Summary
Due to the increasing non-communicable disease burden in Africa, several strategies that target the major lifestyle and physiological risk factors have been implemented to combat such diseases. The Healthy Aging Adult South Africa report card systematically reviews national and regional prevalence data of middle-aged South African adults (45–65 years) published between 2013 and 2020 on diet, physical activity, tobacco use and alcohol consumption, obesity, hypertension, dyslipidaemia and diabetes mellitus. Each indicator was assigned two grades, (1) based on the availability of prevalence data, and (2) based on whether policies have been proposed and implemented for the respective indicators. Alcohol consumption, obesity, hypertension and diabetes received an A grade for the availability of prevalence data. Tobacco use and diet received an A grade for policy and implementation. Gaps have been identified that need to be filled by future research focusing on continued surveillance of all indicators in order to inform and implement effective policies.
Keywords: diet, physical activity, tobacco, alcohol, obesity, hypertension, dyslipidaemia, diabetes
The burden of non-communicable diseases (NCDs) in sub-Saharan Africa is nearly equal to the total burden from communicable, maternal, neonatal and nutritional diseases combined.1 The growing NCD burden is expected to continue to increase with increasing urbanisation, life expectancy and population size, with models estimating that the sub-Saharan population will expand from 13% of the global population in 2017 to 35% in 2100.2
The Sustainable Development Goal (SDG) target of 3.4 is to reduce premature mortality from NCDs by a third by 2030 (relative to 2015), and to promote mental health and wellbeing.3 The implementation of the Strategic Plan for the Prevention and Control of NCDs 2013–2017, in order to achieve certain targets by 2020, and the adoption of the South African Declaration on the Prevention and Control of NCDs, demonstrate South Africa’s commitment to the fight against NCDs.4,5
While being in line with the World Health Organisation’s (WHO) global action plan for the prevention and control of NCDs 2013–2020, the South African strategic plan identifies various areas to be the focus of a multisectoral approach, with the ultimate aim of ensuring ‘a long and healthy life for all’. While recognising the social determinants of health, the strategic plan highlights four major lifestyle risk factors (unhealthy diets, physical inactivity, harmful use of alcohol and tobacco use), as well as four physiological risk factors (overweight and obesity, hypertension, dyslipidaemia and poor glucose control) that can be targeted through NCD prevention strategies to achieve the 10 goals and targets:
reduce relative premature mortality by 25% by 2020
reduce tobacco use by 20% by 2020
reduce the per capita consumption of alcohol by 20% by 2020
reduce the mean population intake of salt by 5 g per day by 2020
reduce the percentage of people who are obese or overweight by 10% by 2020
reducing the prevalence of people with raised blood pressure by 20% by 2020
increase the prevalence of people meeting physical activity recommendations by 10%
reduce the prevalence of cervical cancer
increase the percentage of people controlled for hypertension, diabetes and asthma by 30% by 2020
increase the number of people screened and treated for mental disorders by 30% by 2030.
The unforeseen arrival of the COVID-19 pandemic in South Africa in 2020 has highlighted the risk of NCDs, particularly in settings with high social and economic inequality. One in five people who become infected with COVID-19 are at an increased risk of hospitalisation and mortality, largely as a result of underlying NCDs.6 Furthermore, the restrictions that have been implemented in order to reduce the spread of COVID- 19 have implications for health behaviours that are directly associated with NCD risk, such as diet and physical activity. The interruption of NCD services, as well as public health and surveillance activities, add to the challenge of this pandemic, but the response by governments in rapidly urbanising cities in low- and middle-income countries (LMICs) may also provide opportunities for whole-of-society approaches that can be employed to improve health.7
Data from two recent national surveys have shown that middleaged adults (45–65 years) are at highest risk for many of the NCD risk factors.8,9 Within the life course, middle adulthood is critical, as individuals in this age group are still working and contributing to the economy and society, and in many cases, particularly in LMICs, are also supporting the extended family.10 Healthy aging needs to be prioritised to not only ensure an optimal quality of life, but also to minimise the strain on the health system.
The Healthy Aging Adult South Africa (HAASA) report card provides a systematic review of evidence on middle-aged South African adults (45–65 years), published between 2013 and 2020 on diet, physical activity, tobacco and alcohol use, obesity, hypertension, dyslipidaemia and diabetes. Only national and regional prevalence data are reported and data were included when (1) the mean age was between 45 and 65 years; or (2) the majority of participants were between these ages; or (3) if age-specific data between 45 and 65 years were reported.
Each of the risk factors reviewed in the sections below have been assigned a grade based on the availability of data and whether national policies have been proposed and implemented. A similar grading system to the Healthy Active Kids South Africa (HAKSA) report card was utilised,11 and the criteria presented in Table 1 were employed.
Table 1. Criteria used to grade each risk factor.
| Grade | Prevalence data | Policy and implementation |
| A | Published national and regional prevalence data available for this age group | National policy/ies have been implemented for more than 10 years |
| B | Published national and regional prevalence data available, not specific to age group | National policy/ies have been recently (less than 10 years) implemented |
| C | Only regional prevalence data for this age group | National policy/ies have been proposed but not implemented |
| D | Only regional prevalence data but not specific to this age group | No national policy/ies |
| E | No prevalence data |
Methods
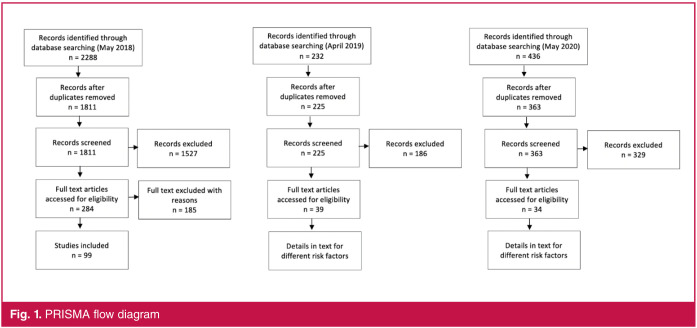
This review was registered in the PROSPERO registry for systematic reviews (registration number: CRD42018093064) and was conducted in accordance with the PRISMA guidelines (see Fig. 1).12
Fig. 1.
PRISMA flow diagram
The search for articles was conducted in May 2018 and then again in April 2019 and May 2020, according to the participants, intervention, comparator and outcomes (PICO) model of formulating a clinical question in the healthcare setting. Restrictions to articles were based on age of participants (between 45 and 65 years), study population (South African) and date of publication (from 1 January 2013 until 30 May 2020). The following databases were used in the search: PubMed, Scopus, Web of Science, EBSCOhost (including Africa Wide, CINHAL, academic search premier and health source). A sample of the PubMed search strategy is available as Table 2.
Meta-analyses, systematic reviews, randomised control trials, cohort studies, case studies, longitudinal and cross-sectional studies, were included. Animal and genetic studies as well as studies not written in English were excluded.
Table 2. PubMed Search strategy, modified as needed for other electronic databases.
| Population: middle-aged adult South Africans | Outcome: metabolic risk factors | ||||
| MeSH terms: | #1 | Middle aged [MeSH] | MeSH terms: | #45 | Diabetes mellitus [MeSH] |
| All fields | #2 | Middle age | #46 | Insulin resistance [MeSH] | |
| #3 | middle aged | #47 | #45 OR #46 | ||
| #4 | midlife | All fields: | #48 | Diabetes | |
| #5 | middle adulthood | #49 | Insulin resistance | ||
| #6 | #2 OR #3 OR #4 OR #5 | #50 | insulin sensitivity | ||
| #7 | #1 OR #6 | #51 | Metabolic syndrome | ||
| MeSH term: | #8 | South Africa [MeSH] | #52 | Syndrome X | |
| #53 | Glucose intolerance | ||||
| All fields | #9 | 'South Africa' OR | #54 | Hyperglycemia | |
| #10 | 'South African' | #55 | #48 OR #49 OR #50 OR #51 OR #52 OR #53 OR #54 | ||
| #11 | #8 OR #9 | #56 | #47 OR #55 | ||
| #12 | #7 OR #10 | ||||
| MeSH terms: | #57 | Overnutrition [MeSH] | |||
| Intervention: | modifiable | lifestyle risk factors | #58 | Overweight [MeSH] | |
| MeSH terms: | #13 | Alcohol drinking [MeSH] | #59 | Body mass index [MeSH] | |
| All fields | #14 | Alcohol consumption | #60 | #57 OR #58 OR #59 | |
| #15 | #13 OR #14 | All fields: | #61 | Obesity | |
| #62 | Overnutrition | ||||
| MeSH terms: | #16 | Exercise [MeSH] | #63 | Overweight | |
| All fields | #17 | Physical activity | #64 | Hypernutrition | |
| #18 | Exercise | #65 | Body mass index | ||
| #19 | Physical exertion | #66 | BMI | ||
| #20 | Physical training | #67 | #61 OR #62 OR #63 OR #64 OR #65 OR #66 | ||
| #21 | Physical fitness | #68 | #60 OR #67 | ||
| #22 | #17 OR #18 OR #19 OR #20 OR #21 | ||||
| #23 | #16 OR #22 | MeSH terms: | #69 | Hypertension [MeSH] | |
| behavior | All fields: | #70 | Hypertension | ||
| MeSH terms: | #24 | Feeding [MeSH] | #71 | High blood pressure | |
| #25 | Diet, food, and nutrition [MeSH] | #72 | #70 OR #71 | ||
| #26 | #24 OR #25 | #73 | #69 OR #72 | ||
| All fields: | #27 | Food habits | MeSH terms: | #74 | |
| #28 | Dietary habits | Dyslipidaemias [MeSH] | |||
| #29 | Eating behavior | All fields: | #75 | Dyslipidaemia | |
| #30 | Dietary behaviour | #76 | Dyslipidaemias | ||
| #31 | Feeding patterns | #77 | Hypercholesterolemia | ||
| #32 | Food preferences | #78 | Hypercholesteremia | ||
| #33 | Dietary fat | #79 | High cholesterol | ||
| #34 | Dietary fats | #80 | Elevated cholesterol | ||
| #35 | Dietary protein | #81 | Raised cholesterol | ||
| #36 | Dietary carbohydrates | #82 | Triglycerides | ||
| #37 | Food consumption | #83 | Lipid profile | ||
| #38 | #27 OR #28 OR #29 OR #30 OR #31 OR #32 OR #33 OR #34 OR #35 OR #36 OR #37 | #84 | #75 OR #76 OR #77 OR #78 OR #79 OR #80 OR #81 OR #82 OR #83 | ||
| #39 | #26 OR #38 | #85 | #74 OR #84 | ||
| MeSH terms: | #40 | Tobacco use [MeSH] | #86 | #15 OR #23 OR #39 OR #56 OR #68 #73 OR #85 AND #7 AND #12 | |
| All fields: | #41 #42 #43 | Smoking Tobacco #41 OR #42 | |||
| #44 | #40 OR #43 | ||||
The results were screened and duplicates were removed, followed by title and abstract screening. After the first search in May 2018, full-text screening was completed by a research assistant who excluded 185 of the 284 full texts (Fig. 1). For the second and third searches in April 2019 and May 2020, respectively, a total of 588 titles and abstracts were screened by one researcher (LM). Full texts of all eligible articles (a total of 73 for both 2019 and 2020) were then accessed and divided among reviewers who were identified as experts in their respective sections. It is worth noting that many of the articles were relevant to more than one risk factor, which is why details of how many of these were included are described in the different sections representing each of the eight risk factors below.
Prevalence data for each of the four lifestyle and four physiological risk factors were extracted by reviewers as follows: AP, physical activity; MM, alcohol consumption; RSM, tobacco use; SW, diet; GM, obesity; AR, dyslipidaemia; LW, hypertension; LM, diabetes. Each reviewer was provided with a standardised data-extraction form with the following headings: reference, study population/sample/setting/design, intervention/ exposure or barriers/facilitators/correlates (brief description of the purpose of the study), outcomes of interest (which included how the risk factor was measured) and main findings (prevalence of the risk factor), and instructed to complete the form for all the studies allocated to their risk factor. The quality of the evidence and risk of bias was not assessed.
Although PRISMA guidelines were closely followed when selecting the articles to be reviewed, the results are presented as a narrative review of the eight different risk factors. Each of the sections is presented separately and structured in such a way to include a brief introduction outlining the global prevalence of the risk factor, as well as the prevalence in LMICs, if available. Each reviewer then briefly described the global and national targets, as well as the contribution of the specific risk factor to the South African NCD burden, where applicable. This is followed by a description of how articles were included or excluded, and then national and regional prevalence data are described for the selected articles.
If national data have not been published in any of the selected articles, the data were obtained from the respective reports, and include, where appropriate, the South African Demographic and Health Survey (SADHS) 2003 and 2016, as well as the South African National Health and Nutrition Examination survey (SANHANES), the report of which was published in 2013.8,9,13 Each of the sections ends with a brief description of whether there are national policies, and if available, whether these have been shown to be effective. Each section then concludes with a grade and some recommendations.
Results
Several studies have made an important contribution to this literature, including the World Health Organisation’s Study on global AGEing and adult health (WHO-SAGE),14 the National Income Dynamics Study (NIDS)15 and the Prospective Urban Rural Epidemiology (PURE) study.16 WHO-SAGE is a multinational longitudinal study examining the health and wellbeing of adult populations. The study takes place in six LMICs (Mexico, Russia, India, Ghana, South Africa and China) with wave 1 having taken place in South Africa in 2007–2010, and subsequent waves in 2015–2016 (wave 2) and 2018–2019 (wave 3). Data have been collected in a nationally representative sample of over 50-year-old adults, with a smaller reference group aged 18–49 years, and the sample size for the South African cohort for wave 1 = 4 233, wave 2 = 4 085 and wave 3 = final numbers to be released.17
NIDS was the first national household panel study to be completed in South Africa, the first wave of which took place in 2008, with the last wave taking place in 2017. The core survey has been repeated every two to three years with a nationally representative sample of 28 000 individuals from 7 300 households.
The PURE study is a large epidemiological study of approximately 225 000 individuals between the ages of 35 and 70 years (enrolled between 2003 and 2013) from urban and rural sites in 18 low-, middle- and high-income countries (South Africa, Tanzania, Zimbabwe, Bangladesh, Pakistan, Indian, occupied Palestinian territory, China, Colombia, Iran, Malaysia, Argentina, Turkey, Brazil, Poland, Chile, Saudi Arabia and United Arab Emirates). The aim of the PURE study was to examine the impact of urbanisation, modernisation and globalisation on health behaviours and the development of risk factors for cardiovascular disease (CVD) and NCDs, such as diabetes and cancer.
Physical activity
In 2010, the WHO published Global Recommendations on Physical Activity for Health, which detailed the science of primary prevention of NCDs through physical activity at the population level, and recommended that adults complete at least 150 minutes of moderate- to vigorous-intensity physical activity per week.18 Global estimates show that 27.5% of adults are insufficiently active, and that women are less active than men in most countries.19 Furthermore, physical inactivity has been shown to be responsible for 6% of deaths globally.20 The recently released WHO Guidelines on Physical Activity and Sedentary Behaviour for Children and Adolescents, Adults and Older Adults (2020) have included sedentary behaviour recommendations as well as specific sub-population guidelines, including for those with chronic conditions.21
Increasing the prevalence of people meeting physical activity recommendations by 10% is one of the 10 goals of the South African Strategic Plan for the Prevention and Control of NCDs.4 The SADHS 2003 reported a physical inactivity prevalence of 56% in men and 64% in women aged 45–54 years; and 67% in both men and women aged 55–64 years.13 Physical activity data were not reported in the 2016 SADHS,9 and while the SANHANES8 reports on cardiovascular fitness, physical activity is not included. Therefore, the only national data available report that physical inactivity prevalence is higher in women and increases with age.
Twenty-three studies were selected for title and abstract screening. Of those, 10 studies reported data from WHO-SAGE, and five reported data from the PURE study. Nine of the 23 studies were excluded due to the age range of participants not falling within the selection criteria. Of the remaining 14 studies, a further seven were excluded due to one or more of the following: they did not report data by age group and therefore we could not extract data for the 45- to 65-year age group (n = 5); they reported on multi-country data and did not separate data by country (n = 2); or they did not report physical activity data and only used it as a covariate in analyses (n = 2). Therefore, this narrative discusses the data from the remaining seven studies.22-28
All studies reviewed, except two that included only women participants,22,23 and one that did not compare by gender,24 found that women were less likely than men to use active travel,25 were less physically active than men,25-28 and were therefore less likely to meet physical activity recommendations. Data from the Dikgale Health and Demographic Surveillance System (HDSS) site reported that the prevalence of physical inactivity was significantly higher in women compared to men (70.8 vs 40.7%, respectively),26 while differences between genders were around 10% in other studies.27,28 Furthermore, in all but one study,28 physical activity levels decreased with age.
The prevalence of adults meeting physical activity guidelines across all the studies included in this review ranged from 34 to 75%, with the wide range possibly being attributable to the large variation in age and socio-economic variables. The WHO-SAGE study compared South African data to data from other LMICs (China, Ghana, India, Mexico and the Russian Federation) and found that South African participants (only data for participants over 50 years old included) had the highest prevalence of low physical activity (59.7%), while in the other countries, this ranged from 23 to 38%.25,27 Two studies found that a higher socioeconomic status was associated with lower ambulatory physical activity (walking).22,25 Furthermore, in various cross-sectional studies, physical activity was associated with lower fasting blood insulin and cholesterol levels,22 higher fat-free soft tissue mass,22 lower blood pressure and/or prevalence of hypertension,23,24,28 lower body mass index (BMI)25 and lower waist circumference.25
Five studies used self-reported questionnaires to assess physical activity, while two used objective measurements of physical activity.23,24 The two studies that employed objective measurements tended to show low participation in moderate- to vigorous-intensity physical activity (between two and 20% of the day), yet did not quantify adherence to WHO guidelines.
The newly formed African Academic Consortium on Physical Activity for Health has recently released a number of policies in an attempt to reach the targets set out in the strategic plan, specifically focusing on guidance during the COVID-19 pandemic and beyond.29 Focus is placed on improving safety in order to allow for exercise for all, and on targeting and providing services at the individual, environmental and societal level. Furthermore, the Strategy for the Prevention and Control of Obesity in South Africa (2015–2020) has highlighted that the high prevalence of physical inactivity in South Africa can be attributed to a lack of an inclusive environment and community networks, increased use of technology and time challenges.30
The International Society for Physical Activity and Health has recently released a call to action for policy makers globally, which includes eight areas for investment in order to boost physical activity.31 These include school programmes, active travel, active urban design, healthcare, public education and the media, sport and recreation for all, and workplace and community-wide programmes. Ultimately, in all cases a multisectoral approach to improving physical activity is required.
Given the current available evidence, a grade B has been assigned to physical activity for the availability of prevalence data (Table 3). While national data are available, albeit between 2003 and 2012 only, there are few regional studies in this age group and not all data clearly define age group. Given the available data, it is unclear whether South Africa has reached the national target of increasing physical activity by 10% in 2020, as newer prevalence data do not exist, and previous national reports seem to show worsening of adherence to physical activity guidelines over time, rather than improvement. A grade C has been assigned for policy and implementation as the recent policy brief prepared by the African Physical Activity Network provides recommendations and an implementation framework aligned to the WHO Global Action Plan for Physical Activity, which is promising, although specific implementation plans for aging adults are not clear (Table 3).
Table 3. Grades for the major risk factors in South Africans.
| Risk factor | Prevalence data | Policy and implementation |
| Physical activity | B | C |
| Alcohol consumption | A | C |
| Tobacco use | B | A |
| Diet | C | A |
| Obesity | A | B |
| Dyslipidaemia | C | B |
| Hypertension | A | B |
| Diabetes | A | C |
Alcohol consumption
In 2016, the Global Burden of Disease (GBD) study estimated three million deaths [95% uncertainty intervals (UI) 2.6–3.6] and 131 (119.4–154.4) million disability-adjusted life-years (DALYs) were attributable to harmful alcohol consumption globally. It estimated harmful alcohol consumption to be the seventh leading risk factor for DALYs.32 In 2017, the global prevalence of harmful alcohol use was 6.4% for women and 18.5% for men.33 Data from sub-Saharan Africa report that alcohol was responsible for 6.4% of all deaths and 4.7% of all DALYs lost.34
There are a series of global initiatives focusing on alcohol consumption, including the WHO’s global strategy to reduce harmful alcohol use35 (targets contained within the NCDs global monitoring framework36) and alcohol use has been addressed in the sustainable development goals (SDG).3 The aims of these initiatives, as is the aim of the South African Strategic Plan for the Prevention and Control of NCDs,4 are to reduce harmful alcohol consumption by at least 10%. In 2000, the burden of disease and injury attributed to misuse of alcohol was 7.1% in South Africa, more than double that of the global mortality average, which was 3.2%.37,38
The 2016 SADHS reported that 10.5% of women between 45 and 54 years of age and 11.2% of women between 55 and 64 years of age consumed alcohol (drank alcohol in the last seven days before the survey) compared to 36.7 and 45.1% of men for the same age categories.9 It also reported that 4.4% of women and 27.8% of men between 45 and 54 years of age consumed five or more drinks on a single occasion in the 30 days prior to the survey (defined as risky drinking), and this decreased slightly in the older age group (55–64 years: 3.7% of women, 25.7% of men).9 The 2012 SANHANES did not report a prevalence of problem drinking in this age group,8 while the 2016 SADHS, using the CAGE test, reported problem drinking in approximately 15% of men and approximately 2.5% of women between the ages of 45 and 64 years.9
Data from the WHO-SAGE study (wave 1) reported a 3.7% prevalence of risky alcohol use, defined as heavy drinkers (more than drinks/week) and binge drinkers (more than three drinks/one occasion/week).39
Recent South African data published between 2013 and 2020 regarding alcohol consumption (n = 30) include national surveys (n = 8), regional studies (n = 7) and a global study (n = 1). Of these, 16 studies were excluded due to the lack of focus on alcohol consumption and/or prevalence. Therefore, 14 studies were included in this narrative.
Self-reported alcohol use is the most common method of assessment due to the cost associated with measuring biological biomarkers;40 however, there are concerns with the accuracy of participants’ recall.41 Carbohydrate-deficient transferrin and gamma-glutamyltransferase are suitable biomarkers for identifying alcohol use or abuse in most populations as they are sensitive to high alcohol consumption; 41,42 however, these can be misinterpreted in patients with liver conditions.40,42 The definition of hazardous alcohol consumption may also result in differences in the reported prevalence between studies but is typically defined as a regular average consumption of 20 to 40 g of alcohol a day for women and 40 to 60 g a day for men. Data from the WHO-SAGE study (wave 1) reported that 13.7% of the 3 840 participants over 50 years of age were current users of alcohol, with the prevalence being higher in men compared to women (15 vs 6.9%).43 The Cardiovascular Risk in Black South Africans (CRIBSA) study reported an increase in self-reported alcohol use in both men and women over the age of 45 years between 1990 and 2008/2009, with the prevalence being significantly higher in men at both time points. The prevalence of problem drinking was also significantly higher in men than women in the age group 45 to 54 years (46.6 vs 16.2%) and 55 to 64 years (29.4 vs 15.8%).44
Data from the South African Panel Study of Small Business and Health, a longitudinal study conducted in African townships (n = 2 213), reported a prevalence of problem drinking in 14.5% of the sample over the age of 50 years, and a further 8.9% who reported problem drinking and daily tobacco use.45 Although there was an increase in problem drinking only from baseline to follow up at 12 months in the whole sample (19.6 to 21.1%), longitudinal data are not reported separately for those over 50 years of age.
A study of farm workers in the Western Cape does not report current and problem drinking prevalences for the age group 45 to 65 years. However, their results show that this age group had a similar preference for papsak wine consumption to people between 35 and 44 years of age, and that this was significantly higher than those in the younger age groups.46 Although banned in 2007, papsak refers to cheap white wine packaged in a soft bag, consumed widely by poorer South African communities. This study also noted that alcohol use shifted to misuse as people grew older.
A study in rural Limpopo reported a 9.2% prevalence of alcohol consumption (having had a drink in the last 12 months) in participants between the ages of 45 and 54 years, and a 12.6% consumption in those between 55 and 64 years of age.26 Homebrewed alcohol is also common among poorer communities.47,48
Policy developments over the last 10 years have included the drafting of the Control of Marketing of Alcoholic Beverages Bill and its approval by the national cabinet in 2013. To date the draft bill has still not been gazetted for public comment or tabled in parliament.49 This proposed legislation would ban alcohol advertisements in all places except where alcohol is sold. Other proposals have included reducing the blood alcohol level allowed for drivers to zero, an increase in the legal age of drinking to 21 years of age as part of the draft Liquor Amendment Bill, and stricter regulations around the marketing and sale of alcohol.50
In order for alcohol policies to be implemented and effective, they need government support, which is also critical in continuing to resist industry. There should be a focus on peer influences, and interventions should be multi-pronged, focusing on enforcing laws that prohibit alcohol sales to minors, and ensuring that advertising campaigns that target young people are stopped.51 The COVID-19 pandemic and the impact of subsequent lockdowns on alcohol consumption has highlighted the harmful effects of alcohol, resulting in an increase in trauma cases presenting at hospitals, gender-based violence and non-natural deaths. Government’s response is to re-visit the Liquor Amendment Bill drafted in 2017, and there has been a recent increase in alcohol tax.
As there is recent national and regional prevalence data on alcohol consumption for this age group, the grade assigned for the availability of prevalence data is an A (Table 2). As several policies have been proposed but not implemented, a grade C is assigned to the policy and implementation component for alcohol (Table 3).
Tobacco use
In 2019, the WHO reported that there were 1.3 billion tobacco users in the world, of which 80% lived in LMICs.52 In line with the Global Action Plan for the Prevention and Control of NCDs,53 the fifth target of the NCDs Global Monitoring Framework established in 2011 aims by 2025 to reduce by 30% the prevalence of current tobacco use in persons of 15 years of age and older.36 In South Africa, it is estimated that 75% of trachea, bronchus and lung cancer deaths, 65% of chronic obstructive pulmonary disease deaths, 18% of CVD deaths and 15% of tuberculosis deaths are attributable to tobacco use.54
In 2013, the South African Declaration for Prevention and Control of NCDs committed to reduce the prevalence of tobacco use, estimated in 2009 at 23.7% (36.7% of men and 10.3% of women), by 20% by 2020.5 Following global trends, national data from South Africa has reported that the prevalence of tobacco smoking among persons aged 15 years and older declined between 2005 and 2015.55 However, high rates of tobacco usage were observed among persons in the 40- to 69-year age range, and particularly in men.8,9,56 The prevalence of tobacco smoking among men and women in the 45 to 65 years age ranges was higher than the adult national prevalence, at approximately 20% in 2012 and approximately 22% in 2016.9,57 In addition, SANHANES 2012 and SADHS 2016 reported gender differences in the prevalence of tobacco smokers in the 45- to 54-year age group (SANHANES 2012: men 35.8% vs women 8.5%; SADHS 2016: men 44.7% vs women 9.1%) and the 55- to 65-year age group (SANHANES 2012: men 29.4% vs women 11.3%; SADHS 2016: men 37.3% vs women 10.1%).57
South African data pertaining to tobacco use, which includes smoke and smoke-free products, published between 2013 and 2020 can mainly be found in cross-sectional surveys including: secondary analyses of nationally representative surveys (n = 6),8,9,57-63 large standardised multi-wave cross-country surveys (n = 19) comprising WHO-SAGE27,39,43,61-66 and the PURE study,23,40,41,47,67-72 a public dataset (n = 1)73 and regional studies (n = 11).26,74-81 Of those, only a few surveys (n = 7) specifically reported information in the 45- to 65-year age group27,28,39,57,73,74,77 and the majority (n = 30) were not designed to focus primarily on tobacco use, its prevalence and aetiology, but rather to investigate the role of tobacco use in NCD risk in broad adult populations, which were only collected using self-reported questionnaires such as the WHO STEPwise questionnaire82 or other tools designed for survey purposes.
The WHO-SAGE wave 1 survey reported smaller gender differences than the national surveys, as well as a lower prevalence in men (25.8% for 50 to 59 years old and 21.4% for 60 to 69 years old), and a higher prevalence in women (17.3% for 50 to 59 years old and 14.9% for 60 to 69 years old)27,39 compared to national data. The use of other tobacco products (hand-rolled cigarettes, pipes, cigars, water-pipes, electronic cigarettes, snuff, chewing tobacco and smokeless tobacco) was also reported to be higher among men than women within the 45- to 65-year age group (~7% in men and 4–6% in women).57
Regional data has reported ethnic disparities in tobacco use in the 45- to 65-year age range. A study of persons who self-identified as Indians living in Durban reported a 20 to 30% prevalence of tobacco use, and a study of self-identified African men and women from a socio-economically deprived neighbourhood in Cape Town reported a prevalence of 35 to 44% in men and approximately 10% in women.74 A case–control study of 481 640 deaths in the South African population reported that the highest smoking-attributed mortality rate was observed in persons who self-identified as coloured (of the 45- to 64-year-old deaths, 60 to 71% were of smokers) and the lowest in persons who self-identified as African (of the 45- to 64-year-old deaths, 42 to 53% were of smokers).73
In South Africa’s Strategic Plan for the Prevention and Control of NCDs (2013–2017),4 which is in line with the six MPOWER measures,83 and the practical and cost-effective interventions of the WHO Framework Convention on Tobacco Control implementation,84 it has been recommended that South Africa monitor tobacco use and tobacco-prevention policies; protect people from tobacco smoke in public places and workplaces; offer help to people who want to stop using tobacco; warn people about the dangers of tobacco; enforce bans on tobacco advertising, promotion and sponsorship; and raise tobacco taxes and prices.4
As members of a household, 45- to 64-year-old adults are likely to be exposed to second-hand smoke, with data from SANHANES reporting that more than half of this age group said that a member of their household smoked inside their homes.57 SANHANES data also reported that 35 to 41% of 45- to 64-year-olds were advised to quit smoking by a healthcare provider, and 49 to 52% of smokers tried to quit smoking.57 In addition, more than 80% of smokers aged 45 to 64 years acknowledged having read health warnings on tobacco packages, but only 47 to 56% of smokers then tried to quit smoking.57 Middle-aged adults are often the head of the household, and tobacco use may significantly reduce their household budgets for food, particularly healthy food.60
Given the evidence available and according to our grading criteria, a grade B is attributed to prevalence data on tobacco use (Table 3). While regular national surveys allow us to track national progress, published national and regional data are needed for the 45- to 65-year age group in order to identify geographical areas and communities needing attention. A grade A is attributed to policy and implementation as since the Tobacco Products Control Act in 1993, South Africa has continuously promulgated and implemented policies to reduce tobacco product consumption, which have proven successful (Table 3).85
To align with the WHO Framework Convention on Tobacco Control, which South Africa ratified in 2005,86 South Africa has committed to complete its legislation on tobacco by developing and implementing evidence-based measures to regulate marketing activities and sales reach, reduce the demand for tobacco, and provide alternatives to agricultural actors involved in the tobacco industry. Overall, the consistent use of WHO tools, indicators and definitions across surveys and studies for the reporting of tobacco use is key in order to track progress towards achieving a 30% reduction in the prevalence of tobacco users.36,82
Diet
The WHO recognises diet as one of the key contributors to several major risk factors for NCDs.87 The diet-specific target for the Global NCD Action Plan and the Global Monitoring Framework for the prevention and control of NCDs is to achieve a 30% relative reduction in salt/sodium intake by the population by 2025.36,53 Alongside this, many of the other NCD targets rely heavily on substantial dietary behavioural change.36 In LMICs such as South Africa, rapid urbanisation and the transition to energy-dense diets high in saturated fat, sugar and salt, and low in micronutrients has been identified as a major driver of obesity and higher rates of NCDs.87 In South Africa, the Strategic Plan for the Prevention and Control of NCDs highlights the need for dietary change to tackle high obesity burdens and micronutrient deficiencies, with a specific focus on reducing the consumption of salt, fried foods and snacks, hard margarines, and sugary foods and beverages, while promoting consumption of whole grains, fruit, vegetables, legumes, lean meat and low-fat dairy products.4
In South Africa, data examining dietary intakes were predominantly from cross-sectional surveys, which used subjective recall-based dietary assessment tools (such as 24-hour recall and food-frequency questionnaires) to report on overall macro- and micronutrient intakes and/or intakes of specific dietary components or food items (e.g. salt intakes). This is an important point to be cognisant of when interpreting dietary data as these approaches may not account for the fact that individual dietary components are consumed in combination, within an overall diet.88 In addition, subjective dietary assessment tools have documented limitations related to the accuracy of portion-size estimation by participants, the varying contribution of individual food items to composite dishes, and the conversion of food items to their individual nutrient components, which may lead to inaccurate quantification of dietary intakes as well as under- and over-reporting, and must be considered when interpreting the dietary data reviewed.88,89
In addition, the adequacy or quality of dietary intakes is often assessed by comparing population mean or median energy, and macro- and micronutrient intakes with national or international guidelines. Since there are no guidelines specific to the South African population, studies most commonly use the USA’s Institute of Medicine’s dietary reference intakes (DRIs);90 however, other guidelines on specific nutrients are also used, for example, the WHO guidelines on added sugar or salt intake. This can add to the complexity of comparing and interpreting dietary intakes and adequacy across studies.
Overall, 27 studies were identified in the review. However, nine studies were excluded due to the age range of participants being outside the selection criteria (45 to 65 years). In addition, three studies were excluded as they presented only pooled multi-country data: one was a methods article with no dietary data presented, one was a review, and one used dietary data in covariate analyses only. Data from the remaining 12 studies are therefore discussed below.27,28,39,91-99 These studies focused on two main areas: namely, (1) overall dietary intakes (macroand micronutrients and/or food items/groups) and comparison with DRIs or recommendations where applicable; and (2) salt consumption. The results are, therefore, presented according to these categories.
Dietary intakes and adequacy
Macro- and micronutrient intakes of middle-aged men and women were reported in three cross-sectional studies. One included adults living in rural KwaZulu-Natal,92 one included urban adults from the CRIBSA study in the Western Cape,91 and the last involved secondary analysis of data from the PURE study.93 These studies showed that, in both the rural and urban settings, energy intakes were either close to, or in excess of, those recommended for middle-aged men and women.91-93 While only two of these studies assessed under- and over-reporting of dietary intake, only one subject was excluded as an ‘overreporter’ by Kolahdooz et al. (2013),92 and only ‘under-reporters’ were excluded by Steyn et al. (2016).91
One recent interventional study showed that women from peri-urban communities in the Free State consumed, on average, substantially lower (3 678–4 504 kJ per day) levels of energy than the estimated average requirement.94 This study also did not clarify whether under- and over-reporting were taken into account during their analyses.
Three studies reported that, overall, carbohydrate intakes exceeded the recommended dietary allowance of < 130 g per day, while fibre intakes were low (< 25 g per day) and total or added sugar intakes were in excess of recommendations (> 25% and > 10% total energy, respectively).91,92,94 This suggests that middle-aged adults are likely to be consuming high amounts of refined carbohydrates,91,92,94 While the proportion of energy consumed as protein fell within the recommended range in these studies (between 10 and 35% of total energy), it was at the lower end of this range and as much as half of the protein consumed came from plant sources.91,92,94 Rural adults consumed a higher proportion of energy from carbohydrates and a lower proportion of energy from fat compared to their urban counterparts, with urbanisation being linked to higher total, saturated and monounsaturated fat intakes.91,92
In a study by Peer et al. (2018), which used cross-sectional data on semi-urban and urban adults from five of South Africa’s nine provinces, 47 to 55% of men and 38 to 48% of women regularly consumed high-fat foods, with more regular consumption in those between 45 and 54 years of age than those between 55 and 64 years of age.28 Steyn et al. (2016) also showed that, while the proportion of energy consumed as fat fell within the recommended range of 20 to 35% for urban adults from Cape Town, saturated fat intakes exceeded recommendations (> 7% total energy).91
Data from the PURE study assessed dietary intakes of urban adults according to alignment with the South African Food- Based Dietary Guidelines.90 The PURE data showed that higher intakes of dairy products, fruit and vegetables, legumes and fish, and lower intakes of meat and meat products (a dietary profile more aligned with the South African Food-Based Dietary Guidelines) was associated with lower energy, fat (saturated, mono-unsaturated and polyunsaturated fat) and sodium, but not fibre, carbohydrate and protein intakes.93
As demonstrated by Kolahdooz et al. (2013) and the CRIBSA study, national fortification of staple foods (maize and wheat flour) with key micronutrients (vitamin A, iron, zinc, folic acid, thiamine, riboflavin, niacin, and vitamin B6) since 2003 has contributed to adequate intakes of some, but not all of these vitamins and minerals.91,92 In a cross-sectional study of peri-urban middle-aged adults in KwaZulu-Natal, the most commonly consumed food items were identified as sugar, maize meal porridge, bread, tea, rice, hard margarine, legumes, cordial squash, non-dairy creamer and milk.96 Both this study and cross-sectional data on urban and rural adults from the Western Cape showed the variety and frequency of fruit and vegetable consumption to be low, with apples and bananas being the most commonly consumed fruit, and cabbage and mixed vegetables being the most commonly consumed vegetables.96,97
Data from Peer et al. (2018),28 as well as from the WHO-SAGE study,17 showed that between half and two-thirds of middle-aged adults consumed inadequate amounts of fruit and vegetables (less than five portions a day).27,28,39 Lower daily fruit and vegetable intakes were also associated with daily or weekly purchasing of sugar-sweetened beverages (SSBs) in one study of rural and urban adults from the Western Cape, with urban adults purchasing SSBs, snacks and sugar more commonly than those living at the rural site.97 In one study exploring seasonality in dietary intakes of women farm workers in the Western Cape, seasonal fluctuations in employment were associated with lower dietary diversity in the autumn and winter months, that is, the non-farming season.98
Salt consumption
Using data collected from the food labels of packaged foods one year prior to implementation of salt regulations in 2016,91 research showed that approximately two-thirds of foods covered by the regulations were either below or meeting the upper limit of sodium content by the time the regulations were implemented100 Despite this, data collected from self-identified white, black and Indian South Africans prior to the implementation of the regulations demonstrated that 65% of adults consumed salt in excess of the WHO’s < 5 g/d recommendation101 In addition, findings from the WHO-SAGE study on salt-related knowledge and behaviour indicated that approximately 30% of South African middle-aged adults were not aware that high salt consumption could have an impact on their health, and 73% perceived the amount that they consumed to be ‘just the right amount’.109 In addition, discretionary salt intake was found to be high, with 79.9% of middle-aged adults adding salt to food during cooking either ‘often’ or ‘always’, and 32.9% regularly adding salt to their meals at the table.109
Peer et al. (2018) showed that in semi-urban and urban settings across South Africa, high intakes of salty foods were evident, with 41.8 to 54.7% of middle-aged men and 38.6 to 49.8% of women regularly consuming high-salt foods8 In addition, a higher percentage of those in the 45- to 54-year age group consumed high-salt foods than those in the 55- to 64-year age group.103 Data from WHO-SAGE has also shown that 91% of all adults fail to meet the daily dietary potassium requirements, and that dietary sodium-to-potassium molar ratios of > 2 are more strongly related to blood pressure as adults age than sodium intakes alone.104 This further emphasises the need for a greater focus on improving overall dietary patterns, rather than limiting research and interventions to single dietary components in isolation.
As mentioned above, in 2003, national fortification of staple foods was introduced in South Africa to reduce the prevalence of micronutrient deficiencies at a population level.105,106 Since then, regulations aimed at minimising trans fatty acid intakes and reducing salt intakes were drafted and published in 2011 and 2013, respectively.101,107 The new regulations108,109 particularly focused on limiting the amount of trans fatty acids and salt in commercially prepared food products.30,110 In 2015, the Strategy for the Prevention and Control of Obesity in South Africa was introduced by the National Department of Health, with goals related to diet particularly focused on improving food environments (including ensuring availability and access to healthy food), as well as educating and mobilising communities and supporting obesity prevention in childhood.30
Sugar-sweetened beverage intake
An SSB tax (the Health Promotion Levy or HPL) was implemented in 2018 at a rate of R0021 per 1 g of sugar, over an initial tax-free threshold of 4 g/100 ml, in order to reduce the sugar content of SSBs and discourage consumer purchasing.103 In a recent longitudinal study, SSB and added sugar intakes of adolescents and adults (n = 617) living in Soweto, Johannesburg, were assessed before, at the time of, and one year after implementation of the HPL.111 Study findings showed that substantial reductions in SSB consumption occurred between 2017 (at the time the intention of an SSB tax was announced) and 2018 (at the time the SSB tax was implemented), particularly by those who consumed higher levels initially. These reduced intakes were maintained in the following year. This is confirmed by data from a sample of South African households, which reported reductions in the sugar, calories and volume of SSB purchases with the implementation of the HPL.112 This is promising, as it supports both supply and demand level benefits of the HPL on SSB and added sugar consumption.
However, data also suggested that the benefits of reduced SSB intake may be mitigated through high sugar consumption from other sources and that improving dietary patterns as a whole is critical to improving health outcomes. While national legislation and fiscal policies are important and documented progress is commendable, there is little evidence of progress on interventions, which would enable improved overall dietary patterns at a population level. This is important as targeting individual dietary components may have a limited impact on overall dietary patterns and, therefore, on obesity and NCD rates. It is increasingly being recognised that there is a need for changes to food environments that simultaneously stimulate demand for, and access to, healthier foods such as fruit and vegetables.
Given the evidence currently available, a grade C is attributed to prevalence data for diet (Table 3). While regional data exist for the 45- to 65-year-old age group, more nationally representative data is needed. Specifically, routine monitoring of salt intakes at national and regional levels in this age group is necessary to track progress towards achieving the target of a 30% reduction in population salt/sodium intakes. In addition, data exploring dietary patterns, as well as their determinants, in this age group are needed to inform interventions and achieve progress towards other NCD targets, which are reliant at population-level dietary behavioural change. For policy and implementation, diet receives a grade A due to the number of national policies that have been implemented since 2003 (Table 3). In future, routine follow-up data to track effectiveness of these policies should be prioritised in order to build on and complement current initiatives.
Obesity
Obesity has become a global epidemic and a major health challenge in both low- and high-income countries.113 Once associated with only developed countries, the prevalence of obesity, along with urbanisation and changes in lifestyle and environment, has increased rapidly in LMICs.114 According to the WHO global estimates, in 2014 more than 1.9 billion adults (39%) were overweight, of whom over 600 million (13%) were obese (BMI > 30.0 kg/m2).115 In the year 2019, it was reported that more than five million people died worldwide as a result of a high BMI.116
While being in line with the WHO’s Global Action Plan for the Prevention and Control of NCDs 2013–2020,53 the South African Strategic Plan targets a reduction in the prevalence of obesity by 10% by 2020.4 The first South African National Burden of Disease study ranked high BMI as the fifth highest risk factor for early death and years of life lived with disability,117 and national statistics reporting trends in the prevalence of overweight and obesity in South Africa between 1980 and 2014 showed that 64% of adult women and 30.7% of adult men were overweight or obese, with the numbers differing quite significantly between the ethnic groups.118 Currently, South Africa’s overweight and obesity prevalence is the highest in sub-Saharan Africa.119
Comparing cross-sectional data from a multi-country study, Ajayi et al. (2016) reported South Africa’s prevalence of obesity (54%) to be the highest compared to Nigeria, Tanzania and Uganda, with age being significantly associated with higher BMI.119 Obesity trends in South Africa have shown an increase between 2003 (SADHS) and 2012 (SANHANES), with the prevalence in women between the ages of 45 and 54 years increasing from 40 to 55%.120,121 The latest SADHS figures reported that the highest prevalence among women was between 45 and 64 years (81–82%), while the prevalence in men of this age group was 42.8% (45–54 years) and 53.2% (55–64 years).9
Forty-two studies were originally selected from the title and abstract search. Fifteen were excluded due to the age range of participants being outside the selection criteria (45–65 years) and because they reported pooled data from other countries. In addition, eight studies were excluded because obesity was not the outcome of interest examined and they did not include prevalence data. Data from the remaining 19 studies are discussed below.26,27,39,62,65,69–71,79,119,121–129
A study by Maimela et al. (2016), in a rural South African community found obesity to be highest in women between 45 and 64 years, with the prevalence showing an increasing trend from 13.6% in 15- to 25-year-olds to 41.9% in 55- to 64-year-olds, while being 45 years or older was associated with a twice as great risk of overweight/obesity among South African adults.26 In addition, as well as reporting age-standardised rates of overweight/obesity of 676 and 686 per 1 000 in adult men and women, respectively, the WHO-SAGE study reported that age was also associated with an increase in the prevalence of central obesity.124
The PURE study reported abdominal obesity in men (mean age 51.9 years) and women (mean age 51.8 years) and although there was no significant change in the rural men, central obesity increased in urban men from 6.1 to 11.7% over five years.71 Furthermore, at baseline, 63.6% of urban women were identified with abdominal obesity compared to 50.5% of rural women and this increased to 69.7% in urban and 55.1% in rural women at follow up.71
The gender difference in obesity prevalence in rural (27.8% women vs 10.6% men, p < 0.001) and urban (43.7% women vs 25.7% men, p < 0.001) South Africans between the ages of 45 and 65 years has been highlighted by several studies.26,69,123 In the study by Ajaya et al. (2016), the BMIs of the South African women were higher than those of the men. As a result, 60% of the women were obese, while only 33% of the men were obese.119 In addition, being a woman was associated with twice the odds of being obese [adjusted odds ratio (AOR) = 2.17; 95% confidence interval (CI): 1.19–4.00] compared with men.119
Although self-identified black South African women are the most affected by obesity, data from Soweto has shown that they are content with their body size or accepting of being obese, which is also aligned with what has been described as the preferred and/or ideal body size in several African populations.71,125 The SADHS 2016 report found that among women who perceived themselves as underweight or normal, 44 and 65%, respectively, were overweight or obese.130
Sociodemographic and socio-economic factors must be considered when trying to understand the aetiology of obesity in South Africa.39,71,79,130 Several studies have shown that living in an urban area, having a higher socio-economic status and being married or cohabiting (married under common law) were significant predictors of obesity in men, but not in women.30,39,71,79,130 However, another study among urban South Africans reported that adults with a low socio-economic status who were overweight/obese were more likely to be older (38.6%), women (84.9%), unemployed (82.9%), non-smokers (45.4%) and 51.0% of them used alcohol.121 The same study showed thar other social determinants of health within South Africa include neighbourhood safety, as it found perceived slow speed from traffic [odds ratio (OR) = 0.41; 95% CI: 0.23–0.72] to be associated with being less likely to be overweight/obese, and high crime during the day (OR = 2.20; 95% CI: 1.04–4.64) to be associated with overweight/obesity in women.121 Living in a household with greater resources or having a higher socioeconomic status in adulthood was significantly associated with obesity in women, while the same was not true for men, as reported by Case and Menendez.131
Findings from the 2016 SADHS report a range in the prevalence of overweight or obesity in adult women of different ethnicities, with the lowest prevalence in white women (67.4%) and the highest in Indian/Asian women (70%).9 In men, the prevalence of overweight or obesity was highest in self-identified white men (75%) and lowest in self-identified black African men (27%).130
It is widely accepted that obesity is a risk factor for cardiometabolic risk, and this has been shown by various studies in South African middle-aged adults.65,69,70,123,126 Data from a study in a self-identified urban black Free State community showed that overweight/obese adults older than 44 years had 25% greater risk of being hypertensive compared to adults with normal or underweight BMI. In order to identify South African men and women at risk of cardiometabolic diseases, Kruger et al. (2017) proposed new BMI cut-off points. These show that a cut-off point of 28 kg/m2 in women and 22 kg/m2 in men is associated with increased odds of various cardiometabolic risk outcomes, including elevated blood pressure, dyslipidaemia and type 2 diabetes.89
In an attempt to reduce the growing prevalence of obesity and raise awareness, the South African government adopted the 2015–2020 Strategy for the Prevention and Control of Obesity, introduced by the National Department of Health. This strategy recognises the need for a population-based approach to tackling obesity and acknowledges the importance of context and an enabling environment. It also emphasises the need to communicate with communities in order to educate and mobilise them.30 Given the current available evidence, a grade A is assigned to the availability of obesity data based on the prevalence grading criteria; however, grade B is assigned to policy and implementation as current policies have only been in place within the last 10 years (Table 3). Although national and regional evidence exists for this age group, it remains to be seen if the current policies will be effective in reducing obesity.
Dyslipidaemia
Raised cholesterol (total cholesterol > 5 mmol/l) is a major cause of CVD and disease burden in both high-income countries and LMICs.132 In 2016, the Global Burden of Disease study reported that high concentrations of total cholesterol caused 4.4 million deaths and over 93 million DALYs, representing the seventh and eight leading risk factors in terms of attributable DALYs globally for women and men, respectively.133 Furthermore, there has been a 15% increase in the deaths (either from ischaemic heart disease or stroke) due to high total cholesterol levels since 2006.133
Global targets to reduce the prevalence of dyslipidaemia are included in the WHO 25 × 25 goal (25% reduction in risk of premature death from NCDs by 2025) and focus on lowering total cholesterol levels to < 5 mmol/l and low-density lipoprotein (LDL) cholesterol to < 3 mmol/l.134 Although the Strategic Plan for the Prevention and Control of NCDs 2013–2017 adopted by South Africa aims to reduce premature mortality by 25% by 2020, it does not include a specific goal targeted at dyslipidaemia.4 In South Africa, it is estimated that since 2000, more than half of all ischaemic heart disease and more than one-quarter of all ischaemic stroke cases were associated with high serum cholesterol levels.38
Following international trends, the SADHS in 2003 reported that more than 5.7 million South Africans had an abnormal lipid profile, of whom less than 50% were on treatment.13 Self-reported high cholesterol levels among women aged over 15 years has increased from 1% (from SADHS 1998) to 4% in 2016 (SADHS 2016).9,135 In contrast, the SANHANES, completed in 2012, revealed that 28% of women and 19% of men older than 18 years had elevated total cholesterol levels, and 52 and 44% of men and women, respectively, had low levels of high-density lipoprotein (HDL) cholesterol.56 Although the national surveys reported the prevalence of abnormal lipid levels in South Africa, it was not specific to the 45- to 65-year age group, but rather included all adult ages from over 18 years.
Dyslipidaemia is characterised by the presence of non-optimal levels of blood lipids. In clinical practice guidelines, dyslipidaemia is defined by elevated total cholesterol (> 5 mmol/l) and/or LDL cholesterol (> 3 mmol/l) levels. However, the definition is often extended to include non-optimal levels of HDL cholesterol (< 1.2 mmol/l ) and elevated circulating triglycerides (> 1.7 mmol/l).136 Of the 18 articles identified on dyslipidaemia, from the title and abstract screening, all were cross-sectional studies, including several articles from the PURE (n = 6)23,40,41,47,68,137 and regional studies (n = 12).26 ,67,69,76,78,122,123,138-142 Of the 18 studies, three reported limited information in the 45- to 65-year age group,67,122,139 while five studies were not designed to focus primarily on dyslipidaemia but rather to investigate various CVD risk factors in the broad adult population.41,47,68,76,137 This narrative therefore discusses the data from the remaining 10 studies.
The prevalence of dyslipidaemia within middle-aged adults reported by various regional South African studies ranges from 31.7 to 67.3%.23,78,140,141 Dyslipidaemia affects both men and women; however, data from the CRIBSA and Dikgale HDSS studies show that lipid irregularities contributing to the overall diagnosis of dyslipidaemia differ between men and women.26,138 In the CRIBSA study, although the prevalence of high total cholesterol levels (> 5 mmol/l) was the same in men and women (38% for both), women had a higher prevalence of low HDL cholesterol (61.3 vs 55.7%) and high LDL cholesterol (62.5 vs 51.6%) compared to men in the 45- to 54-year age range.138 Furthermore, men had a higher prevalence of triglyceride levels > 1.5 mmol/l compared to women in the same age range (28.6 vs 19.0%).
This differed from a study in Limpopo, where the 45- to 54-year-old women had a higher prevalence of high total cholesterol > 5 mmol/l (32.6 vs 21.7%) and raised triglyceride levels > 1.7 mmol/l (27.1 vs 21.7%) compared to men.26 When comparing the two age groups (45–54 vs 55–64 years) in the CRIBSA study, Peer et al., (2014) observed a decrease in the prevalence of high total cholesterol (38–26%), low HDL cholesterol (55.7–55.5%), high LDL cholesterol (51.6–31.1%) and high triglycerides (28.6–26.6%) with age in men; while an increase in all lipid indicators was observed in women: high total cholesterol (38.9– 45.8%), high LDL cholesterol (62.5–66.1%) and high triglycerides (19–25.1%).138
In contrast, the Dikgale HDSS data observed a 7.1% decrease in men and a 15.5% decrease in women in the prevalence of high total cholesterol levels in the 55- to 64-year group compared to the 45- to 54-year group.26 Therefore, dyslipidaemia appears to affect middle-aged men and women differently. Although the prevalence of dyslipidaemia in men and women differs across regions in South Africa, the same factors are strongly associated with dyslipidaemia in both genders, including age, overweight or obesity and waist circumference.23,26,78,138,140,141
In addition to observed gender differences in the prevalence of dyslipidaemia, ethnic differences are evident within South Africa. A regional cross-sectional study showed that selfidentified black African men and women were less likely to have hypercholesterolaemia compared to their self-identified white counterparts (men, OR: 0.64, 95% CI: 0.49–0.84; women, OR: 0.52, 95% CI: 0.43–0.62).123 In addition, self-identified Indian men were more likely to have hypercholesterolaemia than white men (OR: 1.47, 95% CI: 1.05–2.08).123 A general linear pattern was observed for the association between hypercholesterolaemia and BMI category in self-identified black Africans and selfidentified white participants, while no discernible pattern in self-identified Indian or self-identified coloured participants was found. Compared with normal-weight participants, the odds for hypercholesterolaemia were significant for overweight self-identified white participants (OR: 1.51, 95% CI: 1.18–1.94), and obese self-identified white participants (OR: 1.55, 95% CI: 1.19–2.03) and self-identified black Africans (OR: 1.63, 95% CI: 1.25–2.12).123
Of particular concern when reviewing this literature and highlighted by data from the Health and Aging in Africa Longitudinal study (HAALSI) in Agincourt, Mpumalanga, is that only a small proportion of individuals (1.05%) were aware of their dyslipidaemic condition and, of those who were aware, less than 1% (0.73%) are currently on treatment.141 The WHO has reported that a 10% reduction in serum cholesterol level in men aged 40 years and over is associated with a 50% reduction in heart disease within five years.132 Low-cost methods for identifying at-risk individuals exist and treatment with cholesterol-lowering medications in the form of statins is cost effective and is known to have a positive effect. The most recent content review by the Heart and Stroke Foundation South Africa in 2017143 confirmed the current guidelines from the 2016 European Society of Cardiology and European Atherosclerosis Society Guidelines for the Management of Dyslipidaemia, in which the desired lipid targets are a total cholesterol level < 5 mmol/l; LDL cholesterol < 3 mmol/l; HDL cholesterol > 1.2 mmol/l for women and > 1.0 mmol/l for men, and a fasting triglyceride level < 1.7 mmol/l.
Given the current evidence available and according to our prevalence and policy and implementation grading criteria, a grade C and B are attributed to dyslipidaemia, respectively (Table 3). More recent published national data are needed (particularly for the 45- to 65-year age group) in order to highlight the prevalence of dyslipidaemia in middle-aged South African adults, and the consequences thereof. Current guidelines for the management of dyslipidaemia have been updated and currently implemented since 2017, however, regional studies have shown that regardless of this, less than 1% are currently on treatment.
Hypertension
In 2019, the Global Burden of Disease Study estimated that high systolic blood pressure (SBP > 140 mmHg) resulted in approximately 10.8 million deaths globally. However, this estimate excludes individuals with hypertension through elevated diastolic pressure or who are on antihypertensive treatment with an SBP of less than 140 mmHg.116 In 2015, the WHO estimated that the total number of individuals living with hypertension was 1.13 billion, two-thirds of whom lived in LMICs.144 This is of great concern as awareness, treatment and control remain problematic in these regions.145,146
As part of the WHO 25 × 25 target, the United Nations set a target for 25% reduction in the prevalence of SBP > 140 mmHg between 2010 and 2025.147 The South African target as part of the Strategic Plan for the Prevention and Control of NCDs 2013–2017 adopted the broader definition of hypertension, setting a goal of 20% reduction in the prevalence of raised blood pressure by 2020 (by medication and lifestyle), and a 30% increase in individuals controlled for hypertension.4 South African blood pressure data from the Global Burden of Disease study (2015, n = 13 580) showed a > 60% increase in estimated death and disability due to elevated blood pressure between 1990 and 2015.148 Across these 25 years, SBP increased in both men and women and across all older age groups (45–49, 50–54, 55–59 and 60–64 years).
Hypertension is most commonly classified as SBP of 140 mmHg or above, and/or diastolic blood pressure (DBP) of 90 mmHg or above, and/or on hypertension medication. Of 58 articles identified from the title and abstract screening, 18 presented data on hypertension prevalence specifically for South African adults, with results presented for age groups within the 45- to 64-year age range.26-28,43,61,64, 65,148-158 Reasons for exclusion of articles were: hypertension data were combined across countries (n = 5), data were not presented by age group within the target age range (n = 33), or data were presented from selected groups, such as teachers or adults with normal blood pressure only (n = 2).
With the exception of the WHO-SAGE study, which used wrist blood pressure measurement, most other studies presented blood pressure data from automated measurements taken at the brachial artery. The majority of articles reported conducting three measures and blood pressure data were most commonly presented as the average of the second and third readings. However, several studies used methods that could potentially lead to over- or underestimates of blood pressure or hypertension prevalence (averaging all blood pressure measurements taken, using the highest or lowest blood pressure value recorded, or using self-reported data only on hypertension status).
A report produced by the Health Systems Trust in 2015 compares the NIDS hypertension prevalence data between 2008 and 2012, showing that hypertension prevalence remained fairly static in this time period,159 although prevalence had increased since the 1998 SADHS survey.135 However, the 2016 SADHS data9 report that hypertension prevalence overall has nearly doubled since the 1998 survey, with 55% of men and 63% of women aged 45–54 years, and 74 and 78% of men and women aged 55–64 years living with hypertension.
WHO-SAGE wave 1 (2007–2010) data published in 2013 reported that the odds of having hypertension were significantly higher in 40- to 59-year-olds (OR: 2.98, 95% CI: 0.569–15.58; n = 1 134) and in 60- to 79-year-olds (OR: 38.89, 95% CI: 5.549–272.6; n = 360) compared to < 40-year-old adults (n = 569; p < 0.001).150 An analysis of the same dataset but including only those South African adults over 50 years (n = 3 840) showed that around threequarters of adults had hypertension (overall prevalence 77.3%, men 74.4%, women 79.6%) with the highest prevalence observed in women aged 60 to 69 years (81.6%).43 In this nationally representative sample, 38.1% of the adults with hypertension were aware of their hypertension, 32.7% were being treated and 17.1% had their hypertension under control, with four out of five hypertensive older adults showing uncontrolled blood pressure.
Hypertension in the over 50-year-old group did not differ significantly between urban and rural populations in South Africa and was higher than any previous figures for African adults27 and higher than all other WHO-SAGE countries (China, India, Ghana, Mexico, Russia),61 with the lowest levels of hypertension diagnosis across all six countries.64.
In 2016, Irazola et al. reported the prevalence of hypertension from four urban South African communities at 61% in 45- to 54-year-olds and 70% in 55- to 64-year-olds.154 A further 23 and 18% of 45- to 54-year-olds and 55- to 64-year-olds, respectively, were diagnosed with pre-hypertension (SBP between 120 and 139 mmHg and/or DBP between 80 and 89 mmHg in the absence of a diagnosis of hypertension or treatment).154 Data from the CRIBSA study published in 2015 reported a similar prevalence of hypertension, with the highest levels observed in women in both age groups (45- to 54-year-olds: 60.6% in women vs 58.2% in men; and 55- to 64-year-olds: 77.1% in women vs 63.6% in men).157
In another regional study in Cape Town comparing two independent cross-sectional surveys in 2008–2009 and 2014–2016, results showed that in 2008–2009 screen-detected hypertension was highest in ≥ 70-year-olds, while in 2014–2016 the peak was in 40- to 49-year-olds.152 The recorded prevalence of hypertension from the study cohort in 2008–2009 was 33% in 40- to 49-yearolds, 50% in 50- to 59-year-olds and 68% in 60- to 69-year-olds. In 2014–2016, this had increased significantly in all groups to 58% in 40- to 49-year-olds, 71% in 50- to 59-year-olds and 83% in 60- to 69-year-olds. Relying on self-reported hypertension status only, a 2015 survey conducted with 28 007 Gauteng adults reported a hypertension prevalence of 21% in 48-to 57-year-olds, and 32% in 58- to 67-year-olds.155
In 2013, a screening campaign in five of the nine South African provinces reported a hypertension prevalence in selfselected urban and peri-urban adults of 57.3 and 54.1% in 45- to 54-year-old men and women, respectively, and 74.8 and 68.3% in 55- to 64-year-old men and women, respectively.28 Another screening campaign conducted in Zandspruit (outside of Johannesburg), Gauteng, in 2012–2014 reported a prevalence of elevated blood pressure (≥ 140/90 mmHg) as 55% in 45 to 54 year olds (n = 889), and 63% in 55 to 64 year olds (n = 109).158
Treatment use in studies recruiting volunteers as part of a screening campaign report higher prevalences of hypertension than most studies (53.0 and 57.4% in 45- to 54-year-old men and women, respectively; 60.8 and 70.2% in 55- to 64-year-old men and women, respectively), as well as the percentages of those who were controlled on treatment (42.1 and 44.5% in 45- to 54-year-old men and women, respectively; 41.8 and 47.2% in 55- to 64-year-old men and women, respectively).28 These findings may suggest that such screening programmes disproportionately attract individuals who know their diagnosis and are actively seeking to monitor their treatment response.
When examining data for the different ethnic groups collected as part of the screening campaign described above, in comparison to self-identified black women, Indian women were more likely (OR: 1.31, 95% CI: 1.01–1.69) and self-identified white women were less likely (OR: 0.55, 95% CI: 0.44–0.69) to have hypertension. Other factors associated with having hypertension in both men and women were increasing age, being resident in the Eastern Cape or the Free State (compared with the Western Cape), and having multimorbidity (especially having a cardiac problem, stroke, diabetes or being overweight or obese).28
Data from two studies conducted at the Dikgale HDSS site in Limpopo and the Agincourt HDSS site in Mpumalanga have reported quite disparate hypertension prevalences. In Dikgale, a hypertension prevalence of 41% in 40 to 49 year olds, 48% in 50 to 59 year olds and 51% in over 60 year olds were reported.156 While the prevalence of hypertension was similar between men and women in the 55- to 64-year-old group (47 and 46%, respectively), in the younger 45- to 54-year age group more women than men were hypertensive (43 vs 35%).26
Data collected during household visits in Agincourt, Mpumalanga, in 2014–2015 (the HAALSI study) showed hypertension prevalence rates of 45% in 40 to 49 year olds, 64% in 50 to 59 year olds, and 74% in 60 to 69 year olds.151 Interestingly, rates of hypertension awareness were much higher than anywhere reported previously (80% at age 40–49 years, 86% at age 50–59 years, and 94% at age 60–69 years), possibly as a result of continued research and surveillance efforts in the region. However, while many had received antihypertensive treatment (47% at age 40–49 years, 64% at age 50–59 years and 75% at age 60–69 years), fewer adults were continuing to stay on and take their blood pressure medication, particularly in the younger groups (31% at age 40–49 years, 45% at age 50–59 years and 58% at age 60–69 years).
In an attempt to prevent hypertension and reduce population blood pressure, in June 2016 South Africa implemented legislation101 mandating maximum sodium levels in a range of manufactured foods that contribute significantly to salt intake in a population, which was updated with a second level of sodium lowering in 2019.108,109 The diet section in this report discusses the evidence on salt intake in South Africa and, as yet, there are few data to assess the impact of this legislation on population sodium intakes or on blood pressure.
Given the evidence and according to our grading criteria, grades A and B are attributed to the prevalence, and policy and implementation grading criteria for hypertension, respectively (Table 3). Adopting standardised methods of measuring blood pressure is recommended across studies, and as the more immediate changes in blood pressure anticipated as a result of the sodium legislation are expected to be relatively small,160 this attention to measurement technique is critical to not miss these changes in blood pressure. However, the evidence reviewed suggests that, currently, South Africa is not on track to realise either the South African national or global target.
Diabetes
The most recent International Diabetes Federation Atlas (9th edn) has reported an estimated global diabetes prevalence of 9.3%, which is predicted to rise to 10.2% in 2030 and 10.9% in 2045.161 Of the 436 million people with diabetes, 19 million live in sub-Saharan Africa, 60% of whom do not know they have diabetes. The global prevalence is higher in urban compared to rural communities, with 67% of people with diabetes living in urban areas.161 Globally, between the ages of 45 and 65 years, there is a higher prevalence of diabetes in men compared to women, with the prevalence increasing in both genders between these ages.
The latest Global Burden of Disease data (2019) shows that diabetes is one of only three conditions (the other two being HIV/AIDS and musculoskeletal disorders) that have shown large increases in age-standardised DALY rates since 1990, with an increase of 24.4% (95% UI: 18.5–29.7).162 While being the fifth leading cause of global DALYs in 1990 in people between the ages of 50 and 74 years, this has increased to being the third leading cause in 2019.162 The WHO global NCD target has set out to halt the rise in diabetes by 2025, while the diabetes goal outlined in South Africa’s Strategic Plan for the Prevention and Control of NCDs 2013–2017 is to increase the percentage of people controlled for diabetes by 30% by 2020.4
In South Africa, type 2 diabetes was the leading cause of death in 2016 in women (7.2% of deaths).163 SANHANES data published as part of a 12-country study reported the prevalence of diabetes as 14% in men and 17% in women between 40 and 54 years of age, and 23% in men and 30% in women in the 55- to 64-year-old age group.164 The 2016 SADHS, which used adjusted haemoglobin A1c (HbA1c) from dried blood-spot specimens in a sub-sample of nationally representative households, reported a similar prevalence of 12 and 21% in 45- to 54-year-old men and women, respectively, and 23 and 29% for 55- to 64-year-old men and women, respectively.9
Of the 42 articles that were selected based on title and abstract screening, only 19 met the inclusion criteria for this narrative.26,28,63,76,122,123,151,152,155,165-173 Twenty-three articles were excluded and the reasons for exclusion included no diabetesspecific data (n = 8), out of age range (n = 9), only included continuous glucose data (n = 2), only included impaired fasting glucose prevalence (n =1) or diabetes was a correlate with no prevalence data available (n = 1). One reference was a conference abstract and one was a conference poster. Comparisons between the studies is sometimes difficult as various methods and cut-off points are used to define diabetes [self-report, random blood glucose (RBG), HbA1c, fasting blood glucose (FBG)], and the study samples used in the various studies are often self-selected so do not represent a population.
The diabetes prevalence reported by regional studies ranges from 2% in a study by Senekal et al. (n = 517; mean age of 45 years; RBG > 11.1 mmol/l) to 21.4% in 58- to 67-year-old men and women using self-report.122,155 Primary healthcare screening of adults in the Eastern Cape (n = 17 359, over 18 years, 50% older than 45 years) identified 14% of people over the age of 55 years with a RBG ≥ 7.8 mmol/l.165
Bailey et al. (2016), using RBG ≥ 11.1 and self-reported diabetes in a sample of 12 496 individuals from the Western Cape, reported a diabetes prevalence of 11.5% in adults between 40 and 49 years of age, and 18.1% between 50 and 59 years of age.166 The Bellville South study includes of a self-reported coloured population (n =1 294; mean age 47.8 years) and reported a 7.3% prevalence using WHO criteria,152 while another cohort study in South Africa, the HAALSI cohort, reported a 12% prevalence in their sample over the age of 40 years.151 A small study of commercial taxi drivers (98.8% men, mean age 43.3 years) reported a 16% prevalence of diabetes defined as a FBG ≥ 7.0 mmol/l or self-report.167
It is clear from national data as well as regional studies that the prevalence of diabetes increases with age and BMI. This is well illustrated by Shen et al. (2016), who include data from the CRIBSA study as part of a multi-site study, and reported a diabetes prevalence of 13.8% in the whole sample (25–74 years) but a clear increase in prevalence with age and BMI.76 Hird et al. (2016) compared the prevalence of diabetes using the WHO diagnostic criteria for FBG (≥ 7.0 mmol/l), two-hour plasma glucose level from an oral glucose tolerance test [two-hour postprandial glucose (PG) ≥ 11.1 mmol/l] and HbA1c ≥ 6.5%.168 In men between 45 and 54 years, the prevalence was 9.3, 7.0 and 11.6% for FBG, two-hour PG and HbA1c, respectively, and for women of the same age, these were 23.2, 17.2 and 27.2%, respectively. In slightly older adults (55–64 years) these increased to 25.8, 25.8 and 29% for FBG, two-hour PG and HbA1c, respectively, in men, and 34.5, 30.1 and 32.7%, respectively, in women.
Ethnic differences in the prevalence of diabetes have been reported in several regional studies.123,169 Peer et al. (2018) in a sample of 7 711 adults (mean age 47.6 years in men and 48.6 years in women) who attended a screening campaign, compared the prevalence of diabetes between self-identified white, coloured, black and Indian men and women. They showed that compared with self-identified white men and women, selfidentified black men (OR: 2.66, 95% CI: 1.70–4.16) and women (OR: 2.10, 95% CI: 1.49–2.96), self-identified coloured men (OR: 2.28, 95% CI: 1.44–3.60) and women (OR: 2.15, 95% CI: 1.52– 3.05) and self-identified Indian men (OR: 4.38, 95% CI: 2.65–7.26) and women (OR: 3.64, 95% CI: 2.50–5.32) were more likely to have diabetes.123
The prevalence of diabetes was highest in self-identified Indian men and women (26.2 and 26.8%, respectively), followed by self-identified coloured adults (men 12.6 and women 12.2%), then by self-identified black men and women (9.6 and 9.7%, respectively) and then by self-identified white adults (men 9.5% and women 6.8%). A smaller study (n = 317, age 45 ± 9 years) by Malan et al. (2013), reported an HbA1c ≥ 5.7% in 68.8% in self-identified black men, 47.3% in self-identified black women, 44.1% in self-identified white men and 20.7% in self-identified white women.169
Few studies have compared prevalence data between urban and rural populations, with only data from the WHO-SAGE study (n = 3 280; > 50 years of age) reporting a prevalence of self-reported diabetes of 11.1% in urban and 5.6% in rural samples.170 Data from the same study but including adults over 18 years of age (although 95.1% were over 40 years of age), reported a risk ratio for diabetes of 3.36 (1.92–5.74) in urban compared to a rural population.63 The same study also compared a migrant population group to a rural group and found no significant differences in diabetes risk. Using self-reported diabetes data from the Quality of Life Survey from the Gauteng province (n = 280 087; over 18 years of age), Motlhale et al. (2019) showed that being a migrant (internal or external) was associated with a significantly lower risk of diabetes compared to people born in Gauteng.155
Several studies have reported diabetes prevalence data on the > 50-year-old sample from the WHO-SAGE (wave 1) study, all of which describe its association with other diseases or risks. Quashie et al. (2019) reported a diabetes prevalence of 8.9% and showed that diabetes was associated with a four times greater odds of angina. Stubbs et al. (2018) reported a 9.2% diabetes prevalence when examining the association between multimorbidity and stress. Werfalli et al. (2018) also reported a diabetes prevalence of 9.2% and showed that the prevalence was higher in 60- to 69-year-olds (10.6%) compared to 50- to 59-yearolds (7.1%).63,171-173
Gender differences in the prevalence of diabetes have been reported by various studies in middle-aged men and women; however, the findings of these studies differ, with some showing a higher prevalence in women and others showing a higher prevalence in men. Using a FBG cut off of ≥ 7.0 mmol/l, Maimela et al. (2016) reported a higher prevalence in women compared to men between the ages of 45 and 54 years (9.1 vs 4.3%) and 55 to 64 years (15.7 vs 12.2%).26 The screening campaign by Peer et al. (2018) reported a prevalence of diabetes (known or RBG ≥ 11.1 mmol/l) in 13.8% in men and 12.8% in women, with age-specific prevalence also being higher in men than women (45–54 years: men 18.9% vs women 12.9%; 55–64 years: men 21.9% vs women 21.4%).28
As is recommended by various international diabetes organisations, the Society for Endocrinology, Metabolism and Diabetes of South Africa recommends a two-step targeted approach when screening for diabetes. This includes identifying individuals at high risk of diabetes: (1) individuals who are overweight with one or more risk factors for diabetes, such as physical inactivity, hypertension, family history of diabetes, dyslipidaemia, polycystic ovarian syndrome, high-risk ethnic group (South Asian descent), CVD history, gestational diabetes or baby > 4 kg, previous impaired fasting glycaemia or impaired glucose tolerance; or (2) adults ≥ 45 years old.174 This ensures more targeted screening for diabetes, which should ensure that the number of individuals with undiagnosed diabetes is reduced. This guideline highlights that the optimal management should focus on obtaining glycaemic control and reducing other risk factors for macro- and microvascular disease. It advises lifestyle modification and that physical activity is an important component if there are no contra-indications.
Given the prevalence data available, which includes national and regional data highlighting populations most at risk, this section on diabetes should receive an A grading for the prevalence grading criteria (Table 3). However, a significant amount of work still needs to be done with regard to the development and implementation of policies for the management of diabetes in the South African population in order to reach the target outlined by the South African Strategic Plan. For this reason, the policy and implementation grade is a C (Table 3).
Conclusion
It is clear from this systematic review that prevalence data for this age group, both national and regional, is available, with some risk factors, such as obesity, hypertension and diabetes, having more extensive data than others, such as physical activity and diet. What is also evident is the disconnect between the availability of prevalence data, and the proposal and implementation of policies. This is not consistent across the risk factors, with some having higher grades for the availability of prevalence data for this age group, compared to the policy grading (physical activity, alcohol, obesity, hypertension and diabetes) and others having higher grades for policy and implementation compared to grades for prevalence data (tobacco, diet and dyslipidaemia). Perhaps lessons can be learnt and shared in order to achieve full marks for all the risk factors for both grades.
Ensuring that harmonised methodologies are used to collect prevalence data for this age group in order to pool data and more clearly understand national and regional trends should be a priority in order to achieve an A prevalence grading for physical activity, tobacco, diet and dyslipidaemia, while some risk factors such as alcohol and diabetes have extensive prevalence data to draw on that can be used by government to prioritise and support the implementation of policies.
The countdown to achieving SDG 3.4 by 2030 has begun.3 NCD Countdown 2075 collaborators, using cause-specific mortality data from countries that have committed to this goal, show that no country will achieve this by addressing a single disease, and that a combination of multimorbidity prevention strategies and strengthening the health system are necessary.175 Some high-income countries such as Denmark and New Zealand are on track to reach the SDG target if they maintain or improve the average rates of decline recorded between 2010 and 2016.
While these countries focus on the traditional four-by-four approach, which is four major disease groups (cancer, CVD, type 2 diabetes and chronic respiratory disease) linked to four behavioural risk factors (insufficient physical activity, poor diet, tobacco use and harmful use of alcohol), it is now clear that this is a lot more complex in sub-Saharan Africa. The mismatch between local strategic NCD policy agendas that align with global recommendation, and the reality of the local NCD burden and its determinants in the region has meant that many strategic plans may not be effective in meeting their NCD goals. Dover and Lambert stress the importance of recognising the social, environmental, political and economic influences on health behaviours, and that policies and interventions must be designed around an understanding of individual, household and community factors that may influence these behaviours.176
This report card is the first of its kind and clearly describes what is currently known about the prevalence and policy implementation of eight important indicators of NCD risk. It very briefly summarises and grades the prevalence data available, and grades whether policies exist and whether they have been implemented. Future report cards will also include whether current targets set out by the Strategic Plan for the Prevention and Control of NCDs 2013–2017 have been met.
Acknowledgments
We acknowledge Stacy Nelleman for completing the article screening for May 2018 and April 2019, and the librarians at the University of Cape Town Health Sciences Library.
References
- 1.Gouda HN, Charlson F, Sorsdahl K, Ahmadzada S, Ferrari AJ, Erskine H. et al. Burden of non-communicable diseases in sub-Saharan Africa, 1990–2017: results from the Global Burden of Disease Study 2017. Lancet Glob Health. 2019;7(10):e1375–1387. doi: 10.1016/S2214-109X(19)30374-2. [DOI] [PubMed] [Google Scholar]
- 2.Ezeh A, Kissling F, Singer P. Why sub-Saharan Africa might exceed its projected population size by 2100. Lancet. 2020;396(10258):1131–1133. doi: 10.1016/S0140-6736(20)31522-1. [DOI] [PubMed] [Google Scholar]
- 3.Sustainable Development Goals – Goal 3. United Nations Department of Economic and Social Affairs. Retrieved 2020 Nov 5, from https:// sdgs.un.org/goals/goal3 . [Google Scholar]
- 4.Pretoria: National Department of Health (NDoH): 2013. National Department of Health (NDoH). Strategic Plan for the Prevention and Control of Non-Communicable Diseases 2013–+2017. [Accessed 2020 Sep 10]. https://www.iccp-portal.org/south-africa-strategic-planprevention- and-control-non-communicable-diseases-2013-2017. [Google Scholar]
- 5.South African Declaration on the Prevention and Control of Non-Communicable Diseases [Internet]. South African Summit on the Prevention and Control of Non-Communicable Diseases gathered in Gauteng. 2011 http://www.health.uct.ac.za/usr/health/research/groupings/ cdia/downloads/SA_NCD_Declaration.pdf. [Google Scholar]
- 6.Clark A, Jit M, Warren-Gash C, Guthrie B, Wang HHX, Mercer SW. et al. Global, regional, and national estimates of the population at increased risk of severe COVID-19 due to underlying health conditions in 2020: a modelling study. Lancet Glob Health. 2020;8(8):e1003–1017. doi: 10.1016/S2214-109X(20)30264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Oni T, Micklesfield LK, Wadende P, Obonyo CO, Woodcock J, Mogo ERI. et al. Implications of COVID-19 control measures for diet and physical activity, and lessons for addressing other pandemics facing rapidly urbanising countries. Glob Health Action. 2020;13(1):1810415. doi: 10.1080/16549716.2020.1810415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A, Cape Town: HSRC Press: 2013. The South African National Health and Nutrition Examination Survey: SANHANES-1 [2011]. [Google Scholar]
- 9.Pretoria and Rockville, Maryland, USA: NDoH, Stats SA, SAMRC, and ICF; 2019: National Department of Health (NDoH), Statistics South Africa (Stats SA), South African Medical Research Council (SAMRC), ICF. South Africa Demographic and Health Survey 2016. https:// www.samrc.ac.za/reports/sadhs2016. [Google Scholar]
- 10.De Neve J-W, Karlsson O, Coetzee L, Schröder H, Subramanian S, Bärnighausen T. et al. Antiretroviral therapy coverage associated with increased co-residence between elderly people and prime-age adults in Africa. AIDS. 2018;32(14):2051–2057. doi: 10.1097/QAD.0000000000001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Draper CE, Tomaz SA, Harbron J, Kruger HS, Micklesfield LK, Monyeki A. et al. Results from the Healthy Active Kids South Africa 2018 Report Card. S Afr J Child Health. 2019;13(3):130–136. [Google Scholar]
- 12.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JPA. et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. Br Med J. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pretoria: Department of Health, South Africa: MRC South Africa: 2003. National Department of Health (NDoH), South African Medical Research Council (SAMRC). South Africa Demographic and Health Survey 2003. [Google Scholar]
- 14.World Health Organization. WHO Study on global AGEing and adult health (SAGE). World Health Organization. Retrieved 2020 Sep 21, from http://www.who.int/healthinfo/sage/en/ [Google Scholar]
- 15.National Income Dynamics Study (NIDS) [Internet]. N.i.D.S. Retrieved 2020 Sep 21, from http://www.nids.uct.ac.za/ [Google Scholar]
- 16.Teo K, Chow CK, Vaz M, Rangarajan S, Yusuf S. The Prospective Urban Rural Epidemiology (PURE) study: Examining the impact of societal influences on chronic noncommunicable diseases in low-, middle-, and high-income countries. Am Heart J. 2009;158(1):1–7.e1.. doi: 10.1016/j.ahj.2009.04.019. [DOI] [PubMed] [Google Scholar]
- 17.Kowal P, Biritwum R, Haldia K, Isingo R, Kumogola Y, Mathur A. et al. WHO Study on global AGEing and adult health (SAGE). Summary results of a pilot in three countries. Geneva, Switzerland: World Health Organization, 2012: 14. (SAGE Working Paper 2). [Google Scholar]
- 18.World Health Organization. Global Recommendations on Physical Activity for Health. Geneva, Switzerland: World Health Organization, 2010: 1. [PubMed] [Google Scholar]
- 19.Guthold R, Stevens GA, Riley LM, Bull FC. Worldwide trends in insufficient physical activity from 2001 to 2016: a pooled analysis of 358 population-based surveys with 1·9 million participants. Lancet Glob Health. 2018;6(10):e1077–1086. doi: 10.1016/S2214-109X(18)30357-7. [DOI] [PubMed] [Google Scholar]
- 20.Geneva, Switzerland: World Health Organization: 2009. World Health Organization. Global health risks: mortality and burden of disease attributable to selected major risks. pp. 1–62. [Google Scholar]
- 21.World Health Organization. Draft WHO Guidelines on physical activity and sedentary behaviour for children and adolescents, adults and older adults. World Health Organization, 2020. https://www.who.int/docs/ default-source/physical-activity/call-for-consultation/draft-guidelineon-physical-activity-and-sedentray-behaviour.pdf. [Google Scholar]
- 22.Gradidge PJ-L, Crowther NJ, Chirwa ED, Norris SA, Micklesfield LK. Patterns, levels and correlates of self-reported physical activity in urban black Soweto women. BMC Public Health. 2014;14(1):934. doi: 10.1186/1471-2458-14-934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sotunde OF, Kruger HS, Wright HH, Havemann-Nel L, Mels CMC, Ravyse C. et al. Association of 25-hydroxyvitamin D and parathyroid hormone with the metabolic syndrome in black South African women. Appl Physiol Nutr Metab. 2017;42(4):413–419. doi: 10.1139/apnm-2016-0257. [DOI] [PubMed] [Google Scholar]
- 24.Hamer M, Bruwer EJ, de Ridder JH, Swanepoel M, Kengne AP, Cockeran M. et al. The association between seven-day objectively measured habitual physical activity and 24 h ambulatory blood pressure: the SABPA study. J Hum Hypertens. 2017;31(6):409–414. doi: 10.1038/jhh.2016.93. [DOI] [PubMed] [Google Scholar]
- 25.Laverty AA, Palladino R, Lee JT, Millett C. Associations between active travel and weight, blood pressure and diabetes in six middle income countries: a cross-sectional study in older adults. Int J Behav Nutr Phys Act. 2015;12(1):65. doi: 10.1186/s12966-015-0223-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maimela E, Alberts M, Modjadji SEP, Choma SSR, Dikotope SA, Ntuli TS . et al. The prevalence and determinants of chronic noncommunicable disease risk factors amongst adults in the Dikgale Health Demographic and Surveillance System (HDSS) site, Limpopo Province of South Africa. PLoS One. 2016;11(2):e0147926. doi: 10.1371/journal.pone.0147926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wu F, Guo Y, Chatterji S, Zheng Y, Naidoo N, Jiang Y. et al. Common risk factors for chronic non-communicable diseases among older adults in China, Ghana, Mexico, India, Russia and South Africa: the study on global AGEing and adult health (SAGE)wave 1. BMC Public Health. 2015;15(1):88. doi: 10.1186/s12889-015-1407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peer N, Balakrishna Y, de Villiers A, Crickmore C, Mungal-Singh V. Effectiveness of a screening programme in identifying individuals with increased risk of cardiovascular disease in South Africa. J Public Health. 2018;40(1):e34–45. doi: 10.1093/pubmed/fdx012. [DOI] [PubMed] [Google Scholar]
- 29.Cape Town: African Physical Activity Network (AFPAN): 2020. African Physical Activity Network (AFPAN). Policy Brief: Physical Activity for Health in Africa: Guidance during and beyond the COVID- 19 pandemic. Sep: 20. [Google Scholar]
- 30.Pretoria: National Department of Health (NDoH): 2016. National Department of Health (NDoH. Strategy for the Prevention and Control of Obesity in South Africa 2015–2020. http://www.health.gov.za/ index.php/2014-03-17-09-09-38/policies-and-guidelines/category/327- 2017po?download=1832:strategy-for-the-prevention-and-control-ofobesity- in-south-africa. [Google Scholar]
- 31.ISPAH. 8 Investments. ISPAH. 2020. https://www.ispah.org/resources/ key-resources/8-investments/ [Google Scholar]
- 32.Shield K, Manthey J, Rylett M, Probst C, Wettlaufer A, Parry CDH. et al. National, regional, and global burdens of disease from 2000 to 2016 attributable to alcohol use: a comparative risk assessment study. Lancet Public Health. 2020;5(1):e51–61. doi: 10.1016/S2468-2667(19)30231-2. [DOI] [PubMed] [Google Scholar]
- 33.Seattle, Washington, USA: Institute for Health Metrics and Evaluation (IHME): 2018. Institute for Health Metrics and Evaluation (IHME). Findings from the Global Burden of Disease Study 2017. http://www. healthdata.org/policy-report/findings-global-burden-disease-study-2017. [Google Scholar]
- 34.Ferreira-Borges C, Parry C, Babor T. Harmful use of alcohol: a shadow over sub-Saharan Africa in need of workable solutions. Int J Environ Res Public Health. 2017;14(4):346. doi: 10.3390/ijerph14040346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Geneva, Switzerland: World Health Organization: 2010. World Health Organization, editor. Global strategy to reduce the harmful use of alcohol. pp. 1–38. [Google Scholar]
- 36.World Health Organization. Noncommunicable Diseases Global Monitoring Framework: Indicator Definitions and Specifications. World Health Organization, 2014. http://www.who.int/nmh/global_ monitoring_framework/en/ [Google Scholar]
- 37.Rehm J, Mathers C, Popova S, Thavorncharoensap M, Teerawattananon Y, Patra J. Global burden of disease and injury and economic cost attributable to alcohol use and alcohol-use disorders. Lancet. 2009;373(9682):2223–2233. doi: 10.1016/S0140-6736(09)60746-7. [DOI] [PubMed] [Google Scholar]
- 38.Schneider M, Norman R, Parry C, Bradshaw D, Plüddemann A, and the South African Comparative Risk Assessment Collaborating Group. Estimating the burden of disease attributable to alcohol use in South Africa in 2000. S Afr Med J. 2007;97(8) [PubMed] [Google Scholar]
- 39.Phaswana-Mafuya N, Peltzer K, Chirinda W, Musekiwa A, Kose Z. Sociodemographic predictors of multiple non-communicable disease risk factors among older adults in South Africa. Glob Health Action. 2013;6(1):20680. doi: 10.3402/gha.v6i0.20680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zatu MC, van Rooyen JM, Loots DT, Wentzel-Viljoen E, Greeff M, Schutte AE. Self-reported alcohol intake is a better estimate of 5-year change in blood pressure than biochemical markers in low resource settings: the PURE study. J Hypertens. 2014;32(4):749–755. doi: 10.1097/HJH.0000000000000093. [DOI] [PubMed] [Google Scholar]
- 41.Pisa PT, Vorster HH, Kruger A, Margetts B, Loots DT. Association of alcohol consumption with specific biomarkers: a cross-sectional study in South Africa. J Health Popul Nutr. 2015;33(1):146–156. [PMC free article] [PubMed] [Google Scholar]
- 42.Oosthuizen W, Malan L, Scheepers JD, Cockeran M, Malan NT. The defense response and alcohol intake: A coronary artery disease risk? The SABPA Study. Clin Exper Hypertens. 2016;38(6):526–532. doi: 10.3109/10641963.2016.1163372. [DOI] [PubMed] [Google Scholar]
- 43.Peltzer K, Phaswana-Mafuya N. Hypertension and associated factors in older adults in South Africa. Cardiovasc J Afr. 2013;24(3):66–71. doi: 10.5830/CVJA-2013-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peer N, Lombard C, Steyn K, Levitt N. Rising alcohol consumption and a high prevalence of problem drinking in black men and women in Cape Town: the CRIBSA study. J Epidemiol Commun Health. 2014;68(5):446–452. doi: 10.1136/jech-2013-202985. [DOI] [PubMed] [Google Scholar]
- 45.Peltzer K, Chao L-W, Ramlagan S, Szrek H. Daily tobacco use and problem drinking among urban adults in South Africa: a longitudinal study. Pan Afr Med J. 2019;32(51) doi: 10.11604/pamj.2019.32.51.17256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McLoughlin J-A, Little F, Mazok C, Parry C, London L. Prevalence of and associations with papsak wine consumption among farm workers in the Western Cape Province, South Africa. J. Stud Alcohol Drugs. 2013;74(6):879–888. doi: 10.15288/jsad.2013.74.879. [DOI] [PubMed] [Google Scholar]
- 47.Maritz M, Fourie CMT, van Rooyen JM, Kruger IM, Schutte AE. A health profile associated with excessive alcohol use independently predicts aortic stiffness over 10 years in black South Africans. J Hypertens. 2017;35(11):2268–2275. doi: 10.1097/HJH.0000000000001452. [DOI] [PubMed] [Google Scholar]
- 48.Probst C, Shuper PA, Rehm J. Coverage of alcohol consumption by national surveys in South Africa: Coverage of alcohol use in South Africa. Addiction. 2017;112(4):705–710. doi: 10.1111/add.13692. [DOI] [PubMed] [Google Scholar]
- 49.Bertscher A. [Cape Town]: University of Cape Town: 2017. Exploring the complex policy formulation process of the Draft Control of Marketing of Alcoholic Beverages Bill in South Africa. https://open.uct.ac.za/ bitstream/handle/11427/25197/thesis_hsf_2017_bertscher_adam.pdf. [Google Scholar]
- 50.Morojele NK, Dumbili EW, Obot IS, Parry CDH. Alcohol consumption, harms and policy developments in sub‐Saharan Africa: The case for stronger national and regional responses. Drug Alcohol Rev. 2021;40(3):402–419. doi: 10.1111/dar.13247. [DOI] [PubMed] [Google Scholar]
- 51.Harker Burnhams N, Bharat C, Williams DR, Stein DJ, Myers B. Transitions between lifetime alcohol use, regular use and remission: Results from the 2004 South African Stress and Health Survey. S Afr Med J. 2018;109(1):40. doi: 10.7196/SAMJ.2018.v109i1.13061. [DOI] [PubMed] [Google Scholar]
- 52.3rd edn. Geneva, Switzerland: World Health Organization: 2019. World Health Organization. WHO global report on trends in prevalence of tobacco use 2000–2025. https://www.who.int/publicationsdetail- redirect/who-global-report-on-trends-in-prevalence-of-tobaccouse- 2000-2025-third-edition. [Google Scholar]
- 53.Geneva, Switzerland: World Health Organization: 2013. World Health Organization. Global action plan for the prevention and control of non communicable diseases 2013–2020. http://www.who.int/nmh/events/ncd_ action_plan/en/ [Google Scholar]
- 54.Mathers C. Geneva, Switzerland: World Health Organization: 2012. World Health Organization. WHO global report: mortality attributable to tobacco [Internet]. http://whqlibdoc.who.int/publications/ 2012/9789241564434_eng.pdf. [Google Scholar]
- 55.Geneva, Switzerland: World Health Organization: 2015. World Health Organization. WHO global report on trends in prevalence of tobacco smoking, 2015. http://apps.who.int/iris/bitstre am/10665/156262/1/9789241564922_eng.pdf. [Google Scholar]
- 56.Shisana O, Labadarios D, Rehle T, Simbayi L, Zuma K, Dhansay A. et al. The South African National Health and Nutrition Examination Survey, 2012: SANHANES-1: the health and nutritional status of the nation. Cape Town: HSRC Press; 2014. http://repository.hsrc.ac.za/ handle/20.500.11910/2864. [Google Scholar]
- 57.Reddy P, Zuma K, Shisana O, Kim J, Sewpaul R. Prevalence of tobacco use among adults in South Africa: Results from the first South African National Health and Nutrition Examination Survey. S Afr Med J. 2015;105(8):648. doi: 10.7196/samjnew.7932. [DOI] [PubMed] [Google Scholar]
- 58.Ayo-Yusuf OA, Olutola BG, Agaku IT. Cigarette smoking trends and social disparities among South African adults, 2003–2011. Nicotine Tobacco Res J. 2015;17(8):1049–1055. doi: 10.1093/ntr/ntu264. [DOI] [PubMed] [Google Scholar]
- 59.Lau YK, Tam J, Fleischer NL, Meza R. Neighbourhood deprivation, smoking, and race in South Africa: A cross-sectional analysis. Prev Med Rep. 2018;11:202–208. doi: 10.1016/j.pmedr.2018.07.001. https://doi.org/10.1016/j.pmedr.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chelwa G, Koch SF. The effect of tobacco expenditure on expenditure shares in South African households: A genetic matching approach. PLoS One. 2019;14(9):e0222000. doi: 10.1371/journal.pone.0222000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lloyd-Sherlock P, Beard J, Minicuci N, Ebrahim S, Chatterji S. Hypertension among older adults in low- and middle-income countries: prevalence, awareness and control. Int J Epidemiol. 2014;43(1):116–128. doi: 10.1093/ije/dyt215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shukla A, Kumar K, Singh A. Association between obesity and selected morbidities: a study of BRICS countries. PLoS One. 2014;9(4):e94433. doi: 10.1371/journal.pone.0094433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Oyebode O, Pape UJ, Laverty AA, Lee JT, Bhan N, Millett C. Rural, urban and migrant differences in non-communicable disease risk-factors in middle income countries: a cross-sectional study of WHO-SAGE data. PLoS One. 2015;10(4):e0122747. doi: 10.1371/journal.pone.0122747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Arokiasamy P, Uttamacharya, Kowal P, Capistrant BD, Gildner TE, Thiele E. et al. Chronic noncommunicable diseases in 6 low- and middle-income countries: findings from wave 1 of the World Health Organization’s Study on Global Ageing and Adult Health (SAGE). Am J Epidemiol. 2017;185(6):414–428. doi: 10.1093/aje/kww125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Koyanagi A, Stubbs B, Vancampfort D. Correlates of sedentary behavior in the general population: a cross-sectional study using nationally representative data from six low- and middle-income countries. PLoS One. 2018;13(8):e0202222. doi: 10.1371/journal.pone.0202222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Luo M, Ding D, Bauman A, Negin J, Phongsavan P. Social engagement pattern, health behaviors and subjective well-being of older adults: an international perspective using WHO-SAGE survey data. BMC Public Health. 2020;20(1):99. doi: 10.1186/s12889-019-7841-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ware L, Rennie KL, Kruger HS, Kruger IM, Greeff M, Fourie CMT. et al. Evaluation of waist-to-height ratio to predict 5 year cardiometabolic risk in sub-Saharan African adults. Nutr Metab Cardiovasc Dis. 2014;24(8):900–907. doi: 10.1016/j.numecd.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 68.Zatu MC, van Rooyen JM, Kruger A, Schutte AE. Alcohol intake, hypertension development and mortality in black South Africans. Eur J Prevent Cardiol. 2016;23(3):308–315. doi: 10.1177/2047487314563447. https://doi.org/10.1177/2047487314563447. [DOI] [PubMed] [Google Scholar]
- 69.Kruger HS, Schutte AE, Walsh CM, Kruger A, Rennie KL. Body mass index cut-points to identify cardiometabolic risk in black South Africans. Eur J Nutr. 2017;56(1):193–202. doi: 10.1007/s00394-015-1069-9. [DOI] [PubMed] [Google Scholar]
- 70.Okop KJ, Levitt N, Puoane T. Factors associated with excessive body fat in men and women: cross-sectional data from black South Africans living in a rural community and an urban township. PLoS One. 20158;10(10):e0140153. doi: 10.1371/journal.pone.0140153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nienaber-Rousseau C, Sotunde O, Ukegbu P, Myburgh P, Wright H, Havemann-Nel L. et al. Socio-demographic and lifestyle factors predict 5-year changes in adiposity among a group of black South African adults. Int J Environ Res Public Health. 2017;14(9):1089. doi: 10.3390/ijerph14091089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yusuf S, Joseph P, Rangarajan S, Islam S, Mente A, Hystad P. et al. Modifiable risk factors, cardiovascular disease, and mortality in 155 722 individuals from 21 high-income, middle-income, and low-income countries (PURE): a prospective cohort study. Lancet. 2020;395(10226):795–808. doi: 10.1016/S0140-6736(19)32008-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sitas F, Egger S, Bradshaw D, Groenewald P, Laubscher R, Kielkowski D. et al. Differences among the coloured, white, black, and other South African populations in smoking-attributed mortality at ages 35–74 years: a case-control study of 481 640 deaths. Lancet. 2013;382(9893):685–693. doi: 10.1016/S0140-6736(13)61610-4. [DOI] [PubMed] [Google Scholar]
- 74.Peer N, Lombard C, Steyn K, Levitt N. Differential patterns of tobacco use among black men and women in Cape Town: The Cardiovascular Risk in Black South Africans Study. Nicotine Tobacco Res. 2014;16(8):1104–1111. doi: 10.1093/ntr/ntu042. [DOI] [PubMed] [Google Scholar]
- 75.Scheibe AP, Gloeck NR, Shelly S, Marcus TS, Hugo J. The prevalence and characteristics of moderate- to high-risk regulated and unregulated substance use among patients admitted to four public hospitals in Tshwane, South Africa. S Afr Med J. 2019;109(12):971. doi: 10.7196/SAMJ.2019.v109i12.13870. [DOI] [PubMed] [Google Scholar]
- 76.Shen J, Kondal D, Rubinstein A, Irazola V, Gutierrez L, Miranda JJ. et al. A multiethnic study of pre-diabetes and diabetes in LMIC. Glob Heart. 2016;11(1):61. doi: 10.1016/j.gheart.2015.12.015. [DOI] [PubMed] [Google Scholar]
- 77.Prakaschandra DR, Esterhuizen TM, Motala AA, Gathiram P, Naidoo DP. High prevalence of cardiovascular risk factors in Durban South African Indians: The Phoenix Lifestyle Project. S. Afr Med J. 2016;106(3):284. doi: 10.7196/SAMJ.2016.v106i3.9837. [DOI] [PubMed] [Google Scholar]
- 78.Prakaschandra R, Naidoo DP. Increased waist circumference is the main driver for the development of the metabolic syndrome in South African Asian Indians. Diabetes Metab Syndr Clin Res Rev. 2017;11:S81–85. doi: 10.1016/j.dsx.2016.12.011. [DOI] [PubMed] [Google Scholar]
- 79.Kobayashi LC, Frank S, Riumallo-Herl C, Canning D, Berkman L. Socioeconomic gradients in chronic disease risk behaviors in a population- based study of older adults in rural South Africa. Int J Public Health. 2019;64(1):135–145. doi: 10.1007/s00038-018-1173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Sheehy T, Kolahdooz F, Mtshali TL, Khamis T, Sharma S. Development of a quantitative food frequency questionnaire for use among rural South Africans in KwaZulu-Natal. J Hum Nutr Diet. 2014;27(5):443–449. doi: 10.1111/jhn.12166. [DOI] [PubMed] [Google Scholar]
- 81.Ware LJ, Charlton K, Kruger R, Breet Y, van Rooyen J, Huisman H. et al. Assessing tobacco use in an African population: Serum and urine cotinine cut-offs from South Africa. Drug Alcohol Depend. 2019;195:82–89. doi: 10.1016/j.drugalcdep.2018.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geneva, Switzerland: World Health Organization.: World Health Organization. The WHO STEPwise approach to noncommunicable disease risk factor surveillance (STEPS). Retrieved 2020 Aug 31, from http://www.who.int/ncds/surveillance/steps/riskfactor/en/ [Google Scholar]
- 83.Geneva: World Health Organization: 2008. World Health Organization, Research for International Tobacco Control, eds. WHO report on the global tobacco epidemic, 2008: the MPOWER package. pp. 1–329. [Google Scholar]
- 84.Commar A, Prasad VK, Tursan d’Espaignet E, Wolfenden L. WHO global report on trends in prevalence of tobacco smoking 2000. 2025 World Health Organization; 2018. https://apps.who.int/iris/ handle/10665/272694. [Google Scholar]
- 85.Hofman K, Lee R. Brazzaville, Republic of Congo: World Health Organization – African Regional Office: 2013. Intersectoral case study: successful tobacco legislation in South Africa. pp. 1–12. p. https://www. afro.who.int/sites/default/files/2018-02/19%20Dec%202013_%20 Successful%20Tobacco%20Legislation%20in%20South%20Africa.pdf. [Google Scholar]
- 86.FCTC WHO Framework Convention on Tobacco Control. WHO framework convention on tobacco control [2003, updated 2004 & 2005]. Geneva, Switzerland: World Health Organization, 2005 [Google Scholar]
- 87.Geneva, Switzerland: World Health Organization: 2004. World Health Organization. WHO Global Strategy on Diet, Physical Activity and Health. http://www.who.int/dietphysicalactivity/strategy/eb11344/en/ [Google Scholar]
- 88.Pisa PT, Landais E, Margetts B, Vorster HH, Friedenreich CM, Huybrechts I. et al. Inventory on the dietary assessment tools available and needed in Africa: a prerequisite for setting up a common methodological research infrastructure for nutritional surveillance, research, and prevention of diet-related non-communicable diseases. Crit Rev Food Sci Nutr. 2018;58(1):37–61. doi: 10.1080/10408398.2014.981630. [DOI] [PubMed] [Google Scholar]
- 89.Shim J-S, Oh K, Kim HC. Dietary assessment methods in epidemiologic studies. Epidemiol Health. 2014;36:e2014009. doi: 10.4178/epih/e2014009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Washington DC, USA: Institute of Medicine: 2005. Institute of Medicine. Dietary Reference Intakes for Energy, Carbohydrate, Fiber, Fat, Fatty Acids, Cholesterol, Protein, and Amino Acids [Internet]. https://doi.org/10.17226/10490. [Google Scholar]
- 91.Steyn N, Jaffer N, Nel J, Levitt N, Steyn K, Lombard C. et al. Dietary Intake of the urban black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) study. Nutrients. 2016;8(5):285. doi: 10.3390/nu8050285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolahdooz F, Spearing K, Sharma S. Dietary adequacies among South African adults in rural KwaZulu-Natal. PLoS One. 2013;8(6):e67184. doi: 10.1371/journal.pone.0067184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Solomons N, Kruger HS, Puoane T. Association between dietary adherence, anthropometric measurements and blood pressure in an urban black population, South Africa. S Afr J Clin Nutr. 2020;33(1):1–9. [Google Scholar]
- 94.Oldewage-Theron W, Egal A. The effect of consumption of soy foods on metabolic syndrome in women: a case study from peri-urban Qwa-Qwa, South Africa. S Afr J Clin Nutr. 2019;32(2):40–45. [Google Scholar]
- 95.Vorster HH, Badham JB, Venter CS. An introduction to the revised food-based dietary guidelines for South Africa. S Afr J Clin Nutr. 2013;26(S):S5–12. [Google Scholar]
- 96.Faber M, Laubscher R, Laurie S. Availability of, access to and consumption of fruits and vegetables in a peri-urban area in KwaZulu-Natal, South Africa. Matern Child Nutr. 2013;9(3):409–424. doi: 10.1111/j.1740-8709.2011.00372.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Okop KJ, Ndayi K, Tsolekile L, Sanders D, Puoane T. Low intake of commonly available fruits and vegetables in socio-economically disadvantaged communities of South Africa: influence of affordability and sugary drinks intake. BMC Public Health. 2019;19(1):940. doi: 10.1186/s12889-019-7254-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Devereux, Tavener-Smith. Seasonal food insecurity among farm workers in the Northern Cape, South Africa. Nutrients. 2019;11(7):1535. doi: 10.3390/nu11071535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Pretoria: Government Gazette: 2013. National Department of Health (NDoH). Regulations Relating to the Reduction of Sodium in Certain Foodstuffs and Related Matters » Amendment. In: Government Gazette. [Google Scholar]
- 100.Peters S, Dunford E, Ware L, Harris T, Walker A, Wicks M. et al. The sodium content of processed foods in South Africa during the introduction of mandatory sodium limits. Nutrients. 2017;9(4):404. doi: 10.3390/nu9040404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Webster J, Crickmore C, Charlton K, Steyn K, Wentzel-Viljoen E, Naidoo P. South Africa’s salt reduction strategy: Are we on track, and what lies ahead? S Afr Med J. 2016;107(1):20. doi: 10.7196/SAMJ.2016.v107.i1.12120. [DOI] [PubMed] [Google Scholar]
- 102.Menyanu E, Charlton K, Ware L, Russell J, Biritwum R, Kowal P. Salt use behaviours of Ghanaians and South Africans: a comparative study of knowledge, attitudes and practices. Nutrients. 2017;9(9):932. doi: 10.3390/nu9090939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Peer N, Balakrishna Y, de Villiers A, Crickmore C, Mungal-Singh V. Effectiveness of a screening programme in identifying individuals with increased risk of cardiovascular disease in South Africa. J Public Health. 2018;40(1):e34–5. doi: 10.1093/pubmed/fdx012. [DOI] [PubMed] [Google Scholar]
- 104.Peer LJ, Charlton K, Schutte AE, Cockeran M, Naidoo N, Kowal P. Associations between dietary salt, potassium and blood pressure in South African adults: WHO SAGE Wave 2 Salt & Tobacco. Nutr Metab Cardiovasc Dis. 2017;27(9):784–791. doi: 10.1016/j.numecd.2017.06.017. [DOI] [PubMed] [Google Scholar]
- 105.Pretoria: Republic of South Africa: 2003. National Department of Health (NDoH). Regulations Relating to the Fortification of Certain Foodstuffs. In: Government Gazette. [Google Scholar]
- 106.Food Fortification]. Food Facts 2018. https://foodfacts.org.za/foodfortification/ [Google Scholar]
- 107.Pretoria: Government Gazette: 2011. National Department of Health (NDoH). Regulations Relating to Trans-Fat in Foodstuffs. In: Government Gazette. [Google Scholar]
- 108.Pretoria: Republic of South Africa: 2017. National Department of Health (NDoH). Regulations Relating to the Reduction of Sodium in Certain Foodstuffs and Related Matters, R.214 of 20 March 2013: Amendment. In: Government Gazette. [Google Scholar]
- 109.Pretoria: Republic of South Africa: 2019. National Department of Health (NDoH). Regulations Relating to the Reduction of Sodium in Certain Foodstuffs and Related Matters, Amendment. In: Government Gazette. https://www.greengazette.co.za/notices/foodstuffscosmetics- and-disinfectants-act-54-1972-regulations-relating-to-thereduction- of-sodium-in-certain-foodstuffs-and-related-matters-amendment_ 20190531-GGN-42496-00812. [Google Scholar]
- 110.Charlton K, Ware LJ, Baumgartner J, Cockeran M, Schutte AE, Naidoo N. et al. How will South Africa’s mandatory salt reduction policy affect its salt iodisation programme? A cross-sectional analysis from the WHO-SAGE Wave 2 Salt & Tobacco study. Br Med J Open. 2018;8(3) doi: 10.1136/bmjopen-2017-020404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Wrottesley SV, Stacey N, Mukoma G, Hofman KJ, Norris SA. Assessing sugar-sweetened beverage intakes, added sugar intakes and BMI before and after the implementation of a sugar-sweetened beverage tax in South Africa. Public Health Nutr. 2020;24(10) doi: 10.1017/S1368980020005078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Stacey N, Edoka I, Hofman K, Swart EC, Popkin B, Ng SW. Changes in beverage purchases following the announcement and implementation of South Africa’s Health Promotion Levy: an observational study. Lancet Planet Health. 2021;5(4):e200–e208. doi: 10.1016/S2542-5196(20)30304-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.NCD Risk Factor Collaboration (NCD-RisC). Trends in adult bodymass index in 200 countries from 1975 to 2014: a pooled analysis of 1698 population-based measurement studies with 19.2 million participants. Lancet. 2016;387(10026):1377–1396. doi: 10.1016/S0140-6736(16)30054-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Abrahams Z, Mchiza Z, Steyn NP. Diet and mortality rates in sub- Saharan Africa: Stages in the nutrition transition. BMC Public Health. 2011;11(1):801. doi: 10.1186/1471-2458-11-801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.World Health Organization. Obesity and overweight. WHO. 2020. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight. [Google Scholar]
- 116.Murray CJL, Aravkin AY, Zheng P, Abbafati C, Abbas KM, Abbasi-Kangevari M. et al. Global burden of 87 risk factors in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1223–1249. doi: 10.1016/S0140-6736(20)30752-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Norman R, Bradshaw D, Schneider M, Joubert J, Groenewald P, Lewin S. et al. A comparative risk assessment for South Africa in 2000: Towards promoting health and preventing disease. S. Afr Med J. 2007;97(8):637. [PubMed] [Google Scholar]
- 118.NCD Risk Factor Collaboration (NCD-RisC) – Africa Working Group, Kengne AP, Bentham J, Zhou B, Peer N, Matsha TE. et al. Trends in obesity and diabetes across Africa from 1980 to 2014: an analysis of pooled population-based studies. Int J Epidemiol. 2017;46(5):1421–1432. doi: 10.1093/ije/dyx078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ajayi IOO, Adebamowo C, Adami H-O, Dalal S, Diamond MB, Bajunirwe F. et al. Urban–rural and geographic differences in overweight and obesity in four sub-Saharan African adult populations: a multi-country cross-sectional study. BMC Public Health. 2016;16(1):1126. doi: 10.1186/s12889-016-3789-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Puoane T, Steyn K, Bradshaw D, Laubscher R, Fourie J, Lambert V. et al. Obesity in South Africa: The South African Demographic and Health Survey. Obesity Res. 2002;10(10):1038–1048. doi: 10.1038/oby.2002.141. [DOI] [PubMed] [Google Scholar]
- 121.Malambo P, De Villiers A, Lambert EV, Puoane T, Kengne AP. Associations of perceived neighbourhood safety from traffic and crime with overweight/obesity among South African adults of low-socioeconomic status. PLoS One. 2018;13(10):e0206408. doi: 10.1371/journal.pone.0206408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Senekal M, Seme Z, de Villiers A, Steyn NP. Health status of primary school educators in low socio-economic areas in South Africa. BMC Public Health. 2015;15(1):186. doi: 10.1186/s12889-015-1531-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Peer N, Balakrishna Y, de Villiers A, Naidoo P. Differential associations of cardio-metabolic diseases by population group, gender and adiposity in South Africa. PLoS One. 2018 doi: 10.1371/journal.pone.0202899. (9):e0202899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ogunsina K, Dibaba DT, Akinyemiju T. Association between life-course socio-economic status and prevalence of cardio-metabolic risk factors in five middle-income countries. JGlob Health. 2018;8(2):020405. doi: 10.7189/jogh.08.020405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Gradidge PJ-L, Norris SA, Micklesfield LK, Crowther NJ. The role of lifestyle and psycho-social factors in predicting changes in body composition in black South African women. PLoS One. 2015;10(7):e0132914. doi: 10.1371/journal.pone.0132914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kruger HS, Botha-Ravyse C, Havemann-Nel L, Doubell M, van Rooyen JM.. Agreement between specific measures of adiposity and associations with high blood pressure in black South African women. Am J Hum Biol. 2017;29(6):e23042. doi: 10.1002/ajhb.23042. [DOI] [PubMed] [Google Scholar]
- 127.Lategan R, van den Berg VL, Walsh CM. Body adiposity indices are associated with hypertension in a black, urban Free State community. Afr J Prim Health Care Fam Med. 2014;6(1) doi: 10.4102/phcfm.v6i1.581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Peer N, Lombard C, Steyn K, Gwebushe N, Levitt N. Differing patterns of overweight and obesity among black men and women in Cape Town: the CRIBSA study. PLoS One. 2014;9(9):e107471. doi: 10.1371/journal.pone.0107471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Peer N, Steyn K, Levitt N. Differential obesity indices identify the metabolic syndrome in black men and women in Cape Town: the CRIBSA study. J Public Health. 2016;38(1):175–182. doi: 10.1093/pubmed/fdu115. [DOI] [PubMed] [Google Scholar]
- 130.Micklesfield LK, Kagura J, Munthali R, Crowther NJ, Jaff N, Gradidge P. et al. Demographic, socio-economic and behavioural correlates of BMI in middle-aged black men and women from urban Johannesburg, South Africa. Glob Health Action. 2018;11(suppl 2) doi: 10.1080/16549716.2018.1448250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Case A, Menendez A. Sex differences in obesity rates in poor countries: Evidence from South Africa. Econom Hum Biol. 2009;7(3):271–282. doi: 10.1016/j.ehb.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.World Health Organization. Raised cholesterol. WHO. World Health Organization. Retrieved 2020 Sep 11, from https://www.who.int/gho/ ncd/risk_factors/cholesterol_text/en/ [Google Scholar]
- 133.Gakidou E, Afshin A, Abajobir AA, Abate KH, Abbafati C, Abbas KM. et al. Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390(10100):1345–1422. doi: 10.1016/S0140-6736(17)32366-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Murphy A, Faria-Neto JR, Al-Rasadi K, Blom D, Catapano A, Cuevas A. et al. World Heart Federation Cholesterol Roadmap. Glob Heart. 2017;12(3):179. doi: 10.1016/j.gheart.2017.03.002. [DOI] [PubMed] [Google Scholar]
- 135.Pretoria: National Department of Health (NDoH): 1998. National Department of Health (NDoH), South African Medical Research Council (SAMRC), Demographic and Health Surveys – Macro International Inc. South Africa Demographic and Health Survey 1998. https:// www.samrc.ac.za/sites/default/files/files/2017-07-03/dhsfin1.pdf. [Google Scholar]
- 136.Noubiap JJ, Bigna JJ, Nansseu JR, Nyaga UF, Balti EV, Echouffo- Tcheugui JB. et al. Prevalence of dyslipidaemia among adults in Africa: a systematic review and meta-analysis. Lancet Glob Health. 2018;6(9):e998–1007. doi: 10.1016/S2214-109X(18)30275-4. [DOI] [PubMed] [Google Scholar]
- 137.Richter M, Baumgartner J, Wentzel-Viljoen E, Smuts CM.. Different dietary fatty acids are associated with blood lipids in healthy South African men and women: the PURE study. Int J Cardiol. 2014;172(2):368–374. doi: 10.1016/j.ijcard.2014.01.023. [DOI] [PubMed] [Google Scholar]
- 138.Peer N, Steyn K, Lombard C, Gaziano T, Levitt N. Alarming rise in prevalence of atherogenic dyslipidaemia in the black population of Cape Town: the Cardiovascular Risk in Black South Africans (CRIBSA) study. Eur J Prev Cardiol. 2014;21(12):1549–1556. doi: 10.1177/2047487313497865. [DOI] [PubMed] [Google Scholar]
- 139.Naicker A, Venter CS, MacIntyre UE, Ellis S. Dietary quality and patterns and non-communicable disease risk of an Indian community in KwaZulu-Natal, South Africa. J Health Popul Nutr. 2015;33(1):12. doi: 10.1186/s41043-015-0013-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Peer N, Lombard C, Steyn K, Levitt N. High prevalence of metabolic syndrome in the black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) study. Eur J Prev Cardiol. 2015;22(8):1036–1042. doi: 10.1177/2047487314549744. [DOI] [PubMed] [Google Scholar]
- 141.Reiger S, Jardim TV, Abrahams-Gessel S, Crowther NJ, Wade A, Gomez-Olive FX. et al. Awareness, treatment, and control of dyslipidemia in rural South Africa: The HAALSI (Health and Aging in Africa: A Longitudinal Study of an INDEPTH Community in South Africa) study. PLoS One. 2017;12(10):e0187347. doi: 10.1371/journal.pone.0187347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Blom DJ, Raal F, Amod A, Naidoo P, Lai Y. Management of lowdensity lipoprotein cholesterol levels in South Africa: the International ChoLesterol management Practice Study (ICLPS). Cardiovasc J Afr. 2019;30(1):15–23. doi: 10.5830/CVJA-2018-054. [DOI] [PubMed] [Google Scholar]
- 143.Heart & Stroke Foundation South Africa. Cholesterol. 2017. http:// www.heartfoundation.co.za/cholesterol/ [Google Scholar]
- 144.World Health Organization. Hypertension [factsheet]. WHO. 2019. https://www.who.int/news-room/fact-sheets/detail/hypertension. [Google Scholar]
- 145.Cifkova R, Fodor G, Wohlfahrt P. Changes in hypertension prevalence, awareness, treatment, and control in high-, middle-, and low-income countries: an update. Curr Hypertens Rep. 2016;18(8):62. doi: 10.1007/s11906-016-0669-y. [DOI] [PubMed] [Google Scholar]
- 146.Geldsetzer P, Manne-Goehler J, Marcus M-E, Ebert C, Zhumadilov Z, Wesseh CS. et al. The state of hypertension care in 44 low-income and middle-income countries: a cross-sectional study of nationally representative individual-level data from 1.1 million adults. Lancet. 2019;394(10199):652–662. doi: 10.1016/S0140-6736(19)30955-9. [DOI] [PubMed] [Google Scholar]
- 147.Sacco RL, Roth GA, Reddy KS, Arnett DK, Bonita R, Gaziano TA. et al. The heart of 25 by 25: Achieving the goal of reducing global and regional premature deaths from cardiovascular diseases and stroke: a modeling study from the American Heart Association and World Heart Federation. Circulation. 2016;133(23) doi: 10.1161/CIR.0000000000000395. [DOI] [PubMed] [Google Scholar]
- 148.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L. et al. Global burden of hypertension and systolic blood pressure of at least 110 to 115 mmHg, 1990–2015. J Am Med Assoc. 2017;317(2):165. doi: 10.1001/jama.2016.19043. [DOI] [PubMed] [Google Scholar]
- 149.Maredza M, Bertram MY, Gómez-Olivé XF, Tollman SM.. Burden of stroke attributable to selected lifestyle risk factors in rural South Africa. BMC Public Health. 2016;16(1):143. doi: 10.1186/s12889-016-2805-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Basu S, Millett C. Social epidemiology of hypertension in middleincome countries: determinants of prevalence, diagnosis, treatment, and control in the WHO SAGE study. Hypertension. 2013;62(1):18–26. doi: 10.1161/HYPERTENSIONAHA.113.01374. [DOI] [PubMed] [Google Scholar]
- 151.Chang AY, Gómez-Olivé FX, Manne-Goehler J, Wade AN, Tollman S, Gaziano TA. et al. Multimorbidity and care for hypertension, diabetes and HIV among older adults in rural South Africa. Bull World Health Org. 2019;97(1):10–23. doi: 10.2471/BLT.18.217000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Davids SFG, Matsha TE, Peer N, Erasmus RT, Kengne AP. Increase in blood pressure over a 7-year period in a mixed-ancestry South African population. S Afr Med J. 2019;109(7):503. doi: 10.7196/SAMJ.2019.v109i7.13663. [DOI] [PubMed] [Google Scholar]
- 153.Gómez-Olivé FX, Thorogood M, Clark B, Kahn K, Tollman S. Selfreported health and health care use in an ageing population in the Agincourt sub-district of rural South Africa. Glob Health Action. 2013;6 doi: 10.3402/gha.v6i0.19305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Irazola VE, Gutierrez L, Bloomfield G, Carrillo-Larco RM, Prabhakaran D, Gaziano T. et al. Hypertension prevalence, awareness, treatment, and control in selected LMIC communities: Results from the NHLBI/UHG network of centers of excellence for chronic diseases. Glob Heart. 2016;11(1):47. doi: 10.1016/j.gheart.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Motlhale M, Ncayiyana JR. Migration status and prevalence of diabetes and hypertension in Gauteng province, South Africa: effect modification by demographic and socioeconomic characteristics – a cross-sectional population-based study. Br Med J Open. 2019;9(9):e027427. doi: 10.1136/bmjopen-2018-027427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Ntuli ST, Maimela E, Alberts M, Choma S, Dikotope S. Prevalence and associated risk factors of hypertension amongst adults in a rural community of Limpopo Province, South Africa. Afr J Prim Health Care Fam Med. 2015;7(1) doi: 10.4102/phcfm.v7i1.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Peer N, Steyn K, Lombard C, Gwebushe N, Levitt N. A high burden of hypertension in the urban black population of Cape Town: The Cardiovascular Risk in Black South Africans (CRIBSA) Study. PLoS One. 2013;8(11):e78567. doi: 10.1371/journal.pone.0078567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Rheeder P, Muthembe T, Lawson S, Brink J. Diabetes and hypertension screening in Zandspruit, Johannesburg 2012–2014. S Afr Fam Prac. 2016;58(6):219–224. [Google Scholar]
- 159.Wandai M, Day C. Vol. 103. Westville, South Africa: Health Systems Trust: 2015. Trends in risk factors for non-communicable diseases in South Africa – May 2015. https://www.hst.org.za/publications/HSTPublications/ Trends_NCD_SA_HST_28Aug2015.pdf. [Google Scholar]
- 160.Bertram MY, Steyn K, Wentzel-Viljoen E, Tollman S, Hofman KJ. Reducing the sodium content of high-salt foods: Effect on cardiovascular disease in South Africa. S Afr Med J. 2012;102(9):743–745. doi: 10.7196/samj.5832. [DOI] [PubMed] [Google Scholar]
- 161.Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N. et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9th edn. Diabetes Res Clin Prac. 2019;157:107843. doi: 10.1016/j.diabres.2019.107843. [DOI] [PubMed] [Google Scholar]
- 162.Vos T, Lim SS, Abbafati C, Abbas KM, Abbasi M, Abbasifard M. et al. Global burden of 369 diseases and injuries in 204 countries and territories, 1990–2019: a systematic analysis for the Global Burden of Disease Study 2019. Lancet. 2020;396(10258):1204–1222. doi: 10.1016/S0140-6736(20)30925-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pretoria: Statistics South Africa: 2018. Statistics South Africa. Mortality and causes of death in South Africa, 2016: Findings from death notification. Report No: Vol Statistical Release P0309.3. [Google Scholar]
- 164.Manne-Goehler J, Atun R, Stokes A, Goehler A, Houinato D, Houehanou C. et al. Diabetes diagnosis and care in sub-Saharan Africa: pooled analysis of individual data from 12 countries. Lancet Diabetes Endocrinol. 2016;4(11):903–912. doi: 10.1016/S2213-8587(16)30181-4. [DOI] [PubMed] [Google Scholar]
- 165.Rheeder P, Morris-Paxton AA, Ewing R-MG, Woods D. The noncommunicable disease outcomes of primary healthcare screening in two rural subdistricts of the Eastern Cape Province, South Africa. Afr J Prim Health Care Fam Med. 2017;9(1) doi: 10.4102/phcfm.v9i1.1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Bailey SL, Ayles H, Beyers N, Godfrey-Faussett P, Muyoyeta M, du Toit E. et al. Diabetes mellitus in Zambia and the Western Cape province of South Africa: Prevalence, risk factors, diagnosis and management. Diabetes Res Clin Prac. 2016;118:1–11. doi: 10.1016/j.diabres.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Adedokun AO, Ter Goon D, Owolabi EO, Adeniyi OV, Ajayi AI. Prevalence, awareness, and determinants of type 2 diabetes mellitus among commercial taxi drivers in Buffalo City Metropolitan Municipality South Africa: A cross-sectional survey. Medicine. 2019;98(9):e14652. doi: 10.1097/MD.0000000000014652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hird TR, Pirie FJ, Esterhuizen TM, O’Leary B, McCarthy MI, Young EH. et al. Burden of diabetes and first evidence for the utility of HbA1c for diagnosis and detection of diabetes in urban black South Africans: The Durban Diabetes Study. PLoS One. 2016;11(8):e0161966. doi: 10.1371/journal.pone.0161966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Malan L, Hamer M, Schlaich MP, Lambert GW, Ziemssen T, Reimann M. et al. Defensive active coping facilitates chronic hyperglycaemia and endothelial dysfunction in African men: The SABPA study. Int J Cardiol. 2013;168(2):999–1005. doi: 10.1016/j.ijcard.2012.10.035. [DOI] [PubMed] [Google Scholar]
- 170.Peltzer K, Phaswana-Mafuya N, Pengpid S. Rural–urban health disparities among older adults in South Africa. Afr J Pri Health Care Fam Med. 2019;11(1) doi: 10.4102/phcfm.v11i1.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Quashie NT, D’Este C, Agrawal S, Naidoo N, Kowal P. Prevalence of angina and co-morbid conditions among older adults in six low- and middle-income countries: Evidence from SAGE Wave 1. Int J Cardiol. 2019;285:140–146. doi: 10.1016/j.ijcard.2019.02.068. [DOI] [PubMed] [Google Scholar]
- 172.Stubbs B, Vancampfort D, Veronese N, Schofield P, Lin P-Y, Tseng P-T. et al. Multimorbidity and perceived stress: a population-based crosssectional study among older adults across six low- and middle-income countries. Maturitas. 2018;107:84–91. doi: 10.1016/j.maturitas.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 173.Werfalli M, Kassanjee R, Kalula S, Kowal P, Phaswana-Mafuya N, Levitt NS. Diabetes in South African older adults: prevalence and impact on quality of life and functional disability – as assessed using SAGE Wave 1 data. Glob Health Action. 2018;11(1):1449924. doi: 10.1080/16549716.2018.1449924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.SEMDSA Society for Endocrinology, Metabolism and Diabetes of South Africa. SEMDSA. Retrieved 2020 Sep 28, from https://www. semdsa.org.za/ [Google Scholar]
- 175.NCD Countdown 2030: pathways to achieving Sustainable Development Goal target 3.4. Lancet. 2020;396(10255):918–934. doi: 10.1016/S0140-6736(20)31761-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dover RVH, Lambert EV. ‘Choice Set’ for health behavior in choiceconstrained settings to frame research and inform policy: examples of food consumption, obesity and food security. Int J Equity Health. 2016;15(1):48. doi: 10.1186/s12939-016-0336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]