Abstract
Background
We conducted double-blind, placebo-controlled trials assessing the efficacy and tolerability of favipiravir in acute influenza.
Methods
Otherwise healthy adults with influenza-like symptoms and fever of ≤48 hours were randomized to favipiravir (1800 mg twice daily [BID] on day 1, 800 mg BID on days 2–5) or placebo tablets (1:1 in US316; 3:1 in US317). The primary efficacy endpoint was the time to illness alleviation when 6 influenza symptoms were self-rated as absent or mild and fever was absent in the intention-to-treat, influenza-infected participants.
Results
In US316 (301 favipiravir, 322 placebo), favipiravir was associated with a 14.4-hour reduction (median, 84.2 vs 98.6 hours; P = .004) in time to illness alleviation vs placebo. In US317 (526 favipiravir, 169 placebo), favipiravir did not significantly reduce time to alleviation (median, 77.8 vs 83.9 hours). In both trials favipiravir was associated with reduced viral titers, RNA load area under the curve over days 1–5, and median times to cessation of virus detection (P < .001). Aside from asymptomatic hyperuricemia, no important differences in adverse events were found.
Conclusions
This favipiravir dosing regimen demonstrated significant antiviral efficacy but inconsistent illness alleviation in uncomplicated influenza. Studies of higher doses and antiviral combinations for treating serious influenza and other RNA viral infections are warranted.
Clinical Trials Registration. NCT02026349; NCT02008344.
Keywords: influenza, favipiravir, treatment, antiviral, pharmacokinetics
In two placebo-controlled, double-blind randomized trials, oral favipiravir was generally well-tolerated and demonstrated significant antiviral effects in treating uncomplicated influenza in outpatient adults but inconsistent reductions in the primary clinical endpoint of the time to illness alleviation.
The pyrazine derivative favipiravir (T705; Toyama Chemical Co, Japan) was first reported to inhibit influenza virus replication in vitro and in mice in 2002 [1]. Favipiravir is inhibitory for influenza A, B, and C viruses, including variants resistant to adamantanes or neuraminidase inhibitors [1–5], as well as at higher concentrations against many other RNA viruses [5, 6]. Once ribosylated and phosphorylated intracellularly, the triphosphate acts as a purine nucleoside analogue and functions as a competitive substrate inhibitor of the viral RNA-dependent RNA polymerase leading to chain termination [7]. Another mechanism of action is lethal mutagenesis related to an increased guanosine to adenine mutation frequency causing noninfectious progeny during replication [8].
Clinical studies of various dose regimens have been conducted primarily in adults with acute, uncomplicated influenza [9–11]. One randomized controlled trial (RCT) enrolling 271 influenza-infected participants found that a twice-daily dosing regimen (1800 mg BID on day 1 and 800 mg BID on days 2–5) gave better antiviral and clinical effects than a thrice-daily regimen (2400, 600, 600 mg on day 1 and 600 mg TID on days 2–5). The favipiravir 1800 mg/800 mg BID group also demonstrated significantly faster median time to alleviation of influenza symptoms (difference of 15.0 hours) and viral load reductions compared with the placebo group [9]. The drug was approved in Japan in 2014 for treatment of novel or reemerging influenza virus infections, unresponsive or insufficiently responsive to approved agents, but remains investigational for influenza elsewhere. The current report describes the results of 2 pivotal RCTs that assessed the efficacy and tolerability of oral favipiravir when used for treatment of acute uncomplicated influenza.
METHODS
Trial Design and Participants
The 2 trials were phase 3, randomized, double-blind, placebo-controlled international studies that randomized otherwise healthy adults with acute influenza-like illness to favipiravir or placebo. US316 (NCT02026349) was conducted in 14 countries in Africa, Europe, Asia, Australia and New Zealand, and the United States over 3 influenza seasons between January 2014 and March 2015 (Supplementary Appendix). US317 (NCT02008344) was conducted in 10 countries and territories in the Americas between December 2013 and February 2015. For both trials, eligible adults were aged 18–80 years (or, if in Belgium, 18–70 years) and had at least 2 of 6 influenza symptoms (body aches and pains, cough, fatigue, headache, nasal congestion, and sore throat) that were self-rated as moderate or severe in intensity and of 48 hours’ duration or less; fever (defined as an oral body temperature ≥38.0°C in those aged <65 years or ≥37.8°C in those aged ≥65 years); and positive rapid antigen test for influenza A or B (Veritor, Becton Dickinson or other assay) or known exposure to a documented influenza outbreak or patient. They were excluded from participation if they had taken any anti-influenza drug, received a live attenuated influenza vaccine within 4 weeks, or had underlying respiratory disease or any serious chronic disease (Supplementary Appendix). Because of favipiravir’s teratogenic effects in several species at drug exposures comparable to those in humans, women of childbearing potential were required to have negative pregnancy tests, and contraception was required for both male and female participants.
Individual sites obtained local institutional review board approvals, and all participants provided written informed consent in an appropriate language.
Drug Administration
In US316, eligible participants were stratified by age (<50 years or ≥50 years) and sex and randomized 1:1 to receive either favipiravir or matching placebo tablets for a total of 5 days. Favipiravir 1800 mg (9 tablets) was administered BID on study day 1 (total dose 3600 mg), followed by 800 mg BID on days 2–5 (1600 mg/day). In US317, subjects were randomized 3:1 to the same regimen of favipiravir or placebo. Because of favipiravir pharmacokinetic differences related to ethnicity, weight, and perhaps other factors, the approved dose in Japan (1600 mg BID on day 1 and 600 mg BID thereafter) is lower than the one used in these trials. Acetaminophen tablets or capsules were also provided. A number of other medications were prohibited (Supplementary Appendix).
Monitoring
After enrollment and dosing (day 1), participants returned to the clinic (or were visited at home) on days 2–5 for clinical assessments, collection of nasopharyngeal swabs for virology, and in most, blood samples for favipiravir assay (Supplementary Appendix). Routine laboratory tests for safety evaluation were performed on day 5. A follow-up visit occurred on day 15 (±3 days) and a final study visit on day 22 (+7 days). The participants were asked to complete a study diary 3 times per day up to the day 22 visit. The diary included timing of study drug ingestion, oral temperature, presence, and severity of influenza symptoms, ability to perform normal activities using a visual analogue scale (VAS), and use of any study-supplied acetaminophen. Uric acid is known to increase in some subjects when taking favipiravir, so investigators and subjects were blinded to uric acid results during the study (a medical monitor not involved in the management of the study reviewed all uric acid results in real time).
Sample Size Considerations
In US316, the original sample size of 660 subjects was designed to provide >90% power at the α = .05 level of significance (using a generalized Wilcoxon test) to detect at least a 24-hour difference in median time to alleviation between favipiravir and placebo assuming a 50% confirmed influenza infection rate. The sample size was subsequently increased to 860 subjects to ensure an adequate safety database and greater precision in estimating the treatment effect. For similar reasons in US317, the original sample size of 660 subjects was subsequently increased to 1056 subjects.
Outcomes and Data Analysis
In both trials the intention-to-treat (ITT) and safety populations consisted of those who were randomized and dosed with study drug. The primary efficacy analysis populations comprised participants with reverse-transcription polymerase chain reaction (RT-PCR)–confirmed influenza virus infection (ITTI). The primary efficacy endpoint was the time to alleviation of symptoms and resolution of fever, defined as the first time point when all of the 6 influenza symptoms (body aches and pains, cough, fatigue, headache, nasal congestion, and sore throat) were either absent or rated as mild and fever was absent, with both maintained for at least 21.5 hours.
Secondary clinical endpoints included the time to alleviation of each of the 6 influenza symptoms and time to resolution of fever; acetaminophen use; incidence of physician-diagnosed secondary respiratory tract infections leading to an antibiotic prescription; and time to return to normal activity, as assessed 3 times daily on an 11-point VAS. Secondary virologic endpoints included changes over time, area under the curve (AUC), and time to undetectability of log-transformed viral RNA loads and of infectious virus titers (median tissue culture infectious dose [TCID50]). Safety endpoints included the incidence of treatment-emergent adverse events (TEAEs), changes in clinical laboratory tests, vital sign measurements, and physical examination findings over time. In most participants, peak and trough levels of favipiravir and its major metabolite T-705M1 (hereafter “M1”) were determined.
The statistical methods are detailed in the Supplementary Appendix. Time-to-event analysis was assessed using Kaplan–Meier estimates, and a 2-sided Peto–Peto–Prentice test was used to compare the time to event of the favipiravir and placebo groups. The 95% confidence intervals (CIs) for the median were determined by the Hodges–Lehmann method. No interim analyses nor data monitoring by a data monitoring committee were undertaken.
RESULTS
Enrollment and Patient Characteristics
In US316, 855 participants (426 favipiravir, 429 placebo) comprised the ITT population, of whom 5.8% of placebo and 5.2% of favipiravir recipients failed to complete the study (Supplementary Figure 1A). Of these, 623 (72.9%) had RT-PCR–documented influenza virus infection and were included in the ITTI population (301 favipiravir, 322 placebo). The enrollment characteristics of the favipiravir and placebo groups in the ITTI population were comparable (Table 1). The ITTI population was predominantly female (59.1%), White (78.2%), relatively young (mean age, 41.3 years), and unvaccinated for the current influenza season (77.5%). Most participants were infected with influenza type A (87.3%), with A(H3N2) being the predominant subtype (75.8%).
Table 1.
Enrollment Demographic and Illness Characteristics of the Participants in the Intention-to-Treat, Influenza-Infected Populations
| Characteristic | US316 | US317 | ||
|---|---|---|---|---|
| Placebo (n = 322) |
Favipiravir (n = 301) |
Placebo (n = 169) |
Favipiravir (n = 526) |
|
| Age, y, mean (SD) | 41.3 (14.8) | 41.3 (14.2) | 39.3 (14.19) | 40.1 (13.61) |
| Female sex | 192 (59.6) | 176 (58.5) | 87 (51.5) | 305 (58.0) |
| Race | ||||
| African American | 54 (16.8) | 40 (13.3) | 14 (8.3) | 64 (12.2) |
| American Indian/Alaska Native | 0 (0.0) | 2 (0.7) | 8 (4.7) | 17 (3.2) |
| Asian | 14 (4.3) | 5 (1.7) | 0 (0.0) | 9 (1.7) |
| Native Hawaiian/other Pacific Islander | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| White | 241 (74.8) | 246 (81.7) | 118 (69.8) | 348 (66.2) |
| Multiple | 0 (0.0) | 0 (0.0) | 2 (1.2) | 4 (0.8) |
| Other | 13 (4.0) | 10 (3.4) | 27 (16.0) | 84 (16.0) |
| Ethnicity | ||||
| Hispanic or Latino | 46 (14.3) | 46 (15.3) | 85 (50.3) | 250 (47.5) |
| BMI, kg/m2, mean (SD) | 29.0 (7.0) | 29.0 (7.4) | 28.6 (6.2) | 29.0 (6.4) |
| Weight, kg, mean (SD) | 81.8 (20.8) | 83.2 (22.4) | 80.4 (20.7) | 81.5 (20.8) |
| Influenza vaccine in current season | 68 (21.1) | 64 (21.3) | 20 (11.8) | 45 (8.6) |
| Geographical region | ||||
| Australia and New Zealand | 23 (7.1) | 17 (5.6) | NA | NA |
| Europe | 61 (18.9) | 53 (17.6) | NA | NA |
| North America | 191 (59.3) | 184 (61.1) | 120 (71.0) | 384 (73.0) |
| South Africa | 47 (14.6) | 47 (15.6) | 49 (29.0) | 142 (27.0) |
| Time from symptom onset to first dose, mean (SD) | 29.9 (10.6) | 29.2 (10.5) | 30.2 (10.2) | 29.3 (10.7) |
| Time from symptom onset to first dose <24 h | 96 (29.8) | 90 (29.9) | 42 (24.9) | 164 (31.2) |
| Temperature, °C, mean (SD) | 38.0 (0.9) | 37.9 (0.8) | 37.8 (0.8) | 37.9 (0.8) |
| Baseline symptom score ≥15 | 60 (20.1) | 67 (23.5) | 41 (26.3) | 132 (26.5) |
| Viral titer, log10 TCID50/mL, mean (SD) | 3.0 (1.8) (n = 321) |
2.8 (1.7) (n = 301) |
3.3 (2.0) (n = 169) |
3.4 (1.9) (n = 526) |
| Viral RNA load, log10 viral particles/mL, mean (SD) | 6.8 (1.8) | 6.9 (1.5) | 6.9 (1.5) | 6.9 (1.6) |
| Influenza type | ||||
| A | 281 (87.3) | 263 (87.4) | 130 (76.9) | 399 (75.9) |
| B | 38 (11.8) | 34 (11.3) | 37 (21.9) | 124 (23.6) |
| A + B | 3 (0.9) | 4 (1.3) | 2 (1.2) | 3 (0.6) |
| Influenza A subtype | ||||
| A(H1N1)pdm09 | 38 (11.8) | 23 (7.6) | 57 (33.7) | 159 (30.2) |
| A(H3N2) | 237 (73.6) | 235 (78.1) | 67 (39.6) | 224 (42.6) |
| A(H1) + A(H3) | 2 (0.6) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Missing or negative subtyping (including B, A + B) | 45 (14.0) | 43 (14.3) | 45 (26.6) | 143 (27.2) |
Data are presented as No. (%) unless otherwise indicated. The ITTI population comprised participants with reverse-transcription polymerase chain reaction–confirmed influenza virus infection.
Abbreviations: NA, not applicable; SD, standard deviation; TCID50, median tissue culture infectious dose.
In US317, 1144 participants (863 favipiravir, 281 placebo) comprised the ITT population, of whom 695 (60.8%) were included in the ITTI population (526 favipiravir, 169 placebo) (Supplementary Figure 1B). In addition to a lower frequency of confirmed influenza infection in the ITT population than in US316, the ITTI population had higher proportions of persons of Hispanic or Latinx ethnicity, lower influenza vaccine uptake, and larger proportions with influenza B or A(H1N1) infections (Table 1).
Compliance, defined as taking all scheduled doses of study medication on the first 3 days of dosing (ie, 6 doses), was high in all groups ranging from 95.8% to 97.0% of participants in US316 and 95.5% to 97.2% in US317.
Clinical Efficacy
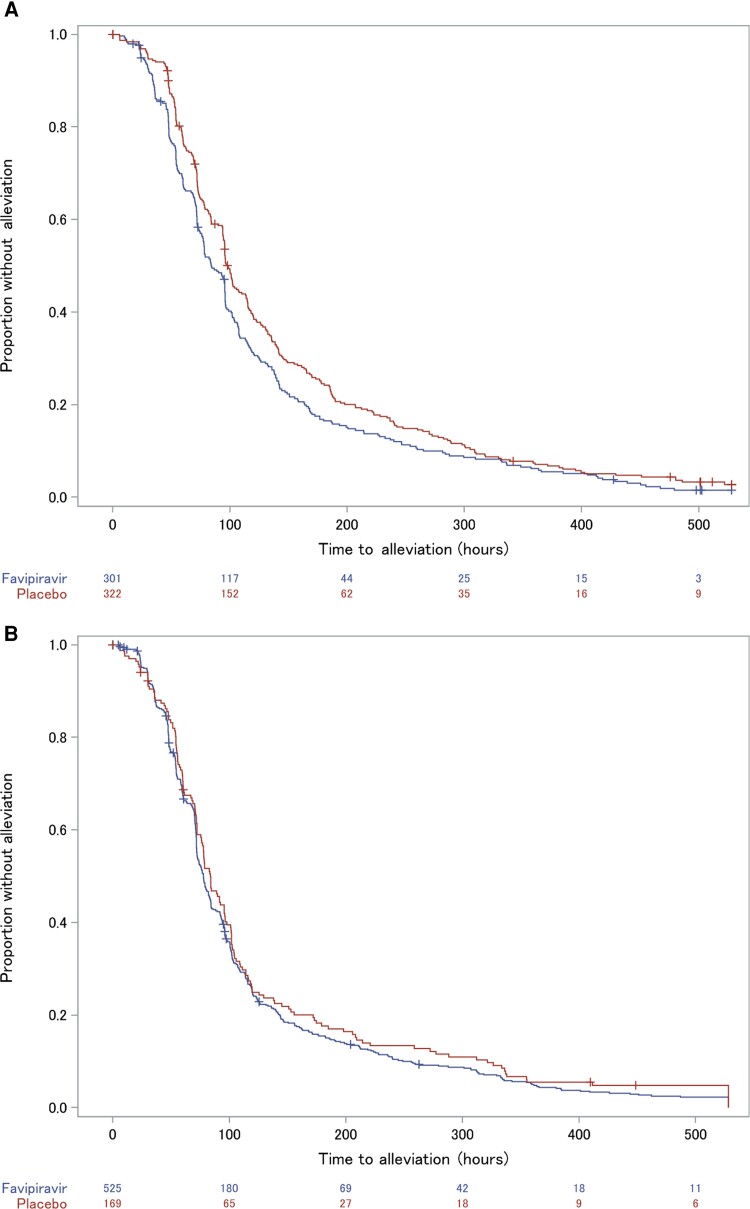
In the ITTI population of US316, favipiravir decreased the time to illness alleviation by a difference of 14.4 hours (median, 84.2 vs 98.6 hours; P = .004) (Figure 1A). Compared to placebo, subset analyses found no important differences in favipiravir-related reductions in time to alleviation in relation to age or sex (Table 2). Those weighing ≥80 kg had <1 hour reduction in median time to alleviation compared to a difference of 16.0 hours in those weighing <80 kg (Table 2). Also the small subset who had received an influenza vaccine in the current season had greater reductions (26.9 hours) compared to those not immunized (5.6 hours). Influenza-infected participants on favipiravir had a nonsignificant 22.9-hour shorter time to return to normal activity (median, 165.3 vs 188.2 hours for placebo). Acetaminophen use was numerically lower in the favipiravir group (mean, 1839 mg per participant) compared to the placebo group (mean, 2287 mg). A small number of participants developed secondary respiratory tract infections leading to antibiotic therapy (3.7% favipiravir, 5.6% placebo).
Figure 1.
Kaplan–Meier estimates for the primary endpoint of time to alleviation of 6 influenza symptoms and resolution of fever in US316 (A) and US317 (B). The estimated probability of persistence of symptoms (failure to have achieved alleviation) as a function of time (hours) from the start of dosing is shown.
Table 2.
Primary Outcome of Time to Illness Alleviation Related to Enrollment Subgroup Characteristics
| Variable | US316 | US317 | ||
|---|---|---|---|---|
| Placebo | Favipiravir | Placebo | Favipiravir | |
| Age, y | ||||
| 18–49 | 100.0 (95.5–113.7) (n = 213) |
88.8 (77.1–95.8) (n = 207) |
78.5 (72.3–91.5) (n = 128) |
77.8 (72.1–83.3) (n = 374) |
| ≥50 | 95.7 (77.1–118.2) (n = 93) |
83.7 (68.3–98.8) (n = 81) |
106.1 (70.7–152.7) (n = 35) |
77.8 (70.8–95.6) (n = 131) |
| Sex | ||||
| Female | 103.8 (95.6–119.8) (n = 179) |
92.8 (74.2–96.6) (n = 169) |
83.3 (71.6–96.3) (n = 85) |
79.7 (75.3–92.3) (n = 289) |
| Male | 94.0 (72.6–100.8) (n = 127) |
82.2 (71.8–96.0) (n = 119) |
84.1 (75.6–101.7) (n = 78) |
72.2 (71.1–80.1) (n = 216) |
| Influenza vaccine | ||||
| No | 95.9 (93.9–105.7) (n = 235) |
90.3 (77.8–95.8) (n = 224) |
83.3 (72.3–95.7) (n = 131) |
77.9 (72.2–83.3) (n = 443) |
| Yes | 102.3 (82.0–126.1) (n = 66) |
75.4 (68.7–101.9) (n = 61) |
102.7 (68.0–145.1) (n = 20) |
73.9 (58.4–97.6) (n = 42) |
| Weight, kg | ||||
| <80 | 100.0 (93.9–115.2) (n = 161) |
84.0 (72.9–95.6) (n = 144) |
84.1 (70.9–101.0) (n = 85) |
76.6 (71.8–83.0) (n = 259) |
| ≥80 | 96.3 (84.4–113.7) (n = 145) |
95.5 (71.8–97.4) (n = 144) |
78.8 (72.3–95.5) (n = 77) |
78.8 (72.0–92.8) (n = 245) |
| Time from onset to first dose, h | ||||
| <24 | 101.4 (95.3–135.3) (n = 91) |
95.7 (77.6–106.9) (n = 87) |
71.7 (59.5–89.1) (n = 40) |
79.0 (71.8–94.4) (n = 155) |
| ≥24 | 96.1 (89.8–107.1) (n = 212) |
79.0 (72.2–95.6) (n = 200) |
84.2 (77.7–101.3) (n = 122) |
76.0 (71.8–82.0) (n = 345) |
| Total symptom score | ||||
| <15 | 95.8 (93.8–102.3) (n = 239) |
78.2 (72.4–95.5) (n = 218) |
78.8 (71.7–92.3) (n = 115) |
75.3 (71.8–80.8) (n = 367) |
| ≥15 | 115.8 (94.2–141.0) (n = 60) |
96.0 (74.7–123.9) (n = 67) |
96.3 (72.3–119.0) (n = 41) |
92.3 (75.6–100.6) (n = 132) |
Results are presented as median (95% confidence interval) hours to illness alleviation. The study was not powered to assess the effect of treatment in subgroups, and no adjustments were made to account for multiplicity. In addition, the number of subjects in some subgroups was small.
In the ITTI population of US317, the favipiravir group had a nonsignificant 6.1-hour shorter time to alleviation (median, 77.8 hours) than the placebo group (median, 83.9 hours) (Figure 1B). No important effects on other measures of illness were found between the groups. The median time to return to normal activity was similar (140.0 hours for favipiravir, 139.3 hours for placebo). The incidence of secondary respiratory illnesses was low (3.0% favipiravir, 3.6% placebo).
In the ITT populations, the difference in median times to illness alleviation was 11.4 hours in US316 and 5.9 hours in US317. No effect of favipiravir on time to illness alleviation was found in the influenza-negative subpopulation in US316 (median, 91.6 vs 95.6 hours in placebo). In contrast, in US317, in which 40% of participants did not have influenza documented, the median time to alleviation was 14.2 hours longer in placebo (95.1 [95% CI, 77.8–102.3]) compared to favipiravir recipients (80.9 [95% CI, 74.8–95.5]).
Virologic Efficacy
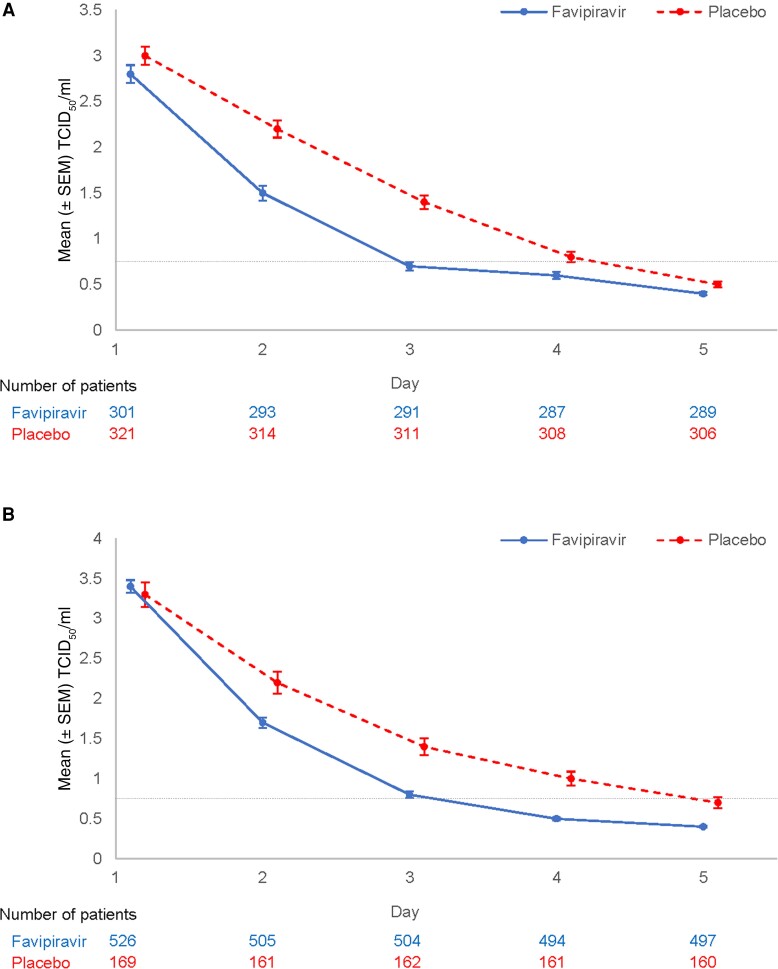
In US316, favipiravir recipients had a 23.2-hour reduction in the median time to cessation of detectable infectious virus compared to placebo (70.7 for placebo vs 47.5 for favipiravir; P < .001; Table 3). Similarly, in US317 the difference in median time to cessation of infectious virus detection was 24.0 hours (71.7 for placebo vs 47.7 for favipiravir; P < .001). Mean viral titers decreased more rapidly in those on favipiravir than in those on placebo from the first assessment time point (24 hours after first dose) (Table 3; Figure 2A and 2B). Viral RNA detectability persisted several days longer than that for infectious virus, and only modest differences between favipiravir and placebo recipients were found in viral RNA load AUC measures (Table 3).
Table 3.
Virologic Outcomes in the Intention-to-Treat, Influenza-Infected Population
| Outcome | US316 | US317 | ||
|---|---|---|---|---|
| Placebo | Favipiravir | Placebo | Favipiravir | |
| Time to cessation of detecting infectious virus, h, median (95% CI) | 70.7 (68.1–71.5) (n = 322) |
47.5 (46.8–47.9) (n = 301) |
71.7 (68.3–72.1) (n = 169) |
47.7 (47.4–47.8) (n = 526) |
| Viral titer change baseline to day 2, log10 TCID50/mL, mean (SD) | −0.8 (1.7) (n = 314) |
−1.3 (1.7) (n = 293) |
−1.1 (1.7) (n = 161) |
−1.7 (1.7) (n = 505) |
| Viral titer change baseline to day 3, log10 TCID50/mL, mean (SD) | −1.6 (2.0) (n = 311) |
−2.1 (1.7) (n = 291) |
−1.9 (2.0) (n = 162) |
−2.6 (1.9) (n = 504) |
| Viral titer AUC for days 1–5, TCID50 × h/mL, mean (SD) | 143.8 (89.0) (n = 318) |
104.1 (64.6) (n = 296) |
152.5 (101.6) (n = 165) |
115.5 (65.9) (n = 509) |
| Viral RNA load AUC, qRT-PCR particles × h/mL, mean (SD) | 506.5 (130.9) (n = 311) |
475.8 (127.0) (n = 290) |
493.1 (138.0) (n = 162) |
456.8 (126.4) (n = 490) |
| Time to undetectable viral RNA, h, median (95% CI) | 98.2 (96.6 to >120) (n = 322) |
100.1 (98.5 to >120) (n = 301) |
>120 (97.4 to >120) (n = 169) |
96.8 (96.4–101.6) (n = 526) |
The ITTI population comprised participants with RT‐PCR–confirmed influenza virus infection. In US316, the observed baseline mean (SD) viral titers on day 1 were 3.0 (1.8) and 2.8 (1.7) log10 TCID50/mL in the placebo and favipiravir groups, respectively, and the mean (SD) log10 viral particles/mL were 6.8 (1.8) and 6.9 (1.5), respectively.
AUC was calculated using the trapezoidal method. Subjects missing a baseline or follow‐up value were not included in the AUC analyses. Time‐to‐event analyses were assessed by using Kaplan–Meier estimates, while the between‐group comparisons were assessed by using a 2–sided Peto–Peto–Prentice test. The lower limit of quantification was defined as <2.18 viral particles/mL in influenza type A and <2.93 viral particles/mL in influenza type B.
Abbreviations: AUC, area under the concentration‐time curve; CI, confidence interval; qRT‐PCR, qualitative reverse-transcription polymerase chain reaction; SD, standard deviation; TCID50, median tissue culture infectious dose.
Figure 2.
Mean ± standard error of the mean (SEM) infectious virus titers (median tissue culture infectious dose [TCID50]/mL) in respiratory tract samples from favipiravir and placebo recipients in US316 (A) and US317 (B). The lower limit of quantification (LLOQ) in the TCID50 assay (0.7852, LLOQ = 0.75 TCID50/mL) is indicated by the dashed horizontal line.
Pharmacokinetics
In both PK populations (363 in US316, 711 in US317), the loading dose of 1800 mg BID resulted in rapid attainment of favipiravir plasma levels. In US316 the mean maximum favipiravir concentration (Cmax) was 47.5 μg/mL by 1 hour after the initial dose, and just prior to dose 3, the mean minimum favipiravir concentration (Cmin) of 36.7 μg/mL remained above the target of 20 μg/mL. The subsequent mean Cmin values were generally maintained above this threshold (Supplementary Figure 2A), although both Cmax (3.5–180 μg/mL) and Cmin (0.0–117 μg/mL) values of individual participants ranged widely over the drug administration period. In US317 the mean Cmax was 42.1 μg/mL by 1 hour after the initial dose, and just prior to dose 3, the mean Cmin of 35.8 μg/mL also remained above the target of 20 μg/mL (Supplementary Figure 2B). In contrast to favipiravir plasma concentrations, the maximum concentration of the major favipiravir metabolite M1, which does not possess anti-influenza activity, occurred after the first dose followed by rapid declines in peak and trough plasma M1 concentrations by dose 3.
Safety and Tolerability
In US316, a numerically higher proportion of placebo (30.7%) than favipiravir (25.9%) subjects experienced a TEAE or a TEAE related to treatment (Table 4). In US317, a slightly higher proportion of subjects in the favipiravir group (28.0%) than in the placebo group (25.1%) experienced 1 or more TEAEs (Table 4). The most commonly reported adverse events were diarrhea, nausea, and urinary tract infection, typically mild or moderate in severity (Supplementary Tables 1A and 1B). In US316, 5 placebo and 1 favipiravir recipient developed pneumonia.
Table 4.
Safety and Tolerability
| Outcome | US316 | US317 | ||
|---|---|---|---|---|
| Placebo (n = 427) |
Favipiravir (n = 428) |
Placebo (n = 283) |
Favipiravir (n = 861) |
|
| Treatment-emergent AE | 131 (30.7) | 111 (25.9) | 71 (25.1) | 241 (28.0) |
| Treatment-related AE | 52 (12.2) | 34 (7.9) | 23 (8.1) | 88 (10.2) |
| SAE | 2 (0.5) | 1 (0.2) | 2 (0.7) | 4 (0.5) |
| AE leading to discontinuation | 8 (1.9) | 8 (1.9) | 1 (0.4) | 10 (1.2) |
Data are presented as No. (%). In US316, 2 subjects received favipiravir despite being randomized to placebo, which accounts for the numerical differences between the safety population and the intention–to–treat population. To prevent inadvertent unblinding of an individual subject’s treatment, uric acid levels were not provided to either the sponsor or the study sites until the safety database was locked. Therefore, elevations in uric acid could not be categorized as AEs.
Abbreviations: AE, adverse event; SAE, serious adverse event.
Serious TEAEs were uncommon in both US316 (breast cancer and malignant melanoma in 2 placebo; pneumonia in 1 favipiravir recipient) and US317 (hypertensive crisis, pyelonephritis in 2 placebo participants; thyroid cancer, asthma exacerbation, pneumonia, colitis, and staphylococcal bacteremia in 4 favipiravir recipients), and none were assessed as related to the study drug by the investigators. In US316, similar proportions of favipiravir (5.1%) and placebo (5.9%) participants discontinued from the study prematurely (1.9% in each group for a TEAE), whereas in US317 a slightly higher proportion of favipiravir (6.6%) than placebo (4.2%) participants discontinued prematurely (1.2% and 0.4%, respectively, for a TEAE). There were no deaths or hospitalizations in either study.
In both studies participants in the favipiravir group showed mild, asymptomatic increases from baseline in mean uric acid levels on day 5 (Supplementary Figure 3A–D). Mean uric acid levels either improved or resolved by the first posttreatment time point analyzed (day 15). No episodes of acute gout were reported during the trials. No other clinically important changes in laboratory values were found.
DISCUSSION
These outpatient trials in uncomplicated influenza found that this twice-daily dose regimen of oral favipiravir was adequately tolerated and associated with significant antiviral effects compared to placebo. Antiviral efficacy was reflected in more rapid reductions in the titers of infectious virus and shortening the duration of infectious virus detectability by approximately 1 day compared to placebo in both trials. However, clinical efficacy in terms of illness alleviation varied between the 2 trials for unclear reasons. Other unexplained differences between the 2 trials are the lower proportion of documented influenza infections in US317 participants and the apparent acceleration of illness alleviation in those without proven influenza in US317 compared to lack of such an effect in US316. Differences found in the demographic characteristics of the populations enrolled in the 2 trials, including the very low proportion with influenza immunization in US317, might have been contributory. Unfortunately, the trials did not perform influenza-specific serologic studies, so that some influenza virus infections were missed, nor testing for other respiratory viruses that might have provided a better understanding of the reasons for these differences.
This dose regimen of favipiravir was generally well-tolerated, and no new safety signals were detected in the current trials. Favipiravir is associated with dose-related elevations in serum uric acid levels that are reversible after drug discontinuation. These elevations have not been associated with acute gout or renal problems in trials to date, but the current trials excluded those with a history of gout or receiving medical treatment for gout or hyperuricemia. Other reported adverse events include mild to moderate diarrhea, asymptomatic increase of transaminases, and uncommonly decreased neutrophil counts [12]. Warnings in the Japanese product label state that favipiravir is contraindicated in women who might be or are pregnant and in lactating women because of its association with embryonic deaths and teratogenicity in animal studies and that men receiving favipiravir need to use the most effective contraceptive methods including condoms during intercourse and not to have intercourse with pregnant women during treatment and for 7 days afterward [13].
Favipiravir has complex, time- and dose-dependent pharmacokinetics that are affected by weight and perhaps ethnicity [11]. Because favipiravir is both metabolized by and inhibits aldehyde oxidase, initial oral loading is required to rapidly reach plasma concentrations predicted to be inhibitory in patients. Two prior phase 2 treatment trials found that favipiravir plasma values maintained above 20 μg/mL were associated with antiviral and clinical benefit (unpublished data). In the current trials the loading dose regimen on day 1 rapidly increased favipiravir plasma concentrations by approximately 1 hour after the first dose. However, there was considerable intersubject variability in both pre- and postdose favipiravir plasma concentrations over time, so that some participants had relatively low favipiravir exposures and trough concentrations below the target of 20 µg/mL. In this regard we undertook a post hoc analysis of the primary clinical endpoint related to favipiravir exposure by determining the proportions of recipients who had average Cmin values over study days 1–5 of <20 µg/mL or >20 µg/mL, a target Cmin determined from murine model and human influenza studies of favipiravir (unpublished observations). In US316 the median (95% CI) time to illness alleviation for 167 favipiravir recipients with average Cmin ≥20 µg/mL was 83.3 (71.8–95.5) hours (P = .003 vs placebo) and 95.7 (77.1–101.1) hours for 134 recipients with average Cmin < 20 µg/mL (P = .157 vs placebo), and 98.6 (94.6–107.1) hours for 322 placebo recipients. These findings suggest that those with higher average exposures had significant reductions in time to illness alleviation compared to placebo, whereas those with lower exposures did not.
Of note, favipiravir recipients weighing ≥80 kg had more modest reduction in time to illness alleviation compared to those weighing less. These observations raise the concern that higher doses may be required in some recipients to reliably obtain inhibitory concentrations. Also critically ill, hospitalized influenza patients receiving the dose regimen used in the current studies have alterations in favipiravir pharmacokinetics that lead to much lower Cmin levels after several days of dosing, possibly related to increased favipiravir metabolism [14]. Consequently, the optimal dose regimens for favipiravir need further study, particularly in heavier patients and in serious influenza.
The current studies did not assess participants for emergence of influenza variants with reduced favipiravir susceptibility. Selection of such variants with reduced favipiravir susceptibility variants during cell culture passage has been difficult [8, 11], although serial passage of an A(H1N1)pdm09 virus led to the emergence of amino acid substitution K229R in the PB1 subunit that was associated with 30-fold reduced susceptibility in a yield reduction assay [15]. Virus with the PB1-K229R substitution had reduced replicative fitness in vitro, but fitness was restored by a compensatory substitution P653L in the PA subunit [15]. Virus with both substitutions is able to infect ferrets and transmit by direct contact and by respiratory droplets to contact ferrets, although the substitution conferring reduced susceptibility, K229R, decreased in frequency over time within ferrets [16]. In 1 Japanese study, viruses with reduced in vitro susceptibility were not isolated from 57 patients treated with favipiravir, although several viruses with amino acid amino acid substitutions in PB1, PB2, and/or PA subunits, perhaps related to the documented mutagenic effects of favipiravir, were detected [17]. Future studies need to look more closely for favipiravir-associated polymerase substitutions and changes in in vitro susceptibility.
Another limitation of these trials is lack of direct comparison to a proven influenza antiviral. The antiviral effects observed in these trials are generally similar to those reported for oseltamivir in uncomplicated influenza but less than observed with baloxavir [11, 18]. An obvious strategy to enhance potency would be the use of antiviral combinations, and in vitro studies indicate that favipiravir shows synergistic effects with oseltamivir for influenza A viruses [4, 19]. Furthermore, in mice with lethal A(H5N1) influenza infection, combination therapy with oseltamivir and favipiravir is effective late in disease. A combination of high-dose oral oseltamivir and favipiravir, when delayed until 72 or 96 hours postinfection, protected 100% of mice from a lethal infection with a highly pathogenic A/Turkey/15/2006 (H5N1) virus, whereas favipiravir treatment alone was less effective [20]. An observational study in severely ill hospitalized patients in China found that a combination of favipiravir and oseltamivir provided greater antiviral effects and somewhat more rapid clinical recovery compared to oseltamivir alone [21]. Further study of this combination in serious influenza appears warranted.
In summary, this favipiravir dosing regimen demonstrated significant antiviral activity in treating influenza in outpatient adults. However, the clinical outcomes, as measured by self-reported symptoms, were inconsistent across the trials for unclear reasons and less than found in similar trials of oseltamivir and baloxavir [18]. In the context of the clinical trial results with approved oral antivirals, the issues around potential reproductive toxicity of favipiravir, and favipiravir’s reversible effects on renal clearance of uric acid, further development of favipiravir for treatment of uncomplicated influenza has not been pursued. However, favipiravir’s remarkable broad-spectrum antiviral activity against many RNA virus threats, including novel influenza viruses, high threshold for resistance emergence, and the potential for study of higher-dose regimens, including intravenous administration and combinations with other antivirals, highlight the need for further clinical studies.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Supplementary Material
Contributor Information
Frederick G Hayden, Division of Infectious Diseases and International Health, Department of Medicine, University of Virginia School of Medicine, Charlottesville, Virginia, USA.
Robert P Lenk, Medivector, Inc, Boston, Massachusetts, USA.
Lucille Stonis, Medivector, Inc, Boston, Massachusetts, USA.
Catherine Oldham-Creamer, Medivector, Inc, Boston, Massachusetts, USA.
Lih Lisa Kang, Medivector, Inc, Boston, Massachusetts, USA.
Carol Epstein, Medivector, Inc, Boston, Massachusetts, USA.
Notes
Acknowledgments. We would like to thank Maggie Neptune, Medivector (currently at Neptune Clinical Consulting, LLC) for her work on clinical operations; Angela Mellon, Medivector (currently at Mellon Clinical Research Consulting, LLC) for organizing and tracking the clinical virology program; and Lisa Cook (University of Virginia) and Moriyoshi Maeda (Fujifilm) for their assistance in manuscript preparation. We also thank the trial participants and the many investigators who enrolled patients into the 2 trials.
Financial support. The US316 and US317 trials were funded under a Department of Defense contract (number HDTRA1-12-C-0031).
References
- 1. Furuta Y, Takahashi K, Fukuda Y, et al. . In vitro and in vivo activities of anti-influenza virus compound T-705. Antimicrob Agents Chemother 2002; 46:977–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Furuta Y, Gowen BB, Takahashi K, Shiraki K, Smee DF, Barnard DL. Favipiravir (T-705), a novel viral RNA polymerase inhibitor. Antiviral Res 2013; 100:446–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Tarbet EB, Vollmer AH, Hurst BL, Barnard DL, Furuta Y, Smee DF. In vitro activity of favipiravir and neuraminidase inhibitor combinations against oseltamivir-sensitive and oseltamivir-resistant pandemic influenza A (H1N1) virus. Arch Virol 2014; 159:1279–91. [DOI] [PubMed] [Google Scholar]
- 4. Kiso M, Takahashi K, Sakai-Tagawa Y, et al. . T-705 (favipiravir) activity against lethal H5N1 influenza A viruses. Proc Natl Acad Sci U S A 2010; 107:882–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Watanabe T, Kiso M, Fukuyama S, et al. . Characterization of H7N9 influenza A viruses isolated from humans. Nature 2013; 501:551–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeLang L, Abdelnabi R, Neyts J. Favipiravir as a potential countermeasure against neglected and emerging RNA viruses. Antiviral Res 2018; 153:85–94. [DOI] [PubMed] [Google Scholar]
- 7. Sangawa H, Komeno T, Nishikawa H, et al. . Mechanism of action of T-705 ribosyl triphosphate against influenza virus RNA polymerase. Antimicrob Agents Chemother 2013; 57:5202–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Baranovich T, Wong SS, Armstrong J, et al. . T-705 (favipiravir) induces lethal mutagenesis in influenza A H1N1 viruses in vitro. J Virol 2013; 87:3741–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. McKimm-Breschkin JL, Fry AM. Meeting report: 4th ISIRV antiviral group conference: novel antiviral therapies for influenza and other respiratory viruses. Antiviral Res 2016; 129:21–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McKimm-Breschkin JL, Jiang S, Hui DS, Beigel JH, Govorkova EA, Lee N. Prevention and treatment of respiratory viral infections: presentations on antivirals, traditional therapies and host-directed interventions at the 5th ISIRV Antiviral Group conference. Antiviral Res 2018; 149:118–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hayden FG, Shindo N. Influenza virus polymerase inhibitors in clinical development. Curr Opin Infect Dis 2019; 32:176–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madelain V, Nguyen TH, Olivo A, et al. . Ebola virus infection: review of the pharmacokinetic and pharmacodynamic properties of drugs considered for testing in human efficacy trials. Clin Pharmacokinet 2016; 55:907–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Avigan tablets 200 mg, PMDA. 5th version, 2018. https://www.sukl.cz/file/92989_1_1/.
- 14. Wang Y, Wu Z, Salame A, et al . Phase 2a, open-label, dose-escalating, multi-center pharmacokinetic study of favipiravir (T-705) in combination with oseltamivir in patients with severe influenza. EBioMedicine 2020; 62:103125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Goldhill DH, Te Velthuis AJ, Fletcher RA, et al. . The mechanism of resistance to favipiravir in influenza. Proc Natl Acad Sci U S A 2018; 115: 11613–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Goldhill DH, Yan A, Frise R, et al. . Favipiravir-resistant influenza A virus shows potential for transmission. PLoS Pathog 2021; 17:e1008937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Takashita E, Ejima M, Ogawa R, et al. . Antiviral susceptibility of influenza viruses isolated from patients pre and post administration of favipiravir. Antiviral Res 2016; 132:170–7. [DOI] [PubMed] [Google Scholar]
- 18. Hayden FG, Sugaya N, Hirotsu N, et al. . Baloxavir marboxil for uncomplicated influenza in adults and adolescents. N Engl J Med 2018; 379:913–23. [DOI] [PubMed] [Google Scholar]
- 19. Tarbet EB, Vollmer AH, Hurst BL, Barnard DL, Furuta Y, Smee DF . In vitro activity of favipiravir and neuraminidase inhibitor combinations against oseltamivir-sensitive and oseltamivir-resistant pandemic influenza A (H1N1) virus. Arch Virol 2014; 159:1279–91. [DOI] [PubMed] [Google Scholar]
- 20. Marathe BM, Wong S-S, Vogel P, et al. . Combinations of oseltamivir and T-705 extend the treatment window for highly pathogenic influenza A(H5N1) virus infection in mice. Sci Rep 2016; 6:26742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yeming W, Fan G, Salam A, et al. . Comparative effectiveness of combined favipiravir and oseltamivir therapy versus oseltamivir monotherapy in critically Ill patients with influenza virus infection. J Infect Dis 2020; 221:1688–98. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.