Abstract
The implantation of foreign material carries a risk of infection which frequently is resistant to all treatment short of removing the implant. We have previously shown that these materials activate neutrophils by contact, leading to production of oxygen free radicals accompanied by release of granule products. Such activation further results in depletion of local host defenses, including the capacity of biomaterial-activated neutrophils to kill bacteria. Among the granule products released from neutrophils are small cationic antibacterial peptides (human neutrophil peptides [HNP]) known as defensins. Here we tested the hypothesis that defensins, released from activated neutrophils onto the surface of biomaterials, might play a role in the deactivation of subsequent neutrophil populations. Incubation of neutrophils with purified HNP resulted in a dose-related impairment of stimulus-induced oxygen radical production and of phagocytic killing. Furthermore, fresh neutrophils added to biomaterial-associated neutrophils exhibited impaired phagocytic killing. This impairment could be abrogated by antibody to HNP but not by an irrelevant antibody. Taken together, these observations support the idea that neutrophils activated at a material surface can create, by means of HNP release, an environment hostile to their microbicidal function and that of their infiltrating brethren.
Implantable biomaterials, virtually indispensable in medical practice (16, 22, 29) and with an excellent overall success rate, have continued to be infection prone, with enormous social and economic consequences (13, 14, 23, 25, 26, 40, 66, 76). These infections tend to be persistent and refractory to antibiotics and often require removal of the implant to clear the infection (14, 22, 40). In this sense, they resemble abscesses, which require surgical drainage in order to be eradicated.
The pathogenic mechanisms of this refractoriness to treatment, however, have remained obscure. Contributory factors include bacterial virulence properties, such as slime production (4, 10, 23, 31, 56, 60), as well as properties of specific biomaterials which increase microbial adherence or alter inflammatory changes (1, 2, 7, 9, 12, 23, 24, 27, 30, 51, 53, 58, 70, 71, 77, 78) and impaired phagocytic host defenses even in the absence of microbial colonization (34–36, 57, 59, 64, 78, 79).
Neutrophils rapidly become associated with any implanted biomaterial in vivo and, under conditions permissive of normal function, should be capable of phagocytic host defense. Previous work by others (2, 15, 21, 27, 30, 39, 41, 52, 53, 57–59, 64, 77–79) and in our laboratory (34–36), however, has shown that biomaterial-associated neutrophils become prematurely activated by contact with the materials themselves and shortly thereafter lose the capacity to become activated in response to normal subsequent stimuli. More importantly, these biomaterial-associated neutrophils appear to actually induce a dysfunctional impaired activation of incoming fresh neutrophils (36, 78, 79).
Normally, neutrophils effect phagocytic host defense by ingesting and killing invading microorganisms (3, 54, 69). The killing of microbes occurs as a consequence of reactive oxygen intermediates (ROI) formed during the process of phagocytosis together with bioactive constituents from granules which discharge into the phagocyte vacuole (5, 62, 63, 72, 73). These constituents include myeloperoxidase, which, when combined with H2O2 and chloride, produces hypochlorous acid within the phagocytic vacuole, as well as small highly cationic peptides (human neutrophil peptides [HNP]), also known as defensins. The defensins HNP1, -2, and -3 comprise 5% of a neutrophil’s total protein (19, 44, 46). HNP contain 29 to 35 amino acid residues, and because of their charge and their tendency to form multimeric subunits, they insinuate themselves into microbial cell membranes (gram-positive and gram-negative bacteria, fungi, and viruses) (11, 45, 47, 61), produce voltage-sensitive channels, and thereby permeabilize the membrane (33). HNP also are known to damage eukaryotic cells, such as tumor cells (48, 65) and human neutrophils (75), and in a cell-free system they have been shown to inhibit NADPH oxidase (67). This cytotoxicity depends in part on the lipid composition of the target cell membrane (28) and requires metabolic activity in the target cells (49). In addition, hydrogen peroxide acts synergistically with HNP to induce cytotoxicity (50). Along with permeabilization, DNA strand breaks in some target cells have been reported (20, 49), and while the mechanism has not been determined, a direct interaction between defensin and DNA is suspected.
Because HNP may be toxic to polymorphonuclear leukocytes (PMN) (67, 75), and because material-activated neutrophils could be expected to release HNP from their granules (5, 62, 63, 72, 73), we proposed that the dysregulated functioning of incoming fresh neutrophils (36) might be induced by these released HNP. To further explore this idea in the context of biomaterial-induced changes, the effect of purified HNP on phagocytic killing by neutrophils was evaluated and demonstrated a dose-related cytotoxicity of HNP1 and HNP2 ameliorated by antibody to HNP. These data support the idea that released HNP contribute to an environment hostile to host defense at the biomaterial surface.
MATERIALS AND METHODS
Cells and reagents.
Blood was obtained from healthy human donors. Neutrophils were isolated by density gradient centrifugation according to the method of Boyum (6), as previously described (37, 43), and suspended in modified Krebs Ringer phosphate buffer, pH 7.4 (16 mM Na2HPO4, 123 mM NaCl, 0.5 mM CaCl2, 0.5 mM MgSO4, 5 mM KCl, and 4.4 mM glucose) (KRPG) (35). In some studies, the KRPG included 10% autologous plasma. Each neutrophil donor provided cells for a single day’s experiments. Cell numbers were determined with Coulter (Hialeah, Fla.) electronic instruments, and the cells were suspended at a concentration of 2 × 106/ml. The preparations were evaluated with DiffQuick-stained cytospin preparations and were uniformly >95% neutrophils. Viability was determined by trypan blue dye exclusion and was uniformly >95%. Stock solutions of formyl methionyl leucyl phenylalanine (fMLP), phorbol myristate acetate (PMA), and opsonized zymosan (OZ) were prepared as previously described (34). Purified human neutrophil defensins HNP1 and HNP2 were obtained from Sigma Chemical Co., St. Louis, Mo., and stored at −20°C until needed. At that time, the HNP were suspended in phosphate-buffered saline (PBS) at a concentration of 25 μg/ml and were diluted in PBS so that the PMN were incubated with 10 to 35 μg of HNP/ml. Monoclonal antibody to HNP (D1-1 immunoglobulin G1, a neutralizing antibody) was a generous gift of T. Ganz, University of California—Los Angeles School of Medicine, Los Angeles. The stock solution of 10,000 μg/ml (10 mg/ml) was diluted in PBS to the desired concentration just before use. Antibody to VCAM-1 (an irrelevant immunoglobulin G1 control antibody) was a generous gift from T. Carlos, University of Pittsburgh. It was in a stock solution of 3,600 μg/ml (3.6 mg/ml) and was appropriately diluted in PBS just before use. Unless specified, all other materials were reagent grade and were obtained from Sigma Chemical Co.
Superoxide release.
Human neutrophils (106) were preincubated with purified human neutrophil defensins and then were placed into triplicate wells of 24-well plates in KRPG. Cytochrome c was added with and without fMLP, PMA, OZ, or Staphylococcus aureus, and the plates were incubated at 37°C for 30 min. Superoxide dismutase was present in a fourth well. The superoxide dismutase-inhibitable cytochrome c reduction was determined after 30 min, at which time the plates were placed in melting ice and centrifuged and the supernatants were transferred to 96-well plates. The data are expressed as nanomoles of O2−/106 cells.
Microbe killing.
The effects of biomaterial association, of antibody to HNP, and of HNP1 and HNP2 on microbe killing were determined by clonal-culture techniques as previously described, using S. aureus D2C as the target organism (38).
The effect of HNP1 and HNP2 on the ability of PMN to kill staphylococci was determined by preincubating PMN in KRPG (106/ml) in 12- by 75-mm test tubes with various concentrations of HNP1 and HNP2 at 37°C for 1 h. The 1-h incubation was based on published data demonstrating that toxicity for mammalian eukaryotic cells requires 1 h for completion and irreversibility (44, 49). The PMN were then centrifuged, washed once with KRPG, and resuspended in KRPG plus 10% plasma; 5 × 105 cells were put in 25-ml Erlenmeyer flasks and placed in a rotary shaking water bath. After 10 min, an inoculum of S. aureus was added (Staphylococcus/neutrophil ratio, 10:1). The samples were swirled to mix them, and a 10-μl sample was removed immediately after the addition of bacteria (the time zero sample). Samples (10 μl) were removed at 2 and 24 h, and the numbers of viable staphylococci were determined.
The effect of biomaterial-associated PMN on the microbicidal activity of a fresh inoculation of PMN was determined by first incubating the PMN in KRPG in 24-well culture dishes for 1 h at 37°C and then adding a similar number of PMN and continuing the incubation for 1 h more. The PMN were removed from the wells after the addition of EDTA to reverse the adhesion phenomenon and then centrifuged and washed prior to being resuspended in KRPG and placed in the wells of a clean 24-well culture dish. For control conditions, PMN were stored at 37°C for 1 h in a test tube and then added to complete buffer containing 10% plasma. S. aureus was added 10 min later, and bacterial colony counts were made at the time of S. aureus addition as well as after 2 and 24 h.
To determine if incubating PMN with antibody to HNP (D1-1) resulted in improved staphylocidal activity of fresh PMN added to biomaterial-associated PMN, various concentrations of the antibody to HNP (2 to 200 μg/ml) or to VCAM at 200 μg/ml were placed in the wells of 24-well plates just prior to the addition of the initial inoculum of PMN. The PMN were in contact with the wells, with and without the presence of antibody, for 1 h at 37°C. In some experiments, 50 and 80% plasma were added instead of antibody. A second inoculation of PMN was added, as described above, and incubation continued for 1 h more at 37°C. These PMN, washed and resuspended in KRPG with 10% plasma, were transferred to a fresh well; staphylococci were then added, and incubation continued for 24 h as described above.
Defensin assay.
The amount of available defensins in PMN before and after exposure to polystyrene was determined by Panyutich’s method. Briefly, this is a “sandwich-type” enzyme immunoassay with a monoclonal antibody for capture and a biotinylated monoclonal antibody for detection. Cetyltrimethyl ammonium bromide was used to counteract nonspecific binding of HNP to surfaces. The assay detects HNP with a range of 0.5 to 16 ng/ml.
Statistical evaluation.
Calculations of statistical significance were done with Student’s t test or the paired t test. Significance was defined as a P value of ≤0.05.
RESULTS
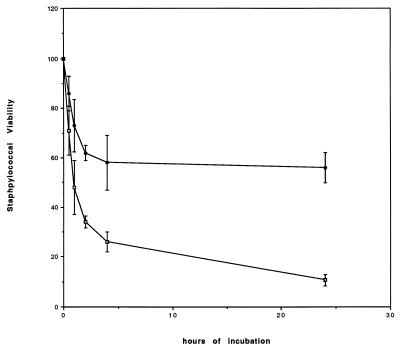
Figure 1 illustrates the effect on bacterial viability of adding a secondary inoculum of fresh neutrophils to polystyrene wells containing the primary inoculum of neutrophils which had been on the polystyrene surface for 1 h. These neutrophils killed 38% ± 4% of the S. aureus organisms in 2 h and killed 41% ± 8% of the organisms by 24 h. In contrast, when the S. aureus organisms were added within 10 min of the primary inoculation of PMN onto the polystyrene, 65% ± 3% of the S. aureus organisms were killed in 2 h and 88% ± 2% were killed by 24 h (P ≤ 0.001). These data indicate that the association of the first inoculum with polystyrene for 1 h down-regulated microbe killing by the secondary inoculum.
FIG. 1.
Effect of polystyrene preincubation on staphylocidal activity. The percent viable staphylococci during 24 h of incubation with neutrophils is shown. Control neutrophils were inoculated with S. aureus at the same time the cells were placed in the polystyrene wells (□). The decrease in the number of viable staphylococci occurred rapidly during the first 2 h of incubation and more slowly after that. These cells killed 65% ± 3% of the inoculum after 2 h and 88% ± 2% of the inoculum after 24 h. The figure also shows the effect on the staphylocidal behavior of fresh neutrophils added to neutrophils that had been preincubated for 1 h in polystyrene wells (●). These cells killed only 38% ± 4% of the inoculum at 2 h and 41% ± 8% of the inoculum at 24 h (P ≤ 0.001 compared to the control at both of these times). The error bars indicate standard errors.
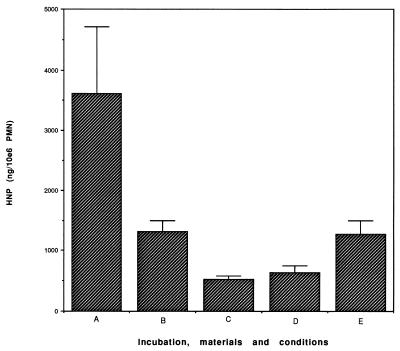
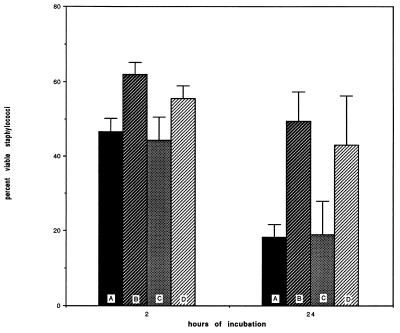
One likely cause for this effect was the release of granule products, such as HNP, with documented (11, 19, 20, 44–50, 61, 65) toxicity against both eukaryotic and prokaryotic cells. We therefore examined the supernatants of polystyrene-associated PMN (after 1 h of association) for the presence of HNP. Less than 1,000 ng was detected, too low a concentration to explain our results based on published data. However, since strongly cationic HNP readily adhere to negatively charged surfaces such as plastic (55) and to cells (17), the poor recoverability of the HNP may have represented an artifact. Residual intracellular HNP from control PMN and from PMN after polystyrene association was then measured and is shown in Fig. 2. In this study, a marked reduction in the detectable HNP content of biomaterial-associated cells was seen, from 3,616 ± 1,098 ng/ml/106 PMN extractable in control cells to 1,313 ± 183 ng/ml extractable 30 min after polystyrene exposure and 886 ± 277 ng/ml extractable at 2 h. We also evaluated defensin content in cells exposed to woven Dacron and Silastic, finding 453 ± 230 and 1,275 ± 215 ng/ml, respectively. These results are consistent with the idea that PMN associated with biomaterials discharge their granular HNP and that the released HNP become associated with or inactivated by the polystyrene. To test this hypothesis, we added purified HNP1 and HNP2 to polystyrene plates in the absence of cells. The supernatants were analyzed for residual HNP. Detectable HNP disappeared within 15 min, with only 2% remaining in the supernatant. These results are consistent with those of others (55), showing rapid association of HNP with plastic.
FIG. 2.
Extractable defensin of control neutrophils (A), cells which had been incubated on polystyrene for 30 min (B) and for 2 h (C), and neutrophils incubated for 2 h on woven Dacron (D) or silastic (E). Exposure to all three materials was associated with significantly reduced defensin content (P ≤ 0.01 under all conditions). The error bars indicate standard errors.
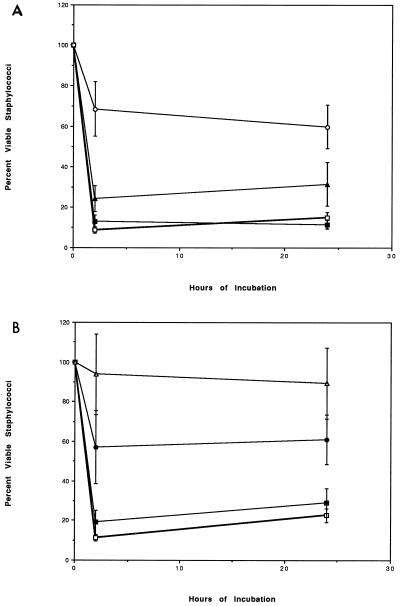
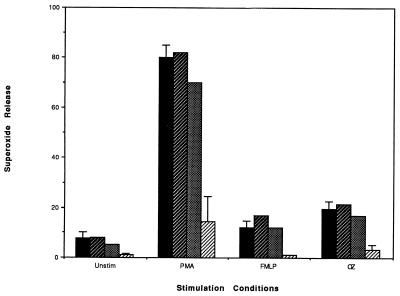
To determine whether HNP might impair PMN staphylocidal activity, commercially available purified HNP were incubated with PMN for 1 h at 37°C. The ability of these incubated and washed PMN to kill S. aureus is shown in Fig. 3. Figure 3A shows that HNP1, at a concentration of 35 μg/ml, impaired the staphylocidal activity of PMN such that only 31% ± 13% and 37% ± 11% of the inoculum was killed after 2 and 24 h of incubation, respectively (P ≤ 0.01 compared with the control or with HNP1 at 10 μg/ml and P ≤ 0.03 compared with HNP1 at 25 μg/ml). Incubation of PMN with HNP1 at 25 μg/ml also compromised staphylocidal activity, but the inhibition was more variable. Figure 3B shows that HNP2 is a more potent inhibitor of PMN staphylocidal activity. The use of 25 μg/ml almost completely abrogated staphylocidal activity after 2 and 24 h of incubation (P ≤ 0.01 compared to the control and to HNP2 at 10 μg/ml). As little as 15 μg of HNP2/ml also significantly impaired staphylocidal activity (P ≤ 0.01 at 2 and 24 h compared to the control and P ≤ 0.05 at 2 and 24 h compared to HNP2 at 10 μg/ml). HNP2, therefore, was far more toxic to PMN than HNP1 was. In addition, Fig. 4 shows that HNP1, at 35 μg/ml, significantly impaired the ability of neutrophils to produce superoxide following stimulation with any of the inducing agents. Parallel experiments were performed with HNP2, which demonstrated complete abrogation of superoxide release at 20 μg/ml (data not shown). These cytotoxic correlations of HNP and the incubation conditions are consistent with the known conditions for cytotoxicity of HNP for eukaryotic cells (18, 32). Despite these inhibitory effects of HNP on microbe killing and O2− release, HNP had no effect on PMN viability as determined by trypan blue dye exclusion during the first 5 h following PMN incubation with 35 μg of HNP1/ml, and viability was 73% after 24 h.
FIG. 3.
(A) Effect of HNP1 on staphylocidal activity. The effect of preincubating neutrophils for 1 h with several concentrations of HNP1 (or buffer control) is shown. The cells were washed and resuspended in KRPG with 10% plasma and then inoculated with staphylococci. The control cells (□) killed 91% ± 1% of the inoculum after 2 h and 88% ± 2% of the inoculum after 24 h. HNP1 at 10 μg/ml (■) did not affect staphylocidal activity. HNP1 at 25 μg/ml (▴) reduced staphylocidal activity to 75% ± 7% killed at 24 h (P ≤ 0.03 compared to the control or HNP1 at 10 μg/ml). HNP1 at 35 μg/ml (○) reduced staphylocidal activity to 31% ± 13% killed at 2 h and 37% ± 13% killed at 24 h (P ≤ 0.01 compared to the control). (B) Effect of HNP2 on staphylocidal activity. The effect of HNP2 on the staphylocidal activity of human PMN is more potent than the effect of HNP1 shown in panel A. Although HNP2 at 10 μg/ml (■) did not significantly affect killing, HNP2 at 15 μg/ml (●) partially inhibited staphylocidal activity: 41% ± 20% of the inoculum was killed at 2 h, and 40% ± 8% was killed at 24 h compared to 89% ± 5% and 85% ± 2% killed at 2 and 24 h by control cells (□) (P ≤ 0.01 at 2 and 24 h). HNP2 at 25 μg/ml (▵) almost completely abrogated staphylocidal activity: 5% ± 15% killed at 2 h and 8% ± 20% killed at 24 h (P ≤ 0.01 at 2 and 24 h compared to the control; P ≤ 0.05 at 2 and 24 h compared to HNP2 at 10 μg/ml).
FIG. 4.
Effect of HNP1 on superoxide release. Incubation of neutrophils with 35 μg of HNP1/ml (▨) significantly reduced the superoxide release of unstimulated (unstim) cells and of cells stimulated with PMA, fMLP, and OZ (P ≤ 0.01) compared to unexposed (control) cells (■). Incubation with 17.5 ( ) and 25 (░⃞) μg of HNP1/ml had little effect (mean of two experiments).
To further evaluate the likelihood that HNP released from polystyrene-associated PMN can act to disable PMN (Fig. 1), antibody to HNP was added to the first inoculum of PMN placed in the polystyrene wells. Figure 5 shows that this antibody reversed the cytotoxic effect at both time points (P ≤ 0.05 at 2 h; P = 0.0001 at 24 h) when killing in the presence and absence of 200 μg of specific anti-HNP antibody/ml were compared. Lower concentrations of antibody (2 to 50 μg/ml) were without effect (data not shown), as was the use of 200 μg of an irrelevant antibody (antibody to VCAM)/ml at 2 and 24 h.
FIG. 5.
Effect of antibody to HNP on polystyrene-associated inhibition of staphylocidal activity. Compared to control cells, in which staphylococci were added without a prior 1-h incubation of PMN on polystyrene (■), staphylocidal activity was impaired when fresh neutrophils were added to the polystyrene-exposed neutrophils ( ), but this is overcome by addition of monoclonal antibody specific for HNP (░⃞) but not by irrelevant antibody to VCAM (▨) prior to the addition of fresh neutrophils. This effect was highly significant at both 2 and 24 h after the inoculation of neutrophils with staphylococci (P = 0.00008).
DISCUSSION
An implanted biomaterial foreign body has paradoxical effects; it induces the accumulation of phagocytes and at the same time can become the nidus of intractable bacterial infection. In fact, the greater the ability of a foreign body to induce a neutrophilic infiltrate, the greater its susceptibility to infection (12, 13, 30). Our previous work has indicated that PMN often become activated at the surfaces of biomaterials (34). The PMN can release chemoattractant substances, which would serve to attract fresh PMN to the surface (36). Such an event would be expected to potentiate host defenses but seems to have the opposite effect. We found that PMN, once in contact with biomaterials, rapidly lose their capacity to generate superoxide and are relatively impotent in their microbicidal activity (36). We subsequently showed that when fresh PMN were added to a surface upon which there had been a prior inoculum of PMN, the fresh PMN became inactive (36). These data, therefore, are consistent with the idea that the infectivity at biomaterial surfaces may be due at least in part to releasant-mediated autacoid and paracoid damage of the PMN themselves, rendering such wounds susceptible to even small inocula. Our present data support and extend our previous observation that fresh PMN, added to polystyrene-associated PMN and then removed and washed free of mediators, acquire a profoundly compromised staphylocidal activity. The data strongly suggest that a cytotoxic agent(s) is released when PMN contact polystyrene. These cytotoxic agents can further compromise the functioning of bystander PMN.
Neutrophils have considerable cytotoxic potential and play a role in many kinds of inflammatory reactions, ranging from autoimmune processes to reperfusion injury. This cytotoxic potential includes the metabolic products derived from the production of oxidative free radicals and the cytoactive granule constituents. The small cationic HNP, the defensins, comprise a potential subset of cytotoxic elements with well-established toxicity against prokaryotic and eukaryotic cells. The toxicity of HNP for bacteria is based on the ability of HNP to permeabilize the outer and inner membranes of susceptible bacteria (44) by the formation of ion channels (33) and by antagonizing intracellular signaling mediated by protein kinase C (8). These actions, however, are not limited to HNP, since Yeaman et al. demonstrated a similar, although not identical, antibacterial action of peptides from platelets (74). While several groups demonstrated that HNP were toxic to eukaryotic as well as to prokaryotic cells, the mechanism of injury to mammalian target cells is less well established. These actions have been reviewed by Kagan et al. (32), who indicated that there are three interdependent actions, including (i) membrane binding due to electrostatic forces, followed by (ii) insertion into the membrane or internalization of the HNP (49). The third step (iii) includes DNA injury (20, 47), inhibition of NADPH oxidase (67, 68), and inhibition of protein kinase C (8). The initial binding becomes irreversible within 1 h of association, and although permeability to trypan blue dye was not observed in our study, the concentration of HNP may not have been high enough, since Lehrer indicated that HNP1 at 50 μg/ml was needed to see this in PMN (personal communication). Mammalian cells have also been shown to require an energized target cell membrane, since metabolic inhibitors which do not effect initial binding do inhibit defensin-induced permeabilization. Yomogida et al. (75) specifically documented some of the results of HNP-induced cytotoxicity in PMN. These included diminished ROI formation, impaired phagocytosis (not due to impaired particle attachment), and increased adherence. Our work supports these findings and also demonstrates that the ability of PMN to kill bacteria is severely compromised.
Wright and Gallin (73) showed that PMN degranulate upon association with artificial materials in vitro. Klock and Bainton demonstrated that PMN associated with nylon wool initially degranulate but subsequently exhibit abnormal bactericidal activity (42). Our finding that PMN in polystyrene wells for 1 h lose intracellular defensin content and concurrently impair the function of subsequently added neutrophils strongly implicates the granular defensins in PMN dysfunction. We demonstrate in this paper that in the presence of antibody to HNP, but not of isotype-identical irrelevant antibody, the PMN placed in the polystyrene wells did not exert a cytotoxic effect on a fresh inoculum of PMN. The staphylococci added with these PMN were killed normally. These data support our hypothesis that HNP (among other possible releasants) from material-associated neutrophils play a significant role in the impairment of cell function at the material surface. Whether or not the defensins, which are apparently readily adsorbed onto polystyrene, exert their anti-PMN function after adsorption is an important question that will require further study.
This work has not addressed the particular mechanisms by which HNP damage PMN in our system. We do not assert that defensin-mediated damage is the only route to neutrophil impairment on biomaterial surfaces, and Tal et al. (68) demonstrated that cationic proteins other than HNP may damage PMN oxidative activity. However, neutrophils also release proinflammatory mediators, such as oxidative free radicals, interleukin-8, and metabolites of arachidonic acid, including leukotrienes and prostaglandins, all of which could serve transiently to augment and potentially harm cell function. Our studies, however, clearly demonstrate that purified HNP incubated with PMN severely compromised phagocytic killing of staphylococci when used at a concentration of 25 μg/ml for HNP2, a concentration determined by others to be toxic to eukaryotic cells, and at a concentration of 35 μg/ml for HNP1. While most of our studies were performed after a 1-h incubation with HNP to ensure that the interaction of HNP with the cell membrane was irreversible, several studies also were performed after a much shorter (15-min) incubation. These studies also demonstrated profound inhibition of the PMN’s killing of staphylococci (data not shown). These concentrations are much lower than HNP concentrations found in phagocytic vacuoles, so they could conceivably be achieved in the microenvironment of the degranulating cell. At lower concentrations which did not diminish phagocytic killing of staphylococci, superoxide production in response to a peptide stimulus (but not to a phagocytic stimulus) appeared to be very slightly greater than it was in the absence of HNP. At higher concentrations (sufficient to impair phagocytic killing), superoxide production was almost totally inhibited. These data are consistent with earlier studies in the 24-well culture plate model system, which demonstrated that the ROI production of secondary-inoculum PMN was diminished (36). The data are consistent with the finding that this biomaterial-associated impairment of ROI production could also be defensin related. It is even conceivable that defensins with intense cationic charge actually scavenge O2− and other antimicrobial radicals, such as ⋅NO. While events on one material should not be extrapolated to occurrences on other materials, preliminary studies have suggested that neutrophils placed on materials commonly used in medical practice, such as woven Dacron (a highly activating material) or Silastic (a less activating material but nevertheless associated with increased infectivity in vivo) also become depleted of defensins, with depletion on woven Dacron much greater than that on Silastic.
Taken together, these studies support our observations that neutrophil dysregulation at a material surface can contribute to microbial survival. It seems clear that released neutrophil products play some role in this dysregulation. These studies further show that one of these products is the granule constituent HNP, which not only plays an important role in host defense against microbial infection but can, under appropriate conditions, create an environment hostile to host defense processes.
ACKNOWLEDGMENTS
We gratefully acknowledge helpful discussions with Robert E. Lehrer, who helped to point us in the right direction.
Expert technical assistance was provided by Amy Sartori and Leo Mortimer, and expert secretarial assistance was provided by Tracy Garchak and Rebecca Pfeifer.
This work was supported by National Institute of Health grant R01-GM-41734.
REFERENCES
- 1.Absolom D R, Lamberti F F, Policova Z, Zingg W, van Oss C J, Neumann A W. Surface thermodynamics of bacterial adhesion. Appl Environ Microbiol. 1983;46:90–97. doi: 10.1128/aem.46.1.90-97.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arizono T, Oga M, Sugioka Y. Increased resistance of bacteria after adherence to polymethyl methacrylate. Acta Orthop Scand. 1992;63:661–664. doi: 10.1080/17453679209169731. [DOI] [PubMed] [Google Scholar]
- 3.Babior B M. Oxidants from phagocytes: agents of defense and destruction. Blood. 1984;64:959–966. [PubMed] [Google Scholar]
- 4.Baddour L M, Christensen G D, Hester M G, Bisno A L. Production of experimental endocarditis by coagulase-negative staphylococci: variability in species virulence. J Infect Dis. 1984;150:721–727. doi: 10.1093/infdis/150.5.721. [DOI] [PubMed] [Google Scholar]
- 5.Borregaard N, Lollike K, Kyeldsen L, Sengelov H, Bastholm L, Nielsen M H, Bainton D F. Human neutrophil granules and secretory vesicles. Eur J Haematol. 1993;51:187–198. doi: 10.1111/j.1600-0609.1993.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 6.Boyum A. Isolation of mononuclear cells and granulocytes from human blood. Scand J Clin Lab Investig. 1968;21:77–89. [PubMed] [Google Scholar]
- 7.Brunstedt M R, Sapatnekar S, Rubin K R, Kieswetter K M, Ziats N P, Merritt K, Anderson J M. Bacteria/blood/material interactions. I. Injected and preseeded slime-forming Staphylococcus epidermidisin flowing blood with biomaterials. J Biomed Mater Res. 1995;29:455–466. doi: 10.1002/jbm.820290405. [DOI] [PubMed] [Google Scholar]
- 8.Charp P A, Rice W G, Raynor R L, Reimund E, Kinkade J M, Ganz T, Selsted M E, Lehrer R I, Kuo J F. Inhibition of protein kinase C by defensins, antibiotic peptides from human neutrophils. Biochem Pharmacol. 1988;37:951–956. doi: 10.1016/0006-2952(88)90187-6. [DOI] [PubMed] [Google Scholar]
- 9.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Adherence of slime-producing strains of Staphylococcus epidermidisto smooth surfaces. Infect Immun. 1982;37:318–326. doi: 10.1128/iai.37.1.318-326.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Christensen G D, Simpson W A, Bisno A L, Beachey E H. Experimental foreign body infections in mice challenged with slime-producing Staphylococcus epidermidis. Infect Immun. 1983;40:407–410. doi: 10.1128/iai.40.1.407-410.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Daher K A, Selsted M E, Lehrer R I. Direct inactivation of viruses by human granulocyte defensins. J Virol. 1986;60:1068–1074. doi: 10.1128/jvi.60.3.1068-1074.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dougherty S H. Pathobiology of infection in prosthetic devices. Rev Infect Dis. 1988;10:1102–1117. doi: 10.1093/clinids/10.6.1102. [DOI] [PubMed] [Google Scholar]
- 13.Dougherty S H, Simmons R L. Infections in bionic man: the pathobiology of infections in prosthetic devices—part I. Curr Probl Surg. 1982;19:221–264. doi: 10.1016/0011-3840(82)90045-4. [DOI] [PubMed] [Google Scholar]
- 14.Dougherty S H, Simmons R L. Endogenous factors contributing to prosthetic device infections. Infect Dis Clin N Am. 1989;3:199–209. [PubMed] [Google Scholar]
- 15.Falck P. Characterization of human neutrophils adherent to organic polymers. Biomaterials. 1995;16:61–66. doi: 10.1016/0142-9612(95)91097-i. [DOI] [PubMed] [Google Scholar]
- 16.Friedman D W, Orland P J, Greco R S. Biomaterials: an historical perspective. In: Greco R S, editor. Implantation biology: the host response and biomedical devices. Boca Raton, Fla: CRC Press; 1994. pp. 1–12. [Google Scholar]
- 17.Ganz T. Extracellular release of antimicrobial defensins by human polymorphonuclear leukocytes. Infect Immun. 1987;55:568–571. doi: 10.1128/iai.55.3.568-571.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ganz T, Lehrer R I. Defensins. Pharmacol Ther. 1995;66:191–205. doi: 10.1016/0163-7258(94)00076-f. [DOI] [PubMed] [Google Scholar]
- 19.Ganz T, Selsted M E, Szklarek D, Harwig S S, Daher K, Bainton D F, Lehrer R I. Defensins: natural peptide antibiotics of human neutrophils. J Clin Investig. 1985;76:1427–1435. doi: 10.1172/JCI112120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gera J R, Lichtenstein A. Human neutrophil peptide defensins induce single strand DNA breaks in target cells. Cell Immunol. 1991;138:108–120. doi: 10.1016/0008-8749(91)90136-y. [DOI] [PubMed] [Google Scholar]
- 21.Gormley I P, Kowolik M J, Cullen R T. The chemiluminescent response of human phagocytic cells to mineral ducts. Br J Exp Pathol. 1985;66:409–416. [PMC free article] [PubMed] [Google Scholar]
- 22.Greco R S. Body parts: in vivo veritas. J Biomed Mater Res. 1997;34:409–410. doi: 10.1002/(sici)1097-4636(19970315)34:4<409::aid-jbm1>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 23.Gristina A G. Biomaterial centered infection: microbial adhesion versus tissue integration. Science. 1987;237:1588–1595. doi: 10.1126/science.3629258. [DOI] [PubMed] [Google Scholar]
- 24.Gristina A G, Costerton J W. Bacterial adherence to biomaterials and tissue. J Bone Joint Surg. 1985;67:264–273. [PubMed] [Google Scholar]
- 25.Gristina A G, Dobbins J J, Giammara B, Lewis J C, DeVries W C. Biomaterial-centered sepsis and the total artificial heart—microbial adhesion versus tissue integration. J Am Med Assoc. 1988;259:870–874. [PubMed] [Google Scholar]
- 26.Guo W. Infections associated with intraperitoneal biomaterials. Bulletin no. 92 of the Department of Surgery, Lund University. Lund, Sweden: Lund University; 1993. pp. 9–12. [Google Scholar]
- 27.Hogt A H, Dankert J, Feijen J. Adhesion of Staphylococcus epidermidis and Staphylococcus saprophyticusto a hydrophobic biomaterial. J Gen Microbiol. 1985;131:2485–2491. doi: 10.1099/00221287-131-9-2485. [DOI] [PubMed] [Google Scholar]
- 28.Hristova K, Selsted M E, White S H. Critical role of lipid composition in membrane permeabilization by rabbit neutrophil defensins. J Biol Chem. 1997;272:24224–24333. doi: 10.1074/jbc.272.39.24224. [DOI] [PubMed] [Google Scholar]
- 29.Hubbell J A. Biomaterials in tissue engineering. Bio/Technology. 1995;13:565–576. doi: 10.1038/nbt0695-565. [DOI] [PubMed] [Google Scholar]
- 30.Hunt J A, Flanagan B F, McLaughlin P J, Strickland I, Williams D F. Effect of biomaterial surface charge on the inflammatory response: evaluation of cellular infiltration and TNF production. J Biomed Mater Res. 1996;31:139–144. doi: 10.1002/(SICI)1097-4636(199605)31:1<139::AID-JBM15>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 31.Johnson G M, Lee D A, Regelmann W E, Gray E D, Peters G, Quie P G. Interference with granulocyte function by Staphylococcus epidermidisslime. Infect Immun. 1986;54:13–20. doi: 10.1128/iai.54.1.13-20.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagan B L, Ganz T, Lehrer R I. Defensins: a family of antimicrobial and cytotoxic peptides. Toxicology. 1994;87:131–149. doi: 10.1016/0300-483x(94)90158-9. [DOI] [PubMed] [Google Scholar]
- 33.Kagan B L, Selsted M E, Ganz T, Lehrer R I. Neutrophil antimicrobial peptides (defensins) form voltage-dependent ionic channels in planar lipid bilayer membranes. Proc Natl Acad Sci USA. 1990;87:210–214. doi: 10.1073/pnas.87.1.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaplan S S, Basford R E, Jeong M H, Simmons R L. Biomaterial-induced alterations of neutrophil superoxide production. J Biomed Mater Res. 1992;26:1039–1051. doi: 10.1002/jbm.820260806. [DOI] [PubMed] [Google Scholar]
- 35.Kaplan S S, Basford R E, Jeong M H, Simmons R L. Mechanisms of biomaterial-induced superoxide release by neutrophils. J Biomed Mater Res. 1994;28:377–386. doi: 10.1002/jbm.820280313. [DOI] [PubMed] [Google Scholar]
- 36.Kaplan S S, Basford R E, Jeong M H, Simmons R L. Biomaterial-neutrophil interactions: dysregulation of oxidative functions of fresh neutrophils induced by prior neutrophil-biomaterial interaction. J Biomed Mater Res. 1996;30:67–75. doi: 10.1002/(SICI)1097-4636(199601)30:1<67::AID-JBM9>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 37.Kaplan S S, Caliguiri L A, Basford R E, Zdziarski U E. Transient chemotactic defect in a child with elevated IgE. Ann Allergy. 1987;59:213–217. [PubMed] [Google Scholar]
- 38.Kaplan S S, Nardi M A. Impairment of leukocyte function during sickle cell crisis. J Reticuloendothel Soc. 1977;22:499–506. [PubMed] [Google Scholar]
- 39.Karlsson C, Nygren H, Braide M. Exposure of blood to biomaterial surfaces liberates substances that activate polymorphonuclear granulocytes. J Lab Clin Med. 1996;128:496–505. doi: 10.1016/s0022-2143(96)90047-5. [DOI] [PubMed] [Google Scholar]
- 40.Katz D A, Greco R S. The pathobiology of infections associated with biomaterials. Probl Gen Surg. 1994;1994(2):209–226. [Google Scholar]
- 41.Katz D A, Haimovich B, Greco R S. Neutrophil activation by expanded polytetrafluoroethylene is dependent on the induction of protein phosphorylation. Surgery. 1994;116:446–455. [PubMed] [Google Scholar]
- 42.Klock J C, Bainton D F. Degranulation and abnormal bactericidal function of granulocytes procured by reversible adhesion to nylon wool. Blood. 1976;48:149–161. [PubMed] [Google Scholar]
- 43.Kuhns D B, Kaplan S S, Basford R E. Hexachlorocyclohexanes, potent stimuli of O2−production and calcium release in human polymorphonuclear leukocytes. Blood. 1986;68:535–540. [PubMed] [Google Scholar]
- 44.Lehrer R I, Barton A, Dahrer K A, Harwig S S L, Ganz T, Selsted M E. Interaction of human defensins with Escherichia coli: mechanism of bactericidal activity. J Clin Investig. 1989;84:553–561. doi: 10.1172/JCI114198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lehrer R I, Daher K, Ganz T, Selsted M E. Direct inactivation of viruses by MCP-1 and MCP-2, natural peptide antibiotics from rabbit leukocytes. J Virol. 1985;54:467–472. doi: 10.1128/jvi.54.2.467-472.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lehrer R I, Ganz T. Antimicrobial polypeptides of human neutrophils. Blood. 1990;76:2169–2181. [PubMed] [Google Scholar]
- 47.Lichtenstein A. Mechanism of mammalian cell lysis mediated by peptide defensins. J Clin Investig. 1991;88:93–100. doi: 10.1172/JCI115310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lichtenstein A, Ganz T, Selsted M E, Lehrer R I. In vitrotumor cell cytolysis mediated by peptide defensins of human and rabbit granulocytes. Blood. 1986;68:1407–1410. [PubMed] [Google Scholar]
- 49.Lichtenstein A K, Ganz T, Nguyen T M, Selsted M E, Lehrer R I. Mechanism of target cytolysis by peptide defensins. Target cell metabolic activities, possibly involving endocytosis, are crucial for expression of cytotoxicity. J Immunol. 1988;140:2686–2694. [PubMed] [Google Scholar]
- 50.Lichtenstein A K, Ganz T, Selsted M E, Lehrer R I. Synergistic cytolysis mediated by hydrogen peroxide combined with peptide defensins. Cell Immunol. 1988;114:104–116. doi: 10.1016/0008-8749(88)90258-4. [DOI] [PubMed] [Google Scholar]
- 51.Malangoni M A, Livingston D H, Peyton J D. The effect of protein binding on the adherence of staphylococci to prosthetic vascular grafts. J Surg Res. 1993;54:168–172. doi: 10.1006/jsre.1993.1027. [DOI] [PubMed] [Google Scholar]
- 52.Marchant R, Hiltner A, Hamlin C, Rabinovitch A, Anderson J M. In vivobiocompatibility studies. I. The cage implant system and a biodegradable hydrogel. J Biomed Mater Res. 1983;2:283–318. doi: 10.1002/jbm.820170209. [DOI] [PubMed] [Google Scholar]
- 53.Marchant R, Miller K, Anderson J M. In vivo biocompatibility studies. V. In vivoleukocyte interactions with biomer. J Biomed Mater Res. 1984;18:1169–1190. doi: 10.1002/jbm.820180917. [DOI] [PubMed] [Google Scholar]
- 54.Miller R A, Britigan B E. The formation and biologic significance of phagocyte-derived oxidants. J Investig Med. 1995;43:39–49. [PubMed] [Google Scholar]
- 55.Panyutich A V, Voitenok N N, Lehrer R I, Ganz T. An enzyme immunoassay for human defensins. J Immunol Methods. 1991;141:149–155. doi: 10.1016/0022-1759(91)90141-2. [DOI] [PubMed] [Google Scholar]
- 56.Peters G, Locci R, Pulverer G. Adherence and growth of coagulase-negative staphylococci on surfaces of intravenous catheters. J Infect Dis. 1982;146:479–482. doi: 10.1093/infdis/146.4.479. [DOI] [PubMed] [Google Scholar]
- 57.Petty W. The effect of methylmethacrylate on bacterial phagocytosis and killing by human polymorphonuclear leukocytes. J Bone Joint Surg. 1978;60A:752–757. [PubMed] [Google Scholar]
- 58.Ratner B D, Johnston A B, Lenk T J. Biomaterial surface. J Biomed Mater Res. 1987;21(Suppl. A):59–87. [PubMed] [Google Scholar]
- 59.Salthouse T N. Cellular enzyme activity at the polymer-tissue interface: a review. J Biomed Mater Res. 1976;10:197–229. doi: 10.1002/jbm.820100204. [DOI] [PubMed] [Google Scholar]
- 60.Schwarzmann S, Boring J R. Antiphagocytic effect of slime from a mucoid strain of Pseudomonas aeruginosa. Infect Immun. 1971;3:762–766. doi: 10.1128/iai.3.6.762-767.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Selsted M E, Szklarek D, Ganz T, Lehrer R I. Activity of rabbit leukocyte peptides against Candida albicans. Infect Immun. 1985;49:202–206. doi: 10.1128/iai.49.1.202-206.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sengelov H, Follin P, Kjeldsen L, Lollike K, Dahlgren C, Borregaard N. Mobilization of granules and secretory vesicles during in vivoexudation of human neutrophils. J Immunol. 1995;154:4157–4165. [PubMed] [Google Scholar]
- 63.Sengelov H, Kjeldsen L, Borregaard N. Control of exocytosis in early neutrophil activation. J Immunol. 1993;150:1535–1543. [PubMed] [Google Scholar]
- 64.Shanbhag A, Yang J, Lilien J, Black J. Decreased neutrophil respiratory burst on exposure to cobalt-chrome alloy and polystyrene in vitro. J Biomed Mater Res. 1992;26:185–195. doi: 10.1002/jbm.820260205. [DOI] [PubMed] [Google Scholar]
- 65.Sheu M J, Baldwin W W, Brunson K W. Cytotoxicity of rabbit macrophage peptides MCP-1 and MCP-2 for mouse tumor cells. Antimicrob Agents Chemother. 1985;28:626–629. doi: 10.1128/aac.28.5.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sugarman B, Young E J. Infections associated with prosthetic devices: magnitude of the problem. Infect Dis Clin N Am. 1989;3:187–198. [PubMed] [Google Scholar]
- 67.Tal T, Aviram I. Defensin interferes with the activation of neutrophil NADPH oxides in a cell-free system. Biochem Biophys Res Commun. 1993;196:636–641. doi: 10.1006/bbrc.1993.2297. [DOI] [PubMed] [Google Scholar]
- 68.Tal T, Sharabani M, Aviram I. Cationic proteins of neutrophil azurophilic granules: protein-protein interaction and blockade of NADPH oxidase activation. J Leukoc Biol. 1998;63:305–311. doi: 10.1002/jlb.63.3.305. [DOI] [PubMed] [Google Scholar]
- 69.Wade B H, Mandell G L. Polymorphonuclear leukocytes: dedicated professional phagocytes. Am J Med. 1983;74:686–693. doi: 10.1016/0002-9343(83)91028-8. [DOI] [PubMed] [Google Scholar]
- 70.Wang I, Anderson J M, Jacobs M R, Marchant R E. Adhesion of Staphylococcus epidermidisto biomedical polymers: contributions of surface thermodynamics and hemodynamic shear conditions. J Biomed Mater Res. 1995;29:485–493. doi: 10.1002/jbm.820290408. [DOI] [PubMed] [Google Scholar]
- 71.Wang I, Anderson J M, Marchant R E. Staphylococcus epidermidisadhesion to hydrophobic biomedical polymer is mediated by platelets. J Infect Dis. 1993;167:329–336. doi: 10.1093/infdis/167.2.329. [DOI] [PubMed] [Google Scholar]
- 72.Wright D G, Bralove D A, Gallin J I. The differential mobilization of human neutrophil granules. Effects of phorbol myristate acetate and ionophore A23187. Am J Pathol. 1977;87:273–284. [PMC free article] [PubMed] [Google Scholar]
- 73.Wright D G, Gallin J I. Secretory responses of human neutrophils: exocytosis of specific (secondary) granules by human neutrophils during adherence in vitro and during exudation in vivo. J Immunol. 1979;123:285–294. [PubMed] [Google Scholar]
- 74.Yeaman M R, Bayer A S, Koo S-P, Foss W, Sullam P M. Platelet microbicidal proteins and neutrophil defensin disrupt the Staphylococcus aureuscytoplasmic membrane by distinct mechanisms of action. J Clin Investig. 1998;101:178–187. doi: 10.1172/JCI562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yomogida S, Nagaoka I, Saito K, Yamashita T. Evaluation of the effects of defensins on neutrophil functions. Inflamm Res. 1996;45:62–67. doi: 10.1007/BF02265117. [DOI] [PubMed] [Google Scholar]
- 76.Young E J, Sugarman B. Infections in prosthetic devices. Surg Clin N Am. 1988;68:167–180. doi: 10.1016/s0039-6109(16)44438-5. [DOI] [PubMed] [Google Scholar]
- 77.Ziats N, Miller K, Anderson J M. In vitro and in vivointeractions of cells with biomaterials. Biomaterials. 1988;9:5–13. doi: 10.1016/0142-9612(88)90063-4. [DOI] [PubMed] [Google Scholar]
- 78.Zimmerli W, Lew P D, Waldvogel F A. Pathogenesis of foreign body infection. Evidence for a local granulocyte defect. J Clin Investig. 1984;73:1191–1200. doi: 10.1172/JCI111305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zimmerli W, Waldvogel F A, Vandaux P, Nydegger V E. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497. doi: 10.1093/infdis/146.4.487. [DOI] [PubMed] [Google Scholar]