Abstract
The use of tick vaccines in mammalian hosts has been shown to be the most promising alternative tick control method to current use of acaricides, which suffers from a number of limitations. However, the success of this method is dependent on the identification, cloning, and in vitro expression of tick molecules involved in the mediation of key physiological roles with respect to the biological success of a tick as a vector and pest. We have sequenced and characterized a Haemaphysalis longicornis tick salivary gland-associated cDNA coding for a 29-kDa extracellular matrix-like protein. This protein is expressed in both unfed and fed immature and mature H. longicornis ticks. The predicted amino acid sequence of p29 shows high homology to sequences of some known extracellular matrix like-proteins with the structural conservation similar to all known collagen proteins. Immunization with the recombinant p29 conferred a significant protective immunity in rabbits, resulting in reduced engorgement weight for adult ticks and up to 40 and 56% mortality in larvae and nymphs that fed on the immunized rabbits. We speculate that this protein is associated with formation of tick cement, a chemical compound that enables the tick to remain attached to the host, and suggest a role for p29 as a candidate tick vaccine molecule for the control of ticks. We have discussed our findings with respect to the search of tick molecules for vaccine candidates.
Ticks are obligate ectoparasites that infest mammals, birds, reptiles, and amphibians and are found in many regions of the world (27). They are the most significant vectors of animal diseases, second to mosquitoes in the number of human diseases they transmit (17, 27). The need to control ticks is the result of their negative economic impact on livestock production. Ticks cause worldwide economic losses in livestock (2). It is estimated that up to 80% of the world’s 1,288 million cattle are infested with ticks, representing $7,500 million in monetary losses for cattle alone (2). Currently the principal tick control method is the application of acaricides. This approach is, however, associated with a number of disadvantages such as chemical pollution of the food chain and the environment as well as the quick development of resistance against acaricides by ticks, thus reducing the effectiveness of acaricides in certain instances (28, 32). In addition, acaricides must be applied at a very high frequency, which makes this approach labor intensive. Because of the fast pace at which ticks develop resistance against acaricides, it is difficult to recover development costs and thus new products are rarely developed. These limitations have necessitated the search for alternative tick control measures. Among the several alternative tick control measures (30) considered to date, only host vaccination against ticks appears promising (5, 28, 38).
Since the report by Trager (33) that mammalian hosts can acquire resistance to tick feeding, host vaccination against tick infestation as an alternative tick control method has received great attention, with some workers achieving significant host protection by using crude tick vaccine antigens (19, 34, 38). Purification and production of sufficient amounts of candidate tick vaccine antigens has over the years been the major limitation to the adoption of host vaccination as an alternative tick control method. With the advent of biotechnology, this limitation is to a large extent no longer valid, and the success of host vaccination as an alternative tick control measure will depend on the identification, cloning, and expression of key physiological tick molecules (5, 10, 37). Recently genes for two Boophilus microplus midgut-associated molecules, Bm 86 and Bm 91, have been cloned and expressed (21, 24). These antigens have been shown to confer a significant protective immunity against B. microplus infestation in cattle (24, 32). The findings from these studies provided important evidence with respect to the reliability and practicality of using biotechnology to identify and produce sufficient amounts of tick vaccine antigen candidates.
We are interested in the salivary gland-associated molecules which regulate the attachment of the tick on to the host, subsequent establishment of the feeding lesion, and uptake of the blood meal as well as transmission of the pathogens to the host (8). The importance of salivary gland-associated molecules with respect to the biological success of ticks as vectors have been covered in several recent reviews (1, 22, 27, 35, 36).
In the present study, we used polyclonal rabbit antitick serum raised against tick saliva molecules from both the immature and mature stages of Haemaphysalis longicornis to probe a cDNA library. This species is a three-host tick and a vector of Theileria spp. as well as Coxiella burnetii commonly infesting cattle, dogs, and humans (31). In Japan and other east Asian countries, this tick is a major vector of Theileria sergenti/buffeli, which causes an economically important disease in cattle (16). We report here on the molecular cloning, sequencing, and characterization of a cDNA coding for an immunogenic extracellular matrix-like protein associated with the salivary gland of the H. longicornis tick and suggest the possible use of the recombinant product for tick control.
MATERIALS AND METHODS
Tick dissection.
Ticks were obtained from a colony of H. longicornis maintained on Japanese White rabbits in our laboratory in Sapporo, Japan. Dissection of ticks was done as described elsewhere (23). Briefly, under a dissection light microscope, partially fed ticks were submerged in autoclaved ice-cold phosphate-buffered saline (PBS; pH 7.4), held down with a pair of soft tissue forceps. The dorsal cuticle was cut out, and the salivary glands were separated from the rest of the organs with the help of 18-gauge needles. Following dissection, the salivary glands were pipetted into microcentrifuge tubes, washed once in PBS, and stored at −80°C until use.
Generation of rabbit anti-tick saliva immune serum.
For the generation of polyclonal rabbit anti-H. longicornis tick saliva immune serum, 4 Japanese White rabbits were used. One rabbit was repeatedly fed on by both immature and mature tick stages (serum for this rabbit is referred to in the text as anti-tick saliva serum). The other three rabbits were each repeatedly fed on by a single tick stage, larva, nymph, or adult (sera from these rabbits are referred to as anti-larval, anti-nymphal, and anti-adult saliva sera). Between tick infestations, rabbits were rested for a fortnight. Antitick rabbit immune serum was collected and stored at −20°C after four or more infestations when rabbit antitick infestation immunity was apparent. Apparent immunity against tick infestation was ascertained basically by the reactivity of the rabbit antitick saliva immune serum on immunoblots of tick antigens.
SDS-PAGE and Western blotting.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) under reducing conditions was performed as described elsewhere (14). For Western blotting, proteins of tick salivary glands dissected as described above were electrophoresed and transferred to polyvinylidene difluoride (PVDF) membranes. The membranes were incubated in the primary antibody, and positive signals were detected with peroxidase-conjugated goat anti-rabbit immunoglobulin G with 3,3-diaminobenzidine tetrahydrochloride and cobalt chloride.
Construction of an expression cDNA library.
Total RNA was extracted from partially fed female adult ticks which had remained attached on the host for 96 h. The ticks were detached from rabbit ears by traction using a pair of forceps, left at room temperature for about 1 h to shed remnants of rabbit pieces of tissue and hair, and then pulverized in liquid nitrogen. Total RNA and subsequently poly(A)+ were isolated by using Trizol reagent (GIBCO BRL, Grand Island, N.Y.) and an mRNA isolation kit (Quick Prep; Pharmacia, Uppsala, Sweden) according to the manufacturers’ instructions. A library of oligo(dT)-primed cDNA with added directional EcoRI/HindIII linkers was constructed from 5 μg of purified mRNA by using a cDNA synthesis kit (Novagen, Madison, Wis.) and ligated to the EcoRI/HindIII arms of the λscreen vector (Novagen). The recombinant phage DNA was packaged by using Phage Maker packaging extracts (Novagen) according to the manufacturer’s instructions, resulting in a primary cDNA library with a size of 1.2 × 105 PFU.
cDNA library screening.
The amplified library was immunoscreened by using the polyclonal rabbit anti-H. longicornis tick saliva immune serum as described elsewhere (26). Prior to sequencing, the cloned cDNAs resulting from immunoscreening were subjected to dot blot hybridization as described elsewhere (4) in order to put them in homogeneous groups. Briefly, cloned cDNAs were amplified by PCR, and subsequently the product was denatured by boiling for 5 min. The denatured products were blotted onto Hybond N+ (Amersham, Little Chalfont, England) by using a dot blotter (San Platech Co. Ltd., Tokyo, Japan). The membranes were dried at room temperature for about 30 min and subsequently fixed in an oven at 120°C for 20 min. Three of the longest cDNAs obtained from immunoscreening were radiolabeled with α-32P by using a Multiprime DNA labeling kit (Amersham) and used as a probe. Hybridization was performed in a buffer containing 0.5 M phosphate buffer (pH 7.2), 1 mM EDTA, and 7% SDS (3) overnight at 65°C. The membranes were washed to a final stringency of 0.1× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate)–0.1% SDS at 65°C for 1 h and then autoradiographed.
To isolate a full sequence cDNA, the library was further subjected to plaque hybridization screening as described elsewhere (26), using the longest cloned cDNA resulting from immunoscreening as a probe. Radiolabeling of the probe and hybridization were carried out as described above. The filters were routinely washed to a final stringency of 0.1× SSC–0.1% SDS and subsequently exposed to an X-ray film overnight at −80°C.
Estimation of the native protein (p29) molecular mass.
To estimate the molecular mass of the native tick protein coded by the cloned cDNA, monospecific antibodies which bound to the products of the recombinant phages were eluted and subsequently used to probe immunoblots of whole larval tick and tick salivary gland protein extracts. The salivary glands were dissected from nymphal and adult H. longicornis ticks, partially fed for 2 and 4 days, respectively. Monospecific antibodies were eluted essentially as described elsewhere (26), with minor modifications. Briefly, cloned phage was grown on 2× YT agar for 6 to 7 h at 37°C followed by transfer of the recombinant polypeptides to Hybond C (Amersham) membranes without isopropyl-β-d-thiogalactopyranoside (IPTG) induction for 3 to 4 h at the same temperature. Nonspecific biding sites were blocked by incubating the membranes for 45 min at room temperature in 3% skim milk diluted in PBS (pH 7.4) containing 0.05% Tween 20 (PBST). Subsequently the filters were incubated for 5 h at room temperature in polyclonal rabbit antitick serum diluted into PBST with 1% skim milk. The filters were washed at room temperature twice, 15 min per wash, with 0.15 M sodium chloride and PBST, respectively. The bound antibodies were eluted in a 100 mM glycine buffer (pH 2.8) at the same temperature with shaking for 20 min. The pH of the eluted antibodies was raised to around 7.0 by addition of 0.1 volume of 1 M Tris base immediately after elution and dialyzed against PBS.
DNA sequencing and analysis.
Using the Dye Terminator cycle sequencing system (Perkin Elmer-Applied Biosystems, Norwalk, Conn.) and automated sequencers (Perkin Elmer-Applied Biosystems 373A and 310 Genetic Analyzers), the nucleotide sequence of the cloned cDNA was determined by using the vector-specific SP6 promoter primer (Promega, Madison, Wis.) plus gene-specific primers where necessary. The template for sequencing was generated by PCR amplification of the cloned phage DNA, using the λscreen vector-specific primers (SP6 promoter and T7 terminator). Sequence analysis was done by using the GENETYX-MAC software package as well as the GenBank and Swissprot databases for comparison of the p29 predicted amino acid sequence with database entries.
Northern blotting analysis.
Total RNA was extracted from partially fed adult female ticks as described above. Thirty μg of total RNA was electrophoresed on a 1% formaldehyde agarose gel in a formamide running buffer as described elsewhere (26). The electrophoresed RNA was routinely transferred to Hybond N+ filters (Amersham) by the capillary transfer method (26) and subsequently UV cross-linked for 3 min with a UV transilluminator (UVP Inc., Uplander Calif.). The cloned cDNA, used as a probe, was radiolabeled with α-32P as described above, and hybridization was carried out overnight at 42°C in a buffer containing 50% formamide, 5× SSPE (1× SSPE is 0.18 M NaCl, 10 mM NaH2PO4, and 1 mM EDTA [pH 7.7]) 2× Denhardt’s salts, 0.1% SDS, and denatured salmon sperm DNA (20 μg/ml). The membranes were washed to a final stringency of 0.1× SSC–0.1% SDS at 65°C for up to 60 min and subsequently exposed to an X-ray film overnight at −80°C.
Southern blotting.
Tick genomic DNA was digested with restriction enzymes XbaI, XhoI, SpeI, and BamHI, (New England Biolabs Inc., Beverly, Mass.), electrophoresed on a 0.8% agarose gel, and blotted onto Hybond N+ (Amersham) by the capillary transfer method (27). The cloned cDNA, used as a probe, was labeled with α-32P by a Multiprime DNA labeling kit (Amersham) according to the manufacturer’s instructions. Hybridization was carried out at 65°C overnight in a buffer containing 0.5 M phosphate (pH 7.2), 1 mM EDTA, and 7% SDS (3), and the membranes were routinely washed to a final stringency of 2× SSC–0.1% SDS at the same temperature for 30 to 60 min.
In vitro expression of recombinant p29.
The predicted mature protein coding sequence for p29 was expressed in Escherichia coli BL21 λDES 3 (a phage λ lysogen) containing the LysS plasmid (Novagen) by using the expression vector pET-32 (c+), which produces a recombinant protein fused with histidine-tagged thioredoxin (Trx). The coding sequence for p29 was initially PCR generated by using the p29 cloned phage cDNA as template and gene-specific primers with added EcoRI/SalI restriction enzyme sites for unidirectional cloning. To improve the cutting efficiency of the restriction enzymes, the PCR product was initially cloned into the pGEMT vector (Promega). The resulting plasmid was transformed and amplified in E. coli DH5α (Promega) and subsequently purified by alkaline lysis as described elsewhere (26). The purified plasmid was digested with appropriate restriction enzymes to create overhangs on the p29 cDNA insert, which was subsequently ligated into EcoRI/SalI cloning site of the pET-32(c+) expression vector (Novagen). For induction of recombinant p29 (rp29) expression, IPTG to a final concentration of between 0.8 to 1 mM was added, and expression was induced for 4 to 6 h at 37°C. The control protein, a 20-kDa histidine-tagged Trx, was produced from E. coli containing the expression vector pET-32(a+) by induction with IPTG. Recombinant polypeptides were purified by Ni affinity chromatography according to the manufacturer’s instructions.
Immunization and challenge infestation.
Ten Japanese White rabbits were used for the immunization and challenge experiment. Of the 10 rabbits, 3 were immunized with the control protein Trx, 6 received rp29, and the remaining rabbit was used as an unimmunized control. The immunized rabbits were divided into three groups (one group for each feeding stage of H. longicornis, larva, nymph, and adult) of three, consisting one Trx- and two rp29-immunized rabbits per group. The unimmunized control rabbit was fed on by adult ticks. One milligram of the control protein or the rp29 was mixed with complete Freund’s adjuvant for the initial injection, and incomplete Freund’s adjuvant for the booster injection was given 2 weeks later. The host immune response to the immunization was analyzed by the reactivity of rp29- and Trx-immunized rabbit sera on immunoblots of salivary glands dissected from adult ticks, Trx, and rp29 10 days after the booster injection.
For the challenge infestation, predetermined numbers of ticks (per rabbit, 1,600 for larvae, 200 to 270 for nymphs, and 40 for adult ticks) were maintained on the rabbit ears with the help of ear bags as described elsewhere (31) 2 weeks after the booster injection. To analyze the effects of the rp29-induced immunity, visual examination was made 24 h after the ticks were put onto the rabbit ears to establish the attachment rates, while the duration of feeding was established by monitoring the period from attachment to collection of the first batch of engorged ticks. The engorgement weights and mortality rates were determined after repletion or termination of the experiment. Postengorgement parameters such as oviposition, hatchability, and moulting rates were not considered in the analysis of rp29-induced immunity. All data are presented as mean ± standard error or percentages where applicable, and differences were considered to be statistically significant if the P value was less than 0.05 in Student’s t test.
Nucleotide sequence accession number.
The GenBank accession number for the H. longicornis salivary gland associated p29 gene is ABO14612.
RESULTS
cDNA library screening and DNA sequence analysis.
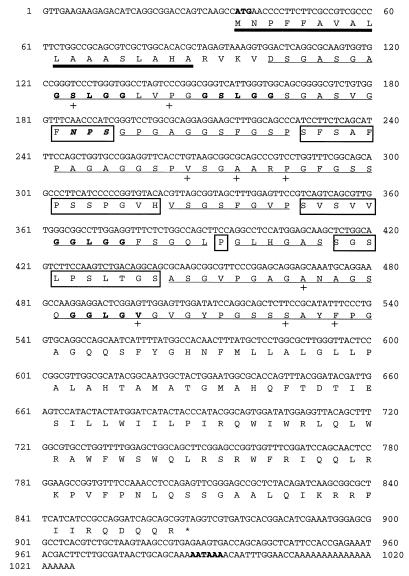
A cDNA library of partially fed adult ticks which had been constructed in a λscreen vector was immunoscreened by using polyclonal rabbit anti-tick saliva immune serum as described in Materials and Methods. A total of 32 cDNA clones ranging in size from 700 to 1,000 bp were isolated (results not shown). The cloned cDNAs were subjected to dot blot hybridization using three of the longest cloned cDNAs as probes, in order to put them in homogeneous groups. Although the dot blot hybridization results were not entirely conclusive, the cloned cDNAs appeared related (results not shown). This observation was confirmed by DNA sequencing of the two longest cloned cDNAs which showed 100% homology. None of the two sequenced cDNA clones appeared to have had a start codon, suggesting that the cloned cDNAs were incomplete (results not shown). To isolate a full-length cDNA coding for the mature protein, the library was further screened by plaque hybridization. Five cDNAs with sizes about 1 kb were isolated and sequenced. DNA sequencing revealed that one of the five cDNAs about 1,100 bp in size had a start codon 36 bases in frame and a polyadenylation signal, AATAAA, 13 bases from the poly(A) tail (Fig. 1).
FIG. 1.
Nucleotide of the cloned cDNA coding for the H. longicornis tick salivary gland-associated 29-kDa protein and its deduced amino acid sequence. The predicted possible cleavable signal peptide (amino acids 1 to 18) is underlined with a thick line. The GX1X2 tripeptide repeat domains are underlined with a thin line. Within the GX1X2 repeat domain, the glycine replacement mutations are indicated by +, the imperfections (non-GX1X2 repeat domains) are boxed, and the GSLGG pentapeptide repeats are indicated in bold letters. The possible N-glycosylation site (NPS) is indicated in bold italics. The start codon (ATG) and the polyadenylation signal (AATAAA) are indicated in bold letters.
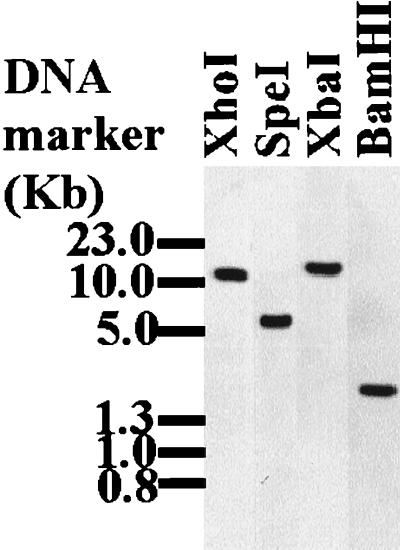
The size of mature mRNA corresponding to the cloned cDNA was confirmed in Northern blotting analysis of total RNA extracted from partially fed adult female H. longicornis ticks. The cloned cDNA hybridized to a band about 1,000 bp in size (Fig. 2). On the Southern blot analysis of tick genomic DNA, the cloned cDNA hybridized to a single band per restriction enzyme digest (Fig. 3), suggesting that the cloned cDNA was a single-copy gene.
FIG. 2.
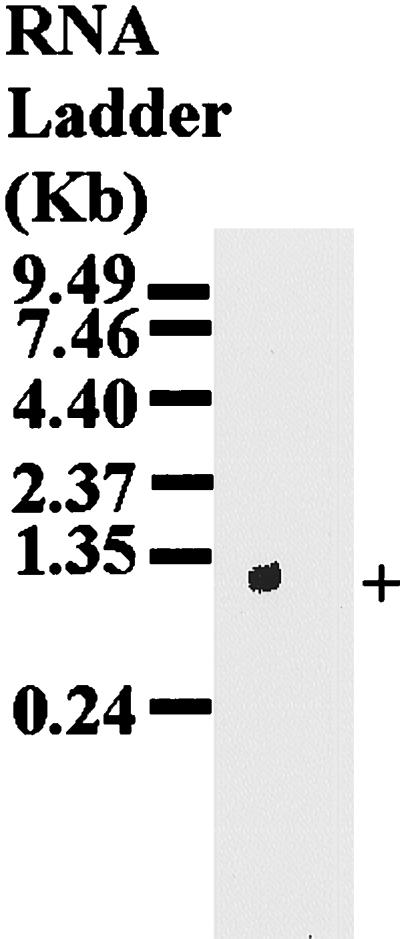
Northern blot analysis of p29 mRNA. Total RNA isolated from partially fed adult female H. longicornis ticks was resolved on an 1% agarose gel containing formaldehyde and transferred to a nylon membrane (Hybond N+; Amersham). The membrane was hybridized with the α-32P-labeled PCR product amplified from the cloned p29 phage DNA by using the λscreen expression vector-specific primers (SP6 promoter and T7 terminator). +, native p29.
FIG. 3.
Southern blot analysis of tick genomic DNA. Tick genomic DNA was extracted and digested with indicated restriction enzymes followed by electrophoresis on a 0.8% agarose gel. Following electrophoresis, the separated DNA was transferred to a nylon membrane (Hybond N+; Amersham). The membrane was probed with the α-32P-labeled PCR product amplified from cloned p29 phage DNA by using the vector-specific primers (SP6 promoter and T7 terminator).
The cloned cDNA has an open reading frame extending from position 36 in frame to position 869 and codes for a mature 277-amino-acid protein with a predicted molecular mass of about 27.9 kDa (Fig. 1). By using PSORT (prediction of protein localization site) and the SignalIP V1.1 programs, the deduced polypeptide appears to have a possible cleavable, 18-amino-acid signal peptide, with the signal peptidase cutting between A and R at position 19 (Fig. 1). The translation product has a single possible N-glycosylation site and appears to be glycine rich. Compared to sequences of proteins in the Swissprot database, the predicted p29 amino acid sequence shows homology to sequences of extracellular matrix proteins such as Petunia hybrida glycine-rich cell wall structural protein (accession no. X04335; 31% amino acid similarity [AAS]), rat keratin (accession no. PNO109; 30% AAS), mouse elastin (accession no. A55721; 33% AAS), major ampullate silk fibroin (accession no. A36068; 27.6% AAS), and mouse alpha collagen 1 (accession no. Z211610; 28% AAS).
Visual examination of the p29 predicted amino acid sequence indicates that the domain structure has conserved structural similarities to most known collagen proteins. The domain structure of the mature p29 appears to have a non-GX1X2-containing N-terminal segment with 25 amino acid residues followed by a central domain containing GX1X2 tripeptide repeats and six interruptions with 130 amino acid residues. The GX1X2 tripeptide repeat domain (hereafter referred to as the collagenous domain) is followed by a non-GX1X2 C terminus with 120 amino acid residues. Within the central collagenous domain, the translation product shows G(S/G/Q)LGG pentapeptide and the GG dipeptide repeats as well as glycine residue replacement mutations (Fig. 1).
Estimation of the molecular mass of the native protein encoded by the cloned cDNA.
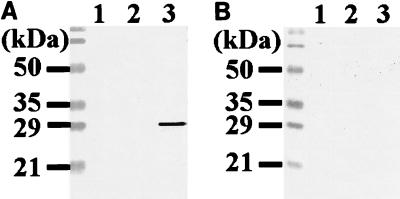
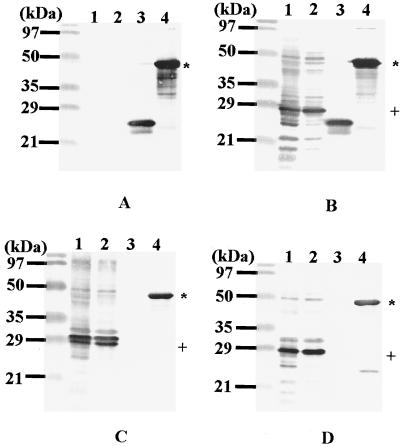
To determine the molecular mass of the tick protein corresponding to the cloned cDNA product, monospecific antibodies eluted from recombinant phage products were used to probe immunoblots of extracts from whole larval tick and salivary glands dissected from partially fed nymphal and adult ticks (Fig. 4A). The eluted monospecific antibodies reacted with a 29-kDa protein which was the most immunodominant protein of the adult tick salivary glands (lane 3), but the reactions against antigens from whole larval ticks and nymphal salivary glands were very faint (lanes 1 and 2). Control sera collected from naive rabbits did not react with the 29-kDa band (Fig. 4B).
FIG. 4.
Determination of the molecular mass of the tick protein corresponding the cloned cDNA product. Whole larval tick extracts (lane 1) and salivary glands dissected from partially fed nymphal and adult ticks (lanes 2 and 3) were electrophoresed on a 12.5% polyacrylamide gel and transferred to a PVDF membrane. The membrane was probed with monospecific antibodies affinity purified by the cloned phage DNA recombinant products as described in Materials and Methods (A) or naive rabbit sera used as a control (B).
In vitro expression and immunogenicity of rp29.
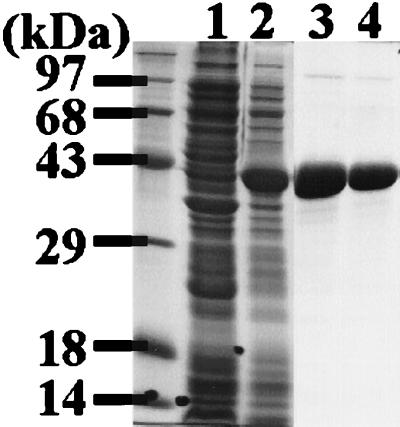
Figure 5 shows in vitro expression rp29, using the pET-32(c+) expression vector and E. coli BL21 λDES 3 (a phage λ lysogen) containing the LysS plasmid. On a 12.5% polyacrylamide gel as shown in Fig. 5, the rp29 appeared to be about 40 kDa, consistent with the expected molecular mass considering that the expression vector produced a recombinant protein fused with Trx of 11.3 kDa.
FIG. 5.
In vitro expression of rp29 in E. coli. The p29 coding sequence initially cloned in the λscreen vector was PCR amplified by using gene-specific primers with added restriction enzyme sites (EcoRI/SalI) for unidirectional cloning. The PCR product processed as described in Materials and Methods was subcloned into the pET-32(c+) expression vector. Induction of rp29 expression and subsequent purification were done as indicated in Materials and Methods. E. coli lysates of both the uninduced control and induced were routinely electrophoresed on 12.5% polyacrylamide gels and stained with Coomassie blue G-250. Lane 1, uninduced E. coli lysate; lane 2, IPTG-induced E. coli lysate; lanes 3 and 4, rp29 fractions purified by Ni affinity chromatography.
Figure 6 summarizes the immunogenicity of rp29 in rabbits. As shown in Fig. 6B to D, lanes 1, 2, and 4, native p29 and rp29 were commonly recognized by sera collected from rabbits immunized with rp29 or rabbits which had been naturally fed on by either larvae or adult H. longicornis ticks. Control serum from rabbits immunized with the Trx control protein reacted only Trx and rp29 a fusion protein fused to Trx (Fig. 6A, lanes 3 and 4), not the native p29 (lanes 1 and 2) indicating the specificity of the rp29 induced immune response.
FIG. 6.
Analysis of rp29 immunogenicity in rabbits. Salivary glands dissected from adult H. longicornis ticks fed for 2 (lane 1), and 4 (lane 2) days, as well as the control proteins, Trx (lane 3) and rp29 (lane 4), were electrophoresed on a 12.5% polyacrylamide gel. Following electrophoresis, the separated proteins were electroblotted on to PVDF membranes as described in Materials and Methods. The membranes were incubated with serum collected from Trx-vaccinated (A) and rp29-vaccinated (B) rabbits and rabbits repeatedly fed on by H. longicornis adult (C) and larval (D) ticks. The positive signal was detected as described in Materials and Methods. +, native p29; *, rp29.
The vaccine effect of rp29 against tick feeding in rabbits.
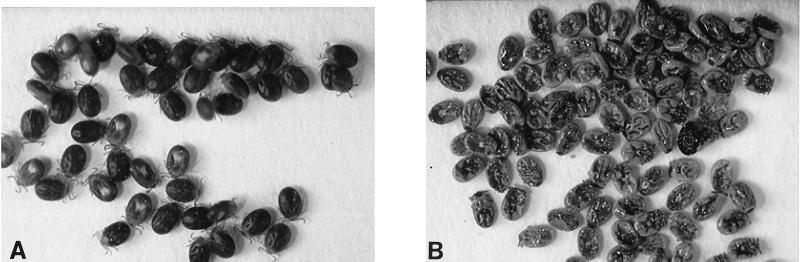
The rp29 vaccine effect on ticks feeding on rabbits immunized with the control protein and rp29 is summarized in Table 1. There was no difference in attachment rates during the initial 24 h after ticks were introduced onto rabbit ears. Except for adult ticks that fed on rp29-immunized rabbits, which had a shorter feeding duration (7 days, compared to 8 days for the control), there was no apparent difference in the feeding duration for larval and nymphal ticks feeding on either rp29-vaccinated rabbits or the control protein-vaccinated rabbits. While only a reduction in the engorgement weight was observed for adult ticks feeding on rp29-vaccinated rabbits compared to the control ticks, mortality of up to 40% for larval ticks and 56% for nymphal ticks was observed. Figure 7 shows the general appearance of larval ticks feeding on the rp29-vaccinated rabbits. Dead ticks (Fig. 7B) recovered from rp29-vaccinated rabbits appeared pale compared to the dark brown live ticks recovered from either rp29-vaccinated (Fig. 7A) or Trx-vaccinated rabbits. These observations were similar to those made on nymphal ticks (results not shown). Other than the observed mortality for the immature ticks and the reduced engorgement weights for the adult ticks, parameters of feeding performance were apparently similar. Behaviors of the hosts during the challenge infestation were comparable, as minimal brooding was observed on both the control protein- and rp29-vaccinated rabbits.
TABLE 1.
Comparison of feeding parameters for ticks feeding on rp29-vaccinated, Trx-vaccinated, and naive rabbits
| Parameter | Unimmunized, Adulta | Immunized with Trx
|
Immunized with p29b
|
||||
|---|---|---|---|---|---|---|---|
| Larva | Nymph | Adult | Larva | Nymph | Adult | ||
| Attachment (%) | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
| Engorged (%) | 85.0 | 83.0 | 93.3 | 77.5c | 85.0 | 94.9 | 72.5c |
| Duration of feeding (days) | 7–11 | 6–9 | 6–9 | 8–12 | 6–9 | 6–9 | 7–11 |
| Engorged weight (mg), mean (standard error) | 344 (6.6)d | NDe | 4.4f | 354 (7.0)d | ND | 4.3f | 295.4 (8.0)d |
| Mortality (%) | 0.0 | 3.5 | 1.1 | 0.0 | 40.0 | 56.0 | 0.0 |
Larva and nymph stages were not examined.
Average of two rabbits.
Ticks crushed by host were not included.
Statistically significant (P < 0.05).
ND, not determined.
Calculated as batch average (batch weight/number of nymphs).
FIG. 7.
General appearance of live (A) and dead (B) larval ticks recovered from a rp29-vaccinated rabbit.
DISCUSSION
The current results describe the cloning, DNA sequencing, and characterization of a cDNA coding for an immunodominant extracellular matrix-like protein associated with the salivary glands of larval, nymphal, and adult H. longicornis ticks. We have used a combination of immunologic and oligonucleotide screening to successfully clone and sequence a full-length cDNA of about 1 kbp. The cloned cDNA has an open reading frame of 833 bp coding for a mature polypeptide with about 28 kDa of predicted molecular mass, consistent with our finding that affinity-purified antibodies recognized a 29-kDa protein on immunoblots of whole larval tick and salivary gland antigens. A recombinant protein expressed in E. coli stimulated a specific protective antitick immune response in rabbits, as shown by the reduced engorgement weights for adult ticks and the observed mortality for immature ticks (larvae and nymph) feeding on rp29-immunized rabbits. The fact that sera raised against both larval and adult tick saliva commonly reacted with rp29 suggests that native p29 is expressed by both immature and mature ticks.
The tick p29 is likely an extracellular matrix-like protein, because of structural homology of its predicted amino acid sequence to sequences of known extracellular matrix proteins in the database. While the overall amino acid sequence homology of p29 to extracellular matrix proteins in the database is low, the domain structure of the translation product shows similarity to the highly conserved structural arrangement of all known collagens of both vertebrates and invertebrates (9, 13, 29, 41). Although the replacements of glycine residues observed in the present study may cause severe disease in vertebrates (18, 20), such events can be tolerated in some invertebrates (6, 15). Kramer (13), in his review on the Caenorhabditis elegans collagens, indicated that the glycine replacement mutations may inhibit triple-helix formation and result in the production of a reduced level of abnormal collagen. The same author (13) indicated that seven of the known C. elegans collagens have glycine replacement mutations; thus, the observed replacement mutations within the p29 collagenous domain are consistent with parasite collagens. Sicot et al. (29) have speculated that the glycine substitutions may allow formation of a less compact triple-helix region permitting cell adhesion to occur. While most known collagens have conserved cysteines (9, 13), the current translation product has none.
The fact that p29 is a salivary gland protein and an extracellular matrix-like protein suggests that native p29 may be associated with the formation of tick cement. Cement is among the initial chemical compounds that all ixodid ticks studied to date secrete and inject into the feeding site 5 to 30 min after the tick proboscis penetrates into the dermis of the host (27, 30). Sonenshine (30) has indicated that establishment of the cement cone precedes the acceleration of protein synthesis and expansion of the salivary glands in the feeding tick. The primary function of cement is to enable the tick to remain attached to the host (30) and prevent host immune response molecules from coming in contact with the tick proboscis. This indicates the importance of cement with respect to the success of the tick both as a vector and as a pest and hence a good target for a host immune response against tick infestation. The chemical composition of cement consists of a mixture of antigenic and nonantigenic proteins, with substantial lipid and carbohydrates in the innermost layers, the outer layers mostly in the form of lipoproteins and glycoproteins. A quarter of the cement protein content are tightly bound and cross-linked (27), which may suggest the presence in cement of helix-forming polypeptides, such as the collagen-like p29 reported here. It will be interesting to define the functional role of p29 in the formation of cement. To this end, characterization of other cement molecules and analysis of their interaction with p29 and host molecules will be necessary.
The observed mortality in immature ticks (nymph and larvae) as well as the reduced engorgement weight in adult ticks feeding on rp29-vaccinated rabbits suggests that p29 may be involved the mediation of key physiological functions in the biological success of H. longicornis and hence an interesting target antigen for a tick vaccine component. Although both the mature and immature ticks commonly express native p29, their sensitivities to the rabbit immune response against rp29 appear to be different. Data from studies by Kemp et al. (11, 12) provided evidence which may support the hypothesis that immature and mature ticks have different sensitivities to host acquired resistance against tick molecules. Kemp et al. (11, 12) found that while there was severe gut damage in both adult female and male B. microplus ticks feeding on cattle vaccinated with B. microplus-derived antigens, there was no effect on larval ticks feeding on the same protected animals. Findings from these studies are therefore consistent with our findings of different vaccine effects on the immature and mature H. longicornis ticks which fed on rp29-vaccinated rabbits. We have not considered the effect of rabbit immune response against rp29 on postattachment parameters such as oviposition and hatchability because we are interested in molecules that mediate the attachment and disease transmission process of hard ticks, and hence consideration of postengorgement parameters is not essential.
Opdebeeck (19) analyzed the merits and demerits of using either exposed (tick saliva proteins injected into the host during tick feeding) or concealed (tick proteins not exposed to host immunity during feeding) antigens as vaccine candidates. Tick concealed antigens produce immunity that inhibits tick fecundity but has utility for effect preventing tick feeding, while the opposite is true for tick saliva proteins (25). Sahibi et al. (25) vaccinated cattle against tick infestation by using either salivary glands or intestinal extracts and found that immunity induced by the former was superior in reducing both attachment rates and engorgement weights whereas the later was superior in reducing fecundity of Hyaloma marginatum marginatum. These findings implied that salivary gland-derived antigens can prevent or minimize pathogen transmission, a basis for tick control better than that provided by intestinal extracts (25).
Our results have emphasized the fact that a single tick molecule, despite its immunodominance, may not confer an inclusive protective immunity. An effective tick vaccine will require a cocktail of target antigens each mediating a physiological function either independently or synergistically (28). Findings by Riding et al. (24) and Willadsen et al. (40) provided evidence that antitick immunity induced by a cocktail antigen vaccine is more effective compared to a single antigen vaccine. These authors (24, 40) found that when the two B. microplus midgut-based antigens Bm 86 and Bm 91 were used as a cocktail to immunize cattle, the anti-B. microplus immunity induced was more effective than when either of the molecules was used alone. Our efforts are currently directed toward characterization and in vitro expression of other tick molecules for use in a cocktail vaccine trial in combination with rp29.
ACKNOWLEDGMENTS
This project was funded by grants from the Ministry of Education, Science, Sports and Culture, Japan.
We thank C. Nkonge, ILRI, for technical assistance.
REFERENCES
- 1.Binnington K C, Kemp D H. Role of the tick salivary glands in feeding and disease transmission. Adv Parasitol. 1980;18:316–340. doi: 10.1016/s0065-308x(08)60403-0. [DOI] [PubMed] [Google Scholar]
- 2.Bowman A S, Dillwith J W, Sauer J R. Tick salivary prostaglandins: presence, origin and significance. Parasitol Today. 1996;12:388–396. doi: 10.1016/0169-4758(96)10061-2. [DOI] [PubMed] [Google Scholar]
- 3.Church M G, Gilbert W. Genomic sequencing; DNA methylation/UV cross linking/filter hybridisation/immunoglobulin genes. Proc Natl Acad Sci USA. 1984;81:1991–1995. doi: 10.1073/pnas.81.7.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casatanzi C, Gillepsie D. Fast blots; immobilization of DNA and RNA from cells. In: Berger S L, Kimmel A R, editors. Methods in enzymology; guide to molecular cloning techniques. New York, N.Y: Academic Press, Inc.; 1987. pp. 582–587. [DOI] [PubMed] [Google Scholar]
- 5.Elvin C M, Kemp D H. Generic approaches to obtaining efficacious antigens from vector arthropods. Int J Parasitol. 1994;24:67–79. doi: 10.1016/0020-7519(94)90060-4. [DOI] [PubMed] [Google Scholar]
- 6.Exposito J Y, Van der Rest M, Garrone R. The complete intron/exon structure of Ephydatia mulleri fibrillar collagen gene suggests a mechanism for evolution of an ancestral gene module. J Mol Evol. 1993;37:254–259. doi: 10.1007/BF00175502. [DOI] [PubMed] [Google Scholar]
- 7.Hopp T P, Woods K R. Prediction of protein antigenic determinants from amino acid sequences. Proc Natl Acad Sci USA. 1981;78:3824–3828. doi: 10.1073/pnas.78.6.3824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jarwoorski C D, Muller M T, Simen F A, Needham G R. Amblyomma americanum: identification of tick salivary gland antigens from unfed and early feeding females with comparisons to Ixodes dammini and Dermancetor variabilis. Exp Parasitol. 1990;70:217–226. doi: 10.1016/0014-4894(90)90102-i. [DOI] [PubMed] [Google Scholar]
- 9.Johnstone I L, Shafi Y, Majeed A, Barry D J. Cuticular collagen genes from the parasitic nematode Ostertagia circumcinta. Mol Biochem Parasitol. 1996;80:103–112. doi: 10.1016/0166-6851(96)02682-5. [DOI] [PubMed] [Google Scholar]
- 10.Kay H B, Kemp D H. Vaccines against arthropods. Am J Trop Med Hyg. 1994;50(Suppl.):87–96. doi: 10.4269/ajtmh.1994.50.87. [DOI] [PubMed] [Google Scholar]
- 11.Kemp D H, Agbede R I S, Johnston L A Y, Gough J M. Immunization of cattle against Boophilus microplus using extracts derived from adult female ticks: feeding and survival of the parasite on vaccinated cattle. Int J Parasitol. 1986;16:115–120. doi: 10.1016/0020-7519(86)90096-2. [DOI] [PubMed] [Google Scholar]
- 12.Kemp D H, Pearson R D, Gough J M, Willadsen P. Vaccination against Boophilus microplus: localization of antigens on the tick gut cells and their interaction with the host immune system. Exp Appl Acarol. 1989;7:43–58. doi: 10.1007/BF01200452. [DOI] [PubMed] [Google Scholar]
- 13.Kramer J M. Structures and functions of collagens in Caenorhabditis elegans. FASEB J. 1994;8:329–336. doi: 10.1096/fasebj.8.3.8143939. [DOI] [PubMed] [Google Scholar]
- 14.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 15.Maan K, Gail F, Timpl R. Amino acid sequence and cell-adhesion activity of a fibril forming collagen from the tube worm Riftia pachyptila living at deep sea hydrothermal vents. Eur J Biochem. 1992;210:255–266. doi: 10.1111/j.1432-1033.1992.tb17487.x. [DOI] [PubMed] [Google Scholar]
- 16.Minami T, Fujinaga T, Furuya K, Ishihara T. Clinico-heamtologic and serological comparison of Japanese and Russian strains of Theileria sergenti. Natl Inst Anim Health Q. 1980;20:44–52. [PubMed] [Google Scholar]
- 17.Obechan F D, Galun R, editors. Physiology of ticks. Oxford, England: Pergamon Press; 1982. [Google Scholar]
- 18.Olsen B R. New insights into the function of collagens from genetic analysis. Curr Opin Cell Biol. 1995;7:720–727. doi: 10.1016/0955-0674(95)80115-4. [DOI] [PubMed] [Google Scholar]
- 19.Opdebeeck J P. Vaccines against blood-sucking arthropods. Vet Parasitol. 1994;54:205–222. doi: 10.1016/0304-4017(94)90091-4. [DOI] [PubMed] [Google Scholar]
- 20.Prockop D J, Kiviriko K I. Collagens: molecular biology diseases and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 21.Rand K N, Moore T, Srikantha A, Spring K, Tellam R, Willadsen P, Cobon G S. Cloning and expression of a protective antigen from the cattle tick Boophilus microplus. Proc Natl Acad Sci USA. 1989;86:9657–9661. doi: 10.1073/pnas.86.24.9657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ribeiro J M C. Role of saliva in blood feeding by arthropods. Annu Rev Entomol. 1987;32:463–478. doi: 10.1146/annurev.en.32.010187.002335. [DOI] [PubMed] [Google Scholar]
- 23.Ribeiro J M C. The midgut hemolysin of Ixodes dammini (Acari: Ixodidae) J Parasitol. 1988;74:532–537. [PubMed] [Google Scholar]
- 24.Riding G A, Jarmey J, McKenna R V, Pearson R, Cobon G S, Willadsen P. A protective concealed antigen from Boophilus microplus: purification, localization and possible function. J Immunol. 1994;153:5158–5166. [PubMed] [Google Scholar]
- 25.Sahibi H, Rhalem A, Barriga O O. Comparative immunizing power of infections, salivary extracts, and intestinal extracts of Hyalomma marginatum marginatum in cattle. Vet Parasitol. 1997;68:359–366. doi: 10.1016/s0304-4017(96)01082-5. [DOI] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sauer J R, McSwain J L, Bowman A S, Essenberg R C. Tick salivary gland physiology. Annu Rev Entomol. 1995;40:245–267. doi: 10.1146/annurev.en.40.010195.001333. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro S Z, Voigt W P, Fujisaki K. Tick antigens recognized by serum from a guinea pig resistant to infestation with the tick Rhipicephalus appendiculatus. J Parasitol. 1986;72:454–463. [PubMed] [Google Scholar]
- 29.Sicot F X, Exposito J Y, Masselot M, Garrone R, Deutsch F. Cloning of an annelid fibrillar-collagen gene and phylogenetic analysis of vertebrate and invertebrate collagens. Eur J Biochem. 1997;246:50–56. doi: 10.1111/j.1432-1033.1997.00050.x. [DOI] [PubMed] [Google Scholar]
- 30.Sonenshine D E. Biology of ticks. Vol. 2. Oxford, England: Oxford University Press; 1993. [Google Scholar]
- 31.Soulsby E J L. Helminths, arthropods and protozoa of domesticated animals. 7th ed. London, England: Bailliere Tindall; 1986. [Google Scholar]
- 32.Tellam R L, Smith D, Kemp D H, Willadsen P. Vaccination against ticks. In: Wong W K, editor. Animal parasite control utilizing biotechnology. Boca Raton, Fla: CRC Press Inc.; 1992. pp. 303–331. [Google Scholar]
- 33.Trager W. Acquired immunity to ticks. J Entomol. 1939;25:57–81. [Google Scholar]
- 34.Wikel S K. Immunological control of hematophagous arthropod vectors: utilization of novel antigens. Vet Parasitol. 1988;29:235–264. doi: 10.1016/0304-4017(88)90127-6. [DOI] [PubMed] [Google Scholar]
- 35.Wikel S K. Host immunity to ticks. Annu Rev Entomol. 1996;41:1–22. doi: 10.1146/annurev.en.41.010196.000245. [DOI] [PubMed] [Google Scholar]
- 36.Wikel S K, Bergman D. Tick immunology: significant advances and challenging opportunities. Parasitol Today. 1997;13:383–389. doi: 10.1016/s0169-4758(97)01126-5. [DOI] [PubMed] [Google Scholar]
- 37.Wikel S K, Whelen C A. Ixodid-host immune interaction. Identification and characterization of relevant antigens and tick induced host immunosuppression. Vet Parasitol. 1986;20:149–174. doi: 10.1016/0304-4017(86)90098-1. [DOI] [PubMed] [Google Scholar]
- 38.Willadsen P. Immunity to ticks. Adv Parasitol. 1980;18:293–313. doi: 10.1016/s0065-308x(08)60402-9. [DOI] [PubMed] [Google Scholar]
- 39.Willadsen P, Bird J, Cobon G S, Hungerford J. Commercialisation of a recombinant vaccine against B. microplus. Parasitology. 1995;110(Suppl):s43–s50. doi: 10.1017/s0031182000001487. [DOI] [PubMed] [Google Scholar]
- 40.Willadsen P, Smith D, Cobon G, McKenna R V. Comparative vaccination of cattle against Boophilus microplus with recombinant Bm86 alone or in combination with recombinant Bm91. Parasite Immunol. 1996;18:241–246. doi: 10.1046/j.1365-3024.1996.d01-90.x. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R Z, Pan C T, Timpl R, Chu M L. Cloning and sequence analysis of cDNAs encoding the α1, α2 and α3 chains of mouse collagen. Biochem J. 1993;291:787–792. doi: 10.1042/bj2910787. [DOI] [PMC free article] [PubMed] [Google Scholar]