Abstract
Myocardial bridge (MB) is the most frequent inborn coronary artery variant in which a portion of the myocardium overlies an epicardial coronary artery segment. Although MB has long been considered a benign entity, a growing body of evidence has suggested its association with angina and adverse cardiac events. However, to date, no data on long-term prognosis are available, nor on therapies improving cardiovascular outcomes. We are currently conducting an ambispective, observational, multicentre, study in which we enrol patients with a clinical indication to undergo coronary angiography (CA) and evidence of MB, aiming to describe the incidence of symptoms and cardiovascular events at baseline and at long-term follow-up (FUP). The role of invasive full-physiology assessment in modifying the discharge therapy and eventually the perceived quality of life and the incidence of major cardiovascular events will be analysed. Basal clinical-instrumental data of eligible and consenting patients have been acquired after CA; FUP was performed 6, 12, and 24 months after the angiographic diagnosis of MB. The primary endpoint of the study is the incidence of major adverse cardiovascular events (MACE), defined as the composite of cardiac death, myocardial infarction, cardiac hospitalization, and target vessel revascularization; the secondary endpoints are the rate of patients with Seattle Angina Questionnaire (SAQ) summary score <70 and the incidence of MACE in patients undergoing invasive intracoronary assessment. Among patients undergone FUP visits, we recorded 31 MACE at 6 months (11.6%), 16 MACE at 12 months (6.5%), and 26 MACE at 24 months (13.5%). The rate of patients with SAQ <70 is 18.8% at 6 months, 20.6% at 12 months, and 21.8% at 24 months. To evaluate the prognostic role of invasive intracoronary assessment, we compared MB patients who underwent only angiographic evaluation (Angio group) to those who underwent acetylcholine (ACH) provocative test with indication to calcium-channel blockers (CCBs) at discharge (Angio + ACH + CCBs group) and those who underwent functional assessment with fractional flow reserve (FFR) with indication to beta-blockers (BBs) at discharge (Angio + FFR + BBs group). After 2 years of FUP, the rate of MACE was significantly reduced in both Angio + ACH + CCBs group (6 vs. 25%, P = 0.029) and Angio + FFR + BBs group (3 vs. 25%, P = 0.005) compared with Angio group. The preliminary results of our study showed that MB may be a cause of angina and adverse cardiac events in patients referred to CA for suspected coronary artery disease (CAD). Full-physiology assessment unmasking MB-related ischaemia mechanisms, allowed to guide the treatment, personalizing the clinical management, improving the quality of life, and cardiovascular outcomes in patients with MB.
Keywords: Myocardial bridge, Myocardial ischaemia, Invasive intracoronary assessment, Intracoronary physiology, Provocative test, Intracoronary imaging, Personalized therapy, Prognosis
Introduction
Myocardial bridge (MB) is the most frequent congenital coronary anomaly in which a portion of the myocardium overlies an epicardial coronary artery segment, most frequently (70–98%) the left anterior descending (LAD).1,2 This tunnelled artery is compressed during systole, resulting in the so-called ‘milking effect’ at coronary angiography (CA).3 However, coronary blood flow occurs primarily during diastole, so the clinical and haemodynamic relevance of MB is still a matter of debate.4 Although most patients affected by MB are asymptomatic, some of them may experience anginal symptoms, angina-equivalents, and, less frequently, acute coronary syndromes (ACS) and arrhythmias.5 Moreover, a growing body of evidence suggested that MB may coexist with concomitant coronary artery disease (CAD), coronary artery spasm (CAS), and coronary microvascular dysfunction (CMD).2,6
Since CA does not provide any information on the haemodynamic significance of MB, nor on functional disorders related to it, the advent of invasive intracoronary assessment tools has strongly improved the understanding of MB pathophysiology.2,7 Imaging tools, such as optical coherence tomography (OCT) and intravascular ultrasound (IVUS), have allowed to better distinguish the morphological features of MB,8–10 and detect the frequent association with native or neo-atherosclerotic plaque.11,12 In addition, invasive pressure wire-based techniques, such as fractional flow reserve (FFR), contrast FFR (cFFR), and instantaneous wave-free ratio (iFR), can be used in the cardiac catheterization laboratory to physiologically describe the relevance of MB with or without concomitant epicardial CAD.2,5,13
Lastly, vasomotor disorders and microvascular dysfunction may represent two further pathophysiological hallmarks of MB-related ischaemia. In fact, previous studies demonstrated that CAS and microvascular dysfunction are more common in patients with than in those without MB.14–20
Our RIALTO (Myocardial Bridge Evaluation Towards Personalized Medicine) registry, by setting up a database of patients affected by MB, has the aim to describe the main clinical and anatomical characteristics of MB patients and the risk of future cardiovascular events. Furthermore, since the poor therapeutic evidence and the lack of agreement on a proposed comprehensive invasive assessment, we sought to evaluate the role of a full-physiology evaluation together with intracoronary imaging and provocative tests in detecting pathophysiological MB endotypes.
At the end of enrolment, the expected results might provide real-world evidence of data-driven decision-making to help patients with MB by modifying the current diagnostic and therapeutic algorithm.
Methods
‘RIALTO’ is an ambispective and observational multicentre registry (ClinicalTrials.gov Identifier: NCT05111418) that currently involves five centres in Italy:
Fondazione policlinico universitario agostino gemelli IRCSS, Roma, ITALIA
Policlinico s. Martino IRCSS, Università di Genova, Genova, ITALIA
Centro cardiologico monzino IRCSS, milano, ITALIA
Arcispedale s. Anna, Azienda Ospedaliero-Università di Ferrara, Ferrara, ITALIA
Fondazione policlinico tor vergata, roma, ITALIA
The inclusion criteria are:
patients referred to undergo invasive CA (for elective or urgent indications) for suspected CAD, found to have MB with or without other coronary lesions;
age above 18 years old;
ability to provide Informed Consent.
Criteria for exclusion from the study are:
patients with life expectancy <12 months;
patients with severe valvular heart disease.
The endpoints of this study are:
PRIMARY ENDPOINT:
Incidence of major adverse cardiovascular events (MACE), defined as the composite of cardiac death, myocardial infarction, cardiac hospitalization, and target vessel revascularization.
SECONDARY ENDPOINT:
Rate of patients with Seattle Angina Questionnaire (SAQ) <70;
The incidence of MACE in patients undergoing invasive intracoronary assessment.
Study procedure
Coronary angiography with or without revascularization and invasive intracoronary assessment are performed according to standard practice and are not part of the present study protocol. After ascertaining the presence of MB on diagnostic CA, clinical-instrumental and laboratory data of eligible and consenting patients are acquired by the principal investigator or his delegates. No indication is given to the operator on how to characterize the significance of the MB: the use of intracoronary imaging and/or the decision to perform a full-physiology assessment and/or provocative test is left to operator’s decision. Clinical-instrumental and laboratory data include: complete demographics assessment; past medical history; cardiovascular risk profile; pharmacological anamnesis with specific data about dosage and time from initiation of beta-blockers (BBs) or calcium-channel blockers (CCBs); present cardiac complaints; cardiac examination; electrocardiogram (ECG); comprehensive echocardiographic evaluation; data about non-invasive evidence of inducible ischaemia (including stress ECG, SPECT, positron emission tomography, magnetic resonance imaging, stress-Echo); coronary CT scan data (including position, length, depth, number of collaterals, angle of entrance, number of curves of the bridged segment); invasive coronary data including results of functional tests (FFR; iFR; cFFR), assessment of coronary microcirculation (coronary flow reserve, CFR; index of microcirculatory resistance, IMR), provocative tests (acetylcholine, ACH), with details on any drugs administered during the assessments, and intracoronary imaging (OCT and IVUS). Patients are contacted 6, 12, and 24 months after the angiographic diagnosis of MB for a telephone interview or for a clinical visit. The data obtained in the follow-up (FUP) visit are included in the study, without adding treatments other than those envisaged in normal clinical practice. The main data collected at the FUP are: pre-specified adverse cardiac events (angina, death for all cause, cardiovascular death, myocardial infarction, cardiac hospitalization, and target lesion revascularization) with the date of occurrence; and SAQ.
Sample size
Since this is an observational study, the sample size is fixed, and no minimum required population is calculated. Some expectations about the final size of the registry can however be made. The incidence of the myocardial bridge in the general population is widely debated with autopsy studies showing this condition in up to 50% of patients while angiographic studies reported rate of detection ranging from 0.5 up to even 16% of patients.1 Considering an average incidence of 10% on around 1500 angiography per year, and accounting for a dropout rate of 30% year due to underreporting of the condition, impossible data retrievement, denial of consent, or exclusion criteria, the final registry size would be around 500 patients.
Statistical analysis
Baseline characteristics are summarized as means and standard and deviations or medians and interquartile ranges according to normality verified by the Kolmogorov-Smirnov test. Difference between categorical variables is compared using the χ2 test or the Mann–Whitney test (if not normal), difference between means will be compared with ANOVA or Kruskal Wallis test according to normality. Time to event data was evaluated using the log-rank test and Kaplan–Meier and with Cox proportional hazard model to identify the significant contributing factors. All analysis is performed on SPSS v23 (IBM Inc.).
Results
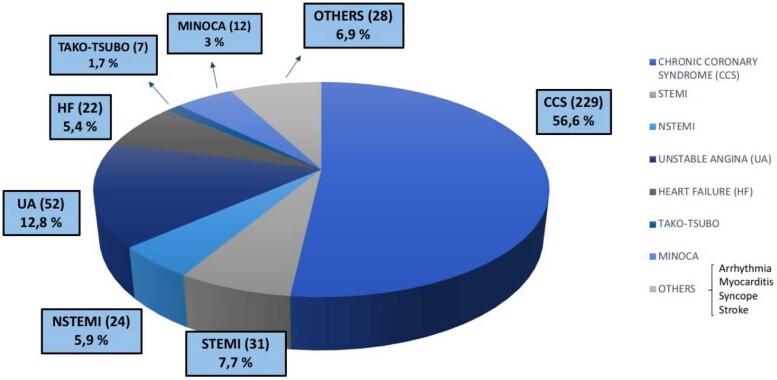
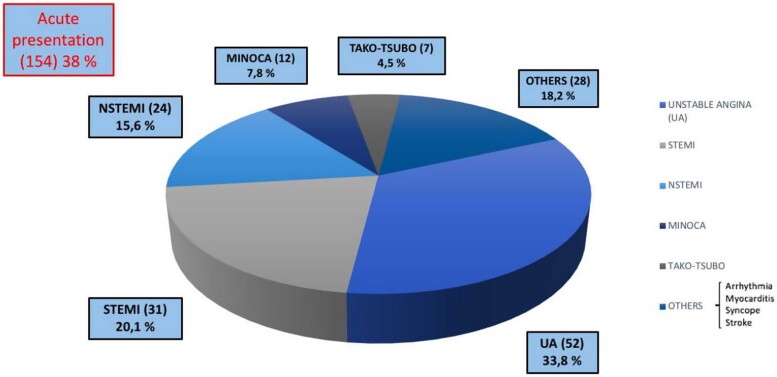
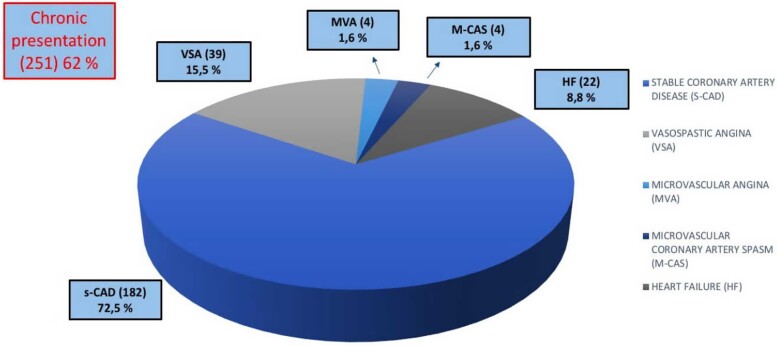
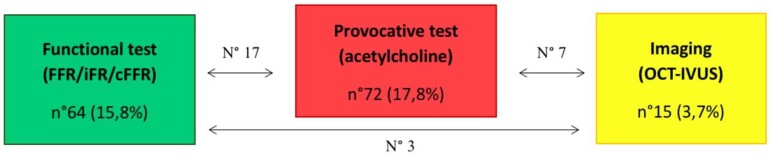
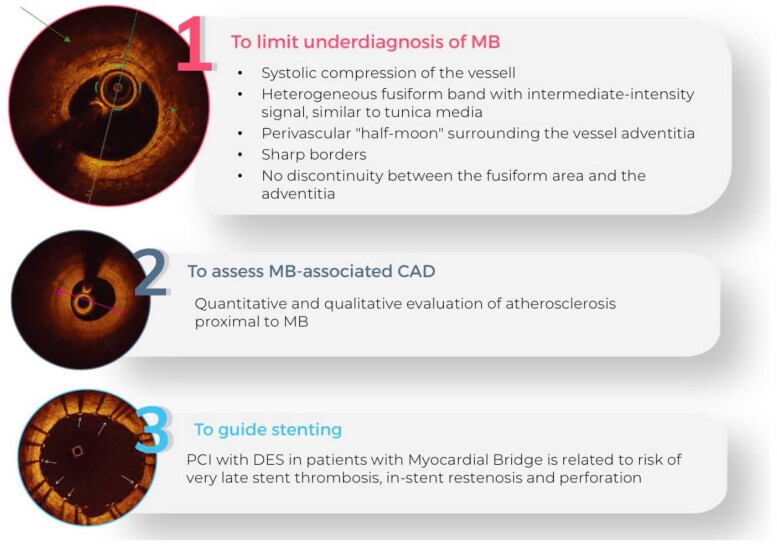
We analysed CA performed between June 2015 and March 2022, using the the dedicated software specific for each centre included in the study. After the screening phase, 405 eligible and consenting patients (estimated incidence of 2%) were included in the study so far. The mean age of patients was 66 ± 14.6 years. All the demographic and clinical characteristics of MB patients are summarized in Table 1. MBs were more frequent among men (291; 72%) than women (114; 28%). We also assessed the prevalence of cardiovascular risk factors and comorbidities, as well as clinical data such as previous percutaneous coronary intervention (PCI) and previous coronary artery bypass graft (Table 1) in patients with MB. Hypertension (62%) and dyslipidaemia (53.8%) were the most prevalent cardiovascular risk factors, found in more than 50% of patients (Table 1). The most frequent location of MB was the LAD coronary artery (96.8% of the study group); other relatively rare locations were the circumflex artery (1.5%), the right coronary artery (0.5%), the posterior interventricular artery (0.5%), the first diagonal artery (0.5%), and the first septal artery (0.2%) (Table 1). Figures 1–3 show that MBs were more frequent in patients with a chronic clinical presentation (62%) than those with acute clinical presentations. Interestingly, a big proportion (154; 38%) of our patients was found to have MB during the occurrence of an ACS (Figure 2). In this acute setting, unstable angina (UA) was the most frequent clinical presentation (52; 33.8%), following by ST-segment elevation myocardial infarction (STEMI) (31; 20.1%); non-STEMI (NSTEMI) (24; 15.6%); myocardial infarction with non-obstructive coronary arteries (MINOCA) (12; 7.8%); and Tako-Tsubo Syndrome (7; 4.5%) (Figure 2). Moreover, 28 patients (18.2%; ‘Others group’) were found to have MB during the occurrence of an atypical clinical presentation, such as arrhythmia, myocarditis, syncope, or stroke (Figure 2). Among patients with chronic clinical presentation, stable CAD was the most frequently detected entity (182; 72.5%) (Figure 3). Provocative test with intracoronary incremental dose of ACH was performed in 72 patients (17.8%) (Figure 4) in order to unmask the presence of CAS, in patients with a clinical presentation suggestive of vasospastic angina (VSA). In 39 of them (54%), ACH test resulted diagnostic for VSA according to the occurrence of usual chest pain, transient ECG ST-segment modifications, and the evidence of transient epicardial CAS.7,21 Among patients with chronic clinical presentation, 22 of them (8.8%) were hospitalized due to clinical symptoms and signs of heart failure (HF) (Figure 3), and they underwent CA to rule out an ischaemic aetiology underlying HF. However, CA did not show obstructive CAD. Finally, a minority of patients (3.2%) were found to have a specific endotype of microvascular angina (Figure 3). In particular, the use of invasive index, such as CFR and IMR, unmasked a structural endotype of microvascular angina when CFR <2 and IMR >25; while the occurrence of typical angina and the development of ischaemic ST-segment changes during ACH test, in the absence of an epicardial coronary diameter reduction ≥90%, were diagnostic for the functional endotype of microvascular angina (microvascular CAS).7 Sixty-four patients underwent invasive physiology evaluation with pressure wire-based techniques in order to assess the haemodynamic significance of MB and proximal stenosis (Figure 4). Fractional flow reserve was assessed in conditions of hyperaemia mediated by intravenous or intracoronary administration of adenosine, or by intracoronary administration of contrast (cFFR); iFR was assessed baseline and after inotropic or chronotropic stimulation (respectively with dobutamine and atropine), to enhance the haemodynamic relevance of MB.13 Finally, 15 patients underwent CA with invasive intracoronary imaging (OCT) to explore the morphological and anatomical characteristics of MB and the quantitative and qualitative features of the atherosclerotic burden detected proximal to MB (Figure 4). In our study, OCT highlighted the presence of a heterogeneous fusiform band with sharp borders and low/intermediate-intensity signal, similar to tunica media, surrounding the vessel adventitia (Figure 5).
Table 1.
Baseline characteristics of the patients (N = 405)
| Demographics | |
| Age—mean years ± SD | 66 ± 14.6 |
| Female sex—n (%) | 114 (28.1) 8/26 (30.8) |
| Body mass index—mean ± SD | 26.3 ± 3.9 |
| Risk factors and medical history [n. (% on total number)] | |
| Hypertension | 251 (62.0) 20/26 (76.92) |
| Diabetes mellitus | 55 (13.6) 0/26 (0) |
| Dyslipidaemia | 218 (53.8) 1/26 (3.85) |
| Former smoker | 109 (26.9) 23/26 (88.46) |
| Active smoker | 70 (15.6) 1/26 (3.85) |
| Previous stroke | 10 (2.6) 1/26 (3.85) |
| Previous MI | 33 (8.1) 10/26 (38.46) |
| Previous CABG | 2 (0.5) 8/26 (30.77) |
| Previous PCI | 58 (14.3) 21/26 (80.77) |
| Myocardial bridge site [n./total n. (%)] | |
| Left anterior descending artery | 392/405 (96.8) 1/26 (3.9) |
| Left circumflex artery | 6/405 (1.5) 11/26 (42.3) |
| Right coronary artery | 2/405 (0.5) |
| Posterior descending artery | 2/405 (0.5) 0/26 (0) |
| First diagonal branch of LAD | 2/405 (0.5) |
| First septal branch of LAD | 1/405 (0.2) |
MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; LAD, left anterior descending artery; n., number; SD, standard deviation.
Figure 1.
Clinical presentation: all patients.
Figure 2.
Clinical presentation: acute setting.
Figure 3.
Clinical presentation: chronic setting.
Figure 4.
Invasive intracoronary assessment. FFR, fractional flow reserve assessment; iFR, instantaneous wave-free ratio; OCT, optical coherence tomography; IVUS, intravascular ultrasound.
Figure 5.
Role of optical coherence tomography in myocardial bridge-assessment. OCT, optical coherence tomography; MB, myocardial bridge; CAD, coronary artery disease; PCI, percutaneous coronary intervention; DES, drug eluting stent.
Among patients who have already undergone FUP visit at 6 months (266; 66%), 12 months (247; 61%), and 24 months (193; 48%), we recorded 31 MACE (11.7%) at 6 months, 16 MACE, at 12 months (6.5%) and 26 MACE (13.5%) at 24 months (Table 2). The rate of patients with SAQ Angina Summary Score <70, which represents an angina affecting patient’s quality of life, is 18.8% (50 patients) at 6 months, 20.6% (51 patients) at 12 months, and 21.8% (42 patients) at 24 months (Table 2).
Table 2.
MACE and SAQ at follow-up
| Follow-up time | 0–6 months | 6–12 months | 12–24 months |
|---|---|---|---|
| Number of patients | 266 | 247 | 193 |
| MACE—n. (%) | 31 (11.7) | 16 (6.5) | 26 (13.5) |
| SAQ < 70—n. (%) | 50 (18.8) | 51 (20.6) | 42 (21.8) |
MACE, major adverse cardiovascular events; SAQ, Seattle Angina Questionnaire.
To evaluate the prognostic impact of invasive intracoronary assessment, we arbitrarily divided the patients undergone FUP visits into three subgroups, based on the invasive intracoronary assessments performed during CA. In particular, we defined the following groups:
‘Angio group’, composed of patients undergone only angiographic evaluation, and not functional or provocative tests;
‘Angio + ACH group’, characterized by patients undergone ACH provocative test during CA;
‘Angio + FFRgroup’, characterized by patients undergone functional tests with FFR, iFR, or cFFR during CA.
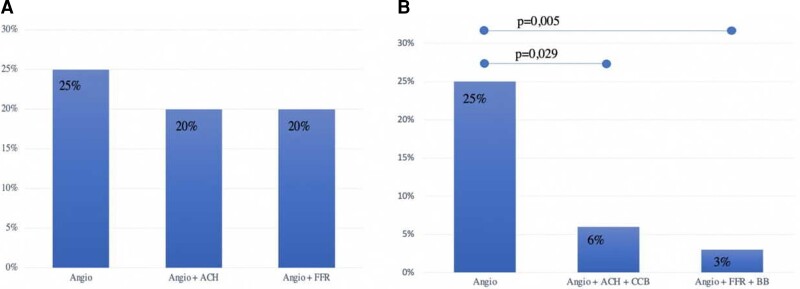
We found no significant differences between the three groups in cardiovascular risk profile, except for the prevalence of female sex in the Angio + ACH group (Table 3). After 2 years of FUP, considering all the MACE occurred during the three FUP intervals (0–6 months; 6–12 months; and 12–24 months), the rate of MACE was higher in Angio group (25% of MACE) compared with Angio + ACH group and Angio + FFR group (both with 20% of MACE), but it did not differ significantly (Figure 6). Nevertheless, the three groups significantly differed in discharge therapy (Table 3). Therefore, in order to evaluate the role of invasive assessment and of discharge therapy in modifying cardiovascular outcomes, we define two new subgroups:
Table 3.
Clinical and therapeutic variables in diagnostic groups
| Angio | Angio + ACH | Angio + FFR | P-value | |
|---|---|---|---|---|
| Clinical features | ||||
| Female sex—n (%) | 40 (22.9%) | 19 (30.4%) | 9 (20.5%) | 0.035 |
| Hypertension | 118 (67.4%) | 29 (61.7%) | 22 (50%) | 0.096 |
| Diabetes mellitus | 24 (13.7%) | 6 (12.8%) | 9 (20.5%) | 0.487 |
| Dyslipidaemia | 96 (54.9%) | 25 (53.2%) | 22 (50%) | 0.843 |
| Smoker | 77 (44%) | 21 (44.7%) | 14 (31.8%) | 0.317 |
| Previous MI | 16 (9.1%) | 3 (6.4%) | 8 (18.2%) | 0.133 |
| Previous PCI | 28 (16%) | 6 (12.8%) | 12 (27.3%) | 0.092 |
| Discharge therapy | ||||
| Beta-blockers | 115 (65.7%) | 16 (34%) | 28 (63.6%) | <0.001 |
| CCB | 36 (20.6%) | 30 (63.8%) | 9 (20.5%) | <0.001 |
| Nitrates | 1 (0.5%) | 2 (4.3%) | 2 (4.5%) | 0.175 |
Angio, coronary angiography; ACH, acetylcholine provocation test; FFR, fractional flow reserve assessment; MI, myocardial infarction; CABG, coronary artery bypass graft; PCI, percutaneous coronary intervention; CCB, calcium-channel blockers.
Figure 6.
Incidence of MACE at 24 months follow-up according to diagnostic assessments (A) and personalized therapy (B). Angio, coronary angiography; ACH, acetylcholine provocation test; FFR, fractional flow reserve assessment; CCB, calcium-channel blockers; BB, beta-blockers.
‘Angio + ACH + CCBs group’, characterized by patients undergone ACH provocative test during CA with indication to CCBs at discharge;
‘Angio + FFR + BBs group’, characterized by patients undergone functional tests with FFR, iFR, or cFFR during CA with indication to BBs at discharge.
Among these subgroups, after 2 years of FUP, the rate of MACE was significantly reduced in both Angio + ACH + CCBs group (6 vs. 25%, P = 0.029) and Angio + FFR + BBs group (3 vs. 25%, P = 0.005) compared with Angio group (Figure 6).
Discussion
Myocardial bridge has been typically considered a bystander since most patients are asymptomatic and only a minority of them undergo CA.1 Nevertheless, recent evidence has suggested its correlation with myocardial ischaemia and cardiac complications.2 The early results of our study confirmed that MB may be associated with angina and MACE. Furthermore, a remarkable proportion of our patients were found to have MB during the occurrence of an ACS or a CCS, highlighting that different mechanisms of ischaemia are not mutually exclusive. In fact, MB coexisted with other ischaemic conditions, such as obstructive CAD, ACS, MINOCA, and ischaemia with non-obstructive coronary artery disease (INOCA). In this regard, we did not exclude from the registry patients with angiographic evidence of coronary thrombosis, critical stenosis, or functional disorders. Surely, this makes it difficult to understand whether reported MACE and symptoms are directly or indirectly attributed to MB. However, as our data confirmed, it is well known that several structural and functional disorders of coronary circulation may coexist, and the evidence of one of them should not limit to perform a comprehensive functional assessment.22 Since MB has been recently recognized as a cause of INOCA by the European Association of Percutaneous Cardiovascular Interventions, invasive intracoronary assessment should be performed once it is detected, allowing to guide the treatment according to the specific pathohistological endotype of myocardial ischaemia.7 In our study, invasive provocative test improved the ability to unmask vasomotor disorders associated with MB, highlighting a paradoxical vasoconstrictive response to ACH. This allowed to prescribe a tailored therapy with CCBs, which improved outcomes (Figure 6). Similarly, invasive physiological evaluation (i.e. FFR, iFR, cFFR, CFR, IMR) improved the ability to assess the haemodynamic significance of MB, or of the atherosclerotic stenosis proximal to MB, and to detect CMD related to MB, allowing to prescribe a tailored therapy (i.e. BBs), which significantly reduced the incidence of MACE after 2 years of FUP (Figure 6). To date, although FFR is considered the gold standard for the physiological assessment of intermediate-grade stenosis (haemodynamically significant stenosis defined as invasive FFR ≤0.80),23,24 it also might be useful to assess dynamic coronary obstructions such as MB.5,13,25 In addition to FFR, diastole-specific index (i.e. iFR) might be used for the same purpose.5 Unlike FFR which measures the trans-lesion pressure gradient in conditions of hyperaemia mediated by intravenous or intracoronary administration of adenosine, iFR is calculated by measuring the resting pressure gradient across a lesion during a portion of diastole, when vascular resistance is low.26 In this regard, Tarantini et al.13 showed that iFR was more consistent with inducible ischaemia compared with FFR in symptomatic MB patients: in particular, iFR at rest was abnormal (<0.89) in a relevant proportion of the patients, with a remarkable lowering after inotropic stimulation. However, validated cut-off does not exist for pressure wire-based index in assessing dynamic coronary obstructions. Nowadays, it is well known that incidence of MB depends on the imaging modality used to identify it, ranging from 2–6% (invasive angiographic series) to 33–42% (autopsy reports).2 Thereby, CA is not completely sensitive for the detection of MB, and invasive imaging tools, such as OCT and IVUS, may be helpful to limit the underdiagnosis of MB.2 In addition to the precision in identifying morphological features of MB, OCT allows to characterize atherosclerotic lesions proximal to it9 and may be useful to guide the percutaneous treatment of both MB and proximal stenosis. In fact, it has been demonstrated that stent implantation in a MB segment might be associated with the occurrence of coronary perforation, in-stent restenosis, and very late stent thrombosis27–29 (Figure 5). In our study, OCT helped to detect MB by highlighting a peculiar morphological pattern that corresponded to the ‘milking effect’ seen using CA (Figure 5).
This study has some limitations: firstly, the observational nature of the study gives an inferior level of evidence; secondly, there is not a big-size population, which may make the result inaccurate, first of all because the FUP data are not complete. Our study is still ongoing, and we hope to maximize the data in order to have a solid comprehension of the clinical and pathophysiological characteristics of MB and to propose an assessment protocol that may indicate a tailored therapy. Meanwhile, further randomized controlled studies are needed to confirm our findings in larger cohorts.
Acknowledgements
We deeply want to thank and remember our beloved and gifted colleague, Salvatore Gervasi, for his vision on this project from the very early phases. We miss Salvatore’s positive attitude and dedication to caring for his patients and linking clinical practice to research, focusing his efforts on the heart diseases of the athletes.
Funding
This paper was published as part of a supplement sponsored by Abbott.
Contributor Information
Domenico D’Amario, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Giuseppe Ciliberti, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Attilio Restivo, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Renzo Laborante, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Stefano Migliaro, Unità di Cardiologia, Aurelia Hospital, Rome 00165, Italy.
Francesco Canonico, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Giuseppe Massimo Sangiorgi, Department of Biomedicine and Prevention, Institute of Cardiology, University of Rome Tor Vergata, Rome 00133, Italy.
Matteo Tebaldi, Cardiologic Center, S. Anna University Hospital, Ferrara 44124, Italy.
Italo Porto, Department of Internal Medicine, University of Genova, Genova 16132, Italy.
Daniele Andreini, Centro Cardiologico Monzino IRCCS, Milan 20138, Italy.
Rocco Vergallo, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Antonio Maria Leone, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Salvatore Gervasi, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Sports Medicine Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Michela Cammarano, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Sports Medicine Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Vincenzo Palmieri, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Sports Medicine Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Francesco Burzotta, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Carlo Trani, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Paolo Zeppilli, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Sports Medicine Unit, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy.
Filippo Crea, Department of Cardiovascular and Thoracic Sciences, Fondazione Policlinico Universitario Agostino Gemelli IRCCS, Rome 00168, Italy; Catholic University of the Sacred Heart, Rome 00168, Italy.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
- 1. Alegria JR, Herrmann J, Holmes DR jr, Lerman A, Rihal CS. Myocardial bridging. Eur Heart J 2005;26:1159–1168. [DOI] [PubMed] [Google Scholar]
- 2. Sternheim D, Power DA, Samtani R, Kini A, Fuster V, Sharma S. Myocardial bridging: diagnosis, functional assessment, and management: JACC state-of-the-art review. J Am Coll Cardiol 2021;78:2196–2212. [DOI] [PubMed] [Google Scholar]
- 3. Möhlenkamp S, Hort W, Ge J, Erbel R. Update on myocardial bridging. Circulation 2002;106:2616–2622. [DOI] [PubMed] [Google Scholar]
- 4. Hayashi T, Ishikawa K. Myocardial bridge: harmless or harmful. Intern Med 2004;43:1097–1098. [DOI] [PubMed] [Google Scholar]
- 5. Tarantini G, Migliore F, Cademartiri F, Fraccaro C, Illiceto S. Left anterior descending artery myocardial bridging: a clinical approach. J Am Coll Cardiol 2016;68:2887–2899. [DOI] [PubMed] [Google Scholar]
- 6. Corban MT, Hung OY, Eshtehardi P, Rasoul-Arzrumly E, McDaniel M, Mekonnen G, Timmins LH, Lutz J, Guyton RA, Samady H. Myocardial bridging: contemporary understanding of pathophysiology with implications for diagnostic and therapeutic strategies. J Am Coll Cardiol 2014;63:2346–2355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kunadian V, Chieffo A, Camici PG, Berry C, Escaned J, Maas AHEM, Prescott E, Karam N, Appelman Y, Fraccaro C, Buchanan GL, Manzo-Silberman S, Al-Lamee R, Regar E, Lansky A, Abbott JD, Badimon L, Duncker DJ, Mehran R, Capodanno D, Baumbach A. An EAPCI expert consensus document on ischaemia with non-obstructive coronary arteries in collaboration with European society of cardiology working group on coronary pathophysiology & microcirculation endorsed by coronary vasomotor disorders international study group. Eur Heart J 2020;41:3504–3520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Yamada R, Tremmel JA, Tanaka S, Lin S, Kobayashi Y, Hollak MB, Yock PG, Fitzgerald PJ, Schnittger I, Honda Y. Functional versus anatomic assessment of myocardial bridging by intravascular ultrasound: impact of arterial compression on proximal atherosclerotic plaque. J Am Heart Assoc 2016;5:e001735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Okamura A, Okura H, Iwai S, Kyodo A, Kamon D, Hashimoto Y, Ueda T, Soeda T, Watanabe M, Saito Y. Detection of myocardial bridge by optical coherence tomography. Int J Cardiovasc Imaging 2022. Online ahead of print. [DOI] [PubMed] [Google Scholar]
- 10. Ye Z, Lai Y, Yao Y, Mintz GS, Liu X. Optical coherence tomography and intravascular ultrasound assessment of the anatomic size and wall thickness of a muscle bridge segment. Catheter Cardiovasc Interv 2019;93:772–778. [DOI] [PubMed] [Google Scholar]
- 11. Corban MT, Hung OY, Timmins LH, Samady H. Myocardial bridging. J Am Coll Cardiol 2014;64:2179–2181. [DOI] [PubMed] [Google Scholar]
- 12. Borovac JA, D'Amario D, Vergallo R, Porto I, Bisignani A, Galli M, Annibali G, Montone RA, Leone AM, Niccoli G, Crea F. Neoatherosclerosis after drug-eluting stent implantation: a novel clinical and therapeutic challenge. Eur Heart J Cardiovasc Pharmacother 2019;5:105–116. [DOI] [PubMed] [Google Scholar]
- 13. Tarantini G, Barioli A, Nai Fovino L, Fraccaro C, Masiero G, Iliceto S, Napodano M. Unmasking myocardial bridge-related ischemia by intracoronary functional evaluation. Circ Cardiovasc Interv 2018;11:e006247. [DOI] [PubMed] [Google Scholar]
- 14. Nam P, Choi BG, Choi SY, Byun JK, Mashaly A, Park Y, Jang WY, Kim W, Choi JY, Park EJ, Na JO, Choi CU, Lim HE, Kim EJ, Park CG, Seo HS, Oh DJ, Rha S-W. The impact of myocardial bridge on coronary artery spasm and long-term clinical outcomes in patients without significant atherosclerotic stenosis. Atherosclerosis 2018;270:8–12. [DOI] [PubMed] [Google Scholar]
- 15. Kim JW, Park CG, Suh SY, Choi CU, Kim EJ, Rha S-W, Seo HS, Oh DJ. Comparison of frequency of coronary spasm in Korean patients with versus without myocardial bridging. Am J Cardiol 2007;100:1083–1086. [DOI] [PubMed] [Google Scholar]
- 16. Teragawa H, Fukuda Y, Matsuda K, Hirao H, Higashi Y, Yamagata T, Oshima T, Matsuura H, Chayama K. Myocardial bridging increases the risk of coronary spasm. Clin Cardiol 2003;26:377–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Montone RA, Gurgoglione FL, Del Buono MG, Rinaldi R, Meucci MC, Iannaccone G, La Vecchia G, Camilli M, D'Amario D, Leone AM, Vergallo R, Aurigemma C, Buffon A, Romagnoli E, Burzotta F, Trani C, Crea F, Niccoli G. Interplay between myocardial bridging and coronary spasm in patients with myocardial ischemia and non-obstructive coronary arteries: pathogenic and prognostic implications. J Am Heart Assoc 2021;10:e020535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sara JDS, Corban MT, Prasad M, Prasad A, Gulati R, Lerman LO, Lerman A. Prevalence of myocardial bridging associated with coronary endothelial dysfunction in patients with chest pain and non-obstructive coronary artery disease. EuroIntervention 2020;15:1262–1268. [DOI] [PubMed] [Google Scholar]
- 19. Pargaonkar VS, Kimura T, Kameda R, Tanaka S, Yamada R, Schwartz JG, Perl L, Rogers IS, Honda Y, Fitzgerald P, Schnittger I, Tremmel JA. Invasive assessment of myocardial bridging in patients with angina and no obstructive coronary artery disease. EuroIntervention 2021;16:1070–1078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. D’Amario D, Migliaro S, Borovac JA, Restivo A, Vergallo R, Galli M, Leone AM, Montone RA, Niccoli G, Aspromonte N, Crea F. Microvascular dysfunction in heart failure with preserved ejection fraction. Front Physiol 2019;10:1347. 10.3389/fphys.2019.01347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Beltrame JF, Crea F, Kaski JC, Ogawa H, Ogawa H, Ong P, Sechtem U, Shimokawa H, Bairey Merz CN. Coronary vasomotion disorders international study group (COVADIS). international standardization of diagnostic criteria for vasospastic angina. Eur Heart J 2017;38:2565–2568. [DOI] [PubMed] [Google Scholar]
- 22. Ang DTY, Berry C. What an interventionalist needs to know about INOCA. Interv Cardiol 2021;16:e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Elgendy IY, Conti CR, Bavry AA. Fractional flow reserve: an updated review. Clin Cardiol 2014; 37:371–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Neumann FJ, Sousa-Uva M, Ahlsson A, Alfonso F, Banning AP, Benedetto U, Byrne RA, Collet JP, Falk V, Head SJ, Jüni P, Kastrati A, Koller A, Kristensen SD, Niebauer J, Richter DJ, Seferovic PM, Sibbingc D, Stefanini GG, Windecker S, Yadav R, Zembala MO and ESC Scientific Document Group . 2018 ESC/EACTS guidelines on myocardial revascularization. Eur Heart J 2019;40:87–165. [DOI] [PubMed] [Google Scholar]
- 25. Gould KL, Johnson NP. Myocardial bridges: lessons in clinical coronary pathophysiology. JACC Cardiovasc Imaging 2015;8:705–709. [DOI] [PubMed] [Google Scholar]
- 26. Davies JE, Sen S, Dehbi HM, Al-Lamee R, Petraco R, Nijjer SS, Bhindi R, Lehman SJ, Walters D, Sapontis J, Janssens L, Vrints CJ, Khashaba A, Laine M, Van Belle E, Krackhardt F, Bojara W, Going O, Härle T, Indolfi C, Niccoli G, Ribichini F, Tanaka N, Yokoi H, Takashima H, Kikuta Y, Erglis A, Vinhas H, Canas Silva P, Baptista SB, Alghamdi A, Hellig F, Koo BK, Nam CW, Shin ES, Doh JH, Brugaletta S, Alegria-Barrero E, Meuwissen M, Piek JJ, van Royen N, Sezer M, Di Mario C, Gerber RT, Malik IS, Sharp ASP, Talwar S, Tang K, Samady H, Altman J, Seto AH, Singh J, Jeremias A, Matsuo H, Kharbanda RK, Patel MR, Serruys P, Escaned J. Use of the instantaneous wave-free ratio or fractional flow reserve in PCI. N Engl J Med 2017;376:1824–1834. [DOI] [PubMed] [Google Scholar]
- 27. Pourhoseini S, Bakhtiari M, Babaee A, Ostovan MA, Eftekhar-Vaghefi SH, Ostovan N, Dehghani P. Increased risk of coronary perforation during percutaneous intervention of myocardial bridge: what histopathology says. J Cardiovasc Thorac Res 2017;9:108–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li W, Li Y, Sheng L, Gong Y. Myocardial bridge: is the risk of perforation increased? Can J Cardiol 2008;24:e80–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jiang Q, Liang C, Wu Z. Myocardial bridging is a potential risk factor of very late stent thrombosis of drug eluting stent. Med Sci Monit 2012;18:HY9–H12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.