Abstract
Emerging plans for travel to Mars and other deep space destinations make it critical for us to understand how spaceflight affects the human brain and behavior. Research over the past decade has demonstrated two co-occurring patterns of spaceflight effects on the brain and behavior: dysfunction and adaptive plasticity. Evidence indicates the spaceflight environment induces adverse effects on the brain, including intracranial fluid shifts, gray matter changes, and white matter declines. Past work also suggests that the spaceflight environment induces adaptive neural effects such as sensory reweighting and neural compensation. Here, we introduce a new conceptual framework to synthesize spaceflight effects on the brain, Spaceflight Perturbation Adaptation Coupled with Dysfunction (SPACeD). We review the literature implicating neurobehavioral dysfunction and adaptation in response to spaceflight and microgravity analogues, and we consider pre-, during-, and post-flight factors that may interact with these processes. We draw several instructive parallels with the aging literature which also suggests co-occurring neurobehavioral dysfunction and adaptive processes. We close with recommendations for future spaceflight research, including: 1) increased efforts to distinguish between dysfunctional versus adaptive effects by testing brain-behavioral correlations, and 2) greater focus on tracking recovery time courses.
Keywords: Spaceflight, Head-down-tilt bed rest, Adaptation, Plasticity, Compensation
1. Introduction
The rapid advances in spaceflight capability make it likely that human space travel will increase in duration, recurrence, and accessibility. Beyond discussions of investigative missions to the Moon and Mars, is the public interest in recreational spaceflight, that is, space tourism. Human curiosity and adventure towards outer space must be grounded in a firm understanding of how spaceflight affects the central nervous system. Indeed, important questions need to be addressed regarding the impact of prolonged body unloading (weightlessness), stress, sleep disruption, radiation exposure, elevated CO2, and other hazards of the space environment on human neural systems and behavioral performance.
The accumulating evidence of brain and behavioral changes that accompany spaceflight suggests two co-occurring patterns: dysfunction and adaptive plasticity. In this review we summarize the literature documenting these two general patterns, consider their potential interactions, and discuss analytic approaches to distinguish between them. We propose a framework to help organize and understand these microgravity effects on the human brain: Spaceflight Perturbation Adaptation Coupled with Dysfunction (SPACeD). We consider instructive parallels between patterns of brain effects associated with spaceflight and observations in human aging neuroscience. We close with recommendations for future spaceflight neuroscience research.
To understand the effects of spaceflight on brain function, it is essential to examine brain measures, behavioral measures, and their relationships. Several decades of human neuroscience research have established that neural measures, such as brain activity, functional and structural connectivity, and volumetric indices must be considered in the context of associated behavioral and cognitive effects in order to understand their functional impact. In other words, to understand whether a change or difference in a particular neural measure is adverse or beneficial requires careful examination of the effects on human performance. For example, musicians show less brain activity in motor regions while performing bimanual tasks compared to non-musicians, a finding thought to be a marker of neural efficiency associated with greater musical expertise (Haslinger et al., 2004). In contrast, greater brain activity has been observed in older adults than younger adults, and when coupled with similar performance for both groups the increased activity in older adults is interpreted as beneficial (cf. Reuter-Lorenz and Lustig, 2005). Thus, interpreting whether a particular neural index is beneficial or not can be aided by evaluating aspects of performance.
Theories and neurally-based hypotheses from the field of brain aging can provide helpful guidance in disambiguating whether spaceflight-induced brain changes reflect dysfunction or adaptive neuroplasticity. For example, numerous studies have documented that older adults exhibit greater brain activity than young adults when performing a given task (cf. Seidler et al., 2010). Debate continues as to whether and under what conditions these brain activation differences reflect dedifferentiation or compensation processes. Dedifferentiation refers to the hypothesis that brain structure-function relationships become less precise with age which can manifest as older adults recruiting additional brain regions compared to young adults (Li and Lindenberger, 1999; Park et al., 2004; Riecker et al., 2006; Langan et al., 2010; Bernard and Seidler, 2012). In contrast, the compensation view posits that recruitment of additional brain areas provides resources and computational support to maintain performance in the face of age-related brain structural and biochemical declines (Cabeza, 2001; Mattay et al., 2002; Ward and Frackowiak, 2003; Reuter-Lorenz and Lustig, 2005; Wu and Hallett, 2005; Naccarato et al., 2006; Heuninckx et al., 2008). The latter interpretation is predicated upon better performance in older adults being associated with higher levels of brain activity. The aging literature has extensively drawn upon brain-behavior associations to interpret whether age-related brain changes are adaptive or maladaptive (for review, see Zahodne and Reuter-Lorenz (2019)). Likewise, we suggest that understanding whether spaceflight induces brain changes and under what conditions brain changes reflect dysfunctional or adaptive responses can also be aided by examining associated changes in behavioral performance.
2. SPACeD: A framework for the interactive effects of neural dysfunction and adaptation with spaceflight
Here we provide an overview of research on brain changes with spaceflight and we examine evidence for brain-behavior associations to show that spaceflight induces both adaptive processes and dysfunction. We propose a conceptual framework, Spaceflight Perturbation Adaptation Coupled with Dysfunction (SPACeD), for studying human brain changes with spaceflight.
The SPACeD framework posits specific factors—both negative (stressors) and positive (resilience)—that drive brain and behavioral changes, and in this respect resembles frameworks from human aging neuroscience (Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Park, 2014). Furthermore, our framework outlines stressors and resilience factors at three stages: pre-flight, in-flight, and post-flight. Each stage includes specific combinations of stressors and resilience factors, which may be external / environmental or internal in nature. Below we provide a brief overview of each stage along with examples of stressors and resilience factors relevant to each. We posit that unique combinations of stressors and resilience during each stage contribute to individual differences in brain changes with flight and to post-flight recovery processes.
Our framework includes not only detrimental effects of spaceflight, but it also draws upon both brain and behavioral evidence suggestive of adaptive plasticity associated with spaceflight. The framework encompasses changes in brain function as well as several measures of brain structure including gray and white matter changes, and intracranial fluid shifts. Critically, the inclusion of behavioral measures aids in the interpretation of whether brain functional or structural changes are adaptive or dysfunctional. The SPACeD framework is summarized in Fig. 1. Tables 1-3 provide specific examples of stressors and resilience factors associated with pre-flight, in-flight, and post-flight stages as well as evidence of accompanying brain dysfunction, brain adaptation, and behavioral changes. This framework is intended to provide a structure for organizing the evidence for concomitant adaptive and dysfunctional brain changes with flight, and to clarify where future work is needed to fully understand the causes and consequences of these changes.
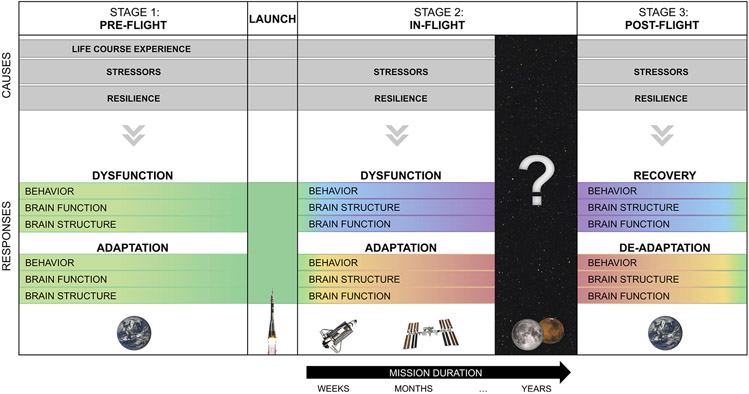
Fig. 1. Overview of spaceflight effects on the brain.
Different stages (pre-flight, in-flight, and post-flight) associated with spaceflight are depicted as a primary organizing principle of the framework. The gray bars represent ongoing positive and negative factors hypothesized to play a causal role in brain and behavioral changes corresponding with each stage. The gradients depicted in the “Responses” represent magnitude of impact (e.g., structural brain changes increase during flight and subsequently recover). Mission duration is shown in black during the in-flight period. Effects of spaceflight durations spanning greater than one year are currently unknown. Each stage corresponds to a section of Tables 1-3, which provides a detailed summary of evidence to date associated with each of these effects. For example, studies pertaining to the effects of stressors and resilience on the pre-flight state (first column in Fig. 1) are highlighted in Section 1 of Table 1.
Table 1.
Stage 1, Pre-Flight: Stressors and resilience factors over the life course that likely contribute to brain changes with spaceflight and over the lifespan.
| STAGE 1 | PRE-FLIGHT | AGING1 |
|---|---|---|
| STRESSORS |
Life Course Experiences
Past Flight Experience
Pre-flight Training
|
|
| RESILIENCE |
Life Course Experiences
Past Flight Experience
Pre-flight Training
|
|
Note. Here we list negative (stressor) and positive (resilience) life course factors that likely influence brain changes with spaceflight and aging. Past research has linked each of these factors to brain function and structure (although not all of these factors have been tested yet with astronauts; see superscript indications). Exceptions are listed factors listed in the pre-flight column without a citation. We hypothesize that these factors elicit brain changes and influence the neural effects of spaceflight; however, these hypotheses have yet to be tested and constitute areas needing future research (Beauchet et al., 2013; Gorman et al., 2016; Maillard et al., 2012; Mårtensson et al., 2012; Meeusen, 2014; Yates et al., 2012; Zwart et al., 2012).
All listed aging factors are adapted from the previously-established Scaffolding Theory of Aging and Cognition-Revised (STAC-r) model where they are referred to as resource enriching and depleting factors, respectively (Reuter-Lorenz and Park, 2014).
review paper.
experimental study.
studies that have tested effects of the listed factor on the brain in healthy adults; indicates an area needing future research in astronauts.
studies that have tested effects of the listed factor on the brain with spaceflight.
Table 3.
Stage 3, Post-Flight: Stressors and resilience factors post-flight and that may contribute to recovery and de-adaptation of brain changes with spaceflight.
| STAGE 3 | POST-FLIGHT | AGING1 | |
|---|---|---|---|
| STRESSORS |
|
||
| RESILIENCE |
|
||
| RECOVERY | Behavior |
|
|
| Brain Structure |
|
||
| Brain Function |
|
||
| DE-ADAPTATION | Behavior |
|
|
| Brain Structure |
|
||
| Brain Function |
|
||
Note. Here we list negative (stressor) and positive (resilience) in-flight factors that likely influence recovery and de-adaptation of spaceflight-induced brain changes. Items without a citation indicate factors hypothesized to affect recovery and de-adaptation; however, these hypotheses are untested and constitute areas for future research. The majority of studies listed here test recovery following exposure to spaceflight analogs, rather than spaceflight. Long duration recovery studies do not yet exist for spaceflight and are a key area in need of future work (Buckey et al., 1996).
The concepts of recovery and de-adaptation do not map well onto known processes in aging and are therefore listed as not applicable (N/A).
experimental study.
studies that have tested effects of the listed factor on the brain in healthy adults; indicates an area where future research is needed completed in astronauts.
studies that have tested effects of the listed factor on the brain with spaceflight.
studies that have tested effects of the listed factor on the brain with spaceflight analog environments (e.g., head-down-tilt bed rest or Antarctic isolation).
In the subsequent sections, we describe the framework’s three stages: pre-flight, in-flight, and post-flight. For each stage, we cite examples of characteristic stressors and resilience factors, and we review relevant literature linking these factors to behavioral changes, brain dysfunction, and brain adaptation. Currently, more is known about the influence of in-flight (Stage 2) stressors and resilience factors on neurophysiology and behavior compared to pre-flight or post-flight effects. Therefore, the in-flight section provides a more comprehensive analysis of spaceflight effects on the human brain and behavior compared to the other two sections. Rather than providing a comprehensive and exhaustive review, we discuss key behavioral and neuroimaging studies with astronaut participants. We refer to spaceflight analog studies in several instances where their findings directly inform our interpretation of prior work with astronauts. In particular, we draw on analog studies that have used identical behavioral assessments or neuroimaging analysis methods as those applied to astronauts. For further review of spaceflight analog effects on the brain, see Van Ombergen et al. (2017a, 2017b).
3. Stage 1: pre-flight
During the pre-flight stage (see Table 1), stressors and resilience factors are shaped by a combination of astronauts’ unique life course experiences, intensive pre-flight training (e.g., novel skill learning), and previous spaceflight experience (e.g., number and length of previous missions). Astronauts represent a unique population of healthy adults who have been carefully screened and selected based on their education, physical and mental health, and physical fitness. NASA’s current basic requirements for applying to the astronaut candidate program include a science, engineering, or math bachelor’s degree plus several years of “related” professional experience (e.g., science graduate work or piloting experience). As lifetime intellectual engagement and education appears to positively affect brain structure (Coffey et al., 1999) and slow onset of brain pathology (e.g., slow β-amyloid plaque deposition; Wirth et al., 2014), astronauts may already benefit from certain life course neuroprotections. Astronauts must also meet basic fitness and health requirements, including corrected 20/20 vision, blood pressure ≤ 140/90 mmHg, and aerobic / muscular strength minimum standards. In general, astronauts are likely in better health than their similarly aged peers; for instance, the 2009 European Space Agency entering astronaut class (n = 45) had fitness levels (i.e., treadmill stress test performance) comparable to the 90th percentile of their age group (Kordi et al., 2013). As a large body of literature has linked aerobic fitness to improved cognition and brain function (Hillman et al., 2008), many astronauts likely also have a neuroprotective advantage due to their higher-than-average fitness levels.
Those selected as astronaut candidates go through approximately two years of training and evaluation which often includes SCUBA certification, military water survival training, geological field work, and flight experience (Brown, 2020). For astronaut candidates who are selected for a specific mission (e.g., travel to the ISS), further training is required such as learning robotics skills, ISS systems, extravehicular activity protocols, as well as completing Russian language training (Brown, 2020). Novel skill acquisition is associated with structural and functional neural plasticity including increased localized gray matter volume and enlarged functional cortical representations when performing the learned task (Chang, 2014). Further, learning a second language is associated with cognitive benefits (i.e., the “bilingual advantage”; Bright and Filippi, 2019). Thus, it is likely that years of intensive astronaut pre-flight training induces positive brain plasticity, which could contribute to resilience during spaceflight in the form of neuroprotection or neural reserve. On the other hand, pre-flight training is also likely stressful as astronauts are tasked with learning many complex and highly technical new skills. Both acute (Weerda et al., 2010) and long-term (Lupien et al., 2009) stress exposure affect brain structure and function. For instance, healthy young adults show increased prefrontal and posterior parietal cortex activity during working memory maintenance after psychosocial stress exposure; this potentially indicates increased neural demand when performing cognitive tasks under stress (Weerda et al., 2010). Thus, while pre-flight stress may be beneficial preparation for the stress that will accompany in-flight experiences, acute stress could adversely affect the brain.
4. Stage 2: In-Flight
4.1. In-flight stressors and resilience factors
In-flight experience brings clear environmental stressors, including microgravity, radiation exposure (Stalport et al., 2019), headward fluid shifts (Leach, 1979; Petersen et al., 2019), and elevated CO2 (Zuj et al., 2012; Hughson et al., 2016), as well as numerous internal stressors, such as emotional stress (Prisniakova, 2004; Strewe and Choukèr, 2012) and lack of sleep (Stoilova et al., 2003; Petit et al., 2019) that will impact the central nervous system. At the same time, resilience factors (including spaceflight euphoria and in-flight exercise programs) could also exert buffering central nervous system effects (see Table 2). Moreover, some factors may result in both positive and negative outcomes; for instance, although there are multiple well established negative consequences of lack of sleep for astronauts (Barger et al., 2014), there is also evidence that microgravity results in a 55% reduction in disordered sleep (i.e., the apnea-hypoxia index) and virtual elimination of snoring (Elliott et al., 2001), which would both be considered beneficial results of microgravity. Together, these multifactorial influences on brain structure and function likely interact and vary over the course of flight. Below we consider these factors in more detail.
Table 2.
Stage 2, In-Flight: Stressors and resilience factors in-flight and in aging that likely contribute to neural dysfunction and adaptations.
| STAGE 2 | IN-FLIGHT | NEW EXPERIENCES AS AN ADULT1 |
|
|---|---|---|---|
| STRESSORS |
|
|
|
| RESILIENCE |
|
|
|
| DYSFUNCTION | Behavior |
|
|
| Brain Structure |
|
|
|
| Brain Function |
|
|
|
| ADAPTATION | Behavior |
|
|
| Brain Structure |
|
Unclear if aging is associated with any structural brain adaptations | |
| Brain Function |
|
|
|
Note. Here we list negative (stressor) and positive (resilience) in-flight factors that likely influence brain changes with spaceflight. Items without citations indicate factors hypothesized to elicit brain changes with spaceflight; however, these hypotheses have yet to be tested and constitute areas for future research (Koppelmans et al., 2015; Manzey et al., 1993; Pagel and Choukèr, 2016; Reschke et al., 2009; Riascos et al., 2019; Stahn et al., 2019; Welch et al., 2009; Wood et al., 2010).
All listed aging factors are adapted from the previously-established Scaffolding Theory of Aging and Cognition-Revised (STAC-r) model where they are referred to as resource enriching and depleting factors, respectively (Reuter-Lorenz and Park, 2014).
review paper.
experimental study.
studies that have tested effects of the listed factor on the brain in healthy adults; indicates an area where research should be conducted in astronauts.
studies that have tested effects of the listed factor on the brain with spaceflight. Most of the factors listed for these spaceflight studies are changes measured post-flight; however, we (and the spaceflight human research community) infer these to be spaceflight-induced changes, rather than changes associated with re-adaptation to Earth’s environment.
studies that have tested effects of the listed factor on the brain with spaceflight analog environments (e.g., head-down-tilt bed rest or Antarctic isolation).
Currently, human spaceflight involves travel on a Russian Soyuz spacecraft to the International Space Station (ISS), where crewmembers participate in missions of approximately six months duration, although some astronauts have stayed for up to one year. While aboard the ISS, which orbits at an altitude of 200–250 miles above Earth, astronauts experience microgravity (i.e., 1 × 10−6 g), rather than zero gravity. That is, even though ISS astronauts appear as if they are in absence of gravity, there is a very small amount of gravitational force acting on them, so this environment is more aptly described as microgravity. In addition to the direct effects of microgravity during spaceflight, other stressors that may impact brain structure and function include sleep disruption, isolation and confinement, a heavy workload (i.e., work days lasting 8 h or more, plus 2.5 h of exercise), space motion sickness, and increased radiation exposure, among other factors. For instance, astronauts sleep fewer hours while aboard the ISS than they do on Earth (Barger et al., 2014). This is potentially due to environmental factors (e.g., microgravity, noise, motion sickness, and uncomfortable temperatures), as well as circadian misalignment (Flynn-Evans et al., 2016). On Earth, light exposure synchronizes circadian rhythms to a 24-h day. However, on the ISS, the natural light-dark cycle lasts only 90 min (i.e., there are 16 sunrises and sunsets per 24-h period on the ISS). Additionally, crewmembers frequently undergo 6- to 12-h shifts in the timing of their sleep-wake cycles due to various mission demands. Although astronauts are usually scheduled for 8-h sleep periods, these factors disrupt circadian rhythms and negatively affect sleep duration and quality. One study found the mean sleep duration aboard the ISS to be 6.4 h during sleep that was aligned with the biological clock, but 5.4 h during sleep misaligned with the biological clock (Flynn-Evans et al., 2016). This work suggests that, even in optimal conditions, astronauts still sleep less than the recommended amount for adults for months at a time aboard the ISS.
Exposure to microgravity induces fluid redistribution within the body, with fluid moving from the lower extremities towards the head (Moore and Thornton, 1987). Crewmembers are also exposed to elevated CO2 levels onboard the ISS where levels are approximately 10 times higher than those on Earth (Moore and Thornton, 1987; Law et al., 2014). Experiments conducted on Earth show that prolonged exposure to elevated CO2 levels, such as that existing on the ISS, results in increased cerebral blood flow and mild performance impairments (Hoffmann et al., 1998; Manzey and Lorenz, 1998; Sliwka et al., 1998). Space Station crewmembers have exhibited symptoms of CO2 exposure at lower concentrations than are typical for their occurrence on Earth (Law et al., 2014), suggesting an interactive effect of CO2 with other factors such as headward fluid shifts. Chronic sleep deprivation also alters dynamic brain activity (Basner et al., 2013).
Despite these reported stressors of spaceflight, astronauts frequently report “spaceflight euphoria”, particularly when viewing Earth from the ISS cupola (Yaden et al., 2016). Emotional exhilaration and euphoria could promote resilience that serves to partially offset negative influences of spaceflight (Kok et al., 2013). Furthermore, astronauts receive various services while on board the ISS to promote their psychological well-being (Beven et al., 2008). Astronauts regularly complete private psychological counseling and receive care packages from their family during resupply. There are several leisure time amenities on board, such as a projector for watching movies as a group and a keyboard guitar. Astronauts also have two “crew discretionary events” per mission, for which they can request to have a private conversation with a person of their choosing, such as a celebrity, musician, or political figure. Astronauts have access to exercise facilities (i.e., a stationary bike, treadmill, and resistance training equipment), and they each exercise for about 2.5 h per day, 6–7 days per week (Hackney et al., 2015). Together, these factors likely contribute to central nervous system resilience during ISS missions. For instance this high-volume exercise likely serves as a resilience factor for both physical and mental well-being, including beneficial effects for cognition and brain health (Hillman et al., 2008). However, in the future, these factors will likely vary during longer missions to more remote deep space locations, so their positive effects may not be generalizable beyond ISS missions.
4.2. Effects of spaceflight on behavior
The negative effects of spaceflight on sensorimotor and cognitive function are well-documented. These include post-flight impairments in posture control (Layne et al., 2001; Cohen et al., 2012) and locomotion (Bloomberg and Mulavara, 2003; Miller et al., 2010; Mulavara et al., 2010; Cohen et al., 2012), as well as increased manual tracking errors under cognitive load (Manzey et al., 2000; Bock et al., 2010) and reduced mass discrimination abilities (i.e., reduced ability to identify differences in the mass of two different objects; Ross et al., 1984). Additionally, negative effects of spaceflight on behavior include in-flight spatial disorientation and dizziness (Young et al., 1984) and changes in gaze holding in response to altered gravity (Clément et al., 1993; Kornilova et al., 1983). Astronauts in microgravity also encounter changes in the perception of self-motion; for instance, one study found immediate alterations in one’s perception of self-motion in the upwards/downwards (pitch) but not left/right (yaw) directions through a virtual tunnel when free-floating in weightlessness—suggesting that weightlessness may negatively affect the early processing stages of self-motion perception (i.e., vestibular and optokinetic function; De Saedeleer et al., 2013).
While negative behavioral changes have been the focus of much research, microgravity exposure also induces adaptive brain processing of visual, vestibular, and proprioceptive information (Paloski et al., 1992, 1994; Reschke et al., 1998). Evidence of this in-flight adaptation is seen as measurable post-flight disturbances in perception, spatial orientation, posture, gait, and eye-head coordination (Reschke et al., 1998). These behavioral changes can provide insight into the underlying drivers of spaceflight’s impact on the brain. Below, we provide a review of evidence for in-flight behavioral impairments followed by a review of adaptive behavioral changes in-flight. Of note, some in-flight effects are inferred based on measurements taken post-flight; however, here we distinguish between in-flight effects (Section 4) and additional changes that occur post-flight when readapting to Earth’s environment (Section 5).
4.2.1. In-flight behavioral impairments
The ability to perform multiple tasks concurrently, referred to as “dual-tasking”, is used by psychological and motor scientists to assess cognitive resource availability and executive function. Dual tasking of cognitive and motor behaviors is significantly impaired during spaceflight (Manzey et al., 1995, 1998; Bock et al., 2010), suggesting that cognitive resources may be reduced. Manzey et al. (1995, 1998) investigated motor skills in space under dual-task conditions. They found interference between a compensatory tracking task and a concurrent memory search task to be greater in space than on Earth. The elevated interference was greatest early in flight, but gradually normalized, reaching the pre-flight baseline only after about three weeks in orbit. Manzey et al. (1995) also found that task interference was independent of the difficulty of the memory search task, suggesting that the critical resources affected were probably not those related to memory, but rather those pertinent to motor programming (both tasks required an immediate motor response). Bock et al. (2010) studied the cognitive demands of human sensorimotor performance and dual tasking during long duration missions and concluded that both stress and scarcity of resources required for sensorimotor adaptation may underlie these deficits during spaceflight. Of note, while these experiments are all classified as “dual tasking” because they require the concurrent performance of two tasks (e.g., a cognitive and motor task), operating in an altered gravitational environment may itself constitute an additional “task” (i.e., a task requiring increased self-perception of body position and orientation). This extra processing demand likely contributes to the negative effects of spaceflight on dual task abilities and may, in part, explain the findings of Manzey et al. (1998) who noted that dual task ability returned to baseline levels after about three weeks in flight. That is, the extra cognitive load of functioning in microgravity eventually decreased (presumably due to adaptive responses), and performance returned to baseline levels after this adaptation to weightlessness.
4.2.2. In-flight adaptive sensory changes
Evidence for sensorimotor adaptation with spaceflight includes post-flight changes in tactile sensitivity of the feet. Post-flight, there is a general reduction in the sensitivity of slow-adapting skin receptors (3 and 25 Hz), which contribute to postural control on Earth by detecting load changes between the foot and ground (Lowrey et al., 2014). It is hypothesized that body unloading (i.e., that one’s muscles are not needed to support their body weight) during flight makes this signaling less vital and it is thus down-weighted by the central nervous system. Approximately half of the astronauts presented with increased sensitivity of fast-acting skin receptors (250 Hz) post-flight. This hypersensitivity has been associated with poorer vestibularly-mediated balance on the first day post-flight. It is hypothesized that this increase in post-flight tactile sensitivity represents adaptive targeted sensory reweighting, in which the altered gravitational environment during flight causes down-weighting of vestibular inputs and up-weighting of signals from fast-acting tactile receptors for balance control. That is, while in microgravity, these tactile receptors may play a larger role in orientation control as compensation for unreliable vestibular inputs. These tactile sensitivity changes seem to represent adaptive, compensatory central nervous system-mediated changes during flight; however, upon return to Earth’s gravitational environment, any residual sensory reweighting becomes detrimental to balance control.
4.3. Effects of spaceflight on the brain
Human and animal work has revealed both negative and positive effects of spaceflight and spaceflight analogs on the central nervous system. Potential negative effects include changes in cerebral blood flow and alterations to brain structure, including evidence for an upward shift of the brain within the skull and disrupted white matter structural connectivity. Potential positive effects include increased motor cortical excitability and structural and functional plasticity, suggestive of sensory reweighting processes with spaceflight. Thus, similar to the behavioral changes that occur, it appears that two broad categories of central nervous system changes occur with spaceflight: 1) structural and functional central nervous system dysfunctions, and 2) adaptive plasticity and sensory reweighting. Examination of these central nervous system changes in conjunction with behavioral changes can help to clarify whether these effects represent dysfunction versus adaptations.
4.3.1. In-flight brain dysfunction
In this section, the functional and structural brain changes that are reviewed are largely interpreted as dysfunctional. In some cases, however, this interpretation is speculative because the neural measures were not accompanied by behavioral measures that could provide converging information about whether or not the changes are detrimental. Future research should include well-chosen sensory and performance measures to inform and constrain interpretations of neural and physiological changes associated with spaceflight.
4.3.1.1. Brain function.
Given the very limited spaceflight functional neuroimaging work to date, the effects of spaceflight on brain activity are largely unknown. A single-subject case study revealed some evidence for dysfunction after six months of flight, including decreased motor and vestibular network connectivity, paired with vestibular ataxia and motor coordination declines (Demertzi et al., 2016). Our past work has noted several examples of neural dysfunction during head-down-tilt bed rest (HDBR; extended periods of HDBR is a frequently-used model for spaceflight that simulates some of the effects of spaceflight on the body). While undergoing HDBR, we have found increased brain activity during vestibular stimulation (Yuan et al., 2018b) and during performance of a cognitive-motor dual task (Yuan et al., 2016), compared to matched controls who did not partake in HDBR1. These findings suggest that extended periods of microgravity simulation might evoke the need for greater neural resources (i.e., reduced neural efficiency) while processing cognitive and sensori-motor information. Future work is needed to determine whether similar patterns emerge in association with spaceflight.
4.3.1.2. Brain structure.
Animal studies have shown that microgravity exposure results in structural brain changes. For example, research with rats has demonstrated that microgravity exposure, results in structural changes particularly in the somatosensory cortex (Krasnov, 1994; Newberg, 1994; D’Amelio et al., 1998; Holstein et al., 1999) and cerebellum (Holstein et al., 1999). These effects include decreased synapses and degeneration of axonal terminals. Hindlimb suspension has been used in animals as a model for body unloading that occurs with spaceflight. One study reported that rodent neural stem cells show reduced proliferation and incomplete differentiation and maturation with hindlimb unloading (Adami et al., 2018). The authors reported no evidence of stress responses but rather attributed the effects to reduced overall movement. Studies of radiation exposure have also shown that radiation-induced neuronal loss seems to differentially affect sensorimotor brain regions (Newberg, 1994; Holstein et al., 1999).
Long-duration HDBR results in similar fluid shifts towards the head and unloading of the body in humans. In this context, we have observed apparent increased brain gray matter volume in posterior parietal cortex and decreases in frontal areas (Koppelmans et al., 2017a). Parallel findings have been reported by Roberts and colleagues (Roberts et al.,2015), including crowding of the cerebrospinal fluid (CSF) around the vertex and an upward shift of the brain within the skull. We have also applied a novel post-processing technique to diffusion MRI scans obtained on HDBR subjects. This technique quantifies “free water,” which is defined as water molecules that are not hindered or restricted by their surroundings (Pasternak et al., 2009). Free water is found in the ventricles, around the brain parenchyma, and in the extracellular space. Free water analysis is therefore an excellent tool to investigate non-invasively cerebral fluid shifts that occur over the course of HDBR and spaceflight. We found free water increases in frontal-temporal regions and decreases in posterior-parietal areas as a result of long duration HDBR (Koppelmans et al., 2017b); these effects were largely recovered two weeks following HDBR. Interestingly, after correcting for these free water shifts, no white matter changes were evident.
Many of these HDBR effects are also evident following human spaceflight (Koppelmans et al., 2016), supporting its status as an appropriate analog. These effects include an upward shift of the brain within the skull, accompanied by reduced gray matter volume in inferior and frontal brain regions, and increases in superior and posterior regions. These changes were found to be larger in individuals who had spent six months on the ISS than in those who spent just a few weeks on a space shuttle mission. With additional analyses on the same dataset, Roberts and colleagues reported narrowing of the central sulcus, increases in ventricular width and volume, and upward displacement of the cerebellar tonsils (Roberts et al., 2017). In combination, these findings suggest compression of adjacent venous structures and impedance of CSF outflow.
Our recent paper reports free water changes in the brain with spaceflight (Lee et al., 2019b). The findings generally recapitulate our earlier findings observed with HDBR (Koppelmans et al., 2017b)—increased free water at the base of the cerebrum and decreases along the posterior vertex. The correspondence between these two data sets suggests the effects are due to mechanical displacements of fluid, due to microgravity in space and reorientation of the head relative to the gravitational vector in HDBR.
After accounting for brain free water shifts with spaceflight, we observed numerous regions of spaceflight-induced white matter changes (Lee et al., 2019b). Crewmembers showed reduced fractional anisotropy, a measure of myelin integrity, in white matter structures implicated in vestibular function, visuospatial processing, and sensorimotor control, namely superior and inferior longitudinal fasciculi, inferior fronto-occipital fasciculus, corticospinal tract, and the cerebellar peduncles. These changes indicate disrupted white matter structural connectivity, which may negatively impact multi-sensory integration and motor behavior. Consistent with this idea, Lee et al. (2019b) showed that crewmembers exhibiting greater post-flight disruptions in white matter structural connectivity in the superior longitudinal fasciculus showed greater declines in balance from pre- to post-flight.
It is not yet clear how these fluid and brain positional shifts resolve over time upon return to Earth, how they affect health, or how they impact astronaut functional performance. These shifts are likely related to spaceflight associated neuro-ocular syndrome, or SANS. This syndrome describes ocular structural changes that have been reported in approximately one third of long duration astronauts, including flattening of the back of the globe, optic disc edema, optic nerve kinking and choroidal folding (Mader et al., 2013; Taibbi et al., 2013; Lee et al., 2016). For example, Alperin and Bagci (2018) found that greater post-flight globe deformation in astronauts was associated with increases in ventricular and orbital CSF volumes (Alperin and Bagci, 2018). Similarly, Van Ombergen et al. (2019) recently reported increases in CSF volume within the lateral and third ventricles following spaceflight, and post-flight increases in lateral ventricular volume were associated with decreases in visual acuity for the left eye (Van Ombergen et al., 2019). Kramer et al. (2020) found evidence for altered CSF hydrodynamics, as well as increased total brain volume and increased CSF volume, with long-duration spaceflight (Kramer et al., 2020). Zwart et al. (2014, 2017, 2018) have proposed a multiple factor model of SANS, in which brain fluid shifts with spaceflight may be a contributing factor. Their work has demonstrated that astronauts who experience signs of SANS have elevated 1-carbon metabolic pathway metabolites, not just post-flight but pre-flight as well (Zwart et al., 2012, 2014). In a subsequent study these authors demonstrated that 1-carbon metabolism genetics and B vitamin status were significant predictors of SANS (Zwart et al., 2016). However, not all astronauts at genetic risk experience SANS, leading Zwart and Smith to propose their multiple hit model. This model suggests that genetics and environmental factors such as elevated CO2 and fluid shifts predispose one to endothelial dysfunction (Zwart et al., 2017; Smith and Zwart, 2018).
Changes in cerebral blood flow as a result of microgravity exposure may also contribute to the brain functional changes described in the preceding paragraph. Following space flight, astronauts have reduced arterial pressure and cerebral blood flow velocity as measured with transcranial Doppler (Bondar et al., 1993). Similarly, Gazenko et al. found that astronauts show reduced cerebral blood flow pulsatility, as measured with impedance rheography, when in a head-down tilt posture following spaceflight (Gazenko et al., 1981; Charles et al., 1996; Watenpaugh and Smith, 1998). Other studies have demonstrated a microgravity dose-dependent effect, with cerebral vasoconstriction following long-term flight remaining unresolved after a period of five weeks (Gazenko et al., 1981; Charles et al., 1996). It is thought that this increased vasoconstriction is an adaptive response to the increased cranial pressures experienced while in the microgravity environment. Blood vessel remodeling can occur relatively quickly. In as little as two weeks of HDBR on Earth, there are increases in vessel wall thickness and vessel diameter in the brain vasculature and concomitant decreases in the lower extremity vasculature (Folkow, 1987; Mao et al., 1999). Elevated CO2 levels have also been shown to increase brain blood flow (Zhou et al., 2008), at least initially.
4.3.2. In-flight adaptive neural changes
Thus far we have discussed the many neural dysfunctions accompanying spaceflight, a topic that receives much attention and has been widely investigated. This section considers a smaller, emerging line of work documenting changes in brain function and structure during spaceflight that may constitute adaptive responses, although, as described above, the limited availability of behavioral data precludes definitive interpretations.
4.3.2.1. Brain function.
During missions, crewmembers experience prolonged periods of reduced somatosensory input and altered vestibular inputs due to the lack of gravity. Accumulating evidence suggests that in-flight sensory attenuation brings about adaptive structural and functional organization of motor and sensory systems, which maintain their capacity for experience-dependent plasticity even in adulthood (for review see Butz et al., 2009). Boyle et al. (2001) have shown upregulation of vestibular inputs in toadfish following shuttle orbital flights. On the first day post-flight, responses of vestibular nerve afferents to lateral movements were three times greater than for control animals. This finding suggests that sensitivity to vestibular input was enhanced in-flight (Boyle et al., 2001).
With body unloading in microgravity, crewmembers experience reductions in lower limb use on the ISS. Similar to the post-flight upregulation observed in the sensory domain, work by Roberts et al. (2007) suggests that motor cortex excitability increases following reductions in lower limb mobility. Transcranial magnetic stimulation was used to assess changes in motor cortex excitability in humans who wore a full leg cast (on Earth) for 10 days. Measures of motor cortex excitability significantly increased following leg cast removal (Roberts et al., 2007). In further support of sensory reweighting, we noted an association between greater brain activation during foot tapping at the end of 70 days of HDBR and better post-HDBR balance and mobility (Yuan et al., 2018a). This suggests a compensatory response in which, in order to sustain smaller reductions in balance and mobility, individuals require greater neural resources for lower limb motor control to compensate for the down-weighting of foot neural representations during HDBR. Further supporting this interpretation of neural compensation during HDBR, in the same subjects, we found that those with the least impairments in balance post-HDBR had the greatest functional connectivity changes with HDBR in a motor network including left primary motor cortex, right postcentral gyrus, and the superior parietal lobule. This brain-behavior relationship suggests that at least some functional connectivity changes with HDBR are adaptive and associated with reduced behavioral declines following HDBR. Similarly, in another study in which subjects underwent 30 days of HDBR combined with elevated CO2, we found further support for adaptive neural changes within the vestibular (Hupfeld et al., 2020a) and spatial working memory (Salazar et al., 2020) systems. For instance, we identified multiple regions in which greater pre- to post-HDBR deactivation of certain vestibular brain regions associated with less balance declines following HDBR (i.e., greater preservation of balance performance). Despite these interesting HDBR findings, there have been few functional brain imaging studies to date with astronauts; we therefore recommend that future studies be conducted to determine if similar effects are seen with spaceflight.
One recent study of 11 cosmonauts tested task-based functional connectivity during plantar stimulation after long-duration spaceflight. This group found connectivity changes within sensorimotor, visual, proprioceptive, and vestibular networks (Pechenkova et al., 2019). Without measures of pre- to post-flight behavioral changes, the functional significance of these results is not fully clear. The authors suggest that such changes represent reorganization of the sensory and vestibular systems and provide some evidence for multisensory reweighting with flight (Pechenkova et al., 2019).
Efforts have been made to use portable neuroimaging methods to assess in-flight measures of brain activity starting in the 1960s (Maulsby, 1966). In-orbit electroencephalography (EEG) studies have suggested that the brain uses dynamic sensory reweighting based on incoming sensory information during spaceflight. Cheron et al. (2006) used EEG to examine alpha and mu brain oscillations in cosmonauts while in eyes-opened and eyes-closed states before, during, and after spaceflight. Alpha EEG rhythm (8–12 Hz) is recorded over occipital and parietal areas, and can also be recorded more anteriorly as the mu rhythm over sensorimotor brain areas. Alpha rhythm is inhibited when the eyes are opened, and is considered an indicator of sensory input inhibition (Pfurtscheller et al., 1996). During in-flight EEG recording sessions, Cheron et al. (2006) found increases in alpha and mu power during trials in which cosmonauts rested in an eyes-closed state. This finding suggests an increase in sensory gain for inputs from other modalities (e.g., vestibular or somatosensory) in the absence of visual input. Cebolla and colleagues (2016) recorded EEG as astronauts performed a visual attention task before, during, and after spaceflight. Astronauts performing the visual task while free-floating on the ISS showed reductions in alpha and mu power over occipital-parietal and central brain regions, respectively. This finding suggests reduced inhibition of other sensory signals when visual input is available in microgravity. Moreover, alpha power within bilateral motor cortices was reduced during the task, again suggesting a release of sensory inhibition during the in-flight task. Such sensory reweighting may reflect increased reliance on somatosensory inputs for adjusting or stabilizing body posture while free-floating (Cebolla et al., 2016). Cheron et al. (2014) have also demonstrated that spaceflight affects early visual processing. EEG data were acquired in-flight while astronauts viewed a two-dimensional checkerboard pattern and a three-dimensional tunnel. During spaceflight, visual evoked potentials triggered by the three-dimensional stimulus were suppressed, and occipital brain areas exhibited reduced alpha band activity. The authors suggested that interactions with brain areas involved in multisensory integration modulate early visual processing, reweighting sensory inputs in the absence of gravitational cues (Cheron et al., 2014). However, these EEG studies were not linked to in-flight behavioral measures so it is not clear whether these effects were adaptive and beneficial for performance.
4.3.2.2. Brain structure.
Using structural brain imaging methods in a population of 27 astronauts who completed either approximately two-week shuttle missions (n = 13) or six-month ISS missions (n = 14), we found increased gray matter volume within medial primary sensorimotor cortex—the area of the brain that represents the lower limbs (Koppelmans et al., 2016). We found similar gray matter volume increases within medial primary sensorimotor cortex following 70 days of HDBR (Koppelmans et al., 2017a). Greater gray matter volumetric increases within this region following HDBR were associated with smaller decrements (and in some cases improvements) in standing balance performance (Koppelmans et al., 2017a). Structural plasticity within lower limb somatosensation and motor control brain areas may reflect a mechanism to increase the gain of somatosensory inputs in microgravity. Interestingly, this finding of structural plasticity in the sensorimotor cortex could relate to the increased alpha and mu oscillations recorded in astronauts (Cheron et al., 2006), as each of the effects identified in this study were reported only in central and parieto-occipital regions, and not in frontal cortex.
The exact mechanisms underlying these functional and structural alterations accompanying spaceflight are unknown; however, studies of sensory loss and deprivation may provide insights. Deprivation or loss of sensory input induces adaptive functional reorganization, including changes in receptive fields or topography, within the somatosensory system (e.g., Pons et al., 1991). Animal models show that even transient deprivation of somatosensory inputs can trigger functional somatosensory reorganization within minutes. Faggin et al. (1997) temporarily deactivated rat somatosensory afferents by subcutaneous anesthetic injection, and found that somatosensory neurons with receptive fields near the injection site began to show large responses to mechanical stimulation–even though those same neurons had not responded to stimulation prior to deafferentation. This suggests that temporary loss of peripheral sensory input can trigger fast reorganization of the somatosensory system (Faggin et al., 1997). Similarly, non-invasive brain stimulation studies involving humans with lower limb amputations show that the motor cortex corresponding to the amputated leg has a lower threshold for muscle activation as well as lower intracortical inhibition compared to the intact side (Chen et al., 1998). Studies such as these suggest that sensory loss may induce modulations of excitatory and inhibitory mechanisms within the sensorimotor system, perhaps through increases in synaptic efficiency via long-term potentiation (e.g., Hess et al., 1996) and/or structural plasticity via formation of new dendritic spines (e.g., Keck et al., 2008). While astronauts do not experience a total loss of afferent input from any given sensory modality during spaceflight, these sensory loss studies suggest possible adaptive mechanisms that may be engaged when astronauts are exposed to reductions in sensory inputs during missions lasting weeks or months.
We and others have reported evidence of structural changes that appear to differ based on flight duration. In particular, one study found pre- to post-flight increases in periventricular white matter hyperintensities and ventricular volumes in astronauts who completed long-duration missions, but not in astronauts who completed shuttle missions (Alperin et al., 2017). We found that twelve months in space generally resulted in larger changes across multiple brain areas involved in sensorimotor processing, compared to six-month missions (Hupfeld et al., 2020b). This duration effect was more apparent for brain fluid shifts than for other structural brain changes, suggesting that brain free water and ventricular volumes may be especially affected by long-duration spaceflight. In another recent study, while we found extensive white matter degeneration post-flight, we also reported that astronauts returning from longer duration missions showed smaller decreases in cerebellar white matter structure in comparison to astronauts returning from shorter flights (Lee et al., 2019b). While seemingly paradoxical, these findings may reflect an adaptive process whereby white matter structural organization is initially disrupted and then becomes more robust over time during spaceflight.
5. Stage 3, post-flight
During post-flight recovery, astronauts experience multiple stressors associated with readapting to Earth’s gravity, readjusting to home, and reintegrating into their family/society. Astronauts likely also experience resilience factors associated with the joy, comfort, and relief of reuniting with family and friends, returning to their pre-flight schedule, and feeling a sense of accomplishment. These factors may influence de-adaptation and recovery of brain changes due to spaceflight (see Table 3).
The majority of studies assume, often implicitly, that measurements acquired within several days of landing reflect brain changes due to spaceflight itself. However, the post-landing delays in obtaining the measurements complicate efforts to disentangle the effects of spaceflight from early readaptation to Earth’s gravitational environment.
Behavioral assessments offer valuable insight into spaceflight-induced brain and performance changes, as these experiments can be performed at the landing site shortly after crewmembers exit the spacecraft. Behavioral experiments have shown that astronauts exhibit deficits in sensorimotor performance following spaceflight, particularly during balance control (Young et al., 1984; Paloski et al., 1992, 1993, 1994; Reschke et al., 1998; Wood et al., 2011, 2015; Cohen et al., 2012; Mulavara et al., 2018; Ozdemir et al., 2018) and locomotion (Young et al., 1984; Paloski et al., 1992, 1993, 1994; Reschke et al., 1998; Wood et al., 2011, 2015; Cohen et al., 2012; Mulavara et al., 2018; Ozdemir et al., 2018). Behavioral impairments are most profound shortly after landing, before remaining in-flight sensory reweighting is resolved (Wood et al., 2011). All crewmembers exhibit postural deficits early post-flight, but there is considerable individual variability in the extent of impairment (Mulavara et al., 2010; Wood et al., 2011). Previous spaceflight experience is currently the best predictor of post-flight behavioral impairments, with experienced astronauts exhibiting less severe post-flight postural impairments compared to novice astronauts. Paloski et al. (1999) performed postural assessments on novice astronauts (n = 17) and veteran astronauts (n = 23) before and after spaceflight. Various postural tests were performed, each one manipulating the availability of sensory inputs (i.e., visual, somatosensory and vestibular inputs). Novice and veteran astronauts groups exhibited comparable pre-flight performance. On landing day, experienced astronauts showed a performance advantage over novices on balance assessments that required reliance on vestibular cues to maintain upright stance. These results suggest that previous flight experience aids astronauts in using vestibular cues for maintaining balance immediately post-flight (Paloski et al., 1999).
Re-adaptation of locomotion and postural control to Earth’s gravity requires between days and weeks (Mulavara et al., 2010; Wood et al., 2011), with recovery time increasing as a function of mission duration (Wood et al., 2011, 2015). For example, Mulavara et al. (2010) used the Functional Mobility Test (an obstacle course) to assess locomotor function of 18 crewmembers upon their return from a 6-month ISS mission. This test involves navigating an obstacle course by executing whole-body movements similar to those required to exit a spacecraft. The first half of the Functional Mobility Test is performed on a hard surface while the second half is built on a compliant foam floor. Following spaceflight, crewmembers showed a 48 % increase in the time required to complete the obstacle course. While crewmembers showed fast, strategic learning across trials on the first post-flight day, they required an average of 15 days for their performance to reach pre-flight levels (Mulavara et al., 2010).
Few neuroimaging studies have assessed post-flight brain changes although—in contrast to behavioral experiments—post-flight neuromaing assessments typically occur days to weeks after the landing date. One such MRI study considered functional brain activity that was collected on average 9.4 days post-flight to represent flight-related changes (Pechenkova et al., 2019). As noted by the authors, this long delay between landing and the MRI scan makes it difficult to interpret whether the results they report were due to the direct effects of flight, neural readaptation to Earth’s gravity, or to a combination of these effects.
Few studies have acquired multiple post-flight measurements to track recovery trajectories of flight-related changes. For instance, one study found ventricular volume increases from pre- to post-flight; in a subset (n = 7) of the cosmonauts tested, these increases had partially recovered, but were still evident at seven months post-flight (Van Ombergen et al., 2019). Another recent study (Kramer et al., 2020) reported persistent elevation of total brain volume and CSF one year after long-duration spaceflight, suggesting long-lasting alterations to brain structure following multi-month missions on the ISS. Similarly, we recently reported recovery of most brain structural changes back to baseline levels by 6-months post-flight for missions lasting from six to twelve months (Hupfeld et al., 2020b); however, lateral ventricular volumes remained substantially elevated at six months post-flight. Interestingly, those with less time between subsequent flights had larger pre-flight ventricles and smaller ventricular volume increases with flight, suggesting that spaceflight-induced ventricular changes may persist for very long periods after flight and influence the magnitude of fluid shifts with subsequent flights.
Across multiple measures in our HDBR work, we have identified recovery of brain changes as rapidly as about two weeks days post-HDBR (Cassady et al., 2016; Yuan et al., 2016, 2018a, 2018b; Koppelmans et al., 2017a, 2017b; Lee et al., 2019a). For instance, we found increased gray matter volume in somatosensory cortex at seven days post-HDBR compared to pre-HDBR, but by 12 days post-HDBR, this difference was no longer significant (Koppelmans et al., 2017a). Ideally, future work should include a post-flight measure immediately after landing (or in-flight metrics, if possible), followed by a series of post-flight measures to better track recovery and readaptation to Earth.
6. Parallels between design and interpretation of aging and spaceflight research
Aging is associated with progressive declines in brain structure and function (Seidler et al., 2010). Like spaceflight, however, there is evidence indicating that age-related declines are partially offset by concomitant neuroplastic and neural compensatory processes. In this section, we review several well-documented age-related effects on the brain and highlight relevant parallels with the effects of spaceflight. Our intention is not to convey that spaceflight has aging-like effects on the brain, but rather to illustrate that concomitant declines and adaptive plasticity accompany both conditions. Thus, approaches used to disentangle these co-constructive processes in aging may also be instructive for advancing our understanding of spaceflight effects on the central nervous system.
Various frameworks have been proposed to conceptualize how dedifferentiation and compensation co-occur and interact in aging, including the Scaffolding Theory of Aging and Compensation (STAC) and STAC-r, which incorporates life course / longitudinal influences on aging (Park and Reuter-Lorenz, 2009; Reuter-Lorenz and Park, 2014). Relative to younger adults, older adults are impacted by “neural challenges” (e.g., structural brain declines such as cortical thinning and atrophy), as well as functional deterioration—maladaptive brain activity changes including dedifferentiation. Both STAC and STAC-r propose that “compensatory scaffolding”, which includes neural mechanisms (e.g., adaptive increased frontal recruitment and bilateral processing), as well as external factors such as new learning, cognitive training, and exercise, can lessen the impacts of these age-related brain changes and ultimately slow cognitive decline. Thus these models explain how the interplay between age-related brain declines and internal, as well as external, enriching and depleting factors contribute to cognitive function and rate of cognitive decline.
6.1. Evidence for central nervous system dysfunction with aging
Older adults often have more diffuse and more bilateral brain activity when performing the same tasks as younger adults (Cabeza et al., 2002; Park et al., 2004). When this overactivation is not related to performance or associated with poorer behavioral performance, it is termed “neural dedifferentiation”. This process, which is assumed to be detrimental, has been demonstrated across multiple systems (Park et al., 2004; Dennis and Cabeza, 2011; Bernard and Seidler, 2012; St-Laurent et al., 2014). We have identified motor dedifferentiation with aging such that older adults show an association between increased motor representation size and slower reaction time (Bernard and Seidler, 2012), as well as a relationship between greater ipsilateral motor cortex recruitment during a unimanual task and poorer task performance (Langan et al., 2010; Bernard and Seidler, 2012). Thus, there is clear evidence for declining neural efficiency with aging.
6.2. Evidence for central nervous system adaptive changes with aging
However, in addition to dedifferentiation, concurrent compensatory processes are also evident in the aging brain. The Posterior-Anterior Shift in Aging (PASA) is one hypothesized phenomenon that captures the interplay of neural dysfunction and compensation with older age (Davis et al., 2008). Aging is associated with a reduction in posterior brain activity paired with a concurrent increase in frontal brain activity (Davis et al., 2008). This decreased posterior activity may represent age-related declines in sensorimotor processing, while the increased frontal activity appears to compensate for these declines. As the magnitude of posterior decreases have been found to correlate with anterior increases, this suggests a direct link between these two processes (Davis et al., 2008). The PASA pattern holds for a variety of tasks, such as attention (Cabeza et al., 2004; Ansado et al., 2012), memory (Rypma and D’Esposito, 2000; Davis et al., 2008), and visual perception tasks (Grady et al., 1994; Davis et al., 2008), and does not seem to relate to task difficulty (Davis et al., 2008).
Similarly, older adults show decreased lateralization of brain activity during cognitive (Cabeza, 2002; Mattay et al., 2006; Cappell et al., 2010) and motor tasks (Mattay et al., 2002; Ward and Frackowiak, 2003; Naccarato et al., 2006; Heuninckx et al., 2008). Several studies have noted that better old adult performers exhibit greater brain activation levels than poorer performers (Heuninckx et al., 2008). The Hemispheric Asymmetry Reduction in Older Adults (HAROLD; Cabeza, 2002) framework suggests that this increased bilateral recruitment represents age-related neural compensation. That is, older adults may recruit more neural resources as compensation, particularly at greater levels of task difficulty, thereby allowing them to perform with similar proficiency as younger adults. Such findings also fit within the Compensation-Related Utilization of Neural Circuits Hypothesis (CRUNCH), which emphasizes that the level or extent of brain activity can vary based on the task difficulty (Reuter-Lorenz and Cappell, 2008). As difficulty increases, brain activity will increase to support task demands, until reaching capacity limits, at which point brain activity either levels off or decreases and behavioral performance also declines (Reuter-Lorenz and Cappell, 2008). Thus, while healthy older adults may be able to adequately perform complex cognitive and motor tasks, this appears to require greater levels and extent of brain activity, and may not be sustainable at the highest levels of task difficulty. Taken together, the PASA, HAROLD, and CRUNCH models exemplify how the healthy aging brain seems to employ functional neural compensatory mechanisms to directly counteract some of the neurobehavioral dysfunction and inefficiency associated with aging.
We suggest that the central neural effects of spaceflight can be conceptualized in a similar manner. That is, we hypothesize that microgravity can have adverse effects on brain structure and function, that are accompanied by adaptive neural responses. For example, as noted in Section 4.3.2.2, we have found some evidence for adaptive structural plasticity post-flight (Koppelmans et al., 2017a). However, the relative lack of functional neuroimaging studies with astronauts to date makes it unclear how functional brain activity adapts during and after spaceflight. Given that neuroplasticity is evident even in much older age (Vance and Crowe, 2006), it is likely that adaptive plasticity processes are also possible in middle age (i.e., the age range of most astronauts). The ability of the central nervous system to adaptively reorganize is critical for responding to environmental perturbations (Sharma et al., 2013); thus it is likely that compensatory neural processes are at work during and after spaceflight. This proposal is further supported by evidence of neural compensation in our HDBR work (Cassady et al., 2016; Yuan et al., 2018a). Further studies are needed to more specifically understand the interplay of neural dysfunction and plasticity / compensation with microgravity exposure and recovery.
7. Conclusions and future directions
Based on the evidence available to date, we propose that spaceflight effects on the brain represent both adaptations as well as impairments. The altered sensory inputs of microgravity provide a unique environment for inducing adaptive plasticity in the central nervous system. These adaptations occur concomitantly with structural and functional neural impairments, including those resulting from altered fluid distribution with flight. While many pre-flight, in-flight, and post-flight factors likely influence the time course, extent, and recovery of these structural and functional brain changes, we posit that these factors can be better identified, measured, and potentially modeled to advance our understanding of human spaceflight.
Going forward, a comprehensive understanding of brain adaptations and dysfunctions with spaceflight and post-flight deadaptation / recovery, will require more thorough measurement of spaceflight factors and more extensive examination of brain-behavior correlations. For instance, future work should provide quantitative measures of radiation exposure to better assess the relationship between radiation and the brain. Further, quantitative measures of exercise (e.g., intensity, form, and schedule), stress (e.g., psychological scales and cortisol measures), and emotion (e.g., quantification of spaceflight euphoria, worry, and homesickness) should be obtained and examined for potential associations with brain changes during spaceflight.
While it is feasible to study functional brain changes during spaceflight using EEG, the results of such studies require careful interpretation in light of brain position shifts, fluid redistribution (Koppelmans et al., 2017a; Roberts et al., 2017) and recording confounds in the ISS environment (Niedermeyer and da Silva, 2005). Brain position shifts are particularly problematic for spaceflight EEG studies using evoked potentials or source localization whether recordings are conducted in-flight or post-flight (Cebolla et al., 2016). Another promising technology for studying brain changes in-flight is functional near-infrared spectroscopy (fNIRS); recent work has shown that collecting fNIRS data is possible in altered gravitational environments, although such technology is still under development (Strangman et al., 2018). Both EEG and fNIRs are also limited in their spatial resolution; thus, while each could provide useful information regarding in-flight brain changes, their clear limitations also should be recognized. Despite these limitations, in-flight measures are not confounded by readaptation processes that will rapidly occur upon return to Earth. That is, even MRI measures collected within several hours of return will likely reflect readaptation to Earth’s gravitational environment, which could complicate interpretations of both functional and structural brain metrics.
It is worth noting that, as space tourism becomes more prevalent, the profile of space travelers will change. Although astronaut classes are becoming more diverse, progress is slow. For example, less than 15 percent of all space travelers as of December 2019 have been female. This limits the range and generalizability of the neurobehavioral changes with spaceflight we have identified. Further, the potential neuroprotective factors resulting from typical astronaut life course experiences and training may not exist for many space tourists. Thus, brain changes due to space tourism are likely to differ from brain changes seen with astronauts and warrant consideration as the space tourism industry progresses.
Taken together, these considerations suggest the need for future research that includes a combination of in-flight brain measures, MRI scans at multiple time points pre- and post-flight, and comprehensive behavioral assessments (Roberts et al., 2020). These advances will permit more accurate mapping of neural changes to the time course of spaceflight, post-flight de-adaptation and recovery, along with a better understanding of how these neural changes affect human performance.
Acknowledgments
The authors would like to acknowledge the members of the University of Florida Neuromotor Behavior Lab, especially Lauren Banker, M.S. and Ana Paula Salazar, PhD, for their constructive discussions of this work.
Funding
During completion of this work KH was supported by training grant T32-NS082128 from the National Institute of Neurological Disorders and Stroke (NINDS), as well as a National Science Foundation (NSF) Graduate Research Fellowship under grants DGE-1315138 and DGE-1842473. HM was supported by a Natural Sciences and Engineering Research Council of Canada (NSERC) postdoctoral fellowship. RS was supported by NASA grants 80NSSC17K0021, 80NSSC18K0783, and 80NSSC17K0461.
Footnotes
Declaration of Competing Interest
The authors report no declarations of interest.
In our HDBR work, subjects are supine in the MRI scanner at all time points pre-, during-, and post-HDBR. Subjects do not maintain the −6 degrees head-down-tilt within the MRI head coil. Thus, findings of differing brain activation while subjects are in HDBR are not due to the physical positioning of the head during scanning.
References
- Adami R, Pagano J, Colombo M, Platonova N, Recchia D, Chiaramonte R, Bottinelli R, Canepari M, Bottai D, 2018. Reduction of movement in neurological diseases: effects on neural stem cells characteristics. Front. Neurosci 12, 336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alperin N, Bagci AM, 2018. Spaceflight-induced visual impairment and globe deformations in astronauts are linked to orbital cerebrospinal fluid volume increase. Acta Neurochir. Suppl 126, 215–219. [DOI] [PubMed] [Google Scholar]
- Alperin N, Bagci AM, Lee SH, 2017. Spaceflight-induced changes in white matter hyperintensity burden in astronauts. Neurology 89, 2187–2191. [DOI] [PubMed] [Google Scholar]
- Ansado J, Monchi O, Ennabil N, Faure S, Joanette Y, 2012. Load-dependent posterior-anterior shift in aging in complex visual selective attention situations. Brain Res. 1454, 14–22. [DOI] [PubMed] [Google Scholar]
- Barger LK, Flynn-Evans EE, Kubey A, Walsh L, Ronda JM, Wang W, Wright KP Jr., Czeisler CA, 2014. Prevalence of sleep deficiency and use of hypnotic drugs in astronauts before, during, and after spaceflight: an observational study. Lancet Neurol. 13, 904–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basner M, Rao H, Goel N, Dinges DF, 2013. Sleep deprivation and neurobehavioral dynamics. Curr. Opin. Neurobiol 23, 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Celle S, Roche F, Bartha R, Montero-Odasso M, Allali G, Annweiler C, 2013. Blood pressure levels and brain volume reduction: a systematic review and meta-analysis. J. Hypertens 31, 1502–1516. [DOI] [PubMed] [Google Scholar]
- Bernard JA, Seidler RD, 2012. Evidence for motor cortex dedifferentiation in older adults. Neurobiol. Aging 33, 1890–1899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beven G, Holland A, Sipes W, 2008. Psychological support for U.S. Astronauts on the International Space Station. Space Med. Assoc. News 79 (12), 1124. [PubMed] [Google Scholar]
- Bloomberg JJ, Mulavara AP, 2003. Changes in walking strategies after spaceflight. IEEE Eng. Med. Biol. Mag 22, 58–62. [DOI] [PubMed] [Google Scholar]
- Bock O, Weigelt C, Bloomberg JJ, 2010. Cognitive demand of human sensorimotor performance during an extended space mission: a dual-task study. Aviat. Space Environ. Med 81, 819–824. [DOI] [PubMed] [Google Scholar]
- Bondar RL, Kassam MS, Stein F, Dunphy PT, 1993. Cerebrovascular response to standing post spaceflight. Aviat. Space Environ. Med 64, 430. [Google Scholar]
- Boyle R, Mensinger AF, Yoshida K, Usui S, Intravaia A, Tricas T, Highstein SM, 2001. Neural readaptation to Earth’s gravity following return from space. J. Neurophysiol 86, 2118–2122. [DOI] [PubMed] [Google Scholar]
- Bright P, Filippi R, 2019. Editorial: perspectives on the “Bilingual advantage”: challenges and opportunities. Front. Psychol 10, 1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K, 2020. Thousands Apply to Join NASA’s Artemis Generation, #BeAnAstronaut. NASA: Humans in Space. Available at: https://www.nasa.gov/press-release/thousands-apply-to-join-nasa-s-artemis-generation-beanastronaut [Accessed August 13, 2020]. [Google Scholar]
- Buckey JC, Lane LD, Levine BD, Watenpaugh DE, Wright SJ, Moore WE, Gaffney FA, Blomqvist CG, 1996. Orthostatic intolerance after spaceflight. J App Physiol 81, 7–18. [DOI] [PubMed] [Google Scholar]
- Butz M, Wörgötter F, van Ooyen A, 2009. Activity-dependent structural plasticity. Brain Res. Rev 60, 287–305. [DOI] [PubMed] [Google Scholar]
- Cabeza R, 2001. Cognitive neuroscience of aging: contributions of functional neuroimaging. Scand. J. Psychol 42, 277–286. [DOI] [PubMed] [Google Scholar]
- Cabeza R, 2002. Hemispheric asymmetry reduction in older adults: the HAROLD model. Psychol. Aging 17, 85–100. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Anderson ND, Locantore JK, McIntosh AR, 2002. Aging gracefully: compensatory brain activity in high-performing older adults. Neuroimage 17, 1394–1402. [DOI] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L, 2004. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb. Cortex 14, 364–375. [DOI] [PubMed] [Google Scholar]
- Cappell KA, Gmeindl L, Reuter-Lorenz PA, 2010. Age differences in prefontal recruitment during verbal working memory maintenance depend on memory load. Cortex 46, 462–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassady K, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Wood S, Castenada RR, Kofman I, Bloomberg J, Mulavara A, Seidler R, 2016. Effects of a spaceflight analog environment on brain connectivity and behavior. Neuroimage 141, 18–30. [DOI] [PubMed] [Google Scholar]
- Cebolla AM, Petieau M, Dan B, Balazs L, McIntyre J, Cheron G, 2016. Cerebellar contribution to visuo-attentional alpha rhythm: insights from weightlessness. Sci. Rep 6, 37824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y, 2014. Reorganization and plastic changes of the human brain associated with skill learning and expertise. Front. Hum. Neurosci 8, 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charles JB, Frey MA, Fritsch-Yelle JM, William Fortner G, 1996. Cardiovascular and cardiorespiratory function. Chapter 3. Space Biology and Medicine - Volume III Books 1 & 2 - Humans in Spaceflight, pp. 63–88. [Google Scholar]
- Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG, 1998. Mechanisms of cortical reorganization in lower-limb amputees. J. Neurosci 18, 3443–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheron G, Leroy A, De Saedeleer C, Bengoetxea A, Lipshits M, Cebolla A, Servais L, Dan B, Berthoz A, McIntyre J, 2006. Effect of gravity on human spontaneous 10-Hz electroencephalographic oscillations during the arrest reaction. Brain Res. 1121, 104–116. [DOI] [PubMed] [Google Scholar]
- Cheron G, Leroy A, Palmero-Soler E, De Saedeleer C, Bengoetxea A, Cebolla A-M, Vidal M, Dan B, Berthoz A, McIntyre J, 2014. Gravity influences top-down signals in visual processing. PLoS One 9 (1), e82371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clément G, Popov KE, Berthoz A, 1993. Effects of prolonged weightlessness on horizontal and vertical optokinetic nystagmus and optokinetic after-nystagmus in humans. Exp. Brain Res 94, 456–462. [DOI] [PubMed] [Google Scholar]
- Coffey CE, Saxton JA, Ratcliff G, Bryan RN, Lucke JF, 1999. Relation of education to brain size in normal aging: implications for the reserve hypothesis. Neurology 53, 189–196. [DOI] [PubMed] [Google Scholar]
- Cohen HS, Kimball KT, Mulavara AP, Bloomberg JJ, Paloski WH, 2012. Posturography and locomotor tests of dynamic balance after long-duration spaceflight. J. Vestib. Res 22, 191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D’Amelio F, Fox RA, Wu LC, Daunton NG, Corcoran ML, 1998. Effects of microgravity on muscle and cerebral cortex: a suggested interaction. Adv. Space Res 22, 235–244. [DOI] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Daselaar SM, Fleck MS, Cabeza R, 2008. Que PASA? The posterior-anterior shift in aging. Cereb. Cortex 18, 1201–1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Saedeleer C, Vidal M, Lipshits M, Bengoetxea A, Cebolla AM, Berthoz A, Cheron G, McIntyre J, 2013. Weightlessness alters up/down asymmetries in the perception of self-motion. Exp. Brain Res 226, 95–106. [DOI] [PubMed] [Google Scholar]
- Demertzi A, Van Ombergen A, Tomilovskaya E, Jeurissen B, Pechenkova E, Di Perri C, Litvinova L, Amico E, Rumshiskaya A, Rukavishnikov I, Sijbers J, Sinitsyn V, Kozlovskaya IB, Sunaert S, Parizel PM, Van de Heyning PH, Laureys S, Wuyts FL, 2016. Cortical reorganization in an astronaut’s brain after long-duration spaceflight. Brain Struct. Funct 221, 2873–2876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Cabeza R, 2011. Age-related dedifferentiation of learning systems: an fMRI study of implicit and explicit learning. Neurobiol. Aging 32, e17–e30, 2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott AR, Shea SA, Dijk DJ, Wyatt JK, Riel E, Neri DF, Czeisler CA, West JB, Prisk GK, 2001. Microgravity reduces sleep-disordered breathing in humans. Am. J. Respir. Crit. Care Med 164, 478–485. [DOI] [PubMed] [Google Scholar]
- Faggin BM, Nguyen KT, Nicolelis MA, 1997. Immediate and simultaneous sensory reorganization at cortical and subcortical levels of the somatosensory system. Proc Natl Acad Sci 94, 9428–9433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn-Evans EE, Barger LK, Kubey AA, Sullivan JP, Czeisler CA, 2016. Circadian misalignment affects sleep and medication use before and during spaceflight. NPJ Microgravity 2, 15019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folkow B, 1987. Structure and function of the arteries in hypertension. Am. Heart J 114, 938–948. [DOI] [PubMed] [Google Scholar]
- Gazenko OG, Genin AM, Egorov AD, 1981. Summary of medical investigations in the U.S.S.R. Manned space missions. Acta Astronaut. 8, 907–917. [DOI] [PubMed] [Google Scholar]
- Gorman JC, Martin MJ, Dunbar TA, Stevens RH, Galloway TL, Amazeen PG, Likens AD, 2016. Cross-level effects between neurophysiology and communication during team training. Hum. Factors 58, 181–199. [DOI] [PubMed] [Google Scholar]
- Grady CL, Maisog JM, Horwitz B, Ungerleider LG, Mentis MJ, Salerno JA, Pietrini P, Wagner E, Haxby JV, 1994. Age-related changes in cortical blood flow activation during visual processing of faces and location. J. Neurosci 14, 1450–1462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hackney KJ, Scott JM, Hanson AM, English KL, Downs ME, Ploutz-Snyder LL, 2015. The astronaut-athlete: optimizing human performance in space. J. Strength Cond. Res 29, 3531–3545. [DOI] [PubMed] [Google Scholar]
- Haslinger B, Erhard P, Altenmüller E, Hennenlotter A, Schwaiger M, Gräfin von Einsiedel H, Rummeny E, Conrad B, Ceballos-Baumann AO, 2004. Reduced recruitment of motor association areas during bimanual coordination in concert pianists. Hum. Brain Mapp 22, 206–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess G, Aizenman CD, Donoghue JP, 1996. Conditions for the induction of long-term potentiation in layer II/III horizontal connections of the rat motor cortex. J. Neurophysiol 75, 1765–1778. [DOI] [PubMed] [Google Scholar]
- Heuninckx S, Wenderoth N, Swinnen SP, 2008. Systems neuroplasticity in the aging brain: recruiting additional neural resources for successful motor performance in elderly persons. J. Neurosci 28, 91–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillman CH, Erickson KI, Kramer AF, 2008. Be smart, exercise your heart: exercise effects on brain and cognition. Nat. Rev. Neurosci 9, 58–65. [DOI] [PubMed] [Google Scholar]
- Hoffmann U, Schöllmann C, Wackerhage H, Leyk D, Wenzel J, 1998. Effects of chronically increased ambient CO2 concentrations on aerobic capacity. Aviat. Space Environ. Med 69, 397–402. [PubMed] [Google Scholar]
- Holstein GR, Kukielka E, Martinelli GP, 1999. Anatomical observations of the rat cerebellar nodulus after 24 hr of spaceflight. J. Gravit. Physiol 6, P47–P50. [PubMed] [Google Scholar]
- Hughson RL, Yee NJ, Greaves DK, 2016. Elevated end-tidal PCO during long-duration spaceflight. Aerosp. Med. Hum. Perform 87, 894–897. [DOI] [PubMed] [Google Scholar]
- Hupfeld KE, Lee JK, Gadd NE, Kofman IS, De Dios YE, Bloomberg JJ, Mulavara AP, Seidler RD, 2020a. Neural correlates of vestibular processing during a spaceflight analog with elevated carbon dioxide (CO2): a pilot study. Front. Syst. Neurosci 13, 80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hupfeld KE, McGregor HR, Lee JK, Beltran NE, Kofman IS, De Dios YE, Reuter-Lorenz PA, Riascos RF, Pasternak O, Wood SJ, Bloomberg JJ, Mulavara, Seidler RD, 2020b. The impact of six and twelve months in space on human brain structure and intracranial fluid shifts. Cereb Cortex Comm. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keck T, Mrsic-Flogel TD, Vaz Afonso M, Eysel UT, Bonhoeffer T, Hübener M, 2008. Massive restructuring of neuronal circuits during functional reorganization of adult visual cortex. Nat. Neurosci 11, 1162–1167. [DOI] [PubMed] [Google Scholar]
- Kok BE, Coffey KA, Cohn MA, Catalino LI, Vacharkulksemsuk T, Algoe SB, Brantley M, Fredrickson BL, 2013. How positive emotions build physical health: perceived positive social connections account for the upward spiral between positive emotions and vagal tone. Psychol. Sci 24, 1123–1132. [DOI] [PubMed] [Google Scholar]
- Koppelmans V, Mulavara AP, Yuan P, Cassady KE, Cooke KA, Wood SJ, Reuter-Lorenz PA, De Dios YE, Stepanyan V, Szecsy DL, Gadd NE, Kofman I, Scott JM, Downs ME, Bloomberg JJ, Ploutz-Snyder L, Seidler RD, 2015. Exercise as potential countermeasure for the effects of 70 days of bed rest on cognitive and sensorimotor performance. Front. Syst. Neurosci 9, 121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Bloomberg JJ, Mulavara AP, Seidler RD, 2016. Brain structural plasticity with spaceflight. NPJ Microgravity 2 (1), 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Bloomberg JJ, De Dios YE, Wood SJ, Reuter-Lorenz PA, Kofman IS, Riascos R, Mulavara AP, Seidler RD, 2017a. Brain plasticity and sensorimotor deterioration as a function of 70 days head down tilt bed rest. PLoS One 12, e0182236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koppelmans V, Pasternak O, Bloomberg JJ, Dios YED, Wood SJ, Riascos R, Reuter-Lorenz PA, Kofman IS, Mulavara AP, Seidler RD, 2017b. Intracranial fluid redistribution but no white matter microstructural changes during a spaceflight analog. Sci. Rep 7, 3154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordi M, Beck A, Damann V, Godard B, Kluge G, Pruett C, Trammer M, Evetts S, 2013. Fitness, blood, and urine measurements from the 2009 European astronaut selection medical examination. Aviat. Space Environ. Med 84, 1286–1290. [DOI] [PubMed] [Google Scholar]
- Kornilova LN, Kreĭdich Iu V., Tarasov IK, Iakovleva I.Ia, 1983. Opto-kinetic nystagmus and optokinetic resistance of cosmonauts in preflight and postflight periods. Kosm. Biol. Aviakosm. Med 17 (4), 12–15. [PubMed] [Google Scholar]
- Kramer LA, Hasan KM, Stenger MB, Sargsyan A, Laurie SS, Otto C, Ploutz-Snyder RJ, Marshall-Goebel K, Riascos RF, Macias BR, 2020. Intracranial effects of microgravity: a prospective longitudinal MRI study. Radiology 295, 640–648. [DOI] [PubMed] [Google Scholar]
- Krasnov IB, 1994. Gravitational neuromorphology. Chapter 4 Advances in Space Biology and Medicine, pp. 85–110. [PubMed] [Google Scholar]
- Langan J, Peltier S, Bo J, Fling BW, Welsh RC, Seidler RD, 2010. Functional implications of age differences in motor system connectivity. Front Sys Neuro 4, 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J, Van Baalen M, Foy M, Mason SS, Mendez C, Wear ML, Meyers VE, Alexander D, 2014. Relationship between carbon dioxide levels and reported headaches on the international space station. J. Occup. Environ. Med 56, 477–483. [DOI] [PubMed] [Google Scholar]
- Layne CS, Mulavara AP, McDonald PV, Pruett CJ, Kozlovskaya IB, Bloomberg JJ, 2001. Effect of long-duration spaceflight on postural control during self-generated perturbations. J. Appl. Physiol 90, 997–1006. [DOI] [PubMed] [Google Scholar]
- Leach CS, 1979. A review of the consequences of fluid and electrolyte shifts in weightlessness. Acta Astronaut. 6, 1123–1135. [DOI] [PubMed] [Google Scholar]
- Lee JK, De Dios Y, Kofman I, Mulavara AP, Bloomberg JJ, Seidler RD, 2019a. Head down tilt bed rest plus elevated CO2 as a spaceflight analog: effects on cognitive and sensorimotor performance. Front. Hum. Neuro 13, 355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JK, Koppelmans V, Riascos RF, Hasan KM, Pasternak O, Mulavara AP, Bloomberg JJ, Seidler RD, 2019b. Spaceflight-associated brain white matter microstructural changes and intracranial fluid redistribution. JAMA Neurol. 76 (4), 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee AG, Tarver WJ, Mader TH, Gibson CR, Hart SF, Otto CA, 2016. Neuro-ophthalmology of space flight. J. Neuroophthalmol 36 (1), 85–91. 10.1097/WNO.0000000000000334. [DOI] [PubMed] [Google Scholar]
- Li SC, Lindenberger U, 1999. Cross-level unification: a computational exploration of the link between deterioration of neurotransmitter systems and dedifferentiation of cognitive abilities in old age. Cognitive Neuroscience of Memory. Hogrefe & Huber, pp. 103–146. [Google Scholar]
- Lowrey CR, Perry SD, Strzalkowski NDJ, Williams DR, Wood SJ, Bent LR, 2014. Selective skin sensitivity changes and sensory reweighting following short-duration space flight. J. Appl. Physiol 116, 683–692. [DOI] [PubMed] [Google Scholar]
- Lupien SJ, McEwen BS, Gunnar MR, Heim C, 2009. Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat. Rev. Neurosci 10, 434–445. [DOI] [PubMed] [Google Scholar]
- Mader T, Gibson CR, Pass AF, Lee AG, Killer HE, Hansen HC, Dervay JP, Barratt MR, Tarver WJ, Sargsyan AE, Kramer LA, Riascos R, Bedi DG, Pettit DR, 2013. Optic disc edema in astronaut after repeat long-duration space flight. J. Neuroophthalmol 33 (3), 249–255. [DOI] [PubMed] [Google Scholar]
- Maillard P, Seshadri S, Beiser A, Himali JJ, Au R, Fletcher E, Carmichael O, Wolf PA, DeCarli C, 2012. Effects of systolic blood pressure on white-matter integrity in young adults in the Framingham Heart Study: a cross-sectional study. Lancet Neurol. 11, 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manzey D, Lorenz B, 1998. Joint NASA-ESA-DARA Study. Part three: Effects of chronically elevated CO2 on mental performance during 26 days of confinement. Aviat. Space Environ. Med 69, 506–514. [PubMed] [Google Scholar]
- Manzey D, Lorenz B, Schiewe A, Finell G, Thiele G, 1993. Behavioral aspects of human adaptation to space: analyses of cognitive and psychomotor performance in space during an 8-day space mission. Clin. Investig 71, 725–731. [DOI] [PubMed] [Google Scholar]
- Manzey D, Lorenz B, Schiewe A, Finell G, Thiele G, 1995. Dual-task performance in space: results from a single-case study during a short-term space mission. Hum. Factors 37, 667–681. [DOI] [PubMed] [Google Scholar]
- Manzey D, Lorenz B, Poljakov V, 1998. Mental performance in extreme environments: results from a performance monitoring study during a 438-day spaceflight. Ergonomics 41, 537–559. [DOI] [PubMed] [Google Scholar]
- Manzey D, Lorenz TB, Heuers H, Sangals J, 2000. Impairments of manual tracking performance during spaceflight: more converging evidence from a 20-day space mission. Ergonomics 43, 589–609. [DOI] [PubMed] [Google Scholar]
- Mao QW, Zhang LF, Zhang LN, Ma J, 1999. Ultrastructural changes of arterial wall from different body parts of rats during simulated weightlessness. Space Med. Med. Eng 12, 249–253. [PubMed] [Google Scholar]
- Mårtensson J, Eriksson J, Bodammer NC, Lindgren M, Johansson M, Nyberg L, Lövdén M, 2012. Growth of language-related brain areas after foreign language learning. Neuroimage 63, 240–244. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Das S, Callicott JH, Weinberger DR, 2002. Neurophysiological correlates of age-related changes in human motor function. Neurology 58, 630–635. [DOI] [PubMed] [Google Scholar]
- Mattay VS, Fera F, Tessitore A, Hariri AR, Berman KF, Das S, Meyer-Lindenberg A, Goldberg TE, Callicott JH, Weinberger DR, 2006. Neurophysiological correlates of age-related changes in working memory capacity. Neurosci. Lett 392, 32–37. [DOI] [PubMed] [Google Scholar]
- Maulsby RL, 1966. Electroencephalogram during orbital flight. Aerosp. Med 37, 1022–1026. [PubMed] [Google Scholar]
- Meeusen R, 2014. Exercise, nutrition and the brain. Sport. Med 44, 47–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CA, Peters BT, Brady RR, Richards JR, Ploutz-Snyder RJ, Mulavara AP, Bloomberg JJ, 2010. Changes in toe clearance during treadmill walking after long-duration spaceflight. Aviat. Space Environ. Med 81, 919–928. [DOI] [PubMed] [Google Scholar]
- Moore TP, Thornton WE, 1987. Space shuttle inflight and postflight fluid shifts measured by leg volume changes. Aviat. Space Environ. Med 58, A91–A96. [PubMed] [Google Scholar]
- Mulavara AP, Feiveson AH, Fiedler J, Cohen H, Peters BT, Miller C, Brady R, Bloomberg JJ, 2010. Locomotor function after long-duration space flight: effects and motor learning during recovery. Exp. Brain Res 202, 649–659. [DOI] [PubMed] [Google Scholar]
- Mulavara AP, Peters BT, Miller CA, Kofman IS, Reschke MF, Taylor LC, Lawrence EL, Wood SJ, Laurie SS, Lee SMC, Buxton RE, May-Phillips TR, Stenger MB, Ploutz-Snyder LL, Ryder JW, Feiveson AH, Bloomberg JJ, 2018. Physiological and functional alterations after spaceflight and bed rest. Med. Sci. Sports Exerc 50, 1961–1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naccarato M, Calautti C, Jones PS, Day DJ, Carpenter TA, Baron J-C, 2006. Does healthy aging affect the hemispheric activation balance during paced index-to-thumb opposition task? An fMRI study. Neuroimage 32, 1250–1256. [DOI] [PubMed] [Google Scholar]
- Newberg AB, 1994. Changes in the central nervous system and their clinical correlates during long-term spaceflight. Aviat. Space Environ. Med 65, 562–572. [PubMed] [Google Scholar]
- Niedermeyer E, da Silva FHL, 2005. Electroencephalography: Basic Principles, Clinical Applications, and Related Fields. Lippincott Williams & Wilkins. [Google Scholar]
- Ozdemir RA, Goel R, Reschke MF, Wood SJ, Paloski WH, 2018. Critical role of somatosensation in postural control following spaceflight: vestibularly deficient astronauts are not able to maintain upright stance during compromised somatosensation. Front. Physiol 9, 1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagel JI, Choukèr A, 2016. Effects of isolation and confinement on humans-implications for manned space explorations. J. Appl. Physiol 120, 1449–1457. [DOI] [PubMed] [Google Scholar]
- Paloski WH, Reschke MF, Black FO, Doxey DD, Harm DL, 1992. Recovery of postural equilibrium control following spaceflight. Ann. N. Y. Acad. Sci 656, 747–754. [DOI] [PubMed] [Google Scholar]
- Paloski WH, Black FO, Reschke MF, Calkins DS, Shupert C, 1993. Vestibular ataxia following shuttle flights: effects of microgravity on otolith-mediated sensorimotor control of posture. Am. J. Otol 14, 9–17. [PubMed] [Google Scholar]
- Paloski WH, Bloomberg JJ, Reschke MF, Harm DL, 1994. Spaceflight-induced changes in posture and locomotion. J. Biomech 27, 812. [Google Scholar]
- Paloski WH, Reschke MF, Black FO, Dow RS, 1999. Recovery of Postural Equilibrium Control Following Space Flight. NASA. [DOI] [PubMed] [Google Scholar]
- Park DC, Reuter-Lorenz P, 2009. The adaptive brain: aging and neurocognitive scaffolding. Annu. Rev. Psychol 60, 173–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park DC, Polk TA, Park R, Minear M, Savage A, Smith MR, 2004. Aging reduces neural specialization in ventral visual cortex. Proc. Natl. Acad. Sci 101, 13091–13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pasternak O, Sochen N, Gur Y, Intrator N, Assaf Y, 2009. Free water elimination and mapping from diffusion MRI. Magn. Reson. Med 62, 717–730. [DOI] [PubMed] [Google Scholar]
- Pechenkova E, Nosikova I, Rumshiskaya A, Litvinova L, Rukavishnikov I, Mershina E, Sinitsyn V, Van Ombergen A, Jeurissen B, Jillings S, Laureys S, Sijbers J, Grishin A, Chernikova L, Naumov I, Kornilova L, Wuyts FL, Tomilovskaya E, Kozlovskaya I, 2019. Alterations of functional brain connectivity after long-duration spaceflight as revealed by fMRI. Front. Physiol 10, 761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen LG, Lawley JS, Lilja-Cyron A, Petersen JCG, Howden EJ, Sarma S, Cornwell WK 3rd, Zhang R, Whitworth LA, Williams MA, Juhler M, Levine BD, 2019. Lower body negative pressure to safely reduce intracranial pressure. J. Physiol 597, 237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petit G, Cebolla AM, Fattinger S, Petieau M, Summerer L, Cheron G, Huber R, 2019. Local sleep-like events during wakefulness and their relationship to decreased alertness in astronauts on ISS. NPJ Microgravity 5, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfurtscheller G, Stancák A, Neuper C, 1996. Event-related synchronization (ERS) in the alpha band—an electrophysiological correlate of cortical idling: A review. Int. J. Psychophysiol 24, 39–46. [DOI] [PubMed] [Google Scholar]
- Pons TP, Garraghty PE, Ommaya AK, Kaas JH, Taub E, Mishkin M, 1991. Massive cortical reorganization after sensory deafferentation in adult macaques. Science 252, 1857–1860. [DOI] [PubMed] [Google Scholar]
- Prisniakova LM, 2004. Fitness to work of astronauts in conditions of action of the extreme emotional factors. Adv. Space Res 33, 1381–1385. [DOI] [PubMed] [Google Scholar]
- Reschke MF, Bloomberg JJ, Harm DL, Paloski WH, Layne C, McDonald V, 1998. Posture, locomotion, spatial orientation, and motion sickness as a function of space flight. Brain Res. Brain Res. Rev 28, 102–117. [DOI] [PubMed] [Google Scholar]
- Reschke MF, Bloomberg JJ, Paloski WH, Mulavara AP, Feiveson AH, Harm DL, 2009. Postural reflexes, balance control, and functional mobility with long-duration head-down bed rest. Aviat. Space Environ. Med 80, A45–A54. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Cappell KA, 2008. Neurocognitive aging and the compensation hypothesis. Curr. Dir. Psychol. Sci 17, 177–182. [Google Scholar]
- Reuter-Lorenz PA, Lustig C, 2005. Brain aging: reorganizing discoveries about the aging mind. Curr. Opin. Neurobiol 15, 245–251. [DOI] [PubMed] [Google Scholar]
- Reuter-Lorenz PA, Park DC, 2014. How does it STAC up? Revisiting the scaffolding theory of aging and cognition. Neuropsychol. Rev 24, 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riascos RF, Kamali A, Hakimelahi R, Mwangi B, Rabiei P, Seidler RD, Behzad BB, Keser Z, Kramer LA, Hasan KM, 2019. Longitudinal analysis of quantitative brain MRI in astronauts following microgravity exposure. J. Neuroimaging 29, 323–330. [DOI] [PubMed] [Google Scholar]
- Riecker A, Gröschel K, Ackermann H, Steinbrink C, Witte O, Kastrup A, 2006. Functional significance of age-related differences in motor activation patterns. Neuroimage 32, 1345–1354. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Ricci R, Funke FW, Ramsey P, Kelley W, Carroll JS, Ramsey D, Borckardt JJ, Johnson K, George MS, 2007. Lower limb immobilization is associated with increased corticospinal excitability. Exp. Brain Res 181, 213–220. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Stahn AC, Seidler RD, Wuyts FL, 2020. Towards understanding the effects of spaceflight on the brain. Lancet Neurol. 19 (10), 808. [DOI] [PubMed] [Google Scholar]
- Roberts DR, Zhu X, Tabesh A, Duffy EW, Ramsey DA, Brown TR, 2015. Structural brain changes following long-term 6° head-down tilt bed rest as an analog for spaceflight. AJNR Am. J. Neuroradiol 36, 2048–2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DR, Albrecht MH, Collins HR, Asemani D, Chatterjee AR, Spampinato MV, Zhu X, Chimowitz MI, Antonucci MU, 2017. Effects of spaceflight on astronaut brain structure as indicated on MRI. N. Engl. J. Med 377, 1746–1753. [DOI] [PubMed] [Google Scholar]
- Ross H, Brodie E, Benson A, 1984. Mass discrimination during prolonged weightlessness. Science 225, 219–221. [DOI] [PubMed] [Google Scholar]
- Rypma B, D’Esposito M, 2000. Isolating the neural mechanisms of age-related changes in human working memory. Nat. Neurosci 3, 509–515. [DOI] [PubMed] [Google Scholar]
- Salazar AP, Hupfeld KE, Lee JK, Beltran NE, Kofman IS, De Dios YE, Mulder E, Bloomberg JJ, Mulavara AP, Seidler RD, 2020. Neural working memory changes during a spaceflight analog with elevated carbon dioxide: a pilot study. Front Sys Neuro 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, Kwak Y, Lipps DB, 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev 34, 721–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Classen J, Cohen LG, 2013. Neural plasticity and its contribution to functional recovery. Neurol. Rehabilitation 3–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sliwka U, Krasney JA, Simon SG, Schmidt P, Noth J, 1998. Effects of sustained low-level elevations of carbon dioxide on cerebral blood flow and autoregulation of the intracerebral arteries in humans. Aviat. Space Environ. Med 69, 299–306. [PubMed] [Google Scholar]
- Smith SM, Zwart SR, 2018. Spaceflight-related ocular changes: the potential role of genetics, and the potential of B vitamins as a countermeasure. Curr. Opin. Clin. Nutr. Metab. Care 21, 481–488. [DOI] [PubMed] [Google Scholar]
- Stahn AC, Gunga H-C, Kohlberg E, Gallinat J, Dinges DF, Kühn S, 2019. Brain changes in response to long antarctic expeditions. N. Engl. J. Med 381, 2273–2275. [DOI] [PubMed] [Google Scholar]
- Stalport F, Rouquette L, Poch O, Dequaire T, Chaouche-Mechidal N, Payart S, Szopa C, Coll P, Chaput D, Jaber M, Raulin F, Cottin H, 2019. The photochemistry on space station (PSS) experiment: organic matter under Mars-like surface UV radiation conditions in low Earth orbit. Astrobiology 19, 1037–1052. [DOI] [PubMed] [Google Scholar]
- St-Laurent M, Abdi H, Bondad A, Buchsbaum BR, 2014. Memory reactivation in healthy aging: evidence of stimulus-specific dedifferentiation. J. Neurosci 34, 4175–4186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoilova IM, Zdravev TK, Yanev TK, 2003. How human sleep in space—investigations during space flights. Adv. Space Res 31, 1611–1615. [DOI] [PubMed] [Google Scholar]
- Strangman GE, Ivkovic V, Zhang Q, 2018. Wearable brain imaging with multimodal physiological monitoring. J. Appl. Physiol 124, 564–572. [DOI] [PubMed] [Google Scholar]
- Strewe C, Choukèr A, 2012. Stressed out in Space – Langzeitmissionen als besondere Herausforderungen an menschliche Adaptationsmechanismen. Flugmedizin Tropenmedizin Reisemedizin FTR 19, 254–256. [Google Scholar]
- Van Ombergen A, Demertzi A, Tomilovskaya E, Jeurissen B, Sijbers J, Kozlovskaya IB, Parizel PM, Van de Heyning PH, Sunaert S, Laureys S, Wuyts FL, 2017a. The effect of spaceflight and microgravity on the human brain. J. Neurol 264, 18–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Ombergen A, Laureys S, Sunaert S, Tomilovskaya E, Parizel PM, Wuyts FL, 2017b. Spaceflight-induced neuroplasticity in humans as measured by MRI: What do we know so far? NPJ Microgravity 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taibbi G, Cromwell RL, Kapoor KG, Godley BF, Vizzeri G, 2013. The effect of microgravity on ocular structures and visual function: a review. Survey Ophthalmol. 58 (2), 155–163. [DOI] [PubMed] [Google Scholar]
- Van Ombergen A, et al. , 2019. Brain ventricular volume changes induced by long-duration spaceflight. Proc. Natl. Acad. Sci 116, 10531–10536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vance DE, Crowe M, 2006. A proposed model of neuroplasticity and cognitive reserve in older adults. Act. Adapt. Aging 30, 61–79. [Google Scholar]
- Ward NS, Frackowiak RSJ, 2003. Age-related changes in the neural correlates of motor performance. Brain 126, 873–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watenpaugh DE, Smith ML, 1998. Human cardiovascular acclimation to microgravity. J. Gravit. Physiol 5, P15–P18. [PubMed] [Google Scholar]
- Weerda R, Muehlhan M, Wolf OT, Thiel CM, 2010. Effects of acute psychosocial stress on working memory related brain activity in men. Hum. Brain Mapp 31, 1418–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch RB, Hoover M, Southward EF, 2009. Cognitive performance during prismatic displacement as a partial analogue of “Space Fog.”. Aviat. Space Environ. Med 80, 771–780. [DOI] [PubMed] [Google Scholar]
- Wirth M, Villeneuve S, La Joie R, Marks SM, Jagust WJ, 2014. Gene-environment interactions: lifetime cognitive activity, APOE genotype, and beta-amyloid burden. J. Neurosci 34, 8612–8617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood SJ, Fiedler J, Taylor LC, Kozlovskaya I, Black FO, Paloski WH, 2010. Comparison of Postural Recovery Following Short and Long Duration Spaceflights. NASA Technical Reports Server. [Google Scholar]
- Wood SJ, Loehr JA, Guilliams ME, 2011. Sensorimotor reconditioning during and after spaceflight. NeuroRehabilitation 29, 185–195. [DOI] [PubMed] [Google Scholar]
- Wood SJ, Paloski WH, Clark JB, 2015. Assessing sensorimotor function following ISS with computerized dynamic posturography. Aerosp. Med. Hum. Perform 86, 45–53. [DOI] [PubMed] [Google Scholar]
- Wu T, Hallett M, 2005. The influence of normal human ageing on automatic movements. J. Physiol 562, 605–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaden DB, Iwry J, Slack KJ, Eichstaedt JC, Zhao Y, Vaillant GE, Newberg AB, 2016. The overview effect: awe and self-transcendent experience in space flight. Psychol. Conscious. Theory Res. Pract 3, 1–11. [Google Scholar]
- Yates KF, Sweat V, Yau PL, Turchiano MM, Convit A, 2012. Impact of metabolic syndrome on cognition and brain. Arterioscler. Thromb. Vasc. Biol 32, 2060–2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young L, Oman C, Watt D, Money K, Lichtenberg B, 1984. Spatial orientation in weightlessness and readaptation to earth’s gravity. Science 225, 205–208. [DOI] [PubMed] [Google Scholar]
- Yuan P, Koppelmans V, Reuter-Lorenz PA, De Dios YE, Gadd NE, Wood SJ, Riascos R, Kofman IS, Bloomberg JJ, Mulavara AP, Seidler RD, 2016. Increased brain activation for dual tasking with 70-days head-down bed rest. Front. Syst. Neurosci 10, 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Riascos R, Kofman I, Bloomberg J, Mulavara A, Seidler RD, 2018a. Change of cortical foot activation following 70 days of head-down bed rest. J. Neurophysiol 119, 2145–2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan P, Koppelmans V, Reuter-Lorenz P, De Dios Y, Gadd N, Wood S, Riascos R, Kofman I, Bloomberg J, Mulavara A, Seidler R, 2018b. Vestibular brain changes within 70 days of head down bed rest. Hum. Brain Mapp 39, 2753–2763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahodne LB, Reuter-Lorenz PA, 2019. Compensation and brain aging: a review and analysis of evidence. The Aging Brain: Functional Adaptation Across Adulthood. American Psychological Association, pp. 185–216. [Google Scholar]
- Zhou H, Saidel GM, LaManna JC, 2008. Cerebral blood flow adaptation to chronic hypoxia. Adv. Exp. Med. Biol 371–377. [DOI] [PubMed] [Google Scholar]
- Zuj KA, Arbeille P, Shoemaker JK, Blaber AP, Greaves DK, Xu D, Hughson RL, 2012. Impaired cerebrovascular autoregulation and reduced CO2 reactivity after long duration spaceflight. Am. J. Physiol.-Heart Circul. Physiol 302, H2592–H2598. [DOI] [PubMed] [Google Scholar]
- Zwart SR, Gibson CR, Mader TH, Ericson K, Ploutz-Snyder R, Heer M, Smith SM, 2012. Vision changes after spaceflight are related to alterations in folate–and vitamin B-12–dependent one-carbon metabolism. J Nutrition 142 (3), 427–431. [DOI] [PubMed] [Google Scholar]
- Zwart SR, Gibson CR, Smith SM, 2014. Space flight ophthalmic changes, diet, and vitamin metabolism. Handbook of Nutrition, Diet and the Eye, pp. 393–399. [Google Scholar]
- Zwart SR, Gregory JF, Zeisel SH, Gibson CR, Mader TH, Kinchen JM, Ueland PM, Ploutz-Snyder R, Heer MA, Smith SM, 2016. Genotype, B-vitamin status, and androgens affect spaceflight-induced ophthalmic changes. FASEB J. 30, 141–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwart SR, Gibson CR, Gregory JF, Mader TH, Stover PJ, Zeisel SH, Smith SM, 2017. Astronaut ophthalmic syndrome. FASEB J. 31, 3746–3756. [DOI] [PubMed] [Google Scholar]