Abstract
The adherence of Enterococcus faecalis strains to human T24 cells was examined by scanning electron microscopy. Five highly adhesive strains were identified from 30 strains isolated from the urine of patients with urinary tract infections. No efficiently adhesive strains were found among the 30 strains isolated from the feces of healthy students. The five isolated strains also adhered efficiently to human bladder epithelial cells. Analysis of restriction endonuclease-digested plasmid DNAs and chromosome DNAs showed that the five strains were different strains isolated from different patients. The adhesiveness of these strains was inhibited by treatment with fibronectin or trypsin, implying that a specific protein (adhesin) on the bacterial cell surface mediates adherence to fibronectin on the host cell surfaces, and the adhesin differs from the reported adhesins.
Enterococci are opportunistic pathogens which cause infections in patients compromised by severe underlying disease, such as urinary tract infections, endocarditis, and wound infections (17, 35, 37, 38, 43, 57). Clinical isolates of enterococci are resistant to many antimicrobial agents in common use (17, 35, 38, 44, 53, 57) and thus have a selective advantage in the hospital environment.
Reports describing molecular and genetic studies of enterococci pathogenic factors are limited. However, there are a number of reports that the phenotypes encoded by the Enterococcus faecalis pheromone-responding plasmids are related to pathogenicity. Plasmid pAD1 encodes a β-hemolysin–bacteriocin (Cyl, cytolysin) mediated by the same genetic determinant. A significant number of E. faecalis clinical isolates produce cytolysin (22, 23). More than 50% of the E. faecalis clinical isolates studied carry transferable cytolysin genes (23, 24). More than 90% of these cytolysin plasmids are closely related to pAD1 (24, 33). The cytolysin encoded on pAD1 has been shown to enhance the virulence of E. faecalis in animal models (3, 25, 29). The transfer functions of the E. faecalis pheromone-responding plasmid are induced in the donor strain by the plasmid-specific peptide sex pheromone which is secreted by the potential recipient cell (5–7, 10). The sex pheromone induces the synthesis of a surface aggregation substance (adhesin) that facilitates the formation of a mating aggregate (10, 11, 13, 26). The deduced amino acid sequences of the aggregation substance have extensive homology with sequences of the well-characterized pheromone-responding plasmids pAD1 (6, 8, 16, 26, 49, 52), pCF10 (4, 12, 21), and pPD1 (11, 15, 16, 55). The aggregation substance of strains carrying pAD1 has been shown to enhance adherence to renal tubular cell in a cell culture model (28, 30), and it has also been shown to enhance internalization to cultured intestinal epithelial cells (41). Another study has shown that there are two types of adhesins present on the E. faecalis cell surface (18): (i) a d-mannose-d-glucose-containing adhesin which mediates adherence to human urinary tract epithelial cells and human embryonic kidney cells and (ii) a galactose-containing adhesin which mediates adherence to Girardi heart cells and is expressed by strains isolated from patients with endocarditis (18).
In this study, we used scanning electron microscopy for quantitative analysis of adherence to human culture cells of clinical E. faecalis strains and identified highly efficient adherent strains among the clinical isolates.
MATERIALS AND METHODS
Bacteria, media, and reagents.
Thirty E. faecalis strains isolated from urine samples of patients with chronic urinary tract infections were used in this study. Of the 30 strains, 24 were from Gunma University Hospital and 6 were from a hospital in Ota City, Gunma, Japan. Thirty E. faecalis strains isolated from stool specimens of 30 healthy students were also used. Laboratory strains used were E. faecalis FA2-2 (Rifr Fusr) (8) and E. faecalis OG1X (Smr) (27). Unless otherwise indicated, the media used throughout this study were Oxoid Nutrient Broth 2 (Oxoid, Basingstoke, Hants, England) supplemented with glucose (0.2%) and Tris-hydrochloride (0.1 M, pH 7.7) (N2GT broth). Antibiotic medium 3 (Difco Laboratories, Detroit, Mich.) was used for testing drug resistance. Antibiotic concentrations (micrograms per milliliter) used in selective plates were as follows: erythromycin, 25; streptomycin, 500; spectinomycin, 500; kanamycin, 500; gentamicin, 200; chloramphenicol, 25; tetracycline, 3; rifampin, 25; and fusidic acid, 25. Hemolysin detection was on Todd-Hewitt agar containing 4% rabbit blood (Toyo Serum Co., Tokyo, Japan).
Bacterial growth condition for adherence.
An overnight culture of E. faecalis in N2GT broth was diluted 100-fold with fresh N2GT broth. The diluted bacteria were grown to an optical density of 200 Klett units (Klett-Summerson colorimeter; no. 54 filter) at 37°C with slow shaking, and the culture was used for adherence experiments.
Mating procedure.
Broth mating was performed as previously described (11, 26) with a donor/recipient ratio 1:10. Overnight cultures of 0.05 ml of donor and 0.5 ml of recipient were added to 4.5 ml of fresh broth, and the mixtures were incubated at 37°C with slow shaking for 4 h and then vortexed. Portions of the mixed culture were then plated on solid media with appropriate selective antibiotics, and the plates were incubated at 37°C for 48 h. Filter matings were carried out as described previously (14) with N2GT broth agar plates containing 4% human blood and with an initial ratio of 1 donor per 10 recipients. For the transfer of hemolysin properties, the mating mixtures were diluted by factors of 10−1, 10−2, and 10−4 with fresh N2GT broth. A 0.1-ml sample of each dilution was plated on selective Todd-Hewitt agar plates containing 4% human blood and an appropriate drug for counterselection of the donor strains. After overnight incubation of the plates at 37°C, the colonies of recipients producing a hemolytic zone were counted as hemolytic transconjugants.
Isolation and manipulation of plasmid DNA.
Plasmid DNA was isolated by the alkaline lysis method (42). Plasmid DNA was treated with restriction enzymes and submitted to agarose gel electrophoresis for analysis of DNA fragments, etc. Restriction enzymes were obtained from Nippon Gene (Toyama, Japan) and New England Biolabs, Inc., and were used in accordance with the suppliers’ specifications. Agarose was obtained from Wako Chemicals, Osaka, Japan.
Pulsed-field gel electrophoresis of the chromosomal DNA.
Pulsed-field gel electrophoresis was performed with a CHEF-DRII system (Bio-Rad, Hercules, Calif.). The embedded chromosome DNAs of E. faecalis strains were prepared and digested according to the manufacturer’s protocols, with some modifications. Cells were embedded in 1% agarose (10 mM Tris-HCl [pH 8.0], 10 mM NaCl, 25 mM EDTA) and treated with lysis solution (10 mM Tris-HCl [pH 8.0], 10 mM NaCl, 25 mM EDTA), mutanolysin (150 U/ml; Sigma, St. Louis, Mo.), and lysozyme (8 mg/ml; Sigma) for 2 h. Agarose-embedded chromosome DNA was digested overnight with 50 U of SmaI (Nippon Gene) in 300 μl of a 1× dilution of the recommended reaction buffer.
Clumping assay.
Detection of clumping was done as previously described (10). Pheromone corresponded to a culture filtrate of the strains FA2-2. Generally, 1.0 ml of culture filtrate from the cells in late log phase was mixed with 1.0 ml of fresh N2GT broth and 20 μl of overnight cultured cells to be tested for the ability to respond. The mixtures were cultured for 4 h at 37°C with shaking and were examined for clumping.
Epithelial cells.
The T24 cell line, which is derived from a human urinary bladder carcinoma, was kindly provided by the Health Science Research Resources Bank (Tokyo, Japan). T24 cells were incubated under 5% CO2 for 24 h at 37°C in Eagle’s minimal essential medium (MEM; GIBCO, Grand Island, N.Y.) supplemented with 10% fetal bovine serum (FBS) and grown without antibiotics in a 24-well multidish plate containing a plastic coverslip (Sumitomo Bakelite; Tokyo, Japan). T24 adhered to a plastic coverslip at 40 to 50% confluence.
Specimen of epithelial cells of the human urinary bladder.
A specimen of epithelial cells of the human urinary bladder was obtained by total cystectomy from a 68-year-old patient with bladder carcinoma in Gunma University Hospital. The segment, which had no carcinoma, was washed several times with cold (4°C) phosphate-buffered saline (PBS; pH 7.4), and the mucosal side was retained. A slice of the mucosa was immediately used for adherence experiments.
Adherence analysis.
A previously described method (56) for scanning electroscopic analysis was modified for direct measurement of adherence of bacteria to human epithelial cells. The cells were washed twice with MEM. One milliliter of MEM without FBS and 40 μl of bacterial culture adjusted to 200 Klett units (Klett-Summerson colorimeter; no. 54 filter) were added to each well, and then the plate was incubated for 2 h at 37°C. After incubation, the wells were washed six times with PBS, and the bacteria adhered to cells on the plastic coverslip were fixed with 2.5% glutaraldehyde in PBS for 3 h (T24 cells) or 72 h (epithelial cells of the human bladder) at room temperature; postfixing was in 1% osmium tetroxide for 15 min at room temperature and then for 45 min at 4°C. The samples were dehydrated with ethanol, critical point dried, coated with gold-palladium, and examined with scanning electron microscope (S4100; Hitachi, Tokyo, Japan). Adherence of E. faecalis to T24 cells or the plastic coverslips was observed; 100 T24 cells or 100 fields of each plastic coverslip were randomly chosen, and the bacteria were counted.
Analysis of inhibitor for adherence.
To examine inhibition of the adherence, the samples containing bacteria and the T24 cells were incubated with fibronectin (100 μg/ml) and fibrinogen (500 μg/ml) for 1 h at 37°C. The samples were washed six times with PBS, fixed, and analyzed by scanning electron microscopy. Fibronectin (purified by affinity chromatography of human plasma on gelatin-Sepharose columns) and fibrinogen (purified from human plasma) were purchased from Koken (Tokyo, Japan).
Fibronectin treatment of bacteria.
Fibronectin was dissolved in PBS and added to bacterial culture in a final concentration of 100 μg/ml. After 1 h of incubation at 37°C, the bacteria were washed with PBS and resuspended in PBS. Then 4 μl of the fibronectin-treated bacteria was added to T24 cells in the wells and incubated for 1 h at 37°C. After incubation, the cells were washed, fixed, and analyzed for bacterial adherence to the cells.
Trypsin treatment of bacteria.
Five microliters of bacterial cultures (200 Klett units) was heated at 60°C for 10 min, centrifuged (3,000 × g 15 min), and resuspended in 1 ml of PBS. The suspension was incubated with various concentrations of trypsin for 30 min at 37°C. The reaction was stopped by the addition of pancreatic trypsin inhibitor and subsequent washing.
RESULTS
Adherence of E. faecalis strains to T24 cells.
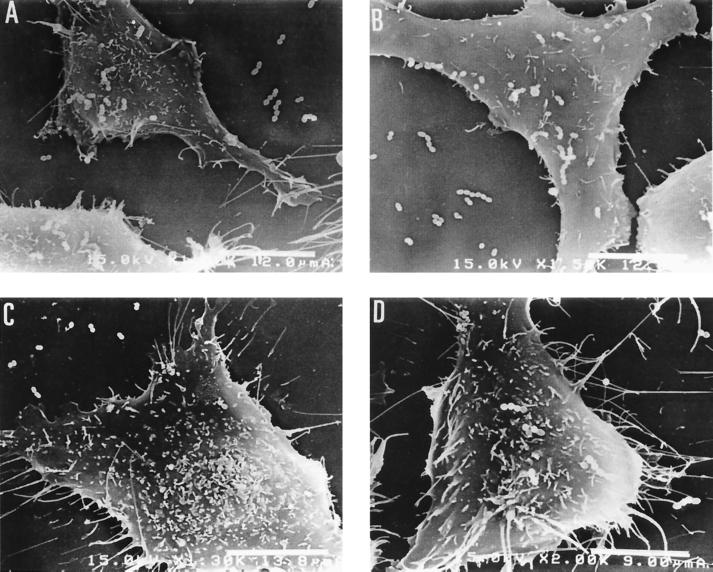
The adherence of E. faecalis strains to T24 cells was examined by scanning electron microscopy. Each group of 30 E. faecalis strains used in this study was isolated from the urine samples of patients with urinary tract infections or the feces of healthy students. For most of the E. faecalis strains derived from urine samples, the number of adherent bacterial cells was less than 40 per T24 cell (Fig. 1A). Five strains (AS11, AS12, AS13, AS14, and AS15) adhered more efficiently than the other strains to T24 cells. Typical results are shown in Fig. 2. The number of adherent cells observed for these strains was more than 230 per 462 μm2 (the average area of a T24 cell). The number of bacterial cells adhering to T24 cells in the efficiently adhesive strains was significantly higher [F(2.57) = 24.85, P < 0.0001 (analysis of variance); P < 0.001 (Fisher’s PLSD)] than those of the inefficiently adhesive strains.
FIG. 1.
Adherence of E. faecalis strains to T24 cells (A) and to plastic coverslips (B). Each circle represents an E. faecalis strain. Laboratory strains used were FA2-2, OG1X, FA2-2(pAD1), and OG1X(pAD1). Total numbers of adherent bacterial cells per 462 μm2 (the average area of a T24 cell) are shown.
FIG. 2.
Adherence of efficiently adhesive strains AS11 (A), AS12 (B), and AS15 (C) to T24 cells.
For strains isolated from the feces of healthy students, the number of adherent cells was less than 20 per 462 μm2 (Fig. 1A). Figure 3 shows typical results for inefficiently adhesive strains isolated from urine and fecal samples.
FIG. 3.
Adherence of inefficiently adhesive strains AS21 (A), AS22 (B), AS23 (C), and AS24 (D). AS21 and AS22 were isolated from urine samples; AS23 and AS24 were isolated from feces.
Adherence of E. faecalis strains to human bladder epithelial cells.
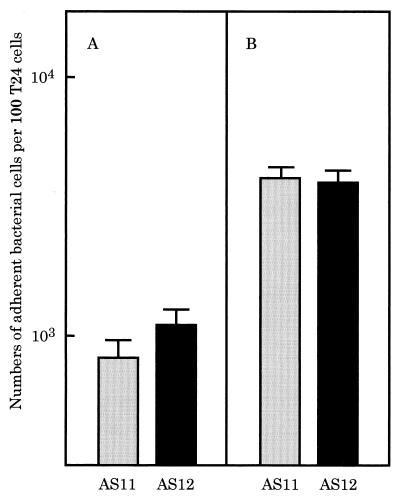
Adherence of the efficiently adhesive strains to epithelial cells of the human urinary bladder was examined as described in Materials and Methods. The efficiently adhesive strains also efficiently adhered to the epithelial cells. Typical results for the adherence of strains AS11 and AS12 are shown in Fig. 4. On the other hand, the inefficiently adhesive strains AS21 and AS23, which were derived from urine and feces, respectively, did not adhere to the epithelial cells (data not shown).
FIG. 4.
Adherence of efficiently adhesive strains AS12 (A) and AS11 (B) to epithelial cells of the human urinary bladder.
Adherence of E. faecalis strains to plastic coverslips.
The adherence of E. faecalis strains to the plastic coverslips used for culture of the T24 cells was also examined by scanning electron microscopy. With a few exceptions, fewer than 20 bacterial cells of each strain per 462 μm2 adhered to the plastic coverslips (Fig. 1B). As indicated above, strains AS11, AS12, AS13, AS14, and AS15 efficiently adhered to T24 cells. On the other hand, the number of these cells adhering to the plastic coverslip was low, ranging from 15 to 25 per 462 μm2. For the efficiently adhesive strains, the number of bacterial cells adhering to T24 cells was significantly higher (t = 27.64, 4 df, P < 0.0001) than the number adhering to the plastic coverslips. These results imply that these strains have a specific mechanism for adherence to the host cell surface.
Clinical surveillance of patients.
We monitored the clinical course of 9 of the 30 patients. Six of the nine patients had been infected with inefficiently adhesive strains, and E. faecalis was not detected in their urine beyond the first month of follow-up. Each of the other three patients had been infected with one of the efficiently adhesive strains (AS13, AS14, or AS15), and E. faecalis was detected in their urine during the follow-up surveillance for a period of 12, 7, or 3 months, respectively. The chromosomal DNA patterns of the E. faecalis isolates obtained from each of the three patients at different times during follow-up were examined by pulsed-field gel electrophoresis. The patterns obtained by pulsed-field gel electrophoresis of SmaI-digested chromosomal DNAs from the E. faecalis isolates obtained from the same patient were identical (data not shown), which implied that the efficiently adhesive strains produced infection for a longer period of time than the inefficiently adhesive strains.
Conjugative plasmids of the efficiently adhesive strains.
Three (AS11, AS12, and AS13) of the efficiently adhesive strains contained plasmids (data not shown). AS11 was a pheromone-responding strain which aggregated by exposure to a culture filtrate of E. faecalis FA2-2 (data not shown). AS13 was a constitutive-clumping strain. The transferabilities of the plasmids to FA2-2 or OG1X were examined by mating experiments.
The plasmid isolated from AS11 had an EcoRI profile almost identical to that of the conjugative cytolysin plasmid pAD1 (60 kb) (data not shown) (8). The transferability of the β-hemolytic trait (cyl) of AS11 was examined as described in Materials and Methods. The β-hemolytic trait transferred to recipient strains at a frequency of approximately 10−3 per donor cell. The tetracycline resistance trait did not transfer by broth mating. The plasmid of the β-hemolytic transconjugant had an EcoRI profile similar to that of the plasmid of the AS11 donor strain. The transconjugant was aggregated by exposure to FA2-2 culture filtrate (pheromone).
AS12 transconjugants were obtained by filter mating and selected on the basis of tetracycline resistance. The EcoRI restriction fragments of the transconjugant plasmid DNAs were examined by agarose gel electrophoresis. The restriction endonuclease digestion patterns of the plasmids showed two different patterns. The molecular size of one plasmid was 45.8 kb, and that of the other plasmid was 61 kb. The 45.8-kb plasmid consists of eight EcoRI fragments with molecular sizes of 12.0, 10.4, 8.9, 5.2, 3.8, 3.4, 1.4, and 0.7 kb. The 61-kb plasmid also consists of eight EcoRI fragments, in this case with molecular sizes of 24.1, 12.0, 10.4, 5.2, 3.8, 3.4, 1.4, and 0.7 kb. The difference in plasmid sizes was due to the 8.9-kb fragment of the 45.8-kb plasmid and the 24.1-kb fragment of the 61-kb plasmid. A second mating experiment with the FA2-2 donor strain containing either the 45.8-kb or the 61-kb plasmid and the recipient strain OG1X was performed. The EcoRI restriction fragments of the 10 tetracycline resistance transconjugants obtained in each mating experiment were examined by agarose gel electrophoresis. Seven of the ten transconjugants derived from the mating experiment with the donor strain containing the 45.8-kb plasmid contained the 45.8-kb plasmid. Three of the ten transconjugants contained the 61-kb plasmid in the corresponding experiment using the 61-kb plasmid. All transconjugants obtained in the mating experiment with the donor strain containing the 61-kb plasmid contained the 61-kb plasmid. These results suggested that AS12 harbored a 45.8-kb conjugative plasmid and a tetracycline resistance conjugative transposon of approximately 15 kb.
The 61-kb plasmid resulted from the insertion of the 15-kb conjugative transposon into the 45.8-kb conjugative plasmid. The E. faecalis strain containing the 45.8- or 61-kb plasmid was not aggregated by exposure to a FA2-2 culture filtrate (pheromone).
The AS13 transconjugants were obtained by broth mating and were selected on the basis of tetracycline resistance. The tetracycline resistance transconjugants of AS13 were isolated at a frequency of 10−2 to 10−3 per donor cell. The transconjugants also showed constitutive clumping and contained the 61.4-kb plasmid.
The molecular size of the plasmid contained in each transconjugant is shown in Table 1. Each of the FA2-2 or OG1X transconjugants was examined for adherence to T24 human culture cells and found not to efficiently adhere to the cells (data not shown).
TABLE 1.
Efficiently adhesive strains
| E. faecalis strain | Phenotype | Plasmid content | Conjugative plasmid identified (size), phenotype conferred by plasmid |
|---|---|---|---|
| AS11 | Cyl Tetr pheromone response | + | pAS11 (60 kb), Cyl pheromone response |
| AS12 | Cyl Tetr | + | pAS12 (45.8 kb) |
| AS13 | Tetr constitutive clumping | + | pAS13 (61.4 kb), Tetr constitutive clumping |
| AS14 | − | ||
| AS15 | Tetr | − |
Restriction endonuclease digestion patterns of the E. faecalis chromosomal DNA.
Pulsed-field electrophoresis was used to compare the efficiently adhesive strains. The restriction endonuclease digestion patterns of the five E. faecalis chromosomal DNAs showed five different patterns (Fig. 5). Two strains, AS11 and AS13, had almost identical restriction endonuclease digestion patterns; however, the sizes of the two largest bands in these plasmids differed slightly. AS11 and AS13 were isolated from different patients in geographically distant hospitals. AS11 was a tetracycline-resistant, β-hemolytic, pheromone-responsive strain; AS13 was a tetracycline-resistant, constitutive-clumping strain. These results indicate that AS11 and AS13 are different strains.
FIG. 5.
Pulsed-field gel electrophoresis of SmaI-digested chromosomal DNAs isolated from efficiently adhesive strains. Lane 1, AS11; lane 2, AS12; lane 3, AS13; lane 4, AS14; lane 5, AS15; lane 6, OG1X; lane 7, FA2-2; lane 8, bacteriophage lambda DNA ladder.
Inhibition of adherence by fibronectin.
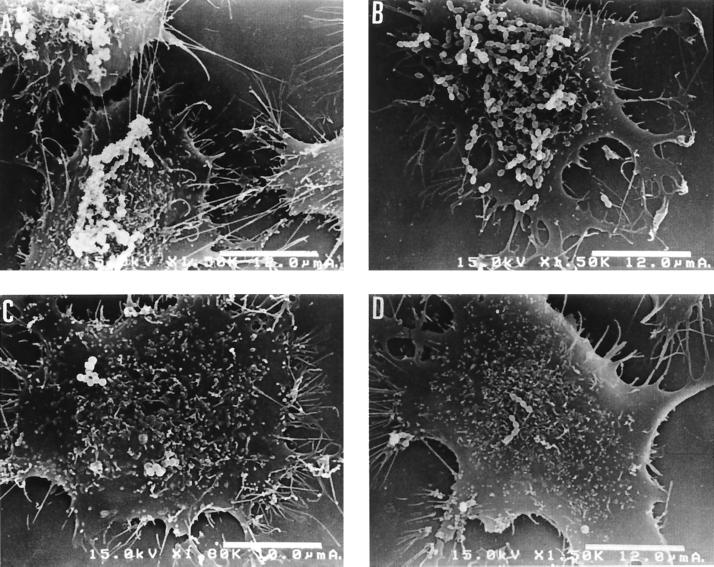
To examine if fibronectin or fibrinogen inhibits adherence of the efficiently adhesive strains, we added each compound to a mixture of E. faecalis AS11 or AS12 and T24 cells, and then examined adherence by scanning electron microscopy. Fibronectin, but not fibrinogen, inhibited the adherence of E. faecalis AS11 or AS12 to T24 cells (data not shown). The adherence of E. faecalis OG1X was not affected by these compounds (data not shown). The effect of fibronectin, an epithelial cell compound, on adherence was examined by pretreatment of E. faecalis strains with fibronectin. When E. faecalis AS11 or AS12 was preincubated with fibronectin, adherence to T24 cells was inhibited (Fig. 6 and 7).
FIG. 6.
Adherence of fibronectin-treated (A) and untreated (B) E. faecalis strains to T24 cells. The efficiently adhesive strains AS11 and AS12 were treated with fibronectin for 1 h at 37°C and then examined for the adherence to T24 cells.
FIG. 7.
Adherence of untreated strains AS11 (A) and AS12 (B) and of fibronectin-treated strains AS11 (C) and AS12 (D) to T24 cells.
Inhibition of adherence by protenase.
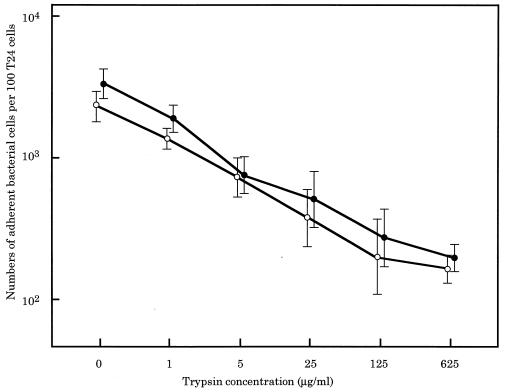
To examine whether protenase affects adherence, E. faecalis AS11 or AS12 was preincubated with trypsin and then examined for adherence. As shown in Fig. 8, the number of trypsin-treated E. faecalis strains adhering to the T24 cells decreased in proportion to the trypsin concentration used in the preincubations.
FIG. 8.
Adherence of trypsin-treated E. faecalis strains to T24 cell. The efficiently adhesive strains AS11 (○) and AS12 (●) were treated with various concentrations of trypsin for 30 min at 37°C and then examined for adherence to T24 cells.
DISCUSSION
E. faecalis strains are frequently isolated from urine and are major causative agents of chronic urinary tract infections. Use of scanning electron microscopy for quantitative analysis of the adherence of E. faecalis clinical strains made it possible to identify from among strains isolated from urinary tract infections those that were highly adhesive to human culture cells. The highly adhesive strains were shown to be different strains isolated from different patients. On the other hand, efficiently adhesive strains were not isolated from the 30 strains derived from feces of healthy students. The isolation frequency of highly adherent strains from the urine of patients with urinary tract infections was significantly greater (P = 0.0261, Fisher’s exact method) than that found for strains derived from the feces of healthy students.
The highly adhesive strains adhered efficiently to the cultured cells and the human bladder cells but not to plastic coverslips. The other strains examined adhered equally to culture cells and plastic coverslips. These observations implied that the highly adhesive strains have specific substances on the bacterial cell surface which produce adherence to the host epithelial cell surface. The gram-positive bacteria Streptococcus pyogenes and Staphylococcus aureus bind to extracellular host proteins that are present on the surface of the target host cells. The host proteins include components of the extracellular matrix, such as fibronectin (1, 20, 31, 32, 39, 45), fibrinogen (9, 36, 54), laminin (34, 48), collagen (46, 47), and vitronectin (2, 50). The binding of bacteria to these host proteins is mediated by specific proteins on the bacterial cell surface (9, 19, 20, 40, 51, 54).
We investigated whether a protein on the bacterial cell surface of the efficiently adhesive strains mediates adherence to the host protein. The adherence of the bacterial cells was inhibited by pretreatment of the bacterial cells with proteinase (trypsin) and fibronectin. The results imply that a specific protein (adhesin) on the bacterial cell surface mediates adherence to fibronectin on the host cell surfaces. These results show that the adhesin differs from the reported E. faecalis adhesins (18, 28, 30), i.e., the d-mannose-d-glucose-containing adhesin (18), the galactose-containing adhesin (18), and the aggregation substance encoded on the pheromone-responsive plasmid (30).
The d-mannose-d-glucose-containing adhesin was expressed in all E. faecalis strains examined which are involved in urinary tract infections and endocarditis (18). Thus, adhesin could be a widespread or general adhesin. In our study, the inefficiently adhesive strains isolated from urine and feces adhered to both culture cells and plastic coverslips to the same degree. It appears that the adherence of these strains is mediated by a general or widespread adhesin, such as the d-mannose-d-glucose-containing adhesin.
The role of the adhesin in the pathogenicity of the efficiently adhesive strains is not clear. The study of E. faecalis strains isolated from patients during clinical surveillance showed that patients who had been infected with efficiently adhesive strains had been infected for a longer period than patients infected with the inefficiently adhesive strains, which implies a correlation between the difficulties observed in the treatment of cases and infection with efficiently adhesive strains.
Two of the highly adhesive strains harbored pheromone-responsive or constitutive aggregation plasmids. Neither of these plasmids, when transferred into the laboratory strain E. faecalis FA2-2 or OG1X, conferred the efficiently adhesive phenotype. These results suggest that the aggregation substance encoded on these plasmids plays little role in efficient adherence, although it is possible that the original host determinant was required to act together with a plasmid determinant for expression of the adherence characteristics of the strains harboring the conjugative plasmids.
ACKNOWLEDGMENTS
This work was supported by grants from the Study of Drug Resistant Bacteria funded by the Ministry of Health and Welfare, Japan, in 1996, 1997, and 1998 and by the Japanese Ministry of Education, Science and Culture.
We thank H. Yamanaka and Y. Fukabori (Department of Urology, Gunma University School of Medicine) for helpful advice and for providing T24 cells, and we thank E. Kamei for helpful advice on the manuscript.
REFERENCES
- 1.Abraham S N, Beachey E H, Simpson W A. Adherence of Streptococcus pyogenes, Escherichia coli, and Pseudomonas aeruginosa to fibronectin-coated and uncoated epithelial cells. Infect Immun. 1983;41:1261–1268. doi: 10.1128/iai.41.3.1261-1268.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chhatwal G S, Preissner K T, Müller-Berghaus G, Blobel H. Specific binding of the human S protein (vitronectin) to streptococci, Staphylococcus aureus, and Escherichia coli. Infect Immun. 1987;55:1878–1883. doi: 10.1128/iai.55.8.1878-1883.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chow J W, Thal L A, Perri M B, Vazquez J A, Donabedian S M, Clewell D B, Zervos M J. Plasmid-associated hemolysin and aggregation substance production contribute to virulence in experimental enterococcal endocarditis. Antimicrob Agents Chemother. 1993;37:2474–2477. doi: 10.1128/aac.37.11.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung J W, Bensing B A, Dunny G M. Genetic analysis of a region of the Enterococcus faecalis pCF10 involved in positive regulation of conjugative transfer function. J Bacteriol. 1995;177:2107–2117. doi: 10.1128/jb.177.8.2107-2117.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Clewell D B. Plasmids, drug resistance, and gene transfer in the genus Streptococcus. Microbiol Rev. 1981;45:409–436. doi: 10.1128/mr.45.3.409-436.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clewell D B. Bacterial sex pheromone-induced plasmid transfer. Cell. 1993;73:9–12. doi: 10.1016/0092-8674(93)90153-h. [DOI] [PubMed] [Google Scholar]
- 7.Clewell D B. Sex pheromones and the plasmid-encoded mating response in Enterococcus faecalis. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 349–367. [Google Scholar]
- 8.Clewell D B, Tomich P K, Gawron-Burke M C, Franke A E, Yagi Y, An F Y. Mapping of Streptococcus faecalis plasmids pAD1 and pAD2 and studies relating to transposition of Tn917. J Bacteriol. 1982;152:1220–1230. doi: 10.1128/jb.152.3.1220-1230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtney H S, Li Y, Dale J B, Hasty D L. Cloning, sequencing, and expression of a fibronectin/fibrinogen-binding protein from group A streptococci. Infect Immun. 1994;62:3937–3946. doi: 10.1128/iai.62.9.3937-3946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dunny G M, Brown B L, Clewell D B. Induced cell aggregation and mating in Streptococcus faecalis, evidence for a bacterial sex pheromone. Proc Natl Acad Sci USA. 1978;75:3479–3483. doi: 10.1073/pnas.75.7.3479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunny G M, Craig R A, Carron R L, Clewell D B. Plasmid transfer in Streptococcus faecalis: production of multiple sex pheromones by recipients. Plasmid. 1979;2:454–465. doi: 10.1016/0147-619x(79)90029-5. [DOI] [PubMed] [Google Scholar]
- 12.Dunny G M, Leonard B A B, Hedberg P J. Pheromone-inducible conjugation in Enterococcus faecalis: interbacterial and host-parasite chemical communication. J Bacteriol. 1995;177:1–2. doi: 10.1128/jb.177.4.871-876.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ehrenfeld E E, Kessler R E, Clewell D B. Identification of pheromone-induced surface proteins in Streptococcus faecalis and evidence of a role for lipoteichoic acid in formation of mating aggregates. J Bacteriol. 1986;168:6–12. doi: 10.1128/jb.168.1.6-12.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franke A, Clewell D B. Evidence for a chromosome-borne resistance transposon in Streptococcus faecalis capable of “conjugal” transfer in the absence of a conjugative plasmid. J Bacteriol. 1981;145:494–502. doi: 10.1128/jb.145.1.494-502.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fujimoto S, Tomita H, Wakamatsu E, Tanimoto K, Ike Y. Physical mapping of the conjugative bacteriocin plasmid pPD1 of Enterococcus faecalis and identification of the determinant related to the pheromone response. J Bacteriol. 1995;177:5574–5581. doi: 10.1128/jb.177.19.5574-5581.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Galli D, Friesengger A, Wirth R. Transcriptional control of sex pheromone-inducible genes on plasmid pAD1 of Enterococcus faecalis and sequence analysis of a third structure gene for (pPD1-encoded) aggregation substance. Mol Microbiol. 1992;6:1297–1308. doi: 10.1111/j.1365-2958.1992.tb00851.x. [DOI] [PubMed] [Google Scholar]
- 17.Gordon S, Swenson J M, Hill B C, Pigott N E, Facklam R R, Cooksey R C, Thornsberry C, Jarvis W R, Tenover F C. Antimicrobial susceptibility patterns of common and unusual species of enterococci causing infections in the United States. Enterococcal Study Group. J Clin Microbiol. 1992;30:2373–2378. doi: 10.1128/jcm.30.9.2373-2378.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guzman C A, Pruzzo C, Lipira G, Calegari L. Role of adherence in pathogenesis of Enterococcus faecalis urinary tract infection and endocarditis. Infect Immun. 1989;57:1834–1838. doi: 10.1128/iai.57.6.1834-1838.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hanski E, Caparon M. Protein F, a fibronectin-binding protein, is an adhesin of the group A streptococcus Streptococcus pyogenes. Proc Natl Acad Sci USA. 1992;89:6172–6176. doi: 10.1073/pnas.89.13.6172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanski E, Horwitz P A, Caparon M G. Expression of protein F, the fibronection-binding protein of Streptococcus pyogenes JRS4, in heterologous streptococcal and enterococcal strains promotes their adherence to respiratory epithelial cells. Infect Immun. 1992;60:5119–5125. doi: 10.1128/iai.60.12.5119-5125.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hedberg P J, Leonard B A, Rufifel R E, Dunny G M. Identification and characterization of the genes of Enterococcus faecalis plasmid pCF10 involved in replication and in negative control of pheromone-inducible conjugation. Plasmid. 1996;35:46–57. doi: 10.1006/plas.1996.0005. [DOI] [PubMed] [Google Scholar]
- 22.Huycke M M, Spiegel C A, Gilmore M S. Bacteremia caused by hemolytic, high-level gentamicin-resistant Enterococcus faecalis. Antimicrob Agents Chemother. 1991;35:1626–1634. doi: 10.1128/aac.35.8.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ike Y, Hashimoto H, Clewell D B. High incidence of hemolysin production by Enterococcus (Streptococcus) faecalis strains associated with human parenteral infection. J Clin Microbiol. 1987;25:1524–1528. doi: 10.1128/jcm.25.8.1524-1528.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ike Y, Clewell D B. Evidence that the hemolysin/bacteriocin phenotype of Enterococcus faecalis subsp. zymogenes can be determined by plasmids in different incompatibility groups as well as by the chromosome. J Bacteriol. 1992;174:8172–8177. doi: 10.1128/jb.174.24.8172-8177.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ike Y, Hashimoto H, Clewell D B. Hemolysin of Streptococcus faecalis subsp. zymogenes contributes to virulence in mice. Infect Immun. 1984;45:528–530. doi: 10.1128/iai.45.2.528-530.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ike Y, Clewell D B. Genetic analysis of the pAD1 pheromone response in Streptococcus faecalis, using transposon Tn917 as an insertional mutagen. J Bacteriol. 1984;158:777–783. doi: 10.1128/jb.158.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ike Y, Craig R A, White B A, Yagi Y, Clewell D B. Modification of Streptococcus faecalis sex pheromones after acquisition of plasmid DNA. Proc Natl Acad Sci USA. 1983;80:5369–5373. doi: 10.1073/pnas.80.17.5369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jett B D, Huycke M M, Gilmore M S. Virulence of enterococci. Clin Microbiol Rev. 1994;7:462–478. doi: 10.1128/cmr.7.4.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jett B D, Jensen H D, Nordquist R E, Gilmore M S. Contribution of the pAD1-encoded cytolysin to the severity of experimental Enterococcus faecalis endophthalmitis. Infect Immun. 1992;60:2445–2452. doi: 10.1128/iai.60.6.2445-2452.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kreft B, Marre R, Schramm U, Wirth R. Aggregation substance of Enterococcus faecalis mediates adhesion to cultured renal tubular cells. Infect Immun. 1992;60:25–30. doi: 10.1128/iai.60.1.25-30.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kuusela P. Fibronectin binds to Staphylococcus aureus. Nature (London) 1978;276:718–720. doi: 10.1038/276718a0. [DOI] [PubMed] [Google Scholar]
- 32.Kuusela P, Vartio T, Vuento M, Myhre E B. Binding sites for streptococci and staphylococci in fibronectin. Infect Immun. 1984;45:433–436. doi: 10.1128/iai.45.2.433-436.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.LeBlanc D J, Lee L N, Clewell D B, Behnke D. Broad geographical distribution of a cytotoxin gene mediating beta-hemolysis and bacteriocin activity among Streptococcus faecalis strains. Infect Immun. 1983;40:1015–1022. doi: 10.1128/iai.40.3.1015-1022.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopes J D, Reis M D, Brentani R R. Presence of laminin receptors in Staphylococcus aureus. Science. 1985;229:275–277. doi: 10.1126/science.3160113. [DOI] [PubMed] [Google Scholar]
- 35.Maki D G, Agger W A. Enterococcal bacteremia: clinical features, the risk of endocarditis, and management. Medicine. 1988;67:248–269. [PubMed] [Google Scholar]
- 36.McDevitt D, Francois P, Vaudaux P, Foster T J. Molecular characterization of the clumping factor (fibrinogen receptor) of Staphylococcus aureus. Mol Microbiol. 1994;11:237–248. doi: 10.1111/j.1365-2958.1994.tb00304.x. [DOI] [PubMed] [Google Scholar]
- 37.Moellering R C., Jr Emergence of Enterococcus as a significant nosocomial pathogen. Clin Infect Dis. 1992;14:1173–1176. doi: 10.1093/clinids/14.6.1173. [DOI] [PubMed] [Google Scholar]
- 38.Murray B E. The life and times of the enterococcus. Clin Microbiol Rev. 1990;3:46–65. doi: 10.1128/cmr.3.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Myhre E B, Kuusela P. Binding of human fibronectin to group A, C, and G streptococci. Infect Immun. 1983;40:29–34. doi: 10.1128/iai.40.1.29-34.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Natanson S, Sela S, Moses A E, Musser J M, Caparon M G, Hanski E. Distribution of fibronectin-binding proteins among group A streptococci of different M types. J Infect Dis. 1995;171:871–878. doi: 10.1093/infdis/171.4.871. [DOI] [PubMed] [Google Scholar]
- 41.Olmsted S B, Dunny G M, Erlandsen S L, Wells C L. A plasmid-encoded surface protein on Enterococcus faecalis augments its internalization by cultured epithelial cells. J Infect Dis. 1994;170:1549–1556. doi: 10.1093/infdis/170.6.1549. [DOI] [PubMed] [Google Scholar]
- 42.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 43.Schaberg D R, Culver D H, Gaynes R P. Major trends in the microbial etiology of nosocomial infections. Am J Med. 1991;91(Suppl. 3B):72–75. doi: 10.1016/0002-9343(91)90346-y. [DOI] [PubMed] [Google Scholar]
- 44.Shiojima M, Tomita H, Tanimoto K, Fujimoto S, Ike Y. High-level plasmid-mediated gentamicin resistance and pheromone response of plasmids present in clinical isolates of Enterococcus faecalis. Antimicrob Agents Chemother. 1997;41:702–705. doi: 10.1128/aac.41.3.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speziale P, Höök M, Switalski L M, Wadström T. Fibronectin binding to a Streptococcus pyogenes strain. J Bacteriol. 1984;157:420–427. doi: 10.1128/jb.157.2.420-427.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Speziale P, Raucci G, Meloni S, Meloni M L, Wadström T. Binding of collagen to group A, B, C, D and G streptococci. FEMS Microbiol Lett. 1987;48:47–51. [Google Scholar]
- 47.Speziale P, Raucci G, Vasai L, Switalski L M, Timpl R, Höök M. Binding collagen to Staphylococcus aureus Cowan 1. J Bacteriol. 1986;167:77–81. doi: 10.1128/jb.167.1.77-81.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Switalski L M, Speziale P, Höök M, Wadström T, Timpl R. Binding of Streptococcus pyogenes to laminin. J Biol Chem. 1984;259:3734–3738. [PubMed] [Google Scholar]
- 49.Tanimoto K, Clewell D B. Regulation of the pAD1-encoded sex pheromone response in Enterococcus faecalis: expression of the positive regulator TraE1. J Bacteriol. 1993;175:1008–1018. doi: 10.1128/jb.175.4.1008-1018.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vallentin-Weigand P, Grulich-Henn J, Chhatwal G S, Müller-Berghaus G, Blobel H, Preissner K T. Mediation of adherence of streptococci to human endothelial cells by complement S protein (vitronectin) Infect Immun. 1988;56:2851–2855. doi: 10.1128/iai.56.11.2851-2855.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang J. M protein mediates streptococcal adhesion to HEp-2 cells. Infect Immun. 1994;62:442–448. doi: 10.1128/iai.62.2.442-448.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weaver K E, Clewell D B. Regulation of the pAD1 sex pheromone response in Enterococcus faecalis: construction and characterization of lacZ transcriptional fusions in a key control region of the plasmid. J Bacteriol. 1988;170:4343–4352. doi: 10.1128/jb.170.9.4343-4352.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wells V D, Wong E S, Murray B E, Coudron P E, Williams D S, Markowtiz S M. Infections due to beta-lactamase-producing, high-level gentamicin-resistant Enterococcus faecalis. Ann Intern Med. 1992;116:285–292. doi: 10.7326/0003-4819-116-4-285. [DOI] [PubMed] [Google Scholar]
- 54.Whitnack E, Beachey E H. Antiopsonic activity of fibrinogen bound to M protein on the surface of group A streptococci. J Clin Investig. 1982;69:1042–1045. doi: 10.1172/JCI110508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yagi Y, Kessler R E, Show J H, Lopatin D E, An F Y, Clewell D B. Plasmid content of Streptococcus faecalis strain 39-5 and identification of a pheromone (cPD1)-induced surface antigen. J Gen Microbiol. 1983;129:1207–1215. doi: 10.1099/00221287-129-4-1207. [DOI] [PubMed] [Google Scholar]
- 56.Yamamoto T, Koyama Y, Matsumoto M, et al. Localized, aggregative, and diffuse adherence to Hela cells, plastic, and human small intestines by Escherichia coli isolated from patients with diarrhea. J Infect Dis. 1992;166:1295–1310. doi: 10.1093/infdis/166.6.1295. [DOI] [PubMed] [Google Scholar]
- 57.Zervos M J, Dembinski S, Mikesell T, Schaberg D R. High-level resistance to gentamicin in Streptococcus faecalis: risk factors and evidence for exogenous acquisition of infection. J Infect Dis. 1986;153:1075–1083. doi: 10.1093/infdis/153.6.1075. [DOI] [PubMed] [Google Scholar]