Abstract
Acne vulgaris is a common dermatosis frequently encountered in general dermatology and presents significant health-related quality of life and psychological challenges. Clinical studies on acne vulgaris in skin of color are limited; thus, it is likely that treatment recommendations to patients with darker skin types are drawn from trial data based on Caucasian skin. The aim of this study was to systematically review the effectiveness and tolerability of treatments used to treat acne vulgaris in patients with skin of color. A literature search was performed in the PubMed, Embase, and Scopus bibliographic databases, with a total of 1,477 retrieved articles, of which 1,316 were excluded after initial screening. Of the 93 studies assessed, 55 studies met our inclusion criteria (28 randomized controlled trials, 4 cohort studies, 6 post-hoc analyses, and 12 other interventional trials). The studies reported a total of 21,202 patients. Most studies explored topical therapies (23 studies) and photodynamic therapy (13 studies). Other treatments included laser/light therapy, systemic therapy, chemical peels, and radiofrequency and microneedling. In general, the different treatment modalities offered an improvement in lesion count and were well tolerated, with no report of major adverse events. However, due to limited evidence, we were unable to draw firm conclusions from the results of this review to guide decisions in practice, particularly with respect to long-term outcomes, in patients with skin of color and acne vulgaris.
Keywords: Acne, medical dermatology, ethnicity
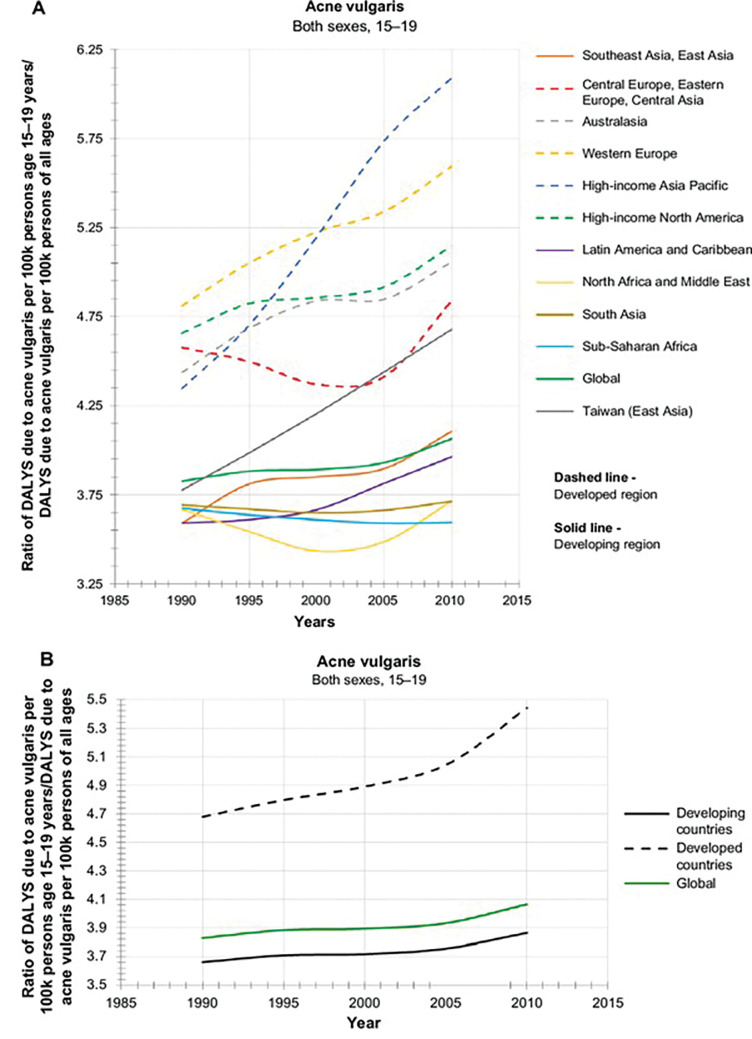
Ethnic dermatology represents a new area of study focusing on common dermatological presentations that can manifest differently in patients with skin of color compared to those of lighter skin types. Ethnic skin, or skin of color, refers to people categorized as having Fitzpatrick Skin Types (FPS) III to VI, typically with African, Native American, Asian, Middle Eastern, or Hispanic backgrounds.1 Acne vulgaris (AV) typically affects the face and torso and is characteristically centered on the pilosebaceous unit.2 AV is among the most common dermatological conditions for which patients with skin of color present for medical attention in primary care.3 In a landmark prevalence study (including female patients only), Perkins et al4 concluded that AV was most prevalent in female patients of African-American descent with FPS V to VI (37%) or Hispanic descent with FPS III to IV (32%), followed by patients of Asian (30%), Caucasian (24%), or Continental Indian (23%) descent. Prevalence of acne subtype was comparable between ethnic groups, except in Asian skin, which had a higher number of inflammatory lesions (IL) compared to comedonal lesions (CL) (20% vs. 10%), and Caucasian skin, in which CL was more prevalent than IL (14% vs. 10%).4 Patients from African American or Hispanic descent showed higher prevalence of hyperpigmentation (65% and 48%, respectively) compared to patients of Caucasian, Asian, or Continental Indian, descent (25%, 18%, and 10%, respectively).4 According to the Global Burden Disease Study, AV is the tenth leading cause of disability-adjusted life years (DALYs) in the 15 to 19-year-old subgroup across developed countries, of which there is a global incline, suggesting an unmet dermatology need globally for AV (Figure 1).5
FIGURE 1.
Global burden of acne vulgaris in adolescents 15 to19-year-olds stratified to developing and developed countries (adapted from Lynn et al5)
The pathophysiology of AV in skin of color is similar to that observed in Caucasian skin, comprising the well-known quartet of excessive sebum production, abnormal follicular keratinisation and plugging, proliferation of Propionibacterium acnes (P. acnes), and exaggerated inflammatory response.3 Nodulocystic acne is more common in Caucasian and Hispanic subjects than in African-American subjects.6 Post-inflammatory hyperpigmentation (PIH), defined as an acquired hypermelanotic process following inflammation or trauma,7 is more pronounced in patients with skin of color, particularly among those with FPS IV to VI; however, PIH frequently goes unrecognized by clinicians, which is likely related to the lack of representation of skin of color in dermatology photography resources and other educational materials.8 The hyperpigmentary changes associated with acne can last substantially longer than the acne lesions themselves, and epidermal lesions can take up to 6 to 12 months to heal, while lesions deeper in the dermis can last for years.9,10 In a study of 30 female African-American patients, Falder et al11 reported that inflammation was histologically evident in all types of acne lesions, of which even simple comedones displayed a mild degree of inflammation. Inflammation was also evident some distance away from index lesions, a condition termed satellite inflammation. Additionally, pomade acne is more common in patients with skin of color,12 due to the frequent use of hair products containing potent acnegens.13 Interestingly, He et al9 provided primary data on a possible association of the CYP17-34T/C polymorphism and the development of severe acne in Chinese subjects, and, in a later study, described two new susceptibility loci—1q24.2 and 11p11.2—that were associated with more severe acne.10 The aim of the current systematic review was to summarize the efficacy and safety of available AV treatments in patients with skin of color. Our review was designed and performed in accordance with the recommendations of the Cochrane Handbook for Systematic Reviews of Interventions,14 as well as the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.15
METHODS
Data sources, search strategy, and design overview. Relevant studies were identified in the PubMed, Embase, and Scopus bibliographic databases, using the search strategy outlined in Appendix 1. Studies were eligible for inclusion if they were published on or after January 1, 2001, were written in English, and involved human participants only. Finally, a manual search was performed by reviewing the references of the included studies.
Selection criteria. Study inclusion criteria were as follows:
Participants diagnosed with AV of the face, head, and/or neck.
Participants with skin of color included in study (using predefined eligibility criteria outlined in Appendix 1).
All grades of acne severity included in study
Treatment comprised topical, mechanical, or systemic agent
Outcome measures assessing efficacy and safety were utilized.
The complete inclusion and exclusion criteria are outlined in Appendix 2.
This systematic review included all FPS, rather than restricting our search to FPS IV to VI. Our search criteria (seen in Appendix 1) included any article that referenced skin of color or any associated terms. FPS was not specified in all retrieved articles; however, this did not lead to exclusion if an article included certain search terms (e.g., non-Caucasian, Japanese, among others). The authors made a conscious decision not to exclude articles that failed to specify FPS as we believe this may have led to the omission of significant findings. Studies in which FPS was specified are presented in Table 1.
TABLE 1.
Effectiveness and tolerability outcomes (all outcomes reported pertain to those at final follow-up visit unless otherwise stated)
| AUTHOR, YEAR | PATIENTS (N) | TREATMENT(S) | EFFECTIVENESS | SAFETY | STUDY LIMITATIONS |
|---|---|---|---|---|---|
| TOPICAL RETINOIDS | |||||
| Cook-Bolden et al,57 2019 | 766 | Tretinoin 0.05% lotion vs. vehicle lotion |
Lesion count
|
Tolerability
|
Need to expand on blinding procedure in original trials; no clear exclusion criteria provided; post-hoc analysis; short duration of follow-up |
| Lain et al 2019,58 |
1,640 | Tretinoin 0.05% lotion vs. vehicle lotion |
Lesion count
|
Not all data reported separately for ethnic vs Caucasian patients Tolerability
|
Short duration of follow-up; ITT analysis; no clear exclusion criteria; post-hoc analysis; safety outcomes poorly reported according to ethnicity; inconsistent reporting of P values especially when statistical significance not achieved; very large numbers of Caucasian patients |
| Kubota et al,25 2012 | 66 | 4/52 of 1% clindamycin phosphate gel 2x/day and 0.1% adapalene gel 1x/day, then 4/52 0.1% adapalene 1x/day vs. 4/52 of 0.1% adapalene for 2/52 |
Lesion count
|
Local AEs
|
Small study size; short duration of follow-up |
| TOPICAL ANTIBIOTICS | |||||
| Kawashima et al,38 2017 |
607 | BPO 2.5% vs. BPO 5% vs. placebo |
Lesion count
|
Percent of patients who experienced AEs
|
No patient satisfaction measures; short duration of follow-up; different denominator for efficacy (n=607) and AEs (n=609) |
| Kawashima et al,20 2017 |
458 | BPO 2.5% vs. BPO |
Lesion count
|
Treatment-emergent AEs
|
Post-hoc analysis; unclear whether ITT used for all outcomes; no analysis of White vs. non-White patients |
| Alexis et al,59 2017 |
136 | Clindamycin phosphate 1.2%/BPO 3.75% vs. vehicle |
Lesion count
|
Percent of patients who experienced AE:
|
Post-hoc analysis; unclear whether ITT used for all outcomes; no analysis of White vs. non-White patients |
| Xu et al,26 2016 |
1,016 | Clindamycin phosphate 1%/BPO 5% vs. clindamycin only |
Lesion count
|
Percent of patients who experienced AE
|
Single blinding; short duration of follow-up; no placebo arm; sex numbers do not match to reported participants |
| Amar et al,63 2015 |
20 | Clindamycin phosphate 1.2%/BPO 2.5% gel |
Lesion count
|
Number of patients who experienced AE
|
Nonblinded study; no control arm; small population sample; short duration of follow-up; no definition of AEs provided |
| Kawashima et al,27 2015 | 800 | Clindamycin phosphate 1.2% / BPO 3.0% OD vs BD vs clindamycin BD |
Lesion count
|
Treatment-emergent AEs
|
Short duration of follow-up; multiple analyses at various timepoints and between subgroups; single-blinding |
| Kawashima et al,39 2014 | 360 | BPO 3% vs. vehicle |
Lesion count
|
Percent of patients who experienced AE
|
Short duration of follow-up; no active comparator |
| Cook-Bolden et al,60 2012 | 458 | Clindamycin phosphate 1.2%/BPO 2.5% vs. clindamycin only vs. BPO only vs vehicle |
Lesion count
|
Local AEs
|
Post-hoc analysis; short duration of follow-up; FPS not reported (Hispanic may include White patients) |
| Callender VD,61 2012 | 797 | Clindamycin phosphate 1.2%/BPO 2.5% vs. vehicle |
Lesion count
|
Local AEs
|
Post-hoc analysis; Comparisons made between FPS subgroups, with little mention of results from vehicle arm; Inclusion of patients with FPS I |
| Jung et al,40 2011 |
34 | 1% nadifloxacin cream vs. vehicle cream |
Lesion count
|
Local AEs
|
Small study size; unclear enrolment process; short duration of follow-up |
| COMBINED TOPICAL RETINOID AND ANTIBIOTIC | |||||
| DuBois et al,64 2019 |
50 | Adapalene 0.3%/BPO 2.5% |
Treatment success
|
Percent of patients who experienced AE
No AEs leading to discontinuation |
Prospective, open-label design; Small number of patients; Single-arm study |
| Hayashi et al,28 2018 | 349 | Clindamycin phosphate 1.2%/BPO 3.0% ON vs. clindamycin 1.2% BD/adapalene 0.1% ON |
Lesion count
|
Percent of patients who experienced AE
|
Single blinding; short duration of follow-up; no placebo arm; multiple subgroup analyses; variable reporting of P values |
| Alexis et al,62 2017 |
286 | Adapalene 0.3%/BPO 2.5% vs. vehicle |
Lesion count
|
Percent of patients who experienced AE
|
Post-hoc analysis; inclusion of FPS I patients and patients w/ darker skin types; multiple subgroup analyses |
| Kim et al,30 2013 | 23 | Adapalene 0.1%/BPO 2.5% vs. Adapalene 0.1% |
Lesion count
|
Local AEs
|
Small study size; short duration of follow-up; single-blinded study; no ITT analysis |
| Takigawa et al,31 2013 | 188 | Adapalene 0.1%/nadifloxacin 1% vs. adapalene 0.1% monotherapy |
Lesion count
|
Local AEs
|
No exploration of limitations within manuscript; per-protocol analysis rather than ITT; short duration of follow-up; no placebo group |
| Callender et al,41 2012 | 33 | Clindamycin phosphate 1.2%/tretinoin 0.025% vs. vehicle |
Lesion count
|
Local AEs
|
Small sample size; short follow-up period; use of cleansing bar and sunscreen as potential confounders; inconsistent reporting of P values |
| Schmidt et al,42 2011 | 2,010 | Clindamycin phosphate 1.2%/tretinoin 0.025% vs. clindamycin only |
Lesion count
|
Local AEs
|
Limitations not explored; unclear where study conducted (multicenter sites not stated); short duration of follow-up; AEs not presented according to FPS |
| TOPICAL DAPSONE | |||||
| Taylor et al,43 2018 |
4,327 | Dapsone 7.5% vs. vehicle |
Lesion count
|
Percent of patients who experienced AE
|
Post-hoc analysis; short duration of follow-up; inconsistent use of P intervals and standard error |
| Draelos et al,44 2017 | 1,850 | Dapsone 7.5% gel OD vs. vehicle |
Lesion count
|
Percent of patients who experienced AE
|
Post-hoc analysis of PIH outcomes; short duration of follow-up; complete inclusion/exclusion criteria from original studies not reported; inconsistent P value reporting |
| Alexis et al,53 2016 |
67 | Dapsone 5% |
Lesion count
|
Percent of patients who experienced AE
|
Different number of patients included for data analysis at different time points; small sample size; short study duration; open-label; no control arm |
| CHEMICAL PEELS | |||||
| How et al,45 2020 | 36 | Jessner's solution peel vs. SA 30% peel |
Lesion count
|
Local AEs
|
Small sample size; short duration of follow-up; ITT and per-protocol analysis performed |
| Sarkar et al,21 2019 |
45 | 35% GA peel vs. 20% SA + 10% mandelic acid peel vs. phytic acid peel |
Lesion count
|
Local AEs
|
Small sample size; short duration of follow-up; evaluator bias due to subjective nature of scoring system; inconsistent reporting of P values; nonspecified population other than "Asian" |
| Kaminaka et al,46 2014 | 25 | 40% GA peel vs. placebo peel |
Lesion count
|
Local AEs
|
Small sample size; short duration of follow-up; raw data unavailable |
| PHOTODYNAMIC THERAPY | |||||
| Choi et al,22 2018 |
21 | ICG-PDT w/ either LED 830nm or diode laser 805nm |
Lesion count
|
NR | Unclear inclusion criteria on acne severity; no details on PDT parameter settings, number of passes or duration; no detail on interval length between treatment or follow-up period; no details on methods of statistical analysis |
| Mokhtari et al,23 2017 | 58 | BPO 5% + 570nm IPL vs. BPO 5% only |
Lesion count
|
Local AEs
|
Small sample size; nonblinding of participants and assessors; per protocol analysis; patients who were sensitive to BPO omitted several doses and recommenced at a lower dose, which introduces heterogeneity of intervention |
| Ma et al,65 2015 |
21 | ALA 5% + LED 633nm |
Lesion count
|
Local AEs
|
Small sample size; short follow-up period; no control group |
| Dong et al,54 2016 |
46 | ALA 10%+543–548nm, and 630±6nm LED |
Lesion count
|
Local AEs
|
Small sample size and short duration of follow-up; single-blinded study; variable endpoint; unclear how patient satisfaction measured |
| Park et al,49 2015 |
1213 | ICG-IPL-PDT |
Treatment success
|
Local AEs
|
Subjective bias due to use of nonvalidated tools; results do not state proportion of patients who had 3, 4, or 5 sessions; no statistical analysis; inconsistent intervention |
| Tao et al,52 2015 |
136 | ALA+LED 633±3nm |
Treatment success:
|
Local AEs
|
Skin was cleansed, oily crusts removed, fluctuant cysts aspirated, and comedones extracted in addition to the study intervention prior to the second and third treatment; reporter bias due to nature of study; short duration of follow-up |
| Song et al,32 2014 | 24 | Chlorophyll-a+430±10nm & 660±10nm LED vs. LED monotherapy |
Lesion count
|
Local AEs
|
Small sample size; single-blinded; histology performed on intervention side only; no chlorophyll-a–only arm |
| Liu et al,24 2014 |
150 | ALA 5%+633nm LED vs. monotherapy w/IPL 420nm vs. LED 415±5nm & 633±6nm |
Lesion count
|
Number of patients who experienced AE
|
Ethical approval not explicitly stated; nonblinded study; short duration of follow-up |
| Asayama-Kosaka et al,55 2014 |
11 | 5% ALA+broadband light 600–1100nm |
Treatment success
|
Local AEs
|
Unclear duration of light therapy; small sample size; no SD given for GAGS scores at 1m and 3m |
| Ma et al,66 2013 |
397 | ALA+LED 633nm for 3–4 sessions |
Treatment success
|
Local AEs
|
Non-randomisation; short duration of study |
| Hong et al,33 2013 |
20 | MAL+red light vs. MAL+IPL 530–750nm |
Lesion count
|
Local AEs
|
Single-blinding only; no placebo arm; small sample size; no ITT analysis; unclear randomisation process |
| Mei et al,34 2013 |
41 | ALA 10%+IPL 420–950nm vs. topical placebo+IPL 420–950nm |
Lesion count
|
Local AEs
|
Blinding of participants only; limited sample size and short duration of follow-up; unclear whether other treatments coadministered during trial period |
| Wang et al,67 2012 |
30 | ALA 3, 5, or 10%+633nm-LED |
Treatment success
|
Local AEs
|
55 patients recruited; results report outcomes for 30 patients only, but some AEs reported out of 55; unclear how moderate-severe acne determined; no validated tools used for primary outcomes; unclear timepoint for outcome measures |
| An et al,74 2011 |
13 | 0.5% liposome-encapsulated 5-ALA+IPL 400–720nm |
Lesion count
|
Local AEs
|
No control arm; small number of patients and short duration of follow-up; inconsistent statistical analysis; no randomization |
| IPL | |||||
| Mohanan et al,69 2012 | 8 | IPL IFL i200 system |
Treatment success
|
Local AEs
|
No follow-up reported; small sample size; no control arm; inconsistent reporting of statistical significance; no randomization; inconsistent #. treatments across participants |
| El-Latif et al,68 2014 |
50 | IPL 530nm vs. 5% BPO |
Lesion count
|
Local AEs
|
No randomisation; no control arm; statistical significance inconsistently reported; small sample size and short duration of follow-up; unclear if ethical approval obtained |
| Lee GS,70 2012 |
18 | IPL 420nm |
Treatment success
|
Local AEs
|
Results for 1 vs. 2 sessions not reported separately; range of follow-up times; no control arm; no measures of statistical significance |
| LED | |||||
| Kwon et al,47 2013 |
35 | LED 420nm blue light and 660nm red light vs. sham device |
Lesion count
|
Local AEs
|
Unclear enrollment process and inclusion criteria; variable duration of LED use/day per patient despite regular adherence checks; small sample size; short duration of follow-up |
| LASERS | |||||
| Kang et al,35 2019 |
9 | Laser one pass 1319nm and one pass 589nm |
Lesion count
|
Local AEs
|
Small sample size; short follow-up, unable to assess sustainability of results; no reporting on number of acne lesions; no statistical significance reported; single-blinded |
| Kwon et al,29 2018 |
25 | 1450nm diode laser in dual mode vs. 1450nm diode laser in high energy mode |
Lesion count
|
Local AEs
|
Single blind; small study group; short follow-up period |
| LASERS AND SYSTEMIC TREATMENT | |||||
| Li et al,36 2021 |
47 | IPL 420nm +isotretinoin 0.5–0.75mg/kg/day |
Lesion count
|
Local AEs
|
Other topical agents also used in both groups (adapalene 0.1% gel and fusidic acid 2% cream); limited sample size and short duration of follow-up; single-blinded study |
| FMR | |||||
| Kwon et al,37 2018 |
26 | FMR w/ 0.8mm and 2.0mm penetration depth and 20–50 intensity vs. 1450nm diode laser |
Lesion count
|
Local AEs
|
Single blinded; short follow-up time; no control arm |
| Lee et al,71 2013 |
20 | FMR w/ 1.0mm or 1.5mm penetration depth for 50ms, or 100ms |
Lesion count
|
Local AEs
|
No control group; small sample size; no histological assessment of sebaceous gland; only one session of treatment |
| Lee et al,50 2012 |
18 | FMR w/ 3.0mm penetration depth and 7 intensity |
Treatment success
|
Local AEs
|
Single blinded; no statistical analysis to determine significance; retrospective assessment from photographs: subjective bias and difficult to assess true skin pattern |
| Suh et al,72 2021 |
12 | Topical gold nanoparticles plus 400nm tip photopneumatic device |
Lesion count
|
Local AEs
|
No control arm; No objectively measured values; Small population size; Short duration of follow-up; Variation of assessment parameters between assessors |
| SYSTEMIC THERAPIES | |||||
| Gan et al,51 2012 |
2,255 | Oral isotretinoin |
Treatment success
|
Local AEs
|
Complete remission and substantial improvement not defined; GAGS used at baseline but not at point of outcome measurement; data on long-term follow-up not available; retrospective study: incomplete documentation; missing data |
| Kim et al,48 2014 |
20 | Topical epidermal growth factor vs. vehicle cream |
Lesion count
|
Local AEs
|
Unclear process of randomization; small sample size; short duration of follow up |
| OTHER THERAPIES | |||||
| Brownell et al,56 2021 |
13 | Topical bakuchiol (UP256) |
Lesion count
|
Local AEs
|
No control arm; no blinding; small sample size; short duration of follow-up |
| Isoda et al,73 2015 |
18 | Mild facial cleanser formulated w/ sodium laureth carboxylate and alkyl carboxylates (AEC/soap) |
Lesion count
|
Local AEs
|
20 patients recruited but only 18 analysed; self-reported as controlled trial, but no method of control identified; limitations of study not explored; inconsistent reporting of statistical significance |
A/BPO: adapalene/benzoyl peroxide; AE: adverse event; ALA: aminolaevulinic acid; BPO: benzoyl peroxide; C/BPO: clindamycin/benzoyl peroxide; CDLQI: Children’s Dermatology Life Quality Index; CI: confidence interval; DL: diode laser; DLQI: Dermatology Life Quality Index; EGSS: Evaluator's Global Severity Score; Excellent or good therapeutic effect: >50% reduction in lesion count); FMR: fractional microneedle radiofrequency; FPS: Fitzpatrick skin type; GA: glycolic acid; GAGS: Global Acne Grading System; GAI: Global Assessment of Improvement; GIS: Global Improvement Score; ICG: idocyanine green; IGA: Investigator’s Global Assessment; IGA: Investigator's Global Assessment; IL: inflammatory lesion count; IPL: Intense pulsed light; ISGA: Investigator's Static Global Assessment; ITT: Intention To Treat; JS: Jessner’s solution; KAGS: Korean Acne Grading System; LED: light emitting diode; LFTs: liver function tests; MAL: Methyl aminolevulinate; NIL: non-inflammatory lesion count; NR: not reported; OR: odds ratio; PIH: post-inflammatory hyperpigmentation; QoL: Quality of Life; rhGEF: topical epidermal growth factor; SA: salicylic acid; SD: standard deviation; TLC: total lesion count
Screening of studies. The titles, abstracts, and full texts of the retrieved articles were screened independently by two reviewers. Randomized, controlled trials (RCTs), cohort studies, case control studies, cross-sectional surveys, and case series with at least three patients were included. Articles were excluded if they focused on acne diagnoses other than AV (e.g. acne rosacea, acne conglobate), if the full text was unavailable, if the outcomes of patients with skin of color were not discussed separately from those of lighter-skinned patients, and if no data pertaining to efficacy were available.
Data collection. Data collection was led by two authors, and disagreements were resolved by discussion and consensus with a third author. The following information was extracted from the included studies: first author, year of publication, study country of origin, study design, number of patients, and primary and secondary outcomes. Extracted data were entered into a pregenerated standard Microsoft® Excel (Microsoft Corporation, Redmond, Washington, USA) file. Due to the heterogeneity of the study designs, participants, interventions, and reported outcomes, a formal meta-analysis was not performed.
Outcome measures. The primary outcome measure was the change in total lesion count (TLC). Secondary outcome measures included the following other validated and nonvalidated measures of effectiveness: Evaluator's Global Severity Score (EGSS), Acne Severity Index (ASI), Acne Global Severity Scale (AGSS), Global Acne Evaluation (GAE), Global Acne Assessment Score (GAAS), and adverse events (AEs). Treatment success was defined by the authors of each individual study according to their study protocols (summarized in Appendix 4).
Assessment of bias. Risk of bias was assessed using the Cochrane Collaboration Tool for RCTs16 and ROBINS-I17 for non-RCTs, and each study was assigned a risk of bias described as low, moderate, serious, or critical (Appendix 3). Assessment of the quality of included studies was based upon the CASP tools18 and Oxford CEBM Levels of Evidence.19
Ethical considerations. Ethical approval was not required for this systematic review. We used publicly accessible data, and the work was conducted in accordance with the standards outlined in the Good Clinical Practice guidelines.
RESULTS
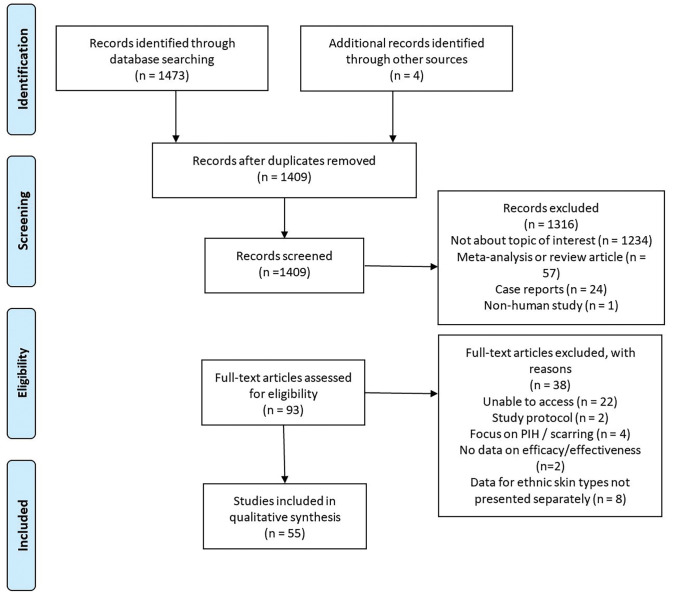
Summary of article types. The literature search produced 1,477 articles; 1,316 were excluded after assessment of the titles and abstracts. Ninety-three studies underwent full review, of which 55 studies met the inclusion criteria. Figure 2 illutrates the PRISMA flow chart for study inclusion. The final studies included 29 RCTs20–48 (5 nonblinded,20–24 13 single-blinded, 25–37 11 double-blinded38–48), four cohort studies49–52 (3 retrospective,49–51 1 prospective52), four pilot studies,53–56 six post-hoc analyses,57–62 and 12 other interventional trials.63–73 Ten studies were of a split-face design.29,30,32,35,37,40,45,46,48,67 The included studies reported a total of 21,202 patients.
FIGURE 2.
PRISMA flow chart
Regarding interventions, 23 studies20,25–28,30,31,38–44,53,57–64 evaluated topical therapies, 14 studies22–24,32–34,49,54,55,66,67,74 evaluated photodynamic therapy, seven studies29,35,36,47,68–70 evaluated laser/light therapy, one study51 evaluated systemic therapy, three studies evaluated chemical peels,21,38,45 three studies evaluated radiofrequency and microneedling,37,50,71 and four studies48,56,72,73 evaluated other therapies.
Outcomes relating to reduction in lesion count were reported in all but 12 studies.24,34,49–52,55,64,66,67,69,70 AEs were reported in most studies. However, no studies reported AEs as per data standards (e.g. the Common Terminology Criteria for Adverse Events (CTCAE) classification75). The main outcomes regarding effectiveness and tolerability are summarized in Table 1. Appendices 4 and 5 summarize study characteristics and participant demographic data, respectively.
Topical retinoids. Retinoids are a class of compounds with a basic core structure of vitamin A and its oxidized metabolites. They are of particular use in skin of color due to their dual action to treat acne and PIH. Three of the included studies25,57,58 explored topical retinoids as monotherapy in skin of color. One post-hoc analysis of two multicenter RCTs7 evaluated lesion count change and reduction in EGSS with tretinoin 0.05%, reporting 60.1-percent reduction in IL count and 53-percent reduction in noninflammatory lesion (NIL) count versus 51.1 percent and 38.7 percent, respectively, with vehicle. Common side effects of topical tretinoin 0.05% lotion included pain, dryness, erythema, scaling, and burning. Another post-hoc analysis by Lain et al58 reported that tretinoin 0.05% lotion resulted in greater mean percent reduction in NIL counts in female patients, compared to baseline data in active treatment and vehicle-only groups. Treatment was significantly more effective in female patients than male patients. Tretinoin 0.05% lotion was well tolerated by both sexes, although there was a higher incidence of treatment-related AEs, especially skin dryness, in female patients. Kubota et al25 evaluated adapalene 0.01% efficacy and safety in 66 Japanese subjects and concluded that twice-weekly application produced similar efficacy results as once-daily application, which may reduce likelihood of AEs in patients with skin of color.
Topical antibiotics. Topical antibiotics provide both antibacterial and anti-inflammatory properties and are typically used concurrently with benzoyl peroxide (BPO) to reduce bacterial resistance. This systematic review yielded 10 studies20,26,27,38–40,59–61,63 that explored antibiotics as a monotherapy in patients with skin of color. Cook-Bolden et al60 reported that, at Week 12, there was a median reduction in IL count of 71.6 percent with clindamycin 1.2%/benzoyl peroxide 3.75% compared to 57.1-percent reduction in the clindamycin-only group, 58.4-percent reduction in benzoyl peroxide-only group, and 47.6-percent reduction in the vehicle group, with excellent tolerability. No patient withdrew due to AEs. Callender et al61 reported that clindamycin 1.2%/BPO 2.5% gel was similarly effective between patients with FPS I to III and those with FPS IV to VI. Similar to the previous study, almost a third of patients reported an IGA of "clear" or "almost clear." In contrast, Amar et al63 presented an open-label, non-RCT study with a small number of patients and nonvalidated definitions of treatment success, reporting a much higher percentage of improvement in LC and IGA scores in patients using clindamycin 1.2%/BPO 3.75%. In a much larger cohort (N=800) of Japanese patients, Kawashima et al27 reported that clindamycin 1.2%/BPO 3.0% once daily was more effective than clindamycin monotherapy in reducing total LC, but was less effective than clindamycin 1.2%/BPO 3.0% twice-daily application. Contact dermatitis was the leading reason for study withdrawal, which was most frequent in the twice-daily clindamycin 1.2%/BPO 3.0% group. Xu et al26 reported significant improvement in LC using clindamycin 1%/BPO 5% once daily, compared to twice-daily application, among Chinese patients with AV. Main adverse events included application site reaction. Alexis et al59 reported clindamycin 1.2%/BPO 3.75% to be superior to vehicle in terms of LC and improvement in EGSS across all FPS types, with infrequent AEs. Hayashi et al28 reported similar efficacy of clindamycin 1.2%/BPO 3% in terms of LC compared to clindamycin 1.2%/adapalene 0.1%, but clindamycin 1.2%/BPO 3% was superior in terms of ISGA score. A study by Kawashima et al,20 which evaluated BPO 2.5% monotherapy in Japanese patients, had the longest follow-up period of 52 weeks. Investigators found BPO 2.5% monotherapy to be effective, with comparable results to BPO 5%, in reducing IL and TL counts, but BPO 2.5% monotherapy was less superior in reducing NIL. In a separate study, Kawashima et al39 reported BPO 3% was effective but was associated with more AEs (58%), compared to vehicle, with contact dermatitis being the most commonly reported. Only one study explored the use of nadifloxacin 1% as monotherapy. Jung et al40 found that IL counts were reduced by 70 percent on nadifloxacin-treated skin and increased by 13.5 percent on vehicle-treated skin; NIL showed reductions of 48.1 and 10.1%, respectively. AEs included mild erythema and dryness, which resolved spontaneously. Treatment duration of eight weeks was chosen to avoid antibiotic resistance; thus, long-term data from this study are not available.
Combination retinoids and antibiotics. Seven studies28,30,31,41,42,62,64 examined combination retinoid and antibiotic treatment, and all authors reported an improvement in LCs. DuBois et al64 reported results from a split-faced, observer-blinded study in Korean subjects, in which a topical combination of adapalene and benzoyl peroxide (A/BPO) was found to be superior to monotherapy. Kim et al30 also reported A/BPO to be superior to monotherapy in Korean patients, and Alexis et al62 reported similar improvements in LCs between patients with lighter skin types and those with darker skin types, all of whom were treated with A/BPO. AEs included erythema and scaling, which were also similar between the FPS I to III and FPS IV to VI groups. Interestingly, 9.7 percent of participants adopted a regimen of alternate-day application due to side effects.
Studies by Callender41 and Schmidt et al42 evaluated clindamycin/tretinoin combination in skin of color patients, reporting excellent responses and minimal side effects. However, it was noted that one patient in Callender’s study withdrew due to periorbital edema.
Takigawa et al31 demonstrated that combination of adapalene 0.1% and nadifloxacin 1% cream had significantly greater efficacy than adapalene monotherapy in reducing IL in patients with moderate or severe AV. Combination use of topical adapalene and nadifloxacin may have additive and complementary effects, resulting in clinical superiority of combination therapy to monotherapy. Furthermore, bacterial examination revealed that no resistance to nadifloxacin was demonstrated among 76 strains of P. acnes isolated from 87 patients.
Dapsone. Taylor et al43 found that dapsone 7.5% gel significantly reduced inflammatory, comedonal, and total lesions in FPS I to III (P<0.001) and IV to VI (P<0.01) groups versus vehicle. ILs responded to treatment first, compared to other lesion types. However, similar to the Alexis et al53 study, evaluation between skin types was not the primary outcome. Draelos et al44 concluded that dapsone 7.5% resulted in similar improvement in LC between skin types compared to vehicle but was superior in terms of GAAS and PIH improvement compared to vehicle. The use of oral dapsone in patients with skin of color has not been practiced widely due to higher incidence of G6PD deficiency in certain ethnic groups, such as African, South Asian, Middle Eastern, and Mediterranean.3
Peels. Chemical peels, similar to retinoids, have dual action against acne and PIH. Sarkar et al21 reported that LCs and PIH improved in all three study groups (5% glycolic acid, 20% salicylic–10% mandelic acid, and phytic acid combination peels) in patients with FPS IV to VI. Data indicate that 20% salicylic–10% mandelic acid had greatest effect in reducing PIH, while glycolic acid was more effective in reducing NIL than IL, according to Kaminaka et al.46 How et al45 reported that Jessner’s and salicylic acid were both equally effective across all outcome measures studied.
Photodynamic therapy (PDT). A range of PDT combinations have been evaluated. Two studies included Korean patients: Choi et al22 (N=21) and Park et al49 (N=1,213). Choi et al evaluated the use of idocyanine green (ICG)-based PDT versus methyl aminolevulinate (MAL)-based PDT in combination with 630nm light-emitting diode (LED), 805nm diode laser, or 830nm (LED).22 Choi et al22 reported no bactericidal effects of MAL-PDT; however, cultured P. acnes were killed using the 805nm diode and 830nm LED lasers in combination with ICG-PDT, though the difference in the efficacy of the 805nm diode laser and 830nm LED was not statistically significant. Park et al49 reported that 39.8 percent of study participants who had received 3 to 5 sessions of ICG-PDT demonstrated excellent improvement in acne lesions (qualified as 76–100% improvement). ICG-based PDT was generally well-tolerated with reports of pain, erythema, scaling and pruritus, which resolved within seven days without further intervention. These studies suggest that ICG-based PDT is an effective treatment option for AV in Korean patients; however, the optimal light source remains equivocal.
Aminolevulinic ccid (ALA)-based PDT was evaluated in nine studies.24,34,52,54,55,65–67,74 Two studies34,74 evaluated 10% ALA, four studies24,55,65,66 evaluated 5% ALA, one study evaluated 3.6% ALA,52 one study evaluated 0.5% ALA,74 and one study67 evaluated three different concentrations (3%, 5% and 10%). The light source in these studies included LED in five studies,52,54,65–67 intense pulsed light (IPL) in two studies,34,74 and broadband light in one study.55 One study24 compared the effectiveness of PDT, IPL, or blue-red LED phototherapy . Three studies52,54,66 reported clearance rates (≥90% reduction in lesion count) of 47.06 percent,52 32.5 percent,66 and 47.83 percent54 with ALA-LED-PDT. An et al74 reported a mean reduction in lesion count of 43.2 poercent with ALA-IPL-PDT. Similarly, Mei et al34 found that ALA-IPL-PDT was more effective than monotherapy with IPL with global lesion count reductions of 75.2 percent and 51.0 percent, respectively. Similarly, Liu et al24 found that 92 percent of participants achieved clearance and moderate improvements (≥60% reduction in lesion count) in the ALA-PDT group versus 58 percent in the IPL only group and 44 percent in the LED only group. Finally, Asayama-Kosaka et al55 found that ALA-PDT with broadband light reduced the Global Acne Grading Scale (GAGS) from 22.1 (standard deviation [SD]±3.8) to 16.3 after one treatment only. Despite the heterogeneity of ALA concentrations and light sources used, it appears that ALA-PDT is an effective treatment for AV in patients with skin of color. However, most patients reported a combination of pain, erythema, and/or oedema after ALA-PDT, which may adversely affect patient adherence. Similarly, the ideal regimen (dose, light source, duration, frequency) requires considerable optimization. However, it should be noted that Wang et al67 reported no significant change in lesion count for a one-unit increase in ALA concentration.
Other PDT regimens included BPO 5% with IPL therapy versus BPO alone, chlorophyll-a-PDT with LED versus LED alone, and MAL-PDT with IPL versus red light.23,32,33 Mokhtari et al23 reported a reduction in total LC from 41.86 (SD±14.17) to 6.95 (SD±6.81) in the BPO-IPL group versus 44.82 (SD±25.36) to 19.65 (SD±9.11) in the topical BPO-only group. Interestingly, Song et al32 reported that chlorophyll-a PDT resulted in a statistically significant reduction in pustule count of 66 percent versus 29 percent in the LED-only group. Hong et al33 reported a 48.7-percent reduction in IL among patients treated with MAL-PDT using red light versus 52.5 percent in the IPL-only group, though these results were not statistically significant. Notably, this study necessitated a reduction of red light dose from 37J/cm2 to 22 J/cm2 due to reports of pain, erythema, and edema. The MAL-PDT and BPO-PDT studies both reported significant numbers of patients experiencing pain, erythema, and edema post-treatment with PDT. Likewise, two33 and six23 patients withdrew from these studies due to the intolerability of these side effects. By contrast, Song et al32 showed that a chlorophyll-a-PDT was well-tolerated, reporting no pain, erythema, or edema.
IPL. The effectiveness of IPL therapy was assessed in two nonrandomized interventional trials68,69 and one preliminary trial.70 El-Latif et al68 compared the efficacy of topical 5% BPO gel applied once nightly for 5/52 with five once-weekly sessions of IPL (530nm) among Egyptian (FPS IV) participants; Lee et al70 and Mohanan et al69 conducted interventional trials evaluating Korean and Indian participants’ responses, respectively, to IPL without a comparator group. El-Latif et al68 reported that BPO and IPL resulted in considerable improvement of acne after 5/52, with a mean 69.40-percent (SD±22.35) reduction of lesions in the BPO group versus 61.56 percent (SD±26.14) in the IPL group, though the difference between the two treatment arms was not considered significant. Lee70 and Mohanan et al69 trials reported 78 percent and 87.5 percent (respectively) of participants achieved an LC rate of at least 50 percent. In all three studies, there were no serious AEs were reported, three reported instances of self-resolving erythema (one in Lee,70 two in Mohanan et al),69 and one report of a "burning sensation" for two hours after sun exposure.68 All studies concluded that IPL is a useful and well-tolerated treatment modality for AV in these patient populations.
LED. One double-blind RCT47 evaluated the efficacy of LED (420nm blue light and 660nm red light) versus a sham device over four weeks. The authors reported a statistically significant reduction in IL and NIL counts of 76.8 percent (from 22.8 to 5.3) and 54 percent (51.2 to 23.7) respectively in the study group. This study also reported histopathological and immunohistochemical changes and noted that LED treatment resulted in reduced sebum output, attenuated inflammatory cell infiltrations and reduced the size of sebaceous glands; these changes were not noted in the control group. The treatment was well-tolerated, with mild erythema and dryness noted in three participants, but no severe AEs reported. Overall, LED appears to be tolerable and effective therapy for acne vulgaris in skin of color patients.
Laser. Two single-blinded, split-face RCTs29,35 evaluated the efficacy of laser therapy. Kang et al35 included nine participants (one of which was White) who were treated with a regimen of one pass with a 1319nm laser followed by one pass with 589nm laser for four sessions at 2 to 3 week intervals to one side of the face, with no intervention on the control side. At the final follow-up (5.4 weeks after the final treatment), IL counts had reduced by 23.1 percent on the treatment side versus an increase of 11.1 percent on the control side. In a study by Kwon et al,29 participants received treatment using a 1450nm diode laser with low-energy stamp mode targeting ILs, followed by moving mode for 4 to 5 passes on the intervention side versus one pass of 1450nm diode laser with conventional high-energy stamp mode on the control side for three sessions at four-week intervals. Twelve weeks after the final treatment, the number of ILs had reduced by 63.5 percent on the dual-mode side versus 39.3 percent on the stamp mode-only side (P<0.05). In both studies, treatment was well-tolerated with no severe AEs reported and a few reports of discomfort during treatment. These studies suggest that laser treatment is a useful monotherapy or adjunct therapy for the treatment of AV in skin of color patients. However, the optimal frequency and mode (dual-mode vs stamp mode) remains undetermined.
Combined IPL and systemic therapy. One RCT36 reported treatment efficacy in Chinese patients (FPS III–IV) with AV using a combined regimen of oral isotretinoin (0.5–0.75mg/kg/day) and biweekly IPL (420nm) versus oral isotretinoin alone. At Week 12, investigators reported a statistically significant (P<0.01) reduction (53%) in the total number of lesions (SD±33.5) in the study group (combined oral isotretinoin plus IPL) compared to 27.2 percent (SD±14.7) in the control (oral isotretinoin only) group. Similarly, the GEA grade reduced from 2.8 at baseline (SD±0.7) to 1.8 (SD±0.8) in the study group vs 2.7 at baseline (SD±0.7) to 2.3 (SD±0.4) in the control group (p< 0.05). No severe AEs were reported; however ,62.5 percent of the participants reported mild-to-moderate pain during the IPL treatment. Other mild AEs included mild erythema immediately post-IPL reported by most participants; one participant developed a 1cm blister post-IPL, which resolved within one week without further intervention. Both groups reported skin dryness, peeling lips, and allergic reactions to coadministered adapalene 0.1% gel without a statistically significant difference observed between the groups. There is little evidence to support the use of combined oral isotretinoin plus IPL (420-nm) for the treatment of AV in Chinese patients (FPS III–IV). However, this single-blind RCT suggests that this treatment regimen might be useful and well-tolerated.
Fractional radiofrequency and microneedling (FRM). FRM was evaluated in three studies.37,50,71 In a prospective interventional study by Lee et al,71 participants received one pass of treatment, whereas participants in Lee et al's50 retrospective cohort study received two FMR passes in each of two sessions, one month apart. Kwon et al37 reported that in a single-blind, split-face RCT, participants received 2 to 3 passes of FMR on one side of the face versus two passes of 1450nm diode laser on the other side. Unfortunately, all three of these studies reported heterogenous outcomes. Lee et al71 reported a reduction in mean total IL from 18 at baseline to 14.1, 19.6, and 17 at Week 2, 4, and 8 respectively, demonstrating an improvement in lesion count after a single treatment and at Week 2, but worsening thereafter. In the retrospective cohort study,50 the mean clinical improvement scores for active lesions and for severity of lesions were Grade 2.6 and Grade 2.4, respectively (Grade 2=26–50% improvement, Grade 3=51–75% improvement). Kwon et al37 reported a statistically significant reduction in total IL count of 39.3 percent on the diode laser side versus 58.2 percent in the FMR side. There were no severe AEs reported among these three studies; however, there were several reports of pain during treatment plus post-treatment bleeding, pain, crusting, and scaling. Kwon et al37 reported no statistically significant difference between the DL and FMR group in terms of AEs. These studies suggest that FMR is an effective treatment option for AV in skin of color patients. However, there remains a need to determine the optimal power settings, number of sessions and interval between sessions.
Systemic therapies. This search found only one study that evaluated the use of systemic treatment for AV in patients with skin of color. Gan et al51 evaluated the effectiveness of oral isotretinoin in a multiracial Asian cohort. This study reported that 93.9 percent of the patients achieved complete remission or substantial improvement; however, these terms were not quantified within the study. The odds ratio for achieving complete remission for patients receiving at least 100mg/kg of isotretinoin was increased to 3.85 when compared to patients receiving a lower dose. Overall, isotretinoin was well-tolerated; however, side effects included cheilitis (64.7%), headache (1.8%), mood change (1.6%), and photosensitivity (1.5%). Importantly, less than five percent of the patients developed abnormal liver function tests and/or raised serum triglycerides. Oral isotretinoin is a well-established treatment for AV; however, there is a paucity of evidence supporting its use in skin of color patients.
Other therapies. Kim et al48 reported that topical recombinant human epidermal growth factor (rhEGF) was effective in reducing IL and NIL in 20 Korean adults with mild-to-moderate AV, with minimal AEs. Topical EGF likely reduces the level of sebum due to its ability to suppress lipogenesis. Possible anti-inflammatory effects include rhEGF’s interference with arachidonic acid metabolism, regulating chemokine expression in keratinocytes, and reduction of keratin plugs in follicles.
Isoda et al73 reported that a sodium laureth carboxylate and alkyl carboxylates (AEC) based-soap was effective and well tolerated in 20 Japanese male patients with mild-to-moderate acne. At Week 4, 25 percent of the patients reported no acne lesions. However, although 20 patients were recruited, only 18 were analyzed with no intention-to-treat analysis, and there was inconsistent reporting of statistical significance.
Brownell et al56 reported that bakuchiol, an ingredient found in the leaves and seeds of the Psoralea corylifolia plant, decreased IL count by 26.9 percent and 28.4 percent at Week 8 and Week 12, respectively, in a single center, open-label pilot study. Thirteen subjects with FPS III to VI and mild or moderate acne received treatment twice daily for 12 weeks. AEs included erythema, dryness, scaling, and oiliness, with no reported discontinuation. However, the lack of control arm, small sample size, and short follow-up duration limit this study's findings.
One nonrandomized interventional trial72 assessed the efficacy of a novel treatment: topical application of gold nanoparticles followed by treatment with a pneumatic device (Isolaz™, Aesthera Corporation, Pleasanton, California) with a flashlamp and vacuum for applying negative pressure. In this trial, Korean patients with moderate-to-severe AV received three successive treatments at 1 to 2 week intervals. According to Assessor A, the average number of pustules decreased from 6.50 to 2.17 after treatment, and the average number of papules decreased from 12.42 to 6.42. Similar results were observed by Assessor B—the average number of pustules decreased from 8.00 to 2.50 after treatment and the average number of papules decreased from 13.33 to 6.50. Histopathological findings reported a decrease in inflammatory cell infiltration and fibrotic changes of the dermis. No serious AEs were reported. In this preliminary trial, gold photothermal therapy showed significant clinical and histological improvements in AV in Asians without serious AEs; however, the very small sample size limit these findings. Randomized, controlled studies with larger patient populations are necessary before firm conclusions can be drawn.
DISCUSSION
This systematic review, based on 55 studies, assesses the effectiveness and tolerability of four main therapies in the treatment of AV in skin of color patients: topical therapies, laser- and light-based therapies, systemic therapies, and miscellaneous therapies. To our knowledge, no previous systematic review or meta-analyses have been performed that specifically evaluate the effectiveness and tolerability of treatments used in patients with AV and skin of color. Previous evidence-based reviews2,76 have collated some data on pharmacological and nonpharmacological therapies when managing AV in skin of color patients. These reviews also noted the paucity of clinical studies that evaluate the tolerability and efficacy of acne treatments specifically among patients with skin of color.
Topical therapies. Retinoids have been the mainstay treatment for AV. Cook Bolden et al57 reported tretinoin 0.05% lotion to be well tolerated and efficacious in Hispanic patients. The investigators interestingly chose to compare tretinoin and vehicle to baseline data, although results would have been more robust if tretinoin was compared directly to vehicle. Furthermore, there was a relatively large discontinuation rate, and a significant side effect profile was reported for tretinoin, which included application site pain, dryness, erythema, scaling, and burning. Almost three quarters of patients expected to see results overnight or in 1 to 2 weeks (81% Hispanics, 68% White); treatment adherence was lower among Hispanic participants. This reinforces the need to manage unrealistic expectations, which may be higher among Hispanics for unexplained reasons. The concern with using retinoids in skin of color is partly due to the fear of an exaggerated PIH response, an element of irritant contact dermatitis. However, more studies recently have concluded that retinoids are better tolerated and efficacious in skin of color when used at lower concentrations.
Adapalene is a third-generation topical retinoid used in the treatment of mild-to-moderate acne. Kawashima et al20 reported a significant incidence of adapalene-related side effects. Tu et al77 compared adapalene 0.1% to tretinoin 0.025% in Chinese patients and found equivalent efficacy but reported higher incidence of local irritation with tretinoin, which was echoed by Goh et al,78 based on data from Chinese, Indian, Malay and Caucasian subjects, although a report on efficacy was not provided. Tazarotene was found to be effective in reducing lesion count among Indian subjects, as reported by Saple et al,79 however as this study had a short follow-up period; hence, long-term conclusions cannot be safely drawn.
The use of topical antibiotics in the treatment of AV is a common practice in all skin types. Cook-Bolden et al60 reported clindamycin phosphate 1.2% in combination with BPO 3.75% to be superior to vehicle, with differences becoming evident at Week 4. Study weaknesses include the short follow-up period and the failure to report results in relation to FPS rather than ethnic group, as even within the Hispanic population, FPS can vary considerably. Amar et al63 echoed the findings of Cook-Bolden et al60 when trialing clindamycin/BPO combination in patients with FPS V to VI with moderate facial acne. The author reported at least 1-grade improvement in IGA in 100 percent of the participants. However, critics may question whether a 1-grade reduction on IGA/PIH scale, is a meaningful outcome in clinical practice. Many authors use at least a 2-grade reduction in the scale as a primary or secondary outcome. Callender et al61 reported clindamycin phosphate 1.2 %/BPO 2.5% to be well tolerated and to have similar efficacy in reducing IL and NIL in patients with FPS I to III, compared to those with FPS IV to VI, at Week 12 (40%, 25.6%, 28.8% vs 40%, 25.7%, 29.4% respectively). Xu et al26 reported that, among 1,020 Chinese subjects with mild-to-moderate acne, more patients achieved a 2-grade or greater improvement in ISGA scores at Week 12 using clindamycin 1%/BPO 5% once daily gel, compared to clindamycin 1% twice-daily gel, though it is not clear why study investigators compared twice daily combination therapy to once daily monotherapy.
Dubois et al64 evaluated adapalene 0.3% in combination with BPO and noted that 56 percent of study participants had IGA 0/1 and 87 percent reported excellent improvement in GAIS. Hyperpigmentation was reduced by 27 percent compared to baseline over a period of 16 weeks. Tolerability of A/BPO 0.1% was similar to A/BPO 0.3%, with no observed increased risk of PIH. Hence, one can argue that adapalene 0.3% is an effective treatment for AV in skin of color and is more tolerable when combined with BPO.
Similarly, in a study by Kim et al30 topical A/BPO was superior to monotherapy in Korean subjects in a split-face model. Lesion count improved but more patients complained of local irritation. However, these findings are limited by the study's single-blinded design. Alexis et al62 performed a randomized, controlled, post-hoc analysis and reported that adapalene 0.3%/BPO 2.5% gel was equally effective among light- and dark-skinned patients (63.6% change in NIL count in lighter skin type vs 61.1% in darker skin typ; similar results in IL count). However, the purpose of the study was to compare differences compared to baseline. The study was insufficiently powered to compare differences between skin types. Callender et al41 found that the clindamycin/tretinoin topical gel combination was well tolerated, causing little to no irritation. However, results cannot be extrapolated to more severe stages of acne as the patient group studied had mild-to-moderate acne. It is, however, commendable that the washout period was mandatory for oral corticosteroids and antibiotics, which was not been explicitly mentioned in many of the other studies. Clindamycin appears to enhance comedolytic activity of tretinoin via its ability to loosen and prevent follicular impactions, and tretinoin may provide greater accessibility and penetration of clindamycin into follicular environment. In terms of AEs, the topical antibiotic was well tolerated but there appeared to be a disproportionally higher incidence of contact dermatitis than with other treatment modalities. Topical dapsone was reported to be efficacious and well tolerated; however, overall recommendations are not possible based on only three studies evaluating distinct formulations. Chemical peels appear to be effective but are likely used in combination with other agents. Large dropout rates seen in chemical peel trials, possibily in part due to the number of treatment sessions required, can be problematic in patients with skin of color, a patient group in which treatment adherence is already known to be low.21 Salicylic acid 20% to 30% seemed to be particularly effective in active LC and PIH in Asian subjects.22,80-81
Light and laser therapies. Regarding ALA-PDT and light-based therapies, most treatments were well-tolerated, and the most frequently reported side-effects included erythema, pain, edema, and hyperpigmentation. However, our most significant finding is the paucity of evidence available to facilitate effective and safe decision-making for the treatment of AV in skin of color patients.
Several studies in this review evaluated the use of light-based therapies in patients with skin of color; however, the quality of these studies was highly variable. Likewise, there was significant variation in the interventions evaluated, such as different photosensitizers, wavelengths, fluences, numbers of sessions, and frequencies of application.
The current systematic review revealed that the number of light-based sessions varied from 1 to 8, with 2 to 4 sessions being the most common. Our analysis indicates that PDT using topical ALA has been the most investigated light-based therapy. Topical ALA’s mechanism of action in PDT is via penetration of the stratum corneum and preferential accumulation in the sebaceous glands where it is metabolized to PpIX, a potent photosensitizer, in sebocytes.55,74 This enhances phototoxicity in preparation for light-based therapies, which leads to the destruction of affected sebaceous glands.55 Eight studies reporting on this treatment showed that ALA-PDT can be successfully used to treat AV in ethnic skin. Three studies52,54,66 reported clearance rates (≥90% reduction in lesion count) of greater than 35 percent, and two studies34,74 reported lesion count reductions greater than 40 percent. However, the ideal regimen (ALA strength, light source, duration, frequency) requires considerable optimization. Higher strengths of ALA may result in increased rates of PIH,66 a particular concern among darker skinned individuals. Notably, Wang et al67 reported that three different ALA concentrations (3%, 5%, 10%) generated similar responses, and Tao et al52 reported that a 3.6% ALA strength with 1.5 hour occlusion may increase patient acceptability of treatment by reducing overall treatment time and side effects. It may, therefore, follow that lower strength ALA preparations would offer maximal therapeutic benefits while attenuating post-treatment side effects in patients with skin of color.
Other photosensitizers evaluated for use in skin of color patients include ICG, MAL, BPO, and chlorophyll-a. ICG-based PDT using 685nm IPL, 830nm LED, and 805nm diode laser had significant clinical effects on reducing acne severity. BPO-PDT was reported to produce a statistically significant reduction in total lesion count compared to BPO monotherapy, and chlorophyll-a PDT was reported to achieve a statistically significant reduction in pustule count compared to LED monotherapy. However, MAL was found to produce no statistically significant reduction in ILs compared to red-light monotherapy. Again, the quality of evidence supporting the effectiveness of these regimens in patients with skin of color is poor. Likewise, the small, single study evaluating photopneumatic therapy with gold particles that combined physical extraction of comedones with that of traditional PDT, cannot reliably be used to inform decision-making for skin of color patients with AV, despite results demonstrating significant clinical and histological improvements.
The light source for PDT remains equivocal. Studies included in this review included IPL, LED, and diode laser as the light source during PDT. Unfortunately, only two studies directly compared a photosensitizer with different light sources—Hong et al33 compared IPL-based MAL-PDT with red light MAL-PDT, and Choi et al22 compared LED-based ICG-PDT with diode laser ICG-PDT. These studies found no statistically significant difference between the two treatment groups.
Dong et al54 concluded that IPL is a useful type of light for PDT due to its ability to increase temperatures in inflamed acne lesions and at the dermal-epidermal junction; and to cause photoactivation and induction of singlet oxygen production—evidenced by the presence of absorption peaks for endogenous porphyrins after IPL-PDT.54 However, Hong et al33 reported that red light was preferable due to the longer wavelength, when compared to IPL, which results in deeper penetration into the dermis to activate porphyrin in deeply-situated sebaceous glands. A similar argument was made by Choi et al82 who suggested that red-light LED is a better treatment option than diode lasers due to its ability to penetrate deeper into the skin and irradiate the entire face and lesions simultaneously. Importantly, in the Hong et al33 study, the red light LED dose was reduced from 37J/cm2 (a protocol accepted in Caucasian patients) to 22J/cm2 because two patients withdrew from the study due to intolerable pain, erythema, and edema. This suggests that smaller doses of red light LED might be preferable in skin of color patients.
Five studies evaluated light-based therapies as monotherapy in skin of color patients. IPL, LED, and laser therapies were found to be effective and well-tolerated treatment options for the treatment of AV. The use of these modalities may offer comparable clinical efficacy to pharmacological therapies, with fewer local AEs and systemic side effects that may complicate oral treatment.47 However, as previously stated, there is an urgent need to optimize treatment protocols in patients with skin of color to determine which therapies offer superior clinical efficacy in this population.
Other therapies. FRM uses insulated needles to target dermal structures using electrothermal energy.71 This may lead to therapeutic effect via thermal damage to sebaceous glands or physical disruption of hyperkeratotic plugs by microneedles.71 FRM led to statistically significant reductions in lesion counts in the three studies in which this treatment was evaluated. FRM may also offer a more tolerated delivery of thermal energy to dermal tissues via microneedles when compared to nonablative radiofrequency devices or ablative fractional lasers.50 Similarly, there is a reduced risk of hyperpigmentation following treatment with FRM due to the reduced energy absorption of melanin pigment.50 Although these studies reported significant results, an overall conclusion based on three studies must be interpreted with caution.
Our search found only one study51 that evaluated the use of systemic therapies for the treatment of AV in skin of color patients. Oral isotretinoin was evaluated in a large cohort study and was found to be effective and well-tolerated, despite the known potential for hepatotoxicity and iatrogenic hypertriglyceridaemia. No studies were found that evaluated the efficacy of other commonly used oral therapies, including oral antibiotics and hormonal therapies. This may be due to our inclusion/exclusion criteria, specifically our exclusion of studies pre-2011; however, despite the widespread use of these agents, their use in skin of color patients is most likely derived from studies evaluating efficacy in Caucasian patients.
One of the most important findings in our review was the lack of studies that evaluated the efficacy and tolerability of treatments among Black, African-American, and Afro-Carribean patients. Out of 55 included studies, only four papers45,53,63,64 exclusively evaluated our primary outcome in patients with FPS IV to VI. The paucity of data pertinent to this population demonstrates the tendency for Black, African American, and Afro-Caribbean patients to be underrepresented in clinical trials.83,84 Participants in clinical trials should reflect the diversity of the population, with a particular focus on those most affected by the disease. The lack of representation of this group in clinical trials has resulted in the adoption of treatment regimens that potentially are less efficacious in this population.
Limitations. An important limitation of our review is the high level of heterogeneity among included studies. There was wide variability in treatment modality, outcome measurement tools, different study characteristics (e.g. parameters, concentrations, number of treatments, follow-up periods) and differences in reporting results (e.g. absolute values, percentages, graphs, and figures). Similarly, the studies we included in our evaluation were performed in different geographical and cultural settings, which may prevent generalization of any results due to factors such as differences in exposure to natural sunlight and baseline variations in FPS. These factors must be taken into account when interpreting our findings.
We also experienced limitations in the inclusion of studies, in particular the inclusion of non-RCT studies. However, this was necessary in order to ensure sufficient data were available for adequate exploration of our primary aim: to assess the effectiveness and tolerability of pharmacological and nonpharmacological therapies in skin of color patients with AV. Several studies had a split-face design; however, it is unclear whether there are systemic effects that light and other therapies may exert on the control side of the face, even if it is not treated directly. Therefore, this review has highlighted the necessity for high-quality RCTs, with inclusion of a control arm in the study design, in order to determine the efficacy of an intervention in patients with skin of color.
The majority of the included studies demonstrated low levels of evidence. The small numbers of participants in most interventional trials indicates that most of the included studies were likely underpowered, which may have resulted in nonstatistically significant results. Secondly, most studies used short follow-up periods, which means that our findings may not be representative of long-term effects. Furthermore, the moderate-to-high risk of bias among the majority of the studies possibly affected the study results. The most common risk of bias was performance bias. In some studies, participants and/or assessing clinicians were not blinded, meaning that participants and/or clinicians were most likely aware of the treatment side. Only a small number of studies ensured blinding integrity by exposing the control arm to a sham or placebo treatment. Furthermore, in most studies, the research integrity was questionable, such as unclear ethical approval or possible conflicts of interest. Finally, many studies did not adequately describe the use of concomitant interventions, which made it difficult to solely evaluate the effectiveness of the investigated therapy.
The authors also acknowledge that this review is likely to have included a biased selection of the evidence, due to exclusion of gray literature and articles without full texts.
Furthermore, to date there is no acne severity assessment tools that have been validated for different ethnic skin types. The lack of standardized assessment tool also presents problems in that data cannot be reliably compared across the literature as these systems are not interchangeable. Development of a universal system is difficult due to the way in which acne can be defined by the multiple lesion types, the changing nature of the lesions, and involvement of multiple body sites other than the face.91 Similarly, lower rates of post-treatment erythema and inflammation as a cause of this erythema are likely due to poor detection with no official assessment tool available for darker skin types.
CONCLUSION
AV seems to share the same pathogenesis regardless of race or ethnicity but has different clinical presentations. In darker skin types, there seems to be a heightened subclinical inflammatory response, even in noninflammatory lesions, which is thought to trigger an exaggerated PIH response and keloid scarring. Our findings are in line with previous reviews regarding the general direction of evidence for the use of current available treatments in AV specific to skin of color.
Our results reinforce the need for standardized outcome measures, larger studies of better quality, and adequate reporting, raised by previous studies on acne in skin of color patients. Due to limited evidence, we are unable to draw firm conclusions from the results of this review to guide decisions in practice, especially those pertaining to long-term outcomes. Our systematic review found a paucity of high-quality clinical trials evaluating the effectiveness and tolerability of therapies for the treatment of AV in darker-skinned patients. Certainly, there is the unmet need for more trial and real-life clinical data on ethnic skin in all areas of dermatology. We would welcome future research, using double-blinded, placebo-controlled study designs with homogeneous data collection and processing to optimize treatment outcomes in this frequently neglected patient group.
APPENDICES
Appendices to this article can be accesed here: https://jcadonline.com/wp-content/uploads/Peterknecht-Appendices.pdf
ACKNOWLEDGMENTS
We thank Tim Ellis (Mid and South Essex NHS Foundation Trust) for his assistance with our initial literature review and Dr. Charlotte Eckhardt (The Blizard Institute, QMUL) for her guidance and scrutiny.
Contributor Information
Pirunthan Pathmarajah, Dr. Pathmarajah is with the Department of Dermatology at Barts Health NHS Trust in London, United Kingdom..
Elizabeth Peterknecht, Dr. Peterknecht is with the Department of General Medicine at Sandwell and West Birmingham Hospitals NHS Trust in Birmingham, United Kingdom..
Karmen Cheung, Dr. Cheung is with the Department of General Medicine at Manchester University NHS Foundation Trust in Manchester, United Kingdom..
Sarak Elyoussfi, Dr. Elyoussfi is with the Department of General Medicine at Salford Royal NHS Foundation Trust in Manchester, United Kingdom..
Vijaytha Muralidharan, Dr. Muralidharan is with the Department of General Medicine at Sandwell and West Birmingham Hospitals NHS Trust in Birmingham, United Kingdom..
Anthony Bewley, Dr. Bewley is with the Department of Dermatology at Barts Health NHS Trust, London, United Kingdom..
REFERENCES
- Kundu RV, Patterson S. Dermatologic conditions in skin of color: part I. Special considerations for common skin disorders. Am Fam Physician. 2013;87(12):850–856. Jun 15. [PubMed] [Google Scholar]
- Williams HC, Dellavalle RP, Garner S. Acne vulgaris. Lancet. 2012;379(9813):361–372. doi: 10.1016/S0140-6736(11)60321-8. [DOI] [PubMed] [Google Scholar]
- Davis EC, Callender VD. A review of acne in ethnic skin. J Clin Aesthet Dermatol. 2010;3(4):24–38. [PMC free article] [PubMed] [Google Scholar]
- Perkins AC, Cheng CE, Hillebrand GG et al. Comparison of the epidemiology of acne vulgaris among Caucasian, Asian, Continental Indian and African American women. J Eur Acad Dermatol Venereol. 2011;25(9):1054–1060. doi: 10.1111/j.1468-3083.2010.03919.x. [DOI] [PubMed] [Google Scholar]
- Lynn DD, Umari T, Dunnick CAC et al. The epidemiology of acne vulgaris in late adolescence. Adolesc Health Med Ther. 2016;7:13–25. doi: 10.2147/AHMT.S55832. Jan 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins JW, Voorhees JJ. Prevalence of nodulocystic acne in white and Negro males. Arch Dermatol. 1970;102(6):631–634. [PubMed] [Google Scholar]
- Silpa-Archa N, Kohli I, Chaowattanapanit S et al. Postinflammatory hyperpigmentation: a comprehensive overview—epidemiology, pathogenesis, clinical presentation, and noninvasive assessment technique. J Am Acad Dermatol. 2017;77(4):591–605. doi: 10.1016/j.jaad.2017.01.035. [DOI] [PubMed] [Google Scholar]
- Louie P, Wilkes R. Representations of race and skin tone in medical textbook imagery. Soc Sci Med. 2018;202:38–42. doi: 10.1016/j.socscimed.2018.02.023. [DOI] [PubMed] [Google Scholar]
- He L, Yang Z, Yu H et al. The relationship between CYP17 -34T/C polymorphism and acne in Chinese subjects revealed by sequencing. Dermatology. 2006;212(4):338–342. doi: 10.1159/000092284. [DOI] [PubMed] [Google Scholar]
- He L, Wu W-J, Yang J-K et al. Two new susceptibility loci 1q24.2 and 11p11.2 confer risk to severe acne. Nat Commun. 2014;5:2870. doi: 10.1038/ncomms3870. [DOI] [PubMed] [Google Scholar]
- Falder RM, Holmes YC, Bridgeman-Shah S et al. A clincohistopathological study of acne vulgaris in black females. Journal of Investigative Dermatology. 1996;4(106):888. [Google Scholar]
- Plewig G, Fulton JE, Kligman AM. Pomade acne. Arch Dermatol. 1970;101(5):580–584. [PubMed] [Google Scholar]
- Kligman AM, Mills OH. Acne cosmetica. Arch Dermatol. 1972;106(6):843–850. [PubMed] [Google Scholar]
- Higgins J, Thomas J, Chandler JC Cochrane Handbook for Systematic Reviews of Interventions. https://training.cochrane.org/handbook/current Version 6.1. 2020. Accessed 1 Feb 2021.
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:b2535. doi: 10.1136/bmj.b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- https://www.riskofbias.info/welcome/rob-2-0-tool/current-version-of-rob-2 Risk of Bias Info website. Revised Cochrane risk-of-bias tool for randomized trials (RoB 2). 2019. Accessed 20 Jun 2021. [DOI] [PubMed]
- Sterne JA, Hernán MA, Reeves BC et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brice R. CASP CHECKLISTS. CASP—Critical Appraisal Skills Programme. 2021. https://casp-uk.net/casp-tools-checklists Accessed17 May 2021.
- https://www.cebm.ox.ac.uk/resources/levels-of-evidence/ocebm-levels-of-evidence OCEBM Levels of Evidence Working Group. The Oxford Levels of Evidence 2. 2011. Accessed 20 Jun 2021.
- Kawashima M, Nagare T, Katsuramaki T. Open-label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long-term use in patients with acne vulgaris: a secondary publication. J Dermatol. 2017;44(6):635–643. doi: 10.1111/1346-8138.13741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar R, Ghunawat S, Garg VK. Comparative study of 35% glycolic acid, 20% salicylic-10% mandelic acid, and phytic acid combination peels in the treatment of active acne and postacne pigmentation. J Cutan Aesthet Surg. 2019;12(3):158–163. doi: 10.4103/JCAS.JCAS_135_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S-H, Seo J-W, Kim K-H. Comparative study of the bactericidal effects of indocyanine green- and methyl aminolevulinate-based photodynamic therapy on Propionibacterium acnes as a new treatment for acne. J Dermatol. 2018;45(7):824–829. doi: 10.1111/1346-8138.14347. [DOI] [PubMed] [Google Scholar]
- Mokhtari F, Gholami M, Siadat AH et al. Efficacy of intense-pulsed light therapy with topical benzoyl peroxide 5% versus benzoyl peroxide 5% alone in mild-to-moderate acne vulgaris: a randomized controlled trial. J Res Pharm Pract. 2017;6(4):199–205. doi: 10.4103/jrpp.JRPP_17_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L-H, Fan X, An Y-X et al. Randomized trial of three phototherapy methods for the treatment of acne vulgaris in Chinese patients. Photodermatol Photoimmunol Photomed. 2014;30(5):246–253. doi: 10.1111/phpp.12098. [DOI] [PubMed] [Google Scholar]
- Kubota Y, Munehiro A, Shirahige Y et al. Effect of sequential application of topical adapalene and clindamycin phosphate in the treatment of Japanese patients with acne vulgaris. J Dermatolog Treat. 2012;23(1):37–45. doi: 10.3109/09546634.2010.519016. [DOI] [PubMed] [Google Scholar]
- Xu JH, Lu QJ, Huang JH et al. A multicentre, randomized, single-blind comparison of topical clindamycin 1%/benzoyl peroxide 5% once-daily gel versus clindamycin 1% twice-daily gel in the treatment of mild to moderate acne vulgaris in Chinese patients. J Eur Acad Dermatol Venereol. 2016;30(7):1176–1182. doi: 10.1111/jdv.13622. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Hashimoto H, Alió Sáenz AB et al. Clindamycin phosphate 1·2%-benzoyl peroxide 3·0% fixed-dose combination gel has an effective and acceptable safety and tolerability profile for the treatment of acne vulgaris in Japanese patients: a phase III, multicentre, randomised, single-blinded, active-controlled, parallel-group study. Br J Dermatol. 2015;172(2):494–503. doi: 10.1111/bjd.13265. [DOI] [PubMed] [Google Scholar]
- Hayashi N, Kurokawa I, Siakpere O et al. Clindamycin phosphate 1.2%/benzoyl peroxide 3% fixed-dose combination gel versus topical combination therapy of adapalene 0.1% gel and clindamycin phosphate 1.2% gel in the treatment of acne vulgaris in Japanese patients: a multicenter, randomized, investigator-blind, parallel-group study. J Dermatol. 2018;45(8):951–962. doi: 10.1111/1346-8138.14497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon HH, Choi SC, Jung JY et al. Comparison of novel dual mode vs conventional single pass of a 1450-nm diode laser in the treatment of acne vulgaris for Korean patients: a 20-week prospective, randomized, split-face study. J Cosmet Dermatol. 2018;17(6):1063–1068. doi: 10.1111/jocd.12788. [DOI] [PubMed] [Google Scholar]
- Kim W-J, Park J-M, Ko H-C et al. A split-faced, observer-blinded comparison study of topical adapalene/benzoyl peroxide and adapalene in the treatment of Asian acne patients. J Drugs Dermatol. 2013;12(2):149–151. [PubMed] [Google Scholar]
- Takigawa M, Tokura Y, Shimada S et al. Clinical and bacteriological evaluation of adapalene 0.1% gel plus nadifloxacin 1% cream versus adapalene 0.1% gel in patients with acne vulgaris. J Dermatol. 2013;40(8):620–625. doi: 10.1111/1346-8138.12189. [DOI] [PubMed] [Google Scholar]
- Song BH, Lee DH, Kim BC et al. Photodynamic therapy using chlorophyll-a in the treatment of acne vulgaris: a randomized, single-blind, split-face study. J Am Acad Dermatol. 2014;71(4):764–771. doi: 10.1016/j.jaad.2014.05.047. [DOI] [PubMed] [Google Scholar]
- Hong JS, Jung JY, Yoon JY, Suh DH. Acne treatment by methyl aminolevulinate photodynamic therapy with red light vs. intense pulsed light. Int J Dermatol. 2013;52(5):614–619. doi: 10.1111/j.1365-4632.2012.05673.x. [DOI] [PubMed] [Google Scholar]
- Mei X, Shi W, Piao Y. Effectiveness of photodynamic therapy with topical 5-aminolevulinic acid and intense pulsed light in Chinese acne vulgaris patients. Photodermatol Photoimmunol Photomed. 2013;29(2):90–96. doi: 10.1111/phpp.12031. [DOI] [PubMed] [Google Scholar]
- Kang A, Lyons A, Herrmann J et al. Treatment of moderate-to-severe facial acne vulgaris with solid-state fractional 589/1,319-nm laser. J Clin Aesthet Dermatol. 2019;12(3):28–31. [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhu J, Zhang Y et al. Isotretinoin plus 420 nm intense pulsed light versus isotretinoin alone for the treatment of acne vulgaris: a randomized, controlled study of efficacy, safety, and patient satisfaction in Chinese subjects. Lasers Med Sci. 2021;36(3):657–665. doi: 10.1007/s10103-020-03113-z. [DOI] [PubMed] [Google Scholar]
- Kwon HH, Park HY, Choi SC et al. Novel device-based acne treatments: comparison of a 1450-nm diode laser and microneedling radiofrequency on mild-to-moderate acne vulgaris and seborrhoea in Korean patients through a 20-week prospective, randomized, split-face study. J Eur Acad Dermatol Venereol. 2018;32(4):639–644. doi: 10.1111/jdv.14714. [DOI] [PubMed] [Google Scholar]
- Kawashima M, Sato S, Furukawa F et al. Twelve-week, multicenter, placebo-controlled, randomized, double-blind, parallel-group, comparative phase II/III study of benzoyl peroxide gel in patients with acne vulgaris: A secondary publication. J Dermatol. 2017;44(7):774–782. doi: 10.1111/1346-8138.13798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima M, Hashimoto H, Alio Sáenz AB et al. Is benzoyl peroxide 3% topical gel effective and safe in the treatment of acne vulgaris in Japanese patients? a multicenter, randomized, double-blind, vehicle-controlled, parallel-group study. J Dermatol. 2014;41(9):795–801. doi: 10.1111/1346-8138.12580. [DOI] [PubMed] [Google Scholar]
- Jung JY, Kwon HH, Yeom KB et al. Clinical and histological evaluation of 1% nadifloxacin cream in the treatment of acne vulgaris in Korean patients. Int J Dermatol. 2011;50(3):350–357. doi: 10.1111/j.1365-4632.2010.04701.x. [DOI] [PubMed] [Google Scholar]
- Callender VD, Young CM, Kindred C et al. Efficacy and safety of clindamycin phosphate 1.2% And tretinoin 0.025% Gel for the treatment of acne and acne-induced post-inflammatory hyperpigmentation in patients with skin of color. J Clin Aesthet Dermatol. 2012;5(7):25–32. [PMC free article] [PubMed] [Google Scholar]
- Schmidt N, Gans EH. Clindamycin 1.2% Tretinoin 0.025% Gel versus clindamycin gel treatment in acne patients: a focus on Fitzpatrick skin types. J Clin Aesthet Dermatol. 2011;4(6):31–40. [PMC free article] [PubMed] [Google Scholar]
- Taylor SC, Cook-Bolden FE, McMichael A et al. Efficacy, safety, and tolerability of topical dapsone gel, 7.5% For treatment of acne vulgaris by Fitzpatrick skin phototype. J Drugs Dermatol. 2018;17(2):160–167. [PubMed] [Google Scholar]
- Draelos ZD, Rodriguez DA, Kempers SE et al. Treatment response with once-daily topical dapsone gel, 7.5% For acne vulgaris: subgroup analysis of pooled data from two randomized, double-blind studies. J Drugs Dermatol. 2017;16(6):591–598. [PubMed] [Google Scholar]
- How KN, Lim PY, Wan Ahmad Kammal WSL et al. Efficacy and safety of Jessner’s solution peel in comparison with salicylic acid 30% peel in the management of patients with acne vulgaris and postacne hyperpigmentation with skin of color: a randomized, double-blinded, split-face, controlled trial. Int J Dermatol. 2020;59(7):804–812. doi: 10.1111/ijd.14948. [DOI] [PubMed] [Google Scholar]
- Kaminaka C, Uede M, Matsunaka H et al. Clinical evaluation of glycolic acid chemical peeling in patients with acne vulgaris: a randomized, double-blind, placebo-controlled, split-face comparative study. Dermatol Surg. 2014;40(3):314–322. doi: 10.1111/dsu.12417. [DOI] [PubMed] [Google Scholar]
- Kwon HH, Lee JB, Yoon JY et al. The clinical and histological effect of home-use, combination blue-red LED phototherapy for mild-to-moderate acne vulgaris in Korean patients: a double-blind, randomized controlled trial. Br J Dermatol. 2013;168(5):1088–1094. doi: 10.1111/bjd.12186. [DOI] [PubMed] [Google Scholar]
- Kim HK, Yeo IK, Li K et al. Topical epidermal growth factor for the improvement of acne lesions: a randomized, double-blinded, placebo-controlled, split-face trial. Int J Dermatol. 2014;53(8):1031–1036. doi: 10.1111/ijd.12488. [DOI] [PubMed] [Google Scholar]
- Park KY, Kim JY, Hyun MY et al. 1,213 cases of treatment of facial acne using indocyanine green and intense pulsed light in Asian Skin. Biomed Res Int. 2015;2015:596161. doi: 10.1155/2015/596161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SJ, Goo JW, Shin J et al. Use of fractionated microneedle radiofrequency for the treatment of inflammatory acne vulgaris in 18 Korean patients. Dermatol Surg. 2012;38(3):400–405. doi: 10.1111/j.1524-4725.2011.02267.x. [DOI] [PubMed] [Google Scholar]
- Gan EY, Koh W-P, Jin AZ et al. Isotretinoin is safe and efficacious in Asians with acne vulgaris. J Dermatolog Treat. 2013;24(5):387–391. doi: 10.3109/09546634.2012.672708. [DOI] [PubMed] [Google Scholar]
- Tao S-Q, Li F, Cao L et al. Low-dose topical 5-aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Cell Biochem Biophys. 2015;73(3):701–706. doi: 10.1007/s12013-015-0627-3. [DOI] [PubMed] [Google Scholar]
- Alexis AF, Burgess C, Callender VD et al. The efficacy and safety of topical dapsone gel, 5% for the treatment of acne vulgaris in adult females with skin of color. J Drugs Dermatol. 2016;15(2):197–204. [PubMed] [Google Scholar]
- Dong Y, Zhou G, Chen J et al. A new LED device used for photodynamic therapy in treatment of moderate to severe acne vulgaris. Photodiagnosis Photodyn Ther. 2016;13:188–195. doi: 10.1016/j.pdpdt.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Asayama-Kosaka S, Akilov OE, Kawana S. Photodynamic therapy with 5% δ-aminolevulinic acid is safe and effective treatment of acne vulgaris in Japanese patients. Laser Ther. 2014;23(2):115–120. doi: 10.5978/islsm.14-OR-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brownell L, Geen S, E Y, Lee W-L. A clinical study evaluating the efficacy of topical bakuchiol (up256) cream on facial acne. J Drugs Dermatol. 2021;20(3):307–310. doi: 10.36849/JDD.5655. [DOI] [PubMed] [Google Scholar]
- Cook-Bolden FE, Weinkle SH, Guenin E et al. Novel tretinoin 0.05% Lotion for once-daily treatment of moderate-to-severe acne vulgaris in a hispanic population. J Drugs Dermatol. 2019;18(1):32–38. [PubMed] [Google Scholar]
- Lain E, Day D, Harper J et al. Tretinoin 0.05% Lotion for the once-daily treatment of moderate-to-severe acne vulgaris: impact of gender and race on efficacy and safety. J Drugs Dermatol. 2019;18(11):1128–1138. [PubMed] [Google Scholar]
- Alexis AF, Cook-Bolden F, Lin T. Treatment of moderate-to-severe acne vulgaris in a Hispanic population: a post-hoc analysis of the efficacy and tolerability of clindamycin 1.2%/Benzoyl peroxide 3.75% Gel. J Clin Aesthet Dermatol. 2017;10(6):36–43. [PMC free article] [PubMed] [Google Scholar]
- Cook-Bolden FE. Treatment of moderate to severe acne vulgaris in a Hispanic population: a post-hoc analysis of efficacy and tolerability of clindamycin phosphate 1.2%/benzoyl peroxide 2.5% gel. J Drugs Dermatol. 2012;11(4):455–459. [PubMed] [Google Scholar]
- Callender VD. Fitzpatrick skin types and clindamycin phosphate 1.2%/benzoyl peroxide gel: efficacy and tolerability of treatment in moderate to severe acne. J Drugs Dermatol. 2012;11(5):643–648. [PubMed] [Google Scholar]
- Alexis AF, Cook-Bolden FE, York JP. Adapalene/benzoyl peroxide gel 0.3%/2.5%: A safe and effective acne therapy in all skin phototypes. J Drugs Dermatol. 2017;16(6):574–581. [PubMed] [Google Scholar]
- Amar L, Kircik LH. Treatment of moderate acne vulgaris in Fitzpatrick skin type v or vi: efficacy and tolerability of fixed combination clindamycin phosphate 1.2%/Benzoyl peroxide 3.75% Gel. J Drugs Dermatol. 2018;17(10):1107–1112. [PubMed] [Google Scholar]
- DuBois J, Ong GCW, Petkar G et al. Patient-reported outcomes in acne patients with skin of color using adapalene 0.3%-Benzoyl peroxide 2.5%: A prospective real-world study. J Drugs Dermatol. 2019;18(5):514. [PubMed] [Google Scholar]
- Ma Y, Liu Y, Wang Q et al. Prospective study of topical 5-aminolevulinic acid photodynamic therapy for the treatment of severe adolescent acne in Chinese patients. J Dermatol. 2015;42(5):504–507. doi: 10.1111/1346-8138.12836. [DOI] [PubMed] [Google Scholar]
- Ma L, Xiang L-H, Yu B et al. Low-dose topical 5-aminolevulinic acid photodynamic therapy in the treatment of different severity of acne vulgaris. Photodiagnosis Photodyn Ther. 2013;10(4):583–590. doi: 10.1016/j.pdpdt.2013.06.007. [DOI] [PubMed] [Google Scholar]
- Wang H-W, Lv T, Zhang L-L et al. Prospective study of topical 5-aminolevulinic acid photodynamic therapy for the treatment of moderate to severe acne vulgaris in Chinese patients. J Cutan Med Surg. 2012;16(5):324–333. doi: 10.1177/120347541201600509. [DOI] [PubMed] [Google Scholar]
- El-Latif AAA, Hassan FAA, Elshahed AR. Intense pulsed light versus benzoyl peroxide 5% gel in treatment of acne vulgaris. Lasers Med Sci. 2014;29(3):1009–1015. doi: 10.1007/s10103-013-1440-0. [DOI] [PubMed] [Google Scholar]
- Mohanan S, Parveen B, Annie Malathy P et al. Use of intense pulse light for acne vulgaris in Indian skin—a case series. Int J Dermatol. 2012;51(4):473–476. doi: 10.1111/j.1365-4632.2011.05295.x. [DOI] [PubMed] [Google Scholar]
- Lee G-S. Inflammatory acne in the asian skin type iii treated with a square pulse, time resolved spectral distribution ipl system: a preliminary study. Laser Ther. 2012;21(2):105–111. doi: 10.5978/islsm.12-OR-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Lee EG, Lee HJ. Assessment of treatment efficacy and sebosuppressive effect of fractional radiofrequency microneedle on acne vulgaris. Lasers Surg Med. 2013;45(10):639–647. doi: 10.1002/lsm.22200. [DOI] [PubMed] [Google Scholar]
- Suh DH, Park TJ, Jeong JY et al. Photothermal therapy using gold nanoparticles for acne in Asian patients: a preliminary study. Dermatol Ther. 2021;34(3):e14918. doi: 10.1111/dth.14918. [DOI] [PubMed] [Google Scholar]
- Isoda K, Takagi Y, Endo K et al. Effects of washing of the face with a mild facial cleanser formulated with sodium laureth carboxylate and alkyl carboxylates on acne in Japanese adult males. Skin Res Technol. 2015;21(2):247–253. doi: 10.1111/srt.12183. [DOI] [PubMed] [Google Scholar]
- An J-S, Kim J-E, Lee D-H et al. 0.5% Liposome-encapsulated 5-aminolevulinic acid (ALA) photodynamic therapy for acne treatment. J Cosmet Laser Ther. 2011;13(1):28–32. doi: 10.3109/14764172.2011.552613. [DOI] [PubMed] [Google Scholar]
- https://www.nlm.nih.gov/research/umls/sourcereleasedocs/current/NCI_CTCAE_5/index.html United States Department of Health and Human Services. UMLS Metathesaurus - NCI_CTCAE_5 (Common Terminology Criteria for Adverse Events 5.0) - Synopsis. 2017. Accessed 4 Jul 2021.
- Govender K, Dlova N. Acne in ethnic skin - part 2: approach to management in patients with skin of colour. Dermatology in practice. 2017;23(2):38–44. [Google Scholar]
- Tu P, Li GQ, Zhu XJ et al. A comparison of adapalene gel 0.1% vs. tretinoin gel 0.025% in the treatment of acne vulgaris in China. J Eur Acad Dermatol Venereol. 2001;15(Suppl 3):31–36. doi: 10.1046/j.0926-9959.2001.00010.x. [DOI] [PubMed] [Google Scholar]
- Goh CL, Tang MBY, Briantais P et al. Adapalene gel 0.1% is better tolerated than tretinoin gel 0.025% among healthy volunteers of various ethnic origins. J Dermatolog Treat. 2009;20(5):282–288. doi: 10.1080/09546630902763164. [DOI] [PubMed] [Google Scholar]
- Saple DG, Torsekar RG, Pawanarkar V et al. An open study to evaluate the efficacy and safety of tazarotene gel (0.1%) in acne vulgaris. Indian J Dermatol Venereol Leprol. 2004;70(2):92–95. [PubMed] [Google Scholar]
- Hashimoto Y, Suga Y, Mizuno Y et al. Salicylic acid peels in polyethylene glycol vehicle for the treatment of comedogenic acne in Japanese patients. Dermatol Surg. 2008;34(2):276–279. doi: 10.1111/j.1524-4725.2007.34055.x. discussion 279. [DOI] [PubMed] [Google Scholar]
- Dainichi T, Ueda S, Imayama S et al. Excellent clinical results with a new preparation for chemical peeling in acne: 30% salicylic acid in polyethylene glycol vehicle. Dermatol Surg. 2008;34(7):891–899. doi: 10.1111/j.1524-4725.2008.34174.x. discussion 899. [DOI] [PubMed] [Google Scholar]
- Choi CW, Lee DH, Kim HS et al. The clinical features of late onset acne compared with early onset acne in women. J Eur Acad Dermatol Venereol. 2011;25(4):454–461. doi: 10.1111/j.1468-3083.2010.03813.x. [DOI] [PubMed] [Google Scholar]
- Taylor K. Why improving inclusion and diversity in clinical trials should be a research priority. Deloitte United States. 2020. https://www2.deloitte.com/us/en/blog/health-care-blog/2019/why-improving-inclusion-and-diversity-in-clinical-trials-should-be-a-research-priority.html Accessed 22 Sep 2021.
- https://clinicalresearchpathways.org/diversity/ Clinical Research Pathways website. Diversity in clinical trials. 2020. Accessed 22 Sep 2021.
- Liu C-H, Shieh C-S, Huang T-L et al. Evaluating the risk factors of post inflammatory hyperpigmentation complications with nd-yag laser toning using lasso-based algorithm. Applied Sciences. 2020;10:2049. [Google Scholar]
- Leal-Silva H. Predicting the risk of postinflammatory hyperpigmentation: the palmar creases pigmentation scale. J Cosmet Dermatol. 2021;20(4):1263–1270. doi: 10.1111/jocd.13968. [DOI] [PubMed] [Google Scholar]
- Hall HI, Rogers JD. Sun protection behaviors among African Americans. Ethn Dis. 1999;9(1):126–131. [PubMed] [Google Scholar]
- Adityan B, Kumari R, Thappa DM. Scoring systems in acne vulgaris. Indian J Dermatol Venereol Leprol. 2009;75(3):323–326. doi: 10.4103/0378-6323.51258. [DOI] [PubMed] [Google Scholar]
- Barratt H, Hamilton F, Car J et al. Outcome measures in acne vulgaris: systematic review. Br J Dermatol. 2009;160(1):132–136. doi: 10.1111/j.1365-2133.2008.08819.x. [DOI] [PubMed] [Google Scholar]
- www.fda.gov/downloads/Drugs/Guidances/UCM071292.pdf United States Food and Drug Administration website. Guidance for industry acne vulgaris: developing drugs for treatment. 2018. 4 Jul 2021.
- Del Rosso JQ. Defining criteria used to evaluate response to treatment of acne vulgaris. Cutis. 2006;78(2):117–121. [PubMed] [Google Scholar]