Abstract
Background
Fear generalization is pivotal for the survival-promoting avoidance of potential danger, but, if too pronounced, it promotes pathological anxiety. Similar to adult patients with anxiety disorders, healthy children tend to show overgeneralized fear responses.
Objective
This study aims to investigate neuro-developmental aspects of fear generalization in adolescence – a critical age for the development of anxiety disorders.
Methods
We compared healthy adolescents (14–17 years) with healthy adults (19–34 years) regarding their fear responses towards tilted Gabor gratings (conditioned stimuli, CS; and slightly differently titled generalization stimuli, GS). In the conditioning phase, CS were paired (CS+) or remained unpaired (CS-) with an aversive stimulus (unconditioned stimuli, US). In the test phase, behavioral, peripheral and neural responses to CS and GS were captured by fear- and UCS expectancy ratings, a perceptual discrimination task, pupil dilation and source estimations of event-related magnetic fields.
Results
Closely resembling adults, adolescents showed robust generalization gradients of fear ratings, pupil dilation, and estimated neural source activity. However, in the UCS expectancy ratings, adolescents revealed shallower generalization gradients indicating overgeneralization. Moreover, adolescents showed stronger visual cortical activity after as compared to before conditioning to all stimuli.
Conclusion
Various aspects of fear learning and generalization appear to be mature in healthy adolescents. Yet, cognitive aspects might show a slower course of development.
Keywords: Fear conditioning, Fear generalization, EEG, MEG, Event-related fields, Adolescence, Brain development
Highlights
-
•
Largely adult-like neural and behavioral generalization gradients in adolescents.
-
•
Child-like shallower generalization gradients in UCS expectancy ratings.
-
•
Higher visual cortical activity after fear conditioning in adolescents versus adults.
-
•
Cognitive aspects of fear learning may develop slower than emotional one.
1. Introduction
It is an important developmental challenge to learn threat and safety associations, i.e. information on contingencies between neutral stimuli (conditioned stimuli, CS) and naturally aversive stimuli (unconditioned stimuli, UCS) or their absence. From an evolutionary perspective, the generalization of conditioned fear responses to stimuli with perceptual similarities to the threat-signaling CS+ (so-called generalization stimuli, GS) may promote survival, as it may help to avoid potential danger. Research on differential fear conditioning in adult humans has consistently revealed that GS can activate emotional responses (e.g. altered skin-conductance response (SCR), fear-potentiated startle reflex (FPS), pupil dilation, and fear ratings) in a parametric fashion along a dimension ranging from the safety-signaling CS- (e.g. a small ring) via perceptually similar GS (e.g., medium sized rings) to the threat-signaling CS+ (e.g., a large ring, Lissek et al., 2008). The level of fear generalization can be characterized by linear and quadratic functions of these generalization parameters (Lissek et al., 2008, Onat and Büchel, 2015, Roesmann et al., 2022b, Schiele et al., 2016, Stegmann et al., 2019), whereby shallower linear gradients may indicate overgeneralization of fear (Lissek et al., 2014, Lissek et al., 2010).
Generalization gradients were shown to become steeper (i.e. more discriminative) with increasing age (Glenn et al., 2012, Reinhard et al., 2021, Schiele et al., 2016). Glenn and colleagues (Glenn et al., 2012) reported that FPS and fear ratings in older children (11–13 years) showed the typical fear generalization pattern (CS- < GS < CS+) as observed in adults. Younger children, by contrast, showed strong FPS responses also to the safety-signaling CS-, leading to shallower generalization gradients. Evidence for shallower generalization gradients in healthy children was further substantiated by Schiele and colleagues (Schiele et al., 2016), who reported higher arousal ratings and SCRs to the GS in children (8–10 years) versus adults (18–50 years). Additionally, Reinhard and colleagues (Reinhard et al., 2021) reported correlational evidence for improved CS+ /CS- discrimination and reduced overgeneralization in UCS expectancy ratings with increasing age in a sample of children and adolescents (8–17 years). In sum, these findings suggest that conditioned fear responses are less specific (shallower generalization gradients) in childhood and might develop to become increasingly specific (steeper or quadratic generalization gradients) throughout adolescence. Yet, evidence for elevated fear responses to safety and generalization stimuli in adolescents versus adults (Klein et al., 2021) suggests that this development might continue until adulthood.
Overgeneralization of fear is not only more pronounced with younger age, but also is a key characteristic in patients with anxiety disorders (AD) (Dymond et al., 2015, Dymond et al., 2014, Kaczkurkin et al., 2017, Greenberg et al., 2013). Given that ADs typically first emerge during childhood (Beesdo et al., 2009), overgeneralization might also contribute to the etiology of AD (Dymond et al., 2015). Evidence for this comes from El-Bar et al. (2017), who investigated perceptual aspects of conditioning and generalization learning in children (9–12 years) and adolescents (13–18 years) with and without AD. Healthy adolescents showed steeper generalization gradients than children, while discrimination abilities did not differ. Adolescents but not children with AD showed overgeneralization of fear compared to their healthy controls. This suggests that deviations from the normal developmental pattern from rather general to more specific fear responses might promote pathological anxiety. Thus, a better understanding of the developmental neurobiological trajectory of fear learning and fear generalization seems pivotal.
Developmental aspects of fear generalization are likely related to the maturation of brain structures that modulate efficient discrimination between danger and safety cues. Animal and human neuroimaging studies have highlighted the role of the prefrontal cortex (PFC), subcortical (amygdala, hippocampus) and cortical visual (temporal, occipito-parietal) areas in fear learning (LeDoux, 2000, Miskovic and Keil, 2012, Shin and Liberzon, 2010) and fear generalization (Dymond et al., 2015, Lissek, 2012, McTeague et al., 2015, Onat and Büchel, 2015, Roesmann et al., 2020, Roesmann et al., 2022a, Roesmann et al., 2022b). Our previous MEG-studies on fear generalization in healthy adults (Roesmann et al., 2022a, Roesmann et al., 2020) and adults with AD (Roesmann et al., 2022b) yielded evidence for reduced neural responses to GS that perceptually resembled the CS+ (vs. the CS-; i.e. negative generalization gradients) in the DLPFC, and for stronger neural responses to GS resembling the CS+ (i.e. positive gradients) in visual (temporal, occipito-parietal) regions. Non-reinforcement of specific CS in experimental paradigms conveying threat (i.e. the CS- in the fear acquisition phase and generalization phase) induces an inhibitory component to these CS (e.g., Haaker et al., 2015). Therefore, negative gradients in frontal structures may reflect fear-inhibitory processes with strongest activations towards stimuli signaling safety, while positive gradients in visual regions likely mirror fear-excitatory processes (e.g. motivated attention (Bradley et al., 2003; Schupp et al., 2006; Vuilleumier, 2005)) with strongest responses to stimuli signaling threat.
Interestingly, these PFC and visual cortex regions are characterized by different developmental trajectories (Casey et al., 2008): “top-down” control-associated structures, like the DLPFC, that have been linked with emotion regulation and fear inhibition (Phillips et al., 2008), tend to mature later than structures supporting bottom-up-processing of relevant stimuli (e.g. subcortical limbic structures, visual cortex). In line with that, evidence from animal models suggests that prefrontal inhibition of fear processes (e.g. during extinction (Kim and Richardson, 2010)) increases with age as the prefrontal cortex matures. In humans, an fMRI study revealed that adolescents (10–17 years) were more likely than adults to engage early-maturing subcortical structures during CS+ /CS- discrimination learning (Lau et al., 2011).
However, so far, it is unclear whether fear generalization mechanisms in adolescents differ from those observed in adults. Therefore, we here investigated fear responses of adolescents (14–17 years) compared to adults (19–34 years) towards conditioned and generalization stimuli. Fear responses were captured by means of fear- and UCS expectancy ratings. Moreover, we used pupil dilation, as a robust psychophysiological readout of fear learning (Finke et al., 2021, Jentsch et al., 2020, Leuchs et al., 2017), and event-related magnetic fields to study the dynamics of neurophysiological generalization gradients. Shallower gradients in behavioral readouts and pupil dilation in adolescents would support that child-like overgeneralization of fear transfers to adolescence. As a neural basis for such differences, we hypothesized group-specific generalization effects in late-maturing frontal structures supporting regulatory top-down control.
2. Methods
2.1. Participants
We included 59 healthy adults (19–34 years) and 61 healthy adolescents (14–17 years) in this study. All participants were recruited via advertisement in public areas including schools, in social media, and in local newspapers. All participants, as well as parents of adolescents, provided written informed consent. Participants were compensated with 10 Euro per hour for their participation. This study was approved by the Ethics Committee of the Medical Faculty of the University of Muenster.
Exclusion criteria for both adult and adolescent participants comprised a current or lifetime mental disorder, (psycho)pharmacological treatment, current or past psychotherapy, neurological or severe somatic diseases, MEG-related exclusion criteria, and pregnancy. In all participants, exclusion criteria were checked in a telephone screening. In adults, this was complemented by a more detailed screening of psychological problems using the Munich-Composite International Diagnostic Interview (M-CIDI/DIA-X (Wittchen and Pfister, 1997)). In adolescents, the telephone screening was conducted with the adolescent and one parent. Moreover, a more detailed assessment of psychological problems was conducted in personal with the adolescent using a German diagnostic interview for adolescents (J-DIPS für DSM-IV-TR, Margraf et al., 2007a, Margraf et al., 2007b).
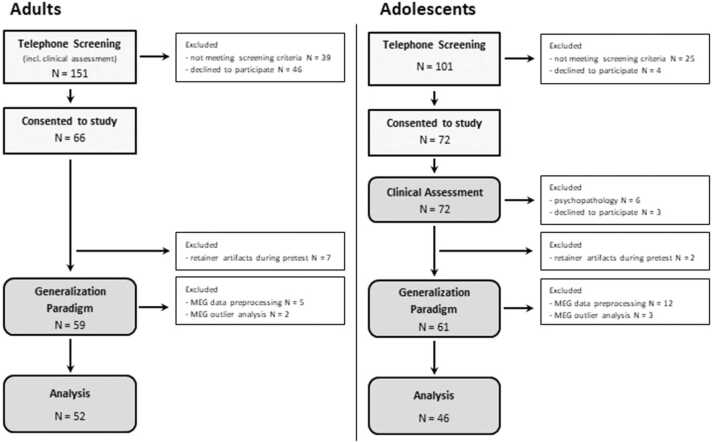
The flowchart presented in Fig. 1 visualizes the recruitment pathway for adults and adolescents, respectively. Detailed information on the rationale of exclusion of participants during MEG preprocessing are outlined below (see 2.4.). Sample characteristics of the final sample entering MEG analyses (52 adults; 46 adolescents) and statistical group comparisons are provided in Table 1. Note that groups were matched regarding depression levels but differed in their levels of self-reported intolerance of uncertainty, with adolescents being less intolerant to uncertainties compared to adults. Both adults and adolescents showed normal levels of anxiety, as revealed by the German State-Trait-Anxiety Inventory, Trait version (Laux and Spielberger, 2001) in adults (N = 51, Raw-value: M = 34.84, SD = 8.29; corresponding T-norm value: M = 50.06, SD = 8.68) and the self- and parent-reports of anxiety symptoms from the German diagnostic system for mental disorders according to ICD-10 and DSM-IV for children and adolescents (DISYPS-II, Döpfner et al., 2008) in adolescents (Self-report, N = 45: Raw-value: M = 20.77 SD = 17.62; corresponding T-norm value: M = 45.23, SD = 9.12; Parent-report, N = 44: Raw-value: M = 9.44, SD = 11.27; corresponding T-norm value: M = 42.67, SD = 11.66). Sample characteristics of the full sample are provided in Supplementary Table S1.
Fig. 1.
Flow-chart visualizing the recruitment pathways. Note: Due to the overall high number of adolescents with dental retainers, participants with retainers were only excluded if retainers caused artifacts in the MEG signal. This was tested by means of an MEG pretest.
Table 1.
Sample characteristics (N = 98).
| Adults | Adolescents | Test-statistic (df) | p-value | |
|---|---|---|---|---|
| Female / Male (N) | 31/21 (52) | 26/20 (46) | Χ2(1) = 0.10 | 0.957 |
| Age (years) | 23.92 (3.41) | 15.43 (1.07) | t(62.09) = 17.05 | < 0.001 |
| Depression (ADS-K)a | 4.27 (3.04) | 4.73 (3.85) | t(95.00) = -0.66 | 0.509 |
| Intolerance of Uncertainty (UI-18)b | ||||
| Total | 40.63 (12.48) | 33.29 (9.52) | t(94.00) = 3.21 | 0.002 |
| Act | 12.24 (4.92) | 10.29 (3.23) | t(87.18) = 2.32 | 0.023 |
| Burden | 8.37 (0.87) | 7.89 (0.75) | t(94.00) = 2.90 | 0.005 |
| Vigilance | 14.47 (5.06) | 11.98 (4.35) | t(94.00) = 2.57 | 0.012 |
Note. Gender is reported in absolute frequencies and all other data are reported as mean and standard deviation (M (SD)). T-tests use Welch-correction for unequal variances if necessary. ADS-K = Allgemeine Depressionsskala Kurzversion (Hautzinger et al., 2012; German short version of the Center for Epidemiologic Depression Studies – Depression Scale, Radloff, 1977). UI-18 = Unsicherheitsintoleranzfragebogen (Intolerance of Uncertainty Questionnaire, Gerlach et al., 2008).
data of one adolescent is missing;
data of one adult and one adolescent are missing.
2.2. Material
2.2.1. Stimuli
2.2.1.1. Conditioned and generalization stimuli (CS and GS)
As conditioned (CS) and generalization (GS) stimuli we employed sinusoidal grating stimuli with different tilt angles. Stimuli were filtered with a Gaussian envelope and had a maximum Michelson contrast of 95% (Gabor gratings). We used four sets of isoluminant black-and-white stimuli (A-D), which each consisted of nine stimuli arranged on a continuum of different orientations. Tilt angles of neighboring stimuli differed by 3°. The two most different stimuli in each set thus differed by 24° from each other and were used as CS+ and CS- stimuli. For each CS+ /CS- pair, seven additional grating stimuli with orientations between CS+ and CS- served as generalization stimuli (GSs). The four sets contained stimuli with orientations between 11° and 35° (set A), 101° and 125° (set B), 56° and 80° (set C), and 146° and 170° (Set D). Prominent orientations (0°, 45°, 90°, 135°) were not included in any of these continua. The assignment of orientations to CS+ and CS- was balanced across participants.
2.2.1.2. Unconditioned stimuli
A picture depicting a threatened female face (NimStim Face Stimulus Set; Tottenham et al., 2009) presented in combination with a female scream from the International Affective Digitized Sounds System (Bradley and Lang, 1999) served as the audiovisual unconditioned stimulus (SOA = 1200 ms). The scream was presented with a sound pressure level of 60 dB above the participants’ individual hearing threshold, corresponding to around 95 dB (range 85–105 dB; t-tests comparing sound pressure levels of adolescents with adults were non-significant for the right and left ear; all t < 1).
2.3. Experimental procedure
2.3.1. General procedure
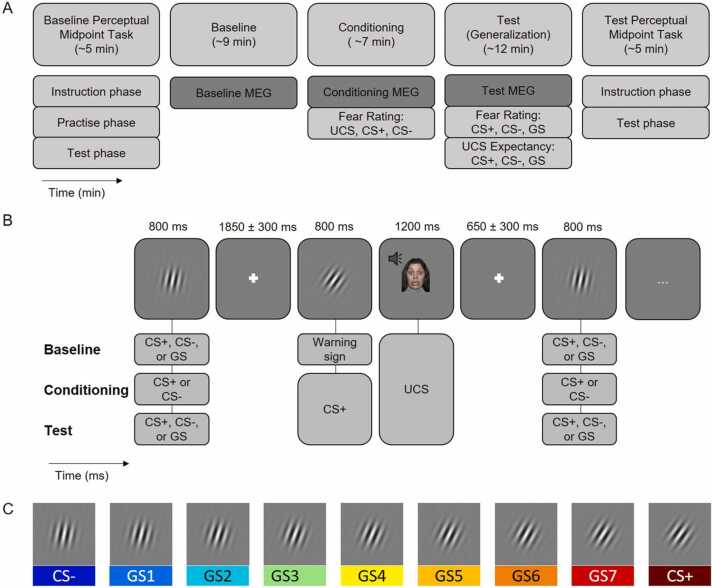
Upon arrival in the MEG laboratory, all participants received written and oral information on the generalization paradigm. They then entered the sound-attenuated and magnetically shielded MEG chamber. Participants were comfortably seated in the MEG scanner with 90 cm distance to the monitor. The experiment consisted of a Baseline MEG phase, a Conditioning MEG phase which terminated with a fear rating of the CS+ , the CS- and the UCS, and a Test MEG phase which terminated with Fear- and UCS expectancy ratings of the CS+ , the CS- and all GS. To assess perceptual aspects such as stimulus discrimination, we employed a Baseline Perceptual Midpoint (PM) Task before and a Test PM Task after MEG assessments. All experimental tasks were programmed and presented using the MATLAB-based Psychophysics Toolbox Version 3 (Kleiner et al., 2007); free software, available at www.psychtoolbox.org, Matlab 2016a).
Fig. 2 gives an overview on the experimental procedure. Overall, the assessment lasted for approximately 2 h.
Fig. 2.
Experimental Procedure. A. The assessment consisted of a Baseline Perceptual Midpoint (PM) Task, an MEG-Baseline phase, an MEG-Conditioning phase which terminated with a fear rating of the CS+ , the CS- and the respective audiovisual USs, an MEG-Test-Phase, which terminated with fear- and UCS expectancy ratings of the CS+ , the CS- and all GS, and – again – the PM Task. B. Sequence of stimulus presentation during the MEG Baseline, Conditioning and Test phase. Stimuli were repeatedly presented. In the Conditioning and Test phase the CS+ predicted the UCS in 33% of the cases while a warning signal predicted all UCS in the Baseline phase. Parallel to the MEG-signal pupil dilation was recorded. C. Example of stimulus set used as CS+ /- and GS (here: orientations between 11° and 35°).
2.3.2. Generalization paradigm
The basic structure of the Baseline, Conditioning and Test MEG phases was based on a prior fMRI study by Onat and Büchel (2015) and adapted for the recording of ERFs (Roesmann et al., 2022a, Roesmann et al., 2022b, 2022c). CS+ , CS-, and – in the Baseline and Test MEG phases – the seven GSs were presented separately for 800 ms in the center of the screen (visual angle = 5.09 degrees, edge to edge) on a grey background. The inter-stimulus interval between individual CSs and GSs had a random duration of 1850 ± 300 ms. In this window, participants were either presented with a fixation cross (1850 ± 300 ms) or with the UCS (1200 ms) which was then replaced by a fixation cross (650 ± 300 ms). MEG and pupil responses to all CSs and GSs were recorded while participants kept their attention on the presented stimuli. No keypress was necessary.
2.3.2.1. Baseline phase
Participants were instructed that they would view differently tilted grating stimuli and – preceded by a “warning symbol” – a screaming face (UCS). In the MEG-Baseline phase, each of the nine differently tilted grating stimuli was presented 21 times in pseudorandomized order. The warning signal (a line drawing of a triangle in black ink, SOA: 800 ms) was presented and replaced by the UCS seven times throughout the Baseline phase, once in every 7th part of the stimulus presentation. As in Onat and Büchel (2015), this setup was employed to keep the number of UCS presentations constant in the Baseline and the Test phase, and to thereby balance overall arousal levels.
2.3.2.2. Conditioning phase
In the Conditioning MEG phase, we explicitly informed participants on key aspects of the following learning phase (translation of instruction: “Now the learning phase starts. You will see two differently tilted gratings. One of these gratings will sometimes be followed by a picture of a face and a loud scream. The tilt of the gratings is critical! In this part, no key pressures are required. Please remove your hand from the response box. If you have any questions, please ask them now. Else, please say that you are ready.”). In this phase, the CS+ as well as the CS- were presented 60 times each in a pseudorandomized order realizing that no stimulus was repeated more than 3 times in a row. The CS+ was followed by the UCS in one third of the cases (20 times, contingency rate of 33%), evenly distributed throughout the whole phase.
After the Conditioning MEG phase, participants were asked to rate the level of fear elicited by the CS+ , the CS- and the UCS on a visual analogue scale with ten discrete values ranging from 1 = “no fear” to 10 = “extreme fear”.
2.3.2.3. Test phase
In the beginning of the Test MEG phase, participants were informed that the previously learned grating stimulus would continue to predict the screaming face and that they would be presented with all grating stimuli they knew from the Baseline phase (translation of instruction: “In the following part, you will again see individual gratings. Please view the gratings attentively. The picture of the face and the loud scream will now be predicted by one grating with a certain tilt angle. In this part, no key pressures are required. Please remove your hand from the response box. If you have any questions, please ask them now. Else, please say that you are ready.”). Like in the Baseline phase, CSs and GSs were pseudorandomly presented 21 times while the UCS appeared seven times in total. In contrast to the Baseline phase, the CS+ (not the warning symbol) predicted the UCS at a contingency rate of 33%. The Test phase terminated with subjective fear and UCS expectancy ratings in response to the CS+ , CS- and all GSs. In both ratings, each of these stimuli were presented three times in a pseudorandomized order (i.e. random order within each of three repetition blocks containing all CS and GS). First, fear ratings were obtained convergent to the fear ratings after the Conditioning phase. Subsequently, patients rated the probability that the UCS would appear following CS+ , CS- and GS (i.e. UCS expectancy). Again, a visual analogue scale was used, now ranging from 1 = very unlikely to 10 = very likely. Fear and UCS expectancy ratings were recorded via button presses on the response box.
2.3.3. Perceptual midpoint (PM) task
The PM task was adopted from research on perceptual learning mechanisms (McMahon and Leopold, 2012) and was employed in our previous experiments on fear generalization (Roesmann et al., 2022a, Roesmann et al., 2022b). Performance in the PM task was measured before (Baseline PM-task) and after (Test PM-task) the Conditioning and Generalization phases (see Fig. 2A) and was employed as an index for different aspects of perceptual discrimination regarding our grating stimuli. Within the task, CS+ and the CS- stimuli were positioned at the left and right side of a monitor screen (RightCS+/LeftCS- or Left-CS+/RightCS- assignment was balanced across participants) and participants had to indicate via forced choice button-press whether a centrally presented GS would rather resemble the CS+ or the CS-. For more details on the PM-task, please consult Roesmann et al. (2022a).
2.4. MEG recording and preprocessing
During all MEG phases, we acquired ERFs using a 275 MEG whole-head sensor system (Omega 275; CTF, VSM MedTech Ltd., Coquitlam, Canada) with first-order axial SQUID gradiometers. Continuous signals in a frequency range between 0 and 150 Hz were recorded using a sampling rate of 600 Hz. Information on the participants’ head shapes was digitized using a 3D tracking device (Polhemus, Colchester, VT, USA; http://www.polh emus.com/). The individual head position in the MEG scanner was tracked by three landmark coils placed on the two ear canals and the nasion.
The preprocessing of MEG data was carried out using the MATLAB-based Electromagnetic Encephalography Software EMEGS (Version 3.0, Peyk et al., 2011). MEG data were filtered offline using a 48 Hz low-pass and a 0.1 Hz high-pass filter and sampled down to 300 Hz. Epochs of 800 ms duration (i.e., 200 ms before to 600 ms after stimulus onset) were extracted, aligned, and baseline-adjusted using a 150 ms pre-stimulus interval as baseline. Single trials were edited and artefacts were corrected following the method for statistical control of artefacts in high-density EEG/MEG data (Junghöfer et al., 2000). This procedure (1) detects artifacts in individual sensors, (2) detects global artifacts; (3), replaces artifact-contaminated sensors by spherical spline interpolations that are statistically weighted on the basis of all remaining sensors; and (4) computes the variance of the signal across trials to document the stability of the averaged waveform. The rejection of artifact-contaminated trials and the interpolation of artifact-contaminated sensors relies on the calculation of statistical parameters for the absolute measured magnetic field amplitudes over time, their standard deviation over time, as well as on the determination of boundaries for each parameter based on their distribution across trials. If the goodness of test topography interpolations based on the residual sensor configuration within a given trial did not reach an a-priori defined minimum criterion (k = 0.01; Roesmann et al., 2022b, Roesmann et al., 2022c, Roesmann et al., 2020; identical for each subject and run) the respective trial was rejected. If more than 30% of the trials in any run of either the Baseline or the Test phase did not meet this criterion (e.g. due to continuous movement artifacts), the respective participant was rejected from the MEG-analysis (5 adults, 12 adolescents, see Fig. 1).
The spatiotemporal signal to different stimulus types was computed separately for each participant and phase. Each category included 21 trials (i.e., the number of repetitions per STIMULUS TYPE (CS+, GS1 to GS7, CS-)) in each PHASE (Baseline, Test). This rather low number of trials per condition resulted from the need to keep runs as short as possible to avoid movements, reduced vigilance, and poor attention performance. To increase the number of trials per category and thus the signal-to-noise ratio, ERFs in reaction to one grating stimulus were merged with those in reaction to its neighboring stimulus (Lissek et al., 2010). ERFs to CS+ were merged with those to GS1 (CS+/GS1), those to GS1 were merged with those GS2 (GS1/GS2), etc. This moving average doubled the number of trials per step and thus enhanced the signal to noise ratio by around 40% (), which was specifically important for a reasonable estimation of the underlying neural sources. It also reduced high-frequency noise along the vector of STIMULUS TYPE, while the resolution of the STIMULUS TYPE gradient function was reduced from nine to eight steps only.
Based on the averaged responses and considering individual sensor positions during MEG recording, cortical sources of the event-related magnetic fields were calculated using the L2-Minimum-Norm-Estimates (L2-MNE) method (Hämäläinen and Ilmoniemi, 1994). The L2-MNE is an inverse modelling technique with which distributed neuronal network activity can be estimated. It does not require a priori specifications of the location and/or number of active current dipoles (Hauk, 2004). A spherical shell with 350 evenly distributed dipole pairs (azimuthal and polar direction) with a source shell radius approximately corresponding to the grey matter depth (i.e., 87% of the individually fitted head) was used as source model. The Tikhonov regularization parameter Lambda was set to 0.1. Topographies of source-direction-independent neural activities – the vector length of the estimated source activities at each position – were calculated for each individual participant, condition, and time point.
To avoid statistical artefacts due to potential outliers in any phase or condition, participants were excluded, if the mean of the standard deviation between experimental conditions across time (2 adults, 3 adolescents) or if the mean number of trials across experimental conditions (1 adolescent, also excluded based on the other outlier criterion) differed from the sample median by more than four standard deviations. The number of remaining trials was evenly distributed across experimental conditions, as confirmed by an 8×2x2 ANOVA with the factors STIMULUS TYPE (CS-&GS1, GS1&2, GS2&3, GS3&4, GS4&5, GS5&6, GS6&7, GS7&CS+), PHASE (Baseline, Test) and GROUP (Adult, Adolescent): STIMULUS TYPE x PHASE, F(7672)= 0.619; p=.741, STIMULUS TYPE x PHASE x GROUP, F(7672)= 1.678; p=.111.
To extract purely perceptual effects elicited by the different tilt angles of the stimuli, difference topographies (Test minus Baseline) of the L2-MNE data were computed and used as the basis for statistical analyses.
2.5. Pupil data recording and preprocessing
Participants’ pupil dilations in response to all stimuli (CS+, GS1 to GS7, CS-) were assessed using the eye tracker EyeLink 1000 Plus (SR Research Ltd., Canada). Pupil data were acquired as an external MEG sensor with the identical sampling rate of 600 Hz. Epochs of 2000 ms duration (−200 to 1800 ms) were extracted and preprocessed and statistically analyzed identically to the MEG, but with a temporal dimension only (i.e., just one sensor and thus temporal cluster analysis instead of spatiotemporal cluster analysis).2
2.6. Statistical analyses
2.6.1. Statistical analyses of subjective ratings and the perceptual midpoint task
Analyses of subjective data were performed using SPSS 24 (IBM Corp., Armonk, NY). All analyses adopted a significance level of α = .05. Whenever applicable and necessary, F-statistics were Greenhouse Geisser corrected and t-statistics were Welch corrected.
2.6.1.1. Fear ratings and UCS expectancy ratings
To estimate potential differences in the aversiveness of the UCS between adults and adolescents, UCS fear ratings were compared using an independent-sample t-test. To confirm effective fear induction in both groups, the CS fear ratings obtained directly after the Conditioning phase were analyzed by a mixed ANOVA with the within-subject factor STIMULUS TYPE (CS+, CS-) and the between subject factor GROUP (Adults, Adolescents). To determine generalization patterns to the GS stimuli, fear and UCS expectancy ratings after the Test phase were analyzed using mixed ANOVAs with the within-subject factor STIMULUS TYPE (CS+, GS1 to GS7, CS-) and the between-subject factor GROUP (Adults, Adolescents). Planned polynomial contrasts tested for linear and quadratic trends and their modulations by GROUP. To test for a potential influence of contingency awareness, generalization analyses on subjective ratings were repeated without participants that were potentially unaware of CS-UCS associations. Following the definition of contingency awareness by Schiele and colleagues (2016), participants were considered aware of the CS-UCS relationship if UCS expectancy ratings were higher for the CS+ than the CS- and if UCS expectancy ratings for the CS- were no higher than 50%.
2.6.1.2. Perceptual midpoint (PM) task
For the PM Task, the relative frequency of classifications as CS+ was determined individually for each GS and separately for each PHASE (Baseline PM Task, Test PM Task). In a first step, a mixed ANOVA with the within-subject factors STIMULUS TYPE (GS1 to GS7) and PHASE (Baseline, Test) and the between-subject factor GROUP (Adults, Adolescents) was computed. Planned polynomial contrasts tested for linear, quadratic, and cubic trends and their modulations by PHASE and GROUP. Based on the conception of the PM task, shifts of the perceptual midpoint should be reflected as changes of cubic trends (see Roesmann et al., 2022a). In a second step, group-specific patterns were investigated using repeated-measures ANOVAs with the within-subject factors STIMULUS TYPE (GS1 to GS7) and PHASE (Baseline, Test) for adults and adolescents separately. Planned polynomial contrasts tested for linear and quadratic trends and their modulations by PHASE.
2.6.2. Statistical analyses of MEG data
Statistical analyses of MEG data used the MATLAB-based Electromagnetic Encephalography Software EMEGS (Version 3.0, Peyk et al., 2011). Continuative statistical analyses used SPSS Statistics (IBM Corp., Armonk, NY). The analyses of MEG data were performed in parallel with the analyses of the fear- and UCS expectancy ratings, however due to the moving average the number of grating steps was reduced from nine to eight (see 2.4.). Note that all statistical MEG analyses were based on the difference topographies (Test minus Baseline).
First, following our previous MEG-studies on fear generalization (Roesmann et al., 2022a, Roesmann et al., 2022b) we set out to identify spatiotemporal clusters reflecting linear generalization gradients. Thus, a linear contrast with the factor STIMULUS TYPE (CS-&GS1, GS1&2, GS2&3, GS3&4, GS4&5, GS5&6, GS6&7, GS7&CS+) was calculated for each time point and dipole. Second, to identify clusters displaying group differences in linear gradients a STIMULUS TYPE x GROUP interaction analysis with an orthogonal linear contrast for adults vs. adolescents (Greenberg et al., 2013, Roesmann et al., 2022b, Roesmann et al., 2022c) was calculated for each time point and dipole. These computations resulted in matrices of T-values for each time point and dipole. Next, we used a non-parametrical statistical testing procedure to correct for multiple comparisons (Maris and Oostenveld, 2007), called cluster permutation analysis, to determine reliably significant spatio-temporal cluster in two time intervals of interest (early TOI: 0–300 ms, late: 300–600 ms, see (Roesmann et al., 2022a, Roesmann et al., 2022b). In each of these TOIs, t-values exceeding the critical alpha level of p=.05 (first level criterion) entered so-called spatio-temporal cluster masses. Cluster masses were then compared against identical analyses based on 1000 permuted drawings of data from the experimental conditions. When the cluster mass of the original analysis (using the correct assignment of experimental conditions) was higher than the critical cluster mass of this permutation distribution corresponding to a p-value = 0.05 (i.e., higher than the 950 highest cluster masses found with the largest cluster of each draw of the random distribution; second level criterion), the cluster was considered significant.
Clusters revealing significant linear generalization gradients, were further analyzed regarding potential modulations of the Factor GROUP. For this purpose, L2-MNE were extracted from significant clusters to ANOVAs testing for main effects of GROUP and/or GROUP by STIMULUS TYPE interactions that followed the predicted linear or quadratic trends.
2.6.3. Statistical analysis of pupil data
The analysis of pupil data was performed in parallel with the MEG analyses, comprising the same linear and orthogonal linear contrasts. Due to strong inter-individual differences of mean pupil diameter, Test minus Baseline differences of each participant were normalized by the overall summed root mean square of individual data within the time interval of interest (0–1800 ms). Cluster permutation analysis was performed for a time interval ranging from 0 to 1800 ms after stimulus onset, with a first and second level criterion of p = .05.
3. Results
3.1. Subjective ratings after conditioning phase
3.1.1. UCS-rating after conditioning phase
Fear ratings in response to the UCS did not differ between groups (Adults: M = 4.33, SD = 2.39; Adolescents: M = 4.52, SD = 1.96; t(95.453) = −0.442, p=.660, d = 0.088).
3.1.2. CS-rating after conditioning phase
After the Conditioning phase, a main effect of the factor STIMULUS TYPE (CS+, CS-) revealed higher fear ratings in response to the CS+ compared to the CS-, F(1,96) = 69.868, p < .001, partial η2 = 0.421, with equivalent effects in both groups (CS+ Adults: M = 3.63, SD = 2.43; CS+ Adolescents: M = 3.50, SD = 2.63; CS- Adults: M = 1.56, SD = 0.96; CS- Adolescents: M = 1.54, SD = 1.05; GROUP x STIMULUS TYPE: F (1,96) = 0.062, p=.803, partial η2 < 0.01; GROUP: F(1,96) = 0.060, p=.808, partial η2 < 0.01).
3.2. Subjective ratings after test phase
3.2.1. Fear ratings of CS and GS after test phase
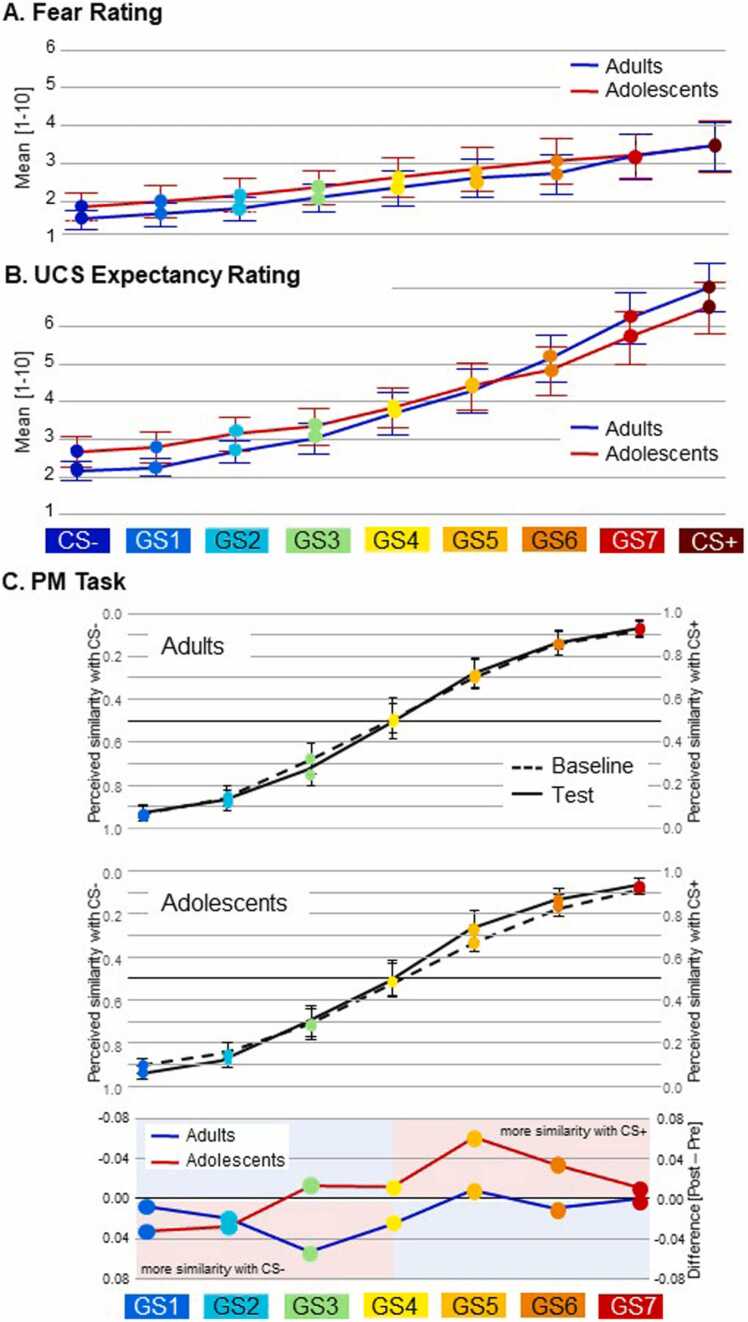
The STIMULUS TYPE (CS–, GS1 to GS7, CS+) GROUP (Adults, Adolescents) ANOVA on the fear ratings showed a main effect of STIMULUS TYPE, F(2.02, 194.22) = 54.89, p < .001, partial η2 = .364 (Fig. 3A). Planned polynomial contrasts revealed that this resulted from a linear increase of fear ratings from CS– to CS+ in both groups, linear trend: F(1, 96) = 81.58, p < .001, partial η2 = .459; quadratic trend: F(1, 96) = 3.24, p=.075, partial η2 = .033. There was neither a main effect of GROUP, F(1, 96) = 0.66, p=.419, partial η2 = .007, nor an interaction between GROUP and STIMULUS TYPE, F(2.02, 194.22) = 0.69, p=.691, partial η2 = .007.
Fig. 3.
Subjective Ratings and Perceptual Midpoint Task. A. Fear ratings indicating subjective fear responses from 1 (no fear) to 10 (extreme fear) in response to the CS+ , CS- and the GS. B. UCS expectancy ratings indicating the perceived probability that the UCS would appear following CS+ , CS- and GS on a scale from 1 = very unlikely to 10 = very likely. C. Perceptual midpoint task. Top and middle: Relative frequencies of each GS to be classified as more similar to CS- (left side) or more similar to CS+ (right side) before (Baseline) and after (Test) the Conditioning phase, respectively. Frequencies are provided separately for adults (top) and adolescents (middle). Bottom: Test minusBaseline differences of relative frequencies. Error bars indicate 95% confidence intervals.
3.2.2. UCS expectancy ratings of CS and GS after test phase
The corresponding ANOVA on the UCS expectancy ratings also showed a main effect of STIMULUS TYPE, F(2.77, 256.63) = 146.29, p < .001, partial η2 = .604 (Fig. 3B). Planned polynomial contrasts revealed that this resulted from a linear and quadratic increase of expectancy ratings from CS– to CS+ in both groups, linear trend: F(1, 96) = 258.62, p < .001, partial η2 = .729; quadratic trend: F(1, 96) = 46.57, p < .001, partial η2 = .327. There was no main effect of GROUP, F(1, 96) = 0.08, p=.783, partial η2 = .001, but a significant GROUP STIMULUS TYPE interaction, F(2.77, 256.63) = 2.86, p=.042, partial η2 = .029. Planned polynomial contrasts revealed group differences regarding linearity, linear trend: F(1, 96) = 4.86, p=.030, partial η2 = .048; quadratic trend: F(1, 96) = 0.73, p=.396, partial η2 = .008. Fig. 3B demonstrates that this effect roots in steeper gradients in adults than in adolescents (Adults linear trend: F(1, 51) = 177.03, p < .001, partial η2 = .776, Adolescents linear trend: F(1, 45) = 91.36, p < .001, partial η2 = .670).
To follow up on the specific contribution of the CS to these findings, an ANOVA with the within subject factor STIMULUS TYPE (CS+, CS-) and the between subject factor GROUP (Adults, Adolescents) was conducted. It revealed a significant STIMULUS TYPE x GROUP interaction, F(1,96) = 3.982; p = .049. Post hoc T-Tests show that Adults had a lower USC-Expectation in response to CS- than Adolescents (Adults: M=1.615, SD=0.997; Adolescents: M=2.130, SD=1.436; T(96) = −2.081; p = .045), while the UCS-Expectation in response to the CS+ did not differ between groups (Adults: M=6.654, SD=2.410; Adolescents: M=6.101, SD=2.357; T(96) = 1.144; p;.255).
Three adults and five adolescents were considered unaware of CS-UCS associations. However, the exclusion of these participants revealed qualitatively equivalent results for UCS expectancy ratings and fear ratings. Therefore, the following analyses were performed on the whole sample.
3.3. Changes in the perceptual midpoint (PM) task from baseline to test phase
Analyses of the PM task examined changes after conditioning in the relative frequency of classifications of the GSs as resembling the CS+ (Fig. 3C). The ANOVA with the factors STIMULUS TYPE (GS 1–7) GROUP (Adults, Adolescents) PHASE (Baseline, Test) revealed a main effect of STIMULUS TYPE, F(1, 2.803) = 653.790, p < .001, partial η2 = 0.872. Planned polynomial contrasts indicated that this resulted from a linear and cubic increase in classifications of the GSs as CS+ , linear trend: F(1, 96) = 2014.676, p < .001, partial η2 = 0.955, quadratic trend: F(1,96) = 0.082, p=.776, partial η2 = 0.001, cubic trend: F(1,96) = 115.234, p < .001, partial η2 = 0.546, reflecting the actual physical similarity. Moreover, there was a STIMULUS TYPE PHASE interaction, F(1, 5.173) = 2.272, p=.044, partial η2 = 0.023, and planned polynomial contrasts indicated that the linear increase became steeper after conditioning, linear trend: F(1, 96) = 6.923, p=.010, partial η2 = 0.067, quadratic trend: F(1, 96) = 0.414, p=.521, partial η = 0.004, cubic trend: F(1, 96) = 3.883, p=.052, partial η2 = 0.039. There were no other significant effects.
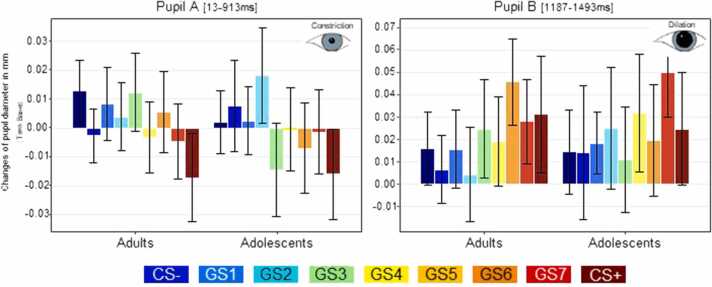
3.4. Changes of pupil dilation from baseline to test phase
The temporal cluster analysis of pupil data searching for linear trends of the factor stimulus across a time interval from 0 to 1800 ms revealed one early time interval from 13 to 913 ms showing a negative linear trend and thus increasing pupil constriction from CS- to CS+ (Pupil A, p-second-level <.001) and one late time interval from 1187 to 1493 ms showing a positive linear trend across groups (Pupil B, p-second-level =.027; Fig. 4). Neither the early nor in the late temporal cluster showed any main effects of GROUP (all Fs < 1). In the early cluster, there was a significant GROUP x STIMULUS TYPE interaction (Pupil A; F(8, 768) = 2.030, p = .05, partial η2 = 0.021), which – however – could not be explained in terms of the predicted linear or quadratic contrasts (all Fs < 1).
Fig. 4.
Changes of Pupil diameter. Significant linear effects in pupil diameter as revealed by the temporal cluster permutation analysis of linear gradients. Group-independent negative effects (constriction from CS- to CS+) were observed in an early time interval (Pupil A: 13–913 ms after stimulus onset), while group-independent positive effects (dilation from CS- to CS+) were observed in a later time interval (Pupil B: 1187–1493 ms after stimulus onset, N = 74). Bar graphs show the change in pupil diameter (Test minus Baseline) for each group (adults, adolescents) within the respective time-intervals. Error bars denote 95% confidence intervals.
The temporal cluster analysis searching for orthogonal linear contrasts, i.e. for differences in linear trends between adolescents and adults revealed no significant effects.
Note that effects of STIMULUS TYPE in pupil data could be shown in the conditioning phase, also, but – as expected – not in the Baseline phase (For further information, see SM2 and Fig. S1). These data suggest that differences in pupil dilation between the CS- and the CS+ emerged already within the first 10 pairings of the CS+ with the UCS.
3.5. Changes of MEG-based, estimated neural activity from baseline to test phase
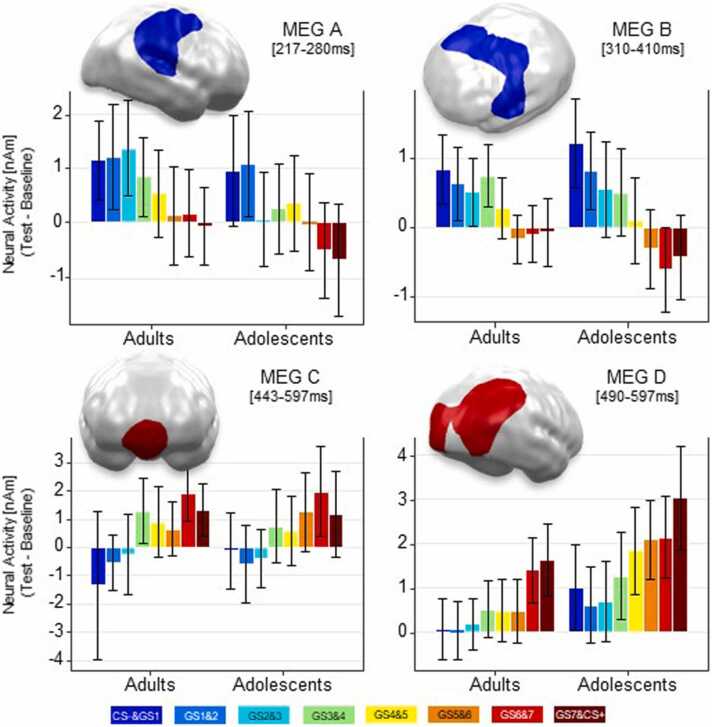
Cluster analyses of MEG data particularly searching for linear trends of the factor stimulus revealed four spatio-temporal clusters showing negative and positive linear trends across groups. Two clusters with negative linear gradients (Fig. 5 top) were observed – one in the early (Cluster MEG A, 217–280 ms, p-cluster=0.028), and one in the late (Cluster MEG B, 310–410 ms, p-cluster=0.011) interval of interest. The early cluster was localized at right lateralized temporo-parietal regions, while the later cluster was localized in similar but left lateralized temporo-parietal regions extending to the right dorsal frontal cortex. Moreover, two further clusters with positive linear trends (Fig. 5 bottom) were observed in the late time interval at a VMPFC region (Cluster C, 443–600 ms, p-cluster=.030) and at bilateral occipital and right parietal cortex areas (Cluster D, 490–597 ms, p-cluster=0.009. Only in Cluster MEG D, the post-hoc ANOVA calculated with the extracted L2-MNE revealed a main effect of GROUP (F(7, 672)= 6.597, p =0.012, partial η2 = 0.064) with stronger neural activity in adolescents than in adults. No other cluster yielded main effects of GROUP or GROUP by STIMULUS TYPE interactions (all F<1.6).
Fig. 5.
MEG Clusters based on Linear Trend Analyses. Clusters revealing negative linear gradients (i.e., CS- > CS+) are indicated in blue (top), clusters revealing positive linear gradients (i.e., CS- < CS+) are indicated in red (bottom). Bar graphs show the regional neural activity (Test minus Baseline in nAM) in the displayed clusters. Error bars denote 95% confidence intervals. For visualization purposes, clusters based on L2-MNE topographies were projected on standard 3D brain models.
Orthogonal contrast analyses of MEG data testing for differences in linear trends between adolescents and adults revealed no significant clusters.
4. Discussion
We set out to investigate subjective, peripheral, and neural correlates of fear generalization in healthy adolescents compared to healthy adults. We observed stable generalization gradients in subjective ratings, with strongest fear ratings and UCS expectancy ratings to the CS+ and weakest ratings to the CS- in both groups. While adolescents and adults showed similar linear and quadratic generalization patterns in their fear ratings, we observed a GROUP-by-STIMULUS TYPE interaction of linear gradients in the UCS expectancy ratings.3 Specifically, adolescents showed a reduced differentiation of CS+ and CS- stimuli, which was predominantly driven by higher UCS expectancies for the CS-. This is in line with evidence in healthy populations showing steeper, i.e. more discriminative, generalization gradients in older compared to younger individuals (Glenn et al., 2012, Klein et al., 2021, Reinhard et al., 2021, Schiele et al., 2016). Discrimination performance, as assessed by the PM-Task, improved after compared to before conditioning (see also Roesmannet al., 2022a), yet, we found no evidence for age-related group differences. Likewise, pupil dilation and MEG data both revealed stable generalization effects, but no evidence for group influences on linear gradients were found. Supporting pupil dilation to be a robust psychophysiological readout of fear learning (Finke et al., 2021, Leuchs et al., 2017), we replicated an increasing early constriction (Roesmann et al., 2022a) and an increasing late dilation (Roesmann et al., 2022a, Roesmann et al., 2022b) to stimuli with increasing similarity to the CS+ . MEG-data revealed negative gradients in left-lateralized temporo-parietal regions that extended to dorsal frontal regions (Roesmann et al., 2022a, Roesmann et al., 2022b, Roesmann et al., 2020) and right-lateralized temporo-parietal regions (Roesmann et al., 2022b), as well as late positive gradients in occipito-parietal regions (Roesmann et al., 2022a, Roesmann et al., 2022b). We found no evidence for the hypothesized age-dependent neural generalization effects – neither in assumed late-maturing frontal structures, nor in other brain areas.
Subjective and psychophysiological data overall provide support for clear conditioning and generalization effects in adolescents. In most outcome measures, – with the exception of UCS expectancy ratings – adolescents showed generalization gradients similar to those observed in adults. This points towards a rather advanced developmental stage in adolescents, in line with findings of a more adult-like generalization pattern in older versus younger children (Glenn et al., 2012) and steeper generalization gradients in healthy adolescents compared to children (El-Bar et al., 2017, Reinhard et al., 2021). In addition to the interpretation that adolescents achieved a level of development that is comparable with adults (in many respects), the lack of group differences in fear ratings might also be grounded in the overall rather low fear ratings - even in response to the CS+ . One might speculate that the non-reinforcement of later CS and GS during the baseline phase (which also included the presentation of UCS) might have induced an inhibitory component to these stimuli (e.g., Haaker et al., 2015). This inhibitory component in turn might have led to floor effects in fear ratings after the conditioning and generalization phase and thereby reduced the chance to find potential group effects. In UCS expectancy ratings, by contrast, we did observe flatter gradients in adolescents compared to adults, which were mainly driven by higher UCS expectancies in response to the CS- in adolescents compared to adults. This corresponds to findings previously reported for children (Glenn et al., 2012, Reinhard et al., 2021, Schiele et al., 2016) and adolescents (Klein et al., 2021) and might point towards a persistence of a reduced discrimination of safety and threat-predicting cues into adolescence and/or towards an overgeneralization of fear (Duits et al., 2015). Importantly, the lack of group differences in the PM-Task argues against a perceptual basis of this effect. Divergent effects in psychophysiological measures and fear ratings on the one hand, and UCS expectancy ratings on the other, might reflect a development-related divergence of emotional compared to more cognitive aspects of fear learning (Lonsdorf et al., 2017), respectively, with an earlier maturation of emotional and later maturation of more cognitive aspects. As a potential neural basis for this divergence, one might have expected that particularly learning related responses of late-maturing structures that support elaborate cognitive (top-down) processes (e.g., DLPFC) should dissociate adolescents from adults (see also Lau et al., 2011).
Yet, surprisingly, interaction patterns observed in UCS expectancy ratings were not accompanied by age-related modulations of neural linear gradients – neither in frontal brain structures known to support top-down mechanisms, nor in sensory structures that are involved in bottom up processing (Casey et al., 2008). Instead, we observed group-independent negative linear gradients covering left DLPFC and temporo-parietal structures, which resemble generalization effects observed in our previous work on healthy adults (Roesmann et al., 2022a, Roesmann et al., 2020) and – driven by responders to exposure therapy – adult patients with AD (Roesmann et al., 2022b). As discussed previously, negative gradients in these structures are supposed to reflect inhibitory mechanisms that support the suppression of fear responses to safe stimuli (especially the CS-) and stimuli resembling the CS- in paradigms conveying threat (Haaker et al., 2015).
In addition to negative gradients peaking at or near the CS-, we here replicated positive fear generalization gradients with stronger activity for stimuli approximating the CS+ in wide-spread brain networks including occipito-parietal brain regions (Lissek et al., 2014, McTeague et al., 2015, Roesmann et al., 2022a, Roesmann et al., 2022b, Roesmann et al., 2020). Occipito-parietal brain networks support processes of so-called motivated attention, which drive a preferential perceptual analyses of emotionally relevant stimuli (Bradley et al., 2003, Schupp et al., 2006, Vuilleumier, 2005).
The lack of a GROUP-by-STIMULUS TYPE interaction in these positive posterior gradients, in combination with the lack of group differences in the PM-Task suggest that attention-related, sensory aspects of fear generalization also operate in a similar manner in adults and adolescents. Interestingly, we found generalization effects in occipito-parietal brain regions to be accompanied by a main effect of group, with overall stronger event-related brain activations in adolescents compared to adults (see Fig. 5, Cluster MEG D). In fact, decreasing brain activity in posterior brain regions and reduced visual event-related potentials with increasing age have previously been reported (Sumich et al., 2012, Taylor et al., 2004, Wessing et al., 2015) and might reflect ongoing brain maturation. Note, however, that previous studies reported age-influences on mean brain activations, while we here report group effects in a difference measure (Test minus Baseline). In particular, adolescents (vs. adults) might display relatively higher levels of brain activity – possibly reflecting motivated attention – to any grating stimulus after (vs. before) fear conditioning, irrespective of its association to the UCS. This effect might reflect generalization of contextual fear (Andreatta et al., 2015) induced during the conditioning procedure, i.e. adolescents might respond more strongly to any stimulus presented in the context of a potentially threatening conditioning procedure (please also refer to Klein et al., 2021). Future research is needed to further investigate neurodevelopmental aspects of such suggested „context generalization“ (as opposed to cue generalization investigated here).
While the observed negative gradients in left DLPFC and bilateral temporo-parietal brain regions, as well as positive gradients in occipito-parietal regions fit with previous research, the observed positive gradient in the VMPFC came as a surprise. Previous fMRI research on fear generalization in healthy populations has consistently revealed negative gradients with strongest activation in response to the CS- (Dymond et al., 2015, Onat and Büchel, 2015) in the VMPFC – a region known to inhibit brain activity in “fear-excitatory” structures (e.g. the amygdala). Reduced negative gradients in the VMPFC or even a reversal to positive gradients, which might reflect inhibitory dysfunctions, have previously been revealed for anxiety patients (Greenberg et al., 2013), non-responders to exposure therapy (Roesmann et al., 2022b) and – following inhibitory, but not sham or excitatory non-invasive VMPFC brain stimulation – also for healthy participants (Roesmann et al., 2022a). Reasons for the unexpected group-independent effect in the current study thus warrant further discussion. First, it might be the case, that sample characteristics of our sample contributed to this effect. Despite the exclusion of participants with pathological anxiety based on clinical interviews and normal average levels of anxiety according to age- and gender adjusted norm groups, average levels of intolerance of uncertainty (and potentially also anxiety levels) were higher in adults than in adolescents. As intolerance of uncertainty is considered a risk-factor for the development of anxiety disorders (Carleton, 2016), the observed effect might reflect inhibitory deficits in both the adolescent (e.g. due to brain maturation processes in frontal structures) and the adult group. On the other hand, the VMPFC consists of various different sub-regions with different functions in the context of fear conditioning and fear extinction (Battaglia et al., 2020). Although extinction of the CS+ is unlikely because we continued to pair it with the UCS during the test phase, it seems possible that the observed VMPFC effects predominantly reflect the acquisition of new safety information and/or the inhibition of fear responses to (unpaired) GS that closely resemble the CS+ (see Fig. 5). Future studies are warranted to disentangle the functional roles of VMPFC subregions regarding fear generalization. A further limitation of our study is that we cannot exclude hormonal influences on the reported effects, as corresponding data were not obtained. Exploratory post-hoc analyses taking sex into account yield some hints that sex might influence some aspects of fear generalization (see SM3, and Table S2), and - in tendency - also its modulation by age. Future studies should therefore investigate the influence of sex hormones and control for the influence of oral contraceptives as a factor that might be confounded with age in females. Additionally, future research is needed to link the observed neural generalization effects with actual anxious behaviors, such as generalized avoidance behaviors (see Klein et al., 2021), which were not measured in this study. Finally, although the reported analyses and findings closely resemble those of our previous studies employing the same paradigm (Roesmann et al., 2022a, Roesmann et al., 2022b), independent preregistered replication studies should be conducted.
Acknowledging these limitations, our study suggests that brain maturation processes underpinning fear generalization from conditioned stimuli to perceptually related cues are largely developed in adolescents. Expected group-differences in frontal structures supporting developmental differences of top-down control mechanisms were not found. Yet, we did observe differences in UCS expectancy ratings, a rather cognitive measure of fear generalization. What might explain the lack of reflections of this effect on a neural level?
First, MEG measures were obtained in a passive viewing task, without the requirement of specific behavioral responses. Thus, it is possible, that cognitive resources needed during the UCS expectancy task, are not reflected in MEG measures. Second, potential neural interaction-effects might have been confounded by different levels of intolerance of uncertainty (IU) in adolescents compared to adults. Both younger age and higher levels of IU are associated with overgeneralization of fear (for IU, see Klein et al., 2021; Morriss et al., 2016). Given that adolescents showed overall lower levels of IU than adults in our sample, potential overgeneralization of fear in adolescence (vs. adulthood) might have been balanced by lower IU levels. However, an exploratory correlational analysis in the four MEG clusters showing linear generalization effects revealed no evidence for associations between individual indices of linearity and IU scores.
5. Conclusions
This study revealed largely similar generalization gradients in adolescents and adults, suggesting that differential fear responses are already maturated in adolescence. However, child-like shallower generalization gradients in adolescents compared to adults were observed specifically in UCS expectancy ratings. We suggest that this reflects a later maturation of cognitive compared to emotional aspects of fear learning (Lonsdorf et al., 2017). Finally, adolescents compared to adults showed stronger visual cortical processing of all stimuli after conditioning. This may reflect neural maturation processes in visual brain regions or a stronger susceptibility for a context-related modulation of fear responses (context conditioning) during adolescence. Further studies are needed to substantiate these findings and elucidate normal and deviant developmental trajectories of fear generalization, not least due to its high clinical relevance.
Disclosure statement
The authors have no conflicts of interest to declare.
Acknowledgements
This work was funded by the German Research Foundation (DFG: JU-445/9-1 and SFB-TRR58-C07), the Interdisciplinary Center for Clinical Research (Ju2/024/15), and the “Innovative Medizinische Forschung” (IMF, grant number RO211907 to KR) of the University of Münster Medical School.
Footnotes
Note that our paradigm was optimized for the collection of MEG data. We employed short SOAs and short ISIs to realize many trials per condition within reasonable time limits of the experiment. This was necessary to increase the SNR, i.e. an important precondition for the estimation of neural sources. By contrast, most studies that focus on pupil dilation typically use much longer SOAs and ISIs (e.g. Leuchs et al., 2017). For the analysis of pupil data, we opted against the often-employed baseline-to-peak analyses, because in our design peaks of the pupil response would be expected after CS offset, i.e. after the (expected) UCS presentation. For further discussion on this procedure, we refer the interested reader to the Supplemental Materials of our previous publications (Roesmann et al., 2022a, Roesmann et al., 2022b). Following recent methodological considerations (Finke et al., 2021), the reported analysis allows to detect response windows directly preceding the UCS, and at the same time enables the detection of even earlier response intervals.
A post-hoc ANOVA with the factors GROUP, STIMULUS TYPE and TASK (fear ratings vs. UCS expectancy rating) revealed a trend-wise significant three-way interaction
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.dcn.2022.101169.
Appendix A. Supplementary material
Supplementary material.
.
Data Availability
Data will be made available on reasonable request.
References
- Andreatta M., Leombruni E., Glotzbach-Schoon E., Pauli P., Mühlberger A. Generalization of contextual fear in humans. Behav. Ther. 2015;46(5):583–596. doi: 10.1016/j.beth.2014.12.008. [DOI] [PubMed] [Google Scholar]
- Battaglia S., Garofalo S., di Pellegrino G., Starita F. Revaluing the role of vmPFC in the acquisition of pavlovian threat conditioning in humans. J. Neurosci. 2020;40(44):8491–8500. doi: 10.1523/JNEUROSCI.0304-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beesdo K., Knappe S., Pine D.S. Anxiety and anxiety disorders in children and adolescents: developmental issues and implications for DSM-V. Psychiatr. Clin. North Am. 2009;32(3) doi: 10.1016/J.PSC.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley M.M., Lang P.J. Technical Report B-2. Gainesville, FL: The Center for Research in Psy- Chophysiology. University of Florida; 1999. International affective digitized sounds (IADS): Stimuli, instruction manual and affective ratings. [Google Scholar]
- Bradley M.M., Sabatinelli D., Lang P.J., Fitzsimmons J.R., King W., Desai P. Activation of the visual cortex in motivated attention. Behav. Neurosci. 2003;117(2):369–380. doi: 10.1037/0735-7044.117.2.369. (Retrieved from http://doi.apa.org/getdoi.cfm?doi=) [DOI] [PubMed] [Google Scholar]
- Carleton R.N. Into the unknown: a review and synthesis of contemporary models involving uncertainty. J. Anxiety Disord. 2016;39:30–43. doi: 10.1016/j.janxdis.2016.02.007. [DOI] [PubMed] [Google Scholar]
- Casey B.J., Jones R.M., Hare T.A. Annals of the New York Academy of Sciences. Blackwell Publishing Inc,; 2008. The adolescent brain. [DOI] [Google Scholar]
- Döpfner M., Görtz-Dorten A., Lehmkuhl G. Huber; Bern: 2008. Diagnostik-System für Psychische Störungen nach ICD-10 und DSM-IV für Kinder und Jugendliche-II (DISYPS-II); Manual. [Google Scholar]
- Duits P., Cath D.C., Lissek S., Hox J.J., Hamm A.O., Engelhard I.M., Baas J.M.P. Updated meta-analysis of classical fear conditioning in the anxiety disorders. Depress Anxiety. 2015;32(4):239–253. doi: 10.1002/da.22353. [DOI] [PubMed] [Google Scholar]
- Dymond S., Schlund M.W., Roche B., Whelan R. The spread of fear: symbolic generalization mediates graded threat-avoidance in specific phobia. Q. J. Exp. Psychol. 2014;67(2):247–259. doi: 10.1080/17470218.2013.800124. [DOI] [PubMed] [Google Scholar]
- Dymond S., Dunsmoor J.E., Vervliet B., Roche B., Hermans D. Fear generalization in humans: systematic review and implications for anxiety disorder research. Behav. Ther. 2015;46(5):561–582. doi: 10.1016/j.beth.2014.10.001. [DOI] [PubMed] [Google Scholar]
- El-Bar N., Laufer O., Yoran-Hegesh R., Paz R. Over-generalization in youth with anxiety disorders. Soc. Neurosci. 2017;12(1):76–85. doi: 10.1080/17470919.2016.1167123. [DOI] [PubMed] [Google Scholar]
- Finke J.B., Roesmann K., Stalder T., Klucken T. Pupil dilation as an index of Pavlovian conditioning. A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2021 doi: 10.1016/j.neubiorev.2021.09.005. [DOI] [PubMed] [Google Scholar]
- Gerlach A.L., Andor T., Patzelt J. Die Bedeutung von Unsicherheitsintoleranz für die generalisierte Angststörung: modellüberlegungen und Entwicklung einer deutschen version der Unsicherheitsintoleranz-Skala. Z. Klin. Psychol. Psychother. 2008;37(3):190–199. doi: 10.1026/1616-3443.37.3.190. [DOI] [Google Scholar]
- Glenn C.R., Klein D.N., Lissek S., Britton J.C., Pine D.S., Hajcak G. The development of fear learning and generalization in 8-13 year-olds. Dev. Psychobiol. 2012;54(7):675–684. doi: 10.1002/dev.20616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg T., Carlson J.M., Cha J., Hajcak G., Mujica-Parodi L.R. Ventromedial prefrontal cortex reactivity is altered in generalized anxiety disorder during fear generalization. Depress Anxiety. 2013;30(3):242–250. doi: 10.1002/da.22016. [DOI] [PubMed] [Google Scholar]
- Haaker J., Lonsdorf T.B., Schümann D., Menz M., Brassen S., Bunzeck N., Kalisch R. Deficient inhibitory processing in trait anxiety: evidence from context-dependent fear learning, extinction recall and renewal. Biol. Psychol. 2015;111:65–72. doi: 10.1016/j.biopsycho.2015.07.010. [DOI] [PubMed] [Google Scholar]
- Hämäläinen M.S.S., Ilmoniemi R.J.J. Interpreting magnetic fields of the brain: minimum norm estimates. Med. Biol. Eng. Comput. 1994;32(1):35–42. doi: 10.1007/BF02512476. [DOI] [PubMed] [Google Scholar]
- Hauk O. Keep it simple: a case for using classical minimum norm estimation in the analysis of EEG and MEG data. NeuroImage. 2004;21(4):1612–1621. doi: 10.1016/j.neuroimage.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Hautzinger M., Bailer M., Hofmeister D., Keller F. Hogrefe Verlag GmbH & Co KG; 2012. Allgemeine Depressions Skala. Manual. 2. überarbeitete und Neu Normierte Auflage. [Google Scholar]
- Jentsch V.L., Wolf O.T., Merz C.J. Temporal dynamics of conditioned skin conductance and pupillary responses during fear acquisition and extinction. Int. J. Psychophysiol. 2020;147:93–99. doi: 10.1016/j.ijpsycho.2019.11.006. [DOI] [PubMed] [Google Scholar]
- Junghöfer M., Elbert T., Tucker D.M., Rockstroh B. Statistical control of artifacts in dense array EEG/MEG studies. Psychophysiology. 2000;37(4):523–532. [PubMed] [Google Scholar]
- Kaczkurkin A.N., Burton P.C., Chazin S.M., Manbeck A.B., Espensen-Sturges T., Cooper S.E., Lissek S. Neural substrates of overgeneralized conditioned fear in PTSD. Am. J. Psychiatry. 2017;174(2):125–134. doi: 10.1176/appi.ajp.2016.15121549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.H., Richardson R. New findings on extinction of conditioned fear early in development: theoretical and clinical implications. Biol. Psychiatry. 2010 doi: 10.1016/j.biopsych.2009.09.003. [DOI] [PubMed] [Google Scholar]
- Klein Z., Berger S., Vervliet B., Shechner T. Fear learning, avoidance, and generalization are more context-dependent for adults than adolescents. Behav. Res. Ther. 2021;147 doi: 10.1016/j.brat.2021.103993. [DOI] [PubMed] [Google Scholar]
- Kleiner M., Brainard D.H., Pelli D.G., Broussard C., Wolf T., Niehorster D. What’s new in psychtoolbox-3? Perception. 2007;36:S14. doi: 10.1068/v070821. [DOI] [Google Scholar]
- Lau J.Y., Britton J.C., Nelson E.E., Angold A., Ernst M., Goldwin M., Pine D.S. Distinct neural signatures of threat learning in adolescents and adults. Proc. Natl. Acad. Sci. USA. 2011;108(11):4500–4505. doi: 10.1073/pnas.1005494108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laux, L., Spielberger, C.D. , 2001. Das state-trait-angstinventar: STAI. Beltz Test.
- LeDoux J.E. Emotion circuits in the brain. Annu. Rev. Neurosci. 2000;23:155–184. doi: 10.1146/annurev.neuro.23.1.155. [DOI] [PubMed] [Google Scholar]
- Leuchs L., Schneider M., Czisch M., Spoormaker V.I. Neural correlates of pupil dilation during human fear learning. Neuroimage. 2017;147:186–197. doi: 10.1016/j.neuroimage.2016.11.072. [DOI] [PubMed] [Google Scholar]
- Lissek S. Toward an account of clinical anxiety predicated on basic, neurally mapped mechanisms of Pavlovian fear-learning: the case for conditioned overgeneralization. Depress Anxiety. 2012;29(4):257–263. doi: 10.1002/da.21922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Biggs A.L., Rabin S.J., Cornwell B.R., Alvarez R.P., Pine D.S., Grillon C. Generalization of conditioned fear-potentiated startle in humans: experimental validation and clinical relevance. Behav. Res. Ther. 2008;46(5):678–687. doi: 10.1016/j.brat.2008.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Rabin S., Randi H.E., Lukenbaugh D., Geraci M., Pine D.S., Grillon C. Overgeneralization of conditioned fear as a pathogenic marker of panic disorder. Am. J. Psychiatry. 2010;167(1):47–55. doi: 10.1176/appi.ajp.2009.09030410.Overgeneralization. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S., Bradford D.E., Alvarez R.P., Burton P., Espensen-Sturges T., Reynolds R.C., Grillon C. Neural substrates of classically conditioned fear-generalization in humans: a parametric fMRI study. Soc. Cogn. Affect. Neurosci. 2014;9(8):1134–1142. doi: 10.1093/scan/nst096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lonsdorf T.B., Menz M.M., Andreatta M., Fullana M.A., Golkar A., Haaker J., Merz C.J. Don’t fear ‘fear conditioning’: methodological considerations for the design and analysis of studies on human fear acquisition, extinction, and return of fear. Neurosci. Biobehav. Rev. 2017;77:247–285. doi: 10.1016/j.neubiorev.2017.02.026. [DOI] [PubMed] [Google Scholar]
- Margraf J., Cwik J.C., Pflug V., Schneider S. Strukturierte klinische Interviews zur Erfassung psychischer Störungen über die Lebensspanne. Z. Klin. Psychol. Psychother. 2007;46(3):176–186. doi: 10.1026/1616-3443/a000430. [DOI] [Google Scholar]
- Margraf, J., Cwik, J.C., Suppinger, A., Schneider, S. , 2007b. J-DIPS: 5., überarbeitete Auflage, gekürzte und modifizierte Jugendversion für das BMBF Projekt “self- injury: treatment, assessment, recovery (STAR)“ (STAR-Assess, PI Tina In-Albon) mit freundlicher Genehmigung der DIPS Autoren.
- Maris E., Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J. Neurosci. Methods. 2007;164(1):177–190. doi: 10.1016/j.jneumeth.2007.03.024. [DOI] [PubMed] [Google Scholar]
- McMahon D.B.T., Leopold D.A. Stimulus timing-dependent plasticity in high-level vision. Curr. Biol. 2012;22(4):332–337. doi: 10.1016/j.cub.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McTeague L.M., Gruss L.F., Keil A. Aversive learning shapes neuronal orientation tuning in human visual cortex. Nat. Commun. 2015;6(1):7823. doi: 10.1038/ncomms8823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miskovic V., Keil A. Acquired fears reflected in cortical sensory processing: a review of electrophysiological studies of human classical conditioning. Psychophysiology. 2012;49(9):1230–1241. doi: 10.1111/j.1469-8986.2012.01398.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morriss J., Macdonald B., Van Reekum C.M. What is going on around here? Intolerance of uncertainty predicts threat generalization. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0154494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onat S., Büchel C. The neuronal basis of fear generalization in humans. Nat. Neurosci. 2015;18(12):1811–1818. doi: 10.1038/nn.4166. [DOI] [PubMed] [Google Scholar]
- Peyk P., De Cesarei A., Junghöfer M. ElectroMagnetoEncephalography software: overview and integration with other EEG/MEG toolboxes. Comput. Intell. Neurosci. 2011;2011 doi: 10.1155/2011/861705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M.L., Ladouceur C.D., Drevets W.C. A neural model of voluntary and automatic emotion regulation: implications for understanding the pathophysiology and neurodevelopment of bipolar disorder. Mol. Psychiatry. 2008;13(9):829–857. doi: 10.1038/mp.2008.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L.S. The CES-D scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1(3):385–401. doi: 10.1177/014662167700100306. [DOI] [Google Scholar]
- Reinhard J., Slyschak A., Schiele M.A., Andreatta M., Kneer K., Reif A., Romanos M. Fear conditioning and stimulus generalization in association with age in children and adolescents. Eur. Child Adolesc. Psychiatry. 2021 doi: 10.1007/s00787-021-01797-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesmann K., Kroker T., Hein S., Rehbein M., Winker C., Leehr E.J., Junghöfer M. Transcranial direct current stimulation of the ventromedial prefrontal cortex modulates perceptual and neural patterns of fear generalization. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7(2):210–220. doi: 10.1016/j.bpsc.2021.08.001. [DOI] [PubMed] [Google Scholar]
- Roesmann K., Leehr E.J., Böhnlein J., Steinberg C., Seeger F., Schwarzmeier H., Junghöfer M. Behavioral and magnetoencephalographic correlates of fear generalization are associated with responses to later virtual reality exposure therapy in spider phobia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging. 2022;7(2):221–230. doi: 10.1101/2021.03.23.21253886. [DOI] [PubMed] [Google Scholar]
- Roesmann K., Toelle J., Leehr E.J., Wessing I., Böhnlein J., Seeger F.R., Junghoefer M. Neural correlates of fear conditioning are associated with treatment-outcomes to behavioral exposure in spider phobia - evidence from magnetoencephalography. NeuroImage Clin. 2022 doi: 10.1016/j.nicl.2022.103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roesmann K., Wiens N., Winker C., Rehbein M.A., Wessing I., Junghoefer M. Fear generalization of implicit conditioned facial features – behavioral and magnetoencephalographic correlates. NeuroImage. 2020;205 doi: 10.1016/j.neuroimage.2019.116302. [DOI] [PubMed] [Google Scholar]
- Schiele M.A., Reinhard J., Reif A., Domschke K., Romanos M., Deckert J., Pauli P. Developmental aspects of fear: comparing the acquisition and generalization of conditioned fear in children and adults. Dev. Psychobiol. 2016;58(4):471–481. doi: 10.1002/dev.21393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schupp H.T., Flaisch T., Stockburger J., Junghoefer M. Emotion and attention: event-related brain potential studies. Prog. Brain Res. 2006;156:31–51. doi: 10.1016/S0079-6123(06)56002-9. [DOI] [PubMed] [Google Scholar]
- Shin L.M., Liberzon I. The neurocircuitry of fear, stress and anxiety disorders. Neuropsychopharmacology. 2010;35(1):169–191. doi: 10.1038/npp.2009.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegmann Y., Schiele M.A., Schümann D., Lonsdorf T.B., Zwanzger P., Romanos M., Pauli P. Individual differences in human fear generalization—pattern identification and implications for anxiety disorders. Transl. Psychiatry. 2019;9(1):1–11. doi: 10.1038/s41398-019-0646-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumich A.L., Sarkar S., Hermens D.F., Ibrahimovic A., Kelesidi K., Wilson D., Rubia K. Sex differences in brain maturation as measured using event-related potentials. Dev. Neuropsychol. 2012;37(5):415–433. doi: 10.1080/87565641.2011.653461. [DOI] [PubMed] [Google Scholar]
- Taylor M.J., Batty M., Itier R.J. The faces of development: a review of early face processing over childhood. J. Cogn. Neurosci. 2004 doi: 10.1162/0898929042304732. (October) [DOI] [PubMed] [Google Scholar]
- Tottenham N., Tanaka J.W., Leon A.C., McCarry T., Nurse M., Hare T.A., Nelson C. The NimStim set of facial expressions: judgments from untrained research participants. Psychiatry Res. 2009;168(3):242–249. doi: 10.1016/j.psychres.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vuilleumier P. How brains beware: neural mechanisms of emotional attention. Trends Cogn. Sci. 2005;9(12):585–594. doi: 10.1016/j.tics.2005.10.011. [DOI] [PubMed] [Google Scholar]
- Wessing I., Rehbein M.A., Romer G., Achtergarde S., Dobel C., Zwitserlood P., Junghöfer M. Cognitive emotion regulation in children: reappraisal of emotional faces modulates neural source activity in a frontoparietal network. Dev. Cogn. Neurosci. 2015;13:1–10. doi: 10.1016/j.dcn.2015.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittchen, H., Pfister, H. , 1997. DIA-X-interviews: manual für screening-Verfahren und interview; Interviewheft. Retrieved from 〈https://pure.mpg.de/pubman/faces/ViewItemOverviewPage.jsp?itemId=item_1646479〉.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.
Data Availability Statement
Data will be made available on reasonable request.