Abstract
Objective:
Elevated vascular risk and beta-amyloid (Aβ) burden have been synergistically associated with cognitive decline in preclinical Alzheimer’s disease (AD), although the underlying mechanisms remain unclear. We examined whether accelerated longitudinal tau accumulation mediates the vascular risk-Aβ interaction on cognitive decline.
Methods:
We included 175 cognitively unimpaired older adults (age 70.5 ± 8.0 years). Baseline vascular risk was quantified using the office-based Framingham Heart Study general cardiovascular disease risk score (FHS-CVD). Baseline Aβ burden was measured with Pittsburgh Compound-B positron emission tomography (PET). Tau burden was measured longitudinally (3.6 ± 1.5 years) with Flortaucipir PET, focusing on inferior temporal cortex (ITC). Cognition was assessed longitudinally (7.0 ± 2.0 years) using the Preclinical Alzheimer’s Cognitive Composite. Linear mixed effects models examined the interactive effects of baseline vascular risk and Aβ on longitudinal ITC tau. Additionally, moderated mediation was used to determine whether tau accumulation mediated the FHS-CVD*Aβ effect on cognitive decline.
Results:
We observed a significant interaction between elevated baseline FHS-CVD and Aβ on greater ITC tau accumulation (p = 0.004), even in individuals with Aβ burden below the conventional threshold for amyloid positivity. Examining individual vascular risk factors, we found elevated systolic blood pressure and body mass index showed independent interactions with Aβ on longitudinal tau (both p < 0.0001). ITC tau accumulation mediated 33% of the interactive association of FHS-CVD and Aβ on cognitive decline.
Interpretation:
Vascular risks interact with subthreshold levels of Aβ to promote cognitive decline, partially by accelerating early neocortical tau accumulation. Our findings support vascular risk reduction, especially treating hypertension and obesity, to attenuate Aβ-related tau pathology and reduce late-life cognitive decline.
Background
Despite recent progress in anti-amyloid therapy,1 there remains an urgent need for safe and effective treatments that can meaningfully alter the course of Alzheimer’s disease (AD). There is growing consensus that elevated beta-amyloid (Aβ) burden is necessary but insufficient to cause AD.2 It is therefore critical to identify, characterize, and target factors that modify the emergence of cognitive decline in concert with Aβ pathology.
Elevated vascular risk and cerebrovascular disease are very common with aging, often co-exist with AD pathology,3 and are increasingly recognized as significant contributors to AD-related cognitive decline.4-8 Elevated systemic vascular risk, particularly when combined with elevated Aβ burden, is associated with accelerated cognitive decline and increased dementia risk in cognitively unimpaired (CU) individuals.7,9 This suggests that interventions to reduce vascular risk may be effective in delaying the onset or progression of cognitive decline. However, the mechanisms underlying this clinically important interaction between vascular risk and Aβ remain to be elucidated.
A previous study demonstrated that higher baseline vascular risk and Aβ were synergistically associated with greater cross-sectional inferior temporal cortex (ITC) tau burden in older CU adults.10 These findings implicate vascular risk related promotion of tau pathology as a potential mechanism linking vascular risk to AD pathology and early AD-related cognitive decline. Better elucidating longitudinal relationships between vascular risk and AD pathology is critical to confirm these cross-sectional findings. If vascular risks are indeed synergistic with Aβ in promoting longitudinal tau accumulation, it would strongly support targeting vascular risk reduction – alone or in combination with AD-pathology focused therapies – as a possible disease-modifying treatment strategy in AD. Additionally, it would support the need to consider vascular risk factors in ongoing and future AD clinical trials, particularly those that use tau positron emission tomography (PET) or cognitive decline as outcomes.
In this context, the present study tested the hypothesis that elevated baseline vascular risk and Aβ burden would be synergistically associated with greater longitudinal ITC tau accumulation, in a deeply phenotyped group of older CU adults participating in the Harvard Aging Brain Study (HABS). We further examined if, and to what extent, tau accumulation mediated the impact of elevated vascular risk on prospective cognitive decline in individuals with elevated Aβ burden.
Methods
Participants
One hundred seventy-five older CU adults were recruited from HABS. We included all participants who had baseline Aβ PET imaging, at least 2 tau PET and cognitive assessments, and sufficient baseline medical information to calculate an algorithmic vascular risk score. At study entry, all participants had global Clinical Dementia Rating11 of 0, education-adjusted Mini-Mental State Examination (MMSE)12 score of 27 or greater, and normal Logical Memory IIa delayed recall performance.13 Exclusion criteria included a modified Hachinski ischemic score greater than 4, and a history of stroke or evidence of infarcts with persistent neurological deficits.14 Data were collected from April 2010 through August 2021. The Mass General Brigham Institutional Review Board approved HABS protocol and procedures, and all participants signed a written informed consent prior to the completion of any study procedures.
Cardiovascular Disease Risk
Our primary measure of cardiovascular disease risk was the office-based Framingham Heart Study cardiovascular disease risk score (FHS-CVD),15 which represents a sex-specific weighted sum of age, antihypertensive treatment (dichotomous), systolic blood pressure (SBP; millimeters of mercury), body mass index (BMI), diabetes status (dichotomous), and cigarette smoking status (dichotomous). The FHS-CVD score provides a 10-year probability of future cardiovascular events, including coronary death, myocardial infarction, coronary insufficiency, angina, ischemic stroke, hemorrhagic stroke, transient ischemic attack, peripheral artery disease, and heart failure. For sensitivity analyses, we calculated the lipid-based FHS-CVD score15 and the atherosclerotic cardiovascular disease (ASCVD) risk score16 for the subset of participants with available serum lipid profiles (n = 155).
PET Imaging
Brain Aβ burden was measured at baseline using 11C-Pittsburgh Compound-B (PiB) PET at the Massachusetts General Hospital using the ECAT EXACT HR+ scanner (Siemens). 18F-Flortaucipir (FTP) PET was introduced into HABS mid-study to measure tau burden, with participants undergoing their first FTP PET at 2.1 ± 1.5 years after baseline visit. Longitudinal tau PET measurements were obtained at year 1 (for those enrolled after FTP PET was introduced), 4, 6, and 9 visits. Detailed Aβ and tau PET protocols have been previously described.17 Aβ PET measurements were represented as a distribution volume ratio (DVR) across a composite of frontal, lateral temporal and parietal, and retrosplenial regions (FLR), defined using FreeSurfer (version 6.0). Tau PET measurements were computed a standardized uptake value ratio (SUVR) within the ITC, an early site of neocortical tau accumulation associated with clinical impairment17 and a region in which cross-sectional associations with vascular risk and Aβ were previously observed.10 Both Aβ and tau PET data used cerebellar gray matter as the reference region and used the geometric transfer matrix method for partial volume correction (PVC).18
Cognitive Measures
Cognition was assessed annually using the Preclinical Alzheimer’s Cognitive Composite-5 (PACC5).19 The PACC5 is a composite that includes the MMSE,12 Wechsler Adult Intelligence Scale–Revised Digit Symbol Coding,20 Wechsler Memory Scale–Revised Logical Memory delayed recall,13 Free and Cued Selective Reminding Test (free recall plus total recall),21 and Category Fluency Test.22
Statistical Analyses
We used R version 4.0.5 for statistical analyses. Linear mixed effects model (“nlme” package) was used to examine the interactive effects of baseline FHS-CVD scores and Aβ on ITC tau burden over time. Time was operationalized as years from the baseline Aβ PET scan. We adjusted for age, sex, APOE ε4 carrier status, their interactions with time, and included random intercepts and slopes. Continuous variables were z-transformed prior to model entry. We performed sensitivity analyses using PiB and FTP PET data without PVC, as well as using the lipid-based FHS-CVD and ASCVD risk scores. For post hoc analyses, we further examined the interactive effects between individual component measures of FHS-CVD and Aβ on longitudinal ITC tau. We applied family-wise error (FWE) correction for multiple comparisons (6 component measures of FHS-CVD), with p < 0.008 for significance.
Next, we examined the FHS-CVD by Aβ interaction using floodlight/Johnson-Neyman analysis (“interactions” package) to determine the level of Aβ at which effects on ITC tau accumulation became significant (p < 0.05). For floodlight analysis, we extracted individual slopes of ITC tau change from unadjusted linear mixed effects models with random slopes and intercepts that have time as the only predictor of ITC tau.
Additionally, we conducted whole-brain, region of interest (ROI)-based exploratory analyses to examine whether the interaction between baseline FHS-CVD risk and Aβ burden on longitudinal tau accumulation extended beyond the ITC. We used FreeSurfer-defined cortical and subcortical ROIs, averaged across both hemispheres. Models were adjusted for age, sex, APOE ε4 carrier status, their interactions with time, and included random intercepts and slopes.
Last, we used moderated mediation analysis (“mediation” package) to examine whether tau accumulation mediated the interactive effects of FHS-CVD and Aβ on PACC5 decline. Individual PACC5 slopes were extracted from unadjusted linear mixed effects model. We conducted moderated mediation analysis with PACC5 slope as outcome, ITC tau slope as mediator, baseline Aβ burden as moderator, and adjusted for age, sex, APOE ε4 carrier status, education (years), and time interval between baseline Aβ and first tau scan. Mediation models were run at both low and high levels of baseline Aβ burden, defined respectively by the mean Aβ burden of amyloid-negative (PiB PVC-DVR = 1.17) and amyloid-positive (PiB PVC-DVR = 1.85) participants, dichotomized using the conventional amyloid-positivity threshold (PiB PVC-DVR of 1.32 in HABS).
Results
The baseline characteristics of the participants and lengths of follow-up are summarized in Table 1. The individual trajectories for ITC tau burden are shown in Figure 1. To more clearly visualize the individual tau trajectories, the data were plotted according to baseline levels of Aβ burden (divided by median split) and FHS-CVD scores (divided by tertiles).
TABLE 1.
Participant Characteristics
| Characteristic | All participants, N = 175 |
|---|---|
| Age at baseline, yr, mean (SD) | 70.5 (8.0) |
| Females, n (%) | 110 (62.9) |
| White race, n (%) | 150 (85.7) |
| Education, yr, mean (SD) | 16.2 (2.9) |
| APOE ε4 carriers, n (%) | 52 (29.7) |
| Baseline FHS-CVD risk score, mean (SD) | 27.3 (18.5) |
| Baseline systolic blood pressure, mean (SD), mmHg | 137.1 (18.7) |
| Baseline body mass index, mean (SD) | 26.8 (4.4) |
| Positive diabetes status at baseline, n (%) | 13 (7.4) |
| Positive smoking status at baseline, n (%) | 7 (4.0) |
| Baseline PiB PET PVC-DVR in FLR regions, mean (SD) | 1.33 (0.4) |
| β-Amyloid positive, n (%) | 43 (24.6) |
| Time interval between baseline and first tau PET scan, mean (SD), yr | 2.1 (1.5) |
| Time interval between baseline and last tau PET scan, mean (SD), yr | 5.6 (2.1) |
| First ITC tau PET SUVR, mean (SD) | 1.45 (0.2) |
| # Longitudinal tau PET scans, mean (SD) | 2.4 (0.6) |
| Duration of tau PET follow-up, mean (SD), yr | 3.6 (1.5) |
| # Longitudinal PACC5 assessments, mean (SD) | 7.9 (2.5) |
| Duration of cognitive follow-up, mean (SD), yr | 7.0 (2.0) |
APOE ε4 = apolipoprotein E ε4 allele; DVR = distribution volume ratio; FHS-CVD = Framingham Heart Study cardiovascular disease risk score; FLR = frontal, lateral temporal and parietal, and retrosplenial regional uptake; ITC = inferior temporal cortex; mm Hg = millimeters of mercury; PACC5 = Preclinical Alzheimer’s Cognitive Composite–5; PET = positron emission tomography; PiB = Pittsburgh Compound–B; PVC = partial volume correction; SUVR = standardized uptake value ratio.
FIGURE 1:
Accumulation of tau pathology as a function of baseline Aβ burden and level of systemic vascular risk. Individual ITC tau burden trajectories from all participants are shown. To allow clear visualization of individual tau trajectories, participants were divided according to baseline Aβ burden (by median split) and the FHS-CVD (by tertiles) using cutoffs shown in the facet labels for the columns and rows, respectively. The trajectories are color coded by baseline Aβ burden according to the color bar, with each line representing one participant. The time of baseline PiB PET was used as the study baseline (time = 0). Timing of the first tau PET scan varied across participants (2.1 ± 1.5 years), as tau PET was introduced mid-study in HABS. Aβ = β-amyloid; FHS-CVD = Framingham Heart Study cardiovascular disease risk score; DVR = distribution volume ratio; HABS = Harvard Aging Brain Study; ITC = inferior temporal cortex; PVC = partial volume correction; PET = positron emission tomography; PiB = Pittsburgh Compound--B; SUVR = standardized uptake value ratio.
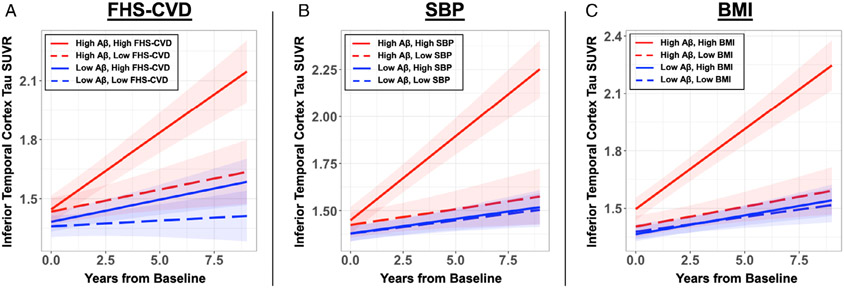
There was no significant association between baseline FHS-CVD scores and Aβ burden, adjusting for age and sex (rpartial = −0.0013, p = 0.99; r = 0.098, p = 0.20 without age/sex adjustment). The primary goal of the study was to investigate whether baseline FHS-CVD and Aβ have an interactive effect on longitudinal ITC tau burden. We found a significant interaction between higher baseline FHS-CVD and elevated Aβ in association with greater tau burden over time (β = 0.05 [0.02–0.09], t = 2.88, p = 0.004; Fig 2A, Table 2). This interaction remained significant in sensitivity analyses using the lipid profile-based FHS-CVD (β = 0.07 [0.03–0.12], t = 3.54, p < 0.001) and ASCVD risk scores (β = 0.06 [0.01–0.10], t = 2.64, p = 0.009). Similarly, the interaction remained significant using Aβ and tau PET data without PVC (β = 0.002 [0.0005–0.004], t = 2.49, p = 0.01).
FIGURE 2:
Elevated baseline cardiovascular risks and Aβ are synergistically associated with accelerated inferior temporal cortex tau accumulation. The FHS-CVD and its component vascular risk factors were modelled separately in linear mixed effects models. There was significant vascular risk*Aβ*time interaction on longitudinal ITC tau burden for (A) FHS-CVD score (β = 0.05 [0.02–0.09], t = 2.88, p = 0.004), (B) SBP (β = 0.09 [0.06–0.12], t = 5.37, p < 0.001), and (C) BMI (β = 0.08 [0.05–0.11], t = 5.43, p < 0.001). To visualize the model results, estimated ITC tau trajectory based on different levels of baseline vascular risk and Aβ are presented. Low and high vascular risk are represented by −1 SD and +1 SD from mean baseline values: FHS-CVD low 8.8%, high 45.8%; SBP low 118.5 mmHg, high 155.8 mmHg; BMI low 22.4, high 31.2. Low and high Aβ levels are represented by the mean partial volume corrected Pittsburgh Compound-B distribution volume ratio (PiB PVC-DVR) of amyloid-negative (1.17) and amyloid-positive (1.85) participants, respectively, dichotomized using the conventional amyloid-positivity threshold (PiB PVC-DVR of 1.32 in our cohort). Aβ = β-amyloid; BMI = body mass index; FHS-CVD = Framingham Heart Study cardiovascular disease risk score; ITC = inferior temporal cortex; SBP = systolic blood pressure; SUVR = standardized uptake value ratio.
TABLE 2.
Summary of Linear Mixed Effects Model Examining the Interactive Effects of Baseline Vascular Risk and Amyloid Burden on Longitudinal Inferior Temporal Tau Burden
| Model: ITC tau ~ FHS-CVD*Aβ*Time + Age*Time + Sex*Time + APOE ε4*Time | ||||
|---|---|---|---|---|
| β Estimate (95% CI) | Standard Error |
t Value | p | |
| FHS-CVD*Aβ*Time | 0.05 [0.2 to 0.9] | 0.02 | 2.88 | 0.004 |
| FHS-CVD*Aβ | −0.02 [−0.16 to 1.32] | 0.07 | −0.21 | 0.84 |
| FHS-CVD*Time | 0.07 [0.02 to 0.11] | 0.02 | 2.77 | 0.01 |
| Aβ*Time | 0.10 [0.06 to 0.12] | 0.02 | 5.78 | <0.001 |
| FHS-CVD | 0.05 [−0.14 to 0.25] | 0.10 | 0.52 | 0.60 |
| Aβ | 0.18 [0.04 to 0.32] | 0.07 | 2.59 | 0.01 |
| Age*Time | −0.02 [−0.06 to 0.02] | 0.02 | −1.08 | 0.28 |
| Sex (F)*Time | 0.12 [0.04 to 0.20] | 0.04 | 2.94 | 0.004 |
| APOE (ε4+)*Time | 0.05 [−0.01 to 0.12] | 0.04 | 1.56 | 0.12 |
| Age | 0.24 [0.08 to 0.40] | 0.08 | 2.95 | 0.004 |
| Sex (F) | 0.02 [−0.30 to 0.34] | 0.16 | 0.14 | 0.89 |
| APOE (ε4+) | −0.06 [−0.34 to 0.22] | 0.14 | −0.41 | 0.68 |
| Time | 0.03 [−0.02 to 0.09] | 0.03 | 1.15 | 0.25 |
All continuous variables were z-transformed prior to model entry.
Aβ = amyloid-beta; APOE ε4 = apolipoprotein E ε4 allele; CI = confidence interval; F= female; FHS-CVD = Framingham Heart Study cardiovascular disease risk score; ITC = inferior temporal cortex.
To explore whether certain vascular risk factors were driving this interaction with Aβ on longitudinal tau, we conducted post hoc analyses to investigate the interactive effects between individual component measures of FHS-CVD and Aβ on longitudinal ITC tau. Of the 6 FHS-CVD components, only SBP (adjusted for antihypertensive use) and BMI showed significant interactions with Aβ on ITC tau burden over time (p < 0.008 for significance for FWE correction), with higher SBP and BMI predicting greater longitudinal ITC tau in setting of elevated baseline Aβ (Table 3; Fig 2B, C). Importantly, there was no significant correlation between baseline SBP and BMI in our sample (r = 0.09, p = 0.23). We further included SBP and BMI in the same linear mixed effects model to test whether their interactions with Aβ and time have independent effects on ITC tau accumulation. Interactions with both measures remained significant, with effect sizes similar to results from their individual models (SBP*Aβ*Time: t = 5.28, p < 0.001; BMI*Aβ*Time: t = 5.08, p = < 0.001). Whereas higher age and having diabetes showed no significant interactive or independent effects on ITC tau burden over time, both were associated with greater baseline ITC tau burden (age: t = 3.769, p < 0.001; diabetes: t = 2.656, p = 0.009).
TABLE 3.
Summary of Linear Mixed Effects Models Examining the Interactive Effects of Baseline Framingham Heart Study Cardiovascular Disease Risk Score Components and Amyloid Burden on Longitudinal Inferior Temporal Tau Burden
| β Estimate (95% CI) | Standard Error | t Value | p | |
|---|---|---|---|---|
| Age*Aβ*Time | 0.02 [−0.02 to 0.06] | 0.02 | 1.01 | 0.31 |
| Sex (F)*Aβ*Time | 0.02 [−0.05 to 0.08] | 0.03 | 0.47 | 0.64 |
| SBP*Aβ*Time | 0.09 [0.06 to 0.12] | 0.02 | 5.37 | <0.001a |
| BMI*Aβ*Time | 0.08 [0.05 to 0.11] | 0.01 | 5.43 | <0.001a |
| Smoking*Aβ*Time | −0.03 [−0.19 to 0.12] | 0.08 | −0.40 | 0.69 |
| Diabetes*Aβ*Time | 0.10 [−0.07 to 0.26] | 0.09 | 1.14 | 0.25 |
Individual vascular risk factors that make up the FHS-CVD score were examined separately for interactive effects with Aβ on ITC tau burden across time. Each vascular risk factor (age, sex, SBP, BMI, smoking, or diabetes) was included in a separate linear mixed effects model and the model results for each vascular risk factor’s interaction with Aβ and time are shown. Models are specified as follows for each vascular risk factor: ITC tau ~ Vascular Risk Factor*Aβ*Time + Age*Time + Sex*Time + APOE ε4*Time. The model for SBP was also adjusted for antihypertensive medication use and its interaction with time
Significant at FWE-corrected threshold of <0.0083.
Aβ = β-amyloid; APOE ε4 = apolipoprotein E ε4 allele; BMI = body mass index; CI = confidence interval; FHS-CVD = Framingham Heart Study cardiovascular disease risk; FWE = family-wise error; ITC = inferior temporal cortex; SBP = systolic blood pressure.
We next conducted floodlight analysis to determine the level of baseline Aβ burden at which interactions with FHS-CVD on ITC tau accumulation became significant (p < 0.05). Results revealed that the FHS-CVD effects on longitudinal ITC tau became significant when baseline Aβ exceeded PiB PVC-DVR of 1.23 (corresponding to 11.8 Centiloids23 in the HABS cohort), which is substantially lower than the conventional threshold for amyloid positivity in this sample (PiB PVC-DVR >1.32 or 18.2 Centiloids).
Additionally, we explored whether the interactive effects between FHS-CVD and Aβ on longitudinal tau burden are limited to the ITC by performing exploratory whole brain analyses using FreeSurfer-based ROIs. Results revealed additional regions with nominally significant synergistic interactions, predominantly in medial temporal and other areas implicated in early neocortical tau spread: amygdala (t = 3.13, p = 0.002), fusiform (t = 2.81, p = 0.005), middle temporal (t = 2.31, p = 0.02), supramarginal (t = 2.72, p = 0.007), and pars orbitalis (t = 2.48, p = 0.01; Fig 3).
FIGURE 3:
Greater systemic vascular risk and baseline Aβ burden are synergistically associated with greater tau accumulation in multiple FreeSurfer-defined regions of interest. We performed an exploratory analysis to assess whether regions beyond the inferior temporal cortex showed greater tau accumulation in the setting of greater vascular risk, as measured by the FHS-CVD, and Aβ burden. Linear mixed effects models revealed significant FHS-CVD*Aβ*time interactions on longitudinal tau burden in the amygdala (t = 3.13, p = 0.002), fusiform (t = 2.81, p = 0.005), middle temporal (t = 2.31, p = 0.02), inferior temporal (t = 2.88, p = 0.004), supramarginal (t = 2.72, p = 0.007), and pars orbitalis (t = 2.48, p = 0.01) regions. Aβ = β-amyloid; FHS-CVD = Framingham Heart Study cardiovascular disease risk score.
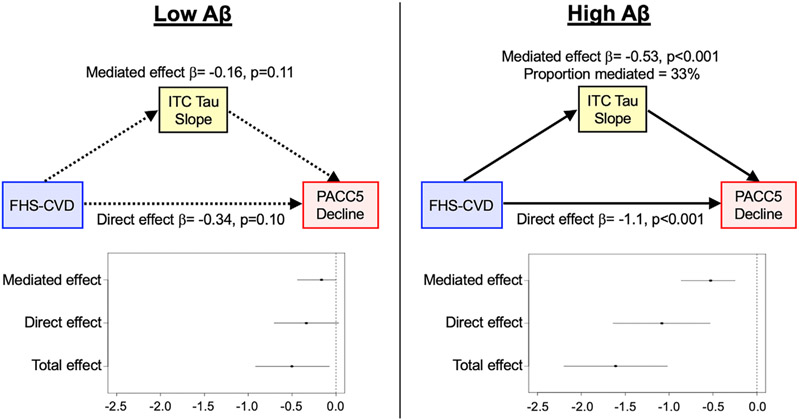
Last, we determined whether ITC tau accumulation may mediate the synergistic association between FHS-CVD and Aβ burden with cognitive decline. Similar to prior work from HABS,7 we observed a significant FHS-CVD*Aβ*Time interaction on longitudinal PACC5 decline (β = −0.04 [−0.06 to −0.02], t = −3.48, p < 0.001). Then, using moderated mediation analysis, in models assuming high baseline Aβ burden (PiB PVC-DVR = 1.85), the slopes of ITC tau change partially mediated the effects of higher FHS-CVD on faster PACC5 decline (mediated effects: β = −0.527 [−0.86 to −0.25], p < 0.001), accounting for 33% of the total effects (Fig 4). In contrast, in the setting of low baseline Aβ (PiB PVC-DVR = 1.17), whereas FHS-CVD was associated with PACC5 decline (β = −0.50, p = 0.03), there was no significant mediation of this effect through ITC tau slopes (β = −0.15, p = 0.11).
FIGURE 4:
Inferior temporal tau accumulation partially mediates elevated systemic vascular risk effects on prospective cognitive decline in individuals with high baseline amyloid burden. Individual ITC tau and PACC5 slopes were extracted from linear mixed effects models for moderated mediation analysis. We modeled FHS-CVD as predictor, ITC tau slope as mediator, and PACC5 decline as outcome, adjusting for age, sex, APOE ε4 status, years of education, and time between study baseline and first tau PET. High and low levels of Aβ burden were represented by the mean partial volume corrected Pittsburgh Compound-B distribution volume ratio (PiB PVC-DVR) of amyloid-positive (1.85) and amyloid-negative (1.17) participants, respectively, dichotomized using the conventional amyloid-positivity threshold (PiB PVC-DVR of 1.32 in the HABS cohort). Results indicate partial mediation of FHS-CVD effects on cognitive decline by ITC tau slope in individuals with elevated baseline Aβ burden (right panel; β = −0.53 [−0.86 to −0.25], p < 0.001), accounting for 33% of the total effects. No statistically significant mediation was observed in the setting of low baseline Aβ burden (left panel; β = −0.16 [−0.38 to 0.03], p = 0.11), though greater baseline FHS-CVD alone continued to be associated with decreasing cognitive performance (total effects: β = −0.50, p = 0.03). Aβ = β-amyloid; FHS-CVD = Framingham Heart Study cardiovascular disease risk score; HABS = Harvard Aging Brain Study; ITC = inferior temporal cortex; PACC5 = Preclinical Alzheimer’s Cognitive Composite-5; PET = positron emission tomography.
Discussion
In a well-characterized cohort of 175 older CU adults with longitudinal tau PET imaging and cognitive follow-up, we observed that higher baseline vascular risk was synergistic with elevated Aβ in predicting greater longitudinal tau burden in the ITC, an area of early neocortical tau spread associated with imminent clinical impairment.24 This interaction was significant at a baseline Aβ level considerably lower than the conventional threshold for amyloid positivity, consistent with growing literature on the deleterious effects of even modestly elevated Aβ.25,26 Additionally, we examined individual components of FHS-CVD risk score and observed that elevated SBP and BMI were significant and independent drivers of this interaction. Last, using moderated mediation analysis, we demonstrated that longitudinal tau accumulation significantly mediated one third of the synergistic effects between baseline vascular risk and Aβ on prospective cognitive decline. Together, our findings suggest that accelerating early neocortical tau accumulation may be one mechanism by which vascular risk interacts with even relatively low levels of Aβ to promote cognitive decline in preclinical AD. This provides strong support for targeting vascular risk reduction as a prevention/treatment strategy to modify the trajectory of AD, and the importance of considering vascular risk in the design, execution, and analysis of data from AD clinical trials.
The current results are consistent with previous findings from HABS demonstrating a significant interaction between baseline FHS-CVD score and Aβ burden on cross-sectional ITC tau.10 The results here further elucidated the temporal ordering of these associations using longitudinal tau PET spanning up to 9.7 years from study baseline. Similar to prior work from HABS, we did not observe an association between baseline Aβ burden and FHS-CVD scores,7,10 consistent with prior work demonstrating late-life Aβ burden was associated with mid-life but not late-life vascular risk factors.27 The synergistic effect of vascular risk and Aβ burden on longitudinal tau was robust and remained significant across sensitivity analyses using the lipid profile-based FHS-CVD15 and ASCVD16 risk scores. To our knowledge, this is the first study to examine the synergistic impact of systemic vascular risk and Aβ burden on longitudinal tau PET in CU older adults. Our results are consistent with a study of longitudinal CSF tau burden in older CU adults,28 which showed higher FHS-CVD scores were associated with greater increase in CSF tau only in those with baseline CSF Aβ and tau pathology, supporting a synergistic interaction between vascular risk and Aβ on longitudinal tau. In contrast, another study of late mid-life CU adults did not find associations between vascular risk and rate of change in CSF tau.29 Several methodological differences may explain the discordant findings. First, a potential moderating effect of Aβ was not explored in that study. Additionally, vascular risk was assessed as a summed composite score of the presence or history (versus absence) of 5 vascular risk factors. This resulted in a limited score range in the study sample (0 to 3), which was then dichotomized prior to analysis. The greater dynamic range of the FHS-CVD scores in our current study and its use as a continuous variable may have enabled greater power to detect significant associations.
Growing evidence indicates that conventional approaches to categorizing individuals as “amyloid positive” or “amyloid negative” may underestimate the deleterious effects of subthreshold levels of Aβ on brain health, tau accumulation, and cognitive decline.25,26 Using floodlight analysis, we demonstrated that the FHS-CVD by Aβ interaction on tau accumulation was significant at an Aβ burden of PiB PVC-DVR >1.23 (corresponding to 11.8 Centiloids), which is substantially lower than the conventional amyloid positivity threshold of 1.32 (18.2 Centiloids) in HABS. This result reinforces the importance of considering subthreshold levels of Aβ in preclinical AD,25,26 and strongly supports the use of Aβ burden as a continuous variable in analyses, rather than dichotomizing into amyloid positive versus negative subgroups using conventional cutoff points.
To better understand the mechanisms through which vascular risk may interact with Aβ to accelerate tau accumulation, we examined individual components of the FHS-CVD score and demonstrated that only SBP and BMI showed significant interactions with Aβ in predicting greater longitudinal ITC tau. This is consistent with evidence linking hypertension and obesity, particularly during midlife, with increased risk of cognitive impairment and dementia, including AD.30-32 A study of mid- to early late-life CU adults showed that hypertension and obesity status were both synergistic with elevated Aβ in predicting faster decline in memory performance.33 In addition, clinical-pathological studies have linked midlife obesity and mid- and late-life hypertension with increased neurofibrillary tangle pathology,34-36, consistent with our findings that these vascular risk factors can moderate AD trajectory through tau. Importantly, our results showed that when included in the same model, the effects of both SBP and BMI remained significant, with essentially unchanged effect sizes. These findings suggest that hypertension and elevated BMI may accelerate tau accumulation independently and through different mechanisms, and support strategies to target these vascular risk factors in AD prevention trials, both individually and in combination.
Mechanistically, Aβ is known to be vasoactive, and therefore may be synergistic with hypertension in promoting vascular dysfunction, as both have been linked to endothelial dysfunction, oxidative stress, neuroinflammation, impaired neuro-vascular coupling, and reduced cerebral blood flow.37-41 In particular, hypoperfusion has been shown to promote tau phosphorylation and aggregation/accumulation in animal studies,42 and has been associated with increased cross-sectional tau PET burden in the temporal lobe.43,44 Obesity, on the other hand, is associated with chronic systemic inflammation, which may act synergistically with Aβ on microglial priming and activation,45,46 which, in turn, has been shown to promote tau phosphorylation and accumulation.47 However, more studies are required to explore this hypothesis, as growing evidence indicates that the relationship between inflammation and AD pathogenesis may be complex and may depend on the disease stage and the specific inflammatory markers examined.48,49
We observed that longitudinal ITC tau change mediated 33% of the FHS-CVD effect on cognitive decline in individuals with elevated Aβ burden. In contrast, in the setting of low baseline Aβ, whereas FHS-CVD was related to cognitive decline, there was no significant mediation by tau change. This is likely due to the relatively low levels of tau in individuals with low levels of Aβ burden. Together, our findings highlight early neocortical tau as one effector mechanism through which Aβ and vascular risk exert their synergistic effects in accelerating cognitive decline. However, given that two thirds of the total FHS-CVD effect was not mediated through ITC tau, future studies investigating additional mechanisms (eg, reduced cerebral blood blow, white matter injury, and cerebral atrophy) are needed to better understand this clinically significant interaction.
The current study has several limitations and the results should be interpreted in context of the cohort studied. HABS excluded participants with baseline uncontrolled hypertension, diabetes, symptomatic stroke, or intracranial hemorrhage. Therefore, individuals with the highest vascular risks are likely under-represented in the current study. In particular, only 7.4% of our participants had diabetes, a risk factor for cognitive impairment and dementia, including AD,50 which likely reduced our power to detect a significant diabetes by Aβ interaction on longitudinal tau. In addition, the HABS sample is overall highly educated and mostly of non-Hispanic European ancestry, which may also limit the generalizability of our findings to other populations.
In summary, our results suggest that elevated systemic vascular risk, particularly higher SBP and BMI, interacts with even subthreshold levels of Aβ to accelerate ITC tau accumulation, which, in turn, partially mediates the synergistic effect between vascular risk and Aβ on prospective cognitive decline. Together with existing literature, these findings strongly support targeting vascular risk factors, especially the treatment of hypertension and obesity in mid- to early late-life, as a strategy to attenuate Aβ-related tau pathology and potentially modify the trajectory of AD. Additionally, treatment strategies targeting both vascular and Aβ pathologies simultaneously may have the greatest impact on those at highest risk for cognitive decline. Future AD clinical trials should assess for vascular risk, given its modifying effect on tau and cognition. Last, AD trials targeting Aβ and tau mechanisms may want to reconsider excluding participants with significant vascular risks/disease, as this may reduce diversity and decrease the synergy between AD and vascular factors, which may be a critical aspect of modulating tau burden and demonstrating clinical benefit.
Acknowledgments
The authors thank the study participants of the Harvard Aging Brain Study. This work was supported by the National Institutes of Health (NIH; [grant numbers P01 AG036694]; Drs. Chhatwal, Sperling, Johnson, Rentz, and Schultz); the American Academy of Neurology, American Brain Foundation and McKnight Brain Research Foundation (McKnight Clinical Translational Research Scholarship in Cognitive Aging and Age-Related Memory Loss; Dr. Yau); Doris Duke Charitable Foundation Clinical Scientist Development Award (Dr. Chhatwal). This research was carried out in part at the Athinoula A. Martinos Center for Biomedical Imaging at the Massachusetts General Hospital, using resources provided by the Center for Functional Neuroimaging Technologies, P41EB015896, a P41 Biotechnology Resource Grant supported by the National Institute of Biomedical Imaging and Bioengineering (NIBIB), NIH. This work also involved the use of instrumentation supported by the NIH Shared Instrumentation Grant Program and/or High-End Instrumentation Grant Program; specifically, grant numbers S10RR021110, S10RR023401, and S10RR023043. Funding sources had no involvement in study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Footnotes
Potential Conflicts of Interests
The authors declare no relevant potential conflicts of interest.
References
- 1.Dunn B, Stein P, Cavazzoni P. Approval of Aducanumab for Alzheimer disease—the FDA’s perspective. JAMA Intern Med 2021;181:1276–1278. 10.1001/jamainternmed.2021.4607. [DOI] [PubMed] [Google Scholar]
- 2.Musiek ES, Holtzman DM. Three dimensions of the amyloid hypothesis: time, space and “wingmen”. Nat Neurosci 2015;18:800–806. 10.1038/nn.4018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 2007;69:2197–2204. 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 4.Schneider JA, Boyle PA, Arvanitakis Z, et al. Subcortical infarcts, Alzheimer’s disease pathology, and memory function in older persons. Ann Neurol 2007;62:59–66. 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- 5.Snyder HM, Corriveau RA, Craft S, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement 2015;11:710–717. 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sweeney MD, Montagne A, Sagare AP, et al. Vascular dysfunction-the disregarded partner of Alzheimer’s disease. Alzheimers Dement 2019;15:158–167. 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabin JS, Schultz AP, Hedden T, et al. Interactive associations of vascular risk and β-amyloid burden with cognitive decline in clinically Normal elderly individuals: findings from the Harvard aging brain study. JAMA Neurol Published online 2018;75:1124–1131. 10.1001/jamaneurol.2018.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vemuri P, Decarli C, Duering M. Imaging markers of vascular brain health: quantification, clinical implications, and future directions. Stroke 2022;53:416–426. 10.1161/strokeaha.120.032611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Shah NS, Vidal JS, Masaki K, et al. Midlife blood pressure, plasma β-amyloid, and the risk for Alzheimer disease. Hypertension 2012;59:780–786. 10.1161/hypertensionaha.111.178962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rabin JS, Yang HS, Schultz AP, et al. Vascular risk and β-amyloid are synergistically associated with cortical tau. Ann Neurol 2019;85:272–279. 10.1002/ana.25399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morris JC. The clinical dementia rating (CDR): current version and scoring rules. Neurology 1993;43:2412–2414. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=8232972&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 12.Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975;12:189–198. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=1202204&retmode=ref&cmd=prlinks. [DOI] [PubMed] [Google Scholar]
- 13.Wechsler D. Wechsier: WMS-R: Wechsler Memory Scale-Revised. 1987th ed. (SA: P Corp., ed.).; 1987. [Google Scholar]
- 14.Dagley A, LaPoint M, Huijbers W, et al. Harvard aging brain study: dataset and accessibility. Neuroimage 2017;144:255–258. 10.1016/j.neuroimage.2015.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.D’Agostino RB, Vasan RS, Pencina MJ, et al. General cardiovascular risk profile for use in primary care: the Framingham heart study. Circulation 2008;117:743–753. 10.1161/circulationaha.107.699579. [DOI] [PubMed] [Google Scholar]
- 16.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association task force on practice guidelines. Published online July 1, 2014. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=24239921&retmode=ref&cmd=prlinks [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson KA, Schultz A, Betensky RA, et al. Tau positron emission tomographic imaging in aging and early Alzheimer disease. Ann Neurol 2016;79:110–119. 10.1002/ana.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rousset OG, Ma Y, Evans AC. Correction for partial volume effects in PET: principle and validation. J Nucl Med 1998;39:904–911. http://eutils.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&id=9591599&retmode=ref&cmd=prlinks. [PubMed] [Google Scholar]
- 19.Papp KV, Rentz DM, Orlovsky I, et al. Optimizing the preclinical Alzheimer’s cognitive composite with semantic processing: the PACC5. Alzheimer’s Dementia Transl Res Clin Interventions 2017;3:668–677. 10.1016/j.trci.2017.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wechsler D. WAIS-R manual: Wechsler adult intelligence scale-revised. New York, NY: Psychological Corporation, 1981. [Google Scholar]
- 21.Grober E, Lipton RB, Hall C, Crystal H. Memory impairment on free and cued selective reminding predicts dementia. Neurology 2000;54:827–832. 10.1212/wnl.54.4.827. [DOI] [PubMed] [Google Scholar]
- 22.Monsch AU, Bondi MW, Butters N, et al. Comparisons of verbal fluency tasks in the detection of dementia of the Alzheimer type. Arch Neurol 1992;49:1253–1258. 10.1001/archneur.1992.00530360051017. [DOI] [PubMed] [Google Scholar]
- 23.Klunk WE, Koeppe RA, Price JC, et al. The Centiloid project: standardizing quantitative amyloid plaque estimation by PET. Alzheimers Dement 2015;11():1–15.e4:1. 10.1016/j.jalz.2014.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol 2007;62:229–234. 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 25.Landau SM, Horng A, Jagust WJ, Initiative F the ADN. Memory decline accompanies subthreshold amyloid accumulation. Neurology 2018;90:e1452–e1460. 10.1212/wnl.0000000000005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Leal SL, Lockhart SN, Maass A, et al. Subthreshold amyloid predicts tau deposition in aging. J Neurosci 2018;38:4482–4489. 10.1523/jneurosci.0485-18.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gottesman RF, Schneider ALC, Zhou Y, et al. Association between midlife vascular risk factors and estimated brain amyloid deposition. JAMA 2017;317:1443–1450. 10.1001/jama.2017.3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bos I, Vos SJB, Schindler SE, et al. Vascular risk factors are associated with longitudinal changes in cerebrospinal fluid tau markers and cognition in preclinical Alzheimer’s disease. Alzheimers Dement 2019;15:1149–1159. 10.1016/j-.jalz.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Besser LM, Alosco ML, Gomez LR, et al. Late-life vascular risk factors and Alzheimer disease neuropathology in individuals with Normal cognition. J Neuropathol Exp Neurol 2016;75:955–962. 10.1093/jnen/nlw072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anstey KJ, Cherbuin N, Budge M, Young J. Body mass index in mid-life and late-life as a risk factor for dementia: a meta-analysis of prospective studies. Obes Rev 2011;12:e426–e437. 10.1111/j.1467-789x.2010.00825.x. [DOI] [PubMed] [Google Scholar]
- 31.Ou YN, Tan CC, Shen XN, et al. Blood pressure and risks of cognitive impairment and dementia: a systematic review and meta-analysis of 209 prospective studies. Hypertension 2020;76:217–225. 10.1161/hypertensionaha.120.14993. [DOI] [PubMed] [Google Scholar]
- 32.McGrath ER, Beiser AS, DeCarli C, et al. Blood pressure from mid- to late life and risk of incident dementia. Neurology 2017;89:2447–2454. 10.1212/wnl.0000000000004741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Clark LR, Koscik RL, Allison SL, et al. Hypertension and obesity moderate the relationship between β-amyloid and cognitive decline in midlife. Alzheimers Dement 2019;15:418–428. 10.1016/j.jalz.2018.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chuang YF, An Y, Bilgel M, et al. Midlife adiposity predicts earlier onset of Alzheimer’s dementia, neuropathology and presymptomatic cerebral amyloid accumulation. Mol Psychiatr 2016;21:910–915. 10.1038/mp.2015.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Petrovitch H, White LR, Izmirilian G, et al. Midlife blood pressure and neuritic plaques, neurofibrillary tangles, and brain weight at death: the HAAS. Neurobiol Aging 2000;21:57–62. 10.1016/s0197-4580(00)00106-8. [DOI] [PubMed] [Google Scholar]
- 36.Arvanitakis Z, Capuano AW, Lamar M, et al. Late-life blood pressure association with cerebrovascular and Alzheimer disease pathology. Neurology 2018;91:e517–e525. 10.1212/wnl.0000000000005951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Paris D, Humphrey J, Quadros A, et al. Vasoactive effects of Aβ in isolated human cerebrovessels and in a transgenic mouse model of Alzheimer’s disease: role of inflammation. Neurol Res 2013;25:642–651. 10.1179/016164103101201940. [DOI] [PubMed] [Google Scholar]
- 38.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci 2011;12:723–738. 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Csiszar A, Tarantini S, Fülöp GA, et al. Hypertension impairs neuro-vascular coupling and promotes microvascular injury: role in exacerbation of Alzheimer’s disease. Geroscience 2017;39:359–372. 10.1007/s11357-017-9991-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin YT, Wu YC, Sun GC, et al. Effect of resveratrol on reactive oxygen species-induced cognitive impairment in rats with angiotensin II-Ind uced early Alzheimer’s disease †. J Clin Med 2018;7:329. 10.3390/jcm7100329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carnevale D, Mascio G, Ajmone-Cat MA, et al. Role of neuroinflammation in hypertension-induced brain amyloid pathology. Neurobiol Aging 2012;33:205.e19–205.e29. 10.1016/j.neurobiolaging.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 42.Laing KK, Simoes S, Baena-Caldas GP, et al. Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain Commun 2020;2:fcaa132. 10.1093/braincomms/fcaa132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Albrecht D, Isenberg AL, Stradford J, et al. Associations between vascular function and tau PET are associated with global cognition and amyloid. J Neurosci 2020;40:8573–8586. 10.1523/jneurosci.1230-20.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rubinski A, Tosun D, Franzmeier N, et al. Lower cerebral perfusion is associated with tau-PET in the entorhinal cortex across the Alzheimer’s continuum. Neurobiol Aging 2021;102:111–118. 10.1016/j.neurobiolaging.2021.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cunningham C. Microglia and neurodegeneration: the role of systemic inflammation. Glia 2013;61:71–90. 10.1002/glia.22350. [DOI] [PubMed] [Google Scholar]
- 46.Drake C, Boutin H, Jones MS, et al. Brain inflammation is induced by co-morbidities and risk factors for stroke. Brain Behav Immun 2011;25:1113–1122. 10.1016/j.bbi.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gorlovoy P, Larionov S, Pham TTH, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J 2009;23:2502–2513. 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 48.Leyns CEG, Gratuze M, Narasimhan S, et al. TREM2 function impedes tau seeding in neuritic plaques. Nat Neurosci 2019;22:1217–1222. 10.1038/s41593-019-0433-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang H, Zhang C, Carlyle BC, et al. Plasma IL-12/IFN-γ axis predicts cognitive trajectories in cognitively unimpaired older adults. Alzheimer’s Dementia 2021;18:645–653. 10.1002/alz.12399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cukierman T, Gerstein HC, Williamson JD. Cognitive decline and dementia in diabetes—systematic overview of prospective observational studies. Diabetologia 2005;48:2460–2469. 10.1007/s00125-005-0023-4. [DOI] [PubMed] [Google Scholar]