Abstract
Background:
The Minamata Convention on Mercury (Article 4) prohibits the manufacture, import, or export of skin-lightening products containing mercury concentrations above . However, there is a lack of knowledge surrounding the global prevalence of mercury-added skin-lightening products.
Objective:
The objective of this study was to increase our understanding of worldwide human mercury exposure from skin-lightening products.
Methods:
A systematic search of peer-reviewed scientific literature was performed for relevant articles in four databases (PubMed, Web of Science Core Collection, Scopus, and TOXLINE). The search strategy, eligibility criteria, and data-extraction methods were established a priori. The search identified 2,303 unique scientific articles, of which 41 were ultimately deemed eligible for inclusion after iterative screens at the title, abstract, and whole-text levels. To facilitate data extraction and synthesis, all papers were organized according to four data groups a) “Mercury in products,” b) “Usage of products,” c) “Human biomarkers of exposure,” and d) “Health impacts.”
Results:
This review was based on data contained in 41 peer-reviewed scientific papers from 22 countries worldwide published between 2000 and 2022. In total, we captured mercury concentration values from 787 skin-lightening product samples [overall pooled central median mercury level was ; interquartile range (IQR): 0.02–5.9] and 1,042 human biomarker measurements from 863 individuals. We also synthesized usage information from 3,898 individuals and self-reported health impacts associated with using mercury-added products from 832 individuals.
Discussion:
This review suggests that mercury widely exists as an active ingredient in many skin-lightening products worldwide and that users are at risk of variable and often high exposures. These synthesized findings identify data gaps and help increase our understanding of the health risks associated with the use of these products. https://doi.org/10.1289/EHP10808
Introduction
Mercury is a global pollutant of concern to human health.1–3 All populations worldwide are likely exposed to varying degrees of mercury.4 Several inorganic and organic forms of mercury exist naturally in the environment (i.e., metallic or elemental), and although there are differences in their toxicity, all forms can adversely impact human health including the nervous, cardiovascular, and immune systems.1,2,4,5
Human exposures to mercury outside of occupational settings (e.g., artisanal and small-scale gold mining and dentistry) are largely realized through the consumption of mercury-contaminated seafood and contact with certain products that contain mercury (e.g., dental amalgam, pesticides, broken fluorescent light bulbs, and batteries).1,4,6 Skin-lightening cosmetics also contain mercury, and there are increasing concerns about the dangers posed by frequent usage of such products.7 In certain cosmetic products, organic mercury compounds, including ethylmercury, methylmercury, and phenyl mercuric salts, may be used as preservatives.8–11 Further, inorganic mercury salts [e.g., mercurous chloride (calomel), mercuric chloride, mercurous oxide, ammoniated mercuric chloride and mercuric iodide, and ammoniated mercury], may be purposefully added into skin-lightening cosmetic products as they interfere with the tyrosinase enzyme inhibiting the skin from producing melanin, resulting in lighter skin pigmentation.12–14 Because mercury is absorbed through the skin, mercury poisoning may arise after use of a skin-lightening product.15 Exposure to inorganic mercury has been associated with renal toxicity, neurological abnormalities, and dermal rashes.16 Many women who use these products are of childbearing age, and the potential transfer of mercury from mother to fetus could have implications resulting in neurological and nephrological disorders.17,18
Skin-lightening products exist in various forms (most commonly creams and soaps), and they are used without medical supervision.11 Use of skin-lightening products is practiced worldwide, particularly in African, Asian, and Caribbean nations, as well as in darker-skinned communities in Europe and North America.3,6,19 Individuals from these communities feel motivated to use skin-lightening products to increase attractiveness, remove existing blemishes, improve skin texture, and impress their peers.20–22 Societal perception of beauty continues to perpetuate the notion that lighter skin is more desirable and creates more social and professional opportunities.23 The market for skin-lightening products is increasing as the demographics of users continues to expand, making it one of the fastest-growing beauty industries globally.24 These products remain easily accessible at beauty supply stores, ethnic markets, and online.25 The illegal nature of skin-lightening products makes it difficult to differentiate the established brands because there is a strong financial motive to produce counterfeit products.26,27 As a result of the decentralized nature of these products, it is difficult to control mercury-added skin-lightening products, because countries often lack infrastructure to monitor their import and export.
The entry into force of the Minamata Convention on Mercury on 16 August 2017 was a global environmental agreement by governments set in place to reduce emissions and releases of mercury and mercury compounds to protect human health and the environment (Article 1).28 Article 4 of the Minamata Convention mandated that parties must ban the manufacture, import, and export of products that include creams and soaps with a mercury content higher than after 2020.28 This concentration of mercury in cosmetics has already formed a basis for regulatory action by organizations such as the U.S. Food and Drug Administration (U.S. FDA)29 and Health Canada.30 Despite such limits (and similar ones set by many governments worldwide), many mercury-added skin-lightening products have mercury levels that exceed .10 Issues detailed in the previous paragraphs, coupled with our limited knowledge concerning the global prevalence of these products and their mercury content, pose challenges for researchers and regulators. Accordingly, the overall objective of this study was to increase our understanding of worldwide human mercury exposure from skin-lightening products. This objective was realized through a systematic review of the peer-reviewed scientific literature centered on the following four data groups: a) “Mercury in products” for the characterization of mercury in products (e.g., concentration, speciation, types of products); b) “Usage of products,” for users of skin-lightening products (e.g., socioeconomic variables, demographics, exposure variables); c) “Human biomarkers of exposure” for human biomarkers of skin-lightening users (e.g., hair, urine, blood); and d) “Health impacts” for associated health outcomes of usage.
Methods
Protocol and Search Strategy
A protocol for the search strategy was developed in July 2018. The search was designed based on a review of key resources [Preferred reporting items for systematic reviews and meta-analyses (PRISMA); http://www.prisma-statement.org/],31 including a systematic review concerning methylmercury exposure from seafood consumption32 and a recent review concerning worldwide mercury exposures as part of the 2018 United Nations (UN) Global Mercury Assessment.4 The protocol defined study selection criteria and the type of data that would be extracted for each study and was uploaded to PROSPERO on 28 April 2020 (CRD42020160299).
A systematic search of peer-reviewed scientific literature was used to identify relevant studies. An electronic search was performed in four databases (PubMed, Web of Science Core Collection, Scopus, and TOXLINE) as outlined in the PRISMA flow diagram (Figure S1). The search strategy included two Boolean search phrases that were combined with AND: #1 – mercury OR Hg OR MeHg OR *Mercur*; and #2 - cosmetic* OR lotion* OR cream* OR soap* OR shampoo* OR makeup OR whitening OR lightening OR bleaching OR mascara. In addition to the systematic search, we considered gray literature and references cited in read works. We focused on works that were written in English, French, Spanish, and Portuguese.
Study Eligibility and Selection Criteria
Scientific papers were identified and reviewed in July 2018, with the search performed again in November 2020 and finalized in April 2022. We restricted studies to those published in or after 2000 and only on original articles. After the initial literature search, duplicates were removed, and all unique references were imported to Mendeley. Next, these publication titles were reviewed to determine their relevance from which unique citations were identified to have their abstracts read. Publications excluded at this “abstract screening” stage were labeled with one of the following primary reasons: “No abstract,” “Background paper,” “Foreign language,” “Wrong drug,” “Wrong outcome,” “Wrong population,” “Wrong population type,” “Wrong study design,” and “Wrong study duration.” Medical case reports were excluded from the main study but were tagged so that they could be qualitatively summarized separately from the formal systematic review. This abstract screening activity was carried out by dividing the total number of publications into two parts, and each part was screened by three reviewers (J.E., V.S., and A.S.).
For full-text screening, the inclusion criteria was whether we could extract data or information (see “Data Extraction and Analysis” section for specific variables) in accordance with four data groups: a) “Mercury in products” for the characterization of mercury in products (e.g., concentration, speciation, types of products); b) “Usage of products,” for users of skin-lightening products (e.g., socioeconomic variables, demographics, exposure variables); c) “Human biomarkers of exposure” for human biomarkers (e.g., mercury measures in hair, urine, or blood) of skin-lightening users; and d) “Health impacts” for associated health outcomes of usage. Based on initial reviews of the available data as well as consideration of the paper’s overall focus (i.e., exposure assessment), we decided to focus the first (“Mercury in products”) and third (“Human biomarkers of exposure”) data groups on papers in which quantitative data could be extracted, and papers from the second (“Usage of products”) and fourth (“Health impacts”) groups on papers in which data could be explored in a semiquantitative manner in support of the overall objective.
Data Extraction and Analysis
To summarize mercury concentrations in skin-lightening product data and human biomarkers, the approach of Sheehan et al.32 was followed. Briefly, we reported on two summary distributions [central (median) and upper] for each skin-lightening product by pooling the respective data together over relevant studies. When studies had multiple measurements for central exposures, median measurements were favored over the mean. Throughout this report, we refer to total mercury as “mercury” measurements in a given skin-lightening product or human biomarker sample type.
“Mercury in products” data group.
Studies were organized by location [country, World Health Organization (WHO) region] of product manufacture and purchase and categorized into type of product (cream, soap, facial cream, other), and method of purchase (in store, online). Cream products were separated into “creams” and “facial creams” because there are differences in the skin’s texture between the body and the face. Products categorized as “other” included scrubs, balms, oils, pills, and personal mixtures as well as products that were not specifically labeled. Personal mixtures of multiple products and at-home remedies are commonly used to increase potency and whitening effect. From each study, data was extracted on sample size (number) and volume of sample (milliliters or grams), along with key information on the mercury measurement including the inorganic (ammoniated mercury) and organic mercury compounds (phenyl mercuric salts, thiomersal), testing instrumentation [cold vapor-atomic absorption spectrophotometry (CVAAS), X-ray fluorescence (XRF), inductively coupled plasma–mass spectrometry (ICP-MS), etc.], analytical quality control (reported detection limit, accuracy, precision), and a measure of the central mercury concentration values (mean or median, as micrograms per gram). Skin-lightening cosmetic samples assessed using XRF instrumentation and commercial screening kits were excluded from the data analysis due to concerns surrounding the reliability of the measures they report, though the information was retained in our database for the user community. Products manufactured in the same geographic location (country, WHO region) and of the same product type were collectively grouped together. We considered mercury as an active ingredient in samples with concentrations greater than . Nondetectable mercury concentration values were entered as half the detection limit as recommended in the U.S. Environmental Protection Agency’s “Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments.”33 In addition to mercury, we took this opportunity to inventory other potentially toxic elements and chemical bleaching agents found in skin-lightening products, as reported in the included studies.
“Usage of products” data group.
A review publication by Sagoe et al.34 was identified and adopted as a framework for this data group. Because usage patterns are irrespective of product ingredients (i.e., mercury, hydroquinone, topical corticosteroids), usage patterns of all skin-lightening product users were considered. We extracted information on study type, type of bleaching products, and demographics of users (i.e., location, sex, age, marital status, occupation, education level, average monthly income), and key factors surrounding exposure characteristics were evaluated: a) application (i.e., face, whole body), b) frequency of application (per day), c) quantity used (grams per month); and d) duration (months).
“Human biomarkers of exposure” data group.
For biomarkers (hair, urine, blood) of mercury exposure, we extracted data on the populations’ characteristics (age, sex, sample size) and location (country, WHO region), as well as mercury exposure measurements [mercury speciation, testing instrumentation (CVAAS, XRF, ICP-MS, etc.)], analytical quality control (reported detection limit, accuracy, precision, testing instrumentation), and a measure of central tendency (mean or median).
“Health impacts” data group.
Reported health impacts associated with the use of mercury-added skin-lightening products were reviewed in a semiquantitative manner. Key metadata (e.g., location, type of study, age, sample size, sex) and measurement information (level of mercury in biomarker, associated health effect) were recorded as available. Individuals reporting health impacts in combination with other mercury-containing products or skin-lightening products not containing mercury were not included.
Quality Assessment
To help increase understanding of the evidence base, we assessed study quality for the articles captured in the “Mercury in products” and “Human biomarkers of exposure” data groups. This assessment was performed in consideration of the framework published by Hu et al.,35 the National Toxicology Program’s Office of Health Assessment and Translation (OHAT) framework,31 as well as the approach taken in the 2018 UN Global Mercury Assessment.4 Study quality was assessed by nine items that were grouped into three categories (Excel Table S1): a) mercury exposure detection bias [i.e., measurement instrument, use of reference material, replicate measures, acceptable limit of detection (LOD)]; b) selection bias (i.e., selection method, sample size, mercury exposure characteristics); and c) other biases (i.e., demographics, key descriptive measures). Each of the nine items was scored as either high, medium, or low quality. One author (A.B.) independently scored each paper and consulted with other authors (J. E., A. S., and V. S.) in cases where additional consensus was required.
Results
Overview of Studies
Through four database searches, 2,303 unique articles were found of which 532 were retained following a review of their titles, and 207 were retained following a review of their abstracts (Figure S1). In the end, this review was based on data contained in 41 peer-reviewed scientific papers from 22 countries published between 2000 and 2022 (Table 1). In total, we captured mercury concentration values from 787 skin-lightening product samples, and 1,042 biomarker measurements from 863 individuals. The average sample size across “Mercury in products” studies was 36 products, with a range of 2 to 191 skin-lightening products reported on in each study. The average sample size for the “Human biomarkers of exposure” group excluding nonusers (i.e., family members, control groups) was 88 biomarker samples, with a range between 11 to 282 biomarker samples per study. In addition, we compiled usage information from 3,898 individuals and self-reported health impacts associated with using mercury-added products from 832 individuals.
Table 1.
Overview and count of studies included in this review.
| Data Group | ||||
|---|---|---|---|---|
| “Mercury in products” | “Usage of products” | “Human biomarkers of exposure” | “Health impacts” | |
| Number of studies included | 25 | 16 | 9 | 5 |
| Study analysis type | Quantitative | Semiquantitative | Quantitative | Semiquantitative |
| Summary of data group | Studies analyzing mercury concentrations in skin-lightening cosmetic samples were collected from 16 countries/territories with sample sizes ranging from 2 to 191 per study. | Studies were collected from nine countries to describe skin-lightening usage patterns (e.g., frequency, duration, application, quantity) among users. | Studies were collected from six countries/territories analyzing mercury concentrations in blood, urine, and hair with sample sizes ranging between 11 and 465 samples per study. | Studies were collected from four countries/territories observing associated health effects from mercury-added skin-lightening products. |
| Total number of items or individuals | 787 | 3,898 | 1,042 | 832 |
| Africa (# of studies) | 5 | 5 | 1 | 0 |
| Americas (# of studies) | 5 | 3 | 3 | 3 |
| Eastern Mediterranean (# of studies) | 7 | 3 | 1 | 0 |
| Europe (# of studies) | 1 | 0 | 0 | 0 |
| South-East Asia (# of studies) | 1 | 1 | 1 | 0 |
| Western Pacific (# of studies) | 6 | 1 | 3 | 2 |
Studies from the two quantitative data groups (i.e., mercury levels in products, and human biomonitoring studies) were largely situated, according to WHO region locations, in the Eastern Mediterranean (27.6%), Western Pacific (24.1%), the Pan American (24.1%), and Africa (17.2%) regions, with fewer studies in South-East Asia (3.4%) and Europe (3.4%) (Table 1). Studies concerning use of products and health impacts were largely situated in Africa (31.3%) and Pan-America (31.3%), Eastern Mediterranean (18.8%), Western Pacific (12.5%), with the fewest in South-East Asia (6.3%) and none from Europe.
Quality Assessment
In terms of exposure detection bias (items 1–4 in the Study Quality Table; Excel Table S1), 1 out of 25 studies in the “Mercury in products” group and 3 out of 9 studies in the “Human biomarkers of exposure” were classified as having a high study quality in these four items (Excel Table S2). When reported, the most commonly used mercury analyzer for skin-lightening products was CVAAS (; 59.2%), followed by atomic absorption spectroscopy (AAS) (; 11.9%), and inductively coupled plasma–optical emission spectrometry (ICP-OES) (; 9.9%) (Table S1). For human biomarker measurements, the most commonly used instrument was particle induced X-ray emission (PIXE) (; 14.2%), followed by advanced mercury analyzer (AMA) (; 6.0%), and AAS (; 3.8%) (Table S2). Notably, a detection instrument was not listed for 768 (73.7%) biomarker measurements. Most “Mercury in products” (; 84%) and “Human biomarkers of exposure” (; 80%) studies considered analytical quality control measures by fully reporting on three of the four key items (measurement instrument, accuracy, precision, and detection limit). In total, eight quantitative studies reported no quality control measures because product and human biomarker samples in these studies were sent to external laboratories for analysis. As a result, these studies were carefully examined and deemed appropriate for inclusion. In terms of selection bias, all skin-lightening products and biomarker samples were conveniently sampled from stores/markets and individuals, respectively.
We note that all studies used convenience sampling methods to obtain human biomarkers and skin-lightening products (item 5 in the Study Quality Table; Excel Table S1). Sixteen out of 25 studies in the “Mercury in products” group and 3 out of 9 studies in the “Human biomarkers of exposure” were classified as having a high study quality, having provided extensive details on how samples were obtained (Excel Table S2). For the “Mercury in products” data group, 4 studies (16%) tested products, 11 studies (44%) tested between 10 and 30 products, and 10 studies (40%) tested products. For the “Human biomarkers of exposure” data group, five studies tested biomarker samples, two studies (22.2%) tested between 50 and 200 biomarkers, and two studies (22.2%) tested biomarker samples. User demographics (i.e., age, sex, location) were reported on in all the “Human biomarkers of exposure” studies; however, most (84%) “Mercury in products” studies did not report on user demographics. Mercury exposure characteristics were only reported in four (16%) “Mercury in products” studies and in five (55.6%) “Human biomarkers of exposure” studies. In terms of other study quality considerations, only three (12%) “Mercury in products” studies did not list any descriptive measures (i.e., mean, upper values), whereas all “Human biomarkers of exposure” studies listed descriptive measures.
“Mercury in Products” Data Group
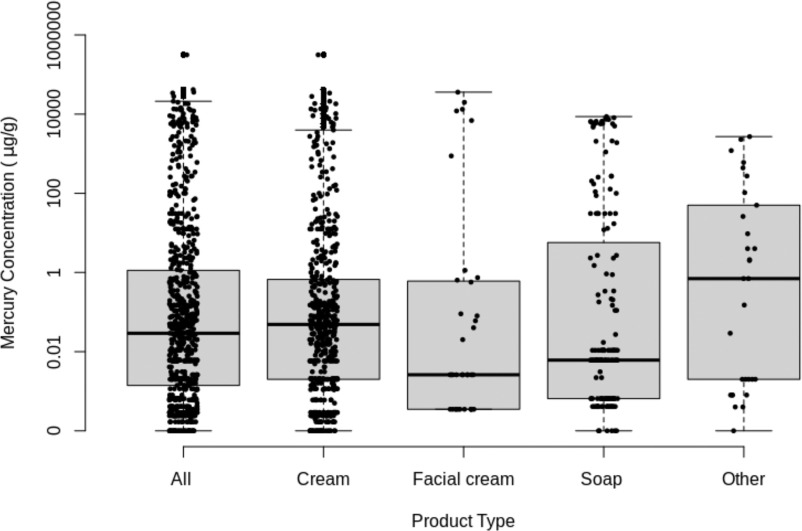
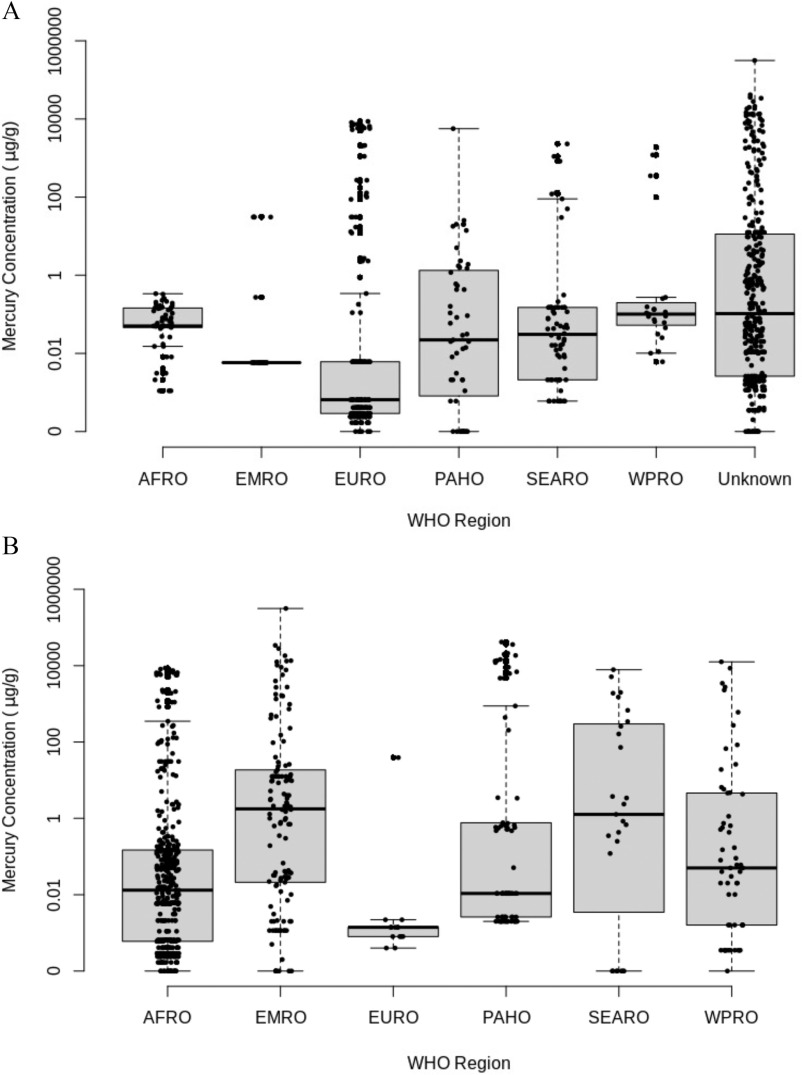
In the analysis of skin-lightening products, we grouped products according to product type (Figure 1), and WHO regions of purchase (Figure 2). A total of 787 skin-lightening products were identified from 25 studies that included creams () (70.1%), soaps () (19.3%), facial cream () (4.6%), and other products () (6.0%) (Table 2; Excel Table S3). The overall pooled central median mercury concentration in skin-lightening products was (IQR: 0.02 and ) (Figure 1). Mercury was an active ingredient (mercury concentration ) in 196 (24.9%) products. Although the values of mercury in the various products overlapped tremendously, the maximum reported values varied with the highest amount found in a cream (), followed by a facial cream (), soap (), and an item from the other products group (). The median mercury content in products manufactured in the Eastern Mediterranean region was more than 10-fold higher than average values reported from the other WHO regions (Figure 2A). In terms of mercury content in products purchased from a specific geographic region, median levels in the Eastern Mediterranean and South-East Asian regions were about 10-fold higher than found in other regions (Figure 2B).
Figure 1.
Box plots showing mercury concentrations (micrograms mercury per gram) found in all 738 skin-lightening products (as well as in creams, facial creams, soaps, and other categorized items). We excluded 49 skin-lightening products (32 creams and 17 “other” items) because mercury concentrations were only available for these as ranges and not discrete values. The box plot midline represents the median value, the box limits represent the IQR, and the lower and upper whiskers are 1.5 times the 25th and 75th percentile values, respectively. See Excel Table S3 for all the data. Note: IQR, interquartile range.
Figure 2.
Box plots showing mercury concentrations (micrograms mercury per gram) in 738 skin-lightening products (creams, facial creams, soaps, other) across six WHO regions where products were (A) manufactured and (B) purchased. The box plot midline represents the median value, the box limits represent the IQR, and the lower and upper whiskers are 1.5 times the 25th and 75th percentile values, respectively. See Excel Table S3 for all the data. Note: AFRO, African Region; EMRO, Eastern Mediterranean Region; EURO, European Region; IQR, interquartile range; PAHO, Pan American Health Region; SEARO, Southeast Asia Region; WHO, World Health Organization; WPRO, Western Pacific Region.
Table 2.
Summary of mercury concentrations from the “mercury in products” data group.
| Reference | Country of purchase | Year of purchase | Number of products | Product types | Median mercury concentration () | Range of mercury concentration () |
|---|---|---|---|---|---|---|
| Harada et al.39 | Japan | 1998 | 14 | Soap | 3,900 | 0.11–7,400 |
| Kinabo41 | Tanzania | 2001–2003 | 60 | Various (cream, soap) | 2.5 | 0–8,665 |
| Murphy et al.26 | USA | 2007 | 19 | Cream | 0.51 | 0.1–12,590 |
| Wang and Zhang67 | China | 2011 | 17 | Other | 0.28 | NA–0.561 |
| Maneli et al.57 | South Africa | 2013 | 22 | Various (cream, soap, other) | 40 | 0.15–2,300 |
| Gbetoh and Amyot68 | Canada | 2013–2014 | 191 | Various (cream, soap) | 0.0083 | 0.000068–8,370 |
| Ho et al.69 | Malaysia | 2014–2015 | 20 | Facial cream | 0.00025 | 0.00025–1.13 |
| Kanwal et al.51 | Japan | 2016 | 10 | Various (cream, soap) | 2.35 | 0.3–34,000 |
| Mohammed et al.70 | Trinidad and Tobago | 2016 | 15 | Cream | 0.575 | 0.051–14,508 |
| Selvaraju et al.71 | Malaysia | 2018 | 15 | Cream | 0.03 | 0.0015–6.5 |
| Agorku et al.72 | Ghana | NA | 62 | Various (cream, soap) | 0.053 | 0.001–0.337 |
| Zainy73 | Saudi Arabia | NA | 19 | Cream | 12.5 | 12.5–5,739 |
| Peregrino et al.60 | Mexico | NA | 16 | Facial cream | 0.0025 | 0.0025–35,824 |
| Al-Saleh et al.74 | Saudi Arabia | NA | 34 | Cream | 0.025 | 0–314,387 |
| Yang et al.75 | Taiwan | NA | 3 | Other | 273 | 0–602 |
| Salama76 | Saudi Arabia | NA | 2 | Various (cream, other) | 0.018 | 0.007–0.029 |
| McKelvey et al.66 | USA | NA | 12 | Various (cream, soap, other) | 5,450 | 3.37–41,600 |
| Ashraf et al.77 | Pakistan | NA | 15 | Cream | 1,781 | 0–18,210 |
| Abbas et al.45 | Indonesia | NA | 27 | Cream | 1.27 | 0–7,834 |
| Voegborlo et al.78 | Ghana | NA | 69 | Cream | 0.013 | 0.0005–0.311 |
| Alqadami et al.79 | Saudi Arabia | NA | 15 | Other | 2 | 0.0019–2,700 |
| Alqadami et al.80 | Saudi Arabia | NA | 34 | Cream | 2.205 | 0.00105–2,745 |
| Ababneh and Al-Momani81 | Jordan | NA | 32 | Cream | 0.66 | NA–26,400 |
| Amponsah et al.82 | Ghana | NA | 50 | Cream | 0.075 | 0.006–0.8 |
| Cristaudo et al.83 | Italy | NA | 14 | Various (cream, soap, other) | 0.0013 | 0.0003–39 |
Note: NA, not available.
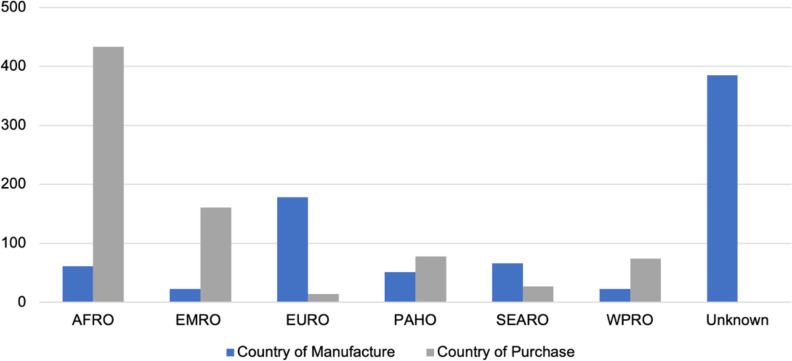
Most skin-lightening products were purchased in stores () (95.3%), with the procurement of the rest not reported by authors or mislabeled. None of the products reported the presence of phenyl mercuric salts or thiomersal, whereas 0.4% of creams reported containing ammoniated mercury. Skin-lightening products were manufactured in 33 countries, mainly in the Europe (; 22.6%), South-East Asia (; 8.4%), and Africa (; 7.8%) regions vs. those in the Pan American (; 6.5%), Western Pacific (; 2.9%), and Eastern Mediterranean (; 2.9%) regions (Figure 3). The manufacture location was unknown or unlisted for nearly half (; 48.9%) of the products. Products were purchased in 20 countries in the Africa (; 55%), Eastern Mediterranean (; 20.5%), Pan American (; 9.9%), Western Pacific (; 9.4%), South East Asian (; 3.4%) and Europe (; 1.8%) regions. Despite Europe manufacturing the largest quantity of products relative to the other regions, only 1.8% of skin-lightening products were purchased in Europe (). In terms of the year that the product was sampled, data was available for 48.7% of the products (). An examination of the results over the 22-y sampling period showed no apparent trends in changing mercury concentrations (Table 2).
Figure 3.
Bar graph showing the number of the 787 skin-lightening products manufactured and purchased according to WHO regions. See Excel Table S3 for all data. Note: AFRO, African Region; EMRO, Eastern Mediterranean Region; EURO, European Region; PAHO, Pan American Health Region; SEARO, Southeast Asia Region; WHO, World Health Organization; WPRO, Western Pacific Region.
A total of 19 out of 25 studies identified at least one skin-lightening product with mercury as an active ingredient (i.e., mercury concentration ). In addition to mercury, several studies have assessed other potentially toxic elements and chemical bleaching agents in skin-lightening products. Specifically, we note that 12 studies captured here evaluated only mercury, whereas 13 studies evaluated mercury in addition to other common active skin-lightening ingredients [e.g., hydroquinone, clobetasol propionate, kojic acid, corticosteroids (e.g., betamethasone, clobetasol propionate)] or trace elements (e.g., aluminum, arsenic, bismuth, cadmium, chromium, cobalt, copper, lead, iron, nickel, manganese, palladium, thallium, titanium, titanium dioxide, zinc) (Table S3).
“Usage of Products” Data Group
After removing duplicate studies identified from our literature search with those found in the Sagoe et al.34 review publication, a total of 16 studies were included here for analysis. A total of 3,898 individuals surveyed from 16 studies were included in the “Usage of products” data group (Excel Table S4). Not all the individuals were surveyed on all four key exposure characteristics (e.g., application, frequency, duration, quantity), and thus there are not 3,898 data points for each of the exposure characteristics. In terms of application, a total of 1,708 individuals from 7 studies reported on application areas (e.g., face, body). Users were most likely to apply skin-lightening products to their face (53.1%) in comparison with “other” application areas (e.g., hands, neck, nonexposed sun areas) (22.4%) and whole body (18.6%). In one study, the sum of percentages exceeds 100%, which may be attributed to individuals reporting on more than one application area.36 In terms of frequency, we reported on information from a total of 2,525 individuals from 10 studies. From these data, most individuals were using products less than one time to three times per day (87.2%), more than three times per day (0.8%), and other unspecified times (8.6%). In terms of duration, we collected information from a total of 2,098 individuals from 10 studies. The duration of usage of skin-lightening products ranged between 1 month to 30 y. Out of 1,187 individuals surveyed from five studies, a total of 385 (32.4%) individuals used skin-lightening products for less than a year,37–40 323 (27.2%) individuals used products for 1 to 5 y,37–41 133 (11.2%) individuals used products for 6 to 10 y,38–41 168 (14.2%) individuals used products for 11 to 20 y,38,40 100 (8.4%) individuals used products for longer than 20 y,38 and 78 (6.6%) individuals used it for an unknown period of time.37,38 A total of 4 studies reported on 1,008 individuals that used skin-lightening products for a duration of 2 months to 30 y,36,42–44 and a total of 157 and 105 individuals from two studies reported a mean duration period of between 20 and 26 months, respectively.42,45 In terms of quantity, we collected information from 1,015 individuals from four studies on the number of skin-lightening products used per month. From a total of 335 individuals, 94 (28%) individuals used less than and up to of product per month, 160 (47.8%) individuals used between 11 to of product per month, and 81 (24.2%) individuals used of product per month.37,45 In two studies, 703 (58.7%) individuals used between 2 and of product per month, with 194 (16.2%) using a median of of product per month and 509 (42.5%) individuals using a median of of product per month.36,46
“Human Biomarkers of Exposure” Data Group
A total of 1,042 biomarker samples [urine () 56.3%, hair () 26.1%, blood () 17.6%] were collected from 863 skin-lightening product users, family members of users, and control groups between 14 and 79 y of age (Table 3). Only three studies41,45,47 included control groups (e.g., nonusers, family members of users) to assess and compare biomarker concentrations.
Table 3.
Summary of mercury concentrations from the “human biomarkers of exposure” data group.
| Reference | Country/Territory | Type of biomarker | User type | Sample size (n) | Age range (median) | Total mercury concentration range (median) | No. of individuals above reference value | No. of individuals below reference value |
|---|---|---|---|---|---|---|---|---|
| McRill47 | USA | Urine | User | 71 | 14–72 (33.3)b | 57 | 14 | |
| McRill47 | USA | Urine | Family members | 18 | — | 9 | 9 | |
| Weldon49 | USA | Urine | User | 203 | 14–79 (32) | 159 | 44 | |
| McKelvey66 | USA | Urine | Usera | 13 | 21–51 | 13 | 0 | |
| Sin and Tsang48 | Hong Kong | Urine | User | 282 | 15–76 (35) | 154 | 128 | |
| Sin and Tsang48 | Hong Kong | Blood | User | 183 | 15–76 (35) | 119 | 64 | |
| Kanwal51 | Japan | Hair | User | 11 | 19–55 | 11 | 0 | |
| Abbas45 | Indonesia | Hair | User | 105 | 19–25 (21) | 95 | 10 | |
| Abbas45 | Indonesia | Hair | Nonuser | 43 | 19–25 (21) | 40 | 3 | |
| Kinabo41 | Tanzania | Hair | User | 20 | 13–33 (29) | 20 | 0 | |
| Kinabo41 | Tanzania | Hair | Nonuser | 20 | 15–33 (21) | 16 | 4 | |
| Harada39 | Japan | Hair | User | 11 | 20–39 (28) | 11 | 0 | |
| Fakour50 | Iran | Hair | User | 36 | 22–43 (27.3)b | b | — | — |
| Fakour50 | Iran | Hair | Nonusers | 26 | 22–43 (27.3)b | b | — | — |
Note: The upper reference values were: urine (), blood (), hair (). —, data not provided.
aIncludes nine known product users and four individuals suspected of using products.
bMean values (in parentheses) used because median values were not available.
Urine mercury levels.
Total mercury levels in urine across 569 skin-lightening product users from Hong Kong48 and U.S.–Mexican border states: Arizona,47 California,49 New Mexico,49 and Texas49 ranged between 0 and . About two-thirds (; 67.3%) of these individuals had urinary mercury levels greater than , which was identified as a reference value by all the studies. Urinary mercury levels were assessed in 18 family members (i.e., husbands and children) of mercury-added skin-lightening product users and 9 (50%) of their concentrations were above the reference value.
Blood mercury levels.
One study48 assessed total mercury levels in blood in addition to urinary mercury levels in 183 skin-lightening product users in Hong Kong. Blood mercury levels in users ranged from 0 to with an overall median of . This study used a reference value of from the Department of Health in Hong Kong to organize biomarker values. A total of 119 (65%) users had blood mercury levels above the reference value.
Hair mercury levels.
Total hair mercury levels across 183 skin-lightening product users in Japan, Indonesia, Tanzania, and Iran ranged between 0 and .39,41,45,50,51 We excluded participants from Iran () because mercury concentrations were not available as discrete values.50 We used hair mercury reference value ranges of , between , and adopted by Abbas et al.45 to organize biomarkers. Out of 147 users, a total of 103 (70.1%) individuals had hair mercury concentrations above , 34 (23.1%) individuals were between 1 to , and 10 (6.8%) individuals were below . The overall pooled central median of hair mercury concentrations was (IQR: 30.3–135.3) across 147 users.39,41,45,51 Out of 63 nonusers, 55 (87.3%) of individuals had hair mercury concentrations between , 7 (11.1%) individuals were below , and 1 (0.7%) individual was above .
“Health Impacts” Data Group
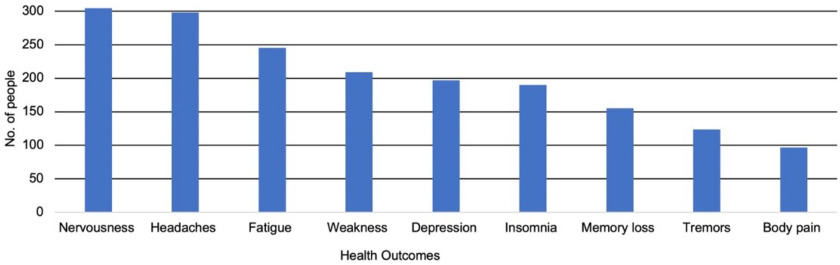
A total of 832 individuals from Kenya, the United States, Jamaica, and Hong Kong39,47–49,52 self-reported symptoms associated with potential mercury exposure. Nine individuals (81.8%) from Kenya39 experienced tremors, lassitude, vertigo, and neurasthenia as reported through a clinical examination. In Jamaica, about 139 individuals self-reported experiencing itchiness (; 39.6%), irritability (; 38.1%), and “other” (headaches, depression, and scars) (; 22.3%).52 In individuals in the United States47,49 and Hong Kong,48 the most self-reported outcomes included fatigue (), nervousness/irritability (), severe headaches (), weakness (), insomnia (), anxiety/depression (), memory loss (), tremors (), and body/joint pain () (Figure 4). Although most participants in the Unites States and Hong Kong had elevated mercury levels in urine and blood, 78% of participants in Hong Kong reported no symptoms.48 One study47 offered clinical evaluations for individuals with urinary mercury concentrations , but those who refused were still advised to seek a medical evaluation. No considerable health abnormalities were identified through the clinical evaluations.
Figure 4.
Graph of self-reported health outcomes from 684 individuals from United States47,49 and Hong Kong48 measuring biomarker samples to determine presence of mercury poisoning and other associated health impacts from a targeted skin-lightening product.
Through our search, we flagged case reports in which health outcomes were observed in association with the use of mercury-added skin-lightening products (Table S4). Although these were not included in our formal analysis, compiling and reporting the information has some value, because such case reports are detailed medical reports that describe novel or unusual occurrence in patients. Here, a total of 16 case report studies were compiled from 8 countries (Barbados, China, the United States, Belgium, the United Kingdom, Philippines, Mexico, and Germany), with 37 individuals (users and nonusers) between 10 months and 50 y old. The skin-lightening products mentioned had mercury concentrations between 1,762 and mercury (vs. , which was the overall pooled central median mercury concentration we calculated here) or contained 0.07%–6.5% mercury. Five adult individuals mentioned using products between 6 wk to 10 y before reporting symptoms. The case reports showed that some individuals had very high levels of total mercury in urine () and blood (), and inorganic mercury in hair (). Individuals mentioned in these case studies presented signs of mercury intoxication, nephrotic syndrome, membranous glomerulonephritis, and hypertension. Four cases of infants under the age of 5 y who presented signs of mercury intoxication after being in contact with their mothers who were using mercury-added skin-lightening products were reported.53–55 The youngest reported case was a 10-month-old toddler who was admitted to the hospital after presenting symptoms, including anorexia, hypertension, and regression in neuromotor skills after his mother used a skin-lightening product locally made in Mexico containing of mercury for 6 wk prior to symptom onset.55
Discussion
To our knowledge, this is the first systematic literature review characterizing the amount of mercury that human populations worldwide may be exposed to through the use of mercury-added skin-lightening products. In doing so, we conclude that mercury is still prevalent in many skin-lightening products in many countries worldwide and that some people and populations worldwide experience relatively high exposures to mercury from the use of such skin-lightening products. Our conclusions are based on the compilation, synthesis, and analysis of a data set that consists of 787 skin-lightening products (manufactured in 33 countries and purchased from 20 countries worldwide) and 1,042 mercury biomarker measurements taken from 863 individuals, along with supportive information on usage patterns from 3,898 individuals and self-reported health impacts from 832 individuals.
Mercury concentrations in skin-lightening products varied greatly among the products themselves, as well as among the studies and countries captured in our analysis. We were able to determine these variations from our review of data from 787 skin-lightening products [(i.e., creams (; 70.1%), soaps (; 19.3%), facial cream (; 4.6%), and other products (; 6.0%)]. These products had mercury levels ranging from 0 to , with an overall pooled central median mercury concentration of (IQR: ). Mercury concentrations in skin-lightening products varied across geographic regions, with the highest median concentrations found in products purchased from countries in the WHO Eastern Mediterranean, South-East Asia, and Western Pacific regions. Although we had a large data set to draw conclusions from, geographical data gaps were evident and there were relatively few studies per region. Most notable was the relative absence of data from Europe, Southeast Asia, and many Latin American countries even though these are regions with populations known to use skin-lightening products.34
In terms of the use of mercury-added skin-lightening products, the Sagoe et al.34 review publication reported that mercury was the second most popular bleaching agent globally after topical corticosteroids. Sagoe et al. reported that individuals age 30 y or younger had the highest prevalence of using skin-lightening products (55.9%), followed by individuals age 31–49 y (25.9%), and individuals age 50 y and above (6.1%). Based on their results, they concluded that the use of skin-bleaching transcends an individual’s education level, relationship/marriage status, employment, and pregnancy stage.34 In our study, we found great variability in skin-lightening product usage patterns across populations [i.e., application (i.e., face, whole body), frequency of application (per day), quantity used (g/month); and duration (months)]. Despite such variability, in general we conclude that most individuals who use these products apply skin-lightening items primarily on their face, typically from to 3 times per day, for less than a year, and in quantities of 11– per month. From this generalized conclusion, we can estimate mercury exposure for 1 y to be mercury through the following calculation: , which was the overall pooled median concentration . This estimate can vary significantly, based on factors including product type, application, and skin characteristics and with the recognition of the great variances in frequency, duration, and quantity of product used, as illustrated by our data.
From the 2018 UN Global Mercury Assessment, it was determined that in populations with background exposures to mercury, levels of the chemical in blood, hair, and urine are generally , , and respectively.4 In the current study, we observed that most of the biomarker measures (i.e., median values) in users of skin-lightening products greatly exceeded the aforementioned levels found in background populations. We draw this conclusion from 1,042 biomarker measurements taken from 863 skin-lightening product users, family members, and control groups. This is a relatively large mercury biomonitoring data set, though not one without challenges. Most of the data sets identified presented their biomarker data in relation to reference values set by their respective health departments.47–49 Although this made it challenging to make specific comparisons, we do note that the highest biomarker concentrations in these studies were well above their respective reference values. The studies with the largest sample sizes were case series studies of individuals with known exposure to different popular skin-lightening products in the United States () individuals from the U.S.–Mexican border region47,49 and () users from Hong Kong.48 In these cases, the studies were conducted after numerous incidents of adverse reactions associated with using these products were reported to health officials, and thus we conclude that these may represent worst-case scenarios. Deriving a control population in these studies is particularly challenging (e.g., there can be diverse sources of mercury exposures within a population, such as seafood consumption and personal dental amalgams), though among users in U.S.–Mexican border states (Arizona, California, New Mexico, and Texas) urinary mercury concentrations in 50% of immediate family members (i.e., husbands, children) were relatively high ().47 This finding illustrates that mercury exposures among those who are in close proximity with users (i.e., breastfeeding, shared sleeping spaces) may also be elevated. Similar cases have been seen among farmers who apply pesticides, where spouses and children of the farmers had detectable levels of glyphosate in their urine without being present during the application process.56 Therefore, in addition to the users themselves, it is important to study family members and individuals who are in close proximity to skin-lightening product users to better understand their exposures and associated health risks.
To characterize mercury exposures through the use of skin-lightening items, it is important to understand the trade of these products. Almost half (; 48.9%) of the products identified [i.e., cream (), soap (), facial cream (), other ()] did not indicate the country that they were manufactured in; thus, we could not identify where a large majority of skin-lightening products originate. When the manufacturing location was noted, Europe, Southeast Asia, and Africa were most prominent. Our review also noted countries in Europe (France, Italy, Spain, and the United Kingdom) as well as the United States as locations where skin-lightening products with high concentrations of mercury are manufactured.39,41,57 Regulating skin-lightening products for banned substances, particularly mercury in low-income countries, is particularly challenging because these regions may not have regulations, and those that do largely do not enforce them.58 In contrast, the European Union Rapid Alert System for dangerous nonfood products (RAPEX) identified about 266 skin-lightening cosmetic import violations across Europe between 2005 and 2018, of which 15.4% contained mercury.59 From our findings, skin-lightening products containing mercury as an active ingredient (mercury concentration ) were mainly manufactured in the Dominican Republic, Mexico, Pakistan, and Thailand, which are areas where the European Union RAPEX and the U.S. FDA have also reported import violations. We found that the skin-lightening products were purchased mainly in Africa, the Eastern Mediterranean, the Americas, and the Western Pacific regions (according to WHO classifications), and that this finding is in line with where these products are most commonly used.27,34,60 We found that the number of countries that manufacture products () is greater than the number of countries in which they were purchased (), supporting the need to strengthen enforcement of existing legislation and increase restrictions on mercury in skin-lightening products.
Despite performing a systematic review and compiling a relatively large data set to draw conclusions from, there are some limitations of this work (for which recommendations are offered). Foremost is that the studies identified in this review tended to focus on geographic regions with known prevalence and concerns surrounding mercury-added skin-lightening products. Our search was also limited to English databases, and it is possible that that we missed relevant publications in other languages, given the worldwide use of these products. Even in well-studied geographic regions, the sample sizes in individual studies were relatively small and data from only a few countries were reported, and these make it difficult to generalize more broadly. Most of the countries in which these products may be problematic are classified as being low- and middle-income, which may experience funding challenges and the likelihood of geographic bias in terms of getting studies published. Further, all studies included sampling bias (nonrandomization), making it difficult to generalize findings. As a result, there are not enough representative data to determine with high certainty the quantity of skin-lightening products containing mercury and the number of affected individuals worldwide. Nonetheless, our approach was able to derive a data set from which we can suggest that mercury is still widely prevalent as an active ingredient (mercury concentration ) in many skin-lightening products worldwide and that exposures among users is relatively high. These represent a good start, and moving forward there is a need for more studies that: a) are designed with careful consideration of pertinent quality control matters (e.g., Excel Table S1), including exposure information from reference populations; b) are situated in regions known to use skin-lightening products (e.g., from Minamata Initial Assessment reports), and also consider their availability online; c) focus on groups vulnerable to mercury, such as women of childbearing age; and d) consider ongoing efforts within the Minamata Convention Secretariat’s office that aim to harmonize the compilation and analysis of data that can help address policy questions that underpin effectiveness evaluation activities.
Most individual biomarker concentrations in studies were either not stated or grouped together based on reference values set out by various agencies and health departments. Aside from the case series studies, biomarker studies generally sampled small groups of individuals likely due to the difficulty in isolating individual users and the negative stigma surrounding use of skin-lightening products. There were very few studies collected that looked at control groups and nonusers in the same areas or in areas where there are no suspected concerns. It may be difficult to isolate mercury exposures from the use of skin-lightening products vs. other notable sources (e.g., dental amalgams).61 To help tease apart exposures to different chemical forms and sources of mercury, detailed survey instruments can be coupled with human biomarker measurements.4,6 Mercury in hair generally reflects exposure to organic methylmercury (usually from the diet), though external contamination by mercury-added skin-lightening products may complicate this measure and necessitate the use of advanced mercury speciation techniques.62 Mercury in urine reflects exposure to inorganic and elemental forms of mercury, and as a noninvasive and relatively inexpensive biomarker to sample, this may be particularly attractive in exposure assessment studies.4 Finally, mercury in whole blood can provide information on recent exposures to both organic and inorganic mercury.63,64 In general, total mercury values are measured in hair, urine, or blood, though studies that employ chemical speciation or mercury stable isotope measures can glean data from which deeper understandings may be realized. With relatively simple training on sampling principles and quality-assurance practices, human biomonitoring studies can be added to health studies in this area to help increase understanding of exposures.
We assessed study quality using the guidance from the framework published by Hu et al.35 and OHAT31 and found numerous issues in terms of exposure detection (e.g., the need for more information on quality control techniques, including the use of reference materials and replicate measures, especially for studies that sent samples to external laboratories for analysis), selection bias (e.g., need studies to carefully report on sampling techniques and breakdown of individuals concentrations of mercury in biomarker samples and include more information on mercury exposure characteristics), and other study quality considerations (e.g., studies need to have more information on user demographics and concentrations of mercury that individuals are using to better understand mercury levels in biomarkers). As an example, a priori we had excluded from our analysis 134 skin-lightening product samples from three studies10,26,52 that used XRF analyzers and commercial screening kits (though retained them in the database). There are many concerns surrounding the validity and reliability of measurements taken from XRF instruments and screening kits because they do not have low detection limits or analytical reliability like CVAAS, ICP-MS, or ICP-OES.65 Moving forward, we advocate that laboratories strengthen their capacity to test these products using higher quality measures.
The Minamata Convention is driven by protecting human health (i.e., Articles 1, 16, 17, 19). To increase public awareness and education (Article 18), one approach is to educate the public on the human health risks associated with exposure to mercury and mercury compounds in collaboration with relevant intergovernmental and nongovernmental organizations.28 Advocacy campaigns run by national health authorities, governments, and members of the public need to work together to educate users about the potential dangers of using mercury-added skin-lightening products. National programs to harmonize biomonitoring of exposed individuals need to be pursued to increase our understanding of human exposures. Although the skin-lightening products industry generates billions of dollars annually, the products identified in this review represent a very small portion of the global inventory, and so there is a need to work collaboratively to better monitor the presence of mercury in such products.24 We suggest that governments collaborate with other stakeholders to create inventories of products with mercury, create (or enforce) legislation, and establish standards (e.g., contact information of manufacturer, country of manufacture, ingredient list) for imported products. We believe that there is a need for all retailers (e.g., stores, vendors, online) to be monitored by law enforcement and required to remove products that contain harmful levels of mercury.
Conclusions
The objective of this study was to increase our understanding of worldwide human mercury exposure from the use of skin-lightening products. To achieve this, we conducted a systematic review to collect and analyze data from 41 peer-reviewed papers, within which we further examined mercury concentrations in human biomarkers and skin-lightening product samples, along with usage patterns and associated health impacts. From this work, we can conclude that mercury widely exists as an active ingredient in skin-lightening products and that there is large variability in human exposures. Although knowledge gaps and limitations exist (e.g., nonrandom selection bias, geographic regions with no data), these synthesized findings help increase our understanding of potential exposures worldwide to mercury through the use of these products. In addition, we believe that the information in this study will be critical in helping regulatory agencies and intergovernmental organizations, particularly the Minamata Convention on Mercury Secretariat and the WHO, better understand the global prevalence of mercury-added skin-lightening products to better promote actions that can help eliminate the production and use of such products.
Supplementary Material
Acknowledgments
The authors thank J. Eng for technical and administrative support.
The authors acknowledge funding support from the Canada Research Chairs Program and the PURE CREATE program funded by Natural Sciences and Engineering Research Council of Canada’s (NSERC).
References
- 1.Eagles-Smith CA, Silbergeld EK, Basu N, Bustamante P, Diaz-Barriga F, Hopkins WA, et al. 2018. Modulators of mercury risk to wildlife and humans in the context of rapid global change. Ambio 47(2):170–197, PMID: , 10.1007/s13280-017-1011-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ha E, Basu N, Bose-O’Reilly S, Dórea JG, McSorley E, Sakamoto M, et al. 2017. Current progress on understanding the impact of mercury on human health. Environ Res 152:419–433, PMID: , 10.1016/j.envres.2016.06.042. [DOI] [PubMed] [Google Scholar]
- 3.WHO (World Health Organization). 2019. Preventing Disease through Healthy Environments: Mercury in Skin Lightening Products. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 4.Basu N, Horvat M, Evers DC, Zastenskaya I, Weihe P, Tempowski J. 2018. A state-of-the-science review of mercury biomarkers in human populations worldwide between 2000 and 2018. Environ Health Perspect 126(10):106001–106014, PMID: , 10.1289/EHP3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO. 2017. Mercury and Health. https://www.who.int/news-room/fact-sheets/detail/mercury-and-health [accessed 28 March 2022].
- 6.UNEP (United Nations Environmental Programme)/WHO. 2008. Guidance for Identifying for Populations at Risk from Mercury Exposure. http://www.who.int/foodsafety/publications/chem/mercuryexposure.pdf [accessed 30 January 2021].
- 7.WHO. 2019. Mercury in Skin Lightening Products. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 8.Al-Saleh I, Al-Doush I. 1997. Mercury content in skin-lightening creams and potential hazards to the health of Saudi women. J Toxicol Environ Health 51(2):123–130, PMID: , 10.1080/00984109708984016. [DOI] [PubMed] [Google Scholar]
- 9.Glahder CM, Appel PWU, Asmund G. 1999. Mercury in Soap in Tanzania: NERI Technical Report No. 306. Aarhus, Denmark: National Environmental Research Institute. [Google Scholar]
- 10.Hamann CR, Boonchai W, Wen L, Sakanashi EN, Chu C-Y, Hamann K, et al. 2014. Spectrometric analysis of mercury content in 549 skin-lightening products: is mercury toxicity a hidden global health hazard? J Am Acad Dermatol 70(2):281–287.e3, PMID: , 10.1016/j.jaad.2013.09.050. [DOI] [PubMed] [Google Scholar]
- 11.Ladizinski B, Mistry N, Kundu RV. 2011. Widespread use of toxic skin lightening compounds: medical and psychosocial aspects. Dermatol Clin 29(1):111–123, PMID: , 10.1016/j.det.2010.08.010. [DOI] [PubMed] [Google Scholar]
- 12.Chen J, Ye Y, Ran M, Li Q, Ruan Z, Jin N. 2020. Inhibition of tyrosinase by mercury chloride: spectroscopic and docking studies. Front Pharmacol 11:81, PMID: , 10.3389/fphar.2020.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Denton CR, Lerner AB, Fitzpatrick TB. 1952. Inhibition of melanin formation by chemical agents. J Invest Dermatol 18(2):119–135, PMID: , 10.1038/jid.1952.16. [DOI] [PubMed] [Google Scholar]
- 14.Lerner AB. 1952. Effect of ions on melanin formation. J Invest Dermatol 18(1):47–52, PMID: , 10.1038/jid.1952.6. [DOI] [PubMed] [Google Scholar]
- 15.Olumide YM, Akinkugbe AO, Altraide D, Mohammed T, Ahamefule N, Ayanlowo S, et al. 2008. Complications of chronic use of skin lightening cosmetics. Int J Dermatol 47(4):344–353, PMID: , 10.1111/j.1365-4632.2008.02719.x. [DOI] [PubMed] [Google Scholar]
- 16.Fisher JF. 2003. Elemental Mercury and Inorganic Mercury Compounds: Human Health Aspects. Geneva, Switzerland: World Health Organization. [Google Scholar]
- 17.Bose-O’Reilly S, McCarty KM, Steckling N, Lettmeier B. 2010. Mercury exposure and children’s health. Curr Probl Pediatr Adolesc Health Care 40(8):186–215, PMID: , 10.1016/j.cppeds.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Counter SA, Buchanan LH. 2004. Mercury exposure in children: a review. Toxicol Appl Pharmacol 198(2):209–230, PMID: , 10.1016/j.taap.2003.11.032. [DOI] [PubMed] [Google Scholar]
- 19.Dadzie OE, Petit A. 2009. Skin bleaching: highlighting the misuse of cutaneous depigmenting agents. J Eur Acad Dermatol Venereol 23(7):741–750, PMID: , 10.1111/j.1468-3083.2009.03150.x. [DOI] [PubMed] [Google Scholar]
- 20.Lewis KM, Robkin N, Gaska K, Njoki LC. 2011. Investigating motivations for women’s skin bleaching in Tanzania. Psychol Women Q 35(1):29–37, 10.1177/0361684310392356. [DOI] [Google Scholar]
- 21.Osei M, Ali M, Owusu A, Baiden F. 2018. Skin-lightening practices among female high school students in Ghana. Public Health 155:81–87, PMID: , 10.1016/j.puhe.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 22.Traore A, Kadeba J-C, Niamba P, Barro F, Ouedraogo L. 2005. Use of cutaneous depigmenting products by women in two towns in Burkina Faso: epidemiologic data, motivations, products and side effects. Int J Dermatol 44(s1):30–32, PMID: , 10.1111/j.1365-4632.2005.02807.x. [DOI] [PubMed] [Google Scholar]
- 23.Peltzer K, Pengpid S, James C. 2016. The globalization of whitening: prevalence of skin lighteners (or bleachers) use and its social correlates among university students in 26 countries. Int J Dermatol 55(2):165–172, PMID: , 10.1111/ijd.12860. [DOI] [PubMed] [Google Scholar]
- 24.Glenn EN. 2008. Yearning for lightness: transnational circuits in the marketing and consumption of skin lighteners. Gend Soc 22(3):281–302, 10.1177/0891243208316089. [DOI] [Google Scholar]
- 25.Uram E, Bischofer BP, Hagermann S. 2010. Market Analysis of Some Mercury Containing Products and Their Mercury-Free Alternatives in Selected Regions. https://ipen.org/sites/default/files/documents/market_analysis_mercury-containing_products_alternatives-en.pdf [accessed 8 November 2022].
- 26.Murphy T, Slotton DG, Irvine K, Sukontason K, Goldman CR. 2009. Mercury contamination of skin whiteners in Cambodia. Hum Ecol Risk Assess 15(6):1286–1303, 10.1080/10807030903306877. [DOI] [Google Scholar]
- 27.Prevodnik A, Willcox A, Lymberidi-Settimo E, Bender M, Lane O. 2018. Mercury-Added Skin Lightening Creams. Brussels, Belgium: European Environmental Bureau. [Google Scholar]
- 28.UNEP. 2019. Minamata Convention Text and Annexes. https://www.mercuryconvention.org/en/resources/minamata-convention-mercury-text-and-annexes [accessed 30 January 2021].
- 29.U.S. Food and Drug Administration (U.S. FDA). 2020. FDA’s Testing of Cosmetics for Arsenic, Cadmium, Chromium, Cobalt, Lead, Mercury, and Nickel Content. https://www.fda.gov/cosmetics/potential-contaminants-cosmetics/fdas-testing-cosmetics-arsenic-cadmium-chromium-cobalt-lead-mercury-and-nickel-content [accessed 30 January 2021].
- 30.Government of Canada. 2012. Guidance on Heavy Metal Impurities in Cosmetics. Government of Canada. https://www.canada.ca/en/health-canada/services/consumer-product-safety/reports-publications/industry-professionals/guidance-heavy-metal-impurities-cosmetics.html#a324 [accessed 30 January 2021].
- 31.OHAT (National Toxicology Program Office of Health Assessment and Translation). 2015. OHAT Risk of Bias Rating Tool for Human and Animal Studies. https://ntp.niehs.nih.gov/ntp/ohat/pubs/riskofbiastool_508.pdf [accessed 8 November 2022].
- 32.Sheehan MC, Burke TA, Navas-Acien A, Breysse PN, McGready J, Fox MA. 2014. Global methylmercury exposure from seafood consumption and risk of developmental neurotoxicity: a systematic review. Bull World Health Organ 92(4):254–269F, PMID: , 10.2471/BLT.12.116152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.U.S. EPA. Regional Guidance on Handling Chemical Concentration Data Near the Detection Limit in Risk Assessments. https://www.epa.gov/risk/regional-guidance-handling-chemical-concentration-data-near-detection-limit-risk-assessments [accessed 10 February 2021].
- 34.Sagoe D, Pallesen S, Dlova NC, Lartey M, Ezzedine K, Dadzie O. 2019. The global prevalence and correlates of skin bleaching: a meta-analysis and meta-regression analysis. Int J Dermatol 58(1):24–44, PMID: , 10.1111/ijd.14052. [DOI] [PubMed] [Google Scholar]
- 35.Hu XF, Singh K, Chan LHM. 2018. Mercury exposure, blood pressure, and hypertension: a systematic review and dose–response meta-analysis. Environ Health Perspect 126(7):076002, PMID: , 10.1289/EHP2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alghamdi KM. 2010. The use of topical bleaching agents among women: a cross-sectional study of knowledge, attitude and practices. J Eur Acad Dermatol Venereol 24(10):1214–1219, PMID: , 10.1111/j.1468-3083.2010.03629.x. [DOI] [PubMed] [Google Scholar]
- 37.Alanzi ME, Alghamdi RA, Alsharif OM, Alghamdi KS, El Sayed SM. 2018. Health knowledge, cosmetic interests, attitude, and the need for health education regarding the use of topical bleaching agents among women in west Saudi Arabia: a cross-sectional study. J Cosmet Sci 69(2):101–120, PMID: . [PubMed] [Google Scholar]
- 38.Brinton LA, Figueroa JD, Ansong D, Nyarko KM, Wiafe S, Yarney J, et al. 2018. Skin lighteners and hair relaxers as risk factors for breast cancer: results from the Ghana breast health study. Carcinogenesis 39(4):571–579, PMID: , 10.1093/carcin/bgy002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harada M, Nakachi S, Tasaka K, Sakashita S, Muta K, Yanagida K, et al. 2001. Wide use of skin-lightening soap may cause mercury poisoning in Kenya. Sci Total Environ 269(1–3):183–187, PMID: , 10.1016/S0048-9697(00)00812-3. [DOI] [PubMed] [Google Scholar]
- 40.Sendrasoa FA, Ranaivo IM, Andrianarison M, Raharolahy O, Razanakoto NH, Ramarozatovo LS, et al. 2017. Misuse of topical corticosteroids for cosmetic purpose in Antananarivo, Madagascar. Biomed Res Int 2017:9637083, PMID: , 10.1155/2017/9637083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kinabo C. 2005. Comparative analysis of mercury content in human hair and cosmetic products used in Dar es Salaam, Tanzania. Tanzania J Sci 31(1):83–90, 10.4314/tjs.v31i1.18412. [DOI] [Google Scholar]
- 42.Atadokpédé F, Adégbidi H, Koudoukpo C, Téclessou J, Aholoukpé C, Degboé B, et al. 2015. Epidemiological and clinical aspects of skin bleaching in secondary school in Bohicon, Benin. J Cosmet Dermatological Sci and Applications 5:1–6, 10.4236/jcdsa.2015.51001. [DOI] [Google Scholar]
- 43.Lartey M, Krampa FD, Abdul-Rahman M, Quarcoo NL, Yamson P, Hagan PG, et al. 2017. Use of skin-lightening products among selected urban communities in Accra, Ghana. Int J Dermatol 56(1):32–39, PMID: , 10.1111/ijd.13449. [DOI] [PubMed] [Google Scholar]
- 44.Ly F, Soko AS, Dione DA, Niang SO, Kane A, Bocoum TI, et al. 2007. Aesthetic problems associated with the cosmetic use of bleaching products. Int J Dermatol 46(suppl 1):15–17, PMID: , 10.1111/j.1365-4632.2007.03456.x. [DOI] [PubMed] [Google Scholar]
- 45.Abbas HH, Sakakibara M, Sera K, Nurgahayu,Andayanie E. 2020. Mercury exposure and health problems of the students using skin-lightening cosmetic products in Makassar, South Sulawesi, Indonesia. Cosmetics 7(3):58, 10.3390/cosmetics7030058. [DOI] [Google Scholar]
- 46.Mahe A, Ly F, Aymard G, Dangou JM. 2003. Skin diseases associated with the cosmetic use of bleaching products in women from Dakar, Senegal. Br J Dermatol 148(3):493–500, PMID: , 10.1046/j.1365-2133.2003.05161.x. [DOI] [PubMed] [Google Scholar]
- 47.McRill C, Boyer LV, Flood TJ, Ortega L. 2000. Mercury toxicity due to use of a cosmetic cream. J Occup Environ Med 42(1):4–7, PMID: , 10.1097/00043764-200001000-00004. [DOI] [PubMed] [Google Scholar]
- 48.Sin KW, Tsang HF. 2003. Large-scale mercury exposure due to a cream cosmetic: community-wide case series. Hong Kong Med J 9(5):329–334, PMID: . [PubMed] [Google Scholar]
- 49.Weldon MM, Smolinski MS, Maroufi A, Hasty BW, Gilliss DL, Boulanger LL, et al. 2000. Mercury poisoning associated with a Mexican beauty cream. West J Med 173(1):15–8; discussion 19, PMID: , 10.1136/ewjm.173.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fakour H, Esmaili-Sari A. 2014. Occupational and environmental exposure to mercury among Iranian hairdressers. J Occup Health 56(1):56–61, PMID: , 10.1539/joh.13-0008-oa. [DOI] [PubMed] [Google Scholar]
- 51.Kanwal S, Yamakawa A, Narukawa T, Yoshinaga J. 2019. Speciation and isotopic characterization of mercury detected at high concentration in Pakistani hair samples. Chemosphere 233:705–710, PMID: , 10.1016/j.chemosphere.2019.05.275. [DOI] [PubMed] [Google Scholar]
- 52.Ricketts P, Knight C, Gordon A, Boischio A, Voutchkov M. 2020. Mercury exposure associated with use of skin lightening products in Jamaica. J Health Pollut 10(26):200601, PMID: , 10.5696/2156-9614-10.26.200601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ori MR, Larsen JB, Shirazi FM. 2018. Mercury poisoning in a toddler from home contamination due to Skin- Lightening cream. J Pediatr 196:314–317.e1, PMID: , 10.1016/j.jpeds.2017.12.023. [DOI] [PubMed] [Google Scholar]
- 54.Copan L, Fowles J, Barreau T, McGee N. 2015. Mercury toxicity and contamination of households from the use of skin creams adulterated with mercurous chloride (calomel). Int J Environ Res Public Health 12(9):10943–10954, PMID: , 10.3390/ijerph120910943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kamani A, Au H, Copes R, Thompson M, Ito S. 2019. Pediatric mercury poisoning from indirect exposure to skin cream. Clin Toxicol 57(10):1011. [Google Scholar]
- 56.Acquavella JF, Alexander BH, Mandel JS, Gustin C, Baker B, Chapman P, et al. 2004. Glyphosate biomonitoring for farmers and their families: results from the farm family exposure study. Environ Health Perspect 112(3):321–326, PMID: , 10.1289/ehp.6667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Maneli MH, Wiesner L, Tinguely C, Davids LM, Spengane Z, Smith P, et al. 2016. Combinations of potent topical steroids, mercury and hydroquinone are common in internationally manufactured skin-lightening products: a spectroscopic study. Clin Exp Dermatol 41(2):196–201, PMID: , 10.1111/ced.12720. [DOI] [PubMed] [Google Scholar]
- 58.Michalek IM, Benn EKT, dos Santos FLC, Gordon S, Wen C, Liu B. 2019. A systematic review of global legal regulations on the permissible level of heavy metals in cosmetics with particular emphasis on skin lightening products. Environ Res 170:187–193, PMID: , 10.1016/j.envres.2018.12.029. [DOI] [PubMed] [Google Scholar]
- 59.Michalek IM, Liu B, Benn EKT, Caetano dos Santos FL. 2019. Skin lightning products’ violations in Europe: an analysis of the rapid alert system for dangerous non-food products 2005–2018. Regul Toxicol Pharmacol 106:50–54, PMID: , 10.1016/j.yrtph.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 60.Peregrino CP, Moreno MV, Miranda SV, Rubio AD, Leal LO. 2011. Mercury levels in locally manufactured Mexican skin-lightening creams. Int J Environ Res Public Health 8(6):2516–2523, PMID: , 10.3390/ijerph8062516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.WHO. 2018. Assessment of Prenatal Exposure to Mercury: Standard Operating Procedures. https://www.who.int/publications/i/item/9789240002845 [accessed 8 November 2022].
- 62.Nuttall KL. 2006. Interpreting hair mercury levels in individual patients. Ann Clin Lab Sci 36(3):248–261, PMID: . [PubMed] [Google Scholar]
- 63.Ye B-J, Kim B-G, Jeon M-J, Kim S-Y, Kim H-C, Jang T-W, et al. 2016. Evaluation of mercury exposure level, clinical diagnosis and treatment for mercury intoxication. Ann Occup Environ Med 28(1):5–8, PMID: , 10.1186/s40557-015-0086-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lindstedt G, Gottberg I, Holmgren B, Jonsson T, Karlsson G. 1979. Individual mercury exposure of chloralkali workers and its relation to blood and urinary mercury levels. Scand J Work Environ Health 5(1):59–69, PMID: , 10.5271/sjweh.2665. [DOI] [PubMed] [Google Scholar]
- 65.McComb JQ, Rogers C, Han FX, Tchounwou PB. 2014. Rapid screening of heavy metals and trace elements in environmental samples using portable X-ray fluorescence spectrometer, a comparative study. Water Air Soil Pollut 225(12):, PMID: , 10.1007/s11270-014-2169-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McKelvey W, Jeffery N, Clark N, Kass D, Parsons PJ. 2011. Population-based inorganic mercury biomonitoring and the identification of skin care products as a source of exposure in New York City. Environ Health Perspect 119(2):203–209, PMID: , 10.1289/ehp.1002396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang L, Zhang H. 2015. Mercury content in marketed cosmetics: analytical survey in Shijiazhuang, China. Cutan Ocul Toxicol 34(4):322–326, PMID: , 10.3109/15569527.2014.994123. [DOI] [PubMed] [Google Scholar]
- 68.Gbetoh MH, Amyot M. 2016. Mercury, hydroquinone and clobetasol propionate in skin lightening products in West Africa and Canada. Environ Res 150:403–410, PMID: , 10.1016/j.envres.2016.06.030. [DOI] [PubMed] [Google Scholar]
- 69.Ho Y, Bin Abdullah NH, Hamsan H, Tan ESS. 2017. Mercury contamination in facial skin lightening creams and its health risks to user. Regul Toxicol Pharmacol 88:72–76, PMID: , 10.1016/j.yrtph.2017.05.018. [DOI] [PubMed] [Google Scholar]
- 70.Mohammed T, Mohammed E, Bascombe S. 2017. The evaluation of total mercury and arsenic in skin bleaching creams commonly used in Trinidad and Tobago and their potential risk to the people of the Caribbean. J Public Health Res 6(3):1097, PMID: , 10.4081/jphr.2017.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Selvaraju A, Abdul Halim ANS, Keyon ASA. 2020. Determination of selected heavy metal concentrations in unregistered face whitening creams sold in Johor Bahru, Johor, Malaysia by using inductively coupled plasma optical emission spectroscopy and their health risk assessment. Malaysian J Anal Sci 24(5):670–681. [Google Scholar]
- 72.Agorku ES, Kwaansa-Ansah EE, Voegborlo RB, Amegbletor P, Opoku F. 2016. Mercury and hydroquinone content of skin toning creams and cosmetic soaps, and the potential risks to the health of Ghanaian women. Springerplus 5:319, PMID: , 10.1186/s40064-016-1967-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Zainy FM. 2015. Determination of heavy metals in 16 bleaching creams and 3 mixtures of bleaching creams from local market of Jeddah. Egypt J Chem 58(3):377–386, 10.21608/EJCHEM.2015.1020. [DOI] [Google Scholar]
- 74.Al-Saleh I, Elkhatib R, Al-Rouqi R, Al-Enazi S, Shinwari N. 2012. The dangers of skin-lightening creams. Toxicol Environ Chem 94(1):195–219, 10.1080/02772248.2011.631925. [DOI] [Google Scholar]
- 75.Yang H-H, Chen P-Y, Ho P-H, Shih Y. 2014. Direct analysis of mercury in cosmetics using screen-printed silver electrodes and flow injection analysis. J Electrochem Soc 161(6):B137–B142, 10.1149/2.057406jes. [DOI] [Google Scholar]
- 76.Salama AK. 2015. Assessment of metals in cosmetics commonly used in Saudi Arabia. Environ Monit Assess 188(10):553, PMID: , 10.1007/s10661-016-5550-6. [DOI] [PubMed] [Google Scholar]
- 77.Ashraf T, Taneez M, Kalsoom S, Irfan T, Shafique MA. 2021. Experimental calculations of metals content in skin-whitening creams and theoretical investigation for their biological effect against tyrosinase enzyme. Biol Trace Elem Res 199(9):3562–3569, PMID: , 10.1007/s12011-020-02441-z. [DOI] [PubMed] [Google Scholar]
- 78.Voegborlo R, Voegborlo S, Buabeng-Acheampong B, Zogli E. 2008. Total mercury content of skin toning creams and the potential risk to the health of women in Ghana. J Sci Technol 28(1):88–96, 10.4314/just.v28i1.33081. [DOI] [Google Scholar]
- 79.Alqadami AA, Naushad M, Abdalla MA, Khan MR, Alothman ZA, Wabaidur SM, et al. 2017. Determination of heavy metals in skin-whitening cosmetics using microwave digestion and inductively coupled plasma atomic emission spectrometry. IET Nanobiotechnol 11(5):597–603, PMID: , 10.1049/iet-nbt.2016.0212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Alqadami AA, Abdalla MA, Alothman ZA, Omer K. 2013. Application of solid phase extraction on multiwalled carbon nanotubes of some heavy metal ions to analysis of skin whitening cosmetics using ICP-AES. Int J Environ Res Public Health 10(1):361–374, PMID: , 10.3390/ijerph10010361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ababneh FA, Al-Momani IF. 2018. Assessments of toxic heavy metals contamination in cosmetic products. Environ Forensics 19(2):134–142, 10.1080/15275922.2018.1448908. [DOI] [Google Scholar]
- 82.Amponsah D, Voegborlo R, Sebiawu GE. 2014. Determination of amount of mercury in some selected skin-lightening creams sold in the Ghanaian market. Int J Eng Res Technol 3(6):344–350, 10.17577/IJERTV3IS060437. [DOI] [Google Scholar]
- 83.Cristaudo A, D’Ilio S, Gallinella B, Mosca A, Majorani C, Violante N, et al. 2013. Use of potentially harmful skin-lightening products among immigrant women in Rome, Italy: a pilot study. Dermatology 226(3):200–206, PMID: , 10.1159/000348706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.