Abstract
CD14 has been implicated as a receptor of lipoteichoic acid (LTA) and other bacterial components as well as lipopolysaccharide (LPS). Since the structures of LTAs from various gram-positive bacteria are heterogeneous, we analyzed the effects of LTAs on the secretion of interleukin-8 (IL-8) by high- and low-CD14-expressing (CD14high and CD14low) human gingival fibroblasts (HGF). While Bacillus subtilis LTA had an IL-8-inducing effect on CD14high HGF which was considerably weaker than that of LPS, Streptococcus sanguis and Streptococcus mutans LTAs had practically no effect on the cells. B. subtilis LTA had only a weak effect on CD14low HGF, as did LPS. S. sanguis and S. mutans LTAs at a 1,000-fold excess each completely inhibited the IL-8-inducing activities of both LPS and a synthetic lipid A on CD14high HGF. The effect of LPS was also inhibited by the presence of an LPS antagonist, synthetic lipid A precursor IVA (LA-14-PP), with a 100-fold higher potency than S. sanguis and S. mutans LTAs and by anti-CD14 monoclonal antibody (MAb). S. sanguis and S. mutans LTAs, LA-14-PP, and anti-CD14 MAb had no significant effect on phorbol myristate acetate-stimulated IL-8 secretion by HGF. These LTAs also inhibited the IL-8-inducing activity of B. subtilis LTA on CD14high HGF, as did LA-14-PP and anti-CD14 MAb. The antagonistic and agonistic functions of LTAs were also observed with human monocytes. Binding of fluorolabeled LPS to human monocytes was inhibited by S. sanguis LTA, although the inhibition was 100 times weaker than that of LPS itself, and anti-CD14 MAb inhibited fluorolabeled LPS and S. sanguis LTA binding. Binding of LTAs to CD14 was also observed with nondenaturing polyacrylamide gel electrophoresis. These results indicate that LTAs act as antagonists or agonists via a CD14-dependent mechanism, probably due to the heterogeneous structure of LTAs, and that an antagonistic LTA might be a useful agent for suppressing the periodontal disease caused by gram-negative bacteria.
Lipoteichoic acids (LTAs) are macroamphiphiles anchored in the cytoplasmic membranes of gram-positive bacteria by hydrophobic interactions and are thought to be counterparts of lipopolysaccharide (LPS) of gram-negative bacteria (8, 37). The structures of LTAs, as proposed by Fischer et al. (9), are not uniform; they vary among the gram-positive bacterial species. LTAs exhibit many biological activities: the induction of interleukin-1 (IL-1), tumor necrosis factor, IL-8, IL-12, and nitric oxide from monocytes or macrophages (4–6, 16); the production of hepatocyte growth factor/scatter factor from human gingival fibroblasts (HGF) (28); and antitumor activities (29, 34, 35, 41). In contrast, purified LTAs from Enterococcus hirae and Staphylococcus aureus and synthetic LTA lacked biological activities in several assays (14, 18, 29, 31). Purified LTA from S. aureus antagonized the activity of LPS (18).
CD14 is a 55-kDa glycosylphosphatidylinositol-anchored glycoprotein on monocytes and neutrophils and also exists in plasma as a soluble protein (soluble CD14 [sCD14]) (33, 39, 40, 45). CD14 functions as a major receptor of LPS and mediates LPS-induced cell activation (33, 39, 40, 45). It was recently shown that CD14 recognizes bacterial components in addition to LPS, such as LTA (6), lipoarabinomannan from Mycobacterium tuberculosis (44), manuronic acid polymers from Pseudomonas species (7), soluble peptidoglycan from S. aureus (36), rhamnose-glucose polymers from Streptococcus mutans (26), insoluble cell walls from several gram-positive bacterial species (24), and lipoproteins from Treponema pallidum and Borrelia burgdorferi (25, 38).
In healthy human gingival sulci, gram-positive cocci are the major morphotype and compose almost two-thirds of the total flora. In accordance with the progression from gingivitis to periodontal disease, the composition of gram-negative black-pigmented bacteria increases considerably in the periodontal pocket (22). This phenomenon leads to the hypothesis that a component of gram-positive bacteria, LTA, may inhibit the activity of LPS, probably via CD14. We have shown recently that HGF are heterogeneous with regard to CD14 expression and that high-CD14-expressing (CD14high) HGF secrete IL-8 in response to LPS via a CD14 pathway (27).
In the present study, we examined the above-mentioned hypothesis and investigated whether LTA can stimulate HGF and human monocytes by interacting with CD14. We found that LTA from Bacillus subtilis stimulates CD14high HGF and monocytes via a CD14 pathway and that LTAs from oral bacteria (S. mutans and Streptococcus sanguis) act as antagonists not only of LPS but also of an agonistic B. subtilis LTA in a CD14-dependent manner.
MATERIALS AND METHODS
Reagents.
LTAs prepared from S. sanguis, S. mutans, and B. subtilis were purchased from Sigma Chemical Co. (St. Louis, Mo.). To examine the possible contamination of these LTA preparations with extraneous LPS and peptidoglycan/β-glucan, a colorimetric Limulus test (Endospecy test; Seikagaku Co., Tokyo, Japan) (23), and a silkworm larva plasma (SLP) test (Wako Pure Chemicals, Osaka, Japan) that makes use of a prophenol oxidase cascade in SLP which is specifically activated by bacterial peptidoglycan and fungal β(1-3)-d-glucan (3) were used. S. sanguis LTA exhibited marked activity in the Limulus test. To obtain purified S. sanguis LTA without LPS and peptidoglycan/β-glucan, the commercial S. sanguis LTA was subjected to hydrophobic interaction chromatography on an octyl-Sepharose CL-4B column (Pharmacia, Uppsala, Sweden) by the method of Fischer et al. (9). As described previously (28), the Limulus activities of the S. sanguis, S. mutans, and B. subtilis LTA preparations used in this study were 0.1, 28.1, and 15.2 ng/mg, respectively. Moreover, the SLP activities of the S. sanguis, S. mutans, and B. subtilis LTA preparations were 0.6, 56.3, and 28.9 μg/mg, respectively. Protein compositions determined by the Lowry method for S. mutans and B. subtilis LTAs were 0.4 and 2.4%, respectively, according to the certificate of analysis from Sigma. An ultrapurified LPS prepared from Salmonella abortusequi (Novo-Pyrexal) (10) was a gift from C. Galanos (Max Planck Institut für Immunbiologie, Freiburg, Germany). A synthetic lipid A, LA-15-PP (compound 506), and synthetic lipid A precursor IVA, LA-14-PP (compound 406), were obtained from Daiichi Chemical Co. (Tokyo, Japan). Anti-CD14 monoclonal antibody (MAb) MY4 (mouse immunoglobulin G2b [IgG2b]) and isotype-matched mouse IgG2b were purchased from Coulter Corporation (Miami, Fla.) and dialyzed against alpha minimal essential medium (α-MEM; Flow Laboratories, McLean, Va.). All other reagents were obtained from Sigma unless otherwise indicated.
Cells.
HGF were prepared from explants of normal human gingival tissues with informed consent as described previously (32). In brief, the explants were cut into pieces and cultured in α-MEM supplemented with 10% fetal calf serum (FCS; Flow Laboratories) in 100-mm-diameter tissue culture dishes (Falcon; Becton Dickinson Labware, Lincoln Park, N.J.). The medium was changed every 3 days for 10 to 15 days until confluent cell monolayers were formed. After three or four subcultures by trypsinization, homogeneous, slim, spindle-shaped cells grown in characteristic swirls were obtained. Cells were used as confluent monolayers at subculture levels 5 through 12.
Human peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood of healthy adult donors by Ficoll-Isopaque density gradient centrifugation (1). The isolated PBMC were washed three times with phosphate-buffered saline (PBS) and suspended in RPMI 1640 medium (Nissui, Tokyo, Japan).
Flow cytometry.
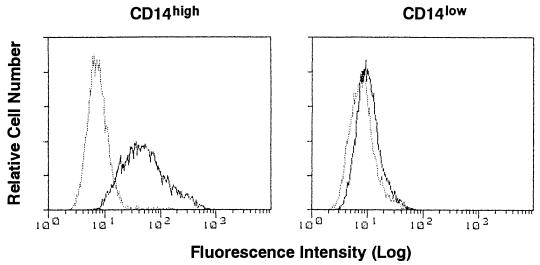
Flow cytometric analyses were performed with a fluorescence-activated cell sorter (FACS) (FACScan; Becton Dickinson, Mountain View, Calif.) (27). For immunofluorescence staining, confluent HGF obtained from different donors were collected by trypsinization and washed in PBS. We determined that trypsinization did not affect the amount of CD14 detected by the FACS. The cells were stained with anti-CD14 MAb MEM-18 (mouse IgG1; Monosan, Uden, The Netherlands) or isotype-matched mouse IgG1 (Coulter) at 4°C for 30 min, followed by incubation with fluorescein isothiocyanate-conjugated goat anti-mouse IgG (BioSource International Inc., Camarillo, Calif.) at 4°C for a further 30 min. Figure 1 shows representative data for CD14 expression of CD14high and low-CD14-expressing (CD14low) HGF obtained from different donors and analyzed by flow cytometry. CD14 expression on the membranes of HGF was correlated with the constitutive expression of CD14 mRNA by the cells (27).
FIG. 1.
Heterogeneous expression of CD14 by HGF. Confluent CD14high and CD14low HGF were collected by trypsinization. The cells were stained with anti-CD14 MAb MEM-18 (solid line) or an isotype control MAb (dotted line) and analyzed with a FACS.
Detection of IL-8 and IL-1β by ELISA.
Confluent HGF were collected by trypsinization and washed in PBS three times. The cells (2 × 104/200 μl) were seeded in α-MEM with 10% FCS in wells of 96-well flat-bottom plates (Falcon; Becton Dickinson Labware). After incubation for 4 days at 37°C in a 5% CO2 atmosphere, confluent monolayers of HGF were washed with α-MEM three times, followed by the addition of a test stimulant in 200 μl of α-MEM with 1% FCS for 24 h. PBMC (2 × 105 cells/well) were incubated with or without a test stimulant in 200 μl of RPMI 1640 medium with 1% FCS for 24 h in 96-well flat-bottom plates (Falcon). For the inhibition experiments, HGF or PBMC in 96-well plates were preincubated with antagonists for 30 min at 37°C and then stimulated with agonists for 24 h at 37°C. After the incubation, the supernatants were collected and the level of interleukin-8 (IL-8) produced from HGF or IL-1β produced from human monocytes in the supernatants was determined with a human IL-8 or IL-1β enzyme-linked immunosorbent assay (ELISA) kit (BioSource).
The cytokine assay with the ELISA kits was performed in accordance with the instructions provided by the manufacturer (BioSource). The concentrations of the cytokines in the supernatants were determined with the Softmax data analysis program (Molecular Devices Corp., Menlo Park, Calif.). Each sample was assayed in triplicate.
Binding of fluorolabeled LPS and LTA to human monocytes.
S. abortusequi LPS and S. sanguis LTA were labeled with BODIPY FL (Molecular Probes, Eugene, Oreg.) as described previously (42). In brief, LPS and LTA were suspended in 0.5 M sodium bicarbonate buffer (pH 9.0) containing labeling reagent for 30 min at room temperature, with frequent sonication. Labeled reagents were extensively dialyzed against distilled water to remove unreacted dye. PBMC (106 cells) were incubated with antagonists in 100 μl of PBS with 1% FCS for 20 min at 37°C, fluorolabeled S. abortusequi LPS (BODIPY-LPS) or S. sanguis LTA (BODIPY-LTA) was added, and the mixture was incubated for an additional 20 min at 37°C and analyzed with a FACS. The mean fluorescence intensity (MFI) of the monocyte population was analyzed by gating on the basis of forward and side scatter characteristics. The minimum concentrations of BODIPY-LPS and BODIPY-LTA which could be used in this method were 0.1 and 1 μg/ml, respectively.
Preparation of sCD14.
Anti-human CD14 MAb D10 (43) was purified from ascitic fluid with a HiTrap protein G column (Pharmacia), and then MAb D10 was coupled to a HiTrap N-hydroxysuccinimide-activated Sepharose column (Pharmacia) to yield 1 mg of IgG/ml of packed gel, in accordance with the manufacturer’s protocol. Five milliliters of normal human serum was passed twice at a flow rate of 0.5 ml/30 min through the column. The column was then washed with 20 volumes of 75 mM Tris-HCl (pH 8.0). The retained sCD14 was eluted with 0.1 M glycine-HCl (pH 2.7). The pH of the eluted material was immediately adjusted to 8.0 with 1 M Tris-HCl (pH 9.0), and the material was dialyzed against PBS. The concentration of sCD14 was determined with a human sCD14 ELISA kit (BioSource).
Gel shift assay.
The gel shift assay was performed by the method of Hailman et al. (13). Briefly, purified sCD14, sonicated S. abortusequi LPS, and sonicated LTAs were diluted in PBS lacking Ca2+ and Mg2+, and 30 μg of sCD14 per ml was incubated with or without LPS and LTAs at 37°C for 2 h. Samples were then mixed with equal volumes of 2× sample buffer (20% glycerol, 125 mM Tris-HCl [pH 6.8], 10 μg of bromophenol blue per ml) and run on 8% Tris-glycine discontinuous nondenaturing polyacrylamide gels (native polyacrylamide gel electrophoresis [PAGE]) in a running buffer containing 192 mM glycine and 25 mM Tris-HCl (pH 8.3) without detergent. Natural silver staining was performed by the method of Morrissey (21).
Data analysis.
All of the experiments in this study were conducted at least three times. The data shown are representative results. Experimental values are given as means ± standard deviations (SD). The statistical significance of differences between two means was evaluated with Student’s unpaired t test, and P values of less than 0.05 were considered significant.
RESULTS
B. subtilis LTA stimulates IL-8 secretion by HGF, but S. sanguis and S. mutans LTAs do not.
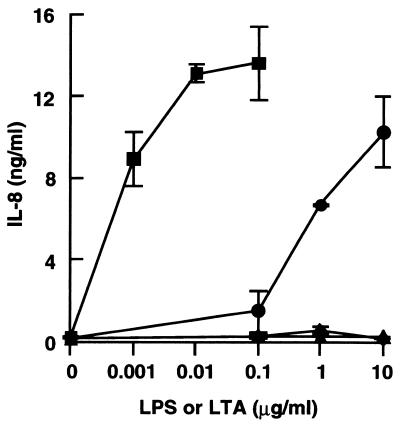
We recently demonstrated that CD14high HGF secrete IL-8 in response to LPS via a CD14 pathway (27). We therefore examined whether LTAs also stimulate CD14high HGF to secrete IL-8. B. subtilis LTA at a concentration of 0.1 μg/ml caused IL-8 secretion, and the secretion was increased at higher concentrations, whereas LTAs of S. sanguis and S. mutans caused no IL-8 secretion when added at concentrations of up to 10 μg/ml (Fig. 2). The reference S. abortusequi LPS at 1 ng/ml stimulated CD14high HGF, and the IL-8 secretion reached a plateau at 10 ng/ml. These results show that B. subtilis LTA has a potent stimulating effect on the secretion of IL-8 from CD14high HGF, although the activity of the LTA is weaker than that of LPS.
FIG. 2.
LTA from B. subtilis stimulated HGF to secrete IL-8, but LTAs from S. sanguis and S. mutans did not. Confluent CD14high HGF were stimulated with various doses of S. abortusequi LPS (■) and LTAs from B. subtilis (●), S. sanguis (▴), and S. mutans (⧫) for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by an ELISA. Error bars indicate SD.
B. subtilis LTA mainly stimulates CD14high HGF.
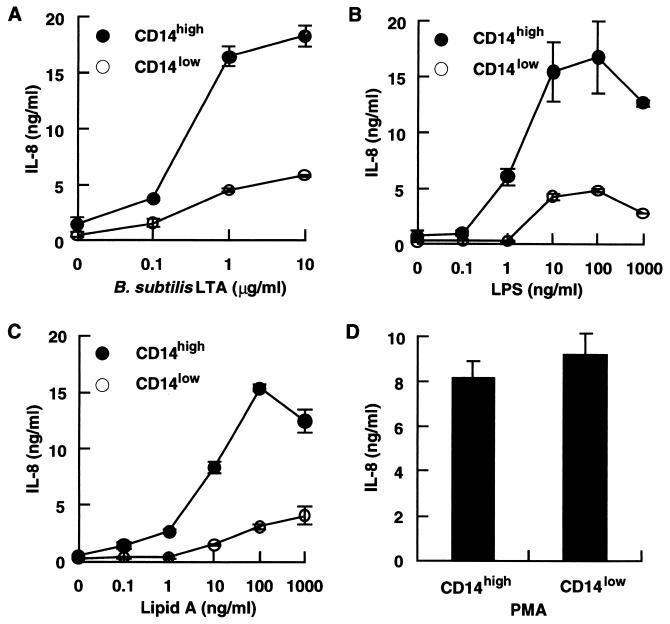
Since HGF are heterogeneous with regard to CD14 expression and can be divided into CD14high and CD14low populations (27), we next examined whether B. subtilis LTA stimulates CD14low as well as CD14high HGF. The CD14high and CD14low HGF shown in Fig. 1 were stimulated with various doses of B. subtilis LTA and the reference LPS. The CD14high HGF secreted a significant amount of IL-8 in response to B. subtilis LTA and LPS, whereas the CD14low HGF showed only a marginal response to B. subtilis LTA as well as LPS (Fig. 3A and B). S. sanguis and S. mutans LTAs had no stimulating effect on CD14low HGF (data not shown). A synthetic lipid A (the bioactive center of LPS [30]) (LA-15-PP) selectively stimulated CD14high HGF to secrete IL-8 (Fig. 3C). The IL-8-producing abilities of the CD14high and CD14low HGF were comparable, because no difference in the IL-8 secretion of these HGF in response to phorbol myristate acetate (PMA) was observed (Fig. 3D).
FIG. 3.
B. subtilis LTA, the reference LPS, and LA-15-PP stimulated mainly CD14high HGF. (A to C) Confluent CD14high and CD14low HGF were stimulated with various doses of B. subtilis LTA (A), S. abortusequi LPS (B), or LA-15-PP (C) for 24 h. (D) Confluent CD14high and CD14low HGF were stimulated with 30 ng of PMA per ml for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by an ELISA. Error bars indicate SD.
S. sanguis and S. mutans LTAs act as antagonists of LPS.
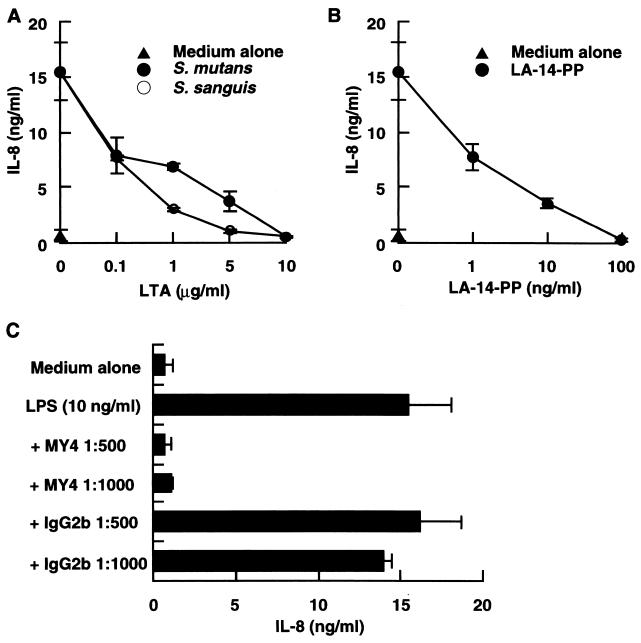
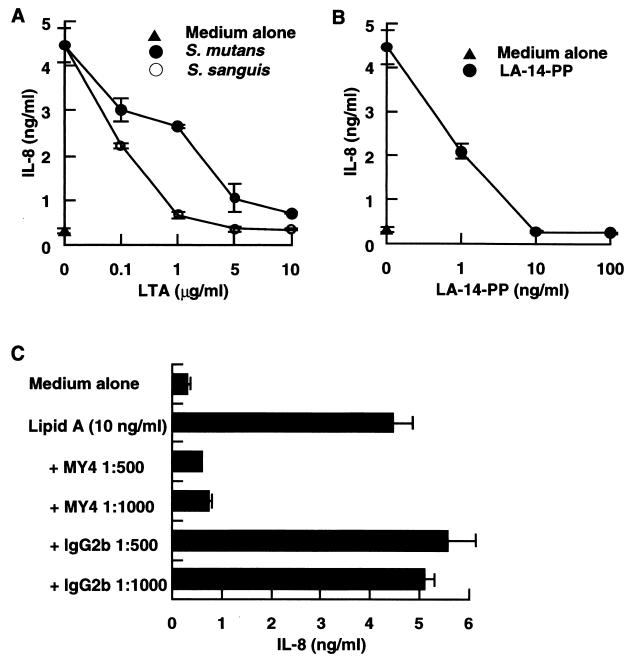
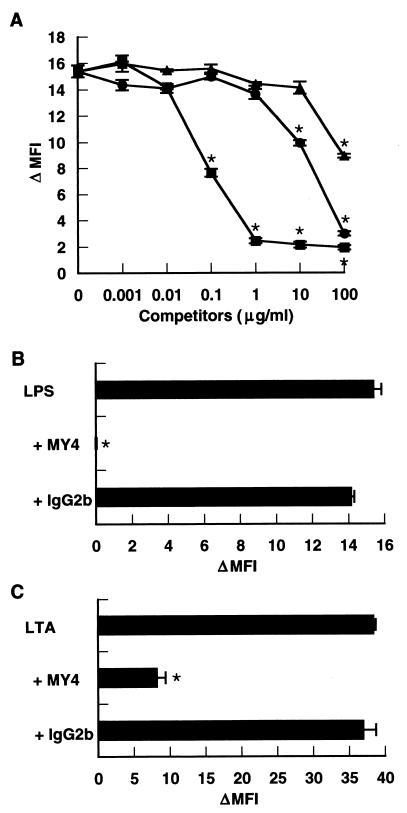
Since LA-14-PP does not stimulate human cells (rather, it acts as an antagonist of LPS [2, 11, 12, 20]), it is possible that no stimulating LTAs antagonize LPS function. To examine this possibility, CD14high HGF were stimulated with 10 ng of LPS per ml in the absence or presence of S. sanguis or S. mutans LTA. Significant inhibition of the LPS effect was observed at a 10- to 100-fold excess of S. sanguis and S. mutans LTAs, and a 1,000-fold excess (10 μg/ml) of either LTA completely inhibited the LPS-induced IL-8 secretion (Fig. 4A). The LPS-induced IL-8 secretion was also markedly inhibited by LA-14-PP and by anti-CD14 MAb MY4 (Fig. 4B and C). These results suggest that, like LA-14-PP, inactive LTA acts as an antagonist of LPS through a CD14-dependent mechanism.
FIG. 4.
LTAs from S. sanguis and S. mutans acted as antagonists of LPS. (A and B) Confluent CD14high HGF were stimulated with 10 ng of S. abortusequi LPS per ml in the absence or presence of various doses of LTA from S. sanguis or S. mutans (A) or LA-14-PP (B) for 24 h. (C) Confluent CD14high HGF were stimulated with 10 ng of LPS per ml in the absence or presence of anti-CD14 MAb MY4 (1:500 and 1:1,000) or isotype-matched mouse IgG2b (1:500 and 1:1,000) for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by an ELISA. Error bars indicate SD.
S. sanguis and S. mutans LTAs act as antagonists of lipid A.
Since lipid A has been shown to be the bioactive center of LPS (30), we next examined whether the activity of lipid A was also inhibited by the antagonistic LTAs. The IL-8-inducing effect of a synthetic lipid A (LA-15-PP) (10 ng/ml) on CD14high HGF was significantly inhibited by a 10- to 100-fold excess of S. sanguis and S. mutans LTAs and completely inhibited by a 1,000-fold excess of either LTA (Fig. 5A). This activity was also completely inhibited by LA-14-PP at 1:1 and by an anti-CD14 MAb (Fig. 5B and C). These results indicate that inactive LTA antagonized not only LPS but also its active moiety, lipid A, through a CD14-dependent mechanism.
FIG. 5.
S. sanguis and S. mutans LTAs acted as antagonists of lipid A. (A and B) Confluent CD14high HGF were stimulated with 10 ng of LA-15-PP per ml in the absence or presence of various doses of S. sanguis or S. mutans LTA (A) or LA-14-PP (B) for 24 h. (C) Confluent CD14high HGF were stimulated with 10 ng of LA-15-PP per ml in the absence or presence of anti-CD14 MAb MY4 (1:500 and 1:1,000) or isotype-matched mouse IgG2b (1:500 and 1:1,000) for 24 h. The culture supernatants were collected and analyzed for the presence of IL-8 by an ELISA. Error bars indicate SD.
Inhibition of the activity of B. subtilis LTA by antagonistic LTAs, LA-14-PP, and an anti-CD14 MAb.
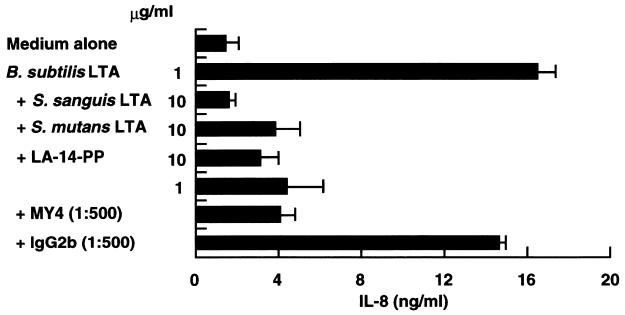
The finding that B. subtilis LTA stimulated mainly CD14high HGF (Fig. 2 and 3A) suggested that the stimulation of CD14high HGF by B. subtilis LTA is mediated by a CD14 pathway. To examine this possibility, CD14high HGF were stimulated with 1 μg of B. subtilis LTA per ml in the presence of LPS antagonists. Figure 6 demonstrates that the secretion of IL-8 from CD14high HGF induced by B. subtilis LTA stimulation was inhibited by S. sanguis and S. mutans LTAs (10:1), LA-14-PP (10:1), and an anti-CD14 MAb (1:500). These results suggest that the stimulation of CD14high HGF by agonistic B. subtilis LTA is mediated in a CD14-dependent manner and that S. sanguis and S. mutans LTAs antagonize not only LPS but also agonistic LTA functions.
FIG. 6.
LPS antagonists inhibited B. subtilis LTA-stimulated IL-8 secretion by HGF. Confluent CD14high HGF were stimulated with 1 μg of B. subtilis LTA per ml in the absence or presence of S. sanguis LTA (10 μg/ml), S. mutans LTA (10 μg/ml), LA-14-PP (1 and 10 μg/ml), anti-CD14 MAb MY4 (1:500), or isotype-matched mouse IgG2b (1:500) for 24 h. The amount of IL-8 in the supernatants was analyzed by an ELISA. Error bars indicate SD.
B. subtilis LTA and the reference LPS stimulated IL-1β secretion by human monocytes, and the stimulation was inhibited by S. sanguis LTA.
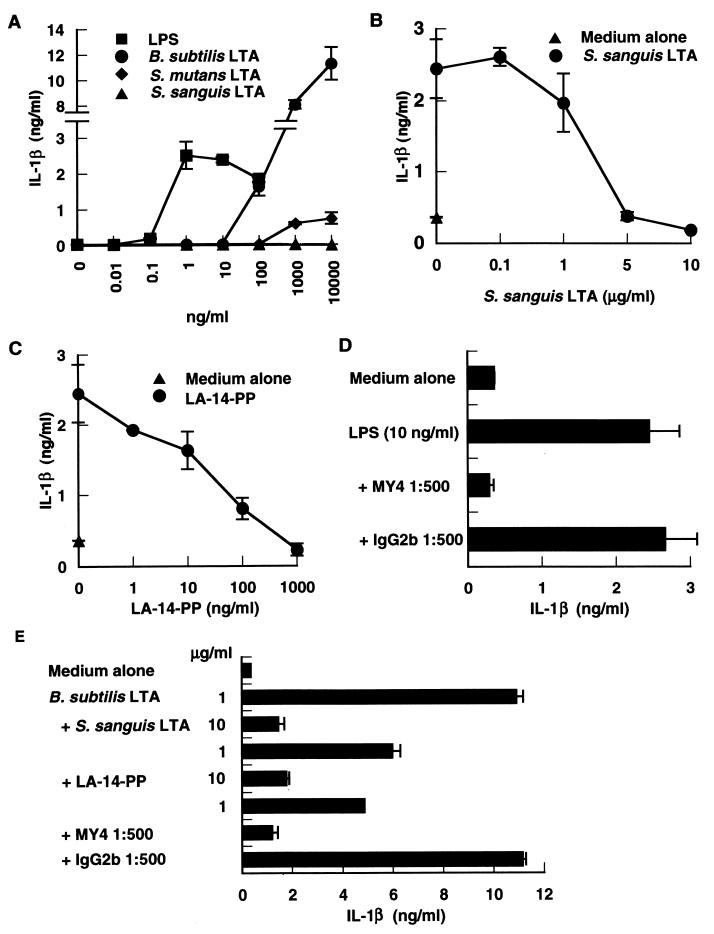
Since CD14 was originally described as a differentiation antigen on monocytes (33, 45), we next analyzed the antagonistic and agonistic effects of LTAs on human monocytes by the determination of monokine (IL-1β) secretion by PBMC cultures. IL-1β secretion by PBMC peaked at 1 ng of the reference LPS per ml and decreased slightly at the higher concentrations of LPS (Fig. 7A). B. subtilis LTA at 100 ng/ml began to stimulate IL-1β secretion and the secretion was markedly increased at 1 and 10 μg of the LTA per ml. In contrast, S. sanguis LTA had no effect and S. mutans LTA had only a marginal effect on IL-1β secretion. The LPS-induced IL-1β secretion was completely inhibited by S. sanguis LTA at a 500- to 1,000-fold excess (Fig. 7B), by LA-14-PP at a 100-fold excess (Fig. 7C), and by an anti-CD14 MAb (Fig. 7D). B. subtilis LTA activity was also inhibited by the presence of S. sanguis LTA (10:1), LA-14-PP (10:1), and an anti-CD14 MAb (Fig. 7E). The antagonistic effect of S. mutans LTA on human monocytes was weaker than that of S. sanguis LTA (data not shown), probably because of the existence of marginal stimulatory activity for human monocytes in the S. mutans LTA preparation. These results indicate that the antagonistic and agonistic functions of LTA are also applicable to human monocytes.
FIG. 7.
B. subtilis LTA and the reference LPS stimulated IL-1β secretion from human monocytes, and the stimulation was inhibited by S. sanguis LTA. (A) PBMC (2 × 105 cells/well) were stimulated with various doses of S. abortusequi LPS and LTAs from B. subtilis, S. sanguis, and S. mutans for 24 h. (B to D) PBMC (2 × 105 cells/well) were stimulated with 10 ng of S. abortusequi LPS per ml in the absence or presence of the indicated concentrations of S. sanguis LTA (B), LA-14-PP (C), or anti-CD14 MAb MY4 or isotype-matched mouse IgG2b (D) for 24 h. (E) PBMC (2 × 105 cells/well) were stimulated with 1 μg of B. subtilis LTA per ml in the absence or presence of the indicated concentration of S. sanguis LTA, LA-14-PP, MY4, or isotype-matched mouse IgG2b for 24 h. The amount of IL-1β in the supernatants was analyzed by an ELISA. Error bars indicate SD.
LPS antagonists do not affect the activity of PMA for HGF and human monocytes.
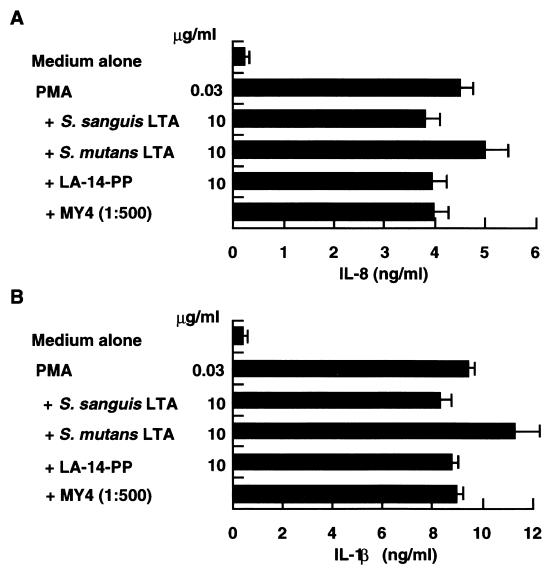
To rule out the possibility that the observed inhibitory activities of S. sanguis and S. mutans LTAs were due to nonspecific inhibition of protein synthesis or cellular secretion, CD14high HGF and PBMC were stimulated with 30 ng of PMA per ml in the absence or presence of S. sanguis and S. mutans LTAs, LA-14-PP, and an anti-CD14 MAb. None of the inhibitors of LPS exerted a significant inhibitory effect on PMA-induced IL-8 and IL-1β secretion from HGF and human monocytes, respectively (Fig. 8). These antagonists also had no effect on phytohemagglutinin-induced T-cell proliferation (data not shown). Thus, the inhibitory effects of S. sanguis and S. mutans LTAs appear to be specific for LPS and agonistic LTA.
FIG. 8.
LPS antagonists did not affect the effect of PMA on HGF and human monocytes. Confluent CD14high HGF (A) and PBMC (2 × 105 cells/well) (B) were stimulated with 30 ng of PMA per ml in the absence or presence of S. sanguis LTA (10 μg/ml), S. mutans LTA (10 μg/ml), LA-14-PP (10 μg/ml), or anti-CD14 MAb MY4 (1:500) for 24 h. The amount of IL-8 from HGF or IL-1β from human monocytes in the supernatants was analyzed by an ELISA. Error bars indicate SD.
Antagonistic LTAs compete with LPS for binding to CD14 of human monocytes.
To determine which step in CD14-mediated cell activation is inhibited by antagonistic LTAs, we examined whether LPS binding is inhibited by antagonistic LTAs by using BODIPY-LPS. Binding of BODIPY-LPS (0.1 μg/ml) to human monocytes was inhibited completely by S. abortusequi LPS at 1 μg/ml and higher concentrations and significantly at 0.1 μg/ml (1:1) (Fig. 9A). LPS binding was also inhibited completely by S. sanguis LTA at 100 μg/ml and significantly at 10 μg/ml, and the amount of S. sanguis LTA required a 100-fold higher concentration than LPS. However, agonistic B. subtilis LTA showed only partial competition with LPS. An anti-CD14 MAb also completely inhibited BODIPY-LPS binding (Fig. 9B) and markedly inhibited BODIPY-LTA binding (Fig. 9C).
FIG. 9.
S. sanguis LTA competes with LPS for binding to CD14 of human monocytes. PBMC (106 cells) were incubated with the indicated concentrations of S. abortusequi LPS (■), LTA of S. sanguis (●), and LTA of B. subtilis (▴) (A) and anti-CD14 MAb MY4 (1:100) or isotype-matched mouse IgG2b (1:100) (B and C) in 100 μl of PBS with 1% FCS for 20 min at 37°C. Then, BODIPY-LPS (0.1 μg/ml) (A and B) or BODIPY-LTA (1 μg/ml) (C) was added, and the mixtures were incubated for an additional 20 min at 37°C and analyzed with a FACS. The MFI of the monocyte populations was analyzed by gating on the basis of forward and side scatter characteristics. The MFI of the untreated monocyte population (3.22 ± 0.16) was subtracted, and the result was expressed as the change in the MFI (ΔMFI). An asterisk indicates a P value of <0.05 for the presence of competitors or MY4 versus BODIPY-LPS or BODIPY-LTA only. The results are expressed as mean ΔMFI ± SD for triplicate cultures.
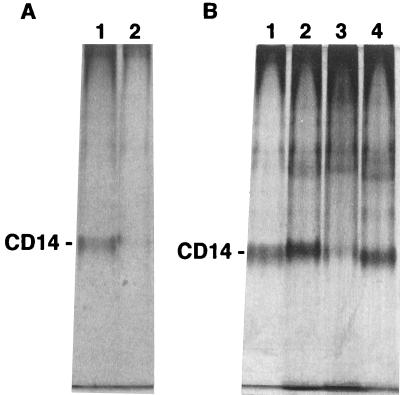
The direct interaction of LTAs with CD14 was examined by a gel shift assay with native PAGE. Incubation of the reference S. abortusequi LPS (500 μg/ml) with purified sCD14 (30 μg/ml) resulted in markedly increased electrophoretic mobility of purified sCD14 to the dye front (Fig. 10A), and LPS at 100 μg/ml induced slightly increased mobility of purified sCD14 (data not shown). The mobilities of purified sCD14 to the dye front were slightly decreased, increased, and slightly increased after incubation with LTAs (100 μg/ml) of B. subtilis, S. sanguis, and S. mutans, respectively (Fig. 10B). These results suggest that the shifts in electrophoretic mobilities were caused by stable binding of LTAs to sCD14.
FIG. 10.
Incubation of LTAs with sCD14 induces shifts in the electrophoretic mobility of CD14 in a gel shift assay with native PAGE. (A) Purified sCD14 (30 μg/ml) was incubated without (lane 1) or with (lane 2) S. abortusequi LPS (500 μg/ml) for 2 h at 37°C. (B) Purified sCD14 (30 μg/ml) was incubated without (lane 1) or with 100 μg of B. subtilis LTA (lane 2), S. sanguis LTA (lane 3), or S. mutans LTA (lane 4) per ml for 2 h at 37°C. Samples were then loaded on a 8% native polyacrylamide gel, which was subsequently stained with silver.
DISCUSSION
LTA is an amphiphile and consists of two parts. One is a polyglycerophosphate which participates in glycerol chain elongation; the other is a glycolipid moiety which anchors the gram-positive bacterial cytoplasmic membrane by hydrophobic interaction in a manner similar to the interaction between the lipid A of LPS and the gram-negative bacterial outer membrane. Since the structures of both parts of LTA vary among gram-positive bacterial species (8), the functional differences (agonistic and antagonistic) in B. subtilis, S. mutans, and S. sanguis LTAs may be due to the different structures of the LTAs of these bacteria. This explanation is supported by the opposite functions of lipid A and LA-14-PP. LA-14-PP lacks the two fatty acid chains (acyl-oxy-acyl structure) of Escherichia coli-type lipid A, and this structural difference leads to the functional difference. LA-14-PP acts as an antagonist against LPS and lipid A on human cells (2, 11, 12, 20) (Fig. 4B, 5B, and 7C).
The chemical structures of B. subtilis LTA and S. sanguis LTA have been described (8, 13). Since the structures of LTAs vary even within bacterial species (8), the structures of the LTAs used in this study have not been clarified. S. sanguis LTA was purified from a commercial preparation by hydrophobic interaction chromatography, and the purity of the LTA preparations used in this study was confirmed by the Limulus test (LPS activity) and the SLP test (peptidoglycan/β-glucan activity) as described in Materials and Methods. Therefore, we considered that the LTA preparations were sufficiently pure to examine their functions and believe that the results of this study represent the functions of the LTAs, although we cannot completely exclude the possibility of the influence of minor constituents in the preparations.
The amount of contamination by LPS in the B. subtilis LTA preparation was smaller than that in the S. mutans LTA preparation (as described in Materials and Methods), and the level of IL-1β induced from human monocytes by B. subtilis LTA was markedly higher than that induced by LPS (Fig. 7A), suggesting that the activity of the LTA specimen was not attributable to LPS contamination. Recent studies indicated that a purified major LTA fraction of E. hirae and the synthetic compounds mimicking the LTA structure had no biological activities in several bioassays (14, 18, 29, 31). Purified LTA of S. aureus antagonized the action of LPS (18), as confirmed with S. sanguis and S. mutans LTAs in this study, and an active molecule of a phenol extract of S. aureus also bound to CD14 (19). These observations suggest that an active component in the B. subtilis LTA preparation may also be distinct from common LTA.
In this study, we compared LTA to LPS based on the weights of these materials. Comparisons based on the molar amounts instead of the weights were difficult since (i) the structure and molecular weight of LTA are heterogeneous not only among gram-positive bacterial species but also within a given species (8) and (ii) the identity of the active moiety of LTA is still controversial, as discussed above. Lipid A is the bioactive center of LPS (30), but the molecular weight of the polysaccharide portion of LPS, including that of S. abortusequi, varies since it is made up of different repeating oligosaccharide units (10).
CD14high HGF secreted IL-8 in response to LPS, and the response was inhibited by the LPS antagonist LA-14-PP and an anti-CD14 MAb (Fig. 4). CD14low HGF showed only a marginal response to LPS, and the responses of the two types of HGF to PMA were not significantly different (Fig. 3). HGF expressed no β2-integrin family, other LPS receptors such as CD11b/CD18 and CD11c/CD18, on the cell surface (27). These results therefore clearly indicate that the response of CD14high HGF to LPS is mediated by a CD14 pathway, as reported previously (27). Aida et al. (2) reported that LA-14-PP and Rhodobacter sphaeroides LPS inhibited the action of E. coli LPS by blocking LPS receptor recognition and/or depleting LPS-binding protein. Golenbock et al. (12) suggested that LA-14-PP competed with the lipid A portion of LPS for the LPS signaling receptor. Jarvis et al. (15) reported that R. sphaeroides diphosphoryl lipid A bound to CD14 and inhibited binding by LPS. In the present study, the binding of fluoro-labeled LPS to human monocytes was inhibited by S. sanguis LTA and by an anti-CD14 MAb, and an anti-CD14 MAb inhibited fluorolabeled S. sanguis LTA binding to human monocytes (Fig. 9). A gel shift assay showed the direct interaction of LTAs with CD14 (Fig. 10). Therefore, inhibition of the LPS response by LTAs of S. sanguis and S. mutans indicates that the antagonistic LTAs compete with LPS for binding to CD14.
B. subtilis LTA as well as the reference LPS selectively stimulated CD14high HGF and human monocytes but not CD14low HGF, and the stimulation was inhibited by LPS antagonists (LA-14-PP and MY4) and LTAs of S. sanguis and S. mutans in patterns similar to those seen with LPS stimulation. Although competition of LPS binding by B. subtilis LTA was 10 times weaker than that by S. sanguis LTA (Fig. 9A), the gel shift assay showed the binding of B. subtilis LTA to CD14 (Fig. 10). These results suggest that an active component in the preparation activates the cells through a CD14-dependent mechanism.
Complete inhibition of the effect of LPS on CD14high HGF and human monocytes was observed at a 1,000-fold excess of S. sanguis and S. mutans LTAs and at a 10- to 100-fold excess of LA-14-PP (Fig. 4, 5, and 7). In contrast, high doses of B. subtilis LTA (1 to 10 μg/ml) were required for cell activation, and the activity was markedly inhibited by a 10-fold excess of the antagonistic LTAs (S. sanguis and S. mutans) and LA-14-PP (Fig. 6 and 7). The inhibition of fluorolabeled LPS binding by S. sanguis LTA was 100 times weaker than that by LPS (Fig. 9). These results suggest that the affinity of LTA for CD14 is weaker than that of LPS; therefore, LTA showed a low efficiency for activating cells compared with LPS and for competing with LPS for CD14 compared with LA-14-PP.
It is possible that the antagonistic function of S. sanguis and S. mutans LTAs was the result of the nonspecific inhibition of protein synthesis or cellular secretion of LPS and B. subtilis LTA under our experimental conditions. However, S. sanguis and S. mutans LTAs (10 μg/ml each), LA-14-PP (10 μg/ml), and MY4 (1:500) did not significantly inhibit PMA-induced IL-8 and IL-1β secretion from HGF and human monocytes, respectively (Fig. 8). They also did not affect phytohemagglutinin-induced T-cell proliferation (data not shown). These results exclude the possibility of nonspecific inhibition and suggest that the LTAs specifically compete with LPS and B. subtilis LTA functions. The removal of the free antagonistic LTAs as well as LPS antagonists (LA-14-PP and MY4) after a 60-min incubation resulted in the partial inhibition of the LPS and B. subtilis LTA effects (data not shown). This evidence suggests that the existence of these antagonists in the culture is required for the efficient inhibition of agonist function. Although the above findings strongly suggested that S. sanguis and S. mutans LTAs are competitive antagonists of LPS, like the reference glycolipid antagonist LA-14-PP (2, 11, 12, 20), the possibility that inhibition by the antagonistic LTAs is due to sequestration of agonists (12) was not completely ruled out in this study.
Finally, the present results indicate that (i) an antagonistic LTA of gram-positive cocci in the oral cavity may inhibit the action of LPS from periodontopathic gram-negative bacteria, possibly resulting in the inhibition of the initiation of periodontal disease, and (ii) an antagonistic LTA may be useful as an agent for suppressing the periodontal disease caused by gram-negative bacteria, since the use of antibiotics may unavoidably cause the induction of drug-resistant bacteria.
ACKNOWLEDGMENTS
We thank Takami Matsuyama (Kagoshima University Medical School, Kagoshima, Japan) and Akiko Sugiyama (University of Tokushima School of Dentistry, Tokushima, Japan) for supplying a hybridoma producing anti-human CD14 MAb D10 and HGF, respectively. We also thank Takeshi Yoshida (Chugai Pharmaceutical Co., Ltd., Tokyo, Japan), Shoichi Kusumoto (Osaka University Graduate School of Science, Osaka, Japan), and Shozo Kotani (Osaka University) for helpful discussions and suggestions; Yuri Togashi for expert editorial assistance; and D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper.
This work was supported in part by grants-in-aid for scientific research from the Ministry of Education, Sports, Science and Culture, Japan (09671843 and 10470378), and by a grant-in-aid for scientific research from Chugai Pharmaceutical Co., Ltd.
REFERENCES
- 1.Abo T, Sugawara S, Amenomori A, Itoh H, Rikiishi H, Moro I, Kumagai K. Selective phagocytosis of gram-positive bacteria and interleukin 1-like factor production by a subpopulation of large granular lymphocytes. J Immunol. 1986;136:3189–3197. [PubMed] [Google Scholar]
- 2.Aida Y, Kusumoto K, Nakatomi K, Takada H, Pabst M J, Maeda K. An analogue of lipid A and LPS from Rhodobacter sphaeroides inhibits neutrophil responses to LPS by blocking receptor recognition of LPS and by depleting LPS-binding protein in plasma. J Leukocyte Biol. 1995;58:675–682. doi: 10.1002/jlb.58.6.675. [DOI] [PubMed] [Google Scholar]
- 3.Ashida M, Yamazaki H I. Biochemistry of the phenoloxidase system in insects: with special reference to its activation. In: Ohnishi E, Ishizaki H, editors. Molting and metamorphosis. Tokyo, Japan: Japan Scientific Societies Press; 1990. pp. 239–265. [Google Scholar]
- 4.Bhakdi S, Klonisch T, Nuber P, Fischer W. Stimulation of monokine production by lipoteichoic acids. Infect Immun. 1991;59:4614–4620. doi: 10.1128/iai.59.12.4614-4620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bucher M, Ittner K P, Zimmermann M, Wolf K, Hobbhahn J, Kurtz A. Nitric oxide synthase isoform III gene expression in rat liver is up-regulated by lipopolysaccharide and lipoteichoic acid. FEBS Lett. 1997;412:511–514. doi: 10.1016/s0014-5793(97)00835-1. [DOI] [PubMed] [Google Scholar]
- 6.Cleveland M G, Gorham J D, Murphy T L, Tuomanen E, Murphy K M. Lipoteichoic acid preparations of gram-positive bacteria induce interleukin-12 through a CD14-dependent pathway. Infect Immun. 1996;64:1906–1912. doi: 10.1128/iai.64.6.1906-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Espevik T, Otterlei M, Skjåk-Bræk G, Ryan L, Wright S D, Sundan A. The involvement of CD14 in stimulation of cytokine production by uronic acid polymers. Eur J Immunol. 1993;23:255–261. doi: 10.1002/eji.1830230140. [DOI] [PubMed] [Google Scholar]
- 8.Fischer W. Bacterial phosphoglycolipids and lipoteichoic acids. In: Kates M, editor. Handbook of lipid research, vol. 6. Glycolipids, phosphoglycolipids, and sulfoglycolipids. New York, N.Y: Plenum Press; 1990. pp. 123–234. [Google Scholar]
- 9.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 10.Galanos C, Lüderitz O, Westphal O. Preparation and properties of a standardized lipopolysaccharide from Salmonella abortus equi (Novo-Pyrexal) Zentbl Bakteriol Mikrobiol Hyg Abt 1 Orig Reihe A. 1979;243:226–244. [PubMed] [Google Scholar]
- 11.Golenbock D T, Hampton R Y, Qureshi N, Takayama K, Raetz C R H. Lipid A-like molecules that antagonize the effect of endotoxins on human monocytes. J Biol Chem. 1991;266:19490–19498. [PubMed] [Google Scholar]
- 12.Golenbock D T, Lynn W A, Hampton R Y, Millham F H, Liu Y. Characterization of LPS signaling pathways with the LPS inhibitors lipid IVa (IA, LA-14-PP) and Rhodobacter sphaeroides lipid A. In: Levin J, Alving C R, Munford R S, Stutz P L, editors. Bacterial endotoxin: recognition and effector mechanisms. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1993. pp. 349–359. [Google Scholar]
- 13.Hailman E, Lichenstein H S, Wurfel M M, Miller D S, Johnson D A, Kelley M, Busse L A, Zukowski M M, Wright S D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994;179:269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hashimoto M, Yasuoka J, Suda Y, Takada H, Yoshida T, Kotani S, Kusumoto S. Structural feature of the major but not cytokine-inducing molecular species of lipoteichoic acid. J Biochem. 1997;121:779–786. doi: 10.1093/oxfordjournals.jbchem.a021653. [DOI] [PubMed] [Google Scholar]
- 15.Jarvis B W, Lichenstein H, Qureshi N. Diphosphoryl lipid A from Rhodobacter sphaeroides inhibits complexes that form in vitro between lipopolysaccharide (LPS)-binding protein, soluble CD14, and spectrally pure LPS. Infect Immun. 1997;65:3011–3016. doi: 10.1128/iai.65.8.3011-3016.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keller R, Fischer W, Keist R, Bassetti S. Macrophage response to bacteria: induction of marked secretory and cellular activities by lipoteichoic acids. Infect Immun. 1992;60:3664–3672. doi: 10.1128/iai.60.9.3664-3672.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kochanowski B, Fischer W, Iida-Tanaka N, Ishizuka I. Isomalto-oligosaccharide-containing lipoteichoic acid of Streptococcus sanguis. Basic structure. Eur J Biochem. 1993;214:747–755. doi: 10.1111/j.1432-1033.1993.tb17976.x. [DOI] [PubMed] [Google Scholar]
- 18.Kusunoki T, Hailman E, Juan T S-C, Lichenstein H S, Wright S D. Molecules from Staphylococcus aureus that bind CD14 and stimulate innate immune responses. J Exp Med. 1995;182:1673–1682. doi: 10.1084/jem.182.6.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kusunoki T, Wright S D. Chemical characteristics of Staphylococcus aureus molecules that have CD14-dependent cell-stimulating activity. J Immunol. 1996;157:5112–5117. [PubMed] [Google Scholar]
- 20.Loppnow H, Brade H, Durrbaum I, Dinarello C A, Kusumoto S, Rietschel E T, Flad H-D. IL-1 induction-capacity of defined lipopolysaccharide partial structures. J Immunol. 1989;142:3229–3238. [PubMed] [Google Scholar]
- 21.Morrissey J H. Silver stain for proteins in polyacrylamide gels: a modified procedure with enhanced uniform sensitivity. Anal Biochem. 1981;117:307–310. doi: 10.1016/0003-2697(81)90783-1. [DOI] [PubMed] [Google Scholar]
- 22.Nisengard R J, Newman M G, Zambon J J. Periodontal disease. In: Nisengard R J, Newman M G, editors. Oral microbiology and immunology. 2nd ed. Philadelphia, Pa: The W. B. Saunders Co.; 1994. pp. 360–384. [Google Scholar]
- 23.Obayashi T, Tamura H, Tanaka S, Ohki M, Takahashi S, Arai M, Masuda M, Kawai T. A new chromogenic endotoxin-specific assay using recombined Limulus coagulation enzymes and its clinical applications. Clin Chim Acta. 1985;149:55–65. doi: 10.1016/0009-8981(85)90273-6. [DOI] [PubMed] [Google Scholar]
- 24.Pugin J, Heumann D, Tomasz A, Kravchenko V V, Akamatsu Y, Nishijima M, Glauser M P, Tobias P S, Ulevitch R J. CD14 is a pattern recognition receptor. Immunity. 1994;1:509–516. doi: 10.1016/1074-7613(94)90093-0. [DOI] [PubMed] [Google Scholar]
- 25.Sellati T J, Bouis D A, Kitchens R L, Darveau R P, Pugin J, Ulevitch R J, Gangloff S C, Goyert S M, Norgard M V, Radolf J D. Treponema pallidum and Borrelia burgdorferi lipoproteins and synthetic lipopeptides activate monocytic cells via a CD14-dependent pathway distinct from that used by lipopolysaccharide. J Immunol. 1998;160:5455–5464. [PubMed] [Google Scholar]
- 26.Soell M, Lett E, Holveck F, Schöller M, Wachsmann D, Klein J-P. Activation of human monocytes by streptococcal rhamnose glucose polymers is mediated by CD14 antigen, and mannan binding protein inhibits TNF-α release. J Immunol. 1995;154:851–860. [PubMed] [Google Scholar]
- 27.Sugawara S, Sugiyama A, Nemoto E, Rikiishi H, Takada H. Heterogeneous expression and release of CD14 by human gingival fibroblasts: characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect Immun. 1998;66:3043–3049. doi: 10.1128/iai.66.7.3043-3049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sugiyama A, Arakaki R, Ohnishi T, Arakaki N, Daikuhara Y, Takada H. Lipoteichoic acid and interleukin 1 stimulate synergistically production of hepatocyte growth factor (scatter factor) in human gingival fibroblasts in culture. Infect Immun. 1996;64:1426–1431. doi: 10.1128/iai.64.4.1426-1431.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takada H, Kawabata Y, Arakaki R, Kusumoto S, Fukase K, Suda Y, Yoshimura T, Kokeguchi S, Kato K, Komuro T, Tanaka N, Saito M, Yoshida T, Sato M, Kotani S. Molecular and structural requirements of a lipoteichoic acid from Enterococcus hirae ATCC 9790 for cytokine-inducing, antitumor, and antigenic activities. Infect Immun. 1995;63:57–65. doi: 10.1128/iai.63.1.57-65.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takada H, Kotani S. Structural requirements of lipid A for endotoxicity and other biological activities. Crit Rev Microbiol. 1989;16:477–523. doi: 10.3109/10408418909104475. [DOI] [PubMed] [Google Scholar]
- 31.Takada H, Kotani S. Immunomodulating activities of streptococcal lipoteichoic acids. In: Masihi N, editor. Immunotherapy of infections. New York, N.Y: Marcel Dekker, Inc.; 1994. pp. 309–328. [Google Scholar]
- 32.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 34.Usami H, Yamamoto A, Sugawara Y, Hamada S, Yamamoto T, Kato K, Kokeguchi S, Takada H, Kotani S. A nontoxic tumor necrosis factor induced by streptococcal lipoteichoic acids. Br J Cancer. 1987;56:797–799. doi: 10.1038/bjc.1987.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Usami H, Yamamoto A, Yamashita W, Sugawara Y, Hamada S, Yamamoto T, Kato K, Kokeguchi S, Ohokuni H, Kotani S. Antitumor effects of streptococcal lipoteichoic acids on Meth A fibrosarcoma. Br J Cancer. 1988;57:70–73. doi: 10.1038/bjc.1988.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weidemann B, Brade H, Rietschel E T, Dziarski R, Bazil V, Kusumoto S, Flad H, Ulmer A J. Soluble peptidoglycan-induced monokine production can be blocked by anti-CD14 monoclonal antibodies and by lipid A partial structures. Infect Immun. 1994;62:4709–4715. doi: 10.1128/iai.62.11.4709-4715.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wicken A J, Knox K W. Bacterial cell surface amphiphiles. Biochim Biophys Acta. 1980;604:1–26. doi: 10.1016/0005-2736(80)90583-0. [DOI] [PubMed] [Google Scholar]
- 38.Wooten R M, Morrison T B, Weis J H, Wright S D, Thieringer R, Weis J J. The role of CD14 in signaling mediated by outer membrane lipoproteins of Borrelia burgdorferi. J Immunol. 1998;160:5485–5492. [PubMed] [Google Scholar]
- 39.Wright S D. CD14 and innate recognition of bacteria. J Immunol. 1995;155:6–9. [PubMed] [Google Scholar]
- 40.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 41.Yamamoto A, Usami H, Nagamuta M, Sugawara Y, Hamada S, Yamamoto T, Kato K, Kokeguchi S, Kotani S. The use of lipoteichoic acid (LTA) from Streptococcus pyogenes to induce a serum factor causing tumour necrosis. Br J Cancer. 1985;51:739–742. doi: 10.1038/bjc.1985.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu B, Wright S D. Catalytic properties of lipopolysaccharide (LPS) binding protein. Transfer of LPS to soluble CD14. J Biol Chem. 1996;271:4100–4105. doi: 10.1074/jbc.271.8.4100. [DOI] [PubMed] [Google Scholar]
- 43.Yu S, Nakashima N, Xu B H, Matsuda T, Izumihara A, Sunahara N, Nakamura T, Tsukano M, Matsuyama T. Pathological significance of elevated soluble CD14 production in rheumatoid arthritis: in the presence of soluble CD14, lipopolysaccharides at low concentrations activate RA synovial fibroblasts. Rheumatol Int. 1998;17:237–243. doi: 10.1007/s002960050041. [DOI] [PubMed] [Google Scholar]
- 44.Zhang Y, Doerfler M, Lee T C, Guillemin B, Rom W N. Mechanisms of stimulation of interleukin-1β and tumor necrosis factor-α by Mycobacterium tuberculosis components. J Clin Investig. 1993;91:2076–2083. doi: 10.1172/JCI116430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ziegler-Heitbrock H W L, Ulevitch R J. CD14: cell surface receptor and differentiation marker. Immunol Today. 1993;14:121–125. doi: 10.1016/0167-5699(93)90212-4. [DOI] [PubMed] [Google Scholar]