Abstract
Genetic, androgenic, epigenetic, environmental, lifestyle, diet, and nutritional factors have influence over promoting or regulating inflammation, immunity, and genetic expression predisposing to hair loss. Oxidative stress is a major mediator for various mechanisms of hair loss, including the release of transforming growth factor-β ( TGF-β). Nutrients counter oxidative stress, repair cellular damage, support cellular functions, and restore hair growth. Nutrients can be synergistic or antagonistic. Covert subclinical nutritional deficiencies are common. Higher dose of nutrients does not mean higher efficiency but may reverse the benefits converting an antioxidant to become prooxidant. Nutrients do not work alone but are supported by accessory micronutrients which ensure biological utilization. Providing proper nutritional environment can neutralize free radicals and perpetuate active hair growth cycles.
Keywords: Hair loss, lifestyle, nutrition, stem cells
Introduction
Hair loss is multifactorial. Antioxidants can block the DHT (dihydrotestosterone)-induced release of TGF-β (transforming growth factor-β), neutralize the reactive oxygen species (ROS), reduce micro-inflammation, build immunity, and improve altered genetic expression which are the main mechanisms of hair loss.[1,2,3,4,5,6,7,8,9] In addition to preventing hair loss, nutrients can induce active hair growth by supporting the high metabolic rate and rapid cell division required by anagen follicles. Hair loss management requires a reliable, consistent, effective, safe long-term care program, without side effects, which is cost effective and shows early improvement. All these criteria can be achieved with nutritional therapy.
Apart from genetics acquired lifestyle can predispose to hair loss
Genetic history predisposes to hair loss. However, prolonged exposure to stress, competitive lifestyle, aggressive fitness regimens, restricted eating, sleep disturbances, smoking, alcohol, pollution, ultraviolet (UV) rays, and electromagnetic radiation disturb the cell metabolism, homeostasis, and work as epigenetic factors influencing genetic expression and create an acquired predisposition to hair loss.[10] There are reports of biological twins expressing different grades of hair loss from exposure to environmental, lifestyle, and epigenetic factors.[11] Genetic research has identified the 5 alpha reductase genes (5AR) but has been unable to link them clinically with the incidence or progression of hair loss.[12,13] In another study, the genetic transfer has been identified as the mitochondrial cell metabolism, inherited completely from the mother’s cells.[14] Therefore, the genetic predisposition also can be hypothesized to be acting through cellular metabolism which is influenced directly by ROS and nutrients. Nutrients influence genetic expression. Several studies have established the influence of micronutrients on genetic expression, gene interaction, gene response to inflammation, immunity, antioxidant activity, and aging.[15] Two new branches of medicine have emerged. Nutrigenetics which studies the role of nutrients on DNA sequence, and nutrigenomics which studies the role of nutrients in gene expression.
Hair loss often presents from causes other than androgens
Nonandrogenic hair loss was observed since the inception of the concept of androgenetic alopecia (AGA). The nonandrogenic hair loss has been described as telogen effluvium (TE). Understanding the role of nutrition in hair loss is like revisiting TE. Any stressful event is followed by TE. Elimination of the causative factor allows spontaneous recovery from TE. Today the factors causing TE, like stress, pollution, lack of sleep, fitness obsession, overwork, restricted eating, smoking, alcohol, erratic lifestyle, environmental, and epigenetic factors, all continue unabated, with no relief, allowing no possibility for spontaneous recovery. There is a continuous derangement of cellular function and loss of internal cellular environment leading to dysregulation of the hair cycles.[10] Not only is the incidence of hair loss rising and occurring at early ages but also thyroid disorders, polycystic ovary syndrome (PCOS), cardiovascular disease, diabetes, and hypertension are all occurring at a younger age, from similar loss of internal environment or homeostasis. All these ailments are commonly associated in hair loss patients. Interestingly, hair loss has been observed to be predisposing to these ailments. Oxidative stress is responsible for these ailments and calls for early intervention to neutralize the free radicals with antioxidants, to repair the DNA and cell damage with amino acids and to restore cell function. Vitamins and minerals are important cofactors and catalysts in cellular metabolism. However, excess nutrients can be counterproductive.[14]
Nutritional supplements can correct hair loss presenting with normal androgens
Androgens are not the only factors responsible for hair loss. Pattern hair loss has been reported in complete androgen insensitivity syndrome.[16] Researchers have reported hair loss with normal DHT and found no correlation between circulating androgens and the incidence, severity, or progress of hair loss.[17] We hypothesize that nonandrogenic factors causing oxidative stress and loss of internal cellular environment have made the cells so weak that they are being affected by normal androgens and cannot recover by mere androgen blockers. Androgens may be termed as opportunistic intruders, affecting the weak follicles. Countering oxidative stress, supporting cell recovery with antioxidants and essential nutrients, can restore cellular functions and deliver active hair growth.[18]
Nutrients counter multiple mechanisms involving reactive oxygen species
Not DHT, but the DHT-induced release of TGF-β causes miniaturization and the release of TGF-β is mediated through ROS, which has been proven to be blocked by a free radical scavenger or antioxidants[1] [Figure 1]. Latent TGF-β binds to extra cellular matrix (ECM) proteins and is activated by ROS.[2] ROS bind to intracellular proteins, changing their immune signature, making them recognized as antigens, thus triggering autoimmune response and inflammation leading to hair loss.[3] The autoimmune response can thus be countered with antioxidants [Figure 2]. The hallmark of micro-inflammation, the periinfundibular lymphocytic infiltration, close to the epidermis, suggests the role of external factors, such as sebum, skin microbes, UV rays, pollution, and heavy metals, which modulate the transduction cascades and alter gene expression through ROS.[4] Even moderate, noncytotoxic, oxidative stress, can downregulate genetic expression.[5] Stress induced substance P inhibits hair shaft elongation and induces catagen through ROS.[6] All these ROS-mediated mechanisms can be prevented by the use of antioxidants and nutrition [Figure 3].
Figure 1.

DHT causes miniaturization through ROS-mediated release of TGF-β which can be blocked with the use of antioxidants thereby countering the action of DHT
Figure 2.
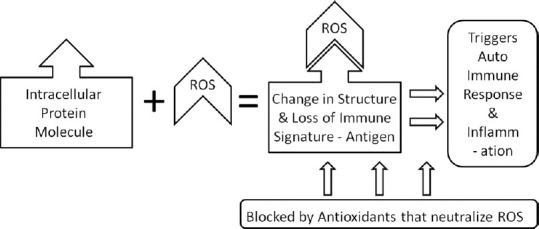
ROS bind intracellular proteins, altering their immune signature, making them act like antigens, triggering autoimmune response and inflammation
Figure 3.
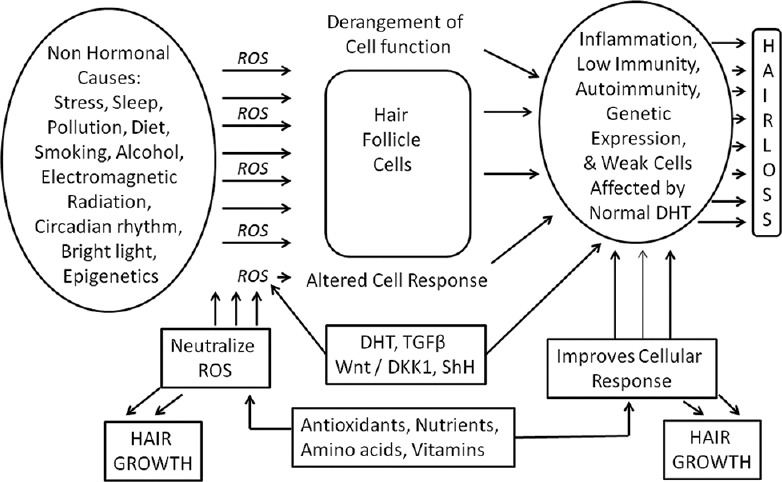
DHT and various mechanisms of hair loss are mediated through ROS. Nutrients and antioxidants can restore cellular function and promote hair growth
Nutrients have anti-inflammatory effect and build immune response
Omega 3 fatty acids and gamma linolenic acid (GLA) are anti-inflammatory.[7] GLA is precursor to anti-inflammatory prostaglandin-1. Fatty acids and polyphenols influence inflammatory gene transcription.[8] Tocotrienol and alpha-tocopherol lead to 34% better hair counts through inhibition of lipid peroxidation and reduction in ROS.[19] Protein energy malnutrition and single nutrient deficiencies are known to compromise immunity. There have been detail studies on nutritional programming of the immune system.[20] Better regulation of inflammation and immunity achieved by nutrients, leads to better functioning of the hair growth cycle.
Nutrients have direct role in promoting hair growth
Unsaturated fatty acids are known to inhibit 5 alpha reductase activity of which gamma linolenic acid (omega 6 fatty acid) has highest inhibitory potency.[21] Vitamin D3 reduces insulin resistance and Rogen levels, regulates hair cycles and has direct action on the dermal papilla cells.[22] Zinc acts as immuno-modulator, inhibits catagen, prevents follicle regression and promotes recovery from telogen.[23] Copper supports the differentiation and proliferation of dermal papilla cells. Arachidonic acid improves the expression of growth factors in the dermal papilla cells, promotes mitosis of matrix keratinocytes, induces angiogenesis and hair elongation.[24] Nutrients are reported to have a direct role in hair growth.
Role of iron is supported by other minerals and vitamins
Hair follicles are known source and storage for ferritin, which is utilized in deficiency states.[25] Iron deficiency is compensated by the arrest of hair growth, ferritin is spared and directed to maintain normal serum levels in the circulation. Thus, on lab tests, the ferritin levels appear normal, despite concomitant deficiency, and hair loss. Iron absorption, binding, transport and activation for erythropoiesis, requires vitamin C, vitamin A, beta carotene, amino acids,[26] zinc, and copper.[27] Associated deficiencies of these accessory nutrients lead to poor utilization of available iron resources, presenting as noniron deficiency anemia and hair loss with apparently normal iron or ferritin levels. Iron is required for conversion of stored thyroid hormone T3 to active T4.[28] All nutrients are interdependent for efficiency; single nutrient assessment cannot represent the complete status of the internal cellular environment. Deficiencies of essential nutrients trigger autophagy or programmed breakdown of intracellular organelles.[29] Autophagy restores the deficiencies and maintains normal nutrient levels in the circulation, again making the lab tests and nutrient levels to appear normal in the presence of clinical hair loss. Hence nutritional support cannot be recommended depending on biochemical tests. It would be too late to wait for detectable failure to guide or indicate the requirement for nutritional support. Clinical hair fall may be the best parameter.
Nutrients support stem cell function
Stem cells are sensitive and are regulated by the availability or deficiency of nutrients, as well as nutrient-induced hormones and factors, like insulin, IGF (insulin like growth factor), steroid hormones, and amino acids.[30,31] Bald scalp retains hair follicle stem cells but lacks hair follicle progenitor cells.[32] Nutrition promotes the proliferation and maintenance of the stem/progenitor cell population.[33,34] Nutrient therapy is effective and augments the effects of conventional hair therapy by stimulating and supporting hair growth.
Conclusion
Nutrients can block the release of DHT-induced TGF-β, counter micro-inflammation, oxidative stress, improve immunity, and improve genetic expression which are the basic mechanisms of hair loss. Apart from genetics, lifestyle and nutritional imbalances can predispose to nongenetic and nonandrogenic hair loss. Nutrients have a direct role in restoring hair growth. Nutrients support the action of one another and also influence stem cell function. Further research is required on the specific role and benefit of nutrients in hair loss management especially in patients presenting with normal androgen levels.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shin H, Yoo HG, Inui S, Itami S, Kim IG, Cho AR, et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013;46:460–4. doi: 10.5483/BMBRep.2013.46.9.228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jobling MF, Mott JD, Finnegan MT, Jurukovski V, Erickson AC, Walian PJ, et al. Isoform-specific activation of latent transforming growth factor beta (LTGF-beta) by reactive oxygen species. Radiat Res. 2006;166:839–48. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 3.Kalkan G, Seçkin HY, Benli İ, Akbaş A, Baş Y, Karakus N, et al. Relationship between manganese superoxide dismutase (MnSODAla-9Val) and glutathione peroxidase (GPx1 Pro 197 Leu) gene polymorphisms and alopecia areata. Int J Clin Exp Med. 2015;8:21533–40. [PMC free article] [PubMed] [Google Scholar]
- 4.Hruza LL, Pentlan AP. Mechanisms of UV-induced inflammation. J Invest Dermatol. 1993;100:35S–41S. doi: 10.1111/1523-1747.ep12355240. [DOI] [PubMed] [Google Scholar]
- 5.Dalton TP, Shertzer HG, Puga A. Regulation of gene expression by reactive oxygen. Annu Rev Pharmacol Toxicol. 1999;39:67–101. doi: 10.1146/annurev.pharmtox.39.1.67. [DOI] [PubMed] [Google Scholar]
- 6.Peters EM, Liotiri S, Bodó E, Hagen E, Bíró T, Arck PC, et al. Probing the effects of stress mediators on the human hair follicle :Substance P holds central position. Am J Pathol. 2007;171:1872–86. doi: 10.2353/ajpath.2007.061206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall R, Ross RP, Fitzgerald GF, Stanton C. Fatty acids from fish:The anti-inflammatory potential of long-chain omega-3 fatty acids. Nutr Rev. 2010;86:280–9. doi: 10.1111/j.1753-4887.2010.00287.x. [DOI] [PubMed] [Google Scholar]
- 8.Sears B, Ricordi C. Role of fatty acids and polyphenols in inflammatory gene transcription and their impact on obesity, metabolic syndrome and diabetes. Eur Rev Med Pharmacol Sci. 2012;16:1137–54. [PubMed] [Google Scholar]
- 9.Rajendrasingh JR. Role of non androgenic factors in hair loss and hair regrowth. J Cosmo Trichol. 2017;3:118. [Google Scholar]
- 10.Gatherwright J, Liu MT, Amirlak B, Gliniak C, Totonchi A, Guyuron B. The contribution of endogenous and exogenous factors to male alopecia:A study of identical twins. Plast Reconstr Surg. 2013;131:794e–801e. doi: 10.1097/PRS.0b013e3182865ca9. [DOI] [PubMed] [Google Scholar]
- 11.Ellis JA, Stebbing M, Harrap SB. Genetic analysis of male pattern baldness and the 5alpha-reductase genes. J Invest Dermatol. 1998;110:849–53. doi: 10.1046/j.1523-1747.1998.00224.x. [DOI] [PubMed] [Google Scholar]
- 12.Ellis JA, Stebbing M, Harrap SB. Polymorphism of the androgen receptor gene is associated with male pattern baldness. J Invest Dermatol. 2001;116:452–5. doi: 10.1046/j.1523-1747.2001.01261.x. [DOI] [PubMed] [Google Scholar]
- 13.Sato M, Sato K. Maternal inheritance of mitochondrial DNA:Degradation of paternal mitochondria by allogeneic organelle autophagy, allophagy. Autophagy. 2012;8:424–5. doi: 10.4161/auto.19243. [DOI] [PubMed] [Google Scholar]
- 14.Rutkowski M, Grzegorczyk K. Adverse effects of antioxidative vitamins. Int J Occup Med Environ Health. 2012;25:105–21. doi: 10.2478/S13382-012-0022-x. [DOI] [PubMed] [Google Scholar]
- 15.Mocchegiani E, Costarelli L, Giacconi R, Piacenza F, Basso A, Malavolta M, et al. Micronutrient (Zn, Cu, Fe)–gene interactions in ageing and inflammatory age-related diseases:Implications for treatments. Ageing Res Rev. 2012;11:297–319. doi: 10.1016/j.arr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 16.Cousen P, Messenger A. Female pattern hair loss in complete androgen insensitivity syndrome. Br J Dermatol. 2010;162:1135–7. doi: 10.1111/j.1365-2133.2010.09661.x. [DOI] [PubMed] [Google Scholar]
- 17.Vierhapper H, Nowotny P, Maier H, Waldhäusl W. Production rates of dihydrotestosterone (DHT) in healthy men and women and in men with male pattern baldness:Determination by stable isotope/dilution and mass spektrometry. J Clin Endocrinol Metab. 2001;86:5762–4. doi: 10.1210/jcem.86.12.8078. [DOI] [PubMed] [Google Scholar]
- 18.Rajput RJ. Controlled clinical trial for evaluation of hair growth with low dose cyclical nutrition therapy in men and women without the use of finasteride. Plast Aesthet Res. 2017;4:161. [Google Scholar]
- 19.Beoy LA, Woei WJ, Hay YK. Effects of tocotrienol supplementation on hair growth in human volunteers. Trop Life Sci Res. 2010;21:91–9. [PMC free article] [PubMed] [Google Scholar]
- 20.Chandra RK. Nutrient regulation of immune functions. Forum Nutr. 2003;56:147–8. [PubMed] [Google Scholar]
- 21.Liang T, Liao S. Inhibition of steroid 5 alpha-reductase by specific aliphatic unsaturated fatty acids. Biochem J. 1992;285:557–62. doi: 10.1042/bj2850557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoi N, Inoue K, Chikanishi T, Fujiki R, Yamamoto H, Kato H, et al. 1α,25-dihydroxyvitamin D3 modulates the hair-inductive capacity of dermal papilla cells:Therapeutic potential for hair regeneration. Stem Cells Transl Med. 2012;1:615–26. doi: 10.5966/sctm.2012-0032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kil MS, Kim CW, Kim SS. Analysis of serum zinc and copper concentrations in hair loss. Ann Dermatol. 2013;25:405–9. doi: 10.5021/ad.2013.25.4.405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Munkhbayar S, Jang S, Cho A-R, Choi S-J, Shin CY, Eun HC, et al. Role of arachidonic acid in promoting hair growth. Ann Dermatol. 2016;28:55–64. doi: 10.5021/ad.2016.28.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deshwali S, Kare PK, Agrawal B.K, Alex A. Study of serum zinc, copper and ferritin levels in alopecia patients. Int J Adv Res Biol Sci. 2015;2:94–6. [Google Scholar]
- 26.Lynch SR. Interaction of iron with other nutrients. Nutr Rev. 1997;55:102–10. doi: 10.1111/j.1753-4887.1997.tb06461.x. [DOI] [PubMed] [Google Scholar]
- 27.Kelkitli E, Ozturk N, Aslan NA, Kilic-Baygutalp N, Bayraktutan Z, Kurt N, et al. Serum zinc levels in patients with iron deficiency anemia and its association with symptoms of iron deficiency anemia. Ann Hematol. 2016;95:751–6. doi: 10.1007/s00277-016-2628-8. [DOI] [PubMed] [Google Scholar]
- 28.Betsy A, Binitha M, Sarita S. Zinc deficiency associated with hypothyroidism:An overlooked cause of severe alopecia. Int J Trichol. 2013;5:40–2. doi: 10.4103/0974-7753.114714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yoshimori T. Autophagy:A regulated bulk degradation process inside cells. Biochem Biophys Res Commun. 2004;313:453–8. doi: 10.1016/j.bbrc.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, McLeod CJ, Jones DL. Regulation of adult stem cell behavior by nutrient signaling. Cell Cycle. 2011;10:2628–34. doi: 10.4161/cc.10.16.17059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ables ET, Laws KM, Drummond-Barbosa D. Control of adult stem cells in vivo by a dynamic physiological environment:Diet-dependent systemic factors in Drosophila and beyond. Wiley Interdiscip Rev Dev Biol. 2012;1:657–74. doi: 10.1002/wdev.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Garza LA, Yang CC, Zhao T, Blatt , HB , Lee M, He H, et al. Bald scalp in men with androgenetic alopecia retains hair follicle stem cells but lacks CD200-rich and CD34-positive hair follicle progenitor cells. J Clin Invest. 2011;121:613–22. doi: 10.1172/JCI44478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dembinska-Kie CA, Polus A, Grzybowska J, Kiec-Wilk B, Balwierz A, Keijer J, et al. Nutritional factors and progenitor cell differentiation. Genes Nutr. 2007;2:115–8. doi: 10.1007/s12263-007-0032-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shim J, Gururaja-Rao S, Banerjee U. Nutritional regulation of stem and progenitor cells in Drosophila. Development. 2013;140:4647–56. doi: 10.1242/dev.079087. [DOI] [PMC free article] [PubMed] [Google Scholar]


