Abstract
Background
Dental erosion is a chemical loss of the mineralized dental tissue caused by exposure to nonbacterial acids. Different treatment protocols have been adopted with the use of fluoride compounds to promote the formation of a layer of mineral precipitation in eroded lesions.
Aim
This systematic review aimed to evaluate the main treatments for dental erosion.
Methodology
This study followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and recorded in the Open Science Framework database (OSF) under DOI 10.17605/OSF.IO/XMFNZ. The searches were conducted in six electronic databases (Pubmed, Embase, Web of Science, Cochrane, Scopus, Lilacs) and two grey literature sources (Google Scholar and OpenGrey). The eligibility criteria included in vitro studies that evaluated eroded teeth under treatment with some topical agent. Risk of bias assessment and qualitative synthesis were performed using the Cochrane collaboration’s tool for assessing risk of bias modified for in vitro studies.
Results
A total of 522 studies were identified, and only four studies that fulfilled our eligibility criteria were included in this review. Among these studies, three were considered to have a low risk of bias, and one to have a high risk of bias. Two studies evaluated the anti-erosion effect of fluoride toothpaste, and the other two assessed the action of casein phosphopeptide–amorphous calcium phosphate (CPP-ACP) on the surface of human teeth. Among the products analyzed, CPP-ACP was the only one that promoted a significant increase in enamel microhardness and reduced tooth wear.
Conclusion
Based on the in vitro studies included in this review, there was no anti-erosion effect after using different fluoride toothpaste. However, it should be considered that one of these studies presented a high risk of bias. On the other hand, studies with CPP-ACP showed anti-erosion efficacy when applied before or after erosive wear.
Keywords: Tooth erosion, Treatment, Systematic review
Introduction
Dental erosion is defined as the chemical loss of dental mineralized tissue caused by exposure to nonbacterial acids (Schlueter et al., 2020). This is a multifactorial condition whose etiology and pathogenesis are related to chemical, biological, and behavioral factors (Kanzow et al., 2016). These factors, acting individually or interacting with each other, play a role in the prevention or progression control of dental erosion (Lussi, 2006).
The early diagnosis of erosive lesions is still challenging for most professionals and is usually identified as the rapid progress of tooth wear where there is already dentin hypersensitivity and the absence of lesion staining (Donovan et al., 2021). Also, other cases of erosive wear occur in the process of slow progression, which does not generate symptoms due to the response mechanism of the dentin-pulp complex that promotes the obliteration of dentinal tubules (Bartlett, 2016).
In this context, direct exposure to acids in the oral cavity promotes the demineralization of hydroxyapatite due to the undersaturation of minerals concerning the surrounding microenvironment (Shellis, Featherstone & Lussi, 2014). Therefore, saliva plays an essential role in the bioavailability of calcium and phosphate ions for the remineralization process. However, the frequent action of acids can overcome the protective effect of saliva, causing mineral dissolution that results in erosive wear (Fernando et al., 2019). To reverse this process, it is necessary to use topical remineralizing agents that contain calcium, phosphate, and mainly fluoride ions (Shen et al., 2018).
The use of fluoride in the form of mouthwash or brushing with toothpaste promotes structural remineralization, forming a mineral layer on the tooth surface and reducing subsequent demineralization, thus helping in the treatment and prevention of dental erosion (Creeth et al., 2015). Although fluoride products are often used, most fluoridated formulations alone have limited preventive effects against tooth erosion as their action on the mineralization process is limited to the surface and the near-surface layers of enamel and is restricted to the demineralized enamel layer (Lussi & Carvalho, 2015).
Numerous vehicles, such as toothpaste, rinse solutions, gels, and varnishes, are currently available as strategies for using fluorides. These products contain different types of active ingredients with distinct anti-erosive properties, and some of them have been demonstrated to be effective preventive therapies against tooth erosion (Buzalaf, Magalhães & Wiegand, 2014; Lussi et al., 2019).
The most appropriate way to access the protective effects of these products would be in vivo studies, with randomized clinical trials considered the gold standard. However, this kind of study has some limitations, such as the low accuracy of available methods for measurement of chemical tooth tissue loss, the need for prolonged studies to assess the rate of progression and the variation of this rate, the lack of control over the exposure to wear, the need for participation of large groups, to minimize contact with other erosive agents, and for several years of follow-up (West, Davies & Amaechi, 2011). Proper clinical methods must be developed and validated to assess dental erosion variables in vivo. Numerous in vitro model studies have been designed to simulate everyday intra-oral conditions as closely as possible (Wiegand & Attin, 2011). Thus, this systematic review was carried out to gather scientific evidence demonstrating the anti-erosion treatments that presented the best results in the remineralization of the tooth structure.
Materials & Methods
Registration
This study was recorded in the Open Science Framework database (OSF) under registration DOI 10.17605/OSF.IO/XMFNZ and performed according to the guidelines of the Preferential Reporting Requirements for Systematic Review (PRISMA) (Page et al., 2021).
Eligibility criteria
This systematic review was guided by the PICO (P = patient or participant, I = intervention, C = comparison, O = outcome) question strategy, covering studies in eroded teeth (P) treated with any topical agent (I) in comparison without treatment (C), whose primary outcome was the change in enamel tissue (O).
The eligibility criteria included studies with human teeth and permanent teeth, in which in vitro data was recorded after tooth erosion and used any topical agent with an active ingredient that could be used for the treatment of dental erosion and compared with the group that used artificial saliva. Studies that did not fall within the PICO were excluded as well as technical papers, clinical cases, literature reviews, uncontrolled studies, guides, letters to the editor and opinion articles. Also, studies that evaluated bovine teeth were excluded because they had different histological characteristics from human teeth. Studies not using specimens placed in artificial saliva as a comparison group were also excluded.
Search and selection of studies
A search strategy composed of keywords and free terms was used to search for studies in the electronic databases Pubmed, Web of Science, Lilacs, Scopus, Embase and Cochrane, as well as the grey literature search through Google Scholar and OpenGrey (Table S1). After that, a reference manager (EndNote, version X9, Thomson Reuters, Philadelphia, USA) was used to exclude duplicate citations.
The first stage of the study selection was carried out by reading the titles and abstracts. The remaining articles were read in full to verify eligibility. From this stage, the papers selected for this review were defined, and a manual search was carried out in the references in order to find other eligible articles. Two independent authors performed the search and selection of studies, and a third reviewer evaluated the data in cases of disagreement.
Data extraction and risk of bias analysis
The following data were extracted from each study: authors, country of publication, study type, tooth type, erosion assessment, erosion treatment, and results. In cases where some data were missing, the authors were contacted by e-mail once a week for a month until the information was obtained.
After the data extraction, two independent researchers performed the risk of bias assessment. At the same time, the disagreements were resolved by a third researcher in consensus with the peers. The Cochrane collaboration’s tool (Higgins et al., 2011) was applied to assess the risk of bias. The selected studies were processed in the Review Manager software (version 5.3, Copenhagen: The Cochrane Collaboration, https://training.cochrane.org/online-learning/core-software/revman).
Randomized sequence creation, allocation concealment, participant blinding, blinding of outcome assessment, inadequate outcome data, and selective reporting are the six major bias domains covered by this instrument. For the final evaluation of each study, the following essential domains were chosen: concealment of selection, blinding of participants, and blinding of outcome evaluation. A study with a high risk of bias was defined as having severe issues in two key domains (Table S2).
Results
Characteristics of the studies
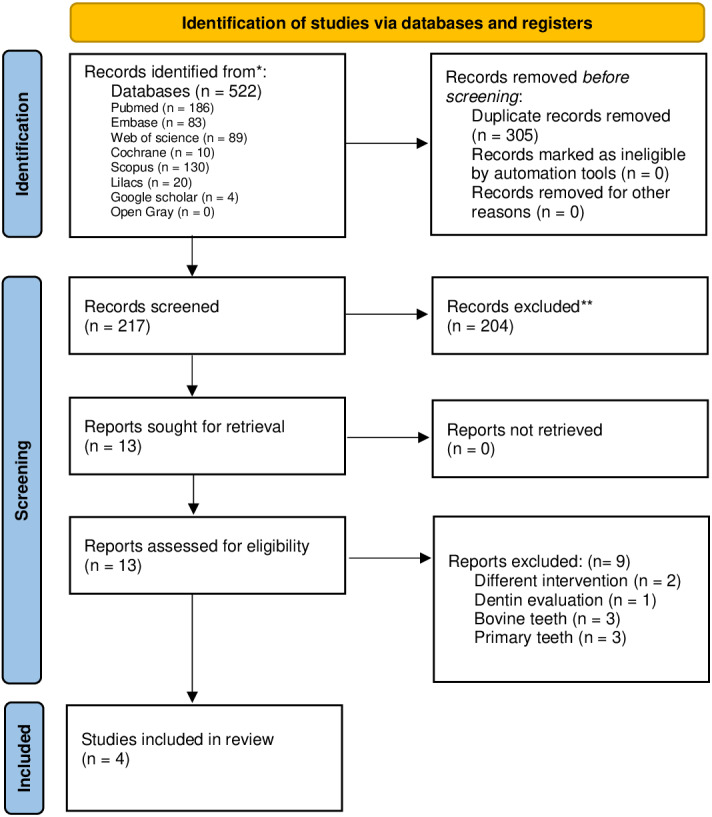
A total of 522 records were identified from the database search, and 305 duplicates were found and removed. The remaining 217 were evaluated by title and abstract according to the eligibility criteria, and as a result, 204 studies were excluded at this stage.
The remaining studies (n = 13) were assessed by full-text reading: three used bovine teeth (Comar et al., 2015; Machado et al., 2019; Scandiffio et al., 2018), and one evaluated dentine (João Souza et al., 2020). Two studies did not realize the intervention group (Austin et al., 2014; Passos, Rodrigues & Santiago, 2018), and three evaluated primary teeth (Elwardani, Harhash & Zaky, 2019; Maden et al., 2017; Rallan et al., 2013), conflicting with the previously established eligibility criteria. Finally, four studies were selected in this systematic review according to the eligibility criteria (Bradna et al., 2015; João Souza et al., 2017; Panich & Poolthong, 2009; Ranjitkar et al., 2009) (Fig. 1).
Figure 1. Flowchart of the study selection process according to the PRISMA protocol.
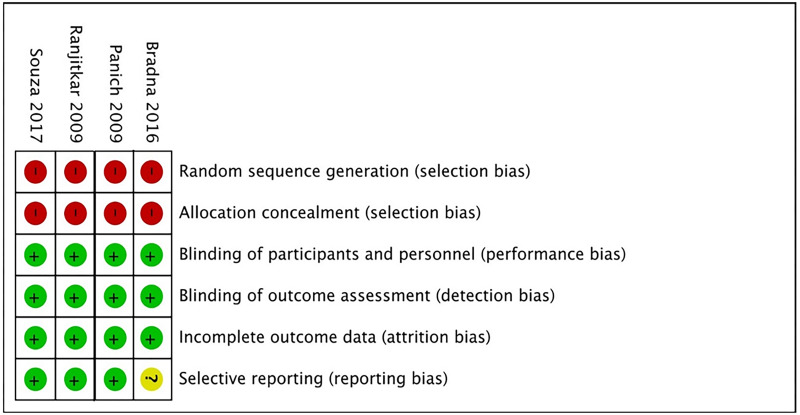
The asterisks (*, **) indicate the number of points attributed to each domain in the quality assessment to each article.
The four included studies were in vitro studies. One was conducted in the Czech Republic (Bradna et al., 2015), one in Brazil (João Souza et al., 2017), one in Thailand (Panich & Poolthong, 2009), and one in the United Kingdom (Ranjitkar et al., 2009). These studies evaluated the surface of eroded tooth enamel and analyzed the effect of different remineralizing treatments.
Individual results of studies
The substances used to induce erosive wear were citric acid solution (Bradna et al., 2015; João Souza et al., 2017; Ranjitkar et al., 2009) and Coca-Cola (Coke®) drink (Panich & Poolthong, 2009). The studies by Bradna et al. (2015) and João Souza et al. (2017) evaluated the effect of various commercial toothpaste compared to the control group that immersed the teeth only in artificial saliva. In the first study mentioned, the treatment groups used fluoride-based (Sensodyne® Pronamel), toothpaste with stannous fluoride (Elmex® Erosion Protection), and toothpaste with calcium (BioRepair plus sensitivity control, SensiShield, Enamel Care). The second study evaluated several desensitizing and/or anti-erosion toothpaste (Table 1). These two studies’ results revealed that toothpaste’s anti-erosion effectiveness is associated with the presence of tin, high concentrations of phosphate and calcium, strong potential to form acid-resistant deposits on the enamel surface, and low potential to create acid-resistant deposits on the enamel surface abrasivity.
Table 1. Data and characteristics and studies included.
| Author (Year)/Country | Size and source of sample | Teeth | Erosion evaluation | Erosion treatment | Results |
|---|---|---|---|---|---|
| Bradna et al. (2015)/ Czech Republic |
N = 35 General University Hospital in Prague, Czech Republic |
Third molars | Microhardness testing | Experimental groups: Sensodyne Pronamel; Elmex erosion protection; BioRepair plus sensitivity control; SensiShield; Enamel care for sensitive teeth Control groups: Artificial saliva Elmex erosion mouth rise |
Enamel Care formed a compact layer of deposits on the enamel surface, while Sensodyne Pronamel and BioRepair Plus Sensitivity Control did not produce any protective deposits. On the other hand, Elmex Erosion Protection and SensiShield showed high abrasivity and consequent low anti-erosion action. |
|
João Souza et al. (2017)/ Brazil |
N = 150 Department of Restorative Dentistry, University of São Paulo, School of Dentistry |
Premolar | Microhardness testing | Experimental groups: Sensodyne Repair and Protect; Elmex Sensitive Professional; Sensodyne Rapid Relief; Blend-a-Med (Oral-B) Pro Expert; Sensodyne Pronamel; Elmex Erosion Protection; Candida Protect Professional; Regenerate Control groups: Artificial Saliva Colgate Caries Protection |
All groups showed progressive surface loss. Sensodyne Pronamel and Elmex Erosion Protection presented the lowest values of enamel surface loss, not differing from the control groups and with no difference between them (p > 0.05). Regenerate and Blend-a-Med Pro Expert showed the highest surface loss values. |
|
Panich & Poolthong (2009)/ Thailand |
N = 40 School of Dentistry, Chulalongkorn University, Bangkok, Thailand |
Central and lateral incisors | Microhardness testing | CPP-ACP (Tooth Mousse) Artificial Saliva |
After remineralization, the mean microhardness increased by 13.27% in the CPP-ACP group and by 2.53% in Artificial Saliva group. Two-way ANOVA results showed no statistically significant interaction among CPP-ACP, artificial saliva and deionized water (p = 0.548). |
|
Ranjitkar et al. (2009)/ United Kingdom |
N = 36 Guy’s Hospital, London, United Kingdom |
Third molars | Profilometry | Tooth Mousse (CPP-ACP) Tooth Mousse (without CPP-ACP) Artificial saliva |
Tooth Mousse significantly reduced enamel wear (p < 0.001). |
Notes.
- APF gel
- Acidulated phosphate fluoride gel
- CPP-ACP
- Casein phosphopeptide-amorphous calcium phosphate
- CPP-ACPF
- Casein phosphopeptide-amorphous calcium phosphate fluoride
- Er,Cr:YSGG
- Erbium yttrium scandium gallium garnet
Two studies included in this systematic review showed the use of fluorinated or non-fluorinated tooth mousses containing casein phosphopeptide-amorphous calcium phosphate (CPP-ACP) (Panich & Poolthong, 2009; Ranjitkar et al., 2009), which is a bioactive agent derived from milk protein that can stabilize calcium phosphate in the tooth structure (Gokkaya et al., 2020). Thus, considering the variability of anti-erosion treatment protocols between the two studies, it can be identified that oral products containing CPP-ACP presented the best results in reducing erosive enamel loss expressed by increasing surface microhardness and reducing surface roughness.
The studies, in general, showed that pastes with a higher concentration of ions such as calcium and phosphate and smaller particle size showed greater effectiveness in protecting against dental erosion so that acids could not degrade the mineral matrix. Among the topical agents, acidulated phosphate fluoride (APF) gel, Elmex® erosion, and Sensodyne® Pronamel showed the best results related to enamel protection (Bradna et al., 2015; João Souza et al., 2017; Ranjitkar et al., 2009).
Nevertheless, Elmex® erosion toothpaste showed high adhesion to enamel due to the high concentration of abrasive agents such as tin, it made the enamel surface a little scratched, compared to Sensodyne® Pronamel, which showed a smoother surface indicating less abrasiveness (Bradna et al., 2015; João Souza et al., 2017).
Risk of bias and qualitative assessment of studies
In the risk of bias analysis using the Cochrane tool (Higgins et al., 2011), although all studies showed methodological problems in the domain of the test machine blinding operator, it was not significant to increase the risk of bias because the sample was large enough, so three of the four selected studies were considered to be at low risk of bias (Panich & Poolthong, 2009; Ranjitkar et al., 2009; João Souza et al., 2017) (Fig. 2). Only one of the studies demonstrated a high risk of bias due to the lack of quantitative data (Bradna et al., 2015).
Figure 2. Risk of bias of the included studies, according to the Cochrane collaboration’s tool for assessing risk of bias.
Discussion
Our systematic review aimed to investigate effective treatments for dental erosion. Although fluoride products showed no significant reduction in enamel surface loss in the studies, there was a remineralizing effect on dental enamel after using CPP-ACP. However, the number and limitations of the included studies must be considered.
Based on the present findings, it was possible to verify that all test products (toothpaste and tooth mousses) had different effects on enamel surface loss. Comparing these products can be difficult, as many intrinsic factors can influence their protective potential, the type of experimental model, and different ways of presenting the products used. The absence of a negative control group with exposure only to water can interfere with the mean difference between groups in erosion/abrasion models. However, when evaluated individually, it is possible to demonstrate that the performance of each product depends on several factors, such as the presence or absence of fluoride; the mode of action of fluoride; the mode of action of other protective agents, such as polyvalent metal ions, amino acids, peptides, proteins and polymers, and CCP-ACP (Lussi & Carvalho, 2015).
The influence of fluoride on dental erosion depends on many factors, such as higher concentration, frequency of application, and vehicle. In these cases, the efficacy of fluoride products is attributed to the formation of precipitates on the tooth surface, which acts as a protective barrier against acid impacts (Schlueter et al., 2020). Although the studies in this review did not show a significant remineralizing effect of fluoride on tooth enamel, other recent studies in the literature have shown that the application of fluoride products after acid challenge to enamel reduced enamel loss (Zanatta et al., 2020; Ionta, dos Santos & Mesquita, 2019). The studies by Bradna et al. (2015) and João Souza et al. (2017) showed no protective effect against dental erosion, although lower enamel surface loss was observed in teeth treated with fluoride dentifrices and/or rinses. These results are limited due to the action of other factors such as calcium and phosphate ion concentration, chemical characteristics of the compounds, and different application protocols.
In addition to these therapeutic approaches, the remineralizing properties of milk protein-based complexes have been investigated, which are used in the form of peptides, among which is the amorphous casein-calcium phosphopeptide complex (CPP-ACP) (Ekambaram, Said & Yiu, 2017). There is evidence that CPP-ACP provides high concentrations of calcium and phosphate ions that, when associated with fluoride, potentiate the remineralizing effect on tooth enamel (Gokkaya et al., 2020; Alexandria et al., 2020; Bejoy et al., 2020). The casein present in the complex stabilizes phosphate and calcium ions and facilitates the formation of mineral deposits on the surface of erosive lesions (Reema, Lahiri & Roy, 2014). Therefore, CPP-ACP effectively prevents erosive lesions when applied before the acid challenge by reducing the percentage of loss of surface microhardness of human enamel (Yu et al., 2018; Bejoy et al., 2020).
Although in vitro studies are very relevant, it should be noted that, no matter how much the methodology used in these studies tries to reproduce in greater detail what happens in the oral cavity, it will never be identical. For example, in dental erosion studies, it is impossible to reproduce the acquired pellicle, which is an essential factor in protecting the tooth surface against erosion. In addition, the studies used in the present systematic review show high heterogeneity in the methodologies used, such as substrate, stages of dental erosion, evaluation times, and erosive protocols, among others. Considering all these factors, the results found in our study need to be evaluated with caution. Moreover, we suggest future well-designed studies with better standardization of the methods, allowing comparison among them and increasing the quality of scientific evidence.
Conclusions
Despite some methodological limitations in in vitro studies, including those discussed in this systematic review, the results provided imply that CPP-ACP therapies can be beneficial for dental erosion. However, these results should be evaluated with caution, and further studies are needed to establish the best treatment for dental erosion with high certainty of evidence.
Supplemental Information
Funding Statement
This study was financed by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior–Brasil (CAPES), Financing Code 001. Rafael Rodrigues Lima is a researcher from Brazilian National Council for Scientific and Technological Development (CNPq) and received grant number 312275/2021-8. The APC was funded by the Pró-Reitoria de Pesquisa e Pós-Graduação from Federal University of Pará. There was no additional external funding received for this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests.
Author Contributions
Yago Gecy de Sousa Né conceived and designed the experiments, performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Deiweson Souza-Monteiro performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Deborah Ribeiro Frazão conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
María Olimpia Paz Alvarenga performed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Walessa Alana Bragança Aragão performed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Nathália Carolina Fernandes Fagundes conceived and designed the experiments, analyzed the data, prepared figures and/or tables, authored or reviewed drafts of the article, and approved the final draft.
Renata Duarte de Souza-Rodrigues analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Rafael Rodrigues Lima conceived and designed the experiments, analyzed the data, authored or reviewed drafts of the article, and approved the final draft.
Data Availability
The following information was supplied regarding data availability:
This is a literature review and does not have raw data.
References
- Alexandria et al. (2020).Alexandria A, Valença A, Cabral LM, Maia LC. Comparative effects of CPP-ACP and Xylitol F-Varnishes on the reduction of tooth erosion and its progression. Brazilian Dental Journal. 2020;31(6):664–672. doi: 10.1590/0103-6440202002985. [DOI] [PubMed] [Google Scholar]
- Austin et al. (2014).Austin RS, Stenhagen KR, Hove LH, Tveit AB, Moazzez RV, Bartlett DW. The effect of single-application fluoride treatment on simulated gastric erosion and erosion-abrasion of enamel in vitro. The International Journal of Prosthodontics. 2014;27(5):425–426. doi: 10.11607/ijp.3956. [DOI] [PubMed] [Google Scholar]
- Bartlett (2016).Bartlett D. A personal perspective and update on erosive tooth wear - 10 years on: part 1 - Diagnosis and prevention. British Dental Journal. 2016;221(3):115–119. doi: 10.1038/sj.bdj.2016.555. [DOI] [PubMed] [Google Scholar]
- Bejoy et al. (2020).Bejoy BM, Sruthi MS, George L, Mathew J, Vineet RP, Joy A. Comparative evaluation of casein phosphopeptide-amorphous calcium phosphate-fluoride paste and sodium fluoride mouthwash in the prevention of dental erosion: an in vitro study. The Journal of Contemporary Dental Practice. 2020;21(3):267–270. [PubMed] [Google Scholar]
- Bradna et al. (2015).Bradna P, Vrbova R, Fialova V, Housova D, Gojisova E. Formation of protective deposits by anti-erosive toothpaste—a microscopic study on enamel with artificial defects. Scanning. 2015;38(5):380–388. doi: 10.1002/sca.21281. [DOI] [PubMed] [Google Scholar]
- Buzalaf, Magalhães & Wiegand (2014).Buzalaf MAR, Magalhães AC, Wiegand A. Alternatives to fluoride in the prevention and treatment of dental erosion. Monographs in Oral Science. 2014;25:244–252. doi: 10.1159/000360557. [DOI] [PubMed] [Google Scholar]
- Comar et al. (2015).Comar LP, Cardoso C de A, Charone S, Grizzo LT, Buzalaf MA, Magalhães AC. TiF4 and NaF varnishes as anti-erosive agents on enamel and dentin erosion progression in vitro. Journal of Applied Oral Science. 2015;23(1):14–18. doi: 10.1590/1678-775720140124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creeth et al. (2015).Creeth JE, Kelly SA, Martinez-Mier EA, Hara AT, Bosma ML, Butler A, Lynch RJM, Zero DT. Dose–response effects of fluoride dentifrice on remineralization and further demineralization of erosive lesions: a randomized in situ clinical study. Journal of Dentistry. 2015;43:823–831. doi: 10.1016/j.jdent.2015.03.008. [DOI] [PubMed] [Google Scholar]
- Donovan et al. (2021).Donovan T, Nguyen-Ngoc C, Alraheam IAbd, Irusa K. Contemporary diagnosis and management of dental erosion. Journal of Esthetic and Restorative Dentistry. 2021;33(1):78–87. doi: 10.1111/jerd.12706. [DOI] [PubMed] [Google Scholar]
- Ekambaram, Mohd Said & Yiu (2017).Ekambaram M, Mohd Said S, Yiu C. A review of enamel remineralisation potential of calcium- and phosphate-based remineralisation systems. Oral Health & Preventive Dentistry. 2017;15(5):415–420. doi: 10.3290/j.ohpd.a38779. [DOI] [PubMed] [Google Scholar]
- Elwardani, Harhash & Zaky (2019).Elwardani GE, Harhash TAH, Zaky AA. Effect of Er,Cr:YSGG on remineralization using CPP-ACPF (MI-Paste Plus) after enamel erosion caused by carbonated soft drink in primary teeth: in-vitro study. Open Access Macedonian Journal of Medical Sciences. 2019;7(7):1184–1192. doi: 10.3889/oamjms.2019.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernando et al. (2019).Fernando JR, Shen P, Sim C, Chen YY, Walker GD, Yuan Y, Reynolds C, Stanton DP, MacRae CM, Reynolds EC. Self-assembly of dental surface nanofilaments and remineralization by SnF2 and CPP-ACP nanocomplexes. Scientific Reports. 2019;9(1):1285. doi: 10.1038/s41598-018-37580-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gokkaya et al. (2020).Gokkaya B, Ozbek N, Guler Z, Akman S, Sarac AS, Kargul B. Effect of a single application of CPP-ACPF varnish on the prevention of erosive tooth wear: an AAS, AFM and SMH study. Oral Health & Preventive Dentistry. 2020;18(1):311–318. doi: 10.3290/j.ohpd.a43365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins et al. (2011).Higgins JP, Altman DG, Gotzsche PC, Juni P, Moher D, Oxman AD, Savovic J, Schulz KF, Weeks L, Sterne JAC, Cochrane Bias Methods Group. Cochrane Statistical Methods Group The Cochrane Collaboration’s tool for assessing risk of bias in randomized trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ionta et al. (2019).Ionta FQ, dos Santos NM, Mesquita IM, Dionísio EJ, Cruvinel T, Honório HM, Rios D. Is the dentifrice containing calcium silicate, sodium phosphate, and fluoride able to protect enamel against chemical mechanical wear? an in situ/ex vivo study. Clinical Oral Investigations. 2019;23:3713–3720. doi: 10.1007/s00784-018-2792-4. [DOI] [PubMed] [Google Scholar]
- João Souza et al. (2017).João Souza SH, Lussi A, Baumann T, Scaramucci T, Aranha ACC, Carvalho TS. Chemical and physical factors of desensitizing and/or anti-erosive toothpaste associated with lower erosive tooth wear. Scientific Reports. 2017;7(1):17909. doi: 10.1038/s41598-017-18154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- João Souza et al. (2020).João Souza SH, Sakae LO, Lussi A, Aranha ACC, Hara A, Baumann T, Scaramucci T, Carvalho TS. Toothpaste factors related to dentine tubule occlusion and dentine protection against erosion and abrasion. Clinical Oral Investigations. 2020;24(6):2051–2060. doi: 10.1007/s00784-019-03069-7. [DOI] [PubMed] [Google Scholar]
- Lussi (2006).Lussi A. Basel, Switzerland: Karger; 2006. Dental erosion: from diagnosis to therapy. [DOI] [Google Scholar]
- Lussi et al. (2019).Lussi A, Buzalaf MAR, Duangthip D, Anttonen V, Ganss C, João Souza SH, Baumann T, Carvalho TS. The use of fluoride for the prevention of dental erosion and erosive tooth wear in children and adolescents. European Archives of Paediatric Dentistry. 2019;20(6):517–527. doi: 10.1007/s40368-019-00420-0. [DOI] [PubMed] [Google Scholar]
- Lussi & Carvalho (2015).Lussi A, Carvalho TS. The future of fluorides and other protective agents in erosion prevention. Caries Research. 2015;49 Suppl 1:18–29. doi: 10.1159/000380886. [DOI] [PubMed] [Google Scholar]
- Machado et al. (2019).Machado AC, Bezerra SJC, João Souza SH, Caetano TM, Russo LC, Carvalho TS, Scaramucci T. Using fluoride mouthrinses before or after toothbrushing: effect on erosive tooth wear. Archives of Oral Biology. 2019;108:104520. doi: 10.1016/j.archoralbio.2019.104520. [DOI] [PubMed] [Google Scholar]
- Maden et al. (2017).Maden EA, Acar Ö, Altun C, Polat GG. The effect of casein phosphopeptide-amorf calcium phosphate and acidulated phosphate fluoride gel on dental erosion in primary teeth: an in vitro study. Journal of Clinical Pediatric Dentistry. 2017;41(4):275–279. doi: 10.17796/1053-4628-41.4.275. [DOI] [PubMed] [Google Scholar]
- Kanzow et al. (2016).Kanzow P, Wegehaupt FJ, Attin T, Wiegand A. Etiology and pathogenesis of dental erosion. Quintessence International (Berlin, Germany: 1985) 2016;47(4):275–278. doi: 10.3290/j.qi.a35625. [DOI] [PubMed] [Google Scholar]
- Page et al. (2021).Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, Shamseer L, Tetzlaff JM, Akl EA, Brennan SE, Chou R, Glanville J, Grimshaw JM, Hróbjartsson A, Lalu MM, Li T, Loder EW, Mayo-Wilson E, McDonald S, McGuinness LA, Stewart LA, Thomas J, Tricco AC, Welch VA, Whiting P, Moher D. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panich & Poolthong (2009).Panich M, Poolthong S. The effect of casein phosphopeptide-amorphous calcium phosphate and a cola soft drink on in vitro enamel hardness. Journal of the American Dental Association. 2009;140(4):455–460. doi: 10.14219/jada.archive.2009.0195. [DOI] [PubMed] [Google Scholar]
- Passos, Rodrigues & Santiago (2018).Passos VF, Rodrigues LKA, Santiago SL. The effect of magnesium hydroxide-containing dentifrice using an extrinsic and intrinsic erosion cycling model. Archives of Oral Biology. 2018;86:46–50. doi: 10.1016/j.archoralbio.2017.11.006. [DOI] [PubMed] [Google Scholar]
- Rallan et al. (2013).Rallan M, Chaudhary S, Goswami M, Sinha A, Arora R, Kishor A. Effect of various remineralizing agents on human eroded enamel of primary teeth. European Archives of Paediatric Dentistry. 2013;14(5):313–318. doi: 10.1007/s40368-013-0085-9. [DOI] [PubMed] [Google Scholar]
- Ranjitkar et al. (2009).Ranjitkar S, Rodriguez JM, Kaidonis JA, Richards LC, Townsend GC, Bartlett DW. The effect of casein phosphopeptide-amorphous calcium phosphate on erosive enamel and dentine wear by toothbrush abrasion. Journal of Dentistry. 2009;37(4):250–254. doi: 10.1016/j.jdent.2008.11.013. [DOI] [PubMed] [Google Scholar]
- Reema, Lahiri & Roy (2014).Reema SD, Lahiri PK, Roy SS. Review of casein phosphopeptides-amorphous calcium phosphate. The Chinese Journal of Dental Research. 2014;17(1):7–14. [PubMed] [Google Scholar]
- Scandiffio et al. (2018).Scandiffio P, Mantilla T, Amaral F, França F, Basting R, Turssi C. Anti-erosive effect of calcium carbonate suspensions. Journal of Clinical and Experimental Dentistry. 2018;10(8):e776–e780. doi: 10.4317/jced.54994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlueter et al. (2020).Schlueter N, Amaechi BT, Bartlett D, Buzalaf MAR, Carvalho TS, Ganss C, Hara AT, Huysmans M-CDNJM, Lussi A, Moazzez R, West NX, Wiegand A, Young A, Lippert F. Terminology of erosive tooth wear: consensus report of a workshop organized by the ORCA and the cariology research group of the IADR. Caries Research. 2020;54:2–6. doi: 10.1159/000503308. [DOI] [PubMed] [Google Scholar]
- Shellis, Featherstone & Lussi (2014).Shellis RP, Featherstone JD, Lussi A. Understanding the chemistry of dental erosion. Monographs in Oral Science. 2014;25:163–179. doi: 10.1159/000359943. [DOI] [PubMed] [Google Scholar]
- Shen et al. (2018).Shen P, Walker GD, Yuan Y, Reynolds C, Stanton DP, Fernando JR, Reynolds EC. Importance of bioavailable calcium in fluoride dentifrices for enamel remineralization. Journal of Dentistry. 2018;78:59–64. doi: 10.1016/j.jdent.2018.08.005. [DOI] [PubMed] [Google Scholar]
- West, Davies & Amaechi (2011).West NX, Davies M, Amaechi BT. In vitro and in situ erosion models for evaluating tooth substance loss. Caries Research. 2011;45 Suppl 1:43–52. doi: 10.1159/000325945. [DOI] [PubMed] [Google Scholar]
- Wiegand & Attin (2011).Wiegand A, Attin T. Design of erosion/abrasion studies—insights and rational concepts. Caries Research. 2011;45 Suppl 1:53–59. doi: 10.1159/000325946. Epub 2011 May 31. [DOI] [PubMed] [Google Scholar]
- Yu et al. (2018).Yu H, Jiang NW, Ye XY, Zheng HY, Attin T, Cheng H. In situ effect of Tooth Mousse containing CPP-ACP on human enamel subjected to in vivo acid attacks. Journal of Dentistry. 2018;76:40–45. doi: 10.1016/j.jdent.2018.05.021. [DOI] [PubMed] [Google Scholar]
- Zanatta et al. (2020).Zanatta RF, Caneppele T, Scaramucci T, El Dib R, Maia LC, Ferreira D, Borges AB. Protective effect of fluorides on erosion and erosion/abrasion in enamel: a systematic review and meta-analysis of randomized in situ trials. Archives of Oral Biology. 2020;120:104945. doi: 10.1016/j.archoralbio.2020.104945. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
This is a literature review and does not have raw data.