Abstract
Background
Total occlusion is the most severe coronary lesion, indicating heavy ischemic burden and poor prognosis. The lipid profile is central to the development of atherosclerotic coronary lesions. Evidence on the optimal lipid measure to be monitored and managed in patients with established coronary artery disease (CAD) is inconclusive.
Methods
Total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), nonhigh-density lipoprotein cholesterol (non-HDL-c), lipoprotein (a) [Lp(a)], apolipoprotein B (apoB), non-HDL-c/HDL-c, and apoB/apoA-1 were analyzed in quintiles and as continuous variables. The associations of lipid measures with total occlusion were tested using logistic regression models, visualized with restricted cubic splines, and compared by areas under the receiver operating characteristic curves (AUROC). Discordance analysis was performed when apoB/apoA-1 and non-HDL-c/HDL-c were not in concordance.
Results
The prospective cohort study included 10,003 patients (mean age: 58 years; women: 22.96%), with 1879 patients having total occlusion. The risks of total occlusion significantly increased with quintiles of Lp(a), non-HDL-c/HDL-c, and apoB/apoA-1 (all p for trend < 0.001). TG had no association with total occlusion. Restricted cubic splines indicate significant positive linear relations between the two ratios and total occlusion [odds ratio per 1-standard deviation increase (95% confidence interval): non-HDL-c/HDL-c: 1.135 (1.095–1.176), p < 0.001; apoB/apoA-1: 2.590 (2.049–3.274), p < 0.001]. The AUROCs of apoB/apoA-1 and non-HDL-c/HDL-c were significantly greater than those of single lipid measures. Elevation in the apoB/apoA-1 tertile significantly increased the risk of total occlusion at a given non-HDL-c/HDL-c tertile but not vice versa.
Conclusion
ApoB/apoA-1 confers better predictive power for total occlusion than non-HDL-c/HDL-c and single lipid measures in established CAD patients.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12944-022-01733-8.
Keywords: Lipids, Lipoproteins, Coronary occlusion, Coronary artery disease
BACKGROUND
Coronary artery disease (CAD) refers to myocardial ischemia due to progressive atherosclerotic lesions narrowing the artery lumen. Total occlusion, caused by acute plaque rupture or atherosclerotic plaque progression, represents the most advanced lesions that completely interrupt coronary blood flow. Acute total occlusion often leads to acute myocardial infarction (AMI) [1], while chronic total occlusion is also associated with adverse prognosis [2, 3]. Treatment of total occlusion is a challenging issue; hence, the prevention of atherosclerotic lesions from developing to total occlusion is crucial in the management of CAD.
Easily measurable blood markers for the prediction of total occlusion in CAD patients have always been a topic of interest. Many hematological, biochemical and inflammatory parameters have been examined to predict acute or chronic total occlusion [4–6]. However, evidence concerning the association of lipid profiles with total occlusion is limited. Lipids, lipoprotein, and apolipoprotein are core factors in the initiation and progression of atherosclerosis. Previous studies have discussed the predictive power of single lipid measures and lipid ratios for the severity of coronary lesions and the risk of fatal myocardial infarction in patients without CAD [7, 8]. We intended to investigate the associations of different lipid measures with total occlusion in a large cohort of patients with established CAD and to determine which measures are more relevant to total occlusion and should be noted in the clinical management of CAD.
Methods
Study design, setting, and participants
The study cohort comprised 10,724 consecutive patients undergoing percutaneous coronary intervention (PCI) from January 2013 to December 2013 at Fuwai Hospital, Chinese Academy of Medical Sciences, Beijing, China. Demographic, clinical and medication information was extracted from the electronic medical records. The cross-sectional study generated post hoc analysis of data from the cohort above and compared total cholesterol (TC), total triglyceride (TG), low-density lipoprotein cholesterol (LDL-c), nonhigh-density lipoprotein cholesterol (non-HDL-c), lipoprotein (a) [Lp(a)], apolipoprotein B (apoB), non-HDL-c/HDL-c, and apoB/apoA-1 in the prediction of total occlusion. Patients with previous coronary artery bypass grafting and missing data on lipid measures were excluded.
Blood sampling and laboratory analysis
Venous blood samples were collected after fasting for at least 12 h and assayed within 24 h of admission. Lipid measures were analyzed using an automatic biochemistry analyzer (Hitachi 7150, Tokyo, Japan). In detail, apoB, apoA-1, and Lp(a) were measured by an immunoturbidimetric method. TC, TG, LDL-c, and HDL-c were measured by an enzymatic method. Non-HDL-c was calculated as TC minus HDL-c. Fasting glucose was assayed by an enzymatic hexokinase method. HbA1c was assayed using a Tosoh Automated Glycohemoglobin Analyzer (HLC-723G8, Tokyo, Japan).
Definitions
Total occlusion was defined as occlusion of the coronary artery with thrombolysis in myocardial infarction (TIMI) grade 0 flow in the distal segment of the completely occluded vessel due to atherosclerosis. A body mass index ≥ 28 kg/m2 was considered to indicate obesity [9]. Diabetes was defined as glycated hemoglobin (HbA1c) > 6.5% or self-reported diabetes. Hypertension was defined as a mean blood pressure ≥ 140/90 mmHg or self-reported hypertension. Dyslipidemia was diagnosed when at least one of the following criteria was met: TC ≥ 6.22 mmol/L; TG ≥ 2.26 mmol/L; LDL-c ≥ 4.14 mmol/L; HDL-c < 1.04 mmol/L; or self-reported lipid-lowering medication use [10].
Statistical analysis
Details on the estimation of the sample size can be found in the Supplemental Methods in Additional File. Lipid measures were analyzed in quintiles and as continuous variables. The distributions of lipid measures are depicted in histograms. Correlation between each pair of lipid measures was assessed using scatterplot (data not shown) and Spearman rank correlation analysis without adjustment. Baseline characteristics across quintiles of each lipid measure and between patients with or without occlusion were compared using Kruskal‒Wallis tests, χ2 tests for trend, Mann‒Whitney U test, or χ2 tests, as appropriate. Categorical variables are expressed as numbers (percentages). Continuous variables are expressed as the median [interquartile range].
The associations of lipid measures with total occlusion were examined using logistic regression models by estimating odds ratios (ORs) and 95% confidence intervals (CIs). Model 1 was unadjusted; Model 2 was adjusted for sex and age; Model 3 was adjusted for sex, age, body mass index, hypertension, diabetes, previous MI, previous PCI, smoking history, and admission presentation. The relations between lipid measure levels and total occlusion were visualized with restricted cubic splines with 4 knots, adjusting for all variables in Model 3. The median of each measure was set as the reference. The receiver operating characteristic curve (ROC) was used to evaluate the predictive power of each lipid measure (continuous) for total occlusion. The areas under the ROC (AUROCs) were compared using a nonparametric approach [11] and the integrated discrimination improvement measure. Subgroup analysis was performed according to four prespecified variables of interest—sex, age, diabetes status, and admission presentation—to calculate the OR (95% CI) and AUROC of each lipid measure (continuous).
A discordance analysis was further performed to quantify the associations of apoB/apoA-1 and non-HDL-c/HDL-c with total occlusion when the two ratios were not in concordance. ApoB/apoA-1 and non-HDL-c/HDL-c were categorized into tertiles (low, middle, high). Concordance was defined as apoB/apoA-1, and non-HDL-c/HDL-c levels were in the same tertile. Discordance was defined as apoB/apoA-1 and non-HDL-c/HDL-c in different tertiles. Baseline characteristics and ORs (95% CIs) across the nine concordance/discordance groups were analyzed as described above.
Since the lipid profile can be influenced by acute stress, all analyses were repeated in patients with acute coronary syndrome (ACS) as a sensitivity analysis to assess the robustness of our findings.
Statistical analyses were conducted with R version 3.6.3 (R Core Team 2020, Vienna, Austria. www.R-project.org). Figures were created by GraphPad Prism version 8.0.2 (GraphPad Software, San Diego, California, USA, www.graphpad.com). Two-tailed P values of < 0.05 were considered statistically significant.
Results
Study population and baseline characteristics
A total of 10,003 CAD patients were included in this analysis after excluding 437 patients with previous coronary artery bypass grafting and 284 patients with missing data for apoB and apoA-1. The mean age of the study population was 58 years (range: 18–91), and 22.96% were women. Supplemental Fig. 1 in Additional file shows the distribution of each lipid measure. TC, LDL-c, non-HDL-c, and apoB were strongly positively correlated with each other. Non-HDL-c/HDL-c strongly positively correlated with apoB/apoA-1. TG had a weak to medium correlation with other measures, while Lp(a) did not correlate with other measures (Table 1).
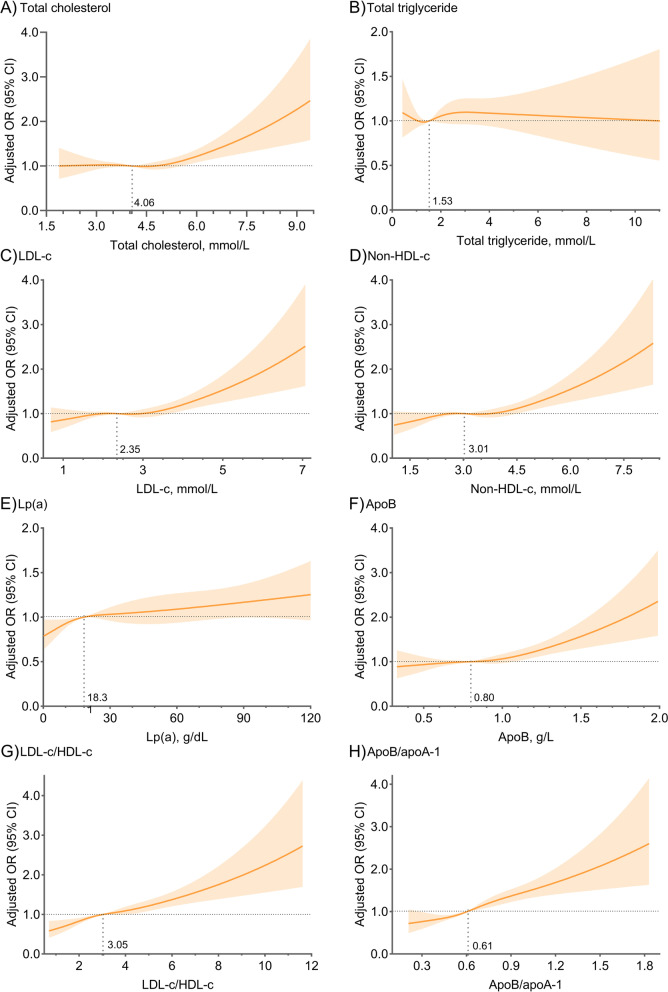
Fig. 1.
Adjusted restricted cubic spline curves of different lipid measures. Adjusted for sex, age, body mass index, hypertension, diabetes, prior myocardial infarction, prior percutaneous coronary intervention, smoking history, and admission presentation. OR, odds ratio; CI, confidence interval; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; Lp(a), lipoprotein (a); apo, apolipoprotein
Table 1.
Spearman correlation coefficients r between each pair of lipid measures
| TC | TG | LDL-c | Non-HDL-c | Lp(a) | ApoB |
Non-HDL-c /HDL-c |
|
|---|---|---|---|---|---|---|---|
| TG | 0.386 | ||||||
| LDL-c | 0.922 | 0.245 | |||||
| Non-HDL-c | 0.954 | 0.491 | 0.922 | ||||
| Lp(a) | 0.124 | -0.088 | 0.165 | 0.113 | |||
| ApoB | 0.860 | 0.439 | 0.859 | 0.892 | 0.200 | ||
|
Non-HDL-c /HDL-c |
0.551 | 0.572 | 0.601 | 0.757 | 0.046 | 0.656 | |
| ApoB/apoA-1 | 0.554 | 0.397 | 0.661 | 0.704 | 0.180 | 0.815 | 0.816 |
r ≤ 0.2: no correlation; 0.2 < r ≤ 0.4: weak correlation; 0.4 < r ≤ 0.6: medium correlation; 0.6 < r ≤ 0.8: high correlation; r > 0.8: strong correlation. LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; lp(a), lipoprotein (a); apo, apolipoprotein
The proportions of women increased with quintiles of all single lipid measures but decreased with quintiles of the two lipid ratios. Patients in higher Lp(a) quintiles were older and had lower body mass index, whereas patients in higher quintiles of other measures were younger and more likely to be obese. Higher quintiles of each lipid measure had more patients presenting with AMI. The proportion of smokers increased with quintiles of TG and lipid ratios and decreased with quintiles of TC, LDL-c, and Lp(a) but had no significant difference among quintiles of apoB and non-HDL-c. The proportion of diabetic patients increased with quintiles of TC and lipid ratios but was comparable among quintiles of other measures. Insulin use did not differ significantly among quintiles of each lipid measure. Patients in higher quintiles of each lipid measure had less use of oral antidiabetic agents and more elevated fasting glucose and HbA1c levels. Patients with higher levels of each lipid measure were less likely to have a history of MI, stroke, or PCI. Other characteristics were not significantly different among quintiles of each lipid measure (see Supplemental Tables 12345678 in Additional File).
Total occlusion was detected by coronary angiography in 1879 patients. Patients with total occlusion were more likely to be men, younger and obese; they were also more likely to present with AMI and have a history of smoking, MI, and PCI. Higher fasting glucose and HbA1c levels, lower estimated glomerular filtration rates and lower left ventricular ejection fractions were observed in the total occlusion group (Table 2). The statistically significant but slight differences in lipid measures between patients with and without total occlusion were probably due to Type I error in large samples, which may yield no clinical significance.
Table 2.
Baseline characteristics according to the presence of total occlusion
| No total occlusion (n = 8124) | Total occlusion (n = 1879) | P | |
|---|---|---|---|
| Sex (Women) | 1956 (24.08) | 341 (18.15) | < 0.001 |
| Age, years | 59 [51, 65] | 57 [49, 64] | < 0.001 |
| ≥ 65 years | 2260 (27.82) | 464 (24.69) | 0.006 |
| BMI, kg/m2 | 25.82 [23.83, 27.76] | 25.95 [24.21, 28.13] | < 0.001 |
| Obesity | 1883 (23.18) | 497 (26.45) | 0.003 |
| Admission presentation | < 0.001 | ||
| Acute myocardial infarction | 1173 (14.43) | 660 (31.12) | |
| Unstable angina | 3571 (43.96) | 617 (32.84) | |
| Chronic coronary syndrome | 3380 (41.61) | 602 (32.04) | |
| Smoking history | 4605 (56.68) | 1215 (64.66) | < 0.001 |
| Diabetes | 3236 (39.83) | 752 (40.02) | 0.880 |
| Oral antidiabetic agents | 1362 (42.09) | 325 (43.22) | 0.572 |
| Insulin | 874 (27.01) | 180 (23.94) | 0.085 |
| Hypertension | 5671 (69.81) | 1270 (67.59) | 0.060 |
| Dyslipidemia | 6113 (75.25) | 1432 (76.21) | 0.381 |
| Peripheral artery disease | 218 (2.68) | 43 (2.29) | 0.333 |
| Chronic obstructive pulmonary disease | 190 (2.34) | 42 (2.24) | 0.788 |
| Prior myocardial infarction | 1416 (17.43) | 452 (24.06) | < 0.001 |
| Prior stroke | 869 (10.70) | 211 (11.23) | 0.503 |
| Prior PCI | 1827 (22.49) | 471 (25.07) | 0.017 |
| eGFR, ml/min/1.73m2 | 118.44 [103.35, 133.66] | 117.55 [101.24, 134.38] | 0.082 |
| LVEF, % | 64 [60, 68] | 61 [56, 65] | < 0.001 |
| HbA1c, % | 6.20 [5.80, 6.90] | 6.20 [5.90, 7.00] | 0.033 |
| Fasting glucose, mmol/L | 5.46 [5.92, 6.54] | 5.65 [5.04, 7.11] | < 0.001 |
| TC, mmol/L | 4.05 [3.45, 4.79] | 4.08 [3.42, 4.88] | 0.358 |
| TG, mmol/L | 1.52 [1.13, 2.09] | 1.57 [1.16, 2.15] | 0.014 |
| LDL-c, mmol/L | 2.34 [1.86, 2.99] | 2.39 [1.89, 3.08] | 0.012 |
| Non-HDL-c, mmol/L | 3.00 [2.42, 3.74] | 3.08 [2.47, 3.84] | < 0.001 |
| HDL-c, mmol/L | 1.00 [0.85, 1.19] | 0.95 [0.81, 1.10] | < 0.001 |
| Lp(a), mg/dL | 17.83 [7.59, 40.21] | 20.20 [8.63, 43.28] | < 0.001 |
| ApoB, g/L | 0.80 [0.66, 0.96] | 0.81 [0.67, 1.00] | < 0.001 |
| ApoA-1, g/L | 1.32 [1.19, 1.50] | 1.26 [1.14, 1.42] | < 0.001 |
| Non-HDL-c/HDL-c | 2.99 [2.29, 3.94] | 3.27 [2.50, 4.27] | < 0.001 |
| ApoB/apoA-1 | 0.60 [0.49, 0.74] | 0.65 [0.52, 0.80] | < 0.001 |
| Lesion location | |||
| Left main coronary artery | 196 (2.41) | 44 (2.34) | 0.856 |
| Left anterior descending artery | 7444 (91.63) | 1617 (86.06) | < 0.001 |
| Left circumflex artery | 1268 (15.61) | 446 (23.74) | < 0.001 |
| Right coronary artery | 1309 (16.11) | 494 (26.29) | < 0.001 |
Values are presented as the median [interquartile range] or number (%)
BMI body mass index, PCI percutaneous coronary intervention, eGFR estimated glomerular filtration rate, LVEF left ventricular ejection fraction, HbA1c glycated hemoglobin
Associations of lipid measures with total occlusion
For Lp(a), non-HDL-c/HDL-c, and apoB/apoA-1, the risks of total occlusion compared with the first quintiles were significantly higher through the subsequent quintiles (all p for trend < 0.001). For LDL-c, non-HDL-c, and apoB, the risks of total occlusion compared with the first quintiles were significantly higher only in the fifth quintiles. The risks of total occlusion in higher quintiles of TC and TG were not significantly different from the first quintiles. The three logistic regression models yielded consistent trends (Table 3).
Table 3.
Associations of different lipid measures as continuous variables and in quintiles with total occlusion
| Number (%) | Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | ||
| TC, mmol/L | |||||||
| Per SD increase | — | 1.050 (1.003, 1.099) | 0.036 | 1.065 (1.017, 1.116) | 0.007 | 1.072 (1.023, 1.123) | 0.004 |
| Q1: < 3.31 | 391 (19.54) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 3.31 ≤ TC < 3.81 | 343 (17.19) | 0.855 (0.728, 1.004) | 0.056 | 0.868 (0.739, 1.019) | 0.084 | 0.884 (0.752, 1.039) | 0.136 |
| Q3: 3.81 ≤ TC < 4.32 | 373 (18.76) | 0.951 (0.812, 1.114) | 0.533 | 0.980 (0.836, 1.148) | 0.803 | 0.980 (0.835, 1.150) | 0.803 |
| Q4: 4.32 ≤ TC < 5.03 | 370 (18.25) | 0.919 (0.785, 1.077) | 0.297 | 0.958 (0.817, 1.123) | 0.593 | 0.975 (0.830, 1.145) | 0.756 |
| Q5: ≥ 5.03 | 402 (20.18) | 1.041 (0.891, 1.216) | 0.612 | 1.090 (0.931, 1.276) | 0.285 | 1.111 (0.948, 1.303) | 0.195 |
| p for trend | — | 0.401 | — | 0.144 | — | 0.097 | — |
| TG, mmol/L | |||||||
| Per SD increase | — | 1.043 (0.998, 1.089) | 0.060 | 1.028 (0.983, 1.075) | 0.223 | 1.016 (0.971, 1.065) | 0.488 |
| Q1: < 1.06 | 361 (17.95) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 1.06 ≤ TG < 1.37 | 338 (17.15) | 0.946 (0.803, 1.114) | 0.506 | 0.938 (0.797, 1.106) | 0.447 | 0.908 (0.769, 1.071) | 0.251 |
| Q3: 1.37 ≤ TG < 1.71 | 388 (19.23) | 1.088 (0.928, 1.275) | 0.298 | 1.080 (0.920, 1.267) | 0.384 | 1.024 (0.871, 1.205) | 0.770 |
| Q4: 1.71 ≤ TG < 2.28 | 382 (19.01) | 1.073 (0.915, 1.258) | 0.385 | 1.053 (0.896, 1.237) | 0.531 | 0.997 (0.847, 1.174) | 0.973 |
| Q5: ≥ 2.28 | 410 (20.56) | 1.183 (1.011, 1.385) | 0.036 | 1.138 (0.969, 1.336) | 0.116 | 1.068 (0.906, 1.258) | 0.436 |
| p for trend | — | 0.011 | — | 0.044 | — | 0.234 | — |
| LDL-c, mmol/L | |||||||
| Per SD increase | — | 1.104 (1.047, 1.165) | < 0.001 | 1.114 (1.056, 1.176) | < 0.001 | 1.119 (1.060, 1.182) | < 0.001 |
| Q1: < 1.76 | 355 (17.84) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 1.76 ≤ LDL-c < 2.16 | 375 (18.66) | 1.056 (0.900, 1.240) | 0.503 | 1.062 (0.904, 1.247) | 0.463 | 1.065 (0.905, 1.252) | 0.448 |
| Q3: 2.16 ≤ LDL-c < 2.59 | 360 (18.12) | 1.019 (0.867, 1.198) | 0.819 | 1.036 (0.881, 1.218) | 0.671 | 1.045 (0.887, 1.231) | 0.599 |
| Q4: 2.59 ≤ LDL-c < 3.19 | 377 (18.64) | 1.055 (0.899, 1.238) | 0.514 | 1.077 (0.917, 1.265) | 0.367 | 1.084 (0.921, 1.276) | 0.331 |
| Q5: ≥ 3.19 | 412 (20.67) | 1.200 (1.025, 1.405) | 0.023 | 1.232 (1.051, 1.444) | 0.010 | 1.243 (1.059, 1.460) | 0.008 |
| p for trend | — | 0.042 | — | 0.017 | — | 0.013 | — |
| Non-HDL-c, mmol/L | |||||||
| Per SD increase | — | 1.110 (1.059, 1.163) | < 0.001 | 1.113 (1.061, 1.167) | < 0.001 | 1.113 (1.061, 1.168) | < 0.001 |
| Q1: < 2.30 | 353 (17.50) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 2.30 ≤ nHDL-c < 2.78 | 375 (19.02) | 1.107 (0.943, 1.300) | 0.216 | 1.106 (0.942, 1.300) | 0.220 | 1.090 (0.926, 1.282) | 0.300 |
| Q3: 2.78 ≤ nHDL-c < 3.28 | 347 (17.09) | 0.972 (0.826, 1.144) | 0.732 | 0.982 (0.834, 1.157) | 0.831 | 0.965 (0.818, 1.139) | 0.674 |
| Q4: 3.28 ≤ nHDL-c < 3.96 | 381 (19.24) | 1.123 (0.957, 1.318) | 0.155 | 1.132 (0.963, 1.330) | 0.132 | 1.120 (0.952, 1.319) | 0.172 |
| Q5: ≥ 3.96 | 423 (21.11) | 1.261 (1.078, 1.476) | 0.004 | 1.275 (1.088, 1.494) | 0.003 | 1.263 (1.076, 1.484) | 0.004 |
| p for trend | — | 0.007 | — | 0.005 | — | 0.006 | — |
| Lp(a), mg/dL | |||||||
| Per SD increase | — | 1.003 (1.001, 1.005) | 0.002 | 1.003 (1.002, 1.005) | < 0.001 | 1.003 (1.002, 1.005) | < 0.001 |
| Q1: < 6.27 | 323 (16.14) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 6.27 ≤ lp(a) < 13.08 | 363 (18.15) | 1.152 (0.977, 1.358) | 0.092 | 1.177 (0.998, 1.389) | 0.052 | 1.168 (0.989, 1.379) | 0.068 |
| Q3: 13.08 ≤ lp(a) < 25.21 | 387 (19.34) | 1.246 (1.059, 1.466) | 0.008 | 1.270 (1.079, 1.496) | 0.004 | 1.263 (1.072, 1.489) | 0.005 |
| Q4: 25.21 ≤ lp(a) < 48.42 | 400 (20.00) | 1.299 (1.105, 1.527) | 0.002 | 1.331 (1.131, 1.565) | 0.001 | 1.340 (1.138, 1.579) | 0.001 |
| Q5: ≥ 48.42 | 406 (20.29) | 1.322 (1.125, 1.554) | 0.001 | 1.391 (1.183, 1.637) | < 0.001 | 1.384 (1.175, 1.631) | 0.001 |
| p for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 | — |
| ApoB, g/L | |||||||
| Per SD increase | — | 1.603 (1.318, 1.949) | < 0.001 | 1.612 (1.323, 1.964) | < 0.001 | 1.605 (1.313, 1.961) | < 0.001 |
| Q1: < 0.63 | 340 (17.03) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 0.63 ≤ apoB < 0.75 | 369 (18.21) | 1.085 (0.923, 1.277) | 0.323 | 1.075 (0.914, 1.265) | 0.384 | 1.078 (0.915, 1.270) | 0.369 |
| Q3: 0.75 ≤ apoB < 0.86 | 336 (17.68) | 1.047 (0.887, 1.236) | 0.587 | 1.041 (0.881, 1.230) | 0.636 | 1.039 (0.878, 1.229) | 0.658 |
| Q4: 0.86 ≤ apoB < 1.02 | 391 (18.93) | 1.138 (0.970, 1.336) | 0.113 | 1.140 (0.970, 1.340) | 0.111 | 1.126 (0.956, 1.325) | 0.155 |
| Q5: ≥ 1.02 | 443 (21.99) | 1.373 (1.174, 1.607) | < 0.001 | 1.375 (1.173, 1.611) | < 0.001 | 1.373 (1.170, 1.613) | < 0.001 |
| p for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 | — |
| Non-HDL-c/HDL-c | |||||||
| Per SD increase | — | 1.168 (1.129, 1.208) | < 0.001 | 1.149 (1.110, 1.190) | < 0.001 | 1.135 (1.095, 1.176) | < 0.001 |
| Q1: < 2.17 | 288 (14.38) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 2.17 ≤ ratio < 2.74 | 328 (16.37) | 1.165 (0.981, 1.384) | 0.081 | 1.136 (0.956, 1.350) | 0.148 | 1.107 (0.930, 1.318) | 0.252 |
| Q3: 2.74 ≤ ratio < 3.39 | 398 (19.92) | 1.481 (1.254, 1.749) | < 0.001 | 1.429 (1.209, 1.689) | < 0.001 | 1.380 (1.165, 1.635) | < 0.001 |
| Q4: 3.39 ≤ ratio < 4.27 | 391 (19.57) | 1.449 (1.226, 1.712) | < 0.001 | 1.390 (1.175, 1.644) | < 0.001 | 1.338 (1.129, 1.587) | < 0.001 |
| Q5: ≥ 4.27 | 474 (23.70) | 1.850 (1.573, 2.175) | < 0.001 | 1.727 (1.465, 2.037) | < 0.001 | 1.619 (1.368, 1.916) | < 0.001 |
| p for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 | — |
| ApoB/apoA-1 | |||||||
| Per SD increase | — | 3.030 (2.419, 3.795) | < 0.001 | 2.762 (2.196, 3.475) | < 0.001 | 2.590 (2.049, 3.274) | < 0.001 |
| Q1: < 0.47 | 288 (14.31) | 1.0 (Ref.) | — | 1.0 (Ref.) | — | 1.0 (Ref.) | — |
| Q2: 0.47 ≤ ratio < 0.57 | 326 (16.48) | 1.181 (0.994, 1.403) | 0.058 | 1.145 (0.963, 1.361) | 0.125 | 1.126 (0.946, 1.341) | 0.181 |
| Q3: 0.57 ≤ ratio < 0.66 | 356 (17.73) | 1.290 (1.089, 1.528) | 0.003 | 1.243 (1.049, 1.474) | 0.012 | 1.209 (1.018, 1.435) | 0.030 |
| Q4: 0.66 ≤ ratio < 0.79 | 410 (20.43) | 1.537 (1.303, 1.813) | < 0.001 | 1.468 (1.243, 1.734) | < 0.001 | 1.427 (1.206, 1.688) | < 0.001 |
| Q5: ≥ 0.79 | 499 (24.97) | 1.993 (1.697, 2.340) | < 0.001 | 1.871 (1.590, 2.202) | < 0.001 | 1.787 (1.514, 2.109) | < 0.001 |
| p for trend | — | < 0.001 | — | < 0.001 | — | < 0.001 | — |
Model 1 was the crude model. Model 2 was adjusted for sex and age. Model 3 was adjusted for sex, age, body mass index, hypertension, diabetes, prior myocardial infarction, prior percutaneous coronary intervention, smoking history, and admission presentation
OR odds ratio, CI confidence interval, SD standard deviation, Q quintile, Ref reference
Restricted cubic spline curves indicate significantly positive linear relations between lipid measure levels and the risk of total occlusion except for TG. Notably, the 95% CIs for the ORs included the null value when either the Lp(a) level was greater than the median or the levels of other single lipid measures were less than the medians (Fig. 1, Table 3). The AUROCs of apoB/apoA-1 and non-HDL-c/HDL-c were significantly greater than those of other measures, with integrated discrimination improvements of 8.1% and 6.6%, respectively (P < 0.001) (Fig. 2).
Fig. 2.
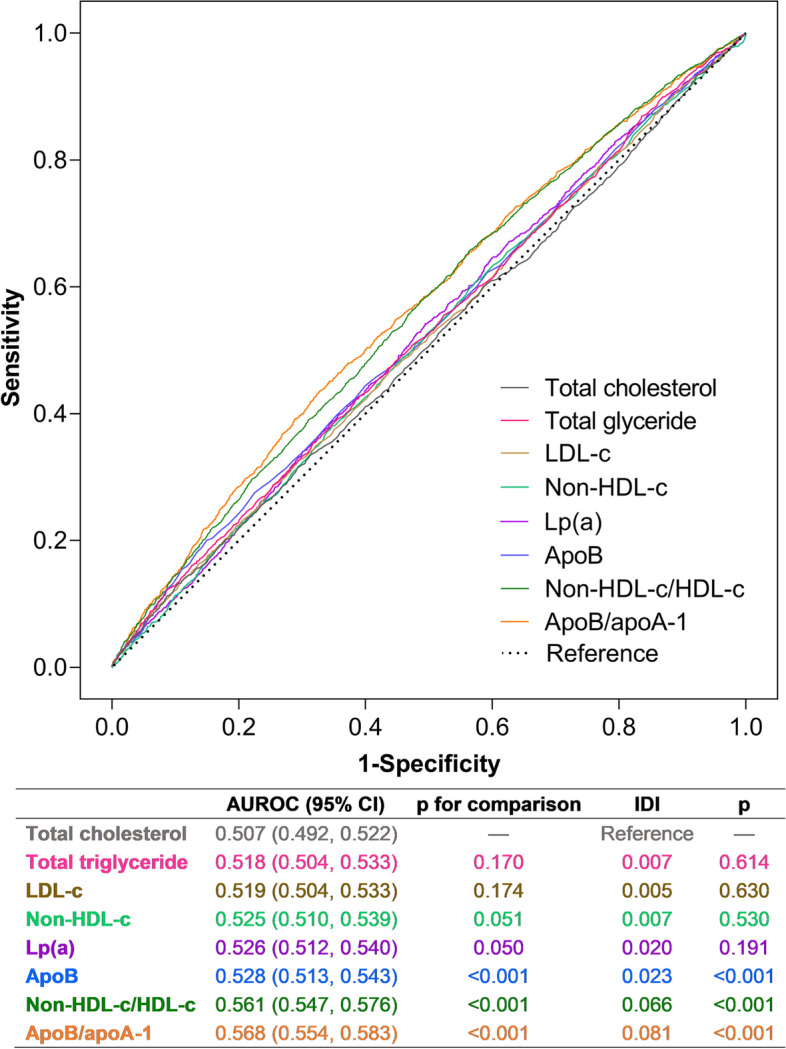
ROC curves of different lipid measures for prediction of total occlusion. ROC, receiver operating characteristic curve; apo, apolipoprotein; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; lp(a), lipoprotein (a); AUROC, area under the receiver operating characteristic curve; IDI, integrated discrimination index; CI, confidence interval
Only ApoB/apoA-1 and non-HDL-c/HDL-c were robustly associated with total occlusion in all subgroups. ROC illustrated similar results that the two lipid ratios confer better predictive power of total occlusion than other single lipid measures in all subgroups (see Supplemental Tables 9–10 in Additional File). Sensitivity analysis including only patients with acute coronary disease yielded consistent results with the main analysis (see Supplemental Tables 11–12 in Additional File).
Discordance analysis of apoB/apoA-1 and non-HDL-c/HDL-c
The above results demonstrated that apoB/apoA-1 and non-HDL-c/HDL-c were better predictors of total occlusion. However, according to our definition, the two ratios were discordant in 3253 (32.52%) patients in the study population.
Elevation in the apoB/apoA-1 tertile significantly increased the incidence and the risk of total occlusion at a given non-HDL-c/HDL-c tertile. A trend that elevation in the non-HDL-c/HDL-c tertile slightly increased the incidence and the risk of total occlusion was observed at the low or middle level of apoB/apoA-1. However, the trend disappeared at the high apoB-apoA-1 level. Patients in the high-apoB/apoA-1-low-non-HDL-c/HDL-c group had the highest incidence and risk of total occlusion (Fig. 3). Models 1 and 2 obtained consistent results (data not shown). Consistent trends were observed in the sensitivity analysis (see Supplemental Table 13 in Additional file).
Fig. 3.
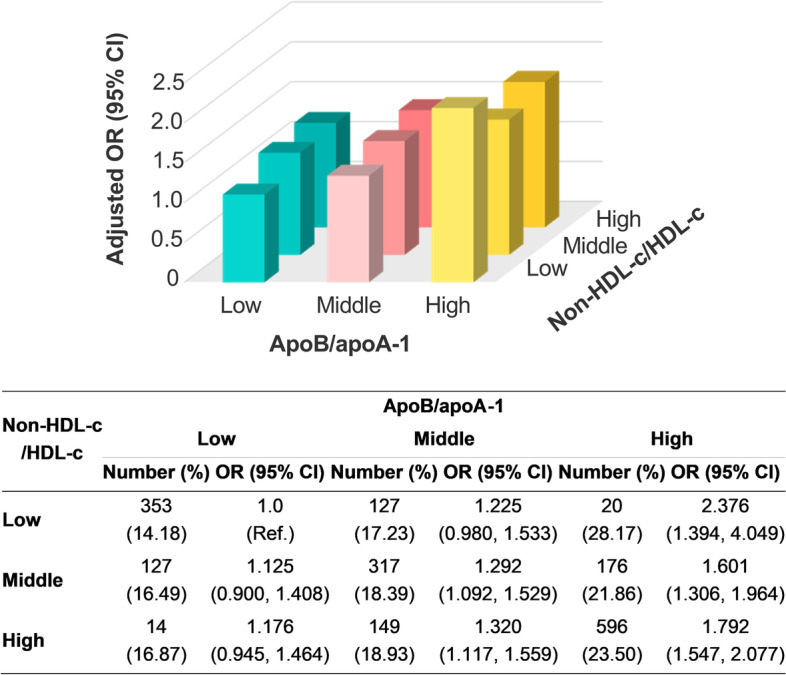
Incidence and adjusted OR of total occlusion by concordance/discordance groups between apoB/apoA-1 and non-HDL-c/HDL-c. Adjusted for sex, age, body mass index, hypertension, diabetes, prior myocardial infarction, prior percutaneous coronary intervention, smoking history, and admission presentation. OR, odds ratio; apo, apolipoprotein; LDL-c, low-density lipoprotein cholesterol; HDL-c, high-density lipoprotein cholesterol; CI, confidence interval; Ref., reference
Discussion
In this study, we investigated the association of lipid profiles with total occlusion in established CAD patients to seek new possibilities for the early diagnosis of total occlusion. We observed that total occlusion was frequent in the study population; patients with total occlusion had significantly higher levels of lipids and lipid ratios; lipid ratios were more stable predictors of total occlusion than single lipid measures; in particular, apoB/apoA-1 was more sensitive than non-HDL-c/HDL-c and should be highlighted in clinical practice.
Single lipid measures and total occlusion
ApoB, LDL-c, and non-HDL-c are strongly correlated with each other. The plasma apoB level is approximately equal to the sum of triglyceride-rich very-low-density lipoprotein, cholesterol-rich LDL, and Lp(a), representing the number of circulating atherogenic particles [12]. We observed that the OR of apoB for total occlusion was higher than that of LDL-c and non-HDL-c. Previous Mendelian randomization studies and discordance analyses have further determined that apoB is more accurate as a marker of coronary calcification and cardiovascular disease than LDL-c or non-HDL-c [12–14]. These findings confirm the widely accepted view that the number of atherogenic particles is more relative to atherosclerosis than the concentration of circulating cholesterol, as only apoB-containing particles < 70 nm in diameter can enter and be retained in the arterial wall, thereby initiating and driving atherosclerosis.
Lp(a) was also observed to be associated with the risk of total occlusion. A possible mechanism is that Lp(a) is related to the progression of low-attenuation plaque (a quantitative marker of necrotic core) on coronary computed tomography angiography in established CAD patients [15]. Low attenuation is an independent predictor of plaque progression to chronic total occlusion [16], and a necrotic core is related to the increased propensity of plaque rupture and subsequent acute total occlusion [15]. However, the OR per 1 SD increase in Lp(a) was only 1.003 (95% CI: 1.002–1.005). This phenomenon could be explained by the fact that the effect of Lp(a) on CAD risk is proportional to the absolute change in plasma Lp(a) levels [17, 18], while a 1-SD change is too small to provide a clinically meaningful reduction in the risk of total occlusion.
Sensitivity analysis in ACS patients generated consistent results with the findings from the overall CAD patients, indicating that transient fluctuation of lipid levels under acute conditions may not change the association of lipid profile with total occlusion. In addition, a study reported high-sensitivity cardiac troponin T as a predictor of acute total occlusion [5]. However, we believe that cardiac troponin, an acute-phase reactant of myocardial injury, is a consequence rather than a cause of acute occlusion.
Lipid ratios and total occlusion
Compared with single lipid measures, apoB/apoA-1 and non-HDL-c/HDL-c had higher and significant ORs, and the superiority of the two ratios was robust in all subgroups. Conversely, single lipid measures lost predictive power in some quintiles or subgroups, suggesting that lipid ratios are more stable indicators of total occlusion. Previous studies support that lipid ratios are superior to single lipid measures in predicting vulnerable plaque and MI [7, 8, 19]. HDL can reverse cholesterol transport and is inversely correlated with cardiovascular risk. An elevation in HDL is associated with obstructive coronary lesions for a given LDL level. ApoA-1 is the major component of HDL. Reduction in apoA-1 potentiates the impact of apoB on major cardiovascular events at any apoB level [20, 21]. Accordingly, lipid ratios reflect the balance between risk and protective factors for atherosclerosis, providing more comprehensive information on atherosclerosis risk than single lipid measures.
For the comparison of apoB/apoA-1 and non-HDL-c/HDL-c, the risk of total occlusion per 1-SD increase in apoB/apoA-1 was over twice as high as non-HDL-c/HDL-c, suggesting that a 1-SD decrease in apoB/apoA-1 can produce a greater benefit than non-HDL-c/HDL-c. The finding was valid in all subgroups. Previous studies have yielded similar results that apoB/apoA-1 is better than other lipid ratios in predicting coronary severity and MI [22–25]. The AUROCs illustrated comparable prediction performance of ApoB/apoA-1 and non-HDL-c/HDL-c. Thus, we used discordance analysis to discriminate between the two strongly correlated ratios. We observed that elevation in the apoB/apoA-1 tertile significantly increased the incidence and the risk of total occlusion at a given non-HDL-c/HDL-c tertile but not vice versa, demonstrating that even at a low non-HDL-c/HDL-c level, a further reduction in apoB/apoA-1 can still lessen the risk of total occlusion. Two possible explanations are as follows. First, only apoB-containing particles can cross the arterial wall; when plasma cholesterol is constant, a higher apoB level means more circulating cholesterol can be transferred, trapped, and deposed. Thus, elevation in apoB aggravates the risk of total occlusion regardless of non-HDL-c level. However, once within the arterial wall, smaller apoB particles with less cholesterol are more hazardous because they have a greater tendency to be trapped than larger cholesterol-rich apoB particles [12, 23], which proves our finding that patients in the high-apoB/apoA-1-low-non-HDL-c/HDL-c group experienced the highest risk of total occlusion. Second, in addition to reversing cholesterol transport, apoA-1 has antioxidation and anti-inflammation abilities and is associated with insulin resistance. Elevation in apoA-1 may bring more protective effects than HDL [26]. Moreover, apoB and apoA-1 are directly assayed and hardly affected by fasting status, making apoB/apoA-1 a superior indicator to non-HDL-c/HDL-c in clinical practice.
Altogether, the lipid profile drives the development of CAD, a continuous and evolving process from lipid deposition and plaque growth to ischemic events. Our work and studies applying coronary severity as the endpoint are mutually supportive. In conclusion, we explored the association of lipid profiles with total occlusion and discovered the advantages of lipid ratios, especially apoB/apoA-1, in predicting total occlusion. Our findings suggest that apoB/apoA-1 should be monitored routinely to identify patients at high risk of total occlusion and to guide lipid-lowering treatment for CAD patients to delay disease progression. In addition, approximately a quarter of non-ST-segment elevation myocardial infarction is caused by a totally occluded culprit vessel [1]. These patients have adverse outcomes and need immediate revascularization. However, the early noninvasive identification of these high-risk patients is unsatisfactory. ApoB/apoA-1 may be a potential indicator for identifying total occlusion of a culprit vessel in non-ST-segment elevation myocardial infarction patients at admission. Furthermore, a study preliminarily proposed three risk levels of apoB/apoA-1: low risk, 0.2–0.6; intermediate risk, 0.61–0.9; and high risk, 0.91–5.0 [21]. A certain cutoff value should be established in future research.
Study strengths and limitations
The study provides evidence on the association between lipid profile and total occlusion in established CAD patients, adding novel insights to the prediction of total occlusion. Other strengths include the large population and low missingness rate (< 2.80%). The study limitations should be noted. First, although we made great efforts to minimize potential confounders, the observational design of this study raises concerns about residual confounding by some unknown and unmeasured factors associated with total occlusion. Second, the single-center nature of this study restricts its generalizability. Third, the lipid profile was assayed only once before the procedure. Repeated measurements estimating long-term average lipid concentrations were unavailable. Thus, we cannot rule out the influence of acute stress on the lipid profile, as lipid levels tend to decrease under acute conditions. Fourth, data on the preadmission use of lipid-modifying drugs were not collected, so we could not adjust for the plaque stabilization effect of lipid-lowering drugs such as statins. Last, we could not distinguish between acute or chronic total occlusion. Nevertheless, our findings are reliable because the precursors of chronic total occlusion and AMI may be more similar than their clinical manifestations [27].
Conclusions
ApoB/apoA-1 confers better predictive power of total occlusion than non-HDL-c/HDL-c and single lipid measures in established CAD patients. Routine apoB/apoA-1 monitoring for CAD patients can help identify individuals at high-risk of total occlusion and guide lipid-lowering therapy to delay disease progression.
Supplementary Information
Additional file 1: Supplemental Methods. Supplemental Table1. Baseline characteristics according to quintiles of total cholesterol. Supplemental Table2. Baseline characteristics according to quintiles of total triglyceride. Supplemental Table3. Baseline characteristics according to quintiles of LDL-c. Supplemental Table4. Baseline characteristics according to quintiles of non-HDL-c. Supplemental Table5. Baseline characteristics according to quintiles of lp(a). Supplemental Table6. Baseline characteristics according to quintiles of apoB. Supplemental Table7. Baseline characteristics according to quintiles of non-HDL-c/HDL-c. Supplemental Table8. Baseline characteristics according to quintiles of apoB/apoA-1. Supplemental Table9. Associations of different lipid measures as continuous variables with total occlusion by subgroups. Supplemental Table10. AUROC of each lipid measure by subgroups. Supplemental Table11. Associations of different lipid measures with total occlusion in patients with acute coronary syndrome. Supplemental Table12. Predictive value of different lipid measures for total occlusion in patients with acute coronary syndrome. Supplemental Table13. Discordance analysis between apoB/apoA-1 and non-HDL-c/HDL-c in patients with acute coronary syndrome. Supplemental Figure1. Distribution of each lipid measure.
Acknowledgements
We thank all staff members for data collection, data entry, and monitoring as part of this study.
Abbreviations
- CAD
Coronary artery disease
- TC
Total cholesterol
- TG
Total triglyceride
- LDL-c
Low-density lipoprotein cholesterol
- HDL-c
High-density lipoprotein cholesterol
- Lp(a)
Lipoprotein (a)
- apo
Apolipoprotein
- AUROC
Area under the receiver operating characteristic curve
- AMI
Acute myocardial infarction
- PCI
Percutaneous coronary intervention
- ROC
Receiver operating characteristic curve
- OR
Odds ratio
- CI
Confidence interval
- SD
Standard deviation
Authors’ contributions
TL: Conceptualization, Methodology, Formal analysis, Writing—Original Draft DY: Formal analysis, Writing—Review & Editing PW: Formal analysis, Visualization SJ: Data Curation CZ: Data Curation PZ: Data Curation YS: Data Curation XT: Data Curation XZ: Validation, Writing—Review & Editing ZG: Validation, Writing—Review & Editing YY: Supervision RG: Supervision BX: Investigation, Resources JY: Conceptualization, Investigation, Supervision, Project administration, Funding acquisition.
Funding
This work was supported by the CAMS Innovation Fund for Medical Sciences [2020-I2 M-C&T-B-049]; the National Clinical Research Center for Cardiovascular Disease, Fuwai Hospital, Chinese Academy of Medical Sciences [NCRC2020013]; and the China International Exchange and Promotion Association for Medical and Healthcare Investigator Sponsored Study [CN174125, DIREGL08735-DAPT].
Availability of data and materials
The data that support the findings of this study are available from the Information Center of Fuwai Hospital, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Information Center of Fuwai Hospital.
Declarations
Ethics approval and consent to participate
The study complied with the Declaration of Helsinki. The Review Board of Fuwai Hospital approved the study protocol before enrollment (No. 2013–449). All participants provided written informed consent before intervention.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Khan AR, Golwala H, Tripathi A, Bin Abdulhak AA, Bavishi C, Riaz H, Mallipedi V, Pandey A, Bhatt DL. Impact of total occlusion of culprit artery in acute non-ST elevation myocardial infarction: a systematic review and meta-analysis. Eur Heart J. 2017;38:3082–3089. doi: 10.1093/eurheartj/ehx418. [DOI] [PubMed] [Google Scholar]
- 2.Claessen BE, Dangas GD, Weisz G, Witzenbichler B, Guagliumi G, Mockel M, Brener SJ, Xu K, Henriques JP, Mehran R, Stone GW. Prognostic impact of a chronic total occlusion in a non-infarct-related artery in patients with ST-segment elevation myocardial infarction: 3-year results from the HORIZONS-AMI trial. Eur Heart J. 2012;33:768–775. doi: 10.1093/eurheartj/ehr471. [DOI] [PubMed] [Google Scholar]
- 3.Gierlotka M, Tajstra M, Gasior M, Hawranek M, Osadnik T, Wilczek K, Olszowski D, Dyrbus K, Polonski L. Impact of chronic total occlusion artery on 12-month mortality in patients with non-ST-segment elevation myocardial infarction treated by percutaneous coronary intervention (from the PL-ACS Registry) Int J Cardiol. 2013;168:250–254. doi: 10.1016/j.ijcard.2012.09.086. [DOI] [PubMed] [Google Scholar]
- 4.Tajstra M, Hawranek M, Desperak P, Cislak A, Gierlotka M, Lekston A, Polonski L, Gasior M. Medium platelet volume as a noninvasive predictor of chronic total occlusion in non-infarct artery in patients with non-ST-segment elevation myocardial infarction and multivessel coronary artery disease. Int J Cardiol. 2017;228:594–598. doi: 10.1016/j.ijcard.2016.11.261. [DOI] [PubMed] [Google Scholar]
- 5.Baro R, Haseeb S, Ordonez S, Costabel JP. High-sensitivity cardiac troponin T as a predictor of acute Total occlusion in patients with non-ST-segment elevation acute coronary syndrome. Clin Cardiol. 2019;42:222–226. doi: 10.1002/clc.23128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koenig W, Khuseyinova N. Biomarkers of atherosclerotic plaque instability and rupture. Arterioscler Thromb Vasc Biol. 2007;27:15–26. doi: 10.1161/01.ATV.0000251503.35795.4f. [DOI] [PubMed] [Google Scholar]
- 7.Deng F, Li D, Lei L, Yang Q, Li Q, Wang H, Deng J, Zheng Q, Jiang W. Association between apolipoprotein B/A1 ratio and coronary plaque vulnerability in patients with atherosclerotic cardiovascular disease: an intravascular optical coherence tomography study. Cardiovasc Diabetol. 2021;20:188. doi: 10.1186/s12933-021-01381-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walldius G, Jungner I, Holme I, Aastveit AH, Kolar W, Steiner E. High apolipoprotein B, low apolipoprotein A-I, and improvement in the prediction of fatal myocardial infarction (AMORIS study): a prospective study. Lancet. 2001;358:2026–2033. doi: 10.1016/S0140-6736(01)07098-2. [DOI] [PubMed] [Google Scholar]
- 9.Zhou B. Coorperative Meta-Analysis Group Of China Obesity Task F: [Predictive values of body mass index and waist circumference to risk factors of related diseases in Chinese adult population] Zhonghua Liu Xing Bing Xue Za Zhi. 2002;23:5–10. [PubMed] [Google Scholar]
- 10.Joint committee issued Chinese guideline for the management of dyslipidemia in a, Chinese guideline for the management of dyslipidemia in adults] Zhonghua Xin Xue Guan Bing Za Zhi. 2016;2016(44):833–853. doi: 10.3760/cma.j.issn.0253-3758.2016.10.005. [DOI] [PubMed] [Google Scholar]
- 11.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 12.Sniderman AD, Thanassoulis G, Glavinovic T, Navar AM, Pencina M, Catapano A, Ference BA. Apolipoprotein B Particles and Cardiovascular Disease: A Narrative Review. JAMA Cardiol. 2019;4:1287–1295. doi: 10.1001/jamacardio.2019.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilkins JT, Li RC, Sniderman A, Chan C, Lloyd-Jones DM. Discordance Between Apolipoprotein B and LDL-Cholesterol in Young Adults Predicts Coronary Artery Calcification: The CARDIA Study. J Am Coll Cardiol. 2016;67:193–201. doi: 10.1016/j.jacc.2015.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Richardson TG, Sanderson E, Palmer TM, Ala-Korpela M, Ference BA, Davey Smith G, Holmes MV. Evaluating the relationship between circulating lipoprotein lipids and apolipoproteins with risk of coronary heart disease: A multivariable Mendelian randomisation analysis. PLoS Med. 2020;17:e1003062. doi: 10.1371/journal.pmed.1003062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser Y, Daghem M, Tzolos E, Meah MN, Doris MK, Moss AJ, Kwiecinski J, Kroon J, Nurmohamed NS, van der Harst P, et al. Association of Lipoprotein(a) With Atherosclerotic Plaque Progression. J Am Coll Cardiol. 2022;79:223–233. doi: 10.1016/j.jacc.2021.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang J, Chun EJ, Park HJ, Cho YS, Park JJ, Kang SH, Cho YJ, Yoon YE, Oh IY, Yoon CH, et al. Clinical and Computed Tomography Angiographic Predictors of Coronary Lesions That Later Progressed to Chronic Total Occlusion. JACC Cardiovasc Imaging. 2019;12:2196–2206. doi: 10.1016/j.jcmg.2018.12.026. [DOI] [PubMed] [Google Scholar]
- 17.Burgess S, Ference BA, Staley JR, Freitag DF, Mason AM, Nielsen SF, Willeit P, Young R, Surendran P, Karthikeyan S, et al. Association of LPA Variants With Risk of Coronary Disease and the Implications for Lipoprotein(a)-Lowering Therapies: A Mendelian Randomization Analysis. JAMA Cardiol. 2018;3:619–627. doi: 10.1001/jamacardio.2018.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parish S, Hopewell JC, Hill MR, Marcovina S, Valdes-Marquez E, Haynes R, Offer A, Pedersen TR, Baigent C, Collins R, et al. Impact of Apolipoprotein(a) Isoform Size on Lipoprotein(a) Lowering in the HPS2-THRIVE Study. Circ Genom Precis Med. 2018;11:e001696. doi: 10.1161/CIRCGEN.117.001696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ridker PM, Rifai N, Cook NR, Bradwin G, Buring JE. Non-HDL cholesterol, apolipoproteins A-I and B100, standard lipid measures, lipid ratios, and CRP as risk factors for cardiovascular disease in women. JAMA. 2005;294:326–333. doi: 10.1001/jama.294.3.326. [DOI] [PubMed] [Google Scholar]
- 20.Kim YG, Cho YR, Park GM, Won KB, Ann SH, Yang DH, Kang JW, Lim TH, Kim HK, Choe J, et al. High-density lipoprotein cholesterol and the risk of obstructive coronary artery disease beyond low-density lipoprotein cholesterol in non-diabetic individuals. Eur J Prev Cardiol. 2020;27:706–714. doi: 10.1177/2047487319844364. [DOI] [PubMed] [Google Scholar]
- 21.Walldius G, de Faire U, Alfredsson L, Leander K, Westerholm P, Malmstrom H, Ivert T, Hammar N. Long-term risk of a major cardiovascular event by apoB, apoA-1, and the apoB/apoA-1 ratio-Experience from the Swedish AMORIS cohort: A cohort study. PLoS Med. 2021;18:e1003853. doi: 10.1371/journal.pmed.1003853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong LF, Yan XN, Fan Y, Wu Q, Luo SH, Yang B, Li JJ. Is the ratio of apoB/apoA-1 the best predictor for the severity of coronary artery lesions in Chinese diabetics with stable angina pectoris? An assessment based on Gensini scores. J Geriatr Cardiol. 2015;12:402–409. doi: 10.11909/j.issn.1671-5411.2015.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parish S, Peto R, Palmer A, Clarke R, Lewington S, Offer A, Whitlock G, Clark S, Youngman L, Sleight P, et al. The joint effects of apolipoprotein B, apolipoprotein A1, LDL cholesterol, and HDL cholesterol on risk: 3510 cases of acute myocardial infarction and 9805 controls. Eur Heart J. 2009;30:2137–2146. doi: 10.1093/eurheartj/ehp221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.McQueen MJ, Hawken S, Wang X, Ounpuu S, Sniderman A, Probstfield J, Steyn K, Sanderson JE, Hasani M, Volkova E, et al. Lipids, lipoproteins, and apolipoproteins as risk markers of myocardial infarction in 52 countries (the INTERHEART study): a case-control study. Lancet. 2008;372:224–233. doi: 10.1016/S0140-6736(08)61076-4. [DOI] [PubMed] [Google Scholar]
- 25.Walldius G, Jungner I, Aastveit AH, Holme I, Furberg CD, Sniderman AD. The apoB/apoA-I ratio is better than the cholesterol ratios to estimate the balance between plasma proatherogenic and antiatherogenic lipoproteins and to predict coronary risk. Clin Chem Lab Med. 2004;42:1355–1363. doi: 10.1515/CCLM.2004.254. [DOI] [PubMed] [Google Scholar]
- 26.Mehta A, Shapiro MD. Apolipoproteins in vascular biology and atherosclerotic disease. Nat Rev Cardiol. 2022;19:168–179. doi: 10.1038/s41569-021-00613-5. [DOI] [PubMed] [Google Scholar]
- 27.Opolski MP. Noninvasive Precursors of Coronary Chronic Total Occlusions: Fantasy or Reality? JACC Cardiovasc Imaging. 2019;12:2207–2209. doi: 10.1016/j.jcmg.2019.01.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Supplemental Methods. Supplemental Table1. Baseline characteristics according to quintiles of total cholesterol. Supplemental Table2. Baseline characteristics according to quintiles of total triglyceride. Supplemental Table3. Baseline characteristics according to quintiles of LDL-c. Supplemental Table4. Baseline characteristics according to quintiles of non-HDL-c. Supplemental Table5. Baseline characteristics according to quintiles of lp(a). Supplemental Table6. Baseline characteristics according to quintiles of apoB. Supplemental Table7. Baseline characteristics according to quintiles of non-HDL-c/HDL-c. Supplemental Table8. Baseline characteristics according to quintiles of apoB/apoA-1. Supplemental Table9. Associations of different lipid measures as continuous variables with total occlusion by subgroups. Supplemental Table10. AUROC of each lipid measure by subgroups. Supplemental Table11. Associations of different lipid measures with total occlusion in patients with acute coronary syndrome. Supplemental Table12. Predictive value of different lipid measures for total occlusion in patients with acute coronary syndrome. Supplemental Table13. Discordance analysis between apoB/apoA-1 and non-HDL-c/HDL-c in patients with acute coronary syndrome. Supplemental Figure1. Distribution of each lipid measure.
Data Availability Statement
The data that support the findings of this study are available from the Information Center of Fuwai Hospital, but restrictions apply to the availability of these data, which were used under license for the current study and are not publicly available. Data are, however, available from the authors upon reasonable request and with permission of the Information Center of Fuwai Hospital.