Abstract
We investigated whether vascular endothelial growth factor (VEGF) production by human pulp cells (HPC) is regulated by lipopolysaccharide (LPS) in relation to the pathogenesis of pulpitis. Although HPC incubated with medium alone only marginally expressed VEGF mRNA and produced a low level of VEGF as detected by enzyme-linked immunosorbent assay, the VEGF mRNA expression and VEGF production were markedly enhanced upon stimulation with LPS from Escherichia coli. Prevotella intermedia LPS, phorbol 12-myristate 13-acetate, and interleukin-6 also induced VEGF mRNA expression in HPC. A simian virus 40-infected HPC line also exhibited increased VEGF mRNA expression in response to E. coli LPS, but lung and skin fibroblasts did not. Fetal bovine serum (FBS) increased the sensitivity of HPC to LPS in a dose-dependent manner. HPC did not express membrane CD14 on their surfaces. However, the anti-CD14 monoclonal antibody MY4 inhibited VEGF induction upon stimulation with LPS in HPC cultures in the presence of 10% FBS but not in the absence of FBS. LPS augmented the VEGF production in HPC cultures in the presence of recombinant human soluble CD14 (sCD14). To clarify the mechanisms of VEGF induction by LPS, we examined the possible activation of the transcription factor AP-1 in HPC stimulated with LPS, by a gel mobility shift assay. AP-1 activation in HPC was clearly observed, whereas that in skin fibroblasts was not. The AP-1 inhibitor curcumin strongly inhibited LPS-induced VEGF production in HPC cultures. In addition, a protein synthesis inhibitor, cycloheximide, inhibited VEGF mRNA accumulation in response to LPS. These results suggest that the enhanced production of VEGF in HPC induced by LPS takes place via an sCD14-dependent pathway which requires new protein synthesis and is mediated in part through AP-1 activation.
An increase of vascular permeability is involved in the acute phase of pulpitis as well as in acute inflammation elsewhere in the body. However, in the case of pulpitis, an excessive increase of vascular permeability easily results in edema and necrosis, due to the specific anatomic characteristics of the pulp tissue. The pulp tissue is enclosed in a rigid structure, and blood is supplied only through a small apical foramen. This foramen is used for both blood supply and drainage. Furthermore, pulp tissue has no collateral blood supply, so it is difficult to rapidly eliminate filtrated fluid. Thus, pulp tissue, having such a limited system of discharge, is susceptible to irreversible pulpitis.
Vasoactive inflammatory substances such as histamine, bradykinin, serotonin, prostaglandins (PGs), and leukotrienes are known as mediators that increase vascular permeability in pulp tissues (14). Vascular endothelial growth factor (VEGF) has also recently attracted attention as a potent inducer of vascular permeability and angiogenesis (7) and is involved in the occurrence and progression of inflammation (12). VEGF is thought of as a mitogenic factor specific for vascular endothelial cells and acts in a paracrine manner. However, we recently demonstrated that VEGF is also produced by human pulp cells (HPC) and that VEGF is mitogenic not only on vascular endothelial cells but also on HPC, in an autocrine manner (17).
Various components and products of bacteria which invade the dentin and root canal are associated with the pathogenesis of pulpitis (19). One of these components, lipopolysaccharide (LPS), is a potent inducer of pulpitis (37). LPS induces many inflammatory cytokines such as interleukin-1 (IL-1), IL-6, IL-8, and tumor necrosis factor alpha (TNF-α) from human peripheral blood mononuclear cells and gingival fibroblasts (16, 28). LPS also induces increased arachidonic acid metabolism in the pulp; LPS was shown to induce PGE2 and PGI2 production, resulting in an increase of vascular permeability and neutrophil infiltration (18). The pulp tissue thus treated with LPS became acutely inflamed. VEGF could increase vascular permeability in pulp tissue, and its activity was 50,000 times stronger than that of histamine (25). However, it is not yet known whether HPC produce VEGF in response to LPS stimulation or whether VEGF is associated with the pathogenesis of pulpitis.
In this study, we first investigated whether LPS can induce VEGF in HPC cultures, and we then attempted to clarify the mechanism of VEGF induction by LPS. We also discuss the possible involvement of VEGF in the pathogenesis of pulpitis.
MATERIALS AND METHODS
Specimens and probes.
LPS was prepared from Prevotella intermedia ATCC 25611 by the hot phenol-water extraction method as described previously (10). LPS extracted with hot phenol-water from Escherichia coli O55:B5, phorbol 12-myristate 13-acetate (PMA), mithramycin A, N-tosyl-l-phenylalanine chloromethyl ketone (TPCK), and cycloheximide were purchased from Sigma Chemical Co. (St. Louis, Mo.). Curcumin was purchased from Nacalai Tesque Inc. (Kyoto, Japan). Recombinant human IL-6 was provided by Ajinomoto Co. (Yokohama, Japan). The fluorescein isothiocyanate (FITC)-conjugated and nonconjugated anti-CD14 monoclonal antibody (MAb) MY4, isotype-matched control antibody mouse immunoglobulin G2b (MSIgG2b), FITC-conjugated anti-CD11b and CD18 MAbs, and isotype-matched control antibody MSIgG1a were purchased from Coulter Immunology (Hialeah, Fla.). A plasmid containing VEGF cDNA (40) was kindly provided by M. Shibuya (Faculty of Pharmaceutical Sciences, University of Tokyo, Tokyo, Japan). A plasmid containing human glyceraldehyde-3-phosphate dehydrogenase (GAPDH) cDNA (33) was a gift from I. Sakiyama (Chiba Cancer Center Research Institute and Hospital, Chiba, Japan). Each plasmid was digested with appropriate restriction enzymes to obtain cDNA probes which were then used for the Northern blot analysis.
Cells.
Specimens of normal human pulp tissue were obtained from first bicuspids extracted for orthodontologic reasons. The explants were cultured in Eagle’s modified minimum essential medium (EMEM; Nissui Pharmaceutical Co., Tokyo, Japan), supplemented with 10% fetal bovine serum (FBS; GIBCO Life Technologies, Inc., Grand Island, N.Y.) in plastic culture dishes with medium changes every 3 days for 10 to 15 days until confluent cell monolayers were formed. After three to four subcultures, homogeneous, slim, spindle-shaped cells (fibroblast-like cells) growing in characteristic swirls were obtained. These primary cultures of HPC (HPC-5 and HPC-10) were used as confluent monolayers at subculture passages 5 through 10. A simian virus 40 (SV40)-infected human pulp fibroblast cell line (LSC) was a gift from A. Sato (Tokyo Medical and Dental University, Tokyo, Japan). Human lung fibroblasts (MRC-5) and human skin fibroblasts (SF-MA) were obtained from the Japanese Cancer Research Resources Bank and used in some experiments as reference cells. HPC-5, HPC-10, and LSC had high alkaline phosphatase activity similar to that of osteoblasts, but MRC-5 and SF-MA did not.
Preparation of recombinant soluble CD14.
A λ ZAPII cDNA library was prepared from synovial cells with a Timesaver cDNA synthesis kit (Pharmacia, Uppsala, Sweden) and Gigapack II Plus packaging extract (Stratagene, La Jolla, Calif.) and then amplified by PCR with Extra DNA polymerase (Takara Shuzo, Tokyo, Japan) and T3 and T7 primers. A mammalian cDNA library was established by ligating PCR products to pcDL-SRa296 vector (29). Cos7 cells were transfected with this library to obtain cDNA encoding the antigen recognized by D10 antibody. The cells by which the antigen was transiently expressed were screened by panning methods (23). One of the positive clones which reacted with D10 antibody was sequenced, and this cDNA was homologous with CD14 cDNA (26). To delete eight C-terminal amino acids including a phosphatidyl-linkage portion, the full CD14 cDNA was used to induce a point mutation, with a site-directed mutagenesis kit (Takara) and an antisense primer (5′-GGGCCCCTTGTTACAGCACCAGG-3′). Cos7 cells were then transiently transfected with mutant CD14 and fed in CHO-S-SFMII, a low-protein and serum-free medium (Gibco BRL). After a 7-day incubation, the supernatant was collected and purified for recombinant human soluble CD14 (rHusCD14) with D10 antibody-coated beads prepared with a Hitrap N-hydroxysuccinimide-activated column (Pharmacia). The purity of the rHusCD14 protein was confirmed in a silver-stained gel after sodium dodecyl sulfate-polyacrylamide gel electrophoresis. The rHusCD14 concentration in the supernatants was determined with a protein assay kit (Bio-Rad, Hercules, Calif.). Finally, 17.0 μg of purified materials per ml was obtained from 1.5 liters of the culture supernatants.
VEGF expression in HPC cultures.
HPC suspensions (5 × 105 cells/ml) were seeded in the wells of flat-bottom microculture plates or 150-cm2 cell culture dishes (Nunc, Roskilde, Denmark) in EMEM supplemented with 10% FBS. After incubation for 4 days at 37°C in a 5% CO2 atmosphere, the cell monolayers were washed with EMEM three times, and 100 μl or 30 ml of EMEM without FBS was added, followed by culture for 24 h. The monolayers were subsequently washed with EMEM three times, and then test stimulants in EMEM with or without FBS were added. After a further incubation for 1 to 72 h, the supernatant and sediment were collected and used in the subsequent experiments. In competition experiments, HPC were incubated for 24 h at 37°C in a 5% CO2 atmosphere with E. coli LPS (1 μg/ml) in EMEM supplemented with 10% FBS in the presence of various concentrations of anti-CD14 MAb MY4. To examine the role of sCD14 in VEGF production induced by LPS, we incubated HPC with E. coli LPS for 24 h at 37°C in a 5% CO2 atmosphere in FBS-free EMEM in the presence of various concentration of rHusCD14. The culture supernatants were collected, and then the VEGF concentration in each supernatant was measured. In some experiments, various concentrations of curcumin, mithramycin A, and TPCK were added simultaneously with LPS to HPC cultures. After incubation for 24 h, the culture supernatants were collected, and the VEGF concentrations in the supernatants were measured.
Northern blot analysis.
The expression of VEGF mRNA by HPC was examined by Northern blot analysis as described previously (30). The total RNA from HPC was prepared by the acid guanidinium-phenol-chloroform procedure (3). Total RNA (20 μg per lane) was resolved by electrophoresis through a 1.2% agarose gel and transferred to a nylon membrane (Zeta-Probe; Bio-Rad Laboratories, Richmond, Calif.) by electroblotting (31). The membrane was hybridized with 32P-labeled cDNA probes (6), then washed and exposed to an imaging plate for 1 h for analysis by a Bio-Imaging analyzer (BAS 1000 Mac; Fuji Photo Film Co., Tokyo, Japan), and exposed to X-ray film (medical X-ray film; Konica, Tokyo, Japan) at −80°C for several days for autoradiography. The results are expressed as the relative mRNA accumulation, with GAPDH mRNA as an internal standard.
Measurement of VEGF protein.
The concentrations of VEGF protein in the culture supernatants were determined at the Tsukuba Research Laboratories of Toagosei Co. (Ibaragi, Japan) by enzyme-linked immunosorbent assay (ELISA).
Flow cytometry analysis.
The analysis of cell surface CD14, CD11b, and CD18 expression was conducted by a FACScan (Becton Dickinson, Mountain View, Calif.). HPC and THP-1 cells were grown in monolayers in cell culture dishes in Dulbecco’s modified minimum essential medium (DMEM) supplemented with 10% FBS. The cells were washed once with serum-free DMEM and detached from the dishes with 0.02% EDTA for 5 min. The detached cells were immediately placed in DMEM supplemented with 10% FBS. The detached HPC and THP-1 cells were washed twice with phosphate-buffered saline. The cells were incubated with the FITC-labeled anti-CD14, CD11b, and CD18 MAbs for 30 min at 4°C. As a negative control, the cells were also incubated with the isotype-matched control antibody MSIgG1a or MSIgG2b.
Analyses of DNA-transcription factor binding.
Nuclear extracts were prepared as described by Jimi et al. (13) and were used for the following experiments.
(i) Gel mobility shift assay.
This assay was conducted as described previously (13). Briefly, DNA binding was assayed with a synthetic oligonucleotide probe for wild-type AP-1 (5′-TTGATGACTCA-3′). Probes were labeled by T4 polynucleotide kinase with [γ-32P]ATP. Nuclear protein extracts (10 μg) were incubated for 30 min with the labeled probe at 30°C, then loaded onto a 5% polyacrylamide gel, and electrophoresed at 175 V for 70 min. After electrophoresis, the gels were dried and autoradiographed by exposure to X-ray film at −80°C for several days.
(ii) Fluorescence polarization analysis.
To test the activations of NF-κB and SP-1, a fluorescence polarization analysis of DNA-protein binding was performed by using a Beacon 2000 fluorescence polarization system (PanVera Co., Madison, Wis.) according to the manufacturer’s instructions. Briefly, DNA FITC-labeled oligonucleotide probes for wild-type SP-1 (5′-GATCGGGGCGGGGC-3′) and NF-κB (5′-AGCTTGGGGACTTTCCGAG-3′) were synthesized at the laboratories of Takara Shuzo. The three double-stranded FITC-labeled oligonucleotides were prepared by annealing in 1 M NaCl–10 mM potassium phosphate–0.1 mM EDTA at 95°C for 10 min. The various concentrations of the nuclear protein extracts (10 μl) were added to 190 μl of binding buffer (20 mM Tris-HCl [pH 7.6], 100 mM KCl, 0.1 mM EDTA, 1 mM dithiothreitol) containing an oligonucleotide (1 nM) in 6- by 50-mm borosilicate test tubes (PanVera Co.). The tubes were incubated at 10°C for 1 h, and then the fluorescence polarization values were determined. The data reported are the values which were deducted from the value in the control tube (binding buffer supplemented with FITC-labeled oligonucleotide alone).
Statistical analysis.
Most assays were carried out in triplicate. The significance of differences between the results of each test and the respective control was determined by one-way analysis of variance (ANOVA) and Scheffé’s F test.
RESULTS
VEGF induction by LPS in HPC cultures.
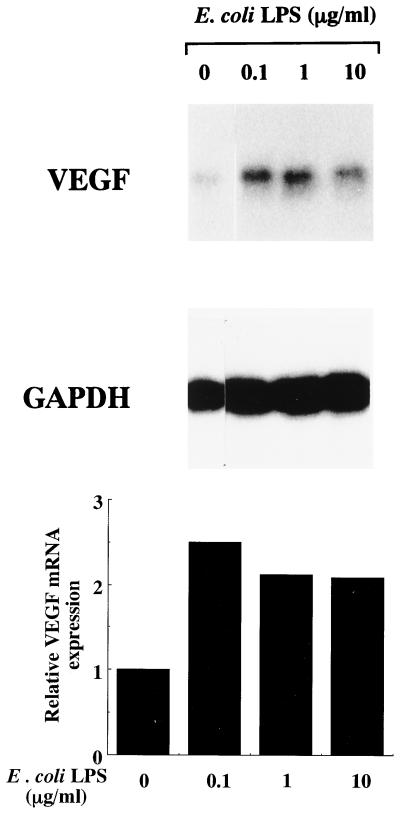
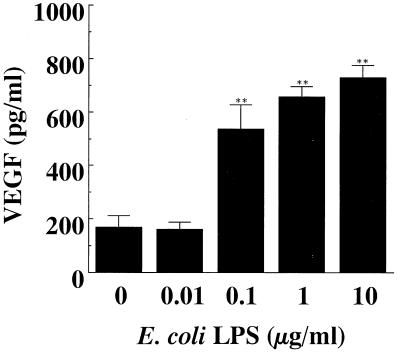
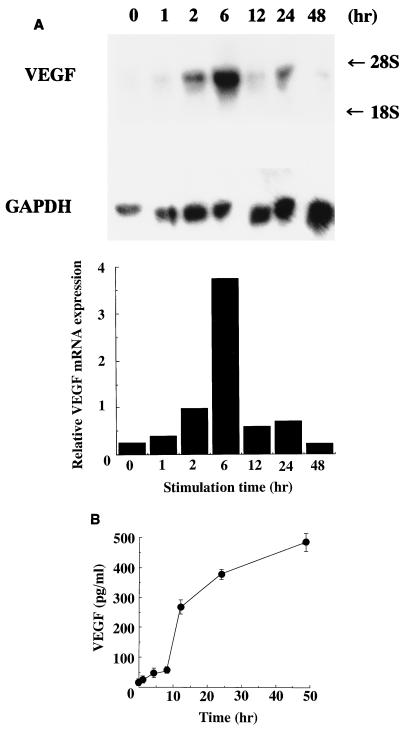
As shown in Fig. 1, E. coli LPS induced VEGF mRNA in HPC cultures, and the strongest expression of VEGF mRNA was observed when HPC were stimulated with 0.1 μg of E. coli LPS per ml. VEGF mRNA was marginally expressed by HPC even when the HPC were cultured with EMEM alone. In accord with the gene expression, VEGF was also detected in the supernatant of HPC cultures by ELISA, and the level was increased in correlation with the addition of LPS (Fig. 2). VEGF mRNA expression by HPC began to increase after 1 h of stimulation with E. coli LPS and reached a maximum level after 6 h of stimulation (Fig. 3A). The induction of VEGF by E. coli LPS was clearly observed from 12 h of cultivation onward (Fig. 3B).
FIG. 1.
Induction of VEGF mRNA by E. coli LPS in HPC. Confluent cultures of HPC-5 were washed and incubated in FBS-free EMEM for 24 h. The cells were washed, fed with EMEM with 1% FBS, and incubated with various concentrations of E. coli LPS for 6 h. Total RNA was extracted and analyzed by Northern blotting. To control for variation in gel loading, the GAPDH mRNA expression in each lane was also analyzed, and blots were quantified by means of a Bio-Imaging analyzer (BAS 1000 Mac). The results are expressed as the relative mRNA accumulation compared with GAPDH mRNA as an initial standard.
FIG. 2.
Enhanced production of VEGF by HPC stimulated with E. coli LPS. Confluent cultures of HPC-5 were washed and incubated in FBS-free EMEM for 24 h. The cells were washed, fed with EMEM with 1% FBS, and incubated with various concentrations of E. coli LPS for 24 h. Triplicate culture supernatants were pooled, and their VEGF concentrations were determined by ELISA. The data are expressed as means ± standard deviations of triplicate assays. The absorbance level was significantly different from that of the control (medium alone) by ANOVA and Scheffé’s F test (∗∗, P < 0.01).
FIG. 3.
Kinetics of LPS effect on VEGF induction on HPC. Confluent cultures of HPC-5 were washed and incubated in FBS-free EMEM for 24 h. The cells were washed, fed with EMEM with 1% FBS, and incubated with 10 μg of E. coli LPS per ml for 0 to 48 h. Total RNA was extracted and analyzed by Northern blotting. To control for variation in gel loading, the GAPDH mRNA expression in each lane also was analyzed. (A) Northern blotting was performed as described in the legend to Fig. 1. (B) ELISA was performed as described in the legend to Fig. 2.
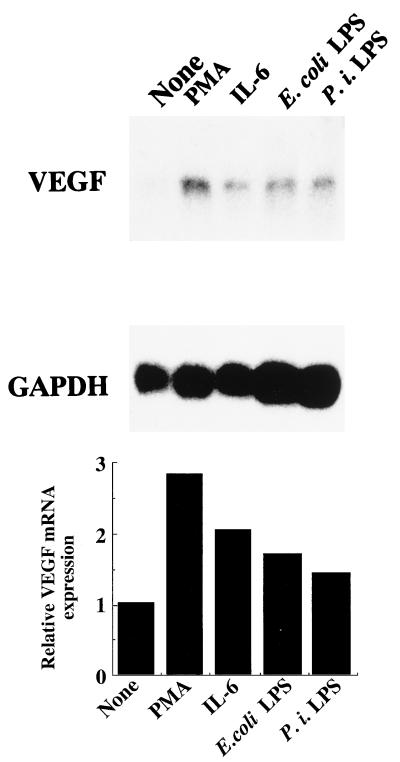
Next, we examined whether other stimulants enhance the expression of VEGF mRNA in HPC cultures. As shown in Fig. 4, LPS from P. intermedia, which is a putative pathogen of pulpitis, strongly induced VEGF mRNA, similarly to E. coli LPS. PMA and IL-6 also induced VEGF mRNA in HPC cultures.
FIG. 4.
Enhancing effects of PMA, IL-6, and E. coli and P. intermedia LPS on VEGF mRNA expression by HPC. Confluent cultures of HPC-5 were washed and incubated in FBS-free EMEM for 24 h. The cells were washed, fed with EMEM with 1% FBS, and incubated with PMA (10 μg/ml), IL-6 (50 U/ml), E. coli LPS (10 μg/ml), or P. intermedia (P. i.) LPS (10 μg/ml) for 6 h. The other procedures are described in the legend to Fig. 1.
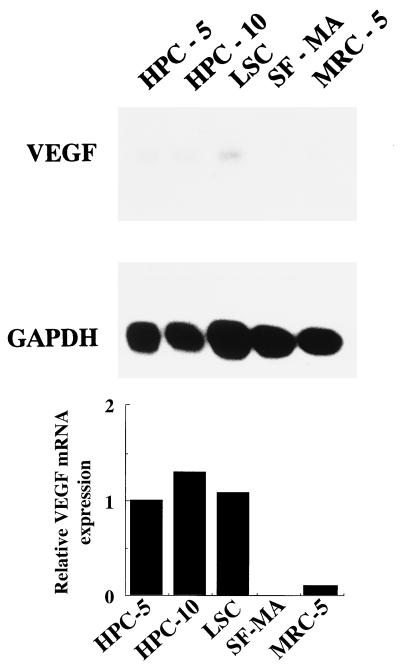
To examine whether other fibroblasts from pulp and other organs also express VEGF upon LPS stimulation, we examined the expression of VEGF mRNA in another primary culture of HPC (HPC-10), an SV40-infected cell line of HPC (LSC), skin fibroblasts (SF-MA), and lung fibroblasts (MRC-5). We found that HPC-10 and LSC as well as HPC-5 expressed VEGF mRNA upon stimulation with E. coli LPS (10 μg/ml), but SF-MA and MRC-5 did not (Fig. 5).
FIG. 5.
VEGF mRNA induction in various fibroblast cell lines by E. coli LPS. Confluent primary cultures of HPC (HPC-5 and HPC-10), SV40-infected human pulp cells (LSC), skin fibroblasts (SF-MA), and lung fibroblasts (MRC-5) were washed and incubated in FCS-free EMEM for 24 h. The cells were washed, fed with EMEM with 1% FCS, and incubated with medium alone or 10 μg of E. coli LPS per ml for 4 h. Total RNA was extracted and analyzed by Northern blotting. To control for variation in gel loading, the GAPDH mRNA expression in each lane was also analyzed.
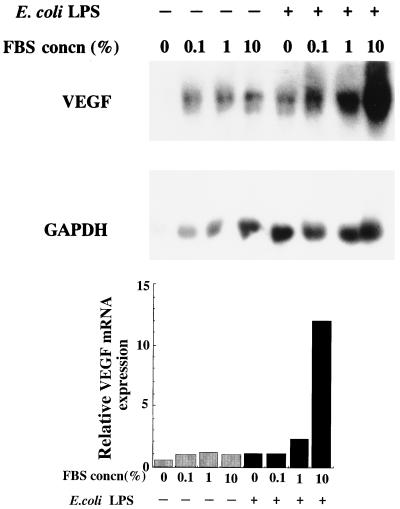
Uchida et al. (34) showed that FBS induces VEGF in glomerular endothelial cell cultures. To examine the effect of FBS on VEGF production by HPC, HPC-5 were stimulated with E. coli LPS in EMEM containing various concentrations of FBS, and then the levels of VEGF mRNA in these cultures were measured. Although VEGF mRNA was expressed even in HPC cultured with EMEM alone for 6 h, the extent of expression was slightly enhanced by the addition of FBS (Fig. 6). The levels of VEGF mRNA induced by E. coli LPS were significantly enhanced by the addition of FBS.
FIG. 6.
Effect of FBS concentration on VEGF production by HPC. Confluent cultures of HPC-5 were washed and incubated in FBS-free EMEM for 24 h. The cells were washed and then fed with EMEM supplemented with various concentrations of FBS (0, 0.1, 1, and 10% [vol/vol]). One microgram of E. coli LPS per milliliter was then added, followed by incubation for 4 h. Other procedures are described in the legend to Fig. 1.
Role of CD14 in the LPS-augmented VEGF expression in HPC cultures.
LPS receptors such as CD14 and β2 integrins (CD11b/CD18 and CD11c/CD18) are crucial for monocytes and macrophages to respond to LPS (11, 38). We investigated whether CD14, CD11b, and CD18 were expressed on the surfaces of HPC and the skin fibroblasts SF-MA. HPC and SF-MA expressed CD18 on the cell surface but not CD11b and CD14 (data not shown).
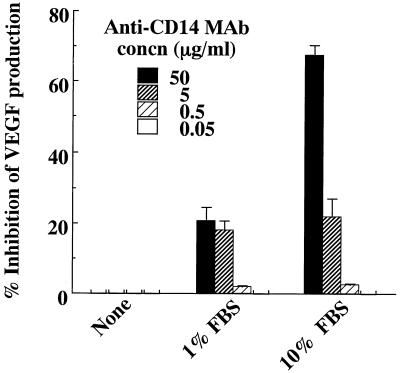
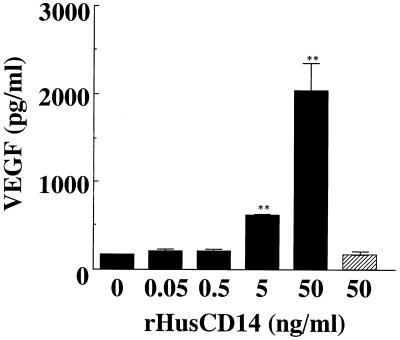
Hayashi et al. (9) reported that the induction of intercellular adhesion molecule 1 by LPS on human gingival fibroblasts is mediated by serum-derived sCD14. To clarify the participation of sCD14 in FBS in the enhanced production of VEGF by HPC stimulated with LPS, we added neutralizing anti-CD14 MAb MY4 to HPC cultures stimulated with LPS. As shown in Fig. 7, MY4 (50 μg/ml) strongly inhibited the VEGF production induced by E. coli LPS in HPC cultures. Furthermore, we also examined the effect of rHusCD14 on LPS-induced VEGF production in HPC cultures. With the addition of 5 or 50 ng of rHusCD14 per ml, the VEGF production induced by E. coli LPS was significantly enhanced in HPC cultures in the absence of FBS (Fig. 8). The rHusCD14 alone did not influence the VEGF production by HPC, and the augmenting effect of rHusCD14 was not observed in SF-MA cultures stimulated with E. coli LPS (data not shown).
FIG. 7.
Effect of anti-CD14 MAb on LPS-elicited VEGF production by HPC. HPC-5 (5 × 105 cells/ml) were incubated with 1 μg of E. coli LPS per ml in EMEM supplemented with 0, 1, or 10% FBS. Various amounts of anti-CD14 MAb were added at 15 min before LPS exposure. After incubation for 24 h, triplicate culture supernatants were pooled, and their VEGF concentrations were determined by ELISA. The data are the means ± standard deviations of triplicate assays.
FIG. 8.
Effect of sCD14 on LPS-elicited VEGF production by HPC. HPC-5 (5 × 105 cells/ml) were incubated with various concentrations of rHusCD14 in EMEM without FCS for 15 min, and E. coli LPS (1 μg/ml) was added (closed bars) or not added (hatched bar). After incubation for 24 h, triplicate culture supernatants were pooled, and their VEGF concentrations were determined by ELISA. The data are the means ± standard deviations of triplicate assays. The absorbance level was significantly different from that of the control (medium alone) by one-way ANOVA and Scheffé’s F test (∗∗, P < 0.01).
Role of nuclear factor AP-1 in the LPS-augmented VEGF expression in HPC cultures.
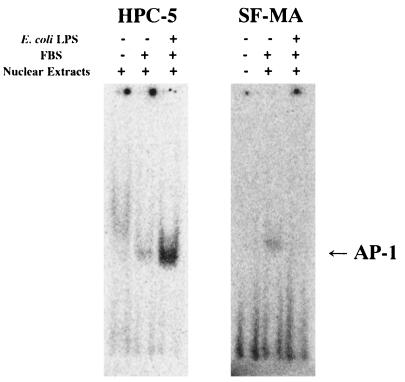
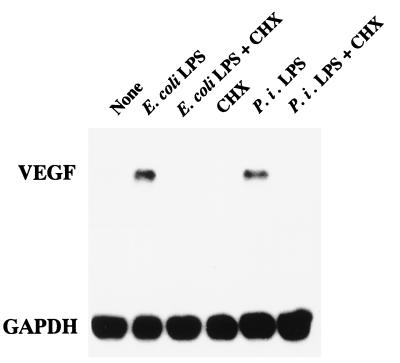
To clarify the signaling pathway of LPS for VEGF induction in HPC, we examined whether LPS modulates the activities of the nuclear factor AP-1 by a gel mobility shift assay. As shown in Fig. 9, HPC cultured in EMEM in the presence of 10% FBS for 4 h showed AP-1 activation, and the activation level was significantly enhanced by the addition of 1 μg of E. coli LPS per ml to the culture. No enhancement of AP-1 activation by LPS was observed in SF-MA cultures, although marginal AP-1 activation was observed in SF-MA cultured in EMEM in the presence of 10% FBS. We also examined the activation of two other nuclear factors, NF-κB and SP-1, in HPC and SF-MA by a fluorescence polarization analysis of DNA-protein binding. NF-κB and SP-1 were also activated in both HPC and the reference SF-MA upon stimulation with E. coli LPS, and their levels of activation in SF-MA were greater than those in HPC (data not shown). To clarify which transcription factors were predominantly associated with the enhanced production of VEGF by HPC stimulated with LPS, we examined the effects of inhibitors of AP-1, SP-1, and NF-κB. VEGF induction by E. coli LPS in HPC cultures was definitely inhibited by the AP-1 inhibitor curcumin in a dose-dependent manner, whereas the SP-1 inhibitor mithramycin A and the NF-κB inhibitor TPCK were inactive in this respect (data not shown). To ascertain whether newly synthesized proteins are necessary for the VEGF mRNA expression induced by LPS, the protein synthesis inhibitor cycloheximide was added to HPC cultures. Cycloheximide at 25 μg/ml was sufficient to inhibit VEGF mRNA accumulation in response to E. coli LPS or P. intermedia LPS (Fig. 10). These results indicate that the expression of VEGF enhanced by LPS required new protein synthesis and appeared to be mediated in part through the transcription factor AP-1.
FIG. 9.
Analysis of AP-1 activation by LPS in HPC by a gel mobility shift assay. Confluent cultures of HPC-5 and SF-MA were preincubated in medium alone for 18 h and then further incubated in medium alone or with E. coli LPS (1 μg/ml) for 4 h. Nuclear extracts were prepared and gel mobility shift assays were carried out as described in the text.
FIG. 10.
Effect of cycloheximide (CHX) on LPS-elicited VEGF mRNA expression by HPC. Confluent cultures of HPC-5 were incubated with or without the following materials in various concentrations in EMEM supplemented with 10% FBS: E. coli LPS (1 μg/ml), P. intermedia (P. i.) LPS (1 μg/ml), and cycloheximide (10 μg/ml) for 4 h. Other procedures are described in the legend to Fig. 1.
DISCUSSION
In this study, we observed that HPC produced more VEGF in response to E. coli LPS, through an sCD14-dependent pathway. The LPS-induced VEGF expression was in part mediated by AP-1 activation. Several potential binding sites for the transcription factors AP-1, AP-2, and SP-1 are localized in the VEGF promoter, and it is thought that these transcription factors are associated with VEGF expression (8, 24). Ryuto et al. (21) reported that basic fibroblast growth factor and TNF-α strongly enhance VEGF mRNA levels in a glioma cell line, and they suggested that this phenomenon is mediated through the transcription factor SP-1. Since HPC also express VEGF mRNA in response to TNF-α (17), the TNF-α-augmented VEGF mRNA expression in HPC cultures may be mediated through SP-1 activation. Another possibility is that LPS induces VEGF mRNA in HPC by a mechanism different from that for TNF-α; TNF-α and LPS induce VEGF gene expression in HPC in part through SP-1 and AP-1, respectively. Further studies are required to reach a final conclusion on this issue. Cycloheximide inhibited the LPS-induced VEGF mRNA expression (Fig. 10). A sufficient supply of c-Fos and c-Jun, which produce AP-1, is essential for the activation of AP-1 (2). LPS induced the expression of c-fos and c-jun genes in human monocytes (5). The VEGF induction by LPS in HPC may therefore be mediated by an ongoing synthesis of c-Fos and c-Jun protein. We recently showed that a human monocytic cell line, THP-1, exhibited increased VEGF production in response to E. coli LPS, and the effect of LPS depended on SP-1 activation (22), suggesting that the intracellular signaling of LPS for VEGF production is different among cell types.
Several studies of macrophages have shown that a membrane glycosylphosphatidylinositol-anchored CD14 molecule (mCD14) mediates LPS-induced cell activation (35, 39). A soluble form of CD14 (sCD14) lacking the glycosylphosphatidylinositol anchor is also present in serum (1), and sCD14 participates in the LPS-induced activation of endothelial and epithelial cells that normally do not express mCD14 (20). Hayashi et al. (9) indicated that although human gingival fibroblasts (HGF) expressed neither CD14 mRNA nor mCD14 on the cell surface, HGF were activated with LPS in a manner dependent on serum-derived sCD14 molecules. In contrast, Watanabe et al. (36) reported mCD14 expression by HGF. Sugawara et al. (27) recently showed heterogeneity of HGF in regard to mCD14 expression. In our study, a flow cytometric analysis showed that mCD14 expression was not present in HPC (data not shown). LPS scarcely enhanced the VEGF mRNA expression in HPC when the HPC were cultured in EMEM without FBS. The presence of FBS allowed HPC, in a dose-dependent manner, to express VEGF mRNA in response to LPS. In fact, the VEGF induction by LPS was inhibited by anti-CD14 MAb MY4, and rHusCD14 reconstituted the VEGF production in response to LPS in HPC cultured in serum-free medium. These findings strongly suggest that HPC respond to LPS in an sCD14-dependent manner. LPS binding protein (LBP) is present in normal sera from many species, including humans and cows, and was suggested to bind LPS to form a complex which strongly binds CD14 (32). Therefore, LPS associates with LBP in FBS, and this complex in turn binds efficiently to sCD14. This LPS-LBP-sCD14 complex might stimulate HPC and strongly induce VEGF.
Besides HPC, reference fibroblasts SF-MA, which originate from skin, also responded to E. coli LPS; SF-MA stimulated with E. coli LPS also showed the activation of NF-κB, AP-1, and SP-1 (data not shown). However, VEGF mRNA was not induced in SF-MA stimulated with LPS (Fig. 5). These findings may be explained as follows: although LPS stimulated SF-MA to activate AP-1, the level of activation was not enough to modulate the VEGF gene expression. In fact, in SF-MA transfected with NF-κB and SP-1 binding-site-deletion (AP-1 binding-site-retained) human immunodeficiency virus long terminal repeat luciferase (luc) gene, luc activity was not enhanced upon stimulation with E. coli LPS, whereas it was enhanced in the corresponding HPC transfectant (15). In addition, HPC and SF-MA which were transfected with NF-κB binding-site-deletion (AP-1 and SP-1 binding-site-retained) HIV long terminal repeat luc gene did not show enhanced luc activity upon stimulation with LPS (15). Since SP-1 was strongly activated in LPS-stimulated SF-MA compared with that in LPS-stimulated HPC in the present study, SP-1 may suppress the VEGF production by fibroblasts stimulated with LPS.
It is reasonable that HPC strongly produce VEGF in response to various stimulants containing LPS to compensate for the limited system of blood circulation in pulp tissue. Since the pulp is enclosed by hard tissue and is supplied with blood only through a small apical foramen, inflammation or injury occurring in pulp tissue followed by poor blood flow might immediately result in necrosis. HPC may therefore have a specific capacity for VEGF production to counteract this vulnerability. VEGF induces not only increased vascular permeability but also the chemotaxis of monocytes/macrophages (4). An upregulated production of VEGF in dental pulp may therefore enhance vascular permeability and the accumulation of inflammatory cells and increase the blood pressure, which results in the irreversible inflammation of dental pulp. However, VEGF also promotes the chemotaxis, proliferation, and differentiation of HPC (15, 16). In the recovery phase of inflamed pulp tissue, a sufficient supply of blood and angiogenesis are required. VEGF thus might also be useful for the cure of inflamed pulp tissue; VEGF promotes angiogenesis in the inflammatory sites of dental pulp and may induce the proliferation and differentiation of HPC and therefore might contribute to the repair of damaged pulp tissue and dentin. If the localization and action of VEGF in pulp tissue in vivo can be ascertained, the role of VEGF in pulp may become clear.
ACKNOWLEDGMENTS
We are indebted to M. Suwa, T. Oyama, T. Tajima, and K. Tomita for experimental assistance. We thank D. Mrozek (Medical English Service, Kyoto, Japan) for reviewing the paper.
This work was supported in part by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan (no. 08771712).
REFERENCES
- 1.Bazil V, Baudys M, Hilgert I, Stefanova I, Low M G, Zbrozek J, Horejsi V. Structural relationship between the soluble and membrane-bound forms of human monocyte surface glycoprotein CD14. Mol Immunol. 1989;26:657–662. doi: 10.1016/0161-5890(89)90048-5. [DOI] [PubMed] [Google Scholar]
- 2.Chen Y, Takeshita A, Ozaki K, Kitano S, Hanazawa S. Transcriptional regulation by transforming growth factor beta of the expression of retinoic acid and retinoid X receptor genes in osteoblastic cells is mediated through AP-1. J Biol Chem. 1996;271:31602–31606. doi: 10.1074/jbc.271.49.31602. [DOI] [PubMed] [Google Scholar]
- 3.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 4.Clauss M, Weich H, Breier G, Knies U, Rockl W, Waltenberger J, Risau W. The vascular endothelial growth factor receptor Flt-1 mediates biological activities. Implications for a functional role of placenta growth factor in monocyte activation and chemotaxis. J Biol Chem. 1996;271:17629–17634. doi: 10.1074/jbc.271.30.17629. [DOI] [PubMed] [Google Scholar]
- 5.Delpedro A D, Barjavel M J, Mamdouh Z, Bakouche O. Activation of human monocytes by LPS and DHEA. J Interferon Cytokine Res. 1998;18:125–135. doi: 10.1089/jir.1998.18.125. [DOI] [PubMed] [Google Scholar]
- 6.Feinberg R F, Sun L H, Ordahl C P, Frankel F R. Identification of glucocorticoid-induced genes in rat hepatoma cells by isolation of cloned cDNA sequences. Proc Natl Acad Sci USA. 1983;80:5042–5046. doi: 10.1073/pnas.80.16.5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ferrara N. The role of vascular endothelial growth factor in pathological angiogenesis. Breast Cancer Res Treat. 1995;36:127–137. doi: 10.1007/BF00666035. [DOI] [PubMed] [Google Scholar]
- 8.Finkenzeller G, Technau A, Marme D. Hypoxia-induced transcription of the vascular endothelial growth factor gene is independent of functional AP-1 transcription factor. Biochem Biophys Res Commun. 1995;208:432–439. doi: 10.1006/bbrc.1995.1356. [DOI] [PubMed] [Google Scholar]
- 9.Hayashi J, Masaka T, Saito I, Ishikawa I. Soluble CD14 mediates lipopolysaccharide-induced intracellular adhesion molecule 1 expression in cultured human gingival fibroblasts. Infect Immun. 1996;64:4946–4951. doi: 10.1128/iai.64.12.4946-4951.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iki K, Kawahara K, Sawamura S, Arakaki R, Sakuta T, Sugiyama A, Tamura H, Sueda T, Hamada S, Takada H. A novel component different from endotoxin extracted from Prevotella intermedia ATCC 25611 activates lymphoid cells from C3H/HeJ mice and gingival fibroblasts from humans. Infect Immun. 1997;65:4531–4538. doi: 10.1128/iai.65.11.4531-4538.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ingalls R R, Golenbock D T. CD11c/CD18, a transmembrane signaling receptor for lipopolysaccharide. J Exp Med. 1995;181:1473–1479. doi: 10.1084/jem.181.4.1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ito A, Hirota S, Mizuno H, Kawasaki Y, Takemura T, Nishiura T, Kanakura Y, Katayama Y, Nomura S, Kitamura Y. Expression of vascular permeability factor (VPF/VEGF) messenger RNA by plasma cells: possible involvement in the development of edema in chronic inflammation. Pathol Int. 1995;45:715–720. doi: 10.1111/j.1440-1827.1995.tb03387.x. [DOI] [PubMed] [Google Scholar]
- 13.Jimi E, Ikebe T, Takahashi N, Hirata M, Suda T, Koga T. Interleukin-1α activates an NF-κB-like factor in osteoclast-like cells. J Biol Chem. 1996;271:4605–4608. doi: 10.1074/jbc.271.9.4605. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, Liu M, Simchon S, Dorscher-Kim J E. Effects of selected inflammatory mediators on blood flow and vascular permeability in the dental pulp. Proc Finn Dent Soc. 1992;88:387–392. [PubMed] [Google Scholar]
- 15.Matsushita, K., and R. Motani. Unpublished data.
- 16.Matsushita K, Nagaoka S, Arakaki R, Kawabata Y, Iki K, Kawagoe M, Takada H. Immunobiological activities of a 55-kilodalton cell surface protein of Prevotella intermedia ATCC 25611. Infect Immun. 1994;62:2459–2469. doi: 10.1128/iai.62.6.2459-2469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Motani R, Matsushita K, Sakuta T, Suwa M, Oyama T, Sakoda Y, Torii M, Nagaoka S. Properties of vascular endothelial growth factor on human pulpal cells. Jpn J Conserv Dent. 1997;40:1121–1130. [Google Scholar]
- 18.Okiji T, Morita I, Sunada I, Murota S. Involvement of arachidonic acid metabolites in increases in vascular permeability in experimental dental pulpal inflammation in the rat. Arch Oral Biol. 1989;34:523–528. doi: 10.1016/0003-9969(89)90090-3. [DOI] [PubMed] [Google Scholar]
- 19.Pekovic D D, Fillery E D. Identification of bacteria in immunopathologic mechanisms of human dental pulp. Oral Surg Oral Med Oral Pathol. 1984;57:652–661. doi: 10.1016/0030-4220(84)90289-5. [DOI] [PubMed] [Google Scholar]
- 20.Read M A, Cordle S R, Veach R A, Carlisle C D, Hawiger J. Cell-free pool of CD14 mediates activation of transcription factor NF-κB by lipopolysaccharide in human endothelial cells. Proc Natl Acad Sci USA. 1993;90:9887–9891. doi: 10.1073/pnas.90.21.9887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ryuto M, Ono M, Izumi H, Yoshida S, Weich H A, Kohno K, Kuwano M. Induction of vascular endothelial growth factor by tumor necrosis factor alpha in human glioma cells. Possible roles of SP-1. J Biol Chem. 1996;271:28220–28228. doi: 10.1074/jbc.271.45.28220. [DOI] [PubMed] [Google Scholar]
- 22.Sakuta, T., K. Matsushita, N. Yamaguchi, T. Koga, K. Abeyama, I. Maruyama, H. Takada, and M. Torii. Submitted for publication.
- 23.Seed B, Aruffo A. Molecular cloning of CD2 antigen, the T-cell erythrocyte receptor, by a rapid immunoselection procedure. Proc Natl Acad Sci USA. 1987;84:3365–3369. doi: 10.1073/pnas.84.10.3365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shima D T, Kuroki M, Deutsch U, Ng Y S, Adamis A P, D’Amore P A. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- 25.Shulman K, Rosen S, Tognazzi K, Manseau E J, Brown L F. Expression of vascular permeability factor (VPF/VEGF) is altered in many glomerular diseases. J Am Soc Nephrol. 1996;7:661–666. doi: 10.1681/ASN.V75661. [DOI] [PubMed] [Google Scholar]
- 26.Simmons D L, Tan S, Tenen D G, Nicholson Weller A, Seed B. Monocyte antigen CD14 is a phospholipid anchored membrane protein. Blood. 1989;73:284–289. [PubMed] [Google Scholar]
- 27.Sugawara S, Sugiyama A, Nemoto E, Rikiishi H, Takada H. Heterogeneous expression and release of CD14 by human gingival fibroblasts: characterization and CD14-mediated interleukin-8 secretion in response to lipopolysaccharide. Infect Immun. 1998;66:3043–3049. doi: 10.1128/iai.66.7.3043-3049.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takada H, Mihara J, Morisaki I, Hamada S. Induction of interleukin-1 and -6 in human gingival fibroblast cultures stimulated with Bacteroides lipopolysaccharides. Infect Immun. 1991;59:295–301. doi: 10.1128/iai.59.1.295-301.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Takebe Y, Seiki M, Fujisawa J, Hoy P, Yokota K, Arai K, Yoshida Y, Arai N. SR alpha promoter: an efficient and versatile mammalian cDNA expression system composed of the simian virus 40 early promoter and R-U5 segment of human T-cell leukemia virus type 1 long terminal repeat. Mol Cell Biol. 1988;8:466–472. doi: 10.1128/mcb.8.1.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tamura M, Tokuda M, Nagaoka S, Takada H. Lipopolysaccharides of Bacteroides intermedius (Prevotella intermedia) and Bacteroides (porphyromonas) gingivalis induce interleukin-8 gene expression in human gingival fibroblast cultures. Infect Immun. 1992;60:4932–4937. doi: 10.1128/iai.60.11.4932-4937.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thomas P S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci USA. 1980;77:5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tobias P S, Soldau K, Kline L, Lee J D, Kato K, Martin T P, Ulevitch R J. Cross-linking of lipopolysaccharide (LPS) to CD14 on THP-1 cells mediated by LPS-binding protein. J Immunol. 1993;150:3011–3021. [PubMed] [Google Scholar]
- 33.Tokunaga K, Nakamura Y, Sakata K, Fujimori K, Ohkubo M, Sawada K, Sakiyama S. Enhanced expression of a glyceraldehyde-3-phosphate dehydrogenase gene in human lung cancers. Cancer Res. 1987;47:5616–5619. [PubMed] [Google Scholar]
- 34.Uchida K, Uchida S, Nitta K, Yumura W, Marumo F, Nihei H. Glomerular endothelial cells in culture express and secrete vascular endothelial growth factor. Am J Physiol. 1994;266:81–88. doi: 10.1152/ajprenal.1994.266.1.F81. [DOI] [PubMed] [Google Scholar]
- 35.Ulevitch R J, Tobias P S. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. doi: 10.1146/annurev.iy.13.040195.002253. [DOI] [PubMed] [Google Scholar]
- 36.Watanabe A, Takeshita A, Kitano S, Hanazawa S. CD14-mediated signal pathway of Porphyromonas gingivalis lipopolysaccharide in human gingival fibroblasts. Infect Immun. 1996;64:4488–4494. doi: 10.1128/iai.64.11.4488-4494.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Warfvinge J, Dahlen G, Bergenholtz G. Dental pulp response to bacterial cell wall material. J Dent Res. 1985;64:1046–1050. doi: 10.1177/00220345850640080401. [DOI] [PubMed] [Google Scholar]
- 38.Wright S D, Jong M T. Adhesion-promoting receptors on human macrophages recognize Escherichia coli by binding to lipopolysaccharide. J Exp Med. 1986;164:1876–1888. doi: 10.1084/jem.164.6.1876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wright S D, Ramos R A, Tobias P S, Ulevitch R J, Mathison J C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990;249:1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- 40.Yamane A, Seetharam L, Yamaguchi S, Gotoh N, Takahashi T, Neufeld G, Shibuya M. A new communication system between hepatocytes and sinusoidal endothelial cells in liver through vascular endothelial growth factor and Flt tyrosine kinase receptor family (Flt-1 and KDR/Flk-1) Oncogene. 1994;9:2683–2690. [PubMed] [Google Scholar]