Abstract
Mycobacterium avium subsp. paratuberculosis infection of cattle results in a chronic granulomatous enteritis. Clinical disease (i.e., cachexia, diarrhea, and high fecal bacterial counts) is preceded by a lengthy subclinical stage of disease. The immunologic mechanisms associated with the progression of infected cattle from subclinical to clinical disease are unclear. In this study, a cell proliferation assay was used in combination with flow cytometry to compare peripheral blood lymphocyte responses of cattle with subclinical paratuberculosis to responses of cattle with clinical paratuberculosis. B cells from cattle with subclinical disease proliferated vigorously upon stimulation with M. avium subsp. paratuberculosis antigen, with up to 12.4% of the total B cells responding. However, B cells from cattle with clinical disease did not proliferate upon antigen stimulation despite good proliferation in response to concanavalin A stimulation. In addition, these animals had high percentages of peripheral blood B cells. B cells from noninfected animals did not proliferate upon M. avium subsp. paratuberculosis antigen stimulation. Thus, it appears that B-cell proliferation is a sensitive indicator of subclinical Johne’s disease. Furthermore, the immunologic mechanisms responsible for the antigen-specific unresponsiveness of peripheral blood B cells may be significant in the eventual progression from subclinical to clinical Johne’s disease in cattle.
Paratuberculosis, or Johne’s disease, is caused by the intracellular bacterium Mycobacterium avium subsp. paratuberculosis (6, 21). In ruminants, the bacterium infects macrophages within the intestinal mucosa and mesenteric lymph nodes, inducing a chronic granulomatous enteritis (9, 27). Ruminants are usually infected at an early age through ingestion of M. avium subsp. paratuberculosis-contaminated milk or feces (6). In field conditions, animals may be infected for ≥3 years without developing clinical signs of disease. During this subclinical stage of disease, the animals generally have undetectable levels of M. avium subsp. paratuberculosis-specific serum antibody and increasing gamma interferon (IFN-γ) responses to M. avium subsp. paratuberculosis and shed undetectable to low numbers of bacteria in feces (2, 19). Clinical disease is characterized by chronic diarrhea, cachexia, and eventual death, with abundant specific serum antibody, decreasing IFN-γ responses, and high numbers of bacteria shed in feces (2, 6, 8, 9, 19). Thus, it appears that cell-mediated immune responses keep bacterial shedding under control, and a switch to a humoral immune response is associated with the progression of cattle to clinical disease and increased bacterial shedding. Similar paradigms have been extensively characterized for other mycobacterial infections (15, 23).
T-cell immune responses are essential in limiting the severity of paratuberculosis infection (1, 7, 14, 24). Clearly, antibody production affords little, if any, protection against this intracellular pathogen. However, B cells can provide support for T-cell responses through antigen presentation and costimulatory function (10, 24). In the present study, we examined in vitro antigen-specific proliferative responses of peripheral blood lymphocyte subsets isolated from M. avium subsp. paratuberculosis-infected or noninfected cattle. Surprisingly, proliferative responses of B cells from cattle with subclinical disease were as much as 6.5 times greater than proliferative responses of T cells from the same animals. Furthermore, animals with clinical signs of disease had severely depressed B- and T-cell proliferative responses and abnormally high percentages of peripheral blood B cells. These findings suggest that the progression of paratuberculosis in cattle from subclinical to clinical disease is associated with peripheral blood lymphocyte unresponsiveness, which is most remarkable within the B-cell subset.
MATERIALS AND METHODS
Animals, bacterial culture, and antigen.
The animal groups used consisted of three noninfected and six M. avium subsp. paratuberculosis-infected Holstein cows. Infection was determined by a standard fecal culture method and following a previously described procedure (20). All animals were housed in American Association for Accreditation of Laboratory Animal Care-accredited facilities (National Animal Disease Center, Ames, Iowa) in temperature- and humidity-controlled rooms. Antigen for use in in vitro assays was prepared by sonication of 1-ml volumes of M. avium subsp. paratuberculosis (strain 19698; 109/ml) at 25 W for 25 min as previously described (20).
Lymphocyte blastogenesis.
Peripheral blood mononuclear cells (PBMC) were isolated from buffy coat fractions of peripheral blood collected in 2× acid citrate dextrose by standard procedures (5). Wells of 96-well round-bottom microtiter plates (Falcon, Becton Dickinson, Lincoln Park, N.J.) were seeded with 2 × 105 PBMC in a total volume of 200 μl/well. The medium was RPMI 1640 (Fisher Scientific, Pittsburgh, Pa.) supplemented with 100 U of penicillin/ml, 0.1 mg of streptomycin/ml, 5 × 10−5 M 2-mercaptoethanol (Sigma, St. Louis, Mo.), and 10% fetal bovine serum (Atlanta Biologics, Atlanta, Ga.). The wells contained either concanavalin A (Con-A) (5 μg/ml; Sigma), M. avium subsp. paratuberculosis antigen (10 μg/ml; whole-cell sonicate), or medium alone (no stimulation). The plates were then incubated at 37°C in a 5% CO2 humidified atmosphere for 4 days. After 4 days, 0.5 μCi of [methyl-3H]thymidine (specific activity, 6.7 Ci mmol−1; Amersham Life Science, Arlington Heights, Ill.) in 10 μl of medium was added to each well, and the plates were incubated for an additional 20 h. The well contents were harvested onto glass fiber filters with a PHD cell harvester (Cambridge Technology, Cambridge, Mass.), and incorporated radioactivity was measured by liquid scintillation counting. Treatments were run in triplicate, and stimulation indices (SI) were calculated by dividing counts min−1 of stimulated wells by counts min−1 from nonstimulated wells. The data are presented as SI ± standard error of the mean SEM.
PKH2 proliferation assay.
The basis for the PKH2 proliferation assay is that upon cell division, cells stained with the green fluorescent dye PKH2 (Sigma) demonstrate a 50% reduction in fluorescence intensity. The assay was performed according to the manufacturer’s instructions. Briefly, 2 × 107 PBMC were centrifuged (400 × g) for 5 min, the supernatants were aspirated, and the cells were resuspended in 1 ml of diluent (Sigma). The cells, in diluent, were added to 1 ml of PKH2 (4 × 10−6 M) and incubated for 5 min followed by a 1-min incubation with 2 ml of FBS to stop the reaction. The cells were then washed three times with RPMI 1640, and a portion of the cells were analyzed by two-color flow cytometry to determine preculture cell surface marker expression and the efficiency of PKH2 staining. The remaining cells were added to triplicate wells of a 96-well round-bottom microtiter plate in medium (no stimulation), medium plus 10 μg of a whole-cell sonicate of M. avium subsp. paratuberculosis antigen/ml, or medium plus 5 μg of Con-A/ml and incubated at 37°C in a 5% CO2 humidified atmosphere for 5 days. The cells were then analyzed by flow cytometry for PKH2 staining as well as cell surface marker expression. Modfit Proliferation Wizard software (Verity Software House Inc., Topsham, Maine) was used for cell proliferation analyses, and Cellquest software (Becton Dickinson, San Jose, Calif.) was used for phenotype analyses. The data are presented as the number of cells proliferating in antigen- or ConA-stimulated wells minus the number of cells proliferating in nonstimulated wells. Proliferation profiles were determined for both gated (i.e., CD3+-, CD4+-, CD8+-, γδ-T-, and B-cell) and ungated (total PBMC) populations and are presented as the number of cells proliferating/5,000 PBMC.
Flow cytometric analysis.
PBMC were analyzed by flow cytometry following standard procedures (26). Briefly, 5 × 105 cells were incubated with primary monoclonal antibody to bovine leukocyte surface antigens (MM1A, CD3; GC50A1, CD4; CACT80A, CD8; CACT61A, γδ T cells; or BAQ155A, B cells) (VMRD, Pullman, Wash.) for 15 min, washed, incubated with phycoerythrin-conjugated goat anti-mouse immunoglobulin (Ig) (Southern Biotechnology Associates, Inc., Birmingham, Ala.) for 15 min, washed, and resuspended for analysis by flow cytometry (FACScan; Becton Dickinson).
IFN-γ assay.
One milliliter of heparinized whole blood was added to individual wells of a 24-well tissue culture plate (Falcon, Becton Dickinson). The wells were then treated with 10 μl of phosphate-buffered saline (PBS) (nonstimulated), 10 μg of Con-A/ml (positive control), 10 μg of M. avium purified protein derivative (PPD; Commonwealth Serum Laboratories, Victoria, Australia)/ml, or 10 μg of a whole-cell sonicate of M. avium subsp. paratuberculosis antigen/ml. The blood cultures were then incubated at 39°C in a 5% CO2 humidified atmosphere for 18 h. The plates were then centrifuged at 1,800 × g for 10 min, and the plasma was harvested and stored at −20°C for later analysis. Plasma IFN-γ concentration was determined by enzyme-linked immunosorbent assay (ELISA) with a commercial kit (Commonwealth Serum Laboratories). Briefly, plasma was incubated in 96-well plates precoated with antibody to bovine IFN-γ for 1 h at room temperature (RT). The wells were then washed six times with PBS, and the secondary antibody (horseradish peroxidase-conjugated mouse anti-bovine IFN-γ) was added to the wells. The plates were incubated for 60 min at RT. The wells were washed with PBS, tetra-methylbenzidine was added to the wells, and the plates were incubated for 30 min at RT. Dilute hydrochloric acid was added to the wells to stop the reaction, and absorbance (A450) readings were obtained on an MR7000 microplate reader (Dynatech, Chantilly, Va.). Absorbance readings of >0.1 for antigen-stimulated cultures compared to nonstimulated cultures were considered positive (the accepted method used by diagnostic laboratories for this kit).
Serum antibody ELISA.
A whole-cell sonicate preparation of M. avium subsp. paratuberculosis (strain 19698, previously described) was diluted in PBS and added to 96-well microtiter plates (Corning, Park Ridge, Ill.). The plates were incubated overnight in a humidified atmosphere at 4°C, washed three times with PBS plus 0.05% Tween 20 (PBST), and incubated for 30 min at 39°C with PBS containing 1% gelatin to block nonspecific binding sites. The plates were washed three times with PBST, test sera were diluted 1:400 in PBS and added to the wells, and the plates were incubated for 1 h at 39°C. The plates were washed three times with PBST, mouse anti-bovine IgM (BIG73A; VMRD) or mouse anti-bovine IgG (BG-18; Sigma) was added to the wells, and the plates were incubated for 1 h at 39°C. The plates were washed three times with PBST, biotinylated F(ab′)2 fragments of sheep anti-mouse Ig (Amersham, Arlington Heights, Ill.) were added to the wells, and the plates were incubated for 2 h at 39°C. The plates were washed three times with PBST, streptavidin peroxidase was added to the wells, and the plates were incubated for 30 min at 39°C. The plates were washed three times with PBST, substrate solution (40 mM ABTS [2,2′-azino-di-ethylbenzthiozoline-6-sulfonic acid] in citrate buffer, pH 4.0) was added to the wells, and the plates were incubated for 10 min at RT. Negative and positive control sera were included for each assay. The absorbances of test samples were read at 405 (test wavelength) and 490 (reference wavelength) nm with a Dynatech MR7000 plate reader.
Statistics.
Statistical evaluation was performed by one-way analysis of variance and either Tukey-Kramer multiple comparison tests or the Mann-Whitney test with a commercially available statistics program (InStat 2.00; GraphPAD Software, San Diego, Calif.).
RESULTS
Bacterial culture and clinical status.
As a measure of clinical disease, animals were examined for fecal shedding of M. avium subsp. paratuberculosis and clinical signs of disease, including diarrhea and cachexia. At the time of the study, animals 167, 5247, and 323 were shedding at least 50 CFU of M. avium subsp. paratuberculosis organisms per g of feces. Prior to the study, animals 10, 107, and 1072 shed small amounts (<10 CFU/g of feces) of M. avium subsp. paratuberculosis organisms, yet several fecal cultures obtained during the study failed to detect any organisms from these three animals. This was consistent with previous observations that animals with subclinical disease are often negative for M. avium subsp. paratuberculosis growth upon fecal culture. Control animals (420, 423, and 477) were negative upon biannual fecal cultures for M. avium subsp. paratuberculosis taken over a 5-year period prior to and including the time of the study. Animals 5247 and 167 had clinical signs of Johne’s disease (e.g., cachexia and diarrhea). Animal 5247 was subsequently euthanized due to clinical deterioration at the conclusion of this study. No signs of clinical Johne’s disease were noted in the other animals.
Proliferative responses and phenotype.
The PKH2 proliferation assay is a new procedure for examining proliferation of PBMC. We compared the results of this assay to results obtained with a standard [3H]thymidine uptake proliferation assay (Fig. 1). With both assays, PBMC from animals 10, 1072, and 323 had strong proliferative responses upon stimulation with M. avium subsp. paratuberculosis antigen. Weak antigen-specific responses were detected for animals 107, 167, and 5247 by the PKH2 assay; however, responses were undetectable for these three animals by the [3H]thymidine uptake assay. PBMC from control animals did not proliferate in response to antigen stimulation. Similar results were obtained in three independent experiments with the same animals plus additional infected and control animals (data not shown).
FIG. 1.
Comparison of two assays for antigen-specific proliferation of PBMC isolated from noninfected (controls) and M. avium subsp. paratuberculosis-infected cattle (identified by number below the bars). The hatched bars represent the number of cells proliferating/5,000 PBMC in response to 10 μg of antigen (whole-cell sonicate of M. avium subsp. paratuberculosis)/ml as measured by the PKH2 assay (see Materials and Methods). The solid bars represent the SI of PBMC in response to antigen as measured by [3H]thymidine uptake (see Materials and Methods).
An advantage of the PKH2 proliferation assay compared to other proliferation assays is the ability to simultaneously detect individual subsets of cells proliferating in response to stimulation. In the present study, all subsets of cells examined (i.e., CD3+, CD4+, CD8+, γδ T, and B cells), from both infected and noninfected animals, proliferated in response to Con-A stimulation (Table 1). In addition, as a group, all subsets of cells tested from M. avium subsp. paratuberculosis-infected animals were capable of proliferation in response to antigen stimulation (Table 1). However, the best antigen-specific responses were detected in B-cell populations, with responses ranging from 0 to 12.4% proliferation (i.e., percent of total B cells within cultures proliferating in response to in vitro antigen stimulation). In comparison, antigen-specific T-cell responses ranged from 0 to 4.1%.
TABLE 1.
Mean number of cells proliferating in response to stimulation with either Con-A or M. avium subsp. paratuberculosis antigena
| Cell type | No. of cells proliferating
|
|||||
|---|---|---|---|---|---|---|
| Ungated | CD3+ | CD4+ | CD8+ | B | γδ T | |
| Con-A stimulated | ||||||
| Controls (n = 3) | 2,033 ± 417 | 773 ± 92 | 186 ± 67 | 210 ± 46 | 913 ± 209 | 423 ± 40 |
| Infected (n = 6) | 2,417 ± 308 | 947 ± 245 | 182 ± 52 | 202 ± 52 | 916 ± 50 | 714 ± 231 |
| Antigen stimulated | ||||||
| Controls (n = 3) | 0 ± 0 | 6.4 ± 5.8 | 5.4 ± 5.4 | 4.2 ± 4.2 | 6.1 ± 6.1 | 14.4 ± 9.8 |
| Infected (n = 6) | *233 ± 88 | 19.1 ± 7.9 | 13.5 ± 8.2 | 11.5 ± 4.3 | 62.2 ± 34.5 | 20.9 ± 6.6 |
PBMC were labeled with PKH2 and incubated in round-bottom 96-well tissue culture plates for 5 days at 37°C and 5% CO2 with or without Con-A (5 μg/ml) or antigen (10 μg/ml) stimulation. The cells were then harvested and analyzed by flow cytometry for expression of cell surface molecules and PKH2 staining. The data represent the mean number of cells proliferating ± SEM in response to either Con-A or antigen stimulation per 5,000 PBMC for ungated or gated (i.e., CD3+-, CD4+-, CD8+-, B-, and γδ-T-cell) samples as analyzed by Modfit Proliferation Wizard software. The mean number of cells proliferating was calculated by subtracting the number of cells proliferating in nonstimulated wells from the number of cells proliferating in either Con-A- or antigen-stimulated wells. *, significantly greater (P < 0.05) than controls.
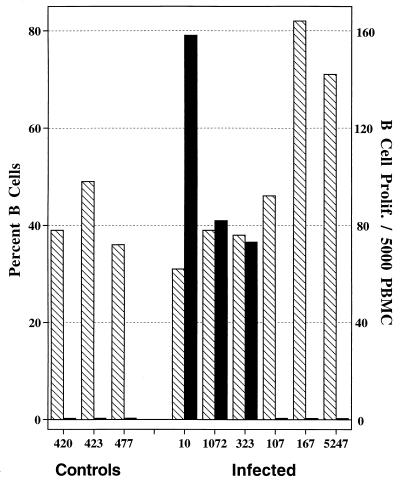
Differences in antigen-specific proliferative responses of animals at different stages of disease were most remarkable for the B-cell subset. Three of six M. avium subsp. paratuberculosis-infected animals (10, 1072, and 323) had strong B-cell antigen-specific proliferative responses (Fig. 2). Proliferation values for B cells from these three animals represented 4.7 to 12.4% of the total population of peripheral blood B cells. B cells from two animals (167 and 5247) with clinical Johne’s disease (i.e., weight loss and high fecal bacterial counts) did not proliferate upon antigen stimulation despite normal proliferation in response to Con-A stimulation. Interestingly, these two animals also had very high percentages of peripheral blood B cells. Elevated percentages of peripheral blood B cells have been associated with bovine leukemia virus infection; however, these animals were negative upon serologic evaluation for bovine leukemia virus-specific antibody (data not shown). Minimal to no B-cell proliferation was detected in noninfected animals upon antigen stimulation. As shown in Fig. 3A to D, the percentage of B cells in the peripheral blood of animals with subclinical disease was similar to percentages of B cells in the peripheral blood of noninfected animals. In addition, cattle with subclinical disease had a higher percentage (11%) of cells proliferating (i.e., decreased intensity of PKH2 staining) in antigen-stimulated cultures (Fig. 3D) compared to PKH2 staining in nonstimulated cultures (Fig. 3C). Upon cell division, the intensity of PKH2 staining of daughter cells diminishes in comparison to that of the parent generation. Cattle with clinical disease had high percentages of B cells yet did not have a diminished intensity of PKH2 staining in antigen-stimulated cultures (Fig. 3F) compared to that in nonstimulated cultures (Fig. 3E).
FIG. 2.
Antigen-specific B-cell proliferation of PBMC isolated from noninfected (controls) and M. avium subsp. paratuberculosis-infected cattle (identified by number below the bars). The hatched bars represent the percentages of PBMC that are B cells as detected by flow cytometry. The solid bars represent the number of B cells proliferating/5,000 PBMC in response to 10 μg of antigen (whole-cell sonicate of M. avium subsp. paratuberculosis)/ml as measured by the PKH2 assay (see Materials and Methods).
FIG. 3.
Flow cytometric analysis of PKH2 staining of B cells from cultures of PBMC from noninfected and M. avium subsp. paratuberculosis-infected cattle. The PBMC were stained with PKH2 prior to culture, cultured with or without antigen (whole-cell sonicate), and harvested for flow cytometric analysis after 5 days of incubation. The cells were examined for expression of BAQ155A (a cell surface marker of bovine B cells) and PKH2 staining. Cells from subclinical animals had a lower intensity of PKH2 staining in antigen-stimulated cultures (D) compared to nonstimulated cultures (C), whereas animals with clinical disease (E and F) and noninfected animals (A and B) had similar PKH2 staining patterns in nonstimulated and antigen-stimulated cultures. The decreased intensity of PKH2 staining (as detected in antigen-stimulated cultures from subclinical animals) indicates cell division, since daughter cells have reduced PKH2 staining within their membranes compared to that of their parent generation. Animals with clinical disease (E and F) had higher percentages of B cells compared to noninfected animals (A and B) and animals with subclinical disease (C and D). PE, phycoerythrin.
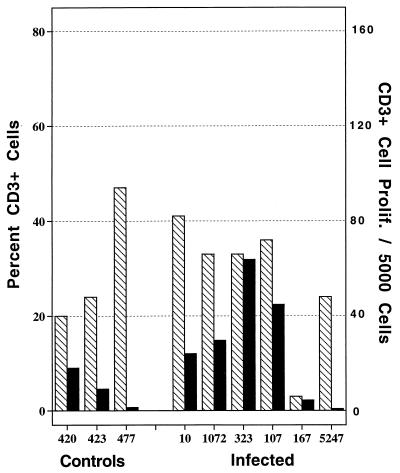
In response to in vitro mycobacterial antigen stimulation, T-cell proliferative responses were weaker than B-cell proliferative responses. However, four of six infected animals and one of three control animals had detectable levels of CD3+-cell proliferation in response to antigen stimulation (Fig. 4). Proliferation values for T cells from these animals represented 0.7 to 4.1% of the total population of peripheral blood T cells. CD3+ cells from the single control animal (420) which proliferated upon antigen stimulation had a concurrent γδ-T-cell proliferative response, suggesting nonspecific proliferation of γδ T cells in response to antigen stimulation. Infected animals had detectable levels of CD4+-, CD8+-, and γδ-T-cell proliferation in response to antigen stimulation (Table 1). Animals 323, 167, and 5247 had the lowest percentages of CD4+ cells (data not shown); interestingly, these three animals were also positive for M. avium subsp. paratuberculosis upon bacteriological culture of their feces. Two of these animals (167 and 5247) also had clinical signs of disease and low B-cell antigen-specific proliferative responses (Fig. 2). In a subsequent experiment, decreased CD4/CD8 ratios were also detected for animals with clinical disease compared to those of animals with subclinical disease (data not shown).
FIG. 4.
Antigen-specific T-cell proliferation of PBMC isolated from control (noninfected) and M. avium subsp. paratuberculosis-infected cattle (identified by number below the bars). The hatched bars represent the percentages of PBMC that are T cells (CD3+) as detected by flow cytometry. The solid bars represent the number of T cells proliferating/5,000 PBMC in response to 10 μg of antigen (whole-cell sonicate of M. avium subsp. paratuberculosis)/ml as measured by the PKH2 assay (see Materials and Methods).
IFN-γ.
As a measure of cell-mediated immunity, the production of IFN-γ by PBMC was measured. Positive responses to Con-A stimulation were detected for all animals (data not shown). Positive responses to M. avium PPD were detected for all infected animals examined except 1072 and one control animal, 420 (Fig. 5). Interpretation of responses to M. avium PPD is often difficult due to cross-reactivity with other mycobacterial antigens (20a). Positive IFN-γ responses to M. avium subsp. paratuberculosis antigen (whole-cell sonicate) were detected for paratuberculosis-infected animals 10 and 323 (Fig. 5). Responses from these two animals were consistently positive at several time points (data not shown). In tests run prior to the present study, positive IFN-γ responses were detected for animals 107, 1072, and 167. Thus, even though inconsistently, infected animals produced IFN-γ in response to antigenic (whole-cell sonicate) stimulation.
FIG. 5.
IFN-γ production of whole-blood cultures from control (noninfected) and M. avium subsp. paratuberculosis-infected cattle (identified by number below the bars). The open bars represent optical density readings from nonstimulated cultures, the hatched bars represent those from M. avium PPD-stimulated cultures, and the solid bars represent those from antigen (10 μg of whole-cell sonicate of M. avium subsp. paratuberculosis/ml)-stimulated cultures as measured by a commercially available ELISA kit. Optical density (OD) readings of >0.1 for antigen-stimulated cultures compared to nonstimulated cultures were considered positive (the accepted method used by diagnostic laboratories for this kit).
Serum antibody.
Positive IgM levels (absorbance values greater than 0.2) were detected for animals 10, 107, and 167 (Table 2). All other animals had insignificant levels of M. avium subsp. paratuberculosis-specific serum IgM. Positive IgG levels were detected for animals 323 and 167 (Table 2). All other animals had insignificant levels of M. avium subsp. paratuberculosis-specific IgG.
TABLE 2.
Serum antibody responsesa
| Animal no. | Status | IgM | IgG |
|---|---|---|---|
| 424 | Control | 0.141 | 0.021 |
| 423 | Control | 0.115 | 0.092 |
| 477 | Control | ND | ND |
| Mean | Control | 0.128 | 0.057 |
| SEM | Control | 0.013 | 0.036 |
| 10 | Infected | 0.247 | 0.141 |
| 1072 | Infected | 0.088 | 0.034 |
| 323 | Infected | 0.161 | 0.537 |
| 107 | Infected | 0.394 | 0.051 |
| 167 | Infected | 0.201 | 0.590 |
| 5247 | Infected | ND | ND |
| Mean | Infected | 0.218 | 0.271 |
| SEM | Infected | 0.051 | 0.121 |
Data represent optical density readings of ELISA (see Materials and Methods) for serum antibody from individual animals. The group means ± SEM are presented in bold face. Serum was diluted 1:400. ND, not determined.
DISCUSSION
The immunologic mechanisms associated with the progression of paratuberculosis from subclinical to clinical disease have not been determined. Animals with clinical signs of disease generally have high levels of antigen-specific serum antibody in conjunction with increased bacterial replication and fecal shedding of M. avium subsp. paratuberculosis (8, 17). Increased serum antibody associated with clinical deterioration and increased bacterial replication suggest that a switch to a humoral immune response signals progression towards clinical disease. The most intriguing finding in this study was that animals with subclinical Johne’s disease demonstrated antigen-specific B-cell proliferative responses while animals with clinical Johne’s disease had minimal to no antigen-specific B-cell proliferative responses. Interestingly, animals with clinical disease had higher percentages of peripheral blood B cells while animals with subclinical disease had percentages of peripheral blood B cells similar to those of control animals. Thus, it appears that progression from subclinical to clinical Johne’s disease is accompanied by increases in peripheral blood B cells and decreases in peripheral blood antigen-specific B-cell proliferative responses.
It is surprising that antigen-specific B-cell proliferative responses are weak in animals with clinical Johne’s disease, since significant levels of serum M. avium subsp. paratuberculosis-specific antibody are detected in these animals. These results imply that antigen-responsive B cells are no longer present in the peripheral blood of clinically affected animals; yet, elsewhere in the body, terminally-differentiated antigen-specific plasma cells are producing antibody. Thus, it is possible that antigen-specific lymphocytes have trafficked to effector sites, resulting in diminished peripheral blood B-cell proliferative responses through lack of peripheral antigen-specific B cells. It is also possible that T cells necessary for B-cell proliferation have redistributed to sites other than the peripheral blood.
T-helper (CD4+) cells produce the majority of IFN-γ in response to M. avium subsp. paratuberculosis infection of cattle and are necessary for antigen-specific proliferation of PBMC from these animals (2). Depletion or inhibition of these cells could lead to a progression from subclinical (cell-mediated immunity) to clinical (humoral immunity) disease (2). Indeed, CD4/CD8 ratios decrease in chronically infected animals, suggesting a relative depletion of peripheral CD4+ cells or, conversely, an increase in CD8+ cells (7, 26a). Also, within tuberculoid-type lesions of M. avium subsp. paratuberculosis-infected sheep (analagous to subclinical disease of cattle) there are increased numbers of CD4+ T cells compared to the number in lepromatous-type lesions of infected sheep (analagous to clinical disease of cattle) (13, 16). Similar redistributions may also occur with paratuberculosis of cattle, resulting in diminished peripheral blood B-cell proliferative responses by animals with clinical disease.
In addition to a redistribution of antigen-specific lymphocytes to sites other than the peripheral blood, decreased B-cell proliferative responses could result from T- and/or B-cell anergy. Mycobacterial infections often induce antigen-specific T-cell anergy, resulting in progression of disease (3, 4, 7, 18, 22, 25). Mechanisms of anergy could include suppression by host serum factors (e.g., antibody or immune complexes), host cell factors (e.g., CD8+ suppressor cells or cytokines), or bacterium-derived factors (3, 11, 12). Clinical Johne’s disease is accompanied by a rise in antigen-specific serum antibody, especially IgG. Thus, it is also possible that reduced B-cell responsiveness of animals with clinical Johne’s disease may be due, at least in part, to antibody-mediated B-cell anergy.
In conclusion, our results indicate that the measurement of B-cell proliferation in response to antigen stimulation is a sensitive indicator of M. avium subsp. paratuberculosis infection as well as clinical progression of paratuberculosis. Cattle with subclinical infection have strong B-cell proliferative responses and normal numbers of peripheral blood B cells, whereas animals with clinical disease have weak B-cell proliferative responses and abnormally high percentages of peripheral blood B cells. The mechanisms of B-cell unresponsiveness in animals with clinical disease remain unclear.
ACKNOWLEDGMENTS
We thank Trudy Bosworth, Andrea Dorn, and Michele Penland for excellent technical assistance.
This research was supported in part by funding provided by the Iowa Livestock Health Advisory Council.
REFERENCES
- 1.Appelberg R, Castro A G, Pedrosa J, Silva R A, Orme I M, Minoprio P. Role of gamma interferon and tumor necrosis factor alpha during T-cell-independent and -dependent phases of Mycobacterium avium infection. Infect Immun. 1994;62:3962–3971. doi: 10.1128/iai.62.9.3962-3971.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bassey E O, Collins M T. Study of T-lymphocyte subsets of healthy and Mycobacterium avium subsp. paratuberculosis-infected cattle. Infect Immun. 1997;65:4869–4872. doi: 10.1128/iai.65.11.4869-4872.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bianca N E. The immunopathology of systemic anergy in infectious diseases: a reappraisal and new perspective. Clin Immunol Immunopathol. 1992;62:253–257. doi: 10.1016/0090-1229(92)90099-a. [DOI] [PubMed] [Google Scholar]
- 4.Bloom B R, Mehra V. Immunological unresponsiveness in leprosy. Immunol Rev. 1984;80:5. doi: 10.1111/j.1600-065x.1984.tb00493.x. [DOI] [PubMed] [Google Scholar]
- 5.Burton J L, Kehrli M E., Jr Effects of dexamethasone on bovine circulating T lymphocyte populations. J Leukoc Biol. 1996;59:90–99. doi: 10.1002/jlb.59.1.90. [DOI] [PubMed] [Google Scholar]
- 6.Chiodini R J, Van Kruiningen H J, Merkal R S. Ruminant paratuberculosis (Johne’s disease): the current status and future prospects. Cornell Vet. 1984;74:217–262. [PubMed] [Google Scholar]
- 7.Chiodini R J, Davis W C. The cellular immunology of bovine paratuberculosis: the predominant response is mediated by cytotoxic gamma/delta T lymphocytes which prevent CD4+ activity. Microb Pathog. 1992;13:447–463. doi: 10.1016/0882-4010(92)90012-d. [DOI] [PubMed] [Google Scholar]
- 8.Clarke C J, Little D. The pathology of ovine paratuberculosis: gross and histological changes in the intestine and other tissues. J Comp Pathol. 1996;114:419–437. doi: 10.1016/s0021-9975(96)80017-x. [DOI] [PubMed] [Google Scholar]
- 9.Clarke C J. The pathology and pathogenesis of paratuberculosis in ruminants and other species. J Comp Pathol. 1997;116:217–261. doi: 10.1016/s0021-9975(97)80001-1. [DOI] [PubMed] [Google Scholar]
- 10.Dunlap N E, Briles D E. Immunology of tuberculosis. Med Clin N Am. 1993;77:1235–1251. doi: 10.1016/s0025-7125(16)30190-0. [DOI] [PubMed] [Google Scholar]
- 11.Hussein J M, Kerr M A, Swanson J, Sheriff M. The mechanism of action of the factor in leprosy serum that inhibits the growth of mitogen-stimulated normal human lymphocytes. Immunology. 1987;61:124–129. [PMC free article] [PubMed] [Google Scholar]
- 12.Kerr M A, Hussein J M, Potts R C, Swanson J, Sheriff M M. Characterization of a factor in leprosy serum that inhibits growth of mitogen-stimulated normal human lymphocytes. Immunology. 1987;61:117–123. [PMC free article] [PubMed] [Google Scholar]
- 13.Little D, Alzuherri H M, Clarke C J. Phenotypic characterization of intestinal lymphocytes in ovine paratuberculosis by immunohistochemistry. Vet Immunol Immunopathol. 1996;55:175–187. doi: 10.1016/s0165-2427(96)05716-9. [DOI] [PubMed] [Google Scholar]
- 14.Orme I M, Furney S K, Roberts A D. Dissemination of enteric Mycobacterium avium infections in mice rendered immunodeficient by thymectomy and CD4 depletion or by prior infection with murine AIDS retrovirus. Infect Immun. 1992;60:4747–4753. doi: 10.1128/iai.60.11.4747-4753.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Orme I M. Immunity to mycobacteria. Curr Opin Immunol. 1993;5:497–502. doi: 10.1016/0952-7915(93)90029-r. [DOI] [PubMed] [Google Scholar]
- 16.Perez V, Garcia Marin J F, Badiola J J. Description and classification of different types of lesions associated with natural paratuberculosis infection of sheep. J Comp Pathol. 1996;114:107–122. doi: 10.1016/s0021-9975(96)80001-6. [DOI] [PubMed] [Google Scholar]
- 17.Perez V, Tellechea J, Badiola J J, Gutierrez M, Garcia Marin J F. Relation between serologic response and pathologic findings in sheep with naturally acquired paratuberculosis. Am J Vet Res. 1997;58:799–803. [PubMed] [Google Scholar]
- 18.Salgame P, Modin R, Bloom B R. On the mechanism of human T cell suppression. Int Immunol. 1989;1:121–129. doi: 10.1093/intimm/1.2.121. [DOI] [PubMed] [Google Scholar]
- 19.Stabel J R. Production of γ-interferon by peripheral blood mononuclear cells: an important diagnostic tool for detection of subclinical paratuberculosis. J Vet Diagn Investig. 1996;8:345–350. doi: 10.1177/104063879600800311. [DOI] [PubMed] [Google Scholar]
- 20.Stabel J R. An improved method for cultivation of Mycobacterium paratuberculosis from bovine fecal samples and comparison to three other models. J Vet Diagn Investig. 1997;9:375–380. doi: 10.1177/104063879700900406. [DOI] [PubMed] [Google Scholar]
- 20a.Stabel, J. R. Unpublished observation.
- 21.Thorel M F, Krichevsky M, Levy-Frebault V V. Numerical taxonomy of mycobactin-dependent mycobacteria, emended description of Mycobacterium avium, and description of Mycobacterium avium subsp. avium subsp. nov., Mycobacterium avium subsp. paratuberculosis subsp. nov., and Mycobacterium avium subsp. silvaticum subsp. nov. Int J Syst Bacteriol. 1990;40:254–260. doi: 10.1099/00207713-40-3-254. [DOI] [PubMed] [Google Scholar]
- 22.Ulrich M, Rodriguez V, Centeno M, Convit J. Differing antibody isotypes in the polar forms of leprosy and cutaneous leishmaniasis characterized by antigen-specific T cell anergy. Clin Exp Immunol. 1995;100:54–58. doi: 10.1111/j.1365-2249.1995.tb03603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Verhagen C E, Wierenga E A, Buffing A A M, Chand M A, Faber W R, Das P K. Reversal reaction in borderline leprosy is associated with a polarized shift to type 1-like Mycobacterium leprae T cell reactivity in lesional skin. J Immunol. 1997;159:4474–4483. [PubMed] [Google Scholar]
- 24.Vordermeier H M, Venkataprasad N, Harris D P, Ivanyi J. Increase of tuberculous infection in the organs of B cell-deficient mice. Clin Exp Immunol. 1996;106:312–316. doi: 10.1046/j.1365-2249.1996.d01-845.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wadee A A, Sher R, Rabson A R. Production of a suppressor factor by human adherent cells treated with mycobacteria. J Immunol. 1980;125:1380–1386. [PubMed] [Google Scholar]
- 26.Waters W R, Harp J A, Nonnecke B J. Phenotypic analysis of peripheral blood lymphocytes and intestinal intra-epithelial lymphocytes in calves. Vet Immunol Immunopathol. 1995;48:249–259. doi: 10.1016/0165-2427(95)05430-e. [DOI] [PubMed] [Google Scholar]
- 26a.Waters, W. R. Unpublished observation.
- 27.Zurbrick B G, Czuprynski C J. Ingestion and intracellular growth of Mycobacterium paratuberculosis within bovine blood monocytes and monocyte-derived macrophages. Infect Immun. 1987;55:1588–1593. doi: 10.1128/iai.55.7.1588-1593.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]