Abstract
Gastric cancer is the fifth most common cancer and the third leading cause of cancer death worldwide, posing a severe threat to human health. Surgical resection remains the most preferred option for gastric cancer treatment. However, for advanced gastric cancer, the curative effect of surgical resection is usually limited by the local recurrence, peritoneal carcinomatosis, or distal metastasis. Intraoperative chemotherapy is an attractive in situ adjuvant treatment strategy to reduce the recurrence and metastasis after surgical resection. Here, we designed a 5-fluorouracil (5-FU) and cis-platinum (DDP) co-delivery system based on a biodegradable temperature-sensitive hydrogel (PDLLA-PEG-PDLLA, PLEL) for intraoperative adjuvant combination chemotherapy of gastric cancer. This 5-FU + DDP/PLEL hydrogel system characterized by a special sol-gel phase transition in response to physiological temperature and presented sustained drug release in vitro and in vivo. A strong synergistic cell proliferation inhibition and apoptosis promotion of 5-FU + DDP/PLEL were observed against gastric cancer MKN45-luc cells. After intraperitoneal injection, the dual-drug loaded hydrogel formulation showed superior anti-tumor effects than the single-drug carrying hydrogels and combination of free 5-FU and DDP on the gastric cancer peritoneal carcinomatosis model. The use of hydrogel for dual-drug delivery had benefited to fewer side effects as well. What's more, we established a mouse model for postsurgical residual tumors and peritoneal carcinomatosis of gastric cancer, in which the intraoperative administration of 5-FU + DDP/PLEL also remarkably inhibited the local recurrence of the orthotopic tumors and the growth of the abdominal metastatic tumors, resulting in an extended lifetime. Hence, this developed dual-drug loaded hydrogel system has great potential in the intraoperative chemotherapy of gastric cancer, that suggests a clinically-relevant and valuable option for postsurgical management of gastric cancer.
Keywords: Thermo-sensitive hydrogel, Localized drug delivery, Sustained release, Intraoperative chemotherapy, Gastric cancer
Graphical abstract
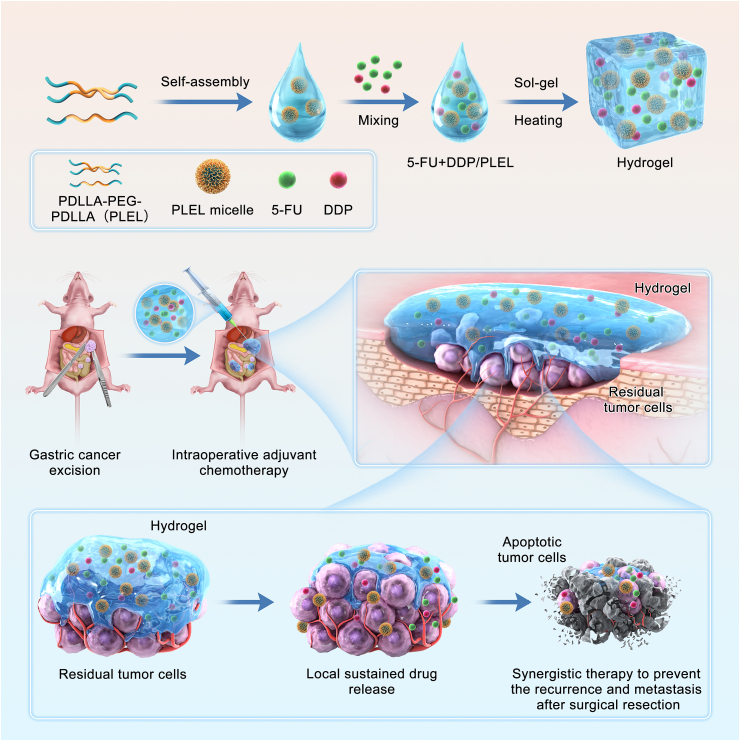
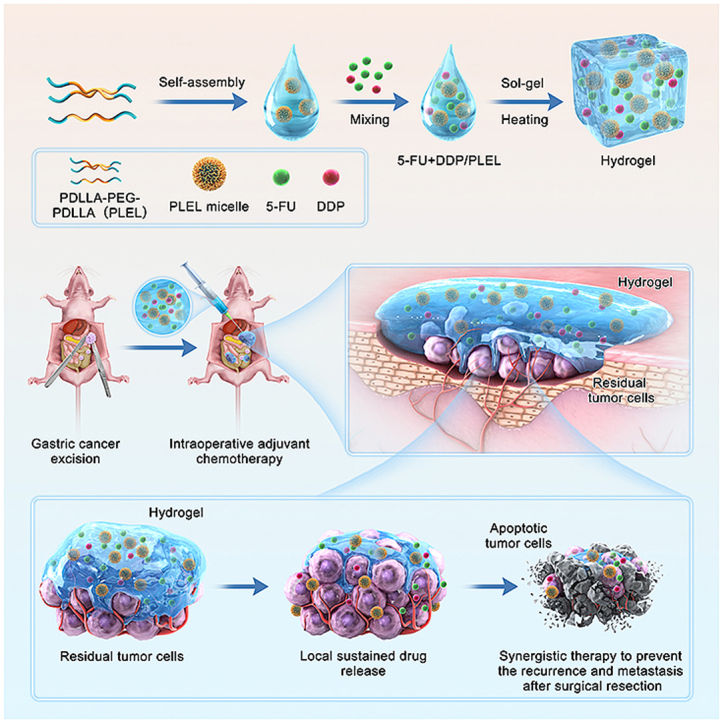
Schematic diagram of sustained co-delivery of 5-Fluorouracil (5-Fu) and cis-platinum (DDP) via biodegradable thermo-sensitive hydrogel, which was used for intraoperative synergistic combination chemotherapy of gastric cancer to inhibit postoperative tumor recurrence and metastasis.
Highlights
-
•
Intraoperative chemotherapy could reduce the recurrence and metastasis after surgical resection of gastroenteric tumors.
-
•
5-FU and DDP co-delivery system based on PLEL was developed for intraoperative combination chemotherapy of gastric cancer.
-
•
This dual-drug loaded hydrogel helped to improve synergistic anti-tumor effects and reduce adverse side effects in vivo.
-
•
5-FU+DDP/PLEL could inhibit recurrence of orthotopic tumors and growth of abdominal metastatic tumors in gastric cancer.
1. Introduction
Gastric cancer is the fifth most common malignant tumor, causing over 760000 new deaths worldwide in 2020 [1]. Although surgical resection is the first-line treatment for stomach cancer, the curative effects of surgical resection are limited by the local relapse from residual tumor cells and peritoneal or distal metastasis, especially for advanced gastric cancer, resulting in significant mortality [2]. Chemotherapy and radiotherapy are important adjuvant therapies to prevent the recurrence and metastasis following surgical resection [3]. However, systemic chemotherapy and radiotherapy are usually administrated 3–4 weeks after surgery due to the post-surgery weak physical condition of patients, which seems to miss the optimal point in time for eliminating residual cancer cells during this period. Besides, serious systemic toxicity and side effects are other limits of systemic chemotherapy and radiotherapy for improving the overall survival after surgery [4,5]. Hence, the need for appropriate strategies remains urgent to prevent tumor recurrence and metastasis after surgical resection of stomach cancer.
Intraoperative chemotherapy presents an attractive option for postoperative management of gastroenteric tumors, since it can inhibit the residual tumor cells timely and directly by providing a higher local drug concentration in abdominal cavity, without severe systemic reaction compared with conventional intravenous chemotherapy [6,7]. As a commonly used intraoperative chemotherapy in clinical practice, hyperthermic intraperitoneal chemotherapy (HIPEC) has been reported to improve the prognosis of patients with gastric cancer significantly [8,9]. Even so, the clinical benefit of HIPEC is still limited by its expensive equipment requirements, short drug maintenance time, and undesirable complications of thermal perfusion [10,11]. In recent years, intraoperative intraperitoneal chemotherapy based on sustained-release drug delivery systems has been considered as a promising alternative, receiving more and more attention. Typically, intraoperative chemotherapy with a commercial sustained-release fluorouracil implant has been shown to reduce the recurrence and prolong the survival time to a certain extent in gastric cancer and colorectal cancer [[12], [13], [14]]. However, its therapeutic effect is still unsatisfactory so far. The main reason is that it is difficult to distribute the implants evenly at the lesion site and abdominal cavity through fixing to the tissue, leading to inexhaustive eradication of the residual cancer cells. In addition, the safety of these fluorouracil implants remains controversy in clinical practice due to its slowly biodegradation and adhesion associated complication, yet resulting in the limited application. Therefore, new safer intraoperative drug delivery systems for gastric cancer will be required soon to improve the local maintenance time of drugs, and inhibit the postoperative tumor recurrence and metastasis.
In the past decades, various drug delivery systems such as microspheres, nanoparticles, nanofibers and hydrogels have been designed for cancer chemotherapy to prolong the retention time of anticancer drugs at the tumor site [[15], [16], [17], [18]]. Among them, injectable in-situ forming hydrogels are ideal biomaterials for medical applications including cancer treatment [[19], [20], [21], [22]]. In particular, biodegradable thermo-sensitive hydrogels with a unique temperature-responsive “sol-gel” transition characteristic are great candidates for local drug delivery and sustained release [[23], [24], [25], [26]]. They are solution at room temperature, allowing the feasible and non-destructive loading of drugs. When injected, the drug loaded composites change into hydrogel spontaneously in response to the physiological temperature and serve as a drug release depot in site. Another attractive advantage of these injectable thermogels is that it’s easy to administrate and distribute uniformly during operation without the need for invasive surgery and implantation procedures [27,28]. All of these peculiarities make thermo-sensitive hydrogels potential sustained-release drug carriers for intraoperative intraperitoneal chemotherapy of gastric cancer.
5-fluorouracil (5-FU) has been widely recognized as a first-line chemotherapy drug in the treatment of gastric cancer [29,30]. However, problems such as short plasma half-life (t1/2 is about 10–20 min), rapid elimination, and drug resistance have some negative effects on the prognosis of patients [31,32]. The combination of chemotherapeutics effectively improves the anti-tumor effect and has been extensively used in the clinical treatment of various cancers. Some studies have shown that cisplatin (DDP) can increase the production of intracellular tetrahydrofolic acid and improve cell sensitivity to 5-FU, playing a synergistic role when combined with 5-FU [33]. Several clinical studies have reported that 5-FU in combination with DDP exhibits tolerable drug toxicity and improves the survival rate of patients with gastric cancer compared with monotherapy [34,35]. However, in order to obtain the best therapeutic effect, systemic injection of each drug for multiple cycles is often necessary, causing serious toxicity and side effects, such as cardiotoxicity, neutropenia, thrombocytopenia, and anemia [36,37].To overcome these problems, a sustained-release drug based on thermo-sensitive hydrogel may be a promising platform for combined local administration of 5-FU and DDP, which hold the chance to prolong the retention time of each drug at the lesion area and minish the adverse effect in the intraoperative chemotherapy of gastric cancer.
Based on the above-mentioned considerations, herein, we proposed a 5-FU and DDP co-delivery system based on a biodegradable temperature-sensitive hydrogel for intraoperative adjuvant combination chemotherapy of gastric cancer (Scheme 1). The injectable thermo-sensitive hydrogel was made of poly(D,l-lactide)-poly(ethylene glycol)-poly(D,l-lactide) (PDLLA-PEG-PDLLA, PLEL) triblock copolymer, whose biocompatibility and potential for local drug sustained delivery has been demonstrated in our several previous studies already [[38], [39], [40]]. This 5-FU and DDP co-loaded composite (5-FU + DDP/PLEL) can be made by a simple physical mixture at room temperature, which means great feasibility and flexibility. Owning to its sensitive sol-gel transition under body temperature, this dual-drug loaded system can be easily distributed at the surgical site, adjacent tissue, and abdominal cavity, where the residual and metastatic cancer cells tend to exist after the surgical resection of gastric cancer. Upon in-site gelation, the encapsulated drugs will release in a sustained way and serve as a long-acting depot, contributing to improving the local maintenance time of drugs and inhibiting the postoperative tumor recurrence and metastasis timely. We studied the efficacy of 5-FU + DDP/PLEL hydrogel in terms of prolonging the local residence time of chemotherapeutics, enhancing the combination therapy effect, and minimizing toxic side effects. Furthermore, the anti-tumor response of 5-FU + DDP/PLEL hydrogel was assessed in a peritoneal carcinomatosis model and a postoperative peritoneal carcinomatosis model of gastric cancer in nude mice.
Scheme 1.
Schematic diagram of sustained co-delivery of 5-fluorouracil (5-FU) and cis-platinum (DDP) via biodegradable thermo-sensitive hydrogel, which was used for intraoperative synergistic combination chemotherapy of gastric cancer to inhibit postoperative tumor recurrence and metastasis.
2. Materials and methods
2.1. Materials and animals
PEG (Mn = 1500), stannous octoate (Sn(Oct)2, 95%), 5-FU(5-Fluorouracil, 99%) and DDP (Cisplatin, 99%) were obtained from Sigma-Aldrich (Saint Louis, USA). D,l-Lactide (D,L-LA) was bought from Daigang chemicals (Jinan, China). Cell Counting Kit-8 (CCK8) was supplied from the Shanghai Haoyuan Biomedical Technology Company (Shanghai, China). Annexin V-FITC/PI Apoptosis Detection Kit and Live & Dead Cytotoxicity Assay Kit were purchased from Jiangsu KGI Biotechnology Company (Nanjing, China). Cy5.5 was provided by the Beijing Fubaike Biotechnology Company (Beijing, China). D-luciferin potassium salt was purchased from Dalian Meilun Biotechnology Company. (Dalian, China). TrypsinEDTA, RPMI 1640 medium were obtained from Shanghai Yuanpei Biotechnology Company (Shanghai, China). And fetal bovine serum (FBS) and penicillin-streptomycin liquid were supplied by Gibco (USA). The other chemical reagents used in this article were analytical grade and could be used without further purification.
Female Balb/c nude mice (6–8 weeks old) were purchased from HFK Biotechnology Company (Beijing, China). They were housed in a specific pathogen-free (SPF) environment with free access to standard food and water. All animal experiments were approved by the Ethics Committee of the Animal Experimental Center of State Key Laboratory of Biotherapy of Sichuan University (Checking number: 20210409028), and were carried out in compliance with the approved guidelines.
2.2. Preparation and sol-gel phase transition behavior of 5-FU + DDP/PLEL hydrogel
2.2.1. Preparation of 5-FU + DDP/PLEL hydrogel
5-FU and DDP loaded PLEL hydrogel (5-FU + DDP/PLEL hydrogel) was prepared in three steps. First, the PDLLA-PEG-PDLLA (PLEL) triblock copolymer was synthesized via ring-opening copolymerization of D,L-lactide initiated by PEG and characterized through nuclear magnetic resonance spectroscopy (1H NMR, Varian 400 spectrometer, USA) and gel permeation chromatography (GPC, Agilent 110 HPLC, USA), according to our previous work [38]. Secondly, the obtained PLEL copolymer was completely dissolved in the phosphate buffered saline (PBS, pH 8.0) at room temperature and stirred well to obtain the PLEL micelle solution. Finally, 5-FU + DDP/hydrogel was prepared by dissolving 5-FU and DDP with the PLEL micelles solution by stir (60 rpm, 25 °C) for about 2 h and ultrasound for about 30 min to form a homogeneous solution. The concentration of the PLEL in the mixed 5-FU + DDP/PLEL here was 10 wt %, 15 wt % and 20 wt %. All drug-loaded samples were filtered with the 0.22 μm membrane for sterilization. The concentration of the PLEL in the mixed 5-FU + DDP/PLEL here was 10 wt %, 15 wt %, 20 wt %, and 25 wt %. The concentration of 5-FU and DDP of 5-FU + DDP/PLEL after filtration was detected by high performance liquid chromatography (HPLC) and inductively coupled plasma (ICP), respectively.
2.2.2. The thermosensitive sol–gel phase transition behavior of 5-FU + DDP/PLEL hydrogel
The thermosensitive sol-gel phase transition behavior of 5-FU + DDP/PLEL hydrogel was observed by the test-tube-inversion method. Add 1 mL 5-FU + DDP/PLEL hydrogel samples to the vial and heat them to 37 °C. Simultaneously observe changes of hydrogels status at room temperature and after heating. Besides, record the phase transition behavior of the blank hydrogel at room temperature and 37 °C for comparison with the drug-loaded hydrogel. To further investigate the injectability and gelation property of the 5-FU + DDP/PLEL hydrogel, we observed the state of the hydrogel when injected with a 1 ml syringe at room temperature and in a 37 °C water bath.
2.2.3. Dynamic rheological study of 5-FU + DDP/PLEL hydrogel
To further understand the effect of PLEL concentration and loaded drugs on the thermosensitive phase transition, dynamic rheology tests of blank hydrogels and 5-FU + DDP/PLEL hydrogels with different concentrations of PLEL copolymer (10 wt%, 15 wt%, 20 wt%, and 25 wt%) were performed using a rheometer (HAAKE RheoStress 6000, Thermo Science, USA). Samples of the drug loaded PLEL hydrogel or blank PLEL hydrogel were fully stabilized at 4 °C and placed between a parallel plate with a diameter of 20 mm and a gap of 1 mm. At the same time, low-viscosity silicone oil was covered around the sample before testing to prevent solvent evaporation. The stress amplitude scanning of each sample was performed at 37 °C and 1 Hz firstly to test the linear viscoelastic region. Then, changes in storage modulus (G′), loss modulus (G″) and viscosity (η) were measured as functions of temperature from 10 to 60 °C. Data were collected under controlled stress of 1Pa and a frequency of 1.0 Hz, and the heating rate was 1 °C/min. In addition, the gel time (Tgel) of drug loaded PLEL hydrogel and blank PLEL hydrogel was measured at 37 °C, where G′ and G″ were performed as a function of time. The data were collected under controlled stress of 1Pa and a frequency of 1.0 Hz.
2.3. Drug release in vitro and in vivo
2.3.1. Drug release in vitro
The release behavior of 5-FU and DDP from 5-FU + DDP/PLEL hydrogel (10 wt%, 15 wt% and 20 wt%) was conducted in vitro and determined by the high performance liquid chromatography (HPLC) and inductively coupled plasma (ICP). First, 1 ml of dual-drug loaded samples (2 mg/mL 5-FU and 0.1 mg/mL DDP) were added into the bottom of the test tube and equilibrated at 37 °C for 30 min to form a stable hydrogel. Secondly, pre-warmed 10 mL PBS (pH 7.4) was gently added to the tube above the gel and incubated at 37 °C with shaking (100 rpm). At pre-determined time points including 2 h, 4 h, 6 h, 8 h, 12 h, 24 h, 48 h, 72 h, 96 h, 168 h, 10d, 14d, 19d and 28d, all release media was harvested and replaced with the pre-warmed fresh PBS solution. Here, the concentration of released 5-FU was determined by high performance liquid chromatography (HPLC) with an octadecyl silane-bonded silica gel at a wavelength of 265 nm. A mixture of 0.05 mol/L phosphoric acid solution at pH 3.5 and methanol (95∶5, v/v) was used as the mobile phase. The cumulative release of DDP was determined by inductively coupled plasma (ICP). All data were subjected to three replicate experiments and averaged.
2.3.2. Drug release in vivo
To evaluate the sustained-release ability of PLEL hydrogels as drug carrier in the abdominal cavity, water-soluble fluorescence dye Cy5.5 was used as the mimetic drug of 5-FU and DDP. Using free Cy5.5 solution as a control, Cy5.5 encapsulated PLEL hydrogels (Cy5.5/PLEL hydrogel, 10 wt%, 15 wt% and 20 wt%) were injected into the abdominal cavity of Balb/c nude mice. Then IVIS Lumina III imaging system (Emission wavelength: 710 nm; Excitation wavelength: 660 nm) were used to record the fluorescence intensity at different time point including 0.3 h, 5 h, 24 h, 48 h, 72 h and 168 h and analyzed the fluorescence images with Living Image software.
2.4. Cellular experiments
2.4.1. In vitro cytotoxicity test and synergy of drug combination
Luciferase labeled human gastric cancer cell line MKN45 (MKN45-luc) was purchased from the American Type Culture Collection (ATCC, Rockville, USA), which was cultured in complete RMPI 1640 growth medium (supplemented with 10% FBS, 1% penicillin, and 1% streptomycin) and maintained at 37 °C with humidified 5% CO2. MKN45-luc cells were seeded in 96-well plates at a density of 4000 cells/well in the presence of 100 μL culture medium and incubated at 37 °C for 24 h. Then, 100 μl RMPI 1640 growth medium containing 5-FU, DDP, or 5-FU and DDP mixture (the drug ratios of 5-FU: DDP were 10:1, 20:1, and 40:1) with different total drug concentrations were added into the 96-well plates (n = 5). After incubation of 24 h and 48 h, CCK8 assays were used to detect cellular viability.
The half maximal inhibitory concentration (IC50) against MKN45-luc cells was calculated using CompuSyn software. The median effect equation was used: Fa = [1 + (IC50/D)m] − 1, where Fa was the fraction of inhibited cells, D was drug concentration and m was the Hill slope. The combination index (CI) analysis according to the Chou-Talalay method was also determined using CompuSyn software [41,42]. Briefly, for different levels of cell inhibition fraction, the CI values for the combination of 5-FU and DDP against MKN45-luc cells were calculated by the following formula: CI = (D)1/(Dx)1 + (D)2/(Dx)2, where (Dx)1 and (Dx)2 represented the concentrations of drug 1 and drug 2 administrated alone, respectively, at a specific drug effect level. D1 and D2 represented the concentrations of drugs in combination to achieve the same drug effect level. The drug effect level represented the fraction of inhibited cells. Simultaneously, combination index (CI) for drug combinations and drug effect level curve was plotted as a function using CompuSyn software. CI < 1, = 1, and >1 indicate synergism, additive effect and antagonism, respectively.
2.4.2. Synergistic effects of dual-drug loaded hydrogel in vitro
The cytotoxicity of drug-loaded hydrogels on MKN45-luc cells was evaluated through a 24-well Transwell (Corning) co-culture system. MKN45-luc cells were seeded in the lower chamber of the Transwell plate (20000 cells per well) and cultivated with 500 μL RMPI 1640 growth medium for 24 h. Then 50 μL PBS, free 5-FU, free DDP, free 5-FU + DDP (the drug ratios of 5-FU: DDP were 10:1, 20:1, and 40:1), PLEL hydrogel solution, 5-FU/PLEL hydrogel, DDP/PLEL hydrogel, 5-FU + DDP/hydrogel (the drug ratios of 5-FU: DDP were 10:1, 20:1, and 40:1) were added to the transwell upper chamber. Notably, the inserts containing hydrogel were kept at 37 °C for 30 min to form micellar cross-linked hydrogels and then placed in medium containing well plates for further co-culture. The concentration of PLEL hydrogel was 20 wt%. To fully simulate in vivo drug clearance, the initial medium in the Transwell well plate was replaced with fresh medium after 24 h. After 24 h or 48 h of co-culture, cell viability was detected by the CCK8 assay (n = 5).
In addition, live dead staining experiments were conducted to explore the antiproliferative effects of drug-load hydrogel further. MKN45-luc cells were cultured via a Transwell co-culture system as described in the previous section, and PLEL hydrogel, free 5-FU + DDP, 5-FU/PLEL hydrogel, DDP/PLEL hydrogel, and 5-FU + DDP/PLEL hydrogel (the drug ratios of 5-FU: DDP were 20:1 and the total drug concentration was 45 μM) were added to the upper chamber, respectively. After another 24 h or 48 h incubation, the MKN45-luc cells were collected by centrifuge and washed twice with PBS. Calcein AM/PI dye was added and cultured at room temperature for 40 min to stain the live and dead cells, then removed completely. Finally, the cell suspension with PBS was added to a clean slide in an appropriate cell concentration and covered with a coverslip. The labeled cells were observed under a fluorescence microscope (Olympus, Tokyo, Japan) and analyzed via ImageJ 7.0 software.
2.4.3. Cell apoptosis analysis by flow cytometry
MKN45-luc cells were co-cultured for 24 h or 48 h according to the method described in the previous section and the same grouping process was performed. Afterward, all the cells in the wells were collected, stained using the Annexin V-FITC/PI Apoptosis Detection Kit according to the manufacturer's instructions, and tested with a flow cytometer (BD FACSCalibur). The data were analyzed with Novoexpress software. All experiments were conducted with three parallel samples (n = 3).
2.5. In vivo anti-tumor experiments
2.5.1. Establishment of gastric cancer models on nude mouse
The peritoneal carcinomatosis (PC) model of gastric cancer was established. Briefly, BALB/c nude mice were intraperitoneally injected with MKN45-luc cells (2 × 106 cells per mouse) that were suspended in 0.2 mL of medium without fetal bovine serum and penicillin-streptomycin liquid. After the inoculation of the gastric cancer cells, the IVIS Lumina III imaging system (PerkinElmer) was used to detect the spread and growth of tumors in the abdominal cavity. Moreover, a mouse model for postsurgical residual tumors and peritoneal carcinomatosis of gastric cancer was established. First, the peritoneal carcinomatosis (PC) model was established according to the previous method. When the tumor signal intensity reached about 109, mice were anesthetized and conducted with laparotomy operation to remove the tumor nodules at the gastric ligaments, while the small tumors on the peritoneum were retained to establish a postoperative peritoneal carcinomatosis model of gastric cancer. At last, the wound was sutured with medical threads.
2.5.2. In vivo anti-tumor efficiency of 5-FU + DDP/PLEL hydrogel
First, we investigated the in vivo anti-tumor effect and safety of the PLEL hydrogel preparations with different drug ratios on the PC model of gastric cancer. The mice were randomly divided into seven groups(n = 5)once the model was established. The tumor-bearing mice were injected intraperitoneally with 200 μL following formulations: Normal saline (NS), free 5-FU + DDP (the ratios of 5-FU: DDP were 40:1, 20:1 and 10:1) and 5-FU + DDP/PLEL (the ratios of 5-FU: DDP were 40:1, 20:1 and 10:1) respectively. The tumor-bearing mice in NS group were intraperitoneally injected with normal saline, which served as the control group without treatment. It should be mentioned in particular that the total dosages of 5-FU and DDP were always maintained at 20 mg/kg every six days for 2 times. Then, the bioluminescence signal of the tumor was acquired every 5 days by the IVIS Lumina III imaging system. After intraperitoneal injection of D-fluorescein potassium salt at a dose of 150 mg/kg for 10 min, anesthetize the mouse with 2% isoflurane and then image to obtain a bioluminescent signal. The body weight was also recorded every two days, simultaneously. For side effect assessment, the mice in each group were sacrificed 4 days after treatment, and all major organs of mice were harvested and fixed in 4% PBS-buffered paraformaldehyde for H&E staining. In addition, the survival of tumor-bearing mice was observed continuously.
Secondly, we studied the in vivo anti-tumor effect of the 5-FU + DDP/PLEL hydrogel under the optimized drug ratio using the PC model of gastric cancer. After the model was established, the tumor-bearing mice were randomly distributed into six groups(n = 7)and injected intraperitoneally with 200 μL different formulations including NS, PLEL solution, free 5-FU and DDP, DDP/PLEL, 5-FU/PLEL and 5-FU + DDP/PLEL. For all chemotherapeutics containing groups, the doses of 5-FU and DDP were maintained at 20 mg/kg and 1 mg/kg every six days for 2 times. Tumor bioluminescence signal was monitored every 3 days via the IVIS Lumina III imaging system. At the same time, the body weight of each mouse was also recorded every other day. On day 13 after treatment, the mice were sacrificed, the tumor and major organs were removed by laparotomy and fixed in 4% PBS-buffered paraformaldehyde for the following study. The lifetime of the remaining tumor-bearing mice was recorded by daily observation of their survival status.
2.5.3. Efficiency evaluation of 5-FU + DDP/PLEL hydrogel to prevent the recurrence and metastasis after surgical resection
Subsequently, the anti-tumor efficacy of drug-loaded hydrogel was also evaluated in a postoperative peritoneal carcinomatosis model. After confirming the build of the PC model, the mice were randomly divided into five groups(n = 8)to carry out the postoperative peritoneal carcinomatosis model as mentioned before. Before the abdomen closure, 200 μl of different formulations were injected on the resection tumor bed and into the abdomen cavity. The formulations included NS, free 5-FU and DDP, DDP/PLEL, 5-FU/PLEL and 5-FU + DDP/PLEL. The dosages of 5-FU and DDP were retained at 20 mg/kg and 1 mg/kg, respectively. To continuously monitor anti-tumor effects, the bioluminescence signal of the tumor was monitored every 3 days through the IVIS Lumina III imaging system. The weight of each mouse was also recorded every other day to evaluate the side effect. On day 13 after treatment, mice were sacrificed and the tumor of each group was harvested to fix in 4% PBS-buffered paraformaldehyde for histology and immunohistochemistry analysis. To evaluate the tumor burden and dissemination, the total number and weight of the peritoneal tumor nodules were measured as well. In addition, the volume of ascites was also analyzed to evaluate the anti-tumor efficacy. Then, the death time of the tumor-bearing mice without sacrifice was recorded by observation of their survival status every day.
2.5.4. Histology and immunohistochemistry analysis
Tumors and major organs (heart, liver, spleen, lung and kidney) fixed with 4% paraformaldehyde were dehydrated by a series of ethanol with different concentrations and then placed in xylene. They were embedded in paraffin wax and then cut into 5 μm slices to the slides, followed by drying in a 45 °C thermostat for later use. To assess the toxic effects of tumor-bearing mice under different treatments, the tissue sections of organs were stained with Hematoxylin-eosin (H&E). In addition, Ki-67 and TUNEL staining were performed according to the manufacturer's protocol to investigate the proliferation and apoptosis of cancer cells in tumor tissue. The images of TUNEL were observed under a fluorescence microscope (Olympus, Tokyo, Japan) and the other images were collected by microscope slide scanner (PANNORAMIC MIDI, 3DHISTECH). All photos were analyzed by CaseViewer software.
2.6. Statistical analysis
All the data were expressed as mean ± standard deviation (SD). Statistical comparisons among the groups were determined by a Student's t-test using GraphPad Prism software. Survival benefits were evaluated using log-rank tests. Statistical significance was set at ∗P < 0.05, ∗∗P < 0.01, and ∗∗∗P < 0.001.
3. Results and discussion
3.1. Preparation and sol-gel phase transition behavior of 5-FU + DDP/PLEL hydrogel
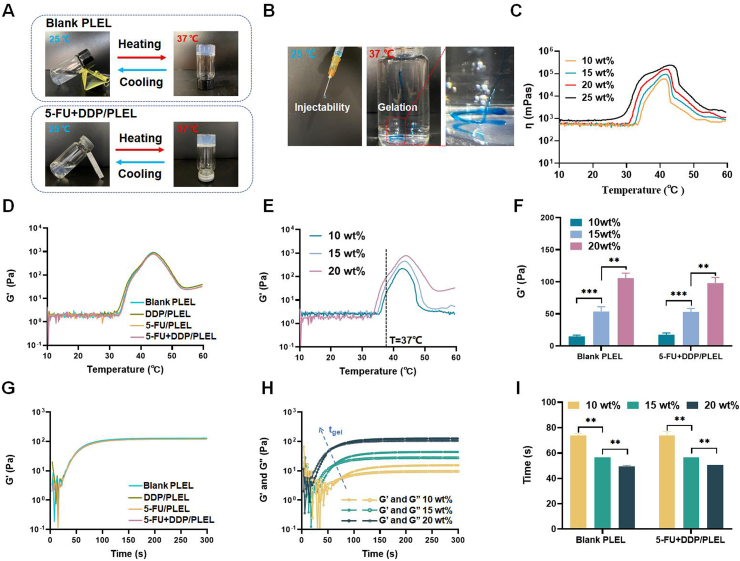
The triblock PLEL copolymer was successfully synthesized via ring-opening copolymerization and the average molecular weight calculated by 1H NMR spectrum was 4650 (PEG/PDLLA ratio: 1500/3150). The molecular weight distribution of PLEL triblock copolymers was further determined via GPC measurements and they showed a unimodal distribution with a polydispersity index (PDI) of 1.69 (Fig. S1). PLEL copolymer could self-assemble into core-shell-like micelles in water on account of its amphipathic property. These micelles are small at room temperature and the aqueous solution seems to be a flexible sol-like suspension. When exposed to the body temperature, a micellar network can spontaneously form due to the increase in micelles size and the sharp aggregation between augmented micelles, resulting in a physical hydrogel [38,39]. PLEL hydrogels with concentrations ranging from 10 wt% to 25 wt% exhibited appropriate gelation temperature and were chosen to prepare drug delivery systems. Taking advantage of the fact that the PLEL hydrogel was liquid at room temperature, a 5-FU and DDP co-loaded hydrogel system (5-FU + DDP/PLEL) was prepared via a simple physical mixing method, which contributed to great operability, flexibility and drug entrapment. Since the water content of the PLEL hydrogel was more than 80%, the composite system was almost a suspension of dissolved drugs and micelles. As shown in Fig. 1A, the resulting 5-FU + DDP/PLEL hydrogel (2 mg/mL 5-FU, 0.1 mg/mL DDP, and 20 wt% PLEL) had good fluidity at 25 °C, and underwent sol-gel transition as the temperature increased to 37 °C, which was super similar to the blank PLEL hydrogel. Considering its expected intraperitoneal administration, the injectability and gelation in situ of 5-FU + DDP/PLEL were evaluated. Injection through 24G syringe was readily performed at 25 °C without the risk of syringe clogging, while quick in situ gelation and stable gel maintenance in water were observed when injected into 37 °C water, implying its potential feasibility of gelation in the peritoneal cavity (Fig. 1B). To obtain sterile formulation, filtration sterilization was used here. However, 5-FU + DDP/PLEL at concentration of 25 wt% was found to be difficult to sterilize by filtration, due to its high viscosity(η) (Fig. 1C). Thus, 5-FU + DDP/PLEL with a concentration of 10 wt% to 20 wt% were selected for further formulation optimization.
Fig. 1.
The sol-gel phase transition behavior of 5-FU + DDP/PLEL hydrogel. (A) Reversible sol-gel phase transition of blank PLEL hydrogel (20 wt%) and 5-FU + DDP/PLEL hydrogel (polymer concentration: 20 wt%; 5-FU loading amount: 2 mg/mL; DDP loading amount: 0.1 mg/mL) between 25 and 37 °C. (B) The injectability and gelation of 5-FU + DDP/PLEL hydrogel. (C) Changes in viscosity (η) of 5-FU + DDP/PLEL hydrogel with different concentrations of PLEL copolymer as a function of temperature. (D) Changes in storage modulus (G′) of blank PLEL hydrogel and drug loaded hydrogel (20 wt%) as a function of temperature. (E) Changes in storage modulus (G′) of 5-FU + DDP/PLEL hydrogel with different concentrations of PLEL copolymer as a function of temperature. (F) The storage modulus (G′) for blank PLEL hydrogel and 5-FU + DDP/PLEL hydrogel at 37 °C. Data are presented as mean ± sd (n = 3). (G) Changes in storage modulus (G′) of blank PLEL hydrogel and drug loaded hydrogel (20 wt%) at 37 °C as a function of time. (H) Changes in storage (G′) and loss modulus (G″) of 5-FU + DDP/PLEL hydrogel with different concentrations of PLEL copolymer at 37 °C as a function of time. tgel means the gelation time of samples. (I) The gelation time of blank PLEL hydrogel and 5-FU + DDP/PLEL hydrogel at 37 °C. Data are presented as mean ± sd (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001.
Furthermore, dynamic rheological measurements were conducted to quantitatively investigated the sol-gel translation and mechanical properties of 5-FU + DDP/PLEL hydrogels. The stress amplitude scanning of each hydrogel sample was performed under 37 °C and 1 Hz firstly. As shown in Fig. S2, the storage modulus (G′) and loss modulus (G″) of all the tested hydrogel samples began to fall when the stress increased to about 50–300 Pa, indicating that the hydrogel was not destroyed under the stress less than 50Pa when the applied frequency is1Hz, which means the linear viscoelastic region of the hydrogel. Therefore, the following tests were all performed at 1Pa and 1 Hz. Results in Fig. 1D and Fig. S3 showed that both the single-drug loaded hydrogel composites and the dual-drug loaded hydrogel went through the similar temperature-responsive gelation behavior compared to blank hydrogel, whose storage modulus (G′) and viscosity (η) were low at low temperature and increased dramatically as the temperature rose to around 37 °C. This result indicated that the loading of drugs didn't change the thermosensitivity of PLEL hydrogel. In addition, the G′ value of PLEL hydrogel systems with or without drugs was found to increase gradually upon the increase of hydrogel concentrations when the gel formed at 37 °C, just as Fig. 1E and F showed.
Moreover, gelation time was also measured under 37 °C and the results indicated that the storage modulus (G′) of drug loaded hydrogels increased rapidly at the beginning 50s and then flattened, which was almost consistent with the blank hydrogel (Fig. 1G). An increase in copolymer concentration was demonstrated to conduce to quicker gelation of 5-FU + DDP/PLEL hydrogels (Fig. 1H and I). All of these studies suggested that the addition of 5-FU and DDP hardly ever changed the temperature sensitivity of the PLEL hydrogel. Among these designed formulations, 5-FU + DDP/PLEL hydrogel at 20 wt% showed higher strength and less gelation time, which may benefit to better shape persistence and more convenient administration during operation.
3.2. Drug release in vitro and in vivo
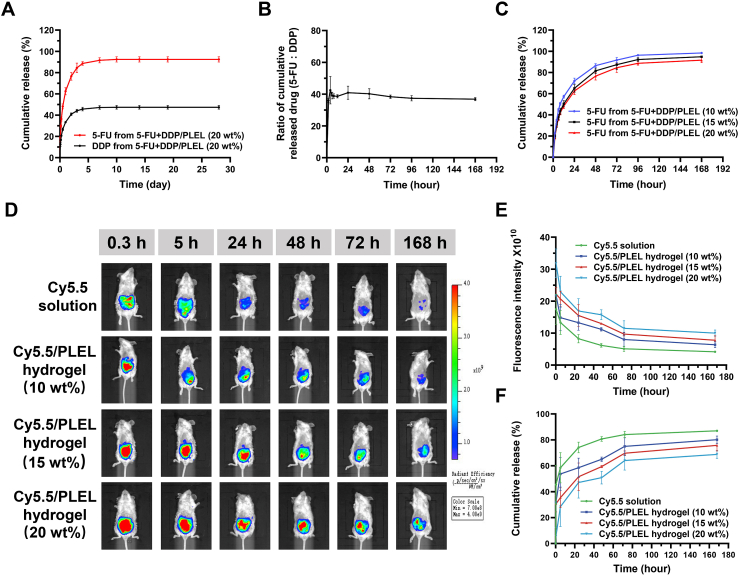
The drug release behavior of 5-FU and DDP from PLEL hydrogel was investigated in vitro. According to Fig. 2A, 5-FU + DDP/PLEL hydrogel released 5-FU and DDP in a sustained manner for up to 1 week. Release of 5-FU and DDP exhibited similar bi-phasic patterns, with a burst release on the first day and a sustained release over the days following. The cumulative release of 5-FU was about 62.43% at 24 h and about 92.42% after 28 days. However, the cumulative release of DDP at 24 h and 28 days was 33.35% and 47.42%, respectively. This difference in release rates may be caused by their different water solubility and diffusion rates in the hydrogel. It was worth mentioning that, although the initial drug ratio of 5-FU and DDP in the drug-loaded hydrogel was 20:1, their cumulative released drug ratio kept at about 40:1 during the 7-day release process, which was very close to the proportion of commonly used 5-FU and DDP combination ratio in clinic (Fig. 2B). Moreover, the cumulative release of 5-FU from PLEL hydrogel systems with different copolymer concentrations varied, as shown in Fig. 2C. 20 wt% 5-FU + DDP/PLEL hydrogel exhibited the lowest cumulative release rate, compared to the ones with 10 wt% and 15 wt% PLEL concentration.
Fig. 2.
Drug release from the thermo-sensitive 5-FU + DDP/PLEL hydrogel both in vivo and in vitro. (A).
In vitro release behavior of 5-FU and DDP from the hydrogel system (20 wt%). (B) The cumulative released drug ratio of 5-FU and DDP from the hydrogel system (20 wt%). (C) Release behaviors of 5-FU from hydrogel systems with different copolymer concentration (5-FU loading amount: 2 mg/mL; DDP loading amount: 0.1 mg/mL). (D) and (E) In vivo extended release intra-abdominal retention of Cy5.5 from PLEL hydrogel in recorded by IVIS. (F) In vivo release of Cy5.5 from PLEL hydrogel. Data are presented as mean ± sd (n = 3).
In addition, noninvasive intravital IVIS Lumina III imaging system was used to study the intra-abdominal retention of drug in vivo after delivered by PLEL hydrogel. A water-soluble fluorescence dye Cy5.5 was used as a substitute for 5-FU and DDP to simulate their retention and distribution in vivo, whose intensity in the abdominal cavity was monitored in real-time. As shown in Fig. 2D, after intraperitoneal injection, the fluorescence intensity of mice treated with Cy5.5 solution decayed sharply within 24 h, almost disappeared by 168 h. In contrast, the fluorescence decayed slowly and preserved for more than 168 h in the case of Cy5.5/PLEL hydrogel with different copolymer concentrations. In particular, mice injected with 20 wt% Cy5.5/PLEL hydrogel maintained the highest fluorescence intensity during monitoring, and the fluorescence signal remained strong even after 168 h (Fig. 2D and E). The in vivo release of Cy5.5 from PLEL hydrogel with different copolymer concentrations was calculated according to the fluorescence intensity as shown in Fig. 2F. The cumulative release rate of free Cy5.5 was the fastest, while 20 wt% 5-FU + DDP/PLEL hydrogel was the slowest among the Cy5.5/PLEL hydrogels with different polymer concentration, which was consistent with the in vivo release results. Both in vitro and in vivo results indicated that the sustained drug release effect of PLEL hydrogel systems enhanced with the increase of hydrogel concentration, which may possibly because the polymer micelle network of the hydrogel was denser and had higher mechanical strength under higher polymer concentration, leading to a certain hindering effect on drug diffusion behavior. The release rate of drug from 20 wt% hydrogel was obviously lower than that of 10 wt% hydrogel, especially in the simulated drug release experiment in vivo, which may also be related to the higher strength of hydrogel can resist the scouring of peritoneal fluid, the mechanical force of intestinal peristalsis, and maintain the integrity of hydrogel better [43]. Therefore, 20 wt% PLEL hydrogel was selected for subsequent study. These results both in vivo and in vitro indicated that using PLEL hydrogel as a local drug carrier can effectively prolong the release of drug in abdominal cavity, which was considered to be quite helpful for intraoperative local therapy of gastrointestinal tumors.
3.3. Synergistic combination chemotherapy of 5-FU + DDP/PLEL hydrogel against gastric cancer cells
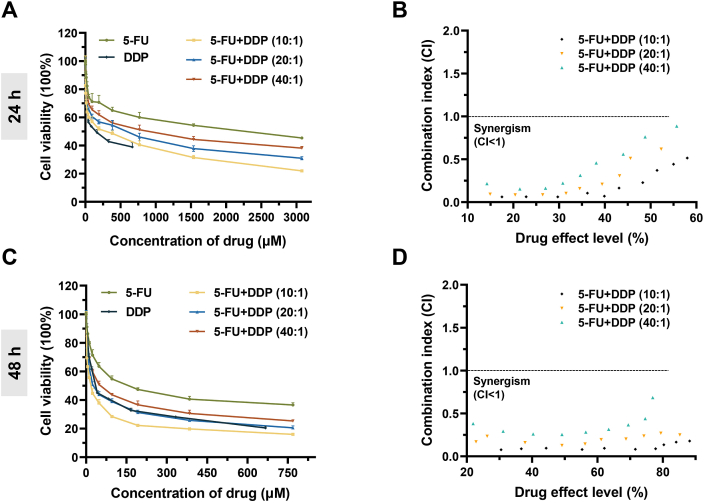
5-FU is a first-line chemotherapy drug for gastric cancer therapy and the combination of DDP has been proven to synergistically enhance the anti-tumor effect both in basic research and in clinical practice [44,45]. Besides, several studies, including ours, had demonstrated that the synergistic activity between combined drugs was influenced by the ratio of drugs, doses, and administration schedule [39,46]. Therefore, we determined the toxicity of combined 5-FU and DDP with a series of total drug concentrations and drug ratios to the human gastric cancer cells MKN45-luc cells via the cell counting kit-8 assay firstly. Referring to the commonly used clinical dosing regimen, 5-FU + DDP at drug ratios of 10:1, 20:1 and 40:1 were studied here [[47], [48], [49]]. Both the single and combined drugs induced cell death in a concentration-dependent manner after incubation of 24 h, and the combined use of 5-FU and DDP enhanced cytotoxicity significantly, as shown in Fig. 3A. The half inhibition concentrations (IC50) of 5-FU + DDP combination at drug ratios of 10:1, 20:1 and 40:1 were about 293.27, 415.95, and 663.62 μM, respectively, which were lower than 5-FU (1189.24 μM) (Table 1). Next, the CI values of different 5-FU + DDP combinations were calculated using Compusyn software [41,42]. As shown in Fig. 3B, all the CI values fell below 1, indicating an obvious synergistic effect between 5-FU and DDP in the drug ratio range of 10:1–40:1.
Fig. 3.
In vitro cytotoxicity of 5-FU + DDP combination against MKN45-luc cells. (A) Cytotoxicity research of 5-FU, DDP, and combined 5-FU + DDP with different drug ratios as a function of the total drug concentration after incubation for 24 h. Data are presented as mean ± sd (n = 5). (B) The CI values of free 5-FU and DDP combination at different drug ratios after co-incubating for 24 h. (C) Cytotoxicity study of 5-FU, DDP, and combined 5-FU + DDP with different drug ratios as a function of the total drug concentration after incubation for 48 h. Data are presented as mean ± sd (n = 5). (D) The CI values of 5-FU and DDP combination at different drug ratios after incubation for 48 h.
Table 1.
IC50 of different drugs against MKN45-luc cells.
| Time | 5-FU (μM) | DDP (μM) | 5-FU:DDP (10:1) (μM) | 5-FU:DDP (20:1) (μM) | 5-FU:DDP (40:1) (μM) |
|---|---|---|---|---|---|
| 24 h | 1189.24 | 133.70 | 293.27 | 415.95 | 663.62 |
| 48 h | 206.48 | 61.04 | 16.17 | 36.06 | 70.01 |
Furthermore, we found that the anti-proliferative effects enhanced significantly when the treatments time up to 48 h, especially the 5-FU + DDP combination treated groups (Fig. 3C). The IC50 value of combined 5-FU + DDP at drug ratios of 10:1 and 20:1 was decreased to 16.17 μM and 36.06 μM, which were notably lower than that of 5-FU (206.48 μM) and DDP (61.04 μM) (Table 1). At the same time, all CI values of 5-FU + DDP combination with different drug ratios were less than 1 as well, even smaller than that of 24 h (Fig. 3D). These results demonstrated that the combination of 5-FU and DDP had good synergistic chemotherapy effect against gastric cancer cells over a wide drug ratio, and this synergy effect seemed to be strengthened with action times.
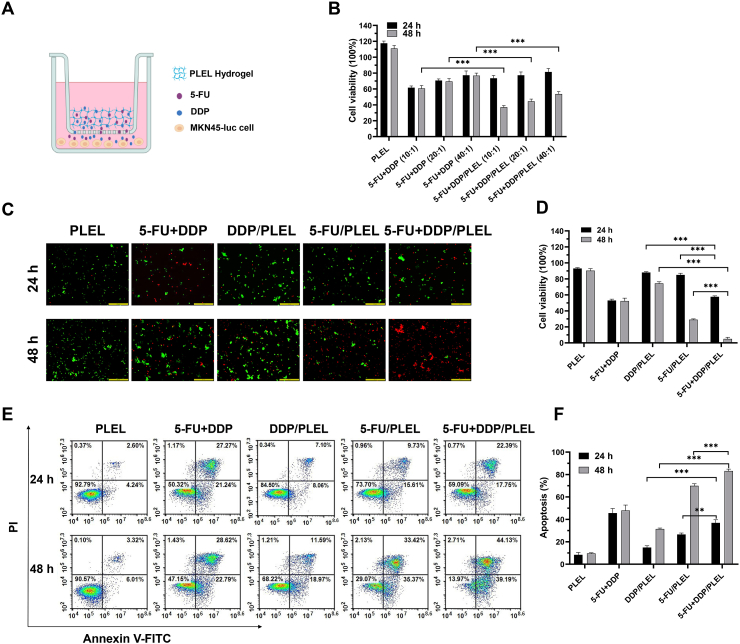
Encouraged by the above synergy effect of 5-FU and DDP, we accessed the anti-proliferative effect of 5-FU and DDP co-delivered hydrogel systems using a Transwell co-culture system to mimic the sustained drug release process, as shown in Fig. 4A. Here, we selected 5-FU and DDP combination with a total drug concentration of 45 μM to study the anti-proliferative effect of dual drug co-delivered hydrogel systems, which was close to the IC50 value of 48 h according to the results of cytotoxicity study. To simulate in vivo drug clearance, the initial medium in the Transwell well system was replaced with fresh medium after 24 h co-incubation. According to the results in Fig. 4B, blank PLEL hydrogel had no obvious cytotoxicity against MKN45-luc cells. Due to the sustained drug release, the antiproliferative effect of 5-FU + DDP/PLEL hydrogels seemed slightly weaker than that of free 5-FU + DDP combinations at the beginning, but it was significantly superior after 48 h co-culture. Besides, we noticed that the cytotoxicity of 5-FU + DDP/PLEL hydrogel was increased with the proportion of DDP, just similar to the free 5-FU + DDP combination shown. Next, to further confirm the synergistic antiproliferative effects of dual-drug loaded hydrogel, the 5-FU + DDP combination with a drug ratio of 20:1 and the total concentration of drug was 45 μM was taken as an example and live/dead cell staining experiment was performed (Fig. 4C and D). Although the medium was refreshed at 24 h, the proportion of living cells treated with the drug-loaded hydrogels decreased with incubation time, while that of the free combination group hardly changed after 24 h. It was apparent that the sustained drug release of drug-loaded hydrogels promoted their sustained anti-cancer activity. In addition, the cell viability after co-incubation with dual-drug delivery hydrogel was notably lower than that of single-drug loaded hydrogel treated cells, indicating great synergistic cellular proliferation inhibition.
Fig. 4.
In vitro cell viability and apoptosis of MKN45-luc cells after treatment with different anti-tumor strategies through a Transwell co-culture system. (A) Schematic illustration of Transwell co-culture system for creating a drug depot. (B) Cell viability of MKN45-luc cells after incubating with blank PLEL hydrogel, free 5-FU + DDP and dual-loaded drug PLEL hydrogel for 24 and 48 h. 5-FU + DDP at drug ratios of 10:1, 20:1 and 40:1. Data are presented as mean ± sd (n = 5). (C) Fluorescent morphology images of the MKN45-luc cells (cells were exposed to blank PLEL, free 5-Fu + DDP and drug-loaded PLEL hydrogel for 24 and 48 h) after live and dead cell staining. The drug ratio of 5-FU: DDP was 20:1 and the total concentration of drug was 45 μM. Scale bar: 200 μm. (D) Quantitative analysis of live/dead staining with various treatments. Data are presented as mean ± sd (n = 3). (E) Cells apoptosis study of MKN45-luc cells by flow cytometry analysis. The drug ratio of 5-FU: DDP was 20:1 and the total concentration of drug was 45 μM. (F) The percentages of apoptotic cells were calculated from the flow cytometry analysis. Data are presented as mean ± sd (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001.
Some studies reported that combining with DDP could improve the cell sensitivity to 5-FU and promoted apoptosis of cancer cells, resulting in synergistic chemotherapy [50,51]. Hence, flow cytometry analysis was conducted as well to explore the influence of 5-FU + DDP/PLEL hydrogel on MKN45-luc cells. As shown in Fig. 4E and F, the apoptosis rate induced by the dual-drug hydrogel was significantly higher than that of 5-FU/PLEL hydrogel and DDP/PLEL hydrogel under the same co-incubation time. This suggested that the synergy effect of 5-FU + DDP on the gastric cancer cell was relevant to its synergistic apoptosis promotion activity. At the same time, apoptosis induced by the drug loaded hydrogels increased with incubation time, indicating that prolonging the drug action time by hydrogel could furtherly promote the apoptosis of cells. To sum up, strong synergistic cellular proliferation inhibition and apoptosis promotion of 5-FU + DDP/PLEL hydrogel were observed against gastric cancer MKN45-luc cells in vitro.
3.4. Formulation optimization and anti-tumor effect assessment of 5-FU + DDP/PLEL hydrogel in vivo
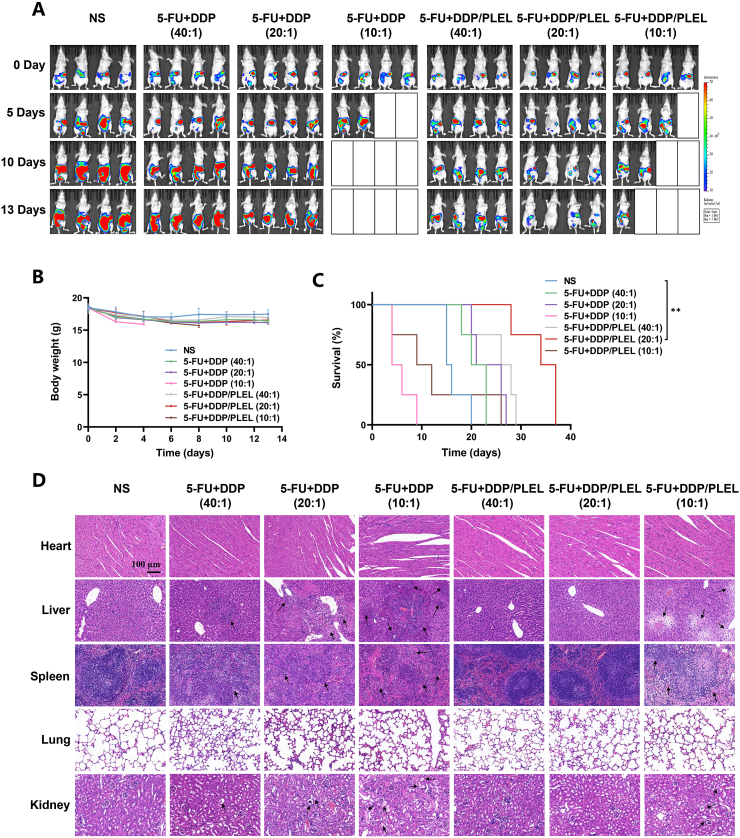
In spite of the cell experiments above had implied the synergistic chemotherapy effect of 5-FU + DDP/PLEL hydrogel increased with the proportion of DDP, the dose-related renal toxicity of DDP should be taken into full consideration at the same time [52]. Therefore, we investigated the in vivo anti-tumor effect and safety of 5-FU + DDP/PLEL hydrogel with different drug ratios on the PC model of gastric cancer, trying to optimize the combination formulation to maximize the synergy effect while minimizing the toxicity. After intraperitoneal administration, changes in tumor burden, body weight and survival status of the mice were studied (Fig. 5A -5C and Fig. S4). The blank boxes in Fig. 5A denoted the missing tumor-bearing mice, which were caused by therapy-related death. The tumor-bearing mice in NS group were intraperitoneally injected with normal saline which served as the control group. Mice in NS group showed the fasted increase in tumor burden among all the groups and started to die 15 day after treatment. All the tumor-bearing mice treated with 5-FU + DDP and 5-FU + DDP/PLEL (5-FU and DDP ratio = 10:1) suffered a quick loss in body weight and died gradually from day 4 after administration, especially the mice treated with free 5-FU + DDP. On the contrary, when the proportion of DDP decreased (5-FU and DDP ratio = 20:1 or 40:1), the weight loss and survival status of mice improved a lot. It should be pointed out that the weight loss and tumor growth of the mice treated with drug loaded hydrogels were less than those treated with the free drug combinations at the same drug ratio. In the free 5-FU and DDP combination groups, the tumor showed a slow to fast growth trend. On the contrary, the tumor signal faded obviously in the drug loaded hydrogel groups (20:1 and 40:1 drug ratios) compared to the control group, indicating great tumor growth inhibition. Especially the mice treated with 5-FU + DDP/PLEL hydrogel at a 20:1 drug ratio showed the weakest tumor fluorescence intensity and the longest lifetime. To identify the side effect caused by free 5-FU + DDP and 5-FU + DDP/PLEL hydrogel with different drug ratios, major organs of mice were harvested, gross examined, and accessed by histological analysis on the fourth day after administration. According to the histological results, mice treated with 5-FU and DDP combination at a 10:1 drug ratio with or without hydrogel both suffered obvious tissue damages, such as liver fat vacuoles, hepatocyte necrosis, unclear spleen cortex and medulla, renal stromal inflammatory cell infiltration, and glomerular atrophy, which has been denoted by black arrows. Meanwhile, a little tissue damage after therapy by free 5-FU + DDP (drug ratio was 20:1 and 40:1) could also be observed. Conversely, organs in the drug loaded hydrogels with low-dose DDP (5-FU and DDP ratio = 40:1, 20:1) were as normal as those in the control group (Fig. 5D). In summary, from the perspective of achieving maximized synergy chemotherapy effect and minimized side effects, 5-FU + DDP/PLEL hydrogel at the drug ratio of 20:1 seemed to hold the most worthy of application.
Fig. 5.
In vivo anti-tumor efficiency and safety of dual-drug loaded hydrogel formulations with different drug ratios. (A) Bioluminescence images of mice after being treated with various formulations. The total dosages of 5-FU and DDP were maintained at 20 mg/kg. The drug ratios of 5-FU: DDP were 40:1, 20:1 and 10:1, and the corresponding doses of DDP were 0.49 mg/kg, 0.95 mg/kg and 1.82 mg/kg, respectively. (B) Body weight curves after different treatments. (C) Survival curves of the mice after different treatments. Data are presented as mean ± sd (n = 4). (D) H&E staining of the main organs of mice after various treatments. The black arrows denote tissue damage. ∗∗p < 0.01.
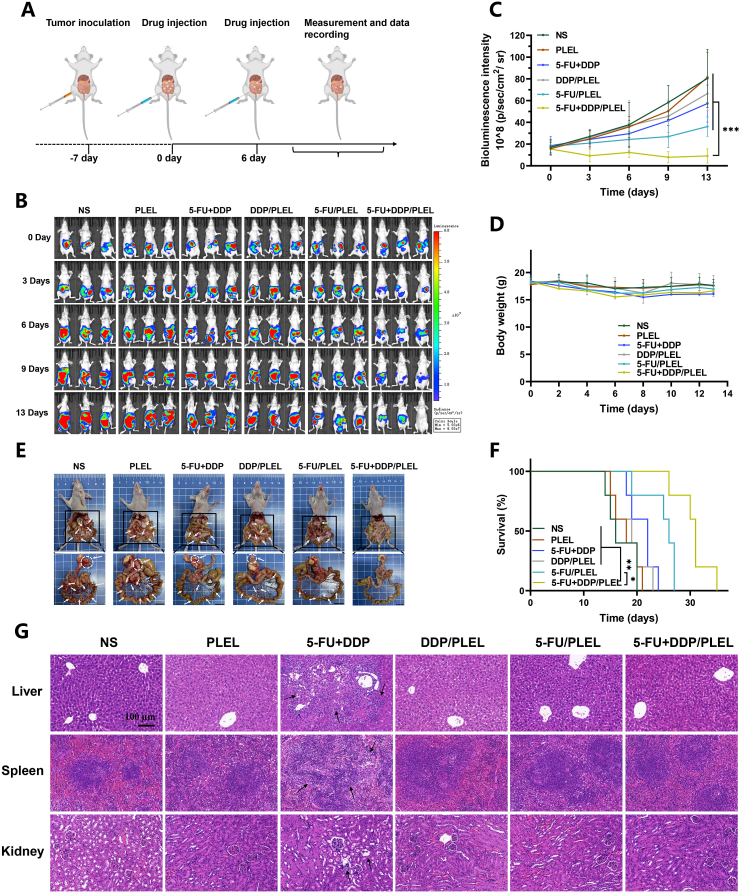
Next, considering the significant advantage of the optimized 5-FU + DDP/PLEL hydrogel (drug ratio 20:1) on the local therapy of gastric cancer, we further designed in vivo experiment as shown in Fig. 6A to explore the combined anti-tumor effect of 5-FU + DDP/PLEL hydrogel in detail. After the establishment of PC model, intraperitoneal administration of different formulations was conducted twice at the interval of 6 days. Bioluminescence signal of tumors in each group was monitored every 3 days up to 13 days, when mice in the NS control group began to die (Fig. 6B and C). Mice treated with NS and PLEL hydrogel showed rapid tumor growth over time. In the case of the free 5-FU and DDP combination group, the tumor showed a slow to rapid growth trend due to the rapid clearance of drugs from the tumor site. In comparison, intraperitoneal injection of the drug loaded hydrogels (DDP/PLEL, 5-FU/PLEL, and 5-FU + DDP/PLEL) persistently reduced the bioluminescence signal of tumors, resulting from the sustained drug release from the hydrogel. Particularly, mice treated with 5-FU + DDP/PLEL displayed the most tumor suppressor effect, which had significant statistical difference compared to other groups (p < 0.001). At the same time, mice treated with drug loaded hydrogels showed less body weight loss when compared to the ones treated with free drug combination, suggesting the positive effect of hydrogel on toxicity reduction as a drug carrier for the local combination chemotherapy (Fig. 6D). According to the autopsy results in Fig. 6E, the least tumor nodules were observed around gastric perigastric and peritoneum after therapy by 5-FU + DDP/PLEL hydrogel, which was consistent with the results of intravital fluorescence imaging before. It was worth noting that the 5-FU + DDP/PLEL hydrogel also prolonged the overall survival time of tumor-bearing mice (approximately 20% of mice were alive on day 31) (Fig. 6F). In addition, histological analyses were performed as well, as shown in Fig. 6G and Fig. S5. Almost all the major organs in the groups of hydrogel systems treated exhibited similar histological morphology to mice in the NS control group, suggesting low systemic toxicity. Conversely, mice treated with free 5-FU and DDP combination showed obvious hepatocyte necrosis, unclear boundaries between the spleen cortex and medulla, and glomerular atrophy. Therefore, 5-FU + DDP/PLEL hydrogel not only improved the antitumor effect of combination chemotherapy of gastric cancer, but also reduced the systemic toxicity effectively, indicating a promising choice for gastric cancer therapy.
Fig. 6.
In vivo anti-tumor efficiency of different treatments on peritoneal carcinomatosis (PC) model of gastric cancer. (A) Schematic diagram of the PC model establishment and therapy schedule. (B) Bioluminescence images of mice after therapy. (C) Quantified bioluminescence intensity of the mice treated with different formulations. Data are presented as mean ± sd (n = 5). (D) Body weight curves after different treatments. Data are presented as mean ± sd (n = 7). (E) Gross examination of peri-gastric and mesenteric tumor nodules on day 13 post-treatment. The white arrows denote tumors. Scale bar: 1 cm. (F) Survival curves of mice after various treatments. Data are presented as mean ± sd (n = 5). (G) H&E staining of the main organs of mice after various treatments on day 13. The black arrows denote abnormal tissue. ∗p < 0.05, ∗∗p < 0.01, ∗∗∗p < 0.001.
3.5. Efficiency evaluation of 5-FU + DDP/PLEL hydrogel to prevent the recurrence and metastasis after surgical resection
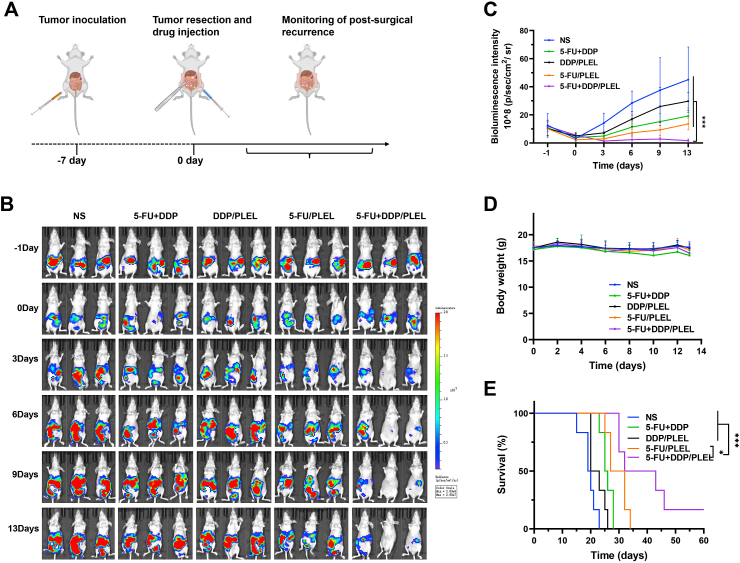
Finally, a mouse model for postsurgical residual tumors and peritoneal carcinomatosis of gastric cancer was established, in which the effects of 5-FU + DDP/PLEL hydrogel to prevent recurrence and metastasis after surgical resection were evaluated. As shown in Fig. 7A and Fig. S6, after the large tumors around the stomach were resected, therapeutic agents were administrated on the surgical site, adjacent tissue, and abdominal cavity, followed by real-time monitoring of the residual tumors in each group through IVIS Lumina III imaging system. Changes in tumor burden after treatment were shown in Fig. 7B and C. Bioluminescence signal of all groups after resection was weakened and remained basically consistent, meaning the uniformity of tumor removal, which could also be proved by the even-sized excised tumor in Fig. S6. Bioluminescent signal intensity of mice treated with normal saline (NS) increased rapidly over time, meaning fast relapse and growth of the residual tumors after surgery. Growth of residual tumors in the groups of free 5-FU + DDP, DDP/PLEL and 5-FU/PLEL were inhibited to varying degrees, but none of them were satisfactory. Fortunately, after being treated with 5-FU + DDP/PLEL hydrogel, mice exhibited rapidly decreased in bioluminescent signal intensity in the first three days, and then remained at a super low level, even tending to zero, suggesting notably effect to prevent recurrence and metastasis after surgical resection. In the meantime, the loss in body weight of mice treated with 5-FU + DDP/PLEL was slight than that of free 5-FU + DDP, meaning less systemic toxicity (Fig. 7D). What's exciting was that, the 5-FU + DDP/PLEL hydrogel formulation markedly extended the overall survival time of mice after surgery (about 20% of the mice survived more than 60 days), while all the mice in the other treatment groups died gradually within 35 days (Fig. 7E).
Fig. 7.
Efficiency evaluation of 5-FU + DDP/PLEL hydrogel on postoperative peritoneal carcinomatosis model of gastric cancer after intraoperative administration. (A) Schematic diagram of the establishment of postoperative peritoneal carcinomatosis gastric cancer model and schedule of intraoperative therapy. (B) Bioluminescence images of mice after various treatments. (C) Quantified bioluminescence intensity of the tumor-bearing mice. Data are presented as mean ± sd (n = 5). (D) Change in mice body weight post-administration. Data are presented as mean ± sd (n = 8). E) Survival curves of mice in each treatment group. Data are presented as mean ± sd (n = 5). ∗p < 0.05, ∗∗∗p < 0.001.
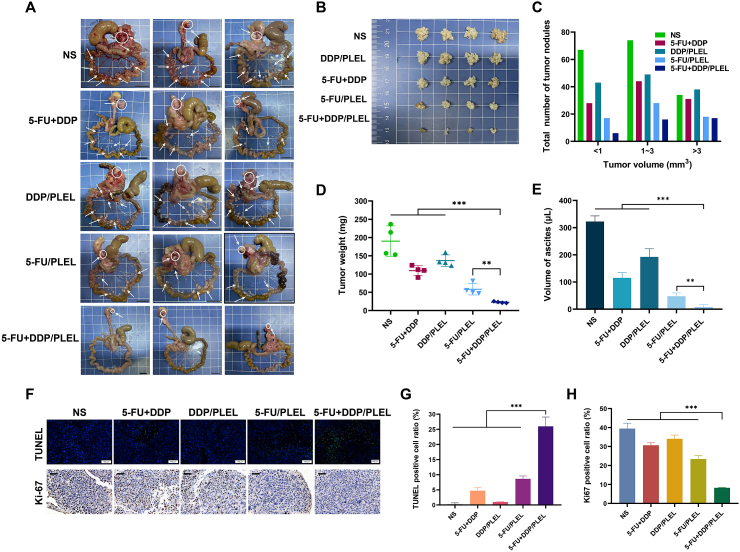
Furthermore, in order to detailedly explore the effect and potential mechanism of 5-FU + DDP/PLEL hydrogel for intraoperative adjuvant combination chemotherapy of gastric cancer, series of experiments such as dissection, gross examined, tumor statistics, and immunohistochemistry analysis were conducted as well. On day 13 after treatment, large peri-gastric tumor nodules (denoted by white circles) and many mesenteric tumor nodules (denoted by white arrows) were observed in the NS, free 5-FU + DDP, and DDP/PLEL treated mice, while the tumor burden of 5-FU/PLEL and 5-FU + DDP/PLEL treated mice were significantly decreased. In particular, mice in the 5-FU + DDP/PLEL treated group had the fewest mesenteric tumor nodules, and some even had disappeared (Fig. 8A). In more detail, tumors in each group were collected, counted, and weighted carefully, as shown in Fig. 8B–D. As expected, mice treated with 5-FU + DDP/hydrogel showed the least number and weight of tumors among all the treatment groups. Based on the final tumor weight calculation, the relative tumor inhibition rate of 5-FU + DDP/PLEL treatment was 88.04%, which was about 2.07 times of combined free 5-FU + DDP (42.55%) (Fig. S7). Meanwhile, the volume of ascites of each group was also detected and that in the 5-FU + DDP/PLEL treated group was still the least and almost tended to normal mice (Fig. 8E). These results demonstrated that intraoperative administration of 5-FU + DDP/PLEL could effectively prevent the relapse of orthotopic tumors, inhibit the growth and number of metastatic tumors, and reduce the volume of ascites as well, resulting great lifetime.
Fig. 8.
Effect and mechanism of intraoperative chemotherapy of gastric cancer by 5-FU + DDP/PLEL hydrogel on the postoperative peritoneal carcinomatosis model of mice. (A) Macroscopic feature of peri-gastric and mesenteric tumor nodules. Peri-gastric tumor nodules (denoted by white circles) and mesenteric tumor nodules (denoted by white arrows). Scale bar: 1 cm. (B) Photographs of tumors in each group. Data are presented as mean ± sd (n = 4). Scale bar: 1 cm. (C) Number of tumor nodules in different tumor volume ranges. (D) Weight of peri-gastric mesenteric tumor nodules on day 13 after different treatments. (E) Volume of ascites on day 13 after different treatments. Data are presented as mean ± sd (n = 4). (F) TUNEL assay and (Scale bar: 100 μm) Ki-67 staining (Scale bar: 50 μm). (G) Percentage of apoptotic cells calculated from the TUNEL results (H) Percentage of Ki67 positive cells after treatment. Data are presented as mean ± sd (n = 3). ∗∗p < 0.01, ∗∗∗p < 0.001.
Moreover, Ki-67 staining and TUNEL staining were used to study the histological feature and fate of cancer cells after intraoperative treatment with 5-FU + DDP/PLEL hydrogel. TUNEL expression showed that the apoptosis rate of tumor cells in the 5-FU + DDP/PLEL hydrogel-treated group was the most (26%), while that in other treatment groups was relatively lower (Fig. 8F and G). Simultaneously, Ki-67 positive cells in the 5-FU + DDP/PLEL hydrogel treatment group (positive rate was 8.21%) were significantly lower than those in the other groups, indicating that tumor cell proliferation was significantly reduced (Fig. 8F and H). Therefore, proliferation inhibition and apoptosis induction of tumor cells were likely to be one of the primary functions of 5-FU + DDP/PLEL hydrogel for intraoperative synergistic combination chemotherapy of gastric cancer.
4. Conclusion
In summary, we have successfully developed a biodegradable temperature-sensitive hydrogel (PDLLA-PEG-PDLLA, PLEL) system to co-delivery 5-fluorouracil (5-FU) and cis-platinum (DDP) for intraoperative adjuvant chemotherapy of gastric cancer. Such a 5-FU + DDP/PLEL hydrogel system exhibited sol-gel phase transition characteristics in response to physiological temperature and presented sustained drug release property. Synergistic cell proliferation inhibition and apoptosis promotion of 5-FU + DDP/PLEL were achieved against gastric cancer cells. Series in vivo studies demonstrated that using PLEL hydrogel as local drug delivery carrier was of great help to the prolonging of local drug retention, improving of anti-tumor effects, and reducing of adverse side effects. Intraoperative administration of 5-FU + DDP/PLEL after the surgical resection of gastric tumor also effectively inhibited the local recurrence of orthotopic tumors and the growth of abdominal metastatic tumors, contributing to a significant extent of the overall survival. Taken together, our developed dual-drug loaded hydrogel system was confirmed to be a logical and effective means for postoperative management of gastric cancer, suggesting a potential choice for the therapy of gastric cancer and other gastrointestinal tumors.
Ethics approval
All animal experiments were approved by the Ethics Committee of the Animal Experimental Center of State Key Laboratory of Biotherapy of Sichuan University (Checking number: 20210409028), and were carried out in compliance with all relevant ethical regulations.
CRediT authorship contribution statement
Wen Chen: Conceptualization, Methodology, Investigation, Data curation, Software, Validation, Formal analysis, Writing - original draft. Kun Shi: Conceptualization, Methodology, Investigation, Validation, Formal analysis, Writing - original draft, Funding acquisition. Jie Liu: Investigation. Peipei Yang: Investigation. Ruxia Han: Investigation. Meng Pan: Investigation. Liping Yuan: Investigation. Chao Fang: Investigation. Yongyang Yu: Investigation. Zhiyong Qian: Conceptualization, Writing - review & editing, Supervision, Funding acquisition.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We gratefully acknowledge financial support from the National Natural Science Foundation of China (U21A20417, 31930067, and 31800797), the Sichuan Science and Technology Program (2022YFS0333, 2022YFS0203), 1⋅3⋅5 project for disciplines of excellence, West China Hospital, Sichuan University (ZYGD18002), and the Post-Doctor Research Project, West China Hospital, Sichuan University (2018HXBH066).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bioactmat.2022.10.004.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global cancer statistics 2020: globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries, CA. Cancer J. Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Hundahl S.A. Staging, stage migration, and patterns of spread in gastric cancer. Semin. Radiat. Oncol. 2002;12(2):141–149. doi: 10.1053/srao.2002.30816. [DOI] [PubMed] [Google Scholar]
- 3.Nccn clinical practice guidelines in oncology. https://www.nccn.org/ Online. 2022. Available from URL:
- 4.Smith P., Lavery A., Turkington R.C. An overview of acute gastrointestinal side effects of systemic anti-cancer therapy and their management. Best Pract. Res. Clin. Gastroenterol. 2020;48–49 doi: 10.1016/j.bpg.2020.101691. [DOI] [PubMed] [Google Scholar]
- 5.Dilalla V., Chaput G., Williams T., Sultanem K. Radiotherapy side effects: integrating a survivorship clinical lens to better serve patients. Curr. Oncol. 2020;27(2) doi: 10.3747/co.27.6233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macrì A., Morabito F. The use of intraperitoneal chemotherapy for gastric malignancies. Expert Rev. Anticancer Ther. 2019;19(10):879–888. doi: 10.1080/14737140.2019.1671189. [DOI] [PubMed] [Google Scholar]
- 7.Kodera Y., Takahashi N., Yoshikawa T., Takiguchi N., Fujitani K., Ito Y., Miyamoto K., Takayama O., Imano M., Kobayashi D., Miyashita Y., Morita S., Sakamoto J. Feasibility of weekly intraperitoneal versus intravenous paclitaxel therapy delivered from the day of radical surgery for gastric cancer: a preliminary safety analysis of the inpact study, a randomized controlled trial. Gastric Cancer. 2017;20(1):190–199. doi: 10.1007/s10120-016-0598-0. [DOI] [PubMed] [Google Scholar]
- 8.Bonnot P.-E., Piessen G., Kepenekian V., Decullier E., Pocard M., Meunier B., Bereder J.-M., Abboud K., Marchal F., Quenet F., Goere D., Msika S., Arvieux C., Pirro N., Wernert R., Rat P., Gagnière J., Lefevre J.H., Courvoisier T., Kianmanesh R., Vaudoyer D., Rivoire M., Meeus P., Passot G., Glehen O. Cytoreductive surgery with or without hyperthermic intraperitoneal chemotherapy for gastric cancer with peritoneal metastases (cyto-chip study): a propensity score analysis. J. Clin. Oncol. 2019;37(23):2028–2040. doi: 10.1200/JCO.18.01688. [DOI] [PubMed] [Google Scholar]
- 9.Yarema R., Mielko J., Fetsych T., Ohorchak M., Skorzewska M., Rawicz-Pruszyński K., Mashukov A., Maksimovsky V., Jastrzębski T., Polkowski W., Gyrya P., Kovalchuk Y., Safiyan V., Karelin I., Kopetskiy V., Kolesnik O., Kondratskiy Y., Paskonis M. Hyperthermic intraperitoneal chemotherapy (hipec) in combined treatment of locally advanced and intraperitonealy disseminated gastric cancer: a retrospective cooperative central-eastern european study. Cancer Med. 2019;8(6):2877–2885. doi: 10.1002/cam4.2204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dedrick R.L., Flessner M.F. Pharmacokinetic problems in peritoneal drug administration: tissue penetration and surface exposure. J. Natl. Cancer Inst. 1997;89(7):480–487. doi: 10.1093/jnci/89.7.480. [DOI] [PubMed] [Google Scholar]
- 11.Tsuyoshi H., Inoue D., Kurokawa T., Yoshida Y. Hyperthermic intraperitoneal chemotherapy (hipec) for gynecological cancer. J. Obstet. Gynaecol. Res. 2020;46(9):1661–1671. doi: 10.1111/jog.14391. [DOI] [PubMed] [Google Scholar]
- 12.Xu Y., Zhang R.P., Li C.F., Sun Z., Deng J.Y., Wang X.N., Ding X.W., Wang B.G., Xue Q., Ke B., Zhan H.J., Liu N., Liu Y., Wang X.J., Liang H., Xue Y.W., Xu H.M. Intraperitoneal chemotherapy using fluorouracil implants combined with radical resection and postoperative adjuvant chemotherapy for stage iii gastric cancer: a multi-center, randomized, open-label, controlled clinical study. Front. Oncol. 2021;11 doi: 10.3389/fonc.2021.670651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ge J., Liu T., Lei T.X., Li X., Song K., Azizi S., Liu H., Tang M. Retrospective cohort study of intraoperative administration of sustained-release 5-fluorouracil implants in advanced gastric cancer patients. Front. Pharmacol. 2021;12 doi: 10.3389/fphar.2021.659258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yuan H., Zheng B.a., Tu S.L. Clinical research of intraperitoneal implantation of sustained-release 5-fluorouracil in advanced colorectal cancer. World J. Surg. Oncol. 2015;13(1):320. doi: 10.1186/s12957-015-0737-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang D.H., Chu Y.H., Qian H.Q., Qian L.Y., Shao J., Xu Q.P., Yu L.X., Li R.T., Zhang Q.A., Wu F.L., Liu B.R., Liu Q. Antitumor activity of thermosensitive hydrogels packaging gambogic acid nanoparticles and tumor-penetrating peptide irgd against gastric cancer. Int. J. Nanomed. 2020;15:735–747. doi: 10.2147/IJN.S231448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li X.L., Yu N., Li J., Bai J.A., Ding D., Tang Q.Y., Xu H. Novel “carrier-free” nanofiber codelivery systems with the synergistic antitumor effect of paclitaxel and tetrandrine through the enhancement of mitochondrial apoptosis. ACS Appl. Mater. Interfaces. 2020;12(9):10096–10106. doi: 10.1021/acsami.9b17363. [DOI] [PubMed] [Google Scholar]
- 17.Liu Q., Zhang D.H., Qian H.Q., Chu Y.H., Yang Y., Shao J., Xu Q.P., Liu B.R. Superior antitumor efficacy of ifn-α2b-incorporated photo-cross-linked hydrogels combined with t cell transfer and low-dose irradiation against gastric cancer. Int. J. Nanomed. 2020;15:3669–3680. doi: 10.2147/IJN.S249174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang X.W., Chen X.B., Wang Y.B., Xu G.H., Yu L., Ding J.D. Sustained release of lipophilic gemcitabine from an injectable polymeric hydrogel for synergistically enhancing tumor chemoradiotherapy. Chem. Eng. J. 2020;396 [Google Scholar]
- 19.Bae W.K., Park M.S., Lee J.H., Hwang J.E., Shim H.J., Cho S.H., Kim D.-E., Ko H.M., Cho C.-S., Park I.-K., Chung I.-J. Docetaxel-loaded thermoresponsive conjugated linoleic acid-incorporated poloxamer hydrogel for the suppression of peritoneal metastasis of gastric cancer. Biomaterials. 2013;34(4):1433–1441. doi: 10.1016/j.biomaterials.2012.10.077. [DOI] [PubMed] [Google Scholar]
- 20.Norouzi M., Nazari B., Miller D.W. Injectable hydrogel-based drug delivery systems for local cancer therapy. Drug Discov. Today. 2016;21(11):1835–1849. doi: 10.1016/j.drudis.2016.07.006. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y.B., Yang X.W., Chen X.B., Wang X., Wang Y., Wang H.C., Chen Z.Y., Cao D.L.G., Yu L., Ding J.D. Sustained release of nitric oxide and cascade generation of reactive nitrogen/oxygen species via an injectable hydrogel for tumor synergistic therapy. Adv. Funct. Mater. 2022;32(36) [Google Scholar]
- 22.Wu X.H., Wang X., Chen X.B., Yang X.W., Ma Q., Xu G.H., Yu L., Ding J.D. Injectable and thermosensitive hydrogels mediating a universal macromolecular contrast agent with radiopacity for noninvasive imaging of deep tissues. Bioact. Mater. 2021;6(12):4717–4728. doi: 10.1016/j.bioactmat.2021.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Darge H.F., Andrgie A.T., Tsai H.-C., Lai J.-Y. Polysaccharide and polypeptide based injectable thermo-sensitive hydrogels for local biomedical applications. Int. J. Biol. Macromol. 2019;133:545–563. doi: 10.1016/j.ijbiomac.2019.04.131. [DOI] [PubMed] [Google Scholar]
- 24.Wu J., Qu Y., Shi K., Chu B.Y., Jia Y.P., Xiao X., He Q.J., Qian Z.Y. Camptothecin@hmsns/thermosensitive hydrogel composite for applications in preventing local breast cancer recurrence. Chin. Chem. Lett. 2018;29(12):1819–1823. [Google Scholar]
- 25.Chen X.B., Wang M.L., Yang X.W., Wang Y.B., Yu L., Sun J., Ding J.D. Injectable hydrogels for the sustained delivery of a her2-targeted antibody for preventing local relapse of her2+ breast cancer after breast-conserving surgery. Theranostics. 2019;9(21):6080–6098. doi: 10.7150/thno.36514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shi J.Y., Yu L., Ding J.D. Peg-based thermosensitive and biodegradable hydrogels. Acta Biomater. 2021;128:42–59. doi: 10.1016/j.actbio.2021.04.009. [DOI] [PubMed] [Google Scholar]
- 27.Han T.S., Hur K., Choi B., Lee J.Y., Byeon S.J., Min J., Yu J., Cho J.K., Hong J., Lee H.J., Kong S.H., Kim W.H., Yanagihara K., Song S.C., Yang H.K. Improvement of anti-cancer drug efficacy via thermosensitive hydrogel in peritoneal carcinomatosis in gastric cancer. Oncotarget. 2017;8(65):108848–108858. doi: 10.18632/oncotarget.22312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Qian H.Q., Qian K.Y., Cai J., Yang Y., Zhu L.J., Liu B.R. Therapy for gastric cancer with peritoneal metastasis using injectable albumin hydrogel hybridized with paclitaxel-loaded red blood cell membrane nanoparticles. ACS Biomater. Sci. Eng. 2019;5(2):1100–1112. doi: 10.1021/acsbiomaterials.8b01557. [DOI] [PubMed] [Google Scholar]
- 29.Digklia A., Wagner A.D. Advanced gastric cancer: current treatment landscape and future perspectives. World J. Gastroenterol. 2016;22(8):2403–2414. doi: 10.3748/wjg.v22.i8.2403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Iwasa S., Nakajima T.E., Nakamura K., Takashima A., Kato K., Hamaguchi T., Yamada Y., Shimada Y. First-line fluorouracil-based chemotherapy for patients with severe peritoneal disseminated gastric cancer. Gastric Cancer. 2012;15(1):21–26. doi: 10.1007/s10120-011-0056-y. [DOI] [PubMed] [Google Scholar]
- 31.Daher G.C., Harris B.E., Diasio R.B. Metabolism of pyrimidine analogues and their nucleosides. Pharmacol. Ther. 1990;48(2):189–222. doi: 10.1016/0163-7258(90)90080-l. [DOI] [PubMed] [Google Scholar]
- 32.Sethy C., Kundu C.N. 5-fluorouracil (5-fu) resistance and the new strategy to enhance the sensitivity against cancer: implication of DNA repair inhibition. Biomed. Pharmacother. 2021;137 doi: 10.1016/j.biopha.2021.111285. [DOI] [PubMed] [Google Scholar]
- 33.Kim R., Tanabe K., Inoue H., Toge T. Mechanism(s) of antitumor action in protracted infusion of low dose 5-fluorouracil and cisplatin in gastric carcinoma. Int. J. Oncol. 2002;20(3):549–555. [PubMed] [Google Scholar]
- 34.Wagner A., Grothe W.W.G., Behl S., Kleber G.G.K., Grothey A.A.G., Haerting J., Fleig W.E. Chemotherapy for advanced gastric cancer. Cochrane Database Syst. Rev. 2005;(2) doi: 10.1002/14651858.CD004064.pub2. [DOI] [PubMed] [Google Scholar]
- 35.Kanetaka K., Enjoji A., Furui J., Nagata Y., Fujioka H., Shiogama T., Miyata A., Kishikawa H., Matsuo S., Iwata T., Kanematsu T., Eguchi S. Effects of intermittent 5-fluorouracil and low-dose cisplatin therapy on advanced and recurrent gastric cancer. Anticancer Res. 2012;32(8):3495. [PubMed] [Google Scholar]
- 36.Peng J.J., Dong C., Wang C., Li W.H., Yu H., Zhang M., Zhao Q., Zhu B., Zhang J., Li W.L., Wang F.H., Wu Q., Zhou W.H., Yuan Y., Qiu M., Chen G. Cardiotoxicity of 5-fluorouracil and capecitabine in Chinese patients: a prospective study. Cancer Commun. 2018;38(1):22. doi: 10.1186/s40880-018-0292-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang L.L., Xing X.L., Meng F.L., Wang Y., Zhong D.S. Oral fluoropyrimidine versus intravenous 5-fluorouracil for the treatment of advanced gastric and colorectal cancer: meta-analysis. J. Gastroenterol. Hepatol. 2018;33(1):209–225. doi: 10.1111/jgh.13845. [DOI] [PubMed] [Google Scholar]
- 38.Shi K., Wang Y.L., Qu Y., Liao J.F., Chu B.Y., Zhang H.P., Luo F., Qian Z.Y. Synthesis, characterization and application of reversible pdlla-peg-pdlla copolymer thermogels in vitro and in vivo. Sci. Rep. 2016;6(1) doi: 10.1038/srep19077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi K., Xue B.X., Jia Y.P., Yuan L.P., Han R.X., Yang F., Peng J.R., Qian Z.Y. Sustained co-delivery of gemcitabine and cis-platinum via biodegradable thermo-sensitive hydrogel for synergistic combination therapy of pancreatic cancer. Nano Res. 2019;12(6):1389–1399. [Google Scholar]
- 40.Jia Y.P., Shi K., Yang F., Liao J.F., Han R.X., Yuan L.P., Hao Y., Pan M., Xiao Y., Qian Z.Y., Wei X.W. Multifunctional nanoparticle loaded injectable thermoresponsive hydrogel as nir controlled release platform for local photothermal immunotherapy to prevent breast cancer postoperative recurrence and metastases. Adv. Funct. Mater. 2020;30(25) [Google Scholar]
- 41.Kaushik S., Shyam H., Sharma R., Balapure A.K. Dietary isoflavone daidzein synergizes centchroman action via induction of apoptosis and inhibition of pi3k/akt pathway in mcf-7/mda mb-231 human breast cancer cells. Phytomedicine. 2018;40:116–124. doi: 10.1016/j.phymed.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 42.Zhang J., Miao L., Guo S.T., Zhang Y., Zhang L., Satterlee A., Kim W.Y., Huang L. Synergistic anti-tumor effects of combined gemcitabine and cisplatin nanoparticles in a stroma-rich bladder carcinoma model. J. Contr. Release. 2014;182:90–96. doi: 10.1016/j.jconrel.2014.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang Y., Wu X.H., Han Y.R., Mo F., Duan Y.R., Li S.M. Novel thymopentin release systems prepared from bioresorbable pla–peg–pla hydrogels. Int. J. Pharm. 2010;386(1):15–22. doi: 10.1016/j.ijpharm.2009.10.045. [DOI] [PubMed] [Google Scholar]
- 44.Cho H., Imada T., Oshima T., Shiozawa M., Rino Y., Takanashi Y. In-vitro effect of a combination of 5-fluorouracil (5-fu) and cisplatin (cddp) on human gastric cancer cell lines: timing of cisplatin treatment. Gastric Cancer. 2002;5(1):43–46. doi: 10.1007/s101200200006. [DOI] [PubMed] [Google Scholar]
- 45.Kondo K., Murase M., Kodera Y., Akiyama S., lto K., Yokoyama Y., Takagi H., Shirasaka T. Feasibility study on protracted infusional 5-fluorouracil and consecutive low-dose cisplatin for advanced gastric cancer. Oncology. 1996;53(1):64–67. doi: 10.1159/000227537. [DOI] [PubMed] [Google Scholar]
- 46.Miao L., Guo S.T., Zhang J., Kim W.Y., Huang L. Nanoparticles with precise ratiometric co-loading and co-delivery of gemcitabine monophosphate and cisplatin for treatment of bladder cancer. Adv. Funct. Mater. 2014;24(42):6601–6611. doi: 10.1002/adfm.201401076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Park S.R., Choi I.J., Kim C.G., Kim Y.-W., Ryu K.W., Lee J.H., Lee J.S., Bae J.-M., Kim H.K. Use of a combination of computed tomography and endoscopy to assess the response to 5-fluorouracil/cisplatin and predict survival in gastric cancer. J. Gastroenterol. 2006;41(4):339–346. doi: 10.1007/s00535-005-1759-9. [DOI] [PubMed] [Google Scholar]
- 48.Bouché O., Ychou M., Burtin P., Bedenne L., Ducreux M., Lebreton G., Baulieux J., Nordlinger B., Martin C., Seitz J.F., Tigaud J.M., Echinard E., Stremsdoerfer N., Milan C., Rougier P. Adjuvant chemotherapy with 5-fluorouracil and cisplatin compared with surgery alone for gastric cancer: 7-year results of the ffcd randomized phase iii trial (8801) Ann. Oncol. 2005;16(9):1488–1497. doi: 10.1093/annonc/mdi270. [DOI] [PubMed] [Google Scholar]
- 49.Al-Batran S.-E., Hartmann J.T., Probst S., Schmalenberg H., Hollerbach S., Hofheinz R., Rethwisch V., Seipelt G., Homann N., Wilhelm G., Schuch G., Stoehlmacher J., Derigs H.G., Hegewisch-Becker S., Grossmann J., Pauligk C., Atmaca A., Bokemeyer C., Knuth A., Jäger E. Phase iii trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the arbeitsgemeinschaft internistische onkologie. J. Clin. Oncol. 2008;26(9):1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 50.Mori S., Kunieda K., Sugiyama Y., Saji S. Prediction of 5-fluorouracil and cisplatin synergism for advanced gastrointestinal cancers using a collagen gel droplet embedded culture. Surg. Today. 2003;33(8):577–583. doi: 10.1007/s00595-003-2569-4. [DOI] [PubMed] [Google Scholar]
- 51.Yim E.K., Lee S.B., Lee K.H., Kim C.J., Park J.S. Analysis of the in vitro synergistic effect of 5-fluorouracil and cisplatin on cervical carcinoma cells. Int. J. Gynecol. Cancer. 2006;16(3):1321–1329. doi: 10.1111/j.1525-1438.2006.00551.x. [DOI] [PubMed] [Google Scholar]
- 52.Pabla N., Dong Z. Cisplatin nephrotoxicity: mechanisms and renoprotective strategies. Kidney Int. 2008;73(9):994–1007. doi: 10.1038/sj.ki.5002786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.