Abstract
Background
Current guidelines for the treatment of human epidermal growth factor receptor 2‒negative (HER2−) advanced breast cancer (ABC) are informed by tumor characteristics and include platinum- and non–platinum-based chemotherapy, chemotherapy plus immunotherapy, endocrine monotherapy, or endocrine therapy plus a targeted therapy. In addition, poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPi) have recently demonstrated improved clinical and patient-reported outcomes and manageable toxicity profiles compared with chemotherapy in patients with germline breast cancer susceptibility gene 1 or 2 (gBRCA1/2)‒mutated HER2− ABC in clinical trials and are now approved to treat this patient population. This study provides complementary real-world data regarding treatment patterns, adverse events, and physician-reported treatment satisfaction in this population.
Methods
This retrospective analysis using the Adelphi Real World ABC Disease Specific Programme in the United States, European Union, and Israel included patients aged ≥18 years receiving therapy for stage IIIb or IV gBRCA1/2-mutated HER2− ABC. Oncologists completed a patient record form detailing patient demographics, clinical assessments, and treatment history and a survey regarding their use of and satisfaction with treatments.
Results
Among the 543 patients, mean age was 55 years, 25% were premenopausal, 70% had hormone receptor‒positive (HR+) ABC, and 30% had triple-negative breast cancer (TNBC). PARPi were used in 5%, 11%, and 12% of first-line, second-line, and third-line therapies, respectively, for patients with HR+ ABC; for TNBC, percentages were 18%, 44%, and 36%. Across treatment lines, neutropenia, anemia, and nausea occurred in 16%, 24%, and 32% of patients receiving PARPi, respectively; 22%, 38%, and 33% of patients receiving platinum chemotherapy; and 20%, 20%, and 33% of patients receiving non–platinum-based chemotherapy. Physician satisfaction was highest with PARPi and with chemotherapy plus immunotherapy.
Conclusions
Findings in this real-world population complement clinical trial observations and provide further support for treatment of patients with PARPi in gBRCA1/2-mutated HER2− ABC.
Keywords: Breast cancer susceptibility gene 1 or 2, Hereditary breast cancer, poly(adenosine diphosphate-ribose) polymerase inhibitors, Real-world, Safety, Treatment patterns
Highlights
-
•
Treatments were evaluated in germline BRCA1/2-mutated HER2− breast cancer patients.
-
•
Physician-reported treatment patterns demonstrated PARPi use in this population.
-
•
Nausea and anemia were the most common adverse events in those receiving PARPi.
-
•
Physician satisfaction was highest with PARPi and chemotherapy plus immunotherapy.
-
•
These real-world findings lend further support for PARPi use in this population.
1. Introduction
Breast cancer (BC) is the most frequently diagnosed cancer type and the leading cause of cancer deaths among women, accounting for approximately 2.1 million diagnoses and approximately 630,000 deaths worldwide in 2018 [1]. Advanced breast cancer (ABC) is defined as locally advanced (stage IIIb/c) or metastatic (stage IV) disease. In the United States and Europe, approximately 5%–10% of all patients with BC present with metastatic disease, and approximately 30% of patients diagnosed with early-stage BC progress to ABC [[2], [3], [4], [5]]. ABC is generally incurable; the goals of treatment are to extend survival without impacting quality of life and improve disease-related symptoms [4].
Breast cancer is categorized into subtypes according to tumor characteristics, including hormone receptor (HR) status and human epidermal growth factor receptor 2 (HER2) overexpression, which support treatment decisions [6,7]. About two-thirds of BCs are HR positive (HR+), 13%–20% are HER2 positive, and 12% are classified as triple-negative BC (TNBC) [[8], [9], [10]]. Another factor that impacts BC risk and treatment options is the presence of germline mutations in BC susceptibility gene 1 or 2 (gBRCA1/2mut). These mutations occur in approximately 5% of BC cases overall and account for approximately 30% of hereditary BCs [[11], [12], [13], [14]]. Individuals with gBRCA1/2mut face substantial lifetime BC risk, estimated at 60%–70% [11,[15], [16], [17]]. According to guidelines, the choice of systemic therapy depends on various tumor and patient characteristics. Tumor characteristics include endocrine receptor and HER2 status and, for TNBC, programmed death ligand 1 (PD-L1) expression. Other factors that inform treatment decisions include disease-free interval from the end of adjuvant therapy, receipt of prior therapies (including presence/absence of persistent toxicities), duration of disease control on prior lines of therapy, sites of metastases, comorbidities, and patient preferences. [18,19] Determination of tumor biomarkers is therefore essential for individual and targeted use of a given therapy.
Current preferred regimens for HER2− ABC include chemotherapy, chemotherapy plus immunotherapy for PD-L1–positive TNBC, and platinum-based chemotherapy for select patients with gBRCA1/2mut TNBC; for HR+ disease, preferred regimens are endocrine monotherapy or endocrine therapy plus a targeted therapy [20]. For patients with HER2− ABC and a gBRCA1/2mut, treatment with poly(adenosine diphosphate-ribose) polymerase inhibitors (PARPi) has also been approved in the United States and European countries based on findings from the OlympiAD [21] and EMBRACA [22] trials, and their use is endorsed by international guidelines [23,24]. The OlympiAD and EMBRACA trials demonstrated improvements in progression-free survival outcomes and patient-reported outcome measures in patients with HER2– ABC with gBRCA1/2mut who received olaparib or talazoparib, respectively, compared with patients who received physician's choice of chemotherapy (capecitabine, vinorelbine, eribulin, or, in EMBRACA only, also included gemcitabine) [21,22,25]. In the trials, PARPi were generally well tolerated, and approximately 98% and 97% of patients who received PARPi or chemotherapy, respectively, experienced any adverse events (AEs) [21,22,25]. Grade ≥3 AEs were reported in 37% and 51% of patients who received PARPi or chemotherapy, respectively, in OlympiAD; grade 3/4 AEs were reported in 26% and 25% of patients in EMBRACA [21,26]. The most common AEs associated with PARPi were anemia, neutropenia, thrombocytopenia, nausea, vomiting, and fatigue [21,22,25].
Although the importance of real-world evidence is widely acknowledged [[27], [28], [29]], limited information is available regarding the use of PARPi in the real-world clinical setting. Patients included in clinical trials represent a small, often nonrepresentative percentage of the target population for a given drug due to strict eligibility criteria (eg, elderly patients or patients with comorbidities are often excluded). Patients treated in the real-world setting may therefore differ from those in clinical trials in numerous ways, including treatment adherence.
Information on how frequently PARPi (or other treatments) are prescribed for patients with gBRCA1/2-mutated HER2− ABC, as well as the AEs experienced by this patient population and overall satisfaction with selected treatments, would be valuable to clinicians when making evidence-based, individualized treatment decisions. To our knowledge, no study has characterized the current multinational treatment landscape for patients with gBRCA1/2-mutated HER2− ABC. This study therefore aimed to assess real-world physician-reported treatment patterns, AEs experienced by patients, and physician-reported satisfaction with treatment in adult patients with gBRCA1/2-mutated HER2− ABC in the United States, 4 EU nations (EU4; ie, Germany, France, Italy, and Spain), and Israel.
2. Methods
2.1. Study design and data source
Data were drawn from the Adelphi Real World ABC Disease Specific Programme (DSP™) during October 2019–March 2020 in the United States, EU4, and Israel. DSPs are large, multinational, point-in-time studies conducted in clinical practices that describe disease burden, disease management strategies, and responses to treatment as assessed by treating physicians [30].
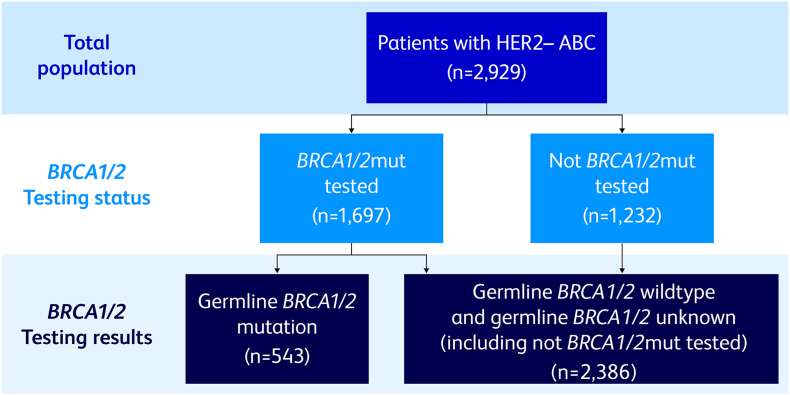
Medical oncologists evaluating ≥5 patients with ABC per month, and who were personally responsible for making treatment decisions, were recruited by local fieldwork teams. Physicians provided patient record forms (PRFs) for the next 8 eligible patients: 4 patients receiving first-line advanced treatment and 4 receiving second- or later-line advanced treatment. Eligible patients were ≥18 years of age with stage IIIb or IV HER2− BC receiving therapy for ABC at the time of data collection. Patients who were participating in a clinical trial at the time of data collection were excluded from this study. Physicians were asked to oversample patients with a gBRCA1/2mut and invited to complete up to 4 additional PRFs for patients who fit the eligibility criteria and had a confirmed gBRCA1/2mut. Patients with unknown HR status, a somatic BRCA1/2mut, or unknown BRCA1/2mut status were excluded from the analysis. Physicians were asked to designate the sample used for testing, and, if reported as done on blood, saliva, or buccal samples, this information was used to confirm that BRCA1/2mut testing was germline. For US-based patients, this was also verified by obtaining the name of the laboratory where the testing was performed; data for laboratory confirmation of test type was not available for the EU4 and Israel (Fig. 1). The PRF included detailed questions regarding patient demographics, clinical assessments, clinical outcomes, AEs experienced at the time of data collection among treated patients, treatment history, and physician-rated satisfaction with treatment. The treating physician completed the survey using patient medical records as well as clinical judgement and diagnostic skills, consistent with the decision-making process during routine clinical practice. Physicians also completed an online survey that included questions related to their patient management and treatment use and perceptions.
Fig. 1.
BRCA1/2mut status testing. ABC = advanced breast cancer; BRCA1/2mut = breast cancer susceptibility gene 1 or 2 mutation; HER2− = human epidermal growth factor receptor 2 negative.
Data were aggregated and de-identified before receipt by Adelphi Real World, and all patients provided informed consent for use of these data for research and scientific publications. This research was approved by the Western Institutional Review Board (study protocol AG8643). Data collection was undertaken in line with European Pharmaceutical Marketing Research Association guidelines [31] and, as such, did not require ethics committee approval. Surveys were administered in accordance with relevant legislation at the time of data collection, including the US Health Insurance Portability and Accountability Act of 1996 [32] and the Health Information Technology for Economic and Clinical Health Act [33].
2.2. Therapy categories
Physicians recorded the current treatment and line of therapy for each patient. Therapies included platinum-based chemotherapy (all platinum-based therapies [ie, carboplatin and cisplatin] alone or in combination with non‒platinum-based chemotherapies), non‒platinum-based chemotherapy (ie, all non‒platinum-based chemotherapies alone or in combination with each other only), chemotherapy plus immunotherapy (ie, any chemotherapy in combination with an immunotherapy/checkpoint inhibitor [all chemotherapies were non-platinum]), PARPi (ie, olaparib and talazoparib monotherapy), endocrine-based therapy (ie, all endocrine regimens [except endocrine monotherapy] plus a targeted agent defined as a cyclin-dependent kinase 4/6 [CDK4/6] inhibitor, mechanistic target of rapamycin [mTOR] inhibitor, or phosphoinositide 3-kinase catalytic subunit alpha [PIK3CA] inhibitor, or any chemotherapy), endocrine monotherapy, and other therapies (ie, therapies not classified by the above categories, such as PARPi plus immunotherapy, vascular endothelial growth factor [VEGF] inhibitor, CDK4/6 inhibitor, immunotherapy, mTOR inhibitor, and PIK3CA inhibitor monotherapies, and chemotherapy plus the following: mTOR inhibitor, VEGF inhibitor, CDK4/6 inhibitor, aromatase inhibitor [AI], AI plus VEGF inhibitor, PARPi, and selective estrogen receptor modulator [all chemotherapies were non-platinum]).
2.3. Statistical analysis
Descriptive summary statistics including means, standard deviations, medians, and ranges were calculated for continuous variables. Frequency counts and percentages were calculated for categorical variables.
Current treatment details are reported by treatment line (first, second, or third) and hormone receptor status (ie, HR+/HER2− or TNBC). AEs experienced by patients at the time of data collection and physician-rated satisfaction with treatment are reported by current treatment type.
Data were entered online using electronic forms, and the design and logic within the online system did not allow for variables to be left blank or skipped. For any remaining missing values, patients were removed from all analyses where that variable was used. There was no imputation of missing data. Analyses were performed using Stata v16.1 or later (StataCorp, College Station, TX, USA).
3. Results
3.1. Study participation and patient characteristics
A total of 192 physicians participated in the study (United States, 24; EU4, 129; Israel, 39). Collectively, these physicians reported data from 543 patients with gBRCA1/2-mutated HER2− ABC. Table 1 shows the demographic and clinical characteristics for the overall population and stratified by the treatment type received. Overall, mean (SD) patient age was 55.1 (12.9) years, and 25% of patients were premenopausal. Twenty percent of patients were currently employed; 19% were on long-term sick leave, and 70% had HR+/HER2− ABC and 30% had TNBC. Eighty-five percent of patients had a BRCA1 mutation, and 57% had a BRCA2 mutation. At collection, 85% of patients had stage IV ABC and 78% had an Eastern Cooperative Oncology Group performance status (ECOG PS) of 0 or 1 (Table 1).
Table 1.
Patient demographics and clinical characteristicsa.
| Patients (N = 543) | Platinum-Based (n = 45) | Non‒Platinum-Based (n = 80) | Chemotherapy + Immunotherapy (n = 11) | PARPi (n = 79) |
Endocrine-Based Therapy (n = 159) | Endocrine Monotherapy (n = 85) | Other (n = 84) | |
|---|---|---|---|---|---|---|---|---|
| Age, y | ||||||||
| Mean (SD) | 55.1 (12.9) | 51.4 (13.0) | 53.1 (13.5) | 46.9 (9.9) | 52.4 (11.2) | 56.7 (12.4) | 60.8 (13.6) | 53.7 (12.1) |
| Median (IQR) | 56.0 (45.0, 66.0) | 51.0 (44.0, 60.0) | 54.5 (41.0, 65.8) | 46.0 (40.0, 58.0) | 52.0 (42.0, 61.0) | 57.0 (48.0, 69.0) | 64.0 (51.5, 72.0) | 53.5 (44.0, 65.0) |
| Ethnic origin | ||||||||
| White | 334 (62) | 38 (84) | 59 (74) | 8 (73) | 54 (68) | 108 (68) | 27 (32) | 40 (48) |
| Other | 209 (38) | 7 (16) | 21 (26) | 3 (27) | 25 (32) | 51 (32) | 58 (68) | 44 (52) |
| Ashkenazi Jewish heritage | ||||||||
| Yes | 107 (20) | 3 (7) | 12 (15) | 2 (18) | 15 (19) | 24 (15) | 25 (29) | 26 (31) |
| No | 400 (74) | 36 (80) | 62 (78) | 9 (82) | 64 (81) | 126 (79) | 48 (56) | 55 (65) |
| Unknown | 36 (7) | 6 (13) | 6 (8) | 0 (0) | 0 (0) | 9 (6) | 12 (14) | 3 (4) |
| Employment status | ||||||||
| Working full-/part-time | 109 (20) | 9 (20) | 17 (21) | 6 (55) | 25 (32) | 31 (19) | 6 (7) | 15 (18) |
| On long-term sick leave | 105 (19) | 16 (36) | 16 (20) | 3 (27) | 19 (24) | 25 (16) | 8 (9) | 18 (21) |
| Homemaker | 149 (27) | 8 (18) | 21 (26) | 2 (18) | 16 (20) | 47 (30) | 30 (35) | 25 (30) |
| Student | 7 (1) | 1 (2) | 2 (3) | 0 (0) | 1 (1) | 2 (1) | 0 (0) | 1 (1) |
| Retired | 112 (21) | 7 (16) | 14 (18) | 0 (0) | 12 (15) | 44 (28) | 19 (22) | 16 (19) |
| Unemployed | 38 (7) | 2 (4) | 8 (10) | 0 (0) | 4 (5) | 7 (4) | 10 (12) | 7 (8) |
| Unknown | 23 (4) | 2 (4) | 2 (3) | 0 (0) | 2 (3) | 3 (2) | 12 (14) | 2 (2) |
| Menopausal status | ||||||||
| Premenopausal | 136 (25) | 13 (30) | 27 (34) | 5 (45) | 24 (31) | 22 (14) | 19 (23) | 26 (31) |
| Family history of BRCA-related cancer | ||||||||
| Yes | 346 (64) | 22 (49) | 43 (54) | 5 (45) | 60 (76) | 103 (65) | 53 (62) | 60 (71) |
| No | 171 (31) | 21 (47) | 33 (41) | 6 (55) | 18 (23) | 53 (33) | 18 (21) | 22 (26) |
| Unknown | 26 (5) | 2 (4) | 4 (5) | 0 (0) | 1 (1) | 3 (2) | 14 (16) | 2 (2) |
| BRCA1/2 mutation status | ||||||||
| BRCA1 mutation | 459 (85) | 38 (84) | 66 (83) | 10 (91) | 63 (80) | 138 (87) | 76 (89) | 68 (81) |
| BRCA2 mutation | 311 (57) | 20 (44) | 41 (51) | 4 (36) | 37 (47) | 97 (61) | 60 (71) | 52 (62) |
| Hormone receptor status | ||||||||
| HR+/HER2− | 381 (70) | 11 (24) | 48 (60) | 2 (18) | 32 (41) | 152 (96) | 78 (92) | 58 (69) |
| Triple-negative breast cancer | 162 (30) | 34 (76) | 32 (40) | 9 (82) | 47 (59) | 7 (4) | 7 (8) | 26 (31) |
| AJCC stage at diagnosis | ||||||||
| 0‒IIIa | 269 (50) | 13 (29) | 44 (55) | 4 (36) | 46 (58) | 72 (45) | 46 (54) | 44 (52) |
| IIIb‒IV | 273 (50) | 32 (71) | 36 (45) | 7 (64) | 33 (42) | 87 (55) | 39 (46) | 39 (46) |
| AJCC stage at time of data collection | ||||||||
| IIIb | 33 (6) | 3 (7) | 11 (14) | 0 (0) | 10 (13) | 3 (2) | 2 (2) | 4 (5) |
| IIIc | 47 (9) | 1 (2) | 9 (11) | 0 (0) | 7 (9) | 9 (6) | 10 (12) | 11 (13) |
| IV | 463 (85) | 41 (91) | 60 (75) | 11 (100) | 62 (78) | 147 (92) | 73 (86) | 69 (82) |
| ECOG PS score at time of data collection | ||||||||
| 0 | 132 (24) | 15 (33) | 24 (30) | 5 (45) | 18 (23) | 49 (31) | 10 (12) | 11 (13) |
| 1 | 293 (54) | 22 (49) | 42 (53) | 5 (45) | 52 (66) | 69 (43) | 51 (60) | 52 (62) |
| 2 | 92 (17) | 6 (13) | 13 (16) | 1 (9) | 8 (10) | 26 (16) | 18 (21) | 20 (24) |
| 3 | 12 (2) | 1 (2) | 0 (0) | 0 (0) | 1 (1) | 7 (4) | 3 (4) | 0 (0) |
| 4 | 14 (3) | 1 (2) | 1 (1) | 0 (0) | 0 (0) | 8 (5) | 3 (4) | 1 (1) |
AJCC = American Joint Committee on Cancer; BRCA = breast cancer susceptibility gene; ECOG PS = Eastern Cooperative Oncology Group performance status; HER2− = human epidermal growth factor receptor 2 negative; HR+ = hormone receptor positive; IQR = interquartile range; PARPi = poly(adenosine diphosphate-ribose) polymerase inhibitors.
All data are n (%) unless specified otherwise.
3.2. Treatment patterns
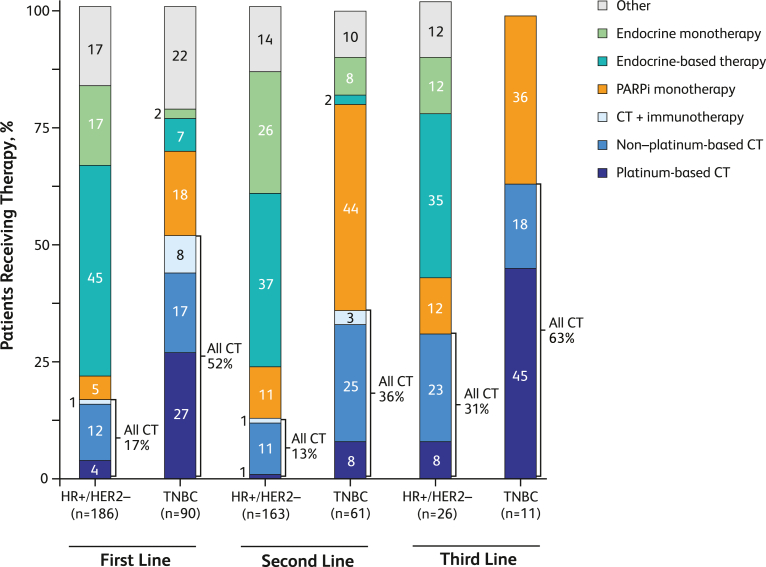
Among the 381 patients with HR+/HER2− ABC, 375 had treatment by line of therapy data available. Endocrine-based treatments were the most common across all lines of therapy, comprising 45%, 37%, and 35% of first-, second-, and third-line therapies, respectively (Fig. 2). The percentage of patients with HR+/HER2− ABC treated with PARPi increased from 5% (first line) to 11% (second line) and 12% (third line); similar treatment patterns were observed for chemotherapy, which increased from 17% to 13% in the first and second lines, respectively, to 31% in the third line. By contrast, among patients with TNBC, the most common treatments differed by line of therapy (Fig. 2). Platinum-based chemotherapy and other therapies were most frequently used as first-line therapies, at 27% and 22%, respectively. PARPi was used in the first-line at 18%. The most common second-line therapy in patients with TNBC was PARPi (44%), followed by non‒platinum-based chemotherapy (25%); the most common third-line therapy was platinum-based chemotherapy (45%) followed by PARPi (36%; Fig. 2). In patients with TNBC, the variety of treatment options narrowed with subsequent therapy lines, with third-line therapy consisting only of platinum-based chemotherapy, non‒platinum-based chemotherapy, or PARPi (Fig. 2).
Fig. 2.
Proportion of patients receiving treatment by line of therapy and hormone receptor status. CT = chemotherapy; HR+ = hormone receptor positive; HER2− = human epidermal growth factor receptor 2 negative; PARPi = poly(adenosine diphosphate-ribose) polymerase inhibitors; TNBC = triple-negative breast cancer.
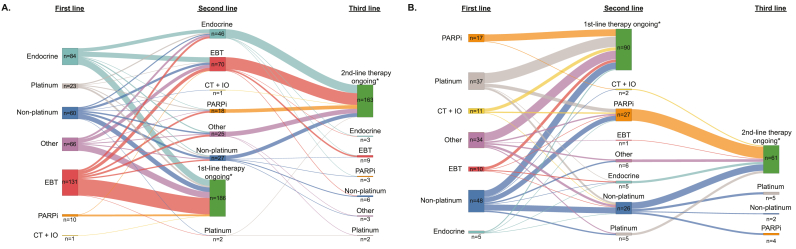
Among the HR+/HER2− ABC patients receiving endocrine-based therapy or endocrine monotherapy as a second-line therapy, the most common therapy received as a first-line treatment was endocrine monotherapy (Fig. 3A). Among those receiving non‒platinum-based chemotherapy as a second-line therapy, the most common therapy received as the first-line treatment was also non‒platinum-based chemotherapy. Of the 18 patients receiving PARPi as a second-line therapy, the most common therapy received as the first-line therapy was endocrine-based therapy. Of the 9 HR+/HER2− ABC patients receiving endocrine-based therapy as a third-line treatment, the most common therapy received as a second-line treatment was endocrine-based therapy. Among the 26 patients with TNBC receiving non‒platinum-based chemotherapy as a second-line therapy, the most common therapy received as the first-line treatment was also non‒platinum-based chemotherapy (Fig. 3B). Of the 27 patients with TNBC receiving PARPi as a second-line therapy, the most common therapies received as a first-line therapies were either non‒platinum-based or platinum-based chemotherapy. Of the 11 patients with TNBC receiving a third-line therapy, all had received non‒platinum-based chemotherapy as a second-line therapy (Fig. 3B). At the time of data collection, for both HR+/HER2− ABC and TNBC patients, a relatively large number of the patients remained on their first- or second-line treatments (Fig. 3A and B). We note that while all current treatment information was known, some patients had unknown line information (e.g. number of lines received) and therefore these were excluded from the treatment overview.
Fig. 3.
Sankey diagrams illustrating the sequence of therapies patients received. The sequence of the first through third lines of therapy for patients with HR+/HER2– advanced breast cancer (A) and TNBC (B). CT + IO = chemotherapy plus immunotherapy; EBT = endocrine-based therapy; endocrine = endocrine monotherapy; PARPi = poly(adenosine diphosphate-ribose) polymerase inhibitors; TNBC = triple-negative breast cancer. *First- or second-line patients are patients that remain on their first- or second-line of therapy, respectively.
3.3. Adverse events experienced at data collection
Table 2 shows AEs experienced by therapy type and median days on treatment across all lines of therapy at the time of data collection. Median days on treatment was relatively high (493 days) for patients receiving endocrine monotherapy. Compared to the patients receiving non–platinum-based chemotherapy (median days on treatment 163), the median days on treatment were relatively low for patients receiving platinum-based chemotherapy (84 days) and PARPi therapy (96 days). The most common AEs experienced by patients who were currently receiving treatment with chemotherapy were anemia, neutropenia, and nausea, which occurred in 38%, 22% and 33% of patients, respectively, treated with platinum-based chemotherapy and 20%, 20% and 33% of patients treated with non‒platinum-based chemotherapy. When stratified by line of therapy, among the most common AEs experienced by patients receiving platinum-based chemotherapy were also anemia, neutropenia, and nausea, which occurred in 38%, 22% and 38% of first-line patients and 83%, 50% and 33% of second-line patients (Supplementary Tables S1 and S2). The most common AEs experienced by patients receiving non–platinum-based chemotherapy as a first-line therapy were nausea (42%), anemia (26%), and neutropenia (26%), and as a second-line therapy were nausea (27%), joint/muscle pain (21%), alopecia (15%), and neutropenia (15%; Supplementary Tables S1 and S2).
Table 2.
All current adverse events experienced by >10% of patients with HER2− ABC with germline BRCA1/2 mutations.
| Adverse Event, n (%) | Chemotherapy |
PARPi n = 79 | Endocrine-Based Therapy n = 159 | Endocrine Monotherapy n = 85 | Other n = 84 | ||
|---|---|---|---|---|---|---|---|
| Platinum-Based n = 45 | Non‒Platinum-Based n = 80 | Chemotherapy + Immunotherapy n = 11 | |||||
| Arthralgia | 0 (0) | 3 (4) | 2 (18) | 10 (13) | 9 (6) | 1 (1) | 3 (4) |
| Joint/muscle pain | 2 (4) | 10 (13) | 2 (18) | 4 (5) | 15 (9) | 13 (15) | 15 (18) |
| Myalgia | 3 (7) | 4 (5) | 0 (0) | 11 (14) | 7 (4) | 2 (2) | 8 (10) |
| Anemiaa | 17 (38) | 16 (20) | 1 (9) | 19 (24) | 18 (11) | 7 (8) | 10 (12) |
| Neutropeniaa | 10 (22) | 16 (20) | 2 (18) | 13 (16) | 30 (19) | 6 (7) | 9 (11) |
| Low platelet count | 5 (11) | 3 (4) | 1 (9) | 9 (11) | 0 (0) | 1 (1) | 2 (2) |
| Diarrhea | 4 (9) | 10 (13) | 1 (9) | 12 (15) | 20 (13) | 8 (9) | 13 (15) |
| Loss of appetite | 5 (11) | 12 (15) | 2 (18) | 15 (19) | 20 (13) | 10 (12) | 15 (18) |
| Nausea | 15 (33) | 26 (33) | 1 (9) | 25 (32) | 45 (28) | 30 (35) | 31 (37) |
| Vomiting | 6 (13) | 10 (13) | 0 (0) | 17 (22) | 23 (14) | 19 (22) | 19 (23) |
| Alopecia | 9 (20) | 13 (16) | 0 (0) | 1 (1) | 4 (3) | 2 (2) | 16 (19) |
| Asthenia | 10 (22) | 7 (9) | 0 (0) | 10 (13) | 17 (11) | 4 (5) | 5 (6) |
| Disturbed sleep | 1 (2) | 2 (3) | 1 (9) | 7 (9) | 3 (2) | 4 (5) | 2 (2) |
| Fatigue | 5 (11) | 12 (15) | 4 (36) | 14 (18) | 39 (25) | 12 (14) | 13 (15) |
| Weight loss | 4 (9) | 9 (11) | 1 (9) | 5 (6) | 5 (3) | 6 (7) | 5 (6) |
| Median days on treatment (min, max) | 84 (0, 3249) | 163 (4, 4316) | 73 (28, 465) | 96 (4, 2857) | 153 (2, 2471) | 493 (3, 2827) | 154 (6, 3275) |
ABC = advanced breast cancer; BRCA1/2 = breast cancer susceptibility gene 1 or 2; HER2− = human epidermal growth factor receptor 2 negative; PARPi = poly(adenosine diphosphate-ribose) polymerase inhibitors.
Anemia and neutropenia were not defined on the patient record questionnaire.
Anemia was also the second most common AE experienced by patients who were currently receiving treatment with PARPi (24% of patients); neutropenia occurred in 16% of these patients (Table 2). When stratified by line of therapy, anemia was common in patients receiving PARPi as a second- or third-line therapy (both 29%) but occurred in only 12% of patients as first-line therapy (Supplementary Tables S1–S3). Nausea was the most common AE experienced by patients receiving PARPi, occurring in 32% of patients overall (Table 2), including 44% of first-line and 31% of second-line treated patients (Supplementary Tables S1 and S2).
Nausea was also the most common AE experienced by patients receiving endocrine-based therapy (28%), endocrine monotherapy (35%), and other therapies (37%; Table 2). The second most common AE experienced by patients receiving endocrine-based therapy was fatigue (25%) and by patients receiving endocrine monotherapy was vomiting (22%; Table 2). Neutropenia was relatively common in patients receiving endocrine-based therapy, occurring in 24% and 15% of patients as first- and second-line therapy, respectively (Supplementary Tables S1 and S2). Fatigue was the most common AE experienced by patients receiving chemotherapy plus immunotherapy (36%), although there were few patients in this group (n = 11), limiting interpretation of this observation.
3.4. Physician satisfaction
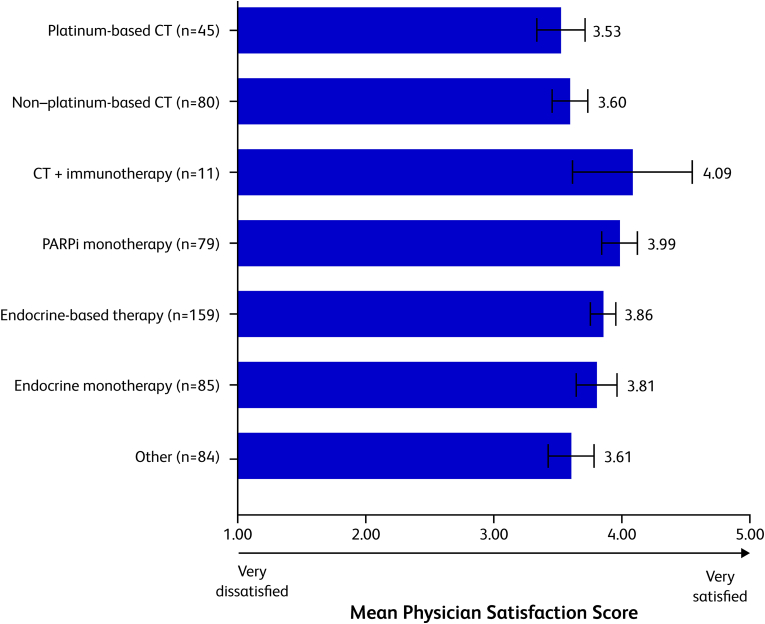
Fig. 4 shows physician satisfaction with each of the treatments across all patients. Overall, physicians reported being least satisfied with platinum-based chemotherapy and non‒platinum-based chemotherapy, with mean satisfaction scores of 3.53 (95% CI, 3.34–3.72) and 3.60 (3.46–3.74), respectively, on a scale of 1 (very dissatisfied) to 5 (very satisfied). The 2 therapies that physicians were most satisfied with were chemotherapy plus immunotherapy (4.09 [3.62–4.56]) and PARPi therapy (3.99 [3.85–4.13]).
Fig. 4.
Physician satisfaction with current therapy. Physicians were asked which of the following best describes their satisfaction with the current treatment for this patient's ABC on a scale of 1 (very dissatisfied) to 5 (very satisfied). Mean physician satisfaction scores are shown. Error bars represent 95% confidence intervals. ABC = advanced breast cancer; CT = chemotherapy; PARPi = poly(adenosine diphosphate-ribose) polymerase inhibitors.
4. Discussion
The safety and efficacy of PARPi in patients with gBRCA1/2-mutated ABC have been established in randomized controlled trials [21,22,25]. In this retrospective analysis of data from the Adelphi Real World DSP, we evaluated patient demographic and clinical characteristics, treatment patterns, AEs experienced, and physician-rated satisfaction with treatment in a real-world population of physicians and their patients with HER2− ABC.
In comparing these real-world data with those obtained during pivotal phase 3 clinical trials, it is important to note that the patients in this study were older (median age, 56 years) at the time of data collection compared with the median ages of patients in the OlympiAD study [21] (44 and 45 years for those treated with olaparib and chemotherapy, respectively) and EMBRACA study [22] (45 and 50 years for those treated with talazoparib and chemotherapy). Additionally, the percentage of patients with an ECOG PS score of 0 was 24% in the current study, whereas corresponding percentages were 72% and 64% in the olaparib- and chemotherapy-treated groups, respectively, in OlympiAD and 53% and 58% in the talazoparib- and chemotherapy-treated groups in EMBRACA [21,22]. Such differences between clinical trial populations and real-world populations highlight the importance of understanding therapeutic treatment patterns and efficacy in routine clinical practice.
Among all patients in the current study, 276 patients received first-line therapy, 224 received second-line therapy, and few had third-line therapy (n = 37). In OlympiAD, patients were eligible if they had undergone ≤2 lines of chemotherapy for metastatic disease, and, in EMBRACA, eligibility was limited to ≤3 lines; as such, the number of lines of therapy received in the current study was on par with both of these trials.
In examining treatment patterns, we found that endocrine therapies (endocrine-based and endocrine monotherapies) were the most common in patients with HR+/HER2− ABC across all lines; used in 62%, 63% and 47% of first-, second-, and third-line patients, respectively. Among patients with HR+/HER2− ABC, chemotherapy use of any type was relatively low as a first- or second-line treatment, 17% and 13% of patients, respectively, but increased to 31% in the third line. PARPi was less commonly used for patients with HR+/HER2− ABC (5%, 11% and 12% in first, second, and third lines, respectively). Among patients with TNBC, platinum-based chemotherapy was the most common therapy in first- and third-line patients, while non-platinum based chemotherapy was the most common therapy in the second-line patients. Endocrine therapies were used in first- and second-line TNBC therapy, but in fewer than 10% of patients. It is unclear why a small proportion of patients with TNBC received endocrine therapy. It is possible that the estrogen receptor status of these patients had changed during the course of treatment; however, we did not capture this information for verification. Among second-line therapies in patients with TNBC, PARPi were used at the highest rate (44%), which was higher than the rate of all chemotherapies (ie, platinum, non-platinum and chemotherapy plus immunotherapy; 36%). In the third-line setting in these patients, PARPi were used at a rate of 36% and chemotherapies (all combined) at 63%. However, the use of PARPi in the first-line was low at 18%. Chemotherapy in combination with immunotherapy was rarely used in patients with HR+/HER2− ABC, and in TNBC patients was used in only 8% of first-line patients and 3% of second-line patients.
Grade ≥3 AEs were reported in 37% and 51% of patients who received PARPi or chemotherapy, respectively, in OlympiAD; grade 3/4 AEs were reported in 26% and 25% of patients in EMBRACA. In the current observational study, AE grades were not standardized across the different sites/countries and thus could not be provided.
In this study, the AEs experienced by patients at the time of data collection with PARPi and chemotherapies were generally consistent in type but somewhat less frequent than those reported in clinical trials. According to a recent meta-analysis of the OlympiAD and EMBRACA trials, an occurrence of any AE was reported in 98% of patients treated with PARPi and 97% of patients treated with chemotherapy [25]. Nausea and anemia were the 2 most common AEs observed in patients treated with PARPi in both the clinical trials and in this study but were more frequent in the clinical trials (53% and 48%, respectively) [25] than in this study (32% and 24%). Neutropenia was the most common AE associated with chemotherapy in the meta-analysis of the OlympiAD and EMBRACA trials, reported at a rate of 46% of the patients receiving chemotherapy and 32% of patients receiving PARPi [25]. In this study, neutropenia was the second most common AE in patients receiving non‒platinum-based chemotherapy, occurring at a rate of 20%, and was reported in 16% of patients receiving PARPi. Fatigue was also relatively common among patients in the clinical trials, reported at rates of 41% and 35% in patients receiving PARPi and chemotherapy. In this study fatigue occurred at rates of 18% and 15% in patients receiving PARPi and chemotherapy, respectively.
Although direct comparisons of AEs experienced by patients between different treatments was not performed in this study, we do note comparable rates of hematologic and gastrointestinal toxicities experienced by patients receiving platinum-based–chemotherapy and PARPi therapy. Anemia, nausea, fatigue, and loss of appetite were experienced by 38%, 33%, 11% and 11%, respectively, of patients receiving platinum-based–chemotherapy and 24%, 32%, 18% and 19% of patients receiving PARPi.
Not surprisingly, given that hormonal therapy is generally better tolerated than chemotherapy, physician satisfaction was higher with endocrine therapy than with chemotherapy (both platinum and non‒platinum-based). Physician satisfaction with PARPi therapy, which was routinely used as a second- or third-line therapy in patients with TNBC, was higher than with platinum-based and non‒platinum-based chemotherapies. The relatively high satisfaction with PARPi likely reflects tolerability and efficacy in patients with HER2− ABC, as observed in clinical trials.
Our findings should be interpreted considering some limitations. Patients selected from the database may not be representative of the general population of patients with BRCA1/2-mutated HER2− ABC, potentially limiting external validity. Patients who visit their physician more frequently, and therefore might be more likely to be included in this study, may be more severely affected. Also, the DSP is not based on true random samples of physicians or patients, and identification of the target patient group is based on physician judgement, not a formalized diagnostic checklist. Additionally, not all countries had access to PARPi at the time of data collection, which may have influenced physicians’ choice of therapy. Furthermore, point-in-time designs do not allow for the determination of causal relationships, such as between specific treatments and observed AEs; however, the identification of associations is possible. As with all retrospective studies, recall bias might have affected participant respondents. Also, AEs are not systematically collected in accordance with a study protocol in real-world studies and as such may be underreported; however, underreporting would be across all respondents. Because AEs were assessed at the time of data collection, they cannot be directly attributed to drug treatment. In addition, physicians reported that 85% and 57% of patients had BRCA1 and BRCA2 mutations, respectively. These data suggest that about 40% of patients had both BRCA1 and BRCA2 mutations; however, the rate of BRCA1 and BRCA2 co-mutation among patients with a BRCA mutation is around 1–2%. Thus, it is likely many physicians did not distinguish between BRCA1 and BRCA2 mutations and incorrectly reported/selected both mutations on the PRF. Finally, physician reported mutation testing in blood was used as a proxy for germline BRCA1/2mut testing. Because blood is used as a source material for testing of circulating tumor DNA, it cannot be verified that all testing done on blood samples was germline testing only. Despite such limitations, real-world studies play an important role in highlighting areas of concern that are not addressed and in identifying treatment patterns, outcomes, and AEs that are not represented, in clinical trials.
5. Conclusions
Based on the efficacy and tolerability of PARPi demonstrated in clinical trials, treatment of patients with gBRCA1/2-mutated ABC with PARPi was approved in the United States and European countries, and international guidelines recommend their use [23,24]. In this real-world population of patients with gBRCA1/2-mutated HER2− ABC from the United States, the EU4, and Israel, PARPi use is reflected in real-world care, especially in TNBC in the second and third line. However, owing to the efficacy and tolerability of PARPi and the high level of physician satisfaction with their use, there is still potential for their increased use for patients with TNBC and HR+/HER2− to avoid or postpone chemotherapy use. Overall AE rates reported during treatment with PARPi were similar to rates in patients treated with chemotherapies, although they were somewhat lower than rates reported in clinical trials. With the exception of chemotherapy in combination with immunotherapy, physician-reported satisfaction with PARPi treatment was higher than with any other type of therapy. Our findings provide further support for the added value of treatment with PARPi in patients with gBRCA1/2-mutated HER2− ABC.
Funding
This study was sponsored by Pfizer Inc. Pfizer was involved in study design, interpretation of data, writing the report, and the decision to submit the report for publication.
Availability of data and materials
Data collection was undertaken by Adelphi Real World as part of an Adelphi Disease Specific Programmes independent survey sponsored by multiple pharmaceutical companies, one of which was Pfizer Inc. Pfizer did not influence the original survey through either contribution to the design of questionnaires or data collection. The study described here using data from the Adelphi Disease Specific Programmes was funded by Pfizer. Publication of study results was not contingent on the sponsor's approval or censorship of the manuscript.
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Katie Lewis at katie.lewis@adelphigroup.com.
Declaration of competing interest
RM contracted research funding from Genentech and acted as consultant for Agendia, Amgen, AstraZeneca, Biotheranostics, Daiichi Sankyo, Eisai, Eli Lilly, Genentech, Immunomedics, Merck, Novartis, Pfizer, Puma, Sanofi, and SeaGen. AN and BA are employees of and own stock in Pfizer Inc. KL, AR, and LM are employees of Adelphi Real World. MPL received honoraria for lectures, consulting, or advisory role for AstraZeneca, Daiichi Sankyo, Eisai, Eli Lilly, Exact Sciences, Gilead, Grünenthal, Medac, MSD, Novartis, Pfizer, PharmaMar, Pierre Fabre, and Roche and travel and accommodations expenses from Pfizer and Roche.
Acknowledgments
This study was funded by Pfizer Inc and Adelphi Real World in accordance with Good Publication Practice (GPP3) guidelines. Medical writing support was provided by John Teiber, PhD, of ICON (Blue Bell, PA).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2022.10.009.
Contributor Information
Reshma Mahtani, Email: rmahtani@baptisthealth.net.
Alexander Niyazov, Email: Alexander.Niyazov@pfizer.com.
Bhakti Arondekar, Email: Bhakti.Arondekar@pfizer.com.
Katie Lewis, Email: katie.lewis@adelphigroup.com.
Alex Rider, Email: Alex.Rider@adelphigroup.com.
Lucy Massey, Email: lucy.massey@adelphigroup.com.
Michael Patrick Lux, Email: M.Lux@vincenz.de.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Bray F., Ferlay J., Soerjomataram I., et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Ginsburg O.M., Fischer H.D., Shah B.R., et al. A population-based study of ethnicity and breast cancer stage at diagnosis in Ontario. Curr Oncol. 2015;22(2):97–104. doi: 10.3747/co.22.2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maclean R., Jeffreys M., Ives A., et al. Primary care characteristics and stage of cancer at diagnosis using data from the national cancer registration service, quality outcomes framework and general practice information. BMC Cancer. 2015;15:500. doi: 10.1186/s12885-015-1497-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Reinert T., Barrios C.H. Optimal management of hormone receptor positive metastatic breast cancer in 2016. Ther Adv Med Oncol. 2015;7(6):304–320. doi: 10.1177/1758834015608993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Walters S., Maringe C., Butler J., et al. Breast cancer survival and stage at diagnosis in Australia, Canada, Denmark, Norway, Sweden and the UK, 2000-2007: a population-based study. Br J Cancer. 2013;108(5):1195–1208. doi: 10.1038/bjc.2013.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hortobagyi G.N., Connolly J.L., D’Orsi C.J., et al. In: AJCC cancer staging manual. 8th ed. Amin M.B., Edge S.B., Greene F.L., et al., editors. Springer International Publishing; 2017. Breast; pp. 58–636. [Google Scholar]
- 7.Hortobagyi G.N., Edge S.B., Giuliano A. New and important changes in the TNM staging system for breast cancer. Am Soc Clin Oncol Educ Book. 2018;38:457–467. doi: 10.1200/EDBK_201313. [DOI] [PubMed] [Google Scholar]
- 8.Rakha E.A., Pinder S.E., Bartlett J.M., et al. Updated UK recommendations for HER2 assessment in breast cancer. J Clin Pathol. 2015;68(2):93–99. doi: 10.1136/jclinpath-2014-202571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wolff A.C., Hammond M.E., Hicks D.G., et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. [DOI] [PubMed] [Google Scholar]
- 10.Nelson D.R., Brown J., Morikawa A., Method M. Breast cancer-specific mortality in early breast cancer as defined by high-risk clinical and pathologic characteristics. PLoS One. 2022;17(2) doi: 10.1371/journal.pone.0264637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Antoniou A., Pharoah P.D., Narod S., et al. Average risks of breast and ovarian cancer associated with BRCA1 or BRCA2 mutations detected in case series unselected for family history: a combined analysis of 22 studies. Am J Hum Genet. 2003;72(5):1117–1130. doi: 10.1086/375033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.King M.C., Marks J.H., Mandell J.B. Breast and ovarian cancer risks due to inherited mutations in BRCA1 and BRCA2. Science. 2003;302(5645):643–646. doi: 10.1126/science.1088759. [DOI] [PubMed] [Google Scholar]
- 13.Peto J., Collins N., Barfoot R., et al. Prevalence of BRCA1 and BRCA2 gene mutations in patients with early-onset breast cancer. J Natl Cancer Inst. 1999;91(11):943–949. doi: 10.1093/jnci/91.11.943. [DOI] [PubMed] [Google Scholar]
- 14.Couch F.J., Hu C., Hart S.N., et al. Age-related breast cancer risk estimates for the general population based on sequencing of cancer predisposition genes in 19,228 breast cancer patients and 20,211 matched unaffected controls from US based cohorts in the CARRIERS study. Cancer Res. 2019;79(4_Supplement) GS2-01. [Google Scholar]
- 15.Begg C.B., Haile R.W., Borg A., et al. Variation of breast cancer risk among BRCA1/2 carriers. JAMA. 2008;299(2):194–201. doi: 10.1001/jama.2007.55-a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gabai-Kapara E., Lahad A., Kaufman B., et al. Population-based screening for breast and ovarian cancer risk due to BRCA1 and BRCA2. Proc Natl Acad Sci U S A. 2014;111(39):14205–14210. doi: 10.1073/pnas.1415979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuchenbaecker K.B., Hopper J.L., Barnes D.R., et al. Risks of breast, ovarian, and contralateral breast cancer for BRCA1 and BRCA2 mutation carriers. JAMA. 2017;317(23):2402–2416. doi: 10.1001/jama.2017.7112. [DOI] [PubMed] [Google Scholar]
- 18.Thill M., Friedrich M., Kolberg-Liedtke C., et al. AGO recommendations for the diagnosis and treatment of patients with locally advanced and metastatic breast cancer: update 2021. Breast Care. 2021;16(3):228–235. doi: 10.1159/000516420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cardoso F., Paluch-Shimon S., Senkus E., et al. 5th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 5) Ann Oncol. 2020;31(12):1623–1649. doi: 10.1016/j.annonc.2020.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Referenced with permission from the NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines®) for Breast Cancer V.4.2022. © National Comprehensive Cancer Network, Inc. 2022 All rights reserved. Accessed July 15, 2022. To view the most recent and complete version of the guideline, go online to NCCN.org. NCCN makes no warranties of any kind whatsoever regarding their content, use or application and disclaims any responsibility for their application or use in any way. [Google Scholar]
- 21.Robson M., Im S.A., Senkus E., et al. Olaparib for metastatic breast cancer in patients with a germline BRCA mutation. N Engl J Med. 2017;377(6):523–533. doi: 10.1056/NEJMoa1706450. [DOI] [PubMed] [Google Scholar]
- 22.Litton J.K., Rugo H.S., Ettl J., et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–763. doi: 10.1056/NEJMoa1802905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cardoso F., Senkus E., Costa A., et al. 4th ESO-ESMO international consensus guidelines for advanced breast cancer (ABC 4) Ann Oncol. 2018;29(8):1634–1657. doi: 10.1093/annonc/mdy192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gradishar W.J., Anderson B.O., Abraham J., et al. Breast cancer, version 3.2020, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Cancer Netw. 2020;18(4):452–478. doi: 10.6004/jnccn.2020.0016. [DOI] [PubMed] [Google Scholar]
- 25.Poggio F., Bruzzone M., Ceppi M., et al. Single-agent PARP inhibitors for the treatment of patients with BRCA-mutated HER2-negative metastatic breast cancer: a systematic review and meta-analysis. ESMO Open. 2018;3(4) doi: 10.1136/esmoopen-2018-000361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Litton J., Rugo H.S., Ettl J., et al. EMBRACA: a phase 3 trial comparing talazoparib, an oral PARP inhibitor, to physician's choice of therapy in patients with advanced breast cancer and a germline BRCA mutation. Cancer Res. 2018;78(4 Supplement) GS6-07. [Google Scholar]
- 27.Corrigan-Curay J., Sacks L., Woodcock J. Real-world evidence and real-world data for evaluating drug safety and effectiveness. JAMA. 2018;320(9):867–868. doi: 10.1001/jama.2018.10136. [DOI] [PubMed] [Google Scholar]
- 28.Khozin S., Blumenthal G.M., Pazdur R. Real-world data for clinical evidence generation in oncology. J Natl Cancer Inst. 2017;109(11):djx187. doi: 10.1093/jnci/djx187. [DOI] [PubMed] [Google Scholar]
- 29.Plueschke K., McGettigan P., Pacurariu A., Kurz X., Cave A. EU-funded initiatives for real world evidence: descriptive analysis of their characteristics and relevance for regulatory decision-making. BMJ Open. 2018;8(6) doi: 10.1136/bmjopen-2018-021864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anderson P., Benford M., Harris N., Karavali M., Piercy J. Real-world physician and patient behaviour across countries: disease-specific programmes - a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. doi: 10.1185/03007990802457040. [DOI] [PubMed] [Google Scholar]
- 31.European Pharmaceutical Marketing Research Association . 2019. EphMRA code of conduct.https://www.ephmra.org/media/2811/ephmra-2019-code-of-conduct-doc-f.pdf Available at: [Google Scholar]
- 32.Atlantic.net. HIPAA compliance: important fundamentals you need to know. https://www.hipaajournal.com/wp-content/uploads/2020/02/HIPAA-Compliance-Fundamentals-Atlantic-Net-Whitepaper-2018.pdf
- 33.Office for Civil Rights OCR privacy brief: summary of the HIPAA privacy rule. https://www.hhs.gov/sites/default/files/ocr/privacy/hipaa/understanding/summary/privacysummary.pdf
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data collection was undertaken by Adelphi Real World as part of an Adelphi Disease Specific Programmes independent survey sponsored by multiple pharmaceutical companies, one of which was Pfizer Inc. Pfizer did not influence the original survey through either contribution to the design of questionnaires or data collection. The study described here using data from the Adelphi Disease Specific Programmes was funded by Pfizer. Publication of study results was not contingent on the sponsor's approval or censorship of the manuscript.
All data that support the findings of this study are the intellectual property of Adelphi Real World. All requests for access should be addressed directly to Katie Lewis at katie.lewis@adelphigroup.com.