Abstract
BACKGROUND
Trypanosoma cruzi shows an exuberant genetic diversity. Currently, seven phylogenetic lineages, called discrete typing units (DTUs), are recognised: TcI-TcVI and Tcbat. Despite advances in studies on T. cruzi and its populations, there is no consensus regarding its heterogeneity.
OBJECTIVES
This study aimed to perform molecular characterisation of T. cruzi strains, isolated in the state of São Paulo, to identify the DTUs involved and evaluate their genetic diversity.
METHODS
T. cruzi strains were isolated from biological samples of chronic chagasic patients, marsupials and triatomines through culture techniques and subjected to molecular characterisation using the fluorescent fragment length barcoding (FFLB) technique. Subsequently, the results were correlated with complementary information to enable better discrimination between the identified DTUs.
FINDINGS
It was possible to identify TcI in two humans and two triatomines; TcII/VI in 19 humans, two marsupials and one triatomine; and TcIII in one human host, an individual that also presented a result for TcI, which indicated the possibility of a mixed infection. Regarding the strains characterised by the TcII/VI profile, the correlation with complementary information allowed to suggest that, in general, these parasite populations indeed correspond to the TcII genotype.
MAIN CONCLUSIONS
The TcII/VI profile, associated with domestic cycles and patients with chronic Chagas disease, was the most prevalent among the identified DTUs. Furthermore, the correlation of the study results with complementary information made it possible to suggest that TcII is the predominant lineage of this work.
Key words: Chagas disease, host-pathogen interactions, molecular biology, Trypanosoma cruzi
The heterogeneity of the protozoan parasite Trypanosoma cruzi, the etiologic agent of Chagas disease, concerning a variety of factors related to morphology, pathogenicity, virulence, genetic content, among others, has been observed for decades and has promoted numerous molecular epidemiology and population genetics studies. Consequently, this parasite has become one of the most notorious models regarding its evolution and population structure. 1
In this scenario, a species identification method was developed in 2008, known as fluorescent fragment length barcoding (FFLB). This technique was initially tested for characterisation and differentiation of African trypanosomatids, 2 and due to its efficiency, it was later used to determine phylogenetic lineages or discrete typing units (DTUs) of American trypanosomatids, having also shown robust results. 3
The FFLB targets specific and polymorphic regions of the 18S and 28S ribosomal RNA (rRNA) genes, classified as 18S1, 18S3, 28S1 and 28S2. Through the polymerase chain reaction (PCR) assay, performed from a set of forward and reverse primers designed for each region, one of which is fluorolabelled, nucleotide sequences of variable lengths are amplified. Subsequently, the amplification products are submitted to capillary electrophoresis and observed, in an automatic sequencer, as fluorescent peaks - electropherograms - of variable size and intensity. Thus, the set of four peaks, one for each amplified region, theoretically constitutes a unique profile for each analysed species, known as a barcode. 2
Currently, seven DTUs for T. cruzi are recognised: TcI-TcVI and Tcbat. 4 - 7 Regarding the main DTU properties, TcI has a wide geographic distribution, extending from the southern United States to northern Argentina and Chile; is frequently isolated in sylvatic cycles, although it is also present in domestic cycles; is responsible for the transmission of Chagas disease in regions located north of the Amazon Basin 4 , 5 , 7 - 9 and has considerable genetic diversity, with possible subdivisions within the lineage. 10 - 12 ) Regarding the other genotypes, TcII, TcV and TcVI are associated with domestic cycles and chronic chagasic patients in Southern Cone countries and Bolivia; TcIII and TcIV are found in rainforest sylvatic cycles; 4 , 5 , 7 - 9 ) and Tcbat was initially identified in bats 13 , 14 and later found in humans. 15 ) Likewise, it is important to note that different DTUs can coexist on the same host. 16 - 18
Despite the multiple advances in the study of T. cruzi and its populations, there is no consensus regarding its diversity. 6 However, the occurrence of genetic recombination events between different lineages of this parasite may lead, at least partially, to the understanding of this process. 5 , 7 , 19 - 22 In this sense, two major theories about the origin of DTUs, accepted by the scientific community, indicate, for example, that TcV and TcVI are hybrid lineages resulting from recombination between TcII and TcIII. 23 , 24
The variety of triatomine vectors and mammalian hosts can play an important role in the distribution of DTUs, especially when related to environmental conditions. This correlation probably maintains the heterogeneity of T. cruzi and promotes the emergence of new variants through natural selection over time. 4
Concerning the state of São Paulo (SP), it was already considered the territory with one of the highest prevalence of Chagas disease in Brazil, with the triatomine Triatoma infestans as the main transmitting species and with wide distribution in the region. 25 , 26 After the implementation of successful actions to control vector transmission by local authorities between the 1950s and 1970s, the state’s epidemiological surveillance service has constantly monitored occurrences involving other triatomine species and the participation of sylvatic reservoirs in the process. 25 In this scenario, Panstrongylus megistus stands out for the following factors: the remarkable ability to colonise artificial environments, with important reports of its finding in households and peridomiciles, especially in condominiums established in the urban areas of the metropolitan region of São Paulo; high infection rates by T. cruzi; and a great anthropophilia. 26 , 27 This conjuncture of factors represents an alert for a possible recurrence of Chagas disease by vector transmission in the state, last reported more than 50 years ago. 28
Contributing to epidemiological surveillance actions within the context of Chagas disease, the Instituto Adolfo Lutz (IAL), Laboratório Central de Saúde Pública do Estado de São Paulo (LACEN-SP), through the Centro de Parasitologia e Micologia (CPM), performs laboratory diagnosis of biological samples from different municipalities and health services and carries out field works in different regions of the state to study the ecoepidemiology of the disease by verifying the participation of sylvatic and domestic animals in its transmission. The CPM-IAL also meets demands for the identification of sylvatic or household triatomines and their possible natural infection by T. cruzi.
Considering the above, this study aimed to perform a molecular characterisation of T. cruzi strains isolated from biological samples analysed by the CPM-IAL from different host profiles, to identify their genotypes and expand knowledge about their diversity.
SUBJECTS AND METHODS
Samples - Twenty-six samples positive for T. cruzi were selected from biological materials sent, processed and examined at CPM-IAL between 2014 and 2018. They originated from 21 chronic chagasic patients (Table I), mostly residing in SP, and periodically attended and followed by different health services - Serviço de Extensão ao Atendimento de Pacientes HIV/Aids (SEAP), Instituto do Coração (Incor) and Serviço de Reumatologia, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (HCFMUSP) -, with different clinical conditions and positive laboratory tests for T. cruzi, by xenodiagnosis and blood culture, two sylvatic animals - marsupials of the Didelphis albiventris and Philander opossumspecies - from the São Paulo municipalities of Santa Fé do Sul and Ilhabela, respectively, with positive xenodiagnosis for T. cruzi, and three sylvatic triatomine specimens of the species P. megistus, collected from dwellings in the São Paulo municipalities of Taboão da Serra, Ilhabela and Itapecerica da Serra, with positive parasitological stool examinations for the presence of trypanosomatids and confirmatory real-time PCR test for T. cruzi.
TABLE I. Information from chronic chagasic patients whose blood samples were processed and examined at CPM-IAL.
| Patient | Age | Provenance (service)* | Provenance municipality (state) | Place of birth municipality (state) | Residence time in SP | Probable infection site municipality (state) | Landscape | Transmission route | Clinical condition | Treatment |
| 1 | 55 | HC-Incor | Guarulhos (SP) | Amargosa (BA) | No data | Amargosa (BA) | No data | Probably vector | Chronic phase - cardiac form | No data |
| 2 | 70 | HC-SEAP | São Paulo (SP) | Iguatu (CE) | 51 years, arrived in 1971 | Iguatu (CE) | Rural | Vector | Chronic phase - megaesophagus and cardiomyopathy | Yes, benznidazole in 2017 |
| 3 | 70 | HC-Incor | Osasco (SP) | Santana dos Garrotes (PB) | No data | Santana dos Garrotes (PB) | No data | Probably vector | Chronic phase - cardiac form | No data |
| 4 | 59 | HC-Incor | São Paulo (SP) | Anápolis (GO) | No data | Anápolis (GO) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 5 | 60 | HC-SEAP | São Paulo (SP) | Cruz das Almas (BA) | 47 years, arrived in 1975 | Cruz das Almas (BA) | Rural | Vector | Chronic phase - megaesophagus | No |
| 6 | 72 | HC-Incor | São Paulo (SP) | João Ramalho (SP) | Stayed in SP | João Ramalho (SP) | No data | Probably vector | Chronic phase - cardiac form | No data |
| 7 | 59 | HC-Incor | São José dos Campos (SP) | São Benedito do Sul (PE) | No data | São Benedito do Sul (PE) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 8 | 75 | HC-SEAP | Araraquara (SP) | Rincão (SP) | Stayed in SP | Rincão (SP) | Rural | Vector | Chronic phase - megacolon, megaesophagus and cardiomyopathy | Yes, benznidazole in 2017, with parasitaemia negativation |
| 9 | 54 | HC-Incor | São Paulo (SP) | Castro Alves (BA) | No data | Castro Alves (BA) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 10 | 61 | HC-Incor | Salvador (BA) | Cruz das Almas (BA) | Non-resident | Cruz das Almas (BA) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 11 | 48 | HC-Reumatologia | São Paulo (SP) | Potosi (Bolivia) | First stay for 12 years, arrived in 1988; second stay since 2017 | Potosi (Bolivia) | No details just a mud house with triatomines on site | Vector | Chronic phase - cardiac form; comorbidity - rheumatoid arthritis in immunosuppression, with intermittent parasitaemia | Yes, benznidazole for 57 days in 2016 |
| 12 | 63 | HC-SEAP | São Paulo (SP) | Riacho de Santana (BA) | 47 years, arrived in 1975 | Riacho de Santana (BA) | Rural | Vector | Chronic phase - indeterminate form | No |
| 13 | 69 | HC-Incor | São Paulo (SP) | Ubaí (MG) | 33 years, arrived in 1989 | Ubaí (MG) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 14 | 44 | HC-Incor | São Paulo (SP) | Monte Azul (MG) | No data | Monte Azul (MG) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 15 | 51 | HC-SEAP | São Paulo (SP) | Sebastião Laranjeiras (BA) | 32 years, arrived in 1990 | Sebastião Laranjeiras (BA) | Rural | Vector | Chronic phase - indeterminate form | No |
| 16 | 68 | HC-SEAP | São Paulo (SP) | Conceição do Canindé (PI) | 40 years, arrived in 1982 | Conceição do Canindé (PI) | Rural | Vector | Chronic phase - megaesophagus and cardiomyopathy | No |
| 17 | 59 | HC-SEAP | São Paulo (SP) | Orobó (PE) | 45 years, arrived in 1977 | Orobó (PE) | Rural | Vector | Chronic phase - indeterminate form; reactivation with Chagas myelitis in 2008 | Yes, benznidazole in 2008 |
| 18 | 70 | HC-Incor | Guarulhos (SP) | Montes Claros (MG) | No data | Montes Claros (MG) | Rural | Probably vector | Chronic phase - cardiac form | No data |
| 19 | 57, when died in 2021 | HC-Reumatologia | São Paulo (SP) | Mundo Novo (BA) | 33 years until his death in 2021, arrived in 1988 | Mundo Novo (BA) | Rural | Vector | Chronic phase - cardiac form; comorbidity - polymyositis in immunosuppression, with intermittent parasitaemia; died in 2021 | Incomplete - benznidazole for 12 days, in 2016, and discontinued due to adverse effect |
| 20 | 62, when died in 2020 | HC-Incor | São Paulo (SP) | Mundo Novo (BA) | No data | Mundo Novo (BA) | Rural | Probably vector | Chronic phase - cardiac form; died in 2020 | No data |
| 21 | 66 | HC-SEAP | São Paulo (SP) | São Paulo (SP) | Stayed in SP | São Paulo (SP) | Urban | Transfusional | Haemophilic patient who received multiple transfusions over time; chronic phase - cardiac form | No |
CPM-IAL: Centro de Parasitologia e Micologia-Instituto Adolfo Lutz. States: BA: Bahia; CE: Ceará; GO: Goiás; MG: Minas Gerais; PB: Paraíba; PE: Pernambuco; PI: Piauí; SP: São Paulo. * Serviço de Extensão ao Atendimento de Pacientes HIV/Aids (SEAP), Instituto do Coração (Incor) and Serviço de Reumatologia, Hospital das Clínicas, Faculdade de Medicina, Universidade de São Paulo (HCFMUSP).
Strain isolation - T. cruzi strains were isolated by blood cultures, xenocultures and cultures of digestive tracts of triatomines. Blood samples from patients were submitted to blood cultures; xenocultures were processed from triatomines of the species Rhodnius neglectus, bred in the CPM-IAL and used in field works involving xenodiagnosis of the marsupials, and the digestive tracts of P. megistus specimens were grown in axenic cultures.
Blood culture - The blood culture technique was used 29 with modifications. Amounts of 10 to 30 mL of venous blood were collected from each patient in heparin tubes. The blood samples were transferred to 50 mL tubes and centrifuged at 2,410 x g at 4ºC for 10 minutes. The sediment of red blood cells, containing the buffy coat, was washed once with 10 mL liver infusion tryptose (LIT) medium, centrifuged at 2,410 x g at 4ºC for 20 minutes and resuspended in 10 mL of fresh medium. Then, this suspension was distributed to six 15 mL tubes containing 4 mL LIT each - about 2 mL suspension/tube - and incubated in a biochemical oxygen demand (BOD) system at 25ºC. Amounts of 10 µL suspension from each tube were examined monthly for 120 days in a common optical microscope at 400X magnification.
Xenoculture/culture of the digestive tract of triatomines - The following technique was used 30 with modifications. The digestive tracts of triatomines, positive for T. cruzi, were cultured in tubes containing a biphasic medium composed of a slanted solid phase of 6 mL blood agar base (BAB) or Ducrey agar from rabbit blood and a liquid phase of 2 mL brain heart infusion (BHI) broth treated with 200 µg/mL gentamicin. The tubes were incubated in a BOD system at 25°C, with the first assessment of the material performed at 4th day of culture, in a common optical microscope at 400X magnification. Subsequent assessments were performed once a week, for 30 days.
Maintenance and multiplication of the isolated strains - Once positivity for T. cruzi was confirmed, the cultures remained in a BOD system at 25°C and the parasites were subjected to periodic repetitions in LIT medium for maintenance and multiplication. Additionally, aliquots of 1 mL of each culture were cryopreserved at -196°C. The T. cruzi strains were kept in these systems until the moment of use in the methodologies for molecular characterisation performed in this work.
DNA extraction from T. cruzi-positive cultures - Procedure performed with the QIAamp® DNA Mini Kit (Qiagen, Valencia, USA) commercial kit. Aliquots of 1 mL, obtained from each T. cruzi-positive culture, were transferred to 1.5 mL tubes and centrifuged at 6,000 x g for 1 minute. The supernatants were removed and the pellets were resuspended in 1 mL phosphate buffered saline (PBS) and centrifuged at 6,000 x g for 1 minute. After this step, 200 µL AL buffer and 20 µL proteinase K were added to each tube, followed by vortexing and incubation in a water bath at 56°C for 30 minutes. Subsequently, DNA purification was performed according to manufacturer’s recommendations.
Quantification and storage of extracted DNAs - The concentrations and quality of the extracted DNAs were evaluated by spectrophotometry in Nanodrop™ ND-100 (Thermo Scientific, Waltham, USA) at 260 and 280 nm. The DNAs were stored at -20ºC until use.
Molecular characterisation by FFLB - The FFLB methodology was used 2 with modifications. PCR assays were performed separately for each of the four primer pairs, with one fluorolabelled primer for each region to be amplified, 2 to a final volume of 15 µL containing 0.5 µL (100 pmoles/µL) forward and reverse primers, 13 µL Invitrogen™ Platinum™ PCR SuperMix (Life Technologies, Carlsbad, USA) and 1 µL DNA template, extracted from each T. cruzi-positive culture. Each PCR assay ran with one positive control for trypanosomatids - Tcon025E - and one negative, without DNA, to ensure the reliability of the results. The reactions were carried out in a Mastercycler™ Nexus Gradient (Eppendorf, Hamburg, Germany) thermocycler, according to the following conditions: 35 cycles of 45 seconds at 95°C, 30 seconds at 62°C and 60 seconds at 72°C, with initial denaturation and final extension of 95°C for 3 minutes and 72°C for 10 minutes, respectively. PCR products, initially, were submitted to electrophoresis in 2% (w/v) agarose gel in Tris-acetate-ethylenediamine tetraacetic acid (TAE) buffer, stained with GelRed® (Biotium, Fremont, USA) and visualised under ultraviolet (UV) transillumination. After having confirmed the presence of bands indicating positivity for trypanosomatids, PCR products were prepared in 96-well plates by adding 9 µL Applied Biosystems™ GeneScan™ 500 ROX™ Size Standard (Life Technologies, Warrington, UK) molecular weight marker with formamide, 1: 30 dilution, and 1.5 µL of amplified product/well followed by vortexing. The plate analysis was processed in an Applied Biosystems™ 3500 Genetic Analyzer (Life Technologies, São Paulo, Brazil) automatic sequencer and the peaks produced, referring to the amplified fragments, were analysed by the Applied Biosystems™ GeneMapper™ (Life Technologies, Foster City, USA) software. Subsequently, the barcodes obtained were compared to those from the original study 3 and from later research, carried out at the Laboratório de Filogenia, Taxonomia e Diagnóstico de Tripanossomatídeos of the Instituto de Ciências Biomédicas da Universidade de São Paulo (ICB-USP), also responsible for the better standardisation of the FFLB, 31 , 32 to identify the DTUs of the T. cruzi strains studied.
Correlation of results with complementary information - The results of molecular characterisation were complemented with information from patients’ charts, presented in Table I, from review works that include distribution maps and graphs of T. cruzi DTUs, 4 - 7 , 9 , 33 and a previous research study 32 to enable better discrimination between the identified genotypes and provide greater robustness to the study’s findings.
Plotting the results in a distribution map - The identified DTUs were plotted on a distribution map by the My Maps (Google LLC) app, according to information from each host.
Ethics - This study was developed under a research project involving human beings, approved by the Ethics Commission for Analysis of Research Projects (CAPPesq) of HCFMUSP on April 20, 2016 (protocol nº 1043/07), which is in agreement with the Helsinki Declaration of 1975, as revised in 1983.
RESULTS
Molecular characterisation by FFLB - The base pairs (bp) size of the amplified fragments and the per-host T. cruzi genotypes identified are shown in Table II. It was possible to identify TcI in four hosts (two humans and two triatomines), TcII/VI in 22 hosts (19 humans, two marsupials and one triatomine) and TcIII in one human host. In the latter case, the individual also presented a result for TcI, which indicated the possibility of a mixed infection. Figure shows the distribution map of T. cruzi DTUs identified in this study, according to the probable infection sites for human hosts and the provenance of the infected hosts belonging to sylvatic fauna. It is important to note here that the DTU identified in patient 21, TcII/VI, may have originated in another municipality or, more broadly, in another state, given that this individual is haemophilic and was infected via blood transfusion. Moreover, the impossibility of determining the origin of the infected blood donor did not allow the definition of this specific case; hence, the identified DTU was plotted only to indicate the probable infection site of the mentioned patient.
TABLE II. Fragments amplified for 18S1, 18S3, 28S1 and 28S2 regions and the identified DTUs per host.
| Host | Fragment length (bp) | Results | |||
| 18S1 | 18S3 | 28S1 | 28S2 | ||
| Patient 1 | 299, 300, 301 | 241, 242 | 349, 350 | 212, 213 | TcII/VI |
| Patient 2 | 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 3 | 300 | 241, 242 | 348, 349, 350, 351 | 212, 213 | TcII/VI |
| Patient 4 | 299, 300 | 241, 242 | 349, 350 | 212, 213 | TcII/VI |
| Patient 5 | 300 | 241, 242 | 346, 347 | 212, 213 | TcII/VI |
| Patient 6 | 299, 300 | 241, 242 | 346, 347, 349, 350, 351 | 212, 213 | TcII/VI |
| Patient 7 | 300 | 241, 242 | 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 8 | 299, 300 | 241, 242 | ●●● | 212, 213 | TcII/VI |
| Patient 9 | 299, 300 | 241, 242 | 346, 347, 348, 350 | 212, 213 | TcII/VI |
| Patient 10 | 299, 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 11 | 304, 305 | 245, 246 | 338, 339, 340, 341 | 196, 197 | TcI |
| Patient 12 | 299, 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 13 | 300 | 241, 242 | 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 14 | 299, 300 | 241, 242 | 349, 350 | 212, 213 | TcII/VI |
| Patient 15 | 300 | 241, 242 | 347, 348, 349, 350 | 211, 213 | TcII/VI |
| Patient 16 | 300 | 241, 242 | 347, 348, 351, 352 | 212, 213 | TcII/VI |
| Patient 17 | 299, 305 | 236, 244, 245 | 333, 334, 336, 338, 339 | 189, 196, 197 | TcI and TcIII |
| Patient 18 | 299, 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 19 | 300 | 241, 242 | 346, 348, 349, 350 | 212, 213 | TcII/VI |
| Patient 20 | 300 | 241, 242 | 346, 347, 349, 350 | 212, 213 | TcII/VI |
| Patient 21 | 299, 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Philander opossum - Ilhabela | 300 | 241, 242 | 348, 349 | 212, 213 | TcII/VI |
| Didelphis albiventris - Santa Fé do Sul | 299, 300 | 241, 242 | 347, 348, 349, 350 | 212, 213 | TcII/VI |
| Panstrongylus megistus - Taboão da Serra | 299, 304, 305 | 244, 245 | 333, 335, 336, 338, 339, 340 | 196 | TcI |
| Panstrongylus megistus - Ilhabela | 299, 300 | 241, 242 | 348, 349, 350, 351 | 212, 213 | TcII/VI |
| Panstrongylus megistus - Itapecerica da Serra | 299, 305 | 244, 245 | 332, 334, 335, 338, 339, 340 | 196, 197 | TcI |
bp: base pairs; ■: fragments corresponding to TcI; ■: fragments corresponding to TcII/VI; ■: fragments corresponding to TcIII; ■: fragments that are common to different discrete typing units (DTUs); ●●●: no fragments were detected.
The distribution map of Trypanosoma cruzi discrete typing units (DTUs) identified in this study according to the probable infection sites for human hosts (patients) and the provenance of the infected sylvatic hosts (marsupials and triatomines). Bar = 100 km.
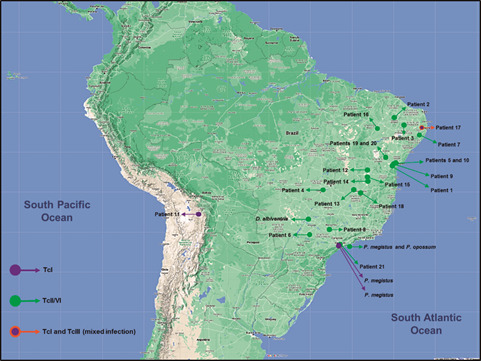
Correlation of results with complementary information - The correlation of molecular characterisation results with information from patients’ charts, reviews containing distribution maps and graphs of T. cruzi DTUs 4 - 7 , 9 , 33 and a previous research study 32 ) made it possible to better discriminate TcII from TcVI and allowed to suggest that, predominantly, the strains identified by the TcII/VI profile indeed correspond to TcII.
DISCUSSION
T. cruzi contemplates an exuberant genetic diversity 4 - 9 and a complex life cycle, with the latter involving extracellular proliferation and differentiation in hematophagous insect - triatomine vectors - and intracellular proliferation and differentiation in a range of mammalian species. The constant transition from an invertebrate host to a vertebrate one sets different pressures, either by the immune response of these hosts or by the new environment of parasite development. 34 , 35 Despite this factor being considered in the attempt to understand the biological characteristics intrinsic to the parasite, there is no consensus, so far, that explains the heterogeneity observed in its populations. 6 However, the emergence of population variants can be derived by the occurrence of genetic recombination events between different T. cruzi lineages throughout the evolutionary process. 5 , 7 , 19 - 22 In this context, there are two major theories, recognised by the scientific community, that propose that the TcV and TcVI genotypes originate from recombinations between the TcII and TcIII genotypes. 23 , 24 ) It is also accepted that TcI and TcII are pure lineages that evolved separately from a common ancestor millions of years ago. 4 , 9 , 24 , 36
The theories reported above can largely explain the difficulty in differentiating parental lines from hybrid lines when using molecular techniques to identify DTUs of T. cruzi strains. This difficulty was corroborated in the results of this work, in which TcII and TcVI could not be discriminated by FFLB, despite the use of highly sensitive primers, as they share DNA fragments of the same size. This limitation and others, exposed by the characterisation of T. cruzi strains by FFLB, were previously addressed by different works. 3 , 31 , 32 However, this molecular technique has advantages compared to other PCR-based trypanosomatids identification methods. Among them are the speed and sensitivity of the method, as it can amplify relatively small regions of DNA, and the capabilities to detect fluorescence and differentiate mixed infections. Furthermore, this method can discriminate species and a range of lineages from the same set of primers and facilitate epidemiological studies and large-scale investigations. 2 , 3 , 32
In the group of strains studied, this work verified the predominance of the TcII/VI profile, represented by 22 hosts, mostly humans. Among the 19 patients within this profile, two were affected by the indeterminate form, 13 by the cardiac form, one by the digestive form - megaesophagus - and three by the mixed form, the latter concomitantly involving cardiomyopathy and mega syndrome. These data are in accordance with the information described in the literature, which relate the transmission pattern and characteristic host profile for these DTUs, to domestic transmission cycles and patients with chronic Chagas disease, respectively, in addition to the variety of clinical pictures promoted, encompassing the cardiac, digestive and mixed forms. 5 , 7 , 9 ) Complementing the results of molecular identification of T. cruzi strains isolated from humans, patients 11 and 17, probably infected in Bolivia and the Brazilian Northeast Region, respectively, harboured TcI, the first being affected by the cardiac form and the second by the indeterminate form. In patient 17, TcIII was also detected, suggesting a mixed infection.
As for the strains isolated from sylvatic fauna, here comprised between marsupials and triatomines, the results corroborate the classical epidemiology of Chagas disease. Specifically, the sylvatic transmission cycles in an environment of balance between vectors and hosts, followed by disturbances and alterations of these cycles by the introduction of buildings and homes in forested areas, and the consequent modification of the transmission dynamics to a domestic or peridomestic pattern. 37 , 38 In this scenario and concerning the TcI genotype, the two specimens of P. megistus from the municipalities of Taboão da Serra and Itapecerica da Serra, which harboured parasites characterised by this DTU, were found and collected inside households located in areas of environmental preservation. A prominent feature of the condominiums that comprise these households is the spatialisation of the residences, which are more distant from each other when compared to urban areas. This environmental transformation can favour the manifestation of synanthropic behaviours by sylvatic reservoirs and the encounter of triatomines inside and around the dwellings. 27
The TcII/VI profile, identified in the strains isolated from marsupials D. albiventris and P. opossum from the municipalities of Santa Fé do Sul and Ilhabela, respectively, and from one of the P. megistus specimens, also from Ilhabela, can be understood by two factors: a) the environment modification, mentioned above, changed the dynamics of transmission cycles and promoted the inclusion of human hosts in this process; and b) synanthropy, characteristic of these sylvatic animals, favoured the displacement of the vector insect to the new location in which these mammals settled, for being their food source. Thus, both animals and triatomine vectors were able to access the urban environment, modifying the cycle and favouring a new transmission pattern. 37 , 38
Such epidemiological aspects raise concerns since a colonisation of triatomines, especially the P. megistus species, has been observed in SP, placing the population at risk of Chagas disease by natural transmission, 27 a phenomenon last reported more than 50 years ago in the region. 28 This scenario is mainly verified in households and peridomiciles in municipalities following the Trecho Oeste and Trecho Sul of the Rodoanel Metropolitano Mário Covas, in locations with natural forest reserves. The municipalities comprised are: Carapicuíba, Cotia, Embu das Artes, Itapecerica da Serra, Osasco, Ribeirão Pires, Santana do Parnaíba, Santo André, São Bernardo do Campo, São Paulo e Taboão da Serra. 39 ) Furthermore, it is important to highlight that the presence of didelphid marsupials in areas of the metropolitan region, close to dwellings, is high, given their ability to adapt to the urban environment. These sylvatic animals have high rates of natural infection by T. cruzi, and probably contribute to maintaining the circulation of the parasite in the region. 27
The complementation of the molecular results with information obtained from patients’ charts, reviews that include distribution maps and graphs of T. cruzi DTUs 4 - 7 , 9 , 33 and a previous research study, 32 made it possible to establish certain discrimination between the TcII and TcVI genotypes, allowing to suggest that, in general, the strains characterised by the TcII/VI profile indeed correspond to TcII. This statement can be further justified if we consider that most of the hosts in this study are humans who, although reside in SP - except for patient 10 - are predominantly from cities in the Brazilian Northeast Region, where there are no reports so far of the TcVI occurrence, 32 and that such individuals were probably infected there. In addition, according to elements provided by the reviews consulted, 4 - 7 , 9 , 33 it is rare or unlikely to find TcVI within the regions that comprise the municipalities of the other hosts approached here, in contrast to the high frequency of TcII. However, future works should promote the differentiation of hybrid DTUs from their evolutionary predecessors to obtain more precise and definitive results through other molecular techniques.
It is important to point out that, except for the technical limitations for discriminating between TcII and TcVI, the FFLB identified the genotypes of all strains isolated in culture media. However, artificial conditions in culture media or even in vivo experimental models represent potential selective pressures for the predominance or elimination of a given parasite population. 6 , 7 This feature had already been observed from the analysis of electrophoretic profiles of DNA from different T. cruzi strains. In one of these experiments, T. cruzi strains were isolated from human hosts by blood culture and inoculated into murine models. After inoculation, these animals were followed for a period of 2 years by schizodeme analysis and the results showed that there were cases in which the initially observed electrophoretic profiles were replaced by others over time, indicating selectivity by the animals’ organism. 40
In other words, it cannot be said that the samples used here and submitted to culture originally harboured a single T. cruzi population. It would be necessary to extract the DNA from the original biological material - blood from vertebrate hosts and triatomine faeces - and proceed with molecular identification to verify the coexistence between different DTUs.
Given the results, we could conclude that: the molecular characterisation of T. cruzi strains identified the TcI, TcII/VI and TcIII genotypes; the TcII/VI profile, associated with domestic cycles and patients with chronic Chagas’ disease, was the most prevalent among the identified genotypes and whose derivation was mostly from human hosts; the complementation of molecular results with additional information allowed us to suggest that TcII is the predominant lineage of this research; two human hosts harboured TcI (patients 11 and 17), where there was a suggestive result for mixed infection in one of them, involving TcI and TcIII (patient 17); and of the strains isolated from sylvatic fauna, two were characterised as TcI (P. megistus specimens from Taboão da Serra and Itapecerica da Serra) and three as TcII/VI (P. megistus and P. opossum from Ilhabela and D. albiventris from Santa Fé do Sul) associated with sylvatic and domestic cycles, respectively.
This work having identified the DTUs of T. cruzi strains isolated from different host profiles, contributes to the epidemiology of Chagas disease in SP, reinforces the attention to the possible recurrence of this disease in the region, from natural transmission by triatomine vectors, and provides a basis for studies on the genetic diversity of this parasite.
ACKNOWLEDGEMENTS
The authors would like to thank Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) for the financial support, the Programa de Pós-Graduação da Coordenadoria de Controle de Doenças da Secretaria de Estado da Saúde de São Paulo (PPG-CCD-SES-SP) for the other subsidies during the period in which this work was developed and the information technology professional at IAL, Mr. Marcio Keiti Guedes Inumaru, for his contribution to the development of the distribution map of T. cruzi DTUs presented in this study.
Footnotes
Financial support: CAPES.
REFERENCES
- 1.Zingales B. Trypanosoma cruzi um parasita, dois parasitas ou vários parasitas da doença de Chagas? Rev Biol. 2011;6b:44–48. [Google Scholar]
- 2.Hamilton PB, Adams ER, Malele II, Gibson WC. A novel, high-throughput technique for species identification reveals a new species of tsetse-transmitted trypanosome related to the Trypanosoma brucei subgenus, Trypanozoon. Infect Genet Evol. 2008;8(1):26–33. doi: 10.1016/j.meegid.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Hamilton PB, Lewis MD, Cruickshank C, Gaunt MW, Yeo M, Llewellyn MS. Identification and lineage genotyping of South American trypanosomes using fluorescent fragment length barcoding. Infect Genet Evol. 2011;11(1):44–51. doi: 10.1016/j.meegid.2010.10.012. [DOI] [PubMed] [Google Scholar]
- 4.Brenière SF, Waleckx E, Barnabé C. Over six thousand Trypanosoma cruzi strains classified into discrete typing units (DTUs) attempt at an Inventory. PLoS Negl Trop Dis. 2016;10(8):1–19. doi: 10.1371/journal.pntd.0004792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zingales B. Trypanosoma cruzi genetic diversity something new for something known about Chagas disease manifestations, serodiagnosis and drug sensitivity. Acta Trop. 2018;184:38–52. doi: 10.1016/j.actatropica.2017.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Jansen AM, Xavier SCDC, Roque ALR. Landmarks of the knowledge and Trypanosoma cruzi biology in the wild environment. Front Cell Infect Microbiol. 2020;10:10–10. doi: 10.3389/fcimb.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zingales B, Bartholomeu DC. Trypanosoma cruzi genetic diversity impact on transmission cycles and Chagas disease. Mem Inst Oswaldo Cruz. 2021;116:e210193. doi: 10.1590/0074-02760210193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brenière SF, Aliaga C, Waleckx E, Buitrago R, Salas R, Barnabé C. Genetic characterization of Trypanosoma cruzi DTUs in wild Triatoma infestans from Bolivia predominance of TcI. PLoS Negl Trop Dis. 2012;6(5):e1650. doi: 10.1371/journal.pntd.0001650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zingales B, Miles MA, Campbell DA, Tibayrenc M, Macedo AM, Teixeira MM. The revised Trypanosoma cruzi subspecific nomenclature rationale, epidemiological relevance and research applications. Infect Genet Evol. 2012;12(2):240–253. doi: 10.1016/j.meegid.2011.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Llewellyn MS, Miles MA, Carrasco HJ, Lewis MD, Yeo M, Vargas J. Genome-scale multilocus microsatellite typing of Trypanosoma cruzi discrete typing unit I reveals phylogeographic structure and specific genotypes linked to human infection. Plos Pathog. 2009;5:e1000410. doi: 10.1371/journal.ppat.1000410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Herrera C, Guhl F, Falla A, Fajardo A, Montilla M, Adolfo Vallejo G. Genetic variability and phylogenetic relationships within Trypanosoma cruzi I Isolated in colombia based on miniexon gene sequences. J Parasitol Res. 2009;2009:897364–897364. doi: 10.1155/2009/897364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cura CI, Mejía-Jaramillo AM, Duffy T, Burgos JM, Rodriguero M, Cardinal MV. Trypanosoma cruzi I genotypes in different geographical regions and transmission cycles based on a microsatellite motif of the intergenic spacer of spliced-leader genes. Int J Parasitol. 2010;40(14):1599–1607. doi: 10.1016/j.ijpara.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcili A, Lima L, Cavazzana M, Junqueira AC, Veludo HH. Maia Da Silva F A new genotype of Trypanosoma cruzi associated with bats evidenced by phylogenetic analyses using SSU rDNA, cytochrome b and histone H2B genes and genotyping based on ITS1 rDNA. Parasitology. 2009;136(6):641–655. doi: 10.1017/S0031182009005861. [DOI] [PubMed] [Google Scholar]
- 14.Ramírez JD, Tapia-Calle G, Muñoz-Cruz G, Poveda C, Rendón LM, Hincapié E. Trypanosome species in neo-tropical bats biological, evolutionary and epidemiological implications. Infect Genet Evol. 2014;22:250–256. doi: 10.1016/j.meegid.2013.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ramírez JD, Hernández C, Montilla M, Zambrano P, Flórez AC, Parra E. First report of human Trypanosoma cruzi infection attributed to Tcbat genotype. Zoonoses Public Health. 2014;61(7):477–479. doi: 10.1111/zph.12094. [DOI] [PubMed] [Google Scholar]
- 16.Brenière SF, Bosseno MF, Telleria J, Bastrenta B, Yacsik N, Noireau F. Different behavior of two Trypanosoma cruzi major clones transmission and circulation in young Bolivian patients. Exp Parasitol. 1998;89(3):285–295. doi: 10.1006/expr.1998.4295. [DOI] [PubMed] [Google Scholar]
- 17.Devillers H, Lobry JR, Menu F. An agent-based model for predicting the prevalence of Trypanosoma cruzi I and II in their host and vector populations. J Theor Biol. 2008;255(3):307–315. doi: 10.1016/j.jtbi.2008.08.023. [DOI] [PubMed] [Google Scholar]
- 18.Dario MA, Rodrigues MS, Barros JH, Xavier SC. D'Andrea PS.Roque AL Ecological scenario and Trypanosoma cruzi DTU characterization of a fatal acute Chagas disease case transmitted orally (Espírito Santo state, Brazil) Parasit Vectors. 2016;9(1):477–477. doi: 10.1186/s13071-016-1754-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gaunt MW, Yeo M, Frame IA, Stothard JR, Carrasco HJ, Taylor MC. Mechanism of genetic exchange in American trypanosomes. Nature. 2003;421(6926):936–939. doi: 10.1038/nature01438. [DOI] [PubMed] [Google Scholar]
- 20.Lewis MD, Llewellyn MS, Gaunt MW, Yeo M, Carrasco HJ, Miles MA. Flow cytometric analysis and microsatellite genotyping reveal extensive DNA content variation in Trypanosoma cruzi populations and expose contrasts between natural and experimental hybrids. Int J Parasitol. 2009;39(12):1305–1317. doi: 10.1016/j.ijpara.2009.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Messenger LA, Miles MA. Evidence and importance of genetic exchange among field populations of Trypanosoma cruzi. Acta Trop. 2015;151:150–155. doi: 10.1016/j.actatropica.2015.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matos GM, Lewis MD, Talavera-López C, Yeo M, Grisard EC, Messenger LA. Microevolution of Trypanosoma cruzi reveals hybridization and clonal mechanisms driving rapid genome diversification. Elife. 2022;11:e75237. doi: 10.7554/eLife.75237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Westenberger SJ, Barnabé C, Campbell DA, Sturm NR. Two hybridization events define the population structure of Trypanosoma cruzi. Genetics. 2005;171(2):527–543. doi: 10.1534/genetics.104.038745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Freitas JM, Augusto-Pinto L, Pimenta JR, Bastos-Rodrigues L, Goncalves VF, Teixeira SM. Ancestral genomes, sex, and the population structure of Trypanosoma cruzi. PLoS Pathog. 2006;2(3):e24. doi: 10.1371/journal.ppat.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Silva EOR, Rodrigues VLCC, Silva RAD, Wanderley DMV. Programa de controle da doença de Chagas no etado de São Paulo, Brasil o controle e a vigilância da transmissão vetorial. Rev Soc Bras Med Trop. 2011;44(2):74–84. doi: 10.1590/s0037-86822011000800012. [DOI] [PubMed] [Google Scholar]
- 26.Silva RAD, Estevão VAO, Duarte AN, Maria PC. Colonization by Panstrongylus megistus (Hemiptera, Reduviidae, Triatominae) in an urban park in the city of São Paulo. Rev Soc Bras Med Trop. 2020;54:e03302020. doi: 10.1590/0037-8682-0330-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Silva RAD, Zaicaner R, Rosa MP, Aun GCG, Muniz JC, Magalhães AC. Colonization of Panstrongylus megistus (Hemiptera Reduvidae: Triatominae) in an urban area and its association with Didelphis marsupialis in the metropolitan region of São Paulo. Rev Soc Bras Med Trop. 2021;54:e04712020. doi: 10.1590/0037-8682-0471-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silva EOR, Dias , Jr J Suspensão do rociado no combate ao Triatoma infestans em áreas do estado de São Paulo, Brasil. Rev Saude Publica. 1969;3(2):173–181. [PubMed] [Google Scholar]
- 29.Chiari E, Dias JCP, de Lana M, Chiari CA. Hemocultures for the parasitological diagnosis of human chronic Chagas' disease. Rev Soc Bras Med Trop. 1989;22(1):9–23. doi: 10.1590/s0037-86821989000100004. [DOI] [PubMed] [Google Scholar]
- 30.Bisugo MC, Araújo MFL, Westphalen EVN, Cunha EA, Oliveira OC, Junior, Guilherme CS. Isolamento de Trypanosoma cruzi por xenocultura após aplicação de xenodiagnóstico in vivo e/ou in vitro em pacientes na fase crônica da doença de Chagas e na co-infecção pelo HIV. Rev Inst Adolfo Lutz. 1998;57(2):89–96. [Google Scholar]
- 31.Lima-Oliveira TM, Fontes FVHM, Lilioso M, Pires-Silva D, Teixeira MMG, Meza JGV. Molecular eco-epidemiology on the sympatric Chagas disease vectors Triatoma brasiliensis and Triatoma petrocchiae ecotopes, genetic variation, natural infection prevalence by trypanosomatids and parasite genotyping. Acta Trop. 2020;201:105188–105188. doi: 10.1016/j.actatropica.2019.105188. [DOI] [PubMed] [Google Scholar]
- 32.Valença-Barbosa C, Finamore-Araujo P, Moreira OC, Vergara-Meza JG, Alvarez MVN, Nascimento JR. Genotypic Trypanosoma cruzi distribution and parasite load differ ecotypically and according to parasite genotypes in Triatoma brasiliensis from endemic and outbreak areas in Northeastern Brazil. Acta Trop. 2021;222:106054–106054. doi: 10.1016/j.actatropica.2021.106054. [DOI] [PubMed] [Google Scholar]
- 33.Jansen AM, Lisboa CV, Dario MA, Xavier SCC. Distribuição das DTUs de Trypanosoma cruzi na natureza. http://chagas.fiocruz.br/biogeografia/
- 34.de Souza W. Basic cell biology of Trypanosoma cruzi. Curr Pharm Des. 2002;8(4):269–285. doi: 10.2174/1381612023396276. [DOI] [PubMed] [Google Scholar]
- 35.Balouz V, Agüero F, Buscaglia CA. Chagas disease diagnostic applications present knowledge and future steps. Adv Parasitol. 2017;97:1–45. doi: 10.1016/bs.apar.2016.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturm NR, Vargas NS, Westenberger SJ, Zingales B, Campbell DA. Evidence for multiple hybrid groups in Trypanosoma cruzi. Int J Parasitol. 2003;33(3):269–279. doi: 10.1016/s0020-7519(02)00264-3. [DOI] [PubMed] [Google Scholar]
- 37.Barretto MP. Trypanosoma cruzi e doença de Chagas. Rio de Janeiro: Guanabara Koogan; 1979. Epidemiologia; pp. 89–151. [Google Scholar]
- 38.Coura JR. Chagas disease: what is known and what is needed - A background article. Mem Inst Oswaldo Cruz. 2007 doi: 10.1590/s0074-02762007000900018. [DOI] [PubMed] [Google Scholar]
- 39.da Silva RA, Estevão VAO, Duarte AN. Triatomíneos na Região Metropolitana de São Paulo vigilância entomológica. BEPA. 2019;16(190):13–18. [Google Scholar]
- 40.Morel CM, Deane MP, Gonçalves AM. The complexity of Trypanosoma cruzi populations revealed by schizodeme analysis. Parasitol Today. 1986;2(4):97–101. doi: 10.1016/0169-4758(86)90038-4. [DOI] [PubMed] [Google Scholar]


