Abstract
Infection with the third-stage larvae (L3) of the filarial nematode Brugia results in a Th2-biased immune response in mice and humans. Previously we have shown that the production of interleukin 4 (IL-4) is critical for down-regulating polyclonal Th1 responses in L3-infected mice. However, the in vitro neutralization of IL-4 did not fully recover the defective polyclonal Th1 responses, nor did it result in the production of any antigen (Ag)-specific Th1 cytokines, suggesting that perhaps infection with L3 does not result in priming of Th1 cells in vivo. In this study, we analyzed the role of IL-10 and Ag-presenting cells (APCs) in the spleen as additional factors controlling the Th2 bias in infected mice. Our data show that IL-10 and APCs also contribute to the suppression of mitogen-driven Th1 responses of spleen cells from infected mice. In addition, the neutralization of IL-10 or the replacement of the resident APC population from spleen cell cultures resulted in the production of Ag-specific Th1 cytokines. Irradiated spleen cells from either L3-infected or uninfected mice were able to restore Ag-specific Th1 responses in vitro. Therefore, it appears that Brugia-reactive Th1 cells are primed following infection with L3, but are actively suppressed in vivo by a mechanism that involves IL-10 and the resident APC population, but not IL-4. These results indicate that a complex interplay of cytokines and cell populations underscores the Th2-polarized response in L3-infected mice.
Infection with the third-stage larvae (L3) of the filarial nematode Brugia results in a Th2-biased immune response in mice (25) and humans (22, 34). Several mechanisms have been suggested to play a role in the impairment of Th1 cell responses following infection, including the overproduction of Th2 cytokines (15, 25, 26), selective Th1 tolerance or deletion (14, 23, 33), suppression by adherent cell populations (3, 17, 18, 29, 30), or the secretion of inhibitory factors directly by the parasite (16, 20, 28).
Mice infected with L3 display a particularly severe impairment of mitogen-driven proliferation and interleukin 2 (IL-2) and gamma interferon (IFN-γ) production and a complete absence of any antigen (Ag)-specific IFN-γ secretion in vitro. Instead, their responses are completely Th2 dominated, as characterized by elevated levels of IL-4, IL-5, and IL-10 in spleen cell cultures in response to concanavalin A (ConA) and Ag. IL-4 appears to be critical, at least in part, for down-regulating the polyclonal Th1 responses in these mice. However, neutralization of IL-4 in vitro did not result in the expression of any Ag-specific Th1 cytokines, nor did it impair the prevailing Th2 cytokine response (25). Furthermore, infection of IL-4 KO mice with L3 (19) did not elicit an Ag-specific Th1 response, suggesting that Ag-specific Th1 cells are not primed as a result of infection with L3. To investigate whether this is indeed the case, or whether Ag-specific Th1 cells are primed following infection, but are suppressed by IL-4-independent mechanisms, we analyzed the role of IL-10 and adherent APCs in the spleen as possible additional factors controlling the Th2 imbalance in L3-infected mice.
MATERIALS AND METHODS
Mice and infection protocols.
Brugia pahangi L3 were harvested from infected Aedes aegyptii mosquitoes at day 9 postinfection (p.i.) as previously described (7). Six-week-old, male BALB/c mice (purchased from Harlan-Olac, Bicester, United Kingdom) were injected with 50 L3 or an equivalent volume of Hanks balanced salt solution (HBSS). In most experiments, mice were injected with L3 by the subcutaneous (s.c.) route in the scruff of the neck, mimicking the natural route of infection. In some experiments, mice were injected by the intraperitoneal (i.p.) route with L3 as an alternative route of Ag entry. The mice were maintained in filter-top cages. At day 12 p.i., mice were sacrificed by CO2 inhalation, and the spleens were removed.
Spleen cell preparation.
Spleens were washed in RPMI (RPMI 1640 Dutch modification with 5 mM HEPES, 5 mM glutamine, 100 U of penicillin per ml, and 100 μg of streptomycin per ml, all from Gibco, Life Technologies, Inc., Gaithersburg, Md.) and teased apart to a single-cell suspension. Erythrocytes were lysed by treatment with 0.83% NH4Cl (pH 7.2). The remaining cells were washed twice in RPMI, and the number of viable lymphocytes was assessed by trypan blue exclusion. The cells were then resuspended in RPMI supplemented with 10% heat-inactivated fetal calf serum (FCS) (Australian, Gibco). The proliferation assays for IL-10 neutralization were carried out by using cells from individual animals (five per group), as were the experiments which investigated the effect of the route of infection. The experiments involving separation of the APC population were performed with spleen cells that were pooled from 5 to 10 animals per group. Experiments were performed at least in triplicate and gave similar results.
Spleen cell cultures.
For proliferation assays, cells were added in triplicate to flat-bottom wells of 96-well plates in a total volume of 200 μl in the presence or absence of 1 μg of ConA per ml (Sigma Chemical Co., St. Louis, Mo.) or 10 μg of adult Ag per ml. The Ag was a soluble extract of B. pahangi adult worms prepared by homogenization of the worms in RPMI on ice. In preliminary experiments, cells were stimulated with a range of concentrations of ConA or Ag to determine the optimal amount of each. After 48 h (ConA) or 72 h (Ag) of incubation at 37°C and 5% CO2, the cells were pulsed for 16 h with 0.5 μCi of [3H]thymidine/well (1 mCi/mmol; Amersham, Buckinghamshire, United Kingdom). The cells were harvested, and the incorporation of [3H]thymidine was measured in a TopCount Microplate scintillation counter (Canberra Packard Instrument Company, Meriden, Conn.).
For cytokine assays, cells were incubated in 1-ml cultures in the presence of ConA (5 μg/ml) or Ag (10 μg/ml), and the supernatants were harvested after 48 h. Levels of IL-2, IFN-γ, IL-4, IL-5, and IL-10 were measured by two-site sandwich enzyme-linked immunosorbent assay (ELISA) using antibody pairs purchased from PharMingen (San Diego, Calif.). The results are expressed as units per milliliter by reference to commercially produced standards of recombinant IL-2 (rIL-2) (Sigma), rIL-4, rIL-5, rIFN-γ (PharMingen), or rIL-10 (Genzyme, Cambridge, Mass.). The limit of detection for each assay was defined as the mean + 3 standard deviations of 16 wells containing medium only.
Neutralizing IL-10 treatment.
In certain experiments, rat anti-mouse IL-10 monoclonal antibody (MAb) JES5-2A5 (a kind gift from R. Grencis) or an immunoglobulin G1 (IgG1) isotype-matched control MAb (R59-40; PharMingen) was added simultaneously with ConA or Ag at a final concentration of 10 μg/ml. After such treatment, the proliferation and cytokine responses of the cells were assessed as described before. IL-10 was assayed for and not detected in any JES5-2A5-treated cultures, demonstrating that the cytokine had been successfully neutralized.
Preparation of selected populations.
Adherent cells were depleted from spleens of L3-infected mice by passing the pooled spleen cell suspension over nylon wool columns. Nylon wool-nonadherent (T-enriched) cells were eluted from the columns, counted, and resuspended in fresh RPMI containing 10% FCS. Two sources of APC were prepared by irradiating pooled spleen cells from uninfected or infected mice; 107 spleen cells/ml in RPMI with 10% FCS were gamma irradiated at 2,500 rads. Following irradiation, the APCs were washed, recounted, and resuspended in fresh RPMI containing 10% FCS. The nylon wool-nonadherent cell population (T cells) and irradiated spleen cell populations (APCs) were then combined at various ratios determined to be optimal in preliminary experiments. For proliferation assays, 4 × 105 T cells and 3 × 105 irradiated spleen cells from infected or uninfected mice were added per well. For cytokine assays, 6 × 106 T cells and 4 × 106 irradiated spleen cells from infected or uninfected mice were added per well. Unseparated spleen cells from infected and uninfected mice were cultured at 7 × 105 cells per well (proliferation) or 1 × 107 cells per well (cytokines). Nylon wool-nonadherent cells or irradiated spleen cells incubated alone were unable to proliferate or to produce significant levels of cytokines in response to stimulation with ConA or Ag, demonstrating that the nylon wool depletion and gamma irradiation treatments had been successful.
Statistical analysis.
The Mann-Whitney U test was used to determine the statistical significance of differences between groups. P < 0.05 was considered to be a significant difference.
RESULTS
In vitro treatment with anti-IL-10 MAb partially restores the defective mitogen-driven Th1 responses of spleen cells from mice infected with L3.
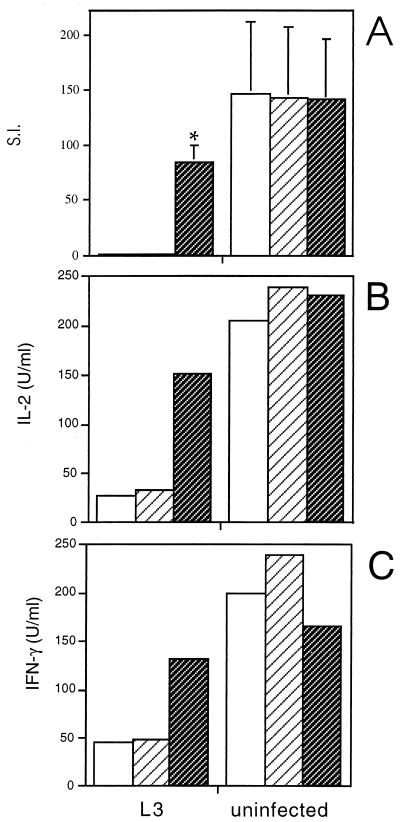
In these experiments, a neutralizing anti-IL-10 MAb (JES5-2A5) or an isotype-matched control MAb (R59-40) was added to ConA-stimulated spleen cell cultures from mice infected with L3 or from uninfected control mice, and the proliferation and cytokine responses were compared. Figure 1 shows that neutralization of IL-10 produced an outcome similar to that previously described following in vitro treatment with anti-IL-4 antibody. There was a significant increase in the ConA-induced proliferative response (stimulation index [SI] for L3-infected mice plus JES5-2A5 of 84.6 compared to the SI for L3-infected mice plus R59-40, 1.2; P = 0.0097), although not to the level of uninfected mice (SI of uninfected plus R59-40 mice of 143.3; P = 0.048) (Fig. 1A). In addition, anti-IL-10 treatment dramatically enhanced ConA-stimulated IL-2 and IFN-γ secretion (Fig. 1B and C), but had no effect on IL-4 or IL-5 production by spleen cells from L3-infected mice (data not shown).
FIG. 1.
Effect of neutralizing anti-IL-10 MAb on ConA-driven responses of spleen cells from mice infected with L3. Mice were injected s.c. with 50 L3 or HBSS, and spleens were removed at day 12 p.i. ConA-stimulated cultures were incubated alone (□) or with 10 μg of either an isotype-matched control (R59-40) (▨) or anti-IL-10 (JES5-2A5) ( ) per ml. After 48 h of incubation, proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured. (A) The means of triplicate wells are expressed as SIs (cpm with ConA/cpm with medium alone). The SIs represent the means ± standard deviations of five animals per group, ∗, significant difference (P < 0.05) between the values obtained with JES5-2A5 and R59-40. SIs were also significantly different (P < 0.05) between the cells of L3-infected mice treated with JES5-2A5 and uninfected mice with no treatment. (B) and (C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
In vitro neutralization of IL-10 allows the expression of Ag-specific Th1 responses in mice infected with L3.
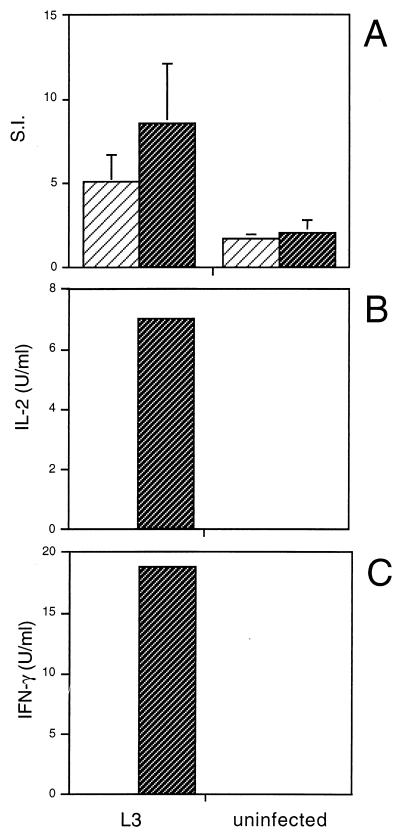
IL-10 has previously been implicated in inhibiting parasite-specific Th1 responses in filariasis. Mice chronically infected with another life cycle stage of the parasite, the microfilariae (mf), or multiply immunized with mf extract generate Th2 responses, characterized by the secretion of Ag-specific IL-4 and IL-5 by CD4+ spleen and lymph node cells (26). Addition of neutralizing anti-IL-10 to these cultures significantly increased Ag-driven IFN-γ production. Therefore, we examined whether IL-10 was performing a similar function in L3-infected mice. As with the analysis of mitogen-driven responses, neutralizing anti-IL-10 MAb was added to Ag-stimulated spleen cell cultures either from mice infected with L3 or from uninfected controls, and the proliferation and cytokine responses were measured. Anti-IL-10 antibody had no effect on the Ag-stimulated proliferation (Fig. 2A) or on the secretion of IL-4 and IL-5 (data not shown) by spleen cells from L3-infected mice. However, the presence of neutralizing anti-IL-10 MAb in the spleen cell cultures from L3-infected mice resulted in the production of IL-2 and IFN-γ in response to Ag (Fig. 2B and C). Spleen cells from L3-infected mice cultured in the presence of the isotype-matched control MAb did not produce any Ag-stimulated IL-2 and IFN-γ.
FIG. 2.
Effect of neutralizing anti-IL-10 MAb on Ag-driven responses of spleen cells from mice infected with L3. Mice were injected as described in the legend to Fig. 1. Ag-stimulated cultures were incubated with 10 μg of either an isotype-matched control (R59-40) (▨) or anti-IL-10 (JES5-2A5) ( ) per ml, and proliferation (A) and IL-2 (B) and IFN-γ (C) production were measured, as described previously. (A) The SIs shown are the means ± standard deviations of five animals per group. (B and C) Cytokine assays were performed with spleen cells pooled from five animals in each group. The results presented were comparable in two additional experiments.
Effect of route of infection on Th responses elicited by infection with L3.
The nature of the Th cell response, including T-cell unresponsiveness, can be influenced by the route of inoculation, which is thought to reflect differential targeting of APC populations (1, 5). Therefore, to examine a possible role for APCs in down-regulating responses, mice were infected by the natural s.c. route or by an alternative (i.p.) route, and the cytokine and proliferative responses of spleen cells from both sets of mice were compared. Uninfected control mice were given an s.c. injection of HBSS.
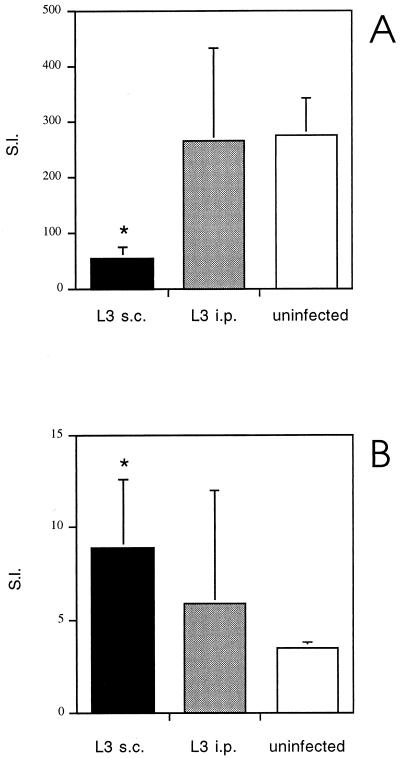
The results of this experiment, shown in Fig. 3, demonstrate that, unlike s.c. infection with L3, i.p. infection fails to down-regulate the ConA-driven proliferative IL-2 and IFN-γ responses of spleen cells (Fig. 3A and Table 1). Consistent with these data, L3-injected i.p. provoked only a weak Th2-type response, as signified by the production of ConA-driven IL-4 and IL-10 (P = 0.004 for both cytokines, compared to uninfected controls), but at significantly lower levels than in mice infected with L3 by the s.c. route (P = 0.016 for IL-4, P = 0.004 for IL-10) (Table 1). In addition, L3 given by the i.p. route failed to generate a significant Ag-specific proliferative response (Fig. 3B) and were unable to stimulate the secretion of IL-4, IL-5, or IL-10 in response to Ag.
FIG. 3.
Proliferative responses of spleen cells from BALB/c mice following s.c. or i.p. injection with L3 to ConA and B. pahangi adult Ag. Mice were injected s.c. or i.p. with 50 L3. Uninfected controls were injected s.c. with HBSS. At day 12 p.i., proliferation of spleen cells from the three groups of mice to ConA (A) or B. pahangi adult Ag (B) was measured. The SIs represent the means ± standard deviations of five animals per group. ∗, significant difference (P < 0.05) compared to uninfected controls.
TABLE 1.
An i.p. infection with L3 does not inhibit polyclonal Th1 cell cytokine responsesa
| Stimulus | Cytokine level (U/ml) in
groupb:
|
||
|---|---|---|---|
| L3
|
Uninfected | ||
| s.c. | i.p. | ||
| IL-2 | |||
| Ag | 1.99 ± 0.9* | 2.3 ± 1.5* | 0 |
| ConA | 37.4 ± 15.3* | 95.6 ± 37.1 | 89.4 ± 20.5 |
| IFN-γ | |||
| Ag | 0 | 0 | 0 |
| ConA | 62.6 ± 31.7* | 200.6 ± 140.1 | 93.1 ± 13.4 |
| IL-4 | |||
| Ag | 0.7 ± 0.3* | 0 | 0 |
| ConA | 20.8 ± 6.6* | 13.5 ± 3.0* | 6.8 ± 0.4 |
| IL-5 | |||
| Ag | 9.6 ± 2.1* | 0 | 0 |
| ConA | 13.5 ± 3.9* | 0 | 0 |
| IL-10 | |||
| Ag | 0.4 ± 0.2* | 0 | 0 |
| ConA | 0.6 ± 0.3* | 0.3 ± 0.07* | 0.2 ± 0.02 |
Mice were injected s.c. or i.p. with 50 L3 or s.c. with HBSS (uninfected). At day 12 p.i., cytokine levels in 48-h supernatants from spleen cells at 107 cells/ml in response to ConA (5 μg/ml) or B. pahangi adult antigen (10 μg/ml) were measured by two-site ELISA with reference to standards of a known concentration (units per milliliter).
A value of 0 indicates a reading below the sensitivity of the assay. The values are means ± standard deviations of five animals per group. ∗, significant difference (P < 0.05) between infected mice and uninfected controls.
Depletion of nylon wool-adherent cells from spleens of L3-infected mice dramatically enhances mitogen-driven Th1 responses.
The results of the previous experiment suggest that APCs do play a functional role in down-regulating polyclonal Th1 responses following s.c. infection with L3. Since adherent cells have been implicated as a major suppressor population in jirds infected with B. pahangi L3 (17, 18, 30) or mice with B. malayi adult worms, mf, or L3 implanted i.p. (3), the role of the adherent APC population in inhibiting Th1 responses in our model was assessed more directly. Spleen cells, pooled from five L3-infected mice, were depleted of adherent cells by passing the cell suspension over a nylon wool column. The nonadherent population, enriched for T cells, was then incubated with irradiated, syngeneic spleen cells from either uninfected mice or L3-infected mice in the presence of ConA or Ag. The ratios of nylon wool-nonadherent cells to irradiated spleen cells used in the proliferation (4:3) and cytokine (3:2) assays were determined to be optimal in preliminary experiments.
Spleen cells depleted of adherent cells were unable to respond to ConA or Ag (data not shown), indicating that the nylon wool-adherent population in the spleens of L3-infected mice contains the APC population. In addition, the irradiated spleen cell populations were unable to proliferate normally or produce significant levels of cytokines in response to ConA or Ag, when cultured alone, demonstrating that the irradiation treatments were successful (data not shown).
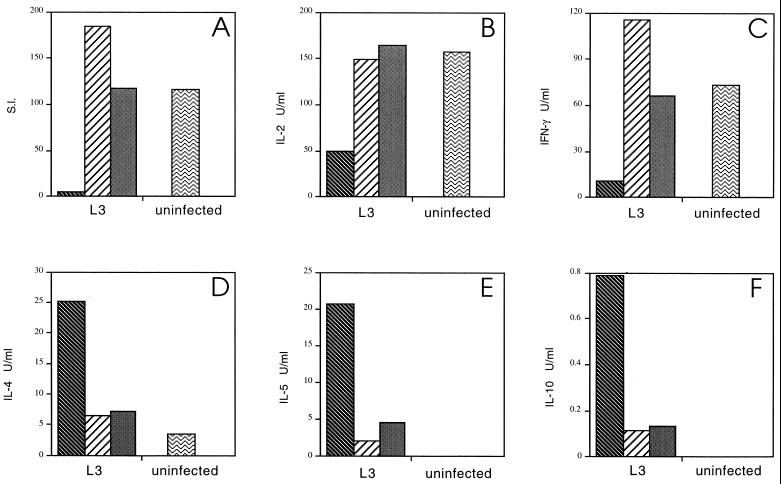
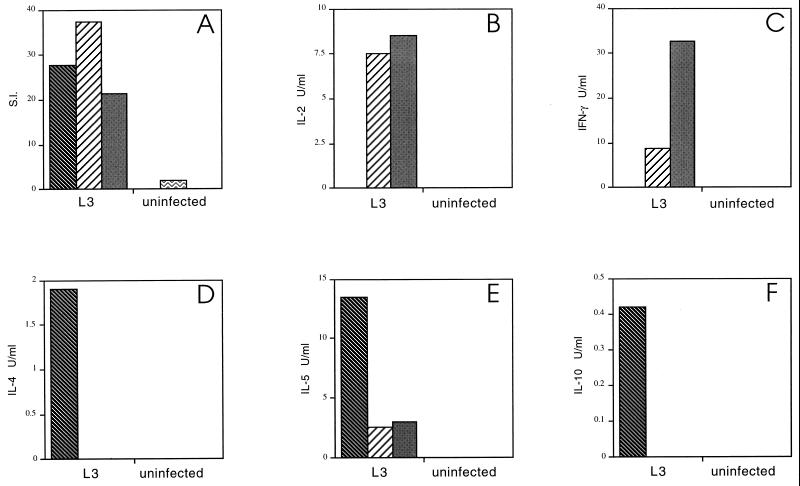
Figure 4A shows that the depletion of nylon wool-adherent cells resulted in a dramatic increase in polyclonal proliferation relative to unseparated spleen cells from the same animals and to a level comparable to that of spleen cells from uninfected animals. This effect was independent of the source of the APC population, since irradiated spleen cells from both uninfected and infected mice restored the defective T-cell proliferative response to mitogen (Fig. 4A). Furthermore, depletion of nylon wool-adherent cells restored ConA-driven IL-2 and IFN-γ (Fig. 4B and C) to the level present in uninfected animals while dramatically reducing the production of IL-4, IL-5, and IL-10 (Fig. 4D, E, and F). Therefore, removal of the resident APC population and replacement with APCs from infected or uninfected mice switched the established phenotype of the in vitro polyclonal response from Th2 to Th1.
FIG. 4.
Effect of depletion of nylon wool-adherent cells from
spleens of L3-infected mice on mitogen-driven responses. Mice were
injected as described in the legend to Fig. 1. Spleen cells from five
mice in each group were pooled. Splenic T cells from L3-infected mice,
purified by passing the pooled spleen cell suspension over nylon wool
columns, were incubated with irradiated syngeneic spleen cells from
uninfected mice
(▨) or with
irradiated spleen cells from L3-infected mice
(▩).
ConA-stimulated proliferation (A) and cytokine production (B to F) were
measured in these cultures and in cultures of unseparated spleen cells
from L3-infected
( ) and
uninfected (
) and
uninfected ( )
mice.
)
mice.
Depletion of nylon wool-adherent cells from spleens of L3-infected mice stimulates Ag-specific Th1 responses.
Because replacement of the adherent cell population switched the in vitro polyclonal response from Th2 to Th1, the involvement of adherent cells in suppressing Ag-specific Th1 responses in these mice was assessed. The ratios of the different cell populations were the same as those for the analysis of mitogen-driven responses.
Figure 5A shows that the removal of the adherent cell population and replacement with irradiated spleen cells from infected or uninfected mice did not effect the level of Ag-specific proliferation of T cells from L3-infected mice. Furthermore, the ability of irradiated spleen cells from both uninfected and L3-infected mice to support the Ag-specific proliferation of T cells from L3-infected mice to levels comparable to that of unseparated spleen cells indicates that both irradiated populations were able to act as a source of functional APCs.
FIG. 5.
Effect of depletion of nylon wool-adherent cells from
spleens of L3-infected mice on Ag-driven responses. Mice were injected
as described in the legend to Fig. 1. Splenic T cells from L3-infected
mice, purified by passing the pooled spleen cell suspension over nylon
wool columns, were incubated with irradiated syngeneic spleen cells
from uninfected mice
(▨) or with
irradiated spleen cells from L3-infected mice
(▩).
Ag-stimulated proliferation (A) and cytokine production (B to F) were
measured in these cultures and in cultures of unseparated spleen cells
from L3-infected
( ) and
uninfected (
) and
uninfected ( )
mice.
)
mice.
Strikingly, the removal of adherent cells and the addition of irradiated spleen cells from either infected or uninfected animals enabled cells from L3-infected mice to produce IL-2 and IFN-γ (Fig. 5B and C). Unseparated spleen cells from L3-infected mice did not secrete IL-2 and IFN-γ in response to Ag. Furthermore, the replacement of the resident adherent APC population reduced the production of Ag-stimulated IL-4 and IL-10 in the cultures to undetectable levels (Fig. 5D and F) and IL-5 to near background levels (Fig. 5E). Therefore, as with the polyclonal response, the removal of the adherent cells from the spleens of L3-infected mice switched the in vitro Ag-driven response of the cultures from Th2 to Th1.
DISCUSSION
The mechanisms by which Th-polarized responses evolve and are maintained following Ag encounter are likely to be both diverse and complex. This complexity clearly applies to the situation in mice and humans following infection with Brugia L3, for which a number of mechanisms have been suggested to underlie the profound Th2-biased immune response. These include selective Th1 clonal deletion, anergy, and suppression. Previously, we have shown that the production of IL-4 is a critical factor in down-regulating polyclonal Th1 responses in L3-infected mice (25). However, it is clear that the direct inhibitory action of IL-4 on Th1 cell activity is not the only immunomodulatory mechanism operating in these mice. The addition of rIL-2 to spleen cell cultures from L3-infected mice also resulted in the recovery of proliferation and IFN-γ production in response to ConA, suggesting that Th1 down-regulation following infection with L3 may operate via a block in IL-2 production (25). In addition, neutralization of IL-4 in vitro only partially recovered the polyclonal Th1 response and was unable to activate any Ag-specific Th1 responsiveness. The aim of the experiments in this study was to investigate the additional mechanisms by which both polyclonal and Ag-specific Th1 responses are suppressed in L3-infected mice.
The results of these experiments clearly demonstrate that, in addition to IL-4, IL-10 and a nylon wool-adherent APC population in the spleens of infected mice play an important role in down-regulating polyclonal Th1 responses. When neutralizing anti-IL-10 MAb was added to ConA-stimulated spleen cells from mice infected with L3, production of IL-2 and IFN-γ was dramatically augmented, while proliferation was partially restored. This result is similar to that previously described following treatment with a neutralizing anti-IL-4 MAb. Indeed, while anti-IL-4 treatment had no effect on mitogen-driven IL-10 (or IL-5) secretion, anti-IL-10 treatment also had no effect on mitogen-driven IL-4 (or IL-5) secretion. The presence of both IL-4 and IL-10 in the treated cultures may explain the inability to fully recover the proliferative response by using anti-IL-4 MAb or anti-IL-10 MAb alone. Alternatively, the proliferative defect may be more profound and thus less easily reversed following short-term in vitro culture than the cytokine impairment. Other studies have demonstrated that proliferation and cytokine production can be differentially regulated (3, 10).
In addition to the role of down-regulatory cytokines, the results presented implicate the adherent cell (APC) population in the suppression of Th1 responses. The first evidence that APCs may be involved in down-regulating Th1 responses in these mice was obtained by infecting mice by alternative routes and comparing the Th cell responses that develop. The route of inoculation can influence the induction of T-cell unresponsiveness due to the utilization of distinct APC populations (1). Mitogen-driven Th1 responses were impaired only when L3 were given by the natural s.c. route; infection with L3 by the i.p. route was unable to down-regulate polyclonal Th1 responses in the spleen even when mitogen-stimulated IL-4 and IL-10 could be detected. However, spleen cells from i.p.-infected mice were able to mount only a weak Th2 response to the parasite in comparison to that of s.c.-infected animals. The results suggest that the magnitude of the Th2 response elicited by L3 may be the critical factor in down-regulating polyclonal Th1 responses in the spleen. Consistent with this observation, mitogen-driven Th1 responses are normal at day 4 after s.c. infection with L3, prior to the induction of a measurable Th2 response (data not shown).
The role of APCs in suppressing Th1 responses was investigated more directly by depleting spleen cell preparations from L3-infected mice of nylon wool-adherent cells and replacing them with irradiated spleen cells from uninfected or infected mice. It is important to note that neither the nylon wool-nonadherent (T enriched) cells nor the irradiated spleen cell populations could respond to ConA or Ag when cultured alone. Therefore, any differences in the proliferative or cytokine responses were due to the combination of T cells and a fresh APC population. In these experiments, it was found that mitogen-stimulated proliferation of splenic T cells from L3-infected mice was restored to that of uninfected controls. Furthermore, the production of ConA-stimulated IL-4, IL-5, and IL-10 was reduced to near background levels, while production of IL-2 and IFN-γ was completely recovered. Therefore, the resident APC population in the spleen following s.c. infection with L3 appears to have a more profound effect on polyclonal Th1 responsiveness than that of IL-4 and IL-10 alone.
Finally, we investigated whether Ag-specific Th1 cells simply do not exist in L3-infected mice or whether they are present but are rendered unresponsive in vivo. In contrast to previous results with anti-IL-4 Mab (25), addition of neutralizing anti-IL-10 MAb to Ag-primed cultures of splenocytes from L3-infected mice resulted in the production of IL-2 and IFN-γ. Furthermore, the replacement of the APC population in the spleen preparations from L3-infected mice completely switched the Ag-specific cytokine response in vitro from Th2 to Th1, indicating the central role played by the resident APC population in maintaining the Th2 imbalance in these mice. The results of these experiments indicate that Brugia-reactive Th1 cells are primed following infection with L3, but are actively suppressed in vivo by a mechanism that involves IL-10 and the resident APC population, but not IL-4. How IL-4, IL-10, and APCs interact in the spleens of infected animals to induce this state of Th1 unresponsiveness is not clear. Previously, IL-4 was thought to directly inhibit the activity of established Th1 cells (27), while IL-10 is known to inhibit T cells indirectly by acting on accessory cells (9). However, a recent study has shown that both IL-4 and IL-10 can directly affect the ability of a macrophage APC population to support the priming of Th1 cells (6). In addition, IL-10 can inhibit T-cell proliferation and IL-2 production in the absence of APCs (8).
The results of this study demonstrate that s.c. infection with L3 has a profound influence on the function of adherent APCs in the spleen resulting in the inhibition of certain Th1 activities (e.g., ConA-stimulated proliferative responsiveness) to such an extent that it is not fully recoverable by neutralizing Th2 cytokines. Various APC-derived factors, in addition to IL-10 (11, 31), are known to suppress T-cell proliferation, including NO, prostaglandin E2, H2O2, and transforming growth factor β (2, 4, 11, 24). The addition of known inhibitors or the appropriate neutralizing antibodies would determine whether any of these mediators are operative in ConA-stimulated spleen cell cultures from L3-infected mice. One clue as to the identity of the suppressor adherent cell population in the spleens of infected mice was provided by the finding that ConA-stimulated proliferation was recovered completely and the Th cell response switched when irradiated spleen cells from L3-infected mice were added back as a source of APCs. Since B cells are more susceptible to the effect of gamma irradiation than other APC types (e.g., dendritic cells) (32), B cells may be the nylon wool-adherent APC population involved in inducing Th1 unresponsiveness in the infected spleen.
The complexity of this infection model is underscored by results from other laboratories. Allen and colleagues (3) have previously highlighted the role of peritoneal exudate APCs in generating defective proliferative responses in a mouse model of filariasis. Their recent results suggest that in vivo, IL-4, but not IL-10, is critical for the induction of the defective APC population in the peritoneal cavity (21). Excreted or secreted molecules of filarial nematodes have also been implicated in a range of suppressive activities. Hartmann et al. (13) demonstrated that a recombinant cystatin cloned from the filarial parasite Acanthocheilonema viteae down-regulated mitogen- and Ag-driven proliferative responses in vitro and up-regulated IL-10 secretion from BALB/c splenocytes, while the studies of Harnett and Harnett have shown that filarial excretory or secretory products containing phosphorylcholine have pronounced down-regulatory effects on B-cell function (12).
While the nature of the cellular and cytokine network following infection with L3 remains to be clarified, the results of this study indicate that the overwhelmingly Th2-biased response in L3-infected mice is maintained by a complex interplay of cytokines and cell populations that actively induce a state of profound Th1 unresponsiveness. Furthermore, the reversibility of this Th1 defect in the face of strong, ongoing Th2 responses provides us with a greater understanding of the possible mechanisms of suppression generated by the parasite in human filarial infection.
ACKNOWLEDGMENTS
This study was funded by a grant from the MRC. E.D. is a Wellcome Trust University Lecturer.
We thank Richard Grencis for supplying the neutralizing anti-IL-10 antibody and Colin Chapman for excellent technical assistance.
REFERENCES
- 1.Aichele P, Brduscha-Riem K, Zinkernagel R M, Hengartner H, Pircher H. T-cell priming versus T-cell tolerance induced by synthetic peptides. J Exp Med. 1995;182:261–266. doi: 10.1084/jem.182.1.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albina J E, Abate J A, Henry W L., Jr Nitric oxide production is required for murine resident macrophages to suppress mitogen-stimulated T cell proliferation. J Immunol. 1991;147:144–148. [PubMed] [Google Scholar]
- 3.Allen J E, Lawrence R A, Maizels R M. APC from mice harbouring the filarial nematode, Brugia malayi, prevent cellular proliferation but not cytokine production. Int Immunol. 1996;8:143–151. doi: 10.1093/intimm/8.1.143. [DOI] [PubMed] [Google Scholar]
- 4.Barral-Netto M, Barral A, Brownell C E, Skeiky Y A W, Ellingsworth L R, Twardzik D R, Reed S G. Transforming growth factor-B in leishmanial infection: a parasite escape mechanism. Science. 1992;257:545–548. doi: 10.1126/science.1636092. [DOI] [PubMed] [Google Scholar]
- 5.Bretscher P A, Wei G, Menon J N, Bielefeldt-Ohmann H. Establishment of stable, cell-mediated immunity that makes “susceptible” mice resistant to Leishmania major. Science. 1992;257:539–542. doi: 10.1126/science.1636090. [DOI] [PubMed] [Google Scholar]
- 6.Cua D J, Coffman R L, Stohlman S A. Exposure to Th2 cytokines in vivo before encounter with antigen selects for T helper subsets via alterations in antigen-presenting cell function. J Immunol. 1996;157:2830–2836. [PubMed] [Google Scholar]
- 7.Devaney E, Jecock R M. The expression of the Mr 30,000 antigen in the third stage larvae of Brugia pahangi. Parasite Immunol. 1991;13:75–87. doi: 10.1111/j.1365-3024.1991.tb00264.x. [DOI] [PubMed] [Google Scholar]
- 8.De Waal Malefyt R, Yssel H, de Vries J E. Direct effects of IL-10 on subsets of human CD4+T cell clones and resting T cells. Specific inhibition of IL-2 production and proliferation. J Immunol. 1993;150:4754–4765. [PubMed] [Google Scholar]
- 9.Ding L, Shevach E M. IL-10 inhibits mitogen-induced T cell proliferation by selectively inhibiting macrophage costimulatory function. J Immunol. 1992;148:3133–3139. [PubMed] [Google Scholar]
- 10.Evavold B D, Allen P M. Separation of IL-4 production from Th cell proliferation by an altered T cell receptor ligand. Science. 1991;252:1308–1310. doi: 10.1126/science.1833816. [DOI] [PubMed] [Google Scholar]
- 11.Haque S, Khan I, Haque A, Kasper L. Impairment of the cellular immune response in acute murine toxoplasmosis: regulation of interleukin 2 production and macrophage-mediated inhibitory effects. Infect Immun. 1994;62:2908–2916. doi: 10.1128/iai.62.7.2908-2916.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harnett W, Harnett M M. Inhibition of murine B cell proliferation and down-regulation of protein kinase C levels by a phosphorylcholine-containing filarial excretory-secretory product. J Immunol. 1993;151:4829–4837. [PubMed] [Google Scholar]
- 13.Hartmann S, Kyewski B, Sonnenburg B, Lucius R. A filarial cysteine protease inhibitor down-regulates T cell proliferation and enhances interleukin-10 production. Eur J Immunol. 1997;27:2253–2260. doi: 10.1002/eji.1830270920. [DOI] [PubMed] [Google Scholar]
- 14.King C L, Kumaraswami V, Poindexter R W, Kumari S, Jayaraman K, Alling D W, Ottesen E A, Nutman T B. Immunological tolerance in lymphatic filariasis. Diminished parasite-specific T and B lymphocyte precursor frequency in the microfilaraemic state. J Clin Investig. 1992;89:1403–1410. doi: 10.1172/JCI115729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.King C L, Mahanty S, Kumaraswami V, Abrams J S, Regunathan J, Jayaraman K, Ottesen E A, Nutman T B. Cytokine control of parasite-specific anergy in human lymphatic filariasis. Preferential induction of a regulatory T helper type 2 lymphocyte subset. J Clin Investig. 1993;92:1667–1673. doi: 10.1172/JCI116752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lal R B, Kumaraswami V, Steel C, Nutman T B. Phosphorylcholine-containing antigens of Brugia malayinonspecifically suppress lymphocyte function. Am J Trop Med Hyg. 1990;42:56–64. doi: 10.4269/ajtmh.1990.42.56. [DOI] [PubMed] [Google Scholar]
- 17.Lammie P J, Katz S P. Immunoregulation in experimental filariasis. I. In vitro suppression of mitogen-induced blastogenesis by adherent cells from jirds chronically infected with Brugia pahangi. J Immunol. 1983;130:1381–1385. [PubMed] [Google Scholar]
- 18.Lammie P J, Katz S P. Immunoregulation in experimental filariasis. IV. Induction of non-specific suppression following in vitro exposure of spleen cells from infected jirds to Brugia pahangiantigen. Immunology. 1984;52:221–229. [PMC free article] [PubMed] [Google Scholar]
- 19.Lawrence R A, Allen J E, Gregory W F, Kopf M, Maizels R M. Infection of IL-4-deficient mice with the parasitic nematode Brugia malayidemonstrates that host resistance is not dependent on a T helper 2-dominated immune response. J Immunol. 1995;154:5995–6001. [PubMed] [Google Scholar]
- 20.Liu L X, Buhlmann J E, Weller P E. Release of prostaglandin E-2 by microfilariae of Wuchereria bancrofti and Brugia malayi. Am J Trop Med Hyg. 1992;46:520–523. doi: 10.4269/ajtmh.1992.46.520. [DOI] [PubMed] [Google Scholar]
- 21.MacDonald A S, Maizels R M, Lawrence R A, Dransfield I, Allen J E. Requirement for in vivo production of IL-4, but not IL-10, in the induction of proliferative suppression by filarial parasites. J Immunol. 1998;160:1304–1312. [PubMed] [Google Scholar]
- 22.Mahanty S, King C L, Kumaraswami V, Regunathan J, Maya A, Jayaraman K, Abrams J S, Ottesen E A, Nutman T B. IL-4 and IL-5 secreting lymphocyte populations are preferentially stimulated by parasite-derived antigens in human tissue invasive nematode infections. J Immunol. 1993;151:3704–3711. [PubMed] [Google Scholar]
- 23.Maizels R M, Lawrence R A. Immunological tolerance: the key feature in human filariasis. Parasitol Today. 1991;7:271–276. doi: 10.1016/0169-4758(91)90093-4. [DOI] [PubMed] [Google Scholar]
- 24.Metzger Z, Hoffeld J T, Oppenheim J J. Macrophage-mediated suppression. I. Evidence for participation of both hydrogen peroxide and prostaglandins in suppression of murine lymphocyte proliferation. J Immunol. 1980;124:983–988. [PubMed] [Google Scholar]
- 25.Osborne J, Hunter S J, Devaney E. Anti-interleukin-4 modulation of the Th2 polarized response to the parasitic nematode Brugia pahangi. Infect Immun. 1996;64:3461–3466. doi: 10.1128/iai.64.9.3461-3466.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pearlman E, Hazlett F E, Jr, Kazura J W, Boom W H. Modulation of murine cytokine responses to mycobacterial antigens by helminth-induced T helper 2 cell responses. J Immunol. 1993;151:4857–4864. [PubMed] [Google Scholar]
- 27.Peleman R, Wu J, Fargeas C, Delespesse G. Recombinant interleukin 4 suppresses the production of interferon γ by human mononuclear cells. J Exp Med. 1989;170:1751–1756. doi: 10.1084/jem.170.5.1751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piessens W F, McGreevy P B, Piessens P W, McGreevy M, Koiman J, Sarsoso H S, Dennis D T. Immune responses in human infections with Brugia malayi. Specific cellular unresponsiveness to filarial antigens. J Clin Investig. 1980;65:172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Piessens W F, Ratiwayanto S, Tuti S, Palmieri J H, Piessens P W, Koiman I, Dennis D T. Antigen-specific suppressor cells and suppressor factors in human filariasis. N Engl J Med. 1980;302:833–837. doi: 10.1056/NEJM198004103021503. [DOI] [PubMed] [Google Scholar]
- 30.Portaro J K, Britton S, Ash L R. Brugia pahangi: depressed mitogen reactivity in filarial infections in the jird, Meriones unguiculatus. Exp Parasitol. 1976;40:438–446. doi: 10.1016/0014-4894(76)90112-0. [DOI] [PubMed] [Google Scholar]
- 31.Silva J S, Morrissey P J, Grabstein K H, Mohler K M, Anderson D, Reed S G. Interleukin 10 and interferon γ regulation of experimental Trypanosoma cruziinfection. J Exp Med. 1992;175:169–174. doi: 10.1084/jem.175.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sopori M L, Cohen D A, Cherian S, Perrone R S, Kaplan A M. Antigen presentation in the rat. II. An Ia+ radiosensitive T cell can present antigen to primed Ia− T cells. J Immunol. 1985;134:1369–1373. [PubMed] [Google Scholar]
- 33.Steele C, Guinea A, McCarthy J S, Ottesen E A. Long-term effect of prenatal exposure to maternal microfilaraemia on immune responsiveness to filarial parasite antigens. Lancet. 1994;343:890–893. doi: 10.1016/s0140-6736(94)90009-4. [DOI] [PubMed] [Google Scholar]
- 34.Yazdanbakhsh M, Sartono E, Kruize Y C M, Kurniawan A, van der Pouw-Kraan T, van der Meide P H, Selkirk M E, Partono F, Hintzen R Q, van Lier R A W, Maizels R M. Elevated levels of T cell activation antigen CD27 and increased interleukin-4 production in human lymphatic filariasis. Eur J Immunol. 1993;23:3312–3317. doi: 10.1002/eji.1830231238. [DOI] [PubMed] [Google Scholar]