Abstract
Ganoderma lucidum is known as lingzhi mushroom, which is said to have medicinal properties by the local residents. This research was focused to assess the antidepressant, anxiolytic, and sedative activities of the mentioned mushroom extracts by means of in vivo and in silico approaches. The antidepressant, anxiolytic, and sedative properties of the methanol extracts of G. lucidum (MEGL) were assessed using the forced swim test hole board, open field test, elevated plus maze, hole cross test, and thiopental sodium-induced sleeping time. The extracts revealed significant antidepressant, anxiolytic, and sedative activities in a dose-dependent manner. Rutin and quercetin were found to be the most effective enzyme inhibitors in the molecular docking study. According to the findings of in vivo and molecular docking study, it could be forecast that, the extract could have substantial antidepressant, anxiolytic, and sedative characteristics and deep molecular strategies on this extracts might create a target for the development of novel therapeutics. Further investigations are needed to appraise the molecular mechanisms implicated and isolate the bioactive components.
Keywords: Mushroom, Antidepressant, Anxiolytic, Sedative, Neuron Receptors, Molecular interaction
Graphical abstract
Highlights
-
•
Rutin and quercetin are reported in Ganoderma lucidum mushroom.
-
•
The mushroom extracts possessed dose-dependent impacts on neural diseases.
-
•
Elements of the Ganoderma lucidum yielded prominent binding affinity to the neural receptors.
-
•
The mushroom extract is non-toxic evident from an acute toxicity study.
1. Introduction
The prevalence of mental diseases is increasing, and the number of people afflicted is rising as well. It is a severe abnormality in an individual's thoughts, emotions, or behavior” is what we mean when we talk about mental illness, and it may pose problems in our daily lives, our employment and our families [1]. Throughout the globe, depression and anxiety are two of the most frequent mental diseases, and they have become a major problem in our society [2]. When it comes to psychiatry, depression has been compared to the “common cold”. It's not uncommon, but it's also present in milder versions, so the similarity extends a little. As a result, in its most extreme manifestations, it may be a huge concern for any patient who is very distressed. Although a severe depressive episode may occur in conjunction with practically any other mental or physiological diagnosis [3]. Symptoms of this disorder may have a profound impact on how a person thinks and acts in their everyday lives, including their ability to eat, sleep, and work. In addition, depression is the second most common chronic disorder in clinical practice, and by 2020, it was assumed to be the world's second most serious public health and disability concern [4,5]. In addition, anxiety disorders are the most common mental illnesses (7.3%) in the USA. A significant degree of comorbidity between anxiety (particularly generalized anxiety disorders or panic disorders) and depressive illnesses makes treatments more challenging [6]. Due to its capacity to block norepinephrine and dopamine reuptake, bupropion is an effective antidepressant with fewer adverse effects than other antidepressants [28]. In order to prevent the upregulation of monoamine metabolism and limit reuptake capacity, typical therapeutic drugs include MAOIs and tricyclic antidepressants (TCAs). However, many of the recognized therapy choices for depression are linked with negative side effects. Consequently, new antidepressant drugs are crucial to prevent these side effects [7]. Ever more medicinal products are being developed from natural resources in recent years. It is possible to discover novel medicinal compounds using mushrooms and their secondary metabolites as a strong source [[7], [8], [9]] because, mushrooms have been a part of people's diets worldwide for decades because of its nutritional values and organoleptic properties [10]. In nature, there are over 150,000 distinct varieties of mushrooms in the environment; only 10% of them are identified and labeled [11]. Notwithstanding, only approximately 2000 species are produced and farmed for nutritive reasons, which is a small percentage of the total. The demand of mushrooms has risen significantly during the last several decades [12]. Mushrooms are delicious foods that are high in proteins, vitamin B, minerals, and most of the necessary amino acids [13].
Ganoderma lucidum, an oriental fungus, has been used for centuries in Japan, China, and other regions in Asia for increasing health and longevity. It's a dark and big mushroom with a woody texture and a glossy exterior. The term lucidus comes from the Latin word lucidus, which means “shiny” or “brilliant,” and states to the glossy appearance of the mushroom's surface. G. lucidum is known in China as lingzhi, whereas the Ganodermataceae family is recognized as reishi or mannentake in Japan. G. lucidum is uncommon among farmed mushrooms in that its medicinal rather than nutritional benefit is essential. G. lucidum powders, tea, and food supplements are among the commercially available G. lucidum items. These are made from a variety of mushroom elements, such as spores, mycelia, and the fruit body. Regulating blood glucose levels, modulating the immune system, hepatoprotection, and bacteriostasis are just a few of the applications and health benefits claimed to lingzhi. Anecdotal evidence, traditional use, and cultural mores are used to support diverse opinions about G. lucidum's health benefits. On the other hand, recent studies back up some of the traditional claims about lingzhi's health benefits. In creating effective therapeutic interventions, the goal is to achieve a compromise of benefit and toxicity [14].
However, no comprehensive analyses of the extracts of this mushroom's antidepressant, anxiolytic, and sedative activities have been documented till the date. So that, we aimed to explore the biochemical and pharmacological properties of this mushroom.
2. Materials and methods
2.1. Chemicals
Square Pharmaceuticals Limited, Dhaka, Bangladesh provided paroxetine, diazepam, and alprazolam. Additional analytical-grade chemicals were obtained from the Department of Pharmacy, Faculty of Biological Science, University of Chittagong, Bangladesh.
2.2. Collection of mushroom and processing
Mushrooms (G. lucidum) were obtained from various parts of the university campus where they were naturally growing. After that, cleansing, water washing, and drying (room dry, woven dry). For efficient extraction, dried mushrooms were ground. G. lucidum. The specimens of mushroom were identified and archived by Dr. Akhter Jahan Kakon, mushroom expert of the Mushroom Research Center in Savar, Dhaka (Accession number: 2019/04/Fungi/CU/DP). The mushroom has been dried and finely powdered. The powder was combined with methanol and distilled water in a 1:4 ratio for seven days at a glass container. After that, filtering paper (Whatman size #1) was used to filter it. The solvent was evaporated using a rotary evaporator to produce methanol. Finally, a crude extract that was blackish in color was obtained.
2.3. Experimental animals
The mice utilized in the experiment were Swiss albino mice of both sex, aged 4–5 weeks and weighing 20–25 g, procured from the BCSIR laboratory in Chittagong. They were kept in the Animal House of the University of Chittagong's Pharmacy Department in clean and only dry polypropylene cages with a 12-h light-dark cycle at 25 ± 2 °C and 45–55% RH. The mice were given a typical laboratory diet and free access to water. Tweleve hours before and throughout the trial, food was withheld. Under the consent number Pharm/P&D/CUDP-16, 2021:08, the Departmental ethical review committee of the Department of Pharmacy at the University of Chittagong, Chittagong 4331 Bangladesh authorized the clinical experiment on animals.
2.4. Experimental design
Twenty-four (24) mice were utilized in each experiment, divided into 4 groups (control, standard, and MEGL) (n = 5). The MEGL group received 200 and 400 mg/kg based on their body weight (BW), while the control group received 1% Tween-80 solution (10 mL/kg, BW). For the hole-board and elevated plus maze, diazepam (1 mg/kg, BW, IP) was used as the standard drug, whereas alprazolam (1 mg/kg, BW, IP) was used for the hole cross and open field, as well as thiopental sodium-induced sleeping period. The paroxetine (10 mg/kg, BW) was given orally for the forced swim test.
2.5. In vivo studies
2.5.1. Acute oral toxicity test
The OECD Regulation 425 was taken into consideration for evaluating the oral route's acute toxicity [15]. Each group consisted of five mice. Male and female mice were used to assess the effects of oral administration of MEGL (1000 mg/kg, 2000 mg/kg, and 3000 mg/kg). Mice in the placebo group received just vehicle treatment (water). For 48 h, each group has been monitored. The mouse's weight, behavioral alterations or indicators of discomfort were monitored and noted [16]. Finally, no physical and psychological appearance yielded in the observation period.
2.5.2. Antidepressant profiling
2.5.2.1. Forced swimming test (FST)
The FST approach was examined for CNS depressant action [17]. Each group was treated according to Section 2.4. The mice were separately inserted and placed on the glass apparatus, which comprised of 25 × 15 × 25 cm3 and was filled with water (15 cm, 25 ± 2°C), 60 min after the treatment. Each mouse was monitored for 6 min, with the first 2 min used for setup and the final 4 min designated as immobility time.
2.5.3. Anxiolytic profiling
2.5.3.1. Hole board test (HBT)
MEGL's anxiolytic activity was determined using the hole-board instrument with minor modification [17,18]. The hole board equipment consists of a wooden chamber (40 × 40 × 25 cm3) with 16 holes evenly spaced on the floor (each measuring 3 cm in diameter). The mice could peep through the holes since the equipment was raised to a height of 25 cm from the ground. Each group was treated according to Section 2.4. During the 5-min observation period 30 min after oral administration, the number and duration of head poking were recorded.
2.5.3.2. Elevated plus maze (EPM) test
At the height of 25 cm, the EPM apparatus consisted of two open and two closed arms connected to a central square in the shape of a plus sign. Each group was treated according to Section 2.4. Each animal was put in the center of the apparatus, facing the closed arms, 30 min after treatment, and the period of movement in both the open and closed arms was monitored for 5 min [18,19].
2.5.4. Sedative activity
2.5.4.1. Hole cross test (HCT)
The hole-cross test for CNS activity was assessed with a small modification [20]. The hole-cross device is built of wood with dimensions of 30 × 20 × 14 cm3 and a height of 7.5 cm. There was a hole in the middle of the box (3 cm). Each group was treated according to Section 2.4. The mice were immediately placed on the board (0, 30, and 60 min interval) after each group treatment and examined for 3 min, with the number of holes crossed being recorded.
2.5.4.2. Open field test (OFT)
This activity of extract of mushrooms was evaluated by means of the protocol with minor modification [18]. The four-sided box has a volume of 60 × 60 × 60 cm3 and 25 equal squares (5 × 5 cm2) (highlighted in black and white). Each group was treated according to Section 2.4. Following each group's treatment, the mouse from each group was placed on the board and observed for 3 min, during which time the number of squares moved was counted. At 30 and 60 min intervals, the square movement was also recorded.
2.5.4.3. Thiopental sodium induced sleeping test
This activity was assessed by Ref. [20] with a small modification and Each group was treated according to Section 2.4. After 30 min, each mouse was administered thiopental sodium (40 mg/kg) to induce sleep. The animals were maintained under observation for the latent period (the time between Thiopental doses and the loss of righting reflex) and sleep duration (the time between the loss and return of righting reflex).
2.6. Molecular docking analysis
2.6.1. Ligand retrieval and preparation
Through a literature review, a total of 17 biological components discovered from the methanol extract of G. lucidum were chosen for molecular docking analysis [21,22]. Among them, 16 compounds were obtained from the PubChem compound repository in.sdf format, namely Thiophene, 2-hexyl; 3-((3-Acetoxythyl)-6-acetoxymethyl-2,4- dimethyl l) pheny l) - 2-methyl-(E)-2-propenyl acetate; 2,7-Diphenylindole; Gallic acid; Theanine; Caffeic acid; Caffeine; Ferulic acid; Theacrine; Catechin; Quercetin; Epigallocatechin; Catechin gallate; Epicatechin gallate; Quercetin hexoside, and Rutin. The remaining compound 5-(2-Bromophenyl)-7-chloro-2, 3-dihydro-1H-1, 4- benzodiazepin-2-one was drawn using ChemDraw version 16.0 (PerkinElmer ChemDraw Professional) and saved in the.sdf format. The structures were neutralized using Epik 2.2 at pH 7.0 2.0, and the force field OPLS 2005 contained in Maestro, v10.1 (Schrödinger suite) was used to minimize them. Per ligand, up to 32 stereoisomers were retained.
2.6.2. Protein preparation
The RCSB PDB (protein data bank) was utilized to obtain three-dimensional crystallographic structures of the proteins used in this investigation [23]. The selected target proteins for the investigation of antidepressant, anxiolytic and sedative activities were human serotonin transporter (PDB ID: 5I6X) [24], potassium channel receptor (PDB ID: 4UUJ) [25], and bromodomain of human BRD4 (PDB ID: 3U5J) [26] respectively. The Protein Preparation Wizard of the Maestro v10.1 (Schrödinger suite) was employed for the preparation and refinement of the crystallographic structures. Charges and bond orders were assigned, selenomethionines were converted to methionines, hydrogens were added to the heavy atoms, and all waters were deleted.
2.6.3. Grid generation and molecular docking
Glide (Schrödinger Suite-Maestro v10.1) was used to generate receptor grids and conduct molecular docking experiments [27,28]. Grids were generated for each protein using the OPLS_2005 force field and the default parameters of van der Waals scaling factor 1.00 and charge cutoff 0.25. A cubic box with specified dimensions centered on the centroid of the active site residues was generated for the receptor, and the box size was fixed to 14 Å × 14 Å × 14 Å for docking study. Docking experiments were carried out by the Standard Precision (SP) scoring function of Glide, and only the best-docked pose with the lowest Glide score was recorded for each ligand [29]. Discovery Studio (DS) version 4.5 was used to visualize the 2D and 3D representations of the selected biological compounds.
3. Statistical analysis
The results were indicated in mean ± standard error mean (SEM) and significance was set to cp < 0.001, bp < 0.01 and ap < 0.05, based on one−way or two−way analysis of variance (ANOVA). Repeated measures were used to assess neuropharmacological activities. The in vivo model utilized five mice per group.
4. Results
4.1. Force swimming test
Fig. 1 summarizes the results of the FST. In comparison to the control, the MEGL at both doses (200 and 400 mg/kg) demonstrates a significant (p < 0.001) result. Similarly, standard paroxetine caused a significant reduction in immobility periods (p < 0.001).
Fig. 1.
Effects of MEGL and diazepam in the FST. Values are presented as the mean ± SEM (n = 5). cp < 0.001 compared with the control group (Dunnett's test). MEGL: methanol extract of G. lucidum.
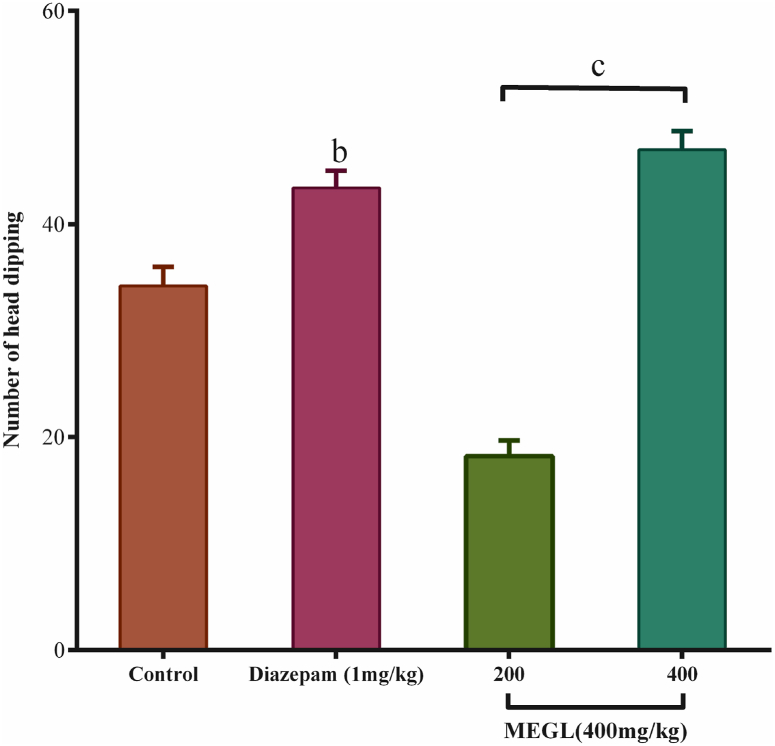
4.2. Hole board test
The MEGL was tested for anxiolytic action using the hole board method. When compared to control, the extract given at doses of 200 mg/kg and 400 mg/kg significantly (p < 0.001) increased head dipping. Standard diazepam produced statistically significant results (p < 0.01) (Fig. 2).
Fig. 2.
Effects of MEGL and diazepam in the hole board test. Values are presented as the mean ± SEM (n = 5). cp < 0.001 and bp < 0.01 compared with the control group (Dunnett's test). MEGL: methanol extract of G. lucidum.
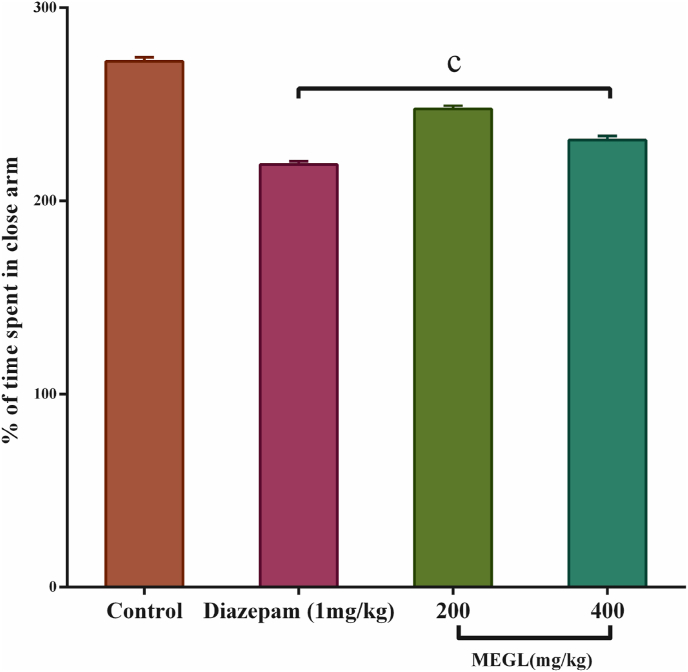
4.3. Elevated plus maze test
On the EPM instrument, the anxiolytic effects of a MEGL at doses of 200 mg/kg and 400 mg/kg were evaluated. When compared to the control, the extract given at a dose of 200 mg/kg and 400 mg/kg had a significant (p < 0.001) outcome. Similarly, standard diazepam produced a significant (p < 0.001) result (Fig. 3).
Fig. 3.
Effects of MEGL and diazepam in the EPM test. Values are presented as the mean ± SEM (n = 5). cp < 0.001 compared with the control group (Dunnett's test). MEGL: methanol extract of G. lucidum.
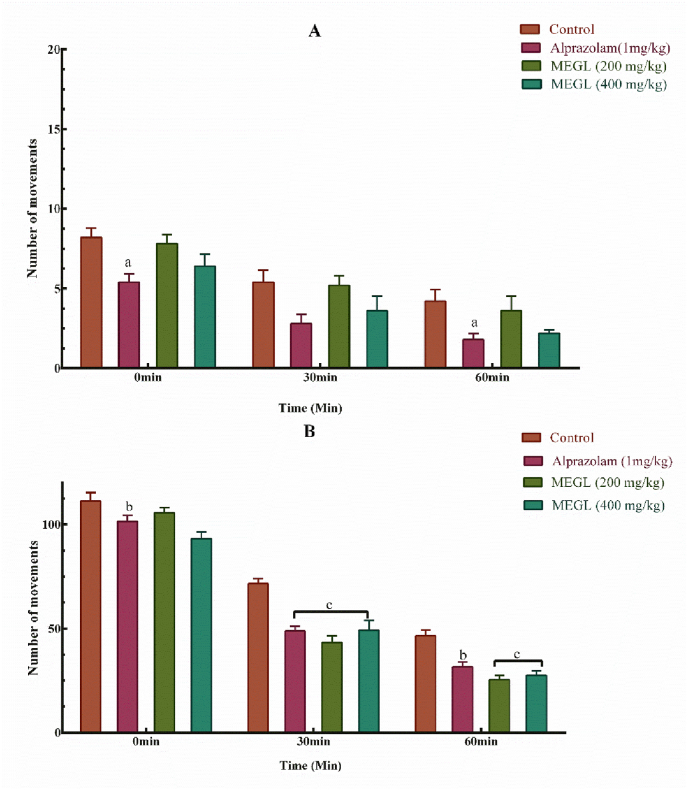
4.4. Hole cross test and open field test
MEGL doses (200 mg/kg and 400 mg/kg) resulted in a considerable decrease in mouse movement in the hole cross open field, which was visible from the first observation (0 min) to the last observation (60 min), compared to standard alprazolam (Fig. 4A). In addition, the open field test revealed that both extracts and standard alprazolam significantly (p < 0.001) reduced hole crossing in a dose-dependent manner (Fig. 4B).
Fig. 4.
Effects of MEGL and alprazolam in the hole cross test (A) and open field test (B). Values are presented as the mean ± SEM (n = 5). cp < 0.001, bp < 0.01 and ap < 0.05 compared with the control group (Dunnett's test). MEGL: methanol extract of G. lucidum.
4.5. Thiopental sodium induced sleeping test
In the thiopental-induced hypnosis experiment, the MEGL at both dosages considerably decreased (p < 0.001) sleep onset in a dose-dependent manner, and the influence of the extract on sleep onset is equal to that of regular medication. In comparison to controls, the extract dosage increased the length of sodium-induced thiopental sleep period in the mice. Table 1.
Table 1.
Thiopental sodium–induced sleeping time.
| Groups | Dose (mg/kg) | Onset of sleep (min) | Duration of sleep (min) |
|---|---|---|---|
| Control | 10 ml/kg | 11.77 ± 4.23 | 9.21 ± 1.33 |
| Alprazolam | 1 | 8.31 ± 4.79cp | 12.59 ± 5.69cp |
| MEGL | 200 | 11.26 ± 1.86cp | 15.23 ± .085cp |
| 400 | 5.42 ± 1.09 cp | 14.23 ± 3.09 cp |
Values are presented as the mean ± SEM (n = 5). cp < 0.001 compared with the control group (Dunnett's test). MEGL: methanol extract of G. lucidum.
4.6. In silico molecular docking simulation for antidepressant, anxiolytic and sedative activities
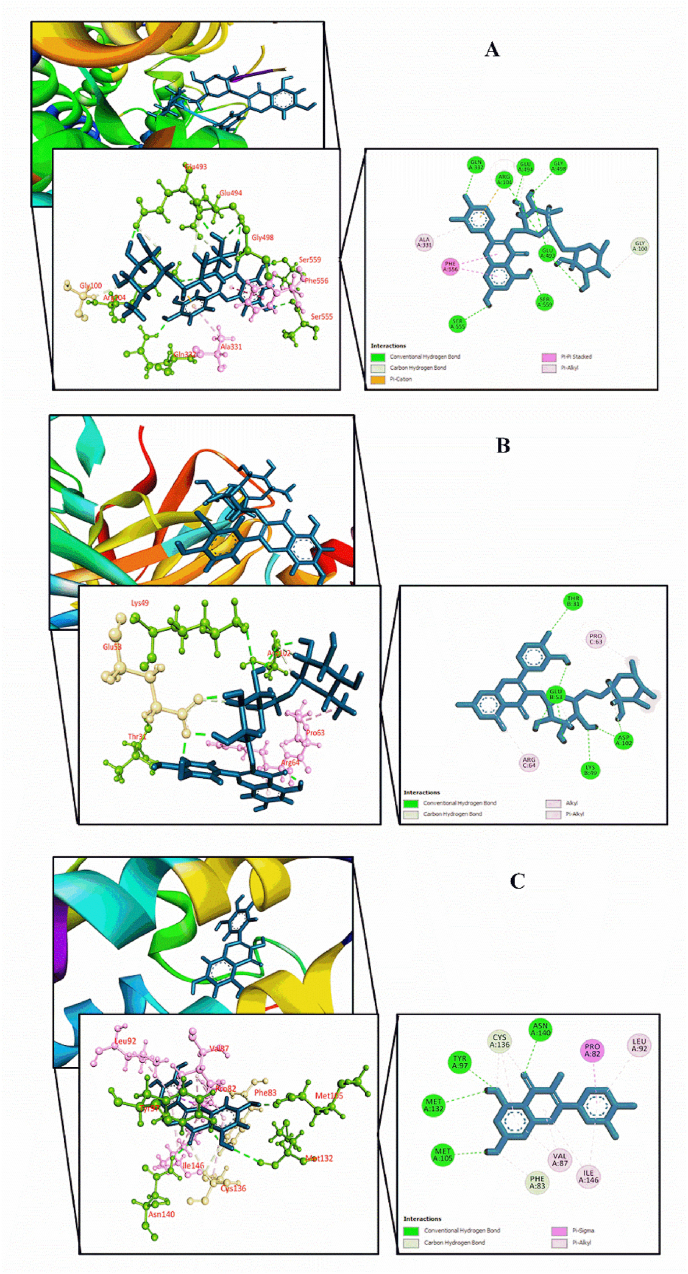
Docking score of the compounds selected from the MEGL against human serotonin transporter (PDB ID: 5I6X), potassium channel receptor (PDB ID: 4UUJ), and bromodomain of human BRD4 receptor (PDB ID: 3U5J) are displayed in Table 2. For each activity, the docking score and interaction analysis of the top five compounds of G. lucidum as well as the reference drug for respective protein targets are represented in Table 3, Table 4, Table 5, Fig. 5, and Supplementary Figs. S1–S3. The amino acid interactions between the selected biological compounds of G. lucidum and human serotonin transporter (PDB ID: 5I6X) were determined for antidepressant activity. All the compounds interacted with our target receptor. Rutin (−7.967 kcal/mol) exposed the utmost binding affinity towards followed by quercetin hexoside (−7.838 kcal/mol), epigallocatechin (−7.698 kcal/mol), epicatechin gallate (−6.771 kcal/mol), and catechin gallate (−6.576 kcal/mol). The reference drug paroxetine exhibited better binding affinity compared to the selected compounds. In anxiolytic activity, all compounds successfully docked with the potassium channel receptor (PDB ID: 4UUJ). Rutin (−5.666 kcal/mol) displayed the utmost binding affinity towards the target receptor and was better compared to the reference drug diazepam (−3.475 kcal/mol). The bonding score of Rutin was followed by ferulic acid (−5.514 kcal/mol), catechin (−5.394 kcal/mol), caffeic acid (−5.30 kcal/mol) and epicatechin gallate (−5.281 kcal/mol). In case of sedative activity, all compounds showed affinities for bromodomain of human BRD4 (PDB ID: 3U5J) receptor. Here, Quercetin (−9.028 kcal/mol) displayed the utmost binding affinity towards the target receptor and was better compared to the reference drug alprazolam (−7.77 kcal/mol). It was followed by rutin (−8.646 kcal/mol), catechin (−8.365 kcal/mol), catechin gallate (−7.873 kcal/mol), and 2, 7-diphenylindole (−7.861 kcal/mol).
Table 2.
Docking score of the selected compounds identified from the methanol extract of G. lucidum against the human serotonin transporter (PDB ID: 5I6X), potassium channel receptor (PDB ID: 4UUJ), and bromodomain of human BRD4 (PDB ID: 3U5J) for antidepressant, anxiolytic, and sedative activity respectively.
| Compounds | PubChem CID | Docking Score (Kcal/mol) |
||
|---|---|---|---|---|
| 5I6X (Antidepressant) | 4UUJ (Anxiolytic) | 3U5J (Sedative) | ||
| Thiophene, 2-hexyl | 87793 | −5.411 | −2.042 | −4.854 |
| 3-((3-Acetoxythyl)-6-acetoxymethyl-2,4- dimethyl)phenyl)-2-methyl-(E)-2-propenyl acetate | 46867877 | −4.576 | −3.784 | −5.693 |
| 2,7-Diphenylindole | 622897 | −6.296 | −2.789 | −7.861 |
| 5-(2-Bromophenyl)-7-chloro-2,3-dihydro-1H-1,4- benzodiazepin-2-one | −6.548 | −3.536 | −5.811 | |
| Gallic acid | 370 | −5.894 | −5.164 | −6.996 |
| Theanine | 46867877 | −4.358 | −3.226 | −4.659 |
| Caffeic acid | 689043 | −4.773 | −5.30 | −6.769 |
| Caffeine | 2519 | −5.824 | −3.946 | −6.582 |
| Ferulic acid | 445858 | −4.807 | −5.514 | −5.971 |
| Theacrine | 75324 | −5.125 | −4.013 | −6.903 |
| Catechin | 9064 | −6.566 | −5.394 | −8.365 |
| Quercetin | 5280343 | −5.626 | −4.963 | −9.028 |
| Epigallocatechin | 72277 | −7.698 | −5.144 | −5.805 |
| Catechin gallate | 6419835 | −6.576 | −4.203 | −7.873 |
| Epicatechin gallate | 107905 | −6.771 | −5.281 | −7.678 |
| Quercetin hexoside | 5378597 | −7.838 | −4.54 | −7.515 |
| Rutin | 5280805 | −7.967 | −5.666 | −8.646 |
| Standard (Paroxetine/Diazepam/Alprazolam) | −8.785 | −3.475 | −7.77 | |
Table 3.
Molecular docking interaction analysis of the five compounds of G. lucidum displaying highest docking scores against human serotonin transporter (PDB ID: 5I6X).
| 5I6X | |||||
|---|---|---|---|---|---|
| Compounds | Docking Score (Kcal/mol) | Hydrogen bond interactions | Hydrophobic Bonds (Pi-alkyl/Alkyl interaction) | Hydrophobic Bonds (Pi-Pi/Pi-sigma/Pi-cation/Pi-anion/Amide-Pi interaction) | Hydrophobic Bonds (Pi-sulfur/carbon-hydrogen interaction) |
| Epigallocatechin | −7.698 | Tyr95, Asp98, Gly100, Phe335, Glu493, Glu494 | Ile172 | Tyr95, Tyr176 | Gly338, Ser438, Gly498 |
| Catechin gallate | −6.576 | Tyr95, Asp98, Ser438, Glu493, Glu494 | Ile172, Phe335, Val501 | Phe335, Phe341 | Gly338 |
| Epicatechin gallate | −6.771 | Gln332, Glu493, Thr497 | Ile172, Val501 | Tyr176, Phe335, Arg104 | – |
| Quercetin hexoside | −7.838 | Asp98, Arg104, Glu493 | Ile172 | Phe335 | Gly100, Glu493, Gly498 |
| Rutin | −7.967 | Arg104, Gln332, Glu493, Glu494, Gly498, Ser555, Ser559 | Ala331 | Arg104, Phe556 | Gly100, Glu493, Glu494 |
| Standard (Paroxetine) | −8.785 | Tyr95, Ala96 | Ile172, Ala173 | Tyr176, Phe341,Ser438 | Ala169, Ser336 |
Bold text indicates the best docking score.
Table 4.
Molecular docking interaction analysis of the five compounds of G. lucidum displaying highest docking scores against potassium channel receptor (PDB ID: 4UUJ).
|
4UUJ | |||||
|---|---|---|---|---|---|
| Compounds | Docking Score (Kcal/mol) | Hydrogen bond interactions | Hydrophobic Bonds (Pi-alkyl/Alkyl interaction) | Hydrophobic Bonds (Pi-Pi/Pi-sigma/Pi-cation/Pi-anion/Amide-Pi interaction) | Hydrophobic Bonds (Pi-sulfur/carbon-hydrogen interaction) |
| Caffeic acid | −5.30 | Lys49, Tyr50, Glu53, Thr61, Arg64, Asp102 | – | Tyr50 | – |
| Ferulic acid | −5.514 | Lys49, Tyr50, Glu53, Thr61, Arg64 | – | Tyr50 | Asp102 |
| Catechin | −5.394 | Lys49, Glu53, Arg100 | Pro63, Arg100 | Tyr50, Asp102 | Asp102 |
| Epicatechin gallate | −5.281 | Lys49, Glu53, Arg64, Asp102 | Pro63, Arg64 | Pro63 | - |
| Rutin | −5.666 | Thr31, Lys49, Glu53, Asp102 | Pro63, Arg64 | - | Glu53 |
| Standard (Diazepam) | −3.475 | Lys49, Tyr104 | – | Tyr50 | Glu53, Asp102 |
Bold text indicates the best docking score.
Table 5.
Molecular docking interaction analysis of the five compounds of G. lucidum displaying highest docking scores against bromodomain of human BRD4 (PDB ID: 3U5J).
| 4UUJ | |||||
|---|---|---|---|---|---|
| Compounds | Docking Score (Kcal/mol) | Hydrogen bond interactions | Hydrophobic Bonds (Pi-alkyl/Alkyl interaction) | Hydrophobic Bonds (Pi-Pi/Pi-sigma/Pi-cation/Pi-anion/Amide-Pi interaction) | Hydrophobic Bonds (Pi-sulfur/carbon-hydrogen interaction) |
| 2,7-Diphenylindole | −7.861 | – | Pro82, Val87, Leu92, Leu94, Cys136, Ile146 | – | – |
| Catechin | −8.365 | Gln85, Met132 | Pro82, Val87, Leu92, Cys136, Ile146 | – | – |
| Quercetin | −9.028 | Tyr97, Met105, Met132, Asn140 | Val87, Leu92, Cys136, Ile146 | Pro82 | Phe83, Cys136 |
| Catechin gallate | −7.873 | Pro82, Gln85, Met105, Met132, Asn140 | Pro82, Val87, Leu92, Leu94, Cys136, Ile146 | – | Phe83 |
| Rutin | −8.646 | Met132, Asn135, Asn140, Asp145 | Pro82, Val87, Leu92, Cys136, Ile146 | Phe83 | Asp145 |
| Standard (Alprazolam) | −7.77 | Asn140 | Trp81, Pro82, Phe83, Val87, Leu92, Cys136, Ile146 | Tyr97, Ile146 | – |
Bold text indicates the best docking score.
Fig. 5.
Molecular docking interaction of compounds displaying highest binding scores against different target receptors: (A) Human serotonin transporter receptor with rutin, (B) Potassium channel receptor interaction with rutin, and (C) Bromodomain of human BRD4 receptor with quercetin.
5. Discussion
Due to the unwanted adverse effects of synthetic medications, herbal therapies are becoming more popular in underdeveloped nations [7]. Plants are now thought to have substantial therapeutic potential due to their distinct characteristics as a key source of medicinal phytocompounds that might aid in developing innovative medication [[30], [31], [32], [33]]. According to the Food and Drug Administration (FDA), a number of pharmacological agents have been authorized for use. Due to the difficulty of synthetic medications, herbal therapies are becoming more popular in third world nations [34]. Considering these concepts, the current study used five neuropharmacological procedures to assess MEGL's CNS depressant actions in mice, including hole-board, forced swimming, elevated plus maze, hole cross and open field, and thiopental sodium-induced sleeping time tests. These methods are frequently employed in neuropharmacological screening models. Certain 5-hydroxytryptamine (5-HT, often known as serotonin) receptors, such as 5-HT6, may have a role in depression mediation. Monoamine oxidase A (MAO-A) inhibitors are being used to treat depression by preventing the MAO-induced catalysis of 5-HT [7]. Another research on quercetin suggests that it has antidepressant-like effects occurs via inhibiting MAO-A, an essential enzyme in the metabolism of 5-HT neurotransmitter [35]. Taraxanthin demonstrated an antidepressant-like effect through the interpretation of 5-HT pathway in mice model. Commonly prescribed antidepressants such as tricyclic antidepressants (TCAs) and MAO inhibitors (5-HT reuptake inhibitors) also have similar mechanisms of action [36]. The FST is a common tool for evaluating antidepressant efficacy in mouse models. Antidepressant activity is shown by a shorter immobility time, whereas CNS depression is indicated by a longer immobility time [37]. Depression is caused by decreased concentration of neurochemicals such as dopamine and norepinephrine. At the same time, any antidepressant medicine acts on at least one of these chemical transmitters, causing them to become more active [[38], [39], [40]]. The FST study, where MEGL reduced the immobility time after administration, implies that the plant extract may elevate at least one of the neurotransmitters involved in depression, whereas CNS depressing effects were also found for standard diazepam in the FST on mice model [39]. In the hole-board test, the MEGL-treated mice showed a significant increase in head-dipping. From the locomotor assay, the hole board equipment measures the exploratory behavior of mice individually [41,42]. In mice, increased head-dipping behavior was previously defined as anxious behavior [43]. Anxiety disorders may be caused by the dysregulation of several neurotransmitters (serotonin, gamma-amino-butyric acid, and dopamine) [44]. Again, the MEGL showed a significant reduction in square movements and crossing of the hole in the hole cross and open field test. The results showed the abridged locomotive action of the extract, confirming the CNS depressant functionality, whilst the locomotor activity was measured using a hole cross and open field test. Any decrease in movement shows the influence of a CNS depressant, and locomotion increment is indicative of the presence of a CNS depressant in the system [45,46]. This reflects the CNS's excitability level, which can be attributed to the plant extract's CNS depressing impact via lowering motor activity [47,48]. The extract significantly reduced the mice's movement in the hole cross and open field test. The antidepressant and anxiolytic properties of MEGL in mice can be produced by the selective 5-HT reuptake inhibitors, tricyclic antidepressants or MAO pathways. The sleeping time test was utilized in the Swiss albino mouse to investigate sedative-hypnotic medications, while thiopental sodium is referred to the group of barbiturates that tempt sleepy mood in humans and mice [48]. As a result of the allosteric alteration of the GABAA receptors, thiopental induces hypnosis via postsynaptic GABA-mediated inhibition. Components of CNS depressants reduce the onset or duration of sleep, or both [49,50]. According to our findings, the greater dose of MEGL had the most CNS depressive impact, and it is presumed that these effects may be mediated by the inhibition of the postsynaptic interpretation of GABA receptor. G. lucidum also contains phytochemicals such as triterpenes, flavonoids, phenolic compounds, glycoproteins, proteoglycan, and polysaccharide [[51], [52], [53], [54]]. Thus, interactions between these phytochemicals and neurotransmitters linked to depressive-like behavior are most likely to be responsible for MEGL's antidepressant and anxiolytic effectiveness. Furthermore, molecular docking is a widely utilized approach for predicting the orientation of small molecule's interaction to an enzyme or receptor [55]. In our present study, rutin displayed the best docking score in antidepressant activity against human serotonin transporter and formed the highest number of hydrogen bonds among the five compounds. Importantly, epigallocatechin and catechin gallate formed a hydrogen bond with Tyr95 residue. It was previously reported that Tyr95 residue is crucial for the potency of an antidepressant drug [24]. The anxiolytic investigation through molecular docking revealed that the best binding interaction was also displayed by rutin and the docking score was better than that of the reference drug diazepam. It has formed binding interactions with Asp102, Lys49, and Glu53 residues which were also displayed by diazepam. Previously, Rutin showed anxiolytic-like responses in the mice model, which was similar to diazepam [56]. Interestingly, the compounds exhibiting upper binding scores for anxiolytic activity have formed hydrogen bonds with Lys49 residue. Caffeic acid, ferulic acid and catechin formed bonds with Tyr50 residue. In molecular docking of sedative activity, quercetin (−9.028 kcal/mol) displayed the utmost binding affinity towards bromodomain of human BRD4 receptor that formed hydrogen bond interactions with Asn140 residue and hydrophobic interactions with Val87, Leu92, Tyr97, Cys136, and Ile146 residues, similar to the reference drug alprazolam. In most bromodomains, Asn140 is a conserved residue serves as a hydrogen bond anchor point for acetyl lysine [26]. As tyrosine acts as a precursor for neurotransmitters such as dopamine, epinephrine, and noradrenaline, this residue is considered to be associated with fear suppression. Additionally, lysine was reported to be involved in anxiety reduction in humans [57]. The identified pharmacological properties were correlated with the computational analysis via molecular docking of G. lucidum phenolic compounds towards various receptors.
6. Conclusions
To sum up, MEGL appears to have antidepressant, anxiolytic, and sedative activities. The secondary metabolites present in the extracts may be responsible for these activities. Rutin and quercetin have higher binding affinity with receptors, according to our computational analysis and in the previous studies, these compounds were claimed to have neuropharmacological properties. These findings suggest that rutin and quercetin may be useful in developing clinical applications in the future. To verify clinical efficacy, in-depth investigations proposed elucidating the possible mechanisms of animal models and humans.
Author contributions
S. M. Moazzem Hossen: conceptualization, designing, acquisition, supervision, writing - review & editing; ATM Yusuf: formal analysis, data curation, writing - review & editing; Nazim Uddin Emon: designing, methodology, formal analysis, writing-original draft, validation, writing-review & editing; Najmul Alam: designing, investigation, methodology, formal analysis, data curation, software, writing - review & editing; Saad Ahmed Sami: investigation, formal analysis, data curation, writing - review & editing; Shajjad Hossain Polash: writing-original draft, writing-review & editing. Md. Arifuzzaman Nur: investigation, formal analysis, data curation, writing - review & editing. Saikat Mitra: methodology, validation, visualization; Mohammad Helal Uddin: methodology, validation, visualization, writing-review & editing, Supervision; Talha Bin Emran: resources, project administration, writing-review & editing. Finally, all the authors reviewed and agreed to publish this research.
Funding
Not applicable.
Institutional review board statement
The “Planning and Development (P&D) Committee of the Department of Pharmacy, University of Chittagong, Chittagong, Bangladesh; approved the study protocol according to government guidelines under the Pharm/P&D/CUDP-16, 2021:08.
Informed consent statement
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
The authors are grateful to the Department of Pharmacy, University of Chittagong, Bangladesh for research facilities and logistic support.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bbrep.2022.101371.
Contributor Information
S.M. Moazzem Hossen, Email: hossen.pharmacy@cu.ac.bd.
Nazim Uddin Emon, Email: nazim7emon@gmail.com.
Talha Bin Emran, Email: talhabmb@bgctub.ac.bd.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
Data availability
The data that has been used is confidential.
References
- 1.Kensbock J.M., Alkærsig L., Lomberg C. The epidemic of mental disorders in business—how depression, anxiety, and stress spread across organizations through employee mobility. Adm. Sci. Q. 2022;67:1–48. [Google Scholar]
- 2.Friedrich M.J. Depression is the leading cause of disability around the world. JAMA. 2017;317 doi: 10.1001/jama.2017.3826. 1517-1517. [DOI] [PubMed] [Google Scholar]
- 3.Goodwin G.M. Depression and associated physical diseases and symptoms. Dialogues Clin. Neurosci. 2022;8(2):259–265. doi: 10.31887/DCNS.2006.8.2/mgoodwin. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schultz J.L., Killoran A., Nopoulos P.C., Chabal C.C., Moser D.J., Kamholz J.A. Evaluating depression and suicidality in tetrabenazine users with Huntington disease. Neurology. 2018;91:e202–e207. doi: 10.1212/WNL.0000000000005817. [DOI] [PubMed] [Google Scholar]
- 5.Zarei M., Mohammadi S., Komaki A., Golipour Choshali Z. Antidepressant-like effects of intra-cerebroventricular microinjection of kaempferol in male rats: involvement of 5-HT2 receptors. Avicenna J. Neuro Psychophysiol. 2022;9:23–30. [Google Scholar]
- 6.Thibaut F. Anxiety disorders: a review of current literature. Dialogues Clin. Neurosci. 2017;19:87–88. doi: 10.31887/DCNS.2017.19.2/fthibaut. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasrin S., Islam M.N., Tayab M.A., Nasrin M.S., Siddique M.A.B., Emran T.B., Reza A.A. Chemical profiles and pharmacological insights of Anisomeles indica Kuntze: an experimental chemico-biological interaction. Biomed. Pharmacother. 2022;149 doi: 10.1016/j.biopha.2022.112842. [DOI] [PubMed] [Google Scholar]
- 8.Wasser S. Medicinal mushroom science: current perspectives, advances, evidences, and challenges. Biomed. J. 2014;37 doi: 10.4103/2319-4170.138318. [DOI] [PubMed] [Google Scholar]
- 9.Devi R., Kaur T., Guleria G., Rana K.L., Kour D., Yadav N., Yadav A.N., Saxena A.K. In: New and Future Developments in Microbial Biotechnology and Bioengineering. Rastegari A.A., Yadav A.N., Yadav N., editors. Elsevier; 2020. Chapter 9 - fungal secondary metabolites and their biotechnological applications for human health; pp. 147–161. [Google Scholar]
- 10.Wang Y X.B. Distribution of antioxidant activities and total phenolic contents in acetone, Ethanol,Water and hot water extracts from 20 edible mushrooms via sequential extraction. Austin J. Nutr. Food Sci. 2014;2(1):5. [Google Scholar]
- 11.Wasser S. Medicinal mushroom science: history, current status, future trends, and unsolved problems. Int. J. Med. Mushrooms. 2010;12:1–16. doi: 10.1615/intjmedmushr.v13.i5.10. [DOI] [PubMed] [Google Scholar]
- 12.Gan C.H., Amira B., Asmah R. Antioxidant analysis of different types of edible mushrooms (Agaricus bisporous and Agaricus brasiliensis) Int. J. Food Res. 2013;20:1095–1102. [Google Scholar]
- 13.Mujic I., Zekovic Z., Lepojević Z., Vidovic S., Živković J. Antioxidant properties of selected edible mushroom species. J. Cent. Eur. Agric. 2010;11:387–391. [Google Scholar]
- 14.Y J., Wachtel-Galor S., Buswell J.A., et al. second ed. CRC Press/Taylor; Boca Raton (FL): 2011. Herbal Medicine: Biomolecular and Clinical Aspects. Francis. (Chapter 9) (2011) [PubMed] [Google Scholar]
- 15.O.f.E. Co-operation, Development . OECD publishing; 2008. Test No. 425: acute oral toxicity: up-and-down procedure. [DOI] [Google Scholar]
- 16.Emon N.U., Rudra S., Alam S., Haidar I.K.A., Paul S., Richi F.T., Shahriar S., Sayeed M.A., Tumpa N.I., Ganguly A. Chemical, biological and protein-receptor binding profiling of Bauhinia scandens L. stems provide new insights into the management of pain, inflammation, pyrexia and thrombosis. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112185. [DOI] [PubMed] [Google Scholar]
- 17.Aziz M.A.I., Barua N., Tareq A.M., Alam N., Prova R.J., Mamun M.N., Sayeed M.A., Chowdhury M.A.U., Emran T.B. Possible neuropharmacological effects of Adenia trilobata (Roxb.) in the Swiss albino mice model. Future J. Pharmaceut. Sci. 2020;6:72. [Google Scholar]
- 18.Hossen M., Islam M., Hossain M., Barua A., Uddin M., Emon N.U. CNS anti-depressant, anxiolytic and analgesic effects of Ganoderma applanatum (mushroom) along with ligand-receptor binding screening provide new insights: multi-disciplinary approaches. Biochem. Biophys. reports. 2021 doi: 10.1016/j.bbrep.2021.101062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goni O., Khan M.F., Rahman M.M., Hasan M.Z., Kader F.B., Sazzad N., Sakib M.A., Romano B., Haque M.A., Capasso R. Pharmacological insights on the antidepressant, anxiolytic and aphrodisiac potentials of Aglaonema hookerianum Schott. J. Ethnopharmacol. 2021;268 doi: 10.1016/j.jep.2020.113664. [DOI] [PubMed] [Google Scholar]
- 20.Chy M.N., Kabir M., Hasanat A., Adnan M., Hossain M., Ahmad S., Islam M. Ficus cunia Buch.-Ham. ex Roxb. (leaves): an experimental evaluation of the cytotoxicity, thrombolytic, analgesic and neuropharmacological activities of its methanol extract. J. Basic Clin. Physiol. Pharmacol. 2019:30. doi: 10.1515/jbcpp-2016-0140. [DOI] [PubMed] [Google Scholar]
- 21.Surahmaida S., Sudarwati T., Junairiah J. Analisis GCMS terhadap senyawa fitokimia ekstrak metanol Ganoderma lucidum. Jurnal Kimia Riset. 2019;3:147. [Google Scholar]
- 22.Vinotha V. Determination of phytocompounds from methanol extracts of Ganoderma lucidum by LC-MS. Int. J. Res. Anal. Rev. 2019;6(1):914–918. R.R. [Google Scholar]
- 23.Berman H.M., Battistuz T., Bhat T.N., Bluhm W.F., Bourne P.E., Burkhardt K., Feng Z., Gilliland G.L., Iype L., Jain S., Fagan P., Marvin J., Padilla D., Ravichandran V., Schneider B., Thanki N., Weissig H., Westbrook J.D., Zardecki C. The protein data bank, acta crystallographica. Acta Crystallogr. Sect. D Biol. Crystallogr. 2002;58:899–907. doi: 10.1107/s0907444902003451. [DOI] [PubMed] [Google Scholar]
- 24.Coleman J.A., Green E.M., Gouaux E. X-ray structures and mechanism of the human serotonin transporter. Nature. 2016;532:334–339. doi: 10.1038/nature17629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lenaeus M.J., Burdette D., Wagner T., Focia P.J., Gross A. Structures of KcsA in complex with symmetrical quaternary ammonium compounds reveal a hydrophobic binding site. Biochemistry. 2014;53:5365–5373. doi: 10.1021/bi500525s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Filippakopoulos P., Picaud S., Fedorov O., Keller M., Wrobel M., Morgenstern O., Bracher F., Knapp S. Benzodiazepines and benzodiazepines as protein interaction inhibitors targeting bromodomains of the BET family. Bioorg. Med. Chem. 2012;20:1878–1886. doi: 10.1016/j.bmc.2011.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 28.Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K., Shaw D.E., Francis P., Shenkin P.S. Glide: a new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 29.Sastry G.M., Adzhigirey M., Day T., Annabhimoju R., Sherman W. Protein and ligand preparation: parameters, protocols, and influence on virtual screening enrichments. J. Comput. Aided Mol. Des. 2013;27:221–234. doi: 10.1007/s10822-013-9644-8. [DOI] [PubMed] [Google Scholar]
- 30.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. [Google Scholar]
- 31.Ağagündüz D., Şahin T.Ö., Yılmaz B., Ekenci K.D., Özer Ş.Duyar, Capasso R. Cruciferous vegetables and their bioactive metabolites: from prevention to novel therapies of colorectal cancer. J. Evid. Based. Integr. Med. 2022;2022 doi: 10.1155/2022/1534083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hossain S., Urbi Z., Karuniawati H., Mohiuddin R.B., Moh Qrimida A., Allzrag A.M.M., Ming L.C., Pagano E., Capasso R. Andrographis paniculata (burm. F.) wall. Ex nees: an updated review of phytochemistry, antimicrobial pharmacology, and clinical safety and efficacy. Life. 2021;11:348. doi: 10.3390/life11040348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fernández J., Silván B., Entrialgo-Cadierno R., Villar C.J., Capasso R., Uranga J.A., Lombó F., Abalo R. Antiproliferative and palliative activity of flavonoids in colorectal cancer. Biomed. Pharmacother. 2021;143 doi: 10.1016/j.biopha.2021.112241. [DOI] [PubMed] [Google Scholar]
- 34.Capasso R., Izzo A.A., Pinto L., Bifulco T., Vitobello C., Mascolo N. Phytotherapy and quality of herbal medicines. Fitoterapia. 2000;71:S58–S65. doi: 10.1016/s0367-326x(00)00173-8. [DOI] [PubMed] [Google Scholar]
- 35.Bandaruk Y., Mukai R., Terao J. Cellular uptake of quercetin and luteolin and their effects on monoamine oxidase-A in human neuroblastoma SH-SY5Y cells. Toxicol Rep. 2014;1:639–649. doi: 10.1016/j.toxrep.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng D., Murtaza G., Ma S., Li L., Li X., Tian F., Zheng J., Lu Y. In Silico prediction of the anti-depression mechanism of a herbal formula (tiansi liquid) containing Morinda officinalis and cuscuta chinensis. Molecules. 2017;22:1614. doi: 10.3390/molecules22101614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Subarnas A., Tadano T., Nakahata N., Arai Y., Kinemuchi H., Oshima Y., Kisara K., Ohizumi Y. A possible mechanism of antidepressant activity of beta-amyrin palmitate isolated from Lobelia inflata leaves in the forced swimming test. Life Sci. 1993;52:289–296. doi: 10.1016/0024-3205(93)90220-w. [DOI] [PubMed] [Google Scholar]
- 38.Southwick S.M., Vythilingam M., Charney D.S. The psychobiology of depression and resilience to stress: implications for prevention and treatment. Annu. Rev. Clin. Psychol. 2005;1:255–291. doi: 10.1146/annurev.clinpsy.1.102803.143948. [DOI] [PubMed] [Google Scholar]
- 39.Osanloo N., Najafi-Abedi A., Jafari F., Javid F., Pirpiran M., Memar Jafari M.R., Mousavi Khosravi S.A., Rahimzadeh Behzadi M., Ranjbaran M., Sahraei H. Papaver rhoeas L. Hydroalcoholic extract exacerbates forced swimming test-induced depression in mice. Basic Clin. Neurosci. 2016;7:195–202. doi: 10.15412/J.BCN.03070304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berton O., Nestler E.J. New approaches to antidepressant drug discovery: beyond monoamines. Nat. Rev. Neurosci. 2006;7:137–151. doi: 10.1038/nrn1846. [DOI] [PubMed] [Google Scholar]
- 41.Takagi K., Watanabe M., Saito H. Studies of the spontaneous movement of animals by the hole cross test; effect of 2-dimethyl-aminoethanol and its acyl esters on the central nervous system. Jpn. J. Pharmacol. 1971;21:797–810. doi: 10.1254/jjp.21.797. [DOI] [PubMed] [Google Scholar]
- 42.Brown G.R., Nemes C. The exploratory behaviour of rats in the hole-board apparatus: is head-dipping a valid measure of neophilia? Behav. Process. 2008;78:442–448. doi: 10.1016/j.beproc.2008.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saitoh A., Hirose N., Yamada M., Yamada M., Nozaki C., Oka T., Kamei J. Changes in emotional behavior of mice in the hole-board test after olfactory bulbectomy. J. Pharmacol. Sci. 2006;102:377–386. doi: 10.1254/jphs.fp0060837. [DOI] [PubMed] [Google Scholar]
- 44.Murrough J.W., Yaqubi S., Sayed S., Charney D.S. Emerging drugs for the treatment of anxiety. Expet Opin. Emerg. Drugs. 2015;20:393–406. doi: 10.1517/14728214.2015.1049996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sousa F.C., Melo C.T., Monteiro A.P., Lima V.T., Gutierrez S.J., Pereira B.A., Barbosa-Filho J.M., Vasconcelos S.M., Fonteles M.F., Viana G.S. Antianxiety and antidepressant effects of riparin III from Aniba riparia (Nees) Mez (Lauraceae) in mice. Pharmacol. Biochem. Behav. 2004;78:27–33. doi: 10.1016/j.pbb.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 46.Gahlot K., Lal V.K., Jha S. Anticonvulsant potential of ethanol extracts and their solvent partitioned fractions from Flemingia strobilifera root. Pharmacogn. Res. 2013;5:265–270. doi: 10.4103/0974-8490.118825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Masur J., Märtz R.M.W., Carlini E.A. Effects of acute and chronic administration of cannabis sativa and (−)Δ9-trans-tetrahydrocannabinol on the behavior of rats in an open-field arena. Psychopharmacologia. 1971;19:388–397. doi: 10.1007/BF00404383. [DOI] [PubMed] [Google Scholar]
- 48.Rakotonirina V.S., Bum E.N., Rakotonirina A., Bopelet M. Sedative properties of the decoction of the rhizome of Cyperus articulatus. Fitoterapia. 2001;72:22–29. doi: 10.1016/s0367-326x(00)00243-4. [DOI] [PubMed] [Google Scholar]
- 49.Nyeem M., Alam M., Awal M., Mostofa M., Uddin S., Islam N., Rouf R. CNS depressant effect of the crude ethanolic extract of the flowering tops of rosa damascena. Iran. J. Pharmacol. Ther. 2006;5 Num 2:5. (ISSN: 1735-2657) Vol. [Google Scholar]
- 50.Raquibul Hasan S.M., Hossain M.M., Akter R., Jamila M., Mazumder E.H., Rahman S. Sedative and anxiolytic effects of different fractions of the Commelina benghalensis Linn. Drug discoveries & therapeutics. 2009;3:221–227. [PubMed] [Google Scholar]
- 51.Boh B., Berovic M., Zhang J., Zhi-Bin L. Ganoderma lucidum and its pharmaceutically active compounds. Biotechnol. Annu. Rev. 2007;13:265–301. doi: 10.1016/S1387-2656(07)13010-6. [DOI] [PubMed] [Google Scholar]
- 52.Cao Y., Xu X., Liu S., Huang L., Gu J. Ganoderma: a cancer immunotherapy review. Front. Pharmacol. 2018;9 doi: 10.3389/fphar.2018.01217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kebaili F.F., Tahar N., Esseddik T.M., Redouane R., Chawki B., Pablo A., Massimiliano P. Antioxidant activity and phenolic content of extracts of wild Algerian lingzhi or reishi medicinal mushroom, Ganoderma lucidum (agaricomycetes) Int. J. Med. Mushrooms. 2021;23:79–88. doi: 10.1615/IntJMedMushrooms.2021038424. [DOI] [PubMed] [Google Scholar]
- 54.Kozarski M., Klaus A., Niksic M., Jakovljevic D., Helsper J.P.F.G., Van Griensven L.J.L.D. Antioxidative and immunomodulating activities of polysaccharide extracts of the medicinal mushrooms Agaricus bisporus, Agaricus brasiliensis, Ganoderma lucidum and Phellinus linteus. Food Chem. 2011;129:1667–1675. [Google Scholar]
- 55.Gohlke H., Hendlich M., Klebe G. Knowledge-based scoring function to predict protein-ligand interactions. JMB (J. Mol. Biol.) 2000;295:337–356. doi: 10.1006/jmbi.1999.3371. [DOI] [PubMed] [Google Scholar]
- 56.Hernandez-Leon A., González-Trujano M.E., Fernández-Guasti A. The anxiolytic-like effect of rutin in rats involves GABAA receptors in the basolateral amygdala. Behav. Pharmacol. 2017;28:303–312. doi: 10.1097/FBP.0000000000000290. [DOI] [PubMed] [Google Scholar]
- 57.Obaidullah A.J., Alanazi M.M., Alsaif N.A., Mahdi W.A., Fantoukh O.I., Tareq A.M., Sami S.A., Alqahtani A.M., Emran T.B. Deeper insights on cnesmone javanica blume leaves extract: chemical profiles, biological attributes, network pharmacology and molecular docking. Plants. 2021:10. doi: 10.3390/plants10040728. Basel, Switzerland. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that has been used is confidential.