Abstract
Accumulating literature has linked poverty to brain structure and function, particularly in affective neural regions; however, few studies have examined associations with structural connections or the importance of developmental timing of exposure. Moreover, prior neuroimaging studies have not used a proximal measure of poverty (i.e., material hardship, which assesses food, housing, and medical insecurity) to capture the lived experience of growing up in harsh economic conditions. The present investigation addressed these gaps collectively by examining the associations between material hardship (ages 1, 3, 5, 9, and 15 years) and white matter connectivity of frontolimbic structures (age 15 years) in a low-income sample. We applied probabilistic tractography to diffusion imaging data collected from 194 adolescents. Results showed that material hardship related to amygdala–prefrontal, but not hippocampus–prefrontal or hippocampus–amygdala, white matter connectivity. Specifically, hardship during middle childhood (ages 5 and 9 years) was associated with greater connectivity between the amygdala and dorsomedial pFC, whereas hardship during adolescence (age 15 years) was related to reduced amygdala–orbitofrontal (OFC) and greater amygdala–subgenual ACC connectivity. Growth curve analyses showed that greater increases of hardship across time were associated with both greater (amygdala–subgenual ACC) and reduced (amygdala–OFC) white matter connectivity. Furthermore, these effects remained above and beyond other types of adversity, and greater hardship and decreased amygdala–OFC connectivity were related to increased anxiety and depressive symptoms. Results demonstrate that the associations between material hardship and white matter connections differ across key prefrontal regions and developmental periods, providing support for potential windows of plasticity for structural circuits that support emotion processing.
INTRODUCTION
In the United States, 16.2%, or approximately 11.9 million, children and adolescents live below the poverty line, and 32% are near poor (within 200% of the poverty line; Semega, Kollar, Creamer, & Mohanty, 2019). Children who grow up in poverty are at an increased risk for adverse outcomes, including behavioral problems, psychopathology, and delayed cognitive development (Brooks-Gunn & Duncan, 1997). Research has shown that neural regions involved in emotion processing such as the pFC, amygdala, and hippocampus are implicated in the effects of poverty and adverse developmental outcomes (Merz, Tottenham, & Noble, 2018; Kim et al., 2013; Luby et al., 2013; Noble, Houston, Kan, & Sowell, 2012; Hanson, Chandra, Wolfe, & Pollak, 2011). Despite evidence linking poverty to brain function (e.g., functional activation and connectivity) and structure (e.g., cortical volume and surface area; Farah, 2017; Johnson, Riis, & Noble, 2016; Brito & Noble, 2014), much less work has focused on its association with structural connectivity (i.e., white matter tracts that facilitate communication between distinct neural structures) in adolescence. Moreover, although the effects of economic hardship differ across development (Green, Stritzel, Smith, Popham, & Crosnoe, 2018; Duncan, Yeung, Brooks-Gunn, & Smith, 1998), most existing investigations on neural correlates of poverty have not considered the critical importance of developmental timing in the experience of economic hardship (Hyde et al., 2020). Thus, more research is needed to examine how economic hardship across development relates to adolescent white matter tracts that link emotion processing regions.
Although most studies examining frontolimbic white matter structures using diffusion MRI (dMRI) have focused on density of major white matter tracts (e.g., fractional anisotropy of the superior longitudinal fasciculus; Rosen, Sheridan, Sambrook, Meltzoff, & McLaughlin, 2018; Noble, Korgaonkar, Grieve, & Brickman, 2013), there has been no examination of white matter microstructures using probabilistic tractography method. As compared with conventional deterministic approaches to tracking white matter fibers, probabilistic tractography has been found to be more sensitive in modeling complex multiple-fiber crossings in diffusion-based data (Behrens, Berg, Jbabdi, Rushworth, & Woolrich, 2007). Using this method to estimate the maximum likelihood of white matter connectivity between seed and target ROIs (Greening & Mitchell, 2015; Behrens et al., 2007), our previous investigation (Goetschius et al., 2019) drew on evidence from tracer studies in nonhuman primates to identify the presence of white matter fibers connecting the amygdala to numerous pFC regions. Results demonstrated that white matter connectivity is particularly widespread from the amygdala to the subgenual ACC (sgACC) BA 25, orbitofrontal (OFC) BA 11 and BA 47, and dorsomedial pFC (dmPFC) BA 10 regions of the pFC—regions that are all implicated in emotion processing (Barbas, 2015; Ghashghaei, Hilgetag, & Barbas, 2007; Barbas, Saha, Rempel-Clower, & Ghashghaei, 2003). Studies have also identified structural connections between these prefrontal regions and the hippocampus (Barbas & Blatt, 1995) as well as robust white matter connections between the hippocampus and amygdala (Colnat-Coulbois et al., 2010). These findings indicate that further examination of white matter connectivity within these frontolimbic circuits is necessary to better understand affective processes relating to socioeconomic hardship.
Though more research is needed to examine the potential impacts of poverty on white matter microstructure across the corticolimbic circuit, it is also important to consider the developmental timing of when children experienced poverty. Recent evidence suggests that activities in specific brain regions (e.g., amygdala) are more sensitive to disadvantaged environment during early childhood whereas others (e.g., pFC) during adolescence (Gard et al., 2021). Although the amygdala and hippocampus develop rapidly during early childhood, pFC undergoes an especially protracted development lasting through adolescence (Blakemore, 2012; Giedd & Rapoport, 2010; Lenroot & Giedd, 2006). Equally important, stress during later childhood and adolescence may also contribute to structural development of frontolimbic regions, especially considering that white matter proliferates in density and mass through adolescence and into adulthood (Giedd et al., 1999). Thus, it is important to consider the timing of exposure to poverty as both the brain and social ecology surrounding poverty are different during early childhood versus adolescence.
Moreover, though the timing of exposure to poverty may be important, the total exposure to poverty across time and how poverty changes over time may have distinct effects. A wealth of literature demonstrates that the cumulative effect and duration of exposure to poverty influences many child developmental outcomes (National Institute of Child Health and Human Development Early Child Care Research Network, 2005; Korenman, Miller, & Sjaastad, 1995). Furthermore, research has found that changes in poverty across time (i.e., trajectory) may be important consideration for its relation to mental health (McLeod & Shanahan, 1996). Thus, improvements or deepening of poverty over time produces qualitatively different experiences for children and are important considerations when examining poverty effects on neural development.
In addition to understanding the impact of timing and cumulative exposure to poverty, work on the neuroscience of poverty should consider measures that capture the lived experience of economic hardship. Measuring whether food, residential, and medical needs are being met (i.e., material hardship) characterizes the immediate challenges and impacts of poverty (Nelson, 2011; Gershoff, Aber, Raver, & Lennon, 2007). Moreover, the effect of family income on child development may vary by region (e.g., cost of living) and other attributes of the family (e.g., accumulated wealth, family support; Gershoff et al., 2007). Although material hardship has been linked to children’s behavioral outcomes (Zilanawala & Pilkauskas, 2012; Gershoff et al., 2007), only one study thus far has examined the association between material hardship with white matter density in school-aged children (Lichtin et al., 2021), and no known study has examined developmental timing effects of material hardship, or how it may relate to frontolimbic white matter connections quantified using probabilistic tractography in adolescents.
This study addressed two aims using open-science preregistered analyses: (1) to examine the association between cumulative material hardship and frontolimbic white matter connectivity (amygdala–pFC, hippocampus–pFC, and hippocampus–amygdala) and (2) to assess longitudinal/developmentally specific associations of material hardship from childhood to adolescence with adolescent white matter connectivity. We predicted that there would be negative associations between cumulative material hardship and white matter connectivity given the existing literature documenting decreased white matter density associated with poverty (Brito & Noble, 2014) and that the associations between material hardship and white matter would differ across developmental periods. Because few studies have examined the developmental timing of poverty with brain structures and both early and late childhood experiences are important for brain development, we did not have a directional hypothesis for timing effects. Finally, in an exploratory analysis, we examined links among material hardship, white matter connectivity, and internalizing symptoms to anchor our brain findings to adolescent affective functioning. Specifically, we assessed the associations between material hardship with symptoms of anxiety and depression in adolescence and the associations between white matter connectivity with anxiety and depressive symptoms.
METHODS
Sample
We addressed these aims in a sample of families with identities and experiences that have been underrepresented in neuroimaging research (e.g., marginalized or non-Western, educated, industrialized, rich, and democratic samples; Falk et al., 2013; Henrich, Heine, & Norenzayan, 2010). Participants were recruited from the Fragile Families and Child Wellbeing Study, a population-based sample of 4,898 children born in large U.S. cities (population over 200,000), with an oversampling of nonmarital births, which led to a high representation of low-income and minority families (Reichman, Teitler, Garfinkel, & McLanahan, 2001). Longitudinal data were collected when the child was 1, 3, 5, 9, and 15 years old through in-home visits and phone calls (Reichman et al., 2001). At 15 years of age, a subsample of families was invited to participate in the Study of Adolescent Neural Development. Out of 237 teens, 52.7% were female with an average age of 15.9 years old, 76% identified as Black, and more than 54% reported family income below $40,000. After exclusions because of missing data and poor imaging quality, this study included 189 participants with amygdala–seed and 191 participants with hippocampus–seed (see Table 1 for characteristics of full sample and included sample). Statistical tests comparing demographic characteristics (age, pubertal development, sex, race, and income) of included sample from the full sample showed no significant differences between the groups (p > .5).
Table 1.
Sample Exclusions and Comparisons
| Reason for Exclusion | Excluded (Amygdala–Seed) | Excluded (Hippocampus–Seed) |
|---|---|---|
| Refused MRI | 16 | 16 |
| Exceeded MRI table weight limit | 3 | 3 |
| Medical restriction | 1 | 1 |
| Braces or other metal in body | 7 | 7 |
| Risk of pregnancy | 1 | 1 |
| Excluded for autism spectrum disorder diagnosis | 2 | 2 |
| Did not complete dMRI scan | 11 | 11 |
| Preprocessing outliers >5% in diffusion dataa | 1 | 3 |
| No probabilistic tractography model convergenceb | 5 | 2 |
| Less than 70% of voxels in pFC masks | 1 | 0 |
| Include vs. Full Sample Comparisonsc | |||
|---|---|---|---|
| Full Sample (n = 237) | Included Sample (Amygdala–Seed; n = 189) | Included Sample (Hippocampus–Seed; n = 191) | |
| Age | M = 15.88 years | M = 15.84 years | M = 15.85 years |
| SD = 0.54 years | SD = 0.52 years | SD = 0.53 years | |
| Pubertal stage | M = 3.24 | M = 3.26 | M = 3.24 |
| SD = 0.59 years | SD = 0.58 years | SD = 0.59 years | |
| Sex | F = 125 (52.7%) | F = 102 (54.0%) | F = 102 (53.4%) |
| M = 112 (47.3%) | M = 87 (46.0%) | M = 89 (46.6%) | |
| Race | Black = 181 (76.4%) | Black = 146 (77.2%) | Black = 148 (77.5%) |
| White = 32 (13.5%) | White = 22 (11.6%) | White = 21 (11.0%) | |
| Hispanic/Latino = 13 (5.5%) | Hispanic/Latino = 12 (6.3%) | Hispanic/Latino = 12 (6.3%) | |
| Other/multiracial = 11 (4.6%) | Other/multiracial = 9 (4.8%) | Other/multiracial = 10 (5.2%) | |
| Annual income | <$15,000 = 57 (24.1%) | <$15,000 = 48 (25.4%) | <$15,000 = 47 (24.6%) |
| $15,000–39,999 = 70 (29.5%) | $15,000–39,999 = 57 (30.2%) | $15,000–39,999 = 58 (30.4%) | |
| $40,000–69,999 = 58 (24.5%) | $40,000–69,999 = 43 (22.8%) | $40,000–69,999 = 44 (23.0%) | |
| >$70,000 = 51 (21.5%) | >$70,000 = 40 (21.2%) | >$70,000 = 41 (21.5%) | |
| Missing/not reported = 1 (0.4%) | Missing/not reported = 1 (0.5%) | Missing/not reported = 1 (0.5%) | |
Outliers detected using dMRI method from Mrtrix (v.3.0.R3). Slices with average intensity 4 SDs or more lower than predicted by eddy’s Gaussian process model were marked as outliers and replaced with model predictions. One participant was excluded from sample due to having more than 5% of total slices replaced.
Zero value connectivity coefficient for at least one seed–target pair.
Demographic characteristics did not significantly differ across samples: age, F(2, 614) = 0.13, p = .878; pubertal stage, F(2, 614) = 0.08, p = .923; sex, F(2, 614) = 0.06, p = .941; race, F(2, 614) = 0.277, p = .758; annual income, F(2, 611) = 0.064, p = .938.
Behavioral Measures
Material Hardship
Longitudinal measurements of material hardship were collected in five waves. During assessments of children ages 1, 3, 5, 9, and 15 years, caregivers indicated whether or not they experienced housing, utility, food, medical, and financial hardship within the past year. The primary caregiver reported yes (1) or no (0) on each of the following eight items: (1) received free meals, (2) did not pay full rent/mortgage, (3) evicted for not paying full rent/mortgage, (4) did not pay full gas/oil/electric bill, (5) borrowed money from family/friends to pay bills, (6) moved in with people because of financial problems, (7) stayed in place not meant for regular housing, and (8) did not receive medical care. These items were drawn from the 1996 Survey of Income and Program Participation; the 1997 and 1999 New York City Social Indicators Survey; and the 1999 Study of Work, Welfare, and Family Well-Being of Iowa families on Iowa’s assistance program and are comparable to past investigations on material hardship and poverty (Bauman, 1999; Mayer & Jencks, 1989). Responses were collected from the mother if the child lived with their mother at least half of the time. Cumulative material hardship score was measured by summing endorsed material hardship items across all waves. Material hardship during early (ages 1 and 3 years) and middle (ages 5 and 9 years) childhood were computed by averaging the age-specific material hardship scores within the developmental period (see Table 2 for zero-order correlations of all hardship variables).
Table 2.
Zero-Order Correlations of Material Hardship Predictors
| Variable | M | SD | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| 1. Hardship at age 1 | 1.02 | 1.30 | ||||
| 2. Hardship at age 3 | 1.06 | 1.33 | .56** | |||
| 3. Hardship at age 5 | 1.27 | 1.40 | .43** | .51** | ||
| 4. Hardship at age 9 | 1.55 | 1.46 | .31** | .40** | .44** | |
| 5. Hardship at age 15 | 1.30 | 1.50 | .27** | .36** | .30** | .53** |
M and SD are used to represent mean and standard deviation, respectively.
p < .01.
Internalizing Symptoms
Anxiety symptoms were measured separately using parent-only and child-only report on the Screen for Child Anxiety Related Disorders (Birmaher et al., 1997) at child age of 15 years. Anxiety was measured using a 3-point scale (0 = almost never, 1 = sometimes, 2 = often), and scores range from 0 to 76 (α = .92). Depressive symptoms were measured separately using parent-only and child-only report on the Mood and Feelings Questionnaire (Angold, Costello, Messer, & Pickles, 1995) at child age of 15 years. Depression was measured using a 3-point scale (0 = not true, 1 = sometimes, 2 = true), and scores range from 0 to 68 (α = .91). Scores on both scales were computed by summing all responses endorsed, with higher scores indicating greater report of adolescent anxiety and depression symptoms.
Covariates
The following measures were added as covariates in subsequent sensitivity analyses: birth city, maternal education, child race, sex, pubertal age, and family structure. Birth city was added as a covariate to account for any confounding sampling differences and was based on the location where child was born and the first interview with mother was conducted. In this study, birth city was classified as either Detroit, MI; Toledo, OH; Chicago, IL; or other (i.e., Baltimore, Indianapolis, Jacksonville, Pittsburgh). Cities under “other” were categorized under one singular variable to minimize the number of variables, and three dummy-coded variables were created to reflect birth city, with Detroit set as the reference group. Maternal education at child’s birth was measured using a 4-point scale (0 = less than high school, 1 = high school or equivalent, 2 = some college/technical school, 3 = college or graduate school) and was added as a covariate to account for any confounding differences stemming from parental education. Ethnoracial identity, a socially constructed category, was utilized as a covariate to adjust for the potential effects of structural or interpersonal racism and the various ways in which these experiences shape development (e.g., neighborhood residence, school access). Ethnoracial identity was measured using three dummy-coded variables to reflect Black (reference variable), White, Hispanic, and other/multiracial and was self-reported at 15 years of age. In the event of missing reports, parent-reported race/ethnicity was utilized (n = 8). Sex was measured through parent report at child age of 1 year using two categories: 1 = male and 0 = female. Pubertal age was measured via youth report at 15 years of age on the Pubertal Development Scale (Petersen, Crockett, Richards, & Boxer, 1988) that measured changes in child height, body hair, skin, facial hair and voice (male participants only), and breast development and menarche (female participants only). Responses were coded on a 4-point scale (1 = no development to 4 = completed development); score was a sum of all items endorsed. Pubertal development was treated as a covariate to account for differences in neural structures stemming from differences in pubertal-related maturation (Giedd et al., 1999). Family structure was included as a covariate to consider household composition and differences in family function between 0 = single and 1 = two-parent families (McLanahan & Sandefur, 1994). A household was considered a two-parent household when the biological father was cohabitating with the biological mother and child at the time of the child’s birth.
Additionally, to account for stress-related factors that may be related to white matter connectivity, as well as examine mechanisms isolated to material hardship above and beyond the effects of other measures of poverty, we adjusted for (1) childhood exposure to violence and social deprivation and (2) annual household income in subsequent sensitivity analyses. Violence exposure and social deprivation were measured using composite standardized scores of exposure at ages 3, 5, and 9 years (Hein et al., 2020). Violence exposure score was based on: (1) parent responses on child physical and emotional abuse items in the Parent–Child Conflict Tactics Scale (Straus, Hamby, Finkelhor, Moore, & Runyan, 1998), (2) child’s exposure to or victimization of violence in the neighborhood report (Zhang & Anderson, 2010), and (3) maternal report of intimate partner violence (physical, emotional, or sexual) in the home (Hunt, Berger,& Slack, 2017). Social deprivation composite score was based on (1) parent responses on child physical and emotional neglect on the Parent–Child Conflict Tactics Scale (Straus et al., 1998) and (2) report of neighborhood social cohesion (Donnelly et al., 2016; Morenoff, Sampson, & Raudenbush, 2001). In both measures, observation was coded as missing if child did not live with parent for at least half the time at each wave. Scores on each measure were first standardized, and z scores across each dimension (violence exposure; social deprivation) across each wave (ages 3, 5, and 9 years) were then summed. Annual household income was reported by primary caregiver at each wave. In instances where annual household income was not reported, the variable was imputed using the partner’s report (if cohabitating) or regression-based imputation if neither caregiver reported household income.
MR Measures
MRI Acquisition
As described in other related publications (Goetschius et al., 2019; 2020; Hein et al., 2018), MRI images were acquired using 3 T GE Discovery MR750 scanner with eight-channel head coil at University of Michigan Functional MRI Laboratory. Head movement was limited through the use of head paddings and detailed instructions provided to participants. T1-weighted gradient-echo images were taken before subsequent scans using same field of view (repetition time = 12 msec, echo time = 5 msec, inversion time = 500 msec, flip angle = 15°, field of view = 26 cm, slice thickness = 1.44 mm, 256 × 192 matrix, 110 slices).
dMRI Processing
dMRI images that were used to identify the microstructural properties of white matter tracts (Jones, Knösche, & Turner, 2013) were captured using spin-echo EPI diffusion sequence. Scan parameters used were as follows: repetition time = 7250 msec, minimum echo time, 128 × 128 acquisition matrix, field of view = 22 cm, 3-mm thick slices (no gap), 40 slices acquired using alternating–increasing order, b value = 1000 s/mm2, 64 nonlinear directions; five b = 0 s/mm2 T2 images (b0) acquired. dMRI data from this sample were utilized in prior work (Goetschius et al., 2019, 2020; Hein et al., 2018), and we used the same processing procedures from previous publications (Goetschius et al., 2019, 2020). dMRI images were visually inspected for quality. Slices with an average intensity lower than 4 SDs or more, as predicted by eddy’s Gaussian, were marked as outliers and replaced with model predictions (Andersson, Graham, Zsoldos, & Sotiropoulos, 2016). Participants were excluded if more than 5% of slices were replaced (n = 1). For included participants, 0–4.35% of slices (median of 0.43%) were replaced. Images for 10 participants with most replaced slices were further visually inspected to ensure there were no additional abnormal artifacts. White matter fibers were mapped using probabilistic tractography method. Probabilistic tractography utilizes a Bayesian algorithm to track pathways between neural regions by creating an individual-level density function representing the probability that specified seed region voxels will reach specified target region voxels (Greening & Mitchell, 2015; Behrens et al., 2007). Using this method, we quantified the likelihood of white matter connections between amygdala–pFC, hippocampus–pFC, and hippocampus–amygdala for each participant. Each lateral hemisphere of the amygdala and hippocampus was utilized as separate seed regions, and each lateral pFC region was utilized as separate targets. pFC targets were BA 9, BA 10, BA 11, BA 24, BA 25, BA 32, and BA 47, corresponding to medial and OFC regions that have been found to be particularly connected to the amygdala and hippocampus, consistent with previous Study of Adolescent Neural Development investigations (Goetschius et al., 2019) and nonhuman primates tracer studies (Barbas & Blatt, 1995).
Raw dMRI images in DICOM format were first converted to NIFTI format using MRIcron (dcm2niix – 2MAY2016) for off-line analysis using MRtrix (v.3.0.R3; Veraart et al., 2016) and FSL (v.5.0.9) FMRIB’s Diffusion Toolbox (v.3.0; Jenkinson, Beckmann, Behrens, Woolrich, & Smith, 2012). The NIFTI diffusion files were corrected for motion, eddy current, and signal dropout with MRTrix dwipreproc using FSL eddy (v.5.0.11; Andersson & Sotiropoulos, 2016).
Bedpostx was performed using standard settings (number of fibers modeled per voxel = 2, multiplicative factor weight = 1, burn in = 1000; Hernández et al., 2013) using Markov chain Monte Carlo sampling at individual voxel (Behrens et al., 2007); FLIRT registration (Jenkinson, Bannister, Brady, & Smith, 2002; Jenkinson & Smith, 2001) was performed to allow linear mapping between diffusion, standard, and structural spaces; probtrackx2 (nsamples per voxel = 5000, nsteps per sample = 2000, step length = 0.5 mm, curvature threshold = 0.2, fibthresh = 0.01, distthresh = 0.1; Hernández-Fernández et al., 2016) then computed the estimated probability of white matter connectivity between target and seed regions (Eickhoff et al., 2010; Behrens et al., 2003, 2007; Johansen-Berg et al., 2004). Individual masks for target and seed regions were then created using WFU Pick Atlas (v.3.0.5b; Maldjian, Laurienti, Kraft, & Burdette, 2003). After individual-level probabilistic tractography mapping was completed, resulting processed images were transformed to Montreal Neurological Institute space for group-level analysis. Each image was divided by the number of samples per voxel (5000), and an average seed image was created. Additionally, peak voxel representing the maximum probability of white matter connectivity between seed and target regions was identified using fslmaths (FMRIB). Amygdala–pFC white matter tracts were analyzed using FSL Version 5.9, whereas hippocampus–pFC and hippocampus–amygdala tracts were analyzed using identical scripts on updated FSL 6.0.
Processed dMRI yielded a likelihood coefficient that seed and target regions were connected. Higher coefficient indicated greater connectivity between seed and target regions. The five connections (within each pair) with the highest white matter connectivity coefficient values were included in subsequent analyses to simplify our model estimates (see Table 3 for zero-order correlations of all white matter connectivity metrics). Amygdala–pFC pairs that were included in our analyses are as follows: left amygdala–BA 25, right amygdala–BA 10, right amygdala–BA 11, right amygdala–BA 25, and right amygdala–BA 47 (Figure 1). The highest five hippocampus–pFC connections that were included in subsequent analyses are as follows: left hippocampus–BA 10, right hippocampus–BA 10, right hippocampus–BA 11, right hippocampus–BA 25, and right hippocampus–BA 47. Both right and left hippocampus–amygdala connectivity were included in our analyses.
Table 3.
Zero-Order Correlations of White Matter Connectivity (Amygdala–Seed)
| Variable | M | SD | 1 | 2 | 3 | 4 |
|---|---|---|---|---|---|---|
| 1. Right amygdala–BA 10 | 0.05 | 0.05 | ||||
| 2. Right amygdala–BA 11 | 0.08 | 0.07 | .51** | |||
| 3. Right amygdala–BA 25 | 0.09 | 0.07 | −.11 | −.24** | ||
| 4. Right amygdala–BA 47 | 0.10 | 0.08 | .23** | .27** | .13 | |
| 5. Left amygdala–BA 25 | 0.08 | 0.06 | −.18* | −.17* | .42** | .04 |
M and SD are used to represent mean and standard deviation, respectively.
p < .05.
p < .01.
Figure 1.
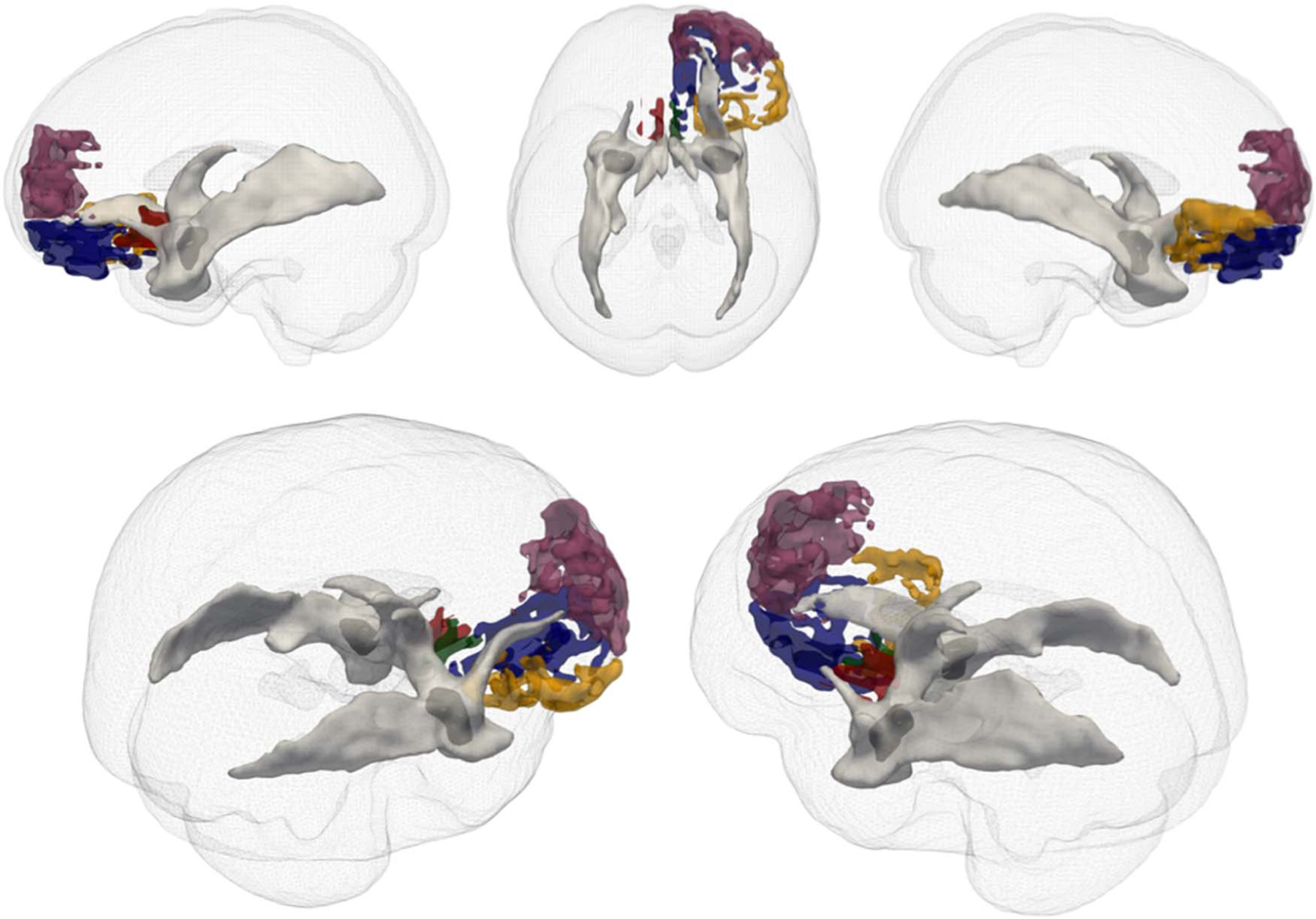
3-D renderings of amygdala to specific pFC targets. Seed amygdala targets in dark gray. pFC targets: left sgACC BA 25 (red); right dmPFC BA 10 (plum); right OFC BA 11 (blue); right sgACC BA 25 (green); right OFC BA 47 (yellow). White matter is illustrated in light gray. White matter in figure depicts all tracts originating from seed amygdala region for illustration purposes.
Statistical Analysis
Preregistered Analyses
Study measures and statistical analyses were preregistered at the Open Science Framework (https://osf.io/c3tf8). Three series of multiple regression models were performed to address our study aims. First, a cumulative score of material hardship was used in three separate multiple regression models to predict amygdala–pFC, hippocampus–pFC, and hippocampus–amygdala white matter connectivity at age 15 years. Second, to examine differential developmental associations between material hardship and white matter connectivity, we performed five multiple regression analyses (one for each prefrontal region) using material hardship summed across developmental stages (early: ages 1 and 3 years; middle: ages 5 and 9 years; adolescent: age 15 years) as separate predictors within the same model. Third, material hardship at each developmental age (1, 3, 5, 9, and 15 years) was included as separate predictors within the same five regression models. To correct for multiple seed–target combinations and avoid false positives, false discovery rate (Benjamini & Hochberg, 1995) correction was performed across each type of analysis (cumulative, developmental stages, and individual ages) for each brain region (five for amygdala–seed analysis: left amydala–BA 25, right amygdala–BA 10, right amygdala–BA 11, right amygdala–BA 25, and right amygdala–BA 47; five for hippocampus–seed analysis: left hippocampus–BA 10, right hippocampus–BA 10, right hippocampus–BA 11, right hippocampus–BA 25, and right hippocampus–BA 47; two for hippocampus–amygdala analysis: left and right hemispheres). Following preregistration, multiple regression analyses with main predictors and outcome variables were performed to test for main effects. If main effects were found, covariates were then added as sensitivity analyses.
Exploratory Analyses
To confirm the results of our preregistered multiple regression analyses, we tested the associations between material hardship and white matter connectivity using structural equation modeling framework. All material hardship predictors at each time point, amygdala–pFC white matter connectivity at each region, and all covariates were examined in the same model using MPlus (Muthén & Muthén, 1998–2011). Furthermore, to examine the trajectory of material hardship exposure over time and its relation to white matter connectivity during adolescence, we performed a latent growth curve analysis using MPlus. Following the approach used by Gard et al. (2021), linear and quadratic trajectories were fit separately on material hardship data over five waves (ages 1, 3, 5, 9, and 15 years), and model fit indices were examined based on standard threshold (Hu & Bentler, 1999). Material hardship at age 1 year was fixed with loading 0 to indicate initial point of trajectory (i.e., the intercept). Loadings for subsequent time points were fixed at 2, 4, 8, and 14, which account for unequal time intervals in between observations (Duncan & Duncan, 2004). Estimated latent slope (change over time) and intercept (initial point) of material hardship were examined before paths were added from latent slope and intercept predicting white matter connectivity metrics. Model fit indices were then reexamined and path coefficients were reported if model fit was deemed adequate. Finally, in an exploratory analysis to anchor findings with affective-related behavioral outcomes, associations between material hardship and white matter connectivity with adolescent anxiety and depression were tested. Results from the regression analyses were utilized to inform these two exploratory analyses, and only regions that were found to have significant associations in previous analyses were examined. Furthermore, to examine any associations via white matter connectivity, indirect effects between hardship and adolescent anxiety and depression symptoms through white matter connectivity were additionally tested using path modeling in MPlus.
RESULTS
Preregistered Analyses
Null Cumulative Effect of Material Hardship
There were no significant relations between cumulative material hardship with amygdala–prefrontal and hippocampus–prefrontal white matter connectivity (Table 4). There was a significant positive relation between cumulative material hardship and left hippocampus–amygdala, but it did not survive correction for multiple comparisons (r = .144, p = .047, padjust = .094).
Table 4.
Zero-Order Correlations between Cumulative Material Hardship with Seed–Target (Amygdala–pFC, Hippocampus–pFC, Amygdala–Hippocampus) White Matter Connectivity
| Model: Seed–Target White Matter Connectivity ~ Cumulative Material Hardship | |||
|---|---|---|---|
| Seed–Target White Matter | r | p | padjust |
| Right amygdala–BA 10 (dmPFC) | .073 | .317 | .396 |
| Right amygdala–BA 11 (OFC) | −.114 | .116 | .396 |
| Right amygdala–BA 25 (sgACC) | −.081 | .270 | .396 |
| Right amygdala–BA 47 (OFC) | −.077 | .291 | .396 |
| Left amygdala–BA 25 (sgACC) | .011 | .881 | .881 |
| Right hippocampus–BA 10 (dmPFC) | .002 | .979 | .979 |
| Right hippocampus–BA 11 (OFC) | −.069 | .346 | .741 |
| Right hippocampus–BA 25 (sgACC) | .039 | .593 | .741 |
| Right hippocampus–BA 47 (OFC) | −.114 | .116 | .580 |
| Left hippocampus–BA 10 (dmPFC) | .054 | .456 | .741 |
| Right hippocampus–amygdala | −.042 | .564 | .564 |
| Left hippocampus–amygdala | .144 | .047 | .094 |
Differential Relations between Material Hardship at Specific Developmental Stages and Specific Amygdala–pFC White Matter Connectivity
Material hardship experienced at specific developmental stages differentially related to white matter connectivity between amygdala and pFC regions (Table 5; Figure 2). Specifically, controlling for other developmental periods, hardship experienced in middle childhood (ages 5 and 9 years) related to increased right amygdala–BA 10 (dmPFC; β = .27, p = .009, padjust = .045) white matter connectivity. Additionally, material hardship during adolescence (age 15 years) was related to increased left amygdala–BA 25 (sgACC; β = .24, p = .009, padjust = .040) and decreased right amygdala–BA 11 (OFC; β = −.22, p = .016, padjust = .040) white matter connectivity, when accounting for other developmental periods. These relations remained significant after false discovery rate correction (Table 5 and adjusted p values reported above) and sensitivity analyses including all covariates (see Table 7). There were significant associations between material hardship at adolescence (age 15 years) and left hippocampus–BA 10, but this association did not survive adjustment for multiple comparison (dmPFC; β = .19, p = .038, padjust = .190). There were no association between material hardship and hippocampus–amygdala white matter connectivity.
Table 5.
Multiple Regression Results Examining Material Hardship Effect at Specific Developmental Stages
| Developmental Stage Specific Material Hardship and Amygdala–Seed White Matter Connectivity | ||||||
|---|---|---|---|---|---|---|
| B | β | SEB | t | p | padjust | |
| Model: Right amygdala–BA 10 (dmPFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | −0.005 | −.138 | 0.004 | −1.428 | .155 | .258 |
| Hardship at ages 5, 9 | 0.010 | .267 | 0.004 | 2.637 | .009 | .045 |
| Hardship at age 15 | −0.005 | −.149 | 0.003 | −1.647 | .102 | .170 |
| Model: Right amygdala–BA 11 (OFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.006 | .090 | 0.006 | 0.934 | .352 | .440 |
| Hardship at ages 5, 9 | −0.005 | −.088 | 0.006 | −0.876 | .382 | .856 |
| Hardship at age 15 | −0.011 | −.220 | 0.005 | −2.441 | .016 | .040 |
| Model: Right amygdala–BA 25 (sgACC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | −0.013 | −.207 | 0.006 | −2.133 | .035 | .175 |
| Hardship at ages 5, 9 | 0.000 | .002 | 0.006 | 0.017 | .987 | .987 |
| Hardship at age 15 | 0.006 | .128 | 0.005 | 1.410 | .161 | .201 |
| Model: Right amygdala–BA 47 (OFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.001 | .010 | 0.007 | 0.097 | .923 | .923 |
| Hardship at ages 5, 9 | −0.004 | −.056 | 0.007 | −0.541 | .589 | .856 |
| Hardship at age 15 | −0.005 | −.093 | 0.005 | −1.009 | .314 | .314 |
| Model: Left amygdala–BA 25 (sgACC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | −0.009 | −.154 | 0.005 | −1.599 | .112 | .258 |
| Hardship at ages 5, 9 | −0.002 | −.041 | 0.005 | −0.407 | .685 | .856 |
| Hardship at age 15 | 0.011 | .239 | 0.004 | 2.641 | .009 | .040 |
| Model: Right hippocampus–BA 10 (dmPFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | −0.005 | −.114 | 0.004 | −1.155 | .250 | .556 |
| Hardship at ages 5, 9 | 0.004 | .094 | 0.004 | 0.922 | .358 | .569 |
| Hardship at age 15 | −0.001 | −.039 | 0.003 | −0.427 | .670 | .670 |
| Model: Right hippocampus–BA 11 (OFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.004 | .126 | 0.003 | 1.288 | .200 | .556 |
| Hardship at ages 5, 9 | −0.004 | −.123 | 0.003 | −1.221 | .224 | .569 |
| Hardship at age 15 | −0.003 | −.137 | 0.002 | −1.505 | .134 | .223 |
| Model: Right hippocampus–BA 25 (sgACC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.002 | .058 | 0.004 | 0.590 | .556 | .556 |
| Hardship at ages 5, 9 | −0.004 | −.111 | 0.004 | −1.091 | .277 | .569 |
| Hardship at age 15 | 0.003 | .105 | 0.003 | 1.138 | .257 | .321 |
| Model: Right hippocampus–BA 47 (OFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.002 | .064 | 0.003 | 0.656 | .513 | .556 |
| Hardship at ages 5, 9 | −0.002 | −.073 | 0.003 | −0.722 | .471 | .569 |
| Hardship at age 15 | −0.003 | −.139 | 0.002 | −1.517 | .131 | .223 |
| Model: Left hippocampus–BA 10 (dmPFC) ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | −0.002 | −.080 | 0.003 | −0.817 | .415 | .556 |
| Hardship at ages 5, 9 | −0.002 | −.058 | 0.003 | −0.571 | .569 | .569 |
| Hardship at age 15 | 0.004 | .191 | 0.002 | 2.097 | .038 | .190 |
| Model: Right hippocampus–amygdala ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.004 | .097 | 0.004 | 0.987 | .325 | .325 |
| Hardship at ages 5, 9 | −0.006 | −.155 | 0.004 | −1.521 | .130 | .260 |
| Hardship at age 15 | −0.001 | −.018 | 0.003 | −0.193 | .847 | .847 |
| Model: Left hippocampus–amygdala ~ developmental stage specific hardship predictors | ||||||
| Hardship at ages 1, 3 | 0.006 | .111 | 0.006 | 1.127 | .262 | .325 |
| Hardship at ages 5, 9 | 0.001 | .019 | 0.005 | 0.190 | .850 | .850 |
| Hardship at age 15 | 0.001 | .024 | 0.004 | 0.259 | .796 | .847 |
Figure 2.
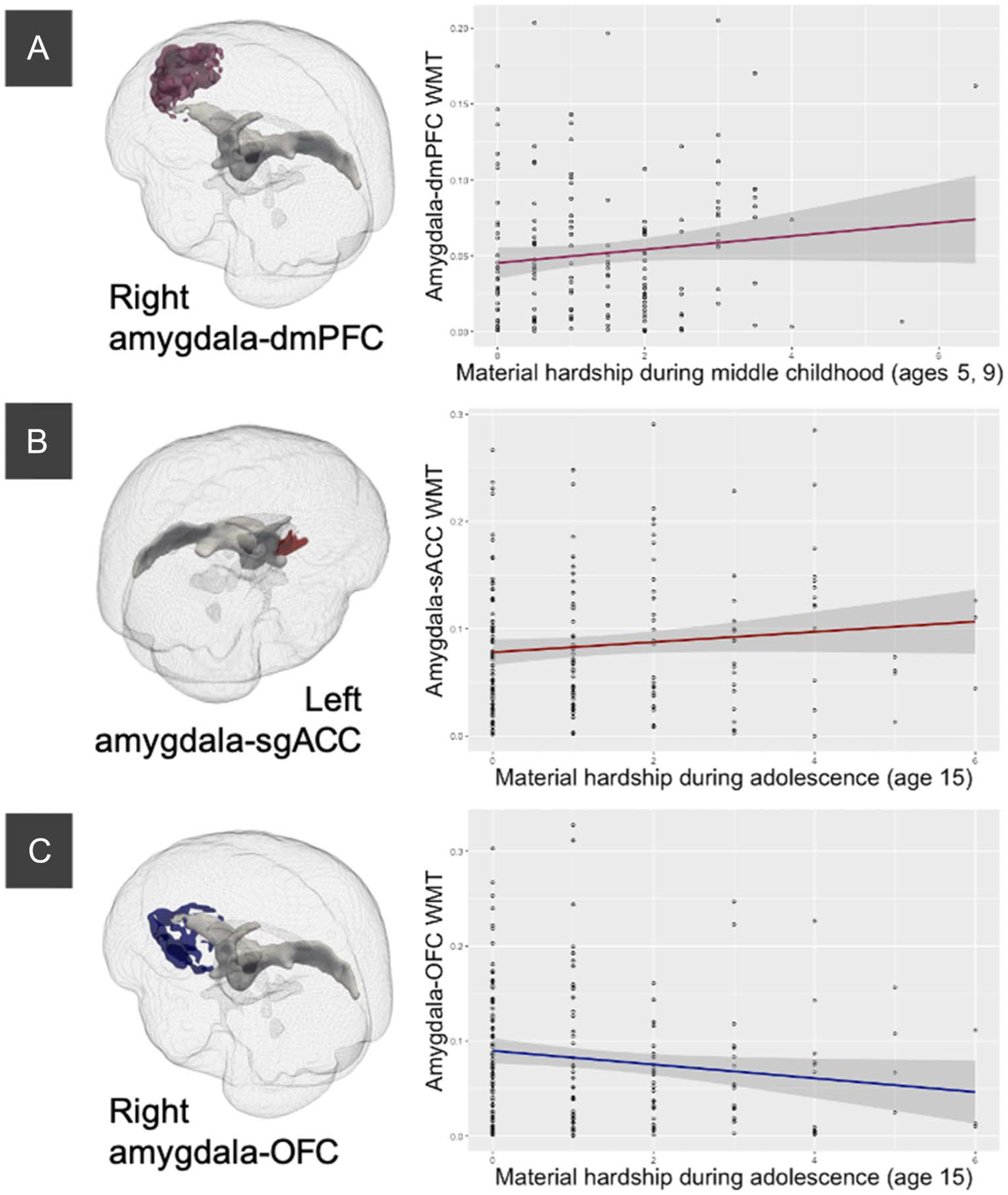
Significant associations between material hardship at specific developmental stages and white matter connectivity after adjusting for other developmental stages. White matter connectivity and target on the left and zero-order plots on the right. (A) Increased material hardship at ages 5 and 9 years was related to increased right amygdala–dmPFC white matter connectivity, adjusting for other developmental stages. (B) Increased material hardship at age 15 years was related to increased left amygdala–sgACC white matter connectivity, adjusting for other developmental stages. (C) Increased material hardship at age 15 years was related to decreased right amygdala–OFC white matter connectivity, adjusting for other developmental stages.
Table 7.
Sensitivity Analyses Results with Covariates
| Sensitivity Analyses Results of White Matter Connectivity Regions with Significant Main Effects with Developmental Stages Specific Material Hardship | |||||
|---|---|---|---|---|---|
| B | β | SEB | t | p | |
| Model: Right amygdala–BA 10 (dmPFC) white matter ~ predictors + covariates | |||||
| Hardship at ages 1, 3 | −0.006 | −.145 | 0.004 | −1.396 | .165 |
| Hardship at ages 5, 9 | 0.008 | .229 | 0.004 | 2.102 | .037 |
| Hardship at age 15 | −0.004 | −.136 | 0.003 | −1.387 | .168 |
| Race/ethnicity (White) | −0.024 | −.172 | 0.013 | −1.858 | .065 |
| Race/ethnicity (Hispanic) | 0.001 | .003 | 0.015 | 0.035 | .972 |
| Race/ethnicity (other/multiracial) | −0.021 | −.102 | 0.018 | −1.152 | .251 |
| Sex | 0.004 | .043 | 0.009 | 0.422 | .674 |
| Pubertal age | 0.007 | .093 | 0.008 | 0.924 | .357 |
| Birth city (Chicago) | −0.001 | −.009 | 0.011 | −0.110 | .913 |
| Birth city (Toledo) | −0.003 | −.024 | 0.011 | −0.256 | .799 |
| Birth city (other) | −0.038 | −.094 | 0.036 | −1.054 | .294 |
| Maternal education | −0.004 | −.105 | 0.004 | −1.104 | .272 |
| Family structure | −0.001 | −.007 | 0.008 | −0.086 | .931 |
| Violence exposure | −0.001 | −.075 | 0.001 | −0.716 | .475 |
| Social deprivation | 0.000 | .042 | 0.001 | 0.421 | .674 |
| Household income at ages 5, 9 | 0.000 | −.009 | 0.000 | −0.084 | .933 |
| Model: Right amygdala–BA 11 (OFC) white matter ~ predictors + covariates | |||||
| Hardship at ages 1, 3 | 0.005 | .084 | 0.007 | 0.814 | .417 |
| Hardship at ages 5, 9 | −0.006 | −.101 | 0.006 | −0.945 | .346 |
| Hardship at age 15 | −0.010 | −.200 | 0.005 | −2.068 | .040 |
| Race/ethnicity (White) | −0.029 | −.128 | 0.021 | −1.390 | .167 |
| Race/ethnicity (Hispanic) | −0.011 | −.036 | 0.025 | −0.437 | .663 |
| Race/ethnicity (other/multiracial) | 0.005 | .016 | 0.029 | 0.176 | .860 |
| Sex | 0.001 | .008 | 0.015 | 0.076 | .939 |
| Pubertal age | −0.010 | −.082 | 0.012 | −0.815 | .416 |
| Birth city (Chicago) | −0.015 | −.069 | 0.018 | −0.799 | .425 |
| Birth city (Toledo) | −0.021 | −.112 | 0.018 | −1.205 | .230 |
| Birth city (other) | −0.084 | −.129 | 0.058 | −1.460 | .146 |
| Maternal education | −0.006 | −.091 | 0.006 | −0.998 | .320 |
| Family structure | 0.024 | .160 | 0.013 | 1.856 | .066 |
| Violence exposure | −0.001 | −.081 | 0.002 | −0.785 | .434 |
| Social deprivation | 0.001 | .059 | 0.002 | 0.590 | .556 |
| Household income at age 15 | 0.000 | .171 | 0.000 | 1.761 | .080 |
| Model: Left amygdala–BA 25 (sgACC) white matter ~ predictors + covariates | |||||
| Hardship at ages 1, 3 | −0.007 | −.129 | 0.006 | −1.273 | .205 |
| Hardship at ages 5, 9 | −0.002 | −.038 | 0.005 | −0.357 | .722 |
| Hardship at age 15 | 0.009 | .209 | 0.004 | 2.199 | .030 |
| Race/ethnicity (White) | 0.008 | .038 | 0.018 | 0.420 | .675 |
| Race/ethnicity (Hispanic) | −0.017 | −.064 | 0.021 | −0.792 | .430 |
| Race/ethnicity (other/multiracial) | 0.002 | .008 | 0.025 | 0.090 | .929 |
| Sex | −0.017 | −.133 | 0.013 | −1.331 | .185 |
| Pubertal age | 0.001 | .013 | 0.011 | 0.136 | .892 |
| Birth city (Chicago) | −0.006 | −.033 | 0.016 | −0.393 | .695 |
| Birth city (Toledo) | 0.018 | .104 | 0.015 | 1.142 | .255 |
| Birth city (other) | 0.098 | .169 | 0.050 | 1.944 | .054 |
| Maternal education | 0.009 | .156 | 0.005 | 1.751 | .082 |
| Family structure | −0.024 | −.179 | 0.011 | −2.116 | .036 |
| Violence exposure | 0.001 | .043 | 0.002 | 0.421 | .675 |
| Social deprivation | −0.002 | −.104 | 0.002 | −1.062 | .290 |
| Household income at age 15 | 0.000 | −.156 | 0.000 | −1.640 | .103 |
| Sensitivity Analyses Results of White Matter Connectivity Regions with Significant Main Effects with Age-Specific Material Hardship | |||||
| B | β | SEB | t | p | |
| Model: Right amygdala–BA 10 (dmPFC) white matter ~ predictors + covariates | |||||
| Hardship at age 1 | −0.003 | −.077 | 0.004 | −0.758 | .450 |
| Hardship at age 3 | −0.005 | −.142 | 0.004 | −1.267 | .207 |
| Hardship at age 5 | 0.010 | .331 | 0.003 | 3.138 | .002 |
| Hardship at age 9 | −0.001 | −.044 | 0.003 | −0.435 | .664 |
| Hardship at age 15 | −0.003 | −.090 | 0.003 | −0.918 | .360 |
| Race/ethnicity (White) | −0.025 | −.180 | 0.012 | −2.003 | .047 |
| Race/ethnicity (Hispanic) | 0.000 | .001 | 0.015 | 0.017 | .986 |
| Race/ethnicity (other/multiracial) | −0.018 | −.086 | 0.018 | −0.981 | .328 |
| Sex | 0.005 | .057 | 0.009 | 0.563 | .574 |
| Pubertal age | 0.007 | .097 | 0.008 | 0.968 | .335 |
| Birth city (Chicago) | −0.001 | −.008 | 0.011 | −0.099 | .921 |
| Birth city (Toledo) | 0.000 | −.001 | 0.011 | −0.015 | .988 |
| Birth city (other) | −0.049 | −.121 | 0.036 | −1.357 | .177 |
| Maternal education | −0.005 | −.128 | 0.004 | −1.391 | .166 |
| Family structure | 0.000 | .003 | 0.008 | 0.030 | .976 |
| Violence exposure | −0.001 | −.049 | 0.001 | −0.470 | .639 |
| Social deprivation | 0.000 | .011 | 0.001 | 0.110 | .913 |
| Household income at age 5 | 0.000 | .016 | 0.000 | 0.161 | .872 |
| Model: Left amygdala–BA 25 (sgACC) white matter ~ predictors + covariates | |||||
| Hardship at age 1 | −0.006 | −.118 | 0.005 | −1.174 | .242 |
| Hardship at age 3 | −0.001 | −.020 | 0.005 | −0.178 | .859 |
| Hardship at age 5 | −0.002 | −.037 | 0.005 | −0.353 | .725 |
| Hardship at age 9 | −0.001 | −.020 | 0.005 | −0.202 | .840 |
| Hardship at age 15 | 0.009 | .205 | 0.004 | 2.107 | .037 |
| Race/ethnicity (White) | 0.008 | .041 | 0.018 | 0.453 | .651 |
| Race/ethnicity (Hispanic) | −0.019 | −.071 | 0.022 | −0.864 | .389 |
| Race/ethnicity (other/multiracial) | 0.001 | .003 | 0.026 | 0.037 | .971 |
| Sex | −0.017 | −.134 | 0.013 | −1.333 | .185 |
| Pubertal age | 0.001 | .011 | 0.011 | 0.114 | .910 |
| Birth city (Toledo) | −0.006 | −.033 | 0.016 | −0.385 | .701 |
| Birth city (Chicago) | 0.017 | .100 | 0.016 | 1.087 | .279 |
| Birth city (other) | 0.102 | .176 | 0.051 | 1.983 | .049 |
| Maternal education | 0.009 | .157 | 0.005 | 1.745 | .083 |
| Family structure | −0.024 | −.184 | 0.011 | −2.151 | .033 |
| Violence exposure | 0.001 | .037 | 0.002 | 0.359 | .720 |
| Social deprivation | −0.001 | −.095 | 0.002 | −0.948 | .345 |
| Household income at age 15 | 0.000 | −.159 | 0.000 | −1.657 | .100 |
Examination of material hardship at individual ages and amygdala–pFC white matter connectivity reflected similar patterns (Table 6). Material hardship at age 5 years was related to increased right amygdala–BA 10 (dmPFC; β = .33, p = .001, padjust = .005) and material hardship at adolescence (age 15 years) was related to increased left amygdala–BA 25 (sgACC; β = .24, p = .010, padjust = .049), when controlling for other age time points. There was also a trending negative association between material hardship and amygdala–BA 11 (OFC; β = −.20, p = .031, padjust = .078) and left hippocampus–BA 10 (dmPFC; β = .20, p = .034, padjust = .170), but results did not survive correction for multiple comparisons. Furthermore, positive associations between hardship at age 5 years and amgydala–BA 10 (dmPFC) as well as hardship at age 15 years and amygdala–BA 25 (sgACC) remained when accounting for all covariates (Table 7).
Table 6.
Multiple Regression Results Examining Material Hardship Effect at Individual Time Points
| Age-Specific Material Hardship with Amygdala-Seed White Matter Connectivity | ||||||
|---|---|---|---|---|---|---|
| B | β | SEB | t | p | padjust | |
| Model: Right amygdala–BA 10 (dmPFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.003 | −.097 | 0.003 | −1.019 | .310 | .673 |
| Hardship at age 3 | −0.003 | −.101 | 0.004 | −0.961 | .338 | .504 |
| Hardship at age 5 | 0.010 | .330 | 0.003 | 3.325 | .001 | .005 |
| Hardship at age 9 | <−0.001 | −.016 | 0.003 | −0.169 | .866 | .866 |
| Hardship at age 15 | −0.003 | −.113 | 0.003 | −1.233 | .219 | .334 |
| Model: Right amygdala–BA 11 (OFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.002 | −.032 | 0.005 | −0.333 | .740 | .740 |
| Hardship at age 3 | 0.006 | .116 | 0.006 | 1.103 | .272 | .504 |
| Hardship at age 5 | 0.003 | .053 | 0.005 | 0.535 | .594 | .944 |
| Hardship at age 9 | −0.009 | −.170 | 0.005 | −1.739 | .084 | .420 |
| Hardship at age 15 | −0.010 | −.200 | 0.005 | −2.174 | .031 | .078 |
| Model: Right amygdala–BA 25 (sgACC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.003 | −.060 | 0.005 | −0.625 | .533 | .673 |
| Hardship at age 3 | −0.008 | −.150 | 0.006 | −1.403 | .162 | .504 |
| Hardship at age 5 | −0.006 | −.123 | 0.005 | −1.223 | .223 | .558 |
| Hardship at age 9 | 0.007 | .132 | 0.005 | 1.338 | .183 | .458 |
| Hardship at age 15 | 0.005 | .103 | 0.005 | 1.114 | .267 | .334 |
| Model: Right amygdala–BA 47 (OFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | 0.004 | .061 | 0.006 | 0.617 | .538 | .673 |
| Hardship at age 3 | −0.004 | −.059 | 0.007 | −0.542 | .589 | .589 |
| Hardship at age 5 | −0.001 | −.022 | 0.006 | −0.212 | .832 | .944 |
| Hardship at age 9 | −0.002 | −.029 | 0.006 | −0.293 | .770 | .866 |
| Hardship at age 15 | −0.005 | −.090 | 0.005 | −0.957 | .340 | .340 |
| Model: Left amygdala–BA 25 (sgACC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.004 | −.090 | 0.005 | −0.924 | .357 | .673 |
| Hardship at age 3 | −0.004 | −.090 | 0.005 | −0.838 | .403 | .504 |
| Hardship at age 5 | <−0.001 | −.010 | 0.004 | −0.070 | .944 | .944 |
| Hardship at age 9 | −0.002 | −.040 | 0.004 | −0.410 | .683 | .866 |
| Hardship at age 15 | 0.011 | .243 | 0.004 | 2.618 | .010 | .049 |
| Age-Specific Material Hardship and Hippocampus–Seed White Matter Connectivity | ||||||
| B | β | SEB | t | p | padjust | |
| Model: Right hippocampus–BA 10 (dmPFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.004 | −.115 | 0.004 | −1.162 | .247 | .612 |
| Hardship at age 3 | 0.000 | −.012 | 0.004 | −0.106 | .916 | .916 |
| Hardship at age 5 | 0.002 | .066 | 0.003 | 0.639 | .523 | .813 |
| Hardship at age 9 | 0.001 | .030 | 0.003 | 0.300 | .765 | .765 |
| Hardship at age 15 | −0.001 | −.037 | 0.003 | −0.400 | .689 | .689 |
| Model: Right hippocampus–BA 11 (OFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.001 | −.020 | 0.003 | −0.210 | .834 | .834 |
| Hardship at age 3 | 0.005 | .166 | 0.003 | 1.529 | .128 | .448 |
| Hardship at age 5 | −0.001 | −.047 | 0.003 | −0.454 | .650 | .813 |
| Hardship at age 9 | −0.003 | −.117 | 0.003 | −1.193 | .235 | .765 |
| Hardship at age 15 | −0.003 | −.133 | 0.002 | −1.443 | .151 | .252 |
| Model: Right hippocampus–BA 25 (sgACC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | 0.004 | .122 | 0.004 | 1.236 | .218 | .618 |
| Hardship at age 3 | −0.002 | −.066 | 0.004 | −0.604 | .546 | .863 |
| Hardship at age 5 | −0.002 | −.064 | 0.003 | −0.622 | .535 | .813 |
| Hardship at age 9 | −0.001 | −.040 | 0.003 | −0.408 | .684 | .765 |
| Hardship at age 15 | 0.003 | .105 | 0.003 | 1.124 | .263 | .329 |
| Model: Right hippocampus–BA 47 (OFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.001 | −.053 | 0.003 | −0.546 | .586 | .733 |
| Hardship at age 3 | 0.004 | .147 | 0.003 | 1.351 | .179 | .448 |
| Hardship at age 5 | −0.002 | −.080 | 0.002 | −0.780 | .436 | .813 |
| Hardship at age 9 | −0.001 | −.031 | 0.002 | −0.319 | .751 | .765 |
| Hardship at age 15 | −0.003 | −.145 | 0.002 | −1.565 | .120 | .252 |
| Model: Left hippocampus–BA 10 (dmPFC) ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | −0.002 | −.058 | 0.003 | −0.588 | .558 | .733 |
| Hardship at age 3 | −0.001 | −.044 | 0.003 | −0.399 | .690 | .863 |
| Hardship at age 5 | 0.000 | .010 | 0.002 | 0.094 | .926 | .926 |
| Hardship at age 9 | −0.002 | −.075 | 0.002 | −0.765 | .445 | .765 |
| Hardship at age 15 | 0.005 | .198 | 0.002 | 2.138 | .034 | .170 |
| Model: Right hippocampus–amygdala ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | 0.006 | .176 | 0.004 | 1.806 | .073 | .073 |
| Hardship at age 3 | −0.004 | −.103 | 0.004 | −0.951 | .343 | .343 |
| Hardship at age 5 | 0.000 | −.010 | 0.003 | −0.095 | .924 | .924 |
| Hardship at age 9 | −0.004 | −.129 | 0.003 | −1.309 | .192 | .311 |
| Hardship at age 15 | 0.000 | −.005 | 0.003 | −0.050 | .960 | .960 |
| Model: Left hippocampus–amygdala ~ age-specific material hardship predictors | ||||||
| Hardship at age 1 | 0.009 | .178 | 0.005 | 1.842 | .067 | .073 |
| Hardship at age 3 | −0.005 | −.111 | 0.005 | −1.031 | .304 | .343 |
| Hardship at age 5 | 0.008 | .168 | 0.005 | 1.647 | .102 | .204 |
| Hardship at age 9 | −0.005 | −.099 | 0.004 | −1.017 | .311 | .311 |
| Hardship at age 15 | 0.002 | .049 | 0.004 | 0.536 | .593 | .960 |
Exploratory Analyses
Differential Associations between Material Hardship at Specific Ages Were Also Reflected Using Structural Equation Modeling Framework
Structural equation model testing the associations between material hardship at all ages and amygdala–pFC white matter connectivity showed consistent findings with multiple regression results. There were positive associations between material hardship at age 5 years with amygdala–BA 10 (dmPFC; β = .37, p < .001) white matter connectivity and material hardship at age 15 years with amygdala–BA 25 (sgACC; β = .21, p = .010; Figure 3). Furthermore, there were trending negative associations between material hardship at 9 and 15 years with amygdala–BA 11 (OFC) white matter connectivity (age 9 years: β = −.15, p = .081; age 15 years: β = −.14, p = .096). All covariates were included in the model, and the model had excellent fit, χ2(45) = 47.931, p = .355 (root mean square error of approximation [RMSEA] = .019 [.000, .053], comparative fit index [CFI] = .980, Tucker–Lewis index [TLI] = .965, standardized root mean square residual [SRMR] = .047).
Figure 3.
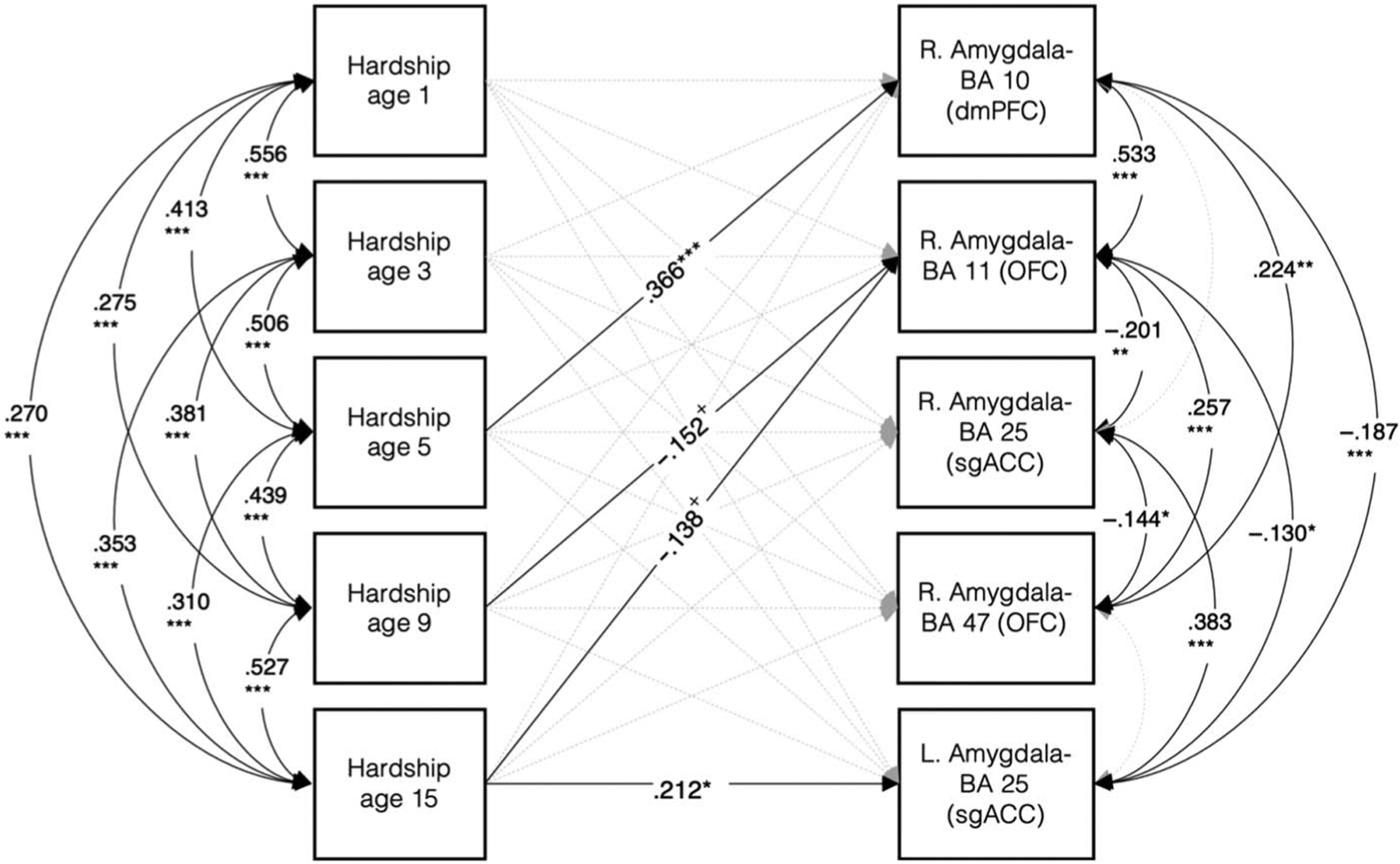
Covariates-adjusted model using structural equation modeling including all material hardship predictors and white matter connectivity targets. Model fit statistics indicate excellent fit: χ2[45] = 47.931, p = .355; RMSEA = .019 [.000, .053], CFI = .980, TLI = .965, SRMR = .047. Consistent with regression results, there were positive associations between material hardship at age 5 years with right amygdala–BA 10 (dmPFC) and hardship at age 15 years with left amygdala–BA 25 (sgACC). Furthermore, there were trending negative associations between material hardship at ages 9 and 15 years with right amygdala–BA 11 (OFC) white matter connectivity. Covariates included in the model: ethnoracial identity, sex, pubertal age, birth city, maternal education, family structure, violence exposure, social deprivation, and annual household income at baseline. + p < .10. * p < .05. ** p < .01. *** p < .001.
Change of Material Hardship Over Time Differentially Related to Specific Amygdala–pFC White Matter Connectivity
We utilized latent growth curve modeling to examine early childhood levels (intercept) and change over time (slope) of material hardship and how they related to amygdala–pFC white matter connectivity. Model fit indices indicated that the linear trajectory of material hardship had moderate fit (Hu & Bentler, 1999), χ2(10) = 21.577, p = .017 (RMSEA = .070 [.028, .111], CFI = .934, TLI = .934, SRMR = .064). Estimated mean for initial levels of material hardship (i.e., intercept) was positive (mean [SE] = 1.01 [0.077], p < .001; variance [SE] = 0.91 [0.166], p < .001), and there was an increasing trajectory of material hardship across time (estimated slope mean [SE] = 0.02 [0.008], p = .009; variance [SE] = 0.01 [0.002], p = .004). Intercept and slope were negatively related to each other (β = −.40, p < .001; Figure 4). A quadratic curve model did not converge, indicating poor model fit of nonlinear trajectory of material hardship.
Figure 4.
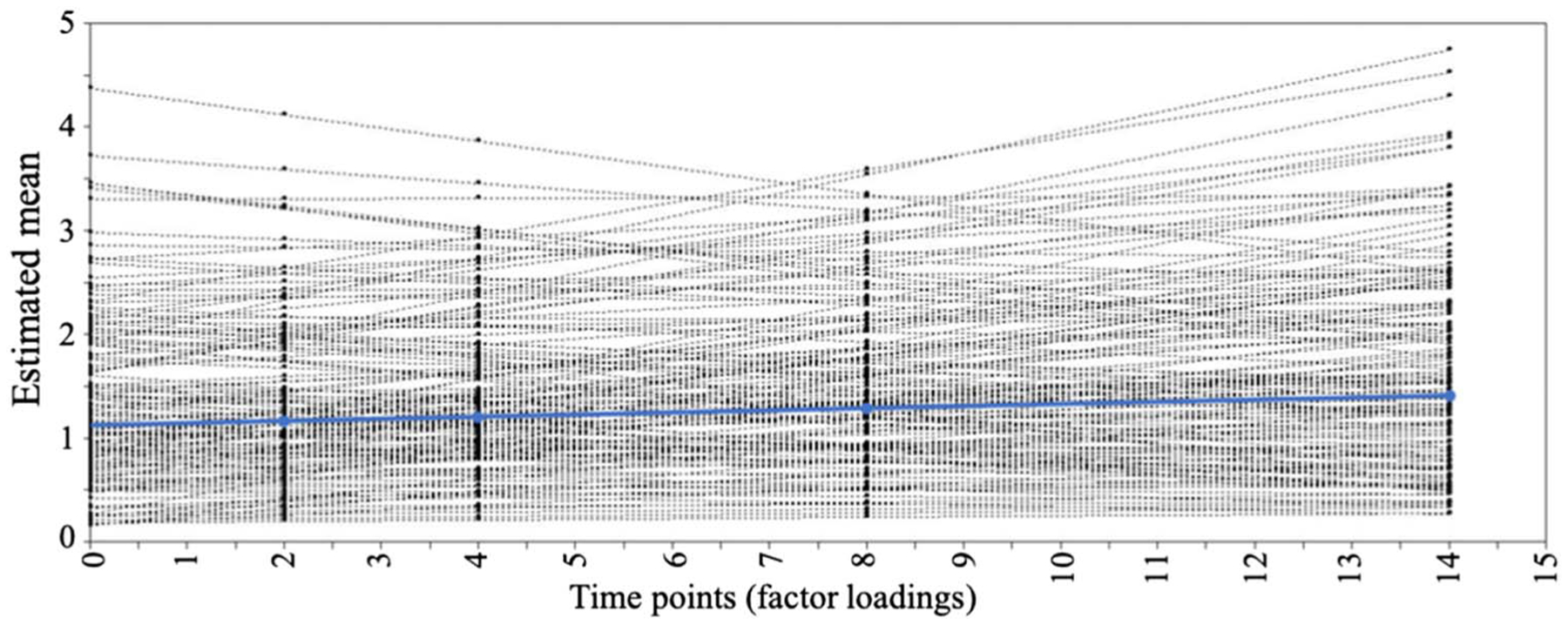
Growth curve trajectory illustrating that material hardship increases with age. Figure shows linear estimated paths for each individual (black lines) as well as mean estimated group-level path trajectory (blue). Material hardship at age 1 year is set as point 0, where estimated mean [SE] for starting point (i.e., intercept) = 1.01 [.077], p < .001; variance [SE] = 0.91 [0.166], p < .001, and estimated mean [SE] change over time (slope) = 0.02 [0.008], p = .009; variance [SE] = 0.01 [0.002], p = .004.
Next, covariates and additional paths from slope and intercept to select amygdala–pFC white matter connectivity regions were added to the base growth curve model. There were significant associations between change in material hardship over time (slope) and two separate amygdala–pFC targets (left BA 25 sgACC and right BA 11 OFC; Figure 5). Specifically, faster increases in material hardship over time were associated with greater left amygdala–sgACC (β = .23, p = .007) and less right amygdala–OFC (β = −.21, p = .017) white matter connectivity (Figure 5). The model also demonstrated moderate but not strong fit: χ2(74) = 102.37, p = .016; RMSEA = .045 [.020, .065], CFI = .920, TLI = .892, SRMR = .062.
Figure 5.
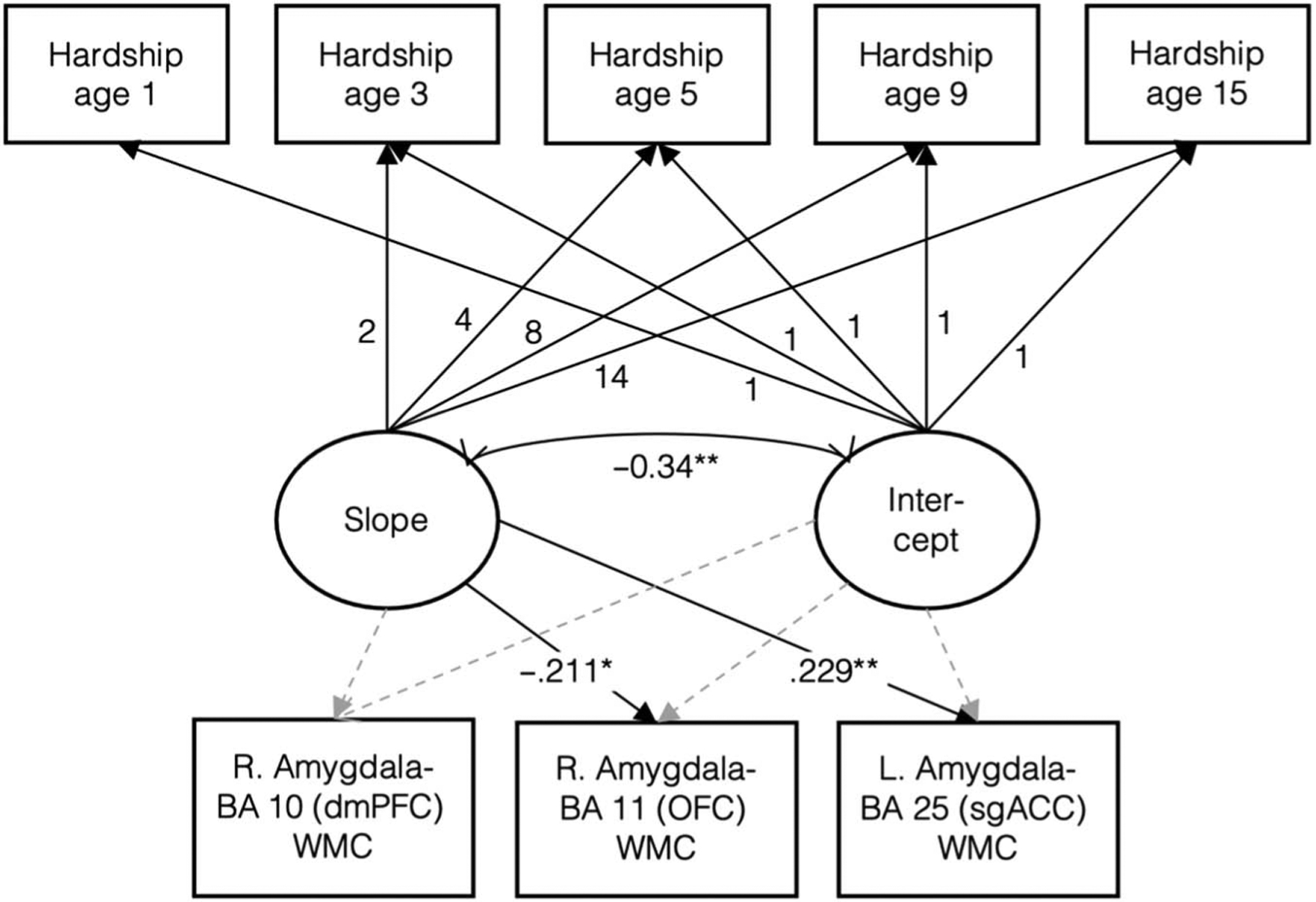
Structural equation path model showing path estimates between material hardship predictors and right amygdala–BA 10, right amygdala–BA 11, and left amygdala–BA 25 white matter connectivity. Material hardship at 1, 3, 5, 9, and 15 loadings estimating latent slope were fixed at 0, 2, 4, 8, and 14, respectively. Covariates included in the model: ethnoracial identity, sex, pubertal age, birth city, maternal education, family structure, violence exposure, social deprivation, and annual household income at baseline. * p < .05. ** p < .01.
Increased Material Hardship Was Related to Increased Anxiety and Depression, and Decreased Amygdala–OFC White Matter Connectivity Was Related to Increased Anxiety and Depression
Material hardship (cumulative, ages 3, 5, 9, and 15 years) was positively related to anxiety and depressive symptoms (Table 8). Moreover, decreased right amygdala–OFC was related to both increased anxiety and depression (anxiety, r = −.16, p = .027; depression, r = −.16, p = .032). There were no significant indirect effects in any models testing white matter connectivity as mediators (Figure 6). Furthermore, these results were observed using parent-reported symptoms, but not youth-reported symptoms (Table 9).
Table 8.
Associations between Material Hardship and White Matter Connectivity with Child Anxiety and Depressive Symptoms Using Parent-Reported Measures
| Zero-Order Correlations between Material Hardship and White Matter Connectivity with Parent-Reported Child Anxiety Symptoms at Age 15 | ||
|---|---|---|
| Predictors | r | p |
| Cumulative hardship | .218 | .003 |
| Hardship at age 1 | .030 | .698 |
| Hardship at age 3 | .196 | .010 |
| Hardship at age 5 | .232 | .002 |
| Hardship at age 9 | .170 | .028 |
| Hardship at age 15 | .198 | .008 |
| Right amygdala–BA 10 (dmPFC) | −.042 | .574 |
| Right amygdala–BA 11 (OFC) | −.164 | .027 |
| Right amygdala–BA 25 (sgACC) | −.071 | .339 |
| Right amygdala–BA 47 (OFC) | −.108 | .145 |
| Left amygdala–BA 25 (sgACC) | .021 | .774 |
| Right hippocampus–BA 10 (dmPFC) | .005 | .946 |
| Right hippocampus–BA 11 (OFC) | −.017 | .818 |
| Right hippocampus–BA 25 (sgACC) | −.069 | .342 |
| Right hippocampus–BA 47 (OFC) | .118 | .103 |
| Left hippocampus–BA 10 (dmPFC) | .045 | .538 |
| Right hippocampus–amygdala | −.002 | .982 |
| Left hippocampus–amygdala | −.054 | .457 |
| Zero-Order Correlations between Material Hardship and White Matter Connectivity with Parent-Reported Child Depressive Symptoms at Age 15 | ||
| Predictors | r | p |
| Cumulative hardship | .255 | <.001 |
| Hardship at age 1 | .103 | .173 |
| Hardship at age 3 | .193 | .010 |
| Hardship at age 5 | .259 | <.001 |
| Hardship at age 9 | .226 | .003 |
| Hardship at age 15 | .254 | <.001 |
| Right amygdala–BA 10 (dmPFC) | .006 | .934 |
| Right amygdala–BA 11 (OFC) | −.157 | .032 |
| Right amygdala–BA 25 (sgACC) | −.060 | .418 |
| Right amygdala–BA 47 (OFC) | −.102 | .166 |
| Left amygdala–BA 25 (sgACC) | .098 | .185 |
| Right hippocampus–BA 10 (dmPFC) | .107 | .142 |
| Right hippocampus–BA 11 (OFC) | .070 | .333 |
| Right hippocampus–BA 25 (sgACC) | −.119 | .101 |
| Right hippocampus–BA 47 (OFC) | .074 | .310 |
| Left hippocampus–BA 10 (dmPFC) | .068 | .349 |
| Right hippocampus–amygdala | .094 | .194 |
| Left hippocampus–amygdala | −.003 | .964 |
Figure 6.
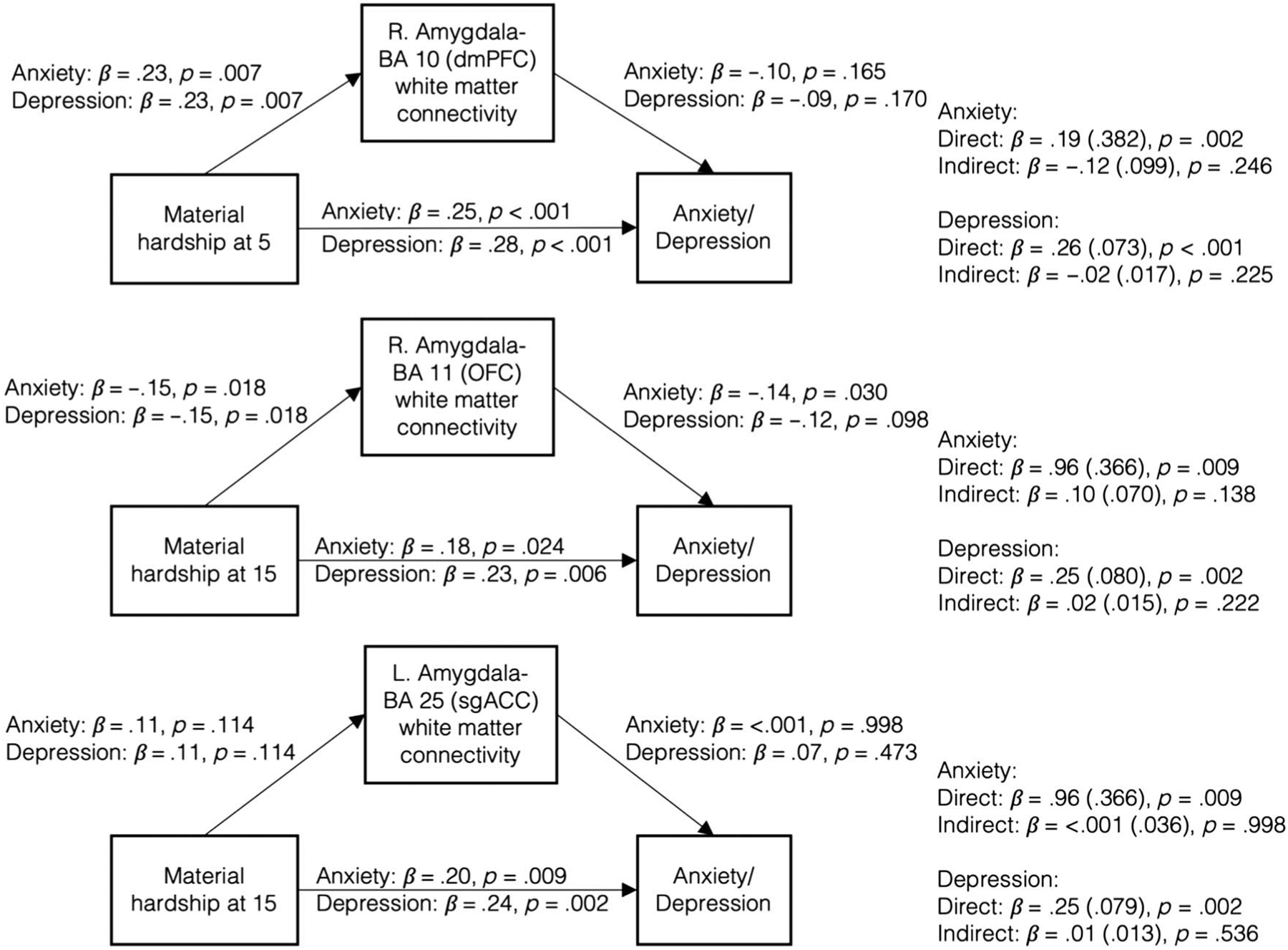
Indirect effects of material hardship through white matter connectivity were nonsignificant for any regions with significant direct associations between material hardship and white matter connectivity.
Table 9.
Associations between Material Hardship and White Matter Connectivity with Child Anxiety and Depressive Symptoms using Child-Reported Measures
| Zero-Order Correlations between Material Hardship and White Matter Connectivity with Child-Reported Child Anxiety Symptoms at Age 15 | ||
|---|---|---|
| Predictors | r | p |
| Cumulative hardship | .103 | .170 |
| Hardship at age 1 | .035 | .654 |
| Hardship at age 3 | .025 | .743 |
| Hardship at age 5 | .081 | .291 |
| Hardship at age 9 | .121 | .123 |
| Hardship at age 15 | .060 | .431 |
| Right amygdala–BA 10 | −.047 | .529 |
| Right amygdala–BA 11 | −.023 | .756 |
| Right amygdala–BA 25 | .095 | .207 |
| Right amygdala–BA 47 | .044 | .562 |
| Left amygdala–BA 25 | .058 | .438 |
| Right hippocampus–BA 10 | −.053 | .479 |
| Right hippocampus–BA 11 | −.005 | .950 |
| Right hippocampus–BA 25 | −.051 | .493 |
| Right hippocampus–BA 47 | −.046 | .542 |
| Left hippocampus–BA 10 | −.058 | .436 |
| Right hippocampus–amygdala | −.072 | .335 |
| Left hippocampus–amygdala | −.024 | .752 |
| Zero-Order Correlations between Material Hardship and White Matter Connectivity with Child-Reported Child Depressive Symptoms at Age 15 | ||
| Predictors | r | p |
| Cumulative hardship | .101 | .179 |
| Hardship at age 1 | .037 | .630 |
| Hardship at age 3 | .021 | .783 |
| Hardship at age 5 | .079 | .300 |
| Hardship at age 9 | .122 | .117 |
| Hardship at age 15 | .052 | .493 |
| Right amygdala–BA 10 | −.047 | .526 |
| Right amygdala–BA 11 | −.033 | .660 |
| Right amygdala–BA 25 | .103 | .168 |
| Right amygdala–BA 47 | .043 | .569 |
| Left amygdala–BA 25 | .061 | .418 |
| Right hippocampus–BA 10 | −.052 | .488 |
| Right hippocampus–BA 11 | −.012 | .873 |
| Right hippocampus–BA 25 | −.050 | .502 |
| Right hippocampus–BA 47 | −.046 | .541 |
| Left hippocampus–BA 10 | −.063 | .400 |
| Right hippocampus–amygdala | −.078 | .297 |
| Left hippocampus–amygdala | −.031 | .681 |
DISCUSSION
This study examined the association between material hardship across developmental stages and emotion-related white matter connectivity in adolescence. Our results indicate that material hardship in middle childhood (particularly at age 5 years) was related to increased amygdala–dmPFC white matter connectivity, whereas material hardship experienced during adolescence (age 15 years) was related to increased amygdala–sgACC and decreased amygdala–OFC white matter connectivity. When examined across development using growth curve modeling, a greater rate of increase in material hardship over time was related to increased amygdala–sgACC white matter connectivity and decreased amygdala–OFC white matter connectivity. Furthermore, in an exploratory analysis, material hardship was related to greater adolescent anxiety and depression, and decreased amygdala–OFC white matter connectivity was also related to increased anxiety and depression. Taken together, these findings demonstrate how the associations between hardship and corticolimbic white matter connectivity, which were developmental timing-specific and were compounded by deepening exposure to hardship across time, are important considerations for adolescent mental health.
These associations between material hardship and white matter connectivity clarify prior inconsistent findings that examined associations of socioeconomic hardship and white matter structures. Although several studies found that greater financial hardship was associated with reduced white matter density (Rosen et al., 2018; Dufford & Kim, 2017; Gianaros, Marsland, Sheu, Erickson, & Verstynen, 2013), one study found a positive association (Simon et al., 2021) and another found no associations between socioeconomic hardship and white matter density (Chiang et al., 2011). Here, utilizing probabilistic tractography in a longitudinal sample, this study suggests that the adversity–brain associations may depend on when hardship was experienced and where in the brain it occurred. In particular, exposure to more material hardship during middle childhood was positively related to white matter connectivity between the amygdala and a prefrontal region associated with higher level social cognition (i.e., dmPFC BA 10; Roca et al., 2011), whereas hardship during adolescence was related to structural connections between the amygdala and prefrontal regions linked to emotion processes (i.e., OFC BA 11; Ueda, Fujimoto, Ubukata, & Murai, 2017) and psychopathology (i.e., sgACC BA 25; Mayberg et al., 2005). Moreover, the present findings echo research on the associations between poverty and reduced white matter density (Brito & Noble, 2014), as well as support potential accelerated maturation relating to adverse experiences (Callaghan & Tottenham, 2016; Gee et al., 2013) that are specific to certain neural regions (e.g., ACC; Thijssen, Collins, & Luciana, 2020). Collectively, these results suggest that certain brain regions may have greater sensitivity for adversity experienced at particular developmental ages. Findings that are specific to both brain regions and developmental timing in this study may have been afforded by the spatial precision of probabilistic tractography as well as the longitudinal approach.
Differential effects of hardship across development are unsurprising when we consider the dissimilar social environment of younger children from their older counterparts. Compared with adolescents, younger children spend considerable time at home with their caregivers (National Institute of Child Health and Human Development Early Child Care Research Network, 2005; Hofferth & Sandberg, 2001). From age 6 years onward, children spend an increasing amount of time in school and outside-the-home activities (Hofferth & Sandberg, 2001). As they enter adolescence, children also become more self-sufficient and demonstrate increased autonomy from parents (Steinberg, 1988). Resources providing nutritional and intellectual enrichment previously available solely through primary caregivers are now accessible through the school system (Bradley, Convyn, Burchinal, McAdoo, & Coll, 2001). As such, exposure to poverty, particularly material hardship, which captures the lack of very specific provisions, may subsequently shape emotion-linked neural structures differentially. Here, our results show that these differences in the environment from early to later childhood and adolescence may also be observed in specific amygdala–pFC (dmPFC, sgACC, and OFC) white matter connectivity. It is noteworthy that we found no early childhood (i.e., ages 1 and 3 years) correlates of material hardship and white matter connectivity. This finding is consistent with research examining material hardship and child behavioral outcomes (internalizing, externalizing problems) in the Fragile Families and Child Wellbeing Study sample (Zilanawala & Pilkauskas, 2012), which, taken together with present findings, suggests that more proximal experiences may pose greater influence on white matter connectivity. Nevertheless, more research is needed to understand long-term consequences of hardship during early childhood on brain development.
Consistent with results showing associations between age-specific material hardship and white matter connectivity, results from the latent growth curve analysis showed that increased material hardship across time also related to white matter connectivity in the same regions (dmPFC, sgACC). These results suggest that worsening exposure to material hardship over time, but not its initial level, may play a role in white matter development. Consistent with prior research, deepening experience of poverty over time may lead to greater impact on children’s health and wellbeing (McLeod & Shanahan, 1996). This pattern of increasing material hardship over time in the sample may be due to the Great Recession (Garfinkel, McLanahan, & Wimer, 2016), which coincided with data collection at age 9 years. Financial burden on families normally diminishes when children enter school age, allowing parents to return to the workforce and bring more income for the family (Traub, Hiltonsmith, & Draut, 2016). However, in the present investigation, parents reported the highest level of material hardship at age 9 years, reflecting the notable impact of the recession on children’s development. Here, our findings demonstrate that faster increases in material hardship with age exhibit greater amygdala–sgACC white matter connectivity and reduced amygdala–OFC white matter. If replicated, this pattern of amygdala–pFC white matter connectivity that arises from worsening economic conditions may reflect neural adaptation that could confer costs and advantages.
The present findings also provide further specificity on how associations between adverse experiences and adolescent white matter may differ across types of adversity. Our previous investigation found that childhood exposure to the combination of violence exposure and social deprivation at ages 3, 5, and 9 years related solely to decreased amygdala–OFC (BA 47) white matter connectivity (Goetschius et al., 2020). In contrast, here, material hardship related to amygdala–pFC white matter tracts in different regions (dmPFC BA 10, OFC BA 11, and sgACC BA 25), and the results remained when adjusting for childhood exposure to violence exposure and social deprivation. Although threat and deprivation are potential mediators through which distal experiences such as material hardship may influence brain development, we controlled for violence exposure and social deprivation in our models to demonstrate hardship associations that are separate from violence and deprivation exposure. Additionally, these material hardship associations with white matter connectivity remained after adjusting for household income, further demonstrating the unique impact of material hardship from other measures of adversity. Collectively, these findings suggest that specific types of adverse experiences have distinct associations with adolescent white matter microstructures and may help prompt the search for additional mechanisms that may connect material hardship to white matter (e.g., lack of specific provisions contributing to parental stress; Hyde et al., 2020).
Although poverty has been extensively linked to greater risk for emotional and behavioral problems in children (Brooks-Gunn & Duncan, 1997), investigations focused specifically on material hardship and internalizing symptoms are relatively limited. Consistent with existing literature, our results found that increased experiences of material hardship across childhood and adolescence were associated with increased adolescent anxiety and depression. Moreover, decreased amygdala–OFC white matter connectivity was related to greater anxiety and depression, suggesting that white matter connectivity is implicated in the potential consequences of material hardship on youth affective processes. Interestingly, anxiety and depression symptoms only related to white matter connectivity where material hardship was linked to less connectivity. This suggests that, although material hardship may lead to both increases and decreases in adolescent white matter connectivity, internalizing problems may be selectively associated with decreases in connectivity. It is notable, however, that we found these associations using only parent-reported and not youth-reported measures of symptoms and that these associations were not corrected for multiple comparisons. Although evidence suggests that both parent and child provide distinct information on adolescent symptoms that are equally meaningful (Bowers et al., 2020), these findings should be interpreted with caution. Furthermore, there were no significant indirect effects between hardship and anxiety or depressive symptoms through any white matter connectivity; thus, more research is needed to further explain these intricate associations between hardship, white matter structures, and internalizing symptoms in adolescence.
Contrary to our hypothesis, our present results found no associations between material hardship and hippocampus–pFC or hippocampus–amygdala white matter connectivity. Despite evidence for direct projections between the hippocampus and prefrontal regions (Barbas & Blatt, 1995), it is possible that communication between pFC and the hippocampus is fully mediated through other neighboring structures, such as the amygdala, and that the hippocampus and the amygdala are too structurally interrelated for their white matter connectivity to be differentially affected by the environment. Given their importance in stress regulation and affective functioning, future examinations on how these structures specifically differ would further clarify underlying processes of poverty effects on the brain.
There are several limitations to this present investigation. First, no data were collected from families between the ages of 10–14 years; thus, we were not able to document material hardship experienced during those transitional years and our measurement of material hardship during adolescence is limited to a single time point. Second, it is not possible to determine the direction of white matter connections between the seed and target regions using our current white matter tract mapping methodology. Although conceptually pFC is commonly believed to be regulating subcortical limbic function in a top–down capacity, communication between amygdala and pFC is bidirectional (Crone & Dahl, 2012). Third, material hardship findings that were specific to ages 9 and 15 years overlap with the dMRI acquisition at 15 years of age; thus, it may not be possible to disentangle the recency effect from a possible sensitive period in our findings.
Conclusion
This study examined longitudinal associations between material hardship and white matter connectivity in adolescents underrepresented in neuroscience research. Although poverty has previously been linked to increased risk for child emotional problems and their neurodevelopmental correlates, our findings extended research on neuroscience of poverty to include measures of lived experience of poverty across a wide span of development. The developmental specific findings indicate that material hardship has potentially distinct associations with brain development depending on age. Furthermore, the increased trajectory of material hardship across development related to increased amygdala–prefrontal white matter connectivity in one region and decreased connectivity in another suggest a nonuniformity in the influence of poverty across emotion processing regions. These findings provide support for heterogeneity in the associations between environment and brain development, which can be explained by both timing and structural regions.
Funding Information
Eunice Kennedy Shriver National Institute of Child Health and Human Development (https://dx.doi.org/10.13039/100009633), grant number: 5T32HD007109-40. NIH Office of the Director (https://dx.doi.org/10.13039/100000052), grant number: 1S10OD012240-01A1. National Institute of Mental Health (https://dx.doi.org/10.13039/100000025), grant number: R01MH103761.
Diversity in Citation Practices
A retrospective analysis of the citations in every article published in this journal from 2010 to 2020 has revealed a persistent pattern of gender imbalance: Although the proportions of authorship teams (categorized by estimated gender identification of first author/last author) publishing in the Journal of Cognitive Neuroscience (JoCN) during this period were M(an)/M = .408, W(oman)/M = .335, M/W = .108, and W/W = .149, the comparable proportions for the articles that these authorship teams cited were M/M = .579, W/M = .243, M/W = .102, and W/W = .076 (Fulvio et al., JoCN, 33:1, pp. 3–7). Consequently, JoCN encourages all authors to consider gender balance explicitly when selecting which articles to cite and gives them the opportunity to report their article’s gender citation balance. The authors of this article report its proportions of citations by gender category to be as follows: M/M = .375; W/M = .232; M/W = .161; W/W = .232.
Footnotes
This article is part of a Special Focus entitled Finances and Feelings: The Affective Neuroscience of Poverty; deriving from a symposium at the 2020 Annual Meeting of the Cognitive Neuroscience Society.
Data Availability Statement
Open Science Framework preregistration can be viewed at https://osf.io/c3tf8. Data will be openly available at https://nda.nih.gov/edit_collection.html?id=2106.
REFERENCES
- Andersson JLR, Graham MS, Zsoldos E, & Sotiropoulos SN (2016). Incorporating outlier detection and replacement into a non-parametric framework for movement and distortion correction of diffusion MR images. Neuroimage, 141, 556–572. 10.1016/j.neuroimage.2016.06.058 [DOI] [PubMed] [Google Scholar]
- Andersson JLR, & Sotiropoulos SN (2016). An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage, 125, 1063–1078. 10.1016/j.neuroimage.2015.10.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Angold A, Costello EJ, Messer SC, & Pickles A (1995). Development of a short questionnaire for use in epidemiological studies of depression in children and adolescents. International Journal of Methods in Psychiatric Research, 5, 237–249. [Google Scholar]
- Barbas H (2015). General cortical and special prefrontal connections: Principles from structure to function. Annual Review of Neuroscience, 38, 269–289. 10.1146/annurev-neuro-071714-033936 [DOI] [PubMed] [Google Scholar]
- Barbas H, & Blatt GJ (1995). Topographically specific hippocampal projections target functionally distinct prefrontal areas in the rhesus monkey. Hippocampus, 5, 511–533. 10.1002/hipo.450050604 [DOI] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, & Ghashghaei T (2003). Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neuroscience, 4, 1–12. 10.1186/1471-2202-4-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman KJ (1999). Shifting family definitions: The effect of cohabitation and other nonfamily household relationships on measures of poverty. Demography, 36, 315–325. 10.2307/2648055 [DOI] [PubMed] [Google Scholar]
- Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MFS, & Woolrich MW (2007). Probabilistic diffusion tractography with multiple fibre orientations: What can we gain? Neuroimage, 34, 144–155. 10.1016/j.neuroimage.2006.09.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrens TEJ, Woolrich MW, Jenkinson M, Johansen-Berg H, Nunes RG, Clare S, et al. (2003). Characterization and propagation of uncertainty in diffusion-weighted MR imaging. Magnetic Resonance in Medicine, 50, 1077–1088. 10.1002/mrm.10609 [DOI] [PubMed] [Google Scholar]
- Benjamini Y, & Hochberg Y (1995). Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society, Series B: Methodological, 57, 289–300. 10.1111/j.2517-6161.1995.tb02031.x [DOI] [Google Scholar]
- Birmaher B, Khetarpal S, Brent D, Cully M, Balach L, Kaufman J, et al. (1997). The Screen for Child Anxiety Related Emotional Disorders (SCARED): Scale construction and psychometric characteristics. Journal of the American Academy of Child and Adolescent Psychiatry, 36, 545–553. 10.1097/00004583-199704000-00018 [DOI] [PubMed] [Google Scholar]
- Blakemore S-J (2012). Imaging brain development: The adolescent brain. Neuroimage, 61, 397–406. 10.1016/j.neuroimage.2011.11.080 [DOI] [PubMed] [Google Scholar]
- Bowers ME, Reider LB, Morales S, Buzzell GA, Miller N, Troller-Renfree SV, et al. (2020). Differences in parent and child report on the screen for child anxiety-related emotional disorders (SCARED): Implications for investigations of social anxiety in adolescents. Journal of Abnormal Child Psychology, 48, 561–571. 10.1007/s10802-019-00609-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley RH, Convyn RF, Burchinal M, McAdoo HP, & Coll CG (2001). The home environments of children in the United States. Part II: Relations with behavioral development through age thirteen. Child Development, 72, 1868–1886. 10.1111/1467-8624.t01-1-00383 [DOI] [PubMed] [Google Scholar]
- Brito NH, & Noble KG (2014). Socioeconomic status and structural brain development. Frontiers in Neuroscience, 8, 276. 10.3389/fnins.2014.00276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks-Gunn J, & Duncan GJ (1997). The effects of poverty on children. Future of Children, 7, 55–71. 10.2307/1602387 [DOI] [PubMed] [Google Scholar]
- Callaghan BL, & Tottenham N (2016). The stress acceleration hypothesis: Effects of early-life adversity on emotion circuits and behavior. Current Opinion in Behavioral Sciences, 7, 76–81. 10.1016/j.cobeha.2015.11.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiang M-C, McMahon KL, de Zubicaray GI, Martin NG, Hickie I, Toga AW, et al. (2011). Genetics of white matter development: A DTI study of 705 twins and their siblings aged 12 to 29. Neuroimage, 54, 2308–2317. 10.1016/j.neuroimage.2010.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colnat-Coulbois S, Mok K, Klein D, Pénicaud S, Tanriverdi T, & Olivier A (2010). Tractography of the amygdala and hippocampus: Anatomical study and application to selective amygdalohippocampectomy: Laboratory investigation. Journal of Neurosurgery, 113, 1135–1143. 10.3171/2010.3.JNS091832 [DOI] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13, 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Donnelly L, McLanahan S, Brooks-Gunn J, Garfinkel I, Wagner BG, Jacobsen WC, et al. (2016). Cohesive neighborhoods where social expectations are shared may have positive impact on adolescent mental health. Health Affairs (Project Hope), 35, 2083–2091. 10.1377/hlthaff.2016.0721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dufford AJ, & Kim P (2017). Family income, cumulative risk exposure, and white matter structure in middle childhood. Frontiers in Human Neuroscience, 11, 547. 10.3389/fnhum.2017.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan TE, & Duncan SC (2004). An introduction to latent growth curve modeling. Behavior Therapy, 35, 333–363. 10.1016/S0005-7894(04)80042-X [DOI] [Google Scholar]
- Duncan GJ, Yeung WJ, Brooks-Gunn J, & Smith JR (1998). How much does childhood poverty affect the life chances of children? American Sociological Review, 63, 406–423. 10.2307/2657556 [DOI] [Google Scholar]
- Eickhoff SB, Jbabdi S, Caspers S, Laird AR, Fox PT, Zilles K, et al. (2010). Anatomical and functional connectivity of cytoarchitectonic areas within the human parietal operculum. Journal of Neuroscience, 30, 6409–6421. 10.1523/JNEUROSCI.5664-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falk EB, Hyde LW, Mitchell C, Faul J, Gonzalez R, Heitzeg MM, et al. (2013). What is a representative brain? Neuroscience meets population science. Proceedings of the National Academy of Sciences, U.S.A, 110, 17615–17622. 10.1073/pnas.1310134110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farah MJ (2017). The neuroscience of socioeconomic status: Correlates, causes, and consequences. Neuron, 96, 56–71. 10.1016/j.neuron.2017.08.034 [DOI] [PubMed] [Google Scholar]
- Gard AM, Maxwell AM, Shaw DS, Mitchell C, Brooks-Gunn J, McLanahan SS, et al. (2021). Beyond family-level adversities: Exploring the developmental timing of neighborhood disadvantage effects on the brain. Developmental Science, 24, e12985. 10.1111/desc.12985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garfinkel I, McLanahan SS, & Wimer C (2016). Children of the Great Recession. Russell Sage Foundation. https://books.google.com/books?id=1LPUDAAAQBAJ [Google Scholar]
- Gee DG, Gabard-Durnam LJ, Flannery J, Goff B, Humphreys KL, Telzer EH, et al. (2013). Early developmental emergence of human amygdala–prefrontal connectivity after maternal deprivation. Proceedings of the National Academy of Sciences, U.S.A, 110, 15638–15643. 10.1073/pnas.1307893110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoff ET, Aber JL, Raver CC, & Lennon MC (2007). Income is not enough: Incorporating material hardship into models of income associations with parenting and child development. Child Development, 78, 70–95. 10.1111/j.1467-8624.2007.00986.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, & Barbas H (2007). Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage, 34, 905–923. 10.1016/j.neuroimage.2006.09.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, & Verstynen TD (2013). Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cerebral Cortex, 23, 2058–2071. 10.1093/cercor/bhs191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, et al. (1999). Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience, 2, 861–863. 10.1038/13158 [DOI] [PubMed] [Google Scholar]
- Giedd JN, & Rapoport JL (2010). Structural MRI of pediatric brain development: What have we learned and where are we going? Neuron, 67, 728–734. 10.1016/j.neuron.2010.08.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius LG, Hein TC, Mattson WI, Lopez-Duran N, Dotterer HL, Welsh RC, et al. (2019). Amygdala–prefrontal cortex white matter tracts are widespread, variable and implicated in amygdala modulation in adolescents. Neuroimage, 191, 278–291. 10.1016/j.neuroimage.2019.02.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goetschius LG, Hein TC, Mitchell C, Lopez-Duran NL, McLoyd VC, Brooks-Gunn J, et al. (2020). Childhood violence exposure and social deprivation predict adolescent amygdala–orbitofrontal cortex white matter connectivity. Developmental Cognitive Neuroscience, 45, 100849. 10.1016/j.dcn.2020.100849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MJ, Stritzel H, Smith C, Popham F, & Crosnoe R (2018). Timing of poverty in childhood and adolescent health: Evidence from the US and UK. Social Science & Medicine, 197, 136–143. 10.1016/j.socscimed.2017.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greening SG, & Mitchell DGV (2015). A network of amygdala connections predict individual differences in trait anxiety. Human Brain Mapping, 36, 4819–4830. 10.1002/hbm.22952 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson JL, Chandra A, Wolfe BL, & Pollak SD (2011). Association between income and the hippocampus. PLoS One, 6, e18712. 10.1371/journal.pone.0018712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Goetschius LG, McLoyd VC, Brooks-Gunn J, McLanahan SS, Mitchell C, et al. (2020). Childhood violence exposure and social deprivation are linked to adolescent threat and reward neural function. Social Cognitive and Affective Neuroscience, 15, 1252–1259. 10.1093/scan/nsaa144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein TC, Mattson WI, Dotterer HL, Mitchell C, Lopez-Duran N, Thomason ME, et al. (2018). Amygdala habituation and uncinate fasciculus connectivity in adolescence: A multi-modal approach. Neuroimage, 183, 617–626. 10.1016/j.neuroimage.2018.08.058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henrich J, Heine SJ, & Norenzayan A (2010). The weirdest people in the world? Behavioral and Brain Sciences, 33, 61–83. 10.1017/S0140525X0999152X [DOI] [PubMed] [Google Scholar]
- Hernández M, Guerrero GD, Cecilia JM, García JM, Inuggi A, Jbabdi S, et al. (2013). Accelerating fibre orientation estimation from diffusion weighted magnetic resonance imaging using GPUs. PLoS One, 8, e61892. 10.1371/journal.pone.0061892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernández-Fernández M, Reguly I, Giles M, Jbabdi S, Smith S, & Sotiropoulos SN (2016). A fast and flexible toolbox for tracking brain connections in diffusion MRI datasets using GPUs—4258 (p. 1).
- Hofferth SL, & Sandberg JF (2001). How American children spend their time. Journal of Marriage and Family, 63, 295–308. 10.1111/j.1741-3737.2001.00295.x [DOI] [Google Scholar]
- Hu L, & Bentler PM (1999). Cutoff criteria for fit indexes in covariance structure analysis: Conventional criteria versus new alternatives. Structural Equation Modeling, 6, 1–55. 10.1080/10705519909540118 [DOI] [Google Scholar]
- Hunt TKA, Berger LM, & Slack KS (2017). Adverse childhood experiences and behavioral problems in middle childhood. Child Abuse & Neglect, 67, 391–402. 10.1016/j.chiabu.2016.11.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde LW, Gard AM, Tomlinson RC, Burt SA, Mitchell C, & Monk CS (2020). An ecological approach to understanding the developing brain: Examples linking poverty, parenting, neighborhoods, and the brain. American Psychologist, 75, 1245–1259. 10.1037/amp0000741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, & Smith S (2002). Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage, 17, 825–841. 10.1006/nimg.2002.1132 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Beckmann CF, Behrens TEJ, Woolrich MW, & Smith SM (2012). FSL. Neuroimage, 62, 782–790. 10.1016/j.neuroimage.2011.09.015 [DOI] [PubMed] [Google Scholar]
- Jenkinson M, & Smith S (2001). A global optimisation method for robust affine registration of brain images. Medical Image Analysis, 5, 143–156. 10.1016/S1361-8415(01)00036-6 [DOI] [PubMed] [Google Scholar]
- Johansen-Berg H, Behrens TEJ, Robson MD, Drobnjak I, Rushworth MFS, Brady JM, et al. (2004). Changes in connectivity profiles define functionally distinct regions in human medial frontal cortex. Proceedings of the National Academy of Sciences, U.S.A, 101, 13335–13340. 10.1073/pnas.0403743101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SB, Riis JL, & Noble KG (2016). State of the art review: Poverty and the developing brain. Pediatrics, 137, e20153075. 10.1542/peds.2015-3075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DK, Knösche TR, & Turner R (2013). White matter integrity, fiber count, and other fallacies: The do’s and don’ts of diffusion MRI. Neuroimage, 73, 239–254. 10.1016/j.neuroimage.2012.06.081 [DOI] [PubMed] [Google Scholar]
- Kim P, Evans GW, Angstadt M, Ho SS, Sripada CS, Swain JE, et al. (2013). Effects of childhood poverty and chronic stress on emotion regulatory brain function in adulthood. Proceedings of the National Academy of Sciences, U.S.A, 110, 18442–18447. 10.1073/pnas.1308240110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korenman S, Miller JE, & Sjaastad JE (1995). Long-term poverty and child development in the United States: Results from the NLSY. Children and Youth Services Review, 17, 127–155. 10.1016/0190-7409(95)00006-X [DOI] [Google Scholar]
- Lenroot RK, & Giedd JN (2006). Brain development in children and adolescents: Insights from anatomical magnetic resonance imaging. Neuroscience & Biobehavioral Reviews, 30, 718–729. 10.1016/j.neubiorev.2006.06.001 [DOI] [PubMed] [Google Scholar]
- Lichtin RD, Merz EC, He X, Desai PM, Simon KR, Melvin SA, et al. (2021). Material hardship, prefrontal cortex-amygdala structure, and internalizing symptoms in children. Developmental Psychobiology, 63, 364–377. 10.1002/dev.22020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luby J, Belden A, Botteron K, Marrus N, Harms MP, Babb C, et al. (2013). The effects of poverty on childhood brain development: The mediating effect of caregiving and stressful life events. JAMA Pediatrics, 167, 1135–1142. 10.1001/jamapediatrics.2013.3139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maldjian JA, Laurienti PJ, Kraft RA, & Burdette JH (2003). An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage, 19, 1233–1239. 10.1016/s1053-8119(03)00169-1 [DOI] [PubMed] [Google Scholar]
- Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. (2005). Deep brain stimulation for treatment-resistant depression. Neuron, 45, 651–660. 10.1016/j.neuron.2005.02.014 [DOI] [PubMed] [Google Scholar]
- Mayer SE, & Jencks C (1989). Poverty and the distribution of material hardship. Journal of Human Resources, 24, 88–114. 10.2307/145934 [DOI] [Google Scholar]
- Merz EC, Tottenham N, & Noble KG (2018). Socioeconomic status, amygdala volume, and internalizing symptoms in children and adolescents. Journal of Clinical Child & Adolescent Psychology, 47, 312–323. 10.1080/15374416.2017.1326122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLanahan S, & Sandefur G (1994). Growing up with a single parent. What hurts, what helps. Cambridge: Harvard University Press. [Google Scholar]
- McLeod JD, & Shanahan MJ (1996). Trajectories of poverty and children’s mental health. Journal of Health and Social Behavior, 37, 207–220. 10.2307/2137292 [DOI] [PubMed] [Google Scholar]
- Morenoff J, Sampson R, & Raudenbush S (2001). Neighborhood inequality, collective efficacy, and the spatial dynamics of urban violence. Criminology, 39, 517–560. 10.1111/j.1745-9125.2001.tb00932.x [DOI] [Google Scholar]
- Muthén LK, & Muthén BO (1998–2011). MPlus user’s guide (6th ed.). Los Angeles: Muthén & Muthén. [Google Scholar]
- National Institute of Child Health and Human Development Early Child Care Research Network. (2005). Duration and developmental timing of poverty and children’s cognitive and social development from birth through third grade. Child Development, 76, 795–810. 10.1111/j.1467-8624.2005.00878.x [DOI] [PubMed] [Google Scholar]
- Nelson G (2011). Measuring poverty: The official U.S. measure and material hardship. Poverty & Public Policy, 3, 1–35. 10.2202/1944-2858.1077 [DOI] [Google Scholar]
- Noble KG, Houston SM, Kan E, & Sowell ER (2012). Neural correlates of socioeconomic status in the developing human brain. Developmental Science, 15, 516–527. 10.1111/j.1467-7687.2012.01147.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noble KG, Korgaonkar MS, Grieve SM, & Brickman AM (2013). Higher education is an age-independent predictor of white matter integrity and cognitive control in late adolescence. Developmental Science, 16, 653–664. 10.1111/desc.12077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett L, Richards M, & Boxer A (1988). A self-report measure of pubertal status: Reliability, validity, and initial norms. Journal of Youth and Adolescence, 17, 117–133. 10.1007/BF01537962 [DOI] [PubMed] [Google Scholar]
- Reichman NE, Teitler JO, Garfinkel I, & McLanahan SS (2001). Fragile families: Sample and design. Children and Youth Services Review, 23, 303–326. 10.1016/S0190-7409(01)00141-4 [DOI] [Google Scholar]
- Roca M, Torralva T, Gleichgerrcht E, Woolgar A, Thompson R, Duncan J, et al. (2011). The role of Area 10 (BA 10) in human multitasking and in social cognition: A lesion study. Neuropsychologia, 49, 3525–3531. 10.1016/j.neuropsychologia.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Rosen ML, Sheridan MA, Sambrook KA, Meltzoff AN, & McLaughlin KA (2018). Socioeconomic disparities in academic achievement: A multi-modal investigation of neural mechanisms in children and adolescents. Neuroimage, 173, 298–310. 10.1016/j.neuroimage.2018.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semega JL, Kollar MA, Creamer J, & Mohanty A (2019). Income and poverty in the United States: 2018. Washington: U. S. Government Printing Office. [Google Scholar]
- Simon KR, Merz EC, He X, Desai PM, Meyer JS, & Noble KG (2021). Socioeconomic factors, stress, hair cortisol, and white matter microstructure in children. Developmental Psychobiology, 63, e22147. 10.1002/dev.22147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L (1988). Reciprocal relation between parent–child distance and pubertal maturation. Developmental Psychology, 24, 122–128. 10.1037/0012-1649.24.1.122 [DOI] [Google Scholar]
- Straus MA, Hamby SL, Finkelhor D, Moore DW, & Runyan D (1998). Identification of child maltreatment with the parent–child conflict tactics scales: Development and psychometric data for a national sample of American parents. Child Abuse & Neglect, 22, 249–270. 10.1016/S0145-2134(97)00174-9 [DOI] [PubMed] [Google Scholar]
- Thijssen S, Collins PF, & Luciana M (2020). Pubertal development mediates the association between family environment and brain structure and function in childhood. Development and Psychopathology, 32, 687–702. 10.1017/S0954579419000580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traub A, Hiltonsmith R, & Draut T (2016). The parent trap: The economic insecurity of families with young children (p. 2100). New York: Demos. [Google Scholar]
- Ueda K, Fujimoto G, Ubukata S, & Murai T (2017). Brodmann areas 11, 46, and 47: Emotion, memory, and empathy. Brain and Nerve = Shinkei Kenkyu No Shinpo, 69, 367–374. 10.11477/mf.1416200753 [DOI] [PubMed] [Google Scholar]
- Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, & Fieremans E (2016). Denoising of diffusion MRI using random matrix theory. Neuroimage, 142, 394–406. 10.1016/j.neuroimage.2016.08.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, & Anderson SG (2010). Low-income single mothers’ community violence exposure and aggressive parenting practices. Children and Youth Services Review, 32, 889–895. 10.1016/j.childyouth.2010.02.010 [DOI] [Google Scholar]
- Zilanawala A, & Pilkauskas NV (2012). Material hardship and child socioemotional behaviors: Differences by types of hardship, timing, and duration. Children and Youth Services Review, 34, 814–825. 10.1016/j.childyouth.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Open Science Framework preregistration can be viewed at https://osf.io/c3tf8. Data will be openly available at https://nda.nih.gov/edit_collection.html?id=2106.


