Abstract
Background:
Very little is known about the risk of developing psychological morbidities among adults living with cerebral palsy (CP) or spina bifida (SB). The objective of this study was to compare the incidence of and adjusted hazards for psychological morbidities among adults with and without CP or SB.
Methods:
Privately-insured beneficiaries were included if they had an ICD-9-CM diagnostic code for CP or SB (n=15,302). Adults without CP or SB were also included (n=1,935,480). Incidence estimates of common psychological morbidities were compared at 4-years of enrollment. Survival models were used to quantify unadjusted and adjusted hazard ratios for incident psychological morbidities.
Results:
Adults living with CP or SB had a higher 4-year incidence of any psychological morbidity (38.8% vs. 24.2%) as compared to adults without CP or SB, and differences were to a clinically meaningful extent. Fully adjusted survival models demonstrated that adults with CP or SB had a greater hazard for any psychological morbidity (Hazard Ratio [HR]: 1.60; 95%CI: 1.55, 1.65), and all but one psychological disorder (alcohol-related disorders), and ranged from HR: 1.32 (1.23, 1.42) for substance disorders, to HR: 4.12 (3.24, 5.25) for impulse control disorders.
Conclusions:
Adults with CP or SB have a significantly higher incidence of and risk for common psychological morbidities, as compared to adults without CP or SB. Efforts are needed to facilitate the development of improved clinical screening algorithms and early interventions to reduce risk of disease onset/progression in these higher risk populations.
Introduction
Cerebral Palsy (CP) is the most common pediatric-onset physical disability with an estimated prevalence ranging from 2.6–3.1 cases per 1,000 live births in the U.S (Maenner et al., 2016). CP is caused by an insult or malformation of the developing brain which affects motor control centers, and causes alterations in growth, development, and overall health throughout the lifespan (Christensen et al., 2014). The population of adults with CP is expanding because of the steady or marginally increased prevalence (Paneth, Hong, & Korzeniewski, 2006) and increases in the childhood survival rate (Brooks et al., 2014) in recent decades. Despite being considered a neurological syndrome caused by a non-progressive insult, the hallmark features of CP represent a highly progressive phenotype of “early aging” (Peterson, Gordon, & Hurvitz, 2013; Peterson, Gordon, Hurvitz, & Burant, 2012; Verschuren et al., 2018).
Spina bifida (SB) is another congenital birth defect that occurs in approximately 3.5 cases per 10,000 live births in the U.S. (Prevention., 2011), and encompasses a spectrum of birth defects (meningomyelocele, myelomeningocele, myelocele, meningocele, and rachischisis), which are the result of an incomplete closure of the spinal column and lead to exposure of or herniation to the spinal cord/meninges (Atta et al., 2016). Although SB has a lower case fatality rate than other neural tube defects, it often results in severe life-long disability and morbidity. Moreover, now that the oldest cohort with SB is nearly 60 years of age, better understanding of age-related changes across the lifespan is critical to inform and improve clinical care for this population (Dennis, Spiegler, & Hetherington, 2000; Ware, Kulesz, Juranek, Cirino, & Fletcher, 2017).
The clinical framework that encompasses healthcare for patients with CP and SB has been largely confined to issues that arise during childhood and adolescence. Despite the shortage of surveillance research to evaluate lifespan health and developmental trajectories in both of these populations, there is ample indication that adults living with CP and SB have significant and progressive functional decline, inadequate muscle and bone development, increased obesity, and risk for secondary chronic disease (Dosa NP, Foley JT, Eckrich M, Woodall-Ruff D, & GS., 2009; Lampe, Grassl, Mitternacht, Gerdesmeyer, & Gradinger, 2006; Lidal, Lundberg Larsen, & Hoff, 2019; Marreiros, Monteiro, Loff, & Calado, 2010; Moreau, Li, Geaghan, & Damiano, 2008; Peterson, Zhang, Haapala, Wang, & Hurvitz, 2015; Polfuss, Bandini, & Sawin, 2017; Trinh et al., 2017; D. Whitney et al., In Press; D. G. Whitney et al., 2018). However, there have been very few studies to examine psychological morbidity among adults living with CP or SB (Bellin et al., 2010; Dicianno et al., 2015; Smith et al., 2018; D. G. Whitney, Warschausky, et al., 2019), and no current studies have examined the longitudinal trends of a broad array of mental health disorders in these populations. The purpose of this study was therefore to examine the incidence of and risk for common psychological morbidities in a large sample of adults with CP or SB, as compared to adults without CP or SB.
Methods
Data Source
This is a retrospective cohort study of adults with congenital CP or SB whose diagnosis could have existed across any patient care setting. This study used a national, private insurance claims database, Clinformatics DataMart Database (OptumInsight, Eden Prairie, MN). This is a de-identified administrative claims database of over 80 million adults and children with commercial insurance representing those on a single, large U.S. private payer who had both medical and pharmacy coverage throughout the enrollment. Enrolled beneficiaries’ emergency department, outpatient, and inpatient encounters are captured. This study was deemed exempt by the University of Michigan Institutional Review Board at the researchers’ institution.
Sample Selection
All individuals 18 years of age and older at the time of their enrollment which could start from 2007 to 2017 were potentially eligible for this analysis. We excluded individuals with less than 12 months of continuous enrollment to require sufficient claim history. All medical claims excluding laboratory and outpatient pharmacy was considered to identify prevalence or treatment for these conditions during the enrollment period.
Identification of Patients with CP and SB
All members with a diagnosis of CP or SB were identified using International Classification of Diseases, Ninth revision, Clinical Modification (ICD-9-CM) (Supplementary Table S1). Members that had CP or SB prior to 2007 were excluded due to poorer coverage of diagnosis codes during 2001 to 2006 in the database. Members without a diagnosis code in any position when they were 18 years or older during enrollment were excluded. Due to lack of clinical feasibility and different disease etiologies, a small number of members were excluded who had both CP and SB during enrollment. To allow adequate longitudinal follow up for all patients with CP or SB, only those that had four or more continuous years of enrollment following their starting date of enrollment within the study period were included.
A comparison cohort of controls without CP or SB were also identified using the same aforementioned inclusion criteria. Additional exclusion criteria for identifying the control cohort included removal of any individual with other physically disabling neurological disorders (e.g., paraplegia, quadriplegia, hemiplegia, traumatic spinal cord injury, and multiple sclerosis). Among remaining members without CP or SB, a 20% simple random sample of members was selected to represent the control group. We performed simple random sampling of the set of controls that had no evidence of CP or SB throughout their enrollment on the insurance plan during the study period. Post-hoc analyses of demographic characteristics were compared between the 20% sample of controls and all controls to ensure no bias in control cohort attributable to random selection (Figure 1).
Figure 1.
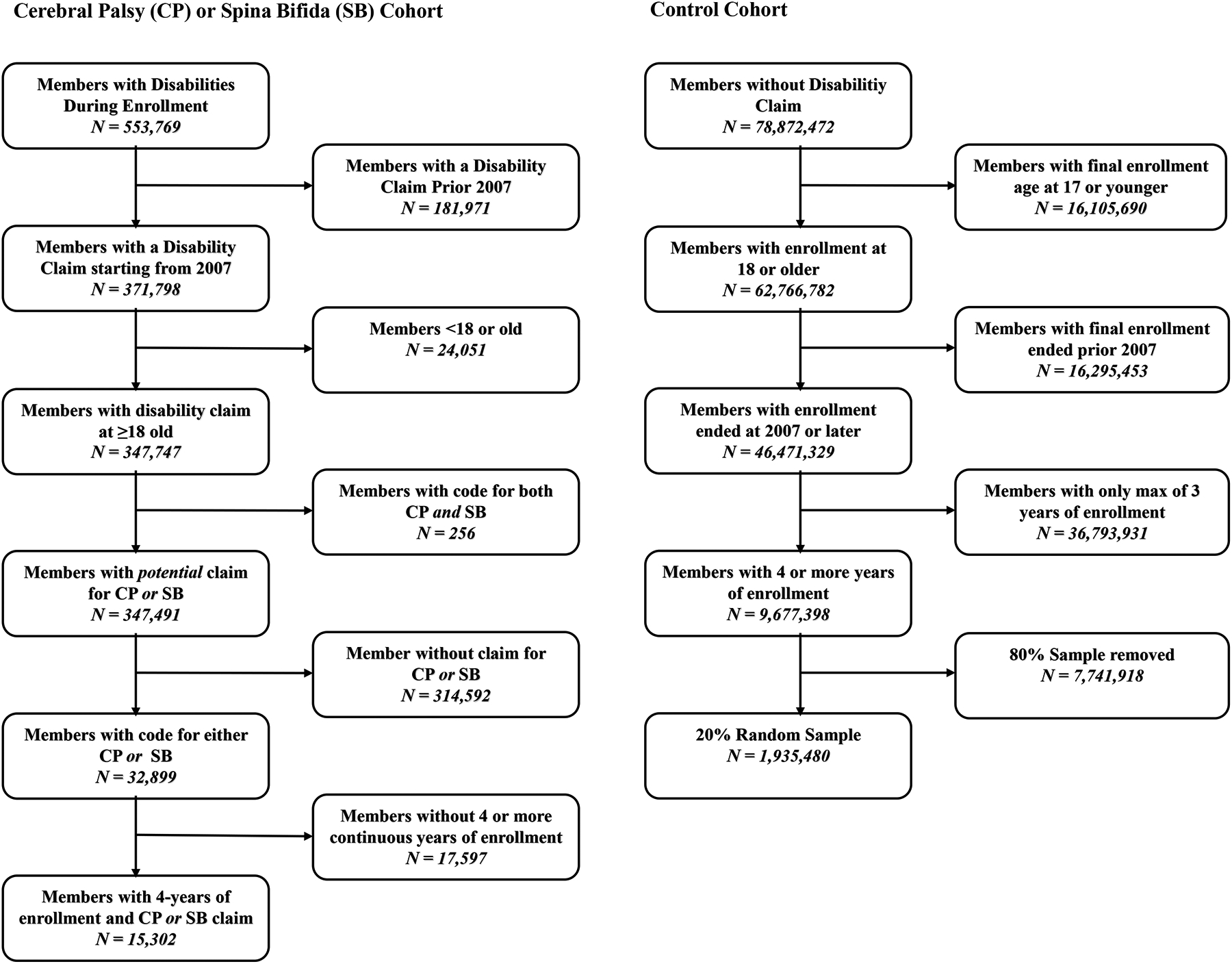
Flow chart of subject inclusion and exclusion for final case and control cohorts.
Psychological Morbidities
Physician-diagnosed psychological health disorders were identified based on a single encounter that included at least one of pertinent ICD-9 or ICD-10 codes (in any position) (see Supplementary Table S1). The primary outcome was time in days to incident psychological morbidity as a composite outcome following enrollment on the plan. Secondary outcomes were component incident psychological morbidity, including: (1) insomnia, (2) adjustment disorders, (3) anxiety disorders and post-traumatic stress disorder (PTSD), (4) delirium/dementia/amnestic or other cognitive disorder, (5) dementias, (6) impulse control disorders, (7) mood disorders, (8) personality disorders, (9) alcohol-related disorders, (10) substance-related disorders, and (11) central pain syndrome.
Covariates
Explanatory covariates included age group split into three categories (18–44, 45–64, 65 or older), sex, race, educational attainment, household net worth, and a modified Elixhauser comorbidity index. The Elixhauser comorbidity index was modified to removed four conditions that would be correlated with incident psychological morbidity: alcohol abuse, drug abuse, psychoses, and depression. Therefore, the revised index only considers 27 comorbidities (Supplemental Table S2).
Statistical Analysis
Bivariate analyses of baseline demographic characteristics between patients with CP or SB and controls were examined. For categorical variables, column percentages were compared between both groups using effect size calculations with Cohen’s h. The Cohen’s h effect size calculation was used since, due to large sample sizes, being statistically overpowered would not provide clinically meaningful differences in proportions between groups. For continuous variables, means and standard deviations as well as medians with upper and lower bounds on interquartile ranges were calculated. Cohen’s d standardized mean differences were calculated for continuous variables to ascertain clinically meaningful differences between groups.
Since CP and SB are congenital conditions, it is assumed that all adults already have the condition at the time of their enrollment by age 18. To capture full comorbidity history within the study period, all patients with sufficient continuous enrollment within the study period of four years were retained to enable sufficient follow-up. The CP/SB cohort, use the first year of enrollment out of the four-year enrollment to capture comorbidity history and to examine if any prevalent psychological outcomes existed.
To examine disease-free survival of individuals with CP or SB compared to controls, those patients that had no evidence of composite psychological morbidity in each group were plotted using Kaplan-Meier product limit survival curves for a three-year period. To establish incidence in claims, we used a one-year lookback period from the index date in each group to obtain evidence of any service utilization with a diagnosis of any psychological morbidity. These patients were excluded from the product-limit survival curves and other subsequent analyses.
To estimate the unadjusted and adjusted hazard of the composite and each psychological morbidity, a series of survival models were developed. For each psychological morbidity, all patients that had evidence of the specific psychological morbidity were excluded from the model. For example, if insomnia was being considered as the incident outcome, all patients with prevalent insomnia in the one year prior to the index date would be excluded from the model. Therefore, sample sizes of patients included for each outcome varied based on evidence of prevalent disease in the one year prior to the index date. Survival models were then used to quantify unadjusted and adjusted hazard ratios for each incident psychological morbidity. Appropriate survival models were based on distributional assumptions that included testing Weibull, lognormal, exponential, gamma, logistic, loglog, and Normal distribution with respect to the follow-up in days by minimizing critical model fit statistics. Critical assessment of Akaiki Information Criterion (AIC) was used as a basis for minimization amongst all candidate distributions. Use of the parametric Weibull regression for incident psychological outcome was applied stepwise. To examine the effects of incremental adjustment on the exposure variable (CP or SB), a series of models for each psychological outcome was evaluated. All patients were right censored if they did not experience the outcome in the follow-up period or disenrolled from the plan. Both unadjusted and all adjusted hazard ratios and 95% confidence intervals for the exposure to CP/SB were calculated.
All analyses were conducted using SAS 9.4 (SAS Institute, Cary, NC). Statistical testing was two-tailed with a significance level of 0.05 and effect sizes used a 0.2 meaningful difference cutoff.
Results
The median time in the plan for eligible enrollees was 7.0 (25th Percentile: 5.1; 75th Percentile: 9.7) and 6.7 (25th Percentile: 5.0; 75th Percentile: 9.3) years for patients with CP or SB vs controls respectively. Adults living with CP or SB had a higher 4-year incidence of any psychological morbidity (38.8% vs. 24.2%) as compared to adults without CP or SB, and differences were to a clinically meaningful extent. Moreover, adults with CP or SB had significantly higher incidence of all of the psychological outcomes, including insomnia (9.6% vs. 5.6%), adjustment disorders (5.2% vs. 3.3%), anxiety disorders (18.9% vs. 11.2%) and PTSD (1.1% vs. 0.4%), delirium/dementia/amnestic or other cognitive disorder (6.7% vs. 2.2%), dementia (2.3% vs. 1.0%), impulse control disorders (0.5% vs. 0.1%), mood disorders (19.6% vs. 10.0%), personality disorders (1.0% vs. 0.2%), alcohol-related disorders (2.3% vs. 1.8%), substance-related disorders (4.9% vs. 2.7%), and central pain syndrome (13.9% vs. 6.1%), as compared to adults without CP or SB (all P<.01 and SMD≥0.2) (Table 2).
Table 2.
Incidence of any and all psychological morbidities among adults with and without CP or SB with one-year clean enrollment period.
| †No Outcome at Baseline | ||
|---|---|---|
| Case/Denominator | Control/Denominator | |
| Any Psychological Morbidity | 4205/10848 (38.8%)* | 395275/1635348 (24.2%) |
| Insomnia | 1402/14655 (9.6%)* | 105331/1884216 (5.6%) |
| Adjustment disorders | 767/14891 (5.2%)* | 63017/1905421 (3.3%) |
| Anxiety disorders | 2568/13560 (18.9%)* | 204060/1819089 (11.2%) |
| PTSD | 163/15193 (1.1%)* | 8416/1931162 (0.4%) |
| Delirium/Dementia/Amnestic/Other Cognitive Disorder | 990/14826 (6.7%)* | 41606/1916260 (2.2%) |
| Dementias | 351/15150 (2.3%)* | 19773/1929124 (1.0%) |
| Impulse control disorders NEC | 79/15236 (0.5%) | 1592/1934754 (0.1%) |
| Mood disorders | 2524/12898 (19.6%)* | 179935/1794787 (10.0%) |
| Personality disorders | 157/15229 (1.0%)* | 4790/1933048 (0.2%) |
| Alcohol-related disorders | 341/15112 (2.3%)* | 33777/1921455 (1.8%) |
| Substance-related disorders | 731/15043 (4.9%)* | 51119/1923254 (2.7%) |
| Central Pain | 1996/14391 (13.9%)* | 115911/1898453 (6.1%) |
P<.01 and standard mean difference (SMD) ≥0.2
Denominators for both cases and controls reflect a one-year clean period during their enrollment for the specific condition. For instance, among cases (CP/SB), there exist 14,655 patients whose first year of enrollment had no evidence of insomnia; therefore, inferred incident insomnia could be estimated for this subset of the full CP/SB cohort. As a result, all patient cohorts’ denominators dynamically change conditional on the incident outcome being measured to ensure a clean period in the first year of enrollment
A Kaplan-Meier curve for the unadjusted disease-free survival for any psychological morbidity in adults with CP or SB and controls are demonstrated in Figure 2. Unadjusted survival models demonstrated a robust increased hazard ratio (HR) for each of the incident psychological morbidities among adults with CP or SB, and ranged from HR: 1.29 (1.16, 1.43) for alcohol-related disorders to HR: 6.32 (4.96, 8.05) (all p<0.001). Fully adjusted survival models demonstrated that adults with CP or SB had a greater hazard for any psychological morbidity (HR: 1.60; 95%CI: 1.55, 1.65) (Supplemental Table S3), and all but one psychological disorder (alcohol-related disorders), and ranged from HR: 1.32 (1.23, 1.42) for substance disorders to HR: 4.12 (3.24, 5.25) for impulse control disorders (Table 3).
Figure 2.
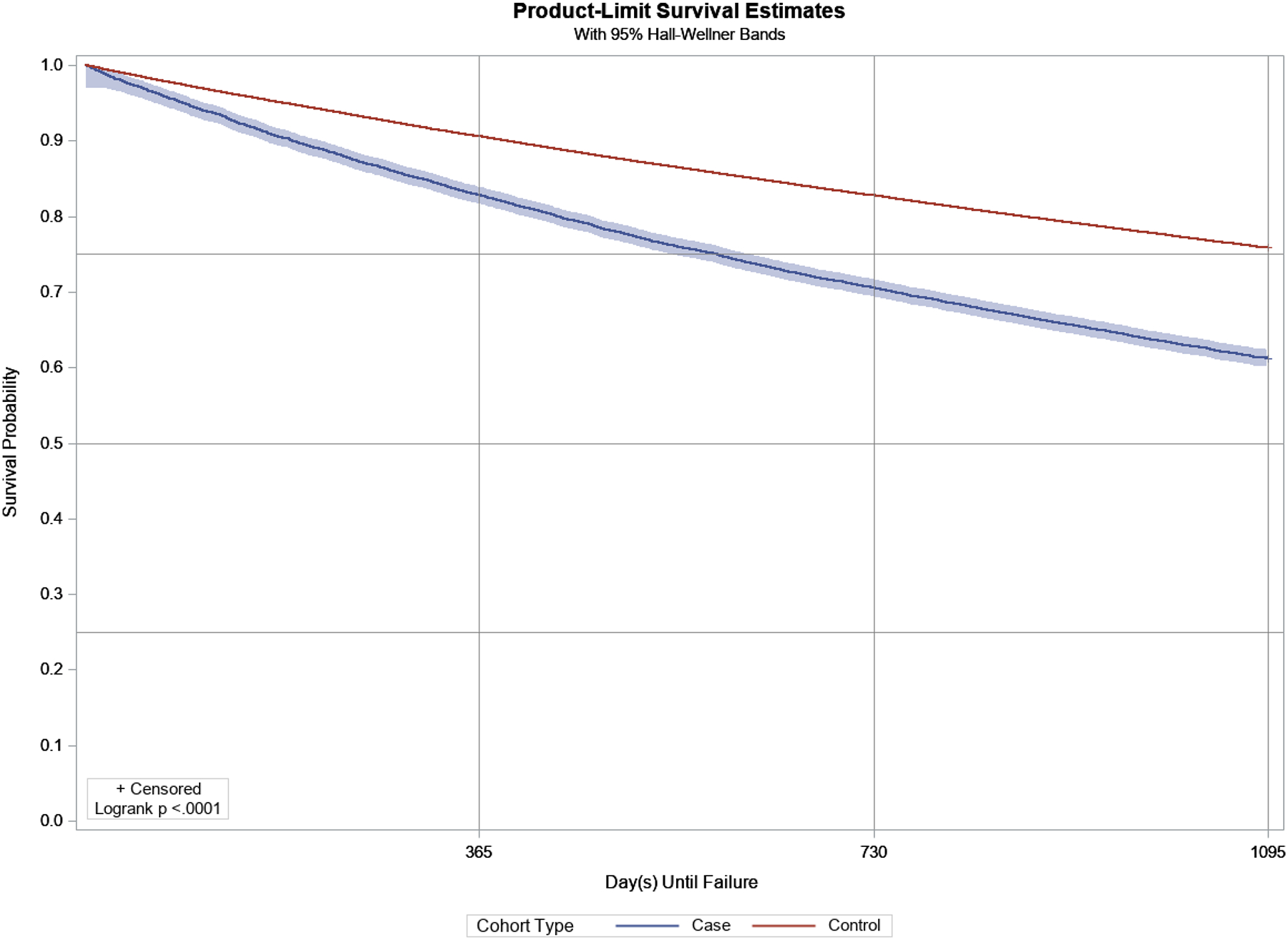
Disease-free survival and Kaplan-Meier product-limit survival curves (3-year) for adults with CP or SB (blue) and without CP or SB (red), for any psychological morbidity.
Table 3.
Survival models with parametric Weibull regression was completed stepwise for each incident psychological outcome to examine the effects of incremental adjustment on the exposure variable (CP or SB).
| Model 1 | Model 2 | Model 3 | Model 4 | |
|---|---|---|---|---|
| Any Psychological | 1.79 (1.74, 1.85)*** | 1.81 (1.76, 1.87)*** | 1.62 (1.57, 1.67)*** | 1.60 (1.55, 1.65)*** |
| Insomnia | 1.75 (1.66, 1.84)*** | 1.78 (1.69, 1.87)*** | 1.51 (1.44, 1.60)*** | 1.50 (1.43, 1.58)*** |
| Adjustment disorders | 1.57 (1.47, 1.69)*** | 1.46 (1.36, 1.57)*** | 1.30 (1.21, 1.40)*** | 1.32 (1.23, 1.42)*** |
| Anxiety disorders | 1.77 (1.70, 1.84)*** | 1.71 (1.65, 1.78)*** | 1.48 (1.43, 1.54)*** | 1.47 (1.42, 1.53)*** |
| PTSD | 2.47 (2.11, 2.89)*** | 2.26 (1.93, 2.64)*** | 1.71 (1.46, 2.00)*** | 1.68 (1.44, 1.97)*** |
| Delirium/Dementia/Amnestic/Other Cognitive Disorder | 3.15 (2.96, 3.36)*** | 4.15 (3.89, 4.43)*** | 3.57 (3.35, 3.81)*** | 3.34 (3.13, 3.56)*** |
| Dementias | 2.28 (2.05, 2.53)*** | 3.34 (3.00, 3.72)*** | 2.84 (2.55, 3.16)*** | 2.56 (2.30, 2.85)*** |
| Impulse control disorders NEC | 6.32 (4.96, 8.05)*** | 6.00 (4.71, 7.64)*** | 4.23 (3.32, 5.39)*** | 4.12 (3.24, 5.25)*** |
| Mood disorders | 2.07 (1.99, 2.15)*** | 2.05 (1.97, 2.13)*** | 1.74 (1.68, 1.81)*** | 1.72 (1.65, 1.79)*** |
| Personality disorders | 4.18 (3.55, 4.92)*** | 3.92 (3.32, 4.61)*** | 2.76 (2.34, 3.25)*** | 2.70 (2.29, 3.18)*** |
| Alcohol-related disorders | 1.29 (1.16, 1.43)*** | 1.30 (1.17, 1.45)*** | 1.11 (1.00, 1.24) | 1.09 (0.98, 1.21) |
| Substance-related disorders | 1.85 (1.72, 1.99)*** | 1.85 (1.72, 1.99)*** | 1.38 (1.28, 1.49)*** | 1.32 (1.22, 1.42)*** |
| Central Pain | 2.38 (2.27, 2.49)*** | 2.46 (2.36, 2.57)*** | 1.92 (1.83, 2.01)*** | 1.88 (1.80, 1.97)*** |
Model 1: Unadjusted
Model 2: Model 1 + Demographic variables (age, sex, race, geographic region).
Model 3: Model 1 + Model 2 + Modified Elixhauser Comorbidity Index
Model 4: Model 1 + Model 2 + Model 3 + Education + Income
Discussion
The principal finding of this study was that adults living with CP or SB had a higher incidence of and adjusted hazard for any and all psychological morbidities than adults without CP or SB. This is the first and largest study to date to examine longitudinal trajectories of psychological morbidity among adults living with CP or SB. Future research and clinical efforts are needed to not only better understand the healthcare burden associated with mental health disorders in the CP and SB populations, as well as across other subpopulations with other neurodevelopmental disabilities, but also to understand the disparities in healthcare access between privately- and federally-insured beneficiaries living with disabilities. Importantly, these findings bolster the need for improved clinical screening and design of early behavioral interventions to reduce risk of psychological disease onset/progression in the CP and SB populations.
Our findings support previous research indicating a higher risk of various psychological morbidities in adults with CP and SB. Adults with CP often experience depression and anxiety at higher prevalence and incidence than the general population (Smith et al., 2018; D. G. Whitney, Warschausky, et al., 2019). In a population representative sample from the UK, more than 16% of younger adults with CP under 30 years of age were found to have depression compared to less than 7% of the general population (Fortuna, Holub, Turk, Meccarello, & Davidson, 2018). Depression and anxiety are also considered to be factors of concern for adults with SB (Mukherjee & Pasulka, 2017). In fact, in a recent cohort study, approximately 26% of adults with SB experienced depressive symptomatology, 36% were on antidepressants to treat depressive symptoms, and 63% of those with clinical symptoms of depression were on antidepressants (Dicianno et al., 2015). The pathophysiology underlying CP and SB consist of insults to or malformations of the central nervous system that may include the frontal cortex, the prefrontal cortex, cerebellum, the limbic system and other brain centers that control behavior, self-regulation, working memory, and executive function may be disrupted, thus predisposing individuals with CP or SB to higher risk of poor cognitive health than typically-developing peers.
In addition to the findings that adults living with CP or SB had higher risk for developing conventional psychological morbidities (e.g., depression and anxiety), we found that risk for central pain was also significantly greater in adults with CP or SB. Indeed, chronic pain is the most commonly reported physical symptomology of CP and SB throughout the lifespan, and yet pain is perhaps the least understood, emphasized, and studied physical comorbidity of these conditions (Alriksson-Schmidt, Josenby, Lindquist, & Westbom, 2018; Blackman, Svensson, & Marchand, 2018; Fehlings, 2017; Wagner et al., 2015). There exists robust literature linking pain and psychological morbidity in the general population (Goesling, Lin, & Clauw, 2018; Tsang et al., 2008); however, there have been virtually no investigations to understand pain phenotype distributions (e.g., including nociceptive, nociplastic, neuropathic) in CP or SB and how they contribute to psychological morbidity onset/progression. Pain is experienced by adults with CP and SB when completing normal tasks, such as dressing and transferring, or other activities that require medical interventions (Fox et al., 2019; Morley CP et al., 2019). Prior research has found that nearly 40% of adults with CP and 45% of those diagnosed with SB commonly experienced pain and severe fatigue (Benner et al., 2017; van Gorp et al., 2019). When compared to individuals without CP, about 70–75% of adults with CP report chronic pain (Fox et al., 2019; van der Slot et al., 2020). Individuals with SB are likely to have pain in all regions of their bodies, with pain in legs (thigh, hip, knee, shin, and ankle) reported most often (Morley CP et al., 2019). Assessment of putative pain mechanisms could provide new insight into the pain experience in adults with CP and SB and inform interventions to address pain and psychological morbidity.
Strengths and Weaknesses
A major strength of this study is the large and longitudinal sample of adults with CP or SB. It can be challenging to gather data on these clinical sub-populations, and very little is known about health outcomes among individuals with CP or SB as they transition throughout adulthood. Moreover, most large administrative claims databases do not contain some socioeconomic indicators such as net worth, race, and location (division). Herein, we provide incidence estimates and adjusted hazards for psychological morbidity while considering numerous sociodemographic variables from samples representing all states in the US. Lastly, while clinical trials may be considered the “gold standard” in clinical research, cohort studies are less expensive, include broader patient populations, and are more efficient. In fact, there is little evidence to support the superiority of clinical trials over observational studies (Benson & Hartz, 2000).
Our study also has several limitations that should be acknowledged. First, we were unable to determine the severity of CP or SB through claims-based data. However, we suspect that our sample may be more reflective of a healthier, higher functioning segment of the CP or SB population (D. G. Whitney, Alford, et al., 2019), because they had to be enrolled in private insurance, either by purchasing their own insurance, or by being covered through employment or marriage to someone who had private insurance. Therefore, results and comparisons to adults without CP or SB are likely conservative estimates, and the true extent of psychological morbidity may be underestimated in this study. Importantly, administrative claims data may be prone to inaccurate coding of medical diagnoses, such as CP or SB, as well as chronic diseases, which may have an effect on our incidence estimates. While validation studies have shown that using >1 claim for a medical condition improves the ability to identify beneficiaries with that medical condition (Kerr, McGlynn, Van Vorst, & Wickstrom, 2000; Reeves et al., 2014), single claim-based algorithms have been reported to have moderate-to-high positive predictive value (~80%) or specificity (up to 96%) (Doktorchik et al., 2019; Leslie, Lix, & Yogendran, 2011; Reeves et al., 2014). However, the accuracy of identifying medical conditions using claims data depends on the number of years for the study period (Leslie et al., 2011) and the medical condition examined (Doktorchik et al., 2019; Leslie et al., 2011; Noyes, Liu, Lyness, & Friedman, 2011; Reeves et al., 2014). Finally, we cannot rule out time-varying confounding since baseline measurements of all covariates were included in our final models. Thus, whether having CP or SB “causes” an elevated risk for earlier-onset psychological morbidity, or if changes in other health parameters (e.g., diabetes, a known predictor of psychological morbidity) themselves, are a cause of poor mental health, is an interesting topic. Thus, we were unable to determine if other competing risks or unmeasured confounding (i.e., other risk factors [e.g., family history of mental health disorders, lack of physical activity, loss of functional independence, etc.]) may have influenced the observed findings. Indeed, unmeasured confounding could also be within proxy variables of appropriate care (PT, OT, etc.) which might mitigate psychological morbidity risk (and were not considered). This would lend credence to additional follow-up work to understand the care pathway to success for these patients.
Conclusion
In conclusion, adults with CP or SB have an elevated risk of developing a variety of psychological morbidities compared to the general adult population of privately insured beneficiaries without CP or SB. Individuals with CP and SB frequently utilize healthcare services as part of their routine clinical care. Therefore, increasing clinical awareness of the mental health disorders experienced and risks among adults with CP and SB, improving clinical screening strategies, and developing efficient referral resources for coordinated care may help reduce the burden of mental health disorders in these population.
Supplementary Material
Supplemental Table S1. Diagnostic codes for cerebral palsy, spina bifida, and all psychological morbidities using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Supplemental Table S2. Modified Elixhauser Index.
Supplemental Table S3. Fully adjusted survival model for any psychological morbidity.
Table 1.
Descriptive characteristics among adults with CP or SB (case) or without CP or SB (control).
| Case | Control | |
|---|---|---|
| Overall | 15,302 (100%) | 1,935,480 (100%) |
| Full Enrollment Length | ||
| Mean (SD) | 7.8 (3.3) | 7.6 (3.3) |
| Median (Q1-Q3) | 7.0 (5.1–9.7) | 6.7 (5.0–9.3) |
| Years Post Eligibility Start Date † | ||
| Mean (SD) | 5.8 (2.2) | 5.5 (2.2) |
| Median (Q1-Q3) | 5.3 (4.0–7.3) | 5.0 (3.7–6.8) |
| Age Group | ||
| 18–44 | 7055 (46.1%) | 798257 (41.2%) |
| 45–64 | 5255 (34.3%) | 617997 (31.9%) |
| 65 or Older | 2992 (19.6%) | 519226 (26.8%) |
| Gender | ||
| Female | 8666 (56.6%) | 1012200 (52.3%) |
| Male | 6636 (43.4%) | 923280 (47.7%) |
| Race | ||
| Asian | 300 (2.0%) | 75437 (3.9%) |
| Black | 1496 (9.8%) | 155609 (8.0%) |
| Hispanic | 1268 (8.3%) | 175966 (9.1%) |
| Unknown/Missing | 3161 (20.7%) | 379802 (19.6%) |
| White | 9077 (59.3%) | 1148666 (59.3%) |
| Education | ||
| <High School Diploma | 86 (0.6%) | 10761 (0.6%) |
| High School Diploma | 4465 (29.2%) | 469829 (24.3%) |
| <Bachelor Degree | 8107 (53.0%) | 1021803 (52.8%) |
| Bachelor Degree | 2238 (14.6%) | 371346 (19.2%) |
| Unknown/Missing | 406 (2.7%) | 61741 (3.2%) |
| Net Worth | ||
| Unknown | 3334 (21.8%) | 346012 (17.9%) |
| <$25K | 3234 (21.1%) | 302790 (15.6%) |
| $25K-$149K | 2695 (17.6%) | 340966 (17.6%) |
| $150K-$249K | 1379 (9.0%) | 196032 (10.1%) |
| $250K-$499K | 2088 (13.6%) | 313883 (16.2%) |
| ≥$500K | 2572 (16.8%) | 435797 (22.5%) |
All adults with CP and SB have their Index Date set the same as start of eligibility date (start of 2007, year when turned 18, or enrollment start date, whichever was the latest)
Acknowledgments
Funding/Support:
This research was developed in part under a grant from the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR #90RTHF0001-01-00).
Footnotes
Conflict of Interest Statement: none
References
- Alriksson-Schmidt A, Josenby AL, Lindquist B, & Westbom L (2018). Pain and health status in adults with myelomeningocele living in Sweden. The Journal of Pediatric Rehabilitation Medicine, 11(4), 255–264. doi: 10.3233/PRM-170517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atta CA, Fiest KM, Frolkis AD, Jette N, Pringsheim T, St Germaine-Smith C, … Metcalfe A (2016). Global Birth Prevalence of Spina Bifida by Folic Acid Fortification Status: A Systematic Review and Meta-Analysis. American Journal of Public Health, 106(1), e24–34. doi: 10.2105/AJPH.2015.302902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellin MH, Zabel TA, Dicianno BE, Levey E, Garver K, Linroth R, & Braun P (2010). Correlates of depressive and anxiety symptoms in young adults with spina bifida. Journal of Pediatric Psychology, 35(7), 778–789. doi: 10.1093/jpepsy/jsp094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benner JL, Hilberink SR, Veenis T, Stam HJ, van der Slot WM, & Roebroeck ME (2017). Long-Term Deterioration of Perceived Health and Functioning in Adults With Cerebral Palsy. Archives of Physical Medicine and Rehabilitation, 98(11), 2196–2205 e2191. doi: 10.1016/j.apmr.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Benson K, & Hartz AJ (2000). A comparison of observational studies and randomized, controlled trials. The New England Journal of Medicine, 342(25), 1878–1886. doi: 10.1056/NEJM200006223422506 [DOI] [PubMed] [Google Scholar]
- Blackman JA, Svensson CI, & Marchand S (2018). Pathophysiology of chronic pain in cerebral palsy: implications for pharmacological treatment and research. Developmental Medicine and Child Neurology. 60(9):861–865. doi: 10.1111/dmcn.13930 [DOI] [PubMed] [Google Scholar]
- Brooks JC, Strauss DJ, Shavelle RM, Tran LM, Rosenbloom L, & Wu YW (2014). Recent trends in cerebral palsy survival. Part I: period and cohort effects. Developmental Medicine and Child Neurology, 56(11), 1059–1064. doi: 10.1111/dmcn.12520 [DOI] [PubMed] [Google Scholar]
- Christensen D, Van Naarden Braun K, Doernberg NS, Maenner MJ, Arneson CL, Durkin MS, … Yeargin-Allsopp M (2014). Prevalence of cerebral palsy, co-occurring autism spectrum disorders, and motor functioning - Autism and Developmental Disabilities Monitoring Network, USA, 2008. Developmental Medicine and Child Neurology, 56(1), 59–65. doi: 10.1111/dmcn.12268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, & Hetherington R (2000). New survivors for the new millennium: cognitive risk and reserve in adults with childhood brain insults. Brain and Cognition, 42(1), 102–105. doi: 10.1006/brcg.1999.1174 [DOI] [PubMed] [Google Scholar]
- Dicianno BE, Kinback N, Bellin MH, Chaikind L, Buhari AM, Holmbeck GN, … Collins DM (2015). Depressive symptoms in adults with spina bifida. Rehabilitation Psychology, 60(3), 246–253. doi: 10.1037/rep0000044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doktorchik C, Patten S, Eastwood C, Peng M, Chen G, Beck CA, … Quan H (2019). Validation of a case definition for depression in administrative data against primary chart data as a reference standard. BMC Psychiatry, 19(1), 9. doi: 10.1186/s12888-018-1990-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosa NP, Foley JT, Eckrich M, Woodall-Ruff D, & GS. L (2009). Obesity across the lifespan among persons with spina bifida. Disability and Rehabilitation, 31(11), 914–920. [DOI] [PubMed] [Google Scholar]
- Fehlings D (2017). Pain in cerebral palsy: a neglected comorbidity. Developmental Medicine and Child Neurology, 59(8), 782–783. doi: 10.1111/dmcn.13477 [DOI] [PubMed] [Google Scholar]
- Fortuna RJ, Holub A, Turk MA, Meccarello J, & Davidson PW (2018). Health conditions, functional status and health care utilization in adults with cerebral palsy. Family Practice, 35(6), 661–670. doi: 10.1093/fampra/cmy027 [DOI] [PubMed] [Google Scholar]
- Fox MA, Ayyangar R, Parten R, Haapala HJ, Schilling SG, & Kalpakjian CZ (2019). Self-report of pain in young people and adults with spastic cerebral palsy: interrater reliability of the revised Face, Legs, Activity, Cry, and Consolability (r-FLACC) scale ratings. Developmental Medicine and Child Neurology, 61(1), 69–74. doi: 10.1111/dmcn.13980 [DOI] [PubMed] [Google Scholar]
- Goesling J, Lin LA, & Clauw DJ (2018). Psychiatry and Pain Management: at the Intersection of Chronic Pain and Mental Health. Current Psychiatry Reports, 20(2), 12. doi: 10.1007/s11920-018-0872-4 [DOI] [PubMed] [Google Scholar]
- Kerr EA, McGlynn EA, Van Vorst KA, & Wickstrom SL (2000). Measuring antidepressant prescribing practice in a health care system using administrative data: implications for quality measurement and improvement. The Joint Commission journal on quality improvement, 26(4), 203–216. [DOI] [PubMed] [Google Scholar]
- Lampe R, Grassl S, Mitternacht J, Gerdesmeyer L, & Gradinger R (2006). MRT-measurements of muscle volumes of the lower extremities of youths with spastic hemiplegia caused by cerebral palsy. Brain & Development, 28(8), 500–506. doi: 10.1016/j.braindev.2006.02.009 [DOI] [PubMed] [Google Scholar]
- Leslie WD, Lix LM, & Yogendran MS (2011). Validation of a case definition for osteoporosis disease surveillance. Osteoporosis International, 22(1), 37–46. doi: 10.1007/s00198-010-1225-2 [DOI] [PubMed] [Google Scholar]
- Lidal IB, Lundberg Larsen K, & Hoff M (2019). 50 Years and older - born with spina bifida: participation, health issues and physical function. Disability and Rehabilitation, 1–10. doi: 10.1080/09638288.2019.1621953 [DOI] [PubMed] [Google Scholar]
- Maenner MJ, Blumberg SJ, Kogan MD, Christensen D, Yeargin-Allsopp M, & Schieve LA (2016). Prevalence of cerebral palsy and intellectual disability among children identified in two US National Surveys, 2011–2013. Annals of Epidemiology, 26(3), 222–226. doi: 10.1016/j.annepidem.2016.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marreiros H, Monteiro L, Loff C, & Calado E (2010). Fractures in children and adolescents with spina bifida: the experience of a Portuguese tertiary-care hospital. Developmental Medicine and Child Neurology, 52(8), 754–759. doi: 10.1111/j.1469-8749.2010.03658.x [DOI] [PubMed] [Google Scholar]
- Moreau NG, Li L, Geaghan JP, & Damiano DL (2008). Fatigue resistance during a voluntary performance task is associated with lower levels of mobility in cerebral palsy. Archives of Physical Medicine and Rehabilitation, 89(10), 2011–2016. doi: 10.1016/j.apmr.2008.03.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morley CP, Struwe S, Pratte MA, Clayton GH, Wilson PE, Dicianno BE, … MA T (2020). Survey of US adults with Spina Bifida. Disability and health journal, 13(2), 1–8. 10.1016/j.dhjo.2019.100833 [DOI] [PubMed] [Google Scholar]
- Mukherjee S, & Pasulka J (2017). Care for Adults with Spina Bifida: Current State and Future Directions. Topics in Spinal Cord Injury Rehabilitation, 23(2), 155–167. doi: 10.1310/sci2302-155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes K, Liu H, Lyness JM, & Friedman B (2011). Medicare beneficiaries with depression: comparing diagnoses in claims data with the results of screening. Psychiatric Services, 62(10), 1159–1166. doi: 10.1176/ps.62.10.pss6210_1159 [DOI] [PubMed] [Google Scholar]
- Paneth N, Hong T, & Korzeniewski S (2006). The descriptive epidemiology of cerebral palsy. Clinics in Perinatology, 33(2), 251–267. doi: 10.1016/j.clp.2006.03.011 [DOI] [PubMed] [Google Scholar]
- Peterson MD, Gordon PM, & Hurvitz EA (2013). Chronic disease risk among adults with cerebral palsy: the role of premature sarcopoenia, obesity and sedentary behaviour. Obesity Reviews, 14(2), 171–182. doi: 10.1111/j.1467-789X.2012.01052.x [DOI] [PubMed] [Google Scholar]
- Peterson MD, Gordon PM, Hurvitz EA, & Burant CF (2012). Secondary muscle pathology and metabolic dysregulation in adults with cerebral palsy. American journal of physiology. Endocrinology and metabolism, 303(9), E1085–1093. doi: 10.1152/ajpendo.00338.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson MD, Zhang P, Haapala HJ, Wang SC, & Hurvitz EA (2015). Greater Adipose Tissue Distribution and Diminished Spinal Musculoskeletal Density in Adults With Cerebral Palsy. Archives of Physical Medicine and Rehabilitation, 96(10), 1828–1833. doi: 10.1016/j.apmr.2015.06.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polfuss M, Bandini LG, & Sawin KJ (2017). Obesity Prevention for Individuals with Spina Bifida. Current Obesity Reports, 6(2), 116–126. doi: 10.1007/s13679-017-0254-y [DOI] [PubMed] [Google Scholar]
- Neural Tube Defect Ascertainment Project. National Birth Defects Prevention: Birth Defects Surveillance, Research, and Prevention. United States. Available online: https://www.nbdpn.org/docs/NTD_Fact_Sheet_11-13_for_website.pdf (accessed on 10 November 2020). [Google Scholar]
- Reeves S, Garcia E, Kleyn M, Housey M, Stottlemyer R, Lyon-Callo S, & Dombkowski KJ (2014). Identifying sickle cell disease cases using administrative claims. Academic Pediatrics, 14(5 Suppl), S61–67. doi: 10.1016/j.acap.2014.02.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith KJ, Peterson MD, O’Connell NE, Victor C, Liverani S, Anokye N, & Ryan JM (2018). Risk of Depression and Anxiety in Adults With Cerebral Palsy. JAMA Neurology. doi: 10.1001/jamaneurol.2018.4147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trinh A, Wong P, Sakthivel A, Fahey MC, Hennel S, Brown J, … Milat F (2017). Fat-Bone Interactions in Adults With Spina Bifida. Journal of the Endocrine Society, 1(10), 1301–1311. doi: 10.1210/js.2017-00258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang A, Von Korff M, Lee S, Alonso J, Karam E, Angermeyer MC, … Watanabe M (2008). Common chronic pain conditions in developed and developing countries: gender and age differences and comorbidity with depression-anxiety disorders. Journal of Pain, 9(10), 883–891. doi: 10.1016/j.jpain.2008.05.005 [DOI] [PubMed] [Google Scholar]
- van der Slot WMA, Benner JL, Brunton L, Engel JM, Gallien P, Hilberink SR, … Roebroeck ME (2020). Pain in adults with cerebral palsy: a systematic review and meta-analysis of individual participant data. Annals of Physical and Rehabilitation Medicine. doi: 10.1016/j.rehab.2019.12.011 [DOI] [PubMed] [Google Scholar]
- van Gorp M, Dallmeijer A, van Wely L, de Groot V, Terwee C, Flens G, … Roebroeck M (2019). Pain, fatigue, depressive symptoms and sleep disturbance in young adults with cerebral palsy. Disability and Rehabilitation, In Press. 10.1080/09638288.2019.1694998 [DOI] [PubMed] [Google Scholar]
- Verschuren O, Smorenburg ARP, Luiking Y, Bell K, Barber L, & Peterson MD (2018). Determinants of muscle preservation in individuals with cerebral palsy across the lifespan: a narrative review of the literature. Journal of Cachexia, Sarcopenia and Muscle, 9(3), 453–464. doi: 10.1002/jcsm.12287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner R, Linroth R, Gangl C, Mitchell N, Hall M, Cady R, & Christenson M (2015). Perception of secondary conditions in adults with spina bifida and impact on daily life. Disability and Health Journal, 8(4), 492–498. doi: 10.1016/j.dhjo.2015.03.012 [DOI] [PubMed] [Google Scholar]
- Ware AL, Kulesz PA, Juranek J, Cirino PT, & Fletcher JM (2017). Cognitive control and associated neural correlates in adults with spina bifida myelomeningocele. Neuropsychology, 31(4), 411–423. doi: 10.1037/neu0000350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney D, Warschausky S, Ng S, Hurvitz E, Kamdar N, & Peterson M (2019). Prevalence of Mental Health Disorders Among Adults With Cerebral Palsy A Cross-sectional Analysis. Annals of Internal Medicine, 171(5):328–333. DOI: 10.7326/M18-3420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitney DG, Alford AI, Devlin MJ, Caird MS, Hurvitz EA, & Peterson MD (2019). Adults With Cerebral Palsy Have Higher Prevalence of Fracture Compared With Adults Without Cerebral Palsy Independent of Osteoporosis and Cardiometabolic Diseases. The Journal of Bone and Mineral Research, 34(7):1240–1247. doi: 10.1002/jbmr.3694 [DOI] [PubMed] [Google Scholar]
- Whitney DG, Hurvitz EA, Devlin MJ, Caird MS, French ZP, Ellenberg EC, & Peterson MD (2018). Age trajectories of musculoskeletal morbidities in adults with cerebral palsy. Bone, 114, 285–291. doi: 10.1016/j.bone.2018.07.002 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Table S1. Diagnostic codes for cerebral palsy, spina bifida, and all psychological morbidities using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and the International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) codes.
Supplemental Table S2. Modified Elixhauser Index.
Supplemental Table S3. Fully adjusted survival model for any psychological morbidity.


