Abstract
Background
Diffuse midline glioma (DMG) which occurs in midline structures and characterized by harboring K27M mutation in genes encoding the histone 3 protein is classified as World Health Organization (WHO) grade IV regardless of histological findings and has a poor prognosis. Nevertheless, because of its relatively rare incidence compared with other high-grade gliomas, a comprehensive description encompassing clinical features and genomic profiles of DMG is still lacking.
Methods
In this study, we analyzed data of 24 patients who were diagnosed as DMG which was confirmed by surgical specimens in both pediatric and adult patients. We described the clinical outcomes of patients with DMG and their genomic profiles through a retrospective analysis of 24 patients with DMG.
Results
The clinical characteristics of the 24 patients with DMG were analyzed. Ten patients (41%) underwent tumor resection and 14 patients (59%) underwent tumor biopsy. The median overall survival was 10.4 months (95% confidence interval [CI], 8.4 to 12.5) and progression free survival was 3.9 months (95% CI, 2.6 to 5.2). Fifteen patients (62%) were accompanied by hydrocephalus. None of the patient, tumor, or treatment factors had any significant associated with survival. In both immunohistochemistry staining (n=24) and targeted next generation sequencing (n=15), TP53 mutation was the most common genetic mutation (25% and 46%, respectively) found in the patients except alterations in histone 3 protein.
Conclusion
Although surgical treatment of patient with DMG does not affect the overall survival prognosis, it can help improve the patient’s accompanying neurological symptoms in some limited cases. Hydrocephalus is often accompanied with DMG and treatment for hydrocephalus is often also required. Multidisciplinary therapeutic approach is needed.
Keywords: Diffuse intrinsic pontine glioma, Histone H3, Biopsy, Chemoradiotherapy, Sequence analysis, Survival
INTRODUCTION
Diffuse midline glioma (DMG) is a newly defined entity in the 4th revised edition of the World Health Organization (WHO) Classification of Tumors of the Central Nervous System (CNS) updated in 2016 [1]. It usually occurs in midline structures including thalamus, brainstem, or spinal cord. It is characterized by substitution of methionine for lysine at site 27 (K27M) in histone 3 protein. Subsequent analyses have demonstrated that histone H3 K27M mutations exist in the majority of high-grade infiltrative astrocytoma arising within midline structures of both pediatric and adult patients [2]. It is classified as WHO grade IV regardless of histological findings, and the prognosis is known to be poor. In the latest 5th edition of the WHO classification, the nomenclature of DMG H3 K27M-mutant has been changed to DMG H3 K27-altered [3,4].
Because of its rarity compared to other high-grade gliomas, a comprehensive description encompassing clinical features and genomic profiles of DMG is still lacking. In this study, we described and analyzed the clinical data of 24 patients who were diagnosed as DMG which was confirmed by surgical specimens in both pediatric and adult patients.
METHODS
Following the approval from the Institutional Review Board (IRB No. 2022-0850) of Asan Medical Center (Seoul, Korea) with a waiver of informed consent, we retrospectively reviewed medical records and images of the 24 patients who were pathological diagnosed as DMG, H3 K27M-altered through biopsy or surgery performed between January 2016 and July 2021 (Table 1). Two patients who were diagnosed as DMG in spinal cord and one foreign patient who returned to his home country immediately after biopsy without any clinical information were excluded from the analysis. Medical records and images were reviewed retrospectively with respect to the patient’s sex and age at diagnosis, symptoms at presentation, tumor location, tumor size, accompanying hydrocephalus, type of treatment, histopathological characteristics, and survival periods.
Table 1. Clinical features and prognosis of diffuse midline glioma of the 24 patients.
| Case No. | Age/sex | Presentation | Location | Size (cm) | Ki-67 LI (%) | Treatment | HC | Tx for HC | PFS (mo) | FU (mo) | Status | Remarks |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 34/M | Seizure | Thalamus | 5.3 | N/A | Biopsy | No | Loss | N/A | N/A | ||
| 2 | 14/M | Motor weakness | Spinal cord | 4.3 | N/A | STR | No | Loss | 5 | Death | ||
| 3 | 49/M | Dizziness | Diffuse | 4.1 | 5 | Biopsy & CCRT | No | 10 | 140 | Alive | ||
| 4 | 25/F | Facial palsy | Thalamus | 3.8 | 50 | Biopsy & CCRT | Yes | VPS | 4 | 14 | Death | |
| 5 | 18/M | Diplopia | Pons | 5.1 | 20 | Biopsy & CCRT | No | 2 | 9 | Death | Previously diagnosed as astrocytoma | |
| 6 | 5/M | Gait disturbance | Pons | 5.4 | N/A | Biopsy & RTx | Yes | ETV | 5 | 9 | Death | |
| 7 | 7/F | Gait disturbance | Pons | 6.1 | 30 | Biopsy & CCRT | Yes | ETV | 5 | 12 | Death | |
| 8 | 24/F | Diplopia | Thalamus | 5.3 | N/A | Biopsy | No | 9 | 9 | Death | ||
| 9 | 16/F | Headache, nausea | Thalamus | 1.5 | 10 | Biopsy & CCRT | Yes | ETV | 3 | 13 | Death | |
| 10 | 7/M | Headache, nausea | Pons | 5.6 | 20 | PR & CCRT | Yes | ETV | 3 | 7 | Death | Tumor bleeding |
| 11 | 64/M | Dizziness | Medulla | 3.6 | N/A | Biopsy | Yes | ETV | 5 | 5 | Death | |
| 12 | 4/F | Dysarthria | Pons | 4.6 | 30 | PR & RTx | No | 9 | 13 | Death | Tumor bleeding | |
| 13 | 26/F | Motor weakness | Pons | 4.6 | 40 | Biopsy & CCRT | Yes | ETV | 0 | 13 | Death | Previously diagnosed as primitive neuroepithelial tumor |
| 14 | 15/F | Motor weakness | Pons | 3.9 | N/A | STR & CCRT | Yes | VPS | 5 | 14 | Death | |
| 15 | 8/F | Headache, nausea | Thalamus | 6.5 | 60 | PR & CCRT | No | 3 | 10 | Death | Leptomeningeal seeding | |
| 16 | 39/M | Seizure | Basal ganglia | 5.4 | 10 | Biopsy & CCRT | No | 1 | 3 | Alive | ||
| 17 | 42/F | Diplopia | Midbrain | 3.1 | N/A | Biopsy & RTx | Yes | ETV | 3 | 7 | Death | Leptomeningeal seeding |
| 18 | 20/F | Motor weakness | Thalamus | 7.1 | 15 | STR & RTx | Yes | Resection | 2 | 7 | Alive | |
| 19 | 8/F | Facial palsy | Cerebellum | 4.9 | 30 | PR & CCRT | Yes | Resection | 1 | 8 | Alive | Previously diagnosed as subependymoma |
| 20 | 7/M | Diplopia | Pons | 4.1 | 5 | PR & RT | Yes | ETV | 3 | 3 | Death | |
| 21 | 73/M | Gait disturbance | Midbrain | 3.1 | N/A | Biopsy & CCRT | Yes | VPS | 1 | 5 | Alive | |
| 22 | 12/M | Headache, nausea | Thalamus | 4.1 | N/A | STR & CCRT | Yes | ETV | - | 4 | Alive | |
| 23 | 38/M | Motor weakness | Spinal cord | 3.2 | 5 | PR & CCRT | - | 2 | Alive | |||
| 24 | 36/F | Seizure | Thalamus | 1.7 | 20 | Biopsy & CCRT | Yes | VPS | - | 3 | Alive |
Ki-67 LI, Ki-67 labeling index; HC, hydrocephalus; PFS, progression free survival; FU, follow up; N/A, not available; CCRT, concurrent chemoradiation therapy; RTx, radiation therapy; ETV, endosopic third ventriculostomy; VPS, ventriculoperitoneal shunt; STR, subtotal resection; RT, radiotherapy; PR, partial resection
The size of the tumor was measured by the longest diameter of the tumor. The location of the tumor was assessed according to the epicenter of the lesion. The surgical extent of DMG was classified into three groups based on the followings: 1) gross total resection (GTR) as absence of a residual lesions, based on postoperative MRI, 2) subtotal resection (STR) as the removal of 50%–90% of tumor volume; and 3) partial resection (PR) as the removal below 50% of tumor volume and biopsy only as biopsy without intent to tumor resection.
The diagnosis of DMG was made by the confirmation of H3K27M mutation utilizing immunostaining for the histone H3 K27 mutation or targeted next generation sequencing (NGS). Targeted NGS was performed in 15 patients. Oncopanel AMC V4, a NGS based assay which identifies genomic alterations in 323 cancer-related genes was used.
Overall survival (OS) and progression-free survival (PFS) were calculated and correlation between survival and other factors such as age, sex, location, size, surgical removal, and Ki-67 labeling index were analyzed. Statistical analysis, especially Kaplan-Meier survival curve and log-rank test, were conducted using IBM SPSS Statistics ver. 21 (IBM Corp., Armonk, NY, USA). Univariate OS and PFS analyses were conducted to identify prognostic factors. Ninetyfive percent confidence interval (CI) was calculated for survival analyses. The p-value of <0.05 was considered statistically significant.
RESULTS
Patient characteristics
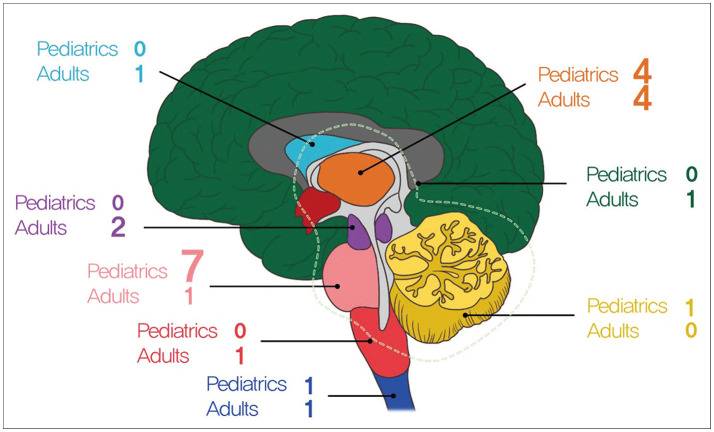
The clinical characteristics of the 24 patients with DMG were summarized in Table 1. There were 12 male and 12 female patients with a mean age of 24 years (range, 4–73 years). Most of the tumor located in brainstem (46%), among which pons were the most common in 8 patients, midbrain in 2 patient and medulla in 1 patient, followed by thalamus in 8 (33%), cerebellum in 1 patient, basal ganglia in 1 patient, spinal cord in 2 patients, and diffuse from thalamus to cerebellum in 1 patient (Fig. 1).
Fig. 1. Age distribution of the patients. Green, diffuse (from thalamus–pons–cerebellum); skyblue, basal ganglia; orange, thalamus; purple, midbrain; pink, pons; dark pink, medulla; blue, spinal cord; yellow, cerebellum.
Distribution of age and tumor location is marked in Fig. 1. There are 13 children, of which 7 had tumors in pons, the most common location. In contrast, only one adult had tumors in the pons. The presenting symptoms of the patients included as follows: cranial nerve palsy in 6 patients (25%), weakness in 5 patients (20%), headache and vomiting in 4 patients (16%), gait disturbance in 3 patients, seizure in 3 patients, dizziness in 2 patients, and dysarthria in 1 patient.
Ten patients (41%) underwent tumor resection and 14 patients (59%) underwent tumor biopsy. Among tumor resection groups, 4 patients underwent STR and 6 patients got PR and there was no GTR case. Fifteen patients (62%) were accompanied by hydrocephalus. Among the hydrocephalus patients, 1 patient got tumor resection and resolved that problem directly and 5 patients underwent ventriculo-peritoneal shunt, 9 patients underwent endoscopic third ventriculostomy. After surgical treatment or biopsy, 15 patients (62%) underwent both chemotherapy and radiotherapy and 5 patients (21%) underwent radiotherapy only and 4 patients (16%) were not treated after pathologic confirmation.
Survival outcome and prognostic factors
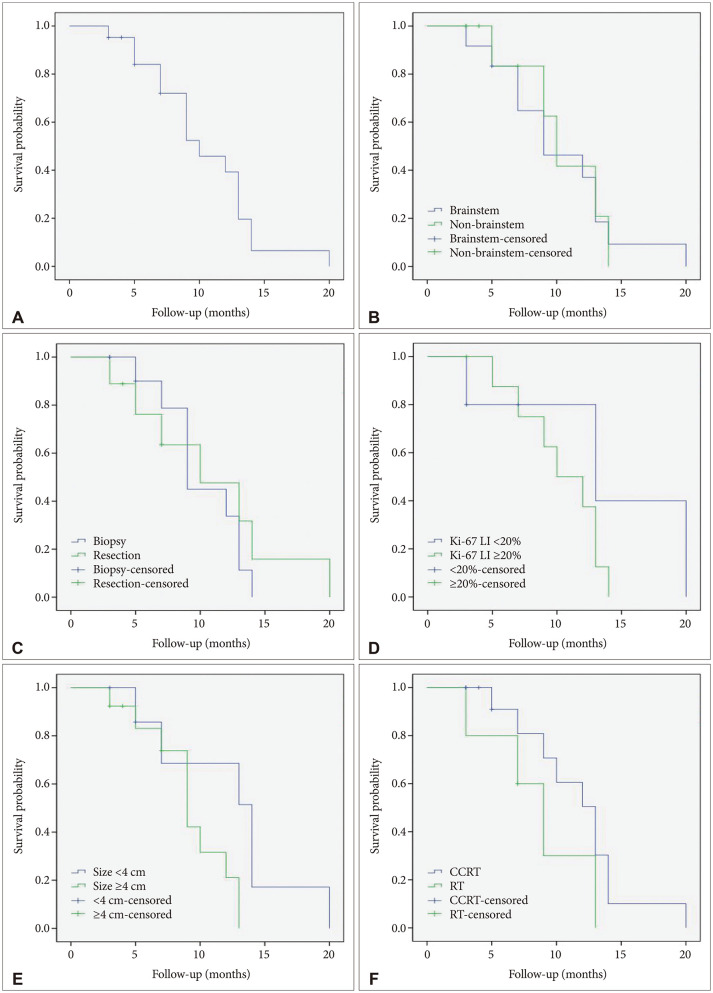
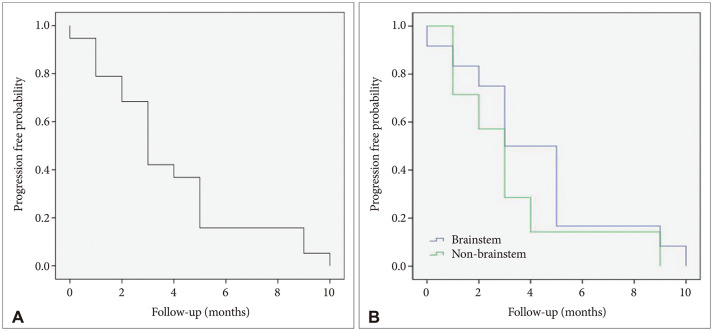
Among 24 patients, 2 patients who were diagnosed as DMG in spinal cord tumor and 1 foreign patient who returned to his own country immediately after biopsy and had no further clinical information were excluded from survival analysis. The median OS was 10.4 months (95% CI, 8.4 to 12.5) and PFS was 3.9 months (95% CI, 2.6 to 5.2) (Figs. 2 and 3).
Fig. 2. Kaplan-Meier survival curves. A: Overall survial curves of the whole cohort (median=10.4 months). B-F: Survival analysis between groups of patients according to tumor locations (p=0.275) (B), surgical treatments (p=0.570) (C), Ki-67 LI of the specimens (p=0.272) (D), tumor sizes and (p=0.054) (E), and adjuvant treatments (p=0.175) (F) reveals no significant differences between the groups (log-rank test). LI, labeling index, CCRT, concurrent chemoradiation therapy; RT, radiation therapy.
Fig. 3. Kaplan-Meier survival curves. A: Progression-free survival curves for the whole cohort (median=3.9 months). B: Analysis of progression-free survial between brainstem group and non-brainstem group shows no significant difference (log-rank test, p=0.275).
The analysis of the correlation between clinical factors and OS and PFS is shown in Table 2. There was no significant difference in survival among patient factors such as age and sex. When considering the size, 13 patients with size of the tumor 4 cm or more had median OS 9.2 months (95% CI, 7.4 to 11.1) and 8 patients with the tumor size less than 4 cm had median OS 12.4 months (95% CI, 8.2 to 16.6), however, the relevance between tumor size and OS was statistically not significant (p=0.054). Considering tumor location, the group of DMG patients located in non-brainstem showed 10.4 months of OS and 3.3 months of PFS (95% CI, 1.2 to 5.3) and the group of DMG located in brainstem showed 10.3 months of OS and 4.3 months of PFS (95% CI, 2.6 to 5.9) (p=0.275).
Table 2. Factors contributing to OS and PFS on DMG.
| Factor (cut-off value) | OS (months) | p-value | PFS (months) | p-value | |||
|---|---|---|---|---|---|---|---|
| Median | 95% CI | Median | 95% CI | ||||
| Age | 0.335 | 0.747 | |||||
| <20 yr | 9.638 | 7.178–12.097 | 4.111 | 2.634–5.588 | |||
| ≥20 yr | 11.349 | 7.706–14.992 | 3.700 | 1.592–5.808 | |||
| Sex | 0.486 | 0.894 | |||||
| Male | 9.407 | 4.666–14.149 | 3.750 | 1.696–5.804 | |||
| Female | 11.114 | 9.277–12.951 | 4.000 | 2.287–5.713 | |||
| Size | 0.054 | 0.387 | |||||
| ≥4 cm | 9.251 | 7.392–11.109 | 3.583 | 1.921–5.245 | |||
| <4 cm | 12.371 | 8.187–16.556 | 4.429 | 2.339–6.518 | |||
| Location | 0.871 | 0.275 | |||||
| Brainstem | 10.296 | 7.506–13.087 | 4.250 | 2.576–5.924 | |||
| Non-brainstem | 10.417 | 7.546–13.287 | 3.286 | 1.247–5.324 | |||
| Operation | 0.570 | 0.423 | |||||
| Resection | 10.905 | 6.780–15.030 | 4.500 | 2.217–6.783 | |||
| Biopsy | 10.175 | 8.232–12.118 | 3.455 | 1.951–4.958 | |||
| KI-67 LI | 0.272 | 0.692 | |||||
| ≥20% | 10.375 | 8.154–12.596 | 3.375 | 1.453–5.297 | |||
| <20% | 13.800 | 6.304–21.296 | 3.800 | 0.676–6.924 | |||
| Adjuvant Tx | 0.604 | 0.387 | |||||
| RTx | 9.667 | 6.047–13.286 | 4.500 | 2.492–6.508 | |||
| CCRTx | 11.525 | 8.710–14.340 | 3.000 | 1.371–4.629 | |||
OS, overall survivial; PFS, progression-free survival; DMG, diffuse midline glioma; CI, confidence interval; LI, labeling index; RTx, radiation therapy; CCRTx, concurrent chemoradiation therapy
Nine patients (37%) underwent surgical resection of tumors and 12 patients (57%) underwent only biopsy of the tumor. Surgical resection was not associated with prolonged OS (median OS was 10.9 months (95% CI, 6.8 to 15.0) for resection group and 10.2 months (95% CI, 8.2 to 12.1) for biopsy group and p value was 0.57. Even when considering the resection range, STR group and non-STR group who had undergone PR or biopsy also had little relationships in OS (median OS was 14 months [95% CI, 14 to 14] for STR group and 10.1 months [95% CI, 7.9 to 12.2] for PR and biopsy group [p=0.254]).
DMG group whose KI-67 labeling index was 20% or more showed median OS as 10.4 months (95% CI, 8.2 to 12.6) and DMG group whose KI-67 labeling index was less than 20% had median OS, 13.8 months (95% CI, 6.3 to 21.3) and two group showed no statistical relevance (p=0.272).
Thirteen patients received adjuvant chemoradiotherapy after pathologic confirm showed no relationship in survival prognosis compared with 6 patients only recieved radiotherapy (median OS was 11.5 months [95% CI, 8.7 to 14.3] for concurrent chemoradiotherapy [CCRT] and 9.7 months [95% CI, 6.0 to 13.3] for RT only and p value was 0.604).
Immunohistochemistry findings and genomic landscape obtained by targeted NGS
Among 24 patients of DMG, immunohistochemistry (IHC) staining found TP53 mutation in 6 patients (25%), ATRX mutation protein in 2 patients (8%) and IDH-1 mutant protein and O6-methylguanine-DNA methyltransferase (MGMT) promoter methylation in 1 patient, respectively (4%).
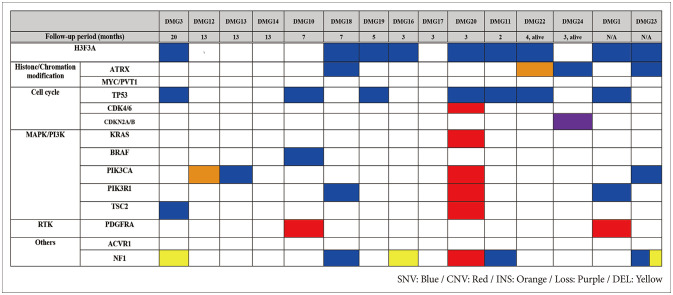
Fifteen patients had available targeted NGS data who included two alive patients and censored patients each other (Fig. 4). Among all patients who were confirmed as H3 K27M mutation in IHC, 9 patients had H3F3A single nucleotide variation. TP53 mutation was the most altered gene (n=7, 46%). Eight samples harbored alteration in cell cycle genes, including TP53, CDK4/6 amplification, and CDKN2A/B homozygous deletion. Alterations in mitogen-activated protein kinase (MAPK)/PI3K pathways, including KRAS, BRAF, NF1, PIK3CA, and PIK3R1, were observed in ten patients (66%). Receptor tyrosine kinase amplification involving PDGFRA and ACVR1 was observed in two patients (13%).
Fig. 4. Genomic landscape of DMG patients obtained by targeted NGS. DMG, diffuse midline glioma; NGS, next generation sequencing; SNV, single-nucleotide variant; CNV, copy number variation; INS, insertion; DEL, deletion.
DISCUSSION
DMG, H3 K27M-mutant is a rare glioma, which appeared from the 4th revised edition of WHO classification of the CNS tumors, later renamed as DMG, H3 K27-altered in the 5th edition to harbor other alterations such as EZHIP overexpression [1,3]. Because the locations where this tumor occurs are usually deep midline structures such as the brainstem or thalamus and the tumor grows diffusely, surgical resection is often impossible, and even biopsy is not feasible in some cases. As the prognosis is also poor, it also makes neurosurgeons less active in performing resection or biopsy. In the case of diffuse intrinsic pontine glioma, which was previously referred to as these tumors occurring in the pons, according to a previous survey, only 13.5% of responders performs biopsy to all patients [5]. Therefore, there is not much data on clinical information of patients who have been pathologically diagnosed with DMG K27M, even after over 5 years have passed since this new diagnosis was added [6,7,8,9,10,11,12,13,14]. Here, we described the clinical factors affecting PFS and OS of DMG and immunohistochemistry findings and genomic landscape obtained by targeted NGS study.
In our case series, we have two patients (8%) who have been diagnosed with other pathology in the past; 8-year-old female previously diagnosed as subependymoma (case 13) and 26-year-old female previously diagnosed as primitive neuroepithelial tumor (case 19). The changes during follow-up did not match the course of the existing disease, therefore resection or biopsy was performed again, and they were newly diagnosed with DMG H3 K27M. As they had already received radiation therapy before their recent surgery, it is not clear whether the effect contributed to the formation of new tumors or whether the past diagnosis was inaccurate. However what is certain is that these cases shows the usefulness of rebiopsy or reoperation to make a clear pathological diagnosis once again even if the location is not feasible if the clinical course of patients is different than expected.
Surgical resection of DMG is often difficult. In our series, 10 (41.7%) patients underwent surgical resection. According to the reports in the existing literature, the surgical extent does not have benefit on survival [6,7,9]. The retrospective study which Bin-Alamer et al. [15] systematically reviewed selected 20 studies describing outcomes and prognostic factors of adult patient with DMG, H3 K27M-altered showed that there was no survival difference between surgical resection versus biopsy. And our study also showed the same result.
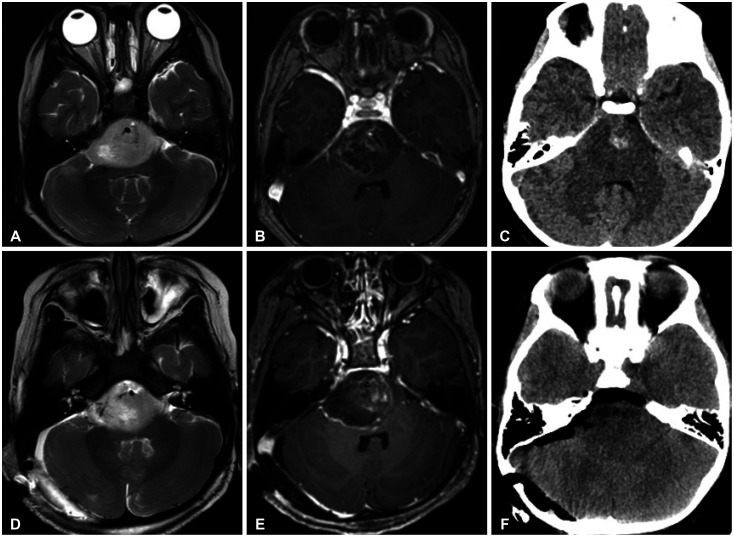
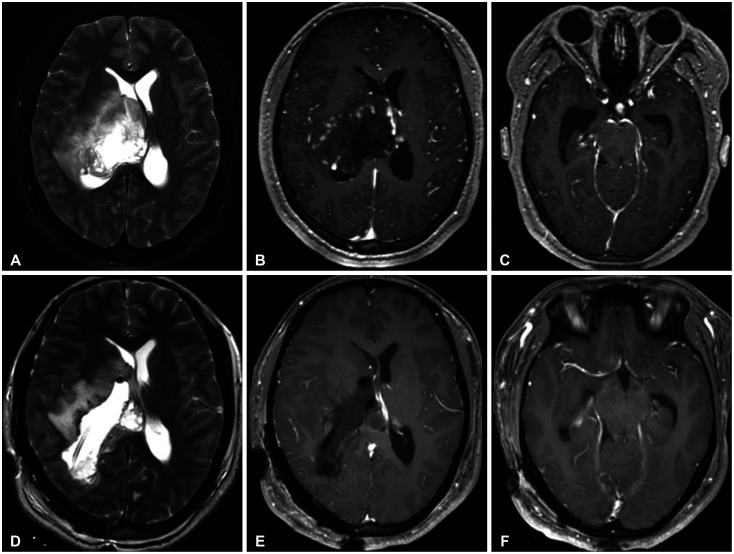
Although the current guideline states that GTR can be attempted if feasible, there are a few cases where it is actually possible [16]. Brainstem and thalamus, where these tumors mainly occur, are critical structures that can cause serious neurological deficits when get injured. Although there is no benefit to survival, there are cases where surgical resection is helpful. Even if the location of tumor is pons and the patient experienced rapid cranial palsies and motor weakness due to tumor bleeding, it could be improved through surgical decompression (case 12) (Fig. 5). In the case of obstructive hydrocephalus caused by mass effect, the hydrocephalus can be resolved spontaneously by direct resection of the tumor (case 18) (Fig. 6). Although these cases are limited, they show that surgical resection is helpful in the treatment of some cases of DMG patients improving neurological status.
Fig. 5. Illustrative case (Case 12). Preoperative MRI and CT images (A-C) and postoperative MRI and CT images (D-F). A 4-year-old female patient presented with left side weakness (grade 3). The day before surgery, motor weakness aggravated to grade 2 and diplopia occurred. Images revealed pontine tumor with tumor bleeding. The tumor was removed partially and the patient was diagnosed as diffuse midline glioma, H3 K27M altered. After resection, diplopia disappeared and weakness also improved to nearly normal for 9 months till progression.
Fig. 6. Illustrative case (Case 18). Preoperative MRI and CT images (A-C) and postoperative MRI and CT images (D-F). A 20-year-old female patient presented with progressive left side weakness (grade 3). Images reveals right thalamic mass with hydrocephalus. The tumor was removed subtotally and the patient was diagnosed as diffuse midline glioma, H3 K27M altered. Note that hydrocephalus is resolved after resection. After resection, weakness improved to grade 4 and hydrocephalus remains resolved for 4 months till progression.
Fifteen patients (62.5%) were accompanied by hydrocephalus with the tumor. This is often obstructive hydrocephalus caused by tumor, therefore endoscopic third ventriculostomy (ETV) is helpful. In our case, 9 patients (60%) who developed HC were able to be treated with ETV. There is an additional advantage that biopsy can be performed simultaneously when tumor is located in the thalamus.
Radiotherapy has been regarded as an important treatment option for brainstem glioma, as is DMG. As for chemoagents, there is no drug that has clearly demonstrated therapeutic effect in DMG, but temozolomide which is used in glioblastoma is often also used in DMG [16,17,18]. In this analysis, there was no significant difference between radiation only and CCRT (OS of the RT: 9.667 months, OS of the CCRT: 11.525 months, p=0.604; PFS of the RT: 4.500 months, PFS of the CCRT: 3.000 months, p=0.387).
Our survival analysis did not find any significant factors related to the prognosis. It is thought that the limitations of our cohort may contribute. First, the number of cases is small to analyze. Second, because collecting rare cases, several factors such as the location of the tumor and the age of the patient are various. The dismal prognosis of DMG is also considered to be a limitation to find differences in the analysis.
In this study, we reviewed result of NGS profile of 15 DMG patients. In all our cases, DMG was diagnosed through immunostaining, but H3F3A was confirmed in NGS only in 9 cases (60%). Inadequacy of the specimen because obtainable tissues are very limited in these DMG cases is thought to be one of reasons. Another possibility is that mutation is present at other sites other than the target. According to the prevalence of concurrent genetic alterations in DMG, H3 K27M-altered mentioned in other studies, mutations in the TP53 genes were the most common genetic mutation. Other common genomic events included ATRX loss or mutations, PDGFRA amplification, ACVR1, PIK3CA, FGFR1, and PPM1D mutations, and MGMT methylation [19]. TP53 mutation was frequently observed throughout this study (7 cases, 28%). ATRX mutations were observed less commonly in our study (4 cases, 26%) than in other studies [13,20,21,22]. The distinct features of DMG from glioblastomas include an extremely rare frequency of IDH1/2 mutation, and only one case (case 13) had found IDH-1 mutant protein [10].
DMG is a rare glioma with and poor prognosis. A definitive pathological diagnosis can help making accurate treatment decisions and expecting proper prognosis. Although surgical treatment does not affect the overall survival prognosis, it can help improve the patient’s accompanying neurological symptoms in some limited cases. Hydrocephalus is often accompanied with DMG and treatment for hydrocephalus is often also required. Therefore, careful multidisciplinary therapeutic approach is needed.
Footnotes
- Conceptualization: Sangjoon Chong.
- Data curation: Sun Woo Jang.
- Formal analysis: Sun Woo Jang.
- Funding acquisition: Sangjoon Chong.
- Investigation: Sun Woo Jang, Sangjoon Chong.
- Methodology: Sangjoon Chong.
- Project administration: Jeong Hoon Kim, Young-Shin Ra, Sangjoon Chong.
- Resources: Sang Woo Song, Young-Hoon Kim, Young Hyun Cho, Seok Ho Hong, Jeong Hoon Kim, Young-Shin Ra, Sangjoon Chong.
- Software: Sun Woo Jang, Sangjoon Chong.
- Supervision: Sang Woo Song, Young-Hoon Kim, Young Hyun Cho, Seok Ho Hong, Jeong Hoon Kim, Young-Shin Ra, Sangjoon Chong.
- Validation: Sangjoon Chong.
- Visualization: Sun Woo Jang, Sangjoon Chong.
- Writing—original draft: Sun Woo Jang, Sangjoon Chong.
- Writing—review & editing: Sang Woo Song, Young-Hoon Kim, Young Hyun Cho, Seok Ho Hong, Jeong Hoon Kim, Young-Shin Ra, Sangjoon Chong.
Conflicts of Interest: The authors have no potential conflicts of interest to disclose.
Funding Statement: None
Availability of Data and Material
All data generated or analyzed during the study are included in this published article.
References
- 1.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol. 2016;131:803–820. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 2.Gielen GH, Gessi M, Hammes J, Kramm CM, Waha A, Pietsch T. H3F3A K27M mutation in pediatric CNS tumors: a marker for diffuse high-grade astrocytomas. Am J Clin Pathol. 2013;139:345–349. doi: 10.1309/AJCPABOHBC33FVMO. [DOI] [PubMed] [Google Scholar]
- 3.Louis DN, Perry A, Wesseling P, Brat DJ, Cree IA, Figarella-Branger D, et al. The 2021 WHO classification of tumors of the central nervous system: a summary. Neuro Oncol. 2021;23:1231–1251. doi: 10.1093/neuonc/noab106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chan KM, Fang D, Gan H, Hashizume R, Yu C, Schroeder M, et al. The histone H3.3K27M mutation in pediatric glioma reprograms H3K27 methylation and gene expression. Genes Dev. 2013;27:985–990. doi: 10.1101/gad.217778.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.El-Khouly FE, Veldhuijzen van Zanten SEM, Santa-Maria Lopez V, Hendrikse NH, Kaspers GJL, Loizos G, et al. Diagnostics and treatment of diffuse intrinsic pontine glioma: where do we stand? J Neurooncol. 2019;145:177–184. doi: 10.1007/s11060-019-03287-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karremann M, Gielen GH, Hoffmann M, Wiese M, Colditz N, Warmuth-Metz M, et al. Diffuse high-grade gliomas with H3 K27M mutations carry a dismal prognosis independent of tumor location. Neuro Oncol. 2018;20:123–131. doi: 10.1093/neuonc/nox149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Solomon DA, Wood MD, Tihan T, Bollen AW, Gupta N, Phillips JJ, et al. Diffuse midline gliomas with histone H3-K27M mutation: a series of 47 cases assessing the spectrum of morphologic variation and associated genetic alterations. Brain Pathol. 2016;26:569–580. doi: 10.1111/bpa.12336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schreck KC, Ranjan S, Skorupan N, Bettegowda C, Eberhart CG, Ames HM, et al. Incidence and clinicopathologic features of H3 K27M mutations in adults with radiographically-determined midline gliomas. J Neurooncol. 2019;143:87–93. doi: 10.1007/s11060-019-03134-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang L, Li Z, Zhang M, Piao Y, Chen L, Liang H, et al. H3 K27M-mutant diffuse midline gliomas in different anatomical locations. Hum Pathol. 2018;78:89–96. doi: 10.1016/j.humpath.2018.04.015. [DOI] [PubMed] [Google Scholar]
- 10.Meyronet D, Esteban-Mader M, Bonnet C, Joly MO, Uro-Coste E, Amiel-Benouaich A, et al. Characteristics of H3 K27M-mutant gliomas in adults. Neuro Oncol. 2017;19:1127–1134. doi: 10.1093/neuonc/now274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schulte JD, Buerki RA, Lapointe S, Molinaro AM, Zhang Y, Villanueva-Meyer JE, et al. Clinical, radiologic, and genetic characteristics of histone H3 K27M-mutant diffuse midline gliomas in adults. Neurooncol Adv. 2020;2:vdaa142. doi: 10.1093/noajnl/vdaa142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kleinschmidt-DeMasters BK, Mulcahy Levy JM. H3 K27M-mutant gliomas in adults vs. children share similar histological features and adverse prognosis. Clin Neuropathol. 2018;37:53–63. doi: 10.5414/NP301085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sturm D, Pfister SM, Jones DTW. Pediatric gliomas: current concepts on diagnosis, biology, and clinical management. J Clin Oncol. 2017;35:2370–2377. doi: 10.1200/JCO.2017.73.0242. [DOI] [PubMed] [Google Scholar]
- 14.Aihara K, Mukasa A, Gotoh K, Saito K, Nagae G, Tsuji S, et al. H3F3A K27M mutations in thalamic gliomas from young adult patients. Neuro Oncol. 2014;16:140–146. doi: 10.1093/neuonc/not144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bin-Alamer O, Jimenez AE, Azad TD, Bettegowda C, Mukherjee D. H3K27M-altered diffuse midline gliomas among adult patients: a systematic review of clinical features and survival analysis. World Neurosurg. 2022;165:e251–e264. doi: 10.1016/j.wneu.2022.06.020. [DOI] [PubMed] [Google Scholar]
- 16.Yoon HI, Wee CW, Kim YZ, Seo Y, Im JH, Dho YS, et al. The Korean Society for Neuro-Oncology (KSNO) guideline for adult diffuse midline glioma: version 2021.1. Brain Tumor Res Treat. 2021;9:1–8. doi: 10.14791/btrt.2021.9.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Banan R, Christians A, Bartels S, Lehmann U, Hartmann C. Absence of MGMT promoter methylation in diffuse midline glioma, H3 K27M-mutant. Acta Neuropathol Commun. 2017;5:98. doi: 10.1186/s40478-017-0500-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abe H, Natsumeda M, Kanemaru Y, Watanabe J, Tsukamoto Y, Okada M, et al. MGMT expression contributes to temozolomide resistance in H3K27M-mutant diffuse midline gliomas and MGMT silencing to temozolomide sensitivity in IDH-mutant gliomas. Neurol Med Chir (Tokyo) 2018;58:290–295. doi: 10.2176/nmc.ra.2018-0044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vuong HG, Le HT, Ngo TNM, Fung KM, Battiste JD, McNall-Knapp R, et al. H3K27M-mutant diffuse midline gliomas should be further molecularly stratified: an integrated analysis of 669 patients. J Neurooncol. 2021;155:225–234. doi: 10.1007/s11060-021-03890-9. [DOI] [PubMed] [Google Scholar]
- 20.Cooney TM, Lubanszky E, Prasad R, Hawkins C, Mueller S. Diffuse midline glioma: review of epigenetics. J Neurooncol. 2020;150:27–34. doi: 10.1007/s11060-020-03553-1. [DOI] [PubMed] [Google Scholar]
- 21.Mackay A, Burford A, Carvalho D, Izquierdo E, Fazal-Salom J, Taylor KR, et al. Integrated molecular meta-analysis of 1,000 pediatric high-grade and diffuse intrinsic pontine glioma. Cancer Cell. 2017;32:520–537.e5. doi: 10.1016/j.ccell.2017.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buczkowicz P, Hoeman C, Rakopoulos P, Pajovic S, Letourneau L, Dzamba M, et al. Genomic analysis of diffuse intrinsic pontine gliomas identifies three molecular subgroups and recurrent activating ACVR1 mutations. Nat Genet. 2014;46:451–456. doi: 10.1038/ng.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during the study are included in this published article.