Abstract
Cancer is a major healthcare burden and cause of death worldwide, with an estimated 19.3 million new cancer cases and 10 million cancer deaths globally only in 2020. While several anticancer therapeutics are available to date, many of these still show low treatment efficacy and high off-target effects and adverse reactions. This prompts a serious need to develop novel therapies that can decrease the side effects and increase treatment efficacy. MicroRNAs (miRNAs) can have a role in tumor development and progression, making them important targets for the improvement of anticancer therapies. In this context, gold nanoparticles have been widely studied for different clinical applications due to their biocompatibility and possibility of customization, and gold nanoconjugates targeting miRNAs are being developed for cancer diagnosis and treatment. Here we summarize the research developed so far and how it can contribute to cancer treatment, discuss how it can be improved, and present the current challenges and future perspectives on their design and application.
Keywords: gold nanoparticles, miRNA nanoconjugates, cancer therapeutics, miRNA therapeutics, nanomedicine
Cancer is a major public health issue and one of the main causes of morbidity and mortality worldwide.1,2 In 2020 there were 19.3 million new cases and 10 million deaths estimated. Breast (2.26 million cases, 11.7%), lung (2.21 million cases, 11.4%), and colorectal (1.93 million cases, 10%) cancers had the most diagnosed new cases, while lung (1.80 million deaths, 18%), colorectal (935 000 deaths, 9.4%) and liver (830 000 deaths, 8.3%) cancers were the most common causes of cancer-related death. The number of cancer cases is expected to rise to 28.4 million cases by 2040.3 The standard approaches for cancer treatment include, among others, surgery, chemotherapy, radiotherapy, and immunotherapy. However, side effects and relapse are often observed following treatment.4,5 Moreover, the discovery of cellular and/or molecular components associated with carcinogenesis and cancer progression, as well as the mechanisms involved, allowed the development of personalized medicines, for example, with the use of microRNAs (miRNAs) for cancer diagnosis or as a target for gene therapies. Regarding this, the use of nanotechnology for both diagnosis and treatment of cancer has been increasingly studied in the past decade.6 The use of nanoconjugates for biosensing of nucleic acids has been reported for different applications, as recently reviewed,7,8 such as the detection of viral RNA or DNA, through colorimetric or fluorescent changes. These can quickly, specifically, and selectively detect the molecules of interest, which can be attained by tuning the biological function of nanoparticles with their optical and plasmonic functions, through the use of differently sizes and shapes, and by modifying their surface, for instance, through the addition of complementary fluorescent nucleic acid molecules, which emit fluorescence after recognizing and binding to their target. Such nanobiosensors could be used for real-time measurements while decreasing the limit of detection of the target molecules, thus becoming alternative methods to other commonly used methods for detecting disease-associated biomarkers.7,8 Moreover, considering the possible applications of nanoconjugates for therapy, the properties of nanoparticles give them a multifunctionality which prompts them to be used as carriers for selective and in situ drug delivery. It has been observed that targeted and nontargeted nanomedicines can efficiently deliver the payloads to the tumors, many times at lower dosages, increasing the therapeutic effect and anticancer efficacy and reducing toxicity when compared to standard therapies.9 miRNA-targeted nanoconjugates are able to overcome challenges found by nucleic acids, such as instability, easy degradation, and limited cellular uptake, improving the down- or upregulation of endogenous miRNAs. Furthermore, effective treatment at the tumor site is essential so that these advantages are achieved. For instance, the use of nanoparticles for photothermal therapy (PTT) and photodynamic therapy (PDT) allows them to improve cancer cell destruction at the site, which also potentially enhances the antitumor immune response, while being detected through their imaging properties.10 Yet, the development of such nanomedicines continues to be challenging in several aspects, which limits their application. Obstacles include the synthesis method used, stability of the nanoparticles, and half-life and toxicity of the molecules and of the particles themselves. Also, many studies still lack in vivo confirmation of the results and the translation to the clinic is the most considerable obstacle for the development of these therapies.10
In this Perspective, we will briefly review the application and use of gold nanoconjugates for cancer therapeutics involving miRNAs. We will also discuss the strategies used so that these nanoconjugates can target different miRNAs, showing how they can be almost fully customizable toward a certain type of cancer or target different targets simultaneously, and discuss the challenges involved in their design and clinical application.
MicroRNAs and Cancer
miRNAs are small (approximately 21–25 nucleotide-long) noncoding endogenous RNAs which participate in gene expression regulation by reducing mRNA stability or by repressing its translation.11 Thus, miRNAs play an essential role in tumorigenesis and tumor progression and metastasis, and disruption of their expression is associated with alterations in different cell processes that can affect these functions. In this context, it has been observed that cancer cells often present abnormal miRNA expression profiles, which can alter their cellular properties and biological processes (e.g., migration, invasion, proliferation)11,12 and the interaction with their microenvironment, namely, with immune cells, by mediating the inflammatory response, thus contributing to tumor initiation and progression, as reviewed recently by Zhang and co-workers.13
These features offer therapeutic potential for miRNAs and for the development of new anticancer strategies. Targeting and modulating their expression by either restoring their function or inhibiting their overexpression will influence their regulatory network, and this can be an approach to halt cancer progression and decrease or overcome drug resistance by combining miRNA therapeutics with other currently used therapies.12,14
Gold Nanoparticles as Anticancer Agents
Nanoparticles have the advantage of being structures that are easily and inexpensively assembled, with a size in the nanometer range that allows their easy diffusion into cells. Moreover, their surface has the possibility to be modified with several types and numbers of moieties, transforming them into delivery vehicles that can selectively target genes, proteins, and other elements at the cell surface and at subcellular locations as well as accumulate at specific tissues. Their structure can also be modified, for example, to release drugs or other components at different pHs and temperatures or upon degradation by enzymes. All these possible nanoparticle designs are made so that the final vehicles are biocompatible and nonimmunogenic, increasing their half-life in the organism and efficiency. The possibility to directly target specific cells or tissues greatly reduces off-target effects, and the use of nanoparticles as vehicles for drug delivery allows a reduction in the dose used and the number of applications, which in turn reduces the side effects that are often observed in anticancer therapies. These and other features of nanoparticles and their applications to drug delivery and medicine have been extensively reviewed recently.15−18 Regarding inorganic nanomaterials, gold nanoparticles have the advantage of being easily synthesized, mostly through the citrate reduction method, and their gold core is mostly inert and nontoxic. Depending on their shape, size, and structure, which includes the different surface modifications, gold nanoparticles have unique optical properties, determined by their surface plasmon resonance (SPR), that can be observed from the visible to the infrared region.19 Moreover, their surface can be functionalized with a wide range of moieties and ligands that influence their stability and targeting and imaging abilities, making these nanoparticles candidate vehicles to be used in several applications, such as therapy, diagnosis, and imaging, and as biosensors.15,20−23 In summary, recent research showed that gold nanomaterials have unique properties that make them very efficient carriers to overcome some of the challenges that will be mentioned throughout this paper.20,21,24−26
Gold Nanoconjugates for miRNA Cancer Therapeutics
The two most used approaches for the development of miRNA-based therapies consist, as previously mentioned, on restoring the loss of miRNA function, or silencing miRNA overexpression (Figure 1). miRNA function can be reestablished with the use of miRNA mimetics, which are synthetic double-stranded RNA molecules that mirror the endogenous miRNAs. However, these nucleotides have a limited cellular uptake and are unstable and easily degraded in the biological systems, creating a barrier for the development of miRNA therapeutics.12 Endogenous miRNA inhibition can be achieved through anti-miRs or antagomiRs, i.e., single-stranded RNAs that are synthesized in accordance to the miRNA mature sequence. These sequences can be chemically modified with fluorophores, thiols, and thiophosphates, for example, to allow tracking inside the cells, to better improve binding to their carrier and stability, and to promote competitive binding with the mature miRNA, increasing the inhibition effect.12 It is, thus, essential, and a challenge, to develop an effective and biocompatible vehicle for the delivery of RNA therapeutics, and here is where nanotechnology, namely, gold nanomaterials, has the potential to be used to substantially improve the miRNA mimics’ and antagomiRs’ stability and cellular uptake, promoting a successful delivery, due to the features previously presented.
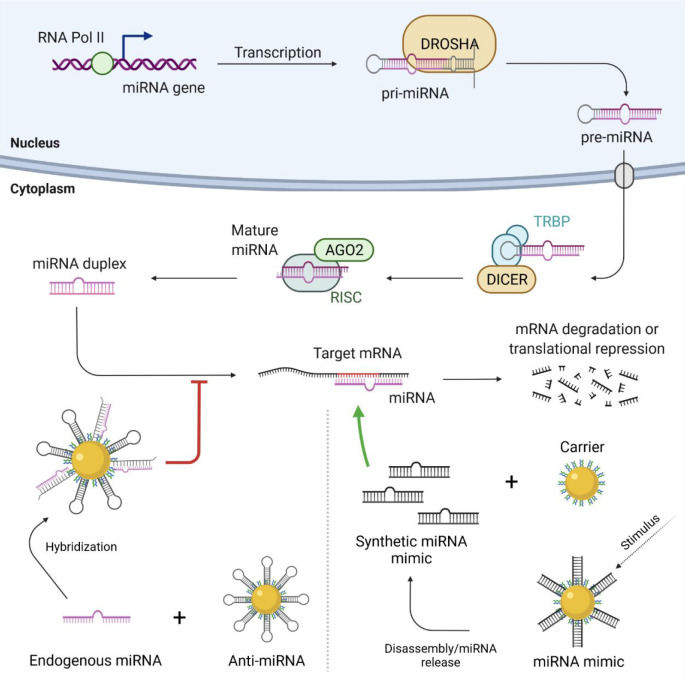
Figure 1.
Schematic representation of miRNA biogenesis and interaction of miRNA nanoconjugates. In the nucleus, the miRNA gene is transcribed by RNA polymerase II (Pol II) forming the double-stranded hairpin structure of the primary miRNA (pri-miRNA). This molecule is processed by DROSHA into a precursor miRNA (pre-miRNA), which is exported to the cytoplasm through an exportin protein. The pre-miRNA hairpin is cleaved by the RNase Dicer and the transactivation response element RNA-binding protein (TRBP) cofactor, and the resulting mature miRNA is further processed by the RNA-induced silencing complex (RISC), which includes the endonuclease Argonaute 2 (AGO2), originating a miRNA duplex. One strand of the miRNA duplex binds to the target mRNA, regulating gene expression by mRNA degradation or by repressing its translation. Anti-miRNA nanoconjugates bind to overexpressed endogenous miRNA, decreasing the number of available molecules to interact with the target mRNA, thus inhibiting or decreasing mRNA degradation and repression (bottom left). Nanoconjugates carrying miRNA mimetics are disassembled or release synthetic miRNA molecules upon a stimulus (e.g., near-infrared light irradiation), increasing the number of miRNA molecules available for interaction with the target mRNAs, thus promoting an increase in the mRNA degradation and repression process (bottom right).
In this context, some studies combining miRNAs with gold nanomaterials as carriers for cancer therapeutics have been reported (Table 1). In some studies, nanoconjugates are being evaluated for gene therapy by delivering miRNAs using miRNA mimics, aiming to restore the expression of tumor-suppressive miRNAs. In other findings, nanoconjugates are being evaluated as miRNA silencers to inhibit overexpressed miRNAs, using antisense oligonucleotides/antagomiRs (Figure 2). Although some of these approaches have been reported as a therapeutic strategy on their own, some authors evaluate them in combination with different standard therapies generally used in cancer treatment, such as chemotherapy or immunotherapy. Due to the properties of gold nanoparticles, many of these nanoconjugates can also be used in theranostics, i.e., used for cancer diagnosis and therapy.
Table 1. Gold Nanoconjugates for miRNA Therapeutics.
| nanomaterial and modifications | target/catalyst mirna | type of cancer | therapy | main results | ref |
|---|---|---|---|---|---|
| spherical gold particle, PEG, miRNA mimetic | hsa-miR-206 | breast | gene therapy | restored miRNA expression; cell cycle arrested in the G0-G1 phase, induction of apoptosis by downregulation of NOTCH 3 | (27) |
| spherical gold shell, PEG, miRNA mimetic | hsa-miR-34a | breast | gene therapy, photothermal therapy | restored miRNA expression; decreased the expression of target genes involved in cell proliferation (CCND1), survival (Bcl2, Sirt1, Survivin), and motility (Nanog), with respective decreased in the cellular metabolic activity, proliferation and migration | (28) |
| silica core with gold shell, PLL, miRNA mimetic | hsa-miR-34a | breast | gene therapy | restored miRNA expression; decreased cell proliferation due to suppression of SIRT1 and Bcl-2 | (29) |
| spherical gold particle, PLL, miRNA mimetic | hsa-miR-708 | breast | gene therapy | restored miRNA expression; increased tumor-suppressive effects and decreased lung metastases | (30) |
| gold rod, PEI, RGD peptide, miRNA mimetic | hsa-miR-320a | lung | gene therapy, photothermal therapy | increased apoptosis; decreased cell proliferation and migration through inhibition of Sp1; increased PTEN expression; Inhibited MMP-9 | (31) |
| spherical gold particles, miRNA mimetic | hsa-miR-326 | liver | gene therapy | restored miRNA expression; increased apoptosis; modulated the EMT phenotype with decrease in migration and invasion; inhibition of tumor growth | (32) |
| hydrogel-embedded spherical gold particles, miRNA mimetic | hsa-miR-96 and hsa-miR-182 | breast | gene therapy, chemotherapy | downregulation of Palladin; decreased cell motility; decreased cancer metastasis | (33) |
| gold cages, PEI, hyaluronic acid, anti-miRNA | hsa-miR-181b | liver | gene therapy, photothermal therapy | downregulation of the miRNA expression; Increased expression of TIMP-3 mRNA; Decreased MMP function; Inhibition of tumor growth | (34) |
| spherical gold particles, polyadenyl linker, AS1411 aptamer, anti-miRNA | hsa-miR-155 | breast | gene therapy | downregulation of the miRNA expression; increased TP53INP1 mRNA, restoring cellular functions | (35) |
| spherical gold particles, nuclear localization signal peptide, AS1411 aptamer, anti-miRNA | hsa-miR-221 | leukemia | gene therapy | downregulation of the miRNA expression; decreased tumor proliferation and clonogenic potential; increased overall survival; decreased and less aggressive metastasis | (36) |
| spherical liposome encapsulated gold particles, PEG, apolipoprotein E, rabies virus glycoprotein, anti-miRNA | hsa-miR-92b | brain | gene therapy | increased systemic delivery and crossing through the blood-brain barrier; increased tumor accumulation; downregulation of the miRNA expression; decreased cell viability | (37) |
| spherical bismuth selenide particles with a gold shell, polyvinylpyrrolidone, fluorophore-labeled pH-sensitive folic acid-mediated cell penetrating peptide, anti-miRNA | hsa-miR-152 | neuroblastoma | gene therapy, photothermal therapy | increased cell targeting; downregulation of the miRNA expression; upregulation of CHUK, resulting in increased death rate; tumor size reduction | (38) |
| spherical gold particles, PEG, folic acid, AS1411 aptamer, doxorubicin, anti-miRNA | hsa-miR-221 | leukemia | gene therapy, chemotherapy | downregulation of the miRNA expression; downregulation of DNMT1; decreased growth of drug-resistant cells; increased sensitization to doxorubicin; decreased P-glycoprotein expression | (41) |
| spherical gold particles, anti-miRNA | hsa-miR-221 | liver | gene therapy, chemotherapy | inhibition of the miR-221/p27/DNMT1 pathway; inhibition of cell proliferation; increased chemo sensitization to sorafenib | (42) |
| gold particles, miRNA mimetic + gold particles, AS1411 aptamer, SN38 | hsa-miR-34a, hsa-miR-182, hsa-miR-137 and hsa-miR-144 | uveal melanoma | gene therapy, chemotherapy | a synergistic effect was observed; increased cytotoxicity; increased inhibition of cell viability | (43) |
| gold rods coated with mesoporous silica particles, PEG, hyaluronic acid, miRNA mimetic, paclitaxel | hsa-let-7 | ovary | gene therapy, chemotherapy | increased specificity to cancer cells; decreased P-glycoprotein expression; decreased cell proliferation; increased chemotherapeutic effect; increased apoptosis; decreased tumor growth | (44) |
| dendrimer-entrapped spherical gold particles, PEG, gemcitabine, anti-miRNA | hsa-miR-21 | pancreas | gene therapy, chemotherapy | enhanced combined therapeutic effect; decreased tumor volume; increased intratumoral blood perfusion; increased tumor apoptosis; increased survival rate | (45) |
| spherical hollow gold particles, PAMAM dedrimers, doxorubicin, anti-miRNA | hsa-miR-21 | breast | gene therapy, photothermal therapy, chemotherapy | sequential delivery increased synergy of two apoptotic signaling pathways; increased tumor accumulation; increased tumor sensitization to chemotherapy; decreased tumor growth; increased anticancer efficacy | (46) |
| spherical gold shell, PEG, RGD peptide, doxorubicin, ALK siRNA, anti-miRNA | hsa-miR-301 | lung | gene therapy, chemotherapy, photothermal therapy | downregulation of ALK expression; increased expression of Bim; increased inhibition of cell proliferation; increased apoptotic effect; increased specificity to EML4-ALK mutated cells; increased tumor accumulation; increased synergistic therapeutic effect; decreased toxicity | (47) |
| gold rods, PEG, folic acid, fluorophore-labeled molecular beacon | hsa-miR-21 | cervix | gene therapy, photothermal therapy | in situ miRNA-responsive imaging of cancer cells; increased apoptosis | (48) |
| gold particles with copper sulfide shell, polydopamine, AS1411 aptamer, fluorophore-labeled hairpin DNA | hsa-miR-21 | cervix | gene therapy, photothermal therapy, photodynamic therapy, chemodynamic therapy | tumor growth inhibition by 50%; imaging and quantitative detection of miRNA-21 in cancer cells | (49) |
| spherical gold–iron oxide particles, β-cyclodextrin-chitosan, PEG-T7 peptide, fluorophore-labeled miRNA mimic, fluorophore-labeled anti-miRNA | hsa-miR-100, hsa-miR-21 | brain | gene therapy, chemotherapy | increased accumulation in the tumor; increased sensitization to temozolomide; increased survival | (50) |
| spherical gold particle-quantum dot complex, fluorophore-labeled DNA, doxorubicin | hsa-miR-21 | cervix | gene therapy, chemotherapy | doxorubicin was effectively released; decreased cell viability | (51) |
| spherical gold particles, DNA/RNA duplexes, doxorubicin | hsa-miR-21, miR-10b | breast | gene therapy, chemotherapy | doxorubicin was released and siRNA was generated in situ after hybridization of the DNA/RNA duplex; downregulation of Bcl-2; decreased cell viability; increased antitumor effect | (52) |
| spherical gold particle core, gold particle satellites, DNA, fluorophore-labeled caspase-3-responsive peptides, doxorubicin | hsa-miR-21 | breast, liver, lung | gene therapy, chemotherapy | doxorubicin was effectively released; increased apoptosis; increased caspase-3 activation | (53) |
| spherical gold particle, PEG, fluorophore-labeled miRNA mimic | hsa-miR-182 | brain | gene therapy | effective delivery through the blood–brain barrier; downregulation of Bcl2L12 and c-Met proteins; increased sensitization to drugs; reduced tumor burden; increased survival; absence of significant adverse side effects and inflammatory responses associated with systemic administration | (65) |
| spherical gold particles, PEG, fluorophore-labeled anti-miRNA | hsa-miR-21 | colorectal | gene therapy | downregulation of miRNA expression; EGFP expression was specifically silenced; no cytotoxicity was observed | (66) |
| spherical gold particles, PEG, fluorophore-labeled miRNA mimic | hsa-miR-375 | liver | gene therapy | suppression of cell proliferation, migration/invasion and colony formation; induction of apoptosis; increased tumor uptake; increased therapeutic efficacy; no toxicity | (67) |
| spherical gold particles on graphene composites, P-glycoprotein antibody, folic acid, miRNA mimic | hsa-miR-122 | liver | gene therapy, photothermal therapy | low toxicity; increased targeting ability; increased permeability of cell membrane to drugs; increased apoptosis; inhibition of tumor growth | (68) |
| spherical gold particle, folic acid, fluorophore-labeled miRNA mimic | hsa-miR-122 | liver | gene therapy | cancer-specific targeting; glutathione-mediated release of the miRNA; induction of apoptosis via activation of the caspase-9 pathway | (69) |
| liposome encapsulated spherical gold particles, fluorophore-labeled molecular beacons | hsa-miR-21 | breast | gene therapy, photothermal therapy | specific imaging of miRNA-21; induction of apoptosis; increased therapeutic effect; no observable side effects | (70) |
| spherical gold particles, PEI, anti-miRNA | EBV-miR-BART7-3p | nasopharynge | gene therapy | reduced cell growth; reduced colony formation; increased PTEN expression; decreased cell growth | (71) |
| spherical gold particle, DNA, doxorubicin | hsa-miR-21 | breast | gene therapy, chemotherapy | fluorescence detection of doxorubicin release and miRNA-21; effective blocking of the endogenous miRNA; increased cell death | (72) |
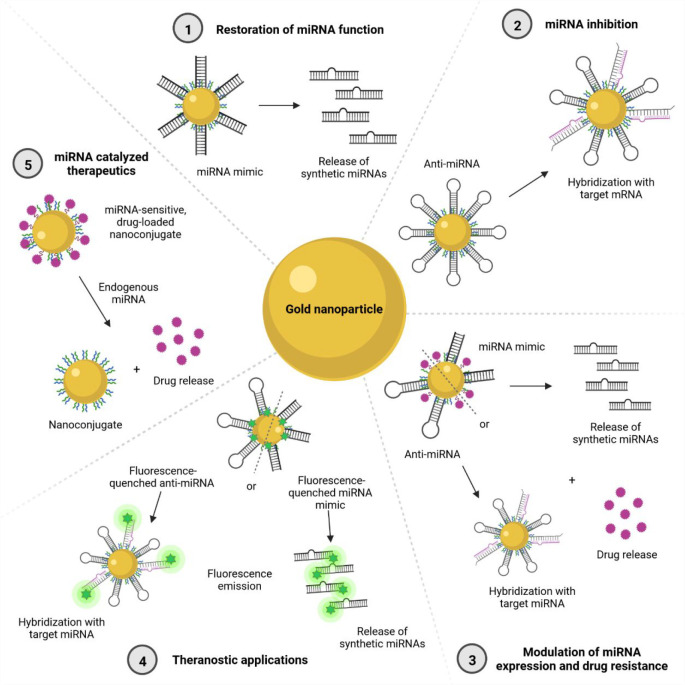
Figure 2.
Possible combinations of using gold nanomaterials for miRNA therapeutics and diagnosis. ① Restoration of miRNA function: a carrier releases miRNA mimics upon exposure to an internal or external stimulus. ② miRNA inhibition: a carrier modified with anti-miRNA molecules binds to the target miRNA, decreasing its functions. ③ Modulation of miRNA expression and drug resistance: a carrier modified with an anti-miRNA or a miRNA mimic and a drug performs its mimicking or inhibitory actions while releasing a drug upon exposure to a stimulus. ④ Theranostic applications: fluorophore-modified carriers perform mimicking or inhibitory actions while allowing for simultaneous detection. ⑤ miRNA catalyzed therapeutics: nanoconjugates release carried drugs upon exposure to a stimulus of endogenous miRNAs.
Gold Nanoconjugates to Restore miRNA Function
Restoring the functions of miRNAs that act as tumor suppressors to their normal is a therapeutic strategy that demonstrated promising results through the use of miRNA mimics or small molecules, as reviewed elsewhere.12 Among other carriers and delivery methods, gold nanoconjugates have been evaluated for gene regulation of cancer through miRNA delivery. Some of the studies, both in vitro using cancer cell lines and in vivo using animal tumor models, will be described below.
For instance, PEGylated gold nanoparticles were conjugated with a miR-206 mimic and tested on breast cancer cells in vitro, showing effective treatment by targeting the NOTCH 3 gene and arresting cells in the G0-G1 phase.27 In another study by Dang et al., the authors designed photoresponsive gold nanoshells conjugated to miR-34a to overcome the lack of specificity and efficacy observed in other studies. These nanocarriers released the miRNA upon stimulation with near-infrared light which led to a regulation of its downstream targets and decreased the function of triple negative breast cancer cells, namely their viability, proliferation, and motility.28 Targeting the same miRNA, Goyal and colleagues reported silica core/gold nanoshells for the intracellular delivery of miR-34a in triple negative breast cancer cells. They used a layer-by layer system by coating negatively charged gold nanoshells with a layer of positive poly-l-lysine (PLL) and another of the negatively charged miRNA. Cellular uptake was facilitated by a final layer of PLL, which also allowed for protection of the miRNA from degradation. This system effectively decreased cell proliferation by suppressing SIRT1 and Bcl-2, both targets of miR-34a.29 Ramchandani and colleagues developed a construct consisting of a gold nanoparticle core to which positively charged PLLs were added and a negatively charged miR-708 mimetic was layered between the PLLs. This layer-by layer design enabled the degradation of the PLLs by proteases upregulated in tumors, such as cathepsin B, and protected the miRNA mimetic from endogenous nuclease degradation, allowing their slow release and increasing their efficacy, which in turn allowed a decrease in the frequency of administration. The construct was evaluated in triple negative breast cancer, both in vitro and in vivo, and was shown to be efficient in restoring the miR-708 expression and tumor-suppressive effects. Moreover, treatment with the nanoconjugate decreased lung metastases.30 Using a gene-targeted therapy that can be combined with laser irradiation for photothermal therapy, Peng and co-workers developed gold nanorods that targeted integrin αvβ3 and were capable of specifically releasing miR-320a in lung cancer cells. Delivery of the miRNA by the nanoconjugate in combination with the laser irradiation increased apoptosis and inhibited cell proliferation and metastasis by inhibiting the Sp1 transcription factor, enhancing the expression of PTEN, and inhibiting matrix metallopeptidase (MMP)-9, both in vitro and in vivo.31 In another study, the authors observed that miR-326 was downregulated in hepatocellular carcinoma cell lines and tissues, which was associated with poor prognosis in patients. In vitro studies using gold nanoparticles to deliver a miR-326 mimic showed that miR-326 expression increased, which promoted apoptosis and modulated the epithelial-to-mesenchymal transition (EMT) phenotype, inhibiting cell invasion and migration. Tumor growth inhibition was also observed in vivo after treatment of a hepatocellular carcinoma model with the nanoconjugate carrying the miR-326 mimic.32 Gilam and co-workers suggested the delivery of miR-96 and miR-182 as a potential antimetastatic drug for breast cancer, as these two miRNAs were found to regulate Palladin, a protein associated to breast cancer motility. By using a vehicle composed of hydrogel-embedded gold nanoparticles, miR-96 and miR-182 were specifically delivered to breast tumors, effectively downregulating Palladin and decreasing cell motility and, thus, cancer metastasis. Also, addition of the chemotherapeutic agent cisplatin to the nanoparticles increased the antimetastatic effect and decreased tumor growth in a mouse model.33
Gold Nanoconjugates for miRNA Silencing
Suppressing miRNAs with oncogenic functions has also been widely evaluated using various delivery methods, showing encouraging results.12 Regarding the use of gold nanoconjugates for gene regulation through the delivery of miRNA inhibitors, some studies have also been performed.
Yan and colleagues devised and evaluated a layer-by-layer method to assemble gold nanocages to polyethyleneimine (PEI), to anti-miR-181b, and to hyaluronic acid. The latter was used to promote a target-specific endocytosis on hepatocellular carcinoma cells, while anti-miR-181b was used to inhibit the expression of the target miRNA, which is often overexpressed in liver cancer. Moreover, the properties of gold nanocages allowed their use for photothermal therapy. The complex showed an effective delivery of the anti-miRNA, downregulating miR-181b expression and increasing expression of TIMP-3 mRNA, which prevents the function of MMPs, inhibiting tumor growth. Also, when compared to single gene therapy or photothermal therapy, the combination of anti-miR181b delivery with near-infrared irradiation increased cell death and exhibited a better overall therapeutic effect in hepatocellular carcinoma cells in vitro.34 Kardani et al. functionalized gold NPs with an antagomiR and a nucleolin-specific aptamer for breast cancer targeted therapy. The AS1411 aptamer promoted the selective targeting of cancer cells, as nucleolin is aberrantly expressed on their surface, while the antagomir promoted the silencing of miR-155, an oncogenic miRNA, and increased the mRNA of its direct target, TP53INP1.35 Using a similar construct, Deng et al. designed a similar nanoconjugate comprising nuclear localization signal peptide-targeted gold nanoparticles loaded with the miR-221 antagomiR and the AS1411 aptamer and observed that leukemia proliferation and clonogenic potential were blocked. Moreover, in an acute myeloid leukemia preclinical animal model, the overall survival increased, the white blood cell count decreased, splenomegaly was reversed, metastasis to the lung was less aggressive, and metastasis to the liver was not found.36 Using peptide apolipoprotein E-conjugated liposome-encapsulated gold nanoparticles, Grafals-Ruiz and co-workers targeted miRNA-92b in brain cells by using functionalization with an oligonucleotide miRNA-92b inhibitor. The conjugation to apolipoprotein E increased the systemic delivery and accumulation to brain tumors in mice models, and aberrant miRNA expression was inhibited by the particles, reducing cell viability.37 In another study, core–shell bismuth selenide–gold nanoparticles were functionalized with polyvinylpyrrolidone to enhance biocompatibility with fluorophore-labeled antagomiR-152 and with a pH-sensitive folic acid-mediated cell penetrating peptide, which enhanced neuroblastoma cell targeting. In this study, downregulation of miR-152 promoted the upregulation of CHUK, a proapoptotic gene, resulting in increased death rate. Also, in vivo studies showed that there was a significant reduction in tumor size after photothermal treatment in the group of mice treated with the nanoparticles, showing that they effectively targeted the tumors and improved the therapeutic effect.38
Gold Nanoconjugates for Simultaneous Modulation of miRNA Expression and Drug-Resistance
A major cause of therapeutic failure is treatment inefficiency due to multidrug resistance (MDR), leading to many patients with different types of cancer relapsing after treatment.39,40 To try and modulate this process, Deng and colleagues designed a multitarget drug-delivery system in which gold nanoparticles were modified with the AS1411 aptamer, for simultaneous delivery of doxorubicin, and a miR-221 antagomiR aiming at reversing multidrug-resistant leukemia. These particles downregulated miR-221 and DNMT1, which was associated with decreased growth of drug-resistant cells and increased sensitization to doxorubicin. Moreover, depletion of miR-221 reduced the expression of P-glycoprotein, frequently overexpressed in multidrug-resistant cancers.41 Another anti-miR-221 gold nanoconjugate was developed by Cai et al., and its synergistic antitumor effects with sorafenib, another chemotherapeutic agent, were evaluated. This antagomiR nanoconjugate inhibited the miR-221/p27/DNMT1 pathway. Synergistic treatment showed an increased effect in the inhibition of cell proliferation, showing that the antagomiR can act as a chemosensitizer and increase the effect of sorafenib on hepatocellular carcinoma cells.42 Moreover, Rois and co-workers functionalized a set of gold nanoparticles with miR-34a, miR-182, miR-137, and miR-144, which are downregulated in uveal melanoma, and another set of nanoparticles with the aptamer AS1411 and the chemotherapeutic drug SN38, a topoisomerase I inhibitor, and evaluated the individual and combined effects of the two gold nanoconjugates on uveal melanoma cells. The molecules delivered by the nanocarriers presented an activity similar to that observed in the free biomolecules, allowing their functions to be carried out without limitations, such as degradation by endonucleases. A synergistic effect was observed and translated to a higher cytotoxicity and inhibition of cell viability when the two types of nanoparticles were evaluated in combination in vitro.43 Targeting ovarian cancer, gold nanorods coated with mesoporous silica nanoparticles were built for a combined delivery of paclitaxel and the miRNA lethal-7a (let-7a), aiming to overcome MDR.44 These nanocomplexes specifically bound to cancer cells, decreased their proliferation ability, and improved the therapeutic effect in vitro. In a mouse model, the authors observed that P-glycoprotein was downregulated by let-7a and that paclitaxel was effectively released, enhancing the chemotherapeutic effect, inducing apoptosis, and inhibiting tumor growth. Lin and colleagues tackled ineffective treatment and drug resistance in pancreatic cancer by reporting PEGylated-dendrimer-entrapped gold NPs for simultaneous loading and delivery of gemcitabine, the standard chemotherapeutic drug administered, and a miR-21 inhibitor, as miR-21 is overexpressed in this type of cancer. In a xenograft model, the combined therapeutic effect was enhanced, as nanoconjugate codelivery showed a lower tumor volume, increased intratumoral blood perfusion, and tumor apoptosis and mice showed an increased survival rate.45 Targeting the same miRNA in triple-negative breast cancer, a near-infrared radiation (NIR)-responsive hollow gold-nanoparticle-based codelivery system for the sequential delivery of a miR-21 inhibitor and doxorubicin was designed. The nanoparticles were modified with polyamidoamine (PAMAM) dendrimers, allowing doxorubicin to be absorbed to the negative surface of the nanoparticles, while the miR-21 inhibitor was condensed to the dendrimers through electrostatic interactions. The nanoconjugate was demonstrated to release first the miR-21 inhibitor and then doxorubicin in a site-specific and timely manner triggered by irradiation, which promoted synergy of two apoptotic signaling pathways, increasing the anticancer efficacy, both in vitro and in vivo, where mice models showed increased tumor accumulation of the nanoconjugates and therefore increased tumor sensitization to chemotherapy and decreased tumor growth effectively after codelivery of both the doxorubicin and the miRNA inhibitor.46 Li and colleagues used a gold nanoshell system to create a multitreatment strategy including chemo-, gene-, and thermal therapy specifically directed for the treatment of the EML4-ALK mutation-positive non-small cell lung cancer. For this, doxorubicin, an ALK siRNA, and a miRNA-301 inhibitor were added to the gold carrier as well as the RGD peptide for cellular targeting and uptake improvement. Treatment of lung cells with the siRNA downregulated ALK expression and increased the expression of Bim, an apoptotic protein. Inhibition of miR-301 also increased Bim expression, which led to a synergistic increased inhibition of cell proliferation and increased apoptotic effects. Moreover, this nanoconjugate was evaluated in different lung cell lines and found to have higher specificity to cells expressing increased levels of the EML4-ALK mutation. Overall, studies using mice models showed that this carrier system effectively accumulated in tumors and had a higher synergistic therapeutic effect, with lower toxicity, when compared to doxorubicin alone.47
Gold Nanoconjugates for Theranostic Applications
As mentioned, gold nanoparticles can be used for theranostic applications by taking advantage of the characteristics, such as photothermal properties and the ability to be functionalized with fluorescent moieties, making their use possible for diagnostic and imaging applications. This can then be combined with their carrier properties for therapeutic uses, creating an all-in-one nanoconjugate that can simultaneously sense cancer-related molecules, such as miRNAs, and image and treat cancer.
For instance, Liu and collaborators designed a fluorescent miR-21-responsive gold nanoprobe that allows imaging and monitoring of the therapeutic effects in cancer. Gold nanorods were assembled with folic acid for targeted delivery and with a fluorophore-labeled molecular beacon. Using this method, fluorescence was detected when the molecular beacon was unfolded by endogenous miRNAs, allowing in situ miRNA-responsive imaging of cancer cells. The nanoprobe also induced cell apoptosis after NIR exposure due to the photothermal effect of the gold nanorods.48 He and colleagues developed a nanodevice for the diagnosis of intracellular miRNA and synergetic therapy of tumor cells. Composed of gold–copper sulfide core–shell particles coated with polydopamine and DNA and presenting photothermal properties while being able to generate reactive oxygen species, the composite allowed multimodal therapy, efficiently inhibiting tumor growth by almost 50%. Also, the nanodevice exhibited surface-enhanced Raman scattering enhancement and elevated fluorescence quenching performance, great properties for a biosensor, which achieved imaging and quantitative detection of miRNA-21 in cancer cells.49 Sukumar and co-workers developed a theranostic platform composed of gold–iron oxide particles conjugated to β-cyclodextrin-chitosan and loaded simultaneously with miRNA-100 mimics and anti-miRNA-21 molecules. This nanoconjugate was designed to promote sensitization of glioblastoma to the chemotherapeutic molecule Temozolomide while allowing imaging of miRNA or anti-miRNA delivery. Intranasal delivery of this conjugate to a mouse model showed effective accumulation in tumor cells by in vivo optical fluorescence, which promoted a survival increase when coadministered with Temozolomide, when compared to controls or to the chemotherapeutic agent alone.50
miRNA Catalyzed Cancer Therapeutics
Studies have also demonstrated that the overexpression of certain miRNAs within cancer cells can be used as a catalyst for the release of molecules by gold nanoconjugates. For example, Luo and colleagues reported a drug release system based on a DNA-functionalized doxorubicin-loaded gold nanoparticle–quantum dot complex catalyzed by miR-21. In this system, trace amounts of endogenous miR-21 promoted a specific disassembly of the nanocomplex and release of doxorubicin. Drug release could be followed due to the emission of photoluminescence by the quantum dot, which was quenched by the gold nanoparticle when the nanocomplex was fully assembled, and this system was shown to be more potent than the free doxorubicin molecules.51 Using a similar method, Yue and co-workers combined chemotherapy and gene therapy by developing a miRNA-responsive drug nanocarrier. Using DNA-functionalized gold nanoparticles in which doxorubicin and DNA/RNA duplexes were assembled, the authors took advantage of the overexpression of miR-21 and miR-10b in triple negative breast cancer cells. The overexpression was used as a catalyst for the disassembly of the nanocarrier, promoting the release of doxorubicin and the hybridization of the DNA/RNA duplex to generate siRNA in situ. This delivery method effectively promoted Bcl-2 downregulation and decreased cell viability in vitro, achieving a higher antitumor effect by combining the two therapies.52 Zhang and co-workers built a multifunctional plasmonic core–satellite nanoprobe composed of a 50 nm gold nanoparticle core, with 13 nm gold nanoparticles working as satellites. These were assembled with DNA sequences for miRNA detection, with fluorophore-labeled caspase-3-responsive peptides and loaded with doxorubicin. Once inside the cells, the DNA sequences hybridized with endogenous miRNA-21, which promoted the disassembly of the carrier by releasing the 13 nm core gold nanoparticles, which resulted in detectable shifts in the localized surface plasmon resonance, thus showing miRNA-21 detection. Also, doxorubicin was released, promoting apoptosis of the cancer cells and caspase-3 activation, which cleaved the peptides and increased the distance between the fluorophore and the nanoparticle, allowing fluorescence detection of the apoptotic process.53
In summary, many different gold nanoconjugates have been designed and functionalized with moieties (Figure 3) targeting genes, proteins, or receptors specific to a certain cell type or tissue, enabling an increase in cell detection, targeting, and therapeutic efficacy. These nanoconjugates have been used to target different types of cancer, either as a monotherapy or in combination with other types of therapies, either given alone or also conjugated to nanoparticles, allowing the authors to target different mechanisms of action to increase the overall therapeutic effect obtained but also to overcome drug resistance. Moreover, miRNAs can also be used for diagnosis and imaging applications, as many of the nanoconjugates allow for the detection of these molecules. Furthermore, miRNAs can also be used to direct the release of molecules from other gold-nanoparticle-based cancer therapeutics.
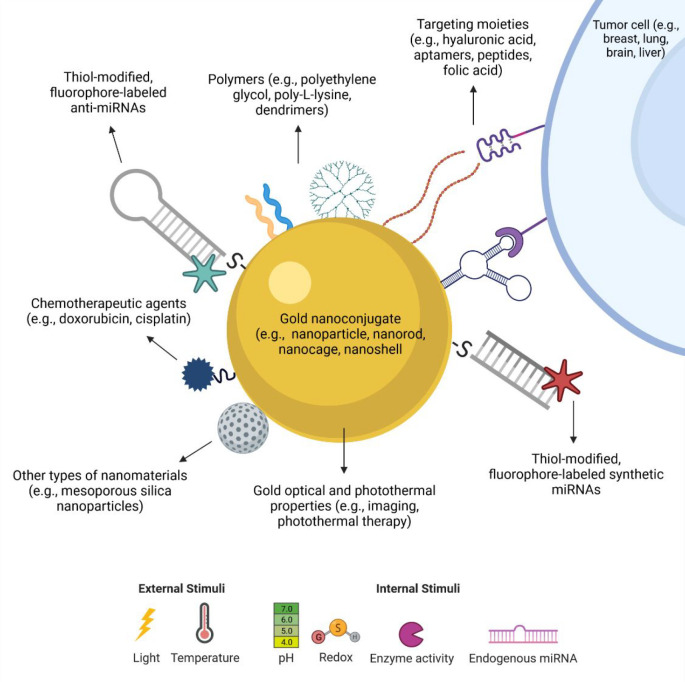
Figure 3.
Schematic representation of the different possibilities of gold nanoparticle modifications used in the development of miRNA therapeutics. Gold nanoparticles of different shapes (e.g., sphere, rod, cage, shell) and with different properties can be functionalized with several types of moieties, such as oligonucleotides (anti-miRNAs and synthetic miRNAs), which can have their own modifications, such as addition of thiols and fluorescent molecules. Moreover, the nanoconjugates can also carry polymers to improve stability and biocompatibility, targeting molecules to improve selectivity toward cancer cells, chemotherapeutic agents, and even other nanomaterials. Also, many of these molecules can be engineered to be released upon different external or internal stimuli, such as light irradiation and pH.
Challenges, Advantages, and Future Directions
Although devising nanoconjugates for miRNA cancer therapeutics may sometimes seem simple, some challenges exist that limit their application and involve different factors from nanoconjugate design to clinical translation.9 Several nanoconjugates have been explored, both in vitro and in vivo, in different types of cancer and for different miRNA applications, as briefly reviewed here. Despite many of them showing promising results in key aspects such as tumor inhibition and modulation of drug resistance, few reached clinical trials.
The first challenges encountered when designing miRNA therapeutics are the delivery efficiency and the low efficacy of the effector molecules due to their low stability. Mimic miRNAs or anti-miRNAs are easily degraded by endogenous nucleases, which will decrease their therapeutic efficacy.12,54 Additionally, the fact that they are negatively charged is an obstacle for cellular internalization. To have a therapeutic effect, the oligonucleotides need to travel across the circulatory system, in the case of systemic delivery, and cross the membranes of different tissues (e.g., blood-brain barrier).12,54 Another obstacle is the selectivity and associated off-target effects, as these oligonucleotides also present poor cellular selectivity, e.g., impossible to discriminate between cancer and healthy cells, and are immunogenic.12,54 For this reason, it is difficult to achieve in vivo efficient, specific, and safe delivery of single therapeutic miRNAs.
To overcome this, researchers started designing delivery systems that include vehicles of many types, such as hydrogels, dendrimers, and polymeric or inorganic nanoparticles, which are based on different processes, such as surface conjugation and encapsulation.55 In this context, gold-nanoparticle-based delivery of miRNAs emerged as an approach for cancer therapy. However, although many of these vehicles are biocompatible, their synthesis method is a key point regarding toxicity. For example, in gold nanoparticles, depending on the reducing agent used in the synthesis and capping agent,56,57 toxicity can be controlled by using citrate. These carriers are also often unstable, and there may be a need to add stabilizing agents, such as polymers (e.g., polyethylene glycol, PEG), which although often used to increase the nanocarrier half-life in circulation58,59 may also present some toxicity, e.g., PLL due to the presence of primary amines on its backbone. However, most of the nanosystems show good biocompatibility and can be easily modified with moieties.60 For instance, regarding oligonucleotides, depending on the carrier, it can be difficult to control the orientation of the sequences that are being attached. However, either miRNA mimics or anti-miRNAs can be easily conjugated to the surface of gold nanoparticles through thiol bonds, a stable connection, and are easily released in proteolytic, acidic, and redox environments, similar to the ones found in tumors.60 This can also be applied to other modifications, and the fact that these miRNA-directed therapies can be targeted greatly helps to decrease damage to the healthy tissue and nonspecific effects, improving efficiency and allowing targeting of different types of cancer. This can be achieved through modification of the nanoconjugate with proteins, or other molecules expressed on tumor cells can be targeted using, for example, aptamers and antibodies, although aptamers present advantages such as lower production cost and lower immunogenicity.61 These modifications contribute to increase the enhanced permeability and retention (EPR) effect, improving the accumulation of nanoparticles at the tumor site and the selected and sustained release of the therapeutic agent for a longer period, allowing a reduction in the frequency of administration. Although most of these processes contribute to a reduction in toxicity, it is important to be aware of miRNA-induced toxicity, as some miRNAs can regulate the expression of drug metabolizing enzymes and a deregulation of this process can alter the metabolism of other drugs and lead to toxicity, especially regarding combination therapies.12,62,63
The different monotherapies available and commonly used as a standard of care are increasingly proving to be more ineffective in cancer treatment. Cancer patients often develop metastasis and MDR, limiting treatment efficacy and causing disease recurrence. In these cases, a combination of different therapies may present itself as a strategy, with nanoconjugates being developed and evaluated as delivery vehicles. As previously mentioned, this alternative would allow a more localized delivery, decreasing the concentration of toxic agents such as chemotherapeutics (e.g., doxorubicin or cisplatin), decreasing side effects, and increasing treatment efficacy, leading to a reduction in relapse and MDR. In fact, studies have reported the increased synergistic effects of combined therapies using gold nanoparticles when compared to the chemotherapeutic compounds alone. Thus, the development of nanoplatforms that can codeliver miRNAs or miRNA inhibitors and other molecules, combining two or even three different therapies in one single vehicle, is an attractive strategy that has been used to target different types of cancer, as mentioned in the previous section regarding miRNA therapy, chemotherapy, and phototherapy. In fact, the properties of gold nanoparticles allow them to be used as photosensitizers,19 enabling the development of multiple therapies in one nanocarrier alone and allowing gene regulation via the application of a light-triggered release of miRNAs.61 These strategies are attractive and often show promising results since different mechanisms of action involving different pathways are targeted sequentially or simultaneously, leading to effective alterations within cells. Furthermore, the possibility of integrating theranostics in one vehicle and having simultaneous tumor imaging and treatment is an effective and critical aspect to prevent under- and overtreatment.
Interestingly, the nucleotide-based therapeutic strategies presented in the previous sections have been proposed for over two decades as effective therapeutic approaches. However, despite encouraging results being often observed in vitro, several challenges need to be addressed:
-
(1)
Many studies still require in vivo confirmation of the results observed, and even when in vivo studies have indeed been performed, their translation to the clinic still represents a major obstacle for the development of these therapies.10,64
-
(2)
Most of the tumor models used are not clinically relevant, as they do not fully recreate the tumor and its microenvironment, as many tumors of internal organs such as breast and lung are still being studied as subcutaneous tumors, which do not effectively show the mechanisms of action.9,10
-
(3)
Therapeutic administrations are often systemic, which leads to nanoparticle diffusion throughout the body and may increase off-target effects, although this can be in part surpassed by the use of active targeting strategies.10
Despite these difficulties, much has been done to address the numerous challenges that have been mentioned when using nanoplatforms to develop therapeutic options for cancer, such as bioavailability, stability, and drug delivery. In fact, the enthusiasm for the development of such therapies using RNA increased with the approval of Nusinersen, an antisense molecule for the treatment of spinal muscular atrophy, and the time record-breaking development and approval of the mRNA vaccines for SARS-CoV-2. Even though researchers in nanoscience are working to surpass many of the challenges mentioned here, much remains undone to close the gap between the scientific advances and the evaluation of these nanoconjugates in clinical trials. On the bench side, the results of the studies should be transparent, accessible, and reproducible, and researchers should provide complete characterizations of the nanoconjugates designed and methodologies used. On the clinical side, clinicians should report what is needed and engage with scientists to design nanoconjugates that can in fact modulate and treat cancer. Lastly, both sides rely on the guidance provided by the regulatory agencies concerning the specific requirements and characteristics (e.g., shape and size) that nanotherapeutic compounds need to achieve and to possess so that they can be evaluated in clinical trials, approved, and commercialized.
Acknowledgments
The authors acknowledge Fundação para a Ciência e a Tecnologia (FCT) for the financial support in the framework of the PhD grant 2020.06638.BD (D.P.S.) and the European Research Council Grant Agreement 848325 (J.C.).
Glossary
Abbreviations
- EMT
epithelial-to-mesenchymal transition
- EPR
enhanced permeability and retention
- EVB
Epstein–Barr virus
- hsa
human miRNA
- MDR
multidrug resistance
- miRNA
microRNA
- MMP
matrix metalloproteinase
- NIR
near-infrared radiation
- PAMAM
polyamidoamine
- PEG
polyethylene glycol
- PEI
polyethyleneimine
- PDT
photodynamic therapy
- PLL
poly-l-lysine
- PTT
photothermal therapy
- SPR
surface plasmon resonance
Author Contributions
All authors wrote, reviewed, and approved the final version of the manuscript.
This work was supported by the FCT PhD Scholarship (2020.06638.BD, Diana P. Sousa) and by the European Research Council Starting Grant ERC-StG-2019-848325 (2019–2024, João Conde).
The authors declare the following competing financial interest(s): J.C. is a co-founder and shareholder of TargTex S.A.
Notes
All figures were created with BioRender.com.
References
- Alvarez E. M.; Force L. M.; Xu R.; Compton K.; Lu D.; Henrikson H. J.; Kocarnik J. M.; Harvey J. D.; Pennini A.; Dean F. E.; Fu W.; Vargas M. T.; Keegan T. H. M.; Ariffin H.; Barr R. D.; Erdomaeva Y. A.; Gunasekera D. S.; John-Akinola Y. O.; Ketterl T. G.; Kutluk T.; Malogolowkin M. H.; Mathur P.; Radhakrishnan V.; Ries L. A. G.; Rodriguez-Galindo C.; Sagoyan G. B.; Sultan I.; Abbasi B.; Abbasi-Kangevari M.; Abbasi-Kangevari Z.; Abbastabar H.; Abdelmasseh M.; Abd-Elsalam S.; Abdoli A.; Abebe H.; Abedi A.; Abidi H.; Abolhassani H.; Abubaker Ali H.; Abu-Gharbieh E.; Achappa B.; Acuna J. M.; Adedeji I. A.; Adegboye O. A.; Adnani Q. E. S.; Advani S. M.; Afzal M. S.; Aghaie Meybodi M.; Ahadinezhad B.; Ahinkorah B. O.; Ahmad S.; Ahmadi S.; Ahmed M. B.; Ahmed Rashid T.; Ahmed Salih Y.; Aiman W.; Akalu G. T.; Al Hamad H.; Alahdab F.; AlAmodi A. A.; Alanezi F. M.; Alanzi T. M.; Alem A. Z.; Alem D. T.; Alemayehu Y.; Alhalaiqa F. N.; Alhassan R. K.; Ali S.; Alicandro G.; Alipour V.; Aljunid S. M.; Alkhayyat M.; Alluri S.; Almasri N. A.; Al-Maweri S. A.; Almustanyir S.; Al-Raddadi R. M.; Alvis-Guzman N.; Ameyaw E. K.; Amini S.; Amu H.; Ancuceanu R.; Andrei C. L.; Andrei T.; Ansari F.; Ansari-Moghaddam A.; Anvari D.; Anyasodor A. E.; Arabloo J.; Arab-Zozani M.; Argaw A. M.; Arshad M.; Arulappan J.; Aryannejad A.; Asemi Z.; Asghari Jafarabadi M.; Atashzar M. R.; Atorkey P.; Atreya A.; Attia S.; Aujayeb A.; Ausloos M.; Avila-Burgos L.; Awedew A. F.; Ayala Quintanilla B. P.; Ayele A. D.; Ayen S. S.; Azab M. A.; Azadnajafabad S.; Azami H.; Azangou-Khyavy M.; Azari Jafari A.; Azarian G.; Azzam A. Y.; Bahadory S.; Bai J.; Baig A. A.; Baker J. L.; Banach M.; Bärnighausen T. W.; Barone-Adesi F.; Barra F.; Barrow A.; Basaleem H.; Batiha A.-M. M.; Behzadifar M.; Bekele N. C.; Belete R.; Belgaumi U. I.; Bell A. W.; Berhie A. Y.; Bhagat D. S.; Bhagavathula A. S.; Bhardwaj N.; Bhardwaj P.; Bhaskar S.; Bhattacharyya K.; Bhojaraja V. S.; Bibi S.; Bijani A.; Biondi A.; Birara S.; Bjørge T.; Bolarinwa O. A.; Bolla S. R.; Boloor A.; Braithwaite D.; Brenner H.; Bulamu N. B.; Burkart K.; Bustamante-Teixeira M. T.; Butt N. S.; Butt Z. A.; Caetano dos Santos F. L.; Cao C.; Cao Y.; Carreras G.; Catalá-López F.; Cembranel F.; Cerin E.; Chakinala R. C.; Chakraborty P. A.; Chattu V. K.; Chaturvedi P.; Chaurasia A.; Chavan P. P.; Chimed-Ochir O.; Choi J.-Y. J.; Christopher D. J.; Chu D.-T.; Chung M. T.; Conde J.; Costa V. M.; Da’ar O. B.; Dadras O.; Dahlawi S. M. A.; Dai X.; Damiani G.; D’Amico E.; Dandona L.; Dandona R.; Daneshpajouhnejad P.; Darwish A. H.; Daryani A.; De la Hoz F. P.; Debela S. A.; Demie T. G. G.; Demissie G. D.; Demissie Z. G.; Denova-Gutiérrez E.; Derbew Molla M.; Desai R.; Desta A. A.; Dhamnetiya D.; Dharmaratne S. D.; Dhimal M. L.; Dhimal M.; Dianatinasab M.; Didehdar M.; Diress M.; Djalalinia S.; Do H. P.; Doaei S.; Dorostkar F.; dos Santos W. M.; Drake T. M.; Ekholuenetale M.; El Sayed I.; El Sayed Zaki M.; El Tantawi M.; El-Abid H.; Elbahnasawy M. A.; Elbarazi I.; Elhabashy H. R.; Elhadi M.; El-Jaafary S. I.; Enyew D. B.; Erkhembayar R.; Eshrati B.; Eskandarieh S.; Faisaluddin M.; Fares J.; Farooque U.; Fasanmi A. O.; Fatima W.; Ferreira de Oliveira J. M. P.; Ferrero S.; Ferro Desideri L.; Fetensa G.; Filip I.; Fischer F.; Fisher J. L.; Foroutan M.; Fukumoto T.; Gaal P. A.; Gad M. M.; Gaewkhiew P.; Gallus S.; Garg T.; Gebremeskel T. G.; Gemeda B. N. B.; Getachew T.; Ghafourifard M.; Ghamari S.-H.; Ghashghaee A.; Ghassemi F.; Ghith N.; Gholami A.; Gholizadeh Navashenaq J.; Gilani S. A.; Ginindza T. G.; Gizaw A. T.; Glasbey J. C.; Goel A.; Golechha M.; Goleij P.; Golinelli D.; Gopalani S. V.; Gorini G.; Goudarzi H.; Goulart B. N. G.; Grada A.; Gubari M. I. M.; Guerra M. R.; Guha A.; Gupta B.; Gupta S.; Gupta V. B.; Gupta V. K.; Haddadi R.; Hafezi-Nejad N.; Hailu A.; Haj-Mirzaian A.; Halwani R.; Hamadeh R. R.; Hambisa M. T.; Hameed S.; Hamidi S.; Haque S.; Hariri S.; Haro J. M.; Hasaballah A. I.; Hasan S. M. M.; Hashemi S. M.; Hassan T. S.; Hassanipour S.; Hay S. I.; Hayat K.; Hebo S. H.; Heidari G.; Heidari M.; Herrera-Serna B. Y.; Herteliu C.; Heyi D. Z.; Hezam K.; Hole M. K.; Holla R.; Horita N.; Hossain M. M.; Hossain M. B.; Hosseini M.-S.; Hosseini M.; Hosseinzadeh A.; Hosseinzadeh M.; Hostiuc M.; Hostiuc S.; Househ M.; Hsairi M.; Huang J.; Hussein N. R.; Hwang B.-F.; Ibitoye S. E.; Ilesanmi O. S.; Ilic I. M.; Ilic M. D.; Innos K.; Irham L. M.; Islam R. M.; Islam S. M. S.; Ismail N. E.; Isola G.; Iwagami M.; Jacob L.; Jadidi-Niaragh F.; Jain V.; Jakovljevic M.; Janghorban R.; Javadi Mamaghani A.; Jayaram S.; Jayawardena R.; Jazayeri S. B.; Jebai R.; Jha R. P.; Joo T.; Joseph N.; Joukar F.; Jürisson M.; Kaambwa B.; Kabir A.; Kalankesh L. R.; Kaliyadan F.; Kamal Z.; Kamath A.; Kandel H.; Kar S. S.; Karaye I. M.; Karimi A.; Kassa B. G.; Kauppila J. H.; Kemp Bohan P. M.; Kengne A. P.; Kerbo A. A.; Keykhaei M.; Khader Y. S.; Khajuria H.; Khalili N.; Khalili N.; Khan E. A.; Khan G.; Khan M.; Khan M. N.; Khan M. A.; Khanali J.; Khayamzadeh M.; Khosravizadeh O.; Khubchandani J.; Khundkar R.; Kim M. S.; Kim Y. J.; Kisa A.; Kisa S.; Kissimova-Skarbek K.; Kolahi A.-A.; Kopec J. A.; Koteeswaran R.; Koulmane Laxminarayana S. L.; Koyanagi A.; Kugbey N.; Kumar G. A.; Kumar N.; Kwarteng A.; La Vecchia C.; Lan Q.; Landires I.; Lasrado S.; Lauriola P.; Ledda C.; Lee S.; Lee W.-C.; Lee Y. Y.; Lee Y. H.; Leigh J.; Leong E.; Li B.; Li J.; Li M.-C.; Lim S. S.; Liu X.; Lobo S. W.; Loureiro J. A.; Lugo A.; Lunevicius R.; Magdy Abd El Razek H.; Magdy Abd El Razek M.; Mahmoudi M.; Majeed A.; Makki A.; Male S.; Malekpour M.-R.; Malekzadeh R.; Malik A. A.; Mamun M. A.; Manafi N.; Mansour-Ghanaei F.; Mansouri B.; Mansournia M. A.; Martini S.; Masoumi S. Z.; Matei C. N.; Mathur M. R.; McAlinden C.; Mehrotra R.; Mendoza W.; Menezes R. G.; Mentis A.-F. A.; Meretoja T. J.; Mersha A. G.; Mesregah M. K.; Mestrovic T.; Miao Jonasson J.; Miazgowski B.; Michalek I. M.; Miller T. R.; Mingude A. B.; Mirmoeeni S.; Mirzaei H.; Misra S.; Mithra P.; Mohammad K. A.; Mohammadi M.; Mohammadi S. M.; Mohammadian-Hafshejani A.; Mohammadpourhodki R.; Mohammed A.; Mohammed S.; Mohammed T. A.; Moka N.; Mokdad A. H.; Molokhia M.; Momtazmanesh S.; Monasta L.; Moni M. A.; Moradi G.; Moradi Y.; Moradzadeh M.; Moradzadeh R.; Moraga P.; Morrison S. D.; Mostafavi E.; Mousavi Khaneghah A.; Mpundu-Kaambwa C.; Mubarik S.; Mwanri L.; Nabhan A. F.; Nagaraju S. P.; Nagata C.; Naghavi M.; Naimzada M. D.; Naldi L.; Nangia V.; Naqvi A. A.; Narasimha Swamy S.; Narayana A. I.; Nayak B. P.; Nayak V. C.; Nazari J.; Nduaguba S. O.; Negoi I.; Negru S. M.; Nejadghaderi S. A.; Nepal S.; Neupane Kandel S.; Nggada H. A.; Nguyen C. T.; Nnaji C. A.; Nosrati H.; Nouraei H.; Nowroozi A.; Nuñez-Samudio V.; Nwatah V. E.; Nzoputam C. I.; Oancea B.; Odukoya O. O.; Oguntade A. S.; Oh I.-H.; Olagunju A. T.; Olagunju T. O.; Olakunde B. O.; Oluwasanu M. M.; Omar E.; Omar Bali A.; Ong S.; Onwujekwe O. E.; Ortega-Altamirano D. V.; Otstavnov N.; Otstavnov S. S.; Oumer B.; Owolabi M. O.; P A M.; Padron-Monedero A.; Padubidri J. R.; Pakshir K.; Pana A.; Pandey A.; Pardhan S.; Pashazadeh Kan F.; Pasovic M.; Patel J. R.; Pati S.; Pattanshetty S. M.; Paudel U.; Pereira R. B.; Peres M. F. P.; Perianayagam A.; Postma M. J.; Pourjafar H.; Pourshams A.; Prashant A.; Pulakunta T.; Qadir M. M. F. F.; Rabiee M.; Rabiee N.; Radfar A.; Radhakrishnan R. A.; Rafiee A.; Rafiei A.; Rafiei S.; Rahim F.; Rahimzadeh S.; Rahman M.; Rahman M. A.; Rahmani A. M.; Rajesh A.; Ramezani-Doroh V.; Ranabhat K.; Ranasinghe P.; Rao C. R.; Rao S. J.; Rashedi S.; Rashidi M.; Rashidi M.-M.; Rath G. K.; Rawaf D. L.; Rawaf S.; Rawal L.; Rawassizadeh R.; Razeghinia M. S.; Regasa M. T.; Renzaho A. M. N.; Rezaei M.; Rezaei N.; Rezaei N.; Rezaeian M.; Rezapour A.; Rezazadeh-Khadem S.; Riad A.; Rios Lopez L. E.; Rodriguez J. A. B.; Ronfani L.; Roshandel G.; Rwegerera G. M.; Saber-Ayad M. M.; Sabour S.; Saddik B.; Sadeghi E.; Sadeghian S.; Saeed U.; Sahebkar A.; Saif-Ur-Rahman K.; Sajadi S. M.; Salahi S.; Salehi S.; Salem M. R.; Salimzadeh H.; Samy A. M.; Sanabria J.; Sanmarchi F.; Sarveazad A.; Sathian B.; Sawhney M.; Sawyer S. M.; Saylan M.; Schneider I. J. C.; Seidu A.-A.; Šekerija M.; Sendo E. G.; Sepanlou S. G.; Seylani A.; Seyoum K.; Sha F.; Shafaat O.; Shaikh M. A.; Shamsoddin E.; Shannawaz M.; Sharma R.; Sheikhbahaei S.; Shetty A.; Shetty B. S. K.; Shetty P. H.; Shin J. Il; Shirkoohi R.; Shivakumar K. M.; Shobeiri P.; Siabani S.; Sibhat M. M.; Siddappa Malleshappa S. K.; Sidemo N. B.; Silva D. A. S.; Silva Julian G.; Singh A. D.; Singh J. A.; Singh J. K.; Singh S.; Sinke A. H.; Sintayehu Y.; Skryabin V. Y.; Skryabina A. A.; Smith L.; Sofi-Mahmudi A.; Soltani-Zangbar M. S.; Song S.; Spurlock E. E.; Steiropoulos P.; Straif K.; Subedi R.; Sufiyan M. B.; Suliankatchi Abdulkader R.; Sultana S.; Szerencsés V.; Szócska M.; Tabaeian S. P.; Tabarés-Seisdedos R.; Tabary M.; Tabuchi T.; Tadbiri H.; Taheri M.; Taherkhani A.; Takahashi K.; Tampa M.; Tan K.-K.; Tat V. Y.; Tavakoli A.; Tbakhi A.; Tehrani-Banihashemi A.; Temsah M.-H.; Tesfay F. H.; Tesfaye B.; Thakur J. S.; Thapar R.; Thavamani A.; Thiyagarajan A.; Thomas N.; Tobe-Gai R.; Togtmol M.; Tohidast S. A.; Tohidinik H. R.; Tolani M. A.; Tollosa D. N.; Touvier M.; Tovani-Palone M. R.; Traini E.; Tran B. X.; Tran M. T. N.; Tripathy J. P.; Tusa B. S.; Ukke G. G.; Ullah I.; Ullah S.; Umapathi K. K.; Unnikrishnan B.; Upadhyay E.; Ushula T. W.; Vacante M.; Valadan Tahbaz S.; Varthya S. B.; Veroux M.; Villeneuve P. J.; Violante F. S.; Vlassov V.; Vu G. T.; Waheed Y.; Wang N.; Ward P.; Weldesenbet A. B.; Wen Y. F.; Westerman R.; Winkler A. S.; Wubishet B. L.; Xu S.; Yahyazadeh Jabbari S. H.; Yang L.; Yaya S.; Yazdi-Feyzabadi V.; Yazie T. S.; Yehualashet S. S.; Yeshaneh A.; Yeshaw Y.; Yirdaw B. W.; Yonemoto N.; Younis M. Z.; Yousefi Z.; Yu C.; Yunusa I.; Zadnik V.; Zahir M.; Zahirian Moghadam T.; Zamani M.; Zamanian M.; Zandian H.; Zare F.; Zastrozhin M. S.; Zastrozhina A.; Zhang J.; Zhang Z.-J.; Ziapour A.; Zoladl M.; Murray C. J. L.; Fitzmaurice C.; Bleyer A.; Bhakta N. The Global Burden of Adolescent and Young Adult Cancer in 2019: A Systematic Analysis for the Global Burden of Disease Study 2019. Lancet Oncol. 2022, 23 (1), 27–52. 10.1016/S1470-2045(21)00581-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kocarnik J. M.; Compton K.; Dean F. E.; Fu W.; Gaw B. L.; Harvey J. D.; Henrikson H. J.; Lu D.; Pennini A.; Xu R.; Ababneh E.; Abbasi-Kangevari M.; Abbastabar H.; Abd-Elsalam S. M.; Abdoli A.; Abedi A.; Abidi H.; Abolhassani H.; Adedeji I. A.; Adnani Q. E. S.; Advani S. M.; Afzal M. S.; Aghaali M.; Ahinkorah B. O.; Ahmad S.; Ahmad T.; Ahmadi A.; Ahmadi S.; Ahmed Rashid T.; Ahmed Salih Y.; Akalu G. T.; Aklilu A.; Akram T.; Akunna C. J.; Al Hamad H.; Alahdab F.; Al-Aly Z.; Ali S.; Alimohamadi Y.; Alipour V.; Aljunid S. M.; Alkhayyat M.; Almasi-Hashiani A.; Almasri N. A.; Al-Maweri S. A. A.; Almustanyir S.; Alonso N.; Alvis-Guzman N.; Amu H.; Anbesu E. W.; Ancuceanu R.; Ansari F.; Ansari-Moghaddam A.; Antwi M. H.; Anvari D.; Anyasodor A. E.; Aqeel M.; Arabloo J.; Arab-Zozani M.; Aremu O.; Ariffin H.; Aripov T.; Arshad M.; Artaman A.; Arulappan J.; Asemi Z.; Asghari Jafarabadi M.; Ashraf T.; Atorkey P.; Aujayeb A.; Ausloos M.; Awedew A. F.; Ayala Quintanilla B. P.; Ayenew T.; Azab M. A.; Azadnajafabad S.; Azari Jafari A.; Azarian G.; Azzam A. Y.; Badiye A. D.; Bahadory S.; Baig A. A.; Baker J. L.; Balakrishnan S.; Banach M.; Bärnighausen T. W.; Barone-Adesi F.; Barra F.; Barrow A.; Behzadifar M.; Belgaumi U. I.; Bezabhe W. M. M.; Bezabih Y. M.; Bhagat D. S.; Bhagavathula A. S.; Bhardwaj N.; Bhardwaj P.; Bhaskar S.; Bhattacharyya K.; Bhojaraja V. S.; Bibi S.; Bijani A.; Biondi A.; Bisignano C.; Bjørge T.; Bleyer A.; Blyuss O.; Bolarinwa O. A.; Bolla S. R.; Braithwaite D.; Brar A.; Brenner H.; Bustamante-Teixeira M. T.; Butt N. S.; Butt Z. A.; Caetano dos Santos F. L.; Cao Y.; Carreras G.; Catalá-López F.; Cembranel F.; Cerin E.; Cernigliaro A.; Chakinala R. C.; Chattu S. K.; Chattu V. K.; Chaturvedi P.; Chimed-Ochir O.; Cho D. Y.; Christopher D. J.; Chu D.-T.; Chung M. T.; Conde J.; Cortés S.; Cortesi P. A.; Costa V. M.; Cunha A. R.; Dadras O.; Dagnew A. B.; Dahlawi S. M. A.; Dai X.; Dandona L.; Dandona R.; Darwesh A. M.; das Neves J.; De la Hoz F. P.; Demis A. B.; Denova-Gutiérrez E.; Dhamnetiya D.; Dhimal M. L.; Dhimal M.; Dianatinasab M.; Diaz D.; Djalalinia S.; Do H. P.; Doaei S.; Dorostkar F.; dos Santos Figueiredo F. W.; Driscoll T. R.; Ebrahimi H.; Eftekharzadeh S.; El Tantawi M.; El-Abid H.; Elbarazi I.; Elhabashy H. R.; Elhadi M.; El-Jaafary S. I.; Eshrati B.; Eskandarieh S.; Esmaeilzadeh F.; Etemadi A.; Ezzikouri S.; Faisaluddin M.; Faraon E. J. A.; Fares J.; Farzadfar F.; Feroze A. H.; Ferrero S.; Ferro Desideri L.; Filip I.; Fischer F.; Fisher J. L.; Foroutan M.; Fukumoto T.; Gaal P. A.; Gad M. M.; Gadanya M. A.; Gallus S.; Gaspar Fonseca M.; Getachew Obsa A.; Ghafourifard M.; Ghashghaee A.; Ghith N.; Gholamalizadeh M.; Gilani S. A.; Ginindza T. G.; Gizaw A. T. T.; Glasbey J. C.; Golechha M.; Goleij P.; Gomez R. S.; Gopalani S. V.; Gorini G.; Goudarzi H.; Grosso G.; Gubari M. I. M.; Guerra M. R.; Guha A.; Gunasekera D. S.; Gupta B.; Gupta V. B.; Gupta V. K.; Gutiérrez R. A.; Hafezi-Nejad N.; Haider M. R.; Haj-Mirzaian A.; Halwani R.; Hamadeh R. R.; Hameed S.; Hamidi S.; Hanif A.; Haque S.; Harlianto N. I.; Haro J. M.; Hasaballah A. I.; Hassanipour S.; Hay R. J.; Hay S. I.; Hayat K.; Heidari G.; Heidari M.; Herrera-Serna B. Y.; Herteliu C.; Hezam K.; Holla R.; Hossain M. M.; Hossain M. B. H.; Hosseini M.-S.; Hosseini M.; Hosseinzadeh M.; Hostiuc M.; Hostiuc S.; Househ M.; Hsairi M.; Huang J.; Hugo F. N.; Hussain R.; Hussein N. R.; Hwang B.-F.; Iavicoli I.; Ibitoye S. E.; Ida F.; Ikuta K. S.; Ilesanmi O. S.; Ilic I. M.; Ilic M. D.; Irham L. M.; Islam J. Y.; Islam R. M.; Islam S. M. S.; Ismail N. E.; Isola G.; Iwagami M.; Jacob L.; Jain V.; Jakovljevic M. B.; Javaheri T.; Jayaram S.; Jazayeri S. B.; Jha R. P.; Jonas J. B.; Joo T.; Joseph N.; Joukar F.; Jürisson M.; Kabir A.; Kahrizi D.; Kalankesh L. R.; Kalhor R.; Kaliyadan F.; Kalkonde Y.; Kamath A.; Kameran Al-Salihi N.; Kandel H.; Kapoor N.; Karch A.; Kasa A. S.; Katikireddi S. V.; Kauppila J. H.; Kavetskyy T.; Kebede S. A.; Keshavarz P.; Keykhaei M.; Khader Y. S.; Khalilov R.; Khan G.; Khan M.; Khan M. N.; Khan M. A. B.; Khang Y.-H.; Khater A. M.; Khayamzadeh M.; Kim G. R.; Kim Y. J.; Kisa A.; Kisa S.; Kissimova-Skarbek K.; Kopec J. A.; Koteeswaran R.; Koul P. A.; Koulmane Laxminarayana S. L.; Koyanagi A.; Kucuk Bicer B.; Kugbey N.; Kumar G. A.; Kumar N.; Kumar N.; Kurmi O. P.; Kutluk T.; La Vecchia C.; Lami F. H.; Landires I.; Lauriola P.; Lee S.; Lee S. W. H.; Lee W.-C.; Lee Y. H.; Leigh J.; Leong E.; Li J.; Li M.-C.; Liu X.; Loureiro J. A.; Lunevicius R.; Magdy Abd El Razek M.; Majeed A.; Makki A.; Male S.; Malik A. A.; Mansournia M. A.; Martini S.; Masoumi S. Z.; Mathur P.; McKee M.; Mehrotra R.; Mendoza W.; Menezes R. G.; Mengesha E. W.; Mesregah M. K.; Mestrovic T.; Miao Jonasson J.; Miazgowski B.; Miazgowski T.; Michalek I. M.; Miller T. R.; Mirzaei H.; Mirzaei H. R.; Misra S.; Mithra P.; Moghadaszadeh M.; Mohammad K. A.; Mohammad Y.; Mohammadi M.; Mohammadi S. M.; Mohammadian-Hafshejani A.; Mohammed S.; Moka N.; Mokdad A. H.; Molokhia M.; Monasta L.; Moni M. A.; Moosavi M. A.; Moradi Y.; Moraga P.; Morgado-da-Costa J.; Morrison S. D.; Mosapour A.; Mubarik S.; Mwanri L.; Nagarajan A. J.; Nagaraju S. P.; Nagata C.; Naimzada M. D.; Nangia V.; Naqvi A. A.; Narasimha Swamy S.; Ndejjo R.; Nduaguba S. O.; Negoi I.; Negru S. M.; Neupane Kandel S.; Nguyen C. T.; Nguyen H. L. T.; Niazi R. K.; Nnaji C. A.; Noor N. M.; Nuñez-Samudio V.; Nzoputam C. I.; Oancea B.; Ochir C.; Odukoya O. O.; Ogbo F. A.; Olagunju A. T.; Olakunde B. O.; Omar E.; Omar Bali A.; Omonisi A. E. E.; Ong S.; Onwujekwe O. E.; Orru H.; Ortega-Altamirano D. V.; Otstavnov N.; Otstavnov S. S.; Owolabi M. O.; P A M.; Padubidri J. R.; Pakshir K.; Pana A.; Panagiotakos D.; Panda-Jonas S.; Pardhan S.; Park E.-C.; Park E.-K.; Pashazadeh Kan F.; Patel H. K.; Patel J. R.; Pati S.; Pattanshetty S. M.; Paudel U.; Pereira D. M.; Pereira R. B.; Perianayagam A.; Pillay J. D.; Pirouzpanah S.; Pishgar F.; Podder I.; Postma M. J.; Pourjafar H.; Prashant A.; Preotescu L.; Rabiee M.; Rabiee N.; Radfar A.; Radhakrishnan R. A.; Radhakrishnan V.; Rafiee A.; Rahim F.; Rahimzadeh S.; Rahman M.; Rahman M. A.; Rahmani A. M.; Rajai N.; Rajesh A.; Rakovac I.; Ram P.; Ramezanzadeh K.; Ranabhat K.; Ranasinghe P.; Rao C. R.; Rao S. J.; Rawassizadeh R.; Razeghinia M. S.; Renzaho A. M. N.; Rezaei N.; Rezaei N.; Rezapour A.; Roberts T. J.; Rodriguez J. A. B.; Rohloff P.; Romoli M.; Ronfani L.; Roshandel G.; Rwegerera G. M.; S M.; Sabour S.; Saddik B.; Saeed U.; Sahebkar A.; Sahoo H.; Salehi S.; Salem M. R.; Salimzadeh H.; Samaei M.; Samy A. M.; Sanabria J.; Sankararaman S.; Santric-Milicevic M. M.; Sardiwalla Y.; Sarveazad A.; Sathian B.; Sawhney M.; Saylan M.; Schneider I. J. C.; Sekerija M.; Seylani A.; Shafaat O.; Shaghaghi Z.; Shaikh M. A.; Shamsoddin E.; Shannawaz M.; Sharma R.; Sheikh A.; Sheikhbahaei S.; Shetty A.; Shetty J. K.; Shetty P. H.; Shibuya K.; Shirkoohi R.; Shivakumar K. M.; Shivarov V.; Siabani S.; Siddappa Malleshappa S. K.; Silva D. A. S.; Singh J. A.; Sintayehu Y.; Skryabin V. Y.; Skryabina A. A.; Soeberg M. J.; Sofi-Mahmudi A.; Sotoudeh H.; Steiropoulos P.; Straif K.; Subedi R.; Sufiyan M. B.; Sultan I.; Sultana S.; Sur D.; Szerencsés V.; Szócska M.; Tabarés-Seisdedos R.; Tabuchi T.; Tadbiri H.; Taherkhani A.; Takahashi K.; Talaat I. M.; Tan K.-K.; Tat V. Y.; Tedla B. A. A.; Tefera Y. G.; Tehrani-Banihashemi A.; Temsah M.-H.; Tesfay F. H.; Tessema G. A.; Thapar R.; Thavamani A.; Thoguluva Chandrasekar V.; Thomas N.; Tohidinik H. R.; Touvier M.; Tovani-Palone M. R.; Traini E.; Tran B. X.; Tran K. B.; Tran M. T. N.; Tripathy J. P.; Tusa B. S.; Ullah I.; Ullah S.; Umapathi K. K.; Unnikrishnan B.; Upadhyay E.; Vacante M.; Vaezi M.; Valadan Tahbaz S.; Velazquez D. Z.; Veroux M.; Violante F. S.; Vlassov V.; Vo B.; Volovici V.; Vu G. T.; Waheed Y.; Wamai R. G.; Ward P.; Wen Y. F.; Westerman R.; Winkler A. S.; Yadav L.; Yahyazadeh Jabbari S. H.; Yang L.; Yaya S.; Yazie T. S. Y.; Yeshaw Y.; Yonemoto N.; Younis M. Z.; Yousefi Z.; Yu C.; Yuce D.; Yunusa I.; Zadnik V.; Zare F.; Zastrozhin M. S.; Zastrozhina A.; Zhang J.; Zhong C.; Zhou L.; Zhu C.; Ziapour A.; Zimmermann I. R.; Fitzmaurice C.; Murray C. J. L.; Force L. M. Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life Years for 29 Cancer Groups From 2010 to 2019. JAMA Oncol. 2022, 8 (3), 420. 10.1001/jamaoncol.2021.6987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sung H.; Ferlay J.; Siegel R. L.; Laversanne M.; Soerjomataram I.; Jemal A.; Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71 (3), 209–249. 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- Higdon M. L.; Atkinson C. J.; Lawrence K. V. Oncologic Emergencies: Recognition and Initial Management. Am. Fam. Physician 2018, 97 (11), 741–748. [PubMed] [Google Scholar]
- Santos-de-Frutos K.; Djouder N. When Dormancy Fuels Tumour Relapse. Commun. Biol. 2021, 4 (1), 747. 10.1038/s42003-021-02257-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebian S.; Rodrigues T.; das Neves J.; Sarmento B.; Langer R.; Conde J. Facts and Figures on Materials Science and Nanotechnology Progress and Investment. ACS Nano 2021, 15 (10), 15940–15952. 10.1021/acsnano.1c03992. [DOI] [PubMed] [Google Scholar]
- Orooji Y.; Sohrabi H.; Hemmat N.; Oroojalian F.; Baradaran B.; Mokhtarzadeh A.; Mohaghegh M.; Karimi-Maleh H. An Overview on SARS-CoV-2 (COVID-19) and Other Human Coronaviruses and Their Detection Capability via Amplification Assay, Chemical Sensing, Biosensing, Immunosensing, and Clinical Assays. Nano-Micro Lett. 2021, 13 (1), 18. 10.1007/s40820-020-00533-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra B. D.; Ali M. A.. Nanomaterials in Biosensors: Fundamentals and Applications. In Nanomaterials for Biosensors; Malhotra B. D., Ali M. A., Eds.; William Andrew Publishing, 2018; Ch. 1, pp 1–74. 10.1016/B978-0-323-44923-6.00001-7 [DOI] [Google Scholar]
- Martins J. P.; das Neves J.; de la Fuente M.; Celia C.; Florindo H.; Günday-Türeli N.; Popat A.; Santos J. L.; Sousa F.; Schmid R.; Wolfram J.; Sarmento B.; Santos H. A. The Solid Progress of Nanomedicine. Drug Delivery Transl. Res. 2020, 10 (3), 726–729. 10.1007/s13346-020-00743-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Z.; Gao L.; Chen K.; Zhang W.; Zhang Q.; Li Q.; Hu K. Nanoparticles: A New Approach to Upgrade Cancer Diagnosis and Treatment. Nanoscale Res. Lett. 2021, 16 (1), 88. 10.1186/s11671-021-03489-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annese T.; Tamma R.; De Giorgis M.; Ribatti D. MicroRNAs Biogenesis, Functions and Role in Tumor Angiogenesis. Front. Oncol. 2020, 10, 581007. 10.3389/fonc.2020.581007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Z.; Wang L.; Li S.; Chen F.; Au-Yeung K. K.-W.; Shi C. MicroRNA as an Important Target for Anticancer Drug Development. Front. Pharmacol. 2021, 12 (August), 736323. 10.3389/fphar.2021.736323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L.; Xu X.; Su X. Noncoding RNAs in Cancer Immunity: Functions, Regulatory Mechanisms, and Clinical Application. Mol. Cancer 2020, 19 (1), 1–12. 10.1186/s12943-020-01154-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He B.; Zhao Z.; Cai Q.; Zhang Y.; Zhang P.; Shi S.; Xie H.; Peng X.; Yin W.; Tao Y.; Wang X. MiRNA-Based Biomarkers, Therapies, and Resistance in Cancer. Int. J. Biol. Sci. 2020, 16 (14), 2628–2647. 10.7150/ijbs.47203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan A. U.; Khan M.; Cho M. H.; Khan M. M. Selected Nanotechnologies and Nanostructures for Drug Delivery, Nanomedicine and Cure. Bioprocess Biosyst. Eng. 2020, 43, 1339. 10.1007/s00449-020-02330-8. [DOI] [PubMed] [Google Scholar]
- Sanità G.; Carrese B.; Lamberti A. Nanoparticle Surface Functionalization: How to Improve Biocompatibility and Cellular Internalization. Front. Mol. Biosci. 2020, 7 (November), 587012. 10.3389/fmolb.2020.587012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes B. B.; Conniot J.; Avital A.; Yao D.; Jiang X.; Zhou X.; Sharf-Pauker N.; Xiao Y.; Adir O.; Liang H.; Shi J.; Schroeder A.; Conde J. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Prim. 2022, 2 (1), 24. 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes B. B.; Sousa D. P.; Conniot J.; Conde J. Nanomedicine-Based Strategies to Target and Modulate the Tumor Microenvironment. Trends in Cancer 2021, 7 (9), 847–862. 10.1016/j.trecan.2021.05.001. [DOI] [PubMed] [Google Scholar]
- Vines J. B.; Yoon J. H.; Ryu N. E.; Lim D. J.; Park H. Gold Nanoparticles for Photothermal Cancer Therapy. Front. Chem. 2019, 7 (APR), 1–16. 10.3389/fchem.2019.00167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J.; Liang X. Progress in Research on Gold Nanoparticles in Cancer Management. Medicine (Baltimore). 2019, 98 (18), e15311. 10.1097/MD.0000000000015311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J.; Oliva N.; Artzi N. Implantable Hydrogel Embedded Dark-Gold Nanoswitch as a Theranostic Probe to Sense and Overcome Cancer Multidrug Resistance. Proc. Natl. Acad. Sci. U. S. A. 2015, 112 (11), E1278–E1287. 10.1073/pnas.1421229112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C.; Conde J.; Curtin J.; Artzi N.; Tian F.; Cui D. Bioresponsive Antisense DNA Gold Nanobeacons as a Hybrid in Vivo Theranostics Platform for the Inhibition of Cancer Cells and Metastasis. Sci. Rep. 2015, 5 (1), 12297. 10.1038/srep12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mendes B. B.; Conniot J.; Avital A.; Yao D.; Jiang X.; Zhou X.; Sharf-Pauker N.; Xiao Y.; Adir O.; Liang H.; Shi J.; Schroeder A.; Conde J. Nanodelivery of Nucleic Acids. Nat. Rev. Methods Prim. 2022, 2 (1), 24. 10.1038/s43586-022-00104-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J.; Dias J. T.; Grazú V.; Moros M.; Baptista P. V.; de la Fuente J. M. Revisiting 30 Years of Biofunctionalization and Surface Chemistry of Inorganic Nanoparticles for Nanomedicine. Front. Chem. 2014, 2 (JUL), 48. 10.3389/fchem.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R. Nanotechnology Based Therapeutic Application in Cancer Diagnosis and Therapy. 3 Biotech 2019, 9 (11), 415. 10.1007/s13205-019-1940-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao C.; Conde J.; Curtin J.; Artzi N.; Tian F.; Cui D. Bioresponsive Antisense DNA Gold Nanobeacons as a Hybrid in Vivo Theranostics Platform for the Inhibition of Cancer Cells and Metastasis. Sci. Rep. 2015, 5 (1), 12297. 10.1038/srep12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari R.; Nasra S.; Meghani N.; Kumar A. MiR-206 Conjugated Gold Nanoparticle Based Targeted Therapy in Breast Cancer Cells. Sci. Rep. 2022, 12 (1), 1–12. 10.1038/s41598-022-08185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dang M. N.; Gomez Casas C.; Day E. S. Photoresponsive MiR-34a/Nanoshell Conjugates Enable Light-Triggered Gene Regulation to Impair the Function of Triple-Negative Breast Cancer Cells. Nano Lett. 2021, 21 (1), 68–76. 10.1021/acs.nanolett.0c03152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyal R.; Kapadia C. H.; Melamed J. R.; Riley R. S.; Day E. S. Layer-by-Layer Assembled Gold Nanoshells for the Intracellular Delivery of MiR-34a. Cell. Mol. Bioeng. 2018, 11 (5), 383–396. 10.1007/s12195-018-0535-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramchandani D.; Lee S. K.; Yomtoubian S.; Han M. S.; Tung C. H.; Mittal V. Nanoparticle Delivery of MiR-708 Mimetic Impairs Breast Cancer Metastasis. Mol. Cancer Ther. 2019, 18 (3), 579–591. 10.1158/1535-7163.MCT-18-0702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng J.; Wang R.; Sun W.; Huang M.; Wang R.; Li Y.; Wang P.; Sun G.; Xie S. Delivery of MiR-320a-3p by Gold Nanoparticles Combined with Photothermal Therapy for Directly Targeting Sp1 in Lung Cancer. Biomater. Sci. 2021, 9 (19), 6528–6541. 10.1039/D1BM01124C. [DOI] [PubMed] [Google Scholar]
- Mo Y.; He L.; Lai Z.; Wan Z.; Chen Q.; Pan S.; Li L.; Li D.; Huang J.; Xue F.; Che S. Gold Nano-Particles (AuNPs) Carrying MiR-326 Targets PDK1/AKT/c-Myc Axis in Hepatocellular Carcinoma. Artif. Cells, Nanomedicine Biotechnol. 2019, 47 (1), 2830–2837. 10.1080/21691401.2018.1489266. [DOI] [PubMed] [Google Scholar]
- Gilam A.; Conde J.; Weissglas-Volkov D.; Oliva N.; Friedman E.; Artzi N.; Shomron N. Local MicroRNA Delivery Targets Palladin and Prevents Metastatic Breast Cancer. Nat. Commun. 2016, 7, 12868. 10.1038/ncomms12868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L.-J.; Guo X.-H.; Wang W.-P.; Hu Y.-R.; Duan S.-F.; Liu Y.; Sun Z.; Huang S.-N.; Li H. Gene Therapy and Photothermal Therapy of Layer-by-Layer Assembled AuNCs /PEI/MiRNA/ HA Nanocomplexes. Curr. Cancer Drug Targets 2019, 19 (4), 330–337. 10.2174/1568009618666181016144855. [DOI] [PubMed] [Google Scholar]
- Kardani A.; Yaghoobi H.; Alibakhshi A.; Khatami M. Inhibition of MiR-155 in MCF-7 Breast Cancer Cell Line by Gold Nanoparticles Functionalized with Antagomir and AS1411 Aptamer. J. Cell. Physiol. 2020, 235 (10), 6887–6895. 10.1002/jcp.29584. [DOI] [PubMed] [Google Scholar]
- Deng R.; Shen N.; Yang Y.; Yu H.; Xu S.; Yang Y. W.; Liu S.; Meguellati K.; Yan F. Targeting Epigenetic Pathway with Gold Nanoparticles for Acute Myeloid Leukemia Therapy. Biomaterials 2018, 167, 80–90. 10.1016/j.biomaterials.2018.03.013. [DOI] [PubMed] [Google Scholar]
- Grafals-Ruiz N.; Rios-Vicil C. I.; Lozada-Delgado E. L.; Quiñones-Díaz B. I.; Noriega-Rivera R. A.; Martínez-Zayas G.; Santana-Rivera Y.; Santiago-Sánchez G. S.; Valiyeva F.; Vivas-Mejía P. E. Brain Targeted Gold Liposomes Improve RNAi Delivery for Glioblastoma. Int. J. Nanomedicine 2020, 15, 2809–2828. 10.2147/IJN.S241055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadniaei M.; Lee T.; Bharate B. G.; Yoon J.; Choi H. K.; Park S.-j.; Kim J.; Kim J.; Choi J.-W. Bifunctional Au@Bi2Se3 Core–Shell Nanoparticle for Synergetic Therapy by SERS-Traceable AntagomiR Delivery and Photothermal Treatment. Small 2018, 14 (38), 1802934. 10.1002/smll.201802934. [DOI] [PubMed] [Google Scholar]
- Wang X.; Zhang H.; Chen X. Drug Resistance and Combating Drug Resistance in Cancer. Cancer Drug Resist. 2019, 2, 141–160. 10.20517/cdr.2019.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansoori B.; Mohammadi A.; Davudian S.; Shirjang S.; Baradaran B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7 (3), 339–348. 10.15171/apb.2017.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng R.; Ji B.; Yu H.; Bao W.; Yang Z.; Yu Y.; Cui Y.; Du Y.; Song M.; Liu S.; Meguellati K.; Yan F. Multifunctional Gold Nanoparticles Overcome MicroRNA Regulatory Network Mediated-Multidrug Resistant Leukemia. Sci. Rep. 2019, 9 (1), 1–11. 10.1038/s41598-019-41866-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai H.; Yang Y.; Peng F.; Liu Y.; Fu X.; Ji B. Gold Nanoparticles-Loaded Anti-Mir221 Enhances Antitumor Effect of Sorafenib in Hepatocellular Carcinoma Cells. Int. J. Med. Sci. 2019, 16 (12), 1541–1548. 10.7150/ijms.37427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rois P. M.; Latorre A.; Diaz C. R.; del Moral Á.; Somoza Á. Reprogramming Cells for Synergistic Combination Therapy with Nanotherapeutics against Uveal Melanoma. Biomimetics 2018, 3 (4), 28. 10.3390/biomimetics3040028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X.; Xiong T.; Cui M.; Li N.; Li Q.; Zhu L.; Duan S.; Wang Y.; Guo Y. A Novel Targeted Co-Delivery Nanosystem for Enhanced Ovarian Cancer Treatment via Multidrug Resistance Reversion and MTOR-Mediated Signaling Pathway. J. Nanobiotechnology 2021, 19 (1), 444. 10.1186/s12951-021-01139-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin L.; Fan Y.; Gao F.; Jin L.; Li D.; Sun W.; Li F.; Qin P.; Shi Q.; Shi X.; Du L. UTMD-Promoted Co-Delivery of Gemcitabine and MiR-21 Inhibitor by Dendrimer-Entrapped Gold Nanoparticles for Pancreatic Cancer Therapy. Theranostics 2018, 8 (7), 1923–1939. 10.7150/thno.22834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Y.; Wang R.; Gao L.; Li K.; Zhou X.; Guo H.; Liu C.; Han D.; Tian J.; Ye Q.; Hu Y. T.; Sun D.; Yuan X.; Zhang N. Sequential Co-Delivery of MiR-21 Inhibitor Followed by Burst Release Doxorubicin Using NIR-Responsive Hollow Gold Nanoparticle to Enhance Anticancer Efficacy. J. Controlled Release 2016, 228, 74–86. 10.1016/j.jconrel.2016.03.008. [DOI] [PubMed] [Google Scholar]
- Li S.; Liu Y.; Rui Y.; Tang L.; Achilefu S.; Gu Y. Dual Target Gene Therapy to EML4-ALK NSCLC by a Gold Nanoshell-Based System. Theranostics 2018, 8 (10), 2621–2633. 10.7150/thno.24469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J.; Zhang L.; Lei J.; Ju H. MicroRNA-Responsive Cancer Cell Imaging and Therapy with Functionalized Gold Nanoprobe. ACS Appl. Mater. Interfaces 2015, 7 (34), 19016–19023. 10.1021/acsami.5b06206. [DOI] [PubMed] [Google Scholar]
- He P.; Han W.; Bi C.; Song W.; Niu S.; Zhou H.; Zhang X. Many Birds, One Stone: A Smart Nanodevice for Ratiometric Dual-Spectrum Assay of Intracellular MicroRNA and Multimodal Synergetic Cancer Therapy. ACS Nano 2021, 15 (4), 6961–6976. 10.1021/acsnano.0c10844. [DOI] [PubMed] [Google Scholar]
- Sukumar U. K.; Bose R. J. C.; Malhotra M.; Babikir H. A.; Afjei R.; Robinson E.; Zeng Y.; Chang E.; Habte F.; Sinclair R.; Gambhir S. S.; Massoud T. F.; Paulmurugan R. Intranasal Delivery of Targeted Polyfunctional Gold–Iron Oxide Nanoparticles Loaded with Therapeutic MicroRNAs for Combined Theranostic Multimodality Imaging and Presensitization of Glioblastoma to Temozolomide. Biomaterials 2019, 218 (March), 119342. 10.1016/j.biomaterials.2019.119342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo X.; Li Z.; Wang G.; He X.; Shen X.; Sun Q.; Wang L.; Yue R.; Ma N. MicroRNA-Catalyzed Cancer Therapeutics Based on DNA-Programmed Nanoparticle Complex. ACS Appl. Mater. Interfaces 2017, 9 (39), 33624–33631. 10.1021/acsami.7b09420. [DOI] [PubMed] [Google Scholar]
- Yue R.; Chen M.; Ma N. Dual MicroRNA-Triggered Drug Release System for Combined Chemotherapy and Gene Therapy with Logic Operation. ACS Appl. Mater. Interfaces 2020, 12 (29), 32493–32502. 10.1021/acsami.0c09494. [DOI] [PubMed] [Google Scholar]
- Zhang D.; Wang K.; Wei W.; Liu Y.; Liu S. Multifunctional Plasmonic Core-Satellites Nanoprobe for Cancer Diagnosis and Therapy Based on a Cascade Reaction Induced by MicroRNA. Anal. Chem. 2021, 93 (27), 9521–9530. 10.1021/acs.analchem.1c01539. [DOI] [PubMed] [Google Scholar]
- Fortunato O.; Iorio M. V. The Therapeutic Potential of MicroRNAs in Cancer: Illusion or Opportunity?. Pharmaceuticals (Basel). 2020, 13 (12), 438. 10.3390/ph13120438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohammadinejad R.; Dehshahri A.; Madamsetty V. S.; Zahmatkeshan M.; Tavakol S.; Makvandi P.; Khorsandi D. In Vivo Gene Delivery Mediated by Non-Viral Vectors for Cancer Therapy. J. Controlled Release 2020, 325 (July), 249–275. 10.1016/j.jconrel.2020.06.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alkilany A. M.; Murphy C. J. Toxicity and Cellular Uptake of Gold Nanoparticles: What We Have Learned so Far?. J. Nanoparticle Res. 2010, 12 (7), 2313–2333. 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enea M.; Pereira E.; Costa J.; Soares M. E.; Dias da Silva D.; Bastos M. de L.; Carmo H. F. Cellular Uptake and Toxicity of Gold Nanoparticles on Two Distinct Hepatic Cell Models. Toxicol. Vitr. 2021, 70, 105046. 10.1016/j.tiv.2020.105046. [DOI] [PubMed] [Google Scholar]
- Capek I. Polymer Decorated Gold Nanoparticles in Nanomedicine Conjugates. Adv. Colloid Interface Sci. 2017, 249, 386–399. 10.1016/j.cis.2017.01.007. [DOI] [PubMed] [Google Scholar]
- Schubert J.; Chanana M. Coating Matters: Review on Colloidal Stability of Nanoparticles with Biocompatible Coatings in Biological Media, Living Cells and Organisms. Curr. Med. Chem. 2018, 25 (35), 4553–4586. 10.2174/0929867325666180601101859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graczyk A.; Pawlowska R.; Jedrzejczyk D.; Chworos A. Gold Nanoparticles in Conjunction with Nucleic Acids as a Modern Molecular System for Cellular Delivery. Molecules 2020, 25 (1), 204. 10.3390/molecules25010204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sibuyi N. R. S.; Moabelo K. L.; Fadaka A. O.; Meyer S.; Onani M. O.; Madiehe A. M.; Meyer M. Multifunctional Gold Nanoparticles for Improved Diagnostic and Therapeutic Applications: A Review. Nanoscale Res. Lett. 2021, 16, 174. 10.1186/s11671-021-03632-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu A.; Tian Y.; Tu M.; Ho P. Y.; Jilek J. L. MicroRNA Pharmacoepigenetics: Posttranscriptional Regulation Mechanisms behind Variable Drug Disposition and Strategy to Develop More Effective Therapy. Drug Metab. Dispos. 2016, 44 (March), 308–319. 10.1124/dmd.115.067470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlíková L.; Šereš M.; Breier A.; Sulová Z. The Roles of MicroRNAs in Cancer Multidrug Resistance. Cancers (Basel). 2022, 14 (4), 1090. 10.3390/cancers14041090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty C.; Sharma A. R.; Sharma G.; Lee S.-S. Therapeutic Advances of MiRNAs: A Preclinical and Clinical Update. J. Adv. Res. 2021, 28, 127–138. 10.1016/j.jare.2020.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouri F. M.; Hurley L. A.; Daniel W. L.; Day E. S.; Hua Y.; Hao L.; Peng C.-Y.; Merkel T. J.; Queisser M. A.; Ritner C.; Zhang H.; James C. D.; Sznajder J. I.; Chin L.; Giljohann D. A.; Kessler J. A.; Peter M. E.; Mirkin C. A.; Stegh A. H. MiR-182 Integrates Apoptosis, Growth, and Differentiation Programs in Glioblastoma. Genes Dev. 2015, 29 (7), 732–745. 10.1101/gad.257394.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conde J.; Rosa J.; de la Fuente J. M.; Baptista P. V. Gold-Nanobeacons for Simultaneous Gene Specific Silencing and Intracellular Tracking of the Silencing Events. Biomaterials 2013, 34 (10), 2516–2523. 10.1016/j.biomaterials.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Xue H. Y.; Liu Y.; Liao J. Z.; Lin J. S.; Li B.; Yuan W. G.; Lee R. J.; Li L.; Xu C. R.; He X. X. Gold Nanoparticles Delivered MiR-375 for Treatment of Hepatocellular Carcinoma. Oncotarget 2016, 7 (52), 86675–86686. 10.18632/oncotarget.13431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Zhang Y.; Liu B.; Wu H.; Kang Y.; Li M.; Zeng X.; He N.; Zhang G. The Effects of Multifunctional MiR-122-Loaded Graphene-Gold Composites on Drug-Resistant Liver Cancer. J. Nanobiotechnology 2015, 13 (1), 1–15. 10.1186/s12951-015-0070-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y.; Zhang X.; Zeng X.; Liu B.; Hu F.; Zhang G. Glutathione-Mediated Release of Functional MiR-122 from Gold Nanoparticles for Targeted Induction of Apoptosis in Cancer Treatment. J. Nanosci. Nanotechnol. 2014, 14 (8), 5620–5627. 10.1166/jnn.2014.8735. [DOI] [PubMed] [Google Scholar]
- Qian R. C.; Cao Y.; Long Y. T. Binary System for MicroRNA-Targeted Imaging in Single Cells and Photothermal Cancer Therapy. Anal. Chem. 2016, 88 (17), 8640–8647. 10.1021/acs.analchem.6b01804. [DOI] [PubMed] [Google Scholar]
- Cai L.; Li J.; Zhang X.; Lu Y.; Wang J.; Lyu X.; Chen Y.; Liu J.; Cai H.; Wang Y.; Li X. Gold Nano-Particles (AuNPs) Carrying Anti-EBV-MiR-BART7–3p Inhibit Growth of EBV-Positive Nasopharyngeal Carcinoma. Oncotarget 2015, 6 (10), 7838–7850. 10.18632/oncotarget.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X.; Wang X.; Ye S.; Li R.; Li H. A One-Two-Three Multifunctional System for Enhanced Imaging and Detection of Intracellular MicroRNA and Chemogene Therapy. ACS Appl. Mater. Interfaces 2021, 13 (24), 27825–27835. 10.1021/acsami.1c04353. [DOI] [PubMed] [Google Scholar]