Abstract
Extracellular vesicles (EVs) represent a valuable tool in liquid biopsy with tremendous clinical potential in diagnosis, prognosis, and therapeutic monitoring of gliomas. Compared to tissue biopsy, EV-based liquid biopsy is a low-cost, minimally invasive method that can provide information on tumor dynamics before, during, and after treatment. Tumor-derived EVs circulating in biofluids carry a complex cargo of molecular biomarkers, including DNA, RNA, and proteins, which can be indicative of tumor growth and progression. Here, we briefly review current commercial and noncommercial methods for the isolation, quantification, and biochemical characterization of plasma EVs from patients with glioma, touching on whole EV analysis, mutation detection techniques, and genomic and proteomic profiling. We review notable advantages and disadvantages of plasma EV isolation and analytical methods, and we conclude with a discussion on clinical translational opportunities and key challenges associated with the future implementation of EV-based liquid biopsy for glioma treatment.
Keywords: extracellular vesicles, glioma, liquid biopsy, plasma
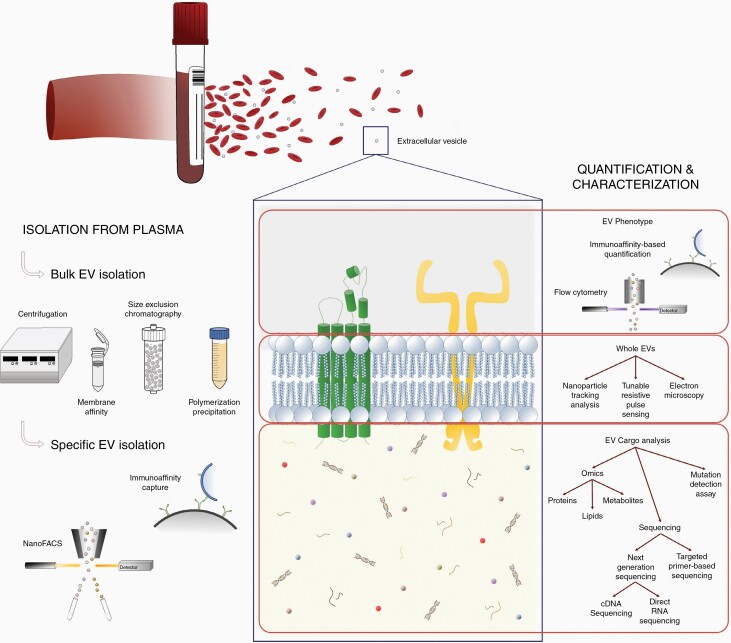
Extracellular vesicles (EVs) are small, membrane-bound nanoparticles released into the extracellular environment via cell shedding and non-apoptotic blebbing.1 EVs can be broadly divided into 3 subtypes by size: exosomes (30-100 nm), microvesicles (50-1000 nm), and oncosomes (1-10 µm)2–4 and further classified by their cargo.5 The biogenesis of EVs results in the encapsulation of DNA, RNA, proteins, and cytosolic compounds within the lipid bilayer, all of which maintain the native configuration from the cell of origin and thus can describe both the physicochemical and biochemical properties of the cell.5 The biological functions of EVs depend on the source cells. However, these membrane-bound nanoparticles play an essential role in cell-to-cell communication via delivering proteins, nucleic acids, and metabolites. In addition, EVs have been shown to regulate a number of cellular processes, including proliferation, apoptosis, and autophagy. Given their membrane-bound nature, EVs remain relatively stable in circulation, increasing the average lifespan of the encapsulated material. There is substantial evidence of glioma pathology represented in EVs circulating in biofluids like cerebrospinal fluid (CSF) and blood, highlighting their potential in liquid biopsy.6,7 Other analytes that can be isolated from biofluids include circulating tumor cells (CTCs), cell-free DNA, and circulating nucleic acids. However, the concentration of EVs isolated from patient biofluid is significantly higher and more likely to be stable in circulation. For instance, CTC isolation requires a starting input of a large volume of fresh blood followed by immediate processing due to rapid deterioration of cell viability. Plasma is a preferred sample type for EV biomarker discovery, given that blood collection is minimally invasive, compared to CSF collection, and unlike serum, plasma does not contain coagulation factors, which potentially confound glioma-specific EV isolation and analysis.8 Here, we review prevalent EV isolation, quantification, and characterization strategies with a focus on plasma-derived EVs from patients with glioma (Figure 1).
Figure 1.
Summary of the main methods for isolation and analysis of glioma-derived extracellular vesicles circulating in patient plasma.
Isolation and Analysis of Whole EVs
The current standards of EV isolation include bulk and specific isolation. Bulk isolation consists of centrifugation, membrane affinity, size exclusion, and polymerization precipitation strategies. Although successful at eliminating debris during isolation, they limit glioma-specific EV enrichment due to the heterogeneous healthy cell-derived EVs in plasma. It is crucial to differentiate tumor-specific EVs from this background. Specific EV isolation methods, such as immunoaffinity (IA) capture and nano-fluorescence activated cell sorting (nanoFACS) harness the EV phenotype to enrich glioma EVs. IA capture isolates EVs based on the expression of target surface proteins, such as EGFRvIII, which bind to antibodies conjugated to magnetic beads or surfaces.9 This in turn allows for isolation of glioma-derived EV subpopulations from plasma and downstream analysis for tumor-specific EV profiling.10 NanoFACS relies on target protein or cargo expression for EV isolation.11 Unlike IA capture, EVs remain in a single EV suspension for downstream analysis.12
Quantification and characterization of whole EVs are broadly categorized into total EV and EV subpopulation studies (Table 1). Analysis of total intact EVs rests on the delineation of total EV population size and concentration via techniques, such as nanoparticle tracking analysis (NTA) and tunable resistive pulse sensing (TRPS). These methods quantify particles based on the Brownian motion of nanoparticles in solution. As such, they are useful for detecting total EV populations. However, proteins and debris of similar sizes may also be detected, producing a nonspecific signal. More specific exploration of EV topographical information (size, shape, morphology) can be performed using transmission electron microscopy (TEM) and scanning electron microscopy (SEM). Analysis of EV subpopulations and single EV events, however, can be achieved using fluorescent labeling of EVs and measured using imaging flow cytometry (IFC) and fluorescence flow cytometry (FFC), as well as IA capture. Unlike total EV analysis, IFC and FFC analyze single events to determine phenotypic prevalence within single EVs and total concentration within the entire EV population.
Table 1.
Analysis of Intact Extracellular Vesicles (EVs) From Plasma of Patients With Glioma
| Biomarker | Method | Study |
|---|---|---|
| EV count, Annexin V | Cryo-electron microscopy (CM) Flow cytometry (FCM) |
Evans et al13 |
| EV count, Annexin V | FCM Electron microscopy (EM) |
Koch et al14 |
| EV count and characterization | Nanoparticle tracking analysis (NTA) | Cumba Garcia et al15 |
| EV count and characterization | NTA Confocal laser scanning microscopy (CLSM) |
Osti et al16 |
| EV count and size | NTA Transmission electron microscopy (TEM) |
Akers et al17 |
| EV size and count, CD63, CD81, CD9 | NTA TEM Imaging flow cytometry (IFCM) Correlative light electron microscopy (CLEM) |
Ricklefs et al18 |
| EGFR, EGFRvIII, PDGFR, PDPN, EphA2 and IDH1, R132H | Size and immunoaffinity Microfluidic nuclear magnetic resonance (µNMR) assay |
Shao et al19 |
| Total protein quantification | FCM TEM |
Muller et al20 |
Enrichment, in both isolation and analysis, of glioma-specific EV subpopulations relies primarily on EV labeling. Labeling using fluorescent markers indicative of intact biological environments (ie, CFDA-SE) has been reported to quantify total EV populations in FC applications. Further analytical depth can be achieved via fluorescent conjugated antibodies for surface protein marker labeling, such as CD63 and CD9, for phenotypic characterization of glioma-derived EVs.18 Similarly, exploration of the cancer-specific EV landscape can be performed via labeling of glioma-related mutations, such as IDH1, EGFRvIII,21 and survivin.22 Although promising, this method of identification is burdened by antibody labeling standardization and processing. Recent studies have explored the use of endogenous fluorophores for the labeling of glioma-produced EVs. Utilizing the downstream features of 5-aminolevulinic acid (5-ALA, a photosensitizer used in fluorescence-guided surgery) metabolism, a subpopulation of EVs produced by 5-ALA-dosed malignant glioma tumors have demonstrated endogenous fluorescence via IFC.23 These fluorescent EVs have successfully been sorted from plasma background.24 The quantification of fluorescence intensity, EV concentration, and EV cargo from plasma provides a venue for EV-based glioma biomarker development.
Phenotype-based EV isolation and quantification methods are considered low throughput, but these modalities provide the highest specificity for glioma EV isolation and enrichment from a heterogeneous background for enhanced downstream analysis.
Isolation and Analysis of EV Cargo
Once glioma-specific EVs are isolated and purified, downstream detection and functional characterization of cargo are achieved by a careful selection of the relevant method. The EV cargo is composed of multiple proteins (tumor-specific antigens, heat shock, transport, and immunogenic proteins) and cytosolic analytes, including nucleic acids (mRNA, lncRNA, microRNAs, and DNA), lipids, and metabolites.
EV Nucleic Acid Detection
Extraction of the encapsulated nucleic acids (RNA, DNA) can be performed using a number of commercially available kits leading to a variation in the yield and size distribution. Of the available techniques, ExoRNeasy (Qiagen) represents the most efficient method of EV RNA extraction from a range of volume of patient plasma with minimal binding to ex-RNA-containing particles like ribonucleoprotein complexes.25 It is the most widely used platform for the extraction of purified EV RNA for downstream mutation detection and genomic interrogation. Inclusion of a standard extraction protocol improves reproducibility and design of large-scale validation studies (intra- and inter-institutional).
Polymerase chain reaction (PCR) methods represent one of the earliest methods of targeted mutation detection using patient-derived plasma. Both real-time and digital PCR methods rely on dye-based fluorescent quantification of cDNA and/or EV DNA via reagents such as the, most commonly using SYBR Green or TaqMan, of cDNA and/or EV DNA. Droplet digital PCR (ddPCR), a more recent platform employs an ultrasensitive fluorescent technique, which has enabled absolute quantification of mutant events in partitioned samples (>10,000 droplets) based on Poisson’s distribution. This approach has several advantages compared to qPCR: measurement of low abundance transcripts, tolerance to PCR inhibitors, less dependence on reference genes, higher signal-to-noise ratio, and higher sensitivity, thereby making digital bioassays a more reliable tool for detection of rare glioma-specific mutations.
IDH1 mutation is a key molecular alteration in gliomas and a noninvasive diagnosis via plasma-based assays will have many clinical applications. The mutant and wild-type IDH1 sequences in extracted EV DNA have been previously detected using the PCR platform.26 Similarly, ddPCR has been used successfully to detect TERT promoter mutations in EV DNA with a sensitivity of >70%.27 EGFRvIII is another important mutation that serves as a reliable diagnostic marker to distinguish glioma from healthy states.28 Studies have reported assays to detect this mutation in EV-derived mRNA from plasma (Table 2). However, the reported sensitivity and specificity have limited its translation in clinical settings. Overall, detecting these mutations in plasma has allowed disease monitoring, surveillance, and tailored treatment approaches. Different miRNA signatures characteristic of glioma have been proposed to allow disease stratification and monitoring of tumor burden over clinical course.
Table 2.
PCR and Sequencing Studies on DNA and RNA Biomarkers From Plasma-Derived Extracellular Vesicles (EVs) in Patients With Glioma
| Biomarker | EV Cargo Analyte | Method | Study |
|---|---|---|---|
| IDH1G395A | DNA |
Conventional PCR Fast COLD-PCR |
García-Romero et al26 |
| TERT promoter | DNA | Droplet digital PCR (ddPCR) | Muralidharan et al27 |
| PD-L1 | DNA | ddPCR | Ricklefs et al29 |
| EGFRwt, EGFRvIII | mRNA | Semi-nested PCR | Manda et al30 |
| EGFRvIII | mRNA |
Herringbone microfluidic device (EVHB-chip) ddPCR |
Reátegui et al9 |
| 24 immune response and glioma progression-related genes (TIMP-1, IL-8, TGF-β, PD-1, etc.) | mRNA | Real-time quantitative reverse transcription PCR (qRT-PCR) | Muller et al20 |
| miR-21, miR-103, miR-24, and miR-125 | microRNA | qRT-PCR | Akers et al17 |
| miR-210, miR-185, miR-5194, and miR-449 | microRNA | qRT-PCR | Tabibkhooei et al31 |
| 54 GBM-specific differentially expressed genes | RNA |
Nextera XT kit (Illumina) HiSeq 2000 |
Reátegui et al9 |
| CD9, CD63, and CD81 | RNA | Quantitative PCR (qPCR) | Ricklefs et al18 |
Genetic profiling of exosomes has elucidated details on the genomic and epigenetic landscape of gliomas. The presence of certain RNA populations, namely mRNAs, miRNAs, and lncRNAs, has been demonstrated using PCR methods. Recently, however, high-throughput transcriptome analysis of EVs has led to the discovery of diverse RNA species, including snRNA, snoRNA, piRNA, scRNA, and SRP-RNA, and their role in mediating the biological effects of EVs on recipient cells.32 However, most of these studies report results based on next-generation sequencing (NGS) of serum and not plasma.33 Currently, plasma is the preferred medium for the isolation and analysis of tumor-specific analytes despite the risk of clotting at room temperature, which increases the risk of EV lysis and degradation.34
Unlike conventional PCR, NGS allows detection and monitoring of both known and unknown molecular alterations.35 The majority of library preparation protocols, however, do contain a PCR amplification step with specific primers to improve the sensitivity of detection and quantification of low-level EV analytes. Additionally, library preparation kits have been tailored for size selection of short vs long RNA fragments.25 Medium length (60-300 nt) RNA sequencing requires the use of kit-free protocols. Common sources of bias in sequencing include adaptor dimers in ligation technique, size selection after cDNA synthesis, choice of sequencing platform, and subsequent bioinformatics analysis.25 Validation of obtained results using additional methods (PCR, Western blot, etc.) can reduce bias and improve robustness.
Given the availability of novel high-throughput methods, it is important to explore the potential use of these technologies for plasma-derived EV cargo transcriptomic and genomic interrogation. Compared to cDNA sequencing, direct RNA sequencing (Oxford Nanopore Technologies [ONT]) offers many opportunities: low input requirement (<1 µg), elimination of PCR bias, detection of ultra-long RNA fragments, and identification of isoforms, gene fusions, and novel transcripts.36 It can also elucidate the role of RNA modifications in gliomagenesis and their interactions with key molecular alterations (IDH, PTEN, MGMT, TERT). For instance, m6A methylation can now be detected and mapped using MeRIP-seq, a technique that combines NGS with m6A-methylated RNA immunoprecipitation.37
EV cargo represents a complex composition of RNA populations of varying lengths. However, a comprehensive overview of different classes of RNA encapsulated in EVs and their functional significance is still lacking. Due to the enrichment of small RNAs in EVs, most studies have focused on miRNA and small non-coding RNA. While most of the RNA is fragmented, little is known about the longer fragments present and their role in glioma progression. This population has not been previously explored due to the inherent limitations of conventional library preparation kits, namely low-depth coverage and low precision of sequencing. ONT, therefore, represents a promising platform for a more comprehensive analysis of coding, non-coding, and regulatory RNA populations in plasma-derived EVs.
EV “Omics” Profiling
Protein components of EVs have been cataloged using a number of mass spectrometry-based modalities (Table 3). It is crucial to consider the influence of isolation protocol and physicochemical properties on proteome content. Western blotting is an immunodetection technique based on affinity binding of a primary and fluorescently labeled secondary antibody to a specific surface antigen in lysed EVs.38 Using this, a study measured the expression of tropomyosin kinase receptor (TrkB) in plasma-derived exosomes and its correlation to aggressiveness and gliomagenesis.39 Furthermore, key cytokines (IL-8, IL-10, IFN-γ) have been similarly identified and shown to be dysregulated in gliomas.15 Some limitations of this method include inability to multiplex, requirement of a large input of EV protein, and limited reproducibility.40
Table 3.
Proteomics-Based Analysis of Biomarkers From Plasma-Derived Extracellular Vesicles in Patients With Glioma
| Biomarker | Method | Study |
|---|---|---|
| Syndecan-1 (SDC1), 12 proteins differed in HGG vs LGG |
Liquid chromatography-mass spectrometry (LC-MS)) Ultrasensitive proximity extension immunoassay (PEA) Enzymelinked immunosorbent assay (ELISA) |
Chandran et al41 |
| Tropomyosin kinase receptor (TrkB) | Western blot | Pinet et al39 |
| GFAP, TAU | Dielectrophoresis(DEP) Immunofluorescence staining (IF) | Lewis et al42 |
| INFγ, IL-10, IL-13, CD80, CD86, B7-1, B7-2, flotillin-1, ICOSL |
Cytokine and checkpoint molecules arrays Western blot ELISA |
Cumba Garcia et al15 |
| 11 differentially expressed proteins |
Sequential window acquisition of all theoretical fragment ion spectra mass spectrometry (SWATH-MS) LC-MS |
Hallal et al43 |
| von Willebrand factor (VWF) | LC-MS | Sabbagh et al44 |
| VWF, APCS, C4B, AMBP, APOD, AZGP1, C4BPB, Serpin3, FTL, C3, and APOE | Liquid chromatography-electrospray ionization-mass spectrometry (LC-ESI-MS) | Osti et al16 |
| Fatty acid synthase (FASN) |
Western blot Imaging flow cytometry (IFCM) |
Ricklefs et al45 |
Another approach utilizes integrated immuno-based microfluidic isolation and protein analysis. Microfluidics devices allow fluorescent antibody-based detection of EVs on a chip rather than on a membrane or magnetic beads,46 allowing for isolation of a broader spectrum of EV antigens and potential biomarkers.47
Mass spectrometry (MS) analysis can be used for global and/or targeted proteomics. The general principle involves digestion of extracted proteins followed by the separation of peptides using gel-based (1D/2D gel electrophoresis) or gel-free platform (liquid chromatography). We can therefore deduce quantity and sequence details. Global (discovery-driven) proteomic approach achieves ionic selection either based on prevalence (data-dependent acquisition [DDA]) or predefined mass range (data-independent acquisition [DIA]).48 Targeted (hypothesis-driven) proteomic analysis is mostly conducted using multiple reaction monitoring (MRM), which allows parallel monitoring of up to a hundred predetermined peptides at different retention times.48 This approach has several advantages: improved sensitivity and specificity, ability to multiplex, and low input requirement.49 The existing studies have highlighted a few candidate proteins, however, a more extensive correlation study between exosome protein levels and glioma cell of origin is needed to delineate disease specific from exosome-enriched proteins.50 To fully harness the clinical potential, we need candidate markers to differentiate between low-grade and high-grade glioma. Syndecan-1 (SDC1), an exosome protein, represents an important example in this application, with the mRNA expression levels measured by MS and enzyme-linked immunosorbent assay (ELISA) were shown to be significantly different in GBM (glioblastoma) vs low-grade glioma cohort.41
No studies have investigated plasma EV-derived lipids or metabolites as putative biomarkers in glioma.
Future Directions
EV-based liquid biopsy has tremendous clinical potential in establishing a minimally invasive and cost-effective platform for characterizing tumors using circulating analytes. However, despite the significant progress in this field, it is yet not recognized as a standard of clinical care. There are a number of factors to consider including the presence of technical and biological variability in preanalytical and analytical stages as outlined in Table 4. The development of multi-analyte tests will further improve the feasibility of using this platform to decipher the tumor genotypic and phenotypic landscape. Lastly, the collaboration between the public and private sectors is essential to improve standardization and reproducibility. With considerable clinical implications, it can be successfully employed as a rapid, reliable, noninvasive clinical decision making tool.
Table 4.
Challenges of Extracellular Vesicle (EV) Isolation and Analysis and Proposed Recommendations
| Method | Challenges | Recommendations |
|---|---|---|
| Isolation of EV cargo | Poor consistency among studies and highly variable isolation protocols Lack of predetermined handling and storage conditions Purification of EV preparations Consideration of the confounding patient-related and environmental variables |
Use of optimized and standardized protocols Standard storage protocols Inclusion of strategies to remove potential contaminants (use of RNase, DNase, proteinase treatment) Careful selection of patient population and controls to minimize the influence of external variables (eg, age-dependent clonal heterogeneity) |
| Mutation detection (PCR) | Low input analyte Limited reproducibility Choice of blood component Low sensitivity and specificity |
Use of ultrasensitive modalities with a lower mutant allele frequency (MAF) detection Large-scale validation studies (intra- and inter-institutional collaboration) More consistent and frequent use of plasma (vs serum) Methods to remove heterogeneous background and reduce the signal-to-noise ratio |
| Sequencing | Size selection bias (eg underrepresentation of medium size RNA) GC content bias Adapter dimers in ligation-based library preparation kits Lack of reproducible and standard bioinformatics pipelines Variability secondary to use of different sequencing platforms Limited reproducibility and clinical translation of findings (novel biomarkers) |
Serial extractions of different-sized populations from the same patient sample. Careful selection of purification kit based on the population of interest. Comparison of different extraction protocols Modification of the kits to reduce ligation bias Use of approved and standard databases for mapping to reduce variability, reliable statistical tools, consistent normalization methods, inclusion of reference genes Use of identical sequencing technologies for accurate inter-study comparisons Validation using reliable techniques (PCR, Western blot, etc.) |
| Proteomics | Variability in isolated EV populations Limited proteome sequence coverage Paucity of information on glioma-specific protein mutations and protein-protein interactions Delineation of glioma-specific EV proteins from non-tumor markers |
Minimize confounding variables (patient-related) and tailor the isolation protocol Use of high-throughput and accurate MS-based methods for a limited quantity of isolated proteins Proteogenomics; integrated approach incorporating targeted proteomics and RNA-seq data Development of sensitive and robust targeted proteomics in combination with downstream validation studies for functional characterization |
Contributor Information
Syeda Maheen Batool, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Tiffaney Hsia, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Sirena K Khanna, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Austin S Gamblin, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Yulia Rosenfeld, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Dong Gil You, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA.
Bob S Carter, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA; Department of Neurosurgery, Harvard Medical School, Boston, Massachusetts, USA.
Leonora Balaj, Department of Neurosurgery, Massachusetts General Hospital, Boston, Massachusetts, USA; Department of Neurosurgery, Harvard Medical School, Boston, Massachusetts, USA.
Funding
This work is supported by grants U01 CA230697 (to B.S.C., L.B.), P01 CA069246 (to B.S.C.), and R01 CA239078, CA237500 (to B.S.C., L.B.). The funding sources had no role in the writing of the manuscript or decision to submit the manuscript for publication. The authors have not been paid to write this article by any entity. The corresponding author has full access and assumes final responsibility for the decision to submit for publication.
Conflict of interest statement. None of the authors report any conflict of interest.
References
- 1. Tricarico C, Clancy J, D’Souza-Schorey C. Biology and biogenesis of shed microvesicles. Small GTPases. 2017;8:220–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cocucci E, Meldolesi J. Ectosomes and exosomes: shedding the confusion between extracellular vesicles. Trends Cell Biol. 2015;25:364–372. [DOI] [PubMed] [Google Scholar]
- 3. Ståhl A-L, Johansson K, Mossberg M, et al. . Exosomes and microvesicles in normal physiology, pathophysiology, and renal diseases. Pediatr Nephrol. 2019;34:11–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Minciacchi VR, You S, Spinelli C, et al. . Large oncosomes contain distinct protein cargo and represent a separate functional class of tumor-derived extracellular vesicles. Oncotarget. 2015;6:11327–11341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Jones J, Nguyen H, Drummond K, et al. . Circulating biomarkers for glioma: a review. Neurosurgery. 2021;88:E221–E230. [DOI] [PubMed] [Google Scholar]
- 6. Andaloussi SEL, Mäger I, Breakefield XO, et al. . Extracellular vesicles: biology and emerging therapeutic opportunities. Nat Rev Drug Discov. 2013;12:347–357. [DOI] [PubMed] [Google Scholar]
- 7. Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med. 2013;91:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Del Bene M, Osti D, Faletti S, et al. . Extracellular vesicles: the key for precision medicine in glioblastoma. Neuro Oncol. 2021;24:184–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Reátegui E, van der Vos KE, Lai CP, et al. . Engineered nanointerfaces for microfluidic isolation and molecular profiling of tumor-specific extracellular vesicles. Nat Commun. 2018;9:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jeong S, Park J, Pathania D, et al. . Integrated magneto-electrochemical sensor for exosome analysis. ACS Nano. 2016;10:1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morales-Kastresana A, Telford B, Musich TA, et al. . Labeling extracellular vesicles for nanoscale flow cytometry. Sci Rep. 2017;7:1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Jones JC, Morales-Kastresana A, Kesarwala AH, et al. . NanoFACS: extracellular vesicle subset sorting for personalized medicine and biodosimetry. Int J Radiat Oncol Biol Phys. 2016;96:S53–S54. [Google Scholar]
- 13. Evans SM, Putt M, Yang XY, et al. . Initial evidence that blood-borne microvesicles are biomarkers for recurrence and survival in newly diagnosed glioblastoma patients. J Neurooncol. 2016;127:391–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Koch CJ, Lustig RA, Yang XY, et al. . Microvesicles as a biomarker for tumor progression versus treatment effect in radiation/temozolomide-treated glioblastoma patients. Transl Oncol. 2014;7:752–758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cumba Garcia LM, Peterson TE, Cepeda MA, et al. . Isolation and analysis of plasma-derived exosomes in patients with glioma. Front Oncol. 2019;9:651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osti D, Del Bene M, Rappa G, et al. . Clinical significance of extracellular vesicles in plasma from glioblastoma patients. Clin Cancer Res. 2019;25:266–276. [DOI] [PubMed] [Google Scholar]
- 17. Akers JC, Ramakrishnan V, Kim R, et al. . miRNA contents of cerebrospinal fluid extracellular vesicles in glioblastoma patients. J Neurooncol. 2015;123:205–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ricklefs FL, Maire CL, Reimer R, et al. . Imaging flow cytometry facilitates multiparametric characterization of extracellular vesicles in malignant brain tumours. J Extracell Vesicles. 2019;8:1588555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Shao H, Chung J, Balaj L, et al. . Protein typing of circulating microvesicles allows real-time monitoring of glioblastoma therapy. Nat Med. 2012;18:1835–1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Muller L, Muller-Haegele S, Mitsuhashi M, et al. . Exosomes isolated from plasma of glioma patients enrolled in a vaccination trial reflect antitumor immune activity and might predict survival. Oncoimmunology. 2015;4:e1008347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Fraser K, Jo A, Giedt J, et al. . Characterization of single microvesicles in plasma from glioblastoma patients. Neuro Oncol. 2019;21:606–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galbo PM Jr, Ciesielski MJ, Figel S, et al. . Circulating CD9+/GFAP+/survivin+ exosomes in malignant glioma patients following survivin vaccination. Oncotarget. 2017;8:114722–114735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Jones PS, Yekula A, Lansbury E, et al. . Characterization of plasma-derived protoporphyrin-IX-positive extracellular vesicles following 5-ALA use in patients with malignant glioma. EBioMedicine. 2019;48:23–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Maas SLN, van Solinge TS, Schnoor R, et al. . Orally administered 5-aminolevulinic acid for isolation and characterization of circulating tumor-derived extracellular vesicles in glioblastoma patients. Cancers. 2020;12:3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Mateescu B, Kowal EJK, van Balkom BWM, et al. . Obstacles and opportunities in the functional analysis of extracellular vesicle RNA—an ISEV position paper. J Extracell Vesicles. 2017;6:1286095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. García-Romero N, Carrión-Navarro J, Esteban-Rubio S, et al. . DNA sequences within glioma-derived extracellular vesicles can cross the intact blood-brain barrier and be detected in peripheral blood of patients. Oncotarget. 2017;8:1416–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Muralidharan K, Yekula A, Small JL, et al. . Promoter mutation analysis for blood-based diagnosis and monitoring of gliomas. Clin Cancer Res. 2021;27:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wikstrand CJ, Reist CJ, Archer GE, et al. . The class III variant of the epidermal growth factor receptor (EGFRvIII): characterization and utilization as an immunotherapeutic target. J Neurovirol. 1998;4:148–158. [DOI] [PubMed] [Google Scholar]
- 29. Ricklefs FL, Alayo Q, Krenzlin H, et al. . Immune evasion mediated by PD-L1 on glioblastoma-derived extracellular vesicles. Sci Adv. 2018;4:eaar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Manda SV, Kataria Y, Tatireddy BR, et al. . Exosomes as a biomarker platform for detecting epidermal growth factor receptor-positive high-grade gliomas. J Neurosurg. 2018;128:1091–1101. [DOI] [PubMed] [Google Scholar]
- 31. Tabibkhooei A, Izadpanahi M, Arab A, et al. . Profiling of novel circulating microRNAs as a non-invasive biomarker in diagnosis and follow-up of high and low-grade gliomas. Clin Neurol Neurosurg. 2020;190:105652. [DOI] [PubMed] [Google Scholar]
- 32. Turchinovich A, Drapkina O, Tonevitsky A. Transcriptome of extracellular vesicles: state-of-the-art. Front Immunol. 2019;10:202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ebrahimkhani S, Vafaee F, Hallal S, et al. . Deep sequencing of circulating exosomal microRNA allows non-invasive glioblastoma diagnosis. NPJ Precis Oncol. 2018;2:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pittella-Silva F, Chin YM, Chan HT, et al. . Plasma or serum: which is preferable for mutation detection in liquid biopsy? Clin Chem. 2020;66:946–957. [DOI] [PubMed] [Google Scholar]
- 35. Schwaederle M, Husain H, Fanta PT, et al. . Detection rate of actionable mutations in diverse cancers using a biopsy-free (blood) circulating tumor cell DNA assay. Oncotarget. 2016;7:9707–9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pyatnitskiy MA, Arzumanian VA, Radko SP, et al. . Oxford nanopore MinION direct RNA-seq for systems biology. Biology. 2021;10:1131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Meyer KD, Saletore Y, Zumbo P, et al. . Comprehensive analysis of mRNA methylation reveals enrichment in 3′ UTRs and near stop codons. Cell. 2012;149:1635–1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Gallagher S, Winston SE, Fuller SA, et al. . Immunoblotting and immunodetection. Curr Protoc Cell Biol. 2011;Chapter 6:Unit6.2. [DOI] [PubMed] [Google Scholar]
- 39. Pinet S, Bessette B, Vedrenne N, et al. . TrkB-containing exosomes promote the transfer of glioblastoma aggressiveness to YKL-40-inactivated glioblastoma cells. Oncotarget. 2016;7:50349–50364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Doyle LM, Wang MZ. Overview of extracellular vesicles, their origin, composition, purpose, and methods for exosome isolation and analysis. Cells. 2019;8:727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chandran VI, Welinder C, Månsson AS, et al. . Ultrasensitive immunoprofiling of plasma extracellular vesicles identifies syndecan-1 as a potential tool for minimally invasive diagnosis of glioma. Clin Cancer Res. 2019;25:3115–3127. [DOI] [PubMed] [Google Scholar]
- 42. Lewis J, Alattar AA, Akers J, et al. . A pilot proof-of-principle analysis demonstrating dielectrophoresis (DEP) as a glioblastoma biomarker platform. Sci Rep. 2019;9:10279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hallal S, Azimi A, Wei H, et al. . A comprehensive proteomic SWATH-MS workflow for profiling blood extracellular vesicles: a new avenue for glioma tumour surveillance. Int J Mol Sci. 2020;21:4754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Sabbagh Q, André-Grégoire G, Alves-Nicolau C, et al. . The von Willebrand factor stamps plasmatic extracellular vesicles from glioblastoma patients. Sci Rep. 2021;11:22792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Ricklefs FL, Maire Cl, Matschke J, et al. . FASN is a biomarker enriched in malignant glioma-derived extracellular vesicles. Int J Mol Sci. 2020;21:1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. He M, Crow J, Roth M, et al. . Integrated immunoisolation and protein analysis of circulating exosomes using microfluidic technology. Lab Chip. 2014;14:3773–3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Li G, Tang W, Yang F. Cancer liquid biopsy using integrated microfluidic exosome analysis platforms. Biotechnol J. 2020;15:e1900225. [DOI] [PubMed] [Google Scholar]
- 48. Schey KL, Luther JM, Rose KL. Proteomics characterization of exosome cargo. Methods. 2015;87:75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Kalra H, Adda CG, Liem M, et al. . Comparative proteomics evaluation of plasma exosome isolation techniques and assessment of the stability of exosomes in normal human blood plasma. Proteomics. 2013;13:3354–3364. [DOI] [PubMed] [Google Scholar]
- 50. Mallawaaratchy DM, Hallal S, Russell B, et al. . Comprehensive proteome profiling of glioblastoma-derived extracellular vesicles identifies markers for more aggressive disease. J Neurooncol. 2017;131:233–244. [DOI] [PMC free article] [PubMed] [Google Scholar]