Comparative Studies on decaffeination of tea and coffee with various solvents. IJLTEMAS. 2014;III(III):145–149. [Google Scholar]
- Datta S.N., O’Trindle C., Illas F. Imperial College Press; London: 2014. Theoretical and Computational Aspects of Magnetic Organic Molecules; p. 224. [Google Scholar]
- Dolgin E. Engineering a better beverage. Nature. 2019;566:S12–13. https://media.nature.com/original/magazine-assets/d41586-019-00400-w/d41586-019-00400-w.pdf URL: [Google Scholar]
- Esmaeili K. Google books; 2015. Viremedy, Homeopathic Remedies, and Energy Healing Remedies as Information – Including Remedies; A Synopsis; p. 43. [Google Scholar]
- Everitt M. 2009. Consumer-targeted sensory quality; pp. 117–128. (Global Issues in Food Science and Technology). [Google Scholar]
- Farah A. 2009. Coffee as a Speciality and Functional Beverage, Functional and Speciality Beverage Technology. [Google Scholar]
- Fauchais P.L., Heberlein J.V.R., Boulos M.I. Springer Science & Business Media; New York: 2014. Thermal Spray Fundamentals from Powder to Part; p. 84. [Google Scholar]
- Garner R.L. vol. 311. Editor-in-Chief, William Marias Malisoff, Professor of Biochemistry at the Polytechnic Institute of Brooklyn, Philosophical Library, Inc.; New York: 1944. Dictionary of Biochemistry and Related Subjects; pp. 530–573. [Google Scholar]
- Girard J.E. third ed. Vol. 99. Jones & Bartlett Learning; USA: 2014. (Principles of Environmental Chemistry). [Google Scholar]
- Hsiao H.H., Huang C.H., Xu B.T., Chen G.T., Ho P.W. Triple narrowband mid-infrared thermal emitter based on a Au grating-assisted nanoscale germanium/titanium dioxide distributed Bragg reflector: implications for molecular sensing. ACS Appl. Nano Mater. 2021;4(9):9344–9352. [Google Scholar]
- Jilani T.N., Preuss C.V., Sharma S. StatPearls. StatPearls Publishing; Treasure Island (FL): 2019. Theophylline. [Google Scholar]
- Joseph M. 2018. Theobromine: Benefits & Side Effects of Cocoa's Interesting Phytochemical, Nutrition Advance. Retrieved 2019-06-27. [Google Scholar]
- Jung D., Bank S., Lee M.L., Wasserman D. Next-generation mid-infrared sources. J. Opt. 2017;19(12) [Google Scholar]
- Keping S., Yu G. Elsevier; UK: 2004. Recent developments in applied electrostatics (ICAES2004) p. 87. (Proceedings of the Fifth International Conference on Applied Electrostatics). [Google Scholar]
- Kowtaluk H. ninth ed. Tata McGraw-Hill Publishing Company Limited; NewDelhi: 2006. Food for Today 9E; p. 456. [Google Scholar]
- Lillo M.P., Sanderson P.N., Wantling E.L., Pudney P.D. Black tea mouthfeel characterisation by NMR analysis and chemometrics. Dev. Food Sci. 2006;43:537–540. [Google Scholar]
- Mahmood T., Ali R., Naeem A., Hamayun M., Aslam M. Potential of used Camellia sinensis leaves as precursor for activated carbon preparation by chemical activation with H3 PO4; optimization using response surface methodology. Process Saf. Environ. Protect. 2017;109 [Google Scholar]
- Marco I.D., Riemma S., Iannone R. Supercritical carbon dioxide decaffeination process: a life cycle assessment study. Chem. Eng. Trans. 2017;57:1699–1704. [Google Scholar]
- Martinez-Pinilla E., Oatibia-Astibia A., Franco R. The relevance of theobromine for the beneficial effects of cocoa consumption. Front. Pharmacol. 2015;6 doi: 10.3389/fphar.2015.00030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan J. second ed. Vol. 19. Alpha science International Ltd.; Harrow, UK: 2004. https://books.google.co.in/books?id=fA08Uy5DR0QC&printsec=frontcover&dq=Jag+Mohan.+Organic+Spectroscopy:+Principles+and+Applications&hl=en&sa=X&ved=0ahUKEwjHpcHUi9fgAhXXFIgKHXvRCpIQ6AEIKjAA#v=onepage&q=Jag%20Mohan.%20Organic%20Spectroscopy%3A%20Principles%20and%20Applications&f=false (Organic Spectroscopy: Principles and Applications). URL: [Google Scholar]
- Nath B., Barbhuiya T.F., Nath A. Synthesis and characterization of nanoparticles from tea leaves. Int. J. Sci. Res. 2014;3(10):1523–1525. [Google Scholar]
- Ngugi K., Aluka P., Bakomeza F., Neumbe B., Kyamuhangire R., Ngabirano H. Sensory and organoleptic cup attributes of Robusta coffee (Coffea canephora Pierre ex A. Froehner) J. Agric. Stud. 2015;4(1):104. [Google Scholar]
- Ogundele O.M., Kayitesi E. Influence of infrared heating processing technology on the cooking characteristics and functionality of African legumes: a review. J. Food Sci. Technol. 2019;56(4):1669–1682. doi: 10.1007/s13197-019-03661-5. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ohno A., Oka K., Sakuma C., Okuda H., Fukuhara K. Characterization of tea cultivated at four different altitudes using 1H NMR analysis coupled with multivariate statistics. J. Agric. Food Chem. 2011;59(10):5181–5187. doi: 10.1021/jf200204y. [DOI] [PubMed] [Google Scholar]
- Park H.S., Im N.G., Kim K.H. Extraction behaviors of caffeine and chlorophylls in supercritical decaffeination of green tea leaves. LWT - Food Sci. Technol. 2012;45(1):73–78. [Google Scholar]
- Perdana B.M., Manihuruk R., Ashyar R., Heriyanti Sutrisno. Evaluation of the effect of roasting process on the energy transition and the crystalline structures of Arabica, Robusta, and Liberica coffee from Jambi Indonesia. IOP Conf. Ser. Mater. Sci. Eng. 2018;345 [Google Scholar]
- Pereira M.F., Shulika O. Springer Science + Business Media B.V; The Netherlands: 2011. Terahertz and MidInfrared Radiation: Generation, Detection and Applications. [Google Scholar]
- Petruzzello M. How is coffee decaffeinated? Britannica.com. https://www.britannica.com/story/how-is-coffee-decaffeinated Website.
- Pople S. Oxford University Press; Oxford: 1999. Complete Physics; p. 166. [Google Scholar]
- Prasad M. Notion Press; Chennai: 2017. Soul, God and Buddha in Language of Science. [Google Scholar]
- Qu F., Qiu F., Zhu X., Ai Z., Ai Y., Ni D. LWT; 2018. Effect of Different Drying Methods on the Sensory Quality and Chemical Components of Black tea. [Google Scholar]
- Roberts E.A.H., Smith R.F. The phenolic substances of manufactured tea liquors. J. Sci. Food Agric. 1963;14:689–700. [Google Scholar]
- Sanders R.H. Cambridge University Press; USA: 2014. Revealing the Heart of the Galaxy; p. 70. [Google Scholar]
- Scanlon V.C., Sanders T. eighth ed. F. A. Davis; Philadelphia: 2019. Essentials of Anatomy and Physiology; p. 33. [Google Scholar]
- Segal L., Creely J.J., Martin A.E., Conrad C.M. An empirical method for estimating the degree of crystallinity of native cellulose using X-ray diffractometer. Textil. Res. J. 1959;29:786–794. [Google Scholar]
- Shankar D.R. Springer-Verlag GmbH; Germany: 2017. Remote Sensing of Soils; p. 268. [Google Scholar]
- Sincore A., Justin C., Tan F., Halawany E., Ahmed, Riggins A., McDaniel S., Cook G., Martyshkin D., Fedorov V.V., Mirov S., Shah L., Abouraddy A., Richardson M., Schepler K. High power single-mode delivery of mid-infrared sources through chalcogenide fiber. Opt Express. 2018;26(6):7313. doi: 10.1364/OE.26.007313. [DOI] [PubMed] [Google Scholar]
- Singh K.C. PHL Learning Private Limited; New Delhi: 2009. Basic Physics; p. 413. [Google Scholar]
- Sivasankar B. PHI Learning Private Limited; Delhi: 2014. Food Processing and Preservation; p. 246. [Google Scholar]
- Smit H.J. Theobromine and the pharmacology of cocoa. Handb. Exp. Pharmacol. 2011;200:201–234. doi: 10.1007/978-3-642-13443-2_7. [DOI] [PubMed] [Google Scholar]
- Sommer A., Caron A., Fecht H.J. Tuning nanoscopic water layers on hydrophobic and hydrophilic surfaces with laser light. Langmuir. 2008;24 doi: 10.1021/la7032737. [DOI] [PubMed] [Google Scholar]
- Sommer A., Zhu D., Mester A., Försterling H.D. Pulsed laser light forces cancer cells to absorb anticancer drugs - the role of water in nanomedicine. Artif. Cells Blood Substit. Immobil. Biotechno. 2011;39:169–173. doi: 10.3109/10731199.2010.516262. [DOI] [PubMed] [Google Scholar]
- Su S.W. Tea or coffee: a study of the beverage choice pattern and its affecting factors at teatime in kaohsiung, taiwan. Asia Pac. Manag. Rev. 2007;12(4):245–257. [Google Scholar]
- Sun J.X., Sun X.F., Zhao H., Sun R.C. Isolation and characterization of cellulose from sugarcane Bagasse. Polym. Degrad. Stabil. 2004;84:331–339. [Google Scholar]
- Takeo T., Oosawa K. vol. 12. 1976. Chagyo Shikenjo; pp. 125–181. [Google Scholar]
- Toci A.T., de Moura Ribeiro M.V., de Toledo P.R., Boralle N., Pezza H.R., Pezza L. Fingerprint and authenticity roasted coffees by (1)H-NMR: the Brazilian coffee case. Food Sci. Biotechnol. 2017;27(1) doi: 10.1007/s10068-017-0243-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toor F., Jackson S., Shang X., Arafin S., Yang H. Mid-infrared lasers for medical applications: introduction to the feature issue. Biomed. Opt Express. 2018;9(12):6255–6257. doi: 10.1364/BOE.9.006255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi L., SharmaGP VermaRC., JainSK MurdiaLK., MathurSM Infrared heating in food processing: an overview. Int. J. Chem. Stud. 2020;8(3):327–336. [Google Scholar]
- Verheest F. Kluwer Academic Publishers; Netherlands: 2000. Waves in Dusty Space Plasmas; p. 89. [Google Scholar]
- Verma R., Kumar L. Characterization of caffeine isolated from camellia sinensis leaves of Sikkim himalayan region. J. Chem. Pharm. Res. 2010;2(4):194–198. [Google Scholar]
- Voora V., Bermúdez S., Larrea C. The International Institute for Sustainable Development; Canada: 2019. Global Market Report: Tea, Sustainable Commodities Marketplace Series 2019.https://www.iisd.org/sites/default/files/publications/ssi-global-market-report-tea.pdf URL: [Google Scholar]
- Weinberg B.A., Bealer B.K. Routledge; New York: 2002. The World of Caffeine: the Science and Culture of the World's Most Popular Drug. [Google Scholar]
- Weinberg B.A., Bealer B.K. Routledge; New York: 2002. The World of Caffeine: the Science and Culture of the World's Most Popular Drug. [Google Scholar]
- Wen H., Lin Y., Seidman D., Schoenung J., Van Rooyen I., Lavernia E. An efficient and cost-effective method for preparing transmission electron microscopy samples from powders. Microsc. Microanal. 2015;21(5):1184–1194. doi: 10.1017/S1431927615014695. [DOI] [PubMed] [Google Scholar]
- Wendish M, Brenguier JL. Airborne Measurements for Environmental Research: Methods and Instruments, Wiley VCH. URL: https://www.books.google.co.uk/books?id=tHdwhn-c5mgC&pg=PT419&dq=A+regularly+oscillating+charge+produces+a+harmonic+electromagnetic+waves+Manfred&hl=en&sa=X&ved=0ahUKEwjBqdv75tvgAhWpSxUIHbQ_D0gQ6AEIKjAA#v=onepage&q=A%20regularly%20oscillating%20charge%20produces%20a%20harmonic%20electromagnetic%20waves%20Manfred&f=false (last accessed on 27.02.2019)
- Wichchukit S., O’Mahony M. The 9-point hedonic scale and hedonic ranking in food science: some reappraisals and alternatives. J. Sci. Food Agric. 2014;95(11):2167–2178. doi: 10.1002/jsfa.6993. [DOI] [PubMed] [Google Scholar]
- Williamson K.L., Masters K.M. sixth ed. Brooks Cole C engage learning; CA: 2011. Macroscale and Microscale Organic Experiments; p. 720. [Google Scholar]
- Wu B., Zhao Z., Wang X., Tian Y., Mi N., Chen P., Xue Z., Liu Z., Zhang P., Shen X., Nie Q., Dai S., Wang R.P. Mid-infrared supercontinuum generation in a suspended-core tellurium-based chalcogenide fiber. Opt. Mater. Express. 2018;8(5):1341. [Google Scholar]
- Yi G.C. Springer-Verlag; Berlin, Heidelberg: 2012. Semiconductor Nanostructures for Optoelectronic Devices: Processing, Characterization and Applications; p. 198. [Google Scholar]
- Yousif E., Haddad R. Photodegradation and photostabilization of polymers, especially polystyrene: review. SpringerPlus. 2013;2:398. doi: 10.1186/2193-1801-2-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data included in article/supp. material/referenced in article.